Abstract
Atrial fibrillation is a significant worldwide contributor to cardiovascular morbidity and mortality. Few studies have investigated the differences in gene expression between the left and right atrial appendages, leaving their characterization largely unexplored. In this study, differential gene expression was investigated in atrial fibrillation and sinus rhythm using left and right atrial appendages from the same patients. RNA sequencing was performed on the left and right atrial appendages from five sinus rhythm (SR) control patients and five permanent AF case patients. Differential gene expression in both the left and right atrial appendages was analyzed using the Bioconductor package edgeR. A selection of differentially expressed genes, with relevance to atrial fibrillation, were further validated using quantitative RT-PCR. The distribution of the samples assessed through principal component analysis showed distinct grouping between left and right atrial appendages and between SR controls and AF cases. Overall 157 differentially expressed genes were identified to be downregulated and 90 genes upregulated in AF. Pathway enrichment analysis indicated a greater involvement of left atrial genes in the Wnt signaling pathway whereas right atrial genes were involved in clathrin-coated vesicle and collagen formation. The differing expression of genes in both left and right atrial appendages indicate that there are different mechanisms for development, support and remodeling of AF within the left and right atria.
Keywords: atrial fibrillation, differential gene expression, right and left atria, RNA sequencing
INTRODUCTION
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia worldwide and a significant contributor to cardiovascular morbidity and mortality. Increased incidence of AF is associated with increasing age and male sex as well as other risk factors, such as hypertension, diabetes, obesity, and smoking, that act to induce structural and histopathological changes to the atrium subsequently increasing susceptibility to AF (39). AF is characterized by high-frequency excitation of the atrium resulting in both dys-synchronous atrial contraction and irregular ventricular excitation (39). Initiation of AF is likely triggered via abnormal calcium handling that causes spontaneous myocyte depolarization in and around the pulmonary veins. Re-entry and ectopic impulse formation then promote the stabilization of AF (23, 39) with the left atria playing a predominant role in AF initiation and maintenance (23). Subsequent changes in atrial structure and function due to remodeling either by altered ion channel expression and/or fibrosis increase re-entry and vulnerability to AF (23, 39).
The genetics of AF is complex with both rare and common variants that increase susceptibility to AF being described (9). Familial AF has been linked to various mutations in K+ and Na+ channel genes and their accessory proteins; for example, mutations in KCNJ2, KCNE1, and SCN5A have been identified (9, 39). More recently, genome-wide association studies (GWAS) have identified around 200 loci, which may also be involved in AF susceptibility (10, 14, 19, 30, 31, 34, 38, 39). For example, the PITX2 locus on chromosome 4q25 has been associated with AF. The PITX2 gene encodes the paired-like homeodomain transcription factor that is one of the isoforms expressed in the heart and may play a role in the transcription regulation of atrial regulation genes (9). Other examples are ZFHX3 and NEURL; decrease in ZFHX3 expression decreases the levels of Ca2+ and shortens the action potential duration (39). The knockdown of NEURL, an E3 ubiquitin ligase, ortholog in zebrafish led to significant prolongation of the atrial action potential (38). Other loci associated with AF include HCN4, KCNN3, and PRRX1 (9, 10, 14, 38, 39)
Previous studies have used either left atrial appendage (LAA) (12, 21, 35, 41) or right atrial appendage (RAA) (3, 8, 15) samples to study gene expression and microRNAs differences in atrial fibrillation. There are limited studies using both the left and right atrial appendages (11, 22). In this study, we use RNA sequencing (RNA-Seq) to assess differential gene expression in AF using paired left and right atrial appendage samples. Understanding any differences in expression may help to further define the pathophysiology of AF.
MATERIALS AND METHODS
Patient selection.
Patients undergoing coronary artery bypass grafting and/or atrial/mitral valve repair or replacement at Barts Heart Centre, Barts Health National Health Service (NHS) Trust gave informed consent for samples of their left and right atrial appendages to be taken during surgery. Initially the samples were collected as part of the Inflammation in Atrial Fibrillation Study at Barts Health NHS Trust [approved by the East London Research Ethics Committee (10/H0704/43)] and latterly in collaboration with the National Institutes for Health Research (NIHR) Barts Biomedical Research Centre Cardiovascular Bio-Registry (approved by the East of England-Cambridge Central Research Ethics Committee (14/EE/0007), https://www.qmul.ac.uk/whri/research/core-facilities/nihr-bioinformatics-and-bio-repository/). All patients were nondiabetic males with a heart rhythm classified as permanent AF or a normal regular heart rhythm, sinus rhythm (SR). Patients in SR who developed postoperative AF were excluded from the study. Samples were immediately immersed in RNAlater to preserve RNA quality. From the samples obtained, two groups were formed. The first group comprised left and right atrial appendage from five SR control patients and five AF patients (Table 1, top). This group was used for RNA-Seq and quantitative RT-PCR validation. A second group that comprised left and right atrial appendage from six SR control patients and seven AF patients (Table 1, bottom) was used for independent replication by quantitative RT-PCR. Due to the difficulty in obtaining left atrial appendage from some AF patients, this independent validation group was formed of four left atrial appendage and seven right atrial appendage samples.
Table 1.
Summary of patient characteristics for group 1 and group 2
| Characteristic | SR | AF |
|---|---|---|
| Group 1 | ||
| Patients, n | 5 | 5 |
| Sex, male/female | 5, 0 | 5, 0 |
| Average age, yr | 62.4 ± 6.87 | 73.6 ± 5.12 |
| CAD, n | 4 | 2 |
| MVD/AVD, n | 1 | 4 |
| CAD + MVD/AVD, n | 0 | 1 |
| Group 2 | ||
| Patients, n | 6 | 7 |
| Sex, male/female | 6, 0 | 7, 0 |
| Average age, yr | 70.5 ± 11.57 | 71.7 ± 9.99 |
| CAD, n | 5 | 0 |
| MVD/AVD, n | 1 | 4 |
| CAD + MVD/AVD, n | 0 | 2 |
| Other, n | 0 | 1 |
SR, sinus rhythm; AF, atrial fibrillation; CAD, coronary artery disease; MVD/AVD, mitral valve disease/atrial valve disease.
Isolation of RNA from human left and right atrial appendage.
RNA was isolated from 30 mg of human left and right atrial appendages, with a RNeasy mini tissue kit (Qiagen). RNA was also subjected to DNase treatment. The resulting concentration of RNA was determined by NanoDrop 1000 or Agilent Bioanalyzer before downstream applications. Only RNA of suitable quality (i.e., RNA integrity number > 8, rRNA ratio (28s/18s) >2, concentration > 50 nl/µl) was used for RNA sequencing.
RNA sequencing and differential gene expression analysis of group 1 samples.
Libraries were generated with TruSeq Stranded Total RNA library prep with RiboZero (Illumina). RNA sequencing was performed at the Queen Mary University of London Genome Centre (http://www.smd.qmul.ac.uk/gc/) with Illumina NextSeq500 with >100 million short reads generated per sample. The quality of the sequencing reads (fastq files) was assessed by FastQC (version 0.11.2). The reads were trimmed for adaptor sequences and poor quality reads with Trim Galore (version 0.3.7). Two trimming phases were applied, the first to remove adaptors and the second to remove poly G sequences. The quality of the trimmed sequences was reassessed with FastQC (version 0.11.2). After satisfactory quality control, trimmed sequences were aligned to the coding regions of the human reference genome (GRCh37) with TopHat2 (version 2.0.13) and bowtie2 (version 2.2.3). Transcript abundance was then calculated by HTSeq-counts software (version 0.6.0) (2). Unadjusted transcript abundance was then exported to the R environment (version 3.1.2) for exploratory data analysis and differential expression analyses. The principal component analysis (PCA) and distance between samples from DESeq2 (version 1.6.3) were used to assess the dispersion and categorization of samples. Differential expression analysis was investigated with edgeR (version_3.8.6) (28). Genes with low counts and expressed in only one sample per category were removed from further analysis (n = 14,823 investigated genes). The calcNormFactors function was used to calculate the normalization factors to account for library sizes. Samples were then investigated for differences either between SR and AF or differences between the left and right atrium. Dispersion was calculated by using the functions estimateCommonDisp and estimateTagwiseDisp. The exact test was applied (exactTest) to obtain genes differentially expressed between SR and AF samples for either LAA or RAA. The data has been deposited in the National Center for Biotechnology Information’s Gene Expression Omnibus (GEO) (13) and are accessible through GEO Series accession number GSE128188 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE128188).
Downstream and enrichment analyses.
Downstream analyses were performed on the differentially expressed genes with a false discovery rate (FDR) of 5% or less. We investigated transcription, pathway, and cell type enrichment with EnrichR (http://amp.pharm.mssm.edu/Enrichr) (5, 25). Furthermore, we have queried the GWAS catalog and the Stanford Global Biobank Engine to identify variants associated to AF that were also differentially expressed in this RNA-Seq study.
Quantitative real-time PCR.
RNA was converted to cDNA with the High Capacity cDNA reverse transcriptase kit (Applied Biosystems). We used 50 ng of cDNA for quantitative RT-PCR, which was performed with customized TaqMan gene expression assays (Applied Biosystems). The following commercially available TaqMan gene expression assays (Thermo Fisher Scientific) were used: MYH7 (Hs01110632_m1), GRIA1 (Hs00181348_m1), KCNJ2 (Hs01876357_s1), CACNA1G (Hs00367969_m1), MT1X (Hs00745167_sH), AGTR1 (Hs00258938_m1), BMP7 (Hs00233476_m1), PPP1R1A (Hs00410058_m1), COMP (Hs00164359_m1), SYTL5 (Hs00371091_m1), ATP1B4 (Hs00201320_m1), KCNK2 (Hs01005159_m1), GREM1 (Hs01879841_s1), GPR183 (Hs00270639_s1), GPR83 (Hs00173906_m1). Each gene was assayed in triplicate. Relative expression was calculated by using the comparative CT method and normalized to GAPDH. Final data are presented as a relative change compared with control. Student’s t-test was used and a P value of <0.05 deemed significant.
RESULTS
Comparison of left and right atrial appendage RNA sequence data across AF cases and SR controls.
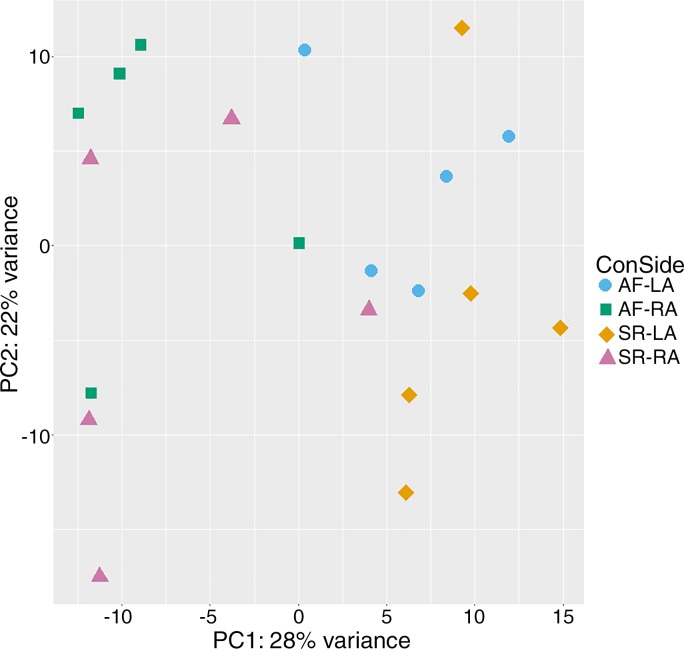
The patient characteristics of group 1 are described in Table 1, top. Differential gene expression analysis using edgeR was performed for AF cases and SR controls. PCA analysis and inspection of the first two principal components illustrate the presence of four groups of samples; corresponding to SR-LAA, SR-RAA, AF-LAA, and AF-RAA (Fig. 1). The PCA plot shows samples from the AF cases are clustered on the top region of the plot and differentiating between left and right atrial appendage, indicating a similarity between AF samples but at the same time a distinction between the two atrial appendages (left and right). The control group, SR-LAA, generally clustered together. However, the SR-RAA samples showed more variability. A similar pattern is shown in the heat map (Supplementary Fig. S1; for all supplementary material see https://figshare.com/s/8483d57e6e79fe95e3c9). Typically, similar biological samples would be expected to cluster together as we see for the AF samples and generally for the SR samples. Further observation of the sample variability can also be visualized by MD plots and volcano plots (Supplementary Figs. S2 and S3).
Fig. 1.
PCA (Principal Component Analysis) plot generated from DeSeq2 showing variation within and between groups. Groups are differentiated by different shapes: atrial fibrillation (AF)-left atrium (LA) (circles), AF-right atrium (RA) (squares), sinus rhythm (SR)-LA (diamonds) and SR-RA (triangles).
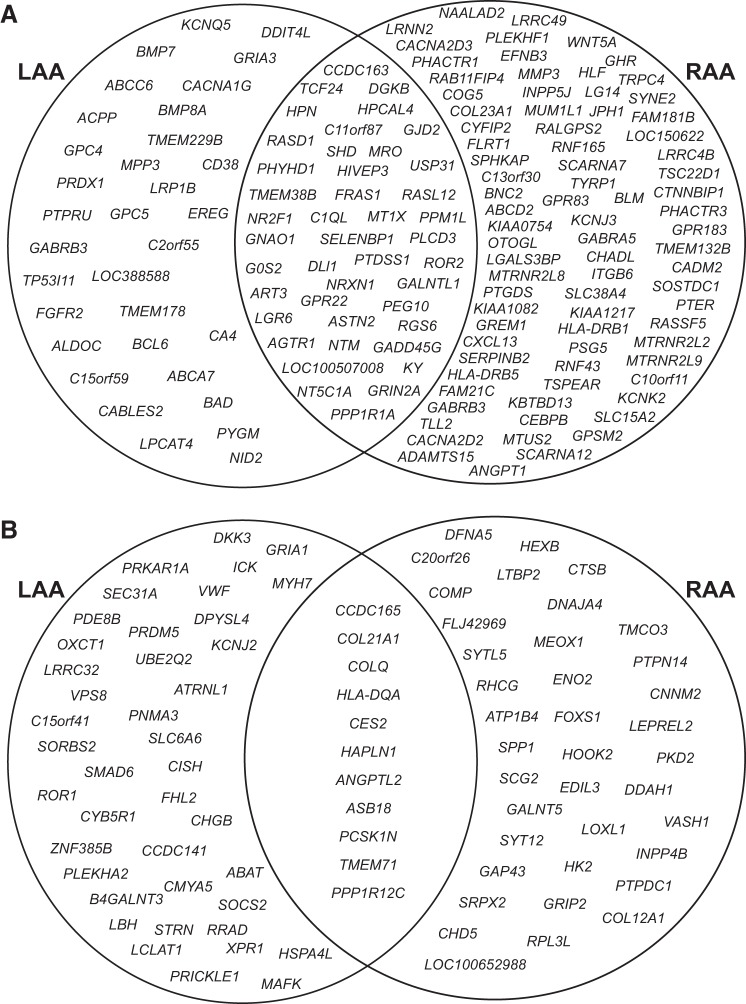
Using a 5% FDR threshold, we found 128 genes to be differentially expressed within the LAA between the SR controls and the AF cases (Supplementary Table S1). Of these 52 genes were upregulated and 76 were downregulated in AF. In the RAA, 173 genes were differentially expressed with a 5% FDR threshold between the SR controls and the AF cases (Supplementary Table S2). Of these, 49 genes were upregulated and 124 genes were downregulated in AF. Overall from both LAA and RAA, 157 genes were found to be downregulated in AF within the 5% FDR threshold. Of these, 27% (43 genes) of these were downregulated in both AF-LAA and AF-RAA. In the AF-LAA, 21% (33 genes) were found to be downregulated, whereas a greater proportion of genes, 52% (81 genes), were found to be downregulated in the AF-RAA. A smaller number of upregulated genes were identified (90 genes) to be within the 5% FDR threshold, and only 12% (11 genes) of these were upregulated in both AF-LAA and AF-RAA. The proportion of genes that were upregulated in either AF-LAA or AF-RAA, 46% (41 genes) vs. 42% (38 genes) respectively was more equally spread (Fig. 2). The gene expression hierarchical clustering of differentially expressed genes [FDR ≤5%, log fold change (LogFC) ≥1] from LAA and RAA is shown in Supplementary Figs. S4 and S5 respectively.
Fig. 2.
Venn diagram showing the genes identified by edgeR within a 5% false discovery rate (FDR) threshold in AF_LAA and AF_RAA. A: downregulated genes in AF_LAA and AF_RAA. Percentage of downregulated genes; AF_LAA = 21%, AF_RAA = 52% and overlapping = 27%. B: upregulated genes in AF_LAA and AF_RAA. Percentage of upregulated genes; AF_LAA = 46%, AF_RAA = 42% and overlapping = 12%. LAA, left atrial appendage; RAA, right atrial appendage.
Validation by quantitative RT-PCR of select differentially expressed genes.
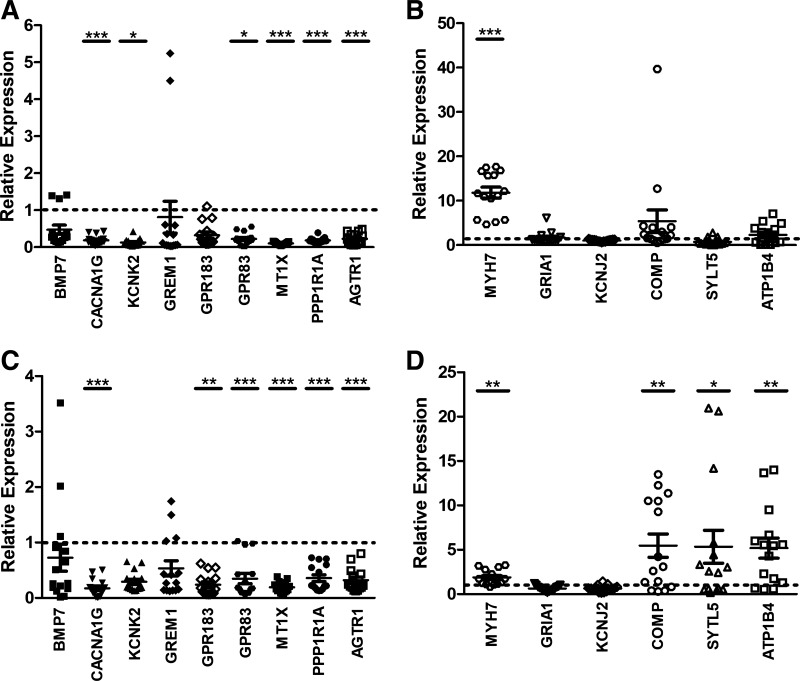
Down- and upregulated genes with relevance to AF differentially expressed below the 5% FDR threshold and with a logFC ≥ 1 (Table 2) were selected for further validation by quantitative RT-PCR in group 1 samples. In brief, downregulated genes in AF in both LAA and RAA (MT1X, AGTR1, and PPP1R1A), LAA (BMP7 and CACNA1G), and RAA (GPR183, GPR83, GREM1, and KCNK2) were selected. Upregulated genes in AF in LAA (MYH7, GRIA1, and KCNJ2) and RAA (ATP1B4, COMP, and STYL5) were also selected. The results of the quantitative RT-PCR generally follow the differential expression observed with the RNA-Seq data (Fig. 3, Table 2). The expression of AGTR1, MT1X ,and PPP1R1A in both AF-LAA and AF-RAA was significantly decreased as was CACNA1G gene expression in AF-LAA and GPR183 and GPR83 gene expression in AF-RAA (Fig. 3, A and C). This was consistent with a significant decrease in the expression of these genes in the edgeR differential expression analysis between the LAA of SR and AF and the RAA of SR and AF. The gene expression of MYH7 in AF-LAA and COMP, STYL5, and ATP1B4 in the AF-RAA was significantly increased, consistent with the increase in gene expression shown by edgeR differential expression analysis between the LAA of SR and AF and the RAA of SR and AF (Fig. 3, B and D). However, there were some exceptions. BMP7 showed upregulation and GRIA1 and KCNJ2 downregulation in AF-LAA in the edgeR differential expression analysis (Table 2), whereas quantitative RT-PCR did not detect any significant changes in expression (Fig. 3, A and B). Similarly the edgeR differential expression analysis indicated that KCNK2 and GREM1 would be significantly downregulated in AF-RAA; however, this was not shown by quantitative RT-PCR (Fig. 3C). Using the counts from edgeR analysis, we find a high level of variation within each SR and AF LAA and RAA set for these genes. Reads per kilobase million (RPKM) values from the GTEx database (18a) indicate that these genes also have relatively low levels of expression within the RAA. Therefore, less sensitive methods of detection such as quantitative RT-PCR may not be able to detect these small levels and their subsequent changes. Interestingly, MYH7 was significantly increased, and GPR83 and CACNA1G were found to be significantly decreased across both AF-LAA and AF-RAA (Fig. 3, A, C, and D).
Table 2.
Genes of interest identified from EdgeR differential expression analysis
| LAA |
RAA |
||||
|---|---|---|---|---|---|
| Genes | Gene Description | logFC | FDR | logFC | FDR |
| ↓ AF | |||||
| AGTR1 | angiotensin II receptor, type 1 | −1.05572565 | 0.0010381 | −1.065913918 | 0.00102674 |
| BMP7 | bone morphogenetic protein 7 | −1.19765233 | 0.03885687 | −0.889386931 | 0.20239137 |
| CACNA1G | calcium channel, voltage-dependent, T type, alpha 1G subunit | −2.18806413 | 0.0001968 | −1.330503299 | 0.06975416 |
| GPR183 | G protein-coupled receptor 183 | −0.93572806 | 0.60331171 | −1.763467958 | 0.01939096 |
| GPR83 | G protein-coupled receptor 83 | −1.1245247 | 0.29476026 | −1.689411363 | 0.01328969 |
| GREM1 | gremlin 1, DAN family BMP antagonist | −0.50658071 | 1 | −3.8855265 | 0.00049114 |
| KCNK2 | potassium channel, two pore domain subfamily K, member 2 | −0.66803768 | 1 | −4.602242229 | 0.00047221 |
| MT1X | metallothionein 1X | −2.16408758 | 3.69E-12 | −2.181138902 | 2.32E-12 |
| PPP1R1A | protein phosphatase 1, regulatory (inhibitor) subunit 1A | −1.09647493 | 0.01272078 | −1.282069342 | 0.00128686 |
| ↑ AF | |||||
| ATP1B4 | ATPase, Na+/K+ transporting, beta 4 polypeptide | 1.783999548 | 0.1494246 | 3.019022579 | 0.00097388 |
| COMP | cartilage oligomeric matrix protein | 1.990304087 | 0.29370875 | 3.801287286 | 0.00131533 |
| GRIA1 | glutamate receptor, ionotropic, AMPA 1 | 1.362915243 | 0.03885687 | 0.902033132 | 0.31616717 |
| KCNJ2 | potassium channel, inwardly rectifying subfamily J, member 2 | 1.155221064 | 0.02582826 | 0.436049293 | 0.83833001 |
| MYH7 | myosin heavy chain, cardiac muscle Beta | 2.671060777 | 8.05E-05 | 0.894323788 | 0.58464821 |
| SYTL5 | synaptotagmin-like 5 | 2.241927172 | 0.17114464 | 3.585616039 | 0.00104004 |
Values in boldface are those below the 5% FDR threshold. LAA, left atrial appendage; RAA, right atrial appendage; FC, fold change; FDR, false discovery rate.
Fig. 3.
Normalized quantitative RT-PCR results of LAA and RAA of group 1. A: left atrial appendage samples with downregulated genes. B: left atrial appendage samples with upregulated genes. C: right atrial appendage samples with downregulated genes. D: right atrial appendage samples with upregulated genes. Individual normalized values for AF samples (n = 5) are represented by different symbols. The mean ± SE for each gene is also represented. The dotted line represents the normalized sinus rhythm value of 1. Student’s t-test was used to calculate significance (*P < 0.05, **P < 0.01, ***P < 0.001).
Independent replication.
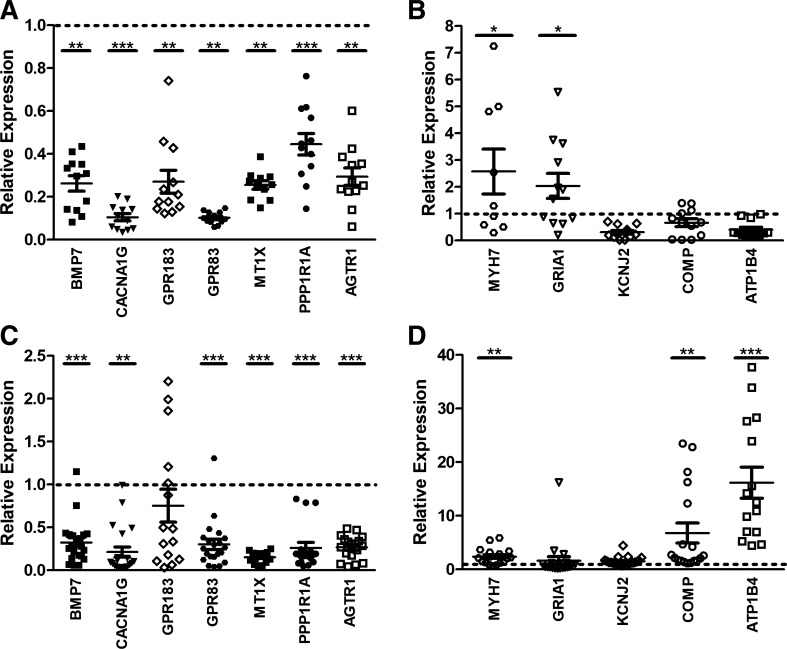
To confirm differential expression of selected candidate genes, we used quantitative RT-PCR to test samples from an independent cohort (group 2, Table 1, bottom). KCNK2, GREM1, and SYTL5 were removed from the candidate genes set due to low levels of expression in the validation cohort and high variability observed in the count data. The expression pattern (Fig. 4) was found to be the same as group 1 for the following genes: MT1X, PPP1R1A, AGTR1, CACNA1G, GPR83, MYH7, COMP, and ATP1B4. However, in group 2, a significant increase in GRIA1 was observed in AF-LAA, while BMP7 was significantly decreased in both AF-LAA and AF-RAA. Expression of both GRIA1 and BMP7 was not observed in the validation group, group 1. However the increased GRIA1 gene expression and decreased BMP7 gene expression observed within this independent group, group 2, are consistent with the edgeR differential expression analysis data. No decrease in expression was observed for GPR183 in AF-RAA in this group. This may be accounted for by varying mRNA levels of these genes in different patient samples.
Fig. 4.
Normalized quantitative RT-PCR results of LAA and RAA of group 2. A: left atrial appendage samples with downregulated genes. B: left atrial appendage samples with upregulated genes. C: right atrial appendage samples with downregulated genes. D: right atrial appendage samples with upregulated genes. Individual normalized values for AF samples (n = 4–7) are represented by different symbols. The mean ± SE for each gene is also represented. The dotted line represents the normalized sinus rhythm value of 1. Student’s t-test was used to calculate significance (*P < 0.05, **P < 0.01, ***P < 0.001).
Enrichment analysis.
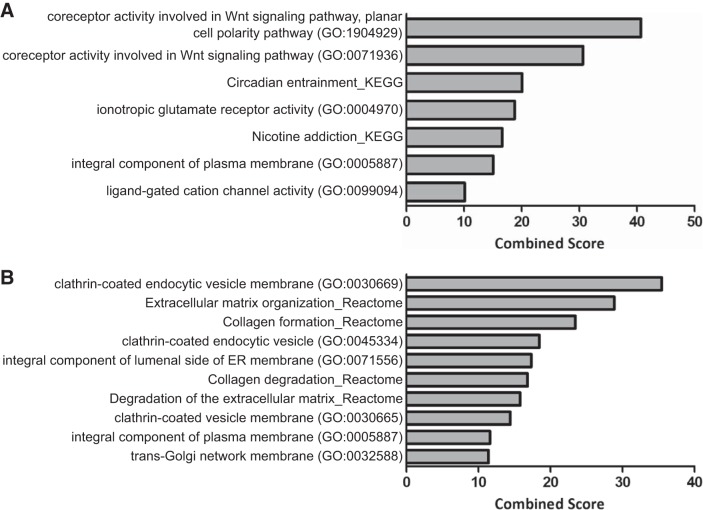
Enrichment analyses were conducted on the genes differentially expressed at a 5% FDR between SR and AF samples for the left and right atrial appendage. Supplementary Table S3 presents the enrichment results with an adjusted P value ≤0.10 for Reactome, KEGG, gene ontology, and MGI mammalian phenotype. The enriched ontologies and pathways differed greatly between appendages, with only five categories shared between the left and the right atrial appendage. These five categories consisted of the neuronal system; the transmission across chemical synapses; the WNT5A-dependent internalization of FZD2, FZD5, and ROR2 Reactome pathways; the integral component of plasma membrane gene ontology term; and the hyperactivity term in the mammalian phenotype models. The differentially expressed genes in the LAA showed enrichment on the circadian entrainment and nicotine addiction pathways and the coreceptor activity involved in the Wnt signaling pathways and ionotropic glutamate receptor gene ontology terms (Fig. 5A). The RAA showed enrichment in the extracellular matrix organization and collagen formation and collagen degradation pathways and the clathrin-coated endocytic vesicle membrane gene ontology term (Fig. 5B). The enriched KEGG pathways were analyzed further by comparing the results between left and right atrial appendage (Supplementary Table S4). As observed in the overall enrichment results, there is much variability between the enrichment observed in the KEGG results for the left and right atrial appendages. In addition, to highlight pathways specific to each atrial appendage, genes that are only differentially expressed between SR and AF in each specific atrial appendage were investigated (i.e., genes only observed to be differentially expressed in the RAA, or only differentially expressed in the LAA). The results were consistent with the overall analysis, where a range of extracellular matrix pathways are enriched in the RAA, including collagen formation and degradation and clathrin-coated endocytic vesicle membrane gene ontology terms. In the LAA, enrichment in the circadian entrainment and nicotine addiction pathways and in the coreceptor activity involved in the Wnt signaling pathway, transforming growth factor-β (TGF-β) receptor binding, and BMP receptor binding gene ontology terms are observed (Supplementary Table S5).
Fig. 5.
Enrichment analysis on differentially expressed genes. A: bar graph of LAA edgeR results with 5% FDR showing the top pathways (Reactome and KEGG) and gene ontology (GO) with a combined Score ≥ 10. B: bar graph of RAA edgeR results with 5% FDR showing the top pathways (Reactome and KEGG) and gene ontology (GO) with a combined Score ≥ 10.
Furthermore, we queried the GWAS catalog and the Stanford Global Biobank Engine for variants reported to be associated with “atrial fibrillation.” More than 250 variants mapping to 212 genes are found to be associated with AF at a genome wide significance threshold of P value ≤5e-08 (Supplementary Table S6). Seven of these genes were found to show differential expression in AF-LAA and/or AF-RAA in this edgeR data set. MYH7 and KCNJ2 were upregulated in AF-LAA; RPL3L was upregulated in AF-RAA; COG5, SYNE2, and C10orf11 were downregulated in AF-RAA; and DGKB was downregulated in both AF-LAA and AF-RAA.
DISCUSSION
In this study, 247 genes were identified to be differentially expressed between SR and AF. The initial cohort consisted of the left and right atrial appendage of five patients with permanent AF and five patients in SR, the control group. Additionally, the average age of the AF group was found to be ~10 yr more than that of the SR group; this was expected as the incidence of AF increases with advancing age (39). All patients in the study were selected to be nondiabetic as there is evidence that pathogenesis of AF is also caused by structural and electrical changes similar to those observed in the hearts of diabetic patients (40). The selection of only nondiabetic patients meant that changes observed would be more likely due to progression of AF.
Most of the studies that have been previously published have been limited by using either left or right atrial appendage (3, 8, 12, 15, 21, 35, 41). One of the main advantages of this study is that it allowed for the direct comparison of genes expressed in the left and right atria in AF. Specifically it revealed significant differential expression in left and right atrial appendages in SR and AF. Only 12 and 27% of genes were up and downregulated respectively in both AF-LAA and AF-RAA, indicating that there are a greater number of genes with altered expression on only one side of the atrium. This supports the potential for significant differences in biology between the two chambers and in how they remodel in AF. Although the left atrium is thought to have a more significant role in the initiation and maintenance of AF (38), overall a greater number of genes were identified to be differentially expressed in the right atrium. In particular, 52% of genes were specifically downregulated in AF-RAA compared with 21% of genes downregulated in AF-LAA. These results could suggest that downregulation of genes, particularly in the right atria, plays a greater role in AF than upregulation. However, the variability observed in the PCA (Fig. 1) within the SR-RAA and AF-RAA samples compared with the SR-LAA and AF-LAA samples may result in a higher number of differentially expressed genes identified in the RAA samples.
Pathway and gene ontology enrichment analysis suggest that there are a multitude of pathways involved that differ between the two sides of the atrial appendages. The RAA demonstrates enrichment in pathways involved in extracellular matrix organization and degradation and collagen formation and degradation. Among the genes identified in these pathways are a member of the matrix metalloprotease family, MMP3, cartilage oligomeric matrix protein, COMP, and collagen-encoding genes, COL12A1 and COL23A1. These could play a key role in atrial fibrosis leading to structural remodeling in the right atria and consequently the promotion of AF. Increased expression in AF-RAA in this study of the COMP gene warrants further investigation as increased levels of COMP have also been found in coronary heart disease patients (44). The LAA also shows enrichment for atrial fibrosis via BMP receptor and TGF-β receptor binding. Genes involved in these pathways, the bone morphogenetic proteins, BMP7, BMP8A, and the inhibitory SMAD6 are all involved in the TGF-β signaling pathway contributing to atrial fibrosis. BMP7 has been shown to have a protective effect on fibrosis (6), and its downregulation in AF-LAA in this study may allow for increased fibrosis to occur in the left atria. Additionally in the LAA, circadian entrainment and nicotine addiction pathways involving the key genes, GRIN2A encoding a NMDA receptor subunit NR2A, and GRIA1 and GRIA3, encoding AMPA-sensitive glutamate receptor subunits GluR1 and GluR3, respectively, were identified. It is possible that nerve endings were harvested with the atrial appendage, and these may reflect genes differentially expressed in this tissue. The role of glutamate receptors in AF is unclear; however, there is evidence that activation of NMDA receptors results in an increase in sympathetic activity, decreased heart rate variability, and promotion of AF (37). There is also evidence that the expression of GluR1 glutamate receptors encoded by GRIA1 are altered in ischemic cardiomyopathy (18), and GluR1-containing complexes are associated with cyclic AMP generation via β-adrenergic stimulation that itself may be involved in the initiation and/or maintenance of AF (45). As GRIA1 is increased only in the AF-LAA this may indicate that it does indeed play a role in AF. Further study into the role of these glutamate receptors in atrial fibrillation is necessary. The noncanonical Wnt signaling pathway was also identified to be enriched in the LAA, and the receptor tyrosine kinase orphan receptor 2, ROR2, gene is significantly downregulated in both AF-LAA and AF-RAA in this study.
Given the large variety of genes that were identified from the differential gene analysis and subsequent enrichment analysis a subset of genes including those previously linked to AF and those who had no links were selected for further validation by quantitative RT-PCR to include genes differentially expressed in either LAA and/or RAA. Selected genes that had previously been implicated in AF included the ion channels, CACNA1G (15), KCNK2 (36) and KCNJ2 (9), signaling proteins and receptors, AGTR1 (17) and PPP1R1A (7), heart structural components, MYH7 (31, 34) and BMP7 (6), and a cardio-protective protein, MT1X (24). Abnormal calcium handling plays an important role in AF (20, 29) and the significantly decreased expression in both AF-LAA and AF-RAA of CACNA1G that encodes a T-type calcium channel, Cav3.1 (32), is worth investigating further. Changes in other calcium channels e.g., voltage-gated calcium channels Cav1.2, Cav 1.3, and Cav 3.2, were not found to be significant. It is known that remodeling Cav1.2 is important in chronic AF (16). Our data suggest that these changes as not as pronounced as others that might be occurring with other calcium handling pathways and that transcriptional remodeling might be modest with other posttranscriptional mechanisms in play. Another gene of particular interest is PPP1R1A that encodes for I-1 and acts as an inhibitor of protein phosphatase 1 (PP1) (8). A link with an increase in PP1 activity and increased phosphorylation of ryanodine receptor 2 (RYR2) leading to AF has been identified (7); therefore, the observed downregulation of PPP1R1A and therefore I-1 could very likely play a role in progression of AF. Of the familial genes commonly found in AF (9), only KCNJ2 showed upregulation in AF-LAA. Overexpression of KCNJ2 has been shown to lead to an increase in IK1 that causes hyperpolarization of the resting membrane potential resulting in stabilization and promotion of re-entry leading to sustained AF (46).
Additionally, a selection of genes that have not been previously implicated in AF were also validated. These reflected our personal interests and included orphan G-protein coupled receptors for example. The genes selected were ATP1B4, COMP, GREM1, GRIA1, GPR183, GPR83, and SYTL5. Expression of ATP1B4 was increased only in AF-RAA and encodes a muscle-specific βM protein found in the inner nuclear membrane that may play a role in the regulation of gene expression through TGF-β (33).
In recent years, the number of genes identified by GWAS to be associated with AF has increased from 30 to around 212 potential genes (Supplementary Table S6) (2, 10, 14, 19, 21, 26, 30, 31, 34, 38, 43). Seven of these potential genes were found to be differentially expressed in this edgeR data set. One of these, MYH7 encodes for β-MyHC protein, the heavy chain subunit of cardiac myosin encoding the adult slow twitch isoform, and has recently been identified to be a functional candidate gene for AF involvement (31, 34). Expression of MYH7 has been shown to be increased in atrial myocytes of patients with chronic AF (4), and variants in the gene have been linked to hypertrophic cardiomyopathy, where AF is a known complication (27). In a rabbit ischemic heart failure model, MYH7 expression was only detectable in the failing left atrium with a heterogeneous distribution (31). The increased presence of β-MyHC could cause the atria to become arrhythmogenic and predispose to permanent AF. Other genes found in this study that have previously been associated with AF include SYNE2, which encodes for synaptic nuclear envelope protein 2 (21, 34), and RPL3L, which encodes a ribosomal protein (ribosome protein like 3L) that is specifically expressed in skeletal muscle and the heart unlike most ribosomal proteins, which are ubiquitously expressed (42).
Limitations of this study include the small number of samples available for RNA-Seq. This was due to difficulties during surgery in obtaining both left and right atrial appendages from each patient. However, the benefit of using paired left and right atrial appendages and a high sequencing depth somewhat counteracts a small sample size. Due to practical limitations only a few differentially expressed genes were able to be validated. However, not all of the genes that were selected for validation could be reliably detected by quantitative RT-PCR. RNA-Seq allows for subtle changes in gene expression to be detected as it identifies novel transcripts, spliced isoforms, and splice sites; these changes may not be observed when using quantitative RT-PCR as it is limited to the detection of known sequences. Further studies are required to understand the significance of these genes in AF.
In summary, using RNA-Seq data from left and right atrial appendages of SR and AF patients can give a further insight into potential new genes and pathways that may be involved in the progression of this complex and multifactorial disease. The left and right atria may play different roles in the development, support, and remodeling of AF as shown by the changes in gene expression levels and their involvement in different pathways between AF-LAA and AF-RAA. These data can be used to study these genes and their potential pathways in further detail.
GRANTS
This work was supported by funding from the NIHR Barts Biomedical Research Centre.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.M.T., P.B.M., and A.T. conceived and designed research; A.M.T. and K.W. performed experiments; A.M.T. and C.P.C. analyzed data; A.M.T., C.P.C., C.A.M., M.R.B., P.B.M., and A.T. interpreted results of experiments; A.M.T. and C.P.C. prepared figures; A.M.T., C.P.C., P.B.M., and A.T. drafted manuscript; A.M.T., C.P.C., P.B.M., and A.T. edited and revised manuscript; A.M.T., C.P.C., M.F., K.L., M.N., R.J.S., K.W., C.A.M., M.R.B., P.B.M., and A.T. approved final version of manuscript.
ACKNOWLEDGMENTS
This works forms part of the research areas contributing to the translational research portfolio of the Cardiovascular Biomedical Research Centre at Barts Health NHS Trust, which is supported and funded by the NIHR. Our thanks go to Victoria Baker as part of the Inflammation in Atrial Fibrillation Study at Barts Health NHS Trust for help identifying suitable patients.
Present address for K. Wood: Labcyte Ltd., Cannock, Staffordshire, WS11 9UU, UK.
REFERENCES
- 2.Anders S, Pyl PT, Huber W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 31: 166–169, 2015. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brundel BJ, Van Gelder IC, Henning RH, Tuinenburg AE, Wietses M, Grandjean JG, Wilde AA, Van Gilst WH, Crijns HJ. Alterations in potassium channel gene expression in atria of patients with persistent and paroxysmal atrial fibrillation: differential regulation of protein and mRNA levels for K+ channels. J Am Coll Cardiol 37: 926–932, 2001. doi: 10.1016/S0735-1097(00)01195-5. [DOI] [PubMed] [Google Scholar]
- 4.Cañón S, Caballero R, Herraiz-Martínez A, Pérez-Hernández M, López B, Atienza F, Jalife J, Hove-Madsen L, Delpón E, Bernad A. miR-208b upregulation interferes with calcium handling in HL-1 atrial myocytes: Implications in human chronic atrial fibrillation. J Mol Cell Cardiol 99: 162–173, 2016. doi: 10.1016/j.yjmcc.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, Clark NR, Ma’ayan A. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14: 128, 2013. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X, Xu J, Jiang B, Liu D. Bone Morphogenetic Protein-7 Antagonizes Myocardial Fibrosis Induced by Atrial Fibrillation by Restraining Transforming Growth Factor-β (TGF-β)/Smads Signaling. Med Sci Monit 22: 3457–3468, 2016. doi: 10.12659/MSM.897560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang DY, Heck AJ, Dobrev D, Wehrens XH. Regulating the regulator: Insights into the cardiac protein phosphatase 1 interactome. J Mol Cell Cardiol 101: 165–172, 2016. doi: 10.1016/j.yjmcc.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiang DY, Zhang M, Voigt N, Alsina KM, Jakob H, Martin JF, Dobrev D, Wehrens XH, Li N. Identification of microRNA-mRNA dysregulations in paroxysmal atrial fibrillation. Int J Cardiol 184: 190–197, 2015. doi: 10.1016/j.ijcard.2015.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christophersen IE, Ellinor PT. Genetics of atrial fibrillation: from families to genomes. J Hum Genet 61: 61–70, 2016. doi: 10.1038/jhg.2015.44. [DOI] [PubMed] [Google Scholar]
- 10.Christophersen IE, Rienstra M, Roselli C, Yin X, Geelhoed B, Barnard J, Lin H, Arking DE, Smith AV, Albert CM, Chaffin M, Tucker NR, Li M, Klarin D, Bihlmeyer NA, Low SK, Weeke PE, Müller-Nurasyid M, Smith JG, Brody JA, Niemeijer MN, Dörr M, Trompet S, Huffman J, Gustafsson S, Schurmann C, Kleber ME, Lyytikäinen LP, Seppälä I, Malik R, Horimoto ARVR, Perez M, Sinisalo J, Aeschbacher S, Thériault S, Yao J, Radmanesh F, Weiss S, Teumer A, Choi SH, Weng LC, Clauss S, Deo R, Rader DJ, Shah SH, Sun A, Hopewell JC, Debette S, Chauhan G, Yang Q, Worrall BB, Paré G, Kamatani Y, Hagemeijer YP, Verweij N, Siland JE, Kubo M, Smith JD, Van Wagoner DR, Bis JC, Perz S, Psaty BM, Ridker PM, Magnani JW, Harris TB, Launer LJ, Shoemaker MB, Padmanabhan S, Haessler J, Bartz TM, Waldenberger M, Lichtner P, Arendt M, Krieger JE, Kähönen M, Risch L, Mansur AJ, Peters A, Smith BH, Lind L, Scott SA, Lu Y, Bottinger EB, Hernesniemi J, Lindgren CM, Wong JA, Huang J, Eskola M, Morris AP, Ford I, Reiner AP, Delgado G, Chen LY, Chen YI, Sandhu RK, Li M, Boerwinkle E, Eisele L, Lannfelt L, Rost N, Anderson CD, Taylor KD, Campbell A, Magnusson PK, Porteous D, Hocking LJ, Vlachopoulou E, Pedersen NL, Nikus K, Orho-Melander M, Hamsten A, Heeringa J, Denny JC, Kriebel J, Darbar D, Newton-Cheh C, Shaffer C, Macfarlane PW, Heilmann-Heimbach S, Almgren P, Huang PL, Sotoodehnia N, Soliman EZ, Uitterlinden AG, Hofman A, Franco OH, Völker U, Jöckel KH, Sinner MF, Lin HJ, Guo X, Dichgans M, Ingelsson E, Kooperberg C, Melander O, Loos RJF, Laurikka J, Conen D, Rosand J, van der Harst P, Lokki ML, Kathiresan S, Pereira A, Jukema JW, Hayward C, Rotter JI, März W, Lehtimäki T, Stricker BH, Chung MK, Felix SB, Gudnason V, Alonso A, Roden DM, Kääb S, Chasman DI, Heckbert SR, Benjamin EJ, Tanaka T, Lunetta KL, Lubitz SA, Ellinor PT; METASTROKE Consortium of the ISGC; Neurology Working Group of the CHARGE Consortium; AFGen Consortium . Large-scale analyses of common and rare variants identify 12 new loci associated with atrial fibrillation. Nat Genet 49: 946–952, 2017. [Erratum in Nat Genet 49: 1286, 2017] doi: 10.1038/ng.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooley N, Cowley MJ, Lin RC, Marasco S, Wong C, Kaye DM, Dart AM, Woodcock EA. Influence of atrial fibrillation on microRNA expression profiles in left and right atria from patients with valvular heart disease. Physiol Genomics 44: 211–219, 2012. doi: 10.1152/physiolgenomics.00111.2011. [DOI] [PubMed] [Google Scholar]
- 12.Deshmukh A, Barnard J, Sun H, Newton D, Castel L, Pettersson G, Johnston D, Roselli E, Gillinov AM, McCurry K, Moravec C, Smith JD, Van Wagoner DR, Chung MK. Left atrial transcriptional changes associated with atrial fibrillation susceptibility and persistence. Circ Arrhythm Electrophysiol 8: 32–41, 2015. doi: 10.1161/CIRCEP.114.001632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30: 207–210, 2002. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellinor PT, Lunetta KL, Albert CM, Glazer NL, Ritchie MD, Smith AV, Arking DE, Müller-Nurasyid M, Krijthe BP, Lubitz SA, Bis JC, Chung MK, Dörr M, Ozaki K, Roberts JD, Smith JG, Pfeufer A, Sinner MF, Lohman K, Ding J, Smith NL, Smith JD, Rienstra M, Rice KM, Van Wagoner DR, Magnani JW, Wakili R, Clauss S, Rotter JI, Steinbeck G, Launer LJ, Davies RW, Borkovich M, Harris TB, Lin H, Völker U, Völzke H, Milan DJ, Hofman A, Boerwinkle E, Chen LY, Soliman EZ, Voight BF, Li G, Chakravarti A, Kubo M, Tedrow UB, Rose LM, Ridker PM, Conen D, Tsunoda T, Furukawa T, Sotoodehnia N, Xu S, Kamatani N, Levy D, Nakamura Y, Parvez B, Mahida S, Furie KL, Rosand J, Muhammad R, Psaty BM, Meitinger T, Perz S, Wichmann HE, Witteman JCM, Kao WHL, Kathiresan S, Roden DM, Uitterlinden AG, Rivadeneira F, McKnight B, Sjögren M, Newman AB, Liu Y, Gollob MH, Melander O, Tanaka T, Stricker BHC, Felix SB, Alonso A, Darbar D, Barnard J, Chasman DI, Heckbert SR, Benjamin EJ, Gudnason V, Kääb S. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet 44: 670–675, 2012. doi: 10.1038/ng.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaborit N, Steenman M, Lamirault G, Le Meur N, Le Bouter S, Lande G, Léger J, Charpentier F, Christ T, Dobrev D, Escande D, Nattel S, Demolombe S. Human atrial ion channel and transporter subunit gene-expression remodeling associated with valvular heart disease and atrial fibrillation. Circulation 112: 471–481, 2005. doi: 10.1161/CIRCULATIONAHA.104.506857. [DOI] [PubMed] [Google Scholar]
- 16.Gan TY, Qiao W, Xu GJ, Zhou XH, Tang BP, Song JG, Li YD, Zhang J, Li FP, Mao T, Jiang T. Aging-associated changes in L-type calcium channels in the left atria of dogs. Exp Ther Med 6: 919–924, 2013. doi: 10.3892/etm.2013.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goette A, Arndt M, Röcken C, Spiess A, Staack T, Geller JC, Huth C, Ansorge S, Klein HU, Lendeckel U. Regulation of angiotensin II receptor subtypes during atrial fibrillation in humans. Circulation 101: 2678–2681, 2000. doi: 10.1161/01.CIR.101.23.2678. [DOI] [PubMed] [Google Scholar]
- 18.Gronich N, Kumar A, Zhang Y, Efimov IR, Soldatov NM. Molecular remodeling of ion channels, exchangers and pumps in atrial and ventricular myocytes in ischemic cardiomyopathy. Channels (Austin) 4: 101–107, 2010. doi: 10.4161/chan.4.2.10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.GTEx Consortium ; Lonsdale J, Thomas J, Salvatore M, Phillips R, Lo E, Shad S, Hasz R, Walters G, Garcia F, Young N, Foster B, Moser M, Karasik E, Gillard B, Ramsey K, Sullivan S, Bridge J, Magazine H, Syron J, Fleming J, Siminoff L, Traino H, Mosavel M, Barker L, Jewell S, Rohrer D, Maxim D, Filkins D, Harbach P, Cortadillo E, Berghuis B, Turner L, Hudson E, Feenstra K, Sobin L, Robb J, Branton P, Korzeniewski G, Shive C, Tabor D, Qi L, Groch K, Nampally S, Buia S, Zimmerman A, Smith A, Burges R, Robinson K, Valentino K, Bradbury D, Cosentino M, Diaz-Mayoral N, Kennedy M, Engel T, Williams P, Erickson K, Ardlie K, Winckler W, Getz G, DeLuca D, MacArthur D, Kellis M, Thomson A, Young T, Gelfand E, Donovan M, Meng Y, Grant G, Mash D, Marcus Y, Basile M, Liu J, Zhu J, Tu Z, Cox NJ, Nicolae DL, Gamazon ER, Im HK, Konkashbaev A, Pritchard J, Stevens M, Flutre T, Wen X, Dermitzakis ET, Lappalainen T, Guigo R, Monlong J, Sammeth M, Koller D, Battle A, Mostafavi S, McCarthy M, Rivas M, Maller J, Rusyn I, Nobel A, Wright F, Shabalin A, Feolo M, Sharopova N, Sturcke A, Paschal J, Anderson JM, Wilder EL, Derr LK, Green ED, Struewing JP, Temple G, Volpi S, Boyer JT, Thomson EJ, Guyer MS, Ng C, Abdallah A, Colantuoni D, Insel TR, Koester SE, Little AR, Bender PK, Lehner T, Yao Y, Compton CC, Vaught JB, Sawyer S, Lockhart NC, Demchok J, Moore HF. The Genotype-Tissue Expression (GTEx) project. Nat Genet 45: 580–585, 2013. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gudbjartsson DF, Sulem P, Helgason H, Gylfason A, Gudjonsson SA, Zink F, Oddson A, Magnusson G, Halldorsson BV, Hjartarson E, Sigurdsson GT, Kong A, Helgason A, Masson G, Magnusson OT, Thorsteinsdottir U, Stefansson K. Sequence variants from whole genome sequencing a large group of Icelanders. Sci Data 2: 150011, 2015. doi: 10.1038/sdata.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hove-Madsen L, Llach A, Bayes-Genís A, Roura S, Rodriguez Font E, Arís A, Cinca J. Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic reticulum in human atrial myocytes. Circulation 110: 1358–1363, 2004. doi: 10.1161/01.CIR.0000141296.59876.87. [DOI] [PubMed] [Google Scholar]
- 21.Hsu J, Gore-Panter S, Tchou G, Castel L, Lovano B, Moravec CS, Pettersson GB, Roselli EE, Gillinov AM, McCurry KR, Smedira NG, Barnard J, Van Wagoner DR, Chung MK, Smith JD. Genetic Control of Left Atrial Gene Expression Yields Insights into the Genetic Susceptibility for Atrial Fibrillation. Circ Genom Precis Med 11: e002107, 2018. doi: 10.1161/CIRCGEN.118.002107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu J, Hanna P, Van Wagoner DR, Barnard J, Serre D, Chung MK, Smith JD. Whole genome expression differences in human left and right atria ascertained by RNA sequencing. Circ Cardiovasc Genet 5: 327–335, 2012. doi: 10.1161/CIRCGENETICS.111.961631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation 124: 2264–2274, 2011. doi: 10.1161/CIRCULATIONAHA.111.019893. [DOI] [PubMed] [Google Scholar]
- 24.Kharlap MS, Goriunova LE, Timofeeva AV, Smolianova GG, Khaspekov GL, Raskin VA, Dzemeshkevich SL, Akchurin RS, Golitsyn SP, Bibilashvili RS. [Gene expression analysis in myocytes of right atrial appendages in patients with atrial fibrillation using cDNA microarray technique]. Kardiologiia 48: 34–42, 2008. [PubMed] [Google Scholar]
- 25.Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, Koplev S, Jenkins SL, Jagodnik KM, Lachmann A, McDermott MG, Monteiro CD, Gundersen GW, Ma’ayan A. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res 44, W1: W90–W97, 2016. doi: 10.1093/nar/gkw377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Low SK, Takahashi A, Ebana Y, Ozaki K, Christophersen IE, Ellinor PT, Ogishima S, Yamamoto M, Satoh M, Sasaki M, Yamaji T, Iwasaki M, Tsugane S, Tanaka K, Naito M, Wakai K, Tanaka H, Furukawa T, Kubo M, Ito K, Kamatani Y, Tanaka T; AFGen Consortium . Identification of six new genetic loci associated with atrial fibrillation in the Japanese population. Nat Genet 49: 953–958, 2017. doi: 10.1038/ng.3842. [DOI] [PubMed] [Google Scholar]
- 27.Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet 381: 242–255, 2013. doi: 10.1016/S0140-6736(12)60397-3. [DOI] [PubMed] [Google Scholar]
- 28.McCarthy DJ, Chen Y, Smyth GK. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res 40: 4288–4297, 2012. doi: 10.1093/nar/gks042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nattel S, Dobrev D. The multidimensional role of calcium in atrial fibrillation pathophysiology: mechanistic insights and therapeutic opportunities. Eur Heart J 33: 1870–1877, 2012. doi: 10.1093/eurheartj/ehs079. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen JB, Fritsche LG, Zhou W, Teslovich TM, Holmen OL, Gustafsson S, Gabrielsen ME, Schmidt EM, Beaumont R, Wolford BN, Lin M, Brummett CM, Preuss MH, Refsgaard L, Bottinger EP, Graham SE, Surakka I, Chu Y, Skogholt AH, Dalen H, Boyle AP, Oral H, Herron TJ, Kitzman J, Jalife J, Svendsen JH, Olesen MS, Njølstad I, Løchen ML, Baras A, Gottesman O, Marcketta A, O’Dushlaine C, Ritchie MD, Wilsgaard T, Loos RJF, Frayling TM, Boehnke M, Ingelsson E, Carey DJ, Dewey FE, Kang HM, Abecasis GR, Hveem K, Willer CJ. Genome-wide Study of Atrial Fibrillation Identifies Seven Risk Loci and Highlights Biological Pathways and Regulatory Elements Involved in Cardiac Development. Am J Hum Genet 102: 103–115, 2018. doi: 10.1016/j.ajhg.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nielsen JB, Thorolfsdottir RB, Fritsche LG, Zhou W, Skov MW, Graham SE, Herron TJ, McCarthy S, Schmidt EM, Sveinbjornsson G, Surakka I, Mathis MR, Yamazaki M, Crawford RD, Gabrielsen ME, Skogholt AH, Holmen OL, Lin M, Wolford BN, Dey R, Dalen H, Sulem P, Chung JH, Backman JD, Arnar DO, Thorsteinsdottir U, Baras A, O’Dushlaine C, Holst AG, Wen X, Hornsby W, Dewey FE, Boehnke M, Kheterpal S, Mukherjee B, Lee S, Kang HM, Holm H, Kitzman J, Shavit JA, Jalife J, Brummett CM, Teslovich TM, Carey DJ, Gudbjartsson DF, Stefansson K, Abecasis GR, Hveem K, Willer CJ. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat Genet 50: 1234–1239, 2018. doi: 10.1038/s41588-018-0171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez-Reyes E, Cribbs LL, Daud A, Lacerda AE, Barclay J, Williamson MP, Fox M, Rees M, Lee JH. Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature 391: 896–900, 1998. doi: 10.1038/36110. [DOI] [PubMed] [Google Scholar]
- 33.Pestov NB, Zhao H, Basrur V, Modyanov NN. Isolation and characterization of BetaM protein encoded by ATP1B4--a unique member of the Na,K-ATPase β-subunit gene family. Biochem Biophys Res Commun 412: 543–548, 2011. doi: 10.1016/j.bbrc.2011.07.112. [DOI] [PubMed] [Google Scholar]
- 34.Roselli C, Chaffin MD, Weng LC, Aeschbacher S, Ahlberg G, Albert CM, Almgren P, Alonso A, Anderson CD, Aragam KG, Arking DE, Barnard J, Bartz TM, Benjamin EJ, Bihlmeyer NA, Bis JC, Bloom HL, Boerwinkle E, Bottinger EB, Brody JA, Calkins H, Campbell A, Cappola TP, Carlquist J, Chasman DI, Chen LY, Chen YI, Choi EK, Choi SH, Christophersen IE, Chung MK, Cole JW, Conen D, Cook J, Crijns HJ, Cutler MJ, Damrauer SM, Daniels BR, Darbar D, Delgado G, Denny JC, Dichgans M, Dörr M, Dudink EA, Dudley SC, Esa N, Esko T, Eskola M, Fatkin D, Felix SB, Ford I, Franco OH, Geelhoed B, Grewal RP, Gudnason V, Guo X, Gupta N, Gustafsson S, Gutmann R, Hamsten A, Harris TB, Hayward C, Heckbert SR, Hernesniemi J, Hocking LJ, Hofman A, Horimoto ARVR, Huang J, Huang PL, Huffman J, Ingelsson E, Ipek EG, Ito K, Jimenez-Conde J, Johnson R, Jukema JW, Kääb S, Kähönen M, Kamatani Y, Kane JP, Kastrati A, Kathiresan S, Katschnig-Winter P, Kavousi M, Kessler T, Kietselaer BL, Kirchhof P, Kleber ME, Knight S, Krieger JE, Kubo M, Launer LJ, Laurikka J, Lehtimäki T, Leineweber K, Lemaitre RN, Li M, Lim HE, Lin HJ, Lin H, Lind L, Lindgren CM, Lokki ML, London B, Loos RJF, Low SK, Lu Y, Lyytikäinen LP, Macfarlane PW, Magnusson PK, Mahajan A, Malik R, Mansur AJ, Marcus GM, Margolin L, Margulies KB, März W, McManus DD, Melander O, Mohanty S, Montgomery JA, Morley MP, Morris AP, Müller-Nurasyid M, Natale A, Nazarian S, Neumann B, Newton-Cheh C, Niemeijer MN, Nikus K, Nilsson P, Noordam R, Oellers H, Olesen MS, Orho-Melander M, Padmanabhan S, Pak HN, Paré G, Pedersen NL, Pera J, Pereira A, Porteous D, Psaty BM, Pulit SL, Pullinger CR, Rader DJ, Refsgaard L, Ribasés M, Ridker PM, Rienstra M, Risch L, Roden DM, Rosand J, Rosenberg MA, Rost N, Rotter JI, Saba S, Sandhu RK, Schnabel RB, Schramm K, Schunkert H, Schurman C, Scott SA, Seppälä I, Shaffer C, Shah S, Shalaby AA, Shim J, Shoemaker MB, Siland JE, Sinisalo J, Sinner MF, Slowik A, Smith AV, Smith BH, Smith JG, Smith JD, Smith NL, Soliman EZ, Sotoodehnia N, Stricker BH, Sun A, Sun H, Svendsen JH, Tanaka T, Tanriverdi K, Taylor KD, Teder-Laving M, Teumer A, Thériault S, Trompet S, Tucker NR, Tveit A, Uitterlinden AG, Van Der Harst P, Van Gelder IC, Van Wagoner DR, Verweij N, Vlachopoulou E, Völker U, Wang B, Weeke PE, Weijs B, Weiss R, Weiss S, Wells QS, Wiggins KL, Wong JA, Woo D, Worrall BB, Yang PS, Yao J, Yoneda ZT, Zeller T, Zeng L, Lubitz SA, Lunetta KL, Ellinor PT. Multi-ethnic genome-wide association study for atrial fibrillation. Nat Genet 50: 1225–1233, 2018. doi: 10.1038/s41588-018-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruan Z, Sun X, Sheng H, Zhu L. Long non-coding RNA expression profile in atrial fibrillation. Int J Clin Exp Pathol 8: 8402–8410, 2015. [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt C, Wiedmann F, Kallenberger SM, Ratte A, Schulte JS, Scholz B, Müller FU, Voigt N, Zafeiriou MP, Ehrlich JR, Tochtermann U, Veres G, Ruhparwar A, Karck M, Katus HA, Thomas D. Stretch-activated two-pore-domain (K2P) potassium channels in the heart: Focus on atrial fibrillation and heart failure. Prog Biophys Mol Biol 130, Pt B: 233–243, 2017. doi: 10.1016/j.pbiomolbio.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Shi S, Liu T, Wang D, Zhang Y, Liang J, Yang B, Hu D. Activation of N-methyl-d-aspartate receptors reduces heart rate variability and facilitates atrial fibrillation in rats. Europace 19: 1237–1243, 2017. [DOI] [PubMed] [Google Scholar]
- 38.Sinner MF, Tucker NR, Lunetta KL, Ozaki K, Smith JG, Trompet S, Bis JC, Lin H, Chung MK, Nielsen JB, Lubitz SA, Krijthe BP, Magnani JW, Ye J, Gollob MH, Tsunoda T, Müller-Nurasyid M, Lichtner P, Peters A, Dolmatova E, Kubo M, Smith JD, Psaty BM, Smith NL, Jukema JW, Chasman DI, Albert CM, Ebana Y, Furukawa T, Macfarlane PW, Harris TB, Darbar D, Dörr M, Holst AG, Svendsen JH, Hofman A, Uitterlinden AG, Gudnason V, Isobe M, Malik R, Dichgans M, Rosand J, Van Wagoner DR, METASTROKE Consortium; AFGen Consortium; Benjamin EJ, Milan DJ, Melander O, Heckbert SR, Ford I, Liu Y, Barnard J, Olesen MS, Stricker BH, Tanaka T, Kääb S, Ellinor PT. Integrating genetic, transcriptional, and functional analyses to identify 5 novel genes for atrial fibrillation. Circulation 130: 1225–1235, 2014. doi: 10.1161/CIRCULATIONAHA.114.009892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH. Atrial Fibrillation: Epidemiology, Pathophysiology, and Clinical Outcomes. Circ Res 120: 1501–1517, 2017. doi: 10.1161/CIRCRESAHA.117.309732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tadic M, Cuspidi C. Type 2 diabetes mellitus and atrial fibrillation: From mechanisms to clinical practice. Arch Cardiovasc Dis 108: 269–276, 2015. doi: 10.1016/j.acvd.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Tan N, Chung MK, Smith JD, Hsu J, Serre D, Newton DW, Castel L, Soltesz E, Pettersson G, Gillinov AM, Van Wagoner DR, Barnard J. Weighted gene coexpression network analysis of human left atrial tissue identifies gene modules associated with atrial fibrillation. Circ Cardiovasc Genet 6: 362–371, 2013. doi: 10.1161/CIRCGENETICS.113.000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thorolfsdottir RB, Sveinbjornsson G, Sulem P, Nielsen JB, Jonsson S, Halldorsson GH, Melsted P, Ivarsdottir EV, Davidsson OB, Kristjansson RP, Thorleifsson G, Helgadottir A, Gretarsdottir S, Norddahl G, Rajamani S, Torfason B, Valgardsson AS, Sverrisson JT, Tragante V, Holmen OL, Asselbergs FW, Roden DM, Darbar D, Pedersen TR, Sabatine MS, Willer CJ, Løchen M-L, Halldorsson BV, Jonsdottir I, Hveem K, Arnar DO, Thorsteinsdottir U, Gudbjartsson DF, Holm H, Stefansson K. Coding variants in RPL3L and MYZAP increase risk of atrial fibrillation. Commun Biol 1: 68, 2018. doi: 10.1038/s42003-018-0068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsai FC, Lin YC, Chang SH, Chang GJ, Hsu YJ, Lin YM, Lee YS, Wang CL, Yeh YH. Differential left-to-right atria gene expression ratio in human sinus rhythm and atrial fibrillation: Implications for arrhythmogenesis and thrombogenesis. Int J Cardiol 222: 104–112, 2016. doi: 10.1016/j.ijcard.2016.07.103. [DOI] [PubMed] [Google Scholar]
- 44.Wang FF, Ha L, Yu HY, Mi L, Han JL, Gao W. Altered serum level of cartilage oligomeric matrix protein and its association with coronary calcification in patients with coronary heart disease. J Geriatr Cardiol 14: 87–92, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Workman AJ. Cardiac adrenergic control and atrial fibrillation. Naunyn Schmiedebergs Arch Pharmacol 381: 235–249, 2010. doi: 10.1007/s00210-009-0474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xia M, Jin Q, Bendahhou S, He Y, Larroque MM, Chen Y, Zhou Q, Yang Y, Liu Y, Liu B, Zhu Q, Zhou Y, Lin J, Liang B, Li L, Dong X, Pan Z, Wang R, Wan H, Qiu W, Xu W, Eurlings P, Barhanin J, Chen Y. A Kir2.1 gain-of-function mutation underlies familial atrial fibrillation. Biochem Biophys Res Commun 332: 1012–1019, 2005. doi: 10.1016/j.bbrc.2005.05.054. [DOI] [PubMed] [Google Scholar]