Abstract
The extracellular vesicles (EVs) are membrane vesicles carrying proteins, nucleic acids, and bioactive lipids of the cell of origin. These vesicles released within the extracellular space and entering into the circulation may transfer their cargo to neighboring or distant cells and induce phenotypical and functional changes that may be relevant in several physiopathological conditions. In an attempt to define the biological properties of EVs, several investigations have focused on their cargo and on the effects elicited in recipient cells. EVs have been involved in modulation of tumor microenvironment and behavior, as well as in the immune and inflammatory response. In the present review, we address the paracrine action of EVs released by stem cells and their potential involvement in the activation of regenerative programs in injured cells.
Keywords: cancer cells, exosomes, immune cells, microvesicles, stem cells
INTRODUCTION
In 2011, the term “extracellular vesicles” (EVs) was proposed to define all of the membrane vesicles containing cytosol enclosed into a lipid bilayer and secreted by cells (66). The secretion of EVs is well preserved throughout evolution; plants, prokaryotic cells, and all eukaryotes from amoeba to mammals can release vesicles into the extracellular space (117). In humans, EVs are present in different body fluids, such as blood, urine, amniotic fluid, breast milk, saliva, cerebrospinal fluid, semen, synovia, and tears. Several studies have shown the EV involvement in many physiological and pathological conditions. In particular, EVs exchange information among cells by carrying different types of molecules, such as proteins, lipids, and genetic materials (RNAs and DNA), which are selectively sorted inside vesicles.
BIOGENESIS AND CLASSIFICATION OF EXTRACELLULAR VESICLES
Vesicles, defined by the generic term of EVs, include heterogeneous populations that have originated from different subcellular compartments. Based on their size and subcellular origin, EVs are distinguished into exosomes and ectosomes (32). Exosomes are intraluminal vesicles with a diameter ranging from 30 to 100 nm, derived from the multivesicular bodies by budding of the endosomal membranes and secretion upon fusion with the cell surface (13). Ectosomes, also known as microvesicles (MVs) or microparticles, include different populations of vesicles, such as those derived from perfectly healthy cells, which are in the nano-range (50–200 nm), and larger vesicles (up to 1,000 nm), which include the preapoptotic vesicles. Ectosomes are generated by plasma membrane budding and are shed in the extracellular space (1, 133). Apoptotic bodies, with a diameter ranging from 1,000 to 5,000 nm, are a class of vesicles released by apoptotic cells that are mainly enriched with nuclear fragments (72).
The mechanisms involved in the biogenesis of different types of EVs are not yet fully understood. The components of the endosomal sorting complex required for transport (ESCRT) machinery and other auxiliary proteins (ALIX, TSG101, and VPS4) have been involved in exosome biogenesis. However, recent studies have shown the involvement of these molecules not exclusively in exosomes, but also in the formation of shedding vesicles. Moreover, an ESCRT-independent mechanism has been described in exosome formation (138). Several components of the RAB family of small GTPase proteins are involved in the interaction of the multivesicular bodies with the plasma membrane during exosome release (6, 50). In the formation of shedding vesicles, a rearrangement of cytoskeletal myosin and actin regulated by Ras-related GTPase ADP-ribosylation factor 6 (ARF6) signaling is implicated (139). Because molecules involved in biogenesis, such as ESCRT and tetraspanins, are commonly expressed by exosomes and ectosomes, these cannot be taken as a criterion of distinction (108). In addition, the same cell may release vesicles in the nano-range, either by exocytosis or by surface membrane budding, making difficult a punctual distinction of the origin of vesicles released by healthy cells. Whereas CD63, CD81, and CD9 members of tetraspanin family are enriched in exosomes, distinctive markers for ectosomes are lacking. However, a recent study suggests that annexin A1 is a marker for ectosomes (77). Surface-expressed molecules may indicate the cellular origin of vesicles. For instance, EVs derived from mesenchymal stem cells express on their surface mesenchymal markers, such as CD44, CD90, CD105, and CD146 (20).
CONTENT OF EXTRACELLULAR VESICLES AND MECHANISMS OF ACTION
The nature and abundance of EV contents vary with respect to cell type, biogenesis, physiologic, or pathological conditions, as well as with the protocol used to purify EVs. Different databases collecting the results of EV content are actually available and publicly accessible: EVpedia (86), Exocarta (103), and Vesiclepedia (82). All available databases include not only the nucleic acid, lipid, and protein content of EVs, but also the isolation procedure used by different researchers. Comparative analyses indicate an enrichment of specific subsets of RNAs, proteins, and lipids in EVs, in respect to the cells of origin (90, 140).
Several studies have characterized the protein cargo of the EV populations produced by different cell types (35, 58, 59, 126, 132). Proteins commonly found in EVs include proteins associated with the endosomal pathway, such as the ESCRT components (e.g., Alix, TSG101), proteins involved in EV formation and release (e.g., RAB27A and ARF 6), proteins involved in signal transduction, different types of tetraspanins, proteins involved in antigen presentation (major histocompatibility complex I and II), transcription factors (Notch and Wnt), growth factors [e.g., hepatocyte growth factor (HGF) and insulin-like growth factor 1 (IGF-1)], and cytokines.
By lipidomic analysis, EVs share common lipid composition with the cells of origin and lipids differentially expressed in respect to cells. For example, EV membranes are enriched in cholesterol, unsaturated lipids, sphingomyelin, ganglioside GM3, phosphatidylserine, and ceramide (96). At variance, EVs contain a reduced amount of diacyl-glycerol and phosphatidylcholine compared with the cell of origin. The ratio of sphingomyelin and phosphatidylcholine in EVs is twice as high as in the producing cells (89). These differences in composition of lipids depend on EV biogenesis.
Various genetic materials are present in EVs. In some cases, genomic (7) and mitochondrial (64, 65) DNAs have been found. A recent study on exosomal subfractions of EVs suggests that DNA is secreted by an autophagy- and multivesicular-endosome-dependent mechanism and not by exosomes (77). In general, the EVs are enriched with RNAs, in particular, with small RNAs. In addition to the ordinarily known RNA types, like mRNAs, microRNAs, and ribosomal RNAs, EVs may contain long- and short-noncoding RNAs, fragments of tRNA, piwi-interacting-RNA, Y-RNA, and vault RNA (30, 40, 70, 73, 112). Several RNAs present in EVs are around 200 nucleotides (9), suggesting that probably they are fragments. Notably, circular RNAs are also present in EVs (92). The encapsulation of nucleic acids within EV membranes confers protection from the degrading enzymes present in the extracellular space. However, it should be noted that RNA-binding proteins not associated with EVs may also confer protection from ectonucleases (49).
After release, EVs may induce a variety of effects in neighboring or distant cells by diffusing in biological fluids. EVs may interact with target cells by different mechanisms, including direct membrane fusion, ligand-receptor interaction, and internalization. The most common mode of entry of EVs into cells seems to be clathrin-dependent or clathrin-independent endocytosis (44, 106, 135). Once entered into the recipient cells, EVs may follow the endocytic pathway, which conducts to lysosomes or they may escape lysosomal digestion and release their content into the cytoplasm of the target cells. However, the modality of cell membrane-EV interaction, the mechanisms allowing transfer of EV-cargo, and the downstream effects triggered by EVs, are only partly understood and depend on the origin of EVs and on the type and activation state of target cells.
CANCER CELL-DERIVED EXTRACELLULAR VESICLES AND TUMOR MICROENVIRONMENT MODULATION
By secreting EVs, cancer cells may communicate with nearby stromal cells, such as endothelial cells, cancer-associated fibroblasts (CAFs), and by entering into the circulation, they may interact with distant cells. Cancer EVs are able to orchestrate several processes, such as angiogenesis (124), extracellular matrix remodeling (110, 125), and induction of epithelial-mesenchymal transition (EMT), promoting tumor aggressiveness, invasiveness, and metastatic potential (48, 54–56, 111, 142). In turn, tumor stroma cells by secreting EVs may influence tumor progression. In fact, CAF-derived EVs are able to stimulate proliferation, motility, and metastatic potential of tumor cells, thus providing evidence for a bidirectional crosstalk between tumor and environmental cells (99, 123).
Recently, it has been shown that tumor-derived EVs play a functional role in the resistance to chemotherapy of prostate, non-small cell lung, breast, and ovarian cancers (29, 36, 41, 115, 143). CAFs, which are innately resistant to chemotherapy, may secrete EVs that contain factors promoting chemoresistance into recipient cancer cells, thus inhibiting tumor cell apoptosis and favoring tumor growth (5, 14, 121).
Different mechanisms are involved in the proangiogenic effect of cancer EVs. Breast cancer cell-derived EVs contain a unique 90-kDa form of vascular endothelial growth factor (VEGF), which is resistant to anti-VEGF antibodies, and activates VEGF receptors on endothelial cells promoting tumor angiogenesis (53). Under hypoxic conditions, EVs from colorectal cancer shuttled Wnt4, a member of the Wnt family. Wnt4 increases β-catenin nuclear translocation and activates Wnt/β-catenin signaling pathway, which triggers endothelial cell proliferation and migration (74). Moreover, cancer EVs may contribute to new blood vessel formation by transferring proangiogenic microRNAs (miRNAs) into recipient fibroblasts and endothelial cells (155). In particular, it has been demonstrated that the exosome expression of miR-155, miR-210, and miR-494 is under regulation of the hypoxia-inducible factor 1α (HIF-1α) (42, 45, 101, 102). In turn, miR-155 and miR-210 stabilize HIF-1α expression under hypoxic conditions, suggesting a positive feedback loop that supports angiogenesis in fibroblasts and endothelial cells (45, 102).
A growing body of evidence shows that EVs are involved not only in primary tumor establishment, but also in metastasis spreading. In fact, tumor-derived EVs can reach distant organs through blood and lymphatic vessels. As showed by Hoshino et al. (71), this process is directed by specific exosome integrin expression, allowing targeting of specific organs, thereby supporting the formation of the so-called “premetastatic niche”. For example, lung metastasis associates with the expression of exosome α6β4 and α6β1 integrins, whereas liver metastasis associates with exosome αvβ5 integrin expression (71). EVs can induce the expression of promigratory and proinflammatory mediators, resulting in stromal cell activation, extracellular matrix deposition, and bone marrow-derived myeloid cell recruitment, which play a crucial role in tumor cell implantation (39, 113).
CANCER STEM CELL-DERIVED EXTRACELLULAR VESICLES
A minor population of cells that is present in cancer responsible for tumor recurrence and resistance to therapy (37, 149) has been defined as cancer stem cells (CSCs). The presence of CSCs has been described in several tumors (22, 37, 46, 79, 149). This population of immature cells may differentiate in all tumor cell types and may favor tumor growth, invasion, and metastases (37). A clonogenic CSC population has been described in renal carcinomas (23). These cells retain self-renewal properties and are capable of differentiating into more mature tumor cells and to favoring metastasis and resistance to therapy. Renal CSCs express several mesenchymal markers, such as CD73, CD90, CD44, CD29, CD146, vimentin, and, in particular, CD105, which has been used to purify this population from primary tumors (22). Moreover, they express the embryonic renal Pax2 and OCT4, NANOG, Nestin, and Musashi embryonic stem cell markers. Renal CSCs are clonogenic, generate spheres in culture, and display tumor-initiating capabilities in vivo. Another characteristic of these cells is to recapitulate the morphological aspects of the tumor of origin and to generate serially transplantable tumors, when implanted in a very low number in SCID mice. Moreover, CSCs have been shown to contribute to vessel formation since several human endothelial cells are detectable within the neo-formed tumor (22). Grange et al. (60) characterized EVs derived from renal CSCs and EVs derived from non-stem cell populations of the same tumors. When compared, these two populations are similar for size (10–100 nm) and zeta potential (22.4 ± 3.5 mV). By FACS analysis, both EV types express CD44 and α5- and α6-integrins, whereas only CSC-derived EVs express CD105. The analysis of RNA content shows an enrichment of small RNAs in both EVs but by miRNoma analysis, CSC-derived EVs express a different pattern in respect to EVs derived from non-stem cell populations (60). In particular, CSC-EVs are enriched in miR-200c, miR-92, and miR-141, which are known to be expressed in several carcinomas (16, 76, 105, 129) and in miR-29a, miR-650, and miR-151, which correlate with tumor and metastases (57, 98, 152). By gene ontology analysis, the authors showed that 24 miRNAs enriched in CSC-derived EVs associate with cell proliferation, cell adhesion, transcription, and several metabolic processes. In addition, EVs derived from CSCs exclusively carry proangiogenic mRNAs, such as fibroblast growth factor 2 (FGF2), angiopoietin 1, MMP2, MMP9, ephrin, and VEGF. EVs derived from CSCs, but not those derived from non-stem renal cancer cells, promote in vitro formation of capillary-like structures in Matrigel, tumor cell invasion, and tumor cell adhesion to the endothelium, and inhibit doxorubicin-induced apoptosis. In vivo, CSC-derived EVs stimulate angiogenesis and induce the formation of premetastatic niche. The latter is associated with an enhanced expression by lung endothelial cells of VEGFR1 and VEGF and MMP2 (60).
Moreover, it has been shown that CSC-EVs may modify phenotype and function of MSCs. MSC interaction with tumor is bivalent. In some instances, it has been reported that MSCs inhibit tumor development, in others, their involvement in tumor progression and angiogenesis (15, 81, 95, 146). CSC-EVs were shown to precondition MSCs toward a protumorigenic and angiogenic phenotype (93). Indeed, COL4A3, MMP1-3, CXCR4, and CXCR7 are significantly upregulated in MSCs pretreated with CSC-EVs.
Recently, it has been shown that osteosarcoma-derived EVs induce epigenetic changes in MSCs, influencing their function in the tumor microenvironment (100). In addition, cancer EVs could interfere with the immune system favoring the immune escape of tumors (130). The EV-mediated repression of innate immune responses may occur through mobilization of the myeloid-derived suppressor cells (27) and activation of tumor-associated macrophages (10) and neutrophils (12).
Tumor EVs promote differentiation of monocytes into monocyte-derived suppressors, which defeat T-cell proliferation and function (137). These EVs have been shown to express membrane PD-L1 and transforming growth factor-β (TGF-β) immunosuppressive cytokine (28, 131, 148) and to inhibit proliferation and cytotoxic activity of natural killer (NK) cells (4, 94, 97). Moreover, cancer EVs suppress adaptive immune responses by repressing antigen-presenting cells (147) and by blocking cytotoxic T-cell activation and proliferation, through the regulation of immune function-related genes (43, 107). In fact, the presence of Fas ligand and tumor necrosis factor-related apoptosis-inducing ligand on colorectal and prostate cancer-derived EVs can induce apoptosis in CD8+ T lymphocytes (2, 75). The immune modulatory activity of EVs is retained by CSC-derived EVs. In fact, it has been shown that CSC-EVs express the immunomodulatory nonclassical human leukocyte antigen G (HLA-G) that suppresses dendritic cell (DC) maturation (61). CSC-EVs reduce the expression of several markers of activation of monocyte-derived DC, such as CD40, CD80, and CD86 costimulatory molecules, CD83, HLA-DR, and several T-cell adhesion molecules. This altered phenotype results in impairment of CD3 + lymphocyte proliferation and associates with an enhanced production of interleukin-10 (IL-10) in respect to control DCs. CSC-EVs also stimulate the release of soluble HLA-G by monocyte-derived DCs. HLA-G has been shown to inhibit natural killer (NK) cells, T cells, and DCs (8) and has been linked to the immune escape of cancer (87). Of interest, CSC-EVs express significantly more HLA-G in respect to cancer cells deprived of the stem cell population. When CSC-EVs were incubated with an anti-HLA-G antibody, the DC phenotype of monocyte-derived cells was restored, and the immune inhibition reverted.
Recently, colorectal cancer stem cells have been shown to secrete EVs by a β-catenin/Tcf-4-activated RAB27B-dependent mechanism, exhibiting a switch of exosome RNAs from retrotransposons to microRNAs (miRNAs) (31). In particular, miR-146a may promote stem-like properties and tumorigenesis in colon cancer cells by targeting Numb. Moreover, these EVs facilitate an immunosuppressive tumor microenvironment.
PARACRINE ACTION OF STEM/PROGENITOR CELL-DERIVED EXTRACELLULAR VESICLES
Stem cell-derived EVs (SC-EVs) contain stem cell-associated transcription factors, such as Nanog and Oct-4 (119), express typical mesenchymal stromal cell (MSC) markers (CD105, CD29, CD73), and other stem cell markers such as CD133 (118) and c-KIT (144). SC-EVs express also Wnt and Hedgehog, which have a possible role in stem cell biology (99). Ratajczak et al. (119) demonstrated that SC-EVs may regulate stemness, self-renewal, and differentiation of stem/progenitor cells. In fact, EVs released from embryonic stem cells sustain the self-renewal of hematopoietic stem cells, by delivering specific proteins and mRNAs, which induce the upregulation of specific genes of pluripotency (119) (Table 1). In addition, EVs derived from other types of stem/progenitor cells shuttle mRNAs that are able to induce reprogramming of target cells. In particular, EVs from endothelial progenitor cells (EPCs) induce a proangiogenic phenotype in terminally differentiated endothelial cells and promote angiogenesis (49).
Table 1.
Biological roles of stem/progenitor cell-derived extracellular vesicles
| Biological Effect | Mechanism of Action | Cell Source | Target | Reference |
|---|---|---|---|---|
| Regulation of stemness, self-renewal, and differentiation | – Transfer of proteins and mRNAs– Upregulation of pluripotency-related gene expression | Mouse embryonic stem cells | Hematopoietic progenitor cells | (119) |
| Proangiogenic effect | – Transfer of specific proteins, mRNAs and miRNAs (miR-126 and miR-296)– Activation of PI3K/Akt pathway– Inhibition of apoptosis | EPCs | Endothelial cells | (24, 49) |
| Improvement of myocardial function and regeneration after IRI | – Transfer of pluripotency-related miRNAs (e.g., miR-294)– Activation of PI3K/Akt pathway– Inhibition of apoptosis– Decreased oxidative stress and increased ATP and NADH production– Reduction of inflammation, infarct size, and fibrosis | MSCs, cardiomyocyte progenitor cells, mouse embryonic stem cells | Cardiomyocites, inflammatory cells | (3, 84, 141) |
| Improvement of renal function and regeneration after AKI | – Transfer of specific proteins, mRNAs, and miRNAs– Transfer of mRNA coding for IGF-1 receptor and HGF which increase cell proliferation– Inhibition of apoptosis– Reduction of inflammation | MSCs from bone marrow, renal glomeruli, Wharton’s jelly, umbilical cord, HLSCs, EPCs, and tubular progenitor cells | Renal tubular epithelial cells, inflammatory cells | (17, 18, 20, 21, 24, 33, 34, 63, 69, 80, 116, 136, 154) |
| Improvement of renal function in experimental anti-Thy1.1 glomerulonephritis | – Transfer of mRNA coding for the complement inhibitors Factor H, CD55 and CD59– Reduction of complement-mediated mesangial injury– Reduction of inflammation and histopathological changes | EPCs | Mesangial cells | (25) |
| Improvement of renal function in CKD (e.g., aristolochic acid-induced kidney fibrosis, DN) | – Transfer of specific miRNAs which downregulate pro-fibrotic genes– Transfer of VEGF, TGF-β1, angiogenin which increase glomerular cell proliferation– Transfer of BMP-7 which inhibits apoptosis– Reduction of fibrosis, inflammation, and histopathological changes | MSCs from bone marrow, urine, HLSCs | Renal fibrosis, podocytes and renal tubular epithelial cells | (62, 78, 85, 109) |
| Stimulation of liver regeneration after acute injury | – Transfer of specific mRNAs– Increase of hepatocyte proliferation and apoptosis resistance | HLSCs | Hepatocytes | (68) |
| Improvement of liver function and regeneration after chronic injury | – Increase of proliferation-related protein expression– Inhibition of apoptosis through upregulation of Bcl-xL– EMT inhibition– Reduction of fibrosis and inflammation | MSCs from umbilical cord and embryonic stem cells | Hepatic fibrosis | (91, 128) |
| Neuroprotective effect (e.g., Alzheimer’s disease, stroke) | – Transfer of specific miRNAs involved in axon regeneration (e.g., miR-17-92, miR-21, miR-133, miR-146a)– Transfer of long non-coding RNAs which increase cell proliferation(e.g., MALAT1)– Transfer of neprilysin which contributes to β-amyloid peptide clearance in the brain– Increase of angiogenesis and neurogenesis– Reduction of neuroinflammation | MSCs from adipose tissue and bone marrow | Glia, hippocampal neurons, retinal ganglion and neuroblastoma cells | (51, 83, 104, 145, 153) |
| Immunomodulation | – Increase of CD4+CD25+FoxP3+ Tregs which inhibit graft-versus-host disease– Reduction of Th17 and NK cells– Downregulation of Th1 responses– Upregulation of anti-inflammatory genes (e.g., TGF-β1 and IL-10)– Downregulation of proinflammatory genes (e.g., IL-1β, IL-12P40, IL-6 and tumor necrosis factor) | MSCs from bone marrow | CD4+ T cells, synovial leukocytes | (26, 150, 151) |
AKI, acute kidney injury; BMP-7, bone morphogenetic protein-7; CKD, chronic kidney disease; DN, diabetic nephropathy; EMT, epithelial-mesenchymal transition; EPCs, endothelial progenitor cells; HLSCs, human liver stem cells; IGF-1, insulin-like growth factor 1; IRI, ischemia-reperfusion injury; MALAT1, metastasis-associated lung adenocarcinoma transcript 1; miRNAs, microRNAs; MSCs, mesenchymal stromal cells; NK, natural killer; PI3K, phosphatidylinositol 3-kinase; TGF-β1, transforming growth factor-β1; VEGF, vascular endothelial growth factor.
Several studies have shown that SC-EVs may mimic the effect of stem cells in various experimental models of tissue injuries (Table 1). In fact, EVs produced by stem cells carry biological messages that can influence the behavior and the fate of the target cells. This observation stimulated a number of studies aimed at evaluating whether SC-EVs may substitute stem cell-based therapies. SC-EVs from various cell sources have been investigated as potential therapeutic agents in cardiovascular diseases. SC-EVs improve heart function and vessel formation by inhibiting cardiomyocyte apoptosis and inflammation, and by promoting angiogenesis. In fact, EVs from MSCs are able to reduce inflammation and ameliorate myocardial functions in the course of ischemia-reperfusion injury (IRI), by triggering phosphatidylinositol 3-kinase (PI3K)/Akt prosurvival signaling and by restoring bioenergetics (3). Moreover, it has been shown that MSC-EVs reduce the infarct size and promote angiogenesis (11). Similarly, EVs secreted by cardiomyocyte progenitor cells (141) and from embryonic stem cells (84) were shown to improve cardiac function by promoting neovascularization of ischemic heart and cardiomyocyte survival. EV treatment also reduces fibrosis after infarction by the delivery to cardiomyocytes of miR-294, which increases cell survival and proliferation (84).
Numerous studies reported that SC-EVs have potent renal-regenerative properties. In particular, MSC-EVs mimic the effect of the cell of origin in inducing renal regeneration in a model of acute kidney injury (AKI) (17). MSC-EVs induce proliferation of renal tubular cells accelerating the reparative process of damaged tubules (17) (Fig. 1) and protect tubular cells from apoptosis (18) through upregulation of specific antiapoptotic genes and downregulation of proapoptotic genes. MSC-EVs shuttled mRNAs that can be translated into proteins, both in vitro and in vivo, stimulating reentry of damaged tubular cells into cell cycle (17, 18). Gradient separation of exosomes and MVs shows a distinct molecular signature and function on renal tubular cells (34), and exosomes retain the majority renal regenerative activity in AKI (20). It has also been reported that MSC-EVs mediate the transfer of human IGF-1 receptor mRNA to tubular cells, increasing the cell sensitivity to the proproliferative actions of IGF-1 (136). In addition, miRNAs carried by MSC-EVs have a relevant role in proregenerative effect of EVs. In fact, miRNA-depleted EVs, released by Drosha-knockdown MSCs, failed to induce renal regeneration in AKI (33). Other sources of SC-EVs have been studied in various experimental models of AKI. In particular, EVs from umbilical cord blood (CB) MSCs (154), EVs obtained from human liver stem cells (HLSCs) (69), and EVs from glomerular MSCs (116) are protective in AKI. EVs obtained from CB-MSCs stimulate tubular cell proliferation by the horizontal transfer of human HGF and by the induction of HGF production (80). Wharton’s Jelly-derived MSC-EVs reduce inflammation and apoptosis via mitochondrial protection (63). EPC-EVs administered after renal IRI prevent the development of renal injury by enhancing tubular cell proliferation and reducing apoptosis and leukocyte infiltration (24). Moreover, EPC-EVs have been tested also in a rat model of experimental anti-Thy1.1 glomerulonephritis induced by a complement-mediated mesangial injury. In this model, EPC-EVs are able to inhibit the infiltration of leukocytes, the activation of mesangial cells, and the activation of serum complement, to decrease proteinuria and to improve renal function (25). EVs derived from endothelial cell-forming colonies, in particular, exosomes, significantly attenuate renal injury in a model of renal IRI (21). SC-EVs have also been studied in different animal models of chronic kidney diseases (CKD). EVs isolated from urinary MSCs are able to reduce the urinary volume and micro-albumin excretion in a rat model of diabetic nephropathy (DN) induced by streptozotocin injection (78). Moreover, the direct injection of MSC-exosomes under the renal capsule in the streptozotocin-induced DN model prompts a rapid improvement of renal morphology (109). EVs derived from MSCs and from HLSCs not only interfere with the progression but also revert to the renal fibrosis in a model of DN induced by streptozotocin. The antifibrotic effect correlates with the downregulation of several profibrotic genes in renal tissues (62, 85). In fact, HLSC-EVs contain a pattern of antifibrotic miRNAs able to downregulate profibrotic genes, restoring normal renal function in different animal models of CKD (62, 85).
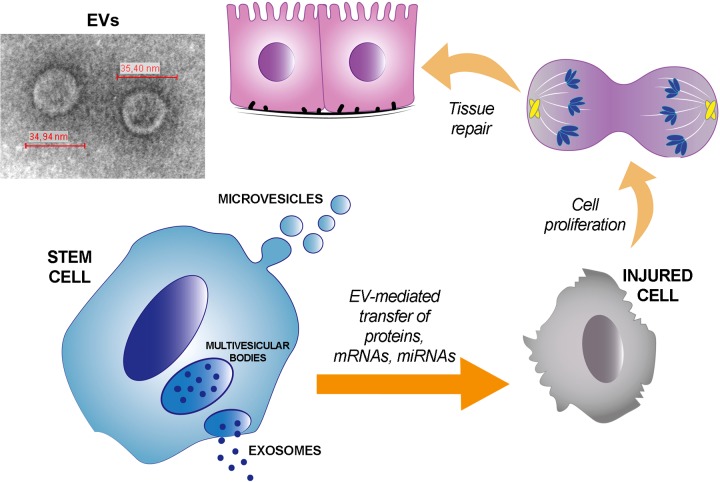
Fig. 1.
Stem cell-derived extracellular vesicles (SC-EVs) contribution to tissue repair. Stem cells release EVs that accumulate at the site of tissue injury and deliver proteins and specific patterns of mRNAs and microRNAs (miRNAs) to injured cells. When incorporated into damaged cells, SC-EVs activate regenerative programs, which accelerate tissue recovery and include cell dedifferentiation, cell cycle reentry, proliferation, and redifferentiation. Inset: representative transmission electron microscopy image of EVs (original magnification ×250,000).
HLSC-EVs participate also in accelerating the morphological and functional rescue of injured liver cells (68). Similarly, injection of EVs produced by human umbilical CB-MSCs diminishes inflammation in fibrotic liver. EVs inhibit hepatic fibrosis and protect hepatocytes by inhibition of EMT (91). Moreover, MSC-EVs stimulate liver regeneration following drug-induced injury (128).
Recent studies have demonstrated that SC-EVs have a neuroprotective effect similar to cells of origin. In particular, MSC-EVs by transfer of miRNAs (miR-133b) are able to promote neural plasticity and recovery of glia cells and of neurons in rats with stroke (145). On the other hand, treatment with EVs derived from neuronal cells under differentiation could induce human MSCs to differentiate into neuron-like cells through the transfer of miR-125b, thus confirming a two-way synergistic interaction (127). EVs obtained from MSCs enhanced endogenous angiogenesis and neurogenesis, and attenuated neuroinflammation in a rat model of traumatic brain injury (153). Moreover, EV-MSCs significantly promote the survival of retinal ganglion cells together with the axon regeneration (104). EVs derived from human adipose stem cells (ADSCs) enhance both neuronal survival and proliferation in vitro (51). Interestingly, in an in vivo model of Alzheimer’s disease, the positive effect of ADSC-EV administration has been attributed to their content in neprilysin, an enzyme involved in degradation of β-amyloid peptide in brain (83).
Many studies indicate that stem cells and, in particular, MSCs secrete immunosuppressive EVs (19, 38, 47, 52, 120). MSC-EVs in vitro increase the CD4+CD25+FoxP3+Treg population, downregulate Th1 responses, and reduce the number of Th17 and NK cells (151). In vivo, the anti-inflammatory effect of MSC-derived EVs is observed in many mouse models of inflammatory and autoimmune diseases (114). The expression of anti-inflammatory transcripts, such as TGF-β1 and IL-10 is upregulated by MSC-EVs, whereas the expression of proinflammatory transcripts, such as IL-1β, IL-12, P40, IL-6, and tumor necrosis factor are reduced. Moreover, MSC-EVs inhibit rejection of allogenic skin graft in mice by inducing an enhanced expression of regulatory T cells (150). This immunosuppressive effect renders MSC-EVs good candidates as surrogate of MSC-based therapy (26, 67, 120). Finally, a first in human study with MSC-derived EVs has shown improvement of graft versus host disease symptoms in a therapy-resistant patient (88).
CONCLUSIONS AND FUTURE PROSPECTS
Overall, the studies summarized in this review indicate an increased awareness of the physiological and pathological role of EVs in different contexts. This newly described mechanism of cell-to-cell communication is based on the transfer of molecular information from an EV donor cell to a recipient cell. Since EV cargo has the signature of the cell of origin, the transfer of bioactive molecules to the recipient cells may induce epigenetic changes and functional modifications in the latter. In the context of tumor microenvironment, a bidirectional exchange of information between stroma and cancer stem cells may create conditions for tumor invasion and progression. Therefore, the search for potential biomarkers in tumor-derived EVs may open diagnostic and prognostic perspectives. Moreover, increasing evidence indicates that EVs are implicated in the physiological and pathological immune and inflammatory responses. A better understanding of these mechanisms may allow the development of new strategies aimed to modulate immunity and inflammation. In fact, EVs may be exploited to either enhance or inhibit immune/inflammatory responses, but this bifunctional role of EVs may also result in unpredictable adverse effects (151). It has been reported that the immune-modulatory action of EVs is related to the different state of maturation and activation of immune cells. For instance, dendritic and antigen-presenting cells may release EVs that modulate positively or negatively the nonspecific and/or antigen-specific immune responses (122). Moreover, the MHC-class I and II, and CD80 and CD86 costimulatory molecule expression may differentially modulate immunogenicity (134). A limitation of all these studies is that purification of EVs has been performed using different methods, and this may influence the characterization of EV content. Moreover, it should be taken into account that many studies described in the literature have been performed with cell lines that do not necessarily reflect the physiopathological conditions.
Several preclinical studies have shown that SC-derived EVs may mimic the beneficial effect of stem cells. It is now accepted that the regenerative potential of adult stem cells is related to paracrine mechanisms and a critical role of their secretome has been described. It may be difficult in this context to discriminate the exact contribution of EVs in respect to the bulk of cytokines and growth factors produced by stem cells. Therefore, a better understanding of the mechanisms related to the EV effects may contribute to the design of new therapeutic strategies. Furthermore, to envisage an EV-based therapy, further studies are needed to develop an upscale production of EVs under good manufacturing practice protocols of isolation and characterization, and to define their pharmacokinetics and pharmacodynamics. Nevertheless, EV-mediated transfer of nucleic acids could permit potentially versatile therapeutic approaches (122). For instance, EVs can be engineered and used as a vehicle for the delivery of miRNAs or of small interfering RNAs (siRNAs) to modulate different biological processes.
GRANTS
This work was supported by a grant from Unicyte AG (Oberdorf NW, Switzerland). This work was supported in part by National Institutes of Health Grants UH2 TR000880 and UH3 TR000880-03S1.
DISCLOSURES
G. Camussi is a member of the Scientific Advisory Board of Unicyte AG. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
G. Chiabotto prepared figures; S.B. and E.F. drafted manuscript; G. Chiabotto, M.C.D., and G. Camussi edited and revised manuscript; S.B., G. Chiabotto, E.F., M.C.D., and G. Camussi approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Valeria Chiabotto for figure realization.
REFERENCES
- 1.Abels ER, Breakefield XO. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol 36: 301–312, 2016. doi: 10.1007/s10571-016-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abusamra AJ, Zhong Z, Zheng X, Li M, Ichim TE, Chin JL, Min WP. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol Dis 35: 169–173, 2005. doi: 10.1016/j.bcmd.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res (Amst) 10: 301–312, 2013. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Ashiru O, Boutet P, Fernández-Messina L, Agüera-González S, Skepper JN, Valés-Gómez M, Reyburn HT. Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes. Cancer Res 70: 481–489, 2010. doi: 10.1158/0008-5472.CAN-09-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Au Yeung CL, Co NN, Tsuruga T, Yeung TL, Kwan SY, Leung CS, Li Y, Lu ES, Kwan K, Wong KK, Schmandt R, Lu KH, Mok SC. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat Commun 7: 11150, 2016. doi: 10.1038/ncomms11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E, Zimmermann P, David G. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol 14: 677–685, 2012. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 7.Balaj L, Lessard R, Dai L, Cho YJ, Pomeroy SL, Breakefield XO, Skog J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun 2: 180, 2011. doi: 10.1038/ncomms1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banas R, Miller C, Guzik L, Zeevi A. Amnion-derived multipotent progenitor cells inhibit blood monocyte differentiation into mature dendritic cells. Cell Transplant 23: 1111–1125, 2014. doi: 10.3727/096368913X670165. [DOI] [PubMed] [Google Scholar]
- 9.Batagov AO, Kurochkin IV. Exosomes secreted by human cells transport largely mRNA fragments that are enriched in the 3′-untranslated regions. Biol Direct 8: 12, 2013. doi: 10.1186/1745-6150-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benito-Martin A, Di Giannatale A, Ceder S, Peinado H. The new deal: a potential role for secreted vesicles in innate immunity and tumor progression. Front Immunol 6: 66, 2015. doi: 10.3389/fimmu.2015.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med (Berl) 92: 387–397, 2014. doi: 10.1007/s00109-013-1110-5. [DOI] [PubMed] [Google Scholar]
- 12.Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC, Ostrowski M, Théry C. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res 72: 4920–4930, 2012. doi: 10.1158/0008-5472.CAN-12-0925. [DOI] [PubMed] [Google Scholar]
- 13.Bobrie A, Colombo M, Krumeich S, Raposo G, Théry C. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J Extracell Vesicles 1: 18397, 2012. doi: 10.3402/jev.v1i0.18397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boelens MC, Wu TJ, Nabet BY, Xu B, Qiu Y, Yoon T, Azzam DJ, Twyman-Saint Victor C, Wiemann BZ, Ishwaran H, Ter Brugge PJ, Jonkers J, Slingerland J, Minn AJ. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell 159: 499–513, 2014. doi: 10.1016/j.cell.2014.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonuccelli G, Avnet S, Grisendi G, Salerno M, Granchi D, Dominici M, Kusuzaki K, Baldini N. Role of mesenchymal stem cells in osteosarcoma and metabolic reprogramming of tumor cells. Oncotarget 5: 7575–7588, 2014. doi: 10.18632/oncotarget.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brase JC, Johannes M, Schlomm T, Fälth M, Haese A, Steuber T, Beissbarth T, Kuner R, Sültmann H. Circulating miRNAs are correlated with tumor progression in prostate cancer. Int J Cancer 128: 608–616, 2011. doi: 10.1002/ijc.25376. [DOI] [PubMed] [Google Scholar]
- 17.Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B, Tetta C, Camussi G. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol 20: 1053–1067, 2009. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, Tetta C, Camussi G. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One 7: e33115, 2012. doi: 10.1371/journal.pone.0033115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruno S, Deregibus MC, Camussi G. The secretome of mesenchymal stromal cells: Role of extracellular vesicles in immunomodulation. Immunol Lett 168: 154–158, 2015. doi: 10.1016/j.imlet.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Bruno S, Tapparo M, Collino F, Chiabotto G, Deregibus MC, Soares Lindoso R, Neri F, Kholia S, Giunti S, Wen S, Quesenberry P, Camussi G. Renal regenerative potential of different extracellular vesicle populations derived from bone marrow mesenchymal stromal cells. Tissue Eng Part A 23: 1262–1273, 2017. doi: 10.1089/ten.tea.2017.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burger D, Viñas JL, Akbari S, Dehak H, Knoll W, Gutsol A, Carter A, Touyz RM, Allan DS, Burns KD. Human endothelial colony-forming cells protect against acute kidney injury: role of exosomes. Am J Pathol 185: 2309–2323, 2015. doi: 10.1016/j.ajpath.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Bussolati B, Bruno S, Grange C, Ferrando U, Camussi G. Identification of a tumor-initiating stem cell population in human renal carcinomas. FASEB J 22: 3696–3705, 2008. doi: 10.1096/fj.08-102590. [DOI] [PubMed] [Google Scholar]
- 23.Bussolati B, Dekel B, Azzarone B, Camussi G. Human renal cancer stem cells. Cancer Lett 338: 141–146, 2013. doi: 10.1016/j.canlet.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Cantaluppi V, Gatti S, Medica D, Figliolini F, Bruno S, Deregibus MC, Sordi A, Biancone L, Tetta C, Camussi G. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int 82: 412–427, 2012. doi: 10.1038/ki.2012.105. [DOI] [PubMed] [Google Scholar]
- 25.Cantaluppi V, Medica D, Mannari C, Stiaccini G, Figliolini F, Dellepiane S, Quercia AD, Migliori M, Panichi V, Giovannini L, Bruno S, Tetta C, Biancone L, Camussi G. Endothelial progenitor cell-derived extracellular vesicles protect from complement-mediated mesangial injury in experimental anti-Thy1.1 glomerulonephritis. Nephrol Dial Transplant 30: 410–422, 2015. doi: 10.1093/ndt/gfu364. [DOI] [PubMed] [Google Scholar]
- 26.Casado JG, Blázquez R, Vela FJ, Álvarez V, Tarazona R, Sánchez-Margallo FM. Mesenchymal stem cell-derived exosomes: immunomodulatory evaluation in an antigen-induced synovitis porcine model. Front Vet Sci 4: 39, 2017. doi: 10.3389/fvets.2017.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, Boireau W, Rouleau A, Simon B, Lanneau D, De Thonel A, Multhoff G, Hamman A, Martin F, Chauffert B, Solary E, Zitvogel L, Garrido C, Ryffel B, Borg C, Apetoh L, Rébé C, Ghiringhelli F. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest 120: 457–471, 2010. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H, Xia H, Man Q, Zhong W, Antelo LF, Wu B, Xiong X, Liu X, Guan L, Li T, Liu S, Yang R, Lu Y, Dong L, McGettigan S, Somasundaram R, Radhakrishnan R, Mills G, Lu Y, Kim J, Chen YH, Dong H, Zhao Y, Karakousis GC, Mitchell TC, Schuchter LM, Herlyn M, Wherry EJ, Xu X, Guo W. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 560: 382–386, 2018. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen WX, Liu XM, Lv MM, Chen L, Zhao JH, Zhong SL, Ji MH, Hu Q, Luo Z, Wu JZ, Tang JH. Exosomes from drug-resistant breast cancer cells transmit chemoresistance by a horizontal transfer of microRNAs. PLoS One 9: e95240, 2014. doi: 10.1371/journal.pone.0095240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng L, Sun X, Scicluna BJ, Coleman BM, Hill AF. Characterization and deep sequencing analysis of exosomal and non-exosomal miRNA in human urine. Kidney Int 86: 433–444, 2014. doi: 10.1038/ki.2013.502. [DOI] [PubMed] [Google Scholar]
- 31.Cheng WC, Liao TT, Lin CC, Yuan LE, Lan HY, Lin HH, Teng HW, Chang HC, Lin CH, Yang CY, Huang SC, Jiang JK, Yang SH, Yang MH, Hwang WL. RAB27B-activated secretion of stem-like tumor exosomes delivers the biomarker microRNA-146a-5p, which promotes tumorigenesis and associates with an immunosuppressive tumor microenvironment in colorectal cancer. Int J Cancer In press. doi: 10.1002/ijc.32338. [DOI] [PubMed] [Google Scholar]
- 32.Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol 25: 364–372, 2015. doi: 10.1016/j.tcb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Collino F, Bruno S, Incarnato D, Dettori D, Neri F, Provero P, Pomatto M, Oliviero S, Tetta C, Quesenberry PJ, Camussi G. AKI recovery induced by mesenchymal stromal cell-derived extracellular vesicles carrying MicroRNAs. J Am Soc Nephrol 26: 2349–2360, 2015. doi: 10.1681/ASN.2014070710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collino F, Pomatto M, Bruno S, Lindoso RS, Tapparo M, Sicheng W, Quesenberry P, Camussi G. Exosome and microvesicle-enriched fractions isolated from mesenchymal stem cells by gradient separation showed different molecular signatures and functions on renal tubular epithelial cells. Stem Cell Rev 13: 226–243, 2017. doi: 10.1007/s12015-016-9713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conde-Vancells J, Rodriguez-Suarez E, Embade N, Gil D, Matthiesen R, Valle M, Elortza F, Lu SC, Mato JM, Falcon-Perez JM. Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. J Proteome Res 7: 5157–5166, 2008. doi: 10.1021/pr8004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corcoran C, Rani S, O’Brien K, O’Neill A, Prencipe M, Sheikh R, Webb G, McDermott R, Watson W, Crown J, O’Driscoll L. Docetaxel-resistance in prostate cancer: evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PLoS One 7: e50999, 2012. doi: 10.1371/journal.pone.0050999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corrò C, Moch H. Biomarker discovery for renal cancer stem cells. J Pathol Clin Res 4: 3–18, 2018. doi: 10.1002/cjp2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cosenza S, Toupet K, Maumus M, Luz-Crawford P, Blanc-Brude O, Jorgensen C, Noël D. Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics 8: 1399–1410, 2018. doi: 10.7150/thno.21072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J, Zhang T, Theilen TM, García-Santos G, Williams C, Ararso Y, Huang Y, Rodrigues G, Shen TL, Labori KJ, Lothe IM, Kure EH, Hernandez J, Doussot A, Ebbesen SH, Grandgenett PM, Hollingsworth MA, Jain M, Mallya K, Batra SK, Jarnagin WR, Schwartz RE, Matei I, Peinado H, Stanger BZ, Bromberg J, Lyden D. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol 17: 816–826, 2015. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crescitelli R, Lässer C, Szabó TG, Kittel A, Eldh M, Dianzani I, Buzás EI, Lötvall J. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles 2: 20677, 2013. doi: 10.3402/jev.v2i0.20677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crow J, Atay S, Banskota S, Artale B, Schmitt S, Godwin AK. Exosomes as mediators of platinum resistance in ovarian cancer. Oncotarget 8: 11,917–11,936, 2017. doi: 10.18632/oncotarget.14440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cui H, Seubert B, Stahl E, Dietz H, Reuning U, Moreno-Leon L, Ilie M, Hofman P, Nagase H, Mari B, Krüger A. Tissue inhibitor of metalloproteinases-1 induces a pro-tumourigenic increase of miR-210 in lung adenocarcinoma cells and their exosomes. Oncogene 34: 3640–3650, 2015. doi: 10.1038/onc.2014.300. [DOI] [PubMed] [Google Scholar]
- 43.Czernek L, Düchler M. Functions of cancer-derived extracellular vesicles in immunosuppression. Arch Immunol Ther Exp (Warsz) 65: 311–323, 2017. doi: 10.1007/s00005-016-0453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Damke H, Baba T, van der Bliek AM, Schmid SL. Clathrin-independent pinocytosis is induced in cells overexpressing a temperature-sensitive mutant of dynamin. J Cell Biol 131: 69–80, 1995. doi: 10.1083/jcb.131.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dang K, Myers KA. The role of hypoxia-induced miR-210 in cancer progression. Int J Mol Sci 16: 6353–6372, 2015. doi: 10.3390/ijms16036353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dawood S, Austin L, Cristofanilli M. Cancer stem cells: implications for cancer therapy. Oncology (Williston Park) 28: 1101–1107, 2014. [PubMed] [Google Scholar]
- 47.Del Fattore A, Luciano R, Pascucci L, Goffredo BM, Giorda E, Scapaticci M, Fierabracci A, Muraca M. Immunoregulatory effects of mesenchymal stem cell-derived extracellular vesicles on T lymphocytes. Cell Transplant 24: 2615–2627, 2015. doi: 10.3727/096368915X687543. [DOI] [PubMed] [Google Scholar]
- 48.Demory Beckler M, Higginbotham JN, Franklin JL, Ham AJ, Halvey PJ, Imasuen IE, Whitwell C, Li M, Liebler DC, Coffey RJ. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol Cell Proteomics 12: 343–355, 2013. doi: 10.1074/mcp.M112.022806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deregibus MC, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone L, Bruno S, Bussolati B, Camussi G. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood 110: 2440–2448, 2007. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- 50.D’Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol 7: 347–358, 2006. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- 51.El Bassit G, Patel RS, Carter G, Shibu V, Patel AA, Song S, Murr M, Cooper DR, Bickford PC, Patel NA. MALAT1 in human adipose stem cells modulates survival and alternative splicing of PKCδII in HT22 cells. Endocrinology 158: 183–195, 2017. doi: 10.1210/en.2016-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Favaro E, Carpanetto A, Lamorte S, Fusco A, Caorsi C, Deregibus MC, Bruno S, Amoroso A, Giovarelli M, Porta M, Perin PC, Tetta C, Camussi G, Zanone MM. Human mesenchymal stem cell-derived microvesicles modulate T cell response to islet antigen glutamic acid decarboxylase in patients with type 1 diabetes. Diabetologia 57: 1664–1673, 2014. doi: 10.1007/s00125-014-3262-4. [DOI] [PubMed] [Google Scholar]
- 53.Feng Q, Zhang C, Lum D, Druso JE, Blank B, Wilson KF, Welm A, Antonyak MA, Cerione RA. A class of extracellular vesicles from breast cancer cells activates VEGF receptors and tumour angiogenesis. Nat Commun 8: 14450, 2017. doi: 10.1038/ncomms14450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Franzen CA, Blackwell RH, Todorovic V, Greco KA, Foreman KE, Flanigan RC, Kuo PC, Gupta GN. Urothelial cells undergo epithelial-to-mesenchymal transition after exposure to muscle invasive bladder cancer exosomes. Oncogenesis 4: e163, 2015. doi: 10.1038/oncsis.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gai C, Pomatto MAC, Grange C, Deregibus MC, Camussi G. Extracellular vesicles in onco-nephrology. Exp Mol Med 51: 29, 2019. doi: 10.1038/s12276-019-0213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galindo-Hernandez O, Serna-Marquez N, Castillo-Sanchez R, Salazar EP. Extracellular vesicles from MDA-MB-231 breast cancer cells stimulated with linoleic acid promote an EMT-like process in MCF10A cells. Prostaglandins Leukot Essent Fatty Acids 91: 299–310, 2014. doi: 10.1016/j.plefa.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 57.Gebeshuber CA, Zatloukal K, Martinez J. miR-29a suppresses tristetraprolin, which is a regulator of epithelial polarity and metastasis. EMBO Rep 10: 400–405, 2009. doi: 10.1038/embor.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gonzalez-Begne M, Lu B, Han X, Hagen FK, Hand AR, Melvin JE, Yates JR. Proteomic analysis of human parotid gland exosomes by multidimensional protein identification technology (MudPIT). J Proteome Res 8: 1304–1314, 2009. doi: 10.1021/pr800658c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Graner MW, Alzate O, Dechkovskaia AM, Keene JD, Sampson JH, Mitchell DA, Bigner DD. Proteomic and immunologic analyses of brain tumor exosomes. FASEB J 23: 1541–1557, 2009. doi: 10.1096/fj.08-122184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, Tetta C, Bussolati B, Camussi G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res 71: 5346–5356, 2011. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- 61.Grange C, Tapparo M, Tritta S, Deregibus MC, Battaglia A, Gontero P, Frea B, Camussi G. Role of HLA-G and extracellular vesicles in renal cancer stem cell-induced inhibition of dendritic cell differentiation. BMC Cancer 15: 1009, 2015. doi: 10.1186/s12885-015-2025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grange C, Tritta S, Tapparo M, Cedrino M, Tetta C, Camussi G, Brizzi MF. Stem cell-derived extracellular vesicles inhibit and revert fibrosis progression in a mouse model of diabetic nephropathy. Sci Rep 9: 4468, 2019. doi: 10.1038/s41598-019-41100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gu D, Zou X, Ju G, Zhang G, Bao E, Zhu Y. Mesenchymal stromal cells derived extracellular vesicles ameliorate acute renal ischemia reperfusion injury by inhibition of mitochondrial fission through miR-30. Stem Cells Int 2016: 2093940, 2016. doi: 10.1155/2016/2093940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guescini M, Guidolin D, Vallorani L, Casadei L, Gioacchini AM, Tibollo P, Battistelli M, Falcieri E, Battistin L, Agnati LF, Stocchi V. C2C12 myoblasts release micro-vesicles containing mtDNA and proteins involved in signal transduction. Exp Cell Res 316: 1977–1984, 2010. doi: 10.1016/j.yexcr.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 65.Guescini M, Genedani S, Stocchi V, Agnati LF. Astrocytes and glioblastoma cells release exosomes carrying mtDNA. J Neural Transm (Vienna) 117: 1–4, 2010. doi: 10.1007/s00702-009-0288-8. [DOI] [PubMed] [Google Scholar]
- 66.György B, Szabó TG, Pásztói M, Pál Z, Misják P, Aradi B, László V, Pállinger E, Pap E, Kittel A, Nagy G, Falus A, Buzás EI. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci 68: 2667–2688, 2011. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harrell CR, Simovic Markovic B, Fellabaum C, Arsenijevic A, Djonov V, Arsenijevic N, Volarevic V. Therapeutic potential of mesenchymal stem cell-derived exosomes in the treatment of eye diseases. Adv Exp Med Biol 1089: 47–57, 2018. doi: 10.1007/5584_2018_219. [DOI] [PubMed] [Google Scholar]
- 68.Herrera MB, Fonsato V, Gatti S, Deregibus MC, Sordi A, Cantarella D, Calogero R, Bussolati B, Tetta C, Camussi G. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J Cell Mol Med 14, 6B: 1605–1618, 2010. doi: 10.1111/j.1582-4934.2009.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Herrera Sanchez MB, Bruno S, Grange C, Tapparo M, Cantaluppi V, Tetta C, Camussi G. Human liver stem cells and derived extracellular vesicles improve recovery in a murine model of acute kidney injury. Stem Cell Res Ther 5: 124–135, 2014. doi: 10.1186/scrt514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hill AF, Pegtel DM, Lambertz U, Leonardi T, O’Driscoll L, Pluchino S, Ter-Ovanesyan D, Nolte-’t Hoen EN. ISEV position paper: extracellular vesicle RNA analysis and bioinformatics. J Extracell Vesicles 2: 22859, 2013. doi: 10.3402/jev.v2i0.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature 527: 329–335, 2015. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hristov M, Erl W, Linder S, Weber PC. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood 104: 2761–2766, 2004. doi: 10.1182/blood-2003-10-3614. [DOI] [PubMed] [Google Scholar]
- 73.Huang X, Yuan T, Tschannen M, Sun Z, Jacob H, Du M, Liang M, Dittmar RL, Liu Y, Liang M, Kohli M, Thibodeau SN, Boardman L, Wang L. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics 14: 319, 2013. doi: 10.1186/1471-2164-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang Z, Feng Y. Exosomes derived from hypoxic colorectal cancer cells promote angiogenesis through Wnt4-induced β-catenin signaling in endothelial cells. Oncol Res 25: 651–661, 2017. doi: 10.3727/096504016X14752792816791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huber V, Fais S, Iero M, Lugini L, Canese P, Squarcina P, Zaccheddu A, Colone M, Arancia G, Gentile M, Seregni E, Valenti R, Ballabio G, Belli F, Leo E, Parmiani G, Rivoltini L. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape. Gastroenterology 128: 1796–1804, 2005. doi: 10.1053/j.gastro.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 76.Iorio MV, Visone R, Di Leva G, Donati V, Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, Calin GA, Ménard S, Croce CM. MicroRNA signatures in human ovarian cancer. Cancer Res 67: 8699–8707, 2007. doi: 10.1158/0008-5472.CAN-07-1936. [DOI] [PubMed] [Google Scholar]
- 77.Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q, Evans R, Fissell WH, Patton JG, Rome LH, Burnette DT, Coffey RJ. Reassessment of exosome composition. Cell 177: 428–445.e18, 2019. doi: 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jiang ZZ, Liu YM, Niu X, Yin JY, Hu B, Guo SC, Fan Y, Wang Y, Wang NS. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res Ther 7: 24–37, 2016. doi: 10.1186/s13287-016-0287-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jin X, Jin X, Kim H. Cancer stem cells and differentiation therapy. Tumour Biol 39: 1010428317729933, 2017. doi: 10.1177/1010428317729933. [DOI] [PubMed] [Google Scholar]
- 80.Ju GQ, Cheng J, Zhong L, Wu S, Zou XY, Zhang GY, Gu D, Miao S, Zhu YJ, Sun J, Du T. Microvesicles derived from human umbilical cord mesenchymal stem cells facilitate tubular epithelial cell dedifferentiation and growth via hepatocyte growth factor induction. PLoS One 10: e0121534, 2015. doi: 10.1371/journal.pone.0121534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jung Y, Kim JK, Shiozawa Y, Wang J, Mishra A, Joseph J, Berry JE, McGee S, Lee E, Sun H, Wang J, Jin T, Zhang H, Dai J, Krebsbach PH, Keller ET, Pienta KJ, Taichman RS. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat Commun 4: 1795, 2013. doi: 10.1038/ncomms2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kalra H, Simpson RJ, Ji H, Aikawa E, Altevogt P, Askenase P, Bond VC, Borràs FE, Breakefield X, Budnik V, Buzas E, Camussi G, Clayton A, Cocucci E, Falcon-Perez JM, Gabrielsson S, Gho YS, Gupta D, Harsha HC, Hendrix A, Hill AF, Inal JM, Jenster G, Krämer-Albers EM, Lim SK, Llorente A, Lötvall J, Marcilla A, Mincheva-Nilsson L, Nazarenko I, Nieuwland R, Nolte-’t Hoen EN, Pandey A, Patel T, Piper MG, Pluchino S, Prasad TS, Rajendran L, Raposo G, Record M, Reid GE, Sánchez-Madrid F, Schiffelers RM, Siljander P, Stensballe A, Stoorvogel W, Taylor D, Thery C, Valadi H, van Balkom BW, Vázquez J, Vidal M, Wauben MH, Yáñez-Mó M, Zoeller M, Mathivanan S. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol 10: e1001450, 2012. doi: 10.1371/journal.pbio.1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Katsuda T, Tsuchiya R, Kosaka N, Yoshioka Y, Takagaki K, Oki K, Takeshita F, Sakai Y, Kuroda M, Ochiya T. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci Rep 3: 1197–1208, 2013. doi: 10.1038/srep01197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P, Mackie AR, Vaughan E, Garikipati VN, Benedict C, Ramirez V, Lambers E, Ito A, Gao E, Misener S, Luongo T, Elrod J, Qin G, Houser SR, Koch WJ, Kishore R. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res 117: 52–64, 2015. doi: 10.1161/CIRCRESAHA.117.305990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kholia S, Herrera Sanchez MB, Cedrino M, Papadimitriou E, Tapparo M, Deregibus MC, Brizzi MF, Tetta C, Camussi G. Human liver stem cell-derived extracellular vesicles prevent aristolochic acid-induced kidney fibrosis. Front Immunol 9: 1639–1655, 2018. doi: 10.3389/fimmu.2018.01639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim DK, Kang B, Kim OY, Choi DS, Lee J, Kim SR, Go G, Yoon YJ, Kim JH, Jang SC, Park KS, Choi EJ, Kim KP, Desiderio DM, Kim YK, Lötvall J, Hwang D, Gho YS. EVpedia: an integrated database of high-throughput data for systemic analyses of extracellular vesicles. J Extracell Vesicles 2: 20384, 2013. doi: 10.3402/jev.v2i0.20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kochan G, Escors D, Breckpot K, Guerrero-Setas D. Role of non-classical MHC class I molecules in cancer immunosuppression. OncoImmunology 2: e26491, 2013. doi: 10.4161/onci.26491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TR, Epple M, Horn PA, Beelen DW, Giebel B. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 28: 970–973, 2014. doi: 10.1038/leu.2014.41. [DOI] [PubMed] [Google Scholar]
- 89.Laulagnier K, Motta C, Hamdi S, Roy S, Fauvelle F, Pageaux JF, Kobayashi T, Salles JP, Perret B, Bonnerot C, Record M. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem J 380: 161–171, 2004. doi: 10.1042/bj20031594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li CC, Eaton SA, Young PE, Lee M, Shuttleworth R, Humphreys DT, Grau GE, Combes V, Bebawy M, Gong J, Brammah S, Buckland ME, Suter CM. Glioma microvesicles carry selectively packaged coding and non-coding RNAs which alter gene expression in recipient cells. RNA Biol 10: 1333–1344, 2013. doi: 10.4161/rna.25281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, Wang M, Zhou Y, Zhu W, Li W, Xu W. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev 22: 845–854, 2013. doi: 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J, Chen D, Gu J, He X, Huang S. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res 25: 981–984, 2015. doi: 10.1038/cr.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lindoso RS, Collino F, Camussi G. Extracellular vesicles derived from renal cancer stem cells induce a pro-tumorigenic phenotype in mesenchymal stromal cells. Oncotarget 6: 7959–7969, 2015. doi: 10.18632/oncotarget.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu C, Yu S, Zinn K, Wang J, Zhang L, Jia Y, Kappes JC, Barnes S, Kimberly RP, Grizzle WE, Zhang HG. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J Immunol 176: 1375–1385, 2006. doi: 10.4049/jimmunol.176.3.1375. [DOI] [PubMed] [Google Scholar]
- 95.Liu S, Ginestier C, Ou SJ, Clouthier SG, Patel SH, Monville F, Korkaya H, Heath A, Dutcher J, Kleer CG, Jung Y, Dontu G, Taichman R, Wicha MS. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res 71: 614–624, 2011. doi: 10.1158/0008-5472.CAN-10-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Llorente A, Skotland T, Sylvänne T, Kauhanen D, Róg T, Orłowski A, Vattulainen I, Ekroos K, Sandvig K. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim Biophys Acta 1831: 1302–1309, 2013. doi: 10.1016/j.bbalip.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 97.Ludwig S, Floros T, Theodoraki MN, Hong CS, Jackson EK, Lang S, Whiteside TL. Suppression of lymphocyte functions by plasma exosomes correlates with disease activity in patients with head and neck cancer. Clin Cancer Res 23: 4843–4854, 2017. doi: 10.1158/1078-0432.CCR-16-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Luedde T. MicroRNA-151 and its hosting gene FAK (focal adhesion kinase) regulate tumor cell migration and spreading of hepatocellular carcinoma. Hepatology 52: 1162–1164, 2010. doi: 10.1002/hep.23854. [DOI] [PubMed] [Google Scholar]
- 99.Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M, Wrana JL. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 151: 1542–1556, 2012. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 100.Mannerström B, Kornilov R, Abu-Shahba AG, Chowdhury IM, Sinha S, Seppänen-Kaijansinkko R, Kaur S. Epigenetic alterations in mesenchymal stem cells by osteosarcoma-derived extracellular vesicles. Epigenetics 14: 352–364, 2019. doi: 10.1080/15592294.2019.1585177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mao G, Liu Y, Fang X, Liu Y, Fang L, Lin L, Liu X, Wang N. Tumor-derived microRNA-494 promotes angiogenesis in non-small cell lung cancer. Angiogenesis 18: 373–382, 2015. doi: 10.1007/s10456-015-9474-5. [DOI] [PubMed] [Google Scholar]
- 102.Matsuura Y, Wada H, Eguchi H, Gotoh K, Kobayashi S, Kinoshita M, Kubo M, Hayashi K, Iwagami Y, Yamada D, Asaoka T, Noda T, Kawamoto K, Takeda Y, Tanemura M, Umeshita K, Doki Y, Mori M. Exosomal miR-155 derived from hepatocellular carcinoma cells under hypoxia promotes angiogenesis in endothelial cells. Dig Dis Sci 64: 792–802, 2019. doi: 10.1007/s10620-018-5380-1. [DOI] [PubMed] [Google Scholar]
- 103.Mathivanan S, Simpson RJ. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics 9: 4997–5000, 2009. doi: 10.1002/pmic.200900351. [DOI] [PubMed] [Google Scholar]
- 104.Mead B, Tomarev S. Bone marrow-derived mesenchymal stem cells-derived exosomes promote survival of retinal ganglion cells through miRNA-dependent mechanisms. Stem Cells Transl Med 6: 1273–1285, 2017. doi: 10.1002/sctm.16-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Motoyama K, Inoue H, Takatsuno Y, Tanaka F, Mimori K, Uetake H, Sugihara K, Mori M. Over- and under-expressed microRNAs in human colorectal cancer. Int J Oncol 34: 1069–1075, 2009. doi: 10.3892/ijo_00000233. [DOI] [PubMed] [Google Scholar]
- 106.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles 3: 24641, 2014. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Muller L, Mitsuhashi M, Simms P, Gooding WE, Whiteside TL. Tumor-derived exosomes regulate expression of immune function-related genes in human T cell subsets. Sci Rep 6: 20254, 2016. doi: 10.1038/srep20254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nabhan JF, Hu R, Oh RS, Cohen SN, Lu Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc Natl Acad Sci USA 109: 4146–4151, 2012. doi: 10.1073/pnas.1200448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nagaishi K, Mizue Y, Chikenji T, Otani M, Nakano M, Konari N, Fujimiya M. Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes. Sci Rep 6: 34842–34858, 2016. doi: 10.1038/srep34842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nawaz M, Shah N, Zanetti BR, Maugeri M, Silvestre RN, Fatima F, Neder L, Valadi H. Extracellular vesicles and matrix remodeling enzymes: the emerging roles in extracellular matrix remodeling, progression of diseases and tissue repair. Cells 7: E167, 2018. doi: 10.3390/cells7100167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nicolson GL. Cell membrane fluid-mosaic structure and cancer metastasis. Cancer Res 75: 1169–1176, 2015. doi: 10.1158/0008-5472.CAN-14-3216. [DOI] [PubMed] [Google Scholar]
- 112.Ogawa Y, Taketomi Y, Murakami M, Tsujimoto M, Yanoshita R. Small RNA transcriptomes of two types of exosomes in human whole saliva determined by next generation sequencing. Biol Pharm Bull 36: 66–75, 2013. doi: 10.1248/bpb.b12-00607. [DOI] [PubMed] [Google Scholar]
- 113.Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 18: 883–891, 2012. [Erratum in Nat Med 22: 1502, 2016.] doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pistoia V, Raffaghello L. Mesenchymal stromal cells and autoimmunity. Int Immunol 29: 49–58, 2017. doi: 10.1093/intimm/dxx008. [DOI] [PubMed] [Google Scholar]
- 115.Qin X, Yu S, Zhou L, Shi M, Hu Y, Xu X, Shen B, Liu S, Yan D, Feng J. Cisplatin-resistant lung cancer cell-derived exosomes increase cisplatin resistance of recipient cells in exosomal miR-100-5p-dependent manner. Int J Nanomedicine 12: 3721–3733, 2017. doi: 10.2147/IJN.S131516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ranghino A, Bruno S, Bussolati B, Moggio A, Dimuccio V, Tapparo M, Biancone L, Gontero P, Frea B, Camussi G. The effects of glomerular and tubular renal progenitors and derived extracellular vesicles on recovery from acute kidney injury. Stem Cell Res Ther 8: 24–39, 2017. doi: 10.1186/s13287-017-0478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200: 373–383, 2013. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rappa G, Mercapide J, Anzanello F, Le TT, Johlfs MG, Fiscus RR, Wilsch-Bräuninger M, Corbeil D, Lorico A. Wnt interaction and extracellular release of prominin-1/CD133 in human malignant melanoma cells. Exp Cell Res 319: 810–819, 2013. doi: 10.1016/j.yexcr.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 20: 847–856, 2006. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 120.Ren K. Exosomes in perspective: a potential surrogate for stem cell therapy. Odontology 107: 271–284, 2019. doi: 10.1007/s10266-018-0395-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Richards KE, Zeleniak AE, Fishel ML, Wu J, Littlepage LE, Hill R. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene 36: 1770–1778, 2017. doi: 10.1038/onc.2016.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol 14: 195–208, 2014. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Santi A, Caselli A, Ranaldi F, Paoli P, Mugnaioni C, Michelucci E, Cirri P. Cancer associated fibroblasts transfer lipids and proteins to cancer cells through cargo vesicles supporting tumor growth. Biochim Biophys Acta 1853: 3211–3223, 2015. doi: 10.1016/j.bbamcr.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 124.Song W, Yan D, Wei T, Liu Q, Zhou X, Liu J. Tumor-derived extracellular vesicles in angiogenesis. Biomed Pharmacother 102: 1203–1208, 2018. doi: 10.1016/j.biopha.2018.03.148. [DOI] [PubMed] [Google Scholar]
- 125.Sung BH, Ketova T, Hoshino D, Zijlstra A, Weaver AM. Directional cell movement through tissues is controlled by exosome secretion. Nat Commun 6: 7164, 2015. doi: 10.1038/ncomms8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sze SK, de Kleijn DP, Lai RC, Khia Way Tan E, Zhao H, Yeo KS, Low TY, Lian Q, Lee CN, Mitchell W, El Oakley RM, Lim SK. Elucidating the secretion proteome of human embryonic stem cell-derived mesenchymal stem cells. Mol Cell Proteomics 6: 1680–1689, 2007. doi: 10.1074/mcp.M600393-MCP200. [DOI] [PubMed] [Google Scholar]
- 127.Takeda YS, Xu Q. Neuronal differentiation of human mesenchymal stem cells using exosomes derived from differentiating neuronal cells. PLoS One 10: e0135111, 2015. doi: 10.1371/journal.pone.0135111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tan CY, Lai RC, Wong W, Dan YY, Lim S, Ho HK. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther 3: 76, 2014. doi: 10.1186/scrt465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol 110: 13–21, 2008. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 130.Taylor DD, Gercel-Taylor C. Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironments. Semin Immunopathol 33: 441–454, 2011. doi: 10.1007/s00281-010-0234-8. [DOI] [PubMed] [Google Scholar]
- 131.Theodoraki MN, Yerneni SS, Hoffmann TK, Gooding WE, Whiteside TL. Clinical Significance of PD-L1+ exosomes in plasma of head and neck cancer patients. Clin Cancer Res 24: 896–905, 2018. doi: 10.1158/1078-0432.CCR-17-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Théry C, Boussac M, Véron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol 166: 7309–7318, 2001. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 133.Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol 2: 569–579, 2002. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 134.Thomson AW, Robbins PD. Tolerogenic dendritic cells for autoimmune disease and transplantation. Ann Rheum Dis 67, Suppl 3: iii90–iii96, 2008. doi: 10.1136/ard.2008.099176. [DOI] [PubMed] [Google Scholar]
- 135.Tian T, Zhu YL, Zhou YY, Liang GF, Wang YY, Hu FH, Xiao ZD. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J Biol Chem 289: 22258–22267, 2014. doi: 10.1074/jbc.M114.588046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tomasoni S, Longaretti L, Rota C, Morigi M, Conti S, Gotti E, Capelli C, Introna M, Remuzzi G, Benigni A. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev 22: 772–780, 2013. doi: 10.1089/scd.2012.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Valenti R, Huber V, Filipazzi P, Pilla L, Sovena G, Villa A, Corbelli A, Fais S, Parmiani G, Rivoltini L. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res 66: 9290–9298, 2006. doi: 10.1158/0008-5472.CAN-06-1819. [DOI] [PubMed] [Google Scholar]
- 138.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19: 213–228, 2018. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 139.Vidal M, Mangeat P, Hoekstra D. Aggregation reroutes molecules from a recycling to a vesicle-mediated secretion pathway during reticulocyte maturation. J Cell Sci 110: 1867–1877, 1997. [DOI] [PubMed] [Google Scholar]
- 140.Villarroya-Beltri C, Baixauli F, Gutiérrez-Vázquez C, Sánchez-Madrid F, Mittelbrunn M. Sorting it out: regulation of exosome loading. Semin Cancer Biol 28: 3–13, 2014. doi: 10.1016/j.semcancer.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vrijsen KR, Sluijter JPG, Schuchardt MWL, van Balkom BW, Noort WA, Chamuleau SA, Doevendans PA. Cardiomyocyte progenitor cell-derived exosomes stimulate migration of endothelial cells. J Cell Mol Med 14: 1064–1070, 2010. doi: 10.1111/j.1582-4934.2010.01081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xiao D, Barry S, Kmetz D, Egger M, Pan J, Rai SN, Qu J, McMasters KM, Hao H. Melanoma cell-derived exosomes promote epithelial-mesenchymal transition in primary melanocytes through paracrine/autocrine signaling in the tumor microenvironment. Cancer Lett 376: 318–327, 2016. doi: 10.1016/j.canlet.2016.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xiao X, Yu S, Li S, Wu J, Ma R, Cao H, Zhu Y, Feng J. Exosomes: decreased sensitivity of lung cancer A549 cells to cisplatin. PLoS One 9: e89534, 2014. doi: 10.1371/journal.pone.0089534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xiao H, Lässer C, Shelke GV, Wang J, Rådinger M, Lunavat TR, Malmhäll C, Lin LH, Li J, Li L, Lötvall J. Mast cell exosomes promote lung adenocarcinoma cell proliferation—role of KIT-stem cell factor signaling. Cell Commun Signal 12: 64, 2014. doi: 10.1186/s12964-014-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG, Chopp M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 30: 1556–1564, 2012. doi: 10.1002/stem.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xu S, Menu E, De Becker A, Van Camp B, Vanderkerken K, Van Riet I. Bone marrow-derived mesenchymal stromal cells are attracted by multiple myeloma cell-produced chemokine CCL25 and favor myeloma cell growth in vitro and in vivo. Stem Cells 30: 266–279, 2012. doi: 10.1002/stem.787. [DOI] [PubMed] [Google Scholar]
- 147.Yang C, Kim SH, Bianco NR, Robbins PD. Tumor-derived exosomes confer antigen-specific immunosuppression in a murine delayed-type hypersensitivity model. PLoS One 6: e22517, 2011. doi: 10.1371/journal.pone.0022517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yen EY, Miaw SC, Yu JS, Lai IR. Exosomal TGF-β1 is correlated with lymphatic metastasis of gastric cancers. Am J Cancer Res 7: 2199–2208, 2017. [PMC free article] [PubMed] [Google Scholar]
- 149.Yu Z, Pestell TG, Lisanti MP, Pestell RG. Cancer stem cells. Int J Biochem Cell Biol 44: 2144–2151, 2012. doi: 10.1016/j.biocel.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang B, Yin Y, Lai RC, Tan SS, Choo ABH, Lim SK. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev 23: 1233–1244, 2014. doi: 10.1089/scd.2013.0479. [DOI] [PubMed] [Google Scholar]
- 151.Zhang B, Yeo RWY, Lai RC, Sim EWK, Chin KC, Lim SK. Mesenchymal stromal cell exosome-enhanced regulatory T-cell production through an antigen-presenting cell-mediated pathway. Cytotherapy 20: 687–696, 2018. doi: 10.1016/j.jcyt.2018.02.372. [DOI] [PubMed] [Google Scholar]
- 152.Zhang X, Zhu W, Zhang J, Huo S, Zhou L, Gu Z, Zhang M. MicroRNA-650 targets ING4 to promote gastric cancer tumorigenicity. Biochem Biophys Res Commun 395: 275–280, 2010. doi: 10.1016/j.bbrc.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 153.Zhang Y, Chopp M, Zhang ZG, Katakowski M, Xin H, Qu C, Ali M, Mahmood A, Xiong Y. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem Int 111: 69–81, 2017. doi: 10.1016/j.neuint.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y, Zhang B, Wang M, Mao F, Yan Y, Gao S, Gu H, Zhu W, Qian H. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther 4: 34–47, 2013. doi: 10.1186/scrt194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhou X, Yan T, Huang C, Xu Z, Wang L, Jiang E, Wang H, Chen Y, Liu K, Shao Z, Shang Z. Melanoma cell-secreted exosomal miR-155-5p induce proangiogenic switch of cancer-associated fibroblasts via SOCS1/JAK2/STAT3 signaling pathway. J Exp Clin Cancer Res 37: 242, 2018. doi: 10.1186/s13046-018-0911-3. [DOI] [PMC free article] [PubMed] [Google Scholar]