Abstract
Smoking is a risk factor for cardiovascular diseases. Prior reports showed a transient increase in blood pressure (BP) following a spontaneous burst of muscle sympathetic nerve activity (MSNA). We hypothesized that this pressor response would be accentuated in smokers. Using signal-averaging techniques, we examined the BP (Finometer) response to MSNA in 18 otherwise healthy smokers and 42 healthy nonsmokers during resting conditions. The sensitivities of baroreflex control of MSNA and heart rate were also assessed. The mean resting MSNA, heart rate, and mean arterial pressure (MAP) were higher in smokers than nonsmokers. The MAP increase following a burst of MSNA was significantly greater in smokers than nonsmokers (Δ3.4 ± 0.3 vs. Δ1.6 ± 0.1 mmHg, P < 0.001). The baroreflex sensitivity (BRS) of burst incidence, burst area, or total activity was not different between the two groups. However, cardiac BRS was lower in smokers than nonsmokers (14.6 ± 1.7 vs. 24.6 ± 1.5 ms/mmHg, P < 0.001). Moreover, the MAP increase following a burst was negatively correlated with the cardiac BRS. These observations suggest that habitual smoking in otherwise healthy individuals raises the MAP increase following spontaneous MSNA and that the attenuated cardiac BRS in the smokers was a contributing factor. We speculate that the accentuated pressor increase in response to spontaneous MSNA may contribute to the elevated resting BP in the smokers.
Keywords: baroreceptor, hemodynamics, nervous system, smoker, sympathetic
INTRODUCTION
Cigarette smoking has a well-established relationship with an increased risk of multiple cardiovascular diseases (2, 31, 36). Although a direct causal linkage between smoking and hypertension remains controversial (10, 30, 48), there are data showing that hypertensive smokers are more likely to develop more severe hypertension (48). Alterations in the autonomic nervous system with chronic smoking are suggested to be important for mediating hemodynamic responses in cardiovascular diseases, including hypertension (17, 20). Some reports have suggested that acute bouts of smoking lead to acute increases in blood pressure (BP) and heart rate (HR) (16, 27, 35, 38). It has been reported that one bout of smoking (16, 38, 39, 42) decreases sympathetic outflow to muscle [muscle sympathetic nerve activity (MSNA)], whereas multiple bouts of smoking raise MSNA (38), in otherwise healthy smokers. Acute nicotine administration also raises catecholamine levels and MSNA in smokers (37). Moreover, resting MSNA is higher in habitual smokers than nonsmokers (20, 40). These effects of smoking on MSNA may be important, because heightened sympathetic nervous system activity is thought to play a role in BP regulation (41). On the other hand, Delius and Wallin and colleagues showed that MSNA is negatively correlated with diastolic BP (DBP) within individuals via a baroreflex-mediated mechanism (8, 45). Furthermore, there is no relationship between interindividual differences in levels of BP and MSNA burst rate in healthy subjects (45, 49). Thus it is unclear how a rise in the levels of resting MSNA would contribute to elevated BP levels in smokers.
An early study by Wallin and Nerhed (51) showed a transient increase in BP following a spontaneous burst of MSNA. A later report (47) showed that sex and aging affect this transient pressor response following a MSNA burst. It was speculated that differences in vascular function, including changes in α-adrenergic receptor responsiveness, may contribute to the amplitude of the transient BP increase following a MSNA burst (47). It is well known that chronic cigarette smoking impairs vascular function (36). Others have shown that smoking impairs endothelial function (19). Based on these reports, we speculated that the transient BP increase following a spontaneous burst of MSNA may be altered in smokers. Additionally, Wallin and Nerhed (51) showed that the transient BP increase begins 1–2 s after a MSNA burst and peaks after five to seven heartbeats. We speculated that, during this period of rising BP, the arterial baroreflex would buffer the BP increase by attenuating the MSNA response and decreasing HR [or suppressing the HR increase that could be induced by other factor(s) (6)] and, in turn, by decreasing cardiac output (or suppressing the increase in cardiac output). If baroreflex sensitivity (BRS) is impaired/attenuated, the baroreflex may be less able to tightly control BP, HR, and MSNA in these individuals than in those with normal BRS. In turn, the peak increase in BP may be accelerated. Our line of reasoning is illustrated in Fig. 1 with representative recordings. In this report we tested the hypothesis that habitual cigarette smoking could alter the BP response to a MSNA burst by increasing vascular responsiveness and by acting to attenuate HR and MSNA adjustment during the period of rising BP.
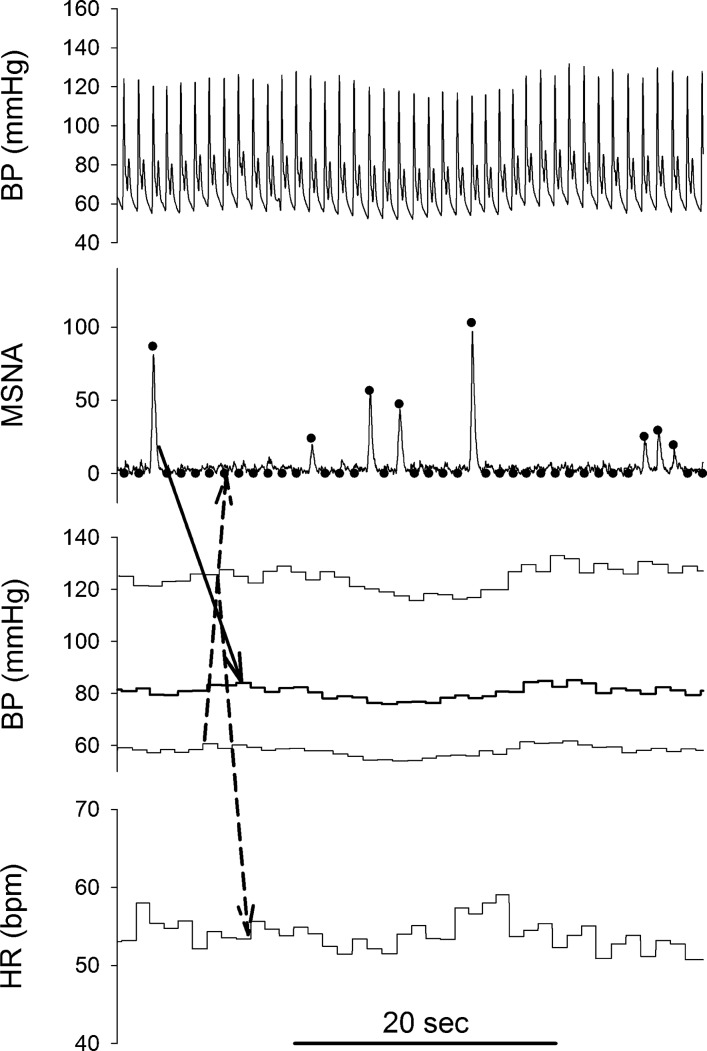
Fig. 1.
Representative original Finometer recording of blood pressure (BP), normalized integrated muscle sympathetic nerve activity (MSNA) neurogram, and beat-by-beat BP [systolic BP, mean arterial pressure (MAP), and diastolic BP] and heart rate (HR, beats/min) recordings. ●, processed beat-by-beat data in MSNA neurogram. When no MSNA was detected in a cardiac cycle, a value of zero was assigned to the cardiac cycle. Solid-line arrow, MAPIncrease following a spontaneous MSNA burst; dashed-line arrows, our speculation that when diastolic and systolic BPs were rising, the baroreflex should inhibit MSNA and tend to decrease HR (or suppress a HR increase that could be induced by other factors).
Existing literature suggests that smoking impairs baroreflex function. Prior studies have shown that acute bouts of smoking impair the baroreflex control of HR (cardiac baroreflex) in smokers (14, 33). Moreover, cardiac BRS is lower in smokers than nonsmokers (13). Finally, it is known that acute bouts of smoking impair the BRS of total MSNA (16). Moreover, the BRS of MSNA burst rate was reported to be lower in female smokers than in nonsmokers during steady-state infusion of phenylephrine and nitroprusside (34). Prior work (26) has suggested that MSNA is controlled at two central “sites”: one that controls MSNA burst incidence via an effect on “gating” and a central site that controls the strength of a sympathetic burst (i.e., burst area). It is speculated that baroreflex input primarily affects gating, whether or not a burst occurs. To our knowledge, there are no reports of BRS of MSNA burst incidence, burst area, and total activity in habitual smokers and nonsmokers during spontaneous BP changes.
Therefore, we hypothesized that the BP responses to the spontaneous MSNA bursts would be accentuated in habitual smokers and that the impaired baroreflex function in smokers would contribute to this accentuated BP response. We further postulated that this accentuated BP increase following MSNA bursts would contribute to elevated resting BP in smokers.
METHODS
Subjects
Data from 18 smokers (13 male and 5 female, 30 ± 2 yr of age) and 42 nonsmokers (30 male and 12 female, 27 ± 1 yr of age) are included in this report. Data from 23 of the 42 nonsmokers were collected previously under the same conditions. These previously collected data were not included in prior publications. To examine if some of our findings are also observed in nonsmokers alone, more nonsmokers than smokers were included in the study. Height, body weight, and body mass index (BMI) of the subjects are listed in Table 1. The smokers reported that they smoked 17 ± 1 cigarettes per day over 13 ± 2 yr. The nonsmokers had no history of smoking. All smokers and nonsmokers were normotensive (Table 1) and in good health, and none were taking medications. The experimental protocols were approved by the Institutional Review Board of the Milton S. Hershey Medical Center and conformed to the Declaration of Helsinki. The purposes and risks of the protocol were explained to each subject before written informed consent was obtained.
Table 1.
Characteristics, hemodynamic variables, and MSNA of smokers and nonsmokers
| Smokers | Nonsmokers | P | |
|---|---|---|---|
| Age, yr | 30 ± 2 | 27 ± 1 | 0.091 |
| Height, cm | 177.5 ± 2.1 | 176.1 ± 1.5 | 0.613 |
| Weight, kg | 81.0 ± 5.2 | 77.7 ± 2.0 | 0.910 |
| BMI, kg/m2 | 22.4 ± 1.3 | 24.9 ± 0.4 | 0.753 |
| SBP, mmHg | 123 ± 4 | 119 ± 2 | 0.272 |
| DBP, mmHg | 78 ± 2* | 66 ± 1 | <0.001 |
| MAP, mmHg | 93 ± 2* | 84 ± 1 | <0.001 |
| HR, beats/min | 65.9 ± 1.7* | 59.9 ± 1.3 | 0.009 |
| MSNABR, bursts/min | 28.3 ± 2.1* | 17.8 ± 1.2 | <0.001 |
| MSNABI, bursts/100 beats | 43.3 ± 3.4* | 29.9 ± 2.0 | <0.001 |
| MSNA total, units/min | 518 ± 54* | 369 ± 28 | 0.017 |
Values are means ± SE; n = 18 smokers and 42 nonsmokers. Systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP) were measured using an automated sphygmomanometer from the brachial artery. Heart rate (HR) was obtained from the electrocardiogram. BMI, body mass index; MSNA, muscle sympathetic nerve activity; MSNABR, MSNA burst rate; MSNABI, MSNA burst incidence.
P < 0.05 vs. nonsmokers.
Measurements
As described previously (5), HR was recorded from a standard three-lead electrocardiogram (Cardicap/5, Datex-Ohmeda, GE Healthcare, Piscataway, NJ). An automated sphygmomanometer (SureSigns Vs3, Philips, Andover, MA) was used to obtain systolic BP (SBP), DBP, and mean arterial pressure (MAP) from the brachial artery. Beat-by-beat BP was recorded from a finger (Finometer, Finapres Medical Systems, Amsterdam, The Netherlands), with resting values verified with the automated sphygmomanometer data from the brachial artery.
As described in our previous reports (4, 5), multifiber recordings of MSNA were obtained with a tungsten microelectrode inserted into the peroneal nerve. A reference electrode was placed subcutaneously 2–3 cm from the recording electrode. The recording electrode was adjusted until a site was found in which muscle sympathetic bursts were clearly identified using previously established criteria (46). The nerve signal was amplified, filtered with a bandwidth of 500–5,000 Hz, integrated with a time constant of 0.1 s (Iowa Bioengineering, Iowa City, IA), and then routed to a loudspeaker and a computer for monitoring. As described in our previous report (4, 5), MSNA bursts were identified in real time by visual inspection of the data, coupled with the burst sound from the audio amplifier, to determine if the MSNA recording was stable during the study.
Protocol
All subjects refrained from caffeine, alcohol, and exercise for 24 h before the study. All subjects had a light breakfast before the study. Smoking was not restricted or limited before the smokers arrived in the laboratory on the morning of the study. No smoking was allowed after the subjects arrived in the laboratory. All subjects were tested in the supine position in a temperature-controlled (23 ± 1°C) room. After instrumentation, the subjects were asked to remain quiet for an acclimation period of ≥5 min or until the hemodynamic variables and MSNA were stable. Subsequently, all variables were continuously recorded for 6 min as the subjects remained supine and still. Automated sphygmomanometer BPs were obtained two or three times during this period. Data collection started ~80–120 min after the subjects arrived in the laboratory, and no smoking was allowed during this period.
Data Analysis
MSNA and hemodynamic variables.
Data were sampled at 200 Hz via a data acquisition system (MacLab, ADInstruments, Castle Hill, Australia). All data files were coded and then analyzed by individuals who were blinded to the group, sex, and age of the subjects. The MSNA bursts were evaluated by a computer program that identified bursts based on fixed criteria, including an appropriate latency following the R wave of the electrocardiogram and a ≥2:1signal-to-noise ratio. The bursts were also manually checked on a beat-by-beat basis. For normalization of integrated MSNA, a value of 100 was assigned to the mean amplitude of the top 10% of the largest bursts during the recording in resting conditions (4, 5). Total MSNA for each cardiac cycle was calculated from burst area of the integrated neurogram. If a burst was not detected for a given cardiac cycle, a value of zero was assigned to that cycle (Fig. 1). Beat-by-beat HR, RR interval (RRI), SBP, DBP, MAP, and MSNA were calculated simultaneously using a computer program (4, 5). Mean MSNA data are reported as burst rate (bursts/min), burst incidence (bursts/100 heartbeats), and total activity (total burst area/min).
MAP response to MSNA bursts.
The relationship between spontaneous bursts of MSNA and the ensuing changes in MAP (47) was characterized using a signal-averaging principle, as described elsewhere (47, 51). The signal-averaging process uses signal summation to progressively increase the signal events correlated with the stimulus while decreasing the amplitude of uncorrelated “stray” events. This signal-averaging method can reveal the mean effect of a series of MSNA bursts on BP responses. Beat-by-beat MAP and HR were sampled at the same frequency (i.e., 200 Hz) with other variables (Fig. 1). For each detected MSNA burst, the MSNA, MAP, and HR trace segments were aligned according to the time of the MSNA burst peak, and the mean course of the responses was calculated. Then the peak MAP responses (i.e., maximal changes) were obtained and are termed the “MAPIncrease” (Fig. 2). This index represents the average peak amplitude of the BP increase following each of the MSNA bursts during the period (e.g., 6 min). The average duration of the peak response is only about one to two beats (47, 51). Similarly, the maximal HR decrease was determined (Fig. 2) and termed the “HRDecrease.” The HRDecrease was obtained around the time that the MAPIncrease occurred. This index differs from the transient HR increase noted by Wallin and Nerhed (51), which was measured just after the burst and before the BP increase.
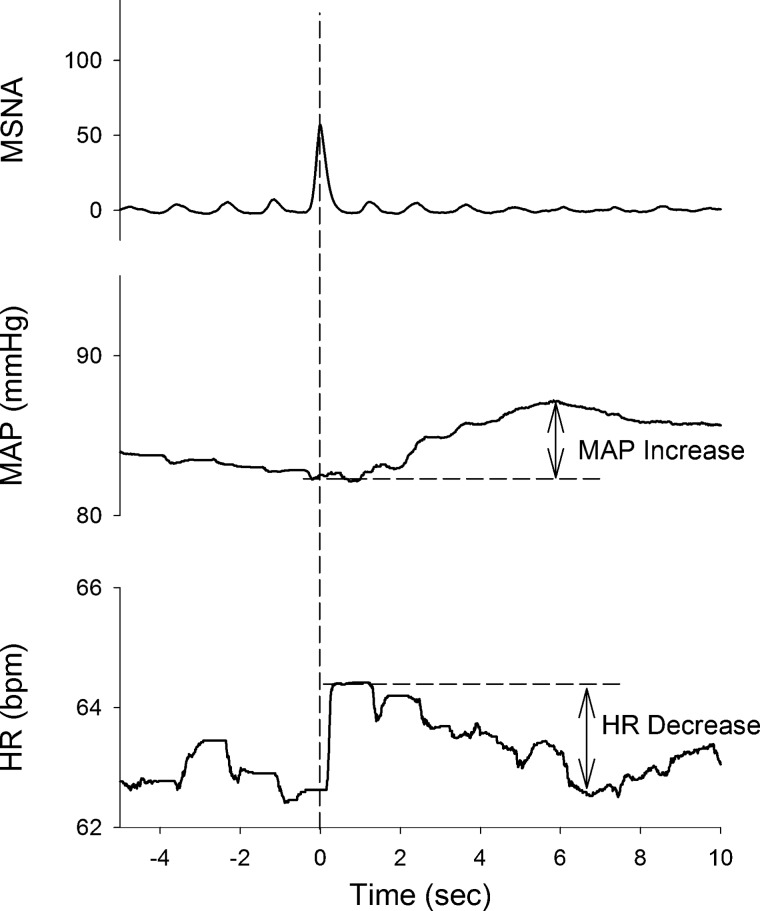
Fig. 2.
Representative averaged traces of mean arterial pressure (MAP) and heart rate (HR, beats/min) following spontaneous bursts of muscle sympathetic nerve activity (MSNA) during 6 min of recording in a smoker. Traces were aligned to the peak time of each identified MSNA burst. In the averaged curves, maximal MAP increase (MAPIncrease) and HR decrease (HRDecrease) occurred ~5–7 s after the peak of MSNA.
Because the MSNA burst rate varies considerably among the individuals, MAPIncrease is unlikely to reflect the effects on BP increase induced by the MSNA bursts in a given period. In an attempt to characterize the effects of the postjunctional responsiveness (i.e., MAPIncrease) and the MSNA burst rate, we multiplied MAPIncrease by MSNA burst rate and termed this index “MAPIncrease*MSNABR.” We also multiplied MAPIncrease by MSNA burst incidence and termed this index “MAPIncrease*MSNABI.”
Baroreflex sensitivity.
As described in previously (5, 25, 26), the baroreflex control of MSNA (burst incidence, burst area, and total activity) was evaluated by analyzing the relationship between beat-by-beat spontaneous variations in DBP and MSNA. Briefly, DBP values for all the cardiac cycles were grouped into 3-mmHg intervals (bins). For a given 3-mmHg DBP bin, “burst incidence” (i.e., 100 × number of MSNA bursts/number of cardiac cycles), “burst area” (i.e., total burst area/number of MSNA bursts), and “total activity” (i.e., total burst area/number of cardiac cycles) were calculated. With all binned data, the slopes of the relationship between mean DBP and burst incidence, burst area, and total MSNA were identified using linear regression analyses. The regression data were accepted when the correlation coefficient (R) between DBP and MSNA burst incidence was >0.7. For all linear regression analyses, the data were weighted for the number of cardiac cycles for each DBP bin. These slopes were used as indexes for the MSNA BRS.
As described previously (5, 14, 33, 44), cardiac baroreflex control was estimated using the sequence technique. The beat-by-beat time series of SBP and RRI were analyzed using HemoLab software (Harald Stauss Scientific). Briefly, sequences of four or more consecutive beats where SBP and RRI changed in the same direction were identified as arterial baroreflex sequences (44). A linear regression was applied to each individual sequence, and only those sequences with R2 > 0.80 were accepted. The slopes of these sequences were calculated as a measure of spontaneous cardiac BRS.
Statistical Analysis
Statistical analysis was determined using SigmaPlot (version 14, Systat Software). An unpaired t-test was employed to examine differences in the variables between the smokers and nonsmokers. When the normality (Shapiro-Wilk) test failed, the Mann-Whitney rank sum test was employed. Pearson’s correlation was used to assess relationships between the variables (e.g., MAPIncrease and cardiac BRS). The level of significance was set at P < 0.05. Values are means ± SE; some data are also presented as box-and-whisker plots.
RESULTS
There was no difference in age, height, body weight, or BMI between smokers and nonsmokers (Table 1). SBP was not different between the groups. DBP, MAP, and HR were significantly higher in smokers than nonsmokers. MSNA burst rate and incidence were significantly higher in smokers than nonsmokers (Table 1).
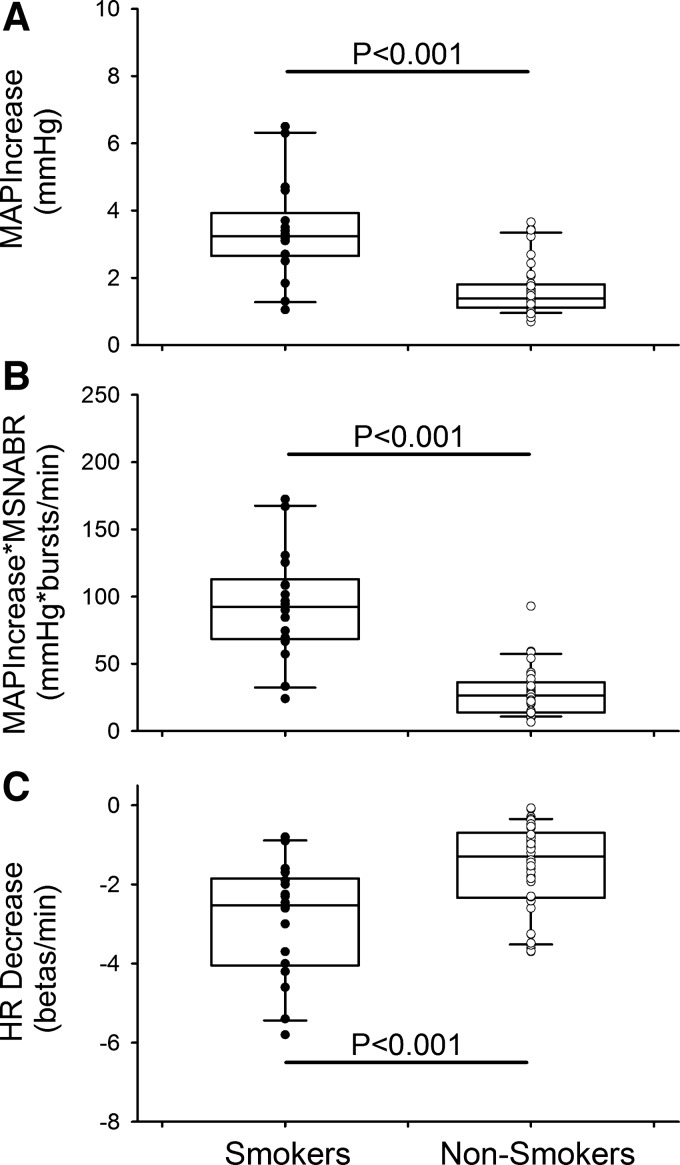
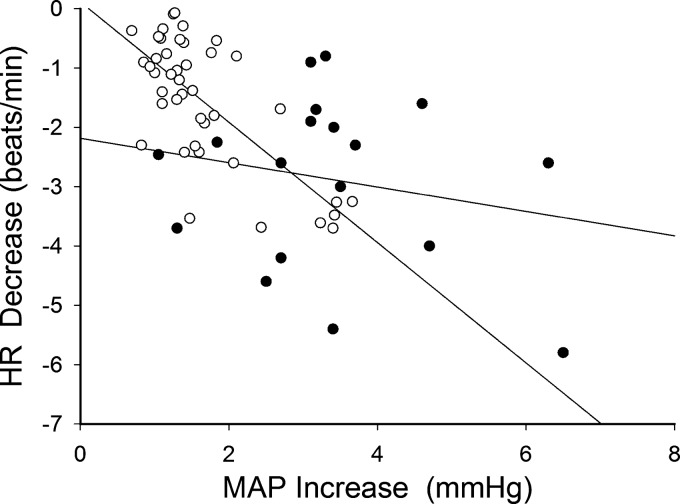
The peak increase in MAP following a spontaneous MSNA burst (i.e., MAPIncrease) was significantly greater in smokers than nonsmokers (3.4 ± 0.3 vs. 1.6 ± 0.1 mmHg, P < 0.001; Fig. 3). The product of the MAPIncrease and the MSNA burst rate (i.e., MAPIncrease*MSNABR) was significantly greater in smokers than nonsmokers (93.0 ± 9.3 vs. 28.5 ± 2.7 mmHg·bursts·min−1, P < 0.001; Fig. 3). Moreover, the product of the MAPIncrease and the MSNA burst incidence (i.e., MAPIncrease*MSNABI) was also significantly greater in smokers than nonsmokers (142.9 ± 15.7 vs. 48.2 ± 4.6 mmHg·bursts·100 beats−1, P < 0.001). The maximal HR decrease (HRDecrease) that accompanied the MAPIncrease was significantly greater in smokers than nonsmokers (−2.9 ± 0.3 vs. −1.6 ± 0.2 beats/min, P < 0.001; Fig. 3). Individual data for HRDecrease were significantly correlated with individual data for MAPIncrease in nonsmokers (R = −0.719, P < 0.001). However, this correlation was loose in smokers (R = −0.206, P = 0.413). Moreover, the slope of the regression line was significantly less steep in smokers than nonsmokers (−0.206 vs. −1.104 beats·min−1·mmHg−1, P = 0.004; Fig. 4).
Fig. 3.
Top: averaged mean arterial pressure (MAP) increase (MAPIncrease) following a spontaneous muscle sympathetic nerve activity (MSNA) burst in smokers and nonsmokers. The averaged MAPIncrease was significantly higher in smokers than nonsmokers. Middle: product of MAPIncrease and MSNA burst rate (MSNABR) was also significantly higher in smokers than nonsmokers. Bottom: averaged heart rate (HR) decrease (HRDecrease) was significantly greater in smokers than nonsmokers. Individual data points for smokers (●, n = 18) and nonsmokers (○, n = 42) are shown on box-and-whisker plots.
Fig. 4.
Relationship between mean arterial pressure (MAP) increase (MAPIncrease) and heart rate (HR) decrease (HRDecrease) following a spontaneous muscle sympathetic nerve activity (MSNA) burst in nonsmokers (R = −0.719, P < 0.001; ○, n = 42) and smokers (R = −0.206, P = 0.413; ●, n = 18). Slope of the regression line was significantly smaller in smokers than nonsmokers (−0.206 vs. −1.104 beats·min−1·mmHg−1, P = 0.004).
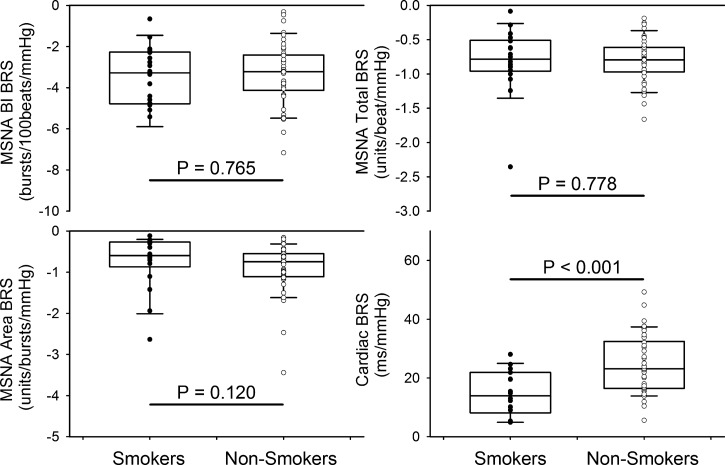
BRS indexes are presented in Fig. 5. The cardiac BRS was significantly lower in smokers than nonsmokers (14.6 ± 1.8 vs. 24.6 ± 1.5 ms/mmHg, P < 0.001). There were no significant differences in the three MSNA BRS indexes between smokers and nonsmokers.
Fig. 5.
Sensitivities of baroreflex control of muscle sympathetic nerve activity (MSNA) burst incidence (BI), burst area, total activity, and heart rate [cardiac baroreflex sensitivity (BRS)]. Individual data points for smokers (●, n = 18) and nonsmokers (○, n = 42) are shown on box-and-whisker plots.
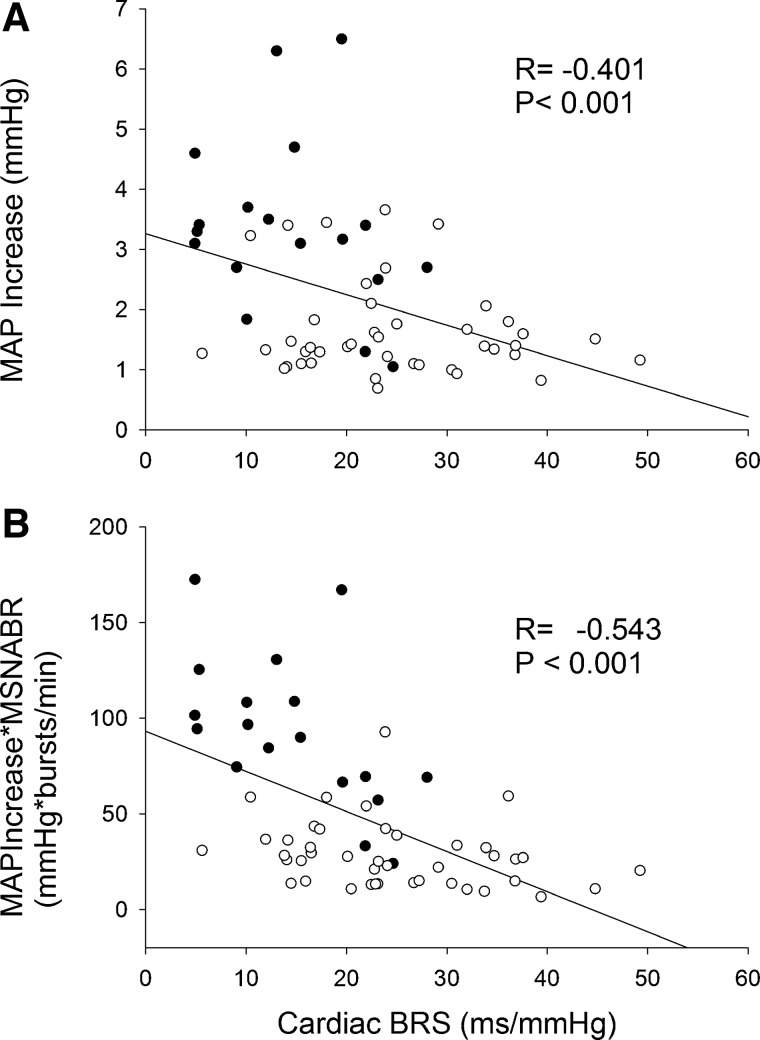
When all data from smokers and nonsmokers were calculated, MAPIncrease negatively correlated with cardiac BRS (Fig. 6). MAPIncrease*MSNABR (Fig. 6) and MAPIncrease*MSNABI (R = −0.496, P < 0.001) also negatively correlated with cardiac BRS. MAPIncrease, MAPIncrease*MSNABR, or MAPIncrease*MSNABI did not correlate with any of the MSNA BRS indexes (all P > 0.05).
Fig. 6.
Top: relationship between cardiac baroreflex sensitivity (BRS) and mean arterial pressure increase (MAPIncrease) following a spontaneous muscle sympathetic nerve activity (MSNA) burst. Bottom: the product of MAPIncrease and MNSA burst rate (MNSABR) also negatively correlated to cardiac BRS. ●, Smokers (n = 18); ○, nonsmokers (n = 42).
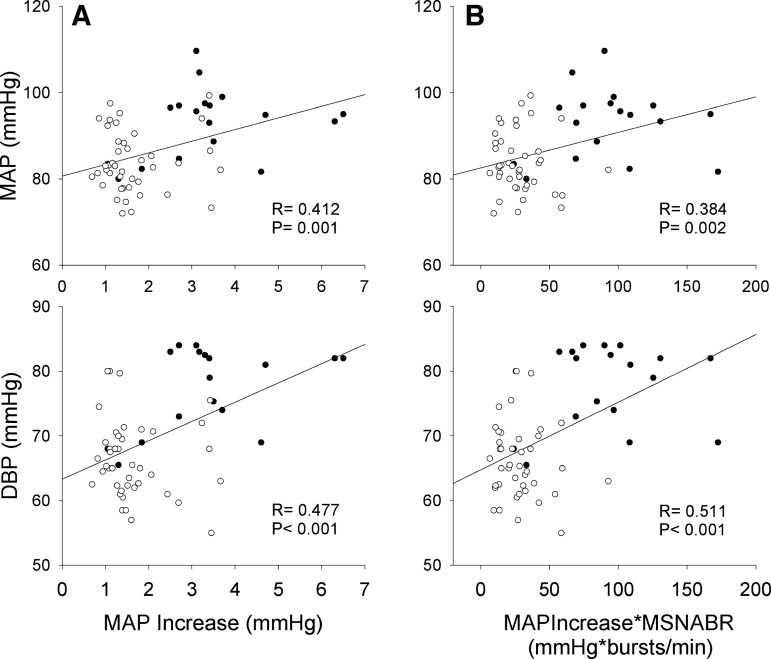
Figure 7 shows positive correlations between resting BPs (i.e., DBP and MAP) and MAPIncrease and MAPIncrease*MSNABR. Moreover, resting DBP (R = 0.449, P < 0.001) and MAP (R = 0.339, P = 0.008) also positively correlated with MAPIncrease*MSNABI. There was no significant (all P > 0.05) correlation between the other measured variables (e.g., SBP) and MAPIncrease.
Fig. 7.
Left: relationship between mean arterial pressure (MAP) increase (MAPIncrease) following a spontaneous muscle sympathetic nerve activity (MSNA) burst and resting diastolic blood pressure (DBP) and MAP. Right: resting DBP and MAP also positively correlated to the product of MAPIncrease and MNSA burst rate (MSNABR). ●, Smokers (n = 18); ○, nonsmokers (n = 42).
DISCUSSION
The important findings of this study are as follows: 1) In otherwise healthy individuals, habitual smoking leads to a larger peak MAP response following a spontaneous MSNA burst. 2) The attenuated cardiac BRS in smokers can be among the factors that contribute to the accentuated MAP increase after a spontaneous MSNA burst. 3) The MSNA BRS is not altered by habitual smoking. 4) The higher MAPIncrease in response to spontaneous MSNA bursts may have contributed to the higher resting BP in smokers than nonsmokers.
Hemodynamic Variables
It has been suggested that acute bouts of smoking lead to acute increases in BP and HR (16, 27, 35, 38), as well as cardiac output (27), but do not alter stroke volume (27). Regarding the chronic effects of smoking, some prior reports showed higher HR (13, 20, 27) and BPs (13) in smokers than nonsmokers, while others found similar HR and/or BPs in smokers and nonsmokers (32, 40). A prior report (27) suggests that the resting cardiac output index and the stroke volume index are not different between nonsmokers and smokers during nonsmoking conditions.
In the present study, resting HR was significantly higher in smokers than nonsmokers. Although the smokers we studied were nonhypertensive, DBP and MAP were significantly higher than in the nonsmokers. We speculate that the differences in the hemodynamic variables could be due to differences in the populations studied (e.g., age, and amount and duration of smoking) in these different reports.
Muscle Sympathetic Nerve Activity
It has been reported that MSNA falls with one bout of smoking in otherwise healthy smokers (16, 38, 39, 42). The decrease in MSNA with smoking was thought to be due to engagement of the baroreflex, as BP actually increased (16, 38). This acute decrease in MSNA with smoking was not seen in older smokers (22) or in patients with coronary disease (42).
In contrast to these prior studies performed during acute smoking, we compared the resting MSNA in habitual, but not acute, smokers and nonsmokers. Our data show higher MSNA burst rate and burst incidence in smokers than nonsmokers. These observations are consistent with prior reports of higher baseline plasma catecholamine concentrations in smokers than nonsmokers (29) and with prior MSNA data collected in otherwise healthy female smokers (40) and hypertensive smokers (20).
Baroreflex Sensitivity
An acute bout of smoking has been shown to lower cardiac BRS in smokers (14, 33). Also, daytime cardiac BRS has been shown to be lower in habitual smokers than nonsmokers (13). The present data are consistent with these prior reports and suggest that habitual smoking impairs baroreflex control of HR even under nonsmoking conditions.
Grassi and colleagues (16) examined the slope of total MSNA as a function of MAP in response to an acute bout of smoking (i.e., MSNA BRS) as well as the slope of this relationship with intravenous infusion of phenylephrine during a nonsmoking period. The data (16) showed lower MSNA BRS during the smoking than the nonsmoking period. In habitual smokers, Middlekauff et al. (34) examined the BRS of MSNA burst rate and cardiac BRS in response to the BP changes evoked by steady infusion of phenylephrine and nitroprusside, respectively. They found that the MSNA responses to changes in BP were less in female habitual smokers than female nonsmokers. However, they found no difference in cardiac BRS between female smokers and nonsmokers (34). Our data show no difference in BRS indexes for MSNA burst incidence, burst area, and total activity between smokers and nonsmokers, although the operating points of baroreflex control of MSNA were shifted. The findings of Middlekauff et al. (34) are different from ours for a number of reasons. First, we used spontaneous BP changes to examine BRS, whereas Middlekauff et al. (34) used steady infusion of phenylephrine and nitroprusside to alter BP in their examination of BRS indexes. Second, we studied men and women, whereas Middlekauff et al. (34) studied only women. Prior reports (21, 35) suggested sex differences in effects of smoking. For example, acute smoking increases BP to a greater extent in women than men (21). Thus, Middlekauff et al. speculated that habitual smoking leads to greater baroreflex impairment in women than men (35). Because of the challenges in recruiting smokers and because there are more male than female smokers in the general population (24, 43), more men than women were studied for this report. The combination of the present results and the prior observations by Middlekauff et al. (35) suggest that habitual smoking may lead to greater baroreflex impairment in women than men.
In our report, cardiac BRS was impaired, but MSNA BRS was not attenuated, in the habitual smokers. A prior study (38) also suggested that acute smoking evoked a greater impairment in cardiac BRS than MSNA BRS. It has been suggested that separate central mechanisms control autonomic outflows directed to skeletal muscles and the heart (50). It is possible that sympathetic nerve activity may be organ-specific and that the sympathetic outflow to varied organs may not change in parallel. Additionally, nicotine may alter sympathetic transmitter release in the heart (28). On the other hand, a number of prior studies have shown that acute and chronic cigarette smoking attenuates vagal tone (9, 18), which may suggest that vagal activity is impaired in smokers. Since vagal activity importantly contributes to the baroreflex-induced rapid changes in HR (23), we speculate that the impaired vagal activities may contribute to impaired cardiac BRS. Thus we believe that there is clear scientific support for the concept that cigarette smoking (acute and chronic) has greater effects on cardiac BRS than MSNA BRS.
BP Increase Following a Spontaneous MSNA Burst and Possible Mechanisms
Our finding that the peak rise in BP following a spontaneous burst of MSNA was significantly greater in smokers than nonsmokers supports our hypothesis. The MAPIncrease for nonsmokers was ~Δ1.6 mmHg, which is lower than the previously reported peak BP increase of ~2.5–3 mmHg (47, 51). The difference between our results and those reported previously could be due to the difference in BP measurements. Beat-by-beat BP was measured using photoplethysmography from a finger (Finometer) in the present study. Although these measurements were validated with automated sphygmomanometer data from an upper arm, the Finometer data could still differ from the arterial line measurements in the previous reports (47, 51).
We did not examine the potential influence of variations in burst size (47) or the burst pattern on MAP responses. Our interest was to examine the sum effects of all MSNA bursts on BP elevation. The MAPIncrease was a mean value of peak BP responses after all MSNA bursts. It does not include information regarding the sum effects of the BP responses over a given period. Thus we calculated the product of MAPIncrease and MSNA burst rate (or burst incidence). Our data showed that MAPIncrease*MSNABR and MAPIncrease*MSNABI were greater in smokers than nonsmokers. These indexes indicate that transient BP elevations also increased in frequency or duration in smokers compared with nonsmokers.
Smokers were not significantly older than nonsmokers. The greater pressor response in smokers cannot be attributed to aging, since a prior study (47) showed that aging decreased the pressor response. We speculate that several factors likely contribute to the accentuated MAP response to discharge of MSNA bursts. First, the prior study (47) postulated that differences in α-adrenergic receptor responsiveness contribute to the BP response to bursts of MSNA. Cardiovascular activation after acute nicotine exposure has been attributed to noradrenergic activation (1). Another prior study (3) showed that smoking raises BP via α-adrenergic receptor-mediated vasoconstriction. Moreover, coronary artery ring studies suggest that vasoconstrictor responses to epinephrine are enhanced by nicotine (smoking) (7). Second, it is possible that more neurotransmitters may be released during the sympathetic engagement in smokers, since plasma catecholamine concentrations have been shown to be higher in smokers than nonsmokers (29). On the other hand, the higher MSNA burst rate in smokers may increase the chances of consecutive bursts, which may be important because, as reported elsewhere (12), multiple consecutive bursts may induce greater vasoconstriction than single bursts. Third, it has been suggested that habitual smoking decreases β-adrenergic receptor density in smokers (29). Finally, other potential contributors to the effects of habitual smoking on the BP response to bursts of MSNA include potential effects of smoking on blood vessel distensibility (11).
Although cardiac BRS was attenuated in smokers, the cardiac baroreflex remained intact (i.e., negative closed loop). Thus a greater BP increase would induce a greater HRDecrease in smokers. On the other hand, our data showed a weaker correlation between MAPIncrease and HRDecrease in smokers than nonsmokers, which may suggest that BP and HR were more loosely controlled in the smokers than nonsmokers. This observation could point to the roles of the baroreflex in evoking the MAPIncrease following a MSNA burst. Indeed, we found a negative correlation between the MAPIncrease following a MSNA burst and cardiac BRS (Fig. 6). This observation suggests that, in individuals with less ability to control BP and HR (e.g., smokers with lower cardiac BRS), the BP response to a burst of MSNA would be greater than in those with a greater ability to more tightly control BP and HR (e.g., healthy individuals with normal BRS). This result suggests that the lower/attenuated cardiac BRS in smokers contributes to the higher MAP in response to a burst of MSNA. This finding supports one of our hypotheses. Additionally, the significant correlations between cardiac BRS and MAPIncrease*MSNABR also support our hypothesis. A prior study (42) showed a negative correlation between the changes in MSNA burst rate in response to acute smoking and cardiac BRS.
Since BP is a product of cardiac output and total peripheral resistance, the changes in cardiac output should also contribute to the BP response following a MSNA burst. A prior report (27) showed that the stroke volume index under a resting (nonsmoking) condition in smokers was not different (i.e., nonsignificantly lower) from that in nonsmokers. Thus the difference in the transient HR response may reflect the difference in the transient response in cardiac output between the two groups in the present study. As discussed above, the smokers may have a reduced ability to control the HR response and, in turn, a reduced ability to control the transient response in cardiac output. Thus we speculate that an impaired cardiac output response may also contribute to the accentuated BP response in smokers. This issue should be examined in further studies.
The impaired cardiac BRS may be only one of the factors that contribute to the accentuated pressor response in smokers. Although the correlation between cardiac BRS and MAPIncrease was significant (P < 0.001), the correlation coefficient was not high. This suggests that other factors (as discussed above) likely also contribute to the accentuated MAP response to discharge of MSNA bursts. The significant correlation between cardiac BRS and MAPIncrease was observed only when data from both groups were combined. No significant correlation was observed for the data of smokers alone (R = −0.243, P = 0.331, n = 18). To examine if this correlation could be observed in normal subjects, data from many more nonsmokers (n = 42) were included in this study. However, no significant correlation was observed for the data from nonsmokers alone (R = −0.156, P = 0.323). The mechanism for this finding is unclear; however, we speculate that the following factors may contribute to it. First, the value ranges for BRS or MAPIncrease within each group were narrower than those in the combined data. Second, the other factors that contribute to MAPIncrease may induce the variations in the data. The combination of these two factors may decrease the correlation coefficient and the statistical significance.
Possible Roles of the Accentuated BP Responses Following Spontaneous MSNA Bursts in Smokers
Our data show larger BP responses following spontaneous MSNA bursts in smokers than nonsmokers. Our data also show a correlation between MAPIncrease and the resting DBP (Fig. 7). Not only the postjunctional responsiveness (indexed with MAPIncrease), but also the MSNA burst rate, could contribute to the elevated BP in smokers. This linkage is supported by the correlation between MAPIncrease*MSNABR and resting DBP. We believe that these observations suggest that sympathetic activation contributes to higher resting BP in smokers. Wallin and Nerhed (51) showed that DBP increases following bursts of MSNA. However, we cannot exclude the possibility that as the baseline BP rises, systemic buffering capacity of the vascular system in response to a vasoconstrictor stimulus is attenuated. Further studies are necessary to examine these potential explanations.
Study Limitations
The MSNA, HR, and BP data obtained from our group of smokers were collected after ~1.5–2 h of nonsmoking. We cannot exclude the possibility that this period of abstinence could have evoked discomfort and irritability. In turn, these factors may have contributed to the level of MSNA and the higher HR. This study was performed in the supine position only. Thus the results cannot be applied to other postures (e.g., upright position), since orthostatic stress can dramatically alter the cardiovascular system.
In this report, only healthy and relatively young (mean 30 yr old) smokers were studied. HR, DBP, and MSNA burst rate were higher in these smokers than in the nonsmoking control subjects. However, the smokers were not hypertensive. Their MSNA burst rates (or incidence) were not as high as those of hypertensive subjects or patients with heart failure (15). Thus we speculate that the results obtained from this study cannot be applied to patient cohorts. Additionally, we cannot exclude the possibility that MSNA BRS may be impaired in older smokers with a longer history of exposure. Further studies of smokers with hypertension and other cardiovascular diseases are needed.
Perspectives and Significance
Cigarette smoking is a major risk factor for atherosclerotic vascular disease. Our data demonstrate that spontaneous sympathetic activity induces greater and more sustained BP elevations in smokers than nonsmokers. Although the smokers in this study remained nonhypertensive, we speculate that the greater and more sustained pressor response to the spontaneous sympathetic activity may contribute to the development of hypertension. We further postulate that this accentuated pressor response may also raise the risk of end-organ damage. Further studies are needed to examine the effects of smoking cessation, smoking cessation products, and other treatment (e.g., antioxidant therapy) on the accentuated pressor response in smokers.
In conclusion, this study suggests that the BP increases following spontaneous sympathetic activity are greater in habitual smokers. A smoking-induced impairment in baroreflex control of HR is one of the reasons for this accentuated pressor response. On the other hand, baroreflex control of MSNA is not altered in otherwise healthy smokers. We speculate that the accentuated pressor responses to spontaneous sympathetic activation contribute to elevated resting BP in smokers.
GRANTS
This study was supported by American Heart Association Grant 15GRNT24480051 (to J. Cui), CURE Tobacco Settlement Bridge Funding (to J. Cui), and National Institutes of Health Grant UL1 TR-002014 (to L. I. Sinoway).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.C. and L.I.S. conceived and designed research; J.C., R.C.D., M.D.M., and C.B. performed experiments; J.C. and V.G. analyzed data; J.C. and L.I.S. interpreted results of experiments; J.C. prepared figures; J.C., R.C.D., M.D.M., and L.I.S. drafted manuscript; J.C., R.C.D., M.D.M., and L.I.S. edited and revised manuscript; J.C., R.C.D., M.D.M., C.B., V.G., and L.I.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to the volunteers for their willingness to participate in the study and to Jennifer L. Stoner for secretarial help in preparing the manuscript.
REFERENCES
- 1.Benowitz NL, Gourlay SG. Cardiovascular toxicity of nicotine: implications for nicotine replacement therapy. J Am Coll Cardiol 29: 1422–1431, 1997. doi: 10.1016/S0735-1097(97)00079-X. [DOI] [PubMed] [Google Scholar]
- 2.Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP, Lloyd-Jones DM. Lifetime risks of cardiovascular disease. N Engl J Med 366: 321–329, 2012. doi: 10.1056/NEJMoa1012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cryer PE, Haymond MW, Santiago JV, Shah SD. Norepinephrine and epinephrine release and adrenergic mediation of smoking-associated hemodynamic and metabolic events. N Engl J Med 295: 573–577, 1976. doi: 10.1056/NEJM197609092951101. [DOI] [PubMed] [Google Scholar]
- 4.Cui J, Blaha C, Moradkhan R, Gray KS, Sinoway LI. Muscle sympathetic nerve activity responses to dynamic passive muscle stretch in humans. J Physiol 576: 625–634, 2006. doi: 10.1113/jphysiol.2006.116640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui J, Boehmer J, Blaha C, Sinoway LI. Muscle sympathetic nerve activity response to heat stress is attenuated in chronic heart failure patients. Am J Physiol Regul Integr Comp Physiol 312: R873–R882, 2017. doi: 10.1152/ajpregu.00355.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Burgh Daly M. Interactions between respiration and circulation, In: Handbook of Physiology. The Respiratory System, edited by Cherniak NS, Widdicombe JG. Bethesda: American Physiological Society, 1986, vol II, p. 529–594. [Google Scholar]
- 7.De Sousa P, Cherian G, Thomas J, Thulesius O. Coronary artery constriction is enhanced with nicotine and propranolol, particularly after endothelial damage. Clin Physiol 11: 143–152, 1991. doi: 10.1111/j.1475-097X.1991.tb00107.x. [DOI] [PubMed] [Google Scholar]
- 8.Delius W, Hagbarth KE, Hongell A, Wallin BG. General characteristics of sympathetic activity in human muscle nerves. Acta Physiol Scand 84: 65–81, 1972. doi: 10.1111/j.1748-1716.1972.tb05157.x. [DOI] [PubMed] [Google Scholar]
- 9.Dinas PC, Koutedakis Y, Flouris AD. Effects of active and passive tobacco cigarette smoking on heart rate variability. Int J Cardiol 163: 109–115, 2013. doi: 10.1016/j.ijcard.2011.10.140. [DOI] [PubMed] [Google Scholar]
- 10.Ezzati M, Henley SJ, Thun MJ, Lopez AD. Role of smoking in global and regional cardiovascular mortality. Circulation 112: 489–497, 2005. doi: 10.1161/CIRCULATIONAHA.104.521708. [DOI] [PubMed] [Google Scholar]
- 11.Failla M, Grappiolo A, Carugo S, Calchera I, Giannattasio C, Mancia G. Effects of cigarette smoking on carotid and radial artery distensibility. J Hypertens 15: 1659–1664, 1997. doi: 10.1097/00004872-199715120-00069. [DOI] [PubMed] [Google Scholar]
- 12.Fairfax ST, Padilla J, Vianna LC, Davis MJ, Fadel PJ. Spontaneous bursts of muscle sympathetic nerve activity decrease leg vascular conductance in resting humans. Am J Physiol Heart Circ Physiol 304: H759–H766, 2013. doi: 10.1152/ajpheart.00842.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerhardt U, Hans U, Hohage H. Influence of smoking on baroreceptor function: 24 h measurements. J Hypertens 17: 941–946, 1999. doi: 10.1097/00004872-199917070-00010. [DOI] [PubMed] [Google Scholar]
- 14.Gerhardt U, Vorneweg P, Riedasch M, Hohage H. Acute and persistant effects of smoking on the baroreceptor function. J Auton Pharmacol 19: 105–108, 1999. doi: 10.1046/j.1365-2680.1999.00123.x. [DOI] [PubMed] [Google Scholar]
- 15.Grassi G, Seravalle G, Bertinieri G, Stella ML, Turri C, Mancia G. Sympathetic response to ventricular extrasystolic beats in hypertension and heart failure. Hypertension 39: 886–891, 2002. doi: 10.1161/01.HYP.0000013265.48954.A5. [DOI] [PubMed] [Google Scholar]
- 16.Grassi G, Seravalle G, Calhoun DA, Bolla GB, Giannattasio C, Marabini M, Del Bo A, Mancia G. Mechanisms responsible for sympathetic activation by cigarette smoking in humans. Circulation 90: 248–253, 1994. doi: 10.1161/01.CIR.90.1.248. [DOI] [PubMed] [Google Scholar]
- 17.Grassi G, Seravalle G, Quarti-Trevano F, Dell’Oro R, Arenare F, Spaziani D, Mancia G. Sympathetic and baroreflex cardiovascular control in hypertension-related left ventricular dysfunction. Hypertension 53: 205–209, 2009. doi: 10.1161/HYPERTENSIONAHA.108.121467. [DOI] [PubMed] [Google Scholar]
- 18.Hayano J, Yamada M, Sakakibara Y, Fujinami T, Yokoyama K, Watanabe Y, Takata K. Short- and long-term effects of cigarette smoking on heart rate variability. Am J Cardiol 65: 84–88, 1990. doi: 10.1016/0002-9149(90)90030-5. [DOI] [PubMed] [Google Scholar]
- 19.Heitzer T, Ylä-Herttuala S, Luoma J, Kurz S, Münzel T, Just H, Olschewski M, Drexler H. Cigarette smoking potentiates endothelial dysfunction of forearm resistance vessels in patients with hypercholesterolemia. Role of oxidized LDL. Circulation 93: 1346–1353, 1996. doi: 10.1161/01.CIR.93.7.1346. [DOI] [PubMed] [Google Scholar]
- 20.Hering D, Kucharska W, Kara T, Somers VK, Narkiewicz K. Smoking is associated with chronic sympathetic activation in hypertension. Blood Press 19: 152–155, 2010. doi: 10.3109/08037051.2010.484150. [DOI] [PubMed] [Google Scholar]
- 21.Hering D, Somers VK, Kara T, Jazdzewski K, Jurak P, Kucharska W, Narkiewicz K. Heightened acute circulatory responses to smoking in women. Blood Press 17: 141–146, 2008. doi: 10.1080/08037050802185780. [DOI] [PubMed] [Google Scholar]
- 22.Hering D, Somers VK, Kara T, Kucharska W, Jurak P, Bieniaszewski L, Narkiewicz K. Sympathetic neural responses to smoking are age dependent. J Hypertens 24: 691–695, 2006. doi: 10.1097/01.hjh.0000217851.95583.57. [DOI] [PubMed] [Google Scholar]
- 23.Higgins CB, Vatner SF, Braunwald E. Parasympathetic control of the heart. Pharmacol Rev 25: 119–155, 1973. [PubMed] [Google Scholar]
- 24.Jamal A, Phillips E, Gentzke AS, Homa DM, Babb SD, King BA, Neff LJ. Current cigarette smoking among adults – United States, 2016. MMWR Morb Mortal Wkly Rep 67: 53–59, 2018. doi: 10.15585/mmwr.mm6702a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keller DM, Cui J, Davis SL, Low DA, Crandall CG. Heat stress enhances arterial baroreflex control of muscle sympathetic nerve activity via increased sensitivity of burst gating, not burst area, in humans. J Physiol 573: 445–451, 2006. doi: 10.1113/jphysiol.2006.108662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol 531: 861–869, 2001. doi: 10.1111/j.1469-7793.2001.0861h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kool MJ, Hoeks AP, Struijker Boudier HA, Reneman RS, Van Bortel LM. Short- and long-term effects of smoking on arterial wall properties in habitual smokers. J Am Coll Cardiol 22: 1881–1886, 1993. doi: 10.1016/0735-1097(93)90773-T. [DOI] [PubMed] [Google Scholar]
- 28.Krüger C, Haunstetter A, Gerber S, Serf C, Kaufmann A, Kübler W, Haass M. Nicotine-induced exocytotic norepinephrine release in guinea-pig heart, human atrium and bovine adrenal chromaffin cells: modulation by single components of ischaemia. J Mol Cell Cardiol 27: 1491–1506, 1995. doi: 10.1016/S0022-2828(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 29.Laustiola KE, Lassila R, Kaprio J, Koskenvuo M. Decreased β-adrenergic receptor density and catecholamine response in male cigarette smokers. A study of monozygotic twin pairs discordant for smoking. Circulation 78: 1234–1240, 1988. doi: 10.1161/01.CIR.78.5.1234. [DOI] [PubMed] [Google Scholar]
- 30.Li G, Wang H, Wang K, Wang W, Dong F, Qian Y, Gong H, Hui C, Xu G, Li Y, Pan L, Zhang B, Shan G. The association between smoking and blood pressure in men: a cross-sectional study. BMC Public Health 17: 797, 2017. doi: 10.1186/s12889-017-4802-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lloyd-Jones DM, Leip EP, Larson MG, D’Agostino RB, Beiser A, Wilson PW, Wolf PA, Levy D. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation 113: 791–798, 2006. doi: 10.1161/CIRCULATIONAHA.105.548206. [DOI] [PubMed] [Google Scholar]
- 32.Mahmud A, Feely J. Effect of smoking on arterial stiffness and pulse pressure amplification. Hypertension 41: 183–187, 2003. doi: 10.1161/01.HYP.0000047464.66901.60. [DOI] [PubMed] [Google Scholar]
- 33.Mancia G, Groppelli A, Di Rienzo M, Castiglioni P, Parati G. Smoking impairs baroreflex sensitivity in humans. Am J Physiol Heart Circ Physiol 273: H1555–H1560, 1997. doi: 10.1152/ajpheart.1997.273.3.H1555. [DOI] [PubMed] [Google Scholar]
- 34.Middlekauff HR, Park J, Agrawal H, Gornbein JA. Abnormal sympathetic nerve activity in women exposed to cigarette smoke: a potential mechanism to explain increased cardiac risk. Am J Physiol Heart Circ Physiol 305: H1560–H1567, 2013. doi: 10.1152/ajpheart.00502.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Middlekauff HR, Park J, Moheimani RS. Adverse effects of cigarette and noncigarette smoke exposure on the autonomic nervous system: mechanisms and implications for cardiovascular risk. J Am Coll Cardiol 64: 1740–1750, 2014. doi: 10.1016/j.jacc.2014.06.1201. [DOI] [PubMed] [Google Scholar]
- 36.Morris PB, Ference BA, Jahangir E, Feldman DN, Ryan JJ, Bahrami H, El-Chami MF, Bhakta S, Winchester DE, Al-Mallah MH, Sanchez Shields M, Deedwania P, Mehta LS, Phan BA, Benowitz NL. Cardiovascular effects of exposure to cigarette smoke and electronic cigarettes: clinical perspectives from the Prevention of Cardiovascular Disease Section Leadership Council and Early Career Councils of the American College of Cardiology. J Am Coll Cardiol 66: 1378–1391, 2015. doi: 10.1016/j.jacc.2015.07.037. [DOI] [PubMed] [Google Scholar]
- 37.Najem B, Houssière A, Pathak A, Janssen C, Lemogoum D, Xhaët O, Cuylits N, van de Borne P. Acute cardiovascular and sympathetic effects of nicotine replacement therapy. Hypertension 47: 1162–1167, 2006. [Erratum in Hypertension 48: e23, 2006.] doi: 10.1161/01.HYP.0000219284.47970.34. [DOI] [PubMed] [Google Scholar]
- 38.Narkiewicz K, van de Borne PJ, Hausberg M, Cooley RL, Winniford MD, Davison DE, Somers VK. Cigarette smoking increases sympathetic outflow in humans. Circulation 98: 528–534, 1998. doi: 10.1161/01.CIR.98.6.528. [DOI] [PubMed] [Google Scholar]
- 39.Niedermaier ON, Smith ML, Beightol LA, Zukowska-Grojec Z, Goldstein DS, Eckberg DL. Influence of cigarette smoking on human autonomic function. Circulation 88: 562–571, 1993. doi: 10.1161/01.CIR.88.2.562. [DOI] [PubMed] [Google Scholar]
- 40.Park J, Middlekauff HR. Altered pattern of sympathetic activity with the ovarian cycle in female smokers. Am J Physiol Heart Circ Physiol 297: H564–H568, 2009. doi: 10.1152/ajpheart.01197.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rowell LB. Arterial baroreflexes, central command, and muscle chemoreflexes: A synthesis. In: Human Cardiovascular Control. New York: Oxford University Press, 1993, p. 441–483. [Google Scholar]
- 42.Shinozaki N, Yuasa T, Takata S. Cigarette smoking augments sympathetic nerve activity in patients with coronary heart disease. Int Heart J 49: 261–272, 2008. doi: 10.1536/ihj.49.261. [DOI] [PubMed] [Google Scholar]
- 43.Sieminska A, Jassem E. The many faces of tobacco use among women. Med Sci Monit 20: 153–162, 2014. doi: 10.12659/MSM.889796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stauss HM, Moffitt JA, Chapleau MW, Abboud FM, Johnson AK. Baroreceptor reflex sensitivity estimated by the sequence technique is reliable in rats. Am J Physiol Heart Circ Physiol 291: H482–H483, 2006. doi: 10.1152/ajpheart.00228.2006. [DOI] [PubMed] [Google Scholar]
- 45.Sundlöf G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol 274: 621–637, 1978. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vallbo AB, Hagbarth K-E, Torebjörk HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev 59: 919–957, 1979. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- 47.Vianna LC, Hart EC, Fairfax ST, Charkoudian N, Joyner MJ, Fadel PJ. Influence of age and sex on the pressor response following a spontaneous burst of muscle sympathetic nerve activity. Am J Physiol Heart Circ Physiol 302: H2419–H2427, 2012. doi: 10.1152/ajpheart.01105.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Virdis A, Giannarelli C, Neves MF, Taddei S, Ghiadoni L. Cigarette smoking and hypertension. Curr Pharm Des 16: 2518–2525, 2010. doi: 10.2174/138161210792062920. [DOI] [PubMed] [Google Scholar]
- 49.Wallin BG. Interindividual differences in muscle sympathetic nerve activity: a key to new insight into cardiovascular regulation? Acta Physiol (Oxf) 190: 265–275, 2007. doi: 10.1111/j.1748-1716.2007.01730.x. [DOI] [PubMed] [Google Scholar]
- 50.Wallin BG, Eckberg DL. Sympathetic transients caused by abrupt alterations of carotid baroreceptor activity in humans. Am J Physiol Heart Circ Physiol 242: H185–H190, 1982. doi: 10.1152/ajpheart.1982.242.2.H185. [DOI] [PubMed] [Google Scholar]
- 51.Wallin BG, Nerhed C. Relationship between spontaneous variations of muscle sympathetic activity and succeeding changes of blood pressure in man. J Auton Nerv Syst 6: 293–302, 1982. doi: 10.1016/0165-1838(82)90002-9. [DOI] [PubMed] [Google Scholar]