Abstract
Corticotropin-releasing factor (CRF) regulates stress responses, and aberrant CRF signals are associated with depressive disorders. Crf expression is responsive to arachidonic acid (AA), where CRF is released from the hypothalamic paraventricular nucleus (PVN) to initiate the hypothalamic-pituitary-adrenal axis, culminating in glucocorticoid stress hormone release. Despite this biological and clinical significance, Crf regulation is unclear. Here, we report that acyloxyacyl hydrolase, encoded by Aoah, is expressed in the PVN, and Aoah regulates Crf through the aryl hydrocarbon receptor (AhR). We previously showed that AOAH-deficient mice mimicked interstitial cystitis/bladder pain syndrome, a condition frequently associated with comorbid anxiety and depression. With the use of novelty-suppressed feeding and sucrose preference assays to quantify rodent correlates of anxiety/depression, AOAH-deficient mice exhibited depressive behaviors. AOAH-deficient mice also had increased CNS AA, increased Crf expression in the PVN, and elevated serum corticosterone, consistent with dysfunction of the hypothalamic-pituitary-adrenal axis. The human Crf promoter has putative binding sites for AhR and peroxisome proliferator-activated receptor (PPARγ). PPARγ did not affect AA-dependent Crf expression in vitro, and conditional Pparγ knockout did not alter the AOAH-deficient depressive phenotype, despite previous studies implicating PPARγ as a therapeutic target for depression. In contrast, Crf induction was mediated by AhR binding sites in vitro and increased by AhR overexpression. Furthermore, conditional Ahr knockout rescued the depressive phenotype of AOAH-deficient mice. Finally, an AhR antagonist rescued the AOAH-deficient depressive phenotype. Together, our results demonstrate that Aoah is a novel genetic regulator of Crf mediated through AhR, and AhR is a therapeutic target for depression.
Keywords: AhR, AOAH, arachidonic acid, CRF, interstitial cystitis, stress
INTRODUCTION
Corticotropin-releasing factor (CRF) is a stress response neuropeptide that has been shown to mediate stress-related behaviors. CRF regulates central stress responses by initiating the hypothalamic-pituitary-adrenal (HPA) axis (42), a cascade that culminates in glucocorticoid stress hormone release and results in physiological responses to stress. First, CRF is synthesized and released from the paraventricular nucleus (PVN) of the hypothalamus into the anterior pituitary gland, causing systemic release of adrenocorticotropic hormone (ACTH) from the pituitary gland (2, 51). ACTH stimulates adrenal secretion of glucocorticoids that feedback to inhibit further CRF secretion (24).
Chronic stress results in sustained HPA axis activation and increased serum cortisol levels. Under such conditions, the negative feedback mechanism that otherwise inhibits CRF secretion becomes unresponsive, and patients that suffer depression have increased serum glucocorticoids and aberrant CRF levels (5, 11, 27, 39, 59). Individuals who have experienced a major stressful event have increased susceptibility to developing depression (30). Additionally, postmortem cerebral spinal fluid from suicide victims shows elevated CRF levels, further supporting an association between CRF, stress, and mood disorders (38). Previous studies have shown that arachidonic acid (AA) and its metabolites can induce Crf expression (8, 12, 28), yet transcriptional mediators of AA-dependent Crf induction remain unknown. Because CRF regulates stress responses and mood, understanding the transcriptional regulation of Crf offers the promise of identifying novel therapeutic targets for depression and other CRF-mediated mood disorders.
Recently, we sought to identify loci mediating symptoms of interstitial cystitis/bladder pain syndrome, a condition marked by chronic pelvic pain and comorbid anxiety/depression (49, 55). A genetic screen in mice identified a single-nucleotide polymorphism near Aoah associated with pain severity in a murine neurogenic cystitis model of interstitial cystitis, and we observed that AOAH-deficient mice develop spontaneous pelvic pain (63). AOAH is a neutrophil lipase that mitigates inflammation by cleaving secondary acyl chains from the lipid A moiety of bacterial LPS (35). Previous studies have shown that AOAH has lipase and acyl transferase activities (35), suggesting that AOAH might also alter host lipids via these activities. Characterization of AOAH distribution revealed AOAH immunoreactivity in several key CNS sites, including the PVN, where CRF initiates the HPA axis. Here, we investigated the potential link between Aoah and Crf by characterizing behaviors associated with mood disorders in AOAH-deficient mice. We then examined the potential mechanism by which AOAH regulates Crf by characterizing a CNS metabolome, revealing increased AA in AOAH-deficient mice. Finally, we examined transcription factor binding sites with the potential to regulate AA-dependent Crf expression downstream of AOAH and found that aryl hydrocarbon receptor (AhR) mediates AA-stimulated Crf.
MATERIALS AND METHODS
Cell Culture
HEK 293T human kidney cells and N42 hypothalamic neurons were maintained at 37°C and 5% CO2 atmosphere in DMEM with 10% FBS (Invitrogen, Carlsbad, CA) and 1% penicillin-streptomycin (CELLutions, Burlington, ON).
Plasmids and Subcloning
A 1-kb fragment of the human Crf promoter (Switchgear Genomics, Menlo Park, CA) was subcloned into the SacI and BglII sites of pGL4.12 (Promega, Madison, WI) to generate phCrf-luc. Human pcDNA-peroxisome proliferator-activated receptor (PPARγ) plasmid and mouse MSCV-IRES-GFP-AhR plasmid were generously provided by Jun Wei and Liang Zhou, respectively. pEGFP-N1 (Takara Bio USA, Mountain View, CA) was used as a control plasmid.
Identification of Putative Transcription Factor Binding Sites
The Molecular Informatics Resource for the Analysis of Gene Expression (MIRAGE) Tfsitescan platform (IFTI.org) was used to to analyze the 1 kb region of the human Crf gene promoter. The proliferator hormone response element (PPRE) site and xenobiotic responsive element (XRE) sites were identified as candidate mediators of AA-dependent Crf expression (see Supplementary Table S1; all supplementary tables are available at https://doi.org/10.17632/gs6r5h34f6.1).
Mutation of PPRE and XRE Sites
Mutations were introduced in PPRE and XRE sites with QuikChange Multi Site-Directed Mutagenesis (Agilent Technologies, Santa Clara, CA) and confirmed by sequence analyses. Primers used to mutate sites were 5′-CGCTGTCTCTTTGCACACCCCTAATATGGCCTTTCATAcTAgGtccgCcATATGTTTTCACACTTGGG-3′ (PPRE), 5′-CGAGCTGTCAAGAGAGCGTCAGCTTATTAGGCAAATGCgcCGgcGTTTTTGAAGAGGGTCGAC-3′ (XRE–57), and 5′-GGCAGGGCCCTATGATTTATGCAGGAGCAGAGGCAGatCGatATCGAGCTGTCAAGAGAGC-3′ (XRE–103), where lower case letters represent nucleotides altered from the wild-type Crf sequence.
Reporter Assays
Reporter activity was quantified using the Dual-Luciferase Reporter Assay (Promega). Briefly, HEK 293T cells were reverse transfected with X-tremeGENE HP (Roche) and 500 ng phCrf-luc/well (or mutants ΔPPRE, ΔXRE1, ΔXRE1, or ΔXRE1/2) and cotransfected with 5 ng/well Renilla RL-TK luciferase. After 24 h, cultures were stimulated with 500 µM N6,2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate and/or 300 µM AA (Sigma). Activity was determined by normalizing luciferase to Renilla. For AhR antagonists, cultures were pretreated with CH-223191 (13, 31, 65) (Sigma) 1 h before stimulation.
Quantifying N42 Crf Expression
Hypothalamic N42 neurons were seeded into 12-well plates in serum-free medium and stimulated with 450 µM AA. Cultures were then harvested, and RNA was extracted by Quick-RNA Mini-Prep (Zymo Research). RNA was converted to cDNA (iScript cDNA synthesis kit, Bio-Rad, Hercules, CA), and quantitative real-time PCR was performed using Bullseye EvaGreen qPCR 2× Mastermix (MIDSCI, Midwest Scientific, Valley Park, MO) and primers specific for murine Crf (forward 5′- TCTCTCTGGATCTCACCTTCCACC-3′ and reverse 5′- AGCTTGCTGAGCTAACTGCTCTGC −3′) and L19 (forward 5′- ATGAAATCGCCAATGCCAACTCCC −3′ and reverse 5′- ACAGGCTGTGATACATATGGCGGT-3′). The ΔΔCT method was used to quantify relative Crf mRNA (32).
Mice
Only 10- to 16-wk-old female mice were employed in experiments. Mice were housed in groups of two to five mice except when specific tests [sucrose preference and LABORAS (Laboratory Animal Behavior Observation, Registration, and Analysis System, Metris, The Netherlands)] required individual housing. Wild-type C57BL/6J mice and breeder pairs of CRF-ires-CRE (B6 (Cg)-Crhtm1(cre)Zjh/J), PPARγloxP (B6.129-Ppargtm2Rev/J), and Ahrfx (Ahrtm3.1Bra/J) mice were purchased from Jackson Laboratory (Bar Harbor, ME). Aoah−/− mice (B6.129S6-Aoahtm1Rsm/J) were received as a generous gift from Dr. Robert S. Munford. To generate conditional knockouts, CRF-ires-CRE, PPARγloxP, and Ahrfx mice were bred onto the AOAH-deficient background. AOAH-deficient Ahr conditioned knockout (cKO) and Pparγ cKO were then generated by mating AOAH-deficient Ahrfx or AOAH-deficient PPARγloxP mice, respectively, with AOAH-deficient CRF-ires-CRE mice for two generations to yield homozygous mice. All animals were maintained at Northwestern University’s Center for Comparative Medicine (CCM) under IACUC-approved protocols. CCM maintains standard housing conditions of 72–74°F in central facilities (the departmental CCM satellite facility maintains temperatures within the range of 70–77°F), 30–70% relative humidity (20–50% in the satellite facility), and 14:10 light:dark cycle. Unless otherwise stated (see below), mice were provided with food and water ad libitum. After transfer from CCM central facilities to the satellite facility, mice were adapted at least one week to the satellite facility before performing any experiments.
Stress-Related Behaviors for Anxiety/Depression
Sucrose preference.
Sucrose preference was used to characterize anhedonia by loss of preference for sucrose water (36, 45, 61). Briefly, mice were housed singly with two identical water bottles; each bottle was weighed daily to calculate consumption, and bottle positions were reversed daily to avoid positional artifacts. Both water bottles contained water on day 1. On day 2, one water bottle was refilled with 0.6% sucrose water. Food was available ad libitum throughout the assay. Mean fluid intake over two 24-h periods was determined based on daily change of bottle mass.
Novelty-suppressed feeding.
Novelty-suppressed feeding was quantified as an alternative depressive-like behavior (17). Briefly, mice were deprived of food 24 h before testing, and water was removed 2 h prior. For the test, a food pellet was placed in the center of a large, square chamber, and a mouse was then placed into a corner of the chamber at which moment a stopwatch was used to manually time the latencies to approach and consume the food pellet.
Quantification of Brain Crf Expression
Brain punches were harvested from 1-mm brain slices using a Pasteur pipette relative to anatomical landmarks (19). RNA was purified with RNeasy Mini (Qiagen, Hilden, Germany) and converted to cDNA (iScript cDNA synthesis kit, Bio-Rad). Quantitative real-time PCR was performed using Bullseye EvaGreen qPCR 2× Mastermix (MIDSCI) and primers specific for Crf and ribosomal protein L19 using the same primers listed under Quantifying N42 Crf Expression. The ΔΔCT method was used to quantify relative Crf mRNA (32).
Corticosterone ELISA
Twelve- to sixteen-week-old female mice were anesthetized by isoflurane and euthanized by cervical dislocation (from 0700 to 1100 h), and serum was collected and frozen until corticosterone was quantified by ELISA (AssayPro) (64).
Metabolomics
Sacral spinal cords were analyzed by LC-MS at the Scripps Center for Metabolomics and Mass Spectrometry using reverse-phase C18 chromatography followed by and an Agilent 6210 to determine m/z values (43). Analyzing data and metabolite databases using XCMS software (53, 54) revealed that Aoah−/− spinal cords had 492 significant peaks (P < 0.05, n = 7).
Metabolite Analyses
Sacral spinal cords were harvested rapidly from female mice and flash frozen. Then 3–5 mg of tissue were homogenized in 10% MeOH spiked with deuterated internal standard and then extracted using Starta-x reverse-phase columns (8B-S100-UBJ, Phenomenex, Torrence, CA). The eluent was dried under vacuum and resolved in 50 µl Solvent A (60:40:0.02, vol/vol/vol, a water/acetonitrile/acetic acid) for LC-MS/MS analysis (60).
Metabolites were separated by reverse-phase liquid chromatography on a C18 column (00B-4462-E0, 4.6 X 50 mm, 2.6µ, Phenomenex) at 500 µl/min. The column was equilibrated in Solvent A, and a 10 µl sample was loaded and eluted with gradients: 0–4 min 0.1–55% in Solvent B (50:50, vol/vol acetonitrile/isopropyl alcohol); 4.0–4.5 min, 55–99% in Solvent B; and 4.5–6.5 min, in 99% Solvent B. Analyses were performed on a QTRAP 6500 (AB Sciex) in multiple reaction-monitoring (MRM) mode. Eicosanoids were detected in negative electrospray ion mode using the following MRM parameters (precursor, product): PGE2 (351, 271), d4-PGE2 (355, 275), PGD2 (351, 271), d9 (360, 280), AA (303, 259), and d8-AA (311, 267) (40, 60). Metabolites were quantified as area-under-the peak relative to internal standards. Data acquisition and quantification were performed using Analyst 1.6.2 (Applied Biosystems, Foster City, CA).
For validation of m/z peak 305.3 as AA, both AA standard and samples were carried out in full scan MS/MS spectrum analyzing the product ions from the precursor ions [M + H]+ 305 to obtain high specificity and sensitivity.
Immunohistochemistry
Mice were perfused with PBS followed by 4% paraformaldehyde, and 4-µm paraffin sections were deparaffinized, rehydrated, and blocked using Fc Receptor Blocker (Innovex, Plymouth, MN) for 30 min and then Background Buster (Innovex) for 30 min. Tissues were incubated overnight with rabbit antibodies against AOAH (1:100, Santa Cruz sc-135110) or AhR (1:100, Santa Cruz sc-5579) and detected with donkey anti-rabbit Alexa Fluor 594 (1:500 A11016, Invitrogen). Sections were counterstained with anti-CRF (1:100, NB110–81721, Novus Biologicals, Centennial, CO) and detected with donkey anti-sheep Alexa Fluor 488 (A21206, Invitrogen) applied at 1:500. Specificity of secondary reagents was confirmed in parallel samples by omitting primary antibodies. For AOAH immunoreactivity, specificity of the primary antiserum was confirmed previously by staining of tissues from AOAH-deficient mice (63).
Treatment of Aoah−/− Mice With AhR Antagonist
Ten- to 12-week-old C57BL/6 mice and AOAH-deficient mice were given 100 µl of corn oil vehicle or 10 mg/kg CH-223191 in corn oil daily for 14 days (31) and continued during the testing periods.
LABORAS Activity Monitoring
LABORAS home cage observation system (Metris) was used to quantify general activity and mobility. Mice were housed singly during the 12-h monitoring period to detect behavior-specific vibrations, weighing food before and after the observation period to determine consumption.
Statistical Analysis
Results were analyzed by Student’s t-test, one-way analysis of variance followed by Dunnett’s multiple comparisons test, or Tukey’s multiple comparisons test with Prism (GraphPad). Differences were considered statistically significant at P < 0.05.
RESULTS
Aoah Is a Genetic Modulator of Depressive-Like Behaviors and Crf
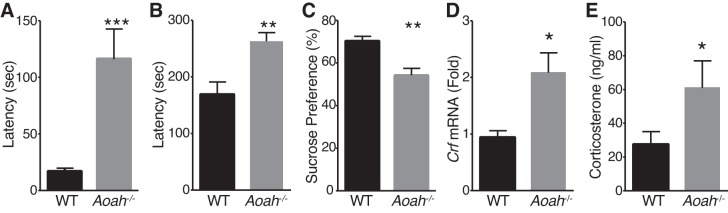
Using a murine neurogenic cystitis model that recapitulates key aspects of interstitial cystitis/bladder pain syndrome, we identified Aoah as a modulator of pelvic pain severity (63). Interstitial cystitis is associated HPA axis dysfunction and comorbid anxiety/depression that affects women disproportionately (20), so female mice were evaluated exclusively in this study. To examine a potential role for AOAH in mood, we evaluated wild-type and AOAH-deficient mice by novelty-suppressed feeding (NSF), where increased latency of a hungry rodent to approach food in a novel environment indicates depressive-like behavior (17). Female Aoah–/– mice exhibited increased latency both to approach and to consume food, (Fig. 1, A and B). To confirm depressive-like behavior in Aoah–/– mice, we assessed sucrose preference, a measure of hedonic activity (61). Aoah−/− mice exhibited reduced preference for sucrose over water, relative to wild-type mice (Fig. 1C). The phenotype of Aoah−/− mice suggested CRF dysregulation, and we observed approximately a twofold increase in Crf mRNA expression in the PVN of Aoah−/− mice as quantified by qRT PCR, compared with wild-type mice (Fig. 1D). To confirm HPA dysregulation in AOAH-deficient mice, we performed ELISA on sera to quantify corticosterone levels. Similar to increased PVN Crf expression, serum corticosterone levels were elevated in Aoah−/− mice approximately twofold relative to wild-type mice (Fig. 1E). Together, these data are consistent with a role for Aoah as a genetic regulator of Crf expression, where AOAH deficiency causes altered depressive-like behaviors and HPA axis dysregulation.
Fig. 1.
Acyloxyacyl hydrolase (AOAH)-deficient mice exhibit depressive-like behavioral phenotypes and corticotropin-releasing factor (Crf) dysregulation. A: female AOAH-deficient mice exhibit increased latency to reach food in novelty suppressed feeding assay (n = 17–18, P = 0.0006, two-tailed Student’s t-test). B: female AOAH-deficient mice consume food pellet (P = 0.0060), compared with wild-type (WT) mice. C: female Aoah−/− mice showed decreased preference for sucrose water in sucrose preference test as compared with B6 mice (n = 10–12, P = 0.0016, two-tailed Student’s t-test,). D: quantitation of Crf mRNA of WT and AOAH-deficient brain punches from the paraventricular nucleus (PVN) normalized to ribosomal protein L19. Female Aoah−/− mice have increased PVN Crf mRNA (n = 14–19, P = 0.0109, two-tailed Student’s t-test). E: female Aoah−/− mice have increased serum corticosterone by ELISA (n = 14–16, P = 0.0148, Mann-Whitney U-test). Data are means ± SE. Differences were considered statistically significant at *P < 0.05; **P < 0.01; ***P < 0.001.
AOAH-Deficient Mice Have Increased Spinal AA
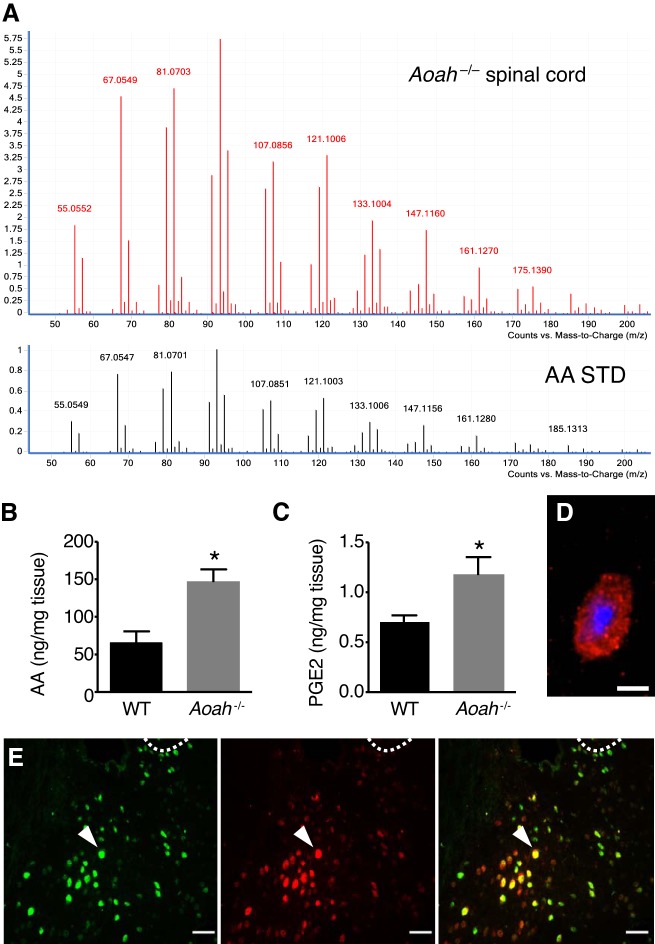
Because AOAH is a lipase, we hypothesized that AOAH deficiency alters CNS lipids. Pilot metabolomic analyses of sacral spinal cords by LC-MS identified 492 peaks with significantly different abundance between wild-type and Aoah−/− mice. The most abundant peak and significantly elevated in Aoah−/− mice had a mass-to-charge ratio (m/z) of 305.3, corresponding to AA by search of the METLIN database. The identity of the putative AA peak at m/z = 305.3 was confirmed by MS-MS analysis, where the 305.3 peak and AA standard shared the same fragmentation pattern (Fig. 2A). To verify altered AA metabolism in AOAH-deficient mice, AA and its metabolites were quantified in sacral spinal cords by mass spectrometry with multiple reaction-monitoring scan. Lipidomic quantification revealed increased AA and PGE2 in AOAH-deficient mice (Fig. 2, B and C), lipids known to drive Crf expression (8, 12, 28). Finally, to characterize AOAH localization relative to CRF, we performed immunostaining in C57BL/6 brain sections. CRF immunoreactivity was detectable and appeared as punctate staining, consistent with prior reports (Fig. 2D) (58). Many PVN cells were immunoreactive for both AOAH and CRF (Fig. 2E). These data suggest Aoah is a novel genetic regulator of Crf and depressive-like behaviors, acting upstream of arachidonic acid-stimulated Crf expression.
Fig. 2.
Acyloxyacyl hydrolase (AOAH)-deficient mice exhibit increased spinal arachidonic acid (AA) levels. A: MS-MS analyses of m/z 305.3 peak and AA standard reveals similar fragment spectra. B: lipidomic analyses in sacral spinal cords of female B6 and Aoah−/− mice shows increased AA (n = 4–6, *P = 0.0107, two-tailed Student’s t-test). C: the test showed increased PGE2 in Aoah−/− mice (n = 6, *P = 0.0329). Data are means ± SE. D: corticotropin-releasing factor immunoreactivity detected in the paraventricular nucleus (PVN) of female B6 mice. Red is CRF staining, and blue is DAPI. E: immunoreactivity of AOAH (green) and CRF (red) in brain sections of female B6 mice shows double-labeled cells (yellow) in the PVN. Dotted line, margin of the third ventricle as a landmark for the PVN.
Identification of AA-Dependent Transcription Regulators of Crf
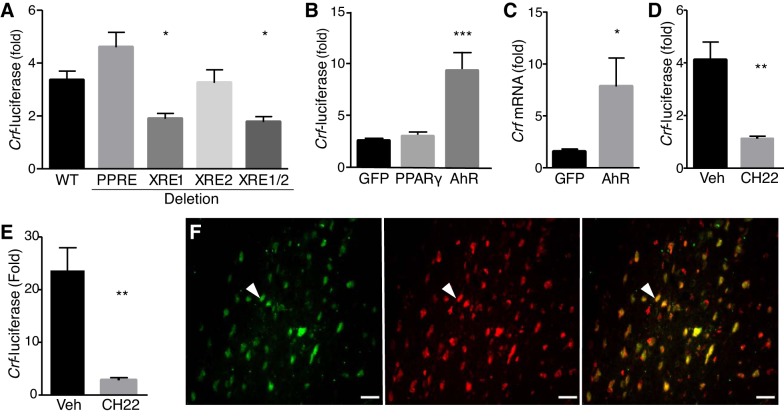
To identify transcriptional regulators downstream of Aoah, we analyzed 1 kb of the human Crf promoter with Tfsitescan of the MIRAGE platform, focusing on transcription factors with potential to bind hydrophobic ligands (see Supplementary Table S1). We identified a putative peroxisome PPRE at –317 and two putative XRE sites, XRE1 at –102 and XRE2 at –56, conserved in humans, rats, and mice (Fig. 3). PPRE is the binding site for PPARγ, which has been shown to regulate gene expression in response to AA and eicosanoids (25, 37). Similarly, XRE sites bind AhR, also known to mediate eicosanoid-induced gene expression (50). As an initial screen to evaluate the roles of putative PPRE and XRE sites in Crf regulation, we mutated these sites in a human Crf-luciferase reporter and quantified luciferase activity in response to AA in HEK 293T cells. AA induced wild-type Crf promoter activity, whereas the Crf XRE1 mutant was expressed significantly less (Fig. 4A). Mutation of XRE2 had no effect, and the XRE1/2 double mutant showed significantly reduced Crf induction, suggesting that XRE1 is the primary mediator of Crf responses to eicosanoids. Mutation of PPRE resulted in insignificant Crf induction in response to AA stimulation. To confirm roles in Crf responses, PPARγ and AhR were overexpressed by cotransfection. Stimulation of wild-type Crf-luciferase resulted in no significant effect of PPARγ over-expression on response to AA (Fig. 4B), but AhR overexpression significantly enhanced AA induction of Crf-luciferase, suggesting Crf responses to eicosanoids are mediated by AhR binding to XRE sites. These findings were corroborated in N42 cells, a hypothalamic neuronal cell line and model of native Crf regulation (18). Overexpression of AhR significantly enhanced AA-induced Crf mRNA accumulation as quantified by qRT-PCR (Fig. 4C). Furthermore, CH-223191, an AhR antagonist (31), significantly reduced AA induction of Crf-luciferase, including when AhR was overexpressed (Fig. 4, D and E). Finally, immunostaining of brain sections revealed colocalization of CRF and AhR in numerous PVN neurons (Fig. 4F). These data are consistent with functional, ligand-induced AhR binding to XREs to mediate eicosanoid induction of Crf in the PVN.
Fig. 3.
Corticotropin-releasing factor (Crf) promoter is highly conserved. Consensus proliferator hormone recepton element (PPRE) and xenobiotic responsive element (XRE) sites are conserved within the Crf promoter of humans, rats, and mice. *Start site.
Fig. 4.
Aryl hydrocarbon receptor (AhR) mediates arachidonic acid (AA)-induced corticotropin-releasing factor (Crf) expression. HEK 293T cells transfected with Crf-luciferase and stimulated with 300 µM AA. A: mutation of xenobiotic responsive element1 (XRE1) reduced AA-mediated Crf promoter activity (F = 8.884, df = 55, one-way ANOVA-Dunnett’s multiple comparisons test). B: 293T cells were also transfected with expression constructs for green fluorescent protein (GFP), peroxisome proliferator-activitor receptor-γ (PPARγ), or AhR. AA-dependent Crf expression was unaltered by PPARγ but was significantly increased by AhR (F = 15.17, df = 33, ANOVA-Dunnett’s multiple comparisons test). C: AhR overexpression in N42 cells showed increased AA-induced Crf mRNA compared with GFP when quantified by qRT-PCR and normalized to L19 (P = 0.0354, two-tailed Student’s t-test). D: in transfected 293T cells, CH-223191 inhibited AA-induced Crf-luciferase (F = 21.37, df = 6, ANOVA-Dunnett’s multiple comparisons test). E: in cultures overexpressing AhR, AA-induced Crf-luciferase is similarly inhibited by CH-223191 (F = 15.08, df = 15, ANOVA-Dunnett’s multiple comparisons test). Data are means ± SE; differences were considered statistically significant at *P < 0.05; **P < 0.01; ***P < 0.001. F: AhR (green) and CRF (red) immunoreactivity in brain sections of female B6 show double-labeled cells (yellow) in the paraventricular nucleus.
AhR Mediates AOAH-Deficient Depressive-Like Behaviors
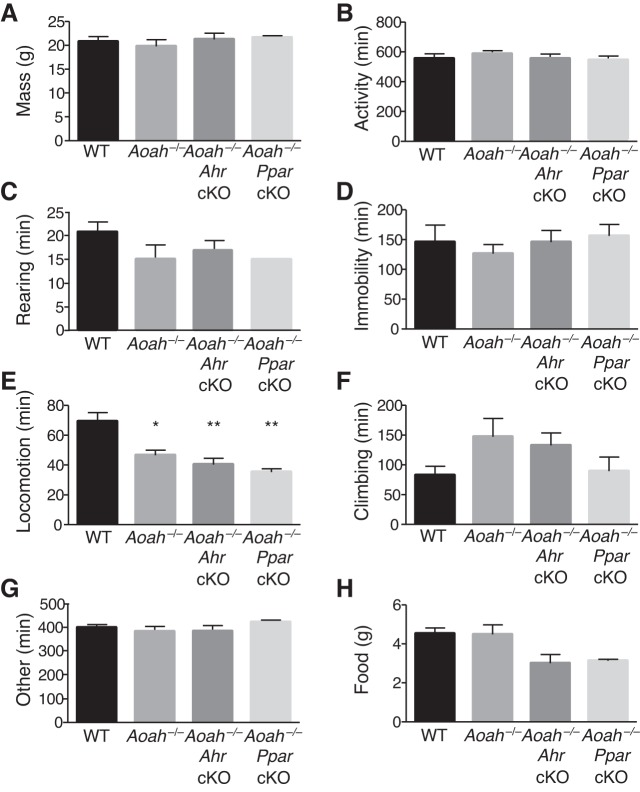
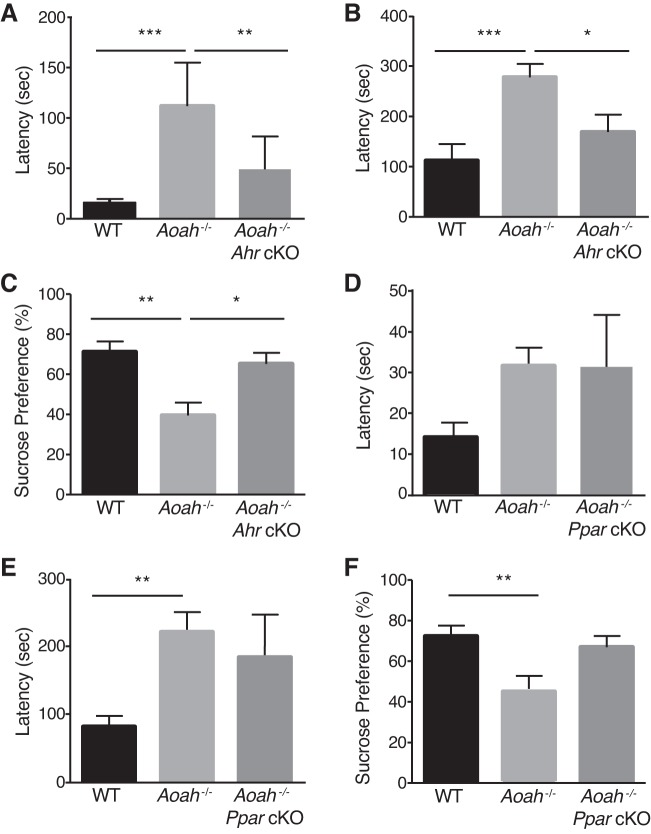
To confirm the role of AhR in vivo, we generated Ahr cKO mice on the AOAH-deficient background by breeding mice expressing Cre recombinase under the control of the Crf promoter with mice with an Ahr allele containing loxP sites flanking exon 2. The resulting Ahr cKO progeny have functional deletion of Ahr in Crf-expressing cells that we hypothesized would rescue Aoah−/− phenotypes. As an initial test of general murine behavior, we quantified several normal behaviors using the LABORAS system (Fig. 5). AOAH-deficient mice and cKO mice exhibited specifically reduced locomotion relative to wild-type mice (Fig. 5E), but combined activities were no different (Fig. 5B), suggesting compensatory increases in other activities (e.g., climbing, Fig. 5F). To assess the effect on mood of AhR as a mediator of the depressive phenotype associated with AOAH deficiency, we performed NSF and sucrose preference tests (Fig. 6, A and B). Aoah−/− mice exhibited increased latency to approach and to consume the food pellet compared with WT mice; however, the conditional knockout of Ahr in the Crf-expressing cells of AOAH-deficient mice resulted in a rescue of anxious/depressive-like behaviors typically seen in AOAH-deficient mice. Supporting the NSF phenotype, AOAH-deficient mice also showed reduced preference for sucrose that was reversed in Aoah−/− Ahr cKO mice, indicating restored hedonic activity (Fig. 6C). Finally, the magnitude of increased NSF latency suggests that the relatively minor and specific locomotion defect in AOAH-deficient mice (Fig. 5E) is unlikely to account for the NSF phenotype of AOAH deficient mice, consistent with anxious/depressive behavior. Furthermore, AhR cKO rescued the NSF phenotype AOAH-deficient mice without altering locomotion, thus dissociating locomotion from the AOAH depressive phenotype. Together, these findings suggest that AOAH-deficient mice have anxious/depressive behaviors mediated by AhR.
Fig. 5.
Overall activity levels are unperturbed by Acyloxyacyl hydrolase (AOAH) deficiency or conditional knockouts. Female wild-type (WT) (n = 4), AOAH-deficient (n = 5), AOAH-deficient Ahr conditioned knockout (Ahr cKO, n = 8), and AOAH-deficient peroxisome proliferator-activated receptorγ (Pparγ) cKO mice (n = 3) were monitored during a 12-h period using LABORAS to determine the number of minutes spent doing each activity. All mouse strains showed similar mass (A), combined activity (locomotion, rearing, climbing) (B), and other rearing (C), or immobility (D). AOAH-deficiency results in decreased locomotion that is not restored by cKO (E). All strains spent a similar amount of time climbing (F) and other activities (G) and consumed similar amounts of chow (H). Statistical analysis was determined by one-way ANOVA followed by Dunnett’s multiple comparisons test. *P < 0.05; **P < 0.01, statistically significant differences. Data are means ± SE.
Fig. 6.
AhR is a mediator of depressive behaviors of AOAH-deficient mice. A–C: Ahr conditional knockout (cKO) in Crf-expressing cells of Aoah−/− mice rescues depression (n = 8 WT, n = 7 Aoah–/–, n = 6 cKO). A: female Aoah−/− mice show increased latency to approach food pellet (F = 17.22, df = 15, ANOVA-Tukey’s multiple comparisons test). B: they consume food pellet (F = 18.10, df = 16) compared with B6 and Aoah−/− Ahr cKO mice, in novelty-suppressed feeding (NSF) assay; outliers were identified using Prism Statistical Software C: female Aoah−/− mice showed decreased sucrose preference compared with B6 and Aoah−/−Ahr cKO in sucrose preference test (F = 9.533, df = 18, ANOVA-Tukey’s multiple comparisons test). D–F: Pparγ conditional knockout in Crf-expressing cells of female Aoah−/− mice does not alter depression-like phenotype (n = 19 WT, n = 9 Aoah–/–, n = 5 cKO). D: female Aoah−/− mice had increased latency to approach food pellet (F = 3.239, df = 20, ANOVA-Tukey’s multiple comparisons test). E: they consume food pellet (F = 6.977, df = 20) compared with B6 mice, in NSF assay. F: female Aoah−/− mice showed decreased preference for sucrose water (F = 7.546, df = 20, ANOVA-Tukey’s multiple comparisons test) compared with B6 mice in sucrose preference test. Data are means ± SE; differences were considered statistically significant at *P < 0.05; **P < 0.01; ***P < 0.001.
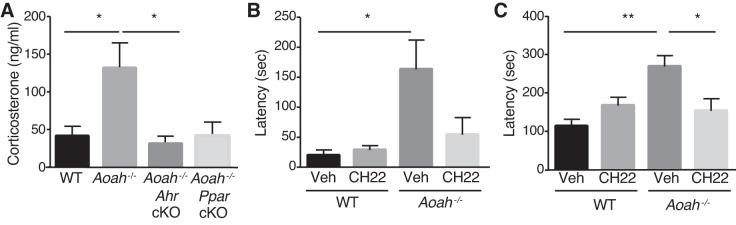
Previous studies showed that a PPARγ agonist decreased depressive symptoms in patients with major depression (14, 15), suggesting PPARγ mediates depressive phenotypes. We also examined the conditional knockout of Pparγ in Crf-expressing cells on the AOAH-deficient background, despite the absence of PPARγ-mediated Crf activation in vitro (Fig. 4). However, unlike the rescue of the AOAH deficiency phenotypes by Ahr cKO, Pparγ cKO resulted in no change in NSF latency, relative to Aoah−/− mice (Figs. 6, D and E). Similarly, Aoah−/− Pparγ cKO mice showed only slight, insignificant increase in sucrose preference (Fig. 6F), indicating Aoah−/− Pparγ cKO mice retained the phenotype of Aoah−/− mice. Furthermore, although both Aoah−/− Pparγ cKO and Aoah−/− Ahr cKO mice had decreased serum corticosterone levels, only the conditional knockout of Ahr in the Crf-expressing cells of Aoah−/− mice resulted in a significant restoration of corticosterone levels (Fig. 7A), demonstrating that HPA axis dysfunction of Aoah−/− mice is mediated predominantly by AhR.
Fig. 7.
AhR is a therapeutic target for AOAH deficiency. A: Ahr conditional knockout in Crf-expressing cells of female Aoah−/− mice rescues HPA axis dysfunction. Female Aoah−/− mice had increased serum corticosterone levels compared with WT and Aoah−/− AhR cKO (n = 5, F = 4.665, df = 41, ANOVA-Tukey’s multiple comparisons test). B–C: AhR inhibition in female Aoah−/− mice rescues NSF depressive-like behaviors. B: female Aoah−/− mice fed CH-223191 (CH22) had decreased latency to approach food pellet (n = 5, F = 5.215, df = 16, ANOVA-Tukey’s multiple comparisons test). C: they consume food pellet (n = 5, F = 6.661, df = 16) compared with vehicle-fed (Veh) Aoah−/− mice. Data are means ± SE; differences were considered statistically significant at *P < 0.05; **P < 0.01.
Finally, because the AhR antagonist CH-223191 blocked Crf induction by AA (Figs. 4, D and E), we evaluated the potential of AhR as a drug target. Administration of the AhR antagonist CH-223191 to Aoah−/− mice significantly reduced NSF latencies, relative to vehicle-treated mice (Figs. 5, B and C), thus rescuing the phenotype of Aoah−/− mice. Hence, AhR mediates AA-dependent Crf expression downstream of AOAH, likely by binding to XRE sites on the Crf promoter. Together, these data demonstrate that AhR mediates HPA axis dysfunction and depressive-like behaviors of AOAH-deficient mice.
DISCUSSION
These studies demonstrate that Aoah is a genetic modulator of Crf and CRF-mediated depressive-like behaviors. AOAH-deficient mice exhibited depressive-like phenotypes relative to wild-type mice by NSF and sucrose preference. To recapitulate the effects of chronic stress, previous studies have shown that repeated exposure to corticosterone induces depression in rodents (34, 66). Here, we find that Aoah−/− mice have increased serum corticosterone and increased Crf mRNA in the PVN. These results are consistent with human studies, which find increased serum cortisol and aberrant CRF levels in patients suffering major depression (4, 38, 39, 59).
CRF-mediated responses are complex, with different stress models resulting in increased Crf in different brain centers. For example, chronic unpredictable stress results in increased Crf in the bed nucleus of stria terminalis (57), while acute restraint stress causes increased Crf in the central nucleus of amygdala (26), but both models result in stress behaviors. These studies suggest differential expression of Crf in specific brain sites depending on the stress paradigm, ultimately manifesting as varying behavioral phenotypes (reviewed in Refs. 21, 23). Furthermore, treatment with a CRF receptor 1 antagonist directed to the central nucleus of amygdala resolves anxiety in rodents (22, 52), consistent with site-specific CRF modulation. We speculate that AOAH and AhR may also be involved in Crf regulation at these different brain centers because AOAH is expressed in many brain structures, including the bed nucleus of stria terminalis (63). Finally, social defeat stress results in increased PVN Crf expression (18), consistent with our findings, as we observed increased PVN Crf and depressive-like phenotypes in Aoah−/− mice. Future studies will explore the role of AOAH in mediating CRF-dependent behavioral phenotypes in different stress paradigms.
As Aoah−/− mice have increased AA in the CNS, we characterized transcription factors that mediate AA-dependent Crf expression. Several studies have shown that eicosanoids induce Crf expression (8, 12, 33), however the transcriptional mediators of AA-dependent Crf expression were previously unknown. We identified PPARγ and AhR as potential transcription factors of AA-dependent Crf expression, consistent with prior studies of these transcription factors (25, 37, 50) and found that AhR is the primary mediator of AA-dependent Crf expression, while PPARγ is a modest inhibitor of AA-dependent Crf expression in vitro.
Previous studies have suggested several possible roles for PPARγ in depression. For instance, PPARγ coactivator-1α (PGC-1α), has been shown to interact with PPARγ to regulate gene expression (46). Mice overexpressing PGC-1α were less likely to exhibit stress behaviors than wild-type mice, and this was mediated by Crf regulation (1). Additionally, exercise induced PGC-1α and PPARγ in the skeletal muscle and protected mice from stress behaviors. Studies have also shown patients with major depression often suffer from insulin resistance (41, 44, 62), and administration of PPARγ agonists in diabetic patients benefits their depressive symptoms (14, 15, 29), suggesting that PPARγ plays an inhibitory role in Crf-mediated depressive phenotypes. Here, we find that conditional knockout of Pparγ in Crf-expressing cells did not rescue stress behaviors or HPA axis dysregulation of Aoah−/− mice. The discrepancy between this study and prior studies may indicate differential roles for PPARγ in regulating insulin homeostasis and Crf.
While no previous studies addressed interactions between AhR and Crf directly, AhR agonists 2,3,7,8-tetrachlorodibenzo-p-dioxin and β-naphthoflavone both stimulated ACTH secretion in vitro (9, 10). Additionally, previous studies have shown that CRF-stimulated ACTH release was blocked by α-naphthoflavone (10), an AhR antagonist, suggesting that AhR mediated release of ACTH from CRF-stimulated cells is, therefore, an inducer of the HPA axis. Here, we find that AhR has an excitatory role in AA-stimulated Crf expression through interactions with XRE sites in the Crf promoter. Thus our findings support previous studies, which showed an AhR antagonist inhibited CRF-dependent end points (9, 10). Furthermore, we confirm the role of AhR by showing that either genetic or pharmacological targeting of Ahr, rescues stress behaviors of Aoah−/− mice.
Previous studies have linked Ahr to caffeine consumption (16), and examination of aggregated genome-wide association data in GWAS Central revealed (7) numerous polymorphisms in and near Aoah (15 SNPs) and Ahr (6 SNPs) that are at least weakly associated with psychiatric diseases (Supplementary Table S2). Because of the essential role that CRF plays in regulating stress responses, as well as autonomic and behavioral responses, future studies should explore the role of AOAH and AhR in modulating other CRF-dependent behaviors. Specifically, CRF plays a fundamental role in modulating bladder function, learning and memory consolidation, fear conditioning, and other stress-associated pathologies (6, 47, 48, 56), so AOAH and AhR may be involved in regulating these behaviors.
Perspectives and Significance
Aoah modulates Crf through AhR, thereby controlling HPA axis function and CRF-mediated behavioral phenotypes. This work also establishes AhR as a novel therapeutic target for psychiatric disease. In addition, the HPA axis and CRF are evolutionarily conserved and central to diverse biologic responses. Likewise, AOAH is evolutionarily conserved, expressed in diverse tissues, and expressed in multiple isoforms. Thus, beyond cognitive function, AOAH potentially modulates diverse biological processes across the animal kingdom.
GRANTS
This work was supported by National Institutes of Health awards F31-AI-106357 from National Institute of Allergy and Infectious Diseases (to L. M. Aguiniga) and U01-DK-82342 from National Institute of Diabetes and Digestive and Kidney Diseases (to D. J. Klumpp and A. J. Schaeffer). Histology services were provided by the Northwestern University Mouse Histology and Phenotyping Laboratory, supported by National Cancer Institute grant P30-CA-060553 (to the Robert H. Lurie Comprehensive Cancer Center).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.M.A., W.Y., A.J.S., and D.J.K. conceived and designed research; L.M.A., W.Y., R.E.Y., A.J.S., and D.J.K. performed experiments; L.M.A., W.Y., R.E.Y., A.J.S., and D.J.K. analyzed data; L.M.A., W.Y., A.J.S., and D.J.K. interpreted results of experiments; L.M.A., R.E.Y., and D.J.K. prepared figures; L.M.A., W.Y., A.J.S., and D.J.K. drafted manuscript; L.M.A., W.Y., A.J.S., D.J.K., and .M.I.A.I.R.N.S.G. edited and revised manuscript; L.M.A., W.Y., R.E.Y., A.J.S., and D.J.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank William Webb of Scripps and Shawn M. Ballard of the Center for Comparative Medicine for expert technical assistance and Dr. Jelena Radulovic for helpful suggestions on the manuscript.
Present address of Lizath M. Aguiniga: School of Pharmacy, University of Southern California, Los Angeles, CA 90089.
APPENDIX
Members of the MAPP Research Network Study Group
MAPP Network.
Executive committee: J. Quentin Clemens, MD, FACS, MSci (Network Chair, 2013–), Philip Hanno, MD, Ziya Kirkali, MD, John W. Kusek, PhD, J. Richard Landis, PhD, M. Scott Lucia, MD, Robert M. Moldwin, MD, Chris Mullins, PhD, Michel A. Pontari, MD; University of Colorado Denver Tissue Analysis & Technology Core: M. Scott Lucia, MD, Core Dir., Adrie van Bokhoven, PhD, Co-Dir., Andrea A. Osypuk, BS, Robert Dayton, Jr, Chelsea S. Triolo, BS, Karen R. Jonscher, PhD, Holly T. Sullivan, BS, R. Storey Wilson, MS, Zachary D. Grasmick, BS; National Institutes of Diabetes & Digestive and Kidney Diseases: Chris Mullins, PhD, John W. Kusek, PhD, Ziya Kirkali, MD, Tamara G. Bavendam, MD; University of Pennsylvania Data Coordinating Core: J. Richard Landis, PhD, Core Dir., Ted Barrell, BA, Ro-Pauline Doe, BA, John T. Farrar, MD, MSCE, PhD, Melissa Fernando, MPH, Laura Gallagher, MPH, CCRP, Philip Hanno, MD, Xiaoling Hou, MS, Tamara Howard, MPH, Thomas Jemielita, MS, Natalie Kuzla, MA, Robert M. Moldwin, MD, Craig Newcomb, MS, Michel A. Pontari, MD, Nancy Robinson-Garvin, PhD, Sandra Smith, AS, Alisa Stephens-Shields, PhD, Yanli Wang, MS, Xingmei Wang, MS.
Discovery sites.
Northwestern University: David J. Klumpp, PhD, Co-Dir., Anthony J. Schaeffer, MD, Co-Dir., Apkar (Vania) Apkarian, PhD, Christina Arroyo, Michael Bass, PhD, David Cella, PhD, Melissa A. Farmer, PhD, Colleen Fitzgerald, MD, Richard Gershon, PhD, James W. Griffith, PhD, Charles J. Heckman II, PhD, Mingchen Jiang, PhD, Laurie Keefer, PhD, Robert Lloyd, PhD, Darlene S. Marko, RN, BSN, CCRC, Jean Michniewicz, Richard Miller, PhD, Todd Parrish, PhD, Frank Tu, MD, MPH, Ryan Yaggi; University of California, LA PAIN Neuroimaging Core: Emeran A. Mayer, MD, Co-Dir., Larissa V. Rodríguez, MD, Co-Dir., Jeffry Alger, PhD, Cody P. Ashe-McNalley, Ben Ellingson, PhD, Nuwanthi Heendeniya, Lisa Kilpatrick, PhD, Cara, Kulbacki, Jason Kutch, PhD, Jennifer S. Labus, PhD, Bruce D. Naliboff, PhD, Fornessa Randal, Suzanne R. Smith, RN, NP; University of Iowa: Karl J. Kreder, MD, MBA, Dir., Catherine S. Bradley, MD, MSCE, Mary Eno, RN, RA, Kris Greiner, BA, Yi Luo, PhD, MD, Susan K. Lutgendorf, PhD, Michael A. O’Donnell, MD, Barbara Ziegler, BA , Andrew Schrepf, PhD, Isabelle Hardy, MBA, Vince Magnotta, PhD, Brad Erickson, MD; University of Michigan: Daniel J. Clauw, MD, Co-Dir. (Network Chair, 2008-2013), J. Quentin Clemens, MD, FACS, MSci (Co-Dir.; Network Chair, 2013–), Suzie As-Sanie, MD, Sandra Berry, MA, Clara Grayhack, , Megan E. Halvorson, BS, CCRP, Richard Harris, PhD, Steve Harte, PhD, Eric Ichesco, BS, Ann Oldendorf, MD, Katherine A. Scott, RN, BSN, David A. Williams, PhD; University of Washington, Seattle: Dedra Buchwald, MD, Dir., Niloofar Afari, PhD, UCSD, Tamara Bacus, BS, Todd Edwards, PhD, John Krieger, MD, Kenneth Maravilla, MD, Jane Miller, MD, Donald Patrick, PhD, Xiaoyan Qin, PhD, Stephanie Richey, BS, Rosana Risques, PhD, Kelly Robertson, BS , Susan O. Ross, RN, MN, Roberta Spiro, MS, Eric Strachan, PhD, TJ Sundsvold, MPH, Suzette Sutherland, MD, Claire C. Yang, MD; Washington University, St. Louis: Gerald L. Andriole, MD, Co-Dir., PI, H. Henry Lai, MD, Co-Dir., PI, Rebecca L. Bristol, BA, BS, Robert W. Gereau IV, PhD, Barry A. Hong, PhD, FAACP, Aleksandra P. Klim, RN, MHS, CCRC, Siobhan Sutcliffe, PhD, ScM, MHS, Joel Vetter, David G. Song, Melissa Milbrandt, Simon Haroutounian, PhD, Pooja Vijairania, Kaveri Parker (Chaturvedi), Tran Hung, Graham Colditz, MD, PH, Vivien C. Gardner, RN, BSN, Jeffrey P Henderson, MD, PhD, Theresa M. Spitznagle, PT, DPT, WCS, Ratna Pakpahan, MHA, Aimee James PhD, MPH, Yan Yan, Marvin Epolian Langston, Barry Hong, PhD, Susan Mueller, Jan Crowley, Sherri Vogt, Scott Hultgren, PhD, Nang Nguyen, PhD, Gabriel Blasche, Chang Shen Qiu, PhD, Lori Cupps, Song Bok, Thomas M. Hooten, MD (U. Miami), Lucy Grullon (U Miami), Nadege Atis(U Miami), Timothy J. Ness, MD, PhD (UAB), Georg Deutsch, PhD (UAB), Jan Den Hollander, PhD (UAB), Beverly D. Corbitt, RN (UAB), Laurence Bradley, PhD (UAB), Carol S. North, MD, MPE (UTSW), Dana Downs, MA (UTSW).
Nonrecruiting discovery sites.
Cedars-Sinai Medical Center: Jennifer Anger, MD, MPH, James Ackerman, MA, A. Lenore Ackerman, MD, PhD, Jeena Cha, BS, CCRP, Karyn Eilber, MD, Michael Freeman, PhD, Vincent Funari, PhD, Jayoung Kim, PhD, Jennifer Van Eyk, PhD, Wei Yang, PhD; Harvard Medical School/Boston Children’s Hospital: Marsha A. Moses, PhD, Dir., Andrew C. Briscoe, David Briscoe, MD, Adam Curatolo, BA, John Froehlich, PhD, Richard S. Lee, MD, Monisha Sachdev, BS, Keith R. Solomon, PhD, Hanno Steen, PhD; Stanford University: Sean Mackey, MD, PhD, Dir., Epifanio Bagarinao, PhD, Lauren C. Foster, BA, Emily Hubbard, BA, Kevin A. Johnson, PhD, RN, Katherine T. Martucci, PhD, Rebecca L. McCue, BA, Rachel R. Moericke, MA, Aneesha Nilakantan, BA, Noorulain Noor, BS; Queens University: J. Curtis Nickel, MD, FRCSC, Dir., Garth D. Ehrlich, PhD (Drexel COM).
REFERENCES
- 1.Agudelo LZ, Femenía T, Orhan F, Porsmyr-Palmertz M, Goiny M, Martinez-Redondo V, Correia JC, Izadi M, Bhat M, Schuppe-Koistinen I, Pettersson AT, Ferreira DMS, Krook A, Barres R, Zierath JR, Erhardt S, Lindskog M, Ruas JL. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 159: 33–45, 2014. [Erratum in Cell 160: P351, 2015]. doi: 10.1016/j.cell.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 2.Aguilera G, Rabadan-Diehl C, Nikodemova M. Regulation of pituitary corticotropin releasing hormone receptors. Peptides 22: 769–774, 2001. doi: 10.1016/S0196-9781(01)00390-4. [DOI] [PubMed] [Google Scholar]
- 4.Alesci S, Martinez PE, Kelkar S, Ilias I, Ronsaville DS, Listwak SJ, Ayala AR, Licinio J, Gold HK, Kling MA, Chrousos GP, Gold PW. Major depression is associated with significant diurnal elevations in plasma interleukin-6 levels, a shift of its circadian rhythm, and loss of physiological complexity in its secretion: clinical implications. J Clin Endocrinol Metab 90: 2522–2530, 2005. doi: 10.1210/jc.2004-1667. [DOI] [PubMed] [Google Scholar]
- 5.Arató M, Bánki CM, Bissette G, Nemeroff CB. Elevated CSF CRF in suicide victims. Biol Psychiatry 25: 355–359, 1989. doi: 10.1016/0006-3223(89)90183-2. [DOI] [PubMed] [Google Scholar]
- 6.Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol 160: 1–12, 1999. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 7.Beck T, Hastings RK, Gollapudi S, Free RC, Brookes AJ. GWAS Central: a comprehensive resource for the comparison and interrogation of genome-wide association studies. Eur J Hum Genet 22: 949–952, 2014. doi: 10.1038/ejhg.2013.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernardini R, Chiarenza A, Calogero AE, Gold PW, Chrousos GP. Arachidonic acid metabolites modulate rat hypothalamic corticotropin-releasing hormone secretion in vitro. Neuroendocrinology 50: 708–715, 1989. doi: 10.1159/000125303. [DOI] [PubMed] [Google Scholar]
- 9.Bestervelt LL, Pitt JA, Nolan CJ, Cai Y, Piper DW, Dybowski JA, Dayharsh GA, Piper WN. In vitro 2,3,7,8-tetrachlorodibenzo-p-dioxin interference with the anterior pituitary hormone adrenocorticortropin. Toxicol Sci 44: 107–115, 1998. doi: 10.1093/toxsci/44.2.107. [DOI] [PubMed] [Google Scholar]
- 10.Bestervelt LL, Pitt JA, Piper WN. Evidence for Ah receptor mediation of increased ACTH concentrations in primary cultures of rat anterior pituitary cells exposed to TCDD. Toxicol Sci 46: 294–299, 1998. doi: 10.1093/toxsci/46.2.294. [DOI] [PubMed] [Google Scholar]
- 11.Binder EB, Nemeroff CB. The CRF system, stress, depression and anxiety-insights from human genetic studies. Mol Psychiatry 15: 574–588, 2010. doi: 10.1038/mp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cambronero JC, Rivas FJ, Borrell J, Guaza C. Role of arachidonic acid metabolism on corticotropin-releasing factor (CRF)-release induced by interleukin-1 from superfused rat hypothalami. J Neuroimmunol 39: 57–66, 1992. doi: 10.1016/0165-5728(92)90174-J. [DOI] [PubMed] [Google Scholar]
- 13.Choi EY, Lee H, Dingle RW, Kim KB, Swanson HI. Development of novel CH223191-based antagonists of the aryl hydrocarbon receptor. Mol Pharmacol 81: 3–11, 2012. doi: 10.1124/mol.111.073643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colle R, de Larminat D, Rotenberg S, Hozer F, Hardy P, Verstuyft C, Fève B, Corruble E. Pioglitazone could induce remission in major depression: a meta-analysis. Neuropsychiatr Dis Treat 13: 9–16, 2016. doi: 10.2147/NDT.S121149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colle R, de Larminat D, Rotenberg S, Hozer F, Hardy P, Verstuyft C, Fève B, Corruble E. PPAR-γ agonists for the treatment of major depression: a review. Pharmacopsychiatry 50: 49–55, 2017. doi: 10.1055/s-0042-120120. [DOI] [PubMed] [Google Scholar]
- 16.Cornelis MC, Monda KL, Yu K, Paynter N, Azzato EM, Bennett SN, Berndt SI, Boerwinkle E, Chanock S, Chatterjee N, Couper D, Curhan G, Heiss G, Hu FB, Hunter DJ, Jacobs K, Jensen MK, Kraft P, Landi MT, Nettleton JA, Purdue MP, Rajaraman P, Rimm EB, Rose LM, Rothman N, Silverman D, Stolzenberg-Solomon R, Subar A, Yeager M, Chasman DI, van Dam RM, Caporaso NE. Genome-wide meta-analysis identifies regions on 7p21 (AHR) and 15q24 (CYP1A2) as determinants of habitual caffeine consumption. PLoS Genet 7: e1002033, 2011. doi: 10.1371/journal.pgen.1002033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dulawa SC, Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci Biobehav Rev 29: 771–783, 2005. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Elliott E, Ezra-Nevo G, Regev L, Neufeld-Cohen A, Chen A. Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat Neurosci 13: 1351–1353, 2010. doi: 10.1038/nn.2642. [DOI] [PubMed] [Google Scholar]
- 19.Paxinos G, Franklin KBJ. Paxinos and Franklin's The Mouse Brain in Stereotaxic Coordinates(4th ed.). Amsterdam: Academic, 2013. [Google Scholar]
- 20.Hanno P. Bladder pain syndrome (interstitial cystitis) and related disorders, in Campbell-Walsh Urology, edited by Wein A, Kavoussi L, Novick A, Partin A, Peters C. Philadelphia, PA: Elsevier, 2016, p. 334–370. [Google Scholar]
- 21.Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J Pharmacol Exp Ther 311: 427–440, 2004. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- 22.Heinrichs SC, Pich EM, Miczek KA, Britton KT, Koob GF. Corticotropin-releasing factor antagonist reduces emotionality in socially defeated rats via direct neurotropic action. Brain Res 581: 190–197, 1992. doi: 10.1016/0006-8993(92)90708-H. [DOI] [PubMed] [Google Scholar]
- 23.Henckens MJ, Deussing JM, Chen A. Region-specific roles of the corticotropin-releasing factor-urocortin system in stress. Nat Rev Neurosci 17: 636–651, 2016. doi: 10.1038/nrn.2016.94. [DOI] [PubMed] [Google Scholar]
- 24.Hinz B, Hirschelmann R. Rapid non-genomic feedback effects of glucocorticoids on CRF-induced ACTH secretion in rats. Pharm Res 17: 1273–1277, 2000. doi: 10.1023/A:1026499604848. [DOI] [PubMed] [Google Scholar]
- 25.Huang JT, Welch JS, Ricote M, Binder CJ, Willson TM, Kelly C, Witztum JL, Funk CD, Conrad D, Glass CK. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature 400: 378–382, 1999. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- 26.Iwasaki-Sekino A, Mano-Otagiri A, Ohata H, Yamauchi N, Shibasaki T. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology 34: 226–237, 2009. doi: 10.1016/j.psyneuen.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Johnson SA, Fournier NM, Kalynchuk LE. Effect of different doses of corticosterone on depression-like behavior and HPA axis responses to a novel stressor. Behav Brain Res 168: 280–288, 2006. doi: 10.1016/j.bbr.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Katafuchi T, Ichijo T, Hori T. Sequential relationship between actions of CRF and PGE2 in the brain on splenic sympathetic nerve activity in rats. J Auton Nerv Syst 67: 200–206, 1997. doi: 10.1016/S0165-1838(97)00115-X. [DOI] [PubMed] [Google Scholar]
- 29.Kemp DE, Ismail-Beigi F, Calabrese JR. Antidepressant response associated with pioglitazone: support for an overlapping pathophysiology between major depression and metabolic syndrome. Am J Psychiatry 166: 619, 2009. doi: 10.1176/appi.ajp.2008.08081195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kendler KS, Kessler RC, Walters EE, MacLean C, Neale MC, Heath AC, Eaves LJ. Stressful life events, genetic liability, and onset of an episode of major depression in women. Am J Psychiatry 152: 833–842, 1995. doi: 10.1176/ajp.152.6.833. [DOI] [PubMed] [Google Scholar]
- 31.Kim SH, Henry EC, Kim DK, Kim YH, Shin KJ, Han MS, Lee TG, Kang JK, Gasiewicz TA, Ryu SH, Suh PG. Novel compound 2-methyl-2H-pyrazole-3-carboxylic acid (2-methyl-4-o-tolylazo-phenyl)-amide (CH-223191) prevents 2,3,7,8-TCDD-induced toxicity by antagonizing the aryl hydrocarbon receptor. Mol Pharmacol 69: 1871–1878, 2006. doi: 10.1124/mol.105.021832. [DOI] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-Delta Delta C(T)] Method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Lyson K, McCann SM. Involvement of arachidonic acid cascade pathways in interleukin-6-stimulated corticotropin-releasing factor release in vitro. Neuroendocrinology 55: 708–713, 1992. doi: 10.1159/000126190. [DOI] [PubMed] [Google Scholar]
- 34.Marks W, Fournier NM, Kalynchuk LE. Repeated exposure to corticosterone increases depression-like behavior in two different versions of the forced swim test without altering nonspecific locomotor activity or muscle strength. Physiol Behav 98: 67–72, 2009. doi: 10.1016/j.physbeh.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 35.Munford RS, Hunter JP. Acyloxyacyl hydrolase, a leukocyte enzyme that deacylates bacterial lipopolysaccharides, has phospholipase, lysophospholipase, diacylglycerollipase, and acyltransferase activities in vitro. J Biol Chem 267: 10116–10121, 1992. [PubMed] [Google Scholar]
- 36.Muscat R, Willner P. Suppression of sucrose drinking by chronic mild unpredictable stress: a methodological analysis. Neurosci Biobehav Rev 16: 507–517, 1992. doi: 10.1016/S0149-7634(05)80192-7. [DOI] [PubMed] [Google Scholar]
- 37.Narala VR, Adapala RK, Suresh MV, Brock TG, Peters-Golden M, Reddy RC. Leukotriene B4 is a physiologically relevant endogenous peroxisome proliferator-activated receptor-alpha agonist. J Biol Chem 285: 22067–22074, 2010. doi: 10.1074/jbc.M109.085118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nemeroff CB, Bissette G, Widerlov E, Beckmann H, Gerner R, Manberg PJ, Lindstrom L, Prange AJ Jr, Gattaz WF. Neurotensin-like immunoreactivity in cerebrospinal fluid of patients with schizophrenia, depression, anorexia nervosa-bulimia, and premenstrual syndrome. J Neuropsychiatry Clin Neurosci 1: 16–20, 1989. doi: 10.1176/jnp.1.1.16. [DOI] [PubMed] [Google Scholar]
- 39.Nemeroff CB, Widerlöv E, Bissette G, Walléus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science 226: 1342–1344, 1984. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- 40.Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MC, Ward AM, Hahn YK, Lichtman AH, Conti B, Cravatt BF. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science 334: 809–813, 2011. doi: 10.1126/science.1209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okamura F, Tashiro A, Utumi A, Imai T, Suchi T, Tamura D, Sato Y, Suzuki S, Hongo M. Insulin resistance in patients with depression and its changes during the clinical course of depression: minimal model analysis. Metabolism 49: 1255–1260, 2000. doi: 10.1053/meta.2000.9515. [DOI] [PubMed] [Google Scholar]
- 42.Orth DN, Jackson RV, DeCherney GS, DeBold CR, Alexander AN, Island DP, Rivier J, Rivier C, Spiess J, Vale W. Effect of synthetic ovine corticotropin-releasing factor. Dose response of plasma adrenocorticotropin and cortisol. J Clin Invest 71: 587–595, 1983. doi: 10.1172/JCI110804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patti GJ, Yanes O, Shriver LP, Courade JP, Tautenhahn R, Manchester M, Siuzdak G. Metabolomics implicates altered sphingolipids in chronic pain of neuropathic origin. Nat Chem Biol 8: 232–234, 2012. doi: 10.1038/nchembio.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pearson S, Schmidt M, Patton G, Dwyer T, Blizzard L, Otahal P, Venn A. Depression and insulin resistance: cross-sectional associations in young adults. Diabetes Care 33: 1128–1133, 2010. doi: 10.2337/dc09-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pothion S, Bizot JC, Trovero F, Belzung C. Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behav Brain Res 155: 135–146, 2004. doi: 10.1016/j.bbr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92: 829–839, 1998. doi: 10.1016/S0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 47.Radulovic J, Fischer A, Katerkamp U, Spiess J. Role of regional neurotransmitter receptors in corticotropin-releasing factor (CRF)-mediated modulation of fear conditioning. Neuropharmacology 39: 707–710, 2000. doi: 10.1016/S0028-3908(99)00185-9. [DOI] [PubMed] [Google Scholar]
- 48.Radulovic J, Rühmann A, Liepold T, Spiess J. Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2. J Neurosci 19: 5016–5025, 1999. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rothrock N, Lutgendorf SK, Kreder KJ. Coping strategies in patients with interstitial cystitis: relationships with quality of life and depression. J Urol 169: 233–236, 2003. doi: 10.1016/S0022-5347(05)64075-X. [DOI] [PubMed] [Google Scholar]
- 50.Seidel SD, Winters GM, Rogers WJ, Ziccardi MH, Li V, Keser B, Denison MS. Activation of the Ah receptor signaling pathway by prostaglandins. J Biochem Mol Toxicol 15: 187–196, 2001. doi: 10.1002/jbt.16. [DOI] [PubMed] [Google Scholar]
- 51.Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci 8: 383–395, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swiergiel AH, Takahashi LK, Kalin NH. Attenuation of stress-induced behavior by antagonism of corticotropin-releasing factor receptors in the central amygdala in the rat. Brain Res 623: 229–234, 1993. doi: 10.1016/0006-8993(93)91432-R. [DOI] [PubMed] [Google Scholar]
- 53.Tautenhahn R, Cho K, Uritboonthai W, Zhu Z, Patti GJ, Siuzdak G. An accelerated workflow for untargeted metabolomics using the METLIN database. Nat Biotechnol 30: 826–828, 2012. doi: 10.1038/nbt.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tautenhahn R, Patti GJ, Rinehart D, Siuzdak G. XCMS Online: a web-based platform to process untargeted metabolomic data. Anal Chem 84: 5035–5039, 2012. doi: 10.1021/ac300698c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.ter Kuile MM, Weijenborg PT, Spinhoven P. Sexual functioning in women with chronic pelvic pain: the role of anxiety and depression. J Sex Med 7: 1901–1910, 2010. doi: 10.1111/j.1743-6109.2009.01414.x. [DOI] [PubMed] [Google Scholar]
- 56.Valentino RJ, Wood SK, Wein AJ, Zderic SA. The bladder-brain connection: putative role of corticotropin-releasing factor. Nat Rev Urol 8: 19–28, 2011. doi: 10.1038/nrurol.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ventura-Silva AP, Pêgo JM, Sousa JC, Marques AR, Rodrigues AJ, Marques F, Cerqueira JJ, Almeida OF, Sousa N. Stress shifts the response of the bed nucleus of the stria terminalis to an anxiogenic mode. Eur J Neurosci 36: 3396–3406, 2012. doi: 10.1111/j.1460-9568.2012.08262.x. [DOI] [PubMed] [Google Scholar]
- 58.Wamsteeker Cusulin JI, Füzesi T, Watts AG, Bains JS. Characterization of corticotropin-releasing hormone neurons in the paraventricular nucleus of the hypothalamus of Crh-IRES-Cre mutant mice. PLoS One 8: e64943, 2013. doi: 10.1371/journal.pone.0064943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang SS, Kamphuis W, Huitinga I, Zhou JN, Swaab DF. Gene expression analysis in the human hypothalamus in depression by laser microdissection and real-time PCR: the presence of multiple receptor imbalances. Mol Psychiatry 13: 786–799, 2008. doi: 10.1038/mp.2008.38. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y, Armando AM, Quehenberger O, Yan C, Dennis EA. Comprehensive ultra-performance liquid chromatographic separation and mass spectrometric analysis of eicosanoid metabolites in human samples. J Chromatogr A 1359: 60–69, 2014. doi: 10.1016/j.chroma.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 93: 358–364, 1987. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 62.Winokur A, Maislin G, Phillips JL, Amsterdam JD. Insulin resistance after oral glucose tolerance testing in patients with major depression. Am J Psychiatry 145: 325–330, 1988. doi: 10.1176/ajp.145.3.325. [DOI] [PubMed] [Google Scholar]
- 63.Yang W, Yaggie RE, Jiang MC, Rudick CN, Done J, Heckman CJ, Rosen JM, Schaeffer AJ, Klumpp DJ. Acyloxyacyl hydrolase modulates pelvic pain severity. Am J Physiol Regul Integr Comp Physiol 314: R353–R365, 2018. doi: 10.1152/ajpregu.00239.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoshida N, Maejima Y, Sedbazar U, Ando A, Kurita H, Damdindorj B, Takano E, Gantulga D, Iwasaki Y, Kurashina T, Onaka T, Dezaki K, Nakata M, Mori M, Yada T. Stressor-responsive central nesfatin-1 activates corticotropin-releasing hormone, noradrenaline and serotonin neurons and evokes hypothalamic-pituitary-adrenal axis. Aging (Albany NY) 2: 775–784, 2010. doi: 10.18632/aging.100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao B, Degroot DE, Hayashi A, He G, Denison MS. CH223191 is a ligand-selective antagonist of the Ah (Dioxin) receptor. Toxicol Sci 117: 393–403, 2010. doi: 10.1093/toxsci/kfq217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao Y, Ma R, Shen J, Su H, Xing D, Du L. A mouse model of depression induced by repeated corticosterone injections. Eur J Pharmacol 581: 113–120, 2008. doi: 10.1016/j.ejphar.2007.12.005. [DOI] [PubMed] [Google Scholar]