Abstract
We hypothesized that the serum from individuals with type 2 diabetes mellitus (T2DM) and impaired glucose tolerance (IGT) would reduce in vitro capillary-like network formation compared with normal glucose tolerance (NGT) serum and that this would occur along with higher serum concentrations of inflammatory cytokines and lower concentrations of angiogenic growth factors. Subjects were sedentary, older (55–65 yr) adults with NGT, IGT, or T2DM (n = 10/group) matched for body mass index. Human retroviral telomerized endothelial cells (HRVT-ECs) or coronary artery endothelial cells (CECs) were used in a capillary-like network formation assay using endothelial basal medium supplemented with 7.5% serum. Quantification of HRVT-EC network length indicated that serum from the T2DM group resulted in 32 and 35% lower network formation than when using serum from the NGT and IGT groups, respectively (P < 0.05). Serum from T2DM subjects resulted in CEC network formation that was 11 and 8% lower than when using serum from NGT and IGT subjects, respectively (P < 0.05). Analysis of serum cytokines indicated that IL-6 was 41% and 49% higher in the IGT and T2DM groups, respectively, compared with the NGT group (P < 0.05) and there was a trend for higher soluble interleukin-6 receptor (sIL-6R; P = 0.06) and IL-8 (P = 0.08) in the T2DM serum compared with NGT. The use of recombinant IL-6 and sIL-6R at concentrations detected in the T2DM serum also reduced capillary network formation compared with NGT concentrations (P < 0.05). These results suggest that IL-6 and sIL-6R present in the serum of T2DM individuals impair in vitro endothelial cell function across different cell lines. Our findings may have implications for the microvascular complications associated with T2DM.
NEW & NOTEWORTHY Higher concentrations of serum factors, specifically Interleukin-6 and its soluble receptor found in individuals with type 2 diabetes (T2DM) appear to impair endothelial cell capillary-like network formation compared with those present in serum from individuals with impaired glucose tolerance and normal glucose tolerance. This may have implications for the vascular complications associated with T2DM.
Keywords: angiogenesis, cytokines, diabetes mellitus, IL-6, impaired glucose tolerance
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is associated with impaired angiogenesis (20, 27, 32), microvascular rarefaction (37), and increased risk for cardiovascular disease (CVD) (19). These vascular complications may, in part, be attributable to endothelial cell dysfunction that is mediated by circulating factors. Hyperglycemia and elevated HbA1c have been inversely related to vascular flow-mediated dilation (6); however, factors other than hyperglycemia may also contribute to endothelial cell dysfunction. Indeed, in vitro experiments have demonstrated a significant reduction in human saphenous vein endothelial cell counts after 3 days of culture with serum from individuals with T2DM compared with healthy serum (35). Importantly, by controlling the ambient glucose concentration, these experiments indicate that the anti-angiogenic properties of T2DM serum are independent of hyperglycemia (35). Thus it appears that T2DM results in imbalances in other circulating factors, such as growth factors or cytokines, that may have an impact on endothelial cell counts and potentially on vascular endothelial function.
Several studies have identified differences in the concentrations of individual proteins in certain disease states, including T2DM. For example, elevated concentrations of circulating inflammatory factors such as IL-6, IL-8, and TNF-α have been found in individuals with T2DM compared with healthy adults (33). These factors may contribute to chronic inflammation in insulin resistance and endothelial cell dysfunction as evidenced by high concentrations of TNF-α that are associated with reduced flow-mediated dilation in T2DM (36). Relationships between serum IL-6 and endothelium-dependent vasodilation have also been reported independent of insulin sensitivity (14). These studies provide evidence for a role of proinflammatory cytokines in endothelial cell dysfunction and suggest that other endothelial cell functions, including angiogenic actions, may also be affected.
Conversely, angiogenic factors such as VEGF and basic (b)FGF have been found to promote capillary-like network formation and are involved in the complicated series of signaling events that regulate angiogenesis (4, 13). Previous reports of reductions in cardiac VEGF expression in individuals with T2DM compared with healthy adults suggest one mechanism behind impaired cardiac collateral vessel formation observed in the diabetic state (11). However, less is understood about the role of circulating VEGF and other angiogenic growth factors in the blood in T2DM and how this may influence endothelial cell proangiogenic functions. Furthermore, few studies have attempted to uncover the role of specific factors (inflammatory or angiogenic) on endothelial cell function or how this may differ across the spectrum of glucose metabolism including normal glucose tolerance (NGT), impaired glucose tolerance (IGT), and T2DM.
The purpose of this study was to determine whether serum from individuals with T2DM or IGT reduces in vitro capillary-like network formation of human retroviral telomerized endothelial cells (HRVT-ECs) and human primary coronary artery endothelial cells (CECs), compared with NGT controls and to determine whether there are differences in angiogenic and inflammatory cytokines that might account for these reductions in capillary-like network formation. We hypothesized that the serum from T2DM and IGT individuals would impair capillary-like network formation and that this would occur in tandem with higher serum concentrations of the inflammatory cytokines IL-6, soluble interleukin-6 receptor (sIL-6R), IL-8, and TNF-α and the soluble VEGF scavenger soluble fms-like tyrosine kinase-1 (sFlt-1), as well as lower serum concentrations of the angiogenic growth factors, VEGF, bFGF, and placental growth factor (PlGF).
METHODS
Subjects and Screening
Subjects were recruited from the Baltimore-Washington metro area and screened for enrollment. Subjects were sedentary (<20 min of aerobic exercise <2× times per week), overweight-to-obese (body mass index >25–35 kg/m2), middle-aged to older (50–75 yr) adults. Subjects were screened by physical examination, medical history, fasting blood chemistry, and graded maximal exercise test. Subjects all had normal, asymptomatic graded treadmill exercise tests and had no history of coronary artery disease, heart failure, peripheral arterial disease, stroke, liver, kidney, or lung disease. All subjects were current nonsmokers; those with a history of smoking had not smoked for >2 yr. The women in the study had not menstruated for at least 1 yr. Subjects in the T2DM group had a previous diagnosis of T2DM and were prescribed medications to control their diabetes. Subjects without known T2DM were given a 75-g oral glucose tolerance test to determine glucose tolerance status (NGT vs. IGT) and grouped according to American Diabetes Association criteria (2). The University of Maryland Baltimore Institutional Review Board approved all study procedures, and subjects provided written informed consent.
Maximal Exercise Test
Maximal oxygen consumption (V̇o2max) was measured by indirect calorimetry (Quark, Cosmed, Chicago, IL) during a graded exercise test on a motorized treadmill. Subjects walked at a constant velocity with the grade initially set to 0% and increasing every 2 min thereafter until volitional exhaustion. V̇o2max was defined as the highest oxygen consumption value obtained for a full 30-s increment and was verified by standard physiological criteria (i.e., respiratory exchange ratio ≥1.10 or a plateau in V̇o2 despite an increase in workload) (1).
Oral Glucose Tolerance Test and Blood Sampling
Subjects reported to the laboratory between 7 and 9 AM after a 12-h, overnight fast. Before and 120 min after consumption of a beverage containing 75 g of glucose, plasma glucose concentrations were analyzed (2300 STAT Plus; YSI, Yellow Springs, OH) for determination of glucose tolerance status. Blood for experiments was taken from the fasted sample before ingestion of the glucose beverage and used for serum collection. T2DM patients not receiving the oral glucose tolerance test reported to the laboratory between 7 and 9 AM after a 12-h overnight fast, and blood was drawn for serum collection.
Plasma Lipoprotein-Lipid Profiles and Blood Pressure
Plasma triglyceride and cholesterol levels were analyzed using enzymatic methods (UniCel DxC880i; Beckman Coulter, Brea, CA), and high-density lipoprotein cholesterol was measured in the supernatant after precipitation with dextran sulfate. Low-density lipoprotein cholesterol was calculated using the Friedewald equation as described previously (23): low-density lipoprotein cholesterol = total cholesterol − (triglyceride/5 + high-density lipoprotein cholesterol). Sitting blood pressure was measured on three occasions and the average reported.
Capillary-Like Network Formation Assay
The capillary-like network formation assay (4, 13, 17, 42) was performed as previously described (29, 30) to assess the effects of serum from NGT, IGT, and T2DM on network formation in HRVT-ECs and human primary CECs from healthy donors. Briefly, culture plates were coated with Matrigel (BD Biosciences) and the Matrigel was left to solidify for 30 min at 37°C and 5% CO2. Each condition was performed in triplicate. In a 96-well plate, each well contained 10,000 HRVT-ECs or CECs and serum-free endothelial basal medium (EBM-2) supplemented with 7.5% serum from NGT, IGT, or T2DM subjects. Additional wells were prepared for both HRVT-EC and CEC assays with a similar amount of fresh EBM-2 free of supplements or serum as a control. Assays were performed using passage 6 and 28 for CECs and HRVT-ECs, respectively. HRVT-EC cultures were then visualized after 6 h, and CEC cultures were visualized 15 h after seeding using a light microscope at ×4 magnification. The entire well was photographed using Nikon software. These images were then coded and blindly assessed for network length using the ImageJ Angiogenesis Analyzer software program (National Institutes of Health) (8). Results are presented as the average of triplicate wells from each condition normalized to the average basal condition.
Measurement of Serum Cytokines
Serum concentrations of IL-6, IL-8, TNF-α, VEGF, bFGF, PlGF, and sFlt-1 were measured in triplicate by multiplex ultra-sensitive sandwich immunoassays (Meso Scale Discovery, Gaithersburg, MD) according to the manufacturer’s instructions. Briefly, the serum sample (or manufacturer-provided standards) and a detection antibody solution were added in sequential steps to 96-well plates precoated with capture antibodies in spatially distinct spots. A buffer was then applied to provide an appropriate electrochemiluminescent signal, and the plate was read on a Meso Scale Discover SECTOR Imager 2400. The average intra-assay coefficients of variation were 3–9% in these assays. Interassay variability for these assays was 1–13%.
Serum concentrations of soluble IL-6 receptor (sIL-6R) were measured in duplicate by singleplex ELISA (ThermoFisher Scientific, Waltham, MA) according to the manufacturer’s instructions. This assay has a sensitivity of 0.01 ng/ml. The average intra-assay coefficient of variation was 4.5%. All samples were assayed on the same plate to avoid interassay variability.
Recombinant IL-6 and sIL-6R Capillary-Like Network Assay
To confirm the direct effects of IL-6 and sIL-6R on HRVT-EC and CEC network formation, the concentrations and proportions of IL-6 and sIL-6R in the serum of the NGT, IGT and T2DM groups were calculated and replicated using recombinant human (rh)IL-6 and sIL-6R in a capillary-like network formation assay. The concentration of each protein to which the HRVT-ECs or CECs were exposed was calculated from ELISA results taking into consideration the dilution factor and volumes used in the 50 μL capillary-like network assay. Based on these estimations, 0.18 pg of rhIL-6 (R&D Systems) and 0.036 μg of rhsIL-6R (R&D Systems) per milliliter of medium were used to simulate the NGT conditions, 0.207 pg of rhIL-6 and 0.042 μg of rhsIL-6R per milliliter of medium were used to match the IGT conditions, and 0.354 pg of rhIL-6 and 0.044 μg of rhsIL-6R per milliliter of medium were used to simulate the T2DM conditions. These proteins were added to HRTV-EC-based and CEC-based capillary-like network formation assays and compared with a control prepared with EBM-2 and a vehicle control. In these experiments, each condition was assessed in samples collected from n = 6 independent cell culture wells from multiple culture plates collected on 3 different days to confirm replication of our findings. All experiments were conducted on cells from the same passage number (P5 for CECs and P27 for HRVT-ECs) for each condition described above. Results are presented as the average of all wells from each condition normalized to the average basal conditions.
Statistical Analysis
A one-way ANOVA with pairwise comparisons was used to analyze data from capillary-like network formation and serum cytokines. Statistical significance was accepted at P ≤ 0.05. Values are expressed as means ± SE.
RESULTS
Subject Characteristics
Subject characteristics can be found in Table 1. As expected, blood glucose levels and HbA1c were significantly higher in the T2DM groups compared with IGT and NGT (P < 0.05 for both). There were no differences in age, body mass index, or cardiovascular fitness levels among groups (P > 0.05 for each).
Table 1.
Subject characteristics
| NGT | IGT | T2DM | |
|---|---|---|---|
| Sex (male/female) | 10 (4/6) | 10 (5/5) | 10 (5/5) |
| Age, yr | 59 ± 1.6 | 66 ± 3 | 61 ± 2.7 |
| BMI, kg/m2 | 31 ± 2.3 | 31 ± 1.2 | 37 ± 1.9 |
| Fasting glucose, mg/dL | 91 ± 2.1 | 95 ± 5 | 141 ± 7.4*# |
| 2-h Postprandial glucose, mg/dL | 96.6 ± 6.3 | 165 ± 10.8* | - |
| Triglycerides, mg/dL | 72 ± 5.6 | 119 ± 24.5 | 130 ± 22.1 |
| Total cholesterol, mg/dL | 180 ± 10.4 | 197 ± 8.2 | 157 ± 13# |
| HDL cholesterol, mg/dL | 57 ± 4.5 | 50 ± 5 | 40 ± 5.5 |
| LDL cholesterol, mg/dL | 109 ± 7.7 | 123 ± 9.1 | 91 ± 9.4# |
| HbA1c, % | 5.5 ± 0.05 | 5.7 ± 0.1 | 7.5 ± 0.3*# |
| Systolic BP, mmHg | 114 ± 4.2 | 127 ± 4.9 | 133 ± 5* |
| Diastolic BP, mmHg | 73 ± 2.8 | 77 ± 2.8 | 71 ± 3.3 |
| V̇o2max, L/min | 2.4 ± 0.2 | 2.04 ± 0.1 | 2.3 ± 0.2 |
| V̇o2max, mL·kg−1·min−1 | 26.1 ± 2.7 | 23.8 ± 1.6 | 22.2 ± 2.1 |
Values are means ± SE. BMI, body mass index; BP, blood pressure; NGT, normal glucose tolerance; IGT, impaired glucose tolerance; T2DM, type 2 diabetes mellitus.
P < 0.05, significantly different from NGT.
P < 0.05, significantly different from IGT.
Capillary-Like Network Formation Assay
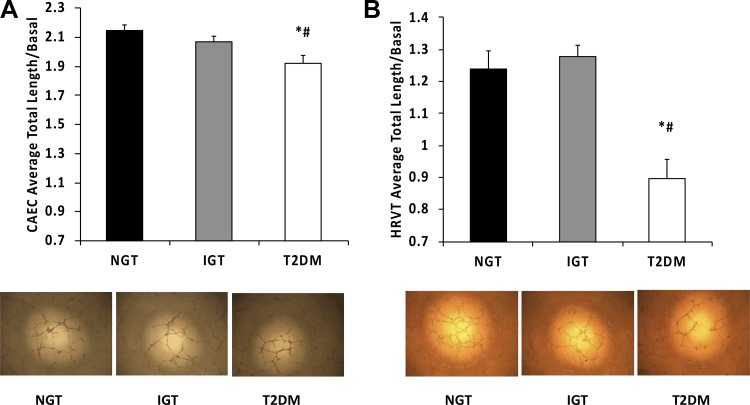
In the CEC assay, serum from T2DM subjects resulted in capillary-like network formation that was 11 and 8% lower than when exposed to serum from NGT and IGT subjects, respectively (Fig. 1A; P < 0.05 for both). In the HRVT-EC assay, network length when exposed to serum from the T2DM group was 32 and 35% lower than when using serum from the NGT and IGT groups, respectively (Fig. 1B; P < 0.05 for both). There were no significant differences in HRVT-EC or CEC network formation between NGT and IGT conditions (P > 0.05). Calculations of the in vitro glucose concentrations exposed to endothelial cells in the capillary-like network formation assays ruled out the effects of hyperglycemia (99.3 ± 0.16, 99.7 ± 0.38, and 103 ± 0.55 mg/dL for NGT, IGT, and T2DM, respectively).
Fig. 1.
Capillary-like network formation and representative images of coronary artery endothelial cells (CAECs; A) and human retroviral telomerized endothelial cells (HRVTs; B) exposed to 7.5% serum from individuals with normal glucose tolerance (NGT), impaired glucose tolerance (IGT), and type 2 diabetes mellitus (T2DM). *P < 0.05, significant difference compared with NGT. #P < 0.05, significant difference compared with IGT.
Serum Cytokines
Growth factors.
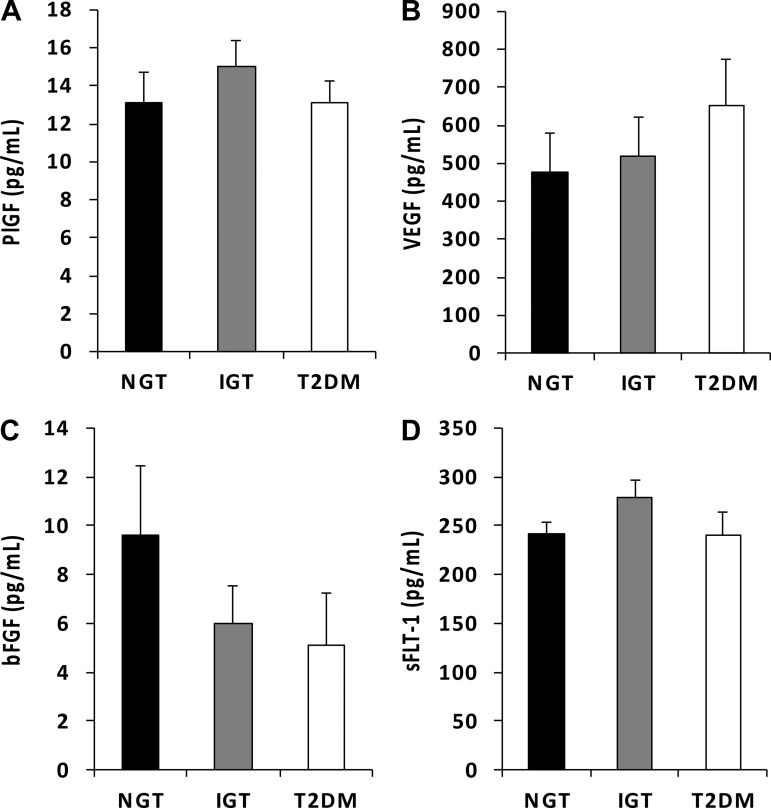
No significant differences were observed between NGT, IGT, and T2DM for serum PlGF or sFlt-1 (P > 0.05 for both; Fig. 2, A and D, respectively). Serum from the T2DM group contained VEGF concentrations that were numerically 26 and 37% higher compared with the IGT and NGT groups but this was not statistically significant (P = 0.4 and P = 0.27, respectively; Fig. 2B). Despite serum from the IGT and T2DM groups exhibiting concentrations of bFGF 37 and 47% lower compared with the NGT group, respectively, this did not reach statistical significance (P = 0.1 and P = 0.2, respectively; Fig. 2C).
Fig. 2.
Concentrations of placental growth factor (PlGF; A), VEGF (B), basic (b)FGF (C), and soluble fms-like tyrosine kinase-1 (sFlt-1; D) present in serum from normal glucose tolerance (NGT), impaired glucose tolerance (IGT), and type 2 diabetes mellitus (T2DM) individuals. No statistically significant differences were observed among groups.
Inflammatory factors.
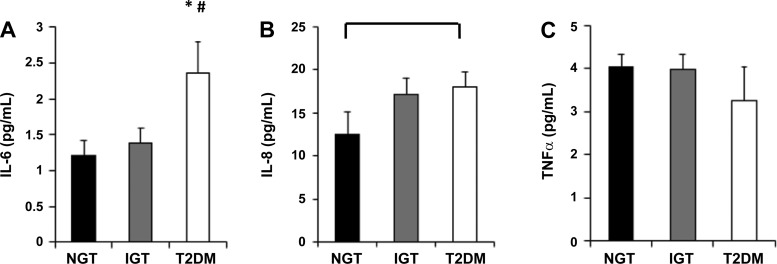
Serum IL-6 concentrations were no different between the NGT and IGT groups (P > 0.05). However, the T2DM group exhibited 41% higher serum IL-6 compared with the IGT group (P = 0.03) and 49% higher serum IL-6 compared with the NGT group (P = 0.01; Fig. 3A). No statistically significant differences were observed among groups for IL-8 (Fig. 3B). However, there was a tendency for higher serum IL-8 in the T2DM group compared with the NGT group (P = 0.08; Fig. 3B). No statistically significant differences were observed among groups for TNF-α (P > 0.05; Fig. 3C).
Fig. 3.
Concentrations of IL-6 (A), IL-8 (B), and TNF-α (C) present in serum from normal glucose tolerance (NGT), impaired glucose tolerance (IGT), and type 2 diabetes mellitus (T2DM) individuals. *P < 0.05, significant difference compared with NGT. #P < 0.05, significant difference compared with IGT.
sIL-6R.
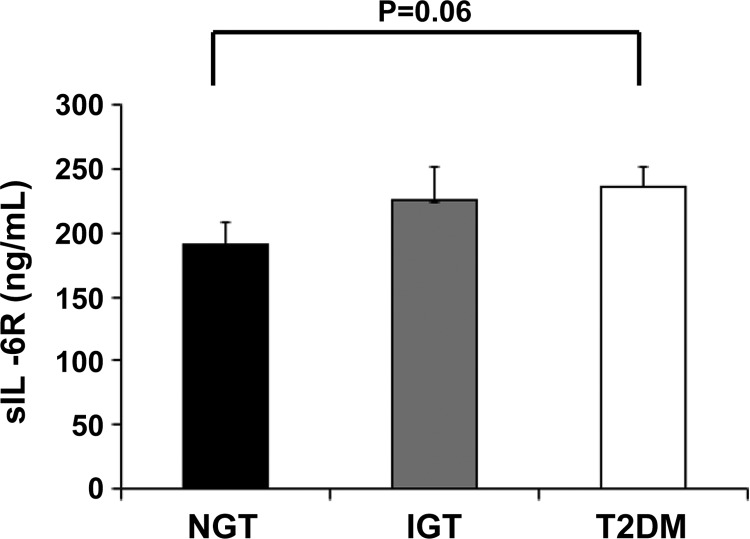
There were no significant differences in sIL-6R concentrations between the NGT and IGT groups or the IGT and T2DM (P > 0.05 for both). Concentrations of sIL-6R in the T2DM serum were 20% higher than those in the NGT serum (P = 0.06; Fig. 4).
Fig. 4.
Concentrations of soluble interleukin-6 receptor (sIL-6R) present in serum from normal glucose tolerance (NGT), impaired glucose tolerance (IGT), and type 2 diabetes mellitus (T2DM) individuals. P = 0.06 NGT vs. T2DM.
Recombinant IL-6 and sIL-6R Capillary-Like Network Assay
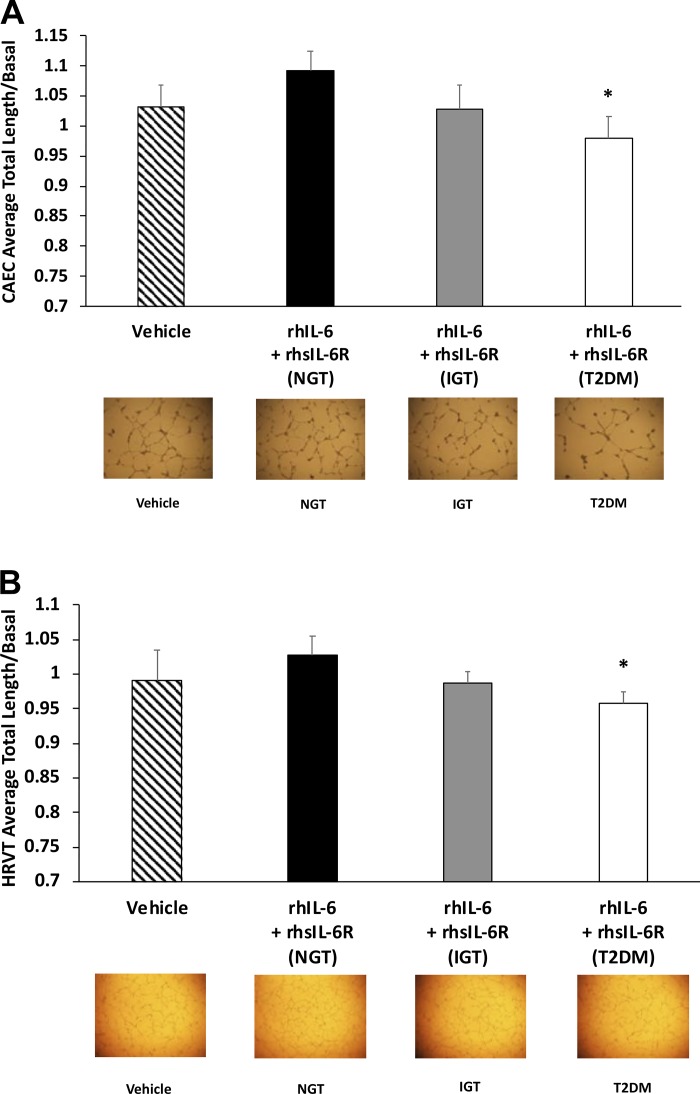
We sought to determine whether the inhibitory effects observed from the T2DM serum on the capillary-like network formation could be explained by IL-6 and its soluble receptor. We repeated the CEC and HRVT-EC experiments using basal medium and rhIL-6 and rhsIL-6R in concentrations and proportions that represented the within-group averages in serum from the NGT, IGT, and T2DM groups. In the CECs, no differences in capillary-like network formation were observed when comparing concentrations of rhIL-6 and rhsIL-6R representing the NGT serum and concentrations representing the IGT serum nor when comparing the IGT and T2DM conditions (P > 0.05 for both, Fig. 5A). However, when using concentrations of rhIL-6 and rhsIL-6R representing the T2DM serum, CEC capillary-like network formation was significantly reduced compared with the NGT condition (P = 0.031; Fig. 5A). There was no significant difference in CEC-based capillary-like network formation between the basal or vehicle conditions (P > 0.05).
Fig. 5.
Experiments using rhIL-6 and rhsIL-6R mimic the effects of the serum from type 2 diabetes mellitus (T2DM) compared with normal glucose tolerance (NGT). Exposure of coronary artery endothelial cells (CAECs; A) and human retroviral telomerized endothelial cells (HRVTs; B) to recombinant human (rh)IL-6 and recombinant human soluble interleukin-6 receptor (rhsIL-6R) levels equivalent to that found in serum from T2DM group impairs capillary-like network formation compared with NGT. Concentrations of IL-6 and sIL-6R were measured in serum and concentrations exposed to the CAECs and HRVTs in the 7.5% serum capillary network assay were applied in this assay as follows: 0.18 pg/mL and 0.036 μg/mL, respectively, for the NGT group, 0.207 pg/mL and 0.042 μg/mL, respectively, for the IGT group and 0.354 and 0.044 μg/mL, respectively, for the T2DM group. In these experiments, each condition was assessed in samples collected from 6 independent cell culture wells from multiple culture plates collected on 3 separate occasions. All experiments were conducted on cells from the same passage number (P5 for CAECs and P27 for HRVTs). *P < 0.05, significantly different compared with NGT condition. P = 0.06 basal vs. NGT in CEC only.
DISCUSSION
In this study, we demonstrate that circulating factors present in the serum of T2DM individuals impair in vitro endothelial cell capillary-like network formation in both primary and telomerized endothelial cell lines. Our data further suggest that elevations in IL-6 and its soluble receptor may be responsible for these alterations in angiogenic potential. These findings suggest IL-6 transsignaling as a potential mechanism underlying vascular impairments and reduced angiogenesis often found in T2DM.
Exposure of endothelial cells to serum from individuals in various disease states has previously resulted in alterations in endothelial cell functions. One study demonstrated that exposure of human umbilical vein endothelial cells to serum from patients who recently had an ST-elevation myocardial infarction resulted in a progressive loss of cell viability over 4 days due to an increase in apoptosis (15). Münzel et al. (35) reported lower cell counts when endothelial cells were exposed to serum from T2DM over 72 h compared with healthy controls. These studies demonstrate that factors contained in serum can have powerful effects on endothelial cell functions; however, few studies to date have attempted to explain what factors present in serum are responsible for these outcomes.
Elevations in proinflammatory cytokines in the serum of individuals with T2DM have consistently been reported in the literature (31, 33, 35). Münzel et al. (35) reported elevations in soluble ICAM-1, sE-selectin, and monocyte chemoattractant protein-1 in serum from T2DM compared with healthy adults. However, the use of pooled samples in this study did not allow for determination of statistical significance. Interestingly, IL-6 and IL-8 concentrations in both healthy and T2DM pooled serum samples were reported as below detection limits by Münzel et al. (35) and were not considered as major contributors to their observed reduction in endothelial cell counts after exposure to T2DM versus healthy serum. In comparison, we detected serum IL-8 levels in the present study that approached statistical significance between NGT and T2DM groups. There have been other reports of elevated IL-8 in serum of people with T2DM compared with NGT (33). While elevated IL-8 has been deemed proangiogenic due to its role in various types of cancer (38), in noncancer pathologies including T2DM, IL-8 has been associated with negative cardiometabolic outcomes (12). Furthermore, reductions in human umbilical vein endothelial cell tube formation after exposure to conditioned media generated from T2DM myotubes has been found to be mediated by IL-8, suggesting that IL-8 may play a role in capillary rarefaction reported in T2DM (3). In the present study, serum IL-6 concentrations were significantly higher in the serum from the T2DM group compared with the NGT and IGT groups. Other studies also showed significantly higher concentrations of serum IL-6 in T2DM compared with healthy controls in similar concentrations as those in the present report (14, 33). Thus IL-6 and perhaps IL-8 serve as viable candidates as cytokines with potential functional roles in T2DM.
The influence of IL-6 on endothelial cells may be affected by the cellular responses to IL-6 transsignaling versus traditional signaling. Indeed, it has been established that endothelial cells do not express the membrane bound receptor for IL-6 preventing traditional IL-6 signaling pathways in endothelial cells (24, 39, 40, 43). Rather, in response to apoptosis, IL-6R can be cleaved from the membrane of IL-6R-expressing cells and be found in circulation in its soluble form (sIL-6R). Binding of IL-6 to the sIL-6R allows for the complex to bind to gp130 on the surface of endothelial cells and induce IL-6 transsignaling. This transsignaling has been described in the proinflammatory actions of IL-6 on endothelial cells (21, 24, 43) in which IL-6/sIL-6R stimulate the expression of monocyte chemoattractant protein-1, VCAM-1, and ICAM-1 and endothelial cell activation, which may impair angiogenic actions (24). Thus the effects of IL-6 on the endothelium are dependent on the presence of sIL-6R. In the present study, we detected a tendency for higher sIL-6R concentrations in the serum of the T2DM group compared with NGT. These physiologically higher sIL-6R concentrations in combination with the higher IL-6 concentrations observed in T2DM serum compared with NGT suggest that IL-6 transsignaling may be responsible for the deleterious effects of T2DM serum on endothelial cell network formation.
We empirically tested the effects of IL-6 and sIL-6R on endothelial cell capillary-like network formation by using concentrations of rhIL-6 and rhsIL-6R that represented the average concentration from NGT, IGT, and T2DM serum that was exposed to HRVT-ECs and CECs in the capillary network formation assay. We demonstrated significantly lower capillary-like network formation in both HRVT-ECs and CECs using concentrations of rhIL-6 and rhsIL-6R that mimicked those in the serum of T2DM compared with concentrations mimicking the NGT condition but no statistically significant decrements in the IGT-simulated condition. Of note, the differences between these groups were not as great as those observed when using serum from these individuals in the same capillary-like network formation assay. Furthermore, although we also observed significantly reduced capillary-like network formation in T2DM versus IGT when using serum from each group, this was not evident when isolating out only the effects of IL-6 using rhIL-6 and rhsIL-6R concentrations from these groups. These findings indicate that although IL-6 and sIL-6R appear to have an important influence on angiogenic actions in T2DM it is likely that other serum factors such as IL-8 or other inflammatory cytokines are also involved in this response and may be playing a more critical role during the progression of IGT to T2DM.
Interestingly, IL-6 was previously reported to promote angiogenesis by inducing local VEGF expression (22). This is most commonly found in cancer lines stimulated with concentrations of IL-6 well outside the range of what is typically found in serum, even in chronic inflammatory disease conditions (16, 22, 25). Furthermore, some studies have found that these high concentrations of IL-6 used in in vitro experiments can promote defective angiogenesis in cancer cell lines through diminished pericyte coverage (5, 16, 25). In the present study, impaired capillary-like network formation was observed when CECs and HRVT-ECs were exposed to IL-6 concentrations mimicking those detected in the T2DM group. Of note, these concentrations were substantially lower than what has been used previously in studies to promote angiogenesis (2.3 pg/mL vs. 50–100 ng/mL). This may be explained by differences in systemic concentrations of IL-6 compared with levels potentially found in a localized cellular microenvironment. Future studies should examine the dose-dependent effects of IL-6 and sIL-6R on angiogenesis across both physiological and supraphysiological concentrations to fully understand the role of IL-6 in T2DM and other chronic diseases with vascular consequences.
Expression of angiogenic growth factors previously was found to be reduced in the cardiac tissue of T2DM patients and rodents (11, 26). Khazaei et al. (26) found elevations in serum sFlt-1 in T2DM rats compared with healthy controls and lower VEGF/sFlt-1 ratios in the diabetic animals that were significantly correlated with lower myocardial capillary density. However, systemic alterations in angiogenic growth factors due to T2DM are not found consistently in the literature. Similar to our findings, there are several other reports demonstrating no significant differences in serum concentrations of angiogenic growth factors including VEGF (7, 18, 26), bFGF (18), and sFlt-1 (7) between healthy individuals and those with T2DM. Thus it is possible that the effects of T2DM may have a larger impact on angiogenic factors at the local level that may not be obvious in the systemic circulation (11, 18). We selected angiogenic growth factors and proinflammatory cytokines that have been previously found to be differentially expressed either at the local or systemic level between healthy individuals and individuals with T2DM and/or have been reported to influence angiogenesis (11, 26, 31, 33). Future experiments are needed to determine differences in concentrations of other factors that may be involved in this process across NGT, IGT, and T2DM and to explore the interplay between these factors and IL-6.
In this study we used rhIL-6 and rhsIL-6R in a stimulation-response approach to confirm the role of these cytokines in isolation on capillary-like network formation. Future approaches including inhibition of IL-6 trans-signaling using pharmacological treatments or sIL-6R inhibitors as well as addition of rhIL-6 and rhsIL-6R to the NGT and IGT serum to levels that replicate the T2DM serum would provide further support as to the role of IL-6 and its soluble receptor in capillary-like network formation. Additionally, the capillary-like network formation assay utilized in this study serves as a comprehensive assay that involves several components of the angiogenic process. Future studies should also determine whether IL-6 and sIL-6R have similar inhibitory effects on functional assays to assess other specific angiogenic actions including endothelial cell migration and proliferation.
Previous studies have compared serum cytokines between healthy individuals and individuals with T2DM (33, 35), as well as the effects of serum from healthy and T2DM individuals on endothelial cell functions (35). However, ours is the first, to our knowledge, to examine differences in serum factors on angiogenic actions across three stages of T2DM (NGT, IGT, and T2DM) and to quantify serum cytokines and growth factors across these stages. This information may help to inform future studies and drive development of therapies to help treat the vascular effects of T2DM at different stages in the development of T2DM to reduce long-term vascular consequences. In this study, information regarding time since development of T2DM was not obtained. Future studies following individuals over time as they progress from NGT to IGT and at different time points since diagnosis of T2DM are needed to confirm our cross-sectional observation. Interestingly, we did not detect differences in serum cytokines between the NGT and IGT groups. This is in agreement with some (10, 28, 41) but not all (28, 34) previous reports. These mixed findings in the literature indicate a need for further investigations to explore other factors associated with IGT that may be explaining these inconsistencies. This study is strengthened by the inclusion of both men and women. However, the small sample size does not currently allow for determination of sex differences. The small sample size may also explain the lack of statistical significance in cytokine levels such as VEGF and bFGF that exhibited notable numerical differences between groups. All individuals with T2DM were currently prescribed medication to control their diabetes. There currently are mixed findings in the literature regarding the effects of diabetes medications such as metformin on serum IL-6 concentrations with most studies reporting no effects or reduction in serum IL-6 (9, 44). Thus it is possible that without medication we would have observed a more profound difference in serum IL-6 between the NGT and T2DM groups. Other prescribed or over the counter medications may have also impacted our findings. Future studies are needed to determine the effects of diabetes medication independent of, or in combination with, other medications on serum-induced capillary network formation and serum cytokines in T2DM. Finally, the use of two cell lines in our study indicates consistency across different parts of the vasculature in both primary and telomerized cells. In this study, both cell lines were from healthy donors. The use of endothelial cells from patients with T2DM in future studies would allow for a better understanding of the effects circulating factors on endothelial cell functions.
In summary, we demonstrate that circulating factors present in the serum of T2DM individuals diminish in vitro endothelial cell capillary-like network formation and that this is consistent across primary and telomerized cell lines. We also provide evidence that elevations in IL-6 and sIL-6R found in T2DM are largely responsible for the impairments in angiogenic actions. Our findings may have implications for the microvascular complications associated with T2DM and may be used to inform future therapies to treat the vascular consequences of T2DM.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant T32-HL-007698, National Institute on Aging Grant K23-AG-040775, Department of Veterans Affairs Grant I01-CX000730, and Baltimore Veterans Affairs Geriatric Research, Education, and Clinical Center.
DISCLAIMERS
All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.Q.L. and S.J.P. conceived and designed research; R.Q.L., J.B.B., and S.J.P. collected data and performed experiments; R.Q.L. and S.J.P. analyzed data; R.Q.L. and S.J.P. interpreted results; R.Q.L. prepared figures and drafted the manuscript; R.Q.L., J.B.B., and S.J.P. edited and revised the final version of the manuscript.
REFERENCES
- 1.American College of Sports Medicine ACSM Guidelines for Exercise Testing and Prescription, edited by Pescatello LS. Philadelphia, PA: Lippincott Williams & Wilkins, 2014. [Google Scholar]
- 2.American Diabetes Association Classification and diagnosis of diabetes: Standards of medical care in diabetes. Diabetes Care 41, Suppl 1: S13–S27, 2018. doi: 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 3.Amir Levy Y, Ciaraldi TP, Mudaliar SR, Phillips SA, Henry RR. Excessive secretion of IL-8 by skeletal muscle in type 2 diabetes impairs tube growth: potential role of PI3K and the Tie2 receptor. Am J Physiol Endocrinol Metab 309: E22–E34, 2015. doi: 10.1152/ajpendo.00513.2014. [DOI] [PubMed] [Google Scholar]
- 4.Arnaoutova I, George J, Kleinman HK, Benton G. The endothelial cell tube formation assay on basement membrane turns 20: state of the science and the art. Angiogenesis 12: 267–274, 2009. doi: 10.1007/s10456-009-9146-4. [DOI] [PubMed] [Google Scholar]
- 5.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro-oncol 7: 452–464, 2005. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bissinger A, Grycewicz T, Grabowicz W, Lubiński A. Endothelial function and left ventricular remodeling in diabetic and non-diabetic patients after acute coronary syndrome. Med Sci Monit 17: CR73–CR77, 2011. doi: 10.12659/MSM.881390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blann AD, Belgore FM, McCollum CN, Silverman S, Lip PL, Lip GY. Vascular endothelial growth factor and its receptor, Flt-1, in the plasma of patients with coronary or peripheral atherosclerosis, or Type II diabetes. Clin Sci (Lond) 102: 187–194, 2002. doi: 10.1042/cs1020187. [DOI] [PubMed] [Google Scholar]
- 8.Carpentier G. G, Martinelli M, Courty J, Cascone I.. Angiogenesis analyzer for ImageJ. 4th ImageJ User and Developer Conference Proceeding Mondorf-les-Bains, Luxembourg, 2012, p. 198–201. [Google Scholar]
- 9.Chen W, Liu X, Ye S. Effects of metformin on blood and urine pro-inflammatory mediators in patients with type 2 diabetes. J Inflamm (Lond) 13: 34, 2016. doi: 10.1186/s12950-016-0142-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi KM, Lee J, Lee KW, Seo JA, Oh JH, Kim SG, Kim NH, Choi DS, Baik SH. Comparison of serum concentrations of C-reactive protein, TNF-α, and interleukin 6 between elderly Korean women with normal and impaired glucose tolerance. Diabetes Res Clin Pract 64: 99–106, 2004. doi: 10.1016/j.diabres.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Chou E, Suzuma I, Way KJ, Opland D, Clermont AC, Naruse K, Suzuma K, Bowling NL, Vlahos CJ, Aiello LP, King GL. Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic States: a possible explanation for impaired collateral formation in cardiac tissue. Circulation 105: 373–379, 2002. doi: 10.1161/hc0302.102143. [DOI] [PubMed] [Google Scholar]
- 12.Cimini FA, Barchetta I, Porzia A, Mainiero F, Costantino C, Bertoccini L, Ceccarelli V, Morini S, Baroni MG, Lenzi A, Cavallo MG. Circulating IL-8 levels are increased in patients with type 2 diabetes and associated with worse inflammatory and cardiometabolic profile. Acta Diabetol 54: 961–967, 2017. doi: 10.1007/s00592-017-1039-1. [DOI] [PubMed] [Google Scholar]
- 13.Csiszar A, Sosnowska D, Tucsek Z, Gautam T, Toth P, Losonczy G, Colman RJ, Weindruch R, Anderson RM, Sonntag WE, Ungvari Z. Circulating factors induced by caloric restriction in the nonhuman primate Macaca mulatta activate angiogenic processes in endothelial cells. J Gerontol A Biol Sci Med Sci 68: 235–249, 2013. doi: 10.1093/gerona/gls158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esteve E, Castro A, López-Bermejo A, Vendrell J, Ricart W, Fernández-Real JM. Serum interleukin-6 correlates with endothelial dysfunction in healthy men independently of insulin sensitivity. Diabetes Care 30: 939–945, 2007. doi: 10.2337/dc06-1793. [DOI] [PubMed] [Google Scholar]
- 15.Forteza MJ, Novella S, Trapero I, Hermenegildo C, Ruiz-Sauri A, Chaustre F, Bonanad C, Oltra R, Palacios L, O’Connor JE, Chorro FJ, Bodi V. Dynamics of serum-induced endothelial cell apoptosis in patients with myocardial infarction. Eur J Clin Invest 44: 46–53, 2014. doi: 10.1111/eci.12189. [DOI] [PubMed] [Google Scholar]
- 16.Gopinathan G, Milagre C, Pearce OM, Reynolds LE, Hodivala-Dilke K, Leinster DA, Zhong H, Hollingsworth RE, Thompson R, Whiteford JR, Balkwill F. Interleukin-6 stimulates defective angiogenesis. Cancer Res 75: 3098–3107, 2015. doi: 10.1158/0008-5472.CAN-15-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groleau J, Dussault S, Haddad P, Turgeon J, Ménard C, Chan JS, Rivard A. Essential role of copper-zinc superoxide dismutase for ischemia-induced neovascularization via modulation of bone marrow-derived endothelial progenitor cells. Arterioscler Thromb Vasc Biol 30: 2173–2181, 2010. doi: 10.1161/ATVBAHA.110.212530. [DOI] [PubMed] [Google Scholar]
- 18.Gui C, Du F, Nong QL, Lian BJ, Zhu LG. Changes of serum angiogenic factors concentrations in patients with diabetes and unstable angina pectoris. Zhonghua Jizhen Yixue Zazhi 12: 34, 2013. doi: 10.1186/1475-2840-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 339: 229–234, 1998. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 20.Hazarika S, Dokun AO, Li Y, Popel AS, Kontos CD, Annex BH. Impaired angiogenesis after hindlimb ischemia in type 2 diabetes mellitus: differential regulation of vascular endothelial growth factor receptor 1 and soluble vascular endothelial growth factor receptor 1. Circ Res 101: 948–956, 2007. doi: 10.1161/CIRCRESAHA.107.160630. [DOI] [PubMed] [Google Scholar]
- 21.Hou T, Tieu BC, Ray S, Recinos A 3rd, Cui R, Tilton RG, Brasier AR. Roles of IL-6-gp130 signaling in vascular inflammation. Curr Cardiol Rev 4: 179–192, 2008. doi: 10.2174/157340308785160570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang SP, Wu MS, Shun CT, Wang HP, Lin MT, Kuo ML, Lin JT. Interleukin-6 increases vascular endothelial growth factor and angiogenesis in gastric carcinoma. J Biomed Sci 11: 517–527, 2004. doi: 10.1007/BF02256101. [DOI] [PubMed] [Google Scholar]
- 23.Joseph LJ, Prigeon RL, Blumenthal JB, Ryan AS, Goldberg AP. Weight loss and low-intensity exercise for the treatment of metabolic syndrome in obese postmenopausal women. J Gerontol A Biol Sci Med Sci 66A: 1022–1029, 2011. doi: 10.1093/gerona/glr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kallen KJ. The role of transsignalling via the agonistic soluble IL-6 receptor in human diseases. Biochim Biophys Acta 1592: 323–343, 2002. doi: 10.1016/S0167-4889(02)00325-7. [DOI] [PubMed] [Google Scholar]
- 25.Kayakabe K, Kuroiwa T, Sakurai N, Ikeuchi H, Kadiombo AT, Sakairi T, Matsumoto T, Maeshima A, Hiromura K, Nojima Y. Interleukin-6 promotes destabilized angiogenesis by modulating angiopoietin expression in rheumatoid arthritis. Rheumatology (Oxford) 51: 1571–1579, 2012. doi: 10.1093/rheumatology/kes093. [DOI] [PubMed] [Google Scholar]
- 26.Khazaei M, Fallahzadeh AR, Sharifi MR, Afsharmoghaddam N, Javanmard SH, Salehi E. Effects of diabetes on myocardial capillary density and serum angiogenesis biomarkers in male rats. Clinics (São Paulo) 66: 1419–1424, 2011. doi: 10.1590/S1807-59322011000800019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med 2012: 1–30, 2012. doi: 10.1155/2012/918267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Konukoglu D, Hatemi H, Bayer H, Bağriaçik N. Relationship between serum concentrations of interleukin-6 and tumor necrosis factor alpha in female Turkish subjects with normal and impaired glucose tolerance. Horm Metab Res 38: 34–37, 2006. doi: 10.1055/s-2006-924974. [DOI] [PubMed] [Google Scholar]
- 29.Landers-Ramos RQ, Sapp RM, Jenkins NT, Murphy AE, Cancre L, Chin ER, Spangenburg EE, Hagberg JM. Chronic endurance exercise affects paracrine action of CD31+ and CD34+ cells on endothelial tube formation. Am J Physiol Heart Circ Physiol 309: H407–H420, 2015. doi: 10.1152/ajpheart.00123.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landers-Ramos RQ, Sapp RM, VandeWater E, Macko J, Robinson S, Wang Y, Chin ER, Spangenburg EE, Prior SJ, Hagberg JM. Investigating the extremes of the continuum of paracrine functions in CD34−/CD31+ CACs across diverse populations. Am J Physiol Heart Circ Physiol 312: H162–H172, 2017. doi: 10.1152/ajpheart.00342.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makino N, Maeda T, Sugano M, Satoh S, Watanabe R, Abe N. High serum TNF-α level in Type 2 diabetic patients with microangiopathy is associated with eNOS down-regulation and apoptosis in endothelial cells. J Diabetes Complications 19: 347–355, 2005. doi: 10.1016/j.jdiacomp.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Med Res Rev 23: 117–145, 2003. doi: 10.1002/med.10024. [DOI] [PubMed] [Google Scholar]
- 33.Mirza S, Hossain M, Mathews C, Martinez P, Pino P, Gay JL, Rentfro A, McCormick JB, Fisher-Hoch SP. Type 2-diabetes is associated with elevated levels of TNF-α, IL-6 and adiponectin and low levels of leptin in a population of Mexican Americans: a cross-sectional study. Cytokine 57: 136–142, 2012. doi: 10.1016/j.cyto.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Müller S, Martin S, Koenig W, Hanifi-Moghaddam P, Rathmann W, Haastert B, Giani G, Illig T, Thorand B, Kolb H. Impaired glucose tolerance is associated with increased serum concentrations of interleukin 6 and co-regulated acute-phase proteins but not TNF-alpha or its receptors. Diabetologia 45: 805–812, 2002. doi: 10.1007/s00125-002-0829-2. [DOI] [PubMed] [Google Scholar]
- 35.Münzel D, Lehle K, Haubner F, Schmid C, Birnbaum DE, Preuner JG. Impact of diabetic serum on endothelial cells: an in-vitro-analysis of endothelial dysfunction in diabetes mellitus type 2. Biochem Biophys Res Commun 362: 238–244, 2007. doi: 10.1016/j.bbrc.2007.06.045. [DOI] [PubMed] [Google Scholar]
- 36.Nyström T, Nygren A, Sjöholm A. Increased levels of tumour necrosis factor-α (TNF-α) in patients with Type II diabetes mellitus after myocardial infarction are related to endothelial dysfunction. Clin Sci (Lond) 110: 673–681, 2006. doi: 10.1042/CS20050353. [DOI] [PubMed] [Google Scholar]
- 37.Prior SJ, McKenzie MJ, Joseph LJ, Ivey FM, Macko RF, Hafer-Macko CE, Ryan AS. Reduced skeletal muscle capillarization and glucose intolerance. Microcirculation 16: 203–212, 2009. doi: 10.1080/10739680802502423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qazi BS, Tang K, Qazi A. Recent advances in underlying pathologies provide insight into interleukin-8 expression-mediated inflammation and angiogenesis. Int J Inflamm 2011: 1–13, 2011. doi: 10.4061/2011/908468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romano M, Sironi M, Toniatti C, Polentarutti N, Fruscella P, Ghezzi P, Faggioni R, Luini W, van Hinsbergh V, Sozzani S, Bussolino F, Poli V, Ciliberto G, Mantovani A. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity 6: 315–325, 1997. doi: 10.1016/S1074-7613(00)80334-9. [DOI] [PubMed] [Google Scholar]
- 40.Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci 8: 1237–1247, 2012. doi: 10.7150/ijbs.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruotsalainen E, Salmenniemi U, Vauhkonen I, Pihlajamäki J, Punnonen K, Kainulainen S, Laakso M. Changes in inflammatory cytokines are related to impaired glucose tolerance in offspring of type 2 diabetic subjects. Diabetes Care 29: 2714–2720, 2006. doi: 10.2337/dc06-0147. [DOI] [PubMed] [Google Scholar]
- 42.Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, Ito A, Liu T, Kamide C, Agrawal H, Perlman H, Qin G, Kishore R, Losordo DW. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res 109: 724–728, 2011. doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 1813: 878–888, 2011. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 44.Xu X, Du C, Zheng Q, Peng L, Sun Y. Effect of metformin on serum interleukin-6 levels in polycystic ovary syndrome: a systematic review. BMC Womens Health 14: 93, 2014. doi: 10.1186/1472-6874-14-93. [DOI] [PMC free article] [PubMed] [Google Scholar]