Abstract
Noncoding RNAs, including microRNAs (miRNAs), long noncoding RNAs (lncRNAs), and circular RNAs (circRNAs) play roles in the development and homeostasis of nearly every tissue of the body, including the regulation of processes underlying heart growth. Cardiac hypertrophy can be classified as either physiological (beneficial heart growth) or pathological (detrimental heart growth), the latter of which results in impaired cardiac function and heart failure and is predictive of a higher incidence of death due to cardiovascular disease. Several miRNAs have a functional role in exercise-induced cardiac hypertrophy, while both miRNAs and lncRNAs are heavily involved in pathological heart growth and heart failure. The latter have the potential to act as an endogenous sponge RNA and interact with specific miRNAs to control cardiac hypertrophy, adding another level of complexity to our understanding of the regulation of cardiac muscle mass. In addition to tissue-specific effects, ncRNA-mediated tissue cross talk occurs via exosomes. In particular, miRNAs can be internalized in exosomes and secreted from various cardiac and vascular cell types to promote angiogenesis, as well as protection and repair of ischemic tissues. ncRNAs hold promising therapeutic potential to protect the heart against ischemic injury and aid in regeneration. Numerous preclinical studies have demonstrated the therapeutic potential of ncRNAs, specifically miRNAs, for the treatment of cardiovascular disease. Most of these studies employ antisense oligonucleotides to inhibit miRNAs of interest; however, off-target effects often limit their potential to be translated to the clinic. In this context, approaches using viral and nonviral delivery tools are promising means to provide targeted delivery in vivo.
Keywords: cardiac hypertrophy, heart, lncRNAs, microRNAs, noncoding RNAs
INTRODUCTION
Noncoding RNAs (ncRNAs) are RNA molecules that do not encode for a protein; rather, ncRNAs contribute to the maintenance of cell and tissue homeostasis through a variety of regulatory processes. The role and regulation of noncoding RNAs in mammalian cells have received considerable attention over the past decades. For example, transfer RNAs and ribosomal RNAs are essential to the production of functional proteins (61). Of particular interest are the more recently described classes of regulatory ncRNAs, comprising small interfering (siRNAs), microRNAs (miRNAs), piwi-associated RNAs (piRNAs), circular RNAs (circRNAs), small nucleolar RNAs (snoRNAs), small nuclear RNAs (snRNAs), and long noncoding RNAs (lncRNAs). ncRNA molecules can directly or indirectly regulate gene expression (34), process other RNA molecules (84), or act as regulators for other RNA species (127). Through their various functions, ncRNA molecules play an essential role in the development and homeostasis of nearly every tissue of the body.
Cardiac pathologies and cardiovascular diseases are among the most common causes of morbidity and mortality worldwide (91). The increasing burden these conditions place on health systems has prompted countless investigations into the physiological and pathological processes regulating cardiac muscle mass. Cardiac muscle mass in adults is largely altered by changes in cardiac myocyte size because it represents 70–80% of the hearts volume; however, in certain settings, myocyte proliferation, myocyte death, and fibrosis can also influence muscle mass (15, 16). Over the past several years, ncRNA molecules have been the focus of an increasing number of studies that, in certain cases, have led to the identification of novel therapeutic targets (24). This minireview aims to summarize our current understanding of ncRNA-mediated regulation of cardiac muscle mass. It will highlight the most important ncRNAs regulating cardiac hypertrophy, with a focus on the widely studied miRNAs and lncRNAs. ncRNA-mediated tissue cross talk will be briefly discussed. Finally, it will provide an insight into the current knowledge and challenges associated to the therapeutic potential of ncRNAs in the context of cardiac medicine.
CLASSES OF NONCODING RNAs
ncRNAs play a central role in regulating the pathophysiological processes underlying heart growth. Among the most vastly studied regulatory ncRNA species are miRNAs, lncRNAs, and circRNAs. circRNAs were first observed in viruses in the 1970s (107), while the existence of miRNAs and lncRNAs was suggested in the early 1990s in nonmammalian and mammalian cells (18, 72, 105). The field has been ever expanding since then.
miRNAs are 20- to 22-nucleotide (nt) single-stranded RNA molecules originating from coding and noncoding parts of the nuclear genome (7, 13). They directly and indirectly regulate gene expression in the cytoplasm (53) and, in some cases, in the mitochondria (32). miRNAs silence protein expression either by degrading specific target mRNA molecules or by directly inhibiting protein translation (7, 13) (Fig. 1). In some cases, they may also stabilize mRNA molecules (120). Each miRNA has the potential to target multiple mRNA transcripts via interactions based on Watson-Crick recognition of a 6- to 8-nt sequence localized at their 5′-end, the “seed” sequence (19). However, it is becoming clearer that noncanonical rules may govern close to 60% of all miRNAs/mRNAs interactions (28, 58). To date, over 4,000 miRNAs have been described in human tissue (miRBase database v.22; Ref. 49).
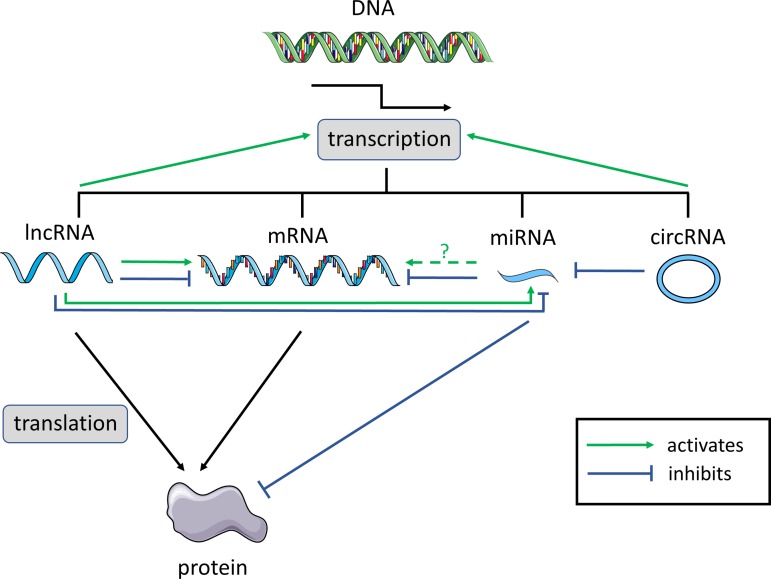
Fig. 1.
Functional interactions between the different classes of noncoding RNAs (ncRNAs). Long noncoding RNAs (lncRNAs), mRNAs, microRNAs (miRNAs), and circular RNAs (circRNAs) are all transcribed from genomic DNA. lncRNAs and mRNAs can be translated into polypeptides. The green arrows indicate “activation.” The blue lines indicate “inhibition.” Dashed lines indicate that the nature of the interaction is still unknown.
lncRNAs are a more heterogeneous class of single- or double-stranded RNA molecules that are arbitrarily defined as longer than 200 nt and shorter than 10,000 nt (127). The presence or absence of a poly-A tail determines lncRNA stability (139). More than 17,000 lncRNA molecules may be encoded by the human genome (134), including the mitochondrial genome (43, 103). lncRNAs have various functions and can act as signal, sensor, stabilization, and decoy molecules for other ncRNAs to regulate gene expression in the cell (127). This class of ncRNAs also directly regulates protein expression (Fig. 1) and activities by providing a scaffold for regulatory proteins, driving allosteric modifications and facilitating histone methylation (34).
Finally, circRNAs constitute an abundant and conserved class of RNA molecules that was originally considered noncoding (102). Recent studies however showed that some circRNAs can be translated into protein in vitro and in vivo (99, 135). circRNAs are covalently closed single-stranded RNA molecules where the 3′- and 5′-ends have been joined together. Bioinformatics coupled to next-generation RNA sequencing tools predict the existence of thousands of circRNAs in the human genome (102). circRNA can regulate nuclear gene expression (89), regulate alternative splicing (4), or act as miRNA sponges by competing against endogenous miRNAs for binding (55) (Fig. 1). However, their biological functions, as well as their localization and degradation, remain mostly unclear.
miRNAs, lncRNAs, and more recently circRNAs are involved in the regulation of nearly every aspect of cellular function (for reviews, see Refs. 53, 89, 127, 140). Aberrant expression of these ncRNA molecules has been consistently linked to disease initiation and progression, including cardiac and cardiovascular conditions (for reviews, see Refs. 13, 56, 88, 97, 140). As such, the role and regulation of these regulatory RNA species constitute one of the most dynamic research topics in the field of molecular medicine.
MOST PROMINENT ncRNAs IN REGULATING CARDIAC MUSCLE MASS
Numerous studies have demonstrated a role of ncRNAs in pathological and physiological cardiac hypertrophy. Of the ncRNAs, the role of miRNAs in regulating cardiac hypertrophy has been extensively studied, whereas much less is known about the role and mechanisms of lncRNAs and circRNAs. Below we summarize the most prominent ncRNAs in regulating cardiac hypertrophy, which are also summarized in Fig. 2.
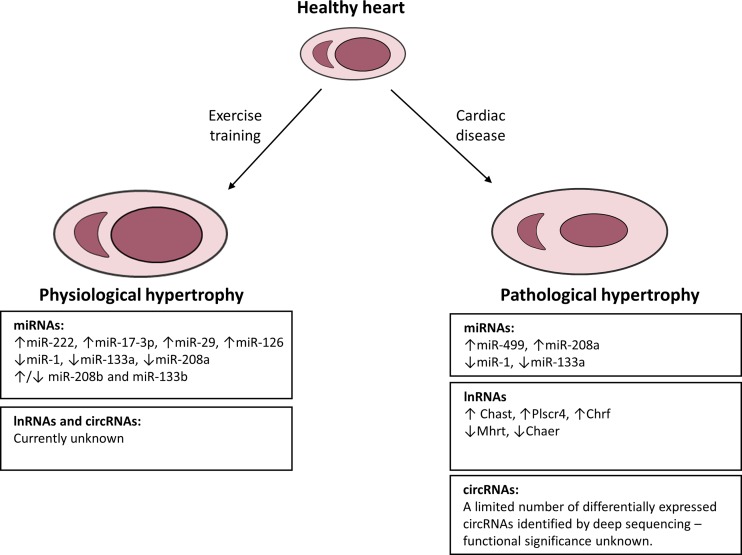
Fig. 2.
Schematic of the most prominent and extensively studied noncoding RNAs in physiological and pathological hypertrophy. Increased workload on the heart leads to heart enlargement that is either physiological (due to exercise) or pathological (due to cardiac disease). A number of ncRNAs [microRNAs and long noncoding RNAs (lncRNAs)] have been identified to play important roles in physiological and pathological hypertrophy, of which the most extensively studied are presented. Little is known about the role of lncRNAs and circular RNAs (circRNAs) in physiological hypertrophy, and the role of circRNAs in pathological hypertrophy requires further investigation.
Physiological Cardiac Hypertrophy–When Big Is Beautiful
Increased heart mass (cardiac hypertrophy) occurs following endurance training and is commonly referred to as physiological cardiac hypertrophy (also called the athlete’s heart in humans), and under these circumstances, resting (nonexercise) cardiac function is either normal or enhanced (100). This is in stark contrast to the bigger heart observed under pathological conditions (termed pathological cardiac hypertrophy) that is most commonly associated with impaired cardiac function and heart failure and predicts a higher incidence of death due to cardiovascular disease (74). There are striking molecular and structural differences between physiological cardiac hypertrophy following endurance exercise training and pathological hypertrophy induced by chronic pressure (i.e., hypertension) or volume overload (16). Unlike physiological hypertrophy, pathological hypertrophy is commonly associated with increased fibrosis, reduced cardiac function, heart failure, and increased mortality (16). The insulin-like growth factor-1 phosphoinositide 3-kinase (PI3K)-Akt signaling pathway is necessary for physiological cardiac hypertrophy, whereas pathological cardiac hypertrophy is regulated in a large part by Gαq signaling (reviewed in Ref. 16).
miRNAs Regulated by Exercise and Settings of Physiological Cardiac Growth
Endurance exercise in rats and mice alters the expression of many miRNA species in the heart that are implicated in cardiac development and growth. Indeed, over 200 miRNA species are differentially expressed in the hearts of rodents following several weeks of endurance training, with ~60% of these species having their expression downregulated (42, 79, 104, 109). Cardiac (and skeletal) muscle is also enriched in several miRNA species, termed myomiRs, and comprise miR-1, miR-133a, miR133b, miR-206, miR-208a, miR-208b, miR-499, and miR-486, with most being downregulated with physiological cardiac hypertrophy. miR-1, miR-133a, and miR-208a levels are downregulated following exercise-induced cardiac hypertrophy (23, 104, 110, 111), and previous studies have shown miR-1 and miR-133a induction acts to repress cardiac hypertrophy and growth (23, 38). There are conflicting reports of miR-208b and miR-133b being upregulated (104) or downregulated (110, 111) following exercise-induced cardiac hypertrophy. The reasons for these conflicting reports for miR-208b are unclear since both studies used similar swim-training protocols of several weeks duration in the same strain of rat (104, 110).
miRNAs also play a role in regulating molecular pathways related to exercise-induced heart adaptations (for reviews, see Refs. 15, 45, 95, 128). Members of the miR-29 family were increased in hypertrophic hearts of swim-trained rats, which was associated with downregulation of collagen gene expression, which may be associated with decreased cardiac fibrosis and improved heart function (111). Enhanced angiogenesis is associated with exercise-induced heart growth and is regulated by the vascular endothelial growth factor pathway. The expression of miR-126 was elevated in swim-trained rodent hearts, which facilitated angiogenic signaling by inhibiting its target genes, Spred1 and PI3KR2, negative regulators of the vascular endothelial growth factor pathway (31). Microarray profiling identified 62 miRNAs that were regulated by PI3K(p110α), a gene essential for exercise-induced heart growth (77). Collectively, these miRNAs target a wide range of genes, some of which are involved in the regulation of fibrosis, apoptosis, autophagy, angiogenesis, and cardiac contraction. Therapeutic inhibition of PI3K-regulated miRNAs using antisense oligonucleotides in cardiac disease mouse models provides therapeutic benefit (9, 11, 12).
Beyond expression profiling, the functional significance of some miRNAs has been established in regulating physiological cardiac hypertrophy. In particular, miR-222 and miR-17-3p were both upregulated in the heart following endurance training and were required for exercise-induced cardiac growth (79, 109, 124). Inhibition of miR-222 prevented the increase in heart mass, cardiomyocyte hypertrophy, and proliferation in mice following endurance training (79, 124). Inhibition of miR-17-3p prevented exercise-induced cardiac hypertrophy and cardiomyocyte hypertrophy and attenuated cardiomyocyte proliferation in mice following endurance training via tissue inhibitor of metalloproteinase 3 and phosphatase and tensin homolog/Akt signaling pathway. However, overexpressing miR-222 or miR-17-3p to levels normally seen following exercise training in the rodent heart did not stimulate the phenotype observed by exercise-induced cardiac hypertrophy (79, 109). Nevertheless, miR-222 and miR-17-3p hold promising therapeutic potential to protect the heart against ischemic injury and aid in regeneration. Indeed, transgenic mice that overexpress miR-222 or agomiR overexpression of miR-17-3p in mice both protected against ischemic injury in vivo (79, 109).
In summary, miRNA species are well established to play a regulatory role in cardiomyocyte proliferation, hypertrophy, and developmental and adult cardiac enlargement. Much less is known about the role of lncRNAs and circRNAs in the regulation of exercise-induced physiological adaptations. miRNAs also hold promising therapeutic potential for the treatment of cardiac conditions. A large number of miRNA species are known to be differentially expressed in the heart following endurance training, and some, such as miR-222 and miR-17-3p, are necessary for the induction of cardiac hypertrophy following endurance training. Understanding the molecular mechanisms as to how a short-term physiological stress can induce such long-term benefits for the heart will provide new targets for the treatment of ischemic injury to the heart and its regeneration.
ncRNAs in Cardiac Hypertrophy and Heart Failure
miRNAs.
A number of miRNAs have pro- or antihypertrophic roles in the heart and have been recently reviewed in detail (76, 97). miR-208a is a heart-enriched miRNA involved in cardiomyocyte hypertrophy, fibrosis, and regulating the shift in myosin heavy chain isoform content during cardiac development and in the adult heart in response to a cardiac stress (21, 119). Furthermore, miR-208a controls the expression of hypertrophy-related signaling components, thyroid hormone activity, and the cardiac conduction system during adaptation to pathological signaling (21, 119). The expression of miR-499 is upregulated in human failing and hypertrophied hearts and in mouse models of pathological hypertrophy. Overexpression of miR-499 in the murine heart accelerated heart failure progression and exacerbated the response to pressure overload through direct and indirect effects on cardiac protein kinases and alterations in protein phosphorylation of proteins in the heart (87). miR-1 is not only downregulated in physiological hypertrophy but also in heart failure. miR-1 regulates cardiomyocyte hypertrophy by negatively regulating genes in the calcium/calmodulin signaling pathway, which controls cardiomyocyte function and growth (65). In a number of cardiac hypertrophy rodent models (pressure overload, transgenic mice with selective cardiac overexpression of a constitutively active mutant of the Akt kinase, and an exercise model), the expression of miR-133a was downregulated (23). miR-133a regulates the hypertrophic gene program by targeting multiple genes including RhoA (an exchange protein regulating cardiac hypertrophy), Cdc42 (a signal transduction kinase implicated in hypertrophy), and Nelf-A/WHSC2 (nuclear factor involved in cardiogenesis, although its role in hypertrophy remains unclear) (23).
lncRNAs.
Two lncRNAs that play a significant role in mouse heart development are Braveheart (Bvht) (68) and Foxof1 adjacent noncoding developmental regulatory RNA (Fendrr) (50). Bvht has an important role in the establishment of the cardiovascular lineage by interacting with SUZ12, suggesting a role in the epigenetic regulation of gene expression programs, and also by activating a number of transcription factors necessary for promoting the cardiac gene expression program in cardiomyocytes (68). Loss of Fendrr during the embryonic stage in mice results in severe cardiac defects and dysfunction (50). Matkovich and colleagues (85) used genome-wide sequencing and bioinformatics to characterize cardiac-enriched lncRNA expression in mouse embryo and adult hearts, where 157 lncRNAs were differentially expressed in embryonic hearts compared with adult hearts. Network analysis revealed a role of these lncRNAs in major cardiac development and metabolic pathways (85).
A number of reports describe a role of lncRNAs in pathological cardiac hypertrophy (78, 123, 136). The cardiac specific lncRNA myosin heavy-chain-associated RNA transcripts (Mhrt) consists of a cluster of RNAs (Mhrt RNAs), which are abundant in adult mouse hearts (54). Mhrt expression was induced during cardiomyocyte maturation and decreased in pressure overload-induced cardiac hypertrophy and heart failure in mice, a profile that correlates with the myosin heavy chain isoform shift during postnatal development and progression to heart failure (54). Reexpression of Mhrt in the heart protected mice against cardiac hypertrophy and heart failure by binding and antagonizing Brahma-related gene 1, suggesting a protective role of Mhrt in cardiovascular disease (54). Transcriptomic analysis of pressure-overload-induced failing hearts in mice identified a lncRNA highly enriched in the heart, which the authors called cardiac-hypertrophy-associated epigenetic regulator (Chaer) (136). Chaer is specifically expressed in cardiomyocytes, and Chaer knockout mice displayed a significantly attenuated cardiac hypertrophic response to pressure overload (136). This was associated with less fibrosis and preserved cardiac function, suggesting a role of Chaer in controlling cardiac remodeling. Chaer controlled cardiac hypertrophy by its direct interaction with polycomb repressor complex 2, preventing histone methylation at the promoter regions of genes implicated in cardiac hypertrophy (136).
Further adding to the complexity surrounding the molecular mechanisms of cardiac hypertrophy, recent studies demonstrate that lncRNAs may act as an endogenous sponge RNA to interact with miRNAs (Fig. 1) to control cardiac hypertrophy (81, 126). Cardiac hypertrophy-related factor (CHRF) was increased in the hearts of mice following pressure overload and in patients with heart failure (126). Chrf acts as a sponge of miR-489, reducing the targeting activity of miRNA-489 on its target gene, myeloid differentiation factor 88 (Myd88), an important component in the Toll-like receptor-4-mediated nuclear factor-κB activation pathway, contributing to the development of cardiac hypertrophy (126). The lncRNA Plscr4 was upregulated in hypertrophic mouse hearts, and overexpression of Plscr4 blunted the hypertrophic response in mice following pressure overload (81). Plscr4 exerts its antihypertrophic effects by sequestering the prohypertrophic miRNA miR-214, which in turn allowed expression of Mitofusin 2 and alleviated hypertrophic growth (81).
A recent study demonstrated that lncRNAs could represent targets for therapeutic intervention in heart failure (123). Cardiac hypertrophy-associated transcript (Chast) was elevated in both mouse and human hypertrophied hearts, and overexpression of Chast induced cardiomyocyte hypertrophy in vitro and in vivo (123). Antisense technology to inhibit Chast (using “GapmeRs”) attenuated cardiac remodeling after pressure overload-induced hypertrophy in mice (123) by targeting pleckstrin homology domain-containing protein family M member 1, which inhibits autophagy and influences hypertrophy (123). The use of gapmeRs (without any signs of toxicology side effects; Ref. 123) highlights the therapeutic potential of lncRNAs.
circRNAs.
Unlike miRNAs and lncRNAs, the role of circRNAs in regulating cardiac muscle mass has been less extensively studied, with most research focusing on identifying those circRNAs associated with heart disease (for review, see Ref. 129). Although deep sequencing studies have identified a number of cardiac-expressed circRNAs in diseased human and rodent hearts (114, 137), only a limited number were differentially expressed. circRNAs were found to be significantly differentially expressed in the rat heart during the developmental transition from embryo to adult (137), but the functional significance of circRNAs in regulating muscle mass remains unclear.
The ncRNA Landscape in Human Cardiac Pathology
Advances in RNA sequencing and genome-wide association studies (GWAS) have allowed the discovery of ncRNAs in human heart disease and have highlighted their potential as cardiac biomarkers. Here we present an overview of ncRNAs implicated in human cardiac pathology.
miRNAs.
Many miRNAs are implicated in human heart disease, often discovered by gene expression microarray profiling. Thum and colleagues (115) profiled the cardiac miRNA and mRNA gene signature in left ventricular tissue from patients with end-stage heart failure and compared it with that of tissue from healthy adult and fetal human hearts. They found that the miRNA expression patterns in fetal and failing human cardiac tissue was similar, suggesting that these miRNAs contribute to the reactivation of the fetal gene program in human heart failure (115). Using a deep sequencing approach, Lepitidis and colleagues (73) identified >250 differential and etiology-specific miRNAs in patients with dilated cardiomyopathy and in hearts from patients with familial hypertrophic cardiomyopathy. Other studies have explored the potential of miRNAs as clinical biomarkers for heart disease (47, 98, 117, 122). Several groups have characterized the levels of miRNAs in the circulation of patients with cardiovascular disease (see Refs. 47, 98, 117, 122; also see reviews in Refs. 29, 101, 148). In some instances, studies suggest that measurement of a panel of circulating miRNAs may be an alternative approach to conventional biomarkers for early detection in acute myocardial infarction (125, 131). While circulating miRNAs may be useful for diagnostics and monitoring purposes, large-scale studies are still required before circulating miRNAs can be successfully used as biomarkers for cardiovascular pathologies.
lncRNAs.
The expression of lncRNAs is altered in a number of human diseases, including cardiovascular disease and is associated with disease development and progression (for review, see Ref. 75). With the use of single nucleotide polymorphism arrays and GWAS, the lncRNA myocardial infarction associated transcript was identified at a susceptible chromosomal location in over 3000 patients with myocardial infarction (compared with control patients) (66). GWAS have identified novel disease-associated DNA regions, including a “hotspot” on human chromosome 9p21, the strongest genetic susceptibility locus for cardiovascular disease (20). The lncRNA gene antisense noncoding RNA in the INK4 locus (ANRIL) resides in this hotspot and is associated with atherosclerosis in patients with suspected coronary artery disease, although its role is yet to be understood (59). The lncRNA long intergenic noncoding RNA (LIPCAR) was identified as a potential biomarker for heart failure from transcriptomic analyses of plasma from patients with cardiac pathology postmyocardial infarction (69). Increased levels of LIPCAR in the plasma of patients with chronic heart failure was also associated with increased risk of future cardiovascular death (69). However, there are currently too few studies to know whether lncRNAs will be suitable biomarkers for cardiovascular disease.
circRNAs.
circRNAs have been less extensively studied in human hearts, with the majority of studies focusing on their potential as biomarkers (for reviews, see Refs. 129, 147). Due to their closed loop structure, they may represent more stable biomarkers than their linear ncRNA counterparts. Patients with high circular ANRIL expression developed less coronary artery disease, suggesting that ANRIL has an atheroprotective role (which is opposite to that of linear ANRIL as described above) (60). To predict patient outcomes following myocardial infarction, Vausort and colleagues (121) identified that patients who had low levels of the circRNA myocardial infarction-associated circular RNA (MICRA) were at higher risk to develop left ventricular cardiac dysfunction. Because of their biological properties, circRNAs have emerged as novel biomarkers; however, more research is needed to elucidate their biological function. It is hoped that identification of circRNAs as biomarkers will aid in the diagnosis of cardiovascular disease and personalized medicine in the future.
ncRNAs IN EXOSOMES
Exosomes are small (30–140 nm), membranous vesicles that are ubiquitously expressed by cells in vivo (51) and play an important role in tissue cross talk (Fig. 3). Their ability to transport peptides, lipids, and genetic material results in functional changes in the recipient tissue or organ (51). Regulatory proteins, mRNAs and miRNAs transported within the exosomal lumen elicit some of these changes (51, 90, 138). miRNAs comprise up to 76.2% of exosomal RNA species (63), along with lncRNAs (3.36%), piRNA (1.31%), snoRNA (0.18%), and snRNA (0.01%) (63). The remaining exosomal RNA includes coding, ribosomal (9.16%), and transfer (1.24%) RNA (63). The role of exosomal lncRNA, piRNA, snoRNA, and snRNA in cardiac hypertrophy has not been investigated thus far. However, the role of exosomal miRNA, which represents a more highly concentrated bioactive pool of circulating miRNAs than riboprotein-bound miRNAs (2, 27), has been extensively researched in heart failure (see review in Ref. 29).
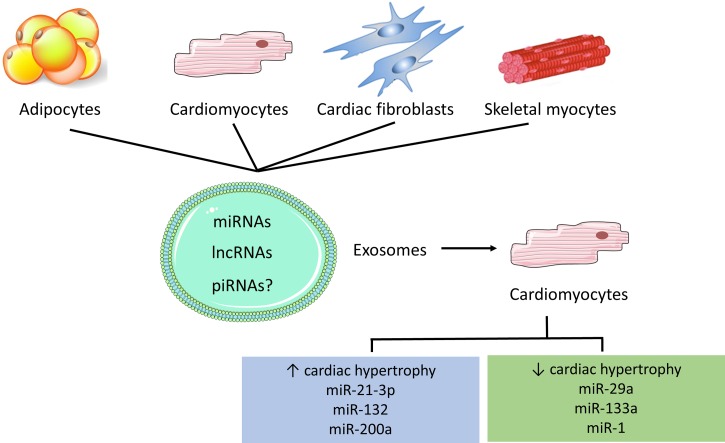
Fig. 3.
Exosomal secretion mediates cross-talk between different tissues. Exosomes contain microRNAs (miRNAs), long noncoding RNAs (lncRNAs), and potentially piwi-associated RNAs (piRNAs). When released in cardiomyocytes, these noncoding RNAs have the potential to promote or protect against cardiac hypertrophy.
Emerging evidence suggests that cardiac and vascular cells, including cardiac fibroblasts (5, 17, 82), cardiomyocytes (17, 132), and H9c2 cells (17, 44), secrete and internalize exosomes (5, 39). These exosomes can influence cardiac function, inducing proliferation, angiogenesis, autophagy, and importantly, cardiac hypertrophy. Exosome release may be constitutive or induced by various stimuli including calcium concentration, mitogens, cytokines, or stress (39). In humans and murine models, both healthy and ischemic cardiomyocytes release exosomes in vivo (106). Exosomes secreted from various cardiac and vascular cell types promote angiogenesis and tissue protection and repair postischemia in animal models (3, 22, 26). Of particular interest, cardiac-derived exosomes can play a positive role in cases of ischemic tissue repair and regeneration (64, 70).
Exosomal content plays an important role in cardiomyocyte health. The exosomal cargo is not merely a reflection of the relative abundance of compounds on the parental cell. Rather, exosomal cargo is selectively secreted or conserved in various pathophysiological conditions (144). By regulating cell proliferation, apoptosis, cytokine production, immune modulation, and metastasis (62), exosomal miRNAs play an integral role in cross talk between cells and tissues in many cardiac pathologies. For example, hypoxia-stimulated C2C12 cells (mouse skeletal myoblasts) secreted miR-29a-enriched exosomes, which were then endocytosed into cardiomyocytes (142).
miR-21-3p provides an example of exosome-derived miRNA species involved in cardiac hypertrophy. miR-21-3p was differentially expressed in aging murine hearts (146) and highly expressed in failing human hearts when compared with nonfailing human hearts (143). Exosomes enriched in miR-21-3p were released from neonatal rat cardiac fibroblasts and internalized by neonatal cardiomyocytes, leading to an increase in cardiomyocyte size in vitro (5), via the regulation of Sorbs2 and Pdlim5. Of interest, pharmacological inhibition of miR-21-3p attenuated pressure overload induced cardiac hypertrophy in a murine model (5), suggesting that manipulation of exosomal cargo may represent a possible treatment pathway.
The miR-212/-132 family has been implicated in pathological cardiac hypertrophy (40) and cardiomyocyte autophagy (118) and is regulated by angiotensin-II (ANG II) (40), a peptide hormone playing an important role in cardiovascular disease (82). ANG II-stimulated exosome release from cardiac fibroblasts (82) increased activation of the ANG II-AT1R axis and pathological cardiac hypertrophy (82), possibly via exosomal proteins (141) and miR-132 (82).
Peroxisome proliferator-activated receptor-γ (PPAR-γ) and miR-200a are suggested to control cross talk between adipose tissue and cardiac tissue. PPAR-γ is a master regulator of adipose tissue signaling (1), and its activation induces cardiac hypertrophy in murine cardiomyocytes (112). Cardiac-specific activation of PPAR-γ in rat and in mouse models induced cardiac hypertrophy (35, 80). There is evidence to suggest that systemic activation of PPAR-γ may also induce cardiac hypertrophy (35, 57), suggesting a role for exosome-mediated cross talk. Activation of PPAR-γ in murine adipocytes stimulated the release of exosomes enriched in miR-200a (41). These exosomes targeted cardiomyocytes and induced cardiac hypertrophy via activation of the mammalian target of rapamycin signaling pathway (41). Treatment with an antagomir for miR-200a blunted cardiomyocyte hypertrophy in vitro (41). This phenomenon was maintained in a mouse model of hypertrophy induced by rosiglitazone treatment (a PPAR-γ agonist), where specific ablation of PPAR-γ in adipocytes blunted cardiac hypertrophy (41). These data provide a possible pathway by which adipocyte-derived exosomes mediate cardiac health and suggest a possible role for exosome-mediated cross talk.
Exosomes released from skeletal muscle also contain many miRNA species that are implicated in cardiac hypertrophy, including miR-1, -133a/b, -222, and -208a (30, 52). In humans, the expression of exosomal miR-1, -222, and -208a was significantly upregulated (30), and miR-133b tended to be upregulated (52) after an acute bout of exercise in vivo. It is well established that cardiomyocytes internalize exosomes and that the species present in exercise-induced exosomes derived from skeletal muscle regulate cardiac hypertrophy. Thus, while there is no direct evidence that these exosomes were internalized by cardiac cells, it is possible these miRNAs were involved in the regulation of cardiac hypertrophy, via exosome-mediated cross talk. However, this is yet to be elucidated and provides an intriguing pathway for future research.
Exosomes are attractive therapeutic targets for many chronic diseases, including cardiac hypertrophy (39). Because of their biophysical properties, the isolation and manipulation of exosomal contents are relatively straightforward (37). In addition, exosomes have a natural ability to cross biological barriers, such as the blood-brain barrier (71). These vesicles therefore constitute possible “trojan horses” to deliver drugs and other therapeutic substances (39), which would otherwise be free in the circulation and susceptible to damage and degradation. Several exosomal miRNA species may play a role in either the attenuation or the pathogenesis of cardiac hypertrophy. By manipulating the expression of these species in exosomes, practitioners may have the potential to treat such pathologies in a targeted and effective manner.
ncRNAs AS POTENTIAL THERAPEUTIC TARGETS FOR CARDIOVASCULAR DISEASE
Of the ncRNAs, the ability of miRNAs to target several genes within a similar signaling network or pathway may give them an advantage as potential therapeutic targets over other ncRNAs. However, this can also be considered a drawback as it may cause undesirable responses. As miRNAs control pathophysiological processes of the heart, including cardiomyocyte cell death, autophagy, contractility, fibrosis, and hypertrophy, investigators have performed numerous preclinical studies demonstrating the therapeutic potential of miRNAs for the treatment of cardiovascular disease, which have been extensively reviewed elsewhere (13, 56).
Despite promising preclinical studies demonstrating therapeutic efficacy of miRNA inhibitors, there are currently no clinical trials for cardiovascular disease. However, there are positive advancements of RNA-based therapeutics (small interfering RNAs and miRNAs) in other areas of disease (24).
Translational Hurdles
miRNAs.
The majority of preclinical and clinical studies employ antisense oligonucleotides that can inhibit the miRNA of interest. These inhibitors are nontissue specific and are taken up by several organs (namely the liver and kidney) upon systemic administration. This may be problematic given the ubiquitous expression of some miRNAs and the different functions of miRNAs in different tissues and/or oncogenic effects. In these instances, a targeted-tissue specific approach may be preferred. Furthermore, with advances in next generation sequencing and systems biology, it has become apparent that cardiac miRNAs that regulate transcription can indirectly regulate other cardiac miRNAs (86, 96). This can lead to unexpected effects of antisense oligonucleotide therapies due to the regulation of the mRNA targets of the secondary miRNAs. Thus a better understanding of precisely how miRNAs molecules interact with one another and regulate complex signaling networks is important for the successful design of miRNA-based therapies.
Other important aspects to consider when considering miRNA-based therapies for the clinic is 1) the potential that miRNA inhibitors may affect RNA species beyond the intended target (96, 113), 2) whether targeting an individual miRNA or miRNA family will confer more therapeutic benefit (9), 3) the use of inhibitors that are specific to the seed region that can block the expression of entire miRNA families (94), and 4) that disease severity and sex can influence therapeutic outcome (8, 14, 36).
lncRNAs.
There are a number of limitations that need to be resolved before lncRNAs can be taken to the clinic (see comprehensive reviews in Refs. 46, 48, 88). The lack of conservation/homology of lncRNAs between different species makes both identification and clinical testing of human lncRNAs challenging and translation from bench to clinic more difficult. The cellular location of lncRNAs needs to be considered before they can be developed as therapeutic targets. lncRNAs reside in multiple cellular extracellular locations, and some pharmacological agents may not penetrate the intracellular compartment of interest (6). Finally, the relationship between lncRNA structure and function is not well understood and needs to be further elucidated.
Other ncRNAs.
The therapeutic potential of targeting other ncRNAs in the heart including piRNAs, circRNAs, and snoRNAs is largely unknown. However, in the cancer field this is being actively explored (83, 92, 145). It is therefore likely that these ncRNAs will also be targeted in the heart in the coming years.
Emerging technologies to deliver ncRNAs to the heart.
The most common approach for targeted delivery is to use adeno-associated viral vectors, and human clinical trials targeting mRNAs have been undertaken without any adverse side effects (149). There are limited reports of using adeno-associated viral vectors for miRNA inhibition in the heart (67), and developing a cardiac-specific approach may prove to be more challenging (10). Other nonviral methods have recently been developed that have the potential to deliver miRNA therapeutics to the heart, including ultrasound microbubbles (133), light-induced miRNA inhibitor activation (a technique which facilitates local delivery) (108), unlockable core-shell nanocomplexes (93), hydrogels (cross-linked polymers capable of carrying and releasing therapeutics after injection in tissues) (130), neutral lipid emulsions (116), and negatively charged calcium phosphate nanoparticles (33). Finally, CRISPR/cas9 technology could be used to edit ncRNAs and has been shown to be an efficient and stable technology for inhibiting miRNAs in vitro and in vivo (25). The emergence of these new technologies may make a cardiac-specific ncRNA drug a reality.
In conclusion, it is clear from the studies conducted in the last two decades that ncRNAs have an important role in physiological and pathological cardiac hypertrophy. miRNAs are able to regulate cardiac hypertrophy by targeting genes in multiple hypertrophic-related signaling pathways, whereas the mechanisms of lncRNAs are more complex: not only can they directly interact with genes, they can act on hypertrophy-related genes by acting as RNA sponges of miRNAs. The role of circRNAs in cardiac hypertrophy remain poorly understood. Preclinical studies suggest that miRNAs and lncRNAs can be promising therapeutic targets for the treatment of cardiovascular disease, and technologies to deliver ncRNAs to the heart are being developed. Future studies are warranted to further understand the molecular mechanisms of ncRNA and their regulatory networks in cardiac hypertrophy and disease.
GRANTS
J. R. McMullen is supported by a National Health and Medical Research Council Senior Research Fellowship Grant 1078985, and B. C. Bernardo is supported by an Alice Baker and Eleanor Shaw Fellowship (The Baker Foundation, Melbourne, Australia).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.C.B. conceived and designed research; G.D.W., S.L., S.E.A., and B.C.B. drafted manuscript; G.D.W., S.L., S.E.A., J.R.M., and B.C.B. edited and revised manuscript; G.D.W., S.L., S.E.A., J.R.M., and B.C.B. approved final version of manuscript; S.L., S.E.A., and B.C.B. prepared figures.
REFERENCES
- 1.Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM. PPARγ signaling and metabolism: the good, the bad and the future. Nat Med 19: 557–566, 2013. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA 108: 5003–5008, 2011. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res (Amst) 10: 301–312, 2013. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N, Kadener S. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell 56: 55–66, 2014. doi: 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 5.Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A, Ponimaskin E, Schmiedl A, Yin X, Mayr M, Halder R, Fischer A, Engelhardt S, Wei Y, Schober A, Fiedler J, Thum T. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest 124: 2136–2146, 2014. doi: 10.1172/JCI70577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell 152: 1298–1307, 2013. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernardo BC, Charchar FJ, Lin RC, McMullen JR. A microRNA guide for clinicians and basic scientists: background and experimental techniques. Heart Lung Circ 21: 131–142, 2012. doi: 10.1016/j.hlc.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Bernardo BC, Gao XM, Tham YK, Kiriazis H, Winbanks CE, Ooi JY, Boey EJ, Obad S, Kauppinen S, Gregorevic P, Du XJ, Lin RC, McMullen JR. Silencing of miR-34a attenuates cardiac dysfunction in a setting of moderate, but not severe, hypertrophic cardiomyopathy. PLoS One 9: e90337, 2014. doi: 10.1371/journal.pone.0090337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernardo BC, Gao XM, Winbanks CE, Boey EJ, Tham YK, Kiriazis H, Gregorevic P, Obad S, Kauppinen S, Du XJ, Lin RC, McMullen JR. Therapeutic inhibition of the miR-34 family attenuates pathological cardiac remodeling and improves heart function. Proc Natl Acad Sci USA 109: 17615–17620, 2012. doi: 10.1073/pnas.1206432109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernardo BC, Gregorevic P, Ritchie RH, McMullen JR. Generation of microRNA-34 sponges and tough decoys for the heart: Developments and challenges. Front Pharmacol 9: 1090, 2018. doi: 10.3389/fphar.2018.01090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernardo BC, Nguyen SS, Gao XM, Tham YK, Ooi JY, Patterson NL, Kiriazis H, Su Y, Thomas CJ, Lin RC, Du XJ, McMullen JR. Inhibition of miR-154 protects against cardiac dysfunction and fibrosis in a mouse model of pressure overload. Sci Rep 6: 22442, 2016. doi: 10.1038/srep22442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernardo BC, Nguyen SS, Winbanks CE, Gao XM, Boey EJ, Tham YK, Kiriazis H, Ooi JY, Porrello ER, Igoor S, Thomas CJ, Gregorevic P, Lin RC, Du XJ, McMullen JR. Therapeutic silencing of miR-652 restores heart function and attenuates adverse remodeling in a setting of established pathological hypertrophy. FASEB J 28: 5097–5110, 2014. doi: 10.1096/fj.14-253856. [DOI] [PubMed] [Google Scholar]
- 13.Bernardo BC, Ooi JY, Lin RC, McMullen JR. miRNA therapeutics: a new class of drugs with potential therapeutic applications in the heart. Future Med Chem 7: 1771–1792, 2015. doi: 10.4155/fmc.15.107. [DOI] [PubMed] [Google Scholar]
- 14.Bernardo BC, Ooi JY, Matsumoto A, Tham YK, Singla S, Kiriazis H, Patterson NL, Sadoshima J, Obad S, Lin RC, McMullen JR. Sex differences in response to miRNA-34a therapy in mouse models of cardiac disease: identification of sex-, disease- and treatment-regulated miRNAs. J Physiol 594: 5959–5974, 2016. doi: 10.1113/JP272512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernardo BC, Ooi JY, Weeks KL, Patterson NL, McMullen JR. Understanding key mechanisms of exercise-induced cardiac protection to mitigate disease: current knowledge and emerging concepts. Physiol Rev 98: 419–475, 2018. doi: 10.1152/physrev.00043.2016. [DOI] [PubMed] [Google Scholar]
- 16.Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther 128: 191–227, 2010. doi: 10.1016/j.pharmthera.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Borosch S, Dahmen E, Beckers C, Stoppe C, Buhl EM, Denecke B, Goetzenich A, Kraemer S. Characterization of extracellular vesicles derived from cardiac cells in an in vitro model of preconditioning. J Extracell Vesicles 6: 1390391, 2017. doi: 10.1080/20013078.2017.1390391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brannan CI, Dees EC, Ingram RS, Tilghman SM. The product of the H19 gene may function as an RNA. Mol Cell Biol 10: 28–36, 1990. doi: 10.1128/MCB.10.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brennecke J, Stark A, Russell RB, Cohen SM. Principles of microRNA-target recognition. PLoS Biol 3: e85, 2005. doi: 10.1371/journal.pbio.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broadbent HM, Peden JF, Lorkowski S, Goel A, Ongen H, Green F, Clarke R, Collins R, Franzosi MG, Tognoni G, Seedorf U, Rust S, Eriksson P, Hamsten A, Farrall M, Watkins H; PROCARDIS consortium . Susceptibility to coronary artery disease and diabetes is encoded by distinct, tightly linked SNPs in the ANRIL locus on chromosome 9p. Hum Mol Genet 17: 806–814, 2008. doi: 10.1093/hmg/ddm352. [DOI] [PubMed] [Google Scholar]
- 21.Callis TE, Pandya K, Seok HY, Tang RH, Tatsuguchi M, Huang ZP, Chen JF, Deng Z, Gunn B, Shumate J, Willis MS, Selzman CH, Wang DZ. MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. J Clin Invest 119: 2772–2786, 2009. doi: 10.1172/JCI36154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campagnolo P, Cesselli D, Al Haj Zen A, Beltrami AP, Kränkel N, Katare R, Angelini G, Emanueli C, Madeddu P. Human adult vena saphena contains perivascular progenitor cells endowed with clonogenic and proangiogenic potential. Circulation 121: 1735–1745, 2010. doi: 10.1161/CIRCULATIONAHA.109.899252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carè A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang M-L, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, Høydal M, Autore C, Russo MA, Dorn GW 2nd, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G. MicroRNA-133 controls cardiac hypertrophy. Nat Med 13: 613–618, 2007. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 24.Chakraborty C, Sharma AR, Sharma G, Doss CGP, Lee SS. Therapeutic miRNA and siRNA: moving from bench to clinic as next generation medicine. Mol Ther Nucleic Acids 8: 132–143, 2017. doi: 10.1016/j.omtn.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang H, Yi B, Ma R, Zhang X, Zhao H, Xi Y. CRISPR/cas9, a novel genomic tool to knock down microRNA in vitro and in vivo. Sci Rep 6: 22312, 2016. doi: 10.1038/srep22312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L, Wang Y, Pan Y, Zhang L, Shen C, Qin G, Ashraf M, Weintraub N, Ma G, Tang Y. Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem Biophys Res Commun 431: 566–571, 2013. doi: 10.1016/j.bbrc.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chevillet JR, Kang Q, Ruf IK, Briggs HA, Vojtech LN, Hughes SM, Cheng HH, Arroyo JD, Meredith EK, Gallichotte EN, Pogosova-Agadjanyan EL, Morrissey C, Stirewalt DL, Hladik F, Yu EY, Higano CS, Tewari M. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc Natl Acad Sci USA 111: 14888–14893, 2014. doi: 10.1073/pnas.1408301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chi SW, Hannon GJ, Darnell RB. An alternative mode of microRNA target recognition. Nat Struct Mol Biol 19: 321–327, 2012. doi: 10.1038/nsmb.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Creemers EE, Tijsen AJ, Pinto YM. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res 110: 483–495, 2012. doi: 10.1161/CIRCRESAHA.111.247452. [DOI] [PubMed] [Google Scholar]
- 30.D’Souza RF, Woodhead JS, Zeng N, Blenkiron C, Merry TL, Cameron-Smith D, Mitchell CJ. Circulatory exosomal miRNA following intense exercise is unrelated to muscle and plasma miRNA abundances. Am J Physiol Endocrinol Metab 315: E723–E733, 2018. doi: 10.1152/ajpendo.00138.2018. [DOI] [PubMed] [Google Scholar]
- 31.DA Silva ND Jr, Fernandes T, Soci UP, Monteiro AW, Phillips MI, DE Oliveira EM. Swimming training in rats increases cardiac MicroRNA-126 expression and angiogenesis. Med Sci Sports Exerc 44: 1453–1462, 2012. doi: 10.1249/MSS.0b013e31824e8a36. [DOI] [PubMed] [Google Scholar]
- 32.Das S, Ferlito M, Kent OA, Fox-Talbot K, Wang R, Liu D, Raghavachari N, Yang Y, Wheelan SJ, Murphy E, Steenbergen C. Nuclear miRNA regulates the mitochondrial genome in the heart. Circ Res 110: 1596–1603, 2012. doi: 10.1161/CIRCRESAHA.112.267732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Mauro V, Iafisco M, Salvarani N, Vacchiano M, Carullo P, Ramírez-Rodríguez GB, Patrício T, Tampieri A, Miragoli M, Catalucci D. Bioinspired negatively charged calcium phosphate nanocarriers for cardiac delivery of MicroRNAs. Nanomedicine (Lond) 11: 891–906, 2016. doi: 10.2217/nnm.16.26. [DOI] [PubMed] [Google Scholar]
- 34.Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F, Xue C, Marinov GK, Khatun J, Williams BA, Zaleski C, Rozowsky J, Röder M, Kokocinski F, Abdelhamid RF, Alioto T, Antoshechkin I, Baer MT, Bar NS, Batut P, Bell K, Bell I, Chakrabortty S, Chen X, Chrast J, Curado J, Derrien T, Drenkow J, Dumais E, Dumais J, Duttagupta R, Falconnet E, Fastuca M, Fejes-Toth K, Ferreira P, Foissac S, Fullwood MJ, Gao H, Gonzalez D, Gordon A, Gunawardena H, Howald C, Jha S, Johnson R, Kapranov P, King B, Kingswood C, Luo OJ, Park E, Persaud K, Preall JB, Ribeca P, Risk B, Robyr D, Sammeth M, Schaffer L, See LH, Shahab A, Skancke J, Suzuki AM, Takahashi H, Tilgner H, Trout D, Walters N, Wang H, Wrobel J, Yu Y, Ruan X, Hayashizaki Y, Harrow J, Gerstein M, Hubbard T, Reymond A, Antonarakis SE, Hannon G, Giddings MC, Ruan Y, Wold B, Carninci P, Guigó R, Gingeras TR. Landscape of transcription in human cells. Nature 489: 101–108, 2012. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duan SZ, Ivashchenko CY, Russell MW, Milstone DS, Mortensen RM. Cardiomyocyte-specific knockout and agonist of peroxisome proliferator-activated receptor-γ both induce cardiac hypertrophy in mice. Circ Res 97: 372–379, 2005. doi: 10.1161/01.RES.0000179226.34112.6d. [DOI] [PubMed] [Google Scholar]
- 36.Eding JE, Demkes CJ, Lynch JM, Seto AG, Montgomery RL, Semus HM, Jackson AL, Isabelle M, Chimenti S, van Rooij E. The efficacy of cardiac anti-miR-208a therapy is stress dependent. Mol Ther 25: 694–704, 2017. doi: 10.1016/j.ymthe.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El-Andaloussi S, Lee Y, Lakhal-Littleton S, Li J, Seow Y, Gardiner C, Alvarez-Erviti L, Sargent IL, Wood MJ. Exosome-mediated delivery of siRNA in vitro and in vivo. Nat Protoc 7: 2112–2126, 2012. doi: 10.1038/nprot.2012.131. [DOI] [PubMed] [Google Scholar]
- 38.Elia L, Contu R, Quintavalle M, Varrone F, Chimenti C, Russo MA, Cimino V, De Marinis L, Frustaci A, Catalucci D, Condorelli G. Reciprocal regulation of microRNA-1 and insulin-like growth factor-1 signal transduction cascade in cardiac and skeletal muscle in physiological and pathological conditions. Circulation 120: 2377–2385, 2009. doi: 10.1161/CIRCULATIONAHA.109.879429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emanueli C, Shearn AIU, Angelini GD, Sahoo S. Exosomes and exosomal miRNAs in cardiovascular protection and repair. Vascul Pharmacol 71: 24–30, 2015. doi: 10.1016/j.vph.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eskildsen TV, Jeppesen PL, Schneider M, Nossent AY, Sandberg MB, Hansen PB, Jensen CH, Hansen ML, Marcussen N, Rasmussen LM, Bie P, Andersen DC, Sheikh SP. Angiotensin II regulates microRNA-132/-212 in hypertensive rats and humans. Int J Mol Sci 14: 11190–11207, 2013. doi: 10.3390/ijms140611190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang X, Stroud MJ, Ouyang K, Fang L, Zhang J, Dalton ND, Gu Y, Wu T, Peterson KL, Huang HD, Chen J, Wang N. Adipocyte-specific loss of PPARγ attenuates cardiac hypertrophy. JCI Insight 1: e89908, 2016. doi: 10.1172/jci.insight.89908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandes T, Hashimoto NY, Magalhães FC, Fernandes FB, Casarini DE, Carmona AK, Krieger JE, Phillips MI, Oliveira EM. Aerobic exercise training-induced left ventricular hypertrophy involves regulatory microRNAs, decreased angiotensin-converting enzyme-angiotensin II, and synergistic regulation of angiotensin-converting enzyme 2-angiotensin (1-7). Hypertension 58: 182–189, 2011. doi: 10.1161/HYPERTENSIONAHA.110.168252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao S, Tian X, Chang H, Sun Y, Wu Z, Cheng Z, Dong P, Zhao Q, Ruan J, Bu W. Two novel lncRNAs discovered in human mitochondrial DNA using PacBio full-length transcriptome data. Mitochondrion 38: 41–47, 2018. doi: 10.1016/j.mito.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Garcia NA, Ontoria-Oviedo I, González-King H, Diez-Juan A, Sepúlveda P. Glucose starvation in cardiomyocytes enhances exosome secretion and promotes angiogenesis in endothelial cells. PLoS One 10: e0138849, 2015. doi: 10.1371/journal.pone.0138849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomes CPC, de Gonzalo-Calvo D, Toro R, Fernandes T, Theisen D, Wang DZ, Devaux Y; Cardiolinc™ network . Non-coding RNAs and exercise: pathophysiological role and clinical application in the cardiovascular system. Clin Sci (Lond) 132: 925–942, 2018. doi: 10.1042/CS20171463. [DOI] [PubMed] [Google Scholar]
- 46.Gomes CPC, Spencer H, Ford KL, Michel LY, Baker AH, Emanueli C, Balligand JL, Devaux Y; Cardiolinc network . The function and therapeutic potential of long non-coding rnas in cardiovascular development and disease. Mol Ther Nucleic Acids 8: 494–507, 2017. doi: 10.1016/j.omtn.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goren Y, Kushnir M, Zafrir B, Tabak S, Lewis BS, Amir O. Serum levels of microRNAs in patients with heart failure. Eur J Heart Fail 14: 147–154, 2012. doi: 10.1093/eurjhf/hfr155. [DOI] [PubMed] [Google Scholar]
- 48.Greco S, Salgado Somoza A, Devaux Y, Martelli F. Long noncoding RNAs and cardiac disease. Antioxid Redox Signal 29: 880–901, 2018. doi: 10.1089/ars.2017.7126. [DOI] [PubMed] [Google Scholar]
- 49.Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res 36: D154–D158, 2008. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grote P, Wittler L, Hendrix D, Koch F, Währisch S, Beisaw A, Macura K, Bläss G, Kellis M, Werber M, Herrmann BG. The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev Cell 24: 206–214, 2013. doi: 10.1016/j.devcel.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guay C, Regazzi R. Exosomes as new players in metabolic organ cross-talk. Diabetes Obes Metab 19, Suppl 1: 137–146, 2017. doi: 10.1111/dom.13027. [DOI] [PubMed] [Google Scholar]
- 52.Guescini M, Canonico B, Lucertini F, Maggio S, Annibalini G, Barbieri E, Luchetti F, Papa S, Stocchi V. Muscle releases alpha-sarcoglycan positive extracellular vesicles carrying miRNAs in the bloodstream. PLoS One 10: e0125094, 2015. doi: 10.1371/journal.pone.0125094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466: 835–840, 2010. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han P, Li W, Lin CH, Yang J, Shang C, Nurnberg ST, Jin KK, Xu W, Lin CY, Lin CJ, Xiong Y, Chien H, Zhou B, Ashley E, Bernstein D, Chen PS, Chen HV, Quertermous T, Chang CP. A long noncoding RNA protects the heart from pathological hypertrophy. Nature 514: 102–106, 2014. doi: 10.1038/nature13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature 495: 384–388, 2013. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 56.Hata A. Functions of microRNAs in cardiovascular biology and disease. Annu Rev Physiol 75: 69–93, 2013. doi: 10.1146/annurev-physiol-030212-183737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He H, Tao H, Xiong H, Duan SZ, McGowan FX Jr, Mortensen RM, Balschi JA. Rosiglitazone causes cardiotoxicity via peroxisome proliferator-activated receptor γ-independent mitochondrial oxidative stress in mouse hearts. Toxicol Sci 138: 468–481, 2014. doi: 10.1093/toxsci/kfu015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Helwak A, Kudla G, Dudnakova T, Tollervey D. Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. Cell 153: 654–665, 2013. doi: 10.1016/j.cell.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holdt LM, Beutner F, Scholz M, Gielen S, Gäbel G, Bergert H, Schuler G, Thiery J, Teupser D. ANRIL expression is associated with atherosclerosis risk at chromosome 9p21. Arterioscler Thromb Vasc Biol 30: 620–627, 2010. doi: 10.1161/ATVBAHA.109.196832. [DOI] [PubMed] [Google Scholar]
- 60.Holdt LM, Stahringer A, Sass K, Pichler G, Kulak NA, Wilfert W, Kohlmaier A, Herbst A, Northoff BH, Nicolaou A, Gäbel G, Beutner F, Scholz M, Thiery J, Musunuru K, Krohn K, Mann M, Teupser D. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat Commun 7: 12429, 2016. doi: 10.1038/ncomms12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hombach S, Kretz M. Non-coding RNAs: classification, biology and functioning. Adv Exp Med Biol 937: 3–17, 2016. doi: 10.1007/978-3-319-42059-2_1. [DOI] [PubMed] [Google Scholar]
- 62.Huang-Doran I, Zhang CY, Vidal-Puig A. Extracellular vesicles: novel mediators of cell communication in metabolic disease. Trends Endocrinol Metab 28: 3–18, 2017. doi: 10.1016/j.tem.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 63.Huang X, Yuan T, Tschannen M, Sun Z, Jacob H, Du M, Liang M, Dittmar RL, Liu Y, Liang M, Kohli M, Thibodeau SN, Boardman L, Wang L. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics 14: 319, 2013. doi: 10.1186/1471-2164-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ibrahim AG, Cheng K, Marbán E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports 2: 606–619, 2014. doi: 10.1016/j.stemcr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ikeda S, He A, Kong SW, Lu J, Bejar R, Bodyak N, Lee KH, Ma Q, Kang PM, Golub TR, Pu WT. MicroRNA-1 negatively regulates expression of the hypertrophy-associated calmodulin and Mef2a genes. Mol Cell Biol 29: 2193–2204, 2009. doi: 10.1128/MCB.01222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ishii N, Ozaki K, Sato H, Mizuno H, Saito S, Takahashi A, Miyamoto Y, Ikegawa S, Kamatani N, Hori M, Saito S, Nakamura Y, Tanaka T. Identification of a novel non-coding RNA, MIAT, that confers risk of myocardial infarction. J Hum Genet 51: 1087–1099, 2006. doi: 10.1007/s10038-006-0070-9. [DOI] [PubMed] [Google Scholar]
- 67.Jeong D, Yoo J, Lee P, Kepreotis SV, Lee A, Wahlquist C, Brown BD, Kho C, Mercola M, Hajjar RJ. miR-25 tough decoy enhances cardiac function in heart failure. Mol Ther 26: 718–729, 2018. doi: 10.1016/j.ymthe.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klattenhoff CA, Scheuermann JC, Surface LE, Bradley RK, Fields PA, Steinhauser ML, Ding H, Butty VL, Torrey L, Haas S, Abo R, Tabebordbar M, Lee RT, Burge CB, Boyer LA. Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell 152: 570–583, 2013. doi: 10.1016/j.cell.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kumarswamy R, Bauters C, Volkmann I, Maury F, Fetisch J, Holzmann A, Lemesle G, de Groote P, Pinet F, Thum T. Circulating long noncoding RNA, LIPCAR, predicts survival in patients with heart failure. Circ Res 114: 1569–1575, 2014. doi: 10.1161/CIRCRESAHA.114.303915. [DOI] [PubMed] [Google Scholar]
- 70.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res (Amst) 4: 214–222, 2010. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 71.Lakhal S, Wood MJ. Exosome nanotechnology: an emerging paradigm shift in drug delivery: exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers. BioEssays 33: 737–741, 2011. doi: 10.1002/bies.201100076. [DOI] [PubMed] [Google Scholar]
- 72.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75: 843–854, 1993. doi: 10.1016/0092-8674(93)90529-Y. [DOI] [PubMed] [Google Scholar]
- 73.Leptidis S, El Azzouzi H, Lok SI, de Weger R, Olieslagers S, Kisters N, Silva GJ, Heymans S, Cuppen E, Berezikov E, De Windt LJ, da Costa Martins P. A deep sequencing approach to uncover the miRNOME in the human heart. PLoS One 8: e57800, 2013. [Erratum in PLoS One 8: 10.1371/annotation/e33f9763-3385-42c7-b31e-d433dc8e499a]. doi: 10.1371/journal.pone.0057800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 322: 1561–1566, 1990. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 75.Li J, Xuan Z, Liu C. Long non-coding RNAs and complex human diseases. Int J Mol Sci 14: 18790–18808, 2013. doi: 10.3390/ijms140918790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Y, Liang Y, Zhu Y, Zhang Y, Bei Y. Noncoding RNAs in cardiac hypertrophy. J Cardiovasc Transl Res 11: 439–449, 2018. doi: 10.1007/s12265-018-9797-x. [DOI] [PubMed] [Google Scholar]
- 77.Lin RC, Weeks KL, Gao XM, Williams RB, Bernardo BC, Kiriazis H, Matthews VB, Woodcock EA, Bouwman RD, Mollica JP, Speirs HJ, Dawes IW, Daly RJ, Shioi T, Izumo S, Febbraio MA, Du XJ, McMullen JR. PI3K(p110 α) protects against myocardial infarction-induced heart failure: identification of PI3K-regulated miRNA and mRNA. Arterioscler Thromb Vasc Biol 30: 724–732, 2010. doi: 10.1161/ATVBAHA.109.201988. [DOI] [PubMed] [Google Scholar]
- 78.Liu L, An X, Li Z, Song Y, Li L, Zuo S, Liu N, Yang G, Wang H, Cheng X, Zhang Y, Yang X, Wang J. The H19 long noncoding RNA is a novel negative regulator of cardiomyocyte hypertrophy. Cardiovasc Res 111: 56–65, 2016. doi: 10.1093/cvr/cvw078. [DOI] [PubMed] [Google Scholar]
- 79.Liu X, Xiao J, Zhu H, Wei X, Platt C, Damilano F, Xiao C, Bezzerides V, Boström P, Che L, Zhang C, Spiegelman BM, Rosenzweig A. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab 21: 584–595, 2015. doi: 10.1016/j.cmet.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu Y, Yan X, Mao G, Fang L, Zhao B, Liu Y, Tang H, Wang N. Metabonomic profiling revealed an alteration in purine nucleotide metabolism associated with cardiac hypertrophy in rats treated with thiazolidinediones. J Proteome Res 12: 5634–5641, 2013. doi: 10.1021/pr400587y. [DOI] [PubMed] [Google Scholar]
- 81.Lv L, Li T, Li X, Xu C, Liu Q, Jiang H, Li Y, Liu Y, Yan H, Huang Q, Zhou Y, Zhang M, Shan H, Liang H. The lncRNA Plscr4 controls cardiac hypertrophy by regulating miR-214. Mol Ther Nucleic Acids 10: 387–397, 2018. doi: 10.1016/j.omtn.2017.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lyu L, Wang H, Li B, Qin Q, Qi L, Nagarkatti M, Nagarkatti P, Janicki JS, Wang XL, Cui T. A critical role of cardiac fibroblast-derived exosomes in activating renin angiotensin system in cardiomyocytes. J Mol Cell Cardiol 89: 268–279, 2015. doi: 10.1016/j.yjmcc.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mannoor K, Liao J, Jiang F. Small nucleolar RNAs in cancer. Biochim Biophys Acta 1826: 121–128, 2012. doi: 10.1016/j.bbcan.2012.1003.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matera AG, Terns RM, Terns MP. Non-coding RNAs: lessons from the small nuclear and small nucleolar RNAs. Nat Rev Mol Cell Biol 8: 209–220, 2007. doi: 10.1038/nrm2124. [DOI] [PubMed] [Google Scholar]
- 85.Matkovich SJ, Edwards JR, Grossenheider TC, de Guzman Strong C, Dorn GW 2nd. Epigenetic coordination of embryonic heart transcription by dynamically regulated long noncoding RNAs. Proc Natl Acad Sci USA 111: 12264–12269, 2014. doi: 10.1073/pnas.1410622111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Matkovich SJ, Hu Y, Dorn GW 2nd. Regulation of cardiac microRNAs by cardiac microRNAs. Circ Res 113: 62–71, 2013. doi: 10.1161/CIRCRESAHA.113.300975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Matkovich SJ, Hu Y, Eschenbacher WH, Dorn LE, Dorn GW 2nd. Direct and indirect involvement of microRNA-499 in clinical and experimental cardiomyopathy. Circ Res 111: 521–531, 2012. doi: 10.1161/CIRCRESAHA.112.265736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McMullen JR, Drew BG. Long non-coding RNAs (lncRNAs) in skeletal and cardiac muscle: potential therapeutic and diagnostic targets? Clin Sci (Lond) 130: 2245–2256, 2016. doi: 10.1042/CS20160244. [DOI] [PubMed] [Google Scholar]
- 89.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, Loewer A, Ziebold U, Landthaler M, Kocks C, le Noble F, Rajewsky N. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495: 333–338, 2013. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 90.Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM, Baty CJ, Gibson GA, Erdos G, Wang Z, Milosevic J, Tkacheva OA, Divito SJ, Jordan R, Lyons-Weiler J, Watkins SC, Morelli AE. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood 119: 756–766, 2012. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation 131: e29–e322, 2015. [Errata in Circulation 131: e535, 2015 and Circulation 133: e417, 2016]. 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 92.Ng KW, Anderson C, Marshall EA, Minatel BC, Enfield KS, Saprunoff HL, Lam WL, Martinez VD. Piwi-interacting RNAs in cancer: emerging functions and clinical utility. Mol Cancer 15: 5, 2016. doi: 10.1186/s12943-016-0491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nie JJ, Qiao B, Duan S, Xu C, Chen B, Hao W, Yu B, Li Y, Du J, Xu FJ. Unlockable nanocomplexes with self-accelerating nucleic acid release for effective staged gene therapy of cardiovascular diseases. Adv Mater 30: 1801570, 2018. doi: 10.1002/adma.201801570. [DOI] [PubMed] [Google Scholar]
- 94.Obad S, dos Santos CO, Petri A, Heidenblad M, Broom O, Ruse C, Fu C, Lindow M, Stenvang J, Straarup EM, Hansen HF, Koch T, Pappin D, Hannon GJ, Kauppinen S. Silencing of microRNA families by seed-targeting tiny LNAs. Nat Genet 43: 371–378, 2011. doi: 10.1038/ng.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ooi JY, Bernardo BC, McMullen JR. The therapeutic potential of miRNAs regulated in settings of physiological cardiac hypertrophy. Future Med Chem 6: 205–222, 2014. doi: 10.4155/fmc.13.196. [DOI] [PubMed] [Google Scholar]
- 96.Ooi JY, Bernardo BC, Singla S, Patterson NL, Lin RC, McMullen JR. Identification of miR-34 regulatory networks in settings of disease and antimiR-therapy: Implications for treating cardiac pathology and other diseases. RNA Biol 14: 500–513, 2017. doi: 10.1080/15476286.2016.1181251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ottaviani L, da Costa Martins PA. Non-coding RNAs in cardiac hypertrophy. J Physiol 595: 4037–4050, 2017. doi: 10.1113/JP273129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ovchinnikova ES, Schmitter D, Vegter EL, Ter Maaten JM, Valente MA, Liu LC, van der Harst P, Pinto YM, de Boer RA, Meyer S, Teerlink JR, O’Connor CM, Metra M, Davison BA, Bloomfield DM, Cotter G, Cleland JG, Mebazaa A, Laribi S, Givertz MM, Ponikowski P, van der Meer P, van Veldhuisen DJ, Voors AA, Berezikov E. Signature of circulating microRNAs in patients with acute heart failure. Eur J Heart Fail 18: 414–423, 2016. doi: 10.1002/ejhf.332. [DOI] [PubMed] [Google Scholar]
- 99.Pamudurti NR, Bartok O, Jens M, Ashwal-Fluss R, Stottmeister C, Ruhe L, Hanan M, Wyler E, Perez-Hernandez D, Ramberger E, Shenzis S, Samson M, Dittmar G, Landthaler M, Chekulaeva M, Rajewsky N, Kadener S. Translation of circRNAs. Mol Cell 66: 9–21.e7, 2017. doi: 10.1016/j.molcel.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pluim BM, Zwinderman AH, van der Laarse A, van der Wall EE. The athlete’s heart. A meta-analysis of cardiac structure and function. Circulation 101: 336–344, 2000. doi: 10.1161/01.CIR.101.3.336. [DOI] [PubMed] [Google Scholar]
- 101.Poller W, Dimmeler S, Heymans S, Zeller T, Haas J, Karakas M, Leistner DM, Jakob P, Nakagawa S, Blankenberg S, Engelhardt S, Thum T, Weber C, Meder B, Hajjar R, Landmesser U. Non-coding RNAs in cardiovascular diseases: diagnostic and therapeutic perspectives. Eur Heart J 39: 2704–2716, 2018. doi: 10.1093/eurheartj/ehx165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Qu S, Yang X, Li X, Wang J, Gao Y, Shang R, Sun W, Dou K, Li H. Circular RNA: A new star of noncoding RNAs. Cancer Lett 365: 141–148, 2015. doi: 10.1016/j.canlet.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 103.Rackham O, Shearwood AM, Mercer TR, Davies SM, Mattick JS, Filipovska A. Long noncoding RNAs are generated from the mitochondrial genome and regulated by nuclear-encoded proteins. RNA 17: 2085–2093, 2011. doi: 10.1261/rna.029405.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ramasamy S, Velmurugan G, Shanmugha Rajan K, Ramprasath T, Kalpana K. MiRNAs with apoptosis regulating potential are differentially expressed in chronic exercise-induced physiologically hypertrophied hearts. PLoS One 10: e0121401, 2015. doi: 10.1371/journal.pone.0121401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 403: 901–906, 2000. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 106.Sahoo S, Losordo DW. Exosomes and cardiac repair after myocardial infarction. Circ Res 114: 333–344, 2014. doi: 10.1161/CIRCRESAHA.114.300639. [DOI] [PubMed] [Google Scholar]
- 107.Sanger HL, Klotz G, Riesner D, Gross HJ, Kleinschmidt AK. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci USA 73: 3852–3856, 1976. doi: 10.1073/pnas.73.11.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schäfer F, Wagner J, Knau A, Dimmeler S, Heckel A. Regulating angiogenesis with light-inducible AntimiRs. Angew Chem Int Ed Engl 52: 13558–13561, 2013. doi: 10.1002/anie.201307502. [DOI] [PubMed] [Google Scholar]
- 109.Shi J, Bei Y, Kong X, Liu X, Lei Z, Xu T, Wang H, Xuan Q, Chen P, Xu J, Che L, Liu H, Zhong J, Sluijter JP, Li X, Rosenzweig A, Xiao J. miR-17-3p contributes to exercise-induced cardiac growth and protects against myocardial ischemia-reperfusion injury. Theranostics 7: 664–676, 2017. doi: 10.7150/thno.15162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Soci UP, Fernandes T, Barauna VG, Hashimoto NY, de Fátima Alves Mota G, Rosa KT, Irigoyen MC, Philips MI, de Oliveira EM. Epigenetic control of exercise training-induced cardiac hypertrophy by miR-208. Clin Sci (Lond) 130: 2005–2015, 2016. doi: 10.1042/CS20160480. [DOI] [PubMed] [Google Scholar]
- 111.Soci UP, Fernandes T, Hashimoto NY, Mota GF, Amadeu MA, Rosa KT, Irigoyen MC, Phillips MI, Oliveira EM. MicroRNAs 29 are involved in the improvement of ventricular compliance promoted by aerobic exercise training in rats. Physiol Genomics 43: 665–673, 2011. doi: 10.1152/physiolgenomics.00145.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Son NH, Park TS, Yamashita H, Yokoyama M, Huggins LA, Okajima K, Homma S, Szabolcs MJ, Huang LS, Goldberg IJ. Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J Clin Invest 117: 2791–2801, 2007. doi: 10.1172/JCI30335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stenvang J, Petri A, Lindow M, Obad S, Kauppinen S. Inhibition of microRNA function by antimiR oligonucleotides. Silence 3: 1, 2012. doi: 10.1186/1758-907X-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tan WL, Lim BT, Anene-Nzelu CG, Ackers-Johnson M, Dashi A, See K, Tiang Z, Lee DP, Chua WW, Luu TD, Li PY, Richards AM, Foo RS. A landscape of circular RNA expression in the human heart. Cardiovasc Res 113: 298–309, 2017. doi: 10.1093/cvr/cvw250. [DOI] [PubMed] [Google Scholar]
- 115.Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW, Doevendans PA, Mummery CL, Borlak J, Haverich A, Gross C, Engelhardt S, Ertl G, Bauersachs J. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation 116: 258–267, 2007. doi: 10.1161/CIRCULATIONAHA.107.687947. [DOI] [PubMed] [Google Scholar]
- 116.Tian Y, Liu Y, Wang T, Zhou N, Kong J, Chen L, Snitow M, Morley M, Li D, Petrenko N, Zhou S, Lu M, Gao E, Koch WJ, Stewart KM, Morrisey EE. A microRNA-Hippo pathway that promotes cardiomyocyte proliferation and cardiac regeneration in mice. Sci Transl Med 7: 279ra38, 2015. doi: 10.1126/scitranslmed.3010841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tijsen AJ, Creemers EE, Moerland PD, de Windt LJ, van der Wal AC, Kok WE, Pinto YM. MiR423-5p as a circulating biomarker for heart failure. Circ Res 106: 1035–1039, 2010. doi: 10.1161/CIRCRESAHA.110.218297. [DOI] [PubMed] [Google Scholar]
- 118.Ucar A, Gupta SK, Fiedler J, Erikci E, Kardasinski M, Batkai S, Dangwal S, Kumarswamy R, Bang C, Holzmann A, Remke J, Caprio M, Jentzsch C, Engelhardt S, Geisendorf S, Glas C, Hofmann TG, Nessling M, Richter K, Schiffer M, Carrier L, Napp LC, Bauersachs J, Chowdhury K, Thum T. The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat Commun 3: 1078, 2012. doi: 10.1038/ncomms2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science 316: 575–579, 2007. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 120.Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science 318: 1931–1934, 2007. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 121.Vausort M, Salgado-Somoza A, Zhang L, Leszek P, Scholz M, Teren A, Burkhardt R, Thiery J, Wagner DR, Devaux Y. Myocardial infarction-associated circular RNA predicting left ventricular dysfunction. J Am Coll Cardiol 68: 1247–1248, 2016. doi: 10.1016/j.jacc.2016.06.040. [DOI] [PubMed] [Google Scholar]
- 122.Vegter EL, Schmitter D, Hagemeijer Y, Ovchinnikova ES, van der Harst P, Teerlink JR, O’Connor CM, Metra M, Davison BA, Bloomfield D, Cotter G, Cleland JG, Givertz MM, Ponikowski P, van Veldhuisen DJ, van der Meer P, Berezikov E, Voors AA, Khan MA. Use of biomarkers to establish potential role and function of circulating microRNAs in acute heart failure. Int J Cardiol 224: 231–239, 2016. doi: 10.1016/j.ijcard.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 123.Viereck J, Kumarswamy R, Foinquinos A, Xiao K, Avramopoulos P, Kunz M, Dittrich M, Maetzig T, Zimmer K, Remke J, Just A, Fendrich J, Scherf K, Bolesani E, Schambach A, Weidemann F, Zweigerdt R, de Windt LJ, Engelhardt S, Dandekar T, Batkai S, Thum T. Long noncoding RNA Chast promotes cardiac remodeling. Sci Transl Med 8: 326ra22, 2016. doi: 10.1126/scitranslmed.aaf1475. [DOI] [PubMed] [Google Scholar]
- 124.Vujic A, Lerchenmüller C, Wu TD, Guillermier C, Rabolli CP, Gonzalez E, Senyo SE, Liu X, Guerquin-Kern JL, Steinhauser ML, Lee RT, Rosenzweig A. Exercise induces new cardiomyocyte generation in the adult mammalian heart. Nat Commun 9: 1659, 2018. doi: 10.1038/s41467-018-04083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang GK, Zhu JQ, Zhang JT, Li Q, Li Y, He J, Qin YW, Jing Q. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur Heart J 31: 659–666, 2010. doi: 10.1093/eurheartj/ehq013. [DOI] [PubMed] [Google Scholar]
- 126.Wang K, Liu F, Zhou LY, Long B, Yuan SM, Wang Y, Liu CY, Sun T, Zhang XJ, Li PF. The long noncoding RNA CHRF regulates cardiac hypertrophy by targeting miR-489. Circ Res 114: 1377–1388, 2014. doi: 10.1161/CIRCRESAHA.114.302476. [DOI] [PubMed] [Google Scholar]
- 127.Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell 43: 904–914, 2011. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang L, Lv Y, Li G, Xiao J. MicroRNAs in heart and circulation during physical exercise. J Sport Health Sci 7: 433–441, 2018. doi: 10.1016/j.jshs.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang L, Meng X, Li G, Zhou Q, Xiao J. Circular RNAs in cardiovascular diseases. Adv Exp Med Biol 1087: 191–204, 2018. doi: 10.1007/978-981-13-1426-1_15. [DOI] [PubMed] [Google Scholar]
- 130.Wang LL, Liu Y, Chung JJ, Wang T, Gaffey AC, Lu M, Cavanaugh CA, Zhou S, Kanade R, Atluri P, Morrisey EE, Burdick JA. Local and sustained miRNA delivery from an injectable hydrogel promotes cardiomyocyte proliferation and functional regeneration after ischemic injury. Nat Biomed Eng 1: 983–992, 2017. doi: 10.1038/s41551-017-0157-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang R, Li N, Zhang Y, Ran Y, Pu J. Circulating microRNAs are promising novel biomarkers of acute myocardial infarction. Intern Med 50: 1789–1795, 2011. doi: 10.2169/internalmedicine.50.5129. [DOI] [PubMed] [Google Scholar]
- 132.Wang X, Huang W, Liu G, Cai W, Millard RW, Wang Y, Chang J, Peng T, Fan GC. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J Mol Cell Cardiol 74: 139–150, 2014. doi: 10.1016/j.yjmcc.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang X, Searle AK, Hohmann JD, Liu AL, Abraham MK, Palasubramaniam J, Lim B, Yao Y, Wallert M, Yu E, Chen YC, Peter K. Dual-targeted theranostic Delivery of miRs arrests abdominal aortic aneurysm development. Mol Ther 26: 1056–1065, 2018. doi: 10.1016/j.ymthe.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang X, Song X, Glass CK, Rosenfeld MG. The long arm of long noncoding RNAs: roles as sensors regulating gene transcriptional programs. Cold Spring Harb Perspect Biol 3: a003756, 2011. doi: 10.1101/cshperspect.a003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang Y, Wang Z. Efficient backsplicing produces translatable circular mRNAs. RNA 21: 172–179, 2015. doi: 10.1261/rna.048272.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang Z, Zhang XJ, Ji YX, Zhang P, Deng KQ, Gong J, Ren S, Wang X, Chen I, Wang H, Gao C, Yokota T, Ang YS, Li S, Cass A, Vondriska TM, Li G, Deb A, Srivastava D, Yang HT, Xiao X, Li H, Wang Y. The long noncoding RNA Chaer defines an epigenetic checkpoint in cardiac hypertrophy. Nat Med 22: 1131–1139, 2016. doi: 10.1038/nm.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Werfel S, Nothjunge S, Schwarzmayr T, Strom TM, Meitinger T, Engelhardt S. Characterization of circular RNAs in human, mouse and rat hearts. J Mol Cell Cardiol 98: 103–107, 2016. doi: 10.1016/j.yjmcc.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 138.Whitham M, Parker BL, Friedrichsen M, Hingst JR, Hjorth M, Hughes WE, Egan CL, Cron L, Watt KI, Kuchel RP, Jayasooriah N, Estevez E, Petzold T, Suter CM, Gregorevic P, Kiens B, Richter EA, James DE, Wojtaszewski JF, Febbraio MA. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab 27: 237–251.e4, 2018. doi: 10.1016/j.cmet.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 139.Wilusz JE, JnBaptiste CK, Lu LY, Kuhn CD, Joshua-Tor L, Sharp PA. A triple helix stabilizes the 3′ ends of long noncoding RNAs that lack poly(A) tails. Genes Dev 26: 2392–2407, 2012. doi: 10.1101/gad.204438.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xiao J. (Editor). Circular RNAs: biogenesis and functions. In: Advances in Experimental Medicine and Biology. Singapore: Springer, vol. 1087, 2018. [Google Scholar]
- 141.Xu J-Y, Chen G-H, Yang Y-J. Exosomes: a rising star in falling hearts. Front Physiol 8: 494, 2017. [Erratum in Front Physiol 8: 494, 2017]. doi: 10.3389/fphys.2017.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yamaguchi T, Izumi Y, Nakamura Y, Yamazaki T, Shiota M, Sano S, Tanaka M, Osada-Oka M, Shimada K, Miura K, Yoshiyama M, Iwao H. Repeated remote ischemic conditioning attenuates left ventricular remodeling via exosome-mediated intercellular communication on chronic heart failure after myocardial infarction. Int J Cardiol 178: 239–246, 2015. doi: 10.1016/j.ijcard.2014.10.144. [DOI] [PubMed] [Google Scholar]
- 143.Yang KC, Yamada KA, Patel AY, Topkara VK, George I, Cheema FH, Ewald GA, Mann DL, Nerbonne JM. Deep RNA sequencing reveals dynamic regulation of myocardial noncoding RNAs in failing human heart and remodeling with mechanical circulatory support. Circulation 129: 1009–1021, 2014. doi: 10.1161/CIRCULATIONAHA.113.003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang J, Li S, Li L, Li M, Guo C, Yao J, Mi S. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics 13: 17–24, 2015. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang M, Xin Y. Circular RNAs: a new frontier for cancer diagnosis and therapy. J Hematol Oncol 11: 21, 2018. doi: 10.1186/s13045-018-0569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang X, Azhar G, Wei JY. The expression of microRNA and microRNA clusters in the aging heart. PLoS One 7: e34688, 2012. doi: 10.1371/journal.pone.0034688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhou Q, Zhang Z, Bei Y, Li G, Wang T. Circular RNAs as Novel Biomarkers for Cardiovascular Diseases. Adv Exp Med Biol 1087: 159–170, 2018. doi: 10.1007/978-981-13-1426-1_13. [DOI] [PubMed] [Google Scholar]
- 148.Zhou SS, Jin JP, Wang JQ, Zhang ZG, Freedman JH, Zheng Y, Cai L. miRNAS in cardiovascular diseases: potential biomarkers, therapeutic targets and challenges. Acta Pharmacol Sin 39: 1073–1084, 2018. doi: 10.1038/aps.2018.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zsebo K, Yaroshinsky A, Rudy JJ, Wagner K, Greenberg B, Jessup M, Hajjar RJ. Long-term effects of AAV1/SERCA2a gene transfer in patients with severe heart failure: analysis of recurrent cardiovascular events and mortality. Circ Res 114: 101–108, 2014. doi: 10.1161/CIRCRESAHA.113.302421. [DOI] [PubMed] [Google Scholar]