Abstract
The purpose of this study was to investigate the underlying cellular basis of muscle atrophy (Placebo) and atrophy reduction (essential amino acid supplementation, EAAs) in total knee arthroplasty (TKA) patients by examining satellite cells and other key histological markers of inflammation, recovery, and fibrosis. Forty-one subjects (53–76 yr) scheduled for TKA were randomized into two groups, ingesting 20 g of EAAs or placebo, twice-daily, for 7 days before TKA and for 6 wk after surgery. A first set of muscle biopsies was obtained from both legs before surgery in the operating room, and patients were randomly assigned and equally allocated to have two additional biopsies at either 1 or 2 wk after surgery. Biopsies were processed for gene expression and immunohistochemistry. Satellite cells were significantly higher in patients ingesting 20 g of essential amino acids twice daily for the 7 days leading up to surgery compared with Placebo (operative leg P = 0.03 for satellite cells/fiber and P = 0.05 for satellite cell proportions for Type I-associated cells and P = 0.05 for satellite cells/fiber for Type II-associated cells.) Myogenic regulatory factor gene expression was different between groups, with the Placebo Group having elevated MyoD expression at 1 wk and EAAs having elevated myogenin expression at 1 wk. M1 macrophages were more prevalent in Placebo than the EAAs Group. IL-6 and TNF-α transcripts were elevated postsurgery in both groups; however, TNF-α declined by 2 wk in the EAAs Group. EAAs starting 7 days before surgery increased satellite cells on the day of surgery and promoted a more favorable inflammatory environment postsurgery.
NEW & NOTEWORTHY Clinical studies by our group indicate that the majority of muscle atrophy after total knee arthroplasty (TKA) in older adults occurs rapidly, within the first 2 wks. We have also shown that essential amino acid supplementation (EAAs) before and after TKA mitigates muscle atrophy; however, the mechanisms are unknown. These results suggest that satellite cell numbers are elevated with EAA ingestion before surgery, and after surgery, EAA ingestion positively influences markers of inflammation. Combined, these data may help inform further studies designed to address the accelerated sarcopenia that occurs in older adults after major surgery.
Keywords: atrophy, clinical trial, muscle stem cell, M1 and M2 macrophage, sarcopenia
INTRODUCTION
Accelerated skeletal muscle atrophy and weakness in older adults due to hospitalization or surgeries, such as total knee arthroplasty (TKA), exacerbate sarcopenia (43)–defined as the gradual loss of muscle mass and function associated with normal (healthy) aging (17, 23, 39, 56). Sarcopenia is directly linked to the onset of physical disability (31), and increased risk of home care (5), nursing home placement (55), and hospitalization (22). Mitigating muscle atrophy following TKA can improve postoperative recovery and alleviate the contribution of surgery to the acceleration of sarcopenia.
Substantial atrophy occurs following surgery. Our group has measured quadriceps volume in TKA patients 6 wk after surgery and found an average volume reduction of 18% and 10% in the operative and nonoperative legs, respectively. Most of that loss happened shortly after surgery; 80% and 100% of the 6-wk loss in the operative leg and nonoperative legs, respectively, happened in the first 2 wk. Surprisingly, muscle atrophy in TKA patients occurs with minimal reductions in calorie or macronutrient intake, while mobility is only partially impaired and patients have no weight-bearing restrictions (15). Our group has shown that essential amino acid supplementation (EAAs) is effective in reducing muscle loss in TKA patients. In a proof-of-principle study, we found that EAAs in TKA patients improved functional outcomes and decreased muscle loss as measured by MRI (16). Our recently published follow-up trial corroborates the efficacy of EAAs in reducing muscle loss in TKA patients (15), and others have used EAAs to enhance functional mobility after hip fracture surgery (2).
Multiple factors can potentially influence muscle atrophy, including satellite cell (muscle stem cell) activation, changes in inflammation, macrophage content, and capillary density, as well as increases in fibrotic tissue, cell death, and central nuclei. How these factors might be influenced by EAAs is not entirely clear. EAA supplementation has been shown to stimulate mTOR and inhibit autophagy (19), a process involving breakdown of muscle tissue. Autophagy and inflammation are directly related [reviewed in Levine (34)]; thus, a consequence of EAAs on autophagy may be the expression of inflammatory cytokines and altered macrophage activity. The inflammatory cytokine, TNFα, through its interaction with p38α kinase, instructs satellite cells to differentiate or proliferate (42). Furthermore, EAA supplementation has been shown to stimulate myogenesis. For example, in cell culture, supplementation with leucine and its breakdown product, hydroxymethylbutyrate, has been shown to increase the size of myotubes (3) and enhance C2C12 differentiation and proliferation (18). In a rat model for muscle recovery following disuse, Alway et al. (1) found that hydroxymethylbutyrate supplementation increased satellite cell proliferation in Type II but not Type I muscle fibers. In human subjects, Hulmi et al. (29) showed that ingestion of 15 g of whey protein immediately following exercise increased expression of myogenic regulatory factors. This supports the efficacy of EAAs in increasing myogenesis in vitro and in vivo.
Despite the evidence suggesting that essential amino acids increase myogenesis, there are studies suggesting this may not be directly applicable to our model of atrophy prevention because of the differences inherent to aged muscle. In older subjects, satellite cells appear to become partially dysfunctional, compromising their ability to facilitate muscle recovery after trauma (8–10). These age-associated deficits in satellite cell activation may (49) or may not (26) be implicated in the development of sarcopenia. Possibly for this reason, different studies have reached differing conclusions regarding the efficacy of EAA supplementation in aging human subjects (12, 20, 37, 38, 41). For example, Snijders et al. (46) found that exercise increased Type I-associated satellite cells in older men following exercise, but they saw no difference in the increase between placebo and a group receiving EAA supplementation. In contrast, Reidy et al. (44) documented an elevation in satellite cells following exercise in a group of aging men who received EAA supplementation.
Our overarching goal is to optimize recovery following TKA by preventing muscle atrophy with EAAs and, in this study, to understand this process of atrophy prevention on a cellular/molecular level. Our previous study (16) involving TKA patients receiving EAAs revealed that the most significant reductions in muscle mass occurred in the first 2 wk after surgery. Therefore, in this study, we examined biopsy tissue from patients during this 2-wk window after surgery. The underlying premise guiding the design of this study was that multiple factors may contribute to muscle loss (such as changes in satellite cell numbers and activation state, changes in inflammation, and increases in fibrotic tissue, cell death, and capillary density), and that EAAs might influence the onset and duration of these processes. Therefore, to identify potential tissue-level differences in the EAAs Group that might influence atrophy in our subjects, EAAs was initiated 1 wk before surgery, and histological parameters and supporting gene expression levels were measured immediately before surgery, and at 1 and 2 wk following surgery to assess tissue differences between groups during this critical 2-wk period of maximum atrophy.
METHODS
Study Approval
This study was approved by the University of Oregon Institutional Review Board and conducted in accordance with Declaration of Helsinki ethical principles. All subjects gave informed written consent before study participation. This study is registered with ClinicalTrials.gov (https://clinicaltrials.gov/ identifier: NCT02145949).
Participants
As reported previously (15), 41 subjects, aged 53–76 yr, scheduled to undergo primary TKA were recruited from the Slocum Center for Orthopedics and Sports Medicine in Eugene, OR. Exclusion criteria included a history of any lower extremity total joint replacement surgery; untreated and/or uncontrolled endocrine disease; significant heart, kidney, liver, blood, or respiratory disease; peripheral vascular disease; active cancer; and recent treatment with anabolic steroids or oral corticosteroids for >1 wk. Patients were randomly assigned to the Placebo or EAAs Group. All researchers collecting and/or analyzing data were blinded to group assignment. All patients were blinded to treatment group. Patient characteristics are summarized in Table 1.
Table 1.
Participant baseline characteristics
| Variable | Placebo | EAA | All |
|---|---|---|---|
| Means ± SE or % | Means ± SE or % | Means ± SE or % | |
| % Female | 77.3% | 52.6% | 65.9% |
| Age, yr | 63.82 ± 1.29 | 64.95 ± 1.35 | 64.34 ± 0.93 |
| Height, m | 1.66 ± 0.02 | 1.71 ± 0.02 | 1.68 ± 0.01 |
| Weight, kg | 89.24 ± 4.85 | 86.90 ± 4.13 | 88.15 ± 3.20 |
| Body mass index, kg/m2 | 30.66 ± 2.13 | 29.71 ± 1.00 | 30.21 ± 1.22 |
| Tourniquet time, min | 58.40 ± 5.35 | 51.25 ± 4.57 | 54.63 ± 3.65 |
| TKA operation time, min | 97.24 ± 4.17 | 93.42 ± 4.96 | 95.64 ± 3.26 |
| Physical activity, kcal/day | 319.71 ± 56.97 | 408.15 ± 45.37 | 361.60 ± 37.12 |
Values are expressed as means ± SE; n = 41. EAA, essential amino acids; TKA, total knee arthroplasty.
Trial Design
A two-arm, parallel trial design was employed with a 1:1 allocation ratio to EAAs versus the Placebo Group (Figs. 1 and 2). Blood chemistry, MRI, strength and functional mobility, and patient-reported outcomes were collected at baseline (1–6 wk before surgery). Follow-up blood chemistry was measured on the day of surgery, at postoperative day 2, and at 2 and 6 wk postsurgery. Functional measures were obtained at 2 and 6 wk after surgery. MRI and patient-reported outcomes were obtained at 6 wk. Patients recorded physical activity via accelerometry and documented caloric intake with 3-day food diaries. (See Muscle Volume, Physical Activity, and Caloric Intake below for further details). The study was conducted from January 2015 to September 2016, and the main clinical outcomes and blood analysis from the trial have been published (15). This report focuses on molecular data derived from muscle biopsies obtained from the same clinical trial participants, detailed below (see Muscle Tissue Collection and Imaging and Analysis).
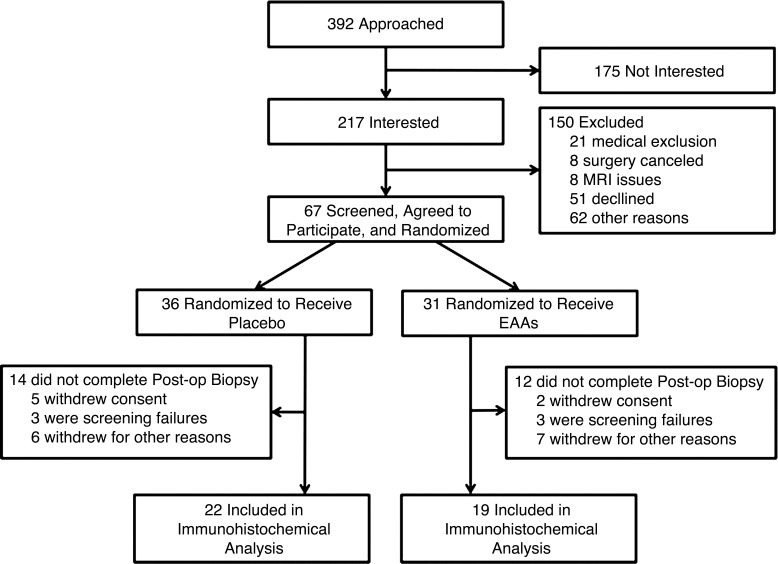
Fig. 1.
CONSORT diagram. CONSORT flow diagram showing the numbers of patients who were randomly assigned to each treatment group, who were excluded or withdrew from the study, and who were included in the analysis.
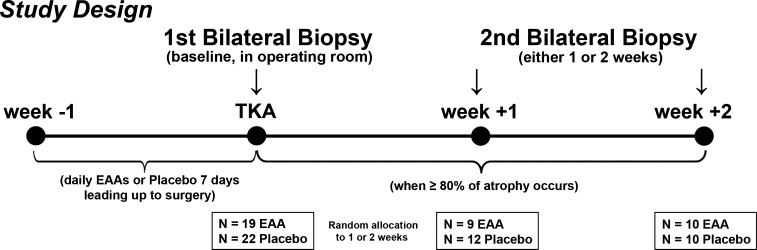
Fig. 2.
Study design. Bilateral biopsies were obtained in the operating room, before total knee arthroplasty (TKA) surgery began, after 7 days of twice-daily ingestion of 20 g of essential amino acid supplement (EAA) or placebo, and at 1 and 2 wk post-TKA (random allocation). Supplements were not taken on the day of surgery, but continued the next day through the end of the trial at 6 wk post-TKA. The rationale for post-TKA biopsy time points was to determine the cell level changes occurring at week 1 and week 2 after TKA, when MRI data from our previously published clinical trial showed that 80% or more of the total muscle atrophy measured at week 6 after TKA. Additionally, the parent study included analysis of functional outcomes, blood analysis, and MRI analysis for 6 wk following surgery, but the results of these measures have been or will be reported in other articles.
Supplementation
Placebo (nonessential amino acid) or EAA supplementation was initiated 7 days before TKA, was discontinued on the day of surgery, was resumed on the first postoperative day, and was continued for 6 wk after surgery, for a total of 49 days. Ingestion of the supplement occurred twice daily, at 10 AM and 2 PM During inpatient hospitalization, the morning supplement was ingested within 1 h of physical therapy. The afternoon supplement was also ingested within 1 h of physical therapy. During outpatient physical therapy, which occurred 3 times/week for the first 2 wk postsurgery and 2 times/week for the next 6 wk, subjects were instructed to ingest the supplement within 1 h after physical therapy on the days they had therapy and at the regular times on all other days. The goal was to maximize the potential anabolic effects of EAA ingestion postexercise (11, 14). The supplement was ingested between meals. Patients were allowed to mix the supplement with any liquid or food. Supplement intake date and time were recorded and, along with empty vials, were used by research personnel to verify compliance. Amino acids were purchased from Ajinomoto U.S.A. Northwest Compounders prepared all of the supplements. Amino acids were added in predefined proportions to achieve a master mix weighing 400 g, which was then aliquoted into blinded 20-g vials and dispensed by study personnel. Patients self-reported taking 99% of the EAA doses and 96% of the placebo doses. Supplement formulation is shown in Table 2.
Table 2.
Supplement composition
| % of Total | Grams | |
|---|---|---|
| EAA | ||
| Histidine | 11 | 2.2 |
| Isoleucine | 10 | 2.0 |
| Leucine | 18 | 3.6 |
| Lysine | 16 | 3.2 |
| Methionine | 3 | 0.6 |
| Phenylalanine | 16 | 3.2 |
| Threonine | 14 | 2.8 |
| Valine | 12 | 2.4 |
| Total | 100 | 20 |
| Placebo | ||
| Alanine | 100 | 20 |
Twenty grams of supplement [essential amino acid (EAA) or placebo] was ingested 2 times/day, at 10 AM and 2 PM, for 7 days before total knee arthroplasty (TKA). Subjects then fasted overnight and surgery was performed the following day. Supplementation resumed on postsurgery day 1. Supplementation continued for 6 wk post-TKA. Each patient was issued a log book in which to record the time of supplement ingestion. This information was verified by research and nursing staff during the in-patient stay and by research staff at each visit.
Total Knee Arthroplasty
On the morning of surgery, subjects were admitted for surgery in a fasting state. Intravenous propofol was used to induce general anesthesia and was maintained with inhalational anesthetic (either desflurane or sevoflurane), with or without muscle relaxant (rocuronium bromide). A 10-cm-wide Zimmer tourniquet was positioned around the proximal third of the thigh and inflated to 300 mmHg to ensure minimal blood flow to the operated leg. After completion of the main components of the surgery, the tourniquet was deflated, allowing for reperfusion of the limb with blood. Total tourniquet time was recorded for each subject. Before closure of the surgical site, a solution containing robivacaine (125 mg), clonidine (40 µg), and ketorolac (15 mg) dissolved in normal saline (20 ml) was injected into the surrounding soft tissues (muscles).
Muscle Tissue Collection
Biopsies were taken from the vastus lateralis of the operative and nonoperative legs at 1) the time of surgery, before tourniquet inflation, and 2) either 1 wk or 2 wk postsurgery. All preoperative study-related functional testing and activities were completed 2 to 6 wk before surgery. Postoperative functional testing and activities were performed (at the earliest) at the 2-wk juncture. Patients were randomly assigned to the 1- or 2-wk groups. Subjects who were randomized to the 2-wk postbiopsy had their biopsy performed before functional testing. All biopsies (pre and post) were obtained in the morning after an overnight fast. However, although no study-related physical testing was performed in the days/hours before obtaining the biopsies, we did not explicitly standardize activity in the immediate days before surgery and 1- and 2-wk sampling time points. Tissues for histology were flash-frozen in liquid nitrogen-cooled isopentane, mounted on cork, and stored at −80°C.
Tissue Preparation for Histology
Tissue sections were cut at 7 µm with a Leica Cryostat (Leica CM 1850UV), mounted on glass slides, air-dried for 1 h, fixed in cold acetone for 7 min, and then rinsed in PBS. Slides were either stained immediately or stored at −80°C until ready for staining. Slides stored at −80°C were air-dried for 1 h at room temperature before staining. Tissue sections were blocked in BSA for 1 h and incubated in primary antibodies overnight at 4°C, and an appropriate secondary antibody was added for 2 h at room temperature.
Imaging and Analysis
Tissue sections were imaged with a Leica fluorescence microscope (DM4000B) equipped with a Leica camera (DFC 360FX). Imaging was performed at ×20 magnification unless otherwise noted. Multiple images of each section were taken systematically to avoid imaging the same region of the section twice. Sections were coded to ensure that the researcher performing the imaging was blinded to treatment group and time point.
Satellite Cells/Fiber and Satellite Cell Proportions
For the satellite cell analysis, a laboratory member blinded to patient identity, treatment allocation, and the timing of the biopsy performed the analysis. A custom routine was developed in ImageJ (National Institutes of Health, Bethesda, MD) to generate a unique numerical identifier for each muscle fiber; this enabled us to track multiple variables (cross-sectional area, satellite cell numbers, fiber type, myonuclei, and centrally located nuclei) and assign the variables to the particular cell based on its identifier. Adobe Photoshop was used to overlay the image with the identifier onto the satellite cell image, and the number of satellite cells associated with each fiber was recorded in a Microsoft Excel spreadsheet.
Pax7+ [Developmental Studies Hybridoma Bank (DSHB); 1:20] was used to label satellite cells. For detection of this antibody, a signal amplification procedure (tyramide signal amplification kit; Thermo Fisher Scientific, Waltham, MA) was performed as follows: the Pax7 primary antibody was tagged with a biotin-conjugated secondary, which was amplified with streptavidin-conjugated horseradish peroxidase (HRP), which, in turn, reacted with Alexa Fluor 555 tyramide to produce enhanced fluorescent signal. Pax7 cells were counted manually and were identified as being associated with Type I or II fibers on the basis of their colocalization with MyHC1 fibers. An MyHC1 antibody (BA-D5, 1:75; Developmental Studies Hybridoma Bank, Iowa City, IA) was used to label Type I fibers. An Alexa Fluor 647-conjugated secondary (Thermo Fisher Scientific; 1:500) was used to label the MyHC1 antibody. Fibers and nuclei were counted manually. Fibers not marked by the MyHC1 antibody were inferred to be Type II fibers. The satellite cell proportion equation was as follows: [satellite cell/(satellite cell + myonuclei)] × 100.
Myonuclei/Fiber and Central Nuclei/Fiber
To determine the number of nuclei per fiber, we used DAPI to label nuclei and BA-D5 antibody (DSHB; 1:75) and an anti-mouse Alexa Fluor 647 secondary antibody (Thermo Fisher Scientific; 1:500) to label Type I fibers. Fibers and nuclei were counted manually. Fibers not marked by BA-D5 were inferred to be Type II fibers. The laminin border was used to determine the fiber with which an individual nuclei was associated. Myonuclear domain was calculated as the mean number of myonuclei per mean cross-sectional area. DAPI-positive cells within but not touching the laminin border were counted as centrally located nuclei.
Capillaries/Fiber
To determine the number of capillaries, blood vessels were labeled with rhodamine-conjugated Ulex europaeus agglutinin (1:200; Vector Laboratories, Burlingame, CA), a lectin associated with blood vessels. We costained with a smooth muscle actin antibody (sc130616, 1:200; Santa Cruz Biotechnology, Dallas, TX) and considered blood vessels expressing smooth muscle actin to be arterioles and, thus, did not count such costaining vessels in the total capillary count. We used laminin (1:1,000; DAKO, Glostrup, Denmark) to determine total fiber numbers. The Capillary-to-Fiber Perimeter Exchange index (CFPE index) was determined in the same way as previous reports (28).
Macrophages
For determination of the number of M1 macrophages, sections were labeled with an antibody to cd86 (no. 53004; 1:200; Abcam, Cambridge, MA), which is considered one of the most reliable markers of M1 macrophages (27) and Alexa Fluor 488 secondary antibody (1:500; Thermo Fisher Scientific). An antibody to cd163 (Abcam no. 156769; 1:200) and Alexa Fluor 555 secondary antibody (1:500; Thermo Fisher Scientific) was used to mark M2c cells. This marker has been traditionally used as an M2 marker, but there is evidence it may label a small subset of other cell types and may not label all M2 cells (4, 32). We used the color thresholding tool in ImageJ to determine the area of overlapping expression between the macrophage marker (either cd86 or cd163) and laminin, thereby normalizing macrophage cell area to overall area of extracellular matrix (which was done by calculating the area occupied by cd86 or cd163 and dividing that area by the area of laminin staining).
Fibroblasts
Fibrotic tissue deposition was examined using TE7 antibody (CBL271, 1:200; EMD Millipore, Burlington, MA). The sections were costained with laminin (1:1,000; DAKO). For measurement of TE7 area, we used the Color Thresholding tool in ImageJ to calculate the area of the image occupied by positive TE7 staining that did not overlap with laminin.
TUNEL+ Cells/Fiber
The number of apoptotic cells was determined using a kit based on the TUNEL method (in situ cell death detection kit; fluorescein; Roche Diagnostics, Minneapolis, MN) following the manufacturer’s instructions. Sections were colabeled with laminin (1:1,000; Developmental Studies Hybridoma Bank) and Alexa Fluor 555 secondary antibody (1:500; Thermo Fisher Scientific) to determine total fiber count.
NCAM/Fiber
To determine the number of fibers expressing neural cell adhesion molecule (NCAM), we used an NCAM antibody (C134863,1:200; LSBio, Seattle, WA) tagged with an Alexa Fluor 555 secondary antibody (1:500; Thermo Fisher Scientific), and the section was costained with laminin (1:1,000; DAKO) to determine total fiber count.
Cytokine Expression Analysis
RNA preparation.
Tissue samples were frozen in liquid nitrogen and stored at −80°C until use. Approximately 50 mg of tissue was weighed and homogenized in 500 µL TRIzol (ThermoFisher, Waltham, MA) using a Spex Geno/Grinder (SPEXSamplePrep; Spex, Liberty, NJ) at 1,500 rpm. Total RNA was purified from tissue homogenate using a Zymo Direct-zol Miniprep kit (Zymo Research, Irvine, CA) and eluted into 50 μL of RNase-free water. RNA quantification was measured on a SpectraMax M5e plate reader (Molecular Devices, San Jose, CA). RNA quality assessment was determined using a Fragment Analyzer (Advanced Analytical, Ames, IA).
Library preparation and sequencing.
Libraries were prepared using the KAPA Stranded mRNA-Seq kit (KAPA Biosystems, Wilmington, MA). Quantity of the libraries was measured in triplicate quantitative PCR using KAPA complete library quantification kit (part no. KK4835) on the StepOnePlus real-time PCR System (Thermo Fisher Scientific). Sequencing was performed with an Illumina HiSeq 500 (Illumina, San Diego, CA). Approximately 400 M single-end, 100-bp reads were generated per sample.
Bioinformatics.
Raw reads were aligned and converted into Ensembl IDs using STAR, which was also used to limit gene counts to protein-coding genes. DESeq2, a package within the statistical package R, was used to determine differential expression across groups.
Muscle Volume, Physical Activity, and Caloric Intake
Detailed methods for muscle volume acquisition and analysis, physical activity status, and caloric intake have been previously published for this sample (15). MRI of both lower extremities was performed with use of a 3T Skyra scanner (Siemens) at the University of Oregon Lewis Center for Neuroimaging. Medical Image Processing, Analysis, and Visualization (or MIPAV) software (v. 7.4.0, https://mipav.cit.nih.gov) was used to calculate midthigh muscle volume. Patients wore an accelerometer for 21 consecutive days, from 1 wk preoperatively through 2 wk postoperatively, as well as between 5 and 6 wk postoperatively. Three-day food diaries on standardized days were completed before surgery, at 1 and 2 days postoperatively, and at 1, 2, and 5 wk postoperatively. Food Processor Nutrition Analysis software (ESHA Research, Salem, OR) was used to calculate caloric intake and other micronutritional and macronutritional measures.
Statistical Analyses
Statistical analysis was performed using SPSS (v. 19.0; IBM, Armonk, NY) software. Descriptive statistics were calculated for all variables to describe the sample and ensure that distributions met the assumptions of the planned analyses. Analysis of covariance models was specified to test for significant treatment group differences and change in muscle histology before and after surgery; separate models were conducted for each variable, and the postsurgery models covaried the presurgery values. In all, we conducted 32 significance tests. With 32 tests, we expected between 0 and 4 false positives (95% confidence bound). Specifically, we anticipated a 48% chance of two or more errors, a 21% chance of three or more errors, and a 7.4% chance of four or more errors.
RESULTS
Demographics and Sample Description
Subject baseline characteristics are provided in Table 2. Data were collected from a total of 41 participants (EAAs = 19; Placebo = 22) and were analyzed by original group assignment (see Fig. 1 for CONSORT diagram and Fig. 2 for the study design). The sample was 66% female and 98% Caucasian, average age was 64 ± 1 yr (means ± SE), average baseline body mass index was 30.21 ± 1.22 kg/m2, and average tourniquet time was 54.63 ± 3.65 min. Treatment groups did not differ significantly for these variables (all P > 0.05).
Physical Activity and Protein Intake
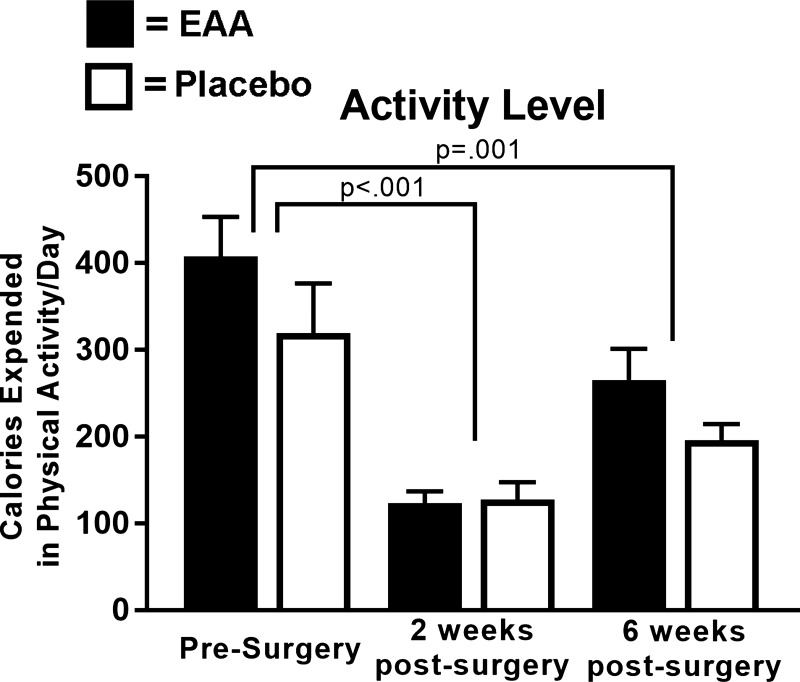
Mean daily calories expended in physical activity, measured by accelerometry, for all subjects overall was reduced by ~65% at 2 wk (P < 0.001) and by ~36% at 6 wk (P = 0.001) from preoperative levels (Fig. 3). The Placebo and EAAs Groups did not significantly differ in terms of average energy expended in physical activity preoperatively (319.71 ± 56.97 compared with 408.15 ± 45.37 kcal/day; P = 0.24) or at 2 and 6 wk postoperatively after covarying baseline values (Fig. 3). Mean daily nutritional protein intake at baseline did not significantly differ across the two groups (P = 0.87), averaging 98.62 ± 13.70 g/day for the Placebo Group and 101.77 ± 12.88 g/day for the EAAs Group—or at 2 and 6 wk postoperatively after covarying baseline values. Across the two groups, protein intake declined significantly from baseline to 2 wk postoperatively (P < 0.05 for all), but not from baseline to 6 wk postoperatively (P > 0.05 for all). These and other outcomes are detailed in a prior report (15).
Fig. 3.
Group activity levels. The histogram shows change in daily calories expended in physical activity for individuals in the EAA and Placebo Groups from presurgery to 2- and 6 wk postsurgery. The histogram presents mean values by group at the three time points.
Muscle Histology
Distributions of the muscle histology variables in this study were normal, with skewness and kurtosis well within assumptions for the statistical tests. Histology variables that measured similar constructs were generally moderately to highly correlated with each other, but typically had low and nonsignificant correlations with other histology variables, providing evidence of discrimination.
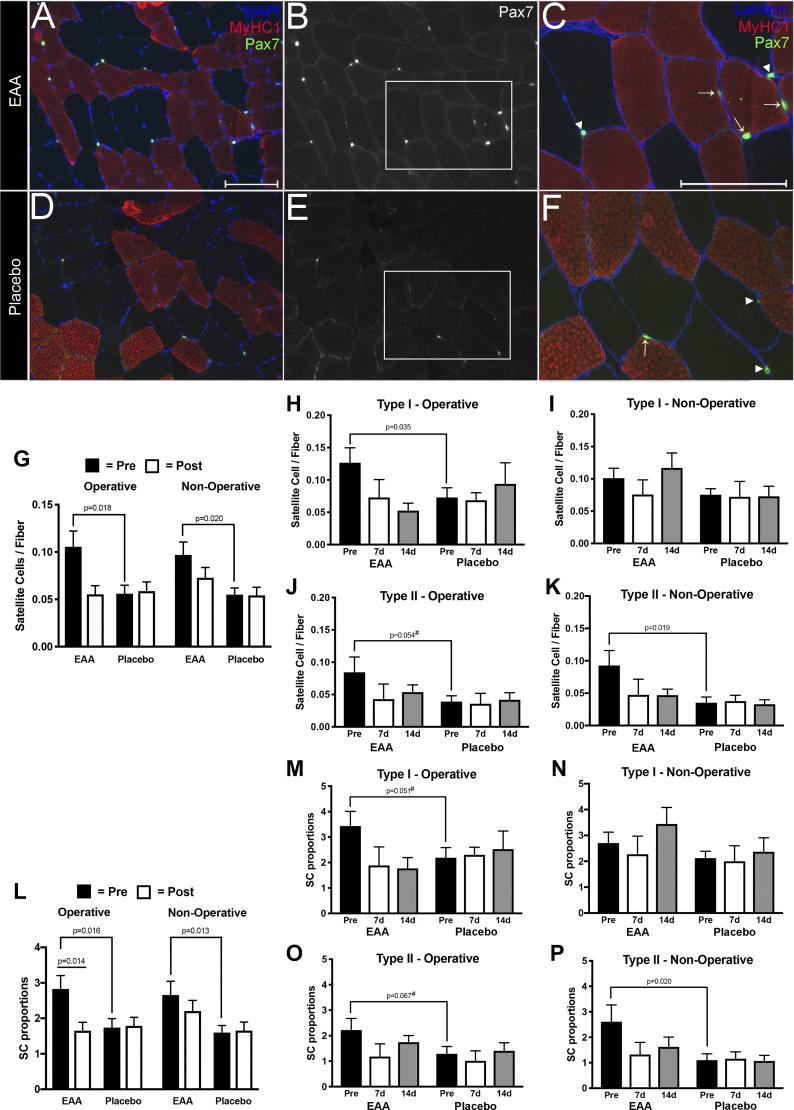
For the satellite cell analysis, we processed and analyzed tissue sections, as previously described (13, 40), with modifications based on methods from Fry et al. (25, 26). Table 3 summarizes the number of muscle fibers by fiber type, treatment group, and time point. Figure 4, A–F shows representative images of muscle biopsies from which we obtained our satellite cell counts. Figure 4, G–P presents results of satellite cell analyses, comparing Pax7 expression between Placebo and EAAs Groups. Overall, the Placebo Group had relatively flat levels over time, while the EAAs group had higher values preoperatively that declined to the level of the Placebo Group at follow-up. Before surgery, the EAAs Group had a significantly higher number of satellite cells per muscle fiber in the operative leg for both Type I (Fig. 3H; P = 0.03) and Type II (Fig. 3J; P = 0.05). This pattern was repeated in the nonoperative leg; the difference was statistically significant for Type II satellite cells (Fig. 3K; P = 0.02), but not for Type I satellite cells (Fig. 3I). Satellite cell proportions showed similar change between groups before surgery in the operative leg (Fig. 3M; P = 0.05 for Type I) and in the nonoperative leg (Fig. 3P; P = 0.02 for Type II).
Table 3.
Number of fibers analyzed by fiber type and treatment group for Pax7 analysis
| Operative | EAA |
Placebo |
Nonoperative | EAA |
Placebo |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Subjects | Average Fiber/Subject | SE | Number of Subjects | Average Fiber/Subject | SE | Number of Subjects | Average Fiber/Subject | SE | Number of Subjects | Average Fiber/Subject | SE | |
| Baseline MHC I | 18 | 61.4 | 12.18 | 21 | 55.9 | 5.87 | 18 | 63.5 | 6.72 | 21 | 47 | 6.33 |
| Baseline MHC II | 18 | 50.55 | 13.05 | 21 | 66 | 7.73 | 18 | 49.06 | 7.19 | 21 | 64 | 7.3 |
| 1 wk MHC I | 7 | 46.57 | 7.11 | 7 | 34 | 8 | 7 | 40 | 11.48 | 9 | 30 | 5 |
| 1 wk MHC II | 7 | 23.09 | 8.73 | 7 | 83 | 8 | 7 | 67.4 | 5.87 | 9 | 56 | 11 |
| 2 wk MHC I | 8 | 60.33 | 11.46 | 5 | 53 | 10.67 | 10 | 48.14 | 6.53 | 7 | 47 | 9.63 |
| 2 wk MHC II | 8 | 66.67 | 8.2 | 5 | 78 | 8.66 | 10 | 49 | 6.24 | 7 | 90 | 20.92 |
EAA, essential amino acids; MHC, myosin heavy chain.
Fig. 4.
Satellite cell content in vastus lateralis tissue of study patients. Representative images from vastus lateralis biopsy sections of EAA (A–C) and Placebo (D–F) Group subjects. Scale bars indicate 150 µm. A and D: satellite cells labeled with anti-Pax7+ (green), fiber types labeled with anti-MyHC Type I (red), and nuclei labeled with DAPI (blue). B and E: Pax7+ cells alone. C and F: expanded view of the lower right corner of the adjacent images with Pax7+ (green), the extracellular matrix surrounding each muscle cell labeled with anti-laminin (blue), and MyHC1 (red). Arrows indicate Pax7+ cells associated with Type I fibers. Arrowheads show Pax7+ cells associated with Type II fibers. G: histograms of satellite cells/fiber in the EAA versus Placebo groups at presurgery versus combined 1- and 2 wk postsurgery in the operative and nonoperative legs. Histograms show satellite cells per Type I fiber in the operative leg (H) and nonoperative leg (I) presurgery and at 1 and 2 wk postsurgery. Histograms show satellite cells per Type II fiber in the operative leg (J) and nonoperative leg (K) leg at baseline and at 1 and 2 wk. L: histograms of satellite cell proportions in the EAA versus Placebo groups presurgery versus combined 1 and 2 wk postsurgery in the operative and nonoperative legs. Histograms show satellite cell proportions per Type I fibers in the operative leg (M) and the nonoperative leg (N) presurgery and at 1 and 2 wk postsurgery. Histograms show satellite proportions per Type II fiber in the operative leg (O) and nonoperative leg (P) presurgery and at 1 and 2 wk postsurgery. *Significance (P) values represent the treatment group main effect in separate analyses of (co)variance. Presurgery values were covaried in models evaluating postsurgery treatment group differences. Values denoted with a # symbol trended toward significance (had a P value between 0.051 and 0.09).
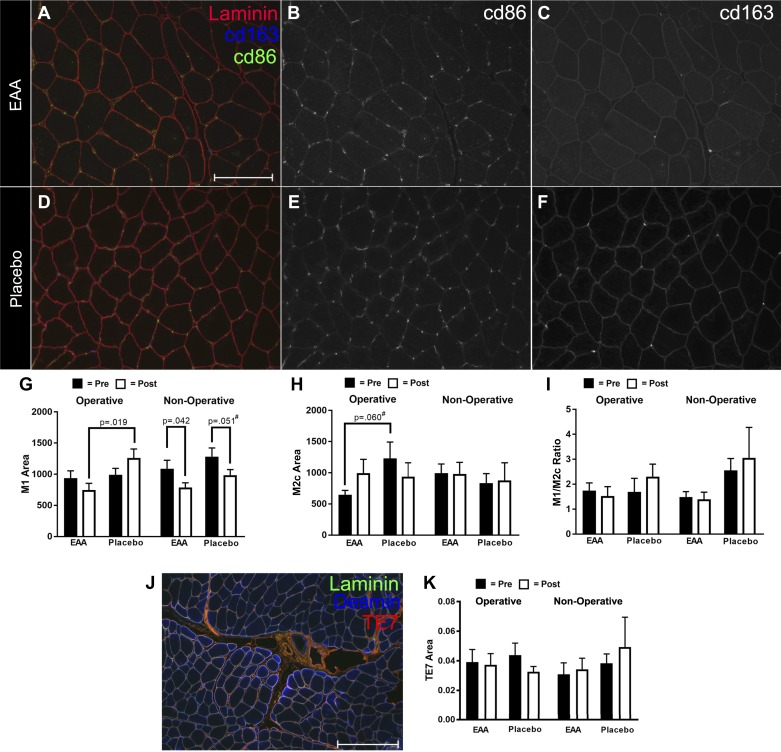
Representative images used for the macrophage analysis are shown in Fig. 5, A–F. Figure 5, G–I presents the results of the analysis, comparing M1 and M2c expression between Placebo and EAAs Groups. EAAs and Placebo had similar M1 area before surgery in the operative leg; however, postsurgery, the M1 area decreased in the EAAs Group and increased in the Placebo Group (Fig. 5G). There was a significant difference (P = 0.019) between Placebo and EAAs Groups postsurgery (Fig. 5G). In the nonoperative leg, both EAAs and Placebo Groups had less M1 area postsurgery (P = 0.042 for EAAs and P = 0.051 in Placebo). M2c area was elevated in the operative leg in the Placebo group compared with the EAAs Group before surgery (Fig. 5H); however, the difference only trended toward significance (P = 0.060). M2c area was flat in both groups in the nonoperative leg (Fig. 5H). Given the link between macrophages and fibrotic tissue deposition, we assessed the amount of fibrotic tissue in our subjects (Fig. 5, J–K). No difference in fibrotic tissue was detected (Fig. 5K).
Fig. 5.
Macrophage content in vastus lateralis tissue of study patients. Representative images of tissue sections from EAA (A–C) and Placebo (D–F) study subjects. Scale bar indicates 150 µm. In A and D, M1 macrophages are labeled with anti-cd86 (green). M2c macrophages are labeled with anti-cd163 (blue). The extracellular matrix is labeled with anti-laminin2 (red). In B and E, cd86-positive staining is shown. In C and F, cd163 is shown. G: histogram showing the changes in M1 area normalized to the area of the extracellular matrix marked with laminin. Black bars indicate average area of the baseline time point, and white bars indicate the combined average of the 1- and 2-wk time points. H: histogram showing the changes in M1 area normalized to the area of the extracellular matrix marked with laminin. Black bars indicate the average area of the baseline time point, and white bars indicate the combined average of the 1- and 2-wk time points. I: ratio of M1 to M2c. J: representative image used for assessment of fibrotic tissue, with TE7 (red), desmin (blue), and laminin (green). K: histogram showing measurements of TE7 area in the various experimental conditions. *Significance (P) values represent the treatment group main effect in separate analyses of (co)variance. Presurgery values were covaried in models evaluating postsurgery treatment group differences. Values denoted with a # symbol trended toward significance (had a P value between 0.051 and 0.099).
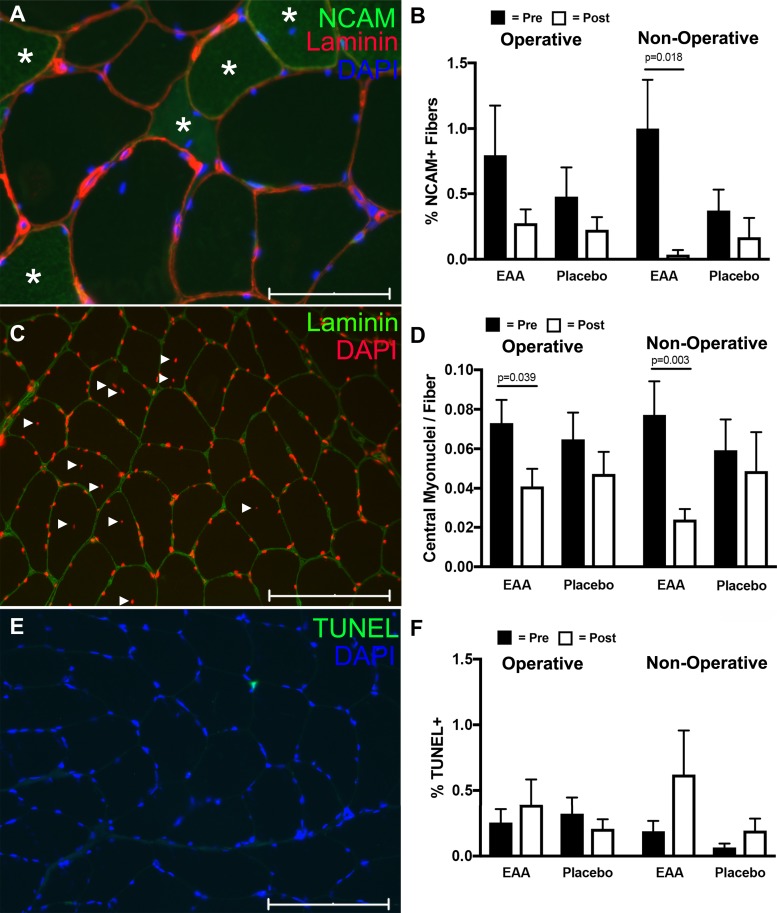
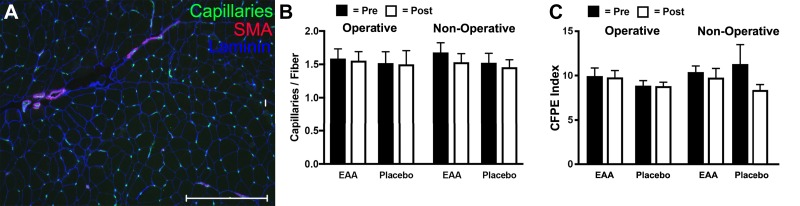
In additional exploratory analyses conducted with other key histology variables (e.g., capillaries/fiber, CFPE index), central myonuclei/fiber, NCAM+ fibers, and percentage of TUNEL positive), there were no significant differences between groups, either presurgery or postsurgery, likely because of the small sample size in the postoperative tissues. These results are summarized in Figs. 6 and 7.
Fig. 6.
NCAM+ fibers, TUNEL and central nuclei assessment in vastus lateralis biopsies of study subjects. A: representative image of NCAM+ fibers (green; positive fibers are marked with an *). The image is costained with laminin (red) and DAPI (blue). Scale bar is 75 µm. B: histogram showing NCAM+ fibers in the various experimental conditions. C: representative image used for determining central nuclei number. Nuclei are labeled with DAPI (red) and laminin (green) marks the extracellular matrix. Solid triangles indicate central myonuclei. Scale bar is 150 µm. D: histogram showing central nuclei distribution in the various experimental conditions. E: TUNEL-positive cell (green) counterstained with DAPI (blue). Scale bar is 150 µm. F: histogram showing the distribution of TUNEL cells across the experimental conditions. *Significance (P) values represent the treatment group main effect in separate analyses of (co)variance. Presurgery values were covaried in models evaluating postsurgery treatment group differences.
Fig. 7.
Capillary numbers per fiber in vastus lateralis biopsies of study subjects. A: representative image of capillaries labeled with Ulex europeaus agglutinin (green), smooth muscle actin (red), and laminin (blue). Scale bar is 300 µm. B: histogram showing the relative numbers of capillaries per fiber across the experimental conditions. C: histogram summarizing the capillary-to-fiber perimeter exchange (CFPE) index across the experimental conditions. *Significance (P) values represent the treatment group main effect in separate analyses of (co)variance. Presurgery values were covaried in models evaluating postsurgery treatment group differences.
Gene Expression Analyses
Cytokine gene expression was measured in our subjects using Next Generation Sequencing. A list of key cytokines and their expression levels is summarized in Table 4. Of the cytokines analyzed, IL-6 and TNF-α showed significantly altered expression after surgery. At 2 wk, IL-6 expression was significantly elevated relative to baseline (P = 0.0147 in Placebo and P = 0.030 in EAAs). TNF-α was not elevated in Placebo at 1 wk but was elevated at 2 wk (P = 0.004). In contrast, TNF-α was elevated in the EAAs Group at 1 wk (P = 0.001) but was not elevated at 2 wk.
Table 4.
Cytokine and myogenic regulatory factor expression profiles
| One Week, Operative |
Two Week, Operative |
One Week, Nonoperative |
Two Week, Nonoperative |
|||||
|---|---|---|---|---|---|---|---|---|
| Altered | log2 Fold Change | Adjusted P Value | log2 Fold Change | Adjusted P Value | log2 Fold Change | Adjusted P Value | log2 Fold Change | Adjusted P Value |
| IL-6 | ||||||||
| Placebo | 1.98 | 0.15 | 3.102 | 0.015 | 2.002 | 0.474 | 2.252 | 0.99988 |
| EAA | −0.601 | 0.749 | 2.894 | 0.03 | 0.448 | NA | 0.391 | 0.99996 |
| TNFα | ||||||||
| Placebo | −0.322 | 0.847 | 2.954 | 0.004 | 0.189 | 0.974 | −0.370 | 0.99988 |
| EAA | 3.095 | 0.001 | 1.359 | 0.262 | −0.752 | NA | 0.062 | 0.99996 |
| Myogenin | ||||||||
| Placebo | 0.599 | 0.168 | 0.954 | 0.022 | −0.173 | 0.917991 | 0.027 | 0.99988 |
| EAA | 1.030 | 0.007 | 1.131 | 0.008 | 0.335 | 0.999576 | 0.247 | 0.999963 |
| MyoD | ||||||||
| Placebo | 0.830 | 0.039 | 1.043 | 0.011 | −0.041 | 0.985121 | 0.128 | 0.99988 |
| EAA | 0.597 | 0.146 | 0.594 | 0.210 | −0.165 | 0.999576 | −0.014 | 0.999963 |
| MRF4 | ||||||||
| Placebo | 0.822 | 0.026 | 0.577 | 0.168 | 0.359 | 0.738109 | 0.106 | 0.99988 |
| EAA | 0.803 | 0.027 | 0.485 | 0.286 | 0.239 | 0.999576 | 0.015 | 0.999963 |
| Unchanged | log2 F Change | Adjusted P Value | log2 Fold Change | Adjusted P Value | log2 Fold Change | Adjusted P Value | log2 Fold Change | Adjusted P Value |
|---|---|---|---|---|---|---|---|---|
| IFNγ | ||||||||
| Placebo | −1.141 | NA | 2.997 | 0.2 | 0.08 | NA | 1.558 | 0.99988 |
| EAA | 2.607 | 0.236 | 0.249 | 0.946 | −0.068 | NA | 3.365 | 0.99996 |
| IL-12 | ||||||||
| Placebo | 0.426 | 0.683 | 1.146 | 0.174 | −0.195 | 0.967 | −0.645 | 0.99988 |
| EAA | 0.416 | 0.658 | −0.211 | 0.867 | 0.402 | NA | 0.15 | 0.99996 |
| IL-4 | ||||||||
| Placebo | −0.77 | NA | 0.777 | NA | 0.007 | NA | −0.017 | 0.99988 |
| EAA | −0.798 | NA | −1.087 | NA | 0.084 | NA | −0.087 | 0.99996 |
| GM-CSF | ||||||||
| Placebo | 0.216 | NA | −0.34 | NA | 0.007 | NA | −0.017 | 0.99988 |
| EAA | −0.022 | NA | 0.925 | NA | 0.101 | NA | −0.391 | 0.99996 |
| IL-10 | ||||||||
| Placebo | 0.841 | NA | 0.632 | NA | −0.37 | NA | −0.032 | 0.99988 |
| EAA | −0.039 | NA | 1.699 | NA | −0.652 | NA | −0.278 | 0.99996 |
| IL-17A | ||||||||
| Placebo | −0.324 | NA | 0.506 | NA | 0.007 | NA | −0.017 | 0.99988 |
| EAA | −0.039 | NA | −0.328 | NA | 0.405 | NA | −0.087 | 0.99996 |
| IL-1A | ||||||||
| Placebo | −0.645 | NA | 0.146 | NA | 0.044 | NA | −0.268 | 0.99988 |
| EAA | 0.113 | NA | 0.786 | NA | 0.084 | NA | 0.201 | 0.99996 |
| IL-1B | ||||||||
| Placebo | 0.893 | 0.526 | 1.442 | 0.264 | 0.211 | 0.97 | 0.983 | 0.99988 |
| EAA | −1.113 | 0.419 | 1.564 | 0.188 | 0.341 | NA | −0.982 | 0.99996 |
| IL-8 | ||||||||
| Placebo | 1.43 | 0.345 | 1.801 | 0.229 | 0.375 | 0.957 | 1.293 | 0.99988 |
| EAA | −0.051 | 0.98 | −0.527 | 0.802 | 1.296 | NA | 1.648 | 0.99996 |
| Myf5 | ||||||||
| Placebo | −0.305 | 0.536 | −0.229 | 0.674 | −0.279 | 0.851 | 0.073 | 0.99988 |
| EAA | −0.149 | 0.773 | −0.037 | 0.955 | −0.063 | 0.9996 | 0.059 | 0.999963 |
| Pax7 | ||||||||
| Placebo | −0.602 | 0.178 | −0.246 | 0.664 | −0.414 | 0.726 | 0.020 | 0.99988 |
| EAA | −0.428 | 0.341 | −0.369 | 0.491 | −0.172 | 0.9996 | −0.061 | 0.999963 |
| Myostatin | ||||||||
| Placebo | 0.366 | 0.567 | −0.494 | 0.425 | 0.465 | 0.749 | −0.316 | 0.99988 |
| EAA | 0.788 | 0.121 | 0.354 | 0.607 | 0.409 | 0.9996 | 0.238 | 0.999963 |
mRNA expression levels of cytokines and myogenic regulatory factors were measured using RNA-seq. log2 fold change and the adjusted P values relative to baseline for the various experimental conditions are shown. NA indicates values for which there was no change in expression. EAA, essential amino acids. Values in bold indicate significant difference.
Myogenic regulatory factor expression was also measured (summarized in Table 4). In the Placebo Group at 1 and 2 wk, MyoD was significantly upregulated (P = 0.08 and P = 0.022, respectively), but no change in MyoD was observed in EAAs. In the EAAs Group, a significant upregulation from baseline to 1 wk was measured for myogenin (P = 0.007), but no change from baseline to 1 wk was observed in myogenin expression in the Placebo Group. Myogenic regulatory factor 4 (MRF4) expression at 1 wk was significantly altered in both the EAAs (P = 0.027) and Placebo (P = 0.026) Groups, but no change from baseline was observed in MRF4 expression at 2 wk.
DISCUSSION
To understand how muscle is preserved in TKA patients receiving EAA supplementation, immunofluorescence methods were used to characterize tissue- and cell-level differences in muscle biopsies obtained from TKA patients who recently completed a randomized, double-blind, placebo-controlled trial. One important finding was that twice daily EAAs for 7 days leading up to surgery increased satellite cell numbers, and proportions in both the operative and nonoperative legs compared with the Placebo Group. Interestingly, the EAA-induced preoperative increase in satellite cells was not maintained after surgery despite continued supplementation. Another notable finding, and potentially more relevant to atrophy prevention, was the difference in M1 macrophages between groups in the operative leg and the timing of cytokine expression favoring subjects in the EAA Group.
Muscle Stem Cells
After 7 days of supplementation, satellite cell numbers at the day of surgery were elevated in the EAA Group compared with Placebo and were higher than in previous reports in aged, healthy subjects. For example, in men aged 70 ± 4 yr (means ± SE), McKay et al. (36) reported that myosin heavy chain (MyHC) Type I fiber satellite cell numbers were 0.060, and Snijders et al. (47) found satellite cell numbers to be 0.078 in men aged 73 ± 4 yr. In our study, satellite cell numbers in Type I fibers from Placebo patients before surgery were 0.07 and 0.08 in the operative and nonoperative legs, respectively. This is in contrast to patients on EAAs treatment for 7 days leading up to surgery; in this group, in the operative and nonoperative legs, Type I satellite cell counts were 0.13 and 0.10, respectively. This difference between the EAAs and Placebo Groups was significant. For MyHC Type II fibers in the same older men, McKay et al. (36) and Snijders et al. (47) found satellite cell numbers to be 0.050 and 0.077, respectively. Values from our Placebo Group were 0.04 in both the operative and nonoperative legs. However, in the EAAs Group, satellite cell numbers in Type II fibers were 0.09 in both legs, a significantly higher value than in Placebo Group. These values also appear well above those reported by McKay et al. and Snijders et al. for older healthy adults in both MyHC Type I and II fibers. When satellite cells are expressed as a proportion of all myonuclei, the findings persist. Although patients in the EAAs Group have elevated satellite cell numbers and proportions before surgery, satellite cell numbers decline to values similar to Placebo at 7 and 14 days post-TKA. It is important to note, however, that while the EAA treatment appears to increase satellite cell numbers in MyHC Type I fibers relative to Placebo Group and older healthy adults (36, 47), the effect of EAAs on MyHC Type II fibers seems to increase satellite cells to approximately the same levels as in healthy, older adults. Notably, satellite cell values in Type II fibers in patients in the Placebo Group are considerably lower than in older healthy adults. However, it is in the EAAs group where satellite cell numbers in Type II fibers appear to normalize to that of healthy older adults, or slightly exceed this level, with treatment for 7 days leading up to surgery.
Whether enhanced satellite cell numbers contribute to atrophy prevention is an open question. Although EAAs increased satellite cell numbers in our study, there is conflicting literature on the relationship between satellite cell number and atrophic muscle. Disuse injury models have revealed the complexity of the relationship between muscle atrophy and satellite cells. In a transgenic mouse model of muscle atrophy, Fry et al. (26) depleted satellite cells and observed an insignificant effect on muscle atrophy. Interestingly, although they observed increased extracellular matrix accumulation (specifically increased collagen and glycosaminoglycan) in older mice that had been chronically depleted of satellite cells (26). Highlighting the interplay between fibroblasts and satellite cells, this study corroborated earlier work by the same group that suggested satellite cells regulate muscle cell microenvironment by signaling to fibroblasts and limiting their accumulation (25). In another study that also called into question the significance of satellite cells during muscle atrophy, Jackson et al. (30) observed in a rat hind limb suspension model that muscle growth occurred upon reloading, even without a contribution from satellite cells. These studies suggest satellite cells may not play a significant role in reducing atrophy. However, satellite cells can be activated in dynamic fashion in response to atrophic stimuli, as illustrated by Ferreira et al. (21), who observed an intense pulse of satellite cell proliferation following 6 h of hind limb suspension (in contrast to other durations that had significantly fewer satellite cells). This suggests that satellite cells do respond to atrophic stimuli but based on Fry and Jackson, it is unclear to what extent the satellite cell proliferation limits atrophy (or whether the kind of pulse of increased satellite cells that Ferreira observed is a common occurrence in muscle put under atrophic conditions). The Ferreira result also underscores the importance of looking at multiple time points to gain a comprehensive understanding of the satellite cell response. In human muscle, the satellite cell response during atrophy also appears to be complex, as varied results from the following studies suggest. Suetta et al. (50) found that satellite cell numbers increased following 14 days of bedrest; Verdijk et al. (52) found satellite numbers were reduced in spinal cord injury patients; and satellite cell numbers did not change following 14 days of immobilization (48) or after 28 days of bed rest (7). Taken together, these results imply that the satellite cell-proliferative response in humans during atrophy is highly context dependent and influenced by the severity and duration of injury/intervention.
Satellite cell number alone (as defined by Pax7+ cells) may not paint a complete picture of the myogenic potential in our muscle biopsies, as studies examining mRNA expression of myogenic regulatory factors (MRFs) in atrophy models suggest. For example, in Snijders et al. (48), although there was no change in satellite cell numbers in the immobilization study mentioned above, mRNA for the myogenic regulatory factor, myogenin [which is initiated in committed satellite cells (reviewed in Wang and Rudnicki (54)], was elevated after 14 days of immobilization, suggesting maturation of myogenic progenitors. Similarly, Brooks et al. (7) measured an increase in Pax7, MyoD, myogenin, and MRF4 mRNA in subjects that had been given EAAs during a 28-day bed rest followed by a 14-day recovery, but these subjects did not have elevated satellite cell counts. In light of these results, we measured MRF expression in our patients. In the EAAs Group in our study, myogenin mRNA expression was significantly elevated relative to baseline at both 1 wk and 2 wk after surgery. In the Placebo Group, myogenin was not elevated at 1 wk but was elevated at 2 wk. Given that myogenin acts in the later stages of myogenesis, these results imply that a larger proportion of the total muscle precursor pool in the biopsy tissue was in a more committed stage of myogenesis at 1 wk and thus, that myogenesis may be accelerated in the EAAs Group. Further supporting this interpretation is that in Placebo versus EAAs Group, MyoD expression [which is initially expressed in activated, proliferating satellite cells before myogenin expression (54)] is not elevated after surgery in the EAAs Group but is elevated in the Placebo Group at 1 and 2 wk. Therefore, the quantitative differences in satellite cells 7 days after the initiation of EAA supplementation and the altered expression of MRFs suggest that EAAs alters myogenic potential in this tissue.
The activation state of satellite cells, and not simply total number, should also be considered when assessing the response of satellite cells during muscle atrophy and growth, and how these responses are influenced by amino acid supplementation. We have attempted to characterize satellite cell activation state by measuring expression of phosphorylated ribosomal protein S6 (p-rpS6), an indicator of mTORC1 activation. p-rpS6 is expressed in satellite cells in our subjects in a manner similar to what has been seen in activated satellite cells in mice (45). However, in this subject pool, no significant between-group difference in intensity of p-rpS6 expression (data not shown) was observed. However, we would expect that p-rpS6 activation would happen in a more acute fashion (hours or days) than in the current study (EAA ingestion began 7 days before baseline biopsies), so we were not entirely surprised that we did not detect a difference between groups. Our future work aims to examine p-rpS6 expression at time points shortly after the start of supplementation in TKA patients. We have also examined whether cell proliferation is influenced by supplementation but were unable to detect a difference in Ki67 expression (data not shown); however, this is very likely also to be an issue of timing of the biopsy occurring after activation and division of satellite cells.
Inflammation
Muscle recovery following injury is also mediated by macrophages, cells of the immune system that have traditionally been considered to fall into two general categories with competing actions, referred to as M1 and M2 macrophages (35). Presurgery, M1 macrophages were roughly equivalent in the two groups in this study. The canonical role of M1 macrophages is to mediate cytotoxicity, and these cells are active during tissue injury. M1 cells in the operative leg of the Placebo Group increased following surgery relative to the EAAs Group, which had decreased M1 cells after surgery. The difference in M1 area postsurgery between the EAAs and Placebo Group in the operative leg was significant (Fig. 5G; P = 0.019). Because the amount of M1 cells does not necessarily provide a complete picture of the inflammatory response, we examined cytokine expression from muscle biopsies, which contains multiple cell types that can potentially produce cytokines. Inflammation is an acute response to muscle damage elicited by TNF-α from M1 macrophages (33) and other cells within muscle tissues. TNF-α, secreted by M1 cells and resident immune cells, is abundantly and rapidly produced in response to injury. Comparing the expression level of TNF-α between treatment groups, we found it increased after surgery in the operative leg such that in the EAAs Group, TNF-α expression was elevated at 1-wk postsurgery but then declined by 2 wk. In contrast, expression in the Placebo Group was not significantly elevated at 1 wk but was significantly elevated at 2 wk. These temporal differences between treatment groups in TNF-α expression, as well as the difference in prevalence of M1 cells, are indications that the immune response is different between groups. In a study involving older subjects on a reduced activity program, muscle atrophy correlated with elevated TNF-α expression, suggesting that elevated TNF-α may be an important component of reduced activity-associated atrophy (and sarcopenia) (6). IL-6 did not increase in these subjects (6), unlike the subjects in our study, who had elevated IL-6 in both the EAAs and Placebo Groups. Because elevated IL-6 expression occurred in both groups, it is likely a consequence of surgery and not EAA consumption. A potential significance of the temporal difference in TNF-α expression between Placebo and EAAs is that IL-6 and TNF-α are coexpressed at a high level at 2 wk in the Placebo Group but not in the EAAs Group (in which it is not changed relative to baseline at 2 wk). Combined upregulation of IL-6 and TNF-α is an acute response of muscle cells to LPS, a potent bacterially derived proinflammatory agent (24); thus, this coexpression may have a negative impact on muscles in our Placebo subjects. Further characterization of how essential amino acids influence TNF-α expression and the importance of IL-6/TNF-α coexpression may provide insight into how essential amino acids preserve muscle mass in older individuals following surgery and how inflammatory mediators interact with factors that promote recovery and/or reduce atrophy.
Recent work in subjects placed on an endurance exercise routine has shed new light on the connection between M2c macrophages and muscle preservation. M2 macrophages suppress the immune response, promote tissue repair, and produce cytokines such as IL-6 that promote recovery (33, 51). Hypertrophy following endurance exercise has been shown to correlate with elevated M2c macrophages, reduced IL-6, and elevated IL-4 (53). Essential amino acids have a similar effect with regard to muscle preservation as we have shown (15, 16), but our results differ from the endurance exercise subjects with regard to IL-6 and IL-4 expression (Table 4). Furthermore, our examination of M2c cells revealed a more complex response compared with the endurance exercise subjects. At baseline, there was significantly less M2c area in the EAAs Group versus the Placebo Group. However, the M1:M2c ratio showed a trend toward an M1 bias in the Placebo Group postsurgery (Fig. 5I), suggesting that EAAs may promote M2c over M1 in our patients. However, this ratio difference was not significant. Therefore, our data do not support a clear relationship between EAAs, M2c cells, and muscle preservation. One limitation of our study is that our marker for M2 cells, cd163, has been shown to mark only M2c cells, and, therefore, does not give a full picture of the entire M2 population (32).
Other Measures
The improvement in muscle preservation is unlikely to be due to changes in capillary numbers, CFPE index, or apoptosis, as these variables did not change during the 2 wk after surgery. Lastly, although there was a change in NCAM+ cells after surgery in the nonoperative leg of the EAAs group, it is not clear how this would have affected preservation of muscle in the operative leg.
We conclude that elevation in satellite cell numbers and proportions between the EAAs and Placebo Groups was significant presurgery. Given these results, we argue that EAAs causes an expansion of satellite cells in the treatment population. The significance of the preoperative satellite cell expansion on postoperative recovery remains to be determined; however, this result and the differences in MyoD and myogenin expression between the Placebo and EAAs Groups argue that essential amino acids influence myogenic potential. Potentially more important in terms of atrophy prevention was the differential response of M1 type macrophages and cytokine expression between groups in the operative leg. Across muscle histology variables, there was a consistent pattern of the EAAs Group having greater changes in means from presurgery to follow-up relative to the Placebo Group. In general, the pattern was that means in the Placebo Group stayed relatively flat over time, while the EAAs Group started with higher levels and declined over time to reach the Placebo level. Results of this study are robust and provide potential mechanisms by which muscle atrophy following TKA may be mitigated and show for the first time how EAAs may stimulate satellite cells, alter the immune response, and potentiate recovery in older adults after major surgery. Future research is needed to document whether EAAs-induced satellite cell expansion before surgery is critical for postoperative atrophy prevention, and whether EAAs work on satellite cells independently of muscle cells (e.g., PI3K/mTORC1 pathways to attenuate autophagy).
Summary
This study presents novel results showing that pre-TKA satellite cell numbers and proportions are positively affected by 7 days of twice-daily ingestion of 20 g of essential amino acids occurring in the lead-up to surgery, and in both the operative and nonoperative legs. Further, we show that changes in inflammatory regulators after surgery may be influenced by EAAs during the acute and subacute period during recovery. Building on two clinical trials demonstrating that essential amino acids are effective in mitigating postoperative muscle atrophy following TKA (15, 16), this report provides potential mechanistic evidence that EAA supplementation has differential effects on the muscle tissue microenvironment before and after surgery, which may be of benefit during recovery. Further studies are needed to achieve conclusive results on the role of satellite cells and inflammation in older adults before and after major surgery.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.C.D. conceived and designed research; J.B.M., D.M.F., N.J.B., T.K.K., B.A.L., S.N.S., C.G.M., and B.A.J. performed experiments; J.B.M., D.M.F., N.J.B., and L.A.S. analyzed data; J.B.M., L.A.S., K.S., E.C.O., and H.C.D. interpreted results of experiments; J.B.M. prepared figures; J.B.M., L.A.S., and H.C.D. drafted manuscript; J.B.M., L.A.S., T.K.K., E.C.O., and H.C.D. edited and revised manuscript; J.B.M., D.M.F., N.J.B., L.A.S., K.S., B.A.L., S.N.S., C.G.M., B.A.J., E.C.O., and H.C.D. approved final version of manuscript.
REFERENCES
- 1.Alway SE, Pereira SL, Edens NK, Hao Y, Bennett BT. β-Hydroxy-β-methylbutyrate (HMB) enhances the proliferation of satellite cells in fast muscles of aged rats during recovery from disuse atrophy. Exp Gerontol 48: 973–984, 2013. doi: 10.1016/j.exger.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Aquilani R, Zuccarelli Ginetto C, Rutili C, Pisano P, Pasini E, Baldissarro E, Verri M, Boschi F. Supplemented amino acids may enhance the walking recovery of elderly subjects after hip fracture surgery. Aging Clin Exp Res 31:157–160, 2019. doi: 10.1007/s40520-018-0941-x. [DOI] [PubMed] [Google Scholar]
- 3.Averous J, Gabillard JC, Seiliez I, Dardevet D. Leucine limitation regulates myf5 and myoD expression and inhibits myoblast differentiation. Exp Cell Res 318: 217–227, 2012. doi: 10.1016/j.yexcr.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 4.Barros MH, Hauck F, Dreyer JH, Kempkes B, Niedobitek G. Macrophage polarisation: an immunohistochemical approach for identifying M1 and M2 macrophages. PLoS One 8: e80908, 2013. doi: 10.1371/journal.pone.0080908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Branch LG, Wetle TT, Scherr PA, Cook NR, Evans DA, Hebert LE, Masland EN, Keough ME, Taylor JO. A prospective study of incident comprehensive medical home care use among the elderly. Am J Public Health 78: 255–259, 1988. doi: 10.2105/AJPH.78.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breen L, Stokes KA, Churchward-Venne TA, Moore DR, Baker SK, Smith K, Atherton PJ, Phillips SM. Two weeks of reduced activity decreases leg lean mass and induces “anabolic resistance” of myofibrillar protein synthesis in healthy elderly. J Clin Endocrinol Metab 98: 2604–2612, 2013. doi: 10.1210/jc.2013-1502. [DOI] [PubMed] [Google Scholar]
- 7.Brooks NE, Cadena SM, Vannier E, Cloutier G, Carambula S, Myburgh KH, Roubenoff R, Castaneda-Sceppa C. Effects of resistance exercise combined with essential amino acid supplementation and energy deficit on markers of skeletal muscle atrophy and regeneration during bed rest and active recovery. Muscle Nerve 42: 927–935, 2010. doi: 10.1002/mus.21780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science 302: 1575–1577, 2003. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- 9.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433: 760–764, 2005. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 10.Cosgrove BD, Gilbert PM, Porpiglia E, Mourkioti F, Lee SP, Corbel SY, Llewellyn ME, Delp SL, Blau HM. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat Med 20: 255–264, 2014. doi: 10.1038/nm.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuthbertson DJ, Babraj JA, Smith K, Wilkes E, Fedele MJ, Esser K, Rennie MJ. Anabolic signalling and protein synthesis in human skeletal muscle after dynamic shortening or lengthening exercise. Am J Physiol Endocrinol Metab 290: E731–E738, 2006. doi: 10.1152/ajpendo.00415.2005. [DOI] [PubMed] [Google Scholar]
- 12.Dirks ML, Tieland M, Verdijk LB, Losen M, Nilwik R, Mensink M, de Groot LCPGM, van Loon LJC. Protein supplementation augments muscle fiber hypertrophy but does not modulate satellite cell content during prolonged resistance-type exercise training in frail elderly. J Am Med Dir Assoc 18: 608–615, 2017. doi: 10.1016/j.jamda.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Dreyer HC, Blanco CE, Sattler FR, Schroeder ET, Wiswell RA. Satellite cell numbers in young and older men 24 hours after eccentric exercise. Muscle Nerve 33: 242–253, 2006. doi: 10.1002/mus.20461. [DOI] [PubMed] [Google Scholar]
- 14.Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, Dhanani S, Volpi E, Rasmussen BB. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab 294: E392–E400, 2008. doi: 10.1152/ajpendo.00582.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dreyer HC, Owen EC, Strycker LA, Smolkowski K, Muyskens JB, Kirkpatrick TK, Christie AD, Kuehl KS, Lantz BA, Shah SN, Mohler CG, Jewett BA. Essential amino acid supplementation mitigates muscle atrophy after total knee arthroplasty: a randomized, double-blind, placebo-controlled trial. JBJS Open Access 3: e0006, 2018. doi: 10.2106/JBJS.OA.18.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dreyer HC, Strycker LA, Senesac HA, Hocker AD, Smolkowski K, Shah SN, Jewett BA. Essential amino acid supplementation in patients following total knee arthroplasty. J Clin Invest 123: 4654–4666, 2013. doi: 10.1172/JCI70160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dreyer HC, Volpi E. Role of protein and amino acids in the pathophysiology and treatment of sarcopenia. J Am Coll Nutr 24: 140S–145S, 2005. doi: 10.1080/07315724.2005.10719455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duan Y, Zeng L, Li F, Wang W, Li Y, Guo Q, Ji Y, Tan B, Yin Y. Effect of branched-chain amino acid ratio on the proliferation, differentiation, and expression levels of key regulators involved in protein metabolism of myocytes. Nutrition 36: 8–16, 2017. doi: 10.1016/j.nut.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Esteban I, Aguado C, Sánchez M, Knecht E. Regulation of various proteolytic pathways by insulin and amino acids in human fibroblasts. FEBS Lett 581: 3415–3421, 2007. doi: 10.1016/j.febslet.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 20.Farup J, Rahbek SK, Knudsen IS, de Paoli F, Mackey AL, Vissing K. Whey protein supplementation accelerates satellite cell proliferation during recovery from eccentric exercise. Amino Acids 46: 2503–2516, 2014. doi: 10.1007/s00726-014-1810-3. [DOI] [PubMed] [Google Scholar]
- 21.Ferreira R, Neuparth MJ, Ascensão A, Magalhães J, Vitorino R, Duarte JA, Amado F. Skeletal muscle atrophy increases cell proliferation in mice gastrocnemius during the first week of hindlimb suspension. Eur J Appl Physiol 97: 340–346, 2006. doi: 10.1007/s00421-006-0197-6. [DOI] [PubMed] [Google Scholar]
- 22.Ferrucci L, Guralnik JM, Pahor M, Corti MC, Havlik RJ. Hospital diagnoses, Medicare charges, and nursing home admissions in the year when older persons become severely disabled. JAMA 277: 728–734, 1997. doi: 10.1001/jama.1997.03540330050034. [DOI] [PubMed] [Google Scholar]
- 23.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M; International Working Group on Sarcopenia . Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 12: 249–256, 2011. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frost RA, Nystrom GJ, Lang CH. Lipopolysaccharide regulates proinflammatory cytokine expression in mouse myoblasts and skeletal muscle. Am J Physiol Regul Integr Comp Physiol 283: R698–R709, 2002. doi: 10.1152/ajpregu.00039.2002. [DOI] [PubMed] [Google Scholar]
- 25.Fry CS, Lee JD, Jackson JR, Kirby TJ, Stasko SA, Liu H, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J 28: 1654–1665, 2014. doi: 10.1096/fj.13-239426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fry CS, Lee JD, Mula J, Kirby TJ, Jackson JR, Liu F, Yang L, Mendias CL, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat Med 21: 76–80, 2015. doi: 10.1038/nm.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gensel JC, Kopper TJ, Zhang B, Orr MB, Bailey WM. Predictive screening of M1 and M2 macrophages reveals the immunomodulatory effectiveness of post spinal cord injury azithromycin treatment. Sci Rep 7: 40144, 2017. doi: 10.1038/srep40144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hepple RT, Mackinnon SL, Goodman JM, Thomas SG, Plyley MJ. Resistance and aerobic training in older men: effects on V̇o2peak and the capillary supply to skeletal muscle. J Appl Physiol (1985) 82: 1305–1310, 1997. doi: 10.1152/jappl.1997.82.4.1305. [DOI] [PubMed] [Google Scholar]
- 29.Hulmi JJ, Kovanen V, Lisko I, Selänne H, Mero AA. The effects of whey protein on myostatin and cell cycle-related gene expression responses to a single heavy resistance exercise bout in trained older men. Eur J Appl Physiol 102: 205–213, 2008. doi: 10.1007/s00421-007-0579-4. [DOI] [PubMed] [Google Scholar]
- 30.Jackson JR, Mula J, Kirby TJ, Fry CS, Lee JD, Ubele MF, Campbell KS, McCarthy JJ, Peterson CA, Dupont-Versteegden EE. Satellite cell depletion does not inhibit adult skeletal muscle regrowth following unloading-induced atrophy. Am J Physiol Cell Physiol 303: C854–C861, 2012. doi: 10.1152/ajpcell.00207.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 50: 889–896, 2002. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 32.Kosmac K, Peck BD, Walton RG, Mula J, Kern PA, Bamman MM, Dennis RA, Jacobs CA, Lattermann C, Johnson DL, Peterson CA. Immunohistochemical identification of human skeletal muscle macrophages. Bio Protoc 8: e2883, 2018. doi: 10.21769/BioProtoc.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lemos DR, Babaeijandaghi F, Low M, Chang CK, Lee ST, Fiore D, Zhang RH, Natarajan A, Nedospasov SA, Rossi FM. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med 21: 786–794, 2015. doi: 10.1038/nm.3869. [DOI] [PubMed] [Google Scholar]
- 34.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature 469: 323–335, 2011. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25: 677–686, 2004. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 36.McKay BR, Ogborn DI, Bellamy LM, Tarnopolsky MA, Parise G. Myostatin is associated with age-related human muscle stem cell dysfunction. FASEB J 26: 2509–2521, 2012. doi: 10.1096/fj.11-198663. [DOI] [PubMed] [Google Scholar]
- 37.Mobley CB, Haun CT, Roberson PA, Mumford PW, Romero MA, Kephart WC, Anderson RG, Vann CG, Osburn SC, Pledge CD, Martin JS, Young KC, Goodlett MD, Pascoe DD, Lockwood CM, Roberts MD. Effects of whey, soy or leucine supplementation with 12 weeks of resistance training on strength, body composition, and skeletal muscle and adipose tissue histological attributes in college-aged males. Nutrients 9: E972, 2017. doi: 10.3390/nu9090972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molsted S, Andersen JL, Harrison AP, Eidemak I, Mackey AL. Fiber type-specific response of skeletal muscle satellite cells to high-intensity resistance training in dialysis patients. Muscle Nerve 52: 736–745, 2015. doi: 10.1002/mus.24633. [DOI] [PubMed] [Google Scholar]
- 39.Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, Cederholm T, Coats AJ, Cummings SR, Evans WJ, Fearon K, Ferrucci L, Fielding RA, Guralnik JM, Harris TB, Inui A, Kalantar-Zadeh K, Kirwan BA, Mantovani G, Muscaritoli M, Newman AB, Rossi-Fanelli F, Rosano GM, Roubenoff R, Schambelan M, Sokol GH, Storer TW, Vellas B, von Haehling S, Yeh SS, Anker SD; Society on Sarcopenia, Cachexia and Wasting Disorders Trialist Workshop . Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc 12: 403–409, 2011. doi: 10.1016/j.jamda.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muyskens JB, Hocker AD, Turnbull DW, Shah SN, Lantz BA, Jewett BA, Dreyer HC. Transcriptional profiling and muscle cross-section analysis reveal signs of ischemia reperfusion injury following total knee arthroplasty with tourniquet. Physiol Rep 4: e12671, 2016. doi: 10.14814/phy2.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olsen S, Aagaard P, Kadi F, Tufekovic G, Verney J, Olesen JL, Suetta C, Kjaer M. Creatine supplementation augments the increase in satellite cell and myonuclei number in human skeletal muscle induced by strength training. J Physiol 573: 525–534, 2006. doi: 10.1113/jphysiol.2006.107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palacios D, Mozzetta C, Consalvi S, Caretti G, Saccone V, Proserpio V, Marquez VE, Valente S, Mai A, Forcales SV, Sartorelli V, Puri PL. TNF/p38α/polycomb signaling to Pax7 locus in satellite cells links inflammation to the epigenetic control of muscle regeneration. Cell Stem Cell 7: 455–469, 2010. doi: 10.1016/j.stem.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phillips SM. Nutrient-rich meat proteins in offsetting age-related muscle loss. Meat Sci 92: 174–178, 2012. doi: 10.1016/j.meatsci.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 44.Reidy PT, Fry CS, Dickinson JM, Drummond MJ, Rasmussen BB. Postexercise essential amino acid supplementation amplifies skeletal muscle satellite cell proliferation in older men 24 hours postexercise. Physiol Rep 5: e13269, 2017. doi: 10.14814/phy2.13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodgers JT, King KY, Brett JO, Cromie MJ, Charville GW, Maguire KK, Brunson C, Mastey N, Liu L, Tsai CR, Goodell MA, Rando TA. mTORC1 controls the adaptive transition of quiescent stem cells from G0 to G(Alert). Nature 510: 393–396, 2014. doi: 10.1038/nature13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Snijders T, Bell KE, Nederveen JP, Saddler NI, Mazara N, Kumbhare DA, Phillips SM, Parise G. Ingestion of a multi-ingredient supplement does not alter exercise-induced satellite cell responses in older men. J Nutr 148: 891–899, 2018. doi: 10.1093/jn/nxy063. [DOI] [PubMed] [Google Scholar]
- 47.Snijders T, Verdijk LB, Smeets JS, McKay BR, Senden JM, Hartgens F, Parise G, Greenhaff P, van Loon LJ. The skeletal muscle satellite cell response to a single bout of resistance-type exercise is delayed with aging in men. Age (Dordr) 36: 9699, 2014. doi: 10.1007/s11357-014-9699-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Snijders T, Wall BT, Dirks ML, Senden JM, Hartgens F, Dolmans J, Losen M, Verdijk LB, van Loon LJ. Muscle disuse atrophy is not accompanied by changes in skeletal muscle satellite cell content. Clin Sci (Lond) 126: 557–566, 2014. doi: 10.1042/CS20130295. [DOI] [PubMed] [Google Scholar]
- 49.Sousa-Victor P, Muñoz-Cánoves P. Regenerative decline of stem cells in sarcopenia. Mol Aspects Med 50: 109–117, 2016. doi: 10.1016/j.mam.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Suetta C, Frandsen U, Mackey AL, Jensen L, Hvid LG, Bayer ML, Petersson SJ, Schrøder HD, Andersen JL, Aagaard P, Schjerling P, Kjaer M. Ageing is associated with diminished muscle re-growth and myogenic precursor cell expansion early after immobility-induced atrophy in human skeletal muscle. J Physiol 591: 3789–3804, 2013. doi: 10.1113/jphysiol.2013.257121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, Yamaguchi M, Ogawa R, Matev MM, Miyagoe-Suzuki Y, Takeda S, Tsujikawa K, Tsuchida K, Yamamoto H, Fukada S. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci 124: 3654–3664, 2011. doi: 10.1242/jcs.086629. [DOI] [PubMed] [Google Scholar]
- 52.Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F, van Loon LJ. Satellite cells in human skeletal muscle: from birth to old age. Age (Dordr) 36: 545–557, 2014. doi: 10.1007/s11357-013-9583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walton RG, Kosmac K, Mula J, Fry CS, Peck BD, Groshong JS, Finlin BS, Zhu B, Kern PA, Peterson CA. Human skeletal muscle macrophages increase following cycle training and are associated with adaptations that may facilitate growth. Sci Rep 9: 969, 2019. doi: 10.1038/s41598-018-37187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang LD, Wagers AJ. Dynamic niches in the origination and differentiation of haematopoietic stem cells. Nat Rev Mol Cell Biol 12: 643–655, 2011. [Erratum in Nat Rev Mol Cell Biol 13: 12, 2011]. doi: 10.1038/nrm3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolinsky FD, Callahan CM, Fitzgerald JF, Johnson RJ. The risk of nursing home placement and subsequent death among older adults. J Gerontol 47: S173–S182, 1992. doi: 10.1093/geronj/47.4.S173. [DOI] [PubMed] [Google Scholar]
- 56.Yarasheski KE. Exercise, aging, and muscle protein metabolism. J Gerontol A Biol Sci Med Sci 58: M918–M922, 2003. doi: 10.1093/gerona/58.10.M918. [DOI] [PubMed] [Google Scholar]