Abstract
We sought to investigate whether the β-adrenergic receptors play a pivotal role in sex-related differences in arterial blood pressure (BP) regulation during isometric exercise. Sixteen volunteers (8 women) performed 2 min of ischemic isometric handgrip exercise (IHE) and 2 min of postexercise circulatory occlusion (PECO). Heart rate (HR) and beat-to-beat arterial BP were continuously measured. Beat-to-beat estimates of stroke volume (ModelFlow) were obtained and matched with HR to calculate cardiac output (Q̇) and total peripheral resistance (TPR). Two trials were randomly conducted between placebo and nonselective β-adrenergic blockade (40 mg propranolol). Under the placebo condition, the magnitude of the BP response in IHE was lower in women compared with men. During PECO, the BP remained elevated and the sex differences persisted. The β-blockade attenuated the BP response during IHE in men (∆57 ± 4 vs. ∆45 ± 7 mmHg, P = 0.025) due to a reduction in Q̇ (∆3.7 ± 0.5 vs. ∆1.8 ± 0.2 L/min, P = 0.012) while TPR was not affected. In women, however, the BP response during IHE was unchanged (∆27 ± 3 vs. ∆28 ± 3 mmHg, P = 0.889), despite attenuated Q̇ (∆2.7 ± 0.4 vs. ∆1.3 ± 0.2 L/min, P = 0.012). These responses were mediated by a robust increase in TPR under β-blockade (∆−0.2 ± 0.4 vs. ∆2.2 ± 0.7 mmHg·L−1·min, P = 0.012). These findings demonstrate that the sex differences in arterial BP regulation during ischemic IHE are mediated by β-adrenergic receptors.
NEW & NOTEWORTHY We found that the blood pressure response during isometric exercise in women is mediated by increases in cardiac output, whereas in men it is mediated by increases in both cardiac output and total peripheral resistance. In addition, women showed a robust increase in total peripheral resistance under β-blockade during isometric exercise and muscle metaboreflex activation. These findings demonstrate that sex differences in blood pressure regulation during isometric exercise are mediated by β-adrenergic receptors.
Keywords: β-adrenergic blockade, cardiac output, total peripheral resistance
INTRODUCTION
During exercise, arterial blood pressure (BP) and heart rate (HR) increase in an intensity-dependent manner, mediated by several neural mechanisms, including central command, the arterial baroreflex, and the skeletal muscle mechano- and metaboreflex (13, 27). Significant attention has been given to the muscle metaboreflex (i.e., the metabolic component of exercise pressor reflex) due to its predominant role in determining sympathetic vasomotor outflow during exercise (1, 12, 39). However, with regards to BP regulation, it is still controversial whether the pressor response to isometric handgrip exercise (IHE) can be attributable to increases in cardiac output (Q̇) and/or neurally induced increases in total peripheral resistance (TPR) (9, 10, 23, 37, 40).
The lack of consistency in previous findings is likely due to considerable individual differences in BP regulation during exercise. Watanabe et al. (41) demonstrated that the magnitude increase in Q̇ or TPR during IHE could be dependent on differences in muscle metaboreflex-mediated responses. Importantly, these studies recruited predominantly men and were not designed to investigate potential sex-related differences in the components of BP regulation during IHE. This becomes important because there is a plethora of studies demonstrating significant sex differences in arterial BP regulation during resting conditions (4, 18, 35). Similar to what has been reported in resting conditions, the current literature has shown that women, different from men, modulate BP responses to IHE through increase in Q̇ rather than TPR (5, 33, 36). However, the underlying mechanisms driving the cardiac reliance in women are not fully understood.
Previous data suggest that β-adrenergic receptor sensitivity is enhanced in young women compared with age-matched men (24). Consequently, young women present blunted vasoconstrictor responses due to concurrent β-adrenergic-mediated vasodilation of sympathetic nerve activity at rest (17). Thus it is possible that β-adrenergic-mediated vasodilation offsets α-adrenergic vasoconstriction associated with sympathetic activity (17). Importantly, all of these studies were conducted at rest and whether these findings could be extrapolated to exercise condition and muscle metaboreflex activation remains to be determined.
Therefore, we sought to investigate whether β-adrenergic receptors play a role in sex-related differences in BP regulation during ischemic IHE. The purpose of using ischemic isometric exercise was to eliminate the potential variations in the forearm perfusion during muscle contraction and to enhance the muscle metaboreflex during exercise. In addition, isolated muscle metaboreflex activation was achieved via postexercise circulatory occlusion (PECO) as the BP response to exercise is tightly linked to this reflex.
METHODS
Ethical approval.
All procedures were approved by the Ethical Committee for Research at the University of Brasília (CAAE: 92350318.0.0000.0030) and conformed to the Declaration of Helsinki. All individuals were informed of the study purpose and potential risks and provided written consent before participation.
Participants.
Eight men and eight women volunteered for the study. Participants were normotensive, nonsmoking, and recreationally active. No participants were using any controlled medications and had no history of cardiopulmonary, metabolic, or neurological diseases. To minimize the potential influences of circulating female sex hormones on BP control, all women were nonusers of oral contraceptive pills for at least 6 mo consecutively and were studied during the early follicular phase of their menstrual cycle (i.e., first 5 days after menstruation onset). All participants were asked to refrain from consuming caffeine/alcohol and from engaging in physical exercise for 6 and 24 h, respectively, before the study visits. Participants were 1-h postprandial upon arrival to the laboratory. To avoid potential diurnal variations, each subject was tested at the same time of day in the same quiet, temperature-controlled room (22–24°C).
Experimental measurements.
HR was measured using a lead II electrocardiogram (DX2022; Dixtal, Manaus, Brazil). BP was continuously measured on a beat-to-beat basis by photoplethysmography using a Finometer device (Human NIBP Controller; ADInstruments, Bella Vista, NSW, Australia) placed at the middle finger of the nondominant hand. In addition, brachial arterial BP of the dominant arm was also measured with an automated digital sphygmomanometer (DX2022; Dixtal) to validate finger photoplethysmography measurements of BP. Respiratory movements were monitored using a pneumatic belt placed around the abdomen (MLT 1132 Piezo Respiratory Belt Transducer; ADInstruments) to ensure that the participants did not perform Valsalva maneuvers during the protocol. The BP waveforms were sampled at 1,000 Hz (Powerlab; AD Instruments), and beat-to-beat values of HR were stored for offline analysis (Chart version 5.2; ADInstruments).
Beat-to-beat left ventricular stroke volume (SV) was estimated from the arterial pressure waveforms using the Modelflow method (Beatscope 1.1a; Finapres Medical Systems, Amsterdam, The Netherlands), which incorporates age, sex, weight, and height. Q̇ was calculated from beat-to-beat HR and SV (Q̇ = HR × SV). TPR was calculated as the ratio between mean BP and Q̇ (TPR = meanBP/Q̇).
Experimental procedures.
Before the experimental visits, participants were familiarized with all equipment and the ischemic IHE and PECO protocols. Weight and height were determined via standard methods, and body mass index was calculated. Thereafter, participants performed a maximal voluntary contraction (MVC) test with a handgrip dynamometer held in the dominant hand. MVC was determined as the highest of three maximal efforts, each separated by >1 min.
The experimental protocol was conducted over two laboratory visits separated by ∼48–72 h. To test our hypothesis that β-adrenergic receptors play a pivotal role in sex-related differences in the BP regulation during ischemic IHE, a single unidentifiable capsule containing either placebo or 40 mg propranolol was orally administered ~50 min before the experimental tasks. The nonselective β-blocker and placebo pill were randomly administered in a double-blind fashion. Experimental procedures were all performed in the supine position. The ischemic IHE protocol consisted of 3 min of baseline ischemia, followed by 2 min of exercise at 30% of MVC and 2 min of PECO. Ischemia was induced before the start of the handgrip exercise to further evoke the muscle metaboreflex and raise BP. Forearm ischemia was induced by inflating a BP cuff placed around the upper portion of the dominant arm to suprasystolic levels (240 mmHg). The IHE intensity was controlled and maintained by visual feedback from a computer screen. Following completion of PECO, the cuff was released and recovery was monitored for 1 min. Participants were requested to evaluate their perception of effort at the end of the exercise on the Borg scale.
Statistical analysis.
All physiological variables were obtained, and averages were calculated at ischemic baseline (3 min). The maximum 30-s average window was detected during IHE (2 min) and PECO (2 min) for the cardiovascular variables. Data were analyzed statistically using IBM SPSS Statistics software (version 20) for Windows. Group data are presented as means ± SE. The normality of all variables was tested by the Shapiro-Wilk’s test. Baseline characteristics between men and women were compared by an unpaired Student’s t-test. Statistical comparisons of resting hemodynamics variables were made using a two-way ANOVA with repeated measures in which sex (men, women) and drug (placebo, β-blockade) were the main factors. Comparison of IHE responses from ischemic rest in physiological variables were made using a 2 × 2 (drug × condition) ANOVA with repeated measures for men and women. Post hoc analysis was employed using Fisher’s test to investigate main effects and interactions. In addition, comparisons of ∆values in hemodynamics variables were made using Friedman ANOVA followed by Wilcoxon-Mann-Whitney tests. Statistical significance was set at P < 0.05.
RESULTS
Ischemic rest.
Men and women were matched for body mass index, although height, body weight, and MVC were all larger in men (all P < 0.01, Table 1).
Table 1.
Subject characteristics
| Men (n = 8) |
Women (n = 8) |
P Value | |
|---|---|---|---|
| Age, yr | 20 ± 0.5 | 24 ± 1.4 | 0.027 |
| Height, cm | 179 ± 0.02 | 164 ± 0.03 | <0.010 |
| Body weight, kg | 77 ± 3 | 63 ± 3 | <0.010 |
| BMI, kg/m2 | 24 ± 1 | 23 ± 0.5 | 0.571 |
| MVC, N | 597 ± 54 | 400 ± 27 | P < 0.01 |
Data are means ± SE. BMI, body mass index; BSA, body surface area; MVC, maximal voluntary contraction.
Resting hemodynamics are shown in Table 2. Women had a higher TPR compared with men under the β-blockade condition. They also had a significantly higher TPR under the β-blockade versus placebo condition. No significant interaction effects were found in any other resting hemodynamic variables under the placebo and β-blockade conditions in men and women (all P > 0.05).
Table 2.
Resting hemodynamic variables
| Men (n = 8) |
Women (n = 8) |
P Value |
|||||
|---|---|---|---|---|---|---|---|
| Hemodynamics Variables | Placebo | β-Blockade | Placebo | β-Blockade | Sex | Drug | Interaction |
| Heart rate, beats/min | 60 ± 2 | 57 ± 3 | 67 ± 2 | 60 ± 2 | 0.160 | 0.038 | 0.341 |
| Systolic BP, mmHg | 119 ± 6 | 116 ± 4 | 108 ± 3 | 106 ± 3 | 0.095 | 0.303 | 0.814 |
| Diastolic BP, mmHg | 63 ± 3 | 60 ± 3 | 63 ± 1 | 63 ± 1 | 0.702 | 0.541 | 0.193 |
| Mean BP, mmHg | 83 ± 4 | 80 ± 3 | 78 ± 2 | 78 ± 2 | 0.373 | 0.407 | 0.379 |
| Stroke volume, ml | 110.8 ± 4.3 | 104.7 ± 4.0 | 82.3 ± 4.6 | 76.7 ± 4.4 | <0.01 | 0.053 | 0.911 |
| Cardiac output, L/min | 6.7 ± 0.4 | 6.1 ± 0.3 | 5.5 ± 0.3 | 4.6 ± 0.2 | 0.039 | 0.022 | 0.443 |
| Total peripheral resistance, mmHg·L−1·min | 12.7 ± 0.7 | 13.5 ± 0.9 | 14.6 ± 0.6 | 17.3 ± 0.7†‡ | 0.037 | 0.020 | 0.019 |
Data are means ± SE. BP, blood pressure.
P < 0.05 vs. placebo (i.e., within group). ‡P < 0.05 vs. men.
Mean BP, Q̇, and TPR during exercise.
Borg scale rating at the end of ischemic IHE was not affected by β-blockade. In addition, there is no difference between men and women under the placebo (men: 16 ± 0.7 units vs. women: 17 ± 0.8 units; P = 0.73) and β-blockade condition (men: 16 ± 0.7 units vs. women: 16 ± 0.8 units; P = 0.73).
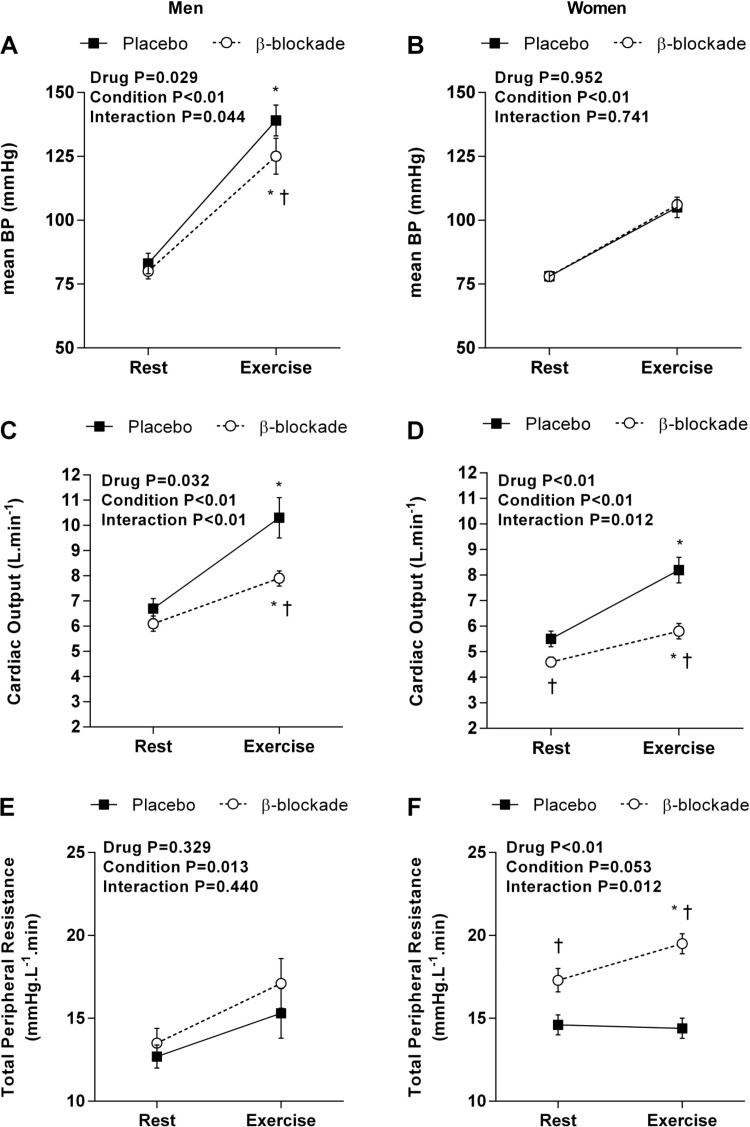
During ischemic IHE under the placebo condition, HR increased similarly from rest in men and women (men: ∆38 ± 3 beats/min vs. women: ∆29 ± 4 beats/min; P = 0.105). In addition, β-blockade attenuated the HR elevation during IHE in both men (∆27 ± 3 beats/min; P = 0.017) and women (∆18 ± 3 beats/min; P = 0.012); Fig. 1 displays the hemodynamic responses during ischemic IHE in men and women.
Fig. 1.
Average values for mean blood pressure (BP; A and B), cardiac output (C and D), and total peripheral resistance (E and F) at rest and during ischemic isometric exercise under the placebo (closed square) and β-blockade (open circles) conditions for men and women. *P < 0.05 vs. rest (i.e., within drug); †P < 0.05 vs. placebo.
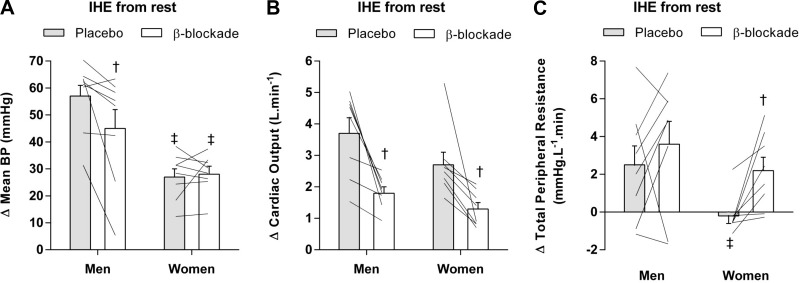
BP increased from ischemic rest under the placebo conditions in men (Fig. 1A) and women (Fig. 1B); however, men reached a higher value compared with women (∆57 ± 4 vs. ∆27 ± 3 mmHg, P < 0.01; Fig. 2A). Change of mean BP from ischemic rest was similar in women under the β-blockade condition (∆28 ± 3 mmHg; P = 0.889; Fig. 2A) but was significantly attenuated in men (∆45 ± 7 mmHg; P = 0.025; Fig. 2A).
Fig. 2.
Individual data (n = 8, lines) and changes from rest during ischemic isometric handgrip exercise (IHE) in mean blood pressure (A), cardiac output (B), and peripheral resistance (C) under the placebo (gray bars) and β-blockade (open bars) conditions for men and women. †P < 0.05 vs. placebo; ‡ P < 0.05 vs. men within drug condition.
Although men and women exhibited increases in mean BP during exercise, the components mediating those pressor responses (i.e., Q̇ and TPR) varied considerably (Fig. 1, C–F). Under the placebo conditions, men and women showed similar increases in Q̇ (men: ∆3.7 ± 0.5 L/min vs. women: ∆2.7 ± 0.4 L/min; P = 0.234; Fig. 2B), yet TPR increased in men but not in women (men: ∆2.5 ± 1.0 mmHg·L−1·min vs. women: ∆−0.2 ± 0.4 mmHg·L−1·min; P = 0.038; Fig. 2C). In addition, β-blockade attenuated the Q̇ increases during ischemic IHE in both men (∆1.8 ± 0.2 L/min; P = 0.012; Fig. 2B) and women (∆1.3 ± 0.2 L/min; P = 0.012; Fig. 2B). Interestingly, the β-blockade condition had no effect on TPR in men (∆3.6 ± 1.2 mmHg·L−1·min; P = 0.326; Fig. 2C), while women showed a robust increase in TPR during exercise (∆2.2 ± 0.7 mmHg·L−1·min; P = 0.012; Fig. 2C). These results indicate that the components contributing to the pressor responses during ischemic IHE differ between men and women and these sex-related differences are mediated by β-adrenergic receptors.
Mean BP, Q̇, and TPR during PECO.
HR fell from end-exercise values during PECO under all conditions (P < 0.01 vs. IHE). Under control condition, HR remained elevated from baseline in men (∆12 ± 5 beats/min; P = 0.04) while women recovered to baseline levels (∆5 ± 3 beats/min; P = 0.15). During β-blockade, HR was unchanged from baseline during PECO in both men (∆6 ± 4 beats/min; P = 0.13) and women (∆1 ± 2 beats/min; P = 0.56).
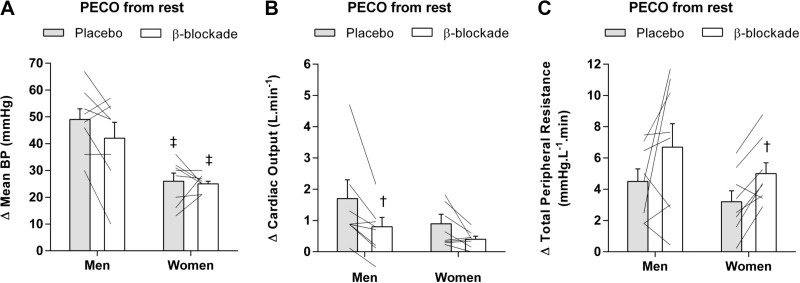
Figure 3 shows the change in hemodynamic responses during PECO from ischemic rest. During PECO following ischemic IHE under the placebo condition, mean BP was higher increased above rest in men compared with women (∆49 ± 4 mmHg vs. ∆26 ± 3 mmHg; P < 0.01; Fig. 3A). β-Blockade did not alter the mean BP response during PECO in men (∆42 ± 6 mmHg; P = 0.161) or women (∆25 ± 1 mmHg; P > 0.99), and thus the sex-based differences were still apparent.
Fig. 3.
Individual data (n = 8, lines) and changes from rest during postexercise circulatory occlusion (PECO) in mean blood pressure mean blood pressure (A), cardiac output (B), and peripheral resistance (C) under the placebo (gray bars) and β-blockade (open bars) conditions for men and women. †P < 0.05 vs. placebo; ‡P < 0.05 vs. men within drug condition.
Under the placebo conditions, Q̇ remained elevated in men and women. The β-blockade attenuated the Q̇ response in men (∆1.7 ± 0.6 vs. ∆0.8 ± 0.3 L/min; P = 0.017; Fig. 3B) but not in women (∆0.9 ± 0.3 vs. ∆0.4 ± 0.1 L/min P = 0.062; Fig. 3B).
TPR was elevated during PECO in men and women under the placebo condition. In addition, the β-blocker had no effect in men (∆4.5 ± 0.8 vs. ∆6.7 ± 1.5 mmHg·L−1·min; P = 0.208; Fig. 3C) yet contributed to a further increase in TPR in women (∆3.2 ± 0.7 vs. ∆5.0 ± 0.7 mmHg·L−1·min; P = 0.017; Fig. 3C).
DISCUSSION
The present study sought to determine whether β-adrenergic receptors play a role in sex-related differences in BP regulation during isometric exercise. To our knowledge, this is the first study examining the components of BP regulation during IHE under β-blockade between men and women. The major finding of this study is that the BP response during IHE in women is mediated by increases in Q̇ whereas in men by increases in both Q̇ and TPR. In contrast, the exercise-induced elevation in BP remains high during isolated muscle metaboreflex activation due to elevated TPR in both men and women. In addition, women showed a robust increase in TPR under β-blockade during IHE and muscle metaboreflex activation while TPR was unaffected in men under β-blockade throughout. Collectively, these findings indicate that sex-related differences in BP regulation during ischemic IHE are mediated by β-adrenergic receptors.
Prior studies investigating the effect of sex on hemodynamic responses during IHE concluded that young women present lower BP response compared with men (11, 20, 33). Our findings are in accordance with these studies demonstrating lower increases in BP response in women compared with men during IHE (Fig. 2A) and during isolated muscle metaboreflex activation (Fig. 3A). These lower BP responses in women could be related, in part, to lower metaboreceptor stimulation. It is known that women produce fewer metabolic by-products during static exercise evoking less metaboreceptor-mediated sympathoexcitation compared with men (11). In addition, the muscle mass (31, 42), the absolute level of strength generated during exercise (7, 29), and polymorphisms in receptors present in skeletal muscle (28) could be other mechanisms contributing to the sex differences in the BP response to exercise. Of note, all volunteers performed ischemic isometric exercise (i.e., blood flow occlusion during handgrip) so that interindividual differences in the skeletal muscle contraction-induced changes in blood flow to the active muscle were eliminated. Furthermore, the Borg scale ratings were similar between men and women throughout the protocols, suggesting a similar central command. Thus more studies are necessary to investigate these contributing factors in pressor responses during exercise, although they are likely driven by differences in metabolite production.
To gain insight into the mechanism(s) underlying the sex-related differences in BP responses to IHE, we examined the components of BP regulation. To our knowledge, only one study has investigated systematically the respective roles of Q̇ and TPR mediating the pressor response to IHE. The results showed large interindividual variability in Q̇ and TPR responses that contributed to the pressor response during isometric exercise (41). However, the majority of the subjects were men (32 men and 7 women) and the study did not investigate sex-related differences in BP regulation during exercise. Thus the present investigation further extends this body of knowledge, demonstrating that the BP response in women during IHE is predominantly regulated by an increased Q̇, while men showed an increase in both Q̇ and TPR. These results are in agreement with previous studies (30, 33, 36, 43), although the mechanism(s) for these sex-related differences in BP regulation during isometric exercise are not fully understood. Watanabe et al. (41) suggest that variations in the muscle metaboreflex-mediated cardioaccelerator and peripheral vasoconstrictor responses are responsible for the marked individual differences in the components of the pressor response to IHE. However, we demonstrated that the exercise-induced elevation in BP remains high during PECO due to elevated Q̇ and TPR in both men and women (Fig. 3). Despite our results being in line with others (3, 25), the muscle metaboreflex likely does not completely explain the sex-related differences in BP regulation during IHE.
Another possibility that we considered in the present study is the lack of rise in TPR in women, which could explain the sex-related difference in BP regulation during IHE. Indeed, it is well established that cardiovascular control is markedly different between men and women (15, 22) in part because of the effects of female sex hormones (i.e., estrogen and progesterone) on the cardiovascular system (26). For example, sympathetic control of TPR plays a role in BP regulation in young men (4, 18), but there is no relationship of sympathetic outflow to TPR in young women (18) at rest. In this sense, it is suggested that muscle sympathetic nerve activity contributes to vasoconstriction in young men but not in young women (18). Several mechanisms may contribute to the lack of relationship between TPR and sympathetic nerve activity in women. Previous works suggested that estrogen has a direct vasodilator effect on the vasculature (14, 34) and also can increase the bioavailability of nitric oxide (38). Furthermore, Kneale et al. (24) indicated that β-adrenergic receptor sensitivity is enhanced in young women compared with men. Collectively, these mechanisms suggest a blunted vasoconstriction response in women due to concurrent β-adrenergic-mediated vasodilatation. Importantly, all of these studies were conducted at rest and whether these findings could be extrapolated to the exercise condition is unknown.
Given this background, we decided to use a nonselective β-adrenergic receptor blocker (i.e., oral propranolol) for all volunteers during exercise and muscle metaboreflex activation. We observed a robust increase in TPR in women during IHE (Fig. 1F) under β-blockade without alteration in the magnitude of BP response (Fig. 1B), unlike the men. Furthermore, during the placebo condition, women were observed to increase TPR only during PECO and these responses were augmented with β-blockade, whereas unaltered in men. Together, these results indicate that the sex-related differences in BP regulation during exercise may be explained by a blunted vasoconstrictor response in women mediated by β-adrenergic receptors.
Limitations.
Several limitations in this present study should be considered. First, ischemic IHE was the only exercise modality used in the present study and care should be taken in extrapolating our findings to different exercise modalities, durations, and intensities, as well as exercising muscle groups. Second, all the participants were young, healthy, and physically active. Thus the results may be delimitated by the population studied because there are several reports of age and/or disease-dependent differences in cardiovascular control (2, 15, 19, 32). However, our study provides new evidence that sex may be an important factor in BP regulation. Third, we cannot exclude the systemic nature of the drug. It is important to note that propranolol is a nonselective β-adrenergic receptor blocker and can also block β1-receptors in the heart. One cardiac effect of propranolol is the reduced HR at rest leading to a lower Q̇. Indeed, the drug can also reduce exercise tachycardia in a dose-dependent manner (6, 21). Noteworthy, is that the dosage in the present study was similar for men and women (i.e., 40 mg), yet the cardiac effect (i.e., SV, HR, and Q̇) could be found in both. The similar magnitude changes in cardiac responses in men compared with women, despite men being of larger size, suggests altered sensitivity to propranolol in men, warranting further investigation. In addition, although propranolol is a nonselective β-adrenergic receptor blocker and can act systemically, the increase in TPR under β-blockade in women is corroborative with the literature, in which intravenous infusion of propranolol can augment forearm vasoconstrictor responsiveness in young women but not in young men (17).
Perspectives.
According to the data presented in this study, there are sex-related differences in BP regulation during isometric exercise that are mediated by β-adrenergic receptors. During exercise, BP increased in men due to augmented Q̇ and TPR while in women only increases in Q̇. Thus we speculate that the lack of ability to increase TPR during exercise in women is related to the greater β-adrenergic receptors activity (17, 24). In this sense, β-receptor blockade (i.e., propranolol) generated a robust increase in TPR during exercise, changing the components regulating BP in women. Studies focusing on sex-related differences in BP have been fundamental in developing what we know about resting BP control. However, little is known about BP responses and regulation during exercise and whether the sex-related differences persisted. Indeed, sex has an important influence on the integrative balance of neural and hemodynamic factors determining the level of BP in a given person. Thus it is raises the interesting possibility to consider the sex differences in BP regulation in pathophysiological conditions (e.g., arterial hypertension, heart failure) in which BP is not regulated appropriately and also to contribute to the specificity of future therapeutic interventions in cardiovascular care.
Conclusion.
In summary, the present findings indicate that sex-related differences in BP regulation during ischemic IHE and muscle metaboreflex activation are mediated by β-adrenergic receptors.
GRANTS
This study was partly supported by an American Physiological Society Arthur C. Guyton Award for Excellence in Integrative Physiology (to L. C. Vianna). M. Samora receives scholarship support from Coordination for the Improvement of Higher Education Personnel (CAPES Finance Code 001). L. C. Vianna receives research support from the National Council for Scientific and Technological Development (CNPq Grants 307686/2016-7 and 431740/2018-6), and A. V. Incognito receives support from the Natural Sciences and Engineering Research Council of Canada Michael Smith Foreign Study Supplement.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.S., A.V.I., and L.C.V. conceived and designed research; M.S., A.V.I., and L.C.V. performed experiments; M.S. and L.C.V. analyzed data; M.S., A.V.I., and L.C.V. interpreted results of experiments; M.S. prepared figures; M.S. and L.C.V. drafted manuscript; M.S., A.V.I., and L.C.V. edited and revised manuscript; M.S., A.V.I., and L.C.V. approved final version of manuscript.
ACKNOWLEDGMENTS
We appreciate the time and effort expended by all volunteer participants in this study.
REFERENCES
- 1.Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89: 372–383, 1937. doi: 10.1113/jphysiol.1937.sp003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker SE, Limberg JK, Ranadive SM, Joyner MJ. Neurovascular control of blood pressure is influenced by aging, sex, and sex hormones. Am J Physiol Regul Integr Comp Physiol 311: R1271–R1275, 2016. doi: 10.1152/ajpregu.00288.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bastos BG, Williamson JW, Harrelson T, Nóbrega AC. Left ventricular volumes and hemodynamic responses to postexercise ischemia in healthy humans. Med Sci Sports Exerc 32: 1114–1118, 2000. doi: 10.1097/00005768-200006000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol 568: 315–321, 2005. doi: 10.1113/jphysiol.2005.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi HM, Stebbins CL, Nho H, Kim KA, Kim C, Kim JK. Skeletal muscle metaboreflex is enhanced in postmenopausal women. Eur J Appl Physiol 112: 2671–2678, 2012. doi: 10.1007/s00421-011-2245-0. [DOI] [PubMed] [Google Scholar]
- 6.Conviser JM, Ng AV, Rockey SS, Thomas DP. Cardio-protection afforded by β-blockade is maintained during resistance exercise. J Sci Med Sport 20: 196–201, 2017. doi: 10.1016/j.jsams.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Cornett JA, Herr MD, Gray KS, Smith MB, Yang QX, Sinoway LI. Ischemic exercise and the muscle metaboreflex. J Appl Physiol (1985) 89: 1432–1436, 2000. doi: 10.1152/jappl.2000.89.4.1432. [DOI] [PubMed] [Google Scholar]
- 8.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 5: 303–311, 1989. [PubMed] [Google Scholar]
- 9.Eisenach JH, Barnes SA, Pike TL, Sokolnicki LA, Masuki S, Dietz NM, Rehfeldt KH, Turner ST, Joyner MJ. Arg16/Gly beta2-adrenergic receptor polymorphism alters the cardiac output response to isometric exercise. J Appl Physiol (1985) 99: 1776–1781, 2005. doi: 10.1152/japplphysiol.00469.2005. [DOI] [PubMed] [Google Scholar]
- 10.Elstad M, Nådland IH, Toska K, Walløe L. Stroke volume decreases during mild dynamic and static exercise in supine humans. Acta Physiol (Oxf) 195: 289–300, 2009. doi: 10.1111/j.1748-1716.2008.01887.x. [DOI] [PubMed] [Google Scholar]
- 11.Ettinger SM, Silber DH, Collins BG, Gray KS, Sutliff G, Whisler SK, McClain JM, Smith MB, Yang QX, Sinoway LI. Influences of gender on sympathetic nerve responses to static exercise. J Appl Physiol (1985) 80: 245–251, 1996. doi: 10.1152/jappl.1996.80.1.245. [DOI] [PubMed] [Google Scholar]
- 12.Fisher JP, Fernandes IA, Barbosa TC, Prodel E, Coote JH, Nóbrega AC, Vianna LC. Diving and exercise: the interaction of trigeminal receptors and muscle metaboreceptors on muscle sympathetic nerve activity in humans. Am J Physiol Heart Circ Physiol 308: H367–H375, 2015. doi: 10.1152/ajpheart.00728.2014. [DOI] [PubMed] [Google Scholar]
- 13.Fisher JP, Young CN, Fadel PJ. Autonomic adjustments to exercise in humans. Compr Physiol 5: 475–512, 2015. doi: 10.1002/cphy.c140022. [DOI] [PubMed] [Google Scholar]
- 14.Gilligan DM, Badar DM, Panza JA, Quyyumi AA, Cannon RO III. Acute vascular effects of estrogen in postmenopausal women. Circulation 90: 786–791, 1994. doi: 10.1161/01.CIR.90.2.786. [DOI] [PubMed] [Google Scholar]
- 15.Hart EC, Charkoudian N. Sympathetic neural regulation of blood pressure: influences of sex and aging. Physiology (Bethesda) 29: 8–15, 2014. doi: 10.1152/physiol.00031.2013. [DOI] [PubMed] [Google Scholar]
- 17.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach J, Joyner MJ. Sex and ageing differences in resting arterial pressure regulation: the role of the β-adrenergic receptors. J Physiol 589: 5285–5297, 2011. doi: 10.1113/jphysiol.2011.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension 53: 571–576, 2009. doi: 10.1161/HYPERTENSIONAHA.108.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hart EC, Joyner MJ, Wallin BG, Charkoudian N. Sex, ageing and resting blood pressure: gaining insights from the integrated balance of neural and haemodynamic factors. J Physiol 590: 2069–2079, 2012. doi: 10.1113/jphysiol.2011.224642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarvis SS, VanGundy TB, Galbreath MM, Shibata S, Okazaki K, Reelick MF, Levine BD, Fu Q. Sex differences in the modulation of vasomotor sympathetic outflow during static handgrip exercise in healthy young humans. Am J Physiol Regul Integr Comp Physiol 301: R193–R200, 2011. doi: 10.1152/ajpregu.00562.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joannides R, Moore N, Iacob M, Compagnon P, Lerebours G, Menard JF, Thuillez C. Comparative effects of ivabradine, a selective heart rate-lowering agent, and propranolol on systemic and cardiac haemodynamics at rest and during exercise. Br J Clin Pharmacol 61: 127–137, 2006. doi: 10.1111/j.1365-2125.2005.02544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joyner MJ, Wallin BG, Charkoudian N. Sex differences and blood pressure regulation in humans. Exp Physiol 101: 349–355, 2016. doi: 10.1113/EP085146. [DOI] [PubMed] [Google Scholar]
- 23.Kiviniemi AM, Frances MF, Tiinanen S, Craen R, Rachinsky M, Petrella RJ, Seppänen T, Huikuri HV, Tulppo MP, Shoemaker JK. α-Adrenergic effects on low-frequency oscillations in blood pressure and R-R intervals during sympathetic activation. Exp Physiol 96: 718–735, 2011. doi: 10.1113/expphysiol.2011.058768. [DOI] [PubMed] [Google Scholar]
- 24.Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol 36: 1233–1238, 2000. doi: 10.1016/S0735-1097(00)00849-4. [DOI] [PubMed] [Google Scholar]
- 25.Lykidis CK, White MJ, Balanos GM. The pulmonary vascular response to the sustained activation of the muscle metaboreflex in man. Exp Physiol 93: 247–253, 2008. doi: 10.1113/expphysiol.2007.039487. [DOI] [PubMed] [Google Scholar]
- 26.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med 340: 1801–1811, 1999. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 27.Mueller PJ, Clifford PS, Crandall CG, Smith SA, Fadel PJ. Integration of central and peripheral regulation of the circulation during exercise: acute and chronic adaptations. Compr Physiol 8: 103–151, 2017. doi: 10.1002/cphy.c160040. [DOI] [PubMed] [Google Scholar]
- 28.Notay K, Klingel SL, Lee JB, Doherty CJ, Seed JD, Swiatczak M, Mutch DM, Millar PJ. TRPV1 and BDKRB2 receptor polymorphisms can influence the exercise pressor reflex. J Physiol 596: 5135–5148, 2018. doi: 10.1113/JP276526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Notay K, Lee JB, Incognito AV, Seed JD, Arthurs AA, Millar PJ. Muscle Strength Influences Pressor Responses to Static Handgrip in Men and Women. Med Sci Sports Exerc 50: 778–784, 2018. doi: 10.1249/MSS.0000000000001485. [DOI] [PubMed] [Google Scholar]
- 30.O’Leary DS, Sheriff DD. Is the muscle metaboreflex important in control of blood flow to ischemic active skeletal muscle in dogs? Am J Physiol 268: H980–H986, 1995. doi: 10.1152/ajpheart.1995.268.3.H980. [DOI] [PubMed] [Google Scholar]
- 31.Petrofsky JS, Lind AR. The blood pressure response during isometric exercise in fast and slow twitch skeletal muscle in the cat. Eur J Appl Physiol Occup Physiol 44: 223–230, 1980. doi: 10.1007/BF00421621. [DOI] [PubMed] [Google Scholar]
- 32.Sabino-Carvalho JL, Teixeira AL, Samora M, Daher M, Vianna LC. Blunted cardiovascular responses to exercise in Parkinson’s disease patients: role of the muscle metaboreflex. J Neurophysiol 120: 1516–1524, 2018. doi: 10.1152/jn.00308.2018. [DOI] [PubMed] [Google Scholar]
- 33.Samora M, Teixeira AL, Sabino-Carvalho JL, Vianna LC. Spontaneous cardiac baroreflex sensitivity is enhanced during post-exercise ischemia in men but not in women. Eur J Appl Physiol 119: 103–111, 2019. doi: 10.1007/s00421-018-4004-y. [DOI] [PubMed] [Google Scholar]
- 34.Scott PA, Tremblay A, Brochu M, St-Louis J. Vasorelaxant action of 17 -estradiol in rat uterine arteries: role of nitric oxide synthases and estrogen receptors. Am J Physiol Heart Circ Physiol 293: H3713–H3719, 2007. doi: 10.1152/ajpheart.00736.2007. [DOI] [PubMed] [Google Scholar]
- 35.Shoemaker JK, Hogeman CS, Khan M, Kimmerly DS, Sinoway LI. Gender affects sympathetic and hemodynamic response to postural stress. Am J Physiol Heart Circ Physiol 281: H2028–H2035, 2001. doi: 10.1152/ajpheart.2001.281.5.H2028. [DOI] [PubMed] [Google Scholar]
- 36.Shoemaker JK, Mattar L, Kerbeci P, Trotter S, Arbeille P, Hughson RL. WISE 2005: stroke volume changes contribute to the pressor response during ischemic handgrip exercise in women. J Appl Physiol (1985) 103: 228–233, 2007. doi: 10.1152/japplphysiol.01334.2006. [DOI] [PubMed] [Google Scholar]
- 37.Stewart JM, Montgomery LD, Glover JL, Medow MS. Changes in regional blood volume and blood flow during static handgrip. Am J Physiol Heart Circ Physiol 292: H215–H223, 2007. doi: 10.1152/ajpheart.00681.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sudhir K, Jennings GL, Funder JW, Komesaroff PA. Estrogen enhances basal nitric oxide release in the forearm vasculature in perimenopausal women. Hypertension 28: 330–334, 1996. doi: 10.1161/01.HYP.28.3.330. [DOI] [PubMed] [Google Scholar]
- 39.Teixeira AL, Daher M, Souza MC, Ramos PS, Fisher JP, Vianna LC. Sympathetically mediated cardiac responses to isolated muscle metaboreflex activation following exercise are modulated by body position in humans. Am J Physiol Heart Circ Physiol 314: H593–H602, 2018. doi: 10.1152/ajpheart.00576.2017. [DOI] [PubMed] [Google Scholar]
- 40.Toska K. Handgrip contraction induces a linear increase in arterial pressure by peripheral vasoconstriction, increased heart rate and a decrease in stroke volume. Acta Physiol (Oxf) 200: 211–221, 2010. doi: 10.1111/j.1748-1716.2010.02144.x. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe K, Ichinose M, Tahara R, Nishiyasu T. Individual differences in cardiac and vascular components of the pressor response to isometric handgrip exercise in humans. Am J Physiol Heart Circ Physiol 306: H251–H260, 2014. doi: 10.1152/ajpheart.00699.2013. [DOI] [PubMed] [Google Scholar]
- 42.Wilson LB, Dyke CK, Parsons D, Wall PT, Pawelczyk JA, Williams RS, Mitchell JH. Effect of skeletal muscle fiber type on the pressor response evoked by static contraction in rabbits. J Appl Physiol (1985) 79: 1744–1752, 1995. doi: 10.1152/jappl.1995.79.5.1744. [DOI] [PubMed] [Google Scholar]
- 43.Wyss CR, Ardell JL, Scher AM, Rowell LB. Cardiovascular responses to graded reductions in hindlimb perfusion in exercising dogs. Am J Physiol Heart Circ Physiol 245: H481–H486, 1983. doi: 10.1152/ajpheart.1983.245.3.H481. [DOI] [PubMed] [Google Scholar]