Abstract
Diabetic bladder dysfunction (DBD) affects up to 50% of all patients with diabetes, characterized by symptoms of both overactive and underactive bladder. Although most diabetic bladder dysfunction studies have been performed using models with type 1 diabetes, few have been performed in models of type 2 diabetes, which accounts for ~90% of all diabetic cases. In a type 2 rat model using a high-fat diet (HFD) and two low doses of streptozotocin (STZ), we examined voiding measurements and functional experiments in urothelium-denuded bladder strips to establish a timeline of disease progression. We hypothesized that overactive bladder symptoms (compensated state) would develop and progress into symptoms characterized by underactive bladder (decompensated state). Our results indicated that this model developed the compensated state at 1 wk after STZ and the decompensated state at 4 mo after STZ administration. Diabetic bladders were hypertrophied compared with control bladders. Increased volume per void and detrusor muscle contractility to exogenous addition of carbachol and ATP confirmed the development of the compensated state. This enhanced contractility to carbachol was not due to increased levels of M3 receptor expression. Decompensation was characterized by increased volume per void, number of voids, and contractility to ATP but not carbachol. Thus, progression from the compensated to decompensated state may involve decreased contractility to muscarinic stimulation. These data suggest that the compensated state of DBD progresses temporally into the decompensated state in the male HFD/STZ model of diabetes; therefore, this male HFD/STZ model can be used to study the progression of DBD.
Keywords: diabetes, diabetic cystopathy, lower urinary tract, smooth muscle
INTRODUCTION
Various forms of urinary bladder or lower urinary tract dysfunction are important standalone medical conditions. Their prevalence increases with age, and they are comorbid with other health issues, including diabetes (5, 6, 8, 29, 31). Diabetes is a disorder of carbohydrate metabolism that affects all organ systems, including the urinary bladder. This complication is referred to as diabetic cystitis or diabetic bladder dysfunction (DBD) and is present in up to 50% of patients with diabetes (9, 15, 17, 18, 20). These patients experience a wide range of symptoms that can be broadly categorized as either symptoms of underactive bladder or overactive bladder. Symptoms of underactivity include difficulty voiding, decreased sensation of bladder fullness, decreased desire to void, decreased bladder contractility, enhanced bladder capacity, and increased postvoid residual volume. Symptoms of overactive bladder include urgency with or without incontinence, increased frequency, nocturia, and increased contractility (9, 15, 17–20). The presence of both underactive bladder and overactive bladder symptoms led Daneshgari et al. (15) in 2009 to propose two states of DBD: an early compensated state and a late decompensated state. The early compensated state is described by storage problems in which patients exhibit symptoms of overactive bladder. In this state, the detrusor muscle layer of the urinary bladder hypertrophies and becomes hypercontractile. This is hypothesized to be caused by early hyperglycemia inducing osmotic polyuria (15, 52). The later decompensated state is described as voiding problems with symptoms of underactive bladder, as the detrusor muscle is hypocontractile. This is hypothesized to occur via the accumulation of oxidative stress products through prolonged hyperglycemia (52).
Many initial studies that focused on the temporal progression of diabetic bladder dysfunction have been in animal models of type 1 diabetes. However, relatively few have studied models of type 2 diabetes, which accounts for 90–95% of all diabetic cases (10–12, 14, 16, 27, 52). Type 2 diabetes is a very complex disease often developing as a result of environmental factors (exercise and diet), hereditary factors, or the result of an interaction between the two (56). The underlying contributing factors for the development of hyperglycemia in patients with type 2 diabetes are 1) insulin resistance and 2) eventual insufficiencies in the production of insulin, in which an individual may have a combination of deficient secretion and action of insulin (56). There are many genetic models with inherited hyperglycemia and obesity used for type 2 diabetic investigations. However, the etiology of these inbred models, and thus their construct validity, is only representative of a small percentage of human cases. They may not provide predictive validity and thus be useful for the pharmaceutical screening of drugs to treat the disease. In addition, a high-fat diet (HFD) has been used to develop insulin resistance in rodents and is used as a model of metabolic syndrome or pre-type 2 diabetes with symptoms of obesity, insulin resistance, and impaired glucose tolerance; it does not, however, develop overt diabetes (1, 4, 25, 41, 51). On the basis of work by Zhang et al. (56) and Zhang et al. (55), which characterized type 2 diabetic animal models, we chose to use a model using the combination of a HFD to induce insulin resistance and low-dose streptozotocin (STZ) injections to decrease insulin synthesis and secretion.
The goal of the present study was to test the development of the compensated and decompensated state of DBD in male rats treated with low-dose STZ coupled with a HFD. More specifically, we used the animal model created by Zhang et al. (56) to determine the effects of type 2 diabetes in male rats on DBD. The previous work of Zhang et al. (55) was performed only in female rats using the HFD/STZ protocol. We hypothesized that the compensated state would develop before 4 mo after STZ based on previous studies using different animal models (15, 55).
METHODS
Animals.
Male Sprague-Dawley rats were obtained from Taconic Biosciences (Rensselaer, NY) and received at 13 wk of age. All rats were given food and water ad libitum and maintained in a temperature- and humidity-controlled room on a 12:12-h light-dark cycle. Animals were housed on site in American Association for Laboratory Animal Care-accredited animal facilities at Drexel University College of Medicine. All protocols were approved by the Drexel University College of Medicine Institutional Animal Care and Use Committee.
HFD/STZ model of diabetes.
We used the method developed by Zhang et al. (56) and Zhang et al. (55) of inducing type 2 diabetes using a HFD (45% fat, diet no. D12451) obtained from Research Diets (New Brunswick, NJ) and two low doses of STZ (Sigma-Aldrich, St. Louis, MO). STZ was solubilized in deionized water immediately before use. Rats randomly assigned to the HFD/STZ group were switched from a normal chow diet (Purina Formulab Diet no. 5008, Purina, St. Louis, MO) to a HFD starting at 15 wk of age. After 1 mo of the HFD, rats were administered two injections of STZ (30 mg/kg ip) 1 wk apart and were kept on the HFD for the duration of the experiment. Rats assigned to the HFD group were switched to a HFD at 15 wk of age, whereas age-matched control groups were maintained on a regular chow diet. All diets were maintained for the duration of the experiment.
Body weight and nonfasting plasma glucose.
Animal weight and blood glucose measurements were obtained once a month. Blood glucose was measured using an AccuCheck Advantage blood glucometer (Roche Diagnostics, Indianapolis, IN) from blood obtained by nicking the tail vein of the rat with a needle. Only HFD/STZ-treated rats with nonfasting blood glucose ≥250 mg/dl, obtained between the hours of 9 AM and 5 PM, were considered diabetic and used in this study.
Voiding behavior.
Voiding behavior was measured before STZ injections, 1 wk after STZ injections, and then every month for 6 mo. Rats were removed from their housing unit and placed in metabolic cages for 6 h, between the hours of 11 AM and 5 PM. Rats were provided water ad libitum for the duration of the metabolic cage experiment. Food was restricted to prevent food particles from entering the urine collection because of the open nature of the wire mesh cage floor. The number of voids and volume per void were measured and recorded for each rat during the 6-h study period. Voiding mass was measured immediately using an analytical balance and converted to volume using an approximate density of 1 g/ml.
Bladder wet weight.
Rat urinary bladder weights from HFD/STZ, HFD, and age-matched control groups were measured on the same days isometric contraction experiments were performed. The urinary bladder was removed from the peritoneal cavity, and any adipose tissue was dissected from the exterior of the bladder. The bladder was then cut open longitudinally, blotted free of excess fluid, and weighed.
Bladder histology.
Whole bladder strips were placed in 15-ml tubes containing 10% formalin and shipped to AML Laboratories (Rosedale, MD) for paraffin embedding, sectioning (5 μm thickness), and Masson’s trichrome staining. Sections were then imaged using bright field on a Zeiss M2 imaging microscope. ImageJ (Bethesda, MD) software was used to measure bladder and smooth muscle thickness.
Isometric contraction measurements.
Rats were euthanized by CO2 inhalation followed by bilateral thoracotomy as approved by the Drexel University College of Medicine Institutional Animal Care and Use Committee. The urinary bladder was stored in ice-cold physiological salt solution (PSS) after removal from the peritoneal cavity. PSS contained 140 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.6 mM CaCl2, 1.2 mM Na2HPO4, 0.02 mM EDTA, 2.3 mM MOPS (pH 7.4), and 5 mM d-glucose. The mucosal/urothelial and serosal layers were removed, leaving a layer composed predominantly of bladder smooth muscle. This detrusor muscle layer was cut into longitudinal strips of ~1.5 × 6 mm in size. The strips were then stored in 4°C PSS until used. All strips were used for experimentation the same day the rat was euthanized.
Bladder smooth muscle strips were suspended between a Grass FT.03 force transducer and a stationary clip located within water-jacketed organ baths. Suspended strips were equilibrated for 45 min in PSS at 37°C and bubbled with 100% O2. PSS was changed every 10 min. A passive force of ~1 g was applied to the mounted tissues by stretch, and tissues were allowed to stress-relax after stretching (40). This was repeated four to five times or until passive force after stress relaxation was equivalent to ~1 g [which approximates preload (Lo)]. Bladder smooth muscle strips were then subjected three to four times to 110 mM KCl solution in PSS (with equimolar substitution of KCl for NaCl) followed by relaxation in PSS until similar levels of maximal force production were attained in response to KCl. Peak force levels in response to KCl stimulation were considered similar when peak force did not change >5% between subsequent stimulations. After relaxation to baseline in PSS, tissues were subjected to carbachol (Sigma-Aldrich) stimulation. Noncumulative concentration response curves to carbachol (100 nM–100 μM) were used. The maximal concentration of stimulus was used to recruit all muscle fibers to produce force so that comparisons between groups could be reliably performed (34). Force results were normalized to maximal force in response to 110 mM KCl. Valid comparisons were made by accounting for the following two aspects: 1) mounting tissues and stretching to the same preload (Lo) and 2) maximally activating tissues (high-KCl solution) (47). Force was defined as the difference between the maximal stable force in response to the stimulus and the basal passive force before the stimulus.
Neurogenic contraction measurements.
Neurogenic contraction measurements followed protocols described by Kendig et al. (23) and Liu and Daneshgari (27). Electrical field stimulation (EFS) was used to release endogenous neurotransmitters and determine their effect on force generation. Briefly, bladder smooth muscle strips were suspended between a Grass FT.03 force transducer and a stationary glass hook with two platinum wire electrodes ~2 mm apart (Radnoti, Monrovia, CA) located within water-jacketed organ baths. The electrode was connected to a Grass S88 stimulator through a Grass SIU-10 stimulus isolation unit. The strips were subjected three to four times to membrane depolarizing KCl-containing solution (110 mM KCl-PSS) followed by relaxation in PSS until similar levels of force production were attained in subsequent stimulations. After relaxation, tissues were exposed to frequency-response curves using the following stimulation settings: 120 V, 8-ms pulse width, 2- to 32-Hz frequency range, and 3-min interval between stimuli. For examination of the muscarinic component of the EFS response, 1 μM atropine was added into the organ bath for 30 min before a frequency-response curve was performed. The portion of force attributable to activation of muscarinic receptors was calculated by subtracting the force in the presence of atropine from force in the absence of atropine. EFS-induced force was normalized to force in response to 110 mM KCl.
Western blot analysis.
A polyacrylamide separating gel was used for SDS-PAGE. Proteins (20 μg) were electrophoresed at 200 V until the dye front reached the bottom of the gel. Proteins were transferred to a nitrocellulose membrane at 100 V for 75 min at 4°C. Membranes were blocked in Odyssey Blocking Buffer for 60 min at room temperature followed by incubation in a 1:1 solution of Odyssey Blocking Buffer (LI-COR Biosciences, Lincoln, NE) and 0.1% (vol/vol) Tris-buffered saline with Tween 20 (TBST)-containing antibodies raised against the muscarinic M3 receptor (1:200, catalog no. AMR-006, RRID:AB_2039997, Alomone Laboratories, Jerusalem, Israel) and α-actin (1:250,000, Sigma-Aldrich) overnight at 4°C. Membranes were washed for 5 min in 0.1% TBST five times. Membranes were incubated in a 1:1 solution of Odyssey Blocking Buffer and 0.1% TBST containing both anti-mouse 800CW and anti-rabbit 680LT fluorescent secondary antibodies (1:10,000 each, LI-COR Biosciences) for 45 min at room temperature. The washes were repeated, and membranes were imaged using an Odyssey Infrared Imager (LI-COR Biosciences). Quantification was performed using Image Studio Lite (version 4.0). Ratios of optical density of M3 receptors to optical density of α-actin from control and diabetic animals were normalized to 1 as seen in the Western blot quantification in Figs. 4C and 5C.
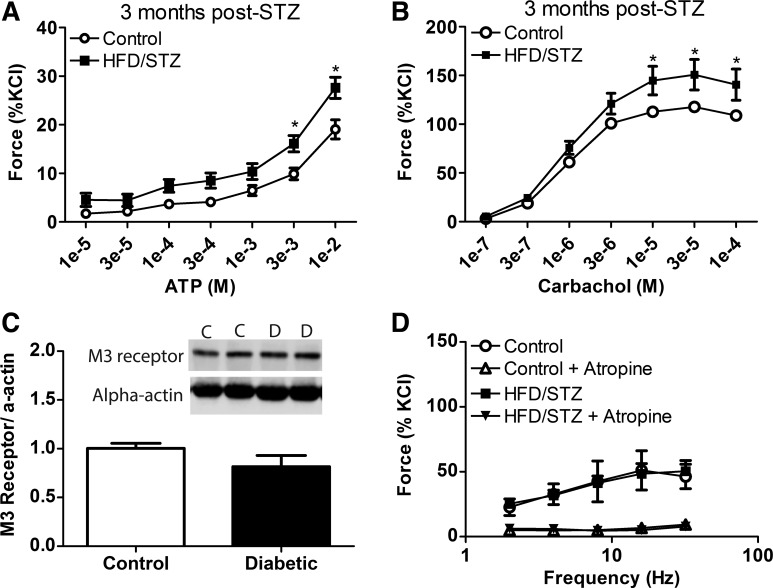
Fig. 4.
Characteristics of the compensated state of diabetic bladder dysfunction at 3 mo after streptozotocin (STZ). A and B: force to exogenous addition of ATP (A) and carbachol (B) was measured in isolated bladder smooth muscles from animals in the high-fat diet (HFD)/STZ and age-matched control groups. The HFD/STZ group exhibited a significant increase in the force response to both ATP and carbachol compared with the age-matched control group. C: muscarinic M3 receptor levels within the urinary smooth muscle were not significantly different between HFD/STZ and age-matched control groups. D: force in response to electrical field stimulation and the effects of atropine were not significantly different between HFD/STZ and age-matched control groups. Statistical comparisons were made using two-way repeated-measures ANOVA and a Bonferroni posttest (n = 4–6). *P ≤ 0.05 compared with the control group.
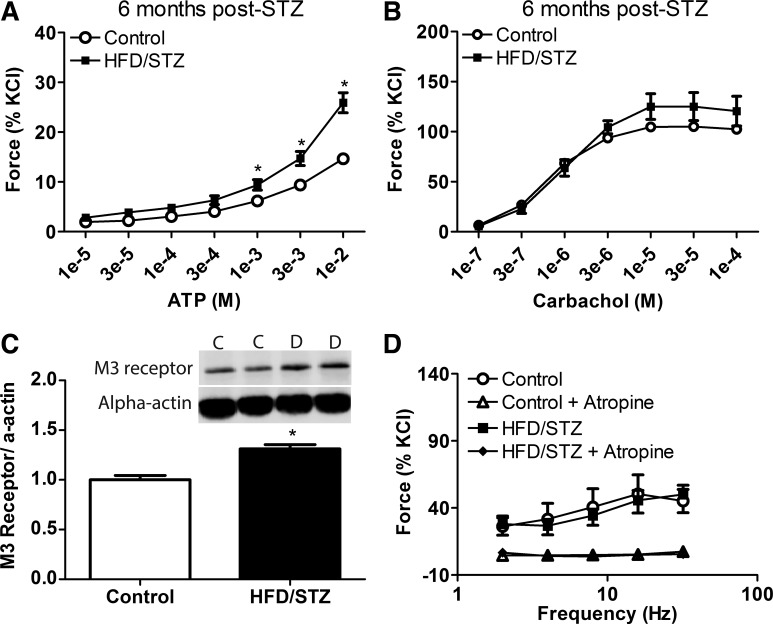
Fig. 5.
Characteristics of the decompensated state of diabetic bladder dysfunction at 6 mo after streptozotocin (STZ). A and B: force to exogenous addition of ATP (A) and carbachol (B) was measured in isolated urinary smooth muscles from animals in the high-fat diet (HFD)/STZ and age-matched control groups at 6 mo after STZ injections. Force in response to ATP was significantly increased in the HFD/STZ group; however, there was no significant difference in the force response to carbachol between the two groups. C: levels of bladder smooth muscle muscarinic M3 receptors within the HFD/STZ and age-matched groups. Expression of the M3 receptor within the urinary bladder of the HFD/STZ group was significantly increased compared with the age-matched control group. D: electrical field stimulation alone and in the presence of atropine measured in urinary bladder smooth muscle between the HFD/STZ and age-matched control groups. Statistical comparisons were made using an unpaired t-test or two-way repeated-measures ANOVA and a Bonferroni posttest (n = 4–6). *P ≤ 0.05 compared with the control group.
Statistical analysis.
Statistical significance was determined by either an unpaired t-test or two-way repeated-measures ANOVA with Bonferroni correction for posttest comparison. All n values refer to the number of animals; one to three strips of bladder smooth muscle from the same animal stimulated with the same agonist were averaged per n. An overall significance level of 0.05 was used for statistical comparisons. On the basis of outcome measures from previous studies (23, 38, 39, 50), power analysis indicates that, if these differences are significant and robust (α = 0.05, β = 0.8), then n = 6 per group will be needed for physiological and biochemical outcomes. Several additional animals were added to each group to account for attrition.
RESULTS
The HFD/STZ rat model exhibits increased nonfasting plasma glucose levels.
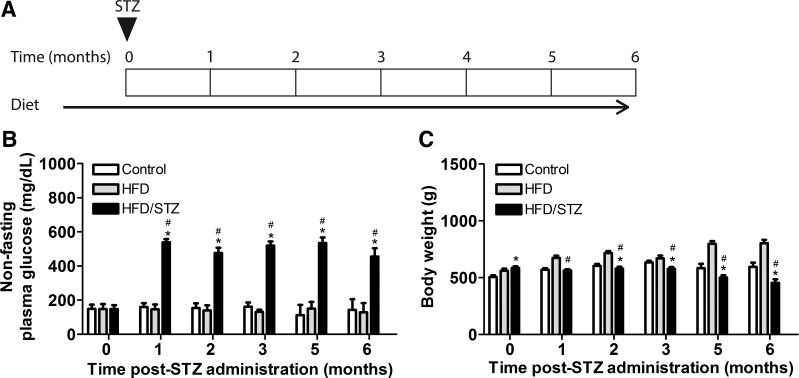
Figure 1A shows the timeline for the HFD/STZ model, including the time of STZ injections and duration of experiment. Nonfasting plasma glucose levels were monitored in three experimental groups (age-matched control, HFD, and HFD/STZ) over a total of 6 mo to determine the extent of diabetes disease progression (Fig. 1B). This allowed identification and utilization of animals with diabetes (nonfasting blood glucose levels of ≥250 mg/dl), which were used in further experiments. As expected, nonfasting plasma glucose levels in the HFD/STZ group increased significantly after STZ administration compared with both the age-matched control and HFD groups. The age-matched control and HFD groups maintained a similar nonfasting glucose level of 150 mg/dl (Fig. 1B).
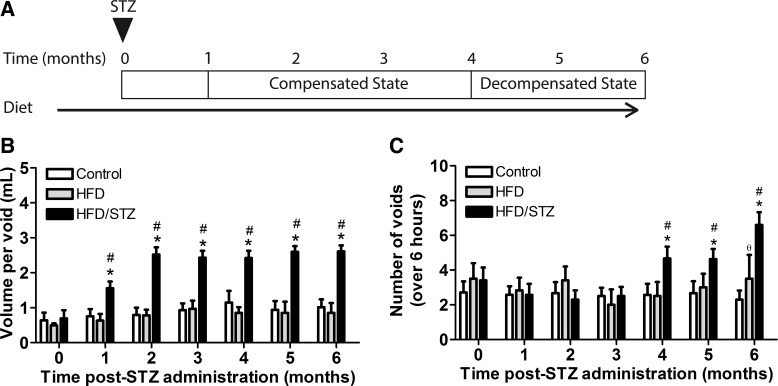
Fig. 1.
Timeline and general characteristics of the high-fat diet (HFD)/streptozotocin (STZ) model over a duration of 6 mo. A: HFD/STZ model timeline. The triangle indicates the timing of STZ intraperitoneal injections. Rats were randomly assigned into control, HFD, or HFD/STZ groups. After 1 mo of the assigned diet, the HFD/STZ group received two low-dose STZ injections (30 mg/kg) 1 wk apart. All experimental groups were maintained through 6 mo after STZ injections. B: nonfasting plasma glucose (in mg/dl) was measured in age-matched control, HFD, and HFD/STZ rats. The HFD/STZ group exhibited significant increases in nonfasting plasma glucose compared with both age-matched control and HFD groups from 1 to 6 mo after STZ. C: body weight (in g) was measured in the age-matched control, HFD, and HFD/STZ groups. Both HFD and HFD/STZ groups had increased body weight compared with the control group at the time of STZ injections. From 1 to 6 mo after STZ, the HFD group exhibited a significant increase in body weight, whereas the HFD/STZ group exhibited a significant decrease, compared with the control group. Statistical comparisons were made using two-way repeated-measures ANOVA and a Bonferroni posttest. *P ≤ 0.05 compared with the control group; #P ≤ 0.05 compared with the HFD group. Values are means ± SE of 12–20 animals from 1–4 mo after STZ and 3–7 animals from 5–6 mo after STZ. Measurements were taken 1 day per month.
Body weight measurements were compared between age-matched control, HFD, and HFD/STZ groups (Fig. 1C). At the time of STZ injections, the HFD/STZ and HFD groups exhibited significant increases in body weight attributable to maintenance on the HFD for 1 mo before injections. At 1 mo after STZ, the HFD group exhibited significant increases in body weight compared with the HFD/STZ group, which was maintained for the duration of the experiment. From 2 to 6 mo after STZ, the control group exhibited a significantly higher body weight compared with the HFD/STZ group.
The HFD/STZ rat model exhibits bladder hypertrophy.
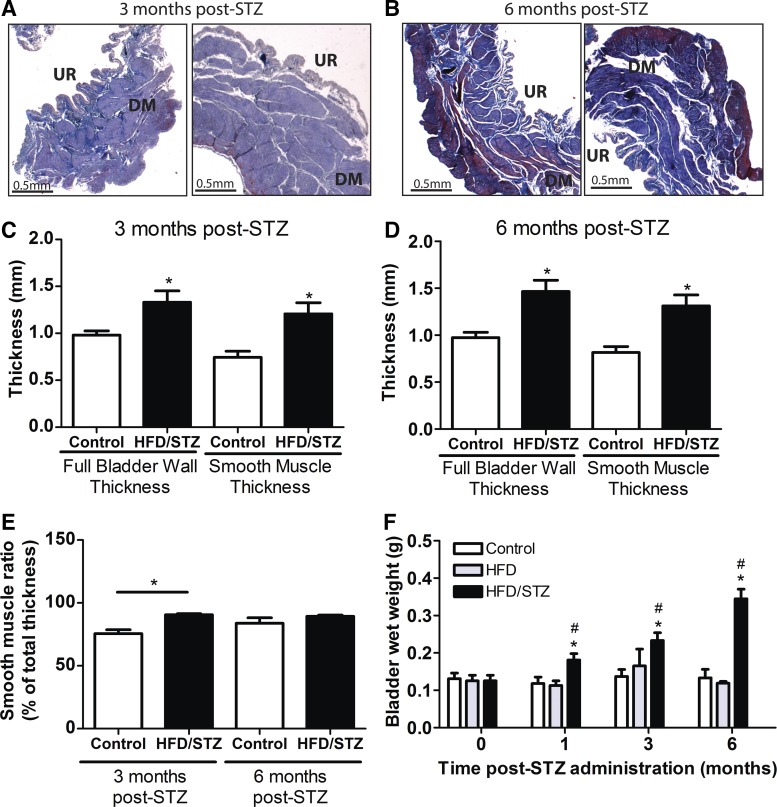
Urinary bladder morphology changes such as bladder hypertrophy are common occurrences within DBD (23, 25, 26, 28, 30, 42, 52, 55). To determine whether the HFD/STZ model induced bladder hypertrophy, intact bladder strips from 3 mo after STZ injections (Fig. 2A) and 6 mo after STZ injections (Fig. 2B) were fixed, sectioned, and stained with Masson’s trichrome. From these stained sections, the full bladder wall and smooth muscle thickness were calculated (Fig. 2, C and D). The HFD/STZ group at 3 and 6 mo after STZ injections exhibited significant increases in both full bladder wall thickness and smooth muscle content compared with the control group (Fig. 2, C and D). To determine whether there was smooth muscle layer hypertrophy in response to HFD/STZ, we measured smooth muscle thickness as a percentage of total tissue thickness (smooth muscle ratio; Fig. 2E). The smooth muscle ratio was significantly increased in the HFD/STZ group at 3 mo but not 6 mo after STZ injections. Bladder wet weight was compared between control, HFD, and HFD/STZ groups (Fig. 2F). From 1 wk to 6 mo after STZ injections, bladder wet weight from the HFD/STZ group was significantly increased compared with both control and HFD alone.
Fig. 2.
Urinary bladder weight and thickness of high-fat diet (HFD)/streptozotocin (STZ) and age-matched control rats. A and B: Masson’s trichrome-stained sections from rats 3 mo after STZ (A) and 6 mo after STZ (B). Left, age-matched control sections; right, HFD/STZ sections. UR, urothelium; DM, detrusor smooth muscle layer. C and D: the full bladder wall and smooth muscle thickness were measured in sections from age-matched control and HFD/STZ groups at 3 mo after STZ (C) and 6 mo after STZ (D). Both the full bladder wall and smooth muscle thickness at 3 and 6 mo after STZ injections were significantly increased in the HFD/STZ group (n = 3–5). E: the smooth muscle ratio (percentage of total thickness) was measured in animals 3 and 6 mo after STZ. The HFD/STZ group at 3 mo after STZ injections was significantly increased over controls (n = 3–5). F: bladder wet weight (in g) was measured over time to 6 mo after STZ injections in age-matched control, HFD, and HFD/STZ rats. HFD/STZ rats exhibited a significantly increased bladder wet weight compared with both age-matched control and HFD groups (n = 5–7). Statistical comparisons were made using an unpaired t-test (C–E) or two-way repeated-measures ANOVA and a Bonferroni posttest (F). *P ≤ 0.05 compared with the control group; #P ≤ 0.05 compared with the HFD group.
The HFD/STZ rat model exhibits a change in voiding behavior reflecting the compensated and decompensated states of DBD.
Daneshgari et al. (15) proposed two different states of DBD based on patient symptoms. Using Daneshgari et al.’s diabetic bladder dysfunction categorization, Zhang et al. (55) determined that the HFD/STZ female rat model developed the decompensated state at 4 mo after STZ injections).
To determine whether disease progression is similar between the sexes and whether both states develop temporally in male HFD/STZ rats, voiding behavior was measured. Figure 3A shows the time course to develop the compensated and decompensated states, based on voiding behavior measurements. Volume per void significantly increased in the HFD/STZ group from 1 mo after STZ injections to 6 mo after STZ injections compared with both the control and HFD groups (Fig. 3B). The number of voids was significantly increased in the HFD/STZ group from 4 mo after STZ injections to 6 mo after STZ injections compared with control and HFD groups (Fig. 4C). From these results, we concluded that the HFD/STZ model developed the compensated state of DBD at 1 mo after STZ as evidenced by an increase in volume per void. The decompensated state of DBD developed at 4 mo after STZ, characterized by an increase in both volume per void and number of voids. To further study the difference between the compensated and decompensated states, the time point at 3 mo after STZ was used to study changes within the compensated state, and 6 mo after STZ was used for the decompensated state.
Fig. 3.
Voiding behavior from high-fat diet (HFD)/streptozotocin (STZ) animals and age-matched control animals over a 6-mo period. A−C: proposed timeline of the compensated and decompensated states of the HFD/STZ model (A) based on volume per void (in ml; B) and number of voids over a 6-h period (C). The HFD/STZ group exhibited a significant increase in volume per void from 1 to 6 mo after STZ compared with both age-matched control and HFD groups (B). The number of voids over a 6-h period was significantly increased from 4 to 6 mo after STZ (C). Statistical comparisons were made using two-way repeated-measures ANOVA and a Bonferroni posttest. *P ≤ 0.05 compared with the control group; #P ≤ 0.05 compared with the HFD group; ϴP ≤ 0.05, HFD group compared with control group. Values are means ± SE of 12–20 animals from 1 wk before STZ to 4 mo after STZ and 3–7 animals from 5–6 mo after STZ. Measurements were taken 1 day per month for 1–6 mo.
The compensated state of DBD results in increased contractility to ATP and carbachol in the absence of changes in neurogenic force.
To determine whether detrusor muscle contractility is altered within the compensated state of DBD, functional reactivity experiments were performed to noncumulative addition of either ATP or carbachol, a purinergic and muscarinic receptor agonist, respectively.
Exogenous addition of ATP to urinary bladder smooth muscle strips resulted in an increase in contractility that was significantly increased in the HFD/STZ group compared with the age-matched control group at 3 mo after STZ (Fig. 4A), which was a similar result to 1 mo after STZ injections (data not shown). Exogenous addition of carbachol to urinary bladder smooth muscle strips also resulted in significantly increased contractility in the HFD/STZ group at 3 mo after STZ (Fig. 4B). Interestingly, this result was different from experiments at 1 mo after STZ, when there was not a significant difference between HFD/STZ and age-matched control groups (data not shown). The main muscarinic receptor responsible for force production in bladder smooth muscle is the M3 receptor subtype (7). Levels of M3 receptor in the urinary bladder smooth muscle (devoid of urothelium) were quantified using Western blot analysis. Levels of the M3 receptor were not significantly different between the control and HFD/STZ groups (Fig. 4C).
To determine whether there were changes in neurogenic force, force in response to EFS was measured alone and in the presence of atropine, a muscarinic receptor antagonist, to study the muscarinic component of neurogenic force (Fig. 4D). Total force generated by EFS was not significantly different between the control and HFD/STZ groups. Similarly, there were no significant differences in the muscarinic component of EFS force between control and HFD/STZ groups.
The decompensated state of DBD results in decreased force to carbachol in the absence of changes in neurogenic force.
Bladder smooth muscle contractility to ATP and carbachol was measured within the decompensated state of DBD at 6 mo after STZ. Exogenous addition of ATP to urinary bladder smooth muscle strips resulted in increased contractility in the HFD/STZ group compared with the age-matched control group (Fig. 5A). Bladder smooth muscle contractility in response to carbachol was not significantly different between control and HFD/STZ groups at 6 mo after STZ (Fig. 5B). This contrasts with contractility in the compensated state at 3 mo after STZ, which was significantly higher compared with age-matched controls (Fig. 4A). The HFD/STZ group exhibited a significant increase in muscarinic M3 receptor levels compared with the control group (Fig. 5C), which differed from findings in the compensated state. Neurogenic force within the decompensated state at 6 mo after STZ was not significantly different between the control and HFD/STZ groups with either EFS alone or in the presence of atropine to examine the muscarinic component (Fig. 5D).
Carbachol-induced force in the HFD/STZ group changes with disease progression.
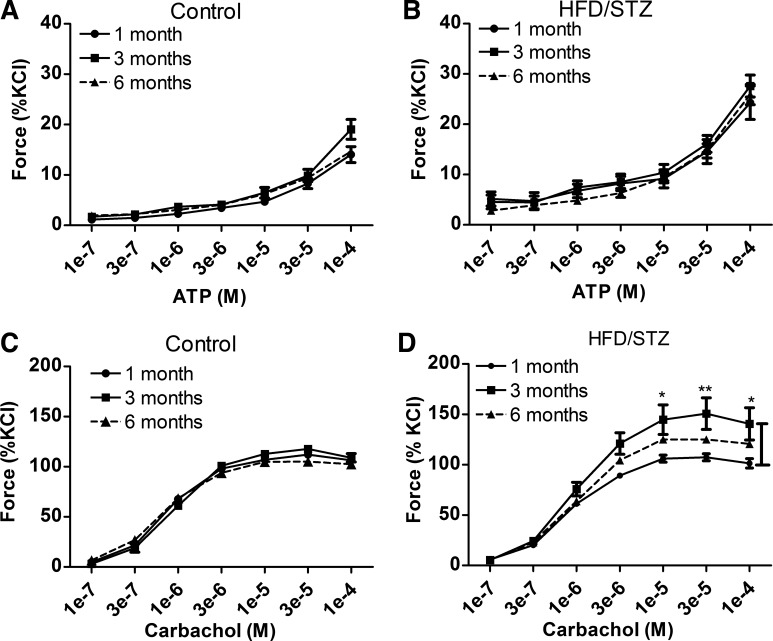
To determine whether there are urinary bladder smooth muscle alterations that correspond with disease progression in the compensated and decompensated states, contractility to exogenous ATP and carbachol was analyzed within the age-matched control or HFD/STZ groups (1, 3, and 6 mo). Exogenous addition of ATP to urinary bladder smooth muscle strips did not significantly alter contractility at different stages of disease progression in either the age-matched control (Fig. 6A) or HFD/STZ groups (Fig. 6B). Exogenous addition of carbachol to urinary bladder smooth muscle strips did not alter contractility in the age-matched control group (Fig. 6C); however, contractility in the HFD/STZ group was significantly increased at 3 mo compared with 1 mo after STZ injections (Fig. 6D). Contractility was not significantly different between groups from 1 and 6 mo after STZ injections. Even though 6 mo contractility in the HFD/STZ group was not significantly different compared with both age-matched control groups at 6 mo or 1 mo after STZ, there was a decrease in contractility within the HFD/STZ group from 3 to 6 mo after STZ injections. This suggests time-dependent changes induced by HFD/STZ treatment during the transition from compensated to decompensated states of dysfunction.
Fig. 6.
Maximal force changes to exogenous addition of ATP and carbachol over disease progression in the high-fat diet (HFD)/streptozotocin (STZ) and age-matched control groups from 1 to 6 mo. To determine changes in maximal force over disease progression in either the HFD/STZ or age-matched control group, 1, 3, and 6 mo after STZ, ATP- and carbachol-induced force were compared. A and B: exogenous additions of ATP-induced force over disease progression in age-matched control (A) and HFD/STZ (B) urinary bladder smooth muscles. ATP-induced force did not change over disease progression in either the HFD/STZ or age-matched control groups. C and D: exogenous additions of carbachol-induced force over disease progression in age-matched control (C) and HFD/STZ (D) urinary bladder smooth muscles. Carbachol-induced force in the control group did not change over disease progression; however, carbachol-induced force in the HFD/STZ group was significantly changed. Bladder smooth muscle force from 3 mo after STZ was significantly increased over 1 mo after STZ. Force between 1 and 6 mo after STZ injections was not significantly different within the HFD/STZ group. Statistical comparisons were made using two-way repeated-measures ANOVA and a Bonferroni posttest. *P ≤ 0.05.
DISCUSSION
DBD, although prevalent, lacks both a clear understanding of the pathogenesis and efficacious and well-tolerated treatments. The goal of the present study was to determine whether the experimental type 2 diabetic model, using the combination of a HFD and two low doses of STZ (30 mg/kg) in male Sprague-Dawley rats, developed both compensated and decompensated states of DBD and to characterize the timeline of development. We also identified distinguishing characteristics of the states to allow for the development of novel treatment options (Table 1). The experimental model uses a HFD to induce insulin resistance and low-dose STZ to weaken β-cells and decrease insulin production (32, 56).
Table 1.
Characteristics of the compensated and decompensated states of diabetic bladder dysfunction in the high-fat diet/STZ rat model
| Compensated State | Decompensated State | |
|---|---|---|
| Description | Storage problems | Voiding problems |
| Start | After STZ injections | 4 mo after STZ injections |
| Characteristics | ||
| Plasma glucose | ↑ | ↑ |
| Urine volume per void | ↑ | ↑ |
| Number of voids | ↔ | ↑ |
| Body weight | ↔ | ↓ |
| Bladder wet weight | ↑ | ↑ |
| Smooth muscle thickness | ↑ | ↑ |
| Smooth muscle ratio | ↑ | ↔ |
| Detrusor muscle changes | ||
| ATP-induced force | ↑ | ↑ |
| Carbachol-induced force | ↑ | ↔ |
| M3 receptor expression | ↔ | ↑ |
STZ, streptozotocin; ↑, increase; ↔, no change; ↓, decrease.
Our results demonstrated the development of the compensated state of DBD, which progressed into the decompensated state in this male rat HFD/STZ animal model. This study resulted in two main conclusions. The first conclusion is that the compensated state of bladder dysfunction in the HFD/STZ type 2 diabetic model developed by 1 mo after STZ administration. This state is characterized by increased nonfasting plasma glucose levels, increased bladder wet weight, and increased volume per void without a change in the number of voids. This state persists through 3 mo after STZ injections. The second conclusion is that the decompensated state of the HFD/STZ model developed by 4 mo after STZ administration, characterized by increased nonfasting plasma glucose levels, increased bladder wet weight, increased volume per void, and increased number of voids. The response to exogenous carbachol also reverted to levels not different than those 1 mo after STZ injections, whereas, during the compensated state at 3 mo, carbachol-induced force was significantly increased. The decompensated state persisted through the end of the experiment at 6 mo after STZ.
In the present work, the HFD/STZ model uses a HFD to induce insulin resistance, simulating the pathology of type 2 diabetes in humans. Insulin resistance and glucose intolerance have been reported to develop within 2 wk of a HFD (32, 36, 37, 55). After 5 wk of a HFD, an increase in body fat percentage and impaired glucose tolerance with hyperinsulemia have been reported (36). Neither 2 nor 5 wk of a HFD was enough time to induce the development of hyperglycemia, similar to our results (36). To control for the effect of diet alone, we used two control groups: one group was fed a regular chow diet and the other group was given a HFD for the duration of the experiment but never received STZ injections. In our model, body weight increased in the HFD/STZ group compared with the age-matched control group after 1 mo on the HFD. Body weight in the HFD group became significantly increased compared with the HFD/STZ group at 1 mo after STZ injections and continued to increase for the duration of the experiment. Although there were stark increases in body weight within the HFD group (Fig. 1C), there were no changes in nonfasting plasma glucose levels (Fig. 1B), bladder wet weight (Fig. 2F), or voiding behavior compared with controls (Fig. 3, B and C). Therefore, we concluded that HFD alone does not alter nonfasting plasma glucose levels or urinary bladder function, at least within the 6-mo duration of our experiment.
In the HFD/STZ type 2 diabetic model, animals exhibited increased glucose levels concurrent with the original studies on the inducible HFD/STZ type 2 model and other models of type 2 diabetes (23, 30, 53), indicating the development of diabetes (32, 55, 56). Initial changes that have been reported with DBD include an increase in voiding behavior, which is similar to symptoms of overactive bladder and hypertrophy of the bladder (15, 19, 20, 54). Increased voiding behavior, or diuresis, is an early sign and symptom of the development of type 1 and type 2 diabetes. This is due to hyperglycemia-induced polyuria, in which the increased presence of glucose acts as an osmotic diuretic. An increase in urine volume chronically increases the workload of the bladder, and, as a result, the bladder hypertrophies to enhance the ability to produce force. We showed that, in the HFD/STZ model of type 2 diabetes in male rats, an increase in plasma glucose correlates with an increase in both voiding volume and bladder hypertrophy. Our results agree with other studies showing that increased voiding behavior induced bladder hypertrophy, which occurred in both type 1 and type 2 diabetes because of high plasma glucose with the presence of glucose in urine as a common symptom (14, 16, 23, 53).
In two different type 1 diabetic models using STZ in mice (60 mg/kg) and rats (65 mg/kg), Daneshgari et al. (14) demonstrated the transition of a compensated to a decompensated bladder of DBD 2–3 mo after STZ administration. The authors concluded that, in type 1 models, the compensated state of DBD occurred 1–2 mo after STZ administration attributable to increased urine output, bladder weight, volume per void, peak voiding pressure, bladder capacity, and residual volume (14). The decompensated state of DBD occurred 3–5 mo after STZ administration characterized by increased void volume, bladder capacity, and residual volume as well as decreased mean voiding pressure and peak voiding pressure (14). Within the diabetic group, urine output increased slightly during the transition from the compensated state to the decompensated state, whereas the mean voided volume remained relatively stable (14). It is important to note that the authors did not make comparisons of different states of DBD within groups. Zhang et al. (15, 55) reported in the HFD/STZ female model of type 2 diabetes that bladder function at 4 mo after STZ was most consistent with hypocontractile bladder and end-state DBD based on cystometric measurements as defined in the temporal theory of DBD. These animals exhibited an increased micturition interval, increased urine volume per void, increased maximal voiding pressure, and increased residual volume at 4 mo after STZ administration (55). We characterized the HFD/STZ model in male rats and showed the compensated and decompensated states of bladder dysfunction through changes in voiding behavior, consistent with Zhang et al. (15, 55). Our study also adds experimental data showing changes in function at the level of the smooth muscle. The transition between compensated and decompensated states in this male rat HFD/STZ type 2 diabetic model begins between 3 and 4 mo after STZ administration, as opposed to 2–3 mo as previously reported by Daneshgari et al. (14, 16) in a type 1 diabetic animal. This may be related to the difference in STZ dosing, which was high in the Daneshgari et al. model (65 mg/kg) and lower in the model used by Zhang et al. and in the present study (30 mg/kg). High-dose STZ induces pancreatic β-cell death, which mimics the pathogenesis of type 1 diabetes. The present HFD/STZ model that incorporates insulin resistance through HFD and lower doses of STZ may present a timeline closer to bladder dysfunction in patients with type 2 diabetes.
Detrusor contractility within the compensated and decompensated states of DBD in this HFD/STZ type 2 diabetic model was also investigated. At 3 mo after STZ administration, the HFD/STZ group is in a compensated state of DBD. Contractility to the exogenous addition of carbachol was significantly increased in the diabetic group compared with age-matched controls. Furthermore, neurogenic force elicited by EFS was not different in our type 2 diabetic model compared with control animals. Our results are in agreement with our previous study (23) in another type 2 diabetic model, the Zucker diabetic fatty rat. In the genetic Zucker diabetic fatty rat model at 6 mo of age, there were no significant differences between diabetic and control EFS-induced force (23). Similarly, in the genetic Goto-Kakizaki rat at 1, 2, and 4 mo of age, there were no significant differences in EFS-induced force (53). Together, these findings may indicate that, in type 2 diabetic animal models, the compensated state does not induce or is not the result of autonomic nerve dysfunction.
At 6 mo after STZ administration, the diabetic group showed, in contrast to 3 mo after STZ administration, no change in contractility to exogenous carbachol compared with the age-matched control group. Not only was contractility to carbachol in the diabetic group no longer significantly different from the control group, but contractility at 6 mo after STZ was not significantly different from 1 mo after STZ. This suggests that there were time-dependent changes in bladder function and that these changes were at least partly mediated by a decrease in the contractility to carbachol from 3 mo after STZ to 6 mo after STZ.
The expression level of muscarinic M3 receptors was significantly increased in diabetic detrusor in the decompensated state at 6 mo after STZ administration. Even though detrusor contractility to exogenous carbachol or ATP in control and diabetic detrusor muscles was not significantly different at this stage, the time-dependent decrease in carbachol-induced force seen from 3 mo after STZ to 6 mo after STZ in the diabetic detrusor muscle suggests a decrease in muscarinic receptor-induced force. This could be the result of a decrease in ACh release from nerves. A decrease in parasympathic innervation has been shown to result in detrusor underactivity and can lead to an upregulation of the postsynaptic M3 receptor as a compensatory mechanism to maintain contractility (44, 45). In fact, our data show this increase in M3 receptor expression in the decompensated state. In the genetic Goto-Kakizaki type 2 diabetic model, Saito et al. (33) showed increases in M3 and M2 mRNA levels at 18 mo of age; however, this increase was accompanied by an increase in carbachol-induced force and an increase in residual volume. There are multiple studies of type 1 diabetes and increased M3 expression. In early type 1 diabetes, Leiria et al. (25) showed increased expression of M3 but not M2 receptor expression, whereas Tong et al. (44–46) showed increased expression of both M3 and M2 receptor expression.
It is important to note that these experiments were completed in denuded bladder strips. Because the urinary bladder is made up of different tissues, it is important to understand the role of each tissue layer and its contribution to DBD symptoms. We focused primarily on the smooth muscle layer; however, it is important to note that other studies have indicated an important role of the urothelium in the regulation of bladder physiology (13, 21, 24, 49).
Although therapeutic options are available to manage diabetes, there are limiting side effects and no curative treatments. New treatment options for type 2 diabetics are continually being investigated; one such therapeutic class comprises Na+-glucose cotransporter 2 (SGLT2) inhibitors [Farxiga (dapagliflozin), AstraZeneca Pharmaceuticals, Wilmington, DE] (22, 48). SGLT2 inhibitors reduce blood glucose levels by increasing the amount of glucose excreted into urine. An increased amount of urinary glucose via SGLT2 inhibition significantly increases urinary output, frequency, urgency, and incidence of nocturia [Steglatro (ertugliflozin), Merck, Sharp, & Dohme, Whitehouse Station, NJ] (43). Furthermore, a recent study (35) concluded that treatment with SGLT2 inhibitors increases daytime frequency in patients with type 2 diabetes without overactive bladder before treatment but had no effect on preexisting overactive bladder. However, more studies are needed to determine the effects of SGLT2 inhibitors on DBD. Presently, the use of SGLT2 inhibitors in patients with overactive or underactive bladder is not a contraindication. The prominent use of this specific drug class makes research into the effects of increased glucose concentrations on bladder dysfunction even more important. Patients may now be spending years of their lives with increased urinary glucose concentrations, which may have chronic effects on bladder function.
Diabetes is a lifelong disease. With an increasing percentage of the population living with long-term diabetes, there will be a corresponding increase in patients with diabetic complications. Furthermore, the increased and chronic use of medications that specifically increase urinary glucose may also increase the number of people dealing with bladder dysfunction symptoms. Our study shows that the HFD/STZ model in male Sprague-Dawley rats exhibits the compensated and decompensated states of DBD and can be used to further study mechanistic changes between the two states of bladder dysfunction and specific therapeutics, such as SGLT2 inhibitors. Understanding the pathophysiology of DBD is of great importance and will contribute to novel bladder dysfunction treatment options and therefore enhanced quality of life for millions of people living with and managing diabetes.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.S.M. conceived and designed research; N.S.K. performed experiments; N.S.K. analyzed data; N.S.K., R.S.M., and D.M.K. interpreted results of experiments; N.S.K. prepared figures; N.S.K. and D.M.K. drafted manuscript; N.S.K. and D.M.K. edited and revised manuscript; N.S.K. and D.M.K. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Sarah Sivilich for help provided in obtaining blood glucose measurements.
REFERENCES
- 1.Ahrén B, Pacini G. Insufficient islet compensation to insulin resistance vs. reduced glucose effectiveness in glucose-intolerant mice. Am J Physiol Endocrinol Metab 283: E738–E744, 2002. doi: 10.1152/ajpendo.00199.2002. [DOI] [PubMed] [Google Scholar]
- 4.Ahrén B, Simonsson E, Scheurink AJ, Mulder H, Myrsén U, Sundler F. Dissociated insulinotropic sensitivity to glucose and carbachol in high-fat diet-induced insulin resistance in C57BL/6J mice. Metabolism 46: 97–106, 1997. doi: 10.1016/S0026-0495(97)90175-X. [DOI] [PubMed] [Google Scholar]
- 5.Altuntas CZ, Daneshgari F, Izgi K, Bicer F, Ozer A, Sakalar C, Grimberg KO, Sayin I, Tuohy VK. Connective tissue and its growth factor CTGF distinguish the morphometric and molecular remodeling of the bladder in a model of neurogenic bladder. Am J Physiol Renal Physiol 303: F1363–F1369, 2012. doi: 10.1152/ajprenal.00273.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersson KE. Storage and voiding symptoms: pathophysiologic aspects. Urology 62, Suppl 2: 3–10, 2003. doi: 10.1016/j.urology.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 7.Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 84: 935–986, 2004. doi: 10.1152/physrev.00038.2003. [DOI] [PubMed] [Google Scholar]
- 8.Andersson KE, Hedlund P. Pharmacologic perspective on the physiology of the lower urinary tract. Urology 60, Suppl 1: 13–20, 2002. doi: 10.1016/S0090-4295(02)01786-7. [DOI] [PubMed] [Google Scholar]
- 9.Brown JS, Wessells H, Chancellor MB, Howards SS, Stamm WE, Stapleton AE, Steers WD, Van Den Eeden SK, McVary KT. Urologic complications of diabetes. Diabetes Care 28: 177–185, 2005. doi: 10.2337/diacare.28.1.177. [DOI] [PubMed] [Google Scholar]
- 10.Chang S, Hypolite JA, DiSanto ME, Changolkar A, Wein AJ, Chacko S. Increased basal phosphorylation of detrusor smooth muscle myosin in alloxan-induced diabetic rabbit is mediated by upregulation of Rho-kinase beta and CPI-17. Am J Physiol Renal Physiol 290: F650–F656, 2006. doi: 10.1152/ajprenal.00235.2005. [DOI] [PubMed] [Google Scholar]
- 11.Chang S, Hypolite JA, Mohanan S, Zderic SA, Wein AJ, Chacko S. Alteration of the PKC-mediated signaling pathway for smooth muscle contraction in obstruction-induced hypertrophy of the urinary bladder. Lab Invest 89: 823–832, 2009. doi: 10.1038/labinvest.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Changolkar AK, Hypolite JA, Disanto M, Oates PJ, Wein AJ, Chacko S. Diabetes induced decrease in detrusor smooth muscle force is associated with oxidative stress and overactivity of aldose reductase. J Urol 173: 309–313, 2005. doi: 10.1097/01.ju.0000141583.31183.7a. [DOI] [PubMed] [Google Scholar]
- 13.Cheng SF, Jiang YH, Kuo HC. Urothelial dysfunction and chronic inflammation are associated with increased bladder sensation in patients with chronic renal insufficiency. Int Neurourol J 22, Suppl 1: S46–S54, 2018. doi: 10.5213/inj.1832814.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daneshgari F, Huang X, Liu G, Bena J, Saffore L, Powell CT. Temporal differences in bladder dysfunction caused by diabetes, diuresis, and treated diabetes in mice. Am J Physiol Regul Integr Comp Physiol 290: R1728–R1735, 2006. doi: 10.1152/ajpregu.00654.2005. [DOI] [PubMed] [Google Scholar]
- 15.Daneshgari F, Liu G, Birder L, Hanna-Mitchell AT, Chacko S. Diabetic bladder dysfunction: current translational knowledge. J Urol 182, Suppl: S18–S26, 2009. doi: 10.1016/j.juro.2009.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daneshgari F, Liu G, Imrey PB. Time dependent changes in diabetic cystopathy in rats include compensated and decompensated bladder function. J Urol 176: 380–386, 2006. doi: 10.1016/S0022-5347(06)00582-9. [DOI] [PubMed] [Google Scholar]
- 17.Frimodt-Møller C. Diabetic cystopathy: epidemiology and related disorders. Ann Intern Med 92: 318–321, 1980. doi: 10.7326/0003-4819-92-2-318. [DOI] [PubMed] [Google Scholar]
- 18.Frimodt-Møller C, Mortensen S. Treatment of diabetic cystopathy. Ann Intern Med 92: 327–328, 1980. doi: 10.7326/0003-4819-92-2-327. [DOI] [PubMed] [Google Scholar]
- 19.Golbidi S, Laher I. Bladder dysfunction in diabetes mellitus. Front Pharmacol 1: 136, 2010. doi: 10.3389/fphar.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez CS, Kanagarajah P, Gousse AE. Bladder dysfunction in patients with diabetes. Curr Urol Rep 12: 419–426, 2011. doi: 10.1007/s11934-011-0214-0. [DOI] [PubMed] [Google Scholar]
- 21.Hanna-Mitchell AT, Ruiz GW, Daneshgari F, Liu G, Apodaca G, Birder LA. Impact of diabetes mellitus on bladder uroepithelial cells. Am J Physiol Regul Integr Comp Physiol 304: R84–R93, 2013. doi: 10.1152/ajpregu.00129.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasan FM, Alsahli M, Gerich JE. SGLT2 inhibitors in the treatment of type 2 diabetes. Diabetes Res Clin Pract 104: 297–322, 2014. doi: 10.1016/j.diabres.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 23.Kendig DM, Ets HK, Moreland RS. Effect of type II diabetes on male rat bladder contractility. Am J Physiol Renal Physiol 310: F909–F922, 2016. doi: 10.1152/ajprenal.00511.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klee NS, McCarthy CG, Lewis S, McKenzie JL, Vincent JE, Webb RC. Urothelial senescence in the pathophysiology of diabetic bladder dysfunction−a novel hypothesis. Front Surg 5: 72, 2018. doi: 10.3389/fsurg.2018.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leiria LO, Sollon C, Calixto MC, Lintomen L, Mónica FZ, Anhê GF, De Nucci G, Zanesco A, Grant AD, Antunes E. Role of PKC and CaV1.2 in detrusor overactivity in a model of obesity associated with insulin resistance in mice. PLoS One 7: e48507, 2012. doi: 10.1371/journal.pone.0048507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lincoln J, Haven AJ, Sawyer M, Burnstock G. The smooth muscle of rat bladder in the early stages of streptozotocin-induced diabetes. Br J Urol 56: 24–30, 1984. doi: 10.1111/j.1464-410X.1984.tb07157.x. [DOI] [PubMed] [Google Scholar]
- 27.Liu G, Daneshgari F. Alterations in neurogenically mediated contractile responses of urinary bladder in rats with diabetes. Am J Physiol Renal Physiol 288: F1220–F1226, 2005. doi: 10.1152/ajprenal.00449.2004. [DOI] [PubMed] [Google Scholar]
- 28.Liu G, Daneshgari F. Temporal diabetes- and diuresis-induced remodeling of the urinary bladder in the rat. Am J Physiol Regul Integr Comp Physiol 291: R837–R843, 2006. doi: 10.1152/ajpregu.00917.2005. [DOI] [PubMed] [Google Scholar]
- 29.Mattiasson A, Andersson KE, Sjögren C, Sundin T, Uvelius B. Supersensitivity to carbachol in the parasympathetically decentralized feline urinary bladder. J Urol 131: 562–565, 1984. doi: 10.1016/S0022-5347(17)50504-2. [DOI] [PubMed] [Google Scholar]
- 30.Oger-Roussel S, Behr-Roussel D, Caisey S, Kergoat M, Charon C, Audet A, Bernabé J, Alexandre L, Giuliano F. Bladder and erectile dysfunctions in the type 2 diabetic Goto-Kakizaki rat. Am J Physiol Regul Integr Comp Physiol 306: R108–R117, 2014. doi: 10.1152/ajpregu.00033.2013. [DOI] [PubMed] [Google Scholar]
- 31.Qiao Z, Xia C, Shen S, Corwin FD, Liu M, Guan R, Grider JR, Qiao LY. Suppression of the PI3K pathway in vivo reduces cystitis-induced bladder hypertrophy and restores bladder capacity examined by magnetic resonance imaging. PLoS One 9: e114536, 2014. doi: 10.1371/journal.pone.0114536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reed MJ, Meszaros K, Entes LJ, Claypool MD, Pinkett JG, Gadbois TM, Reaven GM. A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metabolism 49: 1390–1394, 2000. doi: 10.1053/meta.2000.17721. [DOI] [PubMed] [Google Scholar]
- 33.Saito M, Gotoh M, Kato K, Kondo A. Influence of aging on the rat urinary bladder function. Urol Int 47, Suppl 1: 39–42, 1991. doi: 10.1159/000282247. [DOI] [PubMed] [Google Scholar]
- 34.Schoenfeld BJ. The mechanisms of muscle hypertrophy and their application to resistance training. J Strength Cond Res 24: 2857–2872, 2010. doi: 10.1519/JSC.0b013e3181e840f3. [DOI] [PubMed] [Google Scholar]
- 35.Shikuma J, Ito R, Sasaki-Shima J, Teshima A, Hara K, Takahashi T, Sakai H, Miwa T, Kanazawa A, Odawara M. Changes in overactive bladder symptoms after sodium glucose cotransporter-2 inhibitor administration to patients with type 2 diabetes. Pract Diabetes 35: 47–50, 2018. doi: 10.1002/pdi.2160. [DOI] [Google Scholar]
- 36.Skovsø S. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J Diabetes Investig 5: 349–358, 2014. doi: 10.1111/jdi.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res 52: 313–320, 2005. doi: 10.1016/j.phrs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Stanton MC, Clement M, Macarak EJ, Zderic SA, Moreland RS. Partial bladder outlet obstruction alters Ca2+ sensitivity of force, but not of MLC phosphorylation, in bladder smooth muscle. Am J Physiol Renal Physiol 285: F703–F710, 2003. doi: 10.1152/ajprenal.00162.2003. [DOI] [PubMed] [Google Scholar]
- 39.Su X, Changolkar A, Chacko S, Moreland RS. Diabetes decreases rabbit bladder smooth muscle contraction while increasing levels of myosin light chain phosphorylation. Am J Physiol Renal Physiol 287: F690–F699, 2004. doi: 10.1152/ajprenal.00027.2004. [DOI] [PubMed] [Google Scholar]
- 40.Su X, Stein R, Stanton MC, Zderic S, Moreland RS. Effect of partial outlet obstruction on rabbit urinary bladder smooth muscle function. Am J Physiol Renal Physiol 284: F644–F652, 2003. doi: 10.1152/ajprenal.00274.2002. [DOI] [PubMed] [Google Scholar]
- 41.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes 37: 1163–1167, 1988. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- 42.Szasz T, Wenceslau CF, Burgess B, Nunes KP, Webb RC. Toll-like receptor 4 activation contributes to diabetic bladder dysfunction in a murine model of type 1 diabetes. Diabetes 65: 3754–3764, 2016. doi: 10.2337/db16-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terra SG, Focht K, Davies M, Frias J, Derosa G, Darekar A, Golm G, Johnson J, Saur D, Lauring B, Dagogo-Jack S. Phase III, efficacy and safety study of ertugliflozin monotherapy in people with type 2 diabetes mellitus inadequately controlled with diet and exercise alone. Diabetes Obes Metab 19: 721–728, 2017. doi: 10.1111/dom.12888. [DOI] [PubMed] [Google Scholar]
- 44.Tong YC, Cheng JT. Alteration of M3 subtype muscarinic receptors in the diabetic rat urinary bladder. Pharmacology 64: 148–151, 2002. doi: 10.1159/000056164. [DOI] [PubMed] [Google Scholar]
- 45.Tong YC, Chin WT, Cheng JT. Alterations in urinary bladder M2-muscarinic receptor protein and mRNA in 2-week streptozotocin-induced diabetic rats. Neurosci Lett 277: 173–176, 1999. doi: 10.1016/S0304-3940(99)00871-X. [DOI] [PubMed] [Google Scholar]
- 46.Tong YC, Chin WT, Cheng JT. Role of sorbitol in the up-regulation of urinary bladder M(2) muscarinic receptors in streptozotocin-induced diabetic rats. Neurourol Urodyn 21: 154–159, 2002. doi: 10.1002/nau.10028. [DOI] [PubMed] [Google Scholar]
- 47.Uvelius B. Detrusor smooth muscle in rats with alloxan-induced diabetes. J Urol 136: 949–952, 1986. doi: 10.1016/S0022-5347(17)45138-X. [DOI] [PubMed] [Google Scholar]
- 48.van Baar MJB, van Ruiten CC, Muskiet MHA, van Bloemendaal L, IJzerman RG, van Raalte DH. SGLT2 inhibitors in combination therapy: from mechanisms to clinical considerations in type 2 diabetes management. Diabetes Care 41: 1543–1556, 2018. doi: 10.2337/dc18-0588. [DOI] [PubMed] [Google Scholar]
- 49.Wang CC, Kuo HC. Urothelial dysfunction and chronic inflammation in diabetic patients with overactive bladder. Low Urin Tract Symptoms 9: 151–156, 2017. doi: 10.1111/luts.12126. [DOI] [PubMed] [Google Scholar]
- 50.Wang T, Kendig DM, Smolock EM, Moreland RS. Carbachol-induced rabbit bladder smooth muscle contraction: roles of protein kinase C and Rho kinase. Am J Physiol Renal Physiol 297: F1534–F1542, 2009. doi: 10.1152/ajprenal.00095.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winzell MS, Ahrén B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 53, Suppl 3: S215–S219, 2004. doi: 10.2337/diabetes.53.suppl_3.S215. [DOI] [PubMed] [Google Scholar]
- 52.Xiao N, Wang Z, Huang Y, Daneshgari F, Liu G. Roles of polyuria and hyperglycemia in bladder dysfunction in diabetes. J Urol 189: 1130–1136, 2013. doi: 10.1016/j.juro.2012.08.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yono M, Latifpour J, Yoshida M, Ueda S. Age-related alterations in the biochemical and functional properties of the bladder in type 2 diabetic GK rats. J Recept Signal Transduct Res 25: 147–157, 2005. doi: 10.1080/10799890500210461. [DOI] [PubMed] [Google Scholar]
- 54.Yoshimura N, Chancellor MB, Andersson KE, Christ GJ. Recent advances in understanding the biology of diabetes-associated bladder complications and novel therapy. BJU Int 95: 733–738, 2005. doi: 10.1111/j.1464-410X.2005.05392.x. [DOI] [PubMed] [Google Scholar]
- 55.Zhang H, Qiu X, Shindel AW, Ning H, Ferretti L, Jin X, Lin G, Lin CS, Lue TF. Adipose tissue-derived stem cells ameliorate diabetic bladder dysfunction in a type II diabetic rat model. Stem Cells Dev 21: 1391–1400, 2012. doi: 10.1089/scd.2011.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang M, Lv XY, Li J, Xu ZG, Chen L. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp Diabetes Res 2008: 1–9, 2008. doi: 10.1155/2008/704045. [DOI] [PMC free article] [PubMed] [Google Scholar]