Abstract
The proper function of the organs that make up the urinary tract (kidneys, ureters, bladder, and urethra) depends on their ability to sense and respond to mechanical forces, including shear stress and wall tension. However, we have limited understanding of the mechanosensors that function in these organs and the tissue sites in which these molecules are expressed. Possible candidates include stretch-activated PIEZO channels (PIEZO1 and PIEZO2), which have been implicated in mechanically regulated body functions including touch sensation, proprioception, lung inflation, and blood pressure regulation. Using reporter mice expressing a COOH-terminal fusion of Piezo1 with the sequence for the tandem-dimer Tomato gene, we found that PIEZO1 is expressed in the kidneys, ureters, bladder, and urethra as well as organs in close proximity, including the prostate, seminal vesicles and ducts, ejaculatory ducts, and the vagina. We further found that PIEZO1 expression is not limited to one cell type; it is observed in the endothelial and parietal cells of the renal corpuscle, the basolateral surfaces of many of the epithelial cells that line the urinary tract, the interstitial cells of the bladder and ureters, and populations of smooth and striated muscle cells. We propose that in the urinary tract, PIEZO1 likely functions as a mechanosensor that triggers responses to wall tension.
Keywords: bladder, kidney, mechanotransduction, PIEZO channel, urinary tract
INTRODUCTION
Sensing and responding to mechanical forces, including shear stress and wall tension, are critical for the proper functioning of the urinary tract; for example, the kidney must adjust water and solute flux in accordance with the rates of tubular flow, and the bladder must convey its filling status to the central nervous system to ensure voiding at the right time and place. Putative flow sensors in the kidney include the apically localized and highly ordered brush border of the proximal tubule, which may function by transmitting bending forces to molecules that link the actin cytoskeleton to the plasma membrane, and the primary cilium, which is implicated in Ca2+-stimulated, flow-induced events in the proximal tubule and distal nephron, including the cortical collecting duct (64, 81, 83). Although the nephrons and collecting ducts also experience changes in wall tension, the sensory mechanisms are poorly understood. In the case of the lower urinary tract, both neuronal and nonneuronal cell types are reported to be mechanosensitive, including those associated with the terminals of sensory neurons that innervate the bladder wall and respond to wall distention in the bladder and urethra (i.e., wall mechanoceptors) (20, 36, 73, 88). Despite the presence of putative flow-sensitive apical protrusions in the kidney and wall mechanoceptors in the lower urinary tract, we have limited understanding of the mechanosensors–the specialized molecules that undergo conformational changes in direct response to mechanical stimuli–that initiate mechanotransduction cascades in these organs or their sites of function.
Candidate mechanosensors in other organs include the mechano-gated channels PIEZO1 and PIEZO2 (2,521 and 2,752 amino acids, respectively, in humans), which are evolutionarily conserved but have limited sequence homology with other known channel families or proteins (15, 78). These are large proteins with multiple transmembrane domains, which in the case of PIEZO1, assemble into homotrimers to form a three-bladed, propeller-like structure (32, 91). Several lines of evidence indicate that PIEZO channels act as mechanosensors, including their initial identification as stretch-activated channels in a targeted siRNA screen for such channels in Neuro2A cells (15), and reports that mechanically gated ion transport can be conferred upon cells transfected with Piezo1 or Piezo2 cDNA (15, 17). Telling are the growing number of studies that have implicated these channels in mechanically driven events. In zebrafish, Piezo2b regulates light touch responses (27). In Drosophila, the single dmpiezo gene product may play a role in pain sensation (38). In mammals, PIEZO1 is required for proper vascular development (which depends on blood flow) and cell rearrangements in response to flow (47, 68), stretch-triggered epithelial proliferation and crowd sensing (25, 33), regulation of urinary osmolarity (53), and neuronal stem cell lineage choice (62), whereas PIEZO2 is required for touch sensation in the Merkel cells of skin (34, 86), proprioception in humans and mice (13, 29, 85), and airway stretch and respiration (59). Moreover, both PIEZO1 and PIEZO2 may be required for blood pressure homeostasis and chondrocyte responses to compression (43, 80, 90). Finally, PIEZO channel function is critical as constitutive Piezo 1 or Piezo 2 knockout mice die during embryogenesis, and at birth, respectively (47, 59, 68, 86). In humans, mutations in PIEZO channels lead to multiple diseases, including xerocytosis, congenital lymphatic dysplasia, various forms of distal arthrogryposis, defects in proprioception, and neonatal respiratory insufficiency (1–3, 6, 14, 22, 52, 54, 89).
At present, there is a paucity of information about the expression, localization, and function of PIEZO channels in the urinary tract. Although no studies to date have measured the amount of PIEZO1 or PIEZO2 protein expression in the kidney, relatively low amounts of Piezo1 message have been reported in segments of the rodent nephron and collecting duct (12, 42), and quantitative PCR indicates that Piezo1 message is much greater than that for Piezo2 in the mouse kidney (53, 63). Using a Piezo1 gene-trap/LacZ reporter mouse, Martins et al. (53) reported that Piezo1 expression is concentrated in the inner medulla of the adult mouse. This group further showed that Ksp-Cre-mediated conditional knockout of Piezo1 leads to defects in the acute recovery of urine osmolarity after water restriction or fasting (53). PIEZO1 activity has also been reported in immortalized proximal convoluted tubule cells, where its activity can be modulated through interactions with polycystin-2 (PKD2) (63). In the case of the lower urinary tract, messages for Piezo1 and Piezo2 have been reported in the bladder wall, and Piezo1 message and protein are expressed in the urothelium, possibly in the underlying interstitial cells, and in the serosa (48, 55, 56). Furthermore, treatment with Piezo1-targeted siRNAs impairs the ability of isolated mouse urothelial cells to increase the concentration of intracellular Ca2+ ([Ca2+]i) or release ATP in response to uniaxial stretch or exposure to hypotonic solutions (56). This treatment also reduces hypotonic-induced changes in [Ca2+]i and intracellular Na+ concentration in isolated interstitial cells (48). To date, expression of PIEZO channels in the ureters, urethra, or urethra-associated structures such as the prostate has not been reported. The immediate goal of our analysis was to use genetically modified reporter mice to perform a comprehensive analysis of the expression and localization of PIEZO1 in the organs and tissues that comprise the urinary tract. Long term, these data will reveal which tissues and organs in the urinary tract likely use PIEZO1 to sense changes in tension.
MATERIALS AND METHODS
Animals.
Mice expressing a COOH-terminal fusion of Piezo1 with the sequence for tandem-dimer Tomato (dtT; Piezo1tdT/tdT mice) were generated by Ranade et al. (68). In these animals, the tdT gene and a self-excising pACN neomycin cassette were inserted in frame just before and replacing the endogenous Piezo1 stop codon in exon 51. Heterozygous Piezo1tdT/+ mice, back crossed into the C57BL/6J background, were obtained from The Jackson Laboratory (stock no. 029214, Bar Harbor, ME). Mice were housed in standard caging under a 12:12-h day-night cycle and were fed chow and water ad libitum. Heterozygous Piezo1tdT/+ mice were crossed in house to generate homozygous Piezo1tdT/tdT mice, heterozygous Piezo1tdT/+ mice, and wild-type Piezo1+/+ mice. Genotyping was performed on tail snips collected from 21- to 25-day-old pups. Tail DNA was extracted using the QuickExtract DNA Extraction Solution (catalog no. QE09050, Lucigen, Middleton, WI) and analyzed using the primer sequences and PCR protocol described by The Jackson Laboratory. The primers used were wild-type forward primer (reaction A) 5′-ACGCCAAGCTCATCTTCCT-3′, common primer (reaction A and reaction B) 5′-GTCCCTTTGACAGCAGCATC-3′, and mutant reverse primer (reaction B) 5′-CACCTGTTCCTGTACGGCATGGAC-3′. Two PCRs (reaction A and reaction B) were performed for each sample using the KAPA2G Fast Hot Start Ready Mix with dye (catalog no. KM5610, Kapa Biosystems, Wilmington, MA) and the following PCR cycling conditions: initial denaturation at 94°C for 2 min followed by 10 cycles of 94°C for 20 s, 65°C for 15 s with a decrease of 0.5°C/cycle, and 68°C for 10 s followed by an additional 28 cycles of 94°C for 15 s, 60°C for 15 s, and 72°C for 10 s, and a final step of 72°C for 2 min. The amplicons were separated on 2% agarose gels; the wild-type allele yielded a 227-bp product, whereas the mutant generated a 300-bp product. All experiments were performed with female and male mice between 9 and 24 wk of age. Wild-type Piezo1+/+ littermates were used as controls whenever possible; if not, C57BL/6J mice (stock no. 000664, The Jackson Laboratory) were used instead. All animal experiments were performed in accordance with the relevant guidelines/regulations of the Public Health Service Policy on Humane Care and Use of Laboratory Animals and the Animal Welfare Act and under the approval of the University of Pittsburgh Institutional Animal Care and Use Committee.
Reagents and antibodies.
Unless specified otherwise, all chemicals were obtained from Sigma-Aldrich (St. Louis, MO). The sources of primary antibodies used in this study are shown in Table 1. Minimal cross-reacting goat or donkey secondary antibodies conjugated to Alexa 488, CY3, Alexa 568, or CY5 were purchased from Jackson ImmunoResearch Laboratories (Westgrove, PA). FITC- or rhodamine-labeled phalloidin and TO-PRO-3 were obtained from Molecular Probes (ThermoFisher, Grand Island, NY).
Table 1.
Primary antibodies used in the present study
| Target | Name | Source | Host | Species Reactivity (According to Manufacturer) | Application (Dilution) |
|---|---|---|---|---|---|
| ACTA2 | α-Smooth muscle actin | Proteintech (14395-1-AP) | Rabbit | Human, mouse, rat | IF (1:200) |
| ACTB (horseradish peroxidase conjugated) |
β-Actin | Abcam (Ab4990) | Mouse | Mouse, rat, sheep, rabbit, chicken, guinea pig, hamster, cow, cat, dog, human, pig, carp, monkey | WB (1:60,000) |
| AQP1 | Aquaporin-1 | Abcam (ab15080) | Rabbit | Mouse, rat, sheep, cow, dog, human, pig | IF (1:200) |
| Aquaporin-1 | Millipore (ab2219) | Rabbit | Human, rat, mouse | IF (1:200) | |
| AQP2 | Aquaporin-2 | Alomone (AQP-002) | Rabbit | Human, mouse, rat | IF (1:100) |
| AQP2 | Aquaporin-2 | Santa Cruz Biotechnology (sc-9882; discontinued) | Goat | Mouse, rat, human | IF (1:200) |
| ATP6V1E1 | V-ATPase subunit E1 | Sigma Aldrich (GW22284F; discontinued) | Chicken | Mouse, rat, human | IF (1:500) |
| CDH1 | E-cadherin | BD Transduction Laboratories (610181) | Mouse | Human, mouse, rat, dog | IF (1:100) |
| CUBN | Cubilin | Biorbyt (orb4997) | Rabbit | Human, mouse, pig, rat | IF (1:200) |
| mCherry | MyBioSource (MBS448057) | Goat | Discosoma sp. | IF (1:200) | |
| NPHS2 | Podocin | Proteintech (20384-1-AP) | Rabbit | Human, mouse, rat, zebrafish | IF (1:200) |
| PDGFRA | Platelet-derived growth factor receptor-α | R & D Systems (AF1062) | Goat | Mouse | IF (1:200) |
| PECAM-1 | Platelet endothelial cell adhesion molecule-1 | Millipore (CBL1337-I) | Rat | Mouse | IF (1:200) |
| RFP | Red fluorescent protein | Rockland (600-401-379) | Rabbit | Discosoma sp. | WB (1:1,000) |
| IF (1:200) | |||||
| SLC12A3 | Na+-Cl− cotransporter | StressMarq (SPC-402) | Rabbit | Human, mouse, rat, dog | IF (1:200) |
| SLC26A4 | Pendrin | Wall Laboratory | Rabbit | NA | IF (1:2000) |
| UMOD | Uromodulin | Thermo Fisher (Scientific MA5-24374) | Rat | Mouse | IF (1:200) |
NA, not available; IF, immunofluorescence; WB, Western blot.
Extraction of tissues and sample preparation for Western blot analysis.
Mice were euthanized by inhalation of 100% CO2 followed by a thoracotomy in which a midline incision was made to expose the thoracic cavity. Because red blood cells express PIEZO1, organs were perfused with 10 ml PBS (137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, and 1.5 mM KH2PO4, pH 7.4) warmed to 37°C and injected via a 10-ml syringe connected by Tygon S3 tubing to a 26-gauge needle that was inserted into the heart’s left ventricle. Blood was allowed to escape from the right pulmonary artery, which was transected. In some experiments, the left lung was collected. A midline incision was also made in the lower abdomen, extending through the pelvic bone, to allow ready access to the bladder, ureters, urethra, and kidneys, all of which were collected. The removed organs were rinsed in a 10-cm2 square culture dish filled with 37°C Krebs buffer [110 mM NaCl, 25 mM NaHCO3, 5.8 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 11 mM glucose, and 2 mM CaCl2, pH 7.4, when gassed with 5% (vol/vol) CO2]. Tissue was minced with scalpels and then placed in the following volumes of lysis buffer [100 mM NaCl, 50 mM TEA (pH 8.6), 5 mM EDTA, 0.2% (wt/vol) NaN3, and 0.5% (wt/vol) SDS supplemented with freshly added 0.5 mM PMSF and 1:100 dilution of a protease inhibitor cocktail (catalog no. P8340, Sigma-Aldrich)]: 50 µl for the ureters and urethra, 150 µl for the bladder and lung, and 500 µl for the kidney. Zirconia beads (1.25–1.6 mm, ∼5 for the ureters and urethra, ∼8 for the bladder and lung, and ∼15 for the kidneys, GlenMills, Clifton, NJ) were added, and samples were shaken in a FastPrep 24 device (MP Biomedicals, San Diego, CA) for 20 s. After centrifugation at 14,000 g in an Eppendorf 5415D microfuge (Hauppauge, NY) for 15 min at 4°C, the supernatant was recovered and the protein concentration was determined using a bicinchoninic acid assay kit (Pierce, Rockford, IL).
Samples (typically containing 20–50 µg protein) were mixed with XT Sample Buffer containing freshly added XT reducing agent (BioRad, Hercules, CA), and proteins were resolved by PAGE using Criterion XT 3–8% Tris-acetate polyacrylamide gradient gels (Bio-Rad), bathed in XT Tricine buffer (Bio-Rad), and exposed to 200 V constant current in a Criterion Cell electrophoresis device (Bio-Rad) for 40 min. Proteins in the acrylamide gels were transferred to Immobilon-P (Millipore-Sigma, Burlington, MA) in 100 mM CAPS-NaOH (pH 11.0) buffer at 375-mA constant current for 2 h at room temperature in a Mighty Small Transphor apparatus (Amersham Biosciences, Piscataway, NJ). The membrane was blocked for 1 h at room temperature with 5% (wt/vol) BSA in PBS (pH 7.4) and 0.1% (vol/vol) Tween 20. After incubation with anti-red fluorescent protein antibody [RFP; 1:1,000 diluted in PBS-Tween 20 containing 1% (wt/vol) BSA; Table 1] overnight at 4°C, the Western blots were washed with PBS-Tween 20 (3 × 20 min on a shaker), incubated with horseradish peroxidase-conjugated anti-rabbit or anti-actin antibodies [diluted in Tris-buffered saline-Tween 20 containing 1% (wt/vol) BSA; Table 1] for 45 min at ambient temperature, and washed with PBS-Tween 20 (3 × 20 min on a shaker). Immunoreactive protein species were visualized using SuperSignal West Substrate (Pierce, Rockford, IL). Visualization and image capture were performed using the Chemidoc Touch Imaging System (Bio-Rad), and quantification of digital files was performed using ImageLab (version 5.2.1, Bio-Rad), which was run on an iMac computer (Apple, Cupertino, CA).
For these experiments, organs were obtained from three Piezo1tdT/tdT mice and three Piezo1+/+ mice, and representative blots are presented.
Immunofluorescence and image acquisition.
Mice were euthanized by inhalation of 100% CO2 followed by cervical dislocation. After a midline thoracotomy and midline abdominal incision, the following organs were recovered: lungs, kidneys, ureters, bladder, and urethra. In the case of female mice, the urethra was collected along with the vagina, which in female mice is directly dorsal to the urethra. In the case of male mice, the urethra was recovered along with the seminal vesicles, vas deferens, and prostatic glands as a tissue block.
We tried several tissue preparations, including formalin-fixed, paraffin-embedded tissues (coupled with antigen retrieval), formalin-fixed frozen tissues (with or without antigen retrieval), trichloroacetic acid-fixed frozen tissues, or fresh frozen tissues. Only the latter technique worked reproducibly in our hands. Tissues were placed in cryomolds (15 × 15 × 5 mm, Fisher Scientific) filled with optimal cutting temperature solution (Tissue-Tek, Sakura Finetek, Torrance, CA) and flash frozen by placing the cryomold on a pool of liquid nitrogen. The blocks were stored at −80°C in tightly sealed plastic bags. Cryosections were cut using a Leica Microsystems (Buffalo Grove, IL) CM1950 cryostat (5- to 10-µm sections) and collected on Superfrost Plus glass slides (ThermoFisher Scientific, Pittsburgh, PA). Each experiment contained tissue from control mice (Piezo1+/+) or PIEZO1-tdT-expressing mice (Piezo1tdT/tdT or Piezo1tdT/+), which were treated identically. Sections were immediately placed in 10% neutral buffered formalin [4% (wt/vol) paraformaldehyde dissolved in 29.0 mM NaH2PO4·H2O and 45.8 mM anhydrous Na2HPO4 (pH 7.4)] for 10 min at room temperature, and the unreacted fixative was then quenched by incubating the tissue slices for 10 min at room temperature with quench buffer [75 mM NH4Cl and 20 mM glycine (pH 8.0) dissolved in PBS containing 0.1% (vol/vol Triton X-100]. The tissue was then quickly rinsed three times with PBS and then three times for 5 min in the same buffer followed by an incubation in block solution [PBS containing 0.6% (vol/vol) fish skin gelatin and 0.05% (wt/vol) saponin] supplemented with 5% (vol/vol) donkey or goat serum for 60 min at room temperature. After the block solution had been aspirated, it was replaced with primary antibodies diluted in block solution and incubated for 2 h at room temperature or overnight at 4°C in a humid chamber. Slides were washed three times quickly and three times for 5 min with block solution and then incubated with minimal cross-reactivity and fluorophore-labeled secondary antibodies diluted in block solution for 1 h at room temperature. In some cases, nuclei were counterstained with TO-PRO-3 (1:1,000, ThermoFisher Scientific), and the overall tissue architecture was visualized using TRITC-labeled phalloidin (ThermoFisher Scientific, 1:200). The labeled tissues were then rinsed three times quickly and three times for 5 min with block solution, rinsed with PBS, and then postfixed in 10% neutral-buffered formalin for 5–10 min at room temperature. Slides were rinsed with PBS, excess liquid was aspirated, and a drop of SlowFade Diamond Antifade (Molecular Probes-ThermoFisher) was placed on the tissue. Borosilicate coverslips (no. 1.5, 0.17-mm thickness, 24 × 50 mm, ThermoFisher) were placed above the drop of mounting medium, excess mounting medium was removed by aspiration, the edges of the coverslip were sealed with clear nail polish, and slides were stored at −20°C until image acquisition was performed.
Images were captured using a Leica HCX PL APO CS ×40, 1.25 numerical aperture oil objective and the appropriate laser lines of a Leica TCS SP5 CW-STED confocal microscope (in normal confocal mode). The photomultipliers or HyD detectors were optimized using the Q-LUT option, and 8-bit images collected using eight line averages combined with four frame averages. Collection parameters were optimized for the Piezo1tdT/tdT samples, and identical parameters were then used to collect images for the corresponding control Piezo1+/+ samples. Cross-talk between channels was prevented by use of spectral detectors coupled with sequential scanning. Stacks of images (1,024 × 1,024, 8-bit, typically 3–6 images) were collected using a Z-step of 0.29 μm. Images were imported into Volocity 4-D software (Perkin-Elmer, Waltham, MA) and, after image reconstruction, exported as TIFF files. The contrast of the latter was corrected in Photoshop CC2018 (Adobe, San Jose, CA), and composite images were prepared in Adobe Illustrator CC2018. Representative images are shown in each figure.
For the immunolocalization experiments, we used the following numbers of animals: six female mice with the Piezo1tdT/tdT genotype and six female mice with the Piezo1+/+ genotype as well as four male mice with the Piezo1tdT/tdT genotype and seven male mice with the Piezo1+/+ genotype.
RESULTS
PIEZO1-tdT is expressed in the urinary tract.
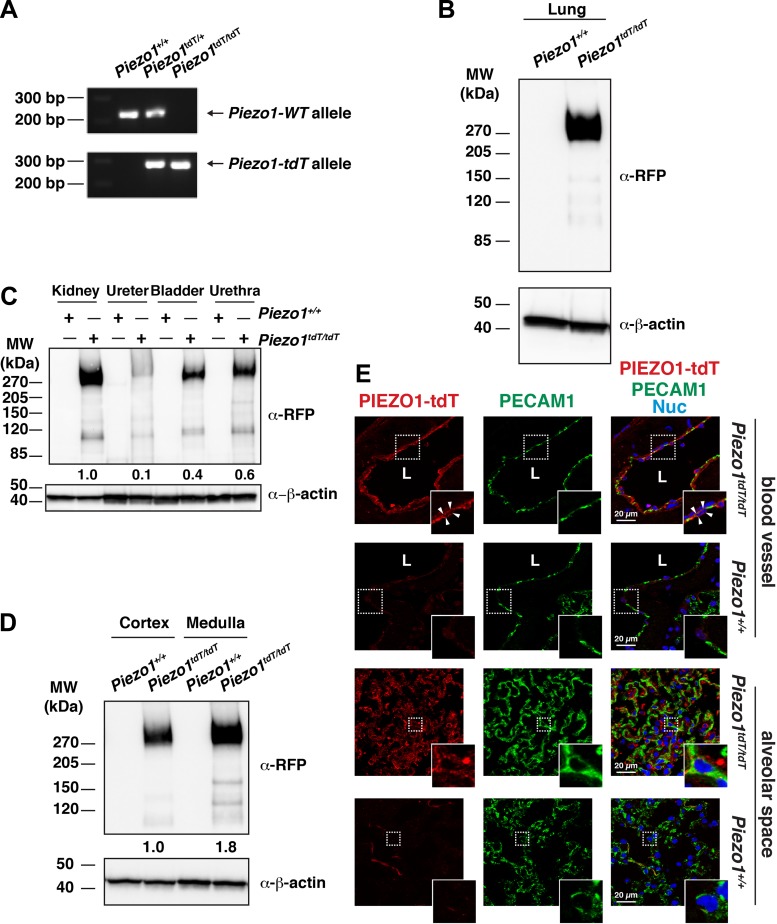
Previous studies have reported that custom-made and commercially available antibodies were not sufficiently sensitive to detect expression of endogenous Piezo1 in a variety of cells and tissues (15, 68). Coupled with our own difficulties confirming the specificity of commercially available antibodies on tissue sections, we made use of transgenic PIEZO1-tdT mice (68). Given that the PIEZO1-tdT fusion protein is expressed from the native Piezo1 promoter and regulatory elements, the levels and pattern of expression are expected to mimic those of the endogenous wild-type channel. Consistent with this possibility, Piezo1-tdT mRNA expression is nearly identical to that for the wild-type Piezo1 gene in several tissues (68). We confirmed that mating Piezo1tdT/+ heterozygous mice generated the expected wild-type, heterozygous, and homozygous genotypes (Fig. 1A).
Fig. 1.
PIEZO1-tandem-dimer Tomato (tdT) expression in the mouse lung and urinary tract. A: genotype analysis of Piezo1-tdT reporter mice. PCR amplification products of offspring resulting from mating heterozygous Piezo1tdT/+ mice using primers that detect the wild-type allele (227 bp; top) or Piezo1-tdT allele (300 bp; bottom) are shown. B: lung tissue lysate (30 µg) prepared from Piezo1+/+ or Piezo1tdT/tdT mice was resolved by SDS-PAGE and Western blots probed with an antibody against red fluorescent protein (α-RFP; which reacts with the tdT moiety). Detection of β-actin was used as the loading control. C: Western blot analysis of PIEZO1-tdT expression in the different organs of the urinary tract. Tissue lysates from the whole kidney (20 µg), ureters (50 µg), bladder (20 µg), or urethra (20 µg) were prepared from Piezo1+/+ or Piezo1tdT/tdT mice, resolved by PAGE, and detected using Western blot analysis. Values for the relative expression of PIEZO1-tdT in each tissue (normalized to the actin loading control and then expressed as a fraction of the kidney) are indicated below the top set of blots (mean value: n = 2). D: expression of PIEZO1-tdT in the cortex (30 µg) and medulla (10 µg) of Piezo1+/+ and Piezo1tdT/tdT mice kidneys was assessed by Western blots. Values of relative PIEZO1-tdT expression (normalized to the actin loading control and, subsequently, the cortex) are indicated below the top set of blots (mean value: n = 2). E: localization of PIEZO1-tdT in the lung. Tissue obtained from Piezo1+/+ or Piezo1tdT/tdT mice was immunolabeled with antibodies that detect PIEZO1-tdT, platelet endothelial cell adhesion molecule (PECAM)-1, or both markers along with TO-PRO-3. Insets show magnified images of the areas enclosed by the white dashed boxes. Top and upper middle insets show that PIEZO1-tdT was expressed at the apical and basolateral surfaces of the endothelial cells lining blood vessels (surfaces are indicated by arrowheads), whereas the bottom and lower middle insets show that PIEZO1-tdT was also expressed in the alveolar space, including in endothelial cells. L, lumen; MW, molecular weight.
Lung tissue can be used as a reference for Piezo1 expression (15, 43), and we confirmed that the PIEZO1-tdT fusion protein was readily detected in lung tissue from Piezo1tdT/tdT mice (Fig. 1B). No signal was observed in lysates obtained from Piezo1+/+ littermates (Fig. 1B). We also confirmed that PIEZO1-tdT protein was expressed in lysates prepared from the kidneys, ureter, bladder, and urethra, with expression in the following rank order: kidney > urethra > bladder > ureter (Fig. 1C). In the case of the ureter, protein staining was discernable above background but was always diffuse and of low levels. Minimal signal was detected in control Piezo1+/+ littermates for any of these organs (Fig. 1C). We also prepared lysates from the cortex and medulla of the kidneys and observed that the medulla had more Piezo1-tdT expression than the cortex (Fig. 1D). Next, we examined lung tissue after immunolabeling, focusing on the vascular system, as PIEZO1 has been reported to be expressed in endothelial cells (46, 47, 68). Because PIEZO1-tdT could not be easily visualized directly in the mouse tissues, we used indirect immunofluorescence using an anti-RFP (or mCherry) primary antibody. Using this approach, PIEZO1-tdT was detected in lung endothelial cells [labeled with the lateral membrane-associated protein platelet endothelial cell adhesion molecule (PECAM)-1; see Ref. 57], which lined the blood vessels of Piezo1tdT/tdT mice but not Piezo1+/+ mice (Fig. 1E). In these endothelial cells, PIEZO1-tdT was found at their apical and basolateral surfaces. PIEZO1-tdT was also detected in the alveolar space, where it was localized in part to PECAM-1-labeled endothelial cells as well as other cells, likely pneumocytes (Fig. 1E).
PIEZO1-tdT localizes to the renal corpuscle and basolateral surfaces of epithelial cells that line the distal nephron, collecting ducts, and renal pelvis of the kidney.
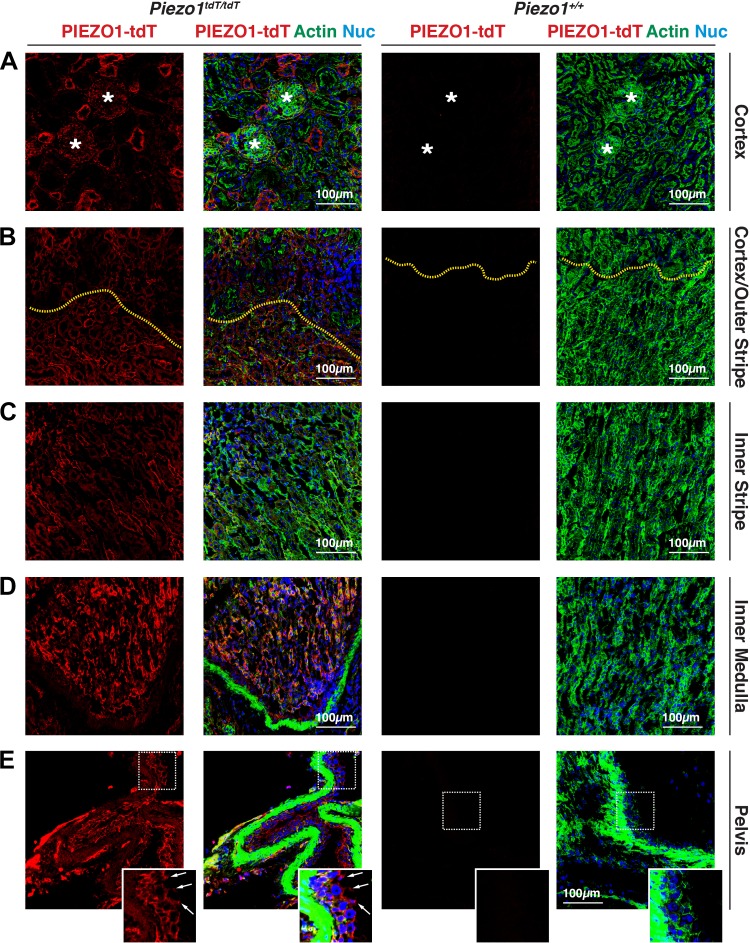
We next characterized the localization of PIEZO1-tdT within the urinary tract, starting with the kidneys of Piezo1tdT/tdT mice (for reference, a diagram of the urinary tract is shown in Fig. 10). In the cortex, PIEZO1-tdT was localized to renal corpuscles (marked with asterisks in Fig. 2A) as well as tubules cut in profile. In the medulla, PIEZO1-tdT was expressed in tubules located in the outer and inner stripes of the outer medulla (Fig. 2, B and C) and, most prominently, in tubules in the inner medulla (Fig. 2D). PIEZO-tdT was also expressed in the renal pelvis, where it was associated with the basal surfaces of the urothelium that lines the pelvis (the position of the junctional complex is marked with arrows in inset in Fig. 2E). Minimal signal was observed in Piezo1+/+ mouse kidneys (Fig. 2, A–E).
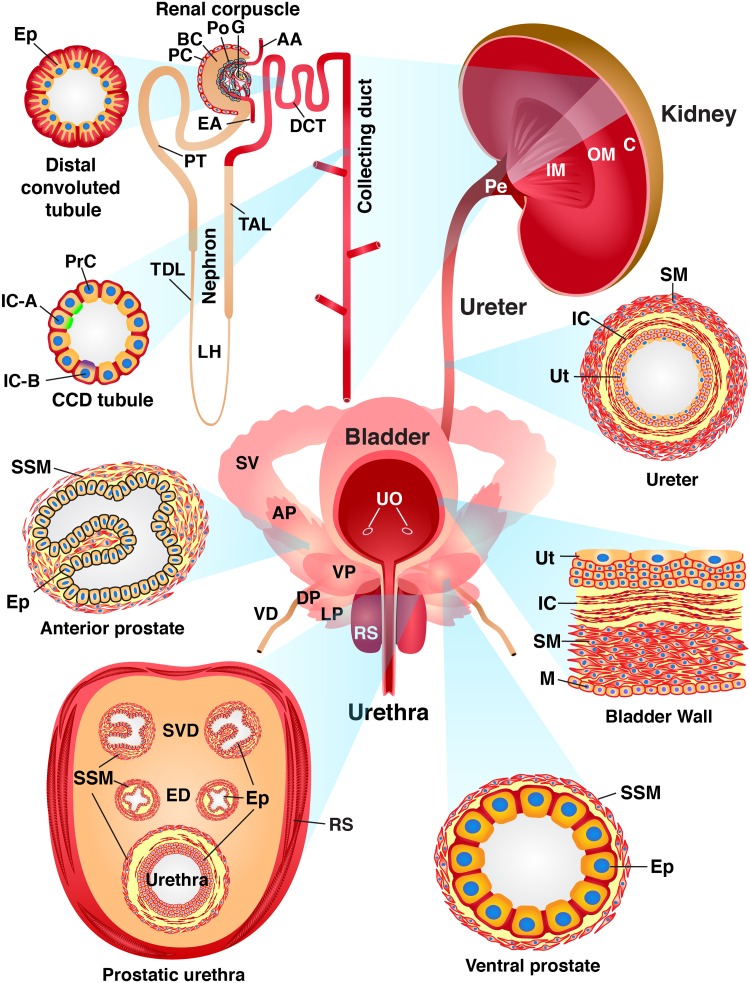
Fig. 10.
Summary of PIEZO1-tandem-dimer Tomato (tdT) expression in the mouse urinary tract. Cross-sections through the walls of the kidney, ureters, bladder, urethra, and urethra-associated organs are shown to highlight the different cell types that express PIEZO1-tdT (red). The kidney is subdivided into the cortex (C), outer medulla (OM; inner and outer stripe regions not depicted), inner medulla (IM), and pelvis (Pe). The functional units of the kidney include the renal corpuscle [comprised of the following: afferent arteriole (AA), Bowman’s capsule (BC), efferent arteriole (EA), glomerulus (G), parietal cell (PC), and podocyte (Po)], nephron [which includes the distal convoluted tubule (DCT), loop of Henle (LH), proximal tubule (PT), thick ascending limb (TAL), and thin descending limb (TDL)], and collecting ducts (CD). The epithelial cells (Ep) that comprise the DCT characteristically have highly folded basolateral membranes and a subapical nucleus. The cortical CD (CCD) comprises principal cells (PrC), type A intercalated cells [IC-A; which express the V-ATPase E1 subunit (ATP6V1E1) apically], and type B intercalated cells [IC-B; which express pendrin (SLC26A4) apically]. Components of the ureter include the urothelium (Ut), lamina propria-associated interstitial cells (IC), smooth muscle cells (SM), and an adventitia (not depicted). Constituents of the bladder include the urothelium (Ut), interstitial cells in the lamina propria (IC), smooth muscles that form the detrusor (SM), and mesothelial cells (M), which line the serosal surface of the organ. In the male mouse ureter, the prostatic urethra is associated with several structures, including the glands and ducts of the four lobes of the mouse prostate: the anterior prostate (AP), ventral prostate (VP), lateral prostate (LP), and dorsal prostate (DP). Both the AP and VP (as well as the LP and DP, which are not depicted) comprise glands comprised of the epithelium (Ep) surrounded by stromal smooth muscle cells (SSM). In addition to the prostate, the male prostatic urethra is associated with the glands (SV) and ducts (SVD) of the seminal vesicles, the terminal ejaculatory ducts (ED) of the vas deferens (VD), and the surrounding rhabdosphincter (RS), which comprises skeletal muscle. Female mice retain the urethra and rhadbosphincter but lack the male accessory glands. Organs, tissues, and cells not drawn to scale. UO, urethral orifices.
Fig. 2.
PIEZO1-tandem-dimer Tomato (tdT) expression and distribution in the mouse kidney. A−E: expression of PIEZO1-tdT in the cortex (A), cortex/outer stripe of the outer medulla (B), inner stripe of the outer medulla (C), inner medulla (D), or renal pelvis (E) of Piezo1tdT/tdT or Piezo1+/+ mice. Tissue was labeled with antibodies that detect PIEZO1-tdT, FITC-phalloidin to label the F-actin cytoskeleton, and TO-PRO-3 to label nuclei. *Renal corpuscles in A. In B, the yellow dashed lines indicate the interface between the cortex (top of image) and outer stripe of the outer medulla (bottom of image). In E, the boxed region of the urothelium lining the renal pelvis is magnified in the inset. Arrows point to the location of the junctional complex, which sits at border between the basolateral and apical plasma membrane domains. The lumen is to the right. The strong F-actin staining (green) is associated with smooth muscle cells that underlie the urothelium.
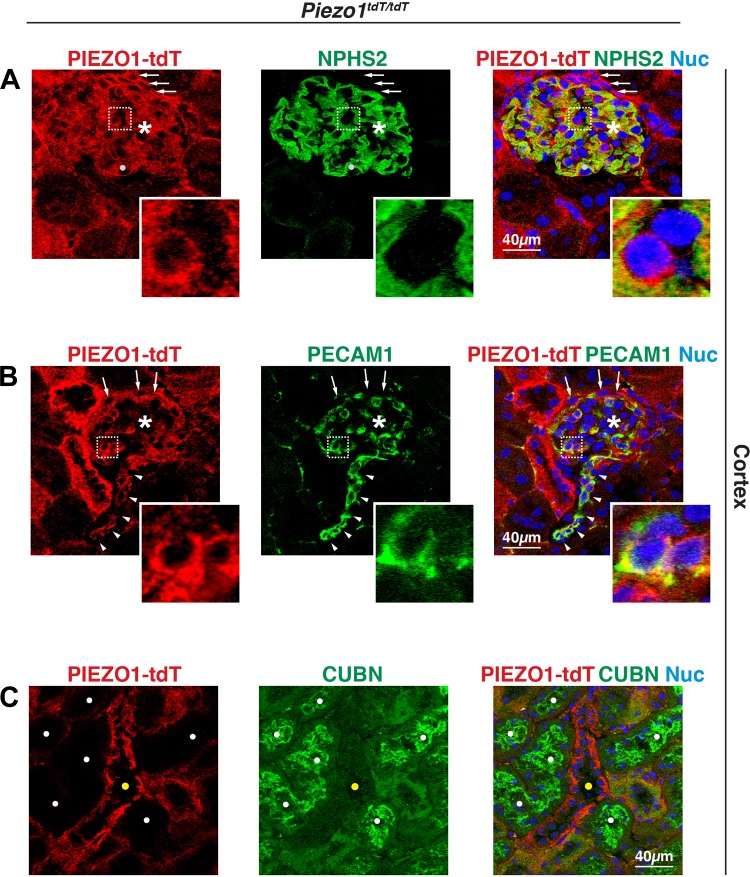
We also defined the nephron segments to which PIEZO1-tdT was localized. We started with the site of filtration: the renal corpuscle. This structure includes Bowman’s capsule, which is composed of a parietal layer (populated by squamous epithelial cells) and a visceral layer (made up of podocytes), and the glomerulus, which is a capillary tuft composed of endothelial cells. PIEZO1-tdT was most clearly localized to the surface and cytoplasm of cells that were surrounded by the podocyte foot processes labeled with podocin (NPHS2) (70), many of which were likely glomerular endothelial cells (Fig. 3A, bottom left cell in the inset). This possibility was confirmed when endothelial cells were labeled with an antibody to PECAM-1, which is expressed along the lateral surfaces of endothelial cells (57), and showed coexpression with PIEZO1-tdT both in the glomerulus (Fig. 3B, inset) and arterioles associated with this structure (arrowheads in Fig. 3B). We also observed that PIEZO1-tdT was diffusely localized to podocytes (and possibly mesangial cells; e.g., see solid white circle in Fig. 3A) but did not obviously localize to the NPHS2-labeled foot processes that comprise the basolateral surfaces of these cells (Fig. 3A). A fraction of PIEZO-tdT was also associated with squamous cells near the edge of Bowman’s capsule, likely parietal cells, where it was distributed at their apical and basolateral surfaces (see arrows in Fig. 3, A and B). Upon exit from Bowman’s capsule, the forming ultrafiltrate enters the proximal tubule. However, proximal tubule epithelial cells, which express the multiligand receptor cubilin (CUBN) (8), did not express PIEZO1-tdT (proximal tubules are marked with white circles in Fig. 3C), whereas adjacent CUBN-negative tubule segments were positive for PIEZO1-tdT (yellow circle in Fig. 3C).
Fig. 3.
Expression of PIEZO1-tandem-dimer Tomato (tdT) in the mouse renal corpuscle but not the proximal tubule. A and B: expression of PIEZO1-tdT in the renal corpuscle of Piezo1tdT/tdT mice immunolabeled with antibodies that detect PIEZO1-tdT and either podocin (NPHS2) or platelet endothelial cell adhesion molecule (PECAM)-1. *Renal corpuscles. Arrows point to the location of parietal cells. Arrowheads point to the location of endothelial cells lining an arteriole. The light gray circle in A is an example of an NPHS2-positive cell whose cytoplasm is PIEZO1-dtT positive. Insets in A show two cells; the left cell expresses PIEZO1-tdT on its surface and is closely associated with the NPHS2-labeled basolateral surface of the adjacent podocyte (see merged image for clarity). The other cell is not PIEZO1-tdT positive and may be associated with a mesangial cell. Insets in B show two PIEZO1-tdT- and PECAM-1-positive endothelial cells. C: expression of PIEZO1-tdT and cubilin (CUBN) in the renal cortex. The CUBN-labeled proximal tubules (indicated by white filled circles) are distinct from the PIEZO1-tdT-labeled tubules (indicated by yellow circles).
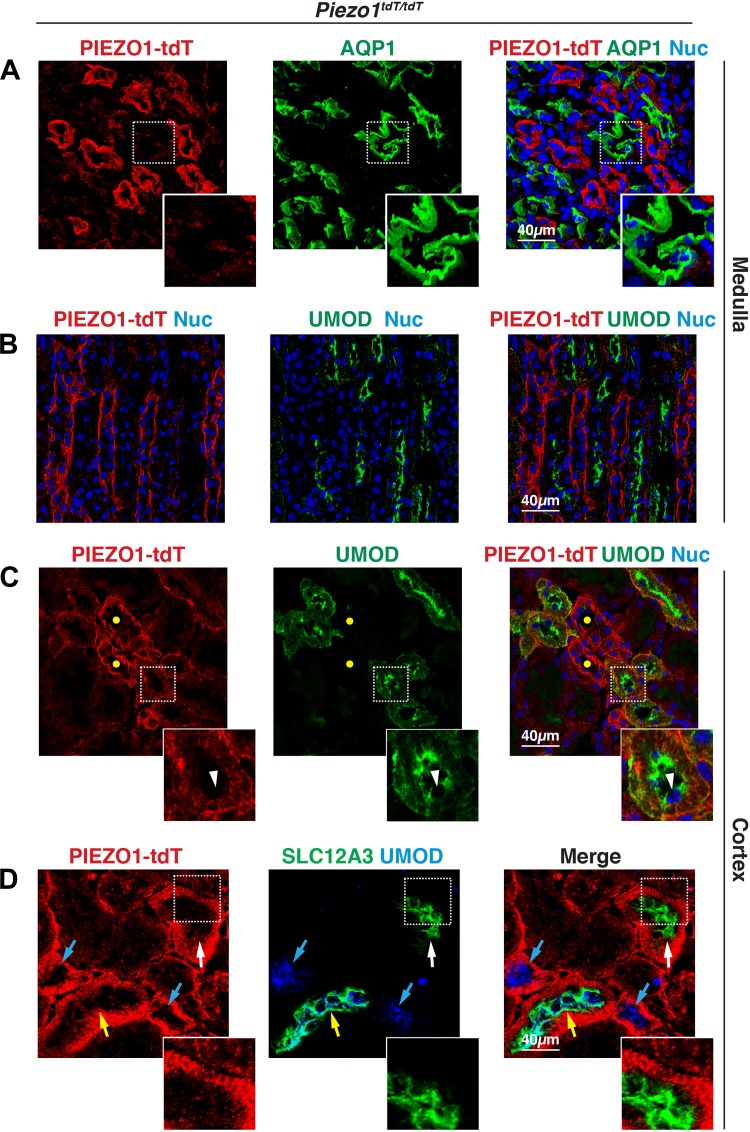
Leading away from the proximal tubules is the thin descending limb of the loop of Henle, a medullary structure that expresses the water channel aquaporin (AQP)1 (71). In the medulla, we observed that PIEZO1-tdT was most concentrated in tubules that were AQP1 negative (Fig. 4A). However, a small amount of PIEZO1-tdT expression was noted at the outermost surfaces of AQP1-positive tubules (Fig. 4A, inset), which we ascribed to PIEZO1-tdT expression by surrounding endothelial cells. AQP1 is also expressed in portions of the proximal tubule (S2 and S3 segments) (71); however, as noted above, PIEZO1-tdT did not localize to proximal tubules, and we observed little colocalization between PIEZO1-tdT and AQP1 in the cortex. We next examined the distribution of uromodulin (UMOD), which in the medulla is localized to the apical surfaces and subapical region of the epithelial cells that comprise the thick ascending limb of the nephron (76). In the medulla, PIEZO1-tdT was not associated with this segment of the nephron (Fig. 4B). However, in the cortex, we observed tubules that contained apically distributed UMOD that coexpressed PIEZO1-tdT, but at the contralateral, basolateral surfaces of these cells (e.g., see Fig. 4C, inset). Although some of these UMOD-positive tubules were likely to be the thick ascending limb, a recent report (76) has indicated that UMOD is also expressed in early segments of the distal convoluted tubule (i.e., DCT1). Consistent with these possibilities, we observed that some of the UMOD-positive tubules were negative for the thiazide-sensitive Na+-Cl− cotransporter (SLC12A3; cyan arrows in Fig. 4D), a well-described marker of the distal convoluted tubule (26, 60), whereas other UMOD-positive tubules were also SLC12A3-positive (yellow arrow in Fig. 4D). We also observed tubules that were SLC12A3 positive but UMOD negative (white arrow in Fig. 4D), which are likely the latter portions of the distal convoluted tubules (i.e., DCT2). In the cortical UMOD-positive tubule segments and SLC12A3-positive tubule segments, PIEZO1-tdT formed a relatively broad band of staining at the base of the tubules (Fig. 4, C and D, insets). In these cells, we observed that in fortuitous sections PIEZO1-tdT was associated with stria that ran perpendicular to the lumen of the tubule. This staining pattern is consistent with the highly convoluted and folded nature of the basolateral surface of the epithelial cells that form the distal convoluted tubule and thick ascending limb. It also accounts for the displacement of the nucleus into the apical pole of these cells (e.g., see arrowhead in Fig. 4C, inset).
Fig. 4.
Distribution of PIEZO1-tandem-dimer Tomato (tdT) in the loop of Henle and distal convoluted tubule (DCT) of the mouse kidney. A and B: localization of PIEZO1-tdT, aquaporin-1 (AQP1), and uromodulin (UMOD) in the kidney medulla. AQP1 is a marker of the thin descending limb of the loop of Henle, whereas UMOD is a marker of the thick ascending limb. Inset in A shows the faint PIEZO1-tdT signal associated with the periphery of AQP1-positive tubules, most likely endothelial cells. In B, there was little colocalization between PIEZO1-tdT and UMOD. C and D: localization of PIEZO1-tdT, UMOD, and thiazide-sensitive Na+-Cl− cotransporter (SLC12A3) in the kidney cortex. In the cortex, UMOD was associated in part with the early segment of the DCT (i.e., DCT1). In C, the yellow circles indicate PIEZO1-tdT-positive tubules that were negative for UMOD expression. The inset in C shows a section through a UMOD-positive tubule demonstrating the stria formed by PIEZO1-tdT localized to the basolateral surface of the cells lining the DCT. Note the apical position of the nucleus (marked with an arrowhead). In D, colored arrows mark cortical tubules that were positive for UMOD alone (likely thick ascending limb; cyan arrow), SLC12A3 alone (likely segments of late DCT2; white arrow), or positivity for both markers (likely DCT1; yellow arrow). The inset in D shows a region of the tubule lining a segment of the DCT that was SLC12A3 positive (but UMOD negative). Note the PIEZO1-tdT-positive stria at the basolateral surfaces of the cells.
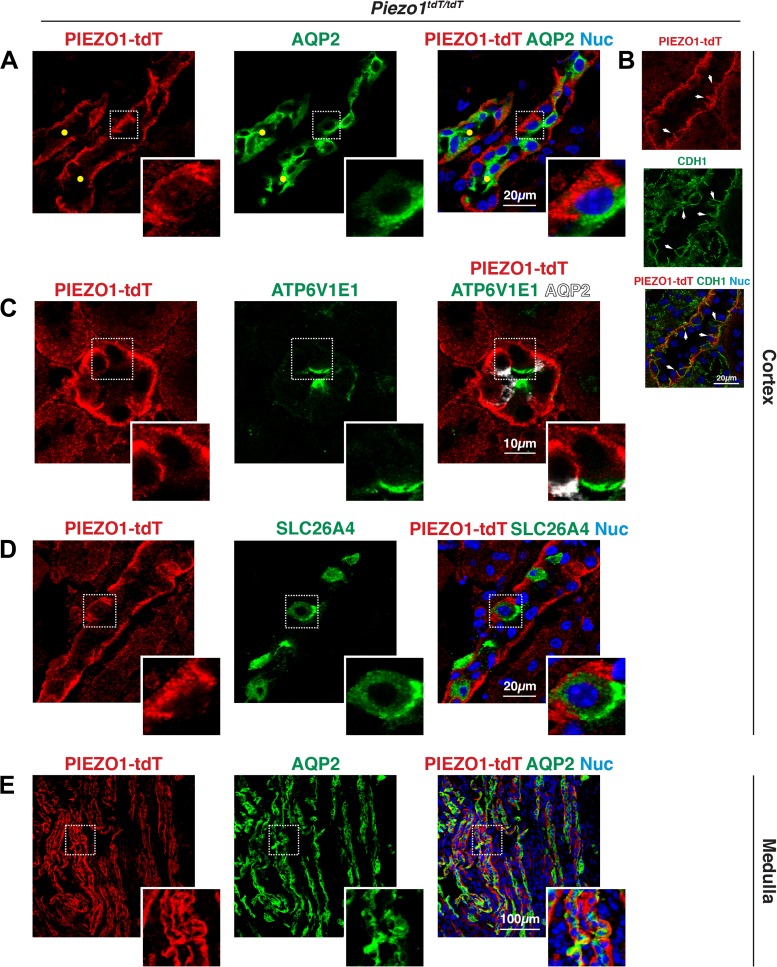
Other tubules in the cortex were characterized by epithelial cells with a relatively thin, bright band of PIEZO1-tdT fluorescence that ran along their basal surfaces and extended up their lateral surfaces (marked with yellow circles in Figs. 3C, 4C, and 5A). In these tubules, PIEZO1-tdT colocalized with the basolateral protein E-cadherin (CDH1), which is associated in part with collecting ducts (Fig. 5B) (66). In fact, PIEZO1-tdT-labeled tubules were positive for the apical water channel AQP2, a marker of the principal cells lining the collecting ducts of the kidney (Fig. 5A) (30). In addition to principal cells, other cells associated with the cortical collecting ducts include type A intercalated cells, which are positive for luminal expression of the V-ATPase E1 subunit (ATP6V1E1) (9), and type B intercalated cells, which express pendrin (SLC26A4) at their apical cell surface (79). In type A ATP6V1E1-positive intercalated cells, PIEZO1-tdT was found at their basolateral surfaces (Fig. 5C, inset). Likewise, PIEZO1-tdT was also localized to the basolateral surfaces of type B SLC26A4-positive intercalated cells (Fig. 5D). Collecting ducts extend from the cortex to the inner medulla, and PIEZO1-tdT was also localized to the basolateral surfaces of the long AQP2-positive tubules found in this region of the kidney (Fig. 5E).
Fig. 5.
PIEZO1-tandem-dimer Tomato (tdT) expression in the collecting ducts (CDs) of the mouse kidney. A–E: localization of PIEZO1-tdT, aquaporin-2 (AQP2), V-ATPase subunit E1 (ATP6V1E1), and pendrin (SLC26A4) in the kidney. In A, the insets show that PIEZO1-tdT was localized to the basolateral surfaces of AQP2-positive principal cells in the cortical CDs (labeled with yellow circles). B: PIEZO1-tdT colocalized with E-cadherin (CDH1) at the basolateral surfaces of CDs. The inset in C shows that PIEZO1-tdT was associated with the basolateral surfaces of an ATP6V1E1-positive type A intercalated cell, which is distinct from AQP2-positive principal cells. The inset in D shows a cell where PIEZO1-tdT was localized to the basolateral surface of type B interstitial cells expressing apical SLC26A4. The inset in E shows that PIEZO1-tdT was localized to the basolateral surfaces of the cells lining the medullary CD.
In summary, PIEZO1-tdT is expressed in the endothelial cells of the glomerulus (and possibly podocytes), parietal cells of Bowman’s capsule, cells lining the distal convoluted tubule, principal and intercalated cells of the collecting ducts, and urothelium lining the renal pelvis. In the distal nephron, collecting ducts, and pelvis-localized urothelium, PIEZO1-tdT was associated with the basolateral surfaces of the epithelial cells forming these tubules.
PIEZO1-tdT is associated with the urothelium, interstitial cells, smooth muscle cells, and mesothelium of the ureters and bladder.
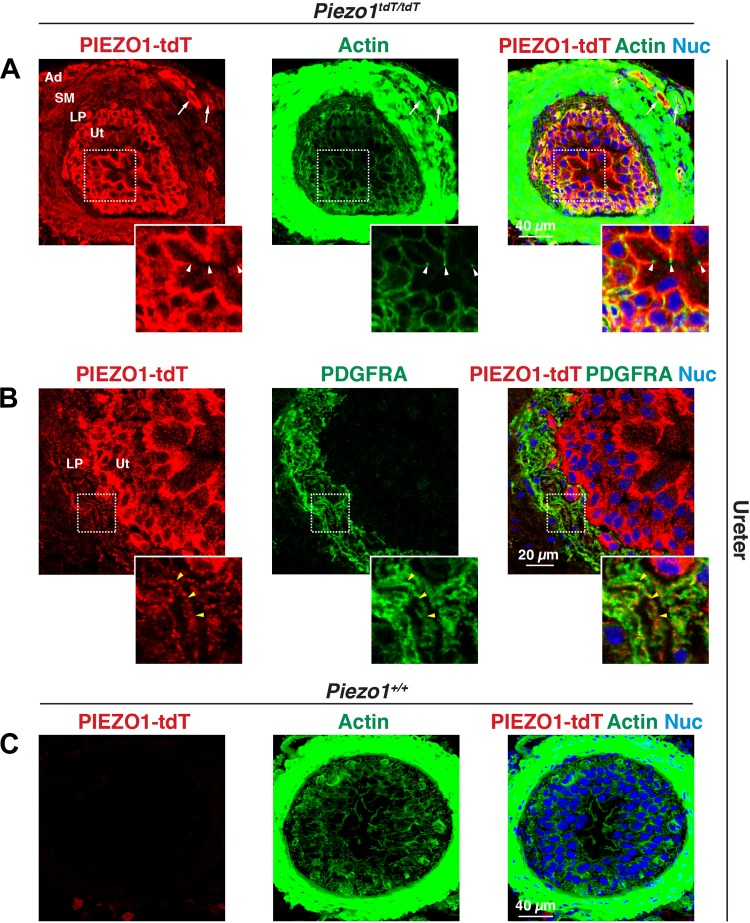
The major layers of the ureter and bladder (starting from the lumen) include the innermost urothelium, lamina propria (which is rich in interstitial cells), smooth muscle layers, and outermost adventitia (ureter) or serosa (bladder). In the ureters of Piezo1tdT/tdT mice, PIEZO1-tdT was expressed in the urothelium, where it was concentrated at the basolateral surface of the superficial umbrella cell layer (arrowheads mark the position of the junctional complex in the inset in Fig. 6A). It was also found at or near the surfaces of the underlying intermediate and basal cell layers. PIEZO1-tdT was also localized to a population of very thin, spindle-shaped cells that were actin positive and were often arranged in wavy, parallel bundles. These cells were identified as interstitial cells based on their coexpression of PDGF receptor-α (PDGFRA; Fig. 6B, inset) (39). Smooth muscle cells also appeared positive for PIEZO1-tdT (Fig. 6A), as did blood vessels in the adventitia (arrows in Fig. 6A). Minimal PIEZO1-tdT staining was observed in ureters taken from wild-type Piezo1+/+ mice (Fig. 6C).
Fig. 6.
PIEZO1-tandem-dimer Tomato (tdT) expression in the mouse ureter. A−C: distribution of PIEZO1-tdT and platelet-derived growth factor-α receptor (PDGFRA) in the ureters of Piezo1tdT/tdT (A and B) and Piezo1+/+ mice (C). The insets in A show that PIEZO1-tdT was localized to the basolateral surfaces of the outermost umbrella cells that face the lumen. Arrowheads indicate the position of the junctional complex that segregates the apical and basolateral surfaces of these cells. The insets in B show that PDGFRA-positive interstitial cells (one of which is indicated by yellow arrowheads) were PIEZO1-tdT positive. Note that the actin signal in A and C was saturated to reveal the relatively weak signal associated with the urothelium. Ad, adventitia; LP, lamina propria; Nuc, nuclei; SM, smooth muscle; Ut, urothelium.
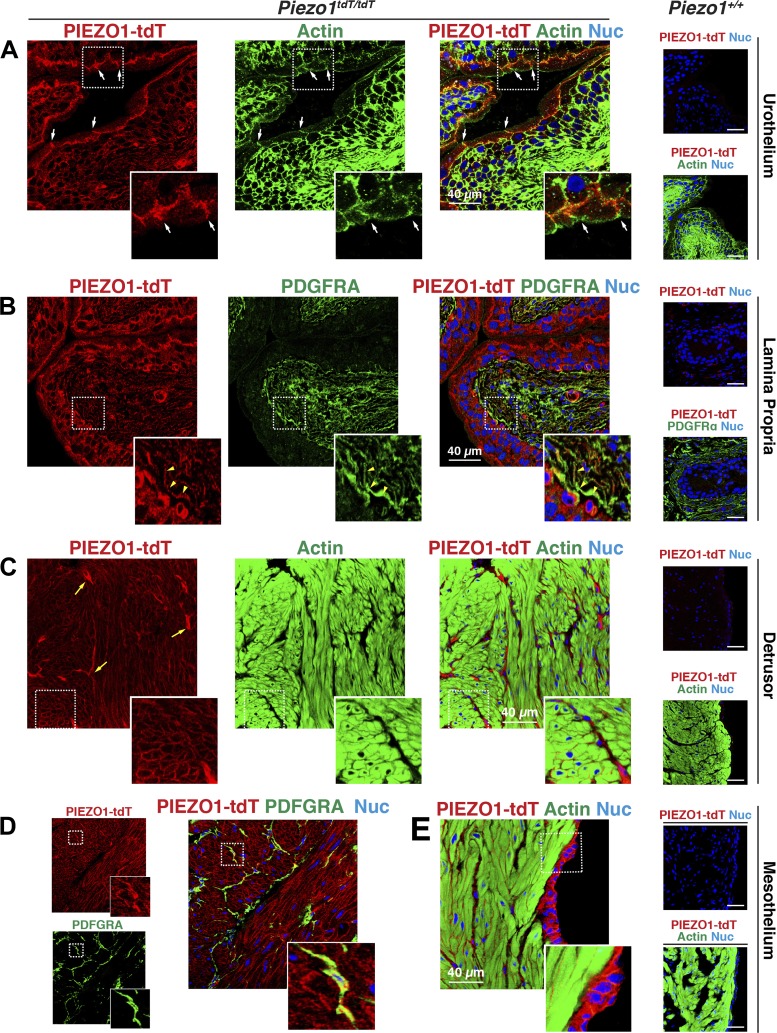
The pattern of PIEZO1-tdT expression in the bladder was similar to that observed in the ureters. In Piezo1tdT/tdT mice, the urothelium was PIEZO1-tdT positive, particularly the basolateral surface of the umbrella cells (arrows mark the position of the junctional complex in Fig. 7A), which exhibited a strong degree of staining. The underlying intermediate and basal cells of the urothelium were also positive, as were PDGFRA-labeled lamina propria-associated interstitial cells, which underlie the urothelium (e.g., see yellow arrowheads in Fig. 7B, inset). In the detrusor muscle, smooth muscle cells were rimmed by a thin band of PIEZO1-tdT (Fig. 7C, inset) that was most likely associated with the sarcolemma of these cells. Surrounding bundles of smooth muscle cells, we observed PIEZO1-tdT positive intermuscular-associated PDGFRA-positive interstitial cells (Fig. 7D; see also the yellow arrows in Fig. 7C). PIEZO1-tdT was also expressed by the mesothelium, an epithelium that separates the bladder wall from the body cavity (Fig. 7E). Minimal staining for PIEZO1-tdT was observed in bladders taken from wild-type Piezo1+/+ mice (Fig. 7, A–C and E, right).
Fig. 7.
PIEZO1-tandem-dimer Tomato (tdT) expression in the mouse bladder. A–E: distribution of PIEZO1-tdT in the urothelium (A), lamina propria (B), detrusor (C and D), and mesothelium (E) of Piezo1tdT/tdT and Piezo1+/+ mice. In A, the position of the junctional complex is indicated by arrows. The inset shows a magnified view of a surface umbrella cell labeled with PIEZO1-tdT at its basolateral surfaces. The inset in B shows that PIEZO1-tdT was associated with platelet-derived growth factor-α receptor (PDGFRA)-positive interstitial cells (one of which is indicated by yellow arrowheads). The inset in C shows that PIEZO1-tdT was localized at the sarcolemma of smooth muscle cells. The brighter-stained, stellate-shaped cells, labeled with yellow arrows, are likely intermuscular interstitial cells (see also D). The inset in D shows that PIEZO1-tdT also labeled intermuscular-associated interstitial cells, and the inset in E shows serosa-associated mesothelial cells. Nuc, nuclei.
In summary, these results indicate that PIEZO1-tdT is expressed in the ureters and bladder, where it is associated with the urothelium and mesothelium, the interstitial cells in the lamina propria and detrusor, and the smooth muscle cells that form the wall of these organs.
PIEZO1-tdT is expressed in the urethra and associated organs of female and male mice.
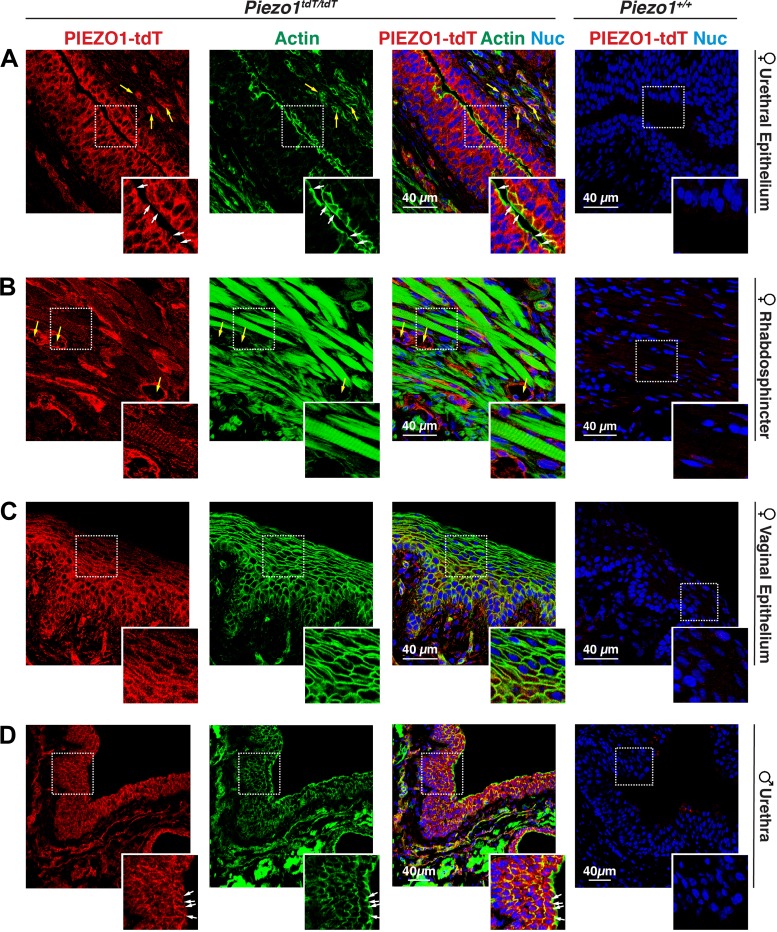
We also analyzed PIEZO1-tdT expression in the pelvic urethra of female mice and the prostatic urethra of male mice. These regions are located just past the bladder neck and in the region of the rhabdosphincter, which is rich in striated muscle fibers. In female Piezo1tdT/tdT mice, PIEZO1-tdT was strongly associated with the stratified cuboidal/columnar epithelium lining the urethral lumen. Using the highly developed cortical F-actin cytoskeleton under the apical surface of the superficial cell layer, PIEZO1-tdT was associated with the contralateral, basolateral surfaces of the superficial cell layer as well as the surfaces of the underlying intermediate and basal cell layers (Fig. 8A, inset). PIEZO1-tdT was also localized to blood vessels (yellow arrows in Fig. 8B) and to the smooth muscle cells (not shown) and striated muscle cells (Fig. 8B) found in this region of the urethra. In the striated muscles, PIEZO1-tdT appeared to associate with structures, consistent with plasma membrane-derived T-tubules that ran perpendicular to the long axis of the myofiber and closely resembled the banding pattern of the myofibril-associated sarcomeres (Fig. 8B, inset). Just dorsal to the female urethra is the vagina. Here, PIEZO1-tdT was localized to all cell layers of the nonkeratinized stratified squamous epithelium lining this organ (Fig. 8C).
Fig. 8.
PIEZO1-tandem-dimer Tomato (tdT) expression in the mouse urethra. A–D: distribution of PIEZO1-tdT in the female urethra (A), female rhabdosphincter (B), female vaginal epithelium (C), or male urethra (D) in Piezo1tdT/tdT and Piezo1+/+ mice. Examples of blood vessels are marked with yellow arrows, and the position of the apicolateral junctional complex is marked with white arrows. The insets in A show the localization of PIEZO1-tdT along the basolateral membranes of surface-localized epithelial cells and the surfaces of the underlying cell layers. The insets in B show that PIEZO1-tdT was expressed by skeletal muscle cells in the rhabdosphincter. The insets in C show the localization of PIEZO1-tdT within the epithelium lining the vagina. The insets in D show the localization of PIEZO1-tdT within the epithelium lining the male urethra. Nuc, nuclei.
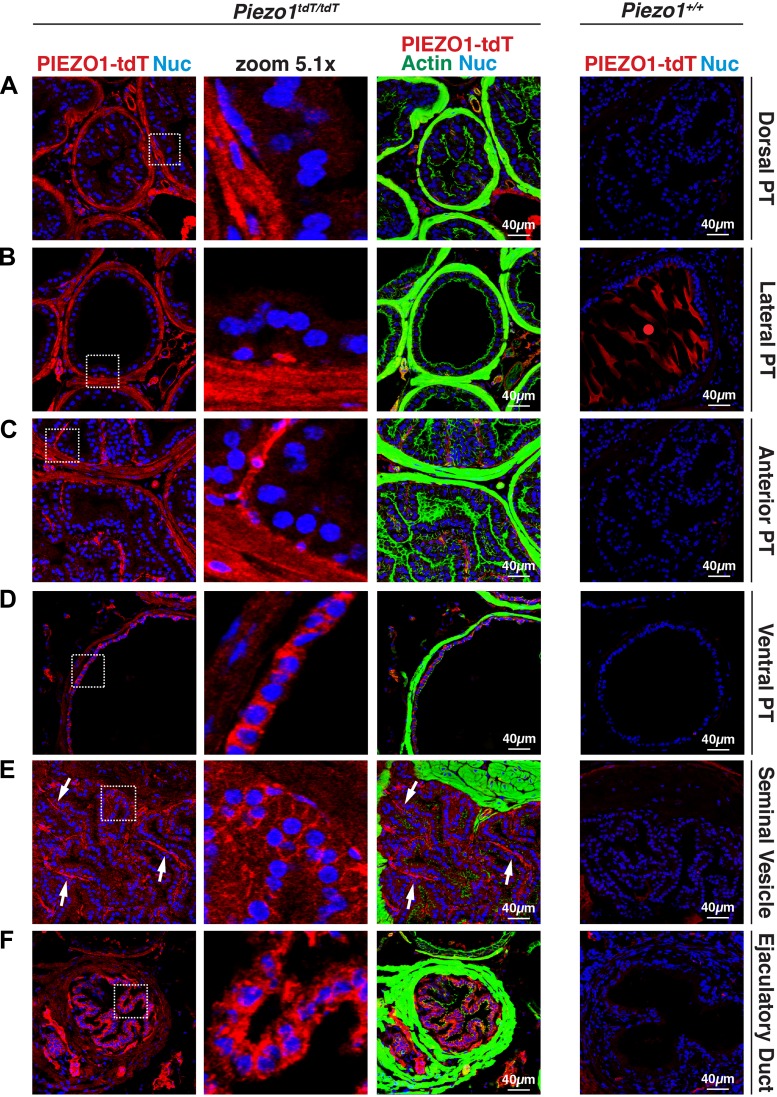
The male prostatic urethra and surrounding tissue is a complex structure characterized by the presence of the urethra and several allied structures, including the ducts and associated glands of the four lobes of the mouse prostate (anterior, ventral, lateral, and dorsal glands), ducts and glands of the seminal vesicles, and ejaculatory ducts (i.e., the terminal portion of ductus deferens; see Fig. 10 for an overview of this region of the ureter) (19, 35, 58, 61, 69). As in female mice, PIEZO1-tdT was strongly expressed in the stratified epithelium lining the male urethra, where it was localized to the basolateral surface of the superficial cells and surfaces of the underlying cell layers (Fig. 8D). The localization of PIEZO1-tdT in the dorsal prostate, lateral prostate, and anterior prostate was similar; PIEZO1-tdT was associated with the thin fibromuscular stroma, comprised of smooth muscle cells and fibroblast-like cells, surrounding each acinus (Fig. 9, A–C) (35). In the case of the ventral prostate, we observed that in addition to PIEZO1-tdT expression in the stroma, PIEZO1-tdT was also expressed on the basolateral surfaces of the simple cuboidal epithelium lining these acini (Fig. 9D). By staining for α-smooth muscle actin (ACTA2), we confirmed that the stromal cells were positive for PIEZO1-tdT expression (data not shown). PIEZO1-tdT was also expressed in the ducts of the seminal vesicles (and glandular portions; not shown) where it was weakly localized to the basolateral surfaces of the columnar epithelium lining these structures (Fig. 9E). In the ducts of the seminal vesicles (and glands), the strongest PIEZO-tdT staining was localized to the thin region of the stroma (comprised of smooth muscle cells and connective tissue) running down the midline of each of the papilla (see arrows in Fig. 9E). PIEZO1-tdT was also strongly expressed in the epithelium lining the ejaculatory ducts and, to a lesser degree, the stromal cells surrounding the duct (Fig. 9F).
Fig. 9.
PIEZO1-tandem-dimer Tomato (tdT) expression in the prostate, seminal vesicles, and ejaculatory ducts of male mice. A–F: distribution of PIEZO1-tdT, actin, and nuclei (Nuc) in the dorsal prostate (PT; A), lateral PT (B), anterior PT (C), ventral PT (D), seminal vesicles (E), and ejaculatory ducts (terminal region of ductus deferens; F) of Piezo1tdT/tdT and Piezo1+/+ mice. The actin signal associated with each organ is saturated to show the relatively weak signal associated with the epithelium of these organs. In the dorsal PT, lateral PT, and anterior PT, PIEZO1-tdT was localized primarily to stromal cells surrounding the epithelium. In the ventral PT, PIEZO1-tdT was localized to the stromal cells as well as the basolateral surface of the cuboidal epithelium that lines this gland of the ventral PT. The red circle in B, right, indicates nonspecific staining observed in glands containing large amounts of prostatic secretions. Arrows in E indicate the stromal regions within the papilla that form the seminal vesicles.
Taken together, our results indicate that PIEZO1-tdT is expressed in the urethra as well as several organs in the close proximity, including the vagina in female mice and the prostate glands, seminal vesicles (and associated ducts), and ejaculatory ducts of male mice. Although it was prominently expressed in the epithelium lining many, but not all, of these organs, PIEZO1-tdT was also expressed in stromal cells and striated muscle cells of the rhabdosphincter.
DISCUSSION
An open question is the identity of the mechanosensors that respond to fluid flow and wall tension in the urinary tract. Here, we report that PIEZO1-tdT is expressed in all of the organs that comprise the upper and lower urinary tracts, where it is associated with a diversity of cell types, including epithelial cells, interstitial cells, and smooth and striated muscle cells. Our observations are summarized in Fig. 10. Thus, PIEZO1 is likely to be a general sensor of mechanical stimuli across multiple cell types and tissues. Our study used the Piezo1tdT/tdT reporter mouse line, which has been previously used to explore PIEZO1 expression by FACS and Western blot (68). We now demonstrate that it can also be exploited to identify sites of PIEZO1 expression and distribution in native mouse tissues using immunofluorescence.
Mechanosensors in the urinary tract.
At present, we have few insights into the molecules that directly sense and respond to mechanical stimuli (i.e., mechanosensors) in the urinary tract. In the case of the kidney, most of the focus has been on responses to flow and the apical protrusions that may sense these events (65, 81, 83). Putative flow sensors in the kidney include the brush border of the proximal tubule cells (81), but the actual molecules that are sensing microvillar movements remain undescribed. The other widely described flow sensor in the kidney is the primary cilium (64). Putative mechanosensors associated with this structure include the polytopic membrane protein polycystin-1 (PKD1) and its allied binding partner and channel PKD2, both of which are mutated in polycystic kidney disease, as well as the PKD2 binding partner transient receptor potential vanilloid 4 (TRPV4) (40, 51). However, it is unclear how PKD2 is gated in situ (74), and TRPV4 has not been shown to be gated directly by stretch (24). Moreover, it was recently proposed that cilia do not directly trigger flow-induced Ca2+ responses (23), which may indicate that other nonciliary mechanosensors are involved. An additional potential mechanosensor is the aldosterone-sensitive distal nephron-localized epithelial Na+ channel (ENaC; comprised of SCNN1A, SCNN1B, and SCNN1G subunits), which is reported to be sensitive to shear stress and thus might also play some role in mechanosensation within the collecting ducts (10, 72). In addition to flow, intravital microscopy has been used to confirm that increasing tubular fluid flow rate by volume expansion (using intravenous injections of saline or treatment with the diuretic furosemide) leads to increases in distal tubular diameter by 50–170% (11). However, there are few insights into how cells in the nephron and collecting ducts detect changes in wall tension.
In the lower urinary tract of rodents, wall “mechanoceptors” (i.e., afferent nerve processes) are found near to and within the urothelium, in the lamina propria, and in and around the detrusor smooth muscle layer (7, 31, 67). They convey information from the bladder wall to the pelvic and hypogastric nerves, whose cell bodies are found in lumbrosacral (L6−S2) and thoracolumbar (T13−L2) dorsal root ganglia, respectively (21). Similar afferent pathways are present in the urethra (20). Despite the presence of these fibers, the mechanosensors involved and their associated downstream mechanotransduction cascades are not well understood. In addition to wall mechanoceptors, other cell types in the wall of lower urinary tract organs are reported to be mechanosensitive, including interstitial cells, smooth muscle cells, and the stratified urothelium, which lines the luminal surface of the renal pelvis, ureters, bladder, and upper portion of the urethra (28, 41, 45, 77, 84). The urothelium releases mediators in response to stretch that are hypothesized to activate a local urothelial:afferent reflex as well as to transmit information to underlying interstitial and smooth muscle cells (4, 37). Again, the mechanosensors involved are unknown, although multiple transient receptor potential family channels as well as ENaC have been implicated in these events (4).
PIEZO1 as a candidate mechanosensor in the urinary tract.
Based on our findings, we hypothesize that PIEZO1 is functioning as a channel-type mechanosensor in the urinary tract and that it is likely to meet all of the criteria for being classified as such (5). The first of these criteria is that the channel should be expressed in relevant cell types, at the correct cellular location, and at the appropriate developmental stage. Martins et al. (53) previously reported Piezo1 expression in the principal cells of the collecting duct, which are known to be sensitive to flow. This is consistent with RNA sequencing analyses of isolated mouse collecting duct cells and rat kidney tubule segments, which have reported relatively low overall abundance of Piezo1 transcripts in the kidney, but with most expression occurring in the collecting duct (12, 42). Our study expands on these earlier studies by documenting the sites of PIEZO1 protein expression in the kidney to include cells associated with the renal corpuscle, distal convoluted tubule, intercalated cells of the cortical collecting duct, and urothelium lining the renal pelvis.
PIEZO1 has also been reported to be expressed in a proximal tubule cell line and possibly in isolated proximal tubules (63). In contrast, RNA sequencing analysis of male rat kidney segments has indicated a paucity of Piezo1 expression in proximal tubules (42), and our analysis appears to exclude PIEZO1 expression in the proximal tubules of the mouse kidney. However, this may depend on the developmental stage of the mouse and/or the physiological state of the kidney (or isolated tubules) during analysis. Here, it is worth noting that there is a difference in the flow-induced Ca2+ responses in newborn mouse cortical collecting ducts versus those in adult mice; in newborn mice, flow triggers a rapid spike in [Ca2+]i, whereas in older mice the spike is followed by a plateau of [Ca2+]i (49). These findings may indicate a change in mechanosensation and possibly PIEZO1 expression in the collecting duct as mice age. Lee et al. (42) have also reported some Piezo1 expression in the long descending limb of the Loop of Henle in the inner medulla and the thin ascending limb; however, PIEZO1 expression in these regions of the nephron was not assessed in our analysis.
In the case of the lower urinary tract, and based on studies of mRNA abundance and immunolocalization using commercially available antibodies, PIEZO1 expression has also been reported in the urothelium and interstitial cells of the bladder (48, 55, 56). Our study confirms these observations and expands the list to other epithelial cells, smooth muscle cells, striated muscle cells, and interstitial cells of the lower urinary tract. The broad expression of PIEZO1 in the urinary tract should serve as a caution to investigators that when designing and interpreting experiments they must take into account the large number of cell types that express PIEZO1 in the urinary tract.
The second and third criteria are that the channel must respond to physiologically relevant stimuli and be required for mechanosensation in the tissue in question. Here, there is evidence in other tissues that PIEZO1 is responsive to both flow and changes in membrane tension (i.e., stretch), the two forces that are likely to be experienced in the urinary tract (15, 47). In the case of the kidney, pressure-induced currents, readily detectable in collecting ducts isolated from control mice, are not detected in cells isolated from conditional KspCre;Piezo1fl/fl knockout mice (53). Furthermore, in these mice, the loss of PIEZO1 affects osmoregulation (53). Both isolated urothelial cells and interstitial cells fail to increase [Ca2+]i in response to tension when treated with Piezo1-specific shRNAs (48, 56). Having identified multiple sites of PIEZO1 expression, it should now be possible to establish whether PIEZO1 channels are physiologically relevant in these tissues.
An additional fourth criterion is the requirement that altering the biophysical properties of the channel should affect its responses in a predictable fashion. Although this has yet to be tested in transgenic animals, there are studies that have characterized the biophysical properties of PIEZO1 channels bearing mutations found in patients with hereditary xerocytosis, a genetic disorder characterized by dehydration of red blood cells as a result of loss of water and K+ (2, 3, 6, 89). In this case, the mutations result in PIEZO1 channels that fail to inactivate properly. The growing list of mutant PIEZO1 proteins that also exhibit altered activity and sensitivity to stretch should provide a rich resource for further understanding how PIEZO1 activity contributes to urinary tract function (16, 82, 87). Finally, the fifth criterion is that the channel should be mechanically gated when heterologously expressed and when reconstituted in liposomes. Here, there is evidence that PIEZO1 channels open in response to membrane tension even in membrane blebs that lack cytoskeletal elements and that PIEZO1 channels are functional (but constitutively active) when incorporated into liposomes or lipid droplet bilayers (15, 17, 18, 44, 75).
PIEZO1 may function to sense changes in wall tension.
With the exception of endothelial cells and parietal cells, which express PIEZO1 at their apical and basolateral surfaces, we found that PIEZO1 is localized to the basolateral surfaces of the epithelial cells that express this protein. This agrees with a previous report (53) that a stretch-activated channel, likely PIEZO1, is active at the basolateral surfaces of isolated medullary collecting ducts. This localization would appear to preclude any direct role for PIEZO1 in sensing flow in these populations of epithelial cells. Coupled with our observation that PIEZO1 is also expressed in cells deep within the walls of the ureter, bladder, and urethra, we hypothesize that a chief function of PIEZO1 in the urinary tract is to sense increases in wall tension as urine flows through the nephron, collecting ducts, ureters, or urethra or when urine collects in the bladder. In the case of the nephron and collecting ducts, the “wall” is limited to the lateral and perhaps basal surfaces of the simple epithelia that form these structures. According to the Young-LaPlace equation (where tension of a cylinder = pressure across the vessel wall × the radius of curvature), as the diameter of the tubule increases, so does wall tension. When isolated cortical collecting ducts are perfused, the associated cells exhibit a rise in [Ca2+]i after an ∼11-s delay. Interestingly, the increase in [Ca2+]i depends in large part on basolateral Ca2+ entry (coupled with internal Ca2+ release) (50). Although the nature of the basolateral entry pathway is currently unknown, PIEZO1 channels, which conduct Ca2+, are possible candidates.
In the lower urinary tract, the changes in wall tension that accompany fluid flow or retention are not limited to the epithelium but also impact tissues deeper in the wall of these organs. Interestingly, in the bladder, PIEZO1 is expressed in the urothelium and interstitial cells of the mucosa, smooth muscles cells of the muscularis externa, and mesothelial cells of the serosa. A similar distribution is noted in the ureters. Assuming PIEZO1 is functional in each of these tissues, it indicates that tension in the bladder wall is sensed not only by central nervous system-associated wall mechanoceptors but by all tissue layers in the bladder wall. Although this concept is already generally accepted in the bladder field, the identification of PIEZO1 as a possible mechanosensor in each of these tissues was not well understood. Our study further indicates that the urethra and closely allied organs, including the rhabdosphincter, vaginal epithelium, seminal vesicles and ducts, ejaculatory ducts, and prostate glands, are also sites of PIEZO1 expression and may similarly use PIEZO1 as a mechanism to sense wall tension. In the case of the prostate and other accessory organs, the localization of PIEZO1-tdT to the thin band of stroma surrounding the glands indicates that it may serve a role in detecting the amounts of glandular content and possibly aiding in promoting the contraction and release of glandular secretions during ejaculation. Future experiments will define whether this is the case.
In summary, there is a paucity of information about the mechanosensors that are expressed in the urinary tract or their sites of action. We used the Piezo1-tdT reporter mouse to identify sites of PIEZO1 expression and found that PIEZO-tdT is expressed throughout the urinary tract, where it is associated with all tissue types. Although definitive functional experiments need to be performed, we believe it is likely that PIEZO1 will serve as a general mechanosensor in the urinary tract, where it will sense changes in wall tension.
GRANTS
This work was supported by the Urology Care Foundation Research Scholar Award Program (to M. G. Dalghi), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant K01-DK-109038 (to M. Al-bataineh), National Institutes of Health Grants R01-DK-104287 and P30-DK-079307 (to G. Apodaca), R01-DK-038470 (to L. M. Satlin and T. R. Kleyman), and U54-DK-104310 and R01-ES-001332 (to W. A. Ricke), and the Kidney Imaging Core of the Pittsburgh Center for Kidney Research (P30-DK-079307).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.G.D., D.R.C., W.G.R., M.M.A.-b., M.D.C., and G.A. conceived and designed research; M.G.D., D.R.C., and W.G.R. performed experiments; M.G.D., D.R.C., W.A.R., and G.A. analyzed data; M.G.D., D.R.C., M.M.A.-b., L.M.S., T.R.K., W.A.R., M.D.C., and G.A. interpreted results of experiments; M.G.D., D.R.C., and G.A. prepared figures; M.G.D. and G.A. drafted manuscript; M.G.D., M.M.A.-b., L.M.S., T.R.K., W.A.R., M.D.C., and G.A. edited and revised manuscript; M.G.D., D.R.C., W.G.R., M.M.A.-b., L.M.S., T.R.K., W.A.R., M.D.C., and G.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Roderick Tan for reading our manuscript and providing useful comments and suggestions and Dr. Susan Wall, Dr. Arohan Subramanya, Dr. Roderick Tan, and Dr. Ora Weisz for providing antibodies.
REFERENCES
- 1.Albuisson J, Murthy SE, Bandell M, Coste B, Louis-Dit-Picard H, Mathur J, Fénéant-Thibault M, Tertian G, de Jaureguiberry JP, Syfuss PY, Cahalan S, Garçon L, Toutain F, Simon Rohrlich P, Delaunay J, Picard V, Jeunemaitre X, Patapoutian A. Dehydrated hereditary stomatocytosis linked to gain-of-function mutations in mechanically activated PIEZO1 ion channels. Nat Commun 4: 1884, 2013. [Erratum in Nat Commun 4: 2440, 2013.] doi: 10.1038/ncomms2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alper SL. Genetic diseases of PIEZO1 and PIEZO2 dysfunction. Curr Top Membr 79: 97–134, 2017. doi: 10.1016/bs.ctm.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Andolfo I, Alper SL, De Franceschi L, Auriemma C, Russo R, De Falco L, Vallefuoco F, Esposito MR, Vandorpe DH, Shmukler BE, Narayan R, Montanaro D, D’Armiento M, Vetro A, Limongelli I, Zuffardi O, Glader BE, Schrier SL, Brugnara C, Stewart GW, Delaunay J, Iolascon A. Multiple clinical forms of dehydrated hereditary stomatocytosis arise from mutations in PIEZO1. Blood 121: 3925–3935, 2013. doi: 10.1182/blood-2013-02-482489. [DOI] [PubMed] [Google Scholar]
- 4.Apodaca G, Balestreire E, Birder LA. The uroepithelial-associated sensory web. Kidney Int 72: 1057–1064, 2007. doi: 10.1038/sj.ki.5002439. [DOI] [PubMed] [Google Scholar]
- 5.Árnadóttir J, Chalfie M. Eukaryotic mechanosensitive channels. Annu Rev Biophys 39: 111–137, 2010. doi: 10.1146/annurev.biophys.37.032807.125836. [DOI] [PubMed] [Google Scholar]
- 6.Bae C, Gnanasambandam R, Nicolai C, Sachs F, Gottlieb PA. Xerocytosis is caused by mutations that alter the kinetics of the mechanosensitive channel PIEZO1. Proc Natl Acad Sci USA 110: E1162–E1168, 2013. doi: 10.1073/pnas.1219777110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahns E, Halsband U, Jänig W. Responses of sacral visceral afferents from the lower urinary tract, colon and anus to mechanical stimulation. Pflugers Arch 410: 296–303, 1987. doi: 10.1007/BF00580280. [DOI] [PubMed] [Google Scholar]
- 8.Birn H, Fyfe JC, Jacobsen C, Mounier F, Verroust PJ, Ørskov H, Willnow TE, Moestrup SK, Christensen EI. Cubilin is an albumin binding protein important for renal tubular albumin reabsorption. J Clin Invest 105: 1353–1361, 2000. doi: 10.1172/JCI8862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown D, Hirsch S, Gluck S. Localization of a proton-pumping ATPase in rat kidney. J Clin Invest 82: 2114–2126, 1988. doi: 10.1172/JCI113833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carattino MD, Liu W, Hill WG, Satlin LM, Kleyman TR. Lack of a role of membrane-protein interactions in flow-dependent activation of ENaC. Am J Physiol Renal Physiol 293: F316–F324, 2007. doi: 10.1152/ajprenal.00455.2006. [DOI] [PubMed] [Google Scholar]
- 11.Carrisoza-Gaytan R, Liu Y, Flores D, Else C, Lee HG, Rhodes G, Sandoval RM, Kleyman TR, Lee FY, Molitoris B, Satlin LM, Rohatgi R. Effects of biomechanical forces on signaling in the cortical collecting duct (CCD). Am J Physiol Renal Physiol 307: F195–F204, 2014. doi: 10.1152/ajprenal.00634.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L, Lee JW, Chou CL, Nair AV, Battistone MA, Păunescu TG, Merkulova M, Breton S, Verlander JW, Wall SM, Brown D, Burg MB, Knepper MA. Transcriptomes of major renal collecting duct cell types in mouse identified by single-cell RNA-seq. Proc Natl Acad Sci USA 114: E9989–E9998, 2017. doi: 10.1073/pnas.1710964114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chesler AT, Szczot M, Bharucha-Goebel D, Čeko M, Donkervoort S, Laubacher C, Hayes LH, Alter K, Zampieri C, Stanley C, Innes AM, Mah JK, Grosmann CM, Bradley N, Nguyen D, Foley AR, Le Pichon CE, Bönnemann CG. The Role of PIEZO2 in Human Mechanosensation. N Engl J Med 375: 1355–1364, 2016. doi: 10.1056/NEJMoa1602812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coste B, Houge G, Murray MF, Stitziel N, Bandell M, Giovanni MA, Philippakis A, Hoischen A, Riemer G, Steen U, Steen VM, Mathur J, Cox J, Lebo M, Rehm H, Weiss ST, Wood JN, Maas RL, Sunyaev SR, Patapoutian A. Gain-of-function mutations in the mechanically activated ion channel PIEZO2 cause a subtype of distal arthrogryposis. Proc Natl Acad Sci USA 110: 4667–4672, 2013. doi: 10.1073/pnas.1221400110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330: 55–60, 2010. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coste B, Murthy SE, Mathur J, Schmidt M, Mechioukhi Y, Delmas P, Patapoutian A. Piezo1 ion channel pore properties are dictated by C-terminal region. Nat Commun 6: 7223, 2015. doi: 10.1038/ncomms8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, Kim SE, Schmidt M, Mathur J, Dubin AE, Montal M, Patapoutian A. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 483: 176–181, 2012. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox CD, Bae C, Ziegler L, Hartley S, Nikolova-Krstevski V, Rohde PR, Ng CA, Sachs F, Gottlieb PA, Martinac B. Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nat Commun 7: 10366, 2016. doi: 10.1038/ncomms10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunha GR, Vezina CM, Isaacson D, Ricke WA, Timms BG, Cao M, Franco O, Baskin LS. Development of the human prostate. Differentiation 103: 24–45, 2018. doi: 10.1016/j.diff.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Groat WC, Griffiths D, Yoshimura N. Neural control of the lower urinary tract. Compr Physiol 5: 327–396, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Groat WC, Yoshimura N. Afferent nerve regulation of bladder function in health and disease. Handb Exp Pharmacol 194: 91–138, 2009. doi: 10.1007/978-3-540-79090-7_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delle Vedove A, Storbeck M, Heller R, Hölker I, Hebbar M, Shukla A, Magnusson O, Cirak S, Girisha KM, O’Driscoll M, Loeys B, Wirth B. Biallelic loss of proprioception-related PIEZO2 causes muscular atrophy with perinatal respiratory distress, arthrogryposis, and scoliosis. Am J Hum Genet 99: 1206–1216, 2016. [Erratum in Am J Hum Genet 99: 1406–1408, 2016.] doi: 10.1016/j.ajhg.2016.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delling M, Indzhykulian AA, Liu X, Li Y, Xie T, Corey DP, Clapham DE. Primary cilia are not calcium-responsive mechanosensors. Nature 531: 656–660, 2016. doi: 10.1038/nature17426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delmas P, Hao J, Rodat-Despoix L. Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nat Rev Neurosci 12: 139–153, 2011. doi: 10.1038/nrn2993. [DOI] [PubMed] [Google Scholar]
- 25.Eisenhoffer GT, Loftus PD, Yoshigi M, Otsuna H, Chien CB, Morcos PA, Rosenblatt J. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature 484: 546–549, 2012. doi: 10.1038/nature10999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellison DH, Biemesderfer D, Morrisey J, Lauring J, Desir GV. Immunocytochemical characterization of the high-affinity thiazide diuretic receptor in rabbit renal cortex. Am J Physiol Renal Physiol 264: F141–F148, 1993. doi: 10.1152/ajprenal.1993.264.1.F141. [DOI] [PubMed] [Google Scholar]
- 27.Faucherre A, Nargeot J, Mangoni ME, Jopling C. piezo2b regulates vertebrate light touch response. J Neurosci 33: 17089–17094, 2013. doi: 10.1523/JNEUROSCI.0522-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes–a possible sensory mechanism? J Physiol 505: 503–511, 1997. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Florez-Paz D, Bali KK, Kuner R, Gomis A. A critical role for Piezo2 channels in the mechanotransduction of mouse proprioceptive neurons. Sci Rep 6: 25923, 2016. doi: 10.1038/srep25923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fushimi K, Uchida S, Harat Y, Hirata Y, Marumo F, Sasaki S. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature 361: 549–552, 1993. doi: 10.1038/361549a0. [DOI] [PubMed] [Google Scholar]
- 31.Gabella G, Davis C. Distribution of afferent axons in the bladder of rats. J Neurocytol 27: 141–155, 1998. doi: 10.1023/A:1006903507321. [DOI] [PubMed] [Google Scholar]
- 32.Ge J, Li W, Zhao Q, Li N, Chen M, Zhi P, Li R, Gao N, Xiao B, Yang M. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature 527: 64–69, 2015. doi: 10.1038/nature15247. [DOI] [PubMed] [Google Scholar]
- 33.Gudipaty SA, Lindblom J, Loftus PD, Redd MJ, Edes K, Davey CF, Krishnegowda V, Rosenblatt J. Mechanical stretch triggers rapid epithelial cell division through Piezo1. Nature 543: 118–121, 2017. doi: 10.1038/nature21407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ikeda R, Gu JG. Piezo2 channel conductance and localization domains in Merkel cells of rat whisker hair follicles. Neurosci Lett 583: 210–215, 2014. doi: 10.1016/j.neulet.2014.05.055. [DOI] [PubMed] [Google Scholar]
- 35.Ittmann M. Anatomy and histology of the human and murine prostate. Cold Spring Harb Perspect Med 8: a030346, 2018. doi: 10.1101/cshperspect.a030346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanai A, Andersson KE. Bladder afferent signaling: recent findings. J Urol 183: 1288–1295, 2010. doi: 10.1016/j.juro.2009.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khandelwal P, Abraham SN, Apodaca G. Cell biology and physiology of the uroepithelium. Am J Physiol Renal Physiol 297: F1477–F1501, 2009. doi: 10.1152/ajprenal.00327.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim SE, Coste B, Chadha A, Cook B, Patapoutian A. The role of Drosophila Piezo in mechanical nociception. Nature 483: 209–212, 2012. doi: 10.1038/nature10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koh BH, Roy R, Hollywood MA, Thornbury KD, McHale NG, Sergeant GP, Hatton WJ, Ward SM, Sanders KM, Koh SD. Platelet-derived growth factor receptor-α cells in mouse urinary bladder: a new class of interstitial cells. J Cell Mol Med 16: 691–700, 2012. doi: 10.1111/j.1582-4934.2011.01506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Köttgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T, Nitschke R, Suzuki M, Kramer-Zucker A, Germino GG, Watnick T, Prenen J, Nilius B, Kuehn EW, Walz G. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol 182: 437–447, 2008. doi: 10.1083/jcb.200805124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee H, Koh BH, Peri LE, Corrigan RD, Lee HT, George NE, Bhetwal BP, Xie Y, Perrino BA, Chai TC, Sanders KM, Koh SD. Premature contractions of the bladder are suppressed by interactions between TRPV4 and SK3 channels in murine detrusor PDGFRα+ cells. Sci Rep 7: 12245, 2017. doi: 10.1038/s41598-017-12561-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee JW, Chou CL, Knepper MA. Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol 26: 2669–2677, 2015. doi: 10.1681/ASN.2014111067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee W, Leddy HA, Chen Y, Lee SH, Zelenski NA, McNulty AL, Wu J, Beicker KN, Coles J, Zauscher S, Grandl J, Sachs F, Guilak F, Liedtke WB. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc Natl Acad Sci USA 111: E5114–E5122, 2014. doi: 10.1073/pnas.1414298111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewis AH, Grandl J. Mechanical sensitivity of Piezo1 ion channels can be tuned by cellular membrane tension. eLife 4: e12088, 2015. doi: 10.7554/eLife.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewis SA, de Moura JL. Incorporation of cytoplasmic vesicles into apical membrane of mammalian urinary bladder epithelium. Nature 297: 685–688, 1982. doi: 10.1038/297685a0. [DOI] [PubMed] [Google Scholar]
- 46.Lhomme A, Gilbert G, Pele T, Deweirdt J, Henrion D, Baudrimont I, Campagnac M, Marthan R, Guibert C, Ducret T, Savineau JP, Quignard JF. Stretch-activated Piezo1 channel in endothelial cells relaxes mouse intrapulmonary arteries. Am J Respir Cell Mol Biol 60: 650–658, 2019. doi: 10.1165/rcmb.2018-0197OC. [DOI] [PubMed] [Google Scholar]
- 47.Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, Sedo A, Hyman AJ, McKeown L, Young RS, Yuldasheva NY, Majeed Y, Wilson LA, Rode B, Bailey MA, Kim HR, Fu Z, Carter DA, Bilton J, Imrie H, Ajuh P, Dear TN, Cubbon RM, Kearney MT, Prasad RK, Evans PC, Ainscough JF, Beech DJ. Piezo1 integration of vascular architecture with physiological force. Nature 515: 279–282, 2014. doi: 10.1038/nature13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Q, Sun B, Zhao J, Wang Q, An F, Hu X, Yang Z, Xu J, Tan M, Li L. Increased Piezo1 channel activity in interstitial Cajal-like cells induces bladder hyperactivity by functionally interacting with NCX1 in rats with cyclophosphamide-induced cystitis. Exp Mol Med 50: 60, 2018. doi: 10.1038/s12276-018-0088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu W, Murcia NS, Duan Y, Weinbaum S, Yoder BK, Schwiebert E, Satlin LM. Mechanoregulation of intracellular Ca2+ concentration is attenuated in collecting duct of monocilium-impaired orpk mice. Am J Physiol Renal Physiol 289: F978–F988, 2005. doi: 10.1152/ajprenal.00260.2004. [DOI] [PubMed] [Google Scholar]
- 50.Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM. Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol 285: F998–F1012, 2003. doi: 10.1152/ajprenal.00067.2003. [DOI] [PubMed] [Google Scholar]
- 51.Liu X, Vien T, Duan J, Sheu SH, DeCaen PG, Clapham DE. Polycystin-2 is an essential ion channel subunit in the primary cilium of the renal collecting duct epithelium. eLife 7: e33183, 2018. doi: 10.7554/eLife.33183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahmud AA, Nahid NA, Nassif C, Sayeed MS, Ahmed MU, Parveen M, Khalil MI, Islam MM, Nahar Z, Rypens F, Hamdan FF, Rouleau GA, Hasnat A, Michaud JL. Loss of the proprioception and touch sensation channel PIEZO2 in siblings with a progressive form of contractures. Clin Genet 91: 470–475, 2017. doi: 10.1111/cge.12850. [DOI] [PubMed] [Google Scholar]
- 53.Martins JR, Penton D, Peyronnet R, Arhatte M, Moro C, Picard N, Kurt B, Patel A, Honoré E, Demolombe S. Piezo1-dependent regulation of urinary osmolarity. Pflugers Arch 468: 1197–1206, 2016. doi: 10.1007/s00424-016-1811-z. [DOI] [PubMed] [Google Scholar]
- 54.McMillin MJ, Beck AE, Chong JX, Shively KM, Buckingham KJ, Gildersleeve HI, Aracena MI, Aylsworth AS, Bitoun P, Carey JC, Clericuzio CL, Crow YJ, Curry CJ, Devriendt K, Everman DB, Fryer A, Gibson K, Giovannucci Uzielli ML, Graham JM Jr, Hall JG, Hecht JT, Heidenreich RA, Hurst JA, Irani S, Krapels IP, Leroy JG, Mowat D, Plant GT, Robertson SP, Schorry EK, Scott RH, Seaver LH, Sherr E, Splitt M, Stewart H, Stumpel C, Temel SG, Weaver DD, Whiteford M, Williams MS, Tabor HK, Smith JD, Shendure J, Nickerson DA, Bamshad MJ; University of Washington Center for Mendelian Genomics . Mutations in PIEZO2 cause Gordon syndrome, Marden-Walker syndrome, and distal arthrogryposis type 5. Am J Hum Genet 94: 734–744, 2014. doi: 10.1016/j.ajhg.2014.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Michishita M, Yano K, Tomita KI, Matsuzaki O, Kasahara KI. Piezo1 expression increases in rat bladder after partial bladder outlet obstruction. Life Sci 166: 1–7, 2016. doi: 10.1016/j.lfs.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 56.Miyamoto T, Mochizuki T, Nakagomi H, Kira S, Watanabe M, Takayama Y, Suzuki Y, Koizumi S, Takeda M, Tominaga M. Functional role for Piezo1 in stretch-evoked Ca2+ influx and ATP release in urothelial cell cultures. J Biol Chem 289: 16565–16575, 2014. doi: 10.1074/jbc.M113.528638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muller WA, Ratti CM, McDonnell SL, Cohn ZA. A human endothelial cell-restricted, externally disposed plasmalemmal protein enriched in intercellular junctions. J Exp Med 170: 399–414, 1989. doi: 10.1084/jem.170.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nicholson TM, Ricke EA, Marker PC, Miano JM, Mayer RD, Timms BG, vom Saal FS, Wood RW, Ricke WA. Testosterone and 17β-estradiol induce glandular prostatic growth, bladder outlet obstruction, and voiding dysfunction in male mice. Endocrinology 153: 5556–5565, 2012. doi: 10.1210/en.2012-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nonomura K, Woo SH, Chang RB, Gillich A, Qiu Z, Francisco AG, Ranade SS, Liberles SD, Patapoutian A. Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature 541: 176–181, 2017. doi: 10.1038/nature20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Obermüller N, Kunchaparty S, Ellison DH, Bachmann S. Expression of the Na-K-2Cl cotransporter by macula densa and thick ascending limb cells of rat and rabbit nephron. J Clin Invest 98: 635–640, 1996. doi: 10.1172/JCI118834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oliveira DS, Dzinic S, Bonfil AI, Saliganan AD, Sheng S, Bonfil RD. The mouse prostate: a basic anatomical and histological guideline. Bosn J Basic Med Sci 16: 8–13, 2016. doi: 10.17305/bjbms.2016.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pathak MM, Nourse JL, Tran T, Hwe J, Arulmoli J, Le DT, Bernardis E, Flanagan LA, Tombola F. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc Natl Acad Sci USA 111: 16148–16153, 2014. doi: 10.1073/pnas.1409802111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peyronnet R, Martins JR, Duprat F, Demolombe S, Arhatte M, Jodar M, Tauc M, Duranton C, Paulais M, Teulon J, Honoré E, Patel A. Piezo1-dependent stretch-activated channels are inhibited by Polycystin-2 in renal tubular epithelial cells. EMBO Rep 14: 1143–1148, 2013. doi: 10.1038/embor.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Praetorius HA. The primary cilium as sensor of fluid flow: new building blocks to the model. A review in the theme: cell signaling: proteins, pathways and mechanisms. Am J Physiol Cell Physiol 308: C198–C208, 2015. doi: 10.1152/ajpcell.00336.2014. [DOI] [PubMed] [Google Scholar]
- 65.Praetorius HA, Spring KR. Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol 191: 69–76, 2003. doi: 10.1007/s00232-002-1042-4. [DOI] [PubMed] [Google Scholar]
- 66.Prozialeck WC, Lamar PC, Appelt DM. Differential expression of E-cadherin, N-cadherin and beta-catenin in proximal and distal segments of the rat nephron. BMC Physiol 4: 10, 2004. doi: 10.1186/1472-6793-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rahnama’i MS, Biallosterski BT, Van Kerrebroeck PE, van Koeveringe GA, Gillespie JI, de Wachter SG. Distribution and sub-types of afferent fibre in the mouse urinary bladder. J Chem Neuroanat 79: 1–11, 2017. doi: 10.1016/j.jchemneu.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 68.Ranade SS, Qiu Z, Woo SH, Hur SS, Murthy SE, Cahalan SM, Xu J, Mathur J, Bandell M, Coste B, Li YS, Chien S, Patapoutian A. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc Natl Acad Sci USA 111: 10347–10352, 2014. doi: 10.1073/pnas.1409233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ricke WA, Timms BG, vom Saal FS. Prostate structure. In: Encyclopedia of Reproduction, edited by Skinner MK. Amsterdam: Elsevier, 2018, p. 315–324. [Google Scholar]
- 70.Roselli S, Gribouval O, Boute N, Sich M, Benessy F, Attié T, Gubler MC, Antignac C. Podocin localizes in the kidney to the slit diaphragm area. Am J Pathol 160: 131–139, 2002. doi: 10.1016/S0002-9440(10)64357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sabolić I, Valenti G, Verbavatz JM, Van Hoek AN, Verkman AS, Ausiello DA, Brown D. Localization of the CHIP28 water channel in rat kidney. Am J Physiol Cell Physiol 263: C1225–C1233, 1992. doi: 10.1152/ajpcell.1992.263.6.C1225. [DOI] [PubMed] [Google Scholar]
- 72.Satlin LM, Sheng S, Woda CB, Kleyman TR. Epithelial Na+ channels are regulated by flow. Am J Physiol Renal Physiol 280: F1010–F1018, 2001. doi: 10.1152/ajprenal.2001.280.6.F1010. [DOI] [PubMed] [Google Scholar]
- 73.Shea VK, Cai R, Crepps B, Mason JL, Perl ER. Sensory fibers of the pelvic nerve innervating the Rat’s urinary bladder. J Neurophysiol 84: 1924–1933, 2000. doi: 10.1152/jn.2000.84.4.1924. [DOI] [PubMed] [Google Scholar]
- 74.Shen PS, Yang X, DeCaen PG, Liu X, Bulkley D, Clapham DE, Cao E. The structure of the polycystic kidney disease channel PKD2 in lipid nanodiscs. Cell 167: 763–773.e11, 2016. doi: 10.1016/j.cell.2016.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Syeda R, Florendo MN, Cox CD, Kefauver JM, Santos JS, Martinac B, Patapoutian A. Piezo1 Channels Are Inherently Mechanosensitive. Cell Reports 17: 1739–1746, 2016. doi: 10.1016/j.celrep.2016.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tokonami N, Takata T, Beyeler J, Ehrbar I, Yoshifuji A, Christensen EI, Loffing J, Devuyst O, Olinger EG. Uromodulin is expressed in the distal convoluted tubule, where it is critical for regulation of the sodium chloride cotransporter NCC. Kidney Int 94: 701–715, 2018. doi: 10.1016/j.kint.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 77.Truschel ST, Wang E, Ruiz WG, Leung SM, Rojas R, Lavelle J, Zeidel M, Stoffer D, Apodaca G. Stretch-regulated exocytosis/endocytosis in bladder umbrella cells. Mol Biol Cell 13: 830–846, 2002. doi: 10.1091/mbc.01-09-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Volkers L, Mechioukhi Y, Coste B. Piezo channels: from structure to function. Pflugers Arch 467: 95–99, 2015. doi: 10.1007/s00424-014-1578-z. [DOI] [PubMed] [Google Scholar]
- 79.Wall SM, Hassell KA, Royaux IE, Green ED, Chang JY, Shipley GL, Verlander JW. Localization of pendrin in mouse kidney. Am J Physiol Renal Physiol 284: F229–F241, 2003. doi: 10.1152/ajprenal.00147.2002. [DOI] [PubMed] [Google Scholar]
- 80.Wang S, Chennupati R, Kaur H, Iring A, Wettschureck N, Offermanns S. Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. J Clin Invest 126: 4527–4536, 2016. doi: 10.1172/JCI87343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang T, Weinbaum S, Weinstein AM. Regulation of glomerulotubular balance: flow-activated proximal tubule function. Pflugers Arch 469: 643–654, 2017. doi: 10.1007/s00424-017-1960-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Y, Chi S, Guo H, Li G, Wang L, Zhao Q, Rao Y, Zu L, He W, Xiao B. A lever-like transduction pathway for long-distance chemical- and mechano-gating of the mechanosensitive Piezo1 channel. Nat Commun 9: 1300, 2018. doi: 10.1038/s41467-018-03570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weinbaum S, Duan Y, Satlin LM, Wang T, Weinstein AM. Mechanotransduction in the renal tubule. Am J Physiol Renal Physiol 299: F1220–F1236, 2010. doi: 10.1152/ajprenal.00453.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wellner MC, Isenberg G. Properties of stretch-activated channels in myocytes from the guinea-pig urinary bladder. J Physiol 466: 213–227, 1993. [PMC free article] [PubMed] [Google Scholar]
- 85.Woo SH, Lukacs V, de Nooij JC, Zaytseva D, Criddle CR, Francisco A, Jessell TM, Wilkinson KA, Patapoutian A. Piezo2 is the principal mechanotransduction channel for proprioception. Nat Neurosci 18: 1756–1762, 2015. doi: 10.1038/nn.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, Stucky CL, Patapoutian A. Piezo2 is required for Merkel-cell mechanotransduction. Nature 509: 622–626, 2014. doi: 10.1038/nature13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu J, Young M, Lewis AH, Martfeld AN, Kalmeta B, Grandl J. Inactivation of mechanically activated Piezo1 ion channels is determined by the C-terminal extracellular domain and the inner pore helix. Cell Reports 21: 2357–2366, 2017. doi: 10.1016/j.celrep.2017.10.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu L, Gebhart GF. Characterization of mouse lumbar splanchnic and pelvic nerve urinary bladder mechanosensory afferents. J Neurophysiol 99: 244–253, 2008. doi: 10.1152/jn.01049.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zarychanski R, Schulz VP, Houston BL, Maksimova Y, Houston DS, Smith B, Rinehart J, Gallagher PG. Mutations in the mechanotransduction protein PIEZO1 are associated with hereditary xerocytosis. Blood 120: 1908–1915, 2012. doi: 10.1182/blood-2012-04-422253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zeng WZ, Marshall KL, Min S, Daou I, Chapleau MW, Abboud FM, Liberles SD, Patapoutian A. PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science 362: 464–467, 2018. doi: 10.1126/science.aau6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhao Q, Zhou H, Chi S, Wang Y, Wang J, Geng J, Wu K, Liu W, Zhang T, Dong MQ, Wang J, Li X, Xiao B. Structure and mechanogating mechanism of the Piezo1 channel. Nature 554: 487–492, 2018. [Erratum in Nature 563: E19, 2018.] doi: 10.1038/nature25743. [DOI] [PubMed] [Google Scholar]