Abstract
Na+/H+ exchanger isoform 3 (NHE3) contributes to Na+/bicarbonate reabsorption and ammonium secretion in early proximal tubules. To determine its role in the diabetic kidney, type 1 diabetic Akita mice with tubular NHE3 knockdown [Pax8-Cre; NHE3-knockout (KO) mice] were generated. NHE3-KO mice had higher urine pH, more bicarbonaturia, and compensating increases in renal mRNA expression for genes associated with generation of ammonium, bicarbonate, and glucose (phosphoenolpyruvate carboxykinase) in proximal tubules and H+ and ammonia secretion and glycolysis in distal tubules. This left blood pH and bicarbonate unaffected in nondiabetic and diabetic NHE3-KO versus wild-type mice but was associated with renal upregulation of proinflammatory markers. Higher renal phosphoenolpyruvate carboxykinase expression in NHE3-KO mice was associated with lower Na+-glucose cotransporter (SGLT)2 and higher SGLT1 expression, indicating a downward tubular shift in Na+ and glucose reabsorption. NHE3-KO was associated with lesser kidney weight and glomerular filtration rate (GFR) independent of diabetes and prevented diabetes-associated albuminuria. NHE3-KO, however, did not attenuate hyperglycemia or prevent diabetes from increasing kidney weight and GFR. Higher renal gluconeogenesis may explain similar hyperglycemia despite lower SGLT2 expression and higher glucosuria in diabetic NHE3-KO versus wild-type mice; stronger SGLT1 engagement could have affected kidney weight and GFR responses. Chronic kidney disease in humans is associated with reduced urinary excretion of metabolites of branched-chain amino acids and the tricarboxylic acid cycle, a pattern mimicked in diabetic wild-type mice. This pattern was reversed in nondiabetic NHE3-KO mice, possibly reflecting branched-chain amino acids use for ammoniagenesis and tricarboxylic acid cycle upregulation to support formation of ammonia, bicarbonate, and glucose in proximal tubule. NHE3-KO, however, did not prevent the diabetes-induced urinary downregulation in these metabolites.
Keywords: albuminuria, diabetes mellitus, diabetic nephropathy, hyperfiltration, Na+-glucose cotransporter 2, proximal tubule, tubular growth
INTRODUCTION
In a subset of patients with early diabetes renal proximal tubules and glomeruli enlarge and glomerular filtration rate (GFR) increases (63). Early kidney growth and glomerular hyperfiltration have been linked to the long-term development of diabetic kidney disease, including the onset and progression of albuminuria, tubulointerstitial fibrosis, glomerular sclerosis, and finally deterioration of renal function (37, 57, 59, 63). Therefore, a better understanding of the mechanisms of diabetes-induced early kidney growth and glomerular hyperfiltration bears the potential to develop new strategies to prevent or attenuate the progression of diabetic nephropathy (DN).
Early in the course of diabetes mellitus, there is a primary increase in proximal reabsorption, which reduces delivery of NaCl and fluid to the macula densa, thereby leading to glomerular hyperfiltration through the normal action of tubuloglomerular feedback with additional contributions from a reduction in Bowman's space pressure (54, 60, 63). It has been confirmed in micropuncture studies that decreased macula densa delivery is a prerequisite for diabetic hyperfiltration, and the association of hyperfiltration with decreased distal delivery has been established in humans using lithium clearance (42, 63). The primary tubular hyperreabsorption can lead to Na+ and fluid retention and contribute to an increase in blood pressure, which is partially mitigated by the increase in GFR (25, 40, 58). Multiple factors seem to contribute to the primary increase in proximal reabsorption in the diabetic kidney. One factor is growth/hypertrophy of the proximal tubule, which results in more tubular machinery for reabsorption (54). Another factor is increased glucose in the glomerular filtrate, which is reabsorbed primarily in proximal tubules along with Na+ through Na+-glucose cotransporter 2 (SGLT2) and Na+-glucose cotransporter 1 (SGLT1) (55, 60, 64).
The proximal tubule isosmotically reabsorbs 60–70% of filtered NaCl and water and reabsorbs 70–90% of filtered bicarbonate (13). A significant portion of proximal tubular Na+ reabsorption occurs through direct or indirect action of apical Na+/H+ exchanger isoform 3 (NHE3), which also happens to be the primary target for hormonal control of proximal Na+ reabsorption (6, 22, 34, 35, 49, 62). NHE3 is also directly responsible for bicarbonate reabsorption and ammonium secretion (35, 39, 49, 68). NHE3 is primarily expressed at the apical membrane of early proximal tubules but is also detected in long thin descending limb as well as in the thick ascending limbs of the loop of Henle (3, 5, 6, 15).
The goal of the present study was to determine the role of tubular NHE3 in the early diabetic kidney. NHE3 may contribute to the primary tubular hyperreabsorption and thereby to diabetes-associated hyperfiltration and a rise in blood pressure (27, 59). Furthermore, studies in human proximal tubular cells have indicated a possible link between NHE3-mediated cellular Na+ uptake and tubular growth (17, 28, 29, 32, 41). NHE3 also participates in the endocytosis mechanism of albumin in proximal tubules (19, 20). Finally, acidosis has been linked to deleterious kidney outcome in patients with chronic kidney disease (CKD) (14), but whether the absence of NHE3 induces deleterious kidney effects remains unclear. To determine the role of NHE3 in the diabetic kidney, we studied type 1 diabetic Akita and control mice with selective knockdown of tubular NHE3.
Previous studies have indicated that the renal compensation during acidosis includes upregulation in tubular branched-chain amino acid (BCAAs) and tricarboxylic acid (TCA) cycle metabolism to increase ammonium and bicarbonate formation in proximal convoluted tubules, a process linked to enhanced tubular gluconeogenesis (1, 2, 23, 36). On the other hand, CKD has been associated with reduced renal BCAA metabolism and BCAA depletion (11). Moreover, urine excretion of metabolites related to mitochondrial function, including BCAA and TCA cycle metabolism, was reduced in patients with diabetes with CKD versus without CKD (50) as well as in nondiabetic patients with CKD versus patients with normal kidney function (24). Therefore, to better understand the impact of tubular NHE3 on the integrated kidney phenotype, we also analyzed urine metabolomics and renal expression of key metabolic genes.
METHODS
Animals.
All animal experimentation was conducted in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD) and was approved by the local Institutional Animal Care and Use Committee (Veterans Affairs San Diego Healthcare System). We generated C57BL/6 mice with NHE3 knockdown along the entire tubular system [NHE3-knockout (KO) mice] by crossing mice with floxed NHE3 (34) and mice heterozygously expressing Cre recombinase under the control of the Pax8 promoter (8). The line was crossed with Akita mice (Ins2+/C96Y; Akita/+; on C57BL/6 background), a genetic murine model of nonobese insulin-dependent type I diabetes mellitus (The Jackson Laboratories, Bar Harbor, ME). The following four groups of littermate male mice were generated and studied: 1) nondiabetic NHE3 wild-type (control WT), 2) nondiabetic NHE3-KO (control NHE3-KO), 3) diabetic NHE3 WT (Akita/+ WT), and 4) diabetic NHE3-KO (Akita/+ NHE3-KO). All animals were housed in the same animal room with a 12:12-h light-dark cycle and free access to standard rodent chow and tap water. All mice that entered the study also completed the experiments. Treatments were not blinded.
Sample collection and measurement of blood pressure and GFR in awake mice.
At 4, 5, 9, and 17 wk of age, body weight and blood glucose (by tail snip in awake mice) were determined. At 13 wk of age, spot urine was collected in a subset of mice to perform urine metabolomics. At 17–18 wk of age, blood pressure was measured in awake mice by an automated tail-cuff system (BP-2000 Blood Pressure Analysis System, Visitech-Systems, Apex, NC) for 6 consecutive days after the appropriate training (58, 61). At 18–20 wk of age, GFR was measured in awake mice by plasma elimination kinetics of FITC-sinistrin (Fresenius-Kabi, Linz, Austria) after retroorbital injection of this GFR marker as previously described (58, 61). At 19–21 wk of age, each mouse was housed singly in a regular cage to determine food and fluid intake for 1–3 days. Subsequently, spot urine was collected, and the animal was anesthetized with isoflurane followed by retroorbital blood collection and subsequent kidney tissue harvesting as previously described (58).
Blood and urine analysis.
Blood glucose levels were determined using an Ascensia Elite XL glucometer (Bayer, Mishawaka, IN). If levels were “high” (>600 mg/dl), glucose concentration was determined by the hexokinase/glucose-6-phosphate dehydrogenase method (Infinity, Thermo Electron, Louisville, CO), which was also used for all urine samples. Urine albumin (Bethyl Laboratories, Montgomery, TX) and ammonium (Pointe Scientific, Canton, MI) were determined using commercial assays. Acid-base analyses were performed in freshly collected blood and urine; bicarbonate concentrations were calculated from pH and CO2 concentrations measured by a blood gas analyzer (OPTI Medical Systems, Roswell, GA) according to the Henderson-Hasselbalch equation. Concentrations in spot urine were normalized to creatinine concentration, which was measured by a kinetic modification of Jaffe's reaction (ThermoFisher Scientific, Waltham, MA). Metabolic cage experiments (performed over 3 days with maintained body weight) confirmed similar urinary creatinine excretion in age-matched male tubular NHE3-KO versus littermate WT mice (797 ± 108 vs. 796 ± 110 µg creatinine/24 h, n = 5–6/group, not significant).
Urine metabolomics.
Urine metabolites were measured as previously described (24, 69). Briefly, aliquots corresponding to 50 nmol creatinine were derivatized and analyzed by gas chromatography-tandem mass spectrometry. A mix of 10 heavy isotope labeled internal standards, corresponding to different groups of metabolites under analysis, were added to aliquots of urine samples before derivatization. Keto acids were oximated with pentafluorobenzylhydroxylamine. After overnight lyophilization, organic acids were isolated by liquid partition chromatography on silica (45% 2-methyl-2-butanol in chloroform). Eluted samples were evaporated under N2, and the dry residue was silylated with 1:1 volumes of N,O-bis(trimethylsilyl)trifluoroacetamide and Tri-Sil HTP for 2 h at 60°C. One microliter of sample was applied onto a 20 m × 0.36 µm column (Agilent DB-5) at 250°C in a gas chromatogram and eluted with helium gas at a temperature gradient of 80–300°C over 20 min. Target metabolites were analyzed by electron ionization and detected on extended dynamic range detector on a triple-quadrupole mass spectrometer (Bruker Scion). Each metabolite was quantified by using an eight-point calibration standard curve (0.1–150 nmol) with R2 > 0.98. The ratio of metabolite peak area to the internal standard peak area was used to determine metabolite concentration.
Western blot analysis.
Western blot analysis was performed using the membrane fraction extracted from whole kidneys as previously described (58, 61). Protein concentration was determined using a DC Protein Assay Kit (Bio-Rad Laboratories, Hercules, CA). Then, 40–60 µg/lane of extracted proteins were resolved on NuPAGE gels in MOPS buffer (Invitrogen, Carlsbad, CA). Gel proteins were transferred to nitrocellulose membranes and immunoblotted with rabbit anti-NHE3 antibody (EMD Millipore, Darmstadt, Germany) (45), rabbit anti-SGLT2 antibody (58, 61), and mouse anti-β-actin antibody (Sigma-Aldrich, St. Louis, MO) (45) followed by treatment with horseradish peroxidase-conjugated secondary antibody. Protein expression was detected autoradiographically by an ECL Plus Kit (Amersham Pharmacia, Piscataway, NJ). Densitometric analysis was performed using ImageJ software (National Institutes of Health). Results were normalized to the control WT group.
Quantitative RT-PCR.
Frozen kidneys were homogenized using a mechanical tissue homogenizer in lysis buffer provided by the RNeasy Plus Mini Kit (catalog no. 74136, Qiagen). RNA was purified using the RNeasy Plus Mini Kit per the manufacturer’s instructions. cDNA was prepared with 5 µg purified RNA using the SuperScript IV First-Strand Synthesis System (catalog no. 18091050, ThermoFisher Scientific) per the manufacturer’s instructions. cDNA reactions were diluted fivefold, and 1 µl was used for quantitative PCR. Real-time PCR was performed in a 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA) using Taqman assays (catalog no. 4331182, ThermoFisher Scientific) and the primers shown in Table 1. Expression levels were expressed as relative fold increases/decreases normalized to the average of two housekeeping genes, β-actin and ribosomal protein 119 (rpl19), which was not different between groups (not shown). Each experiment was performed in duplicate. For some genes and proteins, whole kidney expression was also estimated by considering kidney weight because diabetes had a strong effect on the latter.
Table 1.
Real-time PCR primers used
| Target Gene | Assay ID |
|---|---|
| Actb | Mm00607939_s1 |
| Atp6v1b | Mm00460309_m1 |
| Ccl2 | Mm00441242_m1 |
| Cd14 | Mm00438094_g1 |
| Glud1 | Mm00492353_m1 |
| Glut1 | Mm00441480_m1 |
| Glut2 | Mm00446229_m1 |
| G6pc | Mm00839363_m1 |
| Nhe3 | Mm01352473_m1 |
| Pepck | Mm01247058_m1 |
| Pfkp | Mm00444792_m1 |
| Pkm2 | Mm00834102_gH |
| Renin | Mm02342889_g1 |
| Rhcg | Mm00451199_m1 |
| Rpl19 | Mm02601633_g1 |
| Sglt1 | Mm00451203_m1 |
| Tnfa | Mm00443258_m1 |
| Txnip | Mm01265659_g1 |
See text for the definitions of the abbreviations.
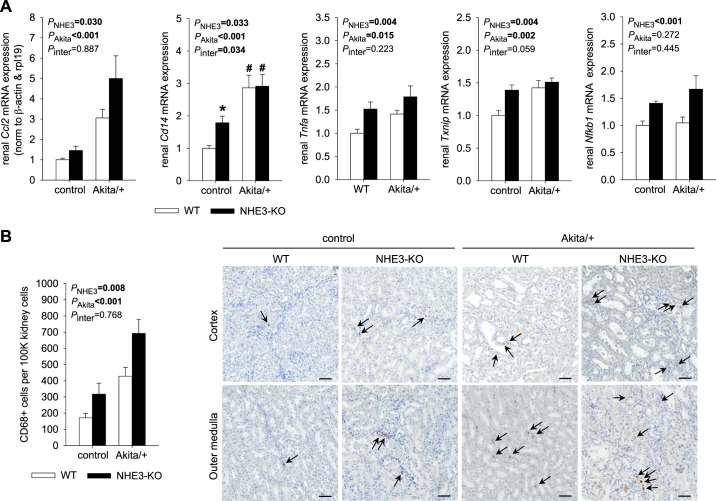
Immunohistochemistry for CD68.
CD68 is a lysosomal/endosomal glycoprotein highly expressed in macrophages and other types of mononuclear phagocytes (12, 26). Paraffin-embedded tissue sections were hydrated, and heat-induced epitope retrieval was performed in 10 mM citrate buffer (pH 6.0). Endogenous peroxidase activity was quenched by an incubation in 3% H2O2 for 5 min, and 2.5% normal goat serum in PBS was used for blocking. Tissues were then incubated with anti-CD68 antibodies (1:1,000, ab125212, Abcam) for overnight at 4°C. After sections had been washed, ImmPRESS secondary antibody horseradish peroxidase-conjugated polymers (MP-7451, Vector Laboratories) were applied as instructed by the manufacturer, and horseradish peroxidase activity was detected using DAB substrate. After nuclei were counterstained via hematoxylin, mounted slides were scanned using an Axio Z1 slide scanner (Carl Zeiss). The number of nuclei and CD68-positive cells was determined by QuPath (4) and ImageJ software using automated analysis and the same parameter settings for all samples.
Statistical analysis.
To analyze for statistical differences between groups, two-way ANOVA was performed to probe for a significant effect of the two specific factors (Akita diabetes or NHE3-KO) and for the interaction between the two factors. If the interaction was statistically significant, then a pair-wise multiple comparison procedure (Holm-Sidak method) was used to identify the significant effects (52). P values of <0.05 were considered statistically significant.
RESULTS
Effective tubular NHE3 gene targeting and unchanged renal NHE3 expression in Akita diabetes.
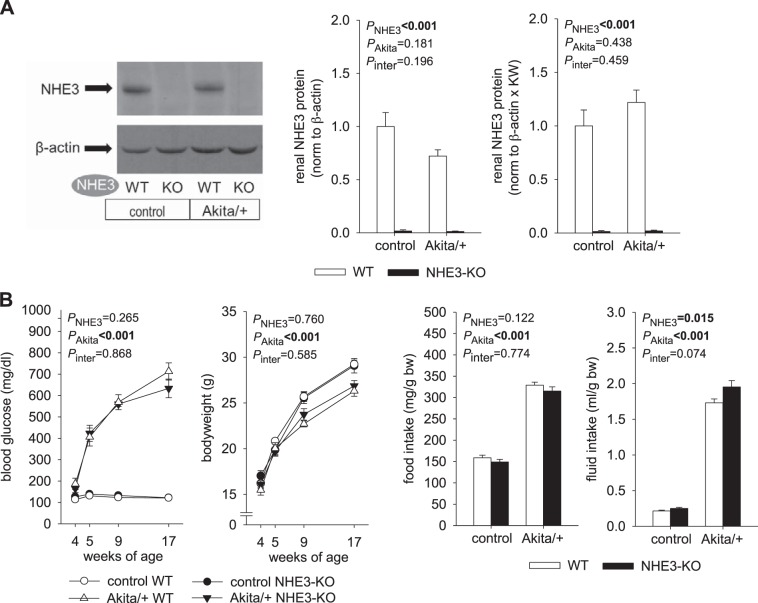
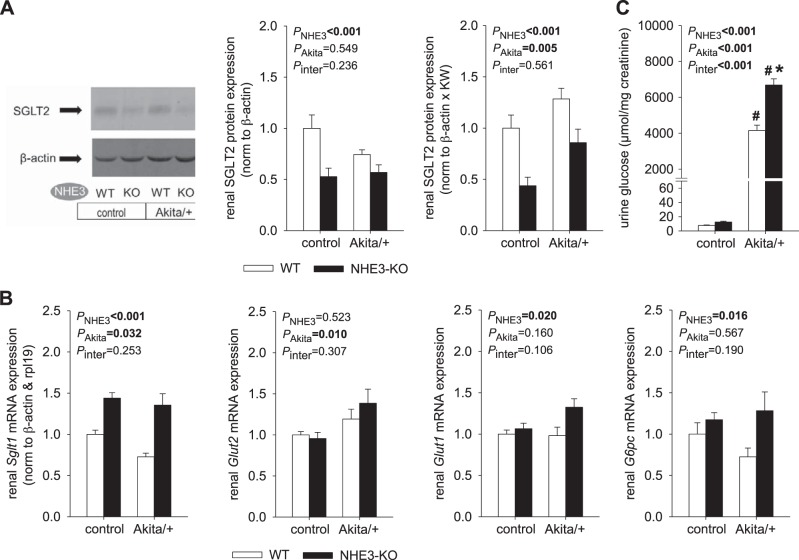
Effective knockdown of kidney NHE3 protein expression in control and Akita diabetic NHE3-KO mice was confirmed (Fig. 1A). In WT mice, renal membrane NHE3 protein expression was numerically lower in Akita diabetes when results were normalized to the housekeeping protein β-actin, but this did not reach statistical significance (Fig. 1A). Since diabetes strongly increased kidney weight (e.g., by 64% in WT mice, see below), normalizing expression to a housekeeping protein may have underestimated total renal expression of NHE3 in diabetes. To better estimate the latter, expression was also normalized to β-actin and multiplied by kidney weight. With the use of this approach, no statistically significant difference was detected in NHE3 expression between diabetic and nondiabetic WT mice (Fig. 1A).
Fig. 1.
Na+/H+ exchanger isoform 3 (NHE3) knockdown [NHE3-knockout (KO)] did not affect hyperglycemia but increased fluid intake in Akita diabetic mice. A: renal membrane NHE3 protein expression was effectively knocked down in NHE3-KO mice. In wild-type (WT) mice, Akita diabetes did not significantly alter renal NHE3 protein expression when normalized to β-actin or, in addition, multiplied by kidney weight (KW) to estimate whole kidney expression (n = 8–10/group for WT mice and 4 mice/group for NHE3-KO mice). B: Akita diabetes-induced changes in blood glucose, body weight, and food intake occurred to a similar extent in NHE3-KO and WT mice. However, fluid intake was higher in NHE3-KO versus WT mice, particularly in Akita diabetes (n = 11–20 mice/group for body weight profile, n = 8–10 mice/group in nondiabetic controls and 23–24 mice/group in Akita diabetes for blood glucose, and n = 16–24 mice/group for daily food and fluid intake). Values are means ± SE. Two-way ANOVA was performed to probe for a significant effect of NHE3-KO (PNHE3), Akita diabetes (PAkita), or the interaction between the two factors (Pinter). If the interaction was statistically significant, then a pair-wise multiple comparison procedure (Holm-Sidak method) identified the significant effects.
NHE3-KO did not affect hyperglycemia in Akita diabetic mice but modestly increased fluid intake.
In nondiabetic control mice, blood glucose, body weight, and food and fluid intake were similar between NHE3-KO and WT mice (Fig. 1B). In WT mice, diabetes increased blood glucose levels and food and fluid intake and attenuated the increase in body weight. These diabetes-induced changes occurred to a similar extent in NHE3-KO mice except that fluid intake was modestly higher in NHE3-KO versus WT Akita diabetic mice (Fig. 1B).
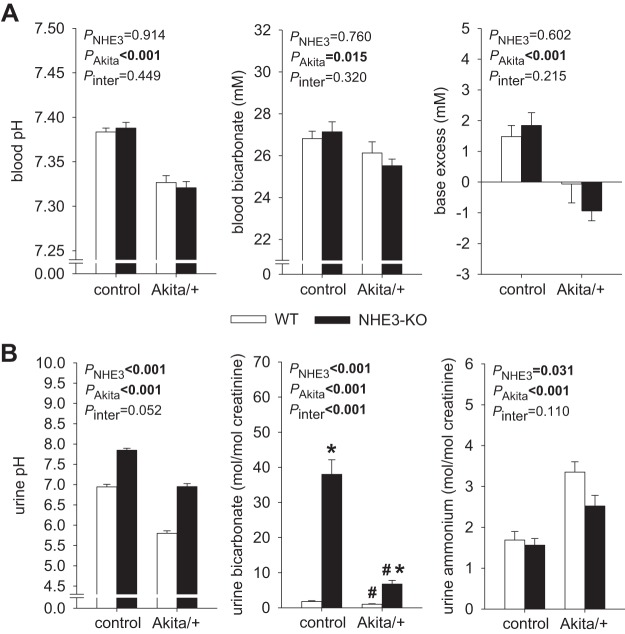
NHE3-KO enhanced urine pH and bicarbonate excretion, associated with compensated blood acid-base status in nondiabetic control and Akita diabetic mice.
Blood pH, bicarbonate concentration, and base excess were less in Akita diabetic mice and not different between WT and NHE3-KO mice (Fig. 2A). The lower pH in Akita diabetic mice could not be fully explained by the lower bicarbonate concentration and included acute CO2 retention, presumably in response to a stronger sedating effect of isoflurane given for blood drawing. Akita diabetic mouse urine had lower pH and contained less bicarbonate and more ammonium than nondiabetic urine (Fig. 2B). Urine titratable acid was not measured but was presumably greater in Akita diabetic mice based on the lower urine pH and greater food intake. Based on the assumption that the available buffer for titratable acid (mainly phosphate) varies in proportion to food intake, the greater net acid excretion in Akita diabetic versus nondiabetic mice can to a large extent be explained by the twofold greater food intake in the former.
Fig. 2.
Compensated acid-base balance in nondiabetic and Akita diabetic Na+/H+ exchanger isoform 3 (NHE3) knockdown [NHE3-knockout (KO)] mice. A: blood bicarbonate concentration was less by ~1 mM in Akita diabetic mice. The reduction in blood pH in these mice may have been overestimated due to sedation-induced acute CO2 retention. NHE3-KO did not significantly change blood acid-base status in the presence or absence of Akita diabetes (n = 16–28 mice/group). B: Akita diabetes lowered urine pH, decreased urine bicarbonate, and increased urine ammonium excretion, possibly reflecting renal compensation of diabetes-induced increases in food intake and acid loading, in both wild-type (WT) and NHE3-KO mice. Urine pH and bicarbonate were increased in nondiabetic and Akita diabetic NHE3-KO versus WT mice, and NHE3-KO attenuated the rise in urinary ammonium excretion observed in WT Akita diabetic mice (n = 16–28 mice/group). Values are means ± SE. Two-way ANOVA was performed to probe for a significant effect of NHE3-KO (PNHE3), Akita diabetes (PAkita), or the interaction between the two factors (Pinter). If the interaction was statistically significant, then a pair-wise multiple comparison procedure (Holm-Sidak method) identified the significant effects. #P < 0.05 vs. control; *P < 0.05 vs. WT mice.
When we compared NHE3-KO with WT mice, urine pH was higher in NHE3-KO mice, commensurate with 10-fold increase in bicarbonate excretion, which applied to Akita diabetic and nondiabetic control mice (Fig. 2B). Urinary ammonium levels were less in NHE3-KO versus WT mice, particularly in Akita diabetes, where urinary ammonium was higher to begin with (Fig. 2B). The combined data on serum and urine bicarbonate, urine pH, and urine ammonium imply that both Akita diabetic and nondiabetic NHE3-KO mice maintain normal serum bicarbonate in the face of modest bicarbonaturia by generating replacement bicarbonate through a mechanism that does not involve enhanced urinary excretion of ammonium.
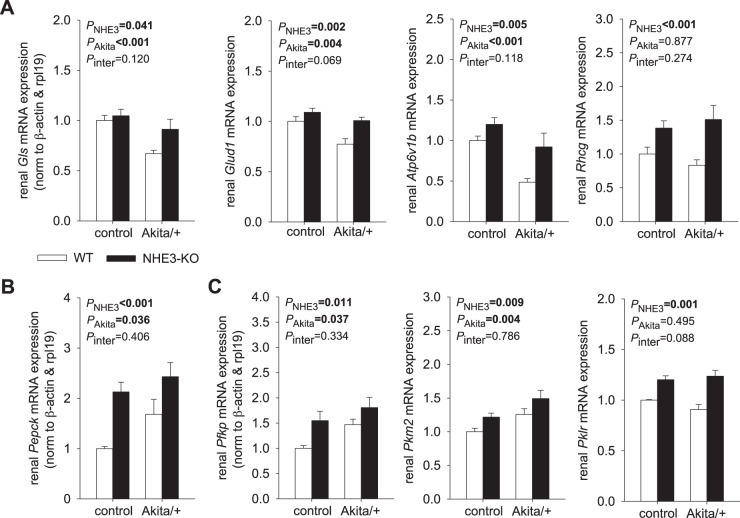
NHE3-KO increased renal mRNA expression for genes associated with ammonium formation and H+ and ammonia secretion.
Glutaminase (GLS) and glutamate dehydrogenase 1 (GLUD1) catalyze the oxidative deamination of glutamine and glutamate, respectively, to form α-ketoglutarate and ammonia, thereby contributing to ammoniagenesis (53). The B1 subunit of H+-ATPase (ATP6V1B1) and the ammonium transporter rhesus C glycoprotein (RHCG) contribute to H+ and ammonium secretion into the lumen of the distal nephron, respectively (16, 67), with the former also facilitating bicarbonate reabsorption at this site. Akita diabetes decreased renal mRNA expression of Gls, Glud1 and Atp6v1b1 and left Rhcg expression unchanged when normalized to housekeeping genes (Fig. 3A). Estimating whole kidney gene expression by also considering kidney weight indicated enhanced mRNA expression of Gls, Glud1, and Rhcg in Akita diabetes, whereas Atp6v1b1 expression remained unchanged (Table 2). NHE3-KO increased renal mRNA expression of Gls, Glud1, Atp6v1b1, and Rhcg, and these effects were more apparent in Akita diabetic mice than in nondiabetic mice (Fig. 3A). These increases likely reflected a compensatory response to the defects in H+ and ammonium secretion caused by eliminating NHE3, which is the usual route for H+ and ammonium secretion (65).
Fig. 3.
Compensatory changes in renal gene expression in nondiabetic and diabetic Na+/H+ exchanger isoform 3 (NHE3) knockdown [NHE3-knockout (KO)] mice. Renal mRNA expression normalized to β-actin and ribosomal protein 119 (rpl19) is shown (n = 9–10 mice/group). A: NHE3-KO increased renal mRNA expression of genes associated with ammonium formation [glutaminase (Gls) and glutamate dehydrogenase (Glud1)], H+ secretion [H+-ATPase B1 subunit (Atp6v1b1)], and ammonia secretion [Rhesus C glycoprotein (Rhcg)]. These effects were more apparent in Akita diabetic mice. WT, wild type. B: both NHE3-KO and Akita diabetes increased renal phosphoenolpyruvate carboxykinase (Pepck) mRNA expression. C: in nondiabetic and diabetic mice, NHE3-KO enhanced renal mRNA expression of phosphofructokinase platelet isoform (Pfkp), pyruvate kinase M2 (Pkm2), and pyruvate kinase liver/red blood cell isoform (Pklr), which are rate-limiting enzymes of glycolysis. Akita diabetes also increased Pfkp and Pkm2. Values are means ± SE. Two-way ANOVA was performed to probe for a significant effect of NHE3-KO (PNHE3), Akita diabetes (PAkita), or the interaction between the two factors (Pinter). If the interaction was statistically significant, then a pair-wise multiple comparison procedure (Holm-Sidak method) identified the significant effects.
Table 2.
Estimation of whole kidney gene expression by considering kidney weight
| Control |
Akita/+ |
P Values |
|||||
|---|---|---|---|---|---|---|---|
| Gene | WT | NHE3-KO | WT | NHE3-KO | PNHE3 | PAkita | Pinter |
| Gls | 1.00 ± 0.05 | 1.33 ± 0.06† | 1.24 ± 0.06‡ | 2.15 ± 0.12†‡ | <0.001* | <0.001* | <0.001* |
| Glud1 | 1.00 ± 0.07 | 1.02 ± 0.08 | 1.46 ± 0.10 | 1.90 ± 0.15 | 0.034* | <0.001* | 0.051 |
| Atp6v1b | 1.00 ± 0.09 | 1.03 ± 0.12 | 0.95 ± 0.10 | 1.55 ± 0.25 | 0.035* | 0.122 | 0.058 |
| Rhcg | 1.00 ± 0.10 | 1.19 ± 0.12 | 1.64 ± 0.16 | 2.47 ± 0.21 | 0.006* | <0.001* | 0.248 |
| Pklr | 1.00 ± 0.05 | 1.08 ± 0.08 | 1.59 ± 0.11‡ | 2.11 ± 0.14†‡ | 0.003* | <0.001* | 0.028* |
| Sglt1 | 1.00 ± 0.05 | 1.33 ± 0.06† | 1.24 ± 0.06‡ | 2.15 ± 0.12†‡ | <0.001* | <0.001* | <0.001* |
| Glut1 | 1.00 ± 0.04 | 0.95 ± 0.07 | 1.56 ± 0.13‡ | 2.20 ± 0.12†‡ | 0.005* | <0.001* | 0.001* |
| G6pc | 1.00 ± 0.15 | 1.00 ± 0.09 | 1.20 ± 0.16 | 1.99 ± 0.32 | 0.051 | 0.005* | 0.053 |
| Renin | 1.00 ± 0.06 | 1.29 ± 0.14 | 0.72 ± 0.11 | 1.15 ± 0.16 | 0.006* | 0.095 | 0.585 |
| Nfkb1 | 1.00 ± 0.08 | 1.19 ± 0.06 | 1.76 ± 0.15 | 2.39 ± 0.22 | 0.004* | <0.001* | 0.468 |
Values are means ± SE. Shown is renal mRNA expression, which was first normalized to the housekeeping genes β-actin and ribosomal protein 119 (rp119) and then multiplied by kidney weight to take into consideration diabetic kidney growth (n = 9–10 mice/group). This additional analysis to estimate total kidney expression was performed when renal gene expression normalized only to β-actin and rpl19 may have obscured the qualitative effect of diabetes due to an increase in kidney weight (see Figs. 3, 5, and 9). Two-way ANOVA was performed to probe for a significant effect of Na+/H+ exchanger isoform 3 (NHE3) knockdown [NHE3-knockout (KO)] (PNHE3), Akita diabetes (PAkita), or the interaction between the two factors (Pinter). If the interaction was statistically significant, then a pair-wise multiple comparison procedure (Holm-Sidak method) identified the significant effects. See text for the definitions of the abbreviations.
Statistically significant difference.
P < 0.05 vs. wild-type (WT) mice;
P < 0.05 vs. control.
NHE3-KO and Akita diabetes enhanced renal gene expression of gluconeogenic and glycolytic enzymes.
Phosphoenolpyruvate carboxykinase (PEPCK) is a principal gluconeogenic enzyme, the activity of which is regulated at the level of transcription (44). Kidneys from Akita diabetic WT mice contained more Pepck mRNA than nondiabetic WT controls (Fig. 3B), consistent with prior observations in Akita mice (58) and with a prior report (21) of increased renal gluconeogenesis in humans with diabetes. NHE3-KO significantly increased renal mRNA expression of Pepck in nondiabetic mice and further increased the expression in Akita diabetic mice (Fig. 3B). This is consistent with the processes of proximal tubular formation of ammonia, bicarbonate, and glucose being functionally linked: α-ketoglutarate formed by ammoniagenesis is used to generate both bicarbonate and glucose by gluconeogenesis. Newly formed bicarbonate is returned to the systemic circulation. Newly formed glucose can be used as an energy substrate in the same cells, delivered to the systemic circulation, or transferred to other cells in the kidney. In accordance, in nondiabetic control mice, NHE3-KO enhanced the renal mRNA expression of the phosphofructokinase platelet isoform (Pfkp), pyruvate kinase M2 (Pkm2), and pyruvate kinase liver/red blood cell isoform (Pklr), which are rate-limiting enzymes of glycolysis (Fig. 3B). Notably, expression of the pyruvate kinase isoforms PKLR and PKM2 appeared to be restricted to proximal tubules and distal tubules, respectively (47). Akita diabetes also increased renal mRNA expression of Pfkp and Pkm2, and NHE3-KO significantly increased expression of Pklr in Akita diabetic mice (Fig. 3C). See Table 2 for the estimation of whole kidney Pklr gene expression. Thus, both NHE3-KO and Akita diabetes enhanced the renal expression of genes involved in gluconeogenesis and glycolysis.
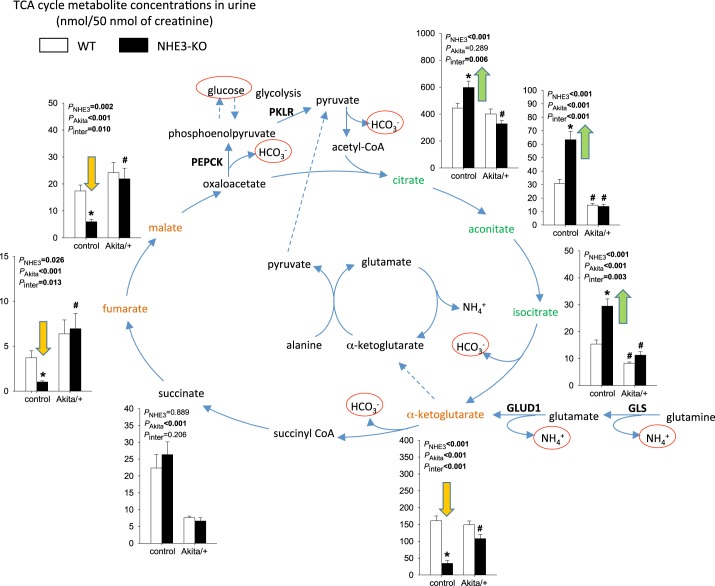
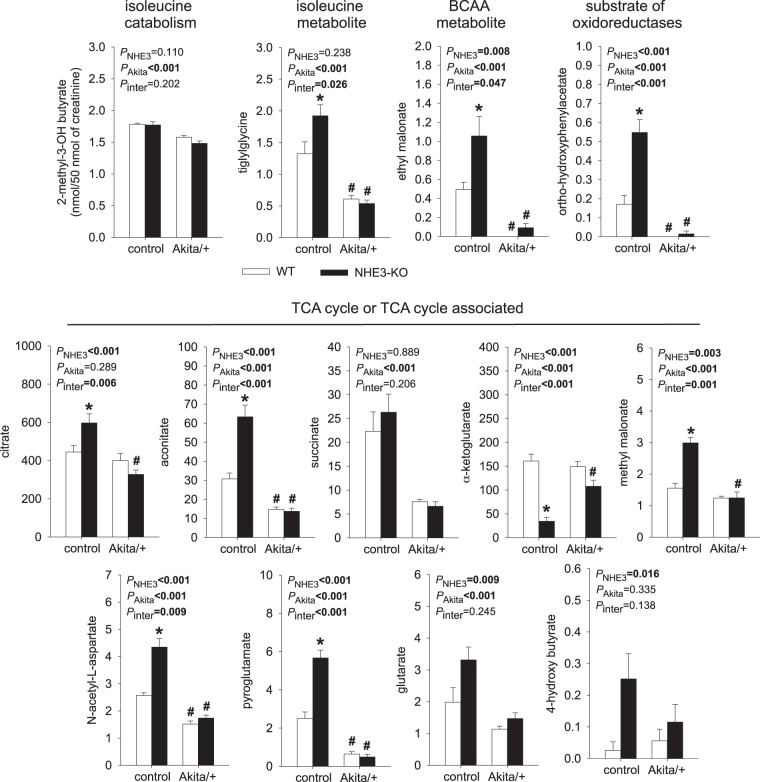
Distinct effects of NHE3-KO and Akita diabetes on urinary excretion of TCA cycle metabolites.
In nondiabetic control mice, NHE3-KO consistently enhanced urinary excretion of the first three metabolites of the TCA cycle, namely, citrate, aconitate, and isocitrate, but reduced the excretion of the next metabolite, α-ketoglutarate (Fig. 4). The latter is consistent with previous studies showing that renal α-ketoglutarate is reduced during compensation for acidosis through activation of α-ketoglutarate dehydrogenase, which converts α-ketoglutarate to succinyly-CoA (36), thereby accelerating the conversion of glutamate to α-ketoglutarate and the formation of ammonium. The lower levels of urinary malate (and fumarate) in NHE3-KO mice are consistent with observations in the rat where activating PEPCK depleted the kidney of oxaloacetate and malate (1, 2, 23).
Fig. 4.
Distinct effects of Na+/H+ exchanger isoform 3 (NHE3) knockdown [NHE3-knockout (KO)] and Akita diabetes on urinary excretion of tricarboxylic acid (TCA) cycle metabolites. In nondiabetic mice, NHE3-KO consistently enhanced the urinary excretion of the first three metabolites of the TCA cycle, namely, citrate, aconitate, and isocitrate, but reduced the excretion of α-ketoglutarate, fumarate, and malate. This pattern is consistent with a compensatory response to acidosis (see text for explanations). The Akita diabetic kidney had potentially reduced mitochondrial energy metabolism, as indicated by reduced urinary excretion of aconitate, isocitrate, and succinate. In Akita diabetic mice, NHE3-KO did not affect the urinary pattern of TCA cycle metabolites. Values are means ± SE. Two-way ANOVA was performed to probe for a significant effect of NHE3-KO (PNHE3), Akita diabetes (PAkita), or the interaction between the two factors (Pinter). If the interaction was statistically significant, then a pair-wise multiple comparison procedure (Holm-Sidak method) identified the significant effects. GLS, glutaminase; GLUD1, glutamate dehydrogenase. #P < 0.05 vs. control; *P < 0.05 vs. wild-type (WT) mice. n = 6 mice/group.
Potentially indicating reduced mitochondrial energy metabolism, Akita diabetes significantly reduced the urinary excretion of the TCA cycle metabolites aconitate, isocitrate, and succinate (Fig. 4). In contrast to the findings in nondiabetic mice, NHE3-KO did not significantly affect the urinary pattern of TCA cycle metabolites in Akita diabetic mice (Fig. 4).
NHE3-KO suppressed renal SGLT2 expression and increased glucosuria in Akita diabetic mice.
Akita diabetes did not significantly alter renal expression of SGLT2 protein when normalized to β-actin but increased estimated whole kidney SGLT2 expression when kidney weight was taken into consideration (Fig. 5A). Akita diabetes increased renal mRNA expression of glucose transporter (Glut)2 (Fig. 5B). Renal mRNA expression of Glut1 and Sglt1 was increased in Akita diabetes only when whole kidney expression was estimated using kidney weight (Table 2). Akita diabetes induced glucosuria (Fig. 5C), indicating that the enhanced glucose load to the tubular system [by increasing blood glucose (Fig. 1B) and GFR; see below] exceeded the glucose reabsorption capacity of SGLT2 and SGLT1.
Fig. 5.
Na+/H+ exchanger isoform 3 (NHE3) knockdown [NHE3-knockout (KO)] reduced renal Na+-glucose cotransporter (SGLT)2 expression and increased glucosuria in Akita diabetic mice. A: NHE3-KO reduced renal SGLT2 protein expression in nondiabetic and Akita diabetic mice when normalized to β-actin or, in addition, multiplied by kidney weight (KW) to estimate whole kidney expression (n = 7–10/group). Figures 1A and 5A are from the same experiment and have the same loading control. B: renal mRNA expression normalized to β-actin and ribosomal protein 119 (rpl19) (n = 9–10 mice/group). NHE3-KO increased renal mRNA expression of Sglt1. Akita diabetic NHE3-KO mice had the numerically highest levels of renal mRNA expression of glucose transporter (Glut)2, Glut1, and glucose-6-phosphatase (G6pc). C: NHE3-KO increased the urine glucose-to-creatinine ratio in Akita diabetic mice (n = 8–11 mice/group in nondiabetic controls and n = 12–14 mice/group in Akita diabetes). Values are means ± SE. Two-way ANOVA was performed to probe for a significant effect of NHE3-KO (PNHE3), Akita diabetes (PAkita), or the interaction between the two factors (Pinter). If the interaction was statistically significant, then a pair-wise multiple comparison procedure (Holm-Sidak method) identified the significant effects. #P < 0.05 vs. control; *P < 0.05 vs. wild-type (WT) mice.
In nondiabetic NHE3-KO mice, the increase in renal mRNA expression of Pepck (Fig. 3B) was associated with reduced renal protein expression of SGLT2 (Fig. 5A), whereas renal mRNA expression of Sglt1 was increased (Fig. 5B). Renal mRNA expression of the basolateral glucose transporters Glut2 and Glut1 was not affected (Fig. 5B), and similar low levels of urinary glucose excretion were observed in nondiabetic NHE3-KO and WT mice (Fig. 5C). In Akita diabetic mice, NHE3-KO further enhanced renal mRNA expression of Pepck (Fig. 3B), associated with reduced renal expression of SGLT2 (Fig. 5A) and a significant increase in urinary glucose excretion versus WT Akita mice (Fig. 5C), despite greater renal mRNA expression of Sglt1 (Fig. 5B) and less filtered glucose (see below) in the former.
These data are consistent with the notion that tubular NHE3-KO enhanced proximal tubular gluconeogenesis, associated with suppressed SGLT2 expression. Downregulation of SGLT2 together with upregulation of SGLT1 suggested a downward tubular shift of Na+ and glucose reabsorption in NHE3-KO mice. Downregulation of SGLT2 is functionally unmasked in diabetic NHE3-KO mice in the form of greater glucosuria. The highest renal mRNA expression of Pepck was observed in Akita diabetic NHE-KO mice (Fig. 3B). This group also showed the highest renal mRNA expression of glucose-6-phosphatase and Glut2 (Fig. 5B). These changes may have facilitated basolateral glucose exit in NHE3-KO mice.
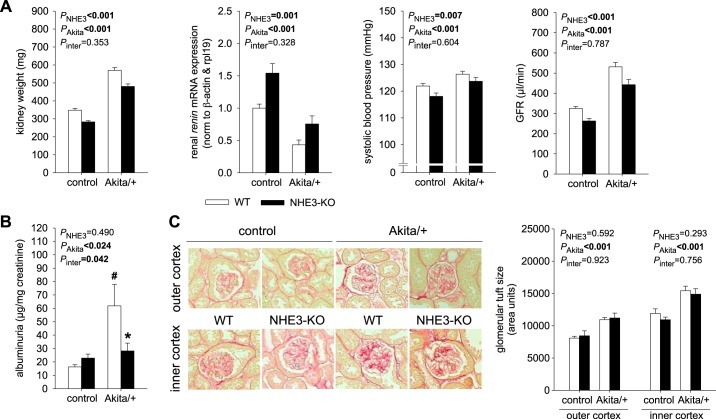
NHE3-KO reduced kidney weight and GFR in nondiabetic and Akita diabetic mice and attenuated diabetes-induced albuminuria but did not prevent diabetes-induced increases in kidney weight and GFR.
In WT, Akita diabetes increased kidney weight. This was associated with reduced renal mRNA expression of renin and higher systolic blood pressure providing evidence for volume retention (Fig. 6A), which may be the consequence of diabetes-induced tubular growth and hyperreabsorption. These changes were associated with an increase in GFR (Fig. 6A), which, together with the increase in blood pressure, is expected to partly counteract the volume retention due to hyperreabsorption.
Fig. 6.
Na+/H+ exchanger isoform 3 (NHE3) knockdown [NHE3-knockout (KO)] reduced kidney weight and glomerular filtration rate (GFR) in nondiabetic and Akita diabetic mice and diabetes-induced albuminuria but did not prevent diabetes-induced increases in kidney weight and GFR. A: in wild-type (WT) mice, Akita diabetes increased kidney weight and reduced renal renin mRNA expression, associated with increased systolic blood pressure and GFR. NHE3-KO induced the opposite effects on these parameters in both nondiabetic and diabetic mice, i.e., kidney weight and GFR were lower in Akita diabetic NHE3-KO vs. WT mice. However, NHE3-KO did not prevent the Akita diabetes-induced increase in kidney weight, GFR, or systolic blood pressure (n = 22–30 mice/group for kidney weight, n = 23–31 mice/group for systolic blood pressure, n = 9–10 mice/group for mRNA, and n = 15–26 mice/group for GFR). B: the diabetes-induced increase in albuminuria was abolished in NHE3-KO mice (n = 9–14 mice/group for albuminuria). C: glomerular tuft size was larger in glomeruli in the inner cortex versus outer cortex, increased in Akita diabetes, and not different between NHE3-KO versus WT mice (n = 5 mice/group; average measurements taken per mouse: 55 and 30 glomeruli in the outer and inner cortex, respectively). Values are means ± SE. Two-way ANOVA was performed to probe for a significant effect of NHE3-KO (PNHE3), Akita diabetes (PAkita), or the interaction between the two factors (Pinter). If the interaction was statistically significant, then a pair-wise multiple comparison procedure (Holm-Sidak method) identified the significant effects. #P < 0.05 vs. control; *P < 0.05 vs. WT mice.
Consistent with a role in tubular growth and reabsorption, NHE3-KO induced opposite effects to Akita diabetes: it reduced kidney weight and systolic blood pressure and increased renal renin mRNA expression, associated with lower GFR (Fig. 6A). These effects of NHE3-KO were observed in both nondiabetic and Akita diabetic mice. Thus, kidney weight and GFR were lower in Akita diabetic NHE3-KO versus WT mice. However, NHE3-KO did not prevent the diabetes-induced increase in kidney weight, systolic blood pressure, and GFR or the decrease in renal renin mRNA expression, indicating that diabetes-induced tubular growth and hyperreabsorption were in part independent of tubular NHE3. On the other hand, the diabetes-induced increase in albuminuria was abolished by NHE3-KO mice (Fig. 6B). Measurement of glomerular tuft size confirmed the expected larger glomeruli in the inner cortex versus outer cortex and the increase in Akita diabetes (Fig. 6C). Glomerular tuft size was not different between NHE3-KO versus WT in nondiabetic or Akita diabetic mice, indicating that the lower kidney weight, GFR, and albuminuria in NHE3-KO mice were not due to smaller glomeruli.
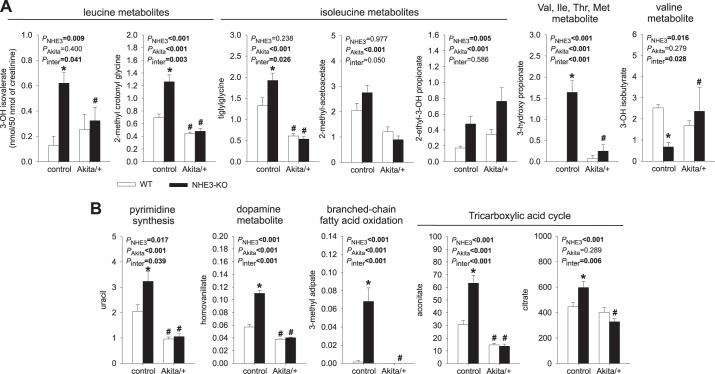
Urinary metabolites previously associated with CKD in humans: distinct effect of NHE3-KO in nondiabetic versus diabetic mice.
Figure 7 shows 12 metabolites that have previously been related to mitochondrial dysfunction and were found to be reduced (ratios to creatinine) in urine of patients with diabetes with CKD versus without CKD (50). A significant portion of these metabolites (7 of 12 metabolites) relate to BCAA metabolism (Fig. 7A). Six of the twelve metabolites (including 3 BCAA metabolites) showed a significant reduction in the urine of WT Akita versus nondiabetic WT control mice. In contrast, urinary excretion of 10 of these 12 metabolites was significantly increased in nondiabetic NHE3-KO mice, including all of the BCAA metabolites except the valine metabolite 3-hydroxy-isobutyrate, which was significantly reduced. Ammoniagenesis from other amino acids than glutamine represents ~20% of ammonia production in normal condition and is increased in response to acidosis (48, 65). Branched chain aminotransferase transfers the α-amino group from leucine, isoleucine, or valine to α-ketoglutarate to form glutamate and BCAA metabolites (10). Moreover, an in vitro study (66) in a renal tubular cell line showed that acidosis stimulated BCAA oxidation by increasing both the amount and activation state of branched-chain α-ketoacid dehydrogenase. Thus, BCAAs contribute to ammoniagenesis during metabolic acidosis, which may explain the upregulation of BCAA metabolites in the urine of nondiabetic NHE3-KO mice. In comparison, the reduction in urinary 3-hydroxy-isobutyrate in NHE3-KO mice may reflect its enhanced use as a gluconeogenic substrate in cortical tubules (33).
Fig. 7.
Urinary metabolites previously associated with chronic kidney disease (CKD) in diabetic patients: distinct effects of Na+/H+ exchanger isoform 3 (NHE3) knockdown [NHE3-knockout (KO)] in nondiabetic versus diabetic mice. Urinary metabolite excretion is expressed as nanomoles of metabolite per 50 nmol creatinine. A and B: many of the metabolites relate to branched-chain amino acid metabolism (A) and mitochondrial function (B), and all have previously been found to be reduced in urine of patients with diabetes with CKD versus those without CKD (50). Urinary excretion of 10 of 12 metabolites was significantly increased in nondiabetic NHE3-KO mice, which may relate to the role of branched-chain amino acids in ammoniagenesis and tricarboxylic acid cycle metabolism in ammoniagenesis, bicarbonate formation, and gluconeogenesis, respectively. Six of 12 metabolites were reduced in wild-type (WT) Akita versus WT control mice and 10 of 12 metabolites in NHE3-KO Akita versus NHE3-KO control mice, indicating that NHE3-KO could not prevent the potentially deleterious metabolite pattern in response to Akita diabetes. Values are means ± SE. Two-way ANOVA was performed to probe for a significant effect of NHE3-KO (PNHE3), Akita diabetes (PAkita), or the interaction between the two factors (Pinter). If the interaction was statistically significant, then a pair-wise multiple comparison procedure (Holm-Sidak method) identified the significant effects. #P < 0.05 vs. control; *P < 0.05 vs. WT mice. n = 6 mice/group.
Notably, Akita diabetes significantly reduced the urinary excretion of 10 of 12 metabolites in NHE3-KO mice. Except for one isoleucine metabolite, urinary concentrations were similar in WT Akita and NHE3-KO Akita mice, indicating that NHE3-KO did not prevent a potentially deleterious urinary metabolite pattern in Akita diabetic mice.
Figure 8 shows 13 metabolites that were previously found to be reduced in the urine of nondiabetic patients with CKD stage 3–4 versus patients with normal kidney function (24). While some metabolites are related to BCAA metabolism, the majority are associated with the TCA cycle. Notably, 9 of 13 metabolites showed a significant reduction in the urine of Akita diabetic versus nondiabetic WT mice. In contrast, urinary excretion of 10 of 13 metabolites was significantly increased in nondiabetic NHE3-KO versus WT mice; one of the exceptions was α-ketoglutarate, which, as described above, was significantly reduced in nondiabetic NHE3-KO versus WT mice.
Fig. 8.
Urinary metabolites previously associated with chronic kidney disease (CKD) in nondiabetic patients: distinct effects of Na+/H+ exchanger isoform 3 (NHE3) knockdown [NHE3-knockout (KO)] in nondiabetic versus diabetic mice. Urinary metabolite excretion is expressed as nanomoles of metabolite per 50 nmol creatinine. Metabolites relate in part to branched-chain amino acid metabolism or tricarboxylic acid (TCA) cycle metabolism, and all have previously been found to be reduced in urine of nondiabetic patients with CKD versus controls (24). Urinary excretion of 10 of 13 metabolites was significantly increased in nondiabetic NHE3-KO mice. Nine of 13 metabolites were reduced in wild-type (WT) Akita versus WT control mice and 11 of 13 metabolites in NHE3-KO Akita versus NHE3-KO control mice, indicating that NHE3-KO did not prevent a potentially deleterious urinary metabolite pattern in Akita diabetic mice. Values are means ± SE. Two-way ANOVA was performed to probe for a significant effect of NHE3-KO (PNHE3), Akita diabetes (PAkita), or the interaction between the two factors (Pinter). If the interaction was statistically significant, then a pair-wise multiple comparison procedure (Holm-Sidak method) identified the significant effects. #P < 0.05 vs. control; *P < 0.05 vs. WT mice. n = 6 mice/group.
Similar as observed in the above series of metabolites, Akita diabetes reduced the urinary excretion of 11 of 13 metabolites in NHE3-KO mice. The urinary concentrations of all 13 metabolites were similar in Akita WT and Akita NHE3-KO mice, indicating again that NHE3-KO did not prevent a potentially deleterious urinary metabolite pattern in Akita diabetic mice.
NHE3-KO increased renal expression of proinflammatory genes and the infiltration of CD68-positive cells.
In WT mice, Akita diabetic kidneys contained more mRNA expression for the proinflammatory molecules chemokine (C-C motif) ligand 2 (Ccl2), Cd14, TNF-α (Tnfa), and thioredoxin-interacting protein (Txnip), which is induced by high glucose or oxidative stress (43) (Fig. 9A). This was associated with an increase in Akita diabetic versus nondiabetic mice of renal CD68-positive cells, a marker of macrophages and other types of mononuclear phagocytes (Fig. 9B) (12, 26). Together, the data pointed to a renal proinflammatory state in WT Akita diabetic mice consistent with the expected phenotype of 4- to 5-mo-old Akita diabetic mice on a C57BL/6 genetic background (9, 58).
Fig. 9.
Na+/H+ exchanger isoform 3 (NHE3) knockdown [NHE3-knockout (KO)] increased renal expression of proinflammatory genes and the infiltration of CD68-positive cells. A and B: renal mRNA expression normalized to β-actin and ribosomal protein 119 (rpl19) (A; n = 9–10 mice/group) and renal fraction of CD68-positive cells (B; n = 6–8 mice/group). CD68-positive cells in the kidney were identified via immunohistochemistry. Arrows indicate CD68-positive cells, and representative images are shown. Scale bars = 50 µm. In NHE3 wild-type (WT) mice, Akita diabetes increased mRNA expression of the proinflammatory markers chemokine (C-C motif) ligand 2 (Ccl2), Cd14, TNF-α (Tnfa), and thioredoxin-interacting protein (Txnip) as well as the infiltration of CD68-positive cells. In nondiabetic mice, NHE3-KO increased renal mRNA expression of Ccl2, Cd14, Tnfa, Txnip, and NF-κ-light-chain-enhancer of activated B cells 1 (Nfkb1) as well as the infiltration of CD68-positive cells. Furthermore, NHE3-KO did not prevent the diabetes-induced increase in these proinflammatory markers. Values are means ± SE. Two-way ANOVA was performed to probe for a significant effect of NHE3-KO (PNHE3), Akita diabetes (PAkita), or the interaction between the two factors (Pinter). If the interaction was statistically significant, then a pair-wise multiple comparison procedure (Holm-Sidak method) identified the significant effects. #P < 0.05 vs. control; *P < 0.05 vs. WT mice.
Somewhat unexpectedly, in nondiabetic control mice, NHE3-KO increased renal mRNA expression of Ccl2, Cd14, Tnfa, Txnip, and NF-κ-light-chain-enhancer of activated B cells 1 (Nfkb1), a prototypical proinflammatory signaling pathway that can be activated by proinflammatory cytokines like TNF-α (Fig. 9A). This was associated with a detectable increase in CD68-positive cells in the kidneys of nondiabetic NHE3-KO versus WT mice (Fig. 9B). Furthermore, NHE3-KO did not prevent the diabetes-induced increase in these proinflammatory markers (Fig. 9, A and B).
DISCUSSION
The present study confirmed the contribution of NHE3 to tubular bicarbonate reabsorption and illustrated some of the compensating mechanisms that likely helped maintain acid-base balance in the absence of tubular NHE3. This included enhanced renal mRNA expression of RHCG, which codes for the key ammonia transporter in the luminal membrane of type A intercalated cells in the distal nephron (67). NHE3-KO mice also showed upregulation of renal mRNA expression of GLS, GLUD1, and ATP6V1B1, particularly when challenged with Akita diabetes: the former two catalyze the formation of ammonia from glutamine and glutamate, respectively, in the proximal tubule and the latter promotes H+ secretion in the distal nephron thereby trapping and excreting ammonia secreted by RHCG (67). The compensation also included enhanced gluconeogenesis as indicated by renal mRNA upregulation of PEPCK. Acidosis-associated renal gluconeogenesis primarily resides in the early proximal tubule and is linked to tubular ammoniagenesis and the formation of new bicarbonate (Fig. 4). Urine metabolomics experiments showed a reduction in α-ketoglutarate and malate in nondiabetic NHE3-KO mice, consistent with reported metabolic acidosis-induced increases in the activity of the downstream enzymes α-ketoglutarate dehydrogenase (36) and PEPCK (1, 2, 23), respectively: while the former by lowering α-ketoglutarate facilitates the deamination of glutamate and thus the formation of ammonium, the latter forms bicarbonate and phosphoenolpyruvate, which is subsequently converted to glucose or pyruvate (Fig. 4). Finally, the reduction in GFR (discussed below) can be considered as a mechanism that limits the urinary loss of filtered bicarbonate in NHE3-KO mice.
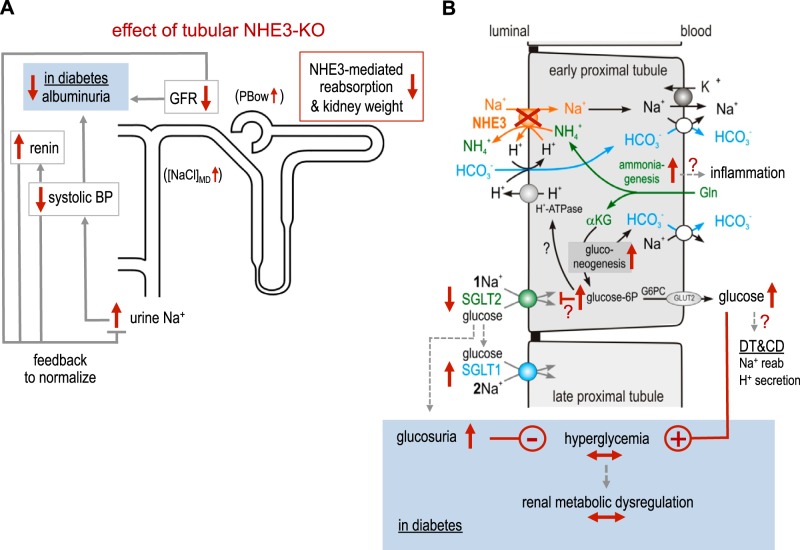
The present study provides evidence for a potential interaction between the formation of glucose and regulation of luminal glucose uptake in the early proximal tubule. One may speculate that enhanced tubular gluconeogenesis in NHE3-KO mice contributed to the observed downregulation of SGLT2 expression, possibly through an intracellular negative feedback loop that regulates SGLT2 to limit the accumulation of glucose in early proximal tubular cells (Fig. 10). Vice versa, SGLT2 inhibition increased renal SGLT2 protein expression in nondiabetic mice (58). Downregulation of SGLT2 could explain the enhanced glucosuria unmasked in NHE3-KO mice by diabetes. In nondiabetic mice, a modest downregulation of SGLT2 in NHE3-KO does not enhance glucosuria, due to the low amounts of filtered glucose and the remaining SGLT2 and SGLT1 transport capacity. Furthermore, NHE3-KO increased renal SGLT1 mRNA expression, possibly to compensate in part for the upstream reduced SGLT2 expression (46). The enhanced glucosuria and urine calorie loss in diabetic NHE3-KO mice did not lower hyperglycemia or induce a compensatory increase in food intake, as observed in response to an SGLT2 inhibitor (58, 61). This could be explained by enhanced renal gluconeogenesis and an associated increased glucose delivery to the systemic circulation, consistent with the observed renal mRNA upregulation of glucose-6-phosphatase, i.e., of the enzyme that dephosphorylates newly formed glucose before it can exit the proximal tubule through GLUT2 (Fig. 10).
Fig. 10.
Proposed consequences of the absence of tubular Na+/H+ exchanger isoform 3 (NHE3). A: in both nondiabetic and diabetic kidneys, tubular NHE3 knockdown [NHE3-knockout (KO)] reduces proximal tubular growth/kidney weight and Na+ reabsorption, which induces a natriuretic tone. The latter reduces systolic blood pressure (BP) and activates the renal renin system, which both attenuate natriuretic tone. The reduced proximal tubular Na+ reabsorption enhances the NaCl concentration at the macula densa ([NaCl]MD) and enhances hydrostatic pressure in Bowman space [tubular back pressure (PBow)], which both lower glomerular filtration rate (GFR)/hyperfiltration and thereby filtered Na+, which helps to normalize urine Na+ excretion. Lower levels of GFR and systolic BP may reduce albuminuria in diabetic NHE3-KO mice. B: in both nondiabetic and diabetic kidneys, NHE3-KO enhances renal ammoniagenesis, likely in the early proximal tubule, in an attempt to compensate for the lack of NHE3-mediated H+ secretion and bicarbonate reabsorption. If NHE3 is decisive for proximal tubular ammonium secretion, then ammonium may accumulate and may trigger inflammation. Ammoniagenesis is associated with gluconeogenesis, which forms bicarbonate as well as glucose. The increase in cytosolic glucose concentration may help drive H+ secretion via luminal H+-ATPase but also inhibit Na+-glucose cotransporter (SGLT)2 to prevent excessive cellular glucose concentrations. Tubular NHE3-KO and the associated reduction in SGLT2 shift Na+ and glucose reabsorption downstream of the early proximal tubule with partial compensation by enhanced SGLT1 reabsorption capacity (the latter transporting 2 rather than 1 Na+ per glucose). The transport capacity by SGLT1 becomes overwhelmed in diabetes and enhanced urinary glucose excretion is observed. The latter does not lower hyperglycemia because enhanced gluconeogenesis delivers more glucose to the systemic circulation thereby inducing hyperglycemia in diabetic mice, which may explain the similar metabolic dysregulation. Part of the glucose may also be used for distal tubular (DT) and collecting duct (CD) Na+ reabsorption and H+ secretion. See text for a further discussion of a potential role of SGLT1 in diabetes-induced increases in kidney weight and GFR in mice with tubular NHE3-KO. αKG, α-ketoglutarate; Gln, glutamine.
NHE3-KO mice showed enhanced renal mRNA expression of PEPCK as well as of rate-limiting enzymes of glycolysis, including PFKP, PKM2, and PKLR. One may speculate that the new formation of glucose is in part metabolized by glycolysis to help drive ATP-consuming processes that facilitate bicarbonate reabsorption or ammonium secretion. This may potentially include luminal H+-ATPase or basolateral Na+-K+-ATPase in the proximal tubule or luminal H+-ATPase in type A intercalated cells. Glucose may reach the latter cells by transcellular transfer from proximal tubules including basolateral entry through GLUT1 (56), the mRNA expression of which was increased in kidneys of diabetic NHE3-KO mice. In accordance, a previous study (47) in the rat kidney has localized PKLR to the proximal tubule, whereas PKM2 was detected in distal tubules. Thus, part of the phosphoenolpyruvate newly formed by PEPCK in proximal tubules may be converted via PKLR to pyruvate, as the last step of glycolysis associated with the formation of ATP, whereas the other part is converted to glucose and delivered to the systemic circulation or used for transcellular glucose transfer to the distal nephron. A previous study (18) in zebrafish has provided the first in vivo evidence that acid-induced PEPCK can provide glucose to support H+ secretion via V-ATPase for acid-base homeostasis (Fig. 10). Upregulation of renal PKM2 expression in NHE3-KO mice may also reflect the shift of Na+ reabsorption from the proximal tubule to more distal tubules and collecting ducts (Fig. 10).
Previous studies have identified different sets of urinary metabolites that were associated with CKD in nondiabetic patients (24) or differentiated patients with diabetes with CKD from those without CKD (50). Most of these metabolites have been related to mitochondrial dysfunction and include BCAA metabolites and TCA cycle-associated compounds, and their urinary excretion was reduced in nondiabetic and diabetic patients with CKD. Notably, in nondiabetic mice, NHE3-KO changed the urinary excretion of most of these metabolites in the opposite direction, i.e., the excretion was increased versus WT controls. Possible explanations include the increasing role of BCAA in the generation of ammonia in acidosis (10, 66). The finding that NHE3-KO increased urinary BCAA metabolites and the expression of genes involved in ammonium formation and secretion, but did not actually increase urinary ammonium excretion compared with WT controls, is consistent with a prominent role of NHE3 in renal ammonium excretion. Furthermore, the upregulation of TCA cycle metabolites may reflect the adaptation of proximal tubular cells to enhance ammonia, bicarbonate, and glucose formation. This may include enhanced pyruvate recycling from phosphoenolpyruvate via PKLR and potentially increased pyruvate dehydrogenase activity, which would enhance proximal tubular formation of citric acid, and could explain the observed increase in urinary excretion of the TCA cycle metabolites citrate, aconitate, and isocitrate in NHE3-KO mice (Fig. 4). Whether the increase in this pattern of urine metabolites is associated with mitochondrial biogenesis is of interest but not evaluated in the present study. One may speculate that the pattern of increased urine metabolites in NHE3-KO mice may be protective against the effect of diabetes. However, tubular knockdown of NHE3 did not prevent Akita diabetes from lowering the urinary excretion of these metabolites. There was also no amelioration in the proinflammatory profile in response to tubular NHE3-KO in diabetic mice, despite a reduction in albuminuria. One explanation could be a dominant diabetes-induced or hyperglycemia-driven impairment in BCAA metabolism and mitochondrial metabolism. The lack of an improvement in the urinary metabolome linked to diabetic kidney disease by tubular NHE3-KO in diabetic mice may be viewed as demonstrating that tubular NHE3 is not a critical regulator of tubular metabolic dysfunction of diabetic kidney disease.
For unclear reasons, NHE3-KO was associated with renal upregulation of proinflammatory markers. Further studies are needed to explore a potential link between impaired tubular NHE3 and proinflammatory signaling in the kidney, which may relate to enhanced tubular gluconeogenesis or glycolysis or a potential increase in proximal tubular synthesis of ammonia that cannot exit across the luminal membrane due to the lack of NHE3. As discussed above, NHE3-KO may enhance SGLT1-mediated glucose reabsorption, which has been recently found to enhance inflammation during recovery from acute kidney injury (38). New insights may also help to better understand potential deleterious effects of acidosis on kidney outcome in patients with CKD (14).
Theoretically, an increase in protein or gene expression in proportion to tubular cell growth may leave the expression unchanged when related to a housekeeping gene or protein. Considering that mean kidney weight was 64% greater in diabetic versus nondiabetic WT mice, total renal protein or gene expression could have been underestimated in diabetic mice. When normalized to housekeeping β-actin, renal NHE3 expression was numerically lower in diabetic versus nondiabetic WT mice, although this did not reach statistical significance. Taking also kidney weight into consideration indicated that whole kidney NHE3 expression was not affected by diabetes. In general, diabetes-associated net acid load and hyperinsulinemia can increase NHE3 expression, whereas proinflammatory factors like TNF-α (30), lack of insulin (30), and volume retention (22) may lower tubular NHE3 expression, respectively. In accordance, renal NHE3 protein expression in response to diabetes has been reported to increase, decline, or be unchanged (31, 59). In the present study, kidney weight and systolic blood pressure were increased and renal mRNA expression of renin was reduced in diabetic mice, whereas TNF-α expression was increased. This is consistent with renal inflammation and an overall enlarged tubular transport machinery that induced volume retention, which, together with hypoinsulinemia, may have prevented a net increase in renal NHE3 expression in diabetic mice despite a diabetes-associated increase in net acid load. Larger kidneys, hyporeninemia, and volume retention have also been described in patients with type 1 diabetes (7, 63).
NHE3-KO reduced blood pressure and enhanced renal renin mRNA expression in nondiabetic and diabetic mice, consistent with a volume-reducing effect of NHE3-KO. The present results also indicated that tubular NHE3 is a determinant of kidney weight and GFR in nondiabetic and diabetic conditions. Possibly as a consequence of the lower levels of GFR and blood pressure in diabetic mice, tubular NHE3-KO also reduced albuminuria. The lower GFR and albuminuria occurred despite unchanged glomerular size, consistent with a functional rather than structural mechanism that reduced GFR. This would be consistent with lowering the reabsorption upstream of the macula densa due to less NHE3-mediated Na+ reabsorption and smaller tubules, which is expected to reduce GFR through tubuloglomerular feedback and increasing tubular back pressure (Fig. 10) (54, 60). This is reminiscent of the effects of SGLT2 inhibition, which also lowers tubular reabsorption and subsequently GFR and blood pressure except that the effect of SGLT2 inhibition is facilitated by hyperglycemia (55, 60, 64). Thus, similar to an SGLT2 inhibitor, pharmacological inhibition of NHE3 would be expected to lower volume retention, glomerular hyperfiltration, and albuminuria in subjects with diabetes. In contrast to SGLT2 inhibition, however, tubular NHE3 inhibition may not improve hyperglycemia despite some downregulation of SGLT2 protein expression and an increase in glucosuria as discussed above.
Kidney weight and GFR were lower in Akita diabetic NHE3-KO versus WT mice. However, NHE3-KO did not prevent the diabetes-induced increase in kidney weight, GFR, and systolic blood pressure or the decrease in renal renin mRNA expression. These results suggest that diabetes-induced tubular growth and hyperreabsorption occurred in part independent of tubular NHE3. Further studies are needed to better define the mechanisms that drive diabetes-induced kidney growth and tubular hyperreabsorption, also in the absence of NHE3. Tubular NHE3-KO did not improve hyperglycemia, which may be a major driving force of diabetic kidney growth (58) as well as of diabetes-induced hyperreabsorption via SGLT2 (64). In addition, tubular NHE3-KO may shift part of the glucose reabsorption from SGLT2 to SGLT1 with the latter reabsorbing two Na+ per glucose and thus reabsorbing twice as much Na+ and using twice as much energy per glucose as SGLT2, which reabsorbs one Na+ per glucose (Fig. 10). This shift could contribute to diabetes-induced hyperreabsorption and tubular growth and thus glomerular hyperfiltration via the tubuloglomerular feedback mechanism in mice lacking tubular NHE3. In accordance, a recent study (51) has indicated a unique role of SGLT1 in diabetes-induced increases in kidney weight and GFR. These recent studies further indicated that the macula densa can sense an increased luminal glucose delivery via SGLT1 expressed in its luminal membrane and that this enhances neuronal nitric oxide synthase expression in the macula densa and the formed NO contributes to diabetic hyperfiltration (51, 70). Whether the increased glucosuria in Akita diabetic NHE3-KO versus WT mice is associated with enhanced macula densa nitric oxide formation and thereby contributes to the maintained diabetes-induced GFR increase will require further experimentation.
In summary, mice with tubular NHE3 knockdown illustrate the close link between renal ammonia, bicarbonate, and glucose handling and the regulation of BCAAs and the TCA cycle. This included an increase in gluconeogenesis associated with a reduction in renal SGLT2 expression and an increase in urinary BCAA and TCA cycle metabolites in nondiabetic NHE3-KO mice, which opposed the pattern previously associated with CKD in nondiabetic and diabetic patients. The absence of tubular NHE3 lowered kidney weight and GFR in nondiabetic and diabetic mice and reduced albuminuria in the latter. The absence of tubular NHE3, however, did not affect the increase in hyperglycemia in diabetic mice. Moreover, tubular NHE3-KO did not prevent the hyperglycemia-associated increase in kidney weight and glomerular hyperfiltration or the drop in urinary BCAA and TCA cycle metabolites. The absence of tubular NHE3 likely shifted Na+ and glucose reabsorption downstream of the early proximal tubule, which, through the actions of SGLT1, may have induced unique consequences in the diabetic kidney and, for unclear reasons, was associated with a proinflammatory renal signal. Further studies are needed to better understand these issues.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01-DK-112042 (to V. Vallon and S. Thomson) and R01-DK-106102 and R01-HL-142814 (V. Vallon); the University of Alabama at Birmingham/University of California-San Diego O’Brien Center of Acute Kidney Injury NIH Grant P30-DK-079337 (to V. Vallon and S. Thomson); Department of Veterans Affairs (to V. Vallon and S. Thomson); and an investigator-initiated research project by Boehringer Ingelheim Pharmaceuticals (to V. Vallon). Support for K. Sharma and M. Darshi was provided by a Veterans Affairs Merit Award.
DISCLOSURES
Over the past 36 mo, V. Vallon has served as a consultant and received honoraria from Astra-Zeneca, Bayer, Boehringer Ingelheim, Janssen Pharmaceutical, Eli Lilly, and Merck and received grant support for investigator-initiated research from Astra-Zeneca, Bayer, Boehringer Ingelheim, Fresenius, and Janssen. K. Sharma has served on advisory boards for Boehringer Ingelheim, Sanofi, and Janssen. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
A.O., M.D., M.S., S.T., K.S., and V.V. conceived and designed research; A.O., Y.F., M.D., M.C.-M., W.H., P.S., R.P., Y.C.K., J.N., B.F., and V.V. performed experiments; A.O., M.D., M.C.-M., W.H., Y.C.K., J.N., B.F., and V.V. analyzed data; A.O., M.D., Y.C.K., S.C.T., K.S., and V.V. interpreted results of experiments; R.P., Y.C.K., and V.V. prepared figures; A.O. and V.V. drafted manuscript; A.O., Y.F., M.D., M.C.-M., W.H., P.S., R.P., Y.C.K., J.N., B.F., M.S., S.C.T., K.S., and V.V. edited and revised manuscript; A.O., Y.F., M.D., M.C.-M., W.H., P.S., R.P., Y.C.K., J.N., B.F., M.S., S.C.T., K.S., and V.V. approved final version of manuscript.
ACKNOWLEDGMENTS
Pax8-Cre mice were kindly provided by Meinrad Busslinger.
REFERENCES
- 1.Alleyne GA. Renal metabolic response to acid-base changes. II. The early effects of metabolic acidosis on renal metabolism in the rat. J Clin Invest 49: 943–951, 1970. doi: 10.1172/JCI106314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alleyne GA, Scullard GH. Renal metabolic response to acid base changes. I. Enzymatic control of ammoniagenesis in the rat. J Clin Invest 48: 364–370, 1969. doi: 10.1172/JCI105993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amemiya M, Loffing J, Lötscher M, Kaissling B, Alpern RJ, Moe OW. Expression of NHE-3 in the apical membrane of rat renal proximal tubule and thick ascending limb. Kidney Int 48: 1206–1215, 1995. doi: 10.1038/ki.1995.404. [DOI] [PubMed] [Google Scholar]
- 4.Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD, McQuaid S, Gray RT, Murray LJ, Coleman HG, James JA, Salto-Tellez M, Hamilton PW. QuPath: open source software for digital pathology image analysis. Sci Rep 7: 16878, 2017. doi: 10.1038/s41598-017-17204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biemesderfer D, Rutherford PA, Nagy T, Pizzonia JH, Abu-Alfa AK, Aronson PS. Monoclonal antibodies for high-resolution localization of NHE3 in adult and neonatal rat kidney. Am J Physiol Renal Physiol 273: F289–F299, 1997. doi: 10.1152/ajprenal.1997.273.2.F289. [DOI] [PubMed] [Google Scholar]
- 6.Bobulescu IA, Moe OW. Luminal Na+/H+ exchange in the proximal tubule. Pflugers Arch 458: 5–21, 2009. doi: 10.1007/s00424-008-0595-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bojestig M, Nystrom FH, Arnqvist HJ, Ludvigsson J, Karlberg BE. The renin-angiotensin-aldosterone system is suppressed in adults with type 1 diabetes. J Renin Angiotensin Aldosterone Syst 1: 353–356, 2000. doi: 10.3317/jraas.2000.065. [DOI] [PubMed] [Google Scholar]
- 8.Bouchard M, Souabni A, Busslinger M. Tissue-specific expression of cre recombinase from the Pax8 locus. Genesis 38: 105–109, 2004. doi: 10.1002/gene.20008. [DOI] [PubMed] [Google Scholar]
- 9.Brosius FC 3rd, Alpers CE, Bottinger EP, Breyer MD, Coffman TM, Gurley SB, Harris RC, Kakoki M, Kretzler M, Leiter EH, Levi M, McIndoe RA, Sharma K, Smithies O, Susztak K, Takahashi N, Takahashi T; Animal Models of Diabetic Complications Consortium . Mouse models of diabetic nephropathy. J Am Soc Nephrol 20: 2503–2512, 2009. doi: 10.1681/ASN.2009070721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brosnan JT, Brosnan ME. Branched-chain amino acids: enzyme and substrate regulation. J Nutr 136, Suppl 1: 207S–211S, 2006. doi: 10.1093/jn/136.1.207S. [DOI] [PubMed] [Google Scholar]
- 11.Cano NJ, Fouque D, Leverve XM. Application of branched-chain amino acids in human pathological states: renal failure. J Nutr 136, Suppl 1: 299S–307S, 2006. doi: 10.1093/jn/136.1.299S. [DOI] [PubMed] [Google Scholar]
- 12.Chistiakov DA, Killingsworth MC, Myasoedova VA, Orekhov AN, Bobryshev YV. CD68/macrosialin: not just a histochemical marker. Lab Invest 97: 4–13, 2017. doi: 10.1038/labinvest.2016.116. [DOI] [PubMed] [Google Scholar]
- 13.Curthoys NP, Moe OW. Proximal tubule function and response to acidosis. Clin J Am Soc Nephrol 9: 1627–1638, 2014. doi: 10.2215/CJN.10391012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubey AK, Sahoo J, Vairappan B, Haridasan S, Parameswaran S, Priyamvada PS. Correction of metabolic acidosis improves muscle mass and renal function in chronic kidney disease stages 3 and 4: a randomized controlled trial. Nephrol Dial Transplant 2018: gfy214, 2018. doi: 10.1093/ndt/gfy214. [DOI] [PubMed] [Google Scholar]
- 15.Fenton RA, Poulsen SB, de la Mora Chavez S, Soleimani M, Dominguez Rieg JA, Rieg T. Renal tubular NHE3 is required in the maintenance of water and sodium chloride homeostasis. Kidney Int 92: 397–414, 2017. doi: 10.1016/j.kint.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finberg KE, Wagner CA, Bailey MA, Paunescu TG, Breton S, Brown D, Giebisch G, Geibel JP, Lifton RP. The B1-subunit of the H+ ATPase is required for maximal urinary acidification. Proc Natl Acad Sci USA 102: 13616–13621, 2005. doi: 10.1073/pnas.0506769102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fine LG, Sakhrani LM. Proximal tubular cells in primary culture. Miner Electrolyte Metab 12: 51–57, 1986. [PubMed] [Google Scholar]
- 18.Furukawa F, Tseng YC, Liu ST, Chou YL, Lin CC, Sung PH, Uchida K, Lin LY, Hwang PP. Induction of phosphoenolpyruvate carboxykinase (PEPCK) during acute acidosis and its role in acid secretion by V-ATPase-expressing ionocytes. Int J Biol Sci 11: 712–725, 2015. doi: 10.7150/ijbs.11827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gekle M, Freudinger R, Mildenberger S. Inhibition of Na+-H+ exchanger-3 interferes with apical receptor-mediated endocytosis via vesicle fusion. J Physiol 531: 619–629, 2001. doi: 10.1111/j.1469-7793.2001.0619h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gekle M, Völker K, Mildenberger S, Freudinger R, Shull GE, Wiemann M. NHE3 Na+/H+ exchanger supports proximal tubular protein reabsorption in vivo. Am J Physiol Renal Physiol 287: F469–F473, 2004. doi: 10.1152/ajprenal.00059.2004. [DOI] [PubMed] [Google Scholar]
- 21.Gerich JE. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med 27: 136–142, 2010. doi: 10.1111/j.1464-5491.2009.02894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Girardi AC, Di Sole F. Deciphering the mechanisms of the Na+/H+ exchanger-3 regulation in organ dysfunction. Am J Physiol Cell Physiol 302: C1569–C1587, 2012. doi: 10.1152/ajpcell.00017.2012. [DOI] [PubMed] [Google Scholar]
- 23.Goodman AD, Fuisz RE, Cahill GF Jr. Renal gluconeogenesis in acidosis, alkalosis, and potassium deficiency: its possible role in regulation of renal ammonia production. J Clin Invest 45: 612–619, 1966. doi: 10.1172/JCI105375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hallan S, Afkarian M, Zelnick LR, Kestenbaum B, Sharma S, Saito R, Darshi M, Barding G, Raftery D, Ju W, Kretzler M, Sharma K, de Boer IH. Metabolomics and gene expression analysis reveal down-regulation of the citric acid (TCA) cycle in non-diabetic CKD patients. EBioMedicine 26: 68–77, 2017. doi: 10.1016/j.ebiom.2017.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hallow KM, Gebremichael Y, Helmlinger G, Vallon V. Primary proximal tubule hyperreabsorption and impaired tubular transport counterregulation determine glomerular hyperfiltration in diabetes: a modeling analysis. Am J Physiol Renal Physiol 312: F819–F835, 2017. doi: 10.1152/ajprenal.00497.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holness CL, Simmons DL. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood 81: 1607–1613, 1993. [PubMed] [Google Scholar]
- 27.Hryciw DH, Lee EM, Pollock CA, Poronnik P. Molecular changes in proximal tubule function in diabetes mellitus. Clin Exp Pharmacol Physiol 31: 372–379, 2004. doi: 10.1111/j.1440-1681.2004.04001.x. [DOI] [PubMed] [Google Scholar]
- 28.Johnson DW, Brew BK, Poronnik P, Cook DI, Field MJ, Györy AZ, Pollock CA. Insulin-like growth factor I stimulates apical sodium/hydrogen exchange in human proximal tubule cells. Am J Physiol Renal Physiol 272: F484–F490, 1997. doi: 10.1152/ajprenal.1997.272.4.F484. [DOI] [PubMed] [Google Scholar]
- 29.Johnson DW, Saunders HJ, Poronnik P, Cook DI, Field MJ, Pollock CA. Physiological changes in extracellular sodium directly control human proximal tubule growth and transport. Pflugers Arch 435: 211–218, 1997. doi: 10.1007/s004240050503. [DOI] [PubMed] [Google Scholar]
- 30.Klisic J, Hu MC, Nief V, Reyes L, Fuster D, Moe OW, Ambühl PM. Insulin activates Na+/H+ exchanger 3: biphasic response and glucocorticoid dependence. Am J Physiol Renal Physiol 283: F532–F539, 2002. doi: 10.1152/ajprenal.00365.2001. [DOI] [PubMed] [Google Scholar]
- 31.Layton AT, Vallon V, Edwards A. Predicted consequences of diabetes and SGLT inhibition on transport and oxygen consumption along a rat nephron. Am J Physiol Renal Physiol 310: F1269–F1283, 2016. doi: 10.1152/ajprenal.00543.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee EM, Pollock CA, Drumm K, Barden JA, Poronnik P. Effects of pathophysiological concentrations of albumin on NHE3 activity and cell proliferation in primary cultures of human proximal tubule cells. Am J Physiol Renal Physiol 285: F748–F757, 2003. doi: 10.1152/ajprenal.00442.2002. [DOI] [PubMed] [Google Scholar]
- 33.Letto J, Brosnan ME, Brosnan JT. Valine metabolism. Gluconeogenesis from 3-hydroxyisobutyrate. Biochem J 240: 909–912, 1986. doi: 10.1042/bj2400909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li HC, Du Z, Barone S, Rubera I, McDonough AA, Tauc M, Zahedi K, Wang T, Soleimani M. Proximal tubule specific knockout of the Na+/H+ exchanger NHE3: effects on bicarbonate absorption and ammonium excretion. J Mol Med (Berl) 91: 951–963, 2013. doi: 10.1007/s00109-013-1015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lorenz JN, Schultheis PJ, Traynor T, Shull GE, Schnermann J. Micropuncture analysis of single-nephron function in NHE3-deficient mice. Am J Physiol Renal Physiol 277: F447–F453, 1999. doi: 10.1152/ajprenal.1999.277.3.F447. [DOI] [PubMed] [Google Scholar]
- 36.Lowry M, Ross BD. Activation of oxoglutarate dehydrogenase in the kidney in response to acute acidosis. Biochem J 190: 771–780, 1980. doi: 10.1042/bj1900771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moriya T, Tsuchiya A, Okizaki S, Hayashi A, Tanaka K, Shichiri M. Glomerular hyperfiltration and increased glomerular filtration surface are associated with renal function decline in normo- and microalbuminuric type 2 diabetes. Kidney Int 81: 486–493, 2012. doi: 10.1038/ki.2011.404. [DOI] [PubMed] [Google Scholar]
- 38.Nespoux J, Patel R, Hudkins KL, Huang W, Freeman B, Kim YC, Koepsell H, Alpers CE, Vallon V. Gene deletion of the Na+-glucose cotransporter SGLT1 ameliorates kidney recovery in a murine model of acute kidney injury induced by ischemia-reperfusion. Am J Physiol Renal Physiol 316: F1201–F1210, 2019. doi: 10.1152/ajprenal.00111.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noonan WT, Woo AL, Nieman ML, Prasad V, Schultheis PJ, Shull GE, Lorenz JN. Blood pressure maintenance in NHE3-deficient mice with transgenic expression of NHE3 in small intestine. Am J Physiol Regul Integr Comp Physiol 288: R685–R691, 2005. doi: 10.1152/ajpregu.00209.2004. [DOI] [PubMed] [Google Scholar]
- 40.Oliva RV, Bakris GL. Blood pressure effects of sodium-glucose co-transport 2 (SGLT2) inhibitors. J Am Soc Hypertens 8: 330–339, 2014. doi: 10.1016/j.jash.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Pollock CA, Field MJ, Bostrom TE, Dyne M, Gyory AZ, Cockayne DJ. Proximal tubular cell sodium concentration in early diabetic nephropathy assessed by electron microprobe analysis. Pflugers Arch 418: 14–17, 1991. doi: 10.1007/BF00370446. [DOI] [PubMed] [Google Scholar]
- 42.Pruijm M, Wuerzner G, Maillard M, Bovet P, Renaud C, Bochud M, Burnier M. Glomerular hyperfiltration and increased proximal sodium reabsorption in subjects with type 2 diabetes or impaired fasting glucose in a population of the African region. Nephrol Dial Transplant 25: 2225–2231, 2010. doi: 10.1093/ndt/gfq008. [DOI] [PubMed] [Google Scholar]
- 43.Qi W, Chen X, Gilbert RE, Zhang Y, Waltham M, Schache M, Kelly DJ, Pollock CA. High glucose-induced thioredoxin-interacting protein in renal proximal tubule cells is independent of transforming growth factor-β1. Am J Pathol 171: 744–754, 2007. doi: 10.2353/ajpath.2007.060813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quinn PG, Yeagley D. Insulin regulation of PEPCK gene expression: a model for rapid and reversible modulation. Curr Drug Targets Immune Endocr Metabol Disord 5: 423–437, 2005. doi: 10.2174/156800805774912962. [DOI] [PubMed] [Google Scholar]
- 45.Rieg T, Gerasimova M, Murray F, Masuda T, Tang T, Rose M, Drucker DJ, Vallon V. Natriuretic effect by exendin-4, but not the DPP-4 inhibitor alogliptin, is mediated via the GLP-1 receptor and preserved in obese type 2 diabetic mice. Am J Physiol Renal Physiol 303: F963–F971, 2012. doi: 10.1152/ajprenal.00259.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rieg T, Masuda T, Gerasimova M, Mayoux E, Platt K, Powell DR, Thomson SC, Koepsell H, Vallon V. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am J Physiol Renal Physiol 306: F188–F193, 2014. doi: 10.1152/ajprenal.00518.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schering B, Reinacher M, Schoner W. Localization and role of pyruvate kinase isoenzymes in the regulation of carbohydrate metabolism and pyruvate recycling in rat kidney cortex. Biochim Biophys Acta 881: 62–71, 1986. doi: 10.1016/0304-4165(86)90097-8. [DOI] [PubMed] [Google Scholar]
- 48.Schoolwerth AC. Regulation of renal ammoniagenesis in metabolic acidosis. Kidney Int 40: 961–973, 1991. doi: 10.1038/ki.1991.301. [DOI] [PubMed] [Google Scholar]
- 49.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282–285, 1998. doi: 10.1038/969. [DOI] [PubMed] [Google Scholar]
- 50.Sharma K, Karl B, Mathew AV, Gangoiti JA, Wassel CL, Saito R, Pu M, Sharma S, You YH, Wang L, Diamond-Stanic M, Lindenmeyer MT, Forsblom C, Wu W, Ix JH, Ideker T, Kopp JB, Nigam SK, Cohen CD, Groop PH, Barshop BA, Natarajan L, Nyhan WL, Naviaux RK. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J Am Soc Nephrol 24: 1901–1912, 2013. doi: 10.1681/ASN.2013020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song P, Huang W, Onishi A, Patel R, Kim YC, van Ginkel C, Fu Y, Freeman B, Koepsell H, Thomson SC, Liu R, Vallon V. Knockout of Na-glucose-cotransporter SGLT1 mitigates diabetes-induced upregulation of nitric oxide synthase-1 in macula densa and glomerular hyperfiltration. Am J Physiol Renal Physiol 317: F207–F217, 2019. doi: 10.1152/ajprenal.00120.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steel RG, Torrie JH, Dickey DA. Principles and Procedures of Statistics. New York: McGraw-Hill, 1997. [Google Scholar]
- 53.Taylor L, Curthoys NP. Glutamine metabolism: role in acid-base balance*. Biochem Mol Biol Educ 32: 291–304, 2004. doi: 10.1002/bmb.2004.494032050388. [DOI] [PubMed] [Google Scholar]
- 54.Thomson SC, Deng A, Bao D, Satriano J, Blantz RC, Vallon V. Ornithine decarboxylase, kidney size, and the tubular hypothesis of glomerular hyperfiltration in experimental diabetes. J Clin Invest 107: 217–224, 2001. doi: 10.1172/JCI10963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomson SC, Rieg T, Miracle C, Mansoury H, Whaley J, Vallon V, Singh P. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol 302: R75–R83, 2012. doi: 10.1152/ajpregu.00357.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thorens B, Lodish HF, Brown D. Differential localization of two glucose transporter isoforms in rat kidney. Am J Physiol Cell Physiol 259: C286–C294, 1990. doi: 10.1152/ajpcell.1990.259.2.C286. [DOI] [PubMed] [Google Scholar]
- 57.Tonneijck L, Muskiet MH, Smits MM, van Bommel EJ, Heerspink HJ, van Raalte DH, Joles JA. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J Am Soc Nephrol 28: 1023–1039, 2017. doi: 10.1681/ASN.2016060666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vallon V, Gerasimova M, Rose MA, Masuda T, Satriano J, Mayoux E, Koepsell H, Thomson SC, Rieg T. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol 306: F194–F204, 2014. doi: 10.1152/ajprenal.00520.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vallon V, Komers R. Pathophysiology of the diabetic kidney. Compr Physiol 1: 1175–1232, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vallon V, Richter K, Blantz RC, Thomson S, Osswald H. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol 10: 2569–2576, 1999. [DOI] [PubMed] [Google Scholar]
- 61.Vallon V, Rose M, Gerasimova M, Satriano J, Platt KA, Koepsell H, Cunard R, Sharma K, Thomson SC, Rieg T. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol 304: F156–F167, 2013. doi: 10.1152/ajprenal.00409.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vallon V, Schwark JR, Richter K, Hropot M. Role of Na+/H+ exchanger NHE3 in nephron function: micropuncture studies with S3226, an inhibitor of NHE3. Am J Physiol Renal Physiol 278: F375–F379, 2000. doi: 10.1152/ajprenal.2000.278.3.F375. [DOI] [PubMed] [Google Scholar]
- 63.Vallon V, Thomson SC. Renal function in diabetic disease models: the tubular system in the pathophysiology of the diabetic kidney. Annu Rev Physiol 74: 351–375, 2012. doi: 10.1146/annurev-physiol-020911-153333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia 60: 215–225, 2017. doi: 10.1007/s00125-016-4157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vinay P, Lemieux G, Gougoux A, Halperin M. Regulation of glutamine metabolism in dog kidney in vivo. Kidney Int 29: 68–79, 1986. doi: 10.1038/ki.1986.9. [DOI] [PubMed] [Google Scholar]
- 66.Wang X, Price SR. Differential regulation of branched-chain α-ketoacid dehydrogenase kinase expression by glucocorticoids and acidification in LLC-PK1-GR101 cells. Am J Physiol Renal Physiol 286: F504–F508, 2004. doi: 10.1152/ajprenal.00296.2003. [DOI] [PubMed] [Google Scholar]
- 67.Weiner ID, Verlander JW. Ammonia transporters and their role in acid-base balance. Physiol Rev 97: 465–494, 2017. doi: 10.1152/physrev.00011.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Woo AL, Noonan WT, Schultheis PJ, Neumann JC, Manning PA, Lorenz JN, Shull GE. Renal function in NHE3-deficient mice with transgenic rescue of small intestinal absorptive defect. Am J Physiol Renal Physiol 284: F1190–F1198, 2003. doi: 10.1152/ajprenal.00418.2002. [DOI] [PubMed] [Google Scholar]
- 69.You YH, Quach T, Saito R, Pham J, Sharma K. Metabolomics reveals a key role for fumarate in mediating the effects of NADPH oxidase 4 in diabetic kidney disease. J Am Soc Nephrol 27: 466–481, 2016. doi: 10.1681/ASN.2015030302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang J, Wei J, Jiang S, Xu L, Wang L, Cheng F, Buggs J, Koepsell H, Vallon V, Liu R. Macula densa SGLT1–NOS1-TGF pathway–a new mechanism for glomerular hyperfiltration during hyperglycemia. J Am Soc Nephrol 30: 578–593, 2019. doi: 10.1681/ASN.2018080844. [DOI] [PMC free article] [PubMed] [Google Scholar]