Abstract
We proposed that circulating metabolites generated by the intestinal microbiota can affect vascular function. One such metabolite, indole 3-propionic acid (IPA), can activate the pregnane X receptor(PXR), a xenobiotic-activated nuclear receptor present in many tissues, including the vascular endothelium. We hypothesized that IPA could regulate vascular function by modulating PXR activity. To test this, Pxr+/+ mice were administered broad-spectrum antibiotics for 2 wk with IPA supplementation. Vascular function was evaluated by bioassay using aorta and pulmonary artery ring tissue from antibiotic-treated Pxr+/+ and Pxr−/−mice, supplemented with IPA, and using aorta tissue maintained in organ culture for 24 h in the presence of IPA. Endothelium-dependent, nitric oxide(NO)-mediated muscarinic and proteinase-activated receptor 2(PAR2)-stimulated vasodilation was assessed. Endothelial nitric oxide synthase (eNOS) abundance was evaluated in intact tissue or in aorta-derived endothelial cell cultures from Pxr+/+ and Pxr−/− mice, and vascular Pxr levels were assessed in tissues obtained from Pxr+/+ mice treated with antibiotics and supplemented with IPA. Antibiotic-treated Pxr+/+ mice exhibited enhanced agonist-induced endothelium-dependent vasodilation, which was phenocopied by tissues from either Pxr−/− or germ-free mice. IPA exposure reduced the vasodilatory responses in isolated and cultured vessels. No effects of IPA were observed for tissues obtained from Pxr−/− mice. Serum nitrate levels were increased in antibiotic-treated Pxr+/+and Pxr−/− mice. eNOS abundance was increased in aorta tissues and cultured endothelium from Pxr−/− mice. PXR stimulation reduced eNOS expression in cultured endothelial cells from Pxr+/+ but not Pxr−/− mice. The microbial metabolite IPA, via the PXR, plays a key role in regulating endothelial function. Furthermore, antibiotic treatment changes PXR-mediated vascular endothelial responsiveness by upregulating eNOS.
Keywords: endothelium, metabolites, microbiota, pregnane X receptor, vasorelaxation
INTRODUCTION
Accumulating evidence indicates that the intestinal microbiome has a substantial effect on cardiovascular function (7). This impact results not only from microbe-host interactions at the site of colonization but also from microbe-derived metabolites that circulate to affect tissue function in a variety of organs. Thus, intestinal microbiome-derived metabolites can play a major role in vascular health and disease (21). Functionally, the endothelium is strategically placed to sense and respond to circulating microbial metabolites. Initially, the impact of microbial metabolites on blood vessel function was focused on short-chain fatty acids, and their activation of the vascular GPCR family of free fatty acid receptors. More recently, attention has turned from the GPCRs to the vascular-expressed steroid hormone-like pregnane X receptor (PXR), a ligand-activated nuclear receptor that senses and responds to a variety of chemical or nutritional stimuli, including microbial metabolites (17–20). The vascular PXR is known to be involved in regulating maternal constrictor and vasodilator responses during pregnancy, which is related to the generation of cytochrome P450 epoxygenase metabolites (6). Thus, it has been proposed that the activation of xenobiotic-responsive receptors in the vasculature by microbial metabolites constitutes a key determinant for the gut microbiota’s homeostatic role in blood vessel regulation. As a consequence of this microbiome-induced metabolite-vascular interplay, it would be expected that marked changes in the microbiota, triggered by antibiotic treatment routinely used for infectious diseases or as a preparation for colon surgery, would be reflected by changes in vascular function.
In keeping with the likelihood that microbiota-derived metabolites can affect vascular function, studies have shown that PXR activation in the endothelium can regulate innate immune receptor expression/function and mediate endothelial detoxification processes by increasing the expression of phase I/phase II metabolic enzymes and various transporters (19). Thus, the vascular PXR could readily sense and respond to circulating indole-3-propionic acid (IPA) (16), a microbiome-derived deamination product of tryptophan (3). Of importance in this regard is that recent human population-based studies have identified an inverse correlation between circulating IPA levels and type 2 diabetes (2), suggesting that this microbial metabolite might also play a role in regulating tissues, including the vasculature and other targets in diabetes.
Based on the information summarized above, we hypothesized that PXR signaling in response to microbiota-derived IPA would regulate endothelial function and that a disruption of the intestinal microbiota by antibiotic treatment and the resulting reduction in IPA abundance would in turn, via the endothelial PXR, change vascular vasodilator properties. Therefore, we assessed the impact on vascular endothelial function of downregulating systemic IPA by treating mice with broad-spectrum antibiotics. The impact of antibiotic treatment on IPA blood levels was assessed by plasma IPA measurements. The antibiotic-treated mice were supplemented or not with exogenously administered IPA in their drinking water to restore IPA levels, and aorta rings obtained from the antibiotic-treated mice were compared with rings obtained from germ-free mice that were supplemented or not with IPA. Data generated with aorta tissues obtained directly from the antibiotic-treated mice were compared with comparable aorta rings treated in organ culture for 24 h with or without IPA. Endothelial function was assessed by measuring an endothelium-dependent vasodilator (EDV) response triggered by activation of either the muscarinic or proteinase-activated receptor-2 (PAR2), both of which are known to stimulate EDV. PAR2 is now recognized as a more physiologically relevant regulator of endothelial function compared with the acetylcholine muscarinic receptor (11). Concurrently, we evaluated a role for PXR in regulating endothelial vasodilator function by comparing aorta vessel responsiveness to vasodilators [muscarinic and proteinase-activated receptor 2 (PAR2) agonists] for tissues derived from wild-type (Pxr+/+) and Pxr-null (Pxr−/−) mice. We then compared the effects of antibiotic treatment-induced downregulation of IPA levels on vascular function in tissues from the Pxr−/− compared with the data obtained using aorta rings from the Pxr+/+ mice and with tissues from germ-free C57Bl/6 mice supplemented or not with IPA. We measured, using quantitative PCR (qPCR), the vascular levels of Pxr mRNA in tissues obtained from Pxr+/+ mice treated or not with antibiotics and supplemented or not with IPA in the drinking water. Finally, we assessed the impact of PXR-activating ligands (IPA and PCN) on the levels of endothelial nitric oxide synthase (eNOS) in aorta-derived cultured endothelial cells isolated from Pxr+/+ and Pxr−/− mice.
MATERIALS AND METHODS
Animal husbandry and treatments.
Pxr−/− mice on a C57Bl/6 genetic background were kindly provided by Dr. Jeff Staudinger (University of Kansas) (14). All comparisons used littermate Pxr−/− and Pxr+/+ mice generated from Pxr+/− breeders. Mice were maintained in individual colonies in the University of Calgary Faculty of Medicine vivarium in accordance with protocols approved by the Health Sciences Animal Care Committee, following the guidelines set forth by the Canadian Council for Animal Care. Animals were either untreated or divided randomly into three groups. Group 1 received an antibiotic cocktail of ampicillin (1 g/l), neomycin (1 g/l), vancomycin (500 mg/l), and metronidazole (1g/l), as described previously (10, 16). Group 2 received IPA in drinking water (200 mg/l) alone, and group 3 received both antibiotics and IPA for a period of 2 wk. IPA supplementation of mice in vivo was achieved by its administration in the drinking water at a concentration of 200 mg/l.
Germ-free C57Bl/6 mice were bred and maintained in flexible film isolators at the International Microbiome Centre (IMC), University of Calgary. Germ-free status was routinely monitored by culture-dependent and -independent methods, and all mice were independently confirmed to be pathogen free. For administration of IPA to germ-free mice, C57Bl/6 animals of age comparable with the Pxr+/+ and Pxr−/− mice (5–6 wk, 20–22 g) were transferred to sterile Isocages within the germ-free IMC facility and maintained in accordance with protocols approved by the Health Sciences Animal Care Committee at the University of Calgary. IPA was sterile-filtered before administration, and confirmation of the germ-free status of the mice was verified at the end of treatment periods through fecal DNA staining and aerobic and anaerobic cultures of fecal material.
Isolation of vascular tissue for bioassays.
Prior to euthanasia, animals were injected with sodium citrate (0.5 ml of 3.5% wt/vol trisodium citrate administered intraperitoneally) and then euthanized 10 min later by cervical dislocation, which was performed under isoflurane anesthesia. Blood vessels were flushed with 0.5 ml of 1 mg/ml sodium citrate transcardially. Descending aorta and pulmonary artery tissues (1st- and 2nd-order pulmonary branches) were dissected free of perivascular adipose and connective tissue in ice-cold Krebs solution (115 mM NaCl, 25 mM NaHCO3, 4.7 mM KCl, 1.2 mM NaH2PO4, 10.0 mM dextrose, and 2.5 mM CaCl2), pH 7.4, and aerated with 95% O2 and 5% CO2. Aorta rings (∼2–3 mm length × 2–3 mm diameter) were either mounted in a Mulvany-Halpern myograph organ bath (610 multimyograph system coupled to Chart5 system software; AD instruments, Colorado Springs, CO) or transferred to culture medium for organ culture procedures as outlined below. All experiments adhered to ARRIVE Guidelines. All procedures involving animals were approved by the University of Calgary’s Health Sciences Animal Care Committee (protocol no. AC15-0034) and follow the guidelines set forth by the Canadian Council on Animal Care.
Wire-myograph vascular bioassays.
Isometric tension studies using a wire myograph were performed, as described previously (5, 8, 13). In brief, descending aorta tissue was cut into 2-mm-long rings as outlined above and mounted in the myograph. For this study we used at least six aortic rings for every treatment. All experiments were performed at 37°C in Krebs bioassay buffer. After a 60-min equilibration period, tissue viability was verified by monitoring a contraction in response to the addition of 80 mM KCl to the organ bath. The integrity of the endothelium was verified by contracting the tissue with phenylephrine (PE; 0.25–2.5 µM) and then monitoring a relaxation caused by acetylcholine (3 µM). Tissues were washed three times after reaching an equilibrium tension and allowed to re-equilibrate in bioassay buffer for 20 min before the next addition of agonists to the organ bath. After the responsiveness of the tissues to constriction and endothelium-dependent relaxation was validated, the following experimental protocols were pursued.
Smooth muscle-dependent contractile responses.
The tissue responses above baseline tension (g of tension) were measured for increasing concentrations of either KCl (5–120 mM) or PE (3–10,000 nM). To assess the impact of spontaneous eNOS-dependent endogenous nitric oxide (NO) release on the PE-induced contractile response, measurements were made in the absence and presence of the eNOS inhibitor Nω-Nitro-L-arginine methyl ester hydrochloride (l-NAME; 0.1 mM) to block NO production either with or without the concurrent presence of 1H-[1,2,4]oxadiazolo[4,3,-a] quinoxalin-1-one (ODQ; 1 μM) to quench residual guanylyl cyclase activity.
Endothelium-dependent vasodilation responses.
Measurements of decreased tension (EDV) in PE-constricted vessels were used to assess the effects of endothelial agonists in our work. Concentration-effect curves for EDV caused by acetylcholine and the proteinase-activated receptor 2 (PAR2)-selective agonist 2-furoyl-Leu-Ile-Gly-Arg-Leu-Orn-amide (2-fLI) were measured upon contracting the tissues to the same tension of ∼1 g with PE (0.25 µM PE for Pxr−/− and 2.5µM for Pxr+/+ tissues), followed by the addition of increasing concentrations of acetylcholine or 2-fLI to the organ bath. Relaxant responses were also evaluated in the presence of inhibitors (l-NAME + ODQ) where tissues were pretreated for 20 min before the tissues were contracted with PE and then an endothelium-dependent vasodilator/relaxant agonist (acetylcholine or 2-fLI) was added to the organ bath. Agonist-mediated relaxation was quantified as a percentage (%relaxation) relative to the tension generated by phenylephrine in the same tissue: %relaxation = [(PE tension − tension after endothelium-dependent relaxation)/original PE tension]} × 100.
Vascular organ culture.
To evaluate the direct impact of IPA on vascular tissue function, organ cultures of aorta rings were done as described in detail elsewhere (5). In brief, aorta rings prepared as described above were subjected to organ culture for 24 h in serum-free Dulbecco’s minimal essential medium (euglycemic DMEM; Thermofisher Scientific, Waltham MA) containing 5 mM glucose in either the absence or presence of 0.1 μM IPA. Rings recovered from organ culture after 24 h were then evaluated by wire myography for endothelium-mediated vasodilation in response to ACh and 2fLI, as outlined above.
qPCR analysis of vascular tissue for Pxr mRNA content.
Excised aortic tissues were cleaned of adventitial fat, weighed, and snap-frozen in liquid nitrogen for further processing. RNA in thawed tissue samples was extracted from aortic tissues using the manufacturer’s protocol (Quick RNA Microprep; Zymo Research). qPCR analysis was done, as described previously (5), using the primers below. Validated primers were purchased from Qiagen (mouse Pxr; gene is Nrli2): Qiagen 330001 PPM04650A, mouse β-actin; Qiagen 330001 PPM02945B, mouse eNOS (Nos3); Qiagen 330001 PPM03801A. Alternatively, primer sequences designed in-house were used. The sequences for Pxr are as follows: forward primer, CATCTCAGCAACCCACACAG; reverse primer, GGGGTCATAGGAGTCATTGG. The signal for Pxr was normalized to the signal for the TATA-binding protein: forward primer, CATCTCAGCAACCCACACAG: reverse primer GGGGTCATAGGAGTCATTGG.
Western blot analysis of eNOS in aorta tissue.
Aorta tissues were analyzed for the expression of eNOS by a Western blot approach, as described previously (5, 9). In brief, freshly isolated aorta tissue was dissected to remove perivascular fat, snap-frozen using solid CO2, and lysed and homogenized in ice-cold lysis buffer [20 mM Tris·HCl, pH 7.5, 100 mM NaCl, 10 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 0.5% NP-40, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 25 mM NaF, 1 mg/ml leupeptin, 1 mg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride, and 1 mM dithiothreitol; to 1 ml of lysis buffer, 10 μl of proteinase inhibitor cocktail was added (Calbiochem cocktail set III, EDTA-free, cat. no. 539134)]. The extract was then cleared by centrifugation (15,000 g for 10 min). Total protein concentration was estimated using the Precision Red reagent (Cytoskeleton). Equivalent amounts of protein from each tissue extract were heat-denatured at 92°C for 6 min in denaturing Laemmli buffer and resolved on 4–20% gradient Novex Tris-Glycine gels (ThermoFisher) run at 120V for 2 h. Transfer of proteins onto PVDF membrane was done using a semidry method. The resolved proteins were transferred to PVDF membrane and blocked for 1 h at room temperature in PBST buffer [phosphate-buffered isotonic saline supplemented with 0.1% (vol/vol) Tween-20] containing 1% ECL Advance Blocking Agent (GE Healthcare, Waukesha, WI). Detection of total eNOS was performed using anti-rabbit total eNOS antibody (cat. no. 9572; Cell Signaling Technology). β-Actin was used as a loading control (sc-47778; Santa Cruz Biotechnology). After the membrane was washed with PBST, the peroxidase activity was detected with the chemiluminescence reagent ECL-Advance (GE Healthcare) on a KODAK Image Station 4000MM. The membrane was then washed again with detergent-containing PBST and reprobed with the β-actin antibody and chemilumiescence detected as above. The mobilities of the β-actin band (42 kDa) and eNOS band (133 kDa) were sufficeintly distinct such that membrane stripping was not required. Furthermore, the only nonspecific signals in the gel present in the 50-kDa region that were not removed by washing did not interfere with quantification of either the eNOS or β-actin signals. Band intensities representing eNOS and β-actin were quantified using the ImageJ quantification software, and the abundance of eNOS was normalized to the signal generated for β-actin in the same gel lane.
qPCR analysis of aortic endothelial cells for eNOS mRNA content.
Endothelial cell cultures were derived from aorta tissue obtained from Pxr+/+ and Pxr−/− mice, as outlined elsewhere (5). In brief, aortic segments were cleaned and placed endothelial side down on a Matrigel (0.5 ml)-coated six-well (35-mm diameter) Corning plastic culture dish. Explants were supplemented with DMEM containing endothelial cell growth supplement (AlfaAesar, Tewksbury MA) for 4 days. Upon removal with 1 mM EDTA-supplemented isotonic phosphate-buffered saline pH 7.4 containing 2.4 U/ml Dispase (Roche), cells were grown for an additional 2 days and later moved to gelatin (2.5 ml 0.1% gelatin; Sigma-Aldrich Cambridge MA)-coated T25 flasks. Cultures were grown to confluency and then treated for 24 h with the Pxr agonists, IPA (1 μM), and PCN (10 μM). Cell monolayers were then rinsed, RNA was extracted using Qiagen RNeasy plus kit, cDNA was prepared, and validated primers were purchased for qPCR analysis of mRNA for eNOS (murine Nos3 primers 330001 PPM03801A; Qiagen, Toronto, ON, Canada) and β-actin (mouse β-actin primers 330001 PPM02945B; Qiagen).
Collection of plasma samples and Griess Assay of plasma nitrite.
Mice were anticoagulated with heparin (0.1 ml of 10,000 U/ml ip) and euthanized 20–30 min later with either ketamine or euthanyl (0.1 ml ip; Phenobarbital-Euthanyl Bimeda-TC, Cambridge, ON, Canada), and their anticoagulated blood (∼0.5 ml) was collected by cardiac puncture. Plasma from the anticoagulated blood samples was collected after centrifugation (1-ml microfuge tube, 9,000 g for 10 min) and stored at −80°C before experiments. Griess assays to quantify serum nitrate concentrations were performed according to the manufacturer’s instructions (Cayman Chemical, Ann Arbor, MI).
Measurement of plasma IPA.
Plasma IPA levels were measured as described previously (16). In brief, plasma samples obtained as for the Griess assay (above) were thawed on ice. A volume of 30 μl of sample was pipetted into a new vial. Volumes of 10 μl of internal standard and 110 μl of methanol were added. The samples were then mixed and centrifuged for 10 min at 14,000 rpm. The supernatants were transferred to an LC sampling vial for analysis. The samples were analyzed with ABsciex 6500+ with an Ace PFP column. A pooled quality control (QC) sample for each sample type (plasma, PBS extraction, and methanol extraction) was added to the sample list. This quality control sample was injected six times for coefficient of variation (CV) calculation for data quality control (for the limited sample volume, quality control samples were run 4 to 5 times).
Peptides and other reagents.
Peptides (>95% purity by HPLC and mass spectral analysis) were prepared by solid-phase synthesis by the University of Calgary peptide synthesis facility. Unless otherwise specified, other reagents and chemicals were purchased from either Millipore-Sigma (Oakville ON, Canada) or VWR International (Radnor PA).
Statistics.
Data points represent the mean ± SE (bars in figures) of five or more independent experiments done on tissue preparations from six different animals. Error bars smaller than the symbols shown in the figures are not visible. Concentration-effect curves were obtained by fitting the data into sigmoidal curves using GraphPad Prism (version 7 for Windows; Graph Pad Software, San Diego, CA). Prior to statistical analysis, all data were assessed for normal distribution using the Shapiro-Wilk test. Statistical comparisons between data points were made using either Student’s unpaired t-test when comparing two groups or a one-way ANOVA, with Tukey’s multiple comparison test when comparing multiple groups. In all cases, a significant difference was considered at P < 0.05.
RESULTS
Antibiotic-treatment enhances agonist-induced EDV in the aorta and pulmonary arteries, an effect that can be reversed by supplementation with the microbial metabolite IPA.
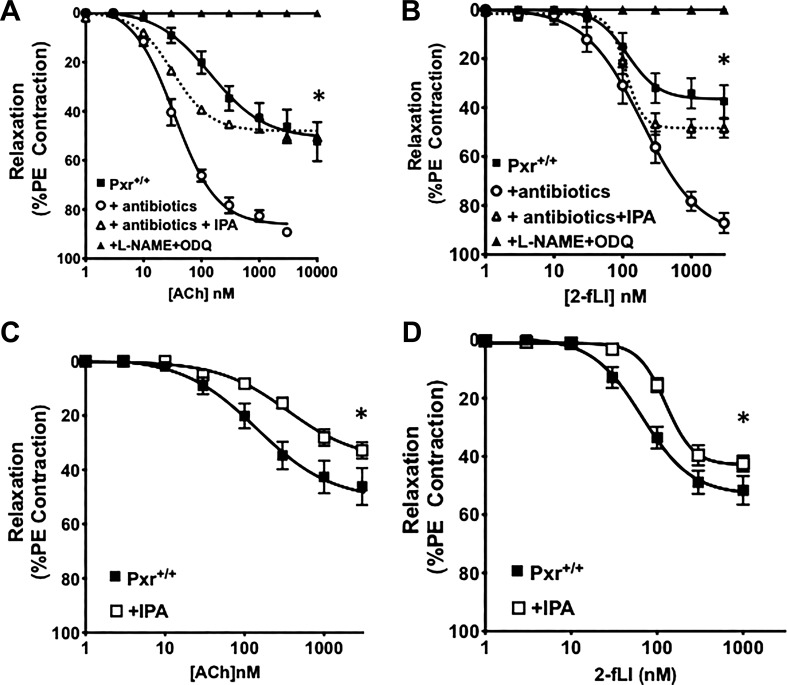
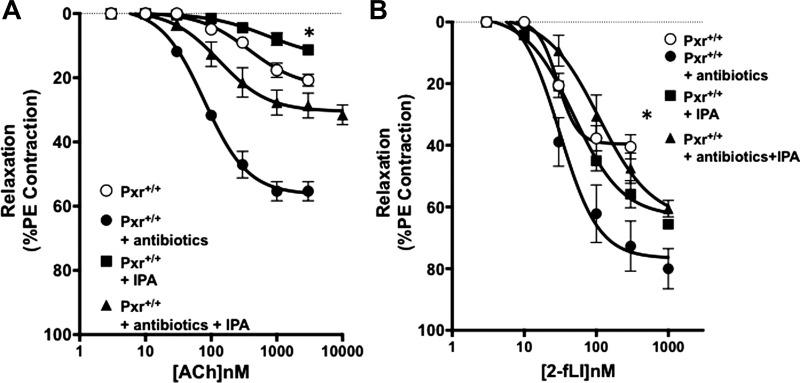
As shown in Fig. 1, aorta tissues derived from the antibiotic-treated Pxr+/+ mice were more sensitive to EDV stimulated by activation of either the muscarinic acetylcholine receptor or PAR2, with a marked increase in the maximum EDV compared with tissues from untreated mice (black squares compared with open circles in Fig. 1, A and B). For the tissues derived from both untreated and antibiotic-treated mice, all of the relaxant activity was eliminated in the presence of L-NAME and ODQ (black triangles in Fig. 1, A and B), demonstrating the NO-dependence of the EDV responses. Strikingly, this increased endothelial vasodilator responsiveness in tissues from the antibiotic-treated mice was reversed by supplementing animals with the microbial metabolite IPA, resulting in a decrease in maximal EDV (open triangles/dotted lines; Fig. 1, A and B). Similarly, supplementing antibiotic-naïve mice with IPA also reduced the sensitivity of the vasculature to the vasodilator actions of ACh and 2fLI (open squares compared with black squares in Fig. 1, C and D). Comparable data were obtained using pulmonary artery rings (Fig. 2). The first- and second-order pulmonary artery branches showed comparable eNOS-dependent EDV responses to muscarinic and PAR2 activation (data combined for all tissues; open circles in Fig. 2, A and B) that were enhanced in tissues from the antibiotic-treated, IPA-depleted mice (black circles, Fig. 2, A and B) and diminished upon supplementing mice with IPA in vivo (black triangles and black squares, Fig. 2, A and B). Thus, the data obtained for the pulmonary artery tissue reflected closely the data obtained for aorta-derived tissues.
Fig. 1.
Broad-spectrum antibiotic treatment sensitizes aorta tissue to agonist-stimulated, endothelium-mediated vasodilation, an effect that can be reversed by administration of indole 3-propionic acid (IPA) in vivo. Concentration-effect curves were obtained for acetylcholine- (Ach; A) and 2-furoyl-Leu-Ile-Gly-Arg-Leu-Orn-amide (2-fLi; B)-induced vasodilation in aorta rings from pregnane X receptor (Pxr)+/+ mice either treated (○) or not (■) with broad-spectrum antibiotics. Antibiotic-treated mice were either supplemented (△) or not (○) with IPA. In all tissues, agonist-stimulated vasodilation was eliminated in the combined presence of Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME) and 1H-[1,2,4]oxadiazolo[4,3,-a] quinoxalin-1-one (ODQ) (▲). C and D: dietary supplementation of microbiome-intact/antibiotic-naive Pxr+/+ animals with IPA (+IPA; □) reduced the vasodilator action of ACh and 2fLI (concentration-effect curves; □) compared with their action in non-IPA-treated mice (■). Data points represent the means ± SE (bars); n = 6 for each group. Two-way ANOVA was used for evaluating the statistical difference between concentration-response curves. *P < 0.05 for differences between tissues from antibiotic-treated vs. nontreated animals (A and B) or for IPA-supplemented vs. nonsupplemented animals (C and D).
Fig. 2.
Broad-spectrum antibiotic treatment sensitizes pulmonary artery tissue to agonist-stimulated endothelium-mediated vasodilation, an effect that can be reversed by administration of indole 3-propionic acid (IPA) in vivo. Concentration-effect curves for acetylcholine- (Ach; A) and 2-furoyl-Leu-Ile-Gly-Arg-Leu-Orn-amide (2-fLi; B)-induced vasodilation were determined in pulmonary vessel rings from pregnane X receptor (Pxr)+/+ mice either treated (■) or not (○) with broad-spectrum antibiotics. Data were pooled from vascular tissues obtained from both 1st- and 2nd-order pulmonary arterial vessels, which did not differ in their responsiveness (not shown). Both untreated and antibiotic-treated Pxr+/+ mice were also supplemented with IPA in vivo (■, untreated + IPA; ▲, antibiotic-treated + IPA). Data points represent means ± SE (bars); n = 6 for each group. Two-way ANOVA was used for evaluating the statistical difference between concentration-response curves. *P < 0.05 for differences between tissues from antibiotic treated vs. antibiotic with IPA (A and B) or for IPA-supplemented.
IPA supplementation can increase systemic IPA levels and induces PXR expression.
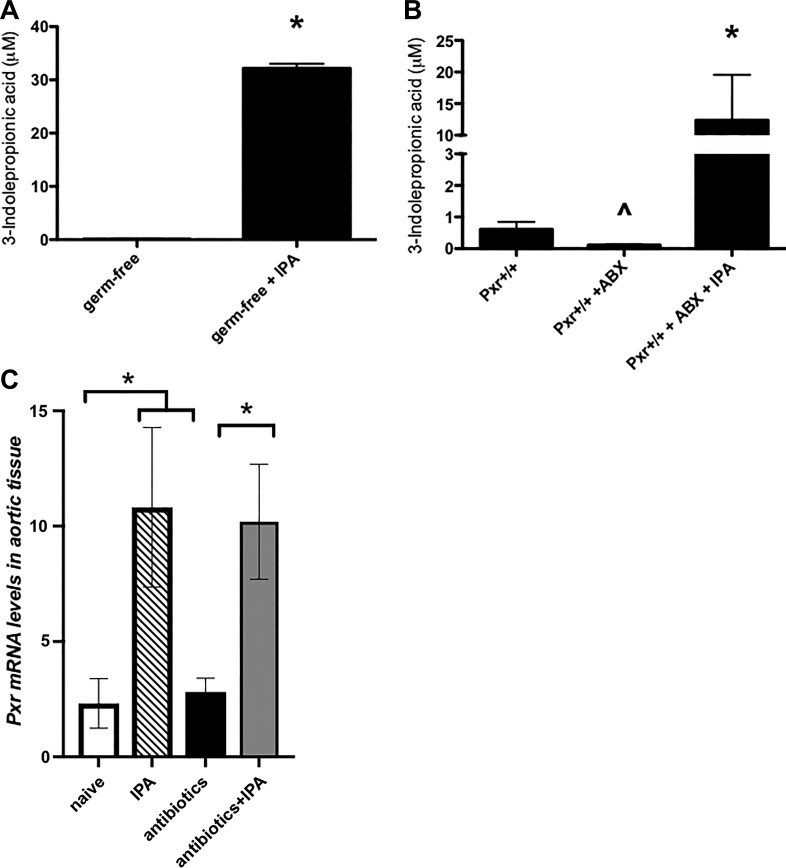
The intestinal microbiota is known to contribute to systemic levels of IPA (3, 12). Given the striking effects of IPA supplementation in our vascular studies, we sought to quantify the serum concentrations of IPA in the mice used in our experiments. Indeed, germ-free mice had undetectable levels of systemic IPA, which could be significantly increased by IPA supplementation in the drinking water (Fig. 3A). Furthermore, antibiotic-treatment significantly reduced the levels of serum IPA in Pxr+/+ mice, an effect that was reversed by IPA supplementation in the drinking water (Fig. 3B, histogram on far right).
Fig. 3.
Indole 3-propionic acid (IPA) supplementation in the drinking water increases systemic IPA levels in germ-free and antibiotic-treated mice. A: plasma from germ-free mice contains negligible levels of IPA (left histogram) that are significantly increased by IPA supplementation in the drinking water (right histogram; 200 mg/l for 2 wk). Histograms are presented as means ± SEM (bars); n = 4–5 for each group; *P < 0.05, Student’s t-test.” B: antibiotic (ABX) treatment of pregnane X receptor (Pxr)+/+ mice results in a significant reduction of serum IPA (middle histogram), which can be significantly increased by IPA supplementation in the drinking water (right histogram; 200 mg/l for the 2-wk duration of antibiotic treatment). Histograms represent means ± SE (bars); n = 6. *P < 0.05 for IPA-supplemented vs. nonsupplemented mice; ∧P < 0.05 for Pxr+/+ + ABX vs. non-ABX-treated Pxr+/+ mice. C: IPA supplementation, but not antibiotic treatment, increases the expression of Pxr mRNA in aortic segments. Quantitative PCR analysis was performed for aortic segments obtained from naïve or antibiotic-treated Pxr+/+ mice treated or not with IPA (200 mg/l for the 2-wk duration of antibiotic treatment). Abundance of Pxr mRNA level was determined relative to β-actin mRNA in each sample. Histograms are presented as means ± SE (bars); n = 4–5 for each group. *P < 0.05, Student’s t-test.
To test the hypothesis that the antibiotic treatment altered the expression of the PXR in vascular tissue, Pxr transcript levels were measured by qPCR in the aorta. While antibiotic treatment had no effect on Pxr expression, IPA supplementation of naïve and antibiotic-treated mice increased its expression (Fig. 3C).
Treating isolated aorta rings with IPA in vitro diminishes EDV in response to ACh and 2fLI.
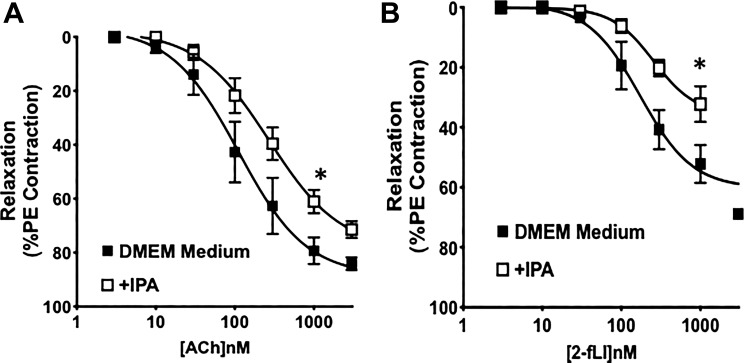
To determine whether the effect on endothelial EDV responses of supplementing the antibiotic-treated mice with IPA in vivo was an indirect or direct effect of IPA on the vascular tissue, we employed an approach described by us previously, using organ cultures of mouse aortic rings to evaluate changes in agonist-mediated EDV responses (5). We cultured isolated aorta rings from the Pxr+/+ mice for 24 h in serum-free DMEM-5 mM glucose in the presence or absence of 0.1 μM IPA and then mounted the rings in the myograph to evaluate EDV in response to ACh and 2fLI. As shown in Fig. 4, A and B (open compared with black symbols), the presence of IPA in the culture medium decreased the EDV responses to both muscarinic and PAR2 activation. This result mimicked the data obtained with the tissues obtained from Pxr+/+ mice (antibiotic treated or not), which had been supplemented or not with IPA. These data suggest that IPA can have a direct effect on the vascular tissues to modify their EDV responses.
Fig. 4.
Organ culture of aorta tissues with indole 3-propionic acid (IPA) alters agonist-stimulated endothelium-mediated vasodilation. Concentration-effect curves were obtained for acetylcholine- (Ach; A) and 2-furoyl-Leu-Ile-Gly-Arg-Leu-Orn-amide (2-fLi; B)-induced vasodilation in aorta rings from pregnane X receptor (Pxr)+/+ mice cultured in in DMEM-5 mM glucose medium for 24 h in the absence or presence of 0.1 μM IPA. IPA treatment reduced the tissue sensitivity to the vasodilator actions of ACh and 2fLI (concentration-effect curves; ■) compared with their actions in untreated tissues (concentration-effect curves; □). Data points represent means ± SE (bars); n = 6 for each group. Two-way ANOVA was used for evaluating the statistical difference between concentration-response curves. *P < 0.05 for IPA-treated tissues vs. nontreated tissues.
Supplementation of germ-free mice with IPA diminishes EDV in response to ACh and 2fLI.
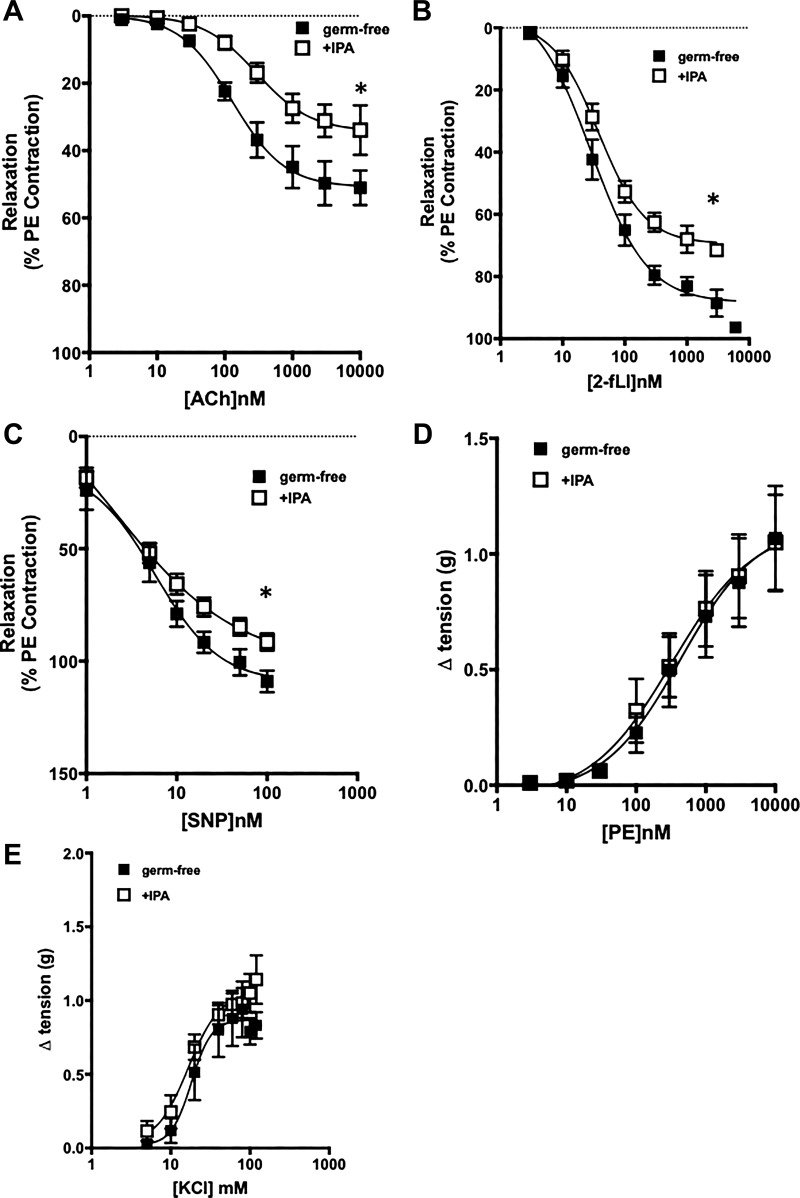
We used germ-free mice, supplemented or not with IPA, to confirm our findings with tissues from the antibiotic-treated microbiome-intact Pxr+/+ mice, implicating the ability of IPA to affect vascular function. Vascular tissue obtained from germ-free animals displayed sensitivities toward ACh and 2fLI (EC50 values) comparable with those of the microbiome-colonized animals that had been treated with antibiotics (open circles in Fig. 1, A and B compared with black squares in Fig. 5, A and B). However, the maximum vasodilator response was smaller for muscarinic receptor activation than for PAR2. Thus, the EC50 values for both agonists to cause vasodilation were in the range of 50 to 100 nM for tissues from the germ-free and antibiotic-treated mice. When the germ-free mice were supplemented with IPA in vivo, the maximal relaxant actions of both ACh and 2fLI were significantly diminished (open compared with black squares in Fig. 5, A and B). This result mirrored the decrease in the maximum vasodilator response to ACh and 2fLI in the vascular tissues obtained from the antibiotic-treated mice that had been supplemented in vivo with IPA (open triangles compared with open circles in Fig. 1, A and B). The reduced responsiveness of the IPA-supplemented germ-free mouse vessels to muscarinic and PAR2 stimulation was in parallel with the reduced sensitivity of the vessels to the vasodilatory action of the NO donor sodium nitroprusside (SNP; Fig. 5C). Nonetheless, the tissues obtained from the germ-free mice, supplemented or not with IPA, showed the same vasoconstrictor responses either to phenylephrine activation of vascular α-adrenoceptors or to depolarization by KCl (Fig. 5, D and E). Thus, the main impact of supplementing the germ-free mice with IPA was to normalize agonist-mediated EDV.
Fig. 5.
Indole 3-propionic acid (IPA) supplementation in vivo reduces vasodilatory responses in germ-free mice. Concentration-response curves were measured for the vasodilator actions of acetylcholine (Ach; A) and 2-furoyl-Leu-Ile-Gly-Arg-Leu-Orn-amide (2-fLi; B) in aorta rings derived from germ-free mice either supplemented (□) or not (■) with IPA in the drinking water. C: vasodilator responses to the nitric oxide donor sodium nitroprusside (SNP) were measured for aorta rings obtained from germ-free mice either supplemented (□) or not (■) with IPA. D and E: vasoconstrictor concentration-response curves were measured for either phenylephrine (PE; D) or KCl (E) for aorta rings obtained from germ-free mice either supplemented (□) or not (■) with IPA. Data represent means ± SE (bars); n = 6 for each group. Two-way ANOVA was used for comparing concentration-response curves, for which P < 0.05 for differences between the curves (□ and ■ in A–C). Curves in D and E are not statistically different. *P < 0.05 for IPA-treated vs. germ-free untreated tissues.
The absence of PXR changes the contractile action of phenylephrine and the vasodilatory effect of an NO donor (SNP).
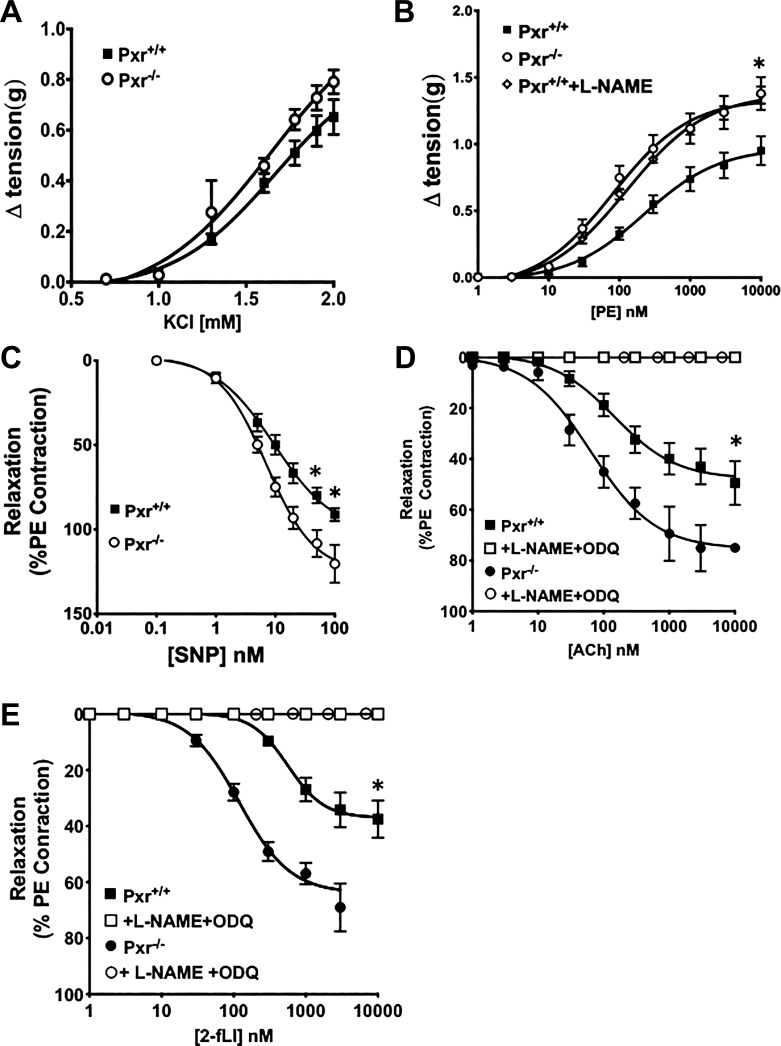
Because the PXR is known to be involved in the regulation of vascular tone during pregnancy (6), and because IPA is known to activate PXR (3, 12, 16), the potential role of PXR in mediating the impact of IPA on vascular EDV was evaluated. To this end, experiments were done with aorta tissues derived from Pxr−/− mice for comparison with Pxr+/+ littermate mice. To assess the impact of the absence of PXR on vascular endothelial function, it was first essential to measure the contractile and relaxant properties of vascular smooth muscle in aorta rings obtained from Pxr−/− animals in comparison with tissues from Pxr+/+ animals. The contractile effect of KCl-mediated tissue depolarization was comparable in aorta rings derived from either Pxr−/− or Pxr+/+ mice (Fig. 6A). Despite the equivalent contractile response to smooth muscle depolarization with KCl in tissues from the Pxr−/− or Pxr+/+ mice, the contractile action of PE was enhanced in endothelium-intact aorta rings from the Pxr−/− mice, compared with tissues from Pxr+/+ animals (open circles, enhanced, compared with Pxr+/+ black squares in Fig. 6B). However, when endothelial NO production was eliminated by treating the Pxr+/+ tissues with l-NAME, there was no difference in the PE contractile concentration-effect curves for either the Pxr+/+ or Pxr−/− tissues (open diamonds compared with open circles in Fig. 6B). The addition of l-NAME to the organ bath did not affect the contractile actions of PE in the Pxr−/− tissues (data not shown). Thus, differences in the contractile actions of PE in the Pxr+/+ tissues, compared with the Pxr−/− tissues, could be attributed to the action eNOS, triggered by PE in the Pxr+/+, but not in the Pxr−/− tissues.
Fig. 6.
Vasoconstrictor and vasodilator responses of aorta tissue from pregnane X receptor (Pxr)−/− mice differ from those observed for tissues from Pxr+/+ mice. Concentration-response curves were obtained for the constrictor actions of either KCl (A) or phenylephrine (PE; B) for aorta rings obtained from either Pxr+/+ (■) or Pxr−/− (□) mice. The response of the Pxr+/+ tissues to PE was measured in the absence (■; B) or presence (◇; B) of 0.1 mM l-NAME. C–E: vasodilator concentration-response curves were measured for rings derived from either Pxr+/+ or Pxr−/− mice for the nitric oxide donor sodium nitroprusside (SNP; C) and for the endothelium-dependent vasodilator (EDV) agonists, acetylcholine (Ach; D), and 2-furoyl-Leu-Ile-Gly-Arg-Leu-Orn-amide (2fLI; E). The vasodilator actions of ACh and 2fLI were measured in the absence (■ and ●; D and E) or presence (□ and ○; D and E) of Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME) + 1H-[1,2,4]oxadiazolo[4,3,-a] quinoxalin-1-one (ODQ). Data points represent means ± SE (bars); n = 6 for each group. Two-way ANOVA was used to compare the concentration response curves. In B–E, *P < 0.05 for differences between the concentration-response curves for tissues from Pxr+/+ compared with Pxr−/− mice.
Because the relaxations of the aorta-derived vascular rings in response to endothelial cell activation are driven mainly by the release of NO generated by eNOS, we next sought to assess the sensitivity of the Pxr+/+ and Pxr−/− tissues to the relaxant action of the NO donor sodium nitroprusside (SNP). As shown in Fig. 6C, the tissues from the Pxr−/− animals were more sensitive to the relaxant action of SNP compared with Pxr+/+ tissues. Thus, the properties of the aorta tissue from the Pxr−/− animals differed from the Pxr+/+ tissue in terms of both reduced PE-induced contractility due to the lack of PE-mediated NO release from the endothelium in the Pxr−/− mice and in terms of an increased sensitivity to SNP-induced relaxation.
Given that the tissues from the Pxr−/− mice were more sensitive to the NO donor SNP, we next evaluated the reactivity of their aorta tissues to the vasodilatory actions of muscarinic and PAR2 receptor activation. The vasodilator response to both of these agonists depends on the release of NO from the endothelium. As shown in Fig. 6, D and E, aorta tissues from the Pxr−/− mice that were constricted to the same tension as Pxr+/+ tissues were much more sensitive to the maximum vasodilatory actions of ACh and 2fLI (solid circles, more sensitive, compared with solid squares in Fig. 6, D and E). Of importance for both sets of tissues is that the vasodilation responses were eliminated by the actions of l-NAME combined with ODQ, which together completely block the synthesis/release and action of endothelium-derived NO (open squares and open circles in Fig. 6, D and E). Taken together, the data indicate that the presence of PXR leads to a decreased agonist-induced vasodilatory response, most likely due to a decreased release of endothelium-derived NO that then acts on tissues that exhibit a lower NO sensitivity compared with Pxr−/− tissues.
The absence of Pxr prevents the effect of IPA supplementation to diminish the EDV responses to ACh and 2fLI.
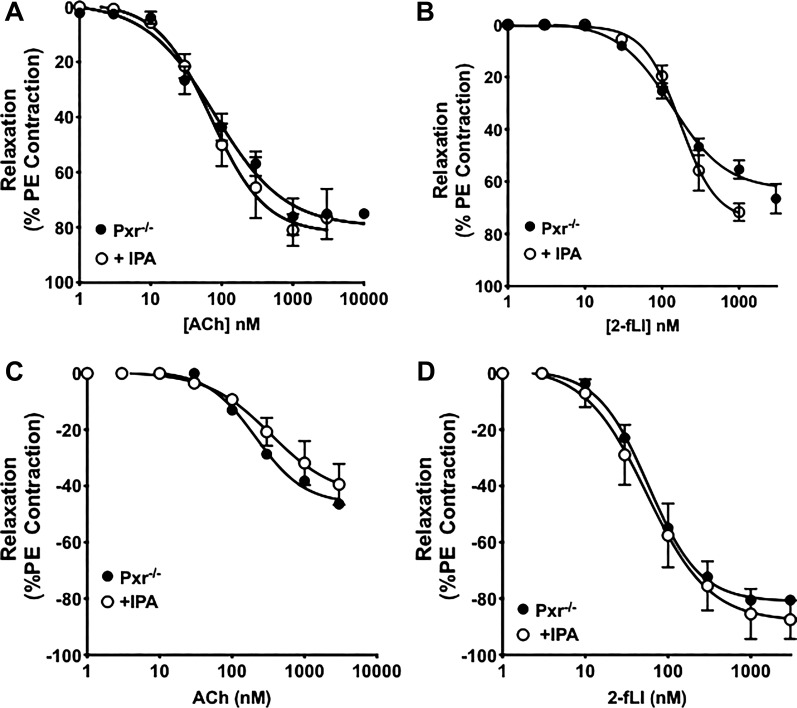
Because IPA supplementation was able to decrease agonist-mediated EDV in aorta and pulmonary artery tissue obtained from antibiotic-treated mice and was also able to able to diminish EDV in antibiotic-naïve and germ-free mice, the impact of IPA supplementation on agonist-mediated EDV was evaluated in the Pxr−/− mice. As shown in Fig. 7, A and B for aorta and in Fig. 7, C and D for pulmonary artery tissue, IPA supplementation in vivo was not able to change the sensitivity of agonist-mediated vasodilation in the tissues derived from the Pxr−/− mice. This result was in contrast with the effect of IPA supplementation to decrease agonist action in tissues from the Pxr+/+ mice (Figs. 1, A and B, and 2, A and B). Thus, Pxr was required for the vascular effect of IPA in vivo in both aorta and pulmonary arterial tissue.
Fig. 7.
Indole 3-propionic acid (IPA) supplementation in vivo has no effect on agonist-stimulated endothelium-mediated vasodilation in aorta and pulmonary arteries in pregnane X receptor (Pxr)−/− mice. Aortic (A and B) and pulmonary artery tissues (C and D) from Pxr−/− mice treated with IPA in their drinking water (○) do not display any alterations in vasodilation at agonist concentrations <1 μM compared with the control non-IPA treated mice (●). Data points are presented as means ± SE (bars); n = 6 for each group. Two-way ANOVA was used to compare concentration-response curves for tissues derived from IPA-supplemented vs. untreated mice, which were not significantly different.
Pxr negatively modulates eNOS expression.
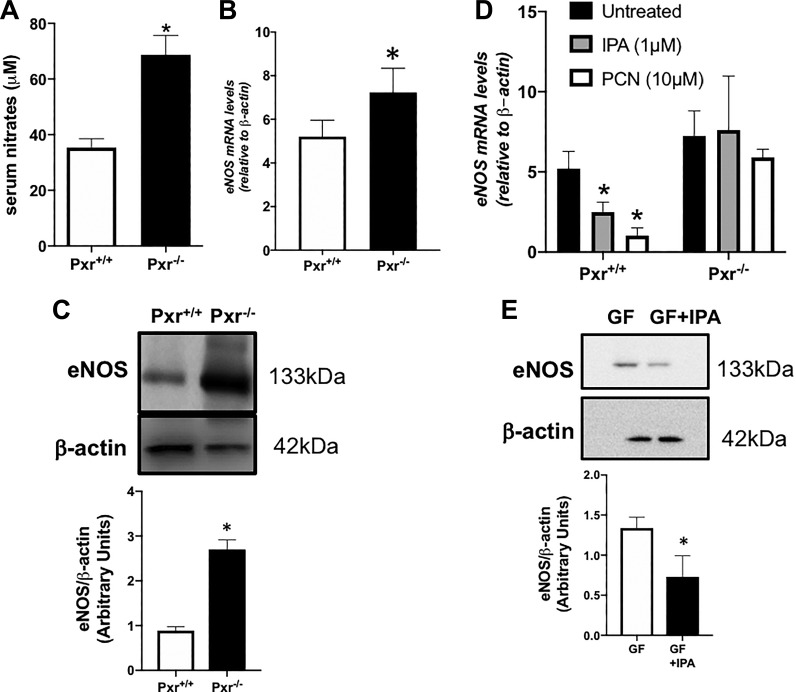
The enhanced vasodilatory actions of ACh and 2fLI in the Pxr−/− tissues, which were blocked by l-NAME, suggested that an interaction between the PXR and eNOS might play a role in vascular responsiveness. This possibility was assessed on two levels. 1) Serum levels of nitrates, indicative of systemic NO release, were measured in the Pxr+/+ and Pxr−/− mice, and 2) eNOS abundance in the vascular tissues from the Pxr+/+ and Pxr−/− mice was measured using qPCR and Western blot approaches. Interestingly, serum nitrates were significantly higher in Pxr−/− mice compared with their Pxr+/+ littermate counterparts (Fig. 8A). This result was associated with an increased expression of eNOS mRNA (Fig. 8B) and protein (Fig. 8C) in Pxr−/− mice compared with their Pxr+/+ littermate counterparts.
Fig. 8.
Serum nitrates and endothelia nitric oxide synthase (eNOS) abundance are increased in pregnane X receptor (Pxr)−/− mice, whereas indole 3-propionic acid (IPA) and the PXR negatively regulate eNOS expression. Serum nitrates (A) and eNOS expression [mRNA (B) and protein (C)] were increased in aortic tissues isolated from Pxr−/− mice. For eNOS expression, the data are expressed relative to β-actin in each sample as quantified by quantitative PCR or Western blot densitometry (histograms below the representative gels). Data points are presented as means ± SE (bars); n = 6 for each group, Student’s t-test was used to compare Pxr+/+ and Pxr−/−. D: eNOS mRNA abundance was assessed in cultured primary aorta-derived endothelial cells isolated from Pxr+/+ and Pxr−/− mice treated with 2 PXR agonists [IPA − 1 μM, pregnenolone 16α-carbonitrile (PCN) − 10 μM] for 24 h. The abundance of eNOS is expressed relative to β-actin in each sample. Histograms are presented as means ± SE (bars); n = 4 for each group. E: Western blot analysis of eNOS abundance was done for aorta tissue from germ-free (GF) mice either supplemented (GF + IPA) or not (GF) with IPA. The abundance of eNOS relative to β-actin in each sample was quantified by densitometry (histograms below the gels, representative of 4 independently conducted analyses). Histograms are presented as means ± SE (bars); n = 4 for each group. *P < 0.05, Student’s t-test.
To determine whether the PXR could directly modulate the expression of eNOS in endothelial cells, cultured aorta-derived endothelial cells isolated from Pxr−/− and Pxr+/+ mice were incubated with PXR agonists (IPA or PCN). As shown in Fig. 8D, both PXR agonists lowered eNOS mRNA levels in the Pxr+/+ cells, but not in the Pxr−/− cells, demonstrating that PXR activation reduces endothelial eNOS expression directly. Furthermore, treating germ-free mice with IPA also significantly reduced eNOS protein expression in aorta tissues (Fig. 8E).
In summary, relative to the Pxr+/+ vessels, the Pxr−/− tissues showed an increased vasodilator response to endothelial agonist activation and an increased sensitivity to the relaxant effect of an NO donor along with an increased abundance of tissue and endothelial cell eNOS. This increase in eNOS in the Pxr−/− tissues relative to the Pxr+/+ littermate controls occurred in concert with an increase in serum nitrite levels, indicating a greater production of NO in Pxr−/− mice. Thus, our data indicate that the presence of PXR and its activation negatively regulate the expression of eNOS and dampens the vasodilator responses to ACh and 2fLI through controlling the agonist-induced production of NO. The data obtained with tissues from the germ-free mice indicated that IPA, a microbial metabolite that can activate PXR, can normalize aberrant vasodilatory responses and concurrently reduce eNOS abundance in vascular tissue. Furthermore, IPA can exhibit a direct effect on tissues, as demonstrated by our tissue organ culture and endothelial cell culture experiments.
DISCUSSION
The main finding of our study was that the PXR, in conjunction with its agonist IPA, a recognized microbiome-derived bacterial metabolite, can regulate aorta and pulmonary vascular vasodilatory function. This regulation is due mainly to changes in eNOS-generated NO production that are suppressed by the IPA-mediated activation of the PXR. Our data are consistent with literature indicating that the intestinal microbiome can play a role in the regulation of cardiovascular function, including blood pressure (7). Although the epidemiological association between aberrant endothelial responses, hypertension, and alterations in the composition of the microbiota has been reported, very few pharmacological mechanisms have been identified. To our knowledge, our data represent the first direct link between a microbiome-derived metabolite known to be altered by antibiotic treatment (IPA) and its corresponding cell signaling target. This link between IPA and the vascular PXR is supported by three key observations. 1) The endothelium-mediated aorta and pulmonary vasodilator responses of tissues from Pxr−/− mice differ substantially from tissues from the Pxr+/+ littermate control animals, demonstrating a role for this receptor in affecting aorta and pulmonary vascular function; 2) reducing the plasma levels of IPA by intensive antibiotic-induced alteration of the gut microbiota is associated with an increase in the vascular responsiveness of the Pxr+/+ tissues that mimics the responsiveness of Pxr−/− tissues; and 3) supplementing either the antibiotic-treated Pxr+/+ animals or germ-free animals with IPA reduces the vascular vasodilator responsiveness caused by ACh and 2fLI toward the levels observed for tissues from Pxr+/+ antibiotic-naive animals. In contrast, IPA supplementation has no effect on the vasodilator responses in the Pxr−/− mice. The data are consistent with the increased expression of eNOS in either the Pxr−/− mice or the germ-free mice lacking the PXR agonist IPA. Moreover, this effect of IPA can be attributed to a direct action on the vascular tissue, since treating the aorta rings or cultured endothelial cells in vitro with IPA mimicked the action of IPA supplementation of mice in vivo.
Therefore, our data reveal a new vascular role for a microbial metabolite that can activate the PXR, a nuclear receptor that has been well characterized as a xenobiotic sensor highly expressed within the liver and intestine. The results obtained for the IPA-mediated, PXR-dependent decreases in agonist-stimulated vasodilation of the aorta tissue can be compared directly with the PXR-dependent increases in mouse mesenteric artery vasodilation caused by progesterone metabolites (6). These apparently diametrically opposed effects of PXR activation on vascular vasodilator function can be linked to two main differences in the targets of PXR-mediated gene transcription: First, in the work described herein, the PXR-mediated downregulation of aorta endothelium-produced eNOS appears to be a major factor to cause decreased vasodilation, whereas the progesterone-triggered PXR-mediated increase in mesenteric artery vasodilator response can be attributed to a PXR-stimulated increase in cytochrome P450 epoxygenase activity. The P450 metabolite of progesterone in turn functions as a vasodilator-inducing and endothelium-derived hyperpolarizing factor (6). The ability of the PXR to regulate the expression of genes like those of the cytochrome P450 family, which is associated with epoxide syntheses as well as drug detoxification and elimination, is well recognized (1). Second, the PXR-mediated increase in arterial vasodilator response caused by 5-β-dihydroprogesterone (6) was observed in mesenteric artery preparations, which are regulated by endothelium-derived hyperpolarizing factors (EDHFs) as well as by NO. In contrast, the aorta and pulmonary artery tissues are regulated by NO alone and not by EDHFs. Thus, the effects of PXR activation on vascular function can be seen to be diverse, depending on the arterial target, the PXR agonist, and the vascular tissue in which the receptor is activated. Of note is that endothelial PXR can also be activated by shear stress to induce gene expression and enhance drug metabolism (19). Thus, as a systemic vascular “sensor,” PXR activation would be able to integrate signals from a variety of sources, including steroid hormone metabolites, ingested xenobiotics (15), vascular flow, and, as shown by the new data described herein, microbiome metabolites. Given the diverse ways in which the endothelium regulates vasodilation (e.g., mainly eNOS in conduit vessels like aorta and pulmonary versus mainly EDHF in resistance vessels like the mesentery), it would be a challenge to interpret the impact of IPA on systemic blood pressure, but an effect on turbulent flow as in the process of atherogenesis at arterial bifurcation points can be predicted.
Our findings bear on the routine clinical use of high-dose antibiotics in humans in settings like the treatment of septic individuals or as a preoperative regimen for abdominal surgery. Furthermore, an unbiased serum metabolomics analysis has revealed a link between low circulating IPA levels and an increased risk of developing type-2 diabetes (2). Because our data clearly link IPA to vascular function, it may be of value in certain circumstances to supplement individuals with IPA or, depending on the setting (e.g., in shock, where mesenteric vasodilation would be detrimental), alternatively, to administer a PXR antagonist.
Although we have yet to identify the exact mechanism through which the absence of PXR increases agonist-induced, endothelial-dependent vasodilation, our functional and biochemical data, in combination with published studies, provide some insight. The vasodilator responses stimulated in the aorta tissue from both the Pxr+/+ and Pxr−/− mice were completely abolished by inhibiting both NO production and soluble guanylyl cyclase activity. That increased serum nitrate levels were observed in Pxr−/− mice points to an increased capacity to generate NO systemically, so as to account for the increased endothelium-mediated vasodilator responses in the Pxr−/− animals. This hypothesis is supported further by the increased expression of eNOS protein in the Pxr−/− relative to the Pxr+/+ tissues. Although as yet there is no reported link between the PXR and eNOS expression or function, the regulation of iNOS mRNA by xenobiotics has been attributed to a PXR response element composed of a direct repeat of two GGTTCA motifs at a distance of four nucleotides (DR4) (15). Of note, the DR4-type response element specifically mediates either the downregulation of iNOS promoter activity by the testosterone metabolite androstanol through constitutive androstane receptor (CAR)-retinoid X receptor (RXR) heterodimers or the upregulation of iNOS mRNA by the xenobiotic drug clotrimazole through PXR-RXR heterodimers. We suggest that eNOS is similarly downregulated via the IPA-mediated activation of a PXR-RXR negative response element mechanism, so as to enable an upregulation of eNOS in the Pxr−/− mice. Our continuing work is focused on exploring this mechanism for IPA and other microbiome-derived metabolites. As is the case for iNOS, it is possible that distinct microbiome metabolites may have a differential impact on the regulation of eNOS, as for the opposing actions of androstanol and clotrimazole, which via the PXR-RXR heterodimer can cause either a downregulation or upregulation of iNOS (15). Therefore, the microbiome metabolite-mediated regulation of endothelial eNOS and possibly brain expression via the PXR, and possibly the constitutive androstane receptor, in tissues other than the vasculature merits further attention.
In summary, the data described herein strongly support a novel role for the microbiome-derived metabolite IPA, via the PXR, to regulate agonist-induced, endothelium-dependent relaxations in conduit vessels like the aorta and pulmonary arteries. It remains to be seen whether IPA, via the PXR, will differentially affect resistance vessels to increase rather than decrease their vasodilator responses, as is the case for progesterone metabolites (18). Therefore, our data provide functional insight into mechanisms whereby microbial-derived metabolites can affect vascular function, so as to provide a stimulus for assessing the impact of IPA and other microbiome metabolites on tissue function in settings, including distinct vascular tissues and tissue sites other than the vasculature.
GRANTS
We also acknowledge the Stable Isotope and Metabolomics Core Facility of the Diabetes Research and Training Center of the Albert Einstein College of Medicine for IPA measurements (supported by National Institutes of Health/National Cancer Institute Grant P60-DK-020541).
This work was also funded by the Dr. Lloyd Sutherland Investigatorship in IBD/GI Research (S. A. Hirota) and the Canadian Institutes of Health Research (M. D. Hollenberg). S. Mani’s laboratory is supported by National Institutes of Health Grants CA161879 and CA222469, the Department of Defense (W81XWH-17-1-0479), and a Broad Medical Research Program-Crohn’s & Colitis Foundation (CCFA) Investigator Award (Proposal No. 262520). The International Microbiome Centre is supported by the Cumming School of Medicine, University of Calgary, Western Economic Diversification and Alberta Economic Development and Trade (Canada).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
V.K.P.V., K.M., Y.-C.T., K.N., L.A., S.M., K.D.M., M.D.H., and S.A.H. conceived and designed research; V.K.P.V., M.S., K.M., Y.-C.T., K.N., L.A., and S.A.H. performed experiments; V.K.P.V., M.S., K.M., Y.-C.T., K.D.M., M.D.H., and S.A.H. analyzed data; V.K.P.V., M.S., K.M., Y.-C.T., K.D.M., M.D.H., and S.A.H. interpreted results of experiments; V.K.P.V., M.D.H., and S.A.H. prepared figures; V.K.P.V., K.D.M., M.D.H., and S.A.H. drafted manuscript; V.K.P.V., S.M., K.D.M., M.D.H., and S.A.H. edited and revised manuscript; V.K.P.V., M.S., K.M., Y.-C.T., K.N., L.A., S.M., K.D.M., M.D.H., and S.A.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank J. Staudinger (University of Kansas, Lawrence, KS) for providing Pxr−/− (Nr1i2−/−) mice. We acknowledge the essential assistance of the staff of the International Microbiome Centre at the University of Calgary for assistance with germ-free mice.
REFERENCES
- 1.Chai X, Zeng S, Xie W. Nuclear receptors PXR and CAR: implications for drug metabolism regulation, pharmacogenomics and beyond. Expert Opin Drug Metab Toxicol 9: 253–266, 2013. doi: 10.1517/17425255.2013.754010. [DOI] [PubMed] [Google Scholar]
- 2.de Mello VD, Paananen J, Lindström J, Lankinen MA, Shi L, Kuusisto J, Pihlajamäki J, Auriola S, Lehtonen M, Rolandsson O, Bergdahl IA, Nordin E, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Landberg R, Eriksson JG, Tuomilehto J, Hanhineva K, Uusitupa M. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci Rep 7: 46337, 2017. doi: 10.1038/srep46337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK, Le A, Cowan TM, Nolan GP, Fischbach MA, Sonnenburg JL. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 551: 648–652, 2017. doi: 10.1038/nature24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Daly M, Pulakazhi Venu VK, Saifeddine M, Mihara K, Kang S, Fedak PWM, Alston LA, Hirota SA, Ding H, Triggle CR, Hollenberg MD. Hyperglycaemic impairment of PAR2-mediated vasodilation: Prevention by inhibition of aortic endothelial sodium-glucose-co-Transporter-2 and minimizing oxidative stress. Vascul Pharmacol 109: 56–71, 2018. doi: 10.1016/j.vph.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Hagedorn KA, Cooke CL, Falck JR, Mitchell BF, Davidge ST. Regulation of vascular tone during pregnancy: a novel role for the pregnane X receptor. Hypertension 49: 328–333, 2007. doi: 10.1161/01.HYP.0000253478.51950.27. [DOI] [PubMed] [Google Scholar]
- 7.Marques FZ, Mackay CR, Kaye DM. Beyond gut feelings: how the gut microbiota regulates blood pressure. Nat Rev Cardiol 15: 20–32, 2018. doi: 10.1038/nrcardio.2017.120. [DOI] [PubMed] [Google Scholar]
- 8.Pulakazhi Venu VK, Saifeddine M, Mihara K, El-Daly M, Belke D, Dean JLE, O’Brien ER, Hirota SA, Hollenberg MD. Heat shock protein-27 and sex-selective regulation of muscarinic and proteinase-activated receptor 2-mediated vasodilatation: differential sensitivity to endothelial NOS inhibition. Br J Pharmacol 175: 2063–2076, 2018. doi: 10.1111/bph.14200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pulakazhi Venu VK, Uboldi P, Dhyani A, Patrini A, Baetta R, Ferri N, Corsini A, Muro AF, Catapano AL, Norata GD. Fibronectin extra domain A stabilises atherosclerotic plaques in apolipoprotein E and in LDL-receptor-deficient mice. Thromb Haemost 114: 186–197, 2015. doi: 10.1160/TH14-09-0790. [DOI] [PubMed] [Google Scholar]
- 10.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118: 229–241, 2004. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Ramachandran R, Noorbakhsh F, Defea K, Hollenberg MD. Targeting proteinase-activated receptors: therapeutic potential and challenges. Nat Rev Drug Discov 11: 69–86, 2012. doi: 10.1038/nrd3615. [DOI] [PubMed] [Google Scholar]
- 12.Roager HM, Licht TR. Microbial tryptophan catabolites in health and disease. Nat Commun 9: 3294, 2018. doi: 10.1038/s41467-018-05470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saifeddine M, El-Daly M, Mihara K, Bunnett NW, McIntyre P, Altier C, Hollenberg MD, Ramachandran R. GPCR-mediated EGF receptor transactivation regulates TRPV4 action in the vasculature. Br J Pharmacol 172: 2493–2506, 2015. doi: 10.1111/bph.13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staudinger J, Liu Y, Madan A, Habeebu S, Klaassen CD. Coordinate regulation of xenobiotic and bile acid homeostasis by pregnane X receptor. Drug Metab Dispos 29: 1467–1472, 2001. [PubMed] [Google Scholar]
- 15.Toell A, Kröncke KD, Kleinert H, Carlberg C. Orphan nuclear receptor binding site in the human inducible nitric oxide synthase promoter mediates responsiveness to steroid and xenobiotic ligands. J Cell Biochem 85: 72–82, 2002. doi: 10.1002/jcb.10104. [DOI] [PubMed] [Google Scholar]
- 16.Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP, Qiu Z, Maher L, Redinbo MR, Phillips RS, Fleet JC, Kortagere S, Mukherjee P, Fasano A, Le Ven J, Nicholson JK, Dumas ME, Khanna KM, Mani S. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 41: 296–310, 2014. doi: 10.1016/j.immuni.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang S, Lei T, Zhang K, Zhao W, Fang L, Lai B, Han J, Xiao L, Wang N. Xenobiotic pregnane X receptor (PXR) regulates innate immunity via activation of NLRP3 inflammasome in vascular endothelial cells. J Biol Chem 289: 30075–30081, 2014. doi: 10.1074/jbc.M114.578781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S, Xie X, Lei T, Zhang K, Lai B, Zhang Z, Guan Y, Mao G, Xiao L, Wang N. Statins attenuate activation of the NLRP3 inflammasome by oxidized LDL or TNFα in vascular endothelial cells through a PXR-dependent mechanism. Mol Pharmacol 92: 256–264, 2017. doi: 10.1124/mol.116.108100. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Fang X, Zhou J, Chen Z, Zhao B, Xiao L, Liu A, Li YS, Shyy JY, Guan Y, Chien S, Wang N. Shear stress activation of nuclear receptor PXR in endothelial detoxification. Proc Natl Acad Sci USA 110: 13174–13179, 2013. doi: 10.1073/pnas.1312065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu H, Chen Z, Ma Z, Tan H, Xiao C, Tang X, Zhang B, Wang Y, Gao Y. Tanshinone IIA protects endothelial cells from H2O2-induced injuries via PXR activation. Biomol Ther (Seoul) 25: 599–608, 2017. doi: 10.4062/biomolther.2016.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WHW, DiDonato JA, Brown JM, Lusis AJ, Hazen SL. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 165: 111–124, 2016. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]