Abstract
Hydrogen sulfide (H2S) attenuates N-methyl-d-aspartate receptor-R1 (NMDA-R1) and mitigates diabetic renal damage; however, the molecular mechanism is not well known. Whereas NMDA-R1 facilitates Ca2+ permeability, H2S is known to inhibit L-type Ca2+ channel. High Ca2+ activates cyclophilin D (CypD), a gatekeeper protein of mitochondrial permeability transition pore (MPTP), thus facilitating molecular exchange between matrix and cytoplasm causing oxidative outburst and cell death. We tested the hypothesis of whether NMDA-R1 mediates Ca2+ influx causing CypD activation and MPTP opening leading to oxidative stress and renal injury in diabetes. We also tested whether H2S treatment blocks Ca2+ channel and thus inhibits CypD and MPTP opening to prevent renal damage. C57BL/6J and Akita (C57BL/6J-Ins2Akita) mice were treated without or with H2S donor GYY4137 (0.25 mg·kg−1·day−1 ip) for 8 wk. In vitro studies were performed using mouse glomerular endothelial cells. Results indicated that low levels of H2S and increased expression of NMDA-R1 in diabetes induced Ca2+ permeability, which was ameliorated by H2S treatment. We observed cytosolic Ca2+ influx in hyperglycemic (HG) condition along with mitochondrial-CypD activation, increased MPTP opening, and oxidative outburst, which were mitigated with H2S treatment. Renal injury biomarker KIM-1 was upregulated in HG conditions and normalized following H2S treatment. Inhibition of NMDA-R1 by pharmacological blocker MK-801 revealed similar results. We conclude that NMDA-R1-mediated Ca2+ influx in diabetes induces MPTP opening via CypD activation leading to increased oxidative stress and renal injury, and H2S protects diabetic kidney from injury by blocking mitochondrial Ca2+ permeability through NMDA-R1 pathway.
Keywords: CypD, H2S, KIM-1, MPTP, NMDA-R1, ROS
INTRODUCTION
Mitochondria, the powerhouse of the cell, participate in multifaceted regulatory pathways in normal and pathophysiological conditions. Mitochondrial permeability transition (MPT) is a process in which the mitochondrial inner membrane becomes permeable to any molecule that is less than 1.5 kDa. Although transient MPT pore (MPTP) opening enhances a protective role, such as by eliminating mitochondrial reactive oxygen species (ROS) (78), prolonged MPTP opening results in swelling of mitochondria, rupture, and eventual cell death (31, 41). MPTP is formed under certain pathological conditions, such as cardiac ischemia-reperfusion injury and chronic kidney diseases (14, 32). Accumulation of calcium (Ca2+) in the mitochondrial matrix is most evident in MPTP (32). The change in Ca2+ concentrations under different physiological conditions leads to the generation of ROS (13). The change in mitochondrial Ca2+ concentrations plays a critical role in MPTP opening, and excessive accumulation of Ca2+ facilitates conformational changes in the mitochondrial inner membrane proteins, resulting is disruption of the electron transport chain and ATP synthesis (32).
A mitochondrial matrix protein, cyclophilin D (CypD), is a modulatory component of MPTP opening (10). High intracellular Ca2+ induces CypD, and overexpression of CypD makes mitochondria more susceptible to Ca2+, thus favoring the pore opening (77). Therefore, the increase in permeability in the inner membrane exerts a colloidal osmotic pressure leading to the swelling of the mitochondrial membrane. This results in loss of matrix components, impairment of mitochondrial functionality, and substantial swelling of the organelle, with consequent outer membrane rupture and release of several proapoptotic proteins that initiate cell death (32). Moreover, opening of the MPTP further induces the production of ROS that also induce cell death (3, 22).
NMDA-R1, a subunit of N-methyl-d-aspartate (NMDA) receptors, has high calcium permeable activity (64). Though it mainly mediates neuronal functions, NMDA-R1 is also expressed in other mammalian tissues, including heart and kidney (35). Several studies showed that NMDA-R1 activation allows Ca2+ to enter into cytosol (52), which in turn induces CypD and therefore opens MPTP to allow molecular exchange between the mitochondrial matrix and cytosol (2). This leads to disorder in mitochondrial function leading to cytochrome C production followed by oxidative outburst (18). In our earlier study, we reported that NMDA-R1 is upregulated in kidney cells including mouse glomerular endothelial cells (MGEC) and proximal tubular epithelial cells following high glucose induction (31). NMDA-R1 was also upregulated in the kidney of diabetic mice C57BL/6J (Akita, Ins2Akita) (30). This upregulation of NMDA-R1 plausibly facilitated mitochondrial dysfunction through MPTP opening and production of ROS in the kidney that lead to chronic renal injury and dysfunction. We also showed that hydrogen sulfide (H2S), a potent vasodilator and physiological mediator, therapy mitigated adverse renal remodeling and improved kidney function (30). However, whether H2S regulates MPTP in diabetes and mitigates kidney injury remains unknown.
H2S is a regulator of the Ca2+ channel under different physiological conditions (7, 15). It modulates Ca2+ concentration and therefore Ca2+-induced MPTP opening (73). In addition, it has been reported that H2S inhibits Ca2+-induced MPTP opening in adult and old rat heart (60, 61). Furthermore, H2S is a strong antioxidant that protects the tissue against pathological matrix turnover, in part, by scavenging ROS (47). Although we have shown that H2S attenuates NMDA-R1 and mitigates diabetic renal damage (30), the molecular mechanism is still unknown. Interestingly, whereas NMDA-R facilitates Ca2+ permeability (55), H2S is known to inhibit the L-type Ca2+ channel (62). High Ca2+ activates CypD, a gatekeeper protein of MPTP, thus facilitating molecular exchange between matrix and cytoplasm causing oxidative outburst and cell death (34).
In the present study, we tested the hypothesis of whether high glucose-mediated NMDA-R1 induction facilitates Ca2+ influx, causing CypD activation and MPTP opening leading to oxidative stress and renal injury. Our findings further suggest that H2S treatment blocks the Ca2+ channel and thus inhibits CypD and MPTP opening and reduces renal damage in diabetes.
MATERIALS AND METHODS
Animal models and treatments.
The Animal Care and Use Committee of the University of Louisville approved all animal procedures including housing and diet. Male C57BL/6 [wild type (WT)]) and C57BL/6 Ins2Akita (diabetic) mice were obtained from Jackson Laboratory (Bar Harbor, ME) and were maintained under 12 h of light and dark cycle with food and water supply ad libitum. C57BL/6 Ins2Akita mice with blood glucose levels >300 mg/dl (measured by One Touch Ultra blood glucose monitoring system) were considered diabetic and were used in this study. Average blood glucose levels of C57BL/6 Ins2Akita mice were 516.25 ± 48.75 mg/dl. Mice aged 18 wk were treated with H2S donor GYY4137 (GYY; 0.25 mg·kg−1·day−1 ip) for 8 wk.
Antibodies and reagents.
The protein lysates that were subjected to Western blotting and immunofluorescence studies with specific antibodies were purchased as follows: Anti-Cav1.2 (MAB-13170) from Millipore (Burlington, MA); Cyclophilin D (PA3-022), NMDA-R1 (PA3-102), and TIM-1 (KIM-1) PA5-20244 from Thermo Fisher Scientific (Waltham, MA); β-actin (SC-47778) and horseradish peroxidase-linked secondary antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse kidney injury molecule-1 (KIM-1) ELISA Kit, T cell immunoglobulin and mucin domain-1 [(TIM-1), ab-119596] and CD31 (ab-9498) were from Abcam (Cambridge, MA), and MK-801 hydrogen maleate (CAS-77086-22-7) was obtained from Sigma-Aldrich (St. Louis, MO). Dihydroethidium (DHE; hydroethidine, cat. no. D1168), MitoTracker Mitochondrion Selective Probes (cat. no. M7511/M7513), Fluo-4 NW Calcium Assay Kit (cat. no. LSF36206), MitoProbe Transition Pore Assay Kit (cat. no. M34153), and Rhodamine 123 (Rh123; cat. no. R302) were procured from Molecular Probes (Eugene, OR). Washington State Probe-1 (WSP-1) was from Cayman Chemicals (CAS no. 1352750-34-5; Ann Arbor, MI). Fluorescent-labeled secondary antibodies were from Molecular Probes, Thermo Fisher Scientific.
Validation and use of antibodies.
All antibodies for immunoblotting were characterized by neutralizing antigens. Control lane using the standard of protein was used in the gel for the blots. To deplete nonspecific binding fractions, the antibodies were incubated with 5% milk to block nonspecific binding. In addition, we used positive and negative control samples, e.g., cells or tissue lysate, either found within the sample or within additional samples, which exhibit the protein of interest or do not express the protein of interest, respectively, to further validate the results. Results of flow cytometry data utilizing antibodies were compared with isotype controls to validate their specificity. Plasma levels of KIM-1 were measured by using the mouse KIM1 ELISA Kit (also known as TIM-1) following manufacturer’s instructions.
Cell culture.
MGECs were purchased from Cell Biologics (Chicago, IL). Cells were cultured in endothelial cell culture medium supplemented with 10% fetal bovine serum, VEGF, heparin, EGF, endothelial cell growth supplement, hydrocortisone, l-glutamine, and antibiotic-antimycotic supplied with the endothelial cell medium supplement kit (Cell Biologics). Cells were maintained at 37°C in a humidified atmosphere with 5% CO2.
Cell treatments.
Cells were seeded onto different culture plates and dishes according to the requirement of the experimental protocols 24 h before the treatment. Cells maintained in medium that contained 5 mM glucose served as NG (normal glucose), and those with 25 mM glucose were designated as HG (high glucose). l-Glucose (25 mM) was added only in the experiments that were performed as an osmotic control for HG levels. Cells were preconditioned with the H2S donor GYY (250 µM; Sigma, St. Louis, MO) before the glucose treatment and incubated at 37°C for 24 h. NMDA-R1 inhibitor MK-801 (50 µM, Sigma Aldrich) was added before glucose treatment.
Protein extraction and analysis.
Cytosolic and mitochondrial protein from control and treated cells were extracted using a mitochondria isolation kit for cultured cells according to the manufacturer’s protocol (Thermo Fisher Scientific). Briefly, after treatment, cells were washed with PBS, trypsinized, and harvested at 1000 g for 5 min. Mitochondrial isolation reagent A with protease inhibitor (100 µl) was added and vortexed at medium speed for 5 s followed by incubation on ice for 2 min. Then 1.25 µl of mitochondrial isolation reagent B was added, vortexed, and incubated on ice for 5 min, with vortexing every minute. Then 100 µl of mitochondrial isolation reagent C with protease inhibitor was added and centrifuged at 2000 g for 10 min at 4°C. The cytosolic fraction was obtained by collecting the supernatant after centrifugation at 13,000 g for 15 min at 4°C. Mitochondrial isolation reagent C with protease inhibitor (100 µl) was added to the pellet and centrifuged at 13,000 g for 5 min at 4°C. Mitochondrial protein was obtained by adding radioimmunoprecipitation assay buffer (with protease inhibitor) to the pellet.
Mouse kidney cortical tissues were lysed with lysis buffer (radioimmunoprecipitation assay buffer with proteinase inhibitor cocktail and PMSF). Tissue lysates were centrifuged at 4°C, 13,000 g for 10 min to pellet debris. Protein concentration was determined by Bradford assay (Bio-Rad Laboratories, Hercules, CA), and an equivalent amount of protein was separated by SDS-PAGE followed by Western blot analysis. Antibodies used are as follows: rabbit polyclonal anti-NMDA-R1 (1:1000), mouse monoclonal anti-Cav1.2 (1:1000), and rabbit polyclonal anti-CypD (1:1000).
Monitoring H2S release.
In MGECs, H2S release was detected by using the reactive disulfide-containing fluorescent probe WSP-1. It selectively reacts with cellular H2S and generates fluorescence. In brief, cells were seeded onto 12-mm glass coverslips and treated with glucose and GYY accordingly. After 24 h, medium from the culture was removed and the cells were incubated with 50 µM WSP-1 in PBS, following which fluorescence of the cells was detected by a confocal microscope (Olympus IX80, Olympus Corporation) and images were taken.
Immunohistochemistry.
Frozen kidney sections 5 µm thick were immunostained following standard immunohistochemistry protocol. Briefly, sections were fixed with 4% paraformaldehyde, and following repeated washing with PBS, slides were blocked with blocking buffer at room temperature. Kidney sections were incubated overnight at 4°C with NMDA-R1 antibody. After repeated washing, sections were incubated with anti-rabbit secondary antibody conjugated with Alexa Fluor 488. Slides were scanned at appropriate filter settings and digital images were captured using Olympus FluoView 1000 (B&B Microscopes, Pittsburgh, PA). By using the ImageJ (https://imagej.nih.gov/ij/) software, the mean fluorescence intensity of NMDA-R1 expression was calculated.
Calcium assay using Fluo-4 AM.
In-cell measurement of calcium signaling was performed using Fluo-4 NW Calcium Assay Kit according to the manufacturer’s protocol (Molecular Probes). Fluo-4 AM is a fluorescent Ca2+ indicator, and its fluorescence is increased upon binding to Ca2+. Briefly, after treatment, medium from the culture plate was removed, and 100 µl of Fluo-4 NW dye loading solution was added to each well quickly. Cells were incubated at 37°C for 30 min, then at room temperature for additional 30 min. The calcium response was measured by measuring the fluorescence using 488-nm argon laser for excitation at 494 nm and emission at 516 nm. Potential fluorescence outside the cells by organic transporters was inhibited by probenecid (Prb), and the baseline signal was reduced.
MPTP assay.
MPTP opening in the normal and treated cells was studied using MitoProbe Transition Pore Assay Kit (Molecular Probes, Eugene, OR). This assay involves a colorless and non- fluorescent esterase substrate, calcein AM, and CoCl2. Calcein AM shows fluorescence upon binding to the Ca2+ that indicates MPTP opening. Changes in the mitochondrial Ca2+ level were selectively observed by quenching the cytosolic Ca2+ signal by CoCl2. Ionomycin was used to stimulate MPTP opening. Cells were harvested by trypsinizing and resuspending in a prewarmed Hank’s balanced salt solution containing Ca2+. For each sample, three aliquots were prepared containing the following: calcein AM (tube 1); calcein AM and CoCl2 (tube 2); and calcein AM, CoCl2 and ionomycin (tube 3). Cells were then incubated at 37°C for 15 min, and flow cytometric analysis was performed using Accuri C6 flow cytometer after washing the cells with Hank’s balanced salt solution/Ca.
Rh123 staining for mitochondrial membrane potential.
Changes in the mitochondrial membrane potential (MMP) were assessed using fluorescent dye Rh123. It is a cationic fluorescent probe that accumulates in healthy mitochondria and releases upon depolarization of the mitochondrial membrane. MMP of the treated cells was analyzed using the confocal microscope. Cells were grown on a 12-mm coverslip (Fisher Scientific). Briefly, cells were washed with PBS and stained with Rh123 (1 µg/ml) for 30 min at room temperature in the dark. Cells were washed again to remove extracellular Rh123. After mounting the coverslip onto a glass slide, cells were observed under a laser scanning confocal microscope (Olympus IX80, Olympus Corporation), and images were taken.
Cytosolic and mitochondrial ROS production using DHE and MitoTracker green.
Superoxide production in MGECs was monitored by DHE and MitoTracker green dye. DHE and MitoTracker form a product that emits a red and green fluorescence, respectively, upon reaction with superoxide anions in cells. Briefly, cells were seeded onto an eight-well chamber slide, washed twice with PBS-BSA, and fixed for 10 min in 1% paraformaldehyde in PBS. Following this, the cells were incubated with 10 µM of DHE in endothelial growth medium at room temperature for 20 min in dark. Fluorescence of the cells was observed, and images were captured using a confocal microscope.
Statistical analysis.
Data obtained from independent experiments were expressed as mean ± SE per group or as stated in the figure legends. Quantitative densitometry for the protein expression was performed by using the ImageJ software (https://imagej.nih.gov/ij/). The data were subjected to one way ANOVA followed by Bonferroni’s multiple comparison tests by using SPSS package (Chicago, IL) for individual comparison between the experimental groups. Statistical differences between groups were considered significant at the level of P < 0.05.
RESULTS
GYY increased H2S levels in diabetic conditions.
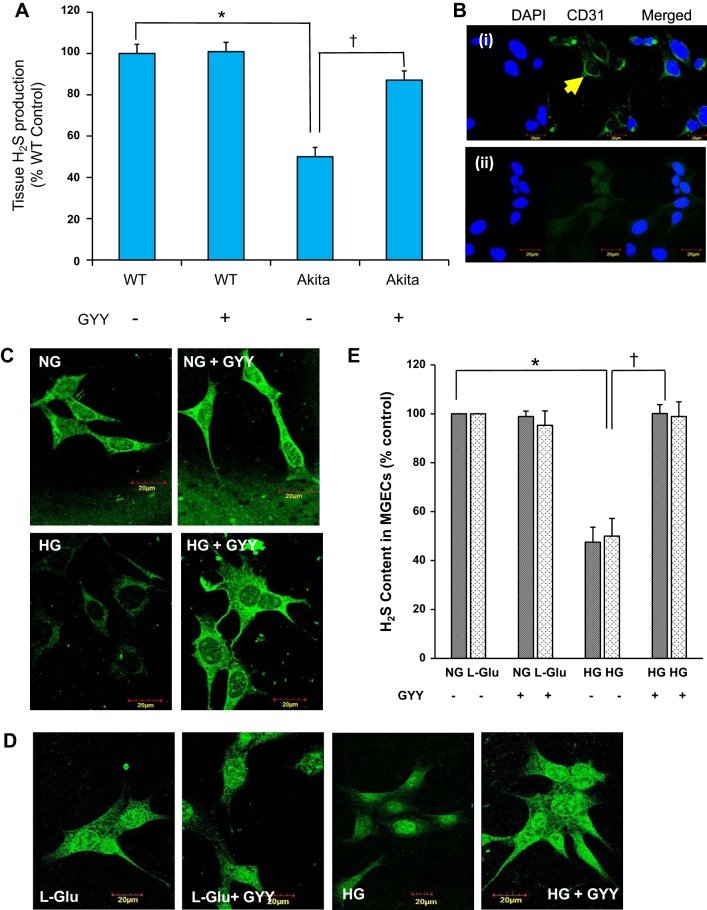
We measured tissue production of H2S in diabetic and nondiabetic kidney, and the results are shown in Fig. 1A. These data indicated that the tissue production of H2S levels in the diabetic kidney was significantly reduced. Interestingly, when Akita mice were treated with GYY for a period of 8 wk, the production of H2S was significantly increased in Akita kidney in comparison with its respective nontreated diabetic group (Fig. 1A). This observed increase of H2S in diabetic kidney following GYY treatment was almost 90% to that of WT control level. No change in the H2S levels was observed in WT kidney with or without GYY treatment (Fig. 1A).
Fig. 1.
A and B: tissue H2S production and cellular H2S release in mouse glomerular endothelial cells (MGECs). A: GYY4137 (GYY) treatment increased H2S production in the kidney cortical tissue of diabetic mice. H2S was measured using 100 μl of isolated kidney cortical tissue extract from mice of different experimental groups within 36 h of sample collection. Data represent mean ± SE, n = 6 or 7/group; *, †P < 0.05. B: confocal images of MGECs fixed in 4% paraformaldehyde and labeled with or without CD31 followed by secondary goat anti-mouse IgG (Alexa Fluor 488). i: CD31-positive cells (green): yellow arrow. ii: negative control cells stained with DAPI in the absence of CD31 and presence of secondary antibody. Nuclear counterstain with DAPI showing blue color. Images were captured at ×100 magnification. C and D: GYY treatment normalized the levels of H2S in MGECs in high-glucose (HG) conditions without or with osmotic control l-Glu (25 mM). Washington State Probe-1 (WSP-1), a reactive disulfide-containing fluorescent probe, was used to detect H2S in cells. E: bar diagram represents percent of fluorescent intensity. Values are mean ± SE, n = 7/group; *, †P < 0.05. Image, original magnification ×100; scale bar, 20 µm. NG, normal glucose; WT, wild type.
Recently, Qi et al. (48) have demonstrated endothelial mitochondrial dysfunction and oxidative stress in diabetic kidney disease-susceptible mice strains including Akita. To examine whether glomerular endothelial cells (GECs) are also susceptible to HG conditions resulting in diminished H2S availability and mitochondrial stress, and whether GYY ameliorates these changes, we performed a number of experiments using MGECs. CD31-positive staining confirmed that the cells, which we have used in our experiments, were in fact endothelial origin (Fig. 1B). In the next set of experiments, we detected a low level of H2S that was significantly reduced under HG conditions in MGECs (Fig. 1, C and E), a similar finding to that of diabetic kidney tissue (Fig. 1A). Furthermore, corroborating with in vivo studies, in vitro GYY treatment also increased the production of H2S in HG conditions toward normal level (Fig. 1, C and E). No changes in H2S level were observed in NG conditions with or without GYY treatment (Fig. 1, C and E).
To determine whether osmotic pressure due to high glucose has any negative impact on H2S levels in HG conditions or not, we used l-glucose (25 mM) to mimic HG conditions (Fig. 1D). Our results indicated that osmotic pressure had no effect on H2S levels, since the levels of H2S in control groups, whether with or without osmotic l-glucose, are comparable (Fig. 1, C–E). In the subsequent experiments, we therefore did not use osmotic controls to substantiate (null) effects of osmotic pressure in HG conditions.
Localization and expression of NMDA-R1 in kidney and in MGECs.
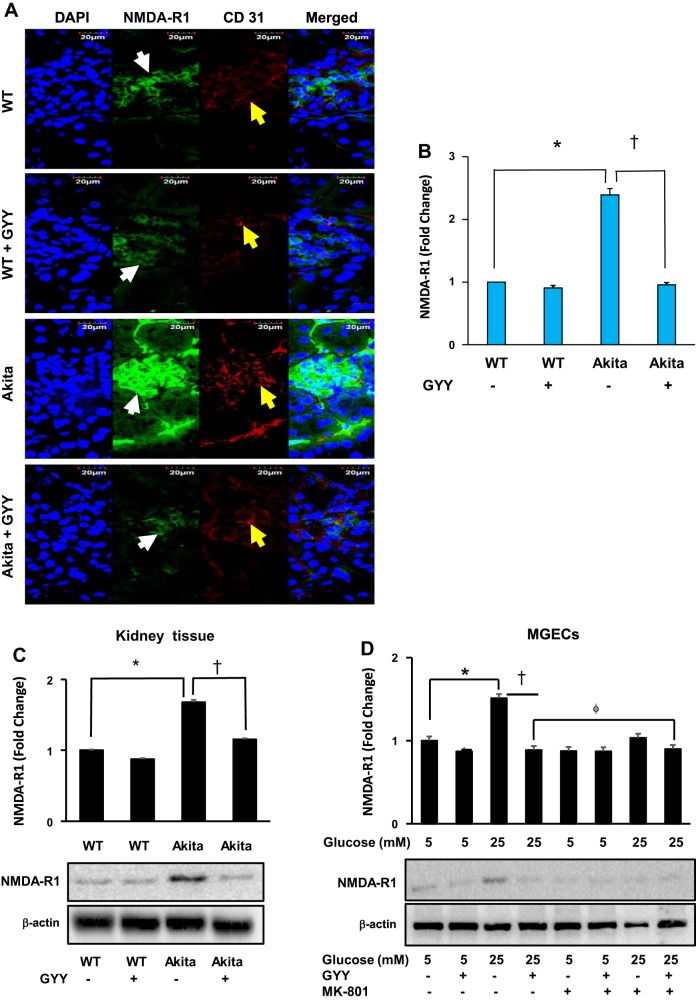
Expression and immuno-localization of NMDA-R1 protein in the glomerulus evidently showed increased expression in diabetic kidney compared with WT control (Fig. 2A). Glomeruli of the diabetic mice treated with GYY showed a significant reduction in the expression of NMDA-R1 compared with nontreated diabetic mice. The expression of NMDA-R1 in the glomerulus of WT kidney with or without GYY treatment remained at the basal level without significant change between the groups (Fig. 2, A and B). Furthermore, to determine whether glomerular expression of NMDA-R1 localized to the endothelium, we co-labeled the same tissue section with endothelial marker CD31, and the results indicated that these two molecules were localized in the glomerulus (Fig. 2A).
Fig. 2.
N-methyl-d-aspartate receptor receptor 1 (NMDA-R1) and CD31 localization and protein expression. A: glomerular localization of NMDA-R1 (white arrows) and CD31 (yellow arrows) counterstained with nuclear stain, DAPI (blue color). Merged images show CD31, NMDA-R1, and DAPI staining. Magnification ×60; scale bar: 20 µm. B: bar diagram represents fluorescence intensity of NMDA-R1 immunostaining. C: expression of NMDA-R1 in mouse kidney cortical tissue. D: mouse glomerular endothelial cells (MGECs) were incubated without or with MK-801, a selective and noncompetitive antagonist of NMDA-R in high-glucose (HG) and normal glucose (NG) conditions, and GYY was administered in the groups as indicated in the figure. Cell lysates were analyzed by immunoblotting against NMDA-R1 antibody. Bar diagrams represent mean ± SE, n = 7 or 8; *,† <0.05 and ϕP < 0.05. GYY, GYY4137; WT, wild type.
Western blot analysis revealed that expression of NMDA-R1 significantly increased in the diabetic kidney tissue compared with nondiabetic control (Fig. 2C). Following GYY treatment in Akita mice, the expression level of NMDA-R1 was mitigated. On the other hand, the expression of NMDA-R1 was at the basal level in WT mice and remained statistically unaltered even after GYY treatment (Fig. 2C).
Similar to in vivo data, in vitro Western blot analysis revealed that expression of NMDA-R1 significantly increased in MGECs under HG conditions compared with NG control, and GYY treatment attenuated NMDA-R1 expression back to the baseline level in HG conditions (Fig. 2D). This reduction of NMDA-R1 expression in HG conditions following GYY treatment was comparable with NG control (Fig. 2D). Although a slight decrease of NMDA-R1 expression was observed in NG conditions following GYY treatment, the difference was nonsignificant when compared with MGECs in NG conditions without GYY (Fig. 2D).
To determine whether the effect of GYY on NMDA-R1 expression in HG is similar to NMDA-R1 blocker, we incubated MGECs for 2 h with MK-801, a specific inhibitor for NMDA-R, and then treated the cells with or without GYY. The results indicated that the expression of NMDA-R1 in MGECs in NG conditions treated without or with GYY along with MK-801 had a basal level of NMDA-R1 expression (Fig. 2D). However, NMDA-R1 expression decreased in MGECs in HG conditions treated with MK-801 compared with nontreated cells, and it was marginally higher than GYY alone (Fig. 2D). Addition of GYY to the MK-801 group further decreased NMDA-R1 expression in HG conditions, although it was nonsignificant compared with GYY alone (Fig. 2D).
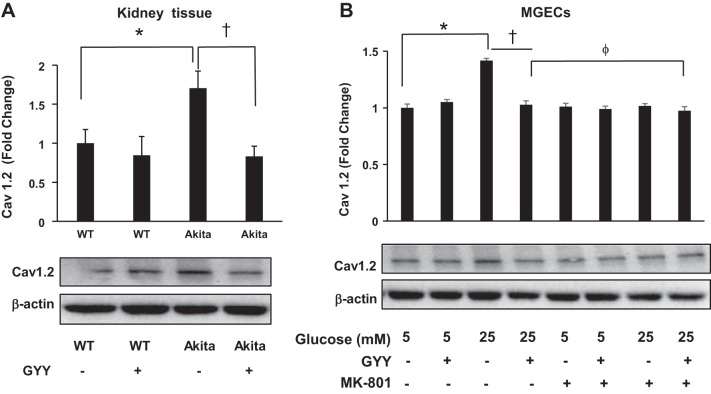
GYY mitigated L-type calcium channel (Cav1.2) expression through NMDA-R1 pathway.
We measured protein expression of Cav1.2 in kidney tissue from experimental groups, and the results indicated that Cav1.2 expression increased in diabetic mice compared with nondiabetic control (Fig. 3A). Interestingly, GYY treatment normalized Cav1.2 expression in diabetic kidney. No statistical differences were observed in the expression level of Cav1.2 in WT mice treated with or without GYY (Fig. 3A). Similar results were also obtained in MGECs, where NG and HG conditions mimicked nondiabetic and diabetic conditions, respectively, in vitro (Fig. 3B). Interestingly, MGECs treated with MK-801 demonstrated a basal level of Cav1.2 expression in HG conditions, a level similar to NG plus MK-801 or NG alone (Fig. 3B).
Fig. 3.
Expression of Cav1.2 protein in kidney tissue and in mouse glomerular endothelial cells (MGECs). A: Cav1.2 expression in wild-type (WT) and Akita kidney cortical tissue with or without GYY4137 (GYY). B: MGECs were incubated without or with MK-801, a selective and noncompetitive antagonist of N-methyl-d-aspartate receptor (NMDA-R) in high-glucose (HG) and normal glucose (NG) conditions, and GYY was administered in the groups as indicated in the figure. Cell lysates were analyzed by immunoblotting against Cav1.2 antibody. Bar diagrams represent mean ± SE, n = 5 or 6; *,† <0.05 and ϕP > 0.05.
GYY mitigated Ca2+ influx in MGECs in HG conditions.
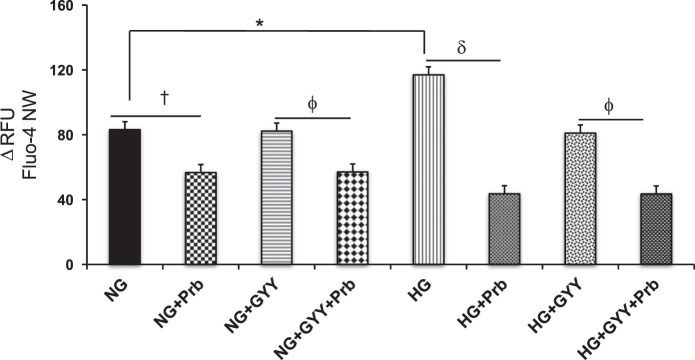
Intracellular Ca2+ measurement showed a 40% increase of fluorescence in HG conditions compared with NG conditions (Fig. 4). Whereas GYY treatment did not change Ca2+ influx in NG conditions compared with NG alone, a significant reduction of Ca2+ influx was measured in NG conditions treated with Prb, an inhibitor of organic anion transporters (Fig. 4). A similar result was also obtained in NG conditions treated with both GYY and Prb. Interestingly, GYY and Prb both mitigated Ca2+ influx in HG conditions compared with HG alone, when treated separately. However, the degree of attenuation was greater in Prb-treated cells compared with GYY treatment in HG conditions (Fig. 4). When HG cells were treated with GYY and Prb together, the Ca2+ influx was also attenuated compared with HG alone; however, this result was not different than the HG + Prb group (Fig. 4).
Fig. 4.
Calcium assay in mouse glomerular endothelial cells (MGECs). Calcium assay was performed using Fluo-4 NW assay kit. Values are mean ± SE, n = 8 or 0; *,†,γ,δ,ϕP < 0.05. Prb, probenecid, an inhibitor of organic anion transporters; RFU, relative fluorescence units.
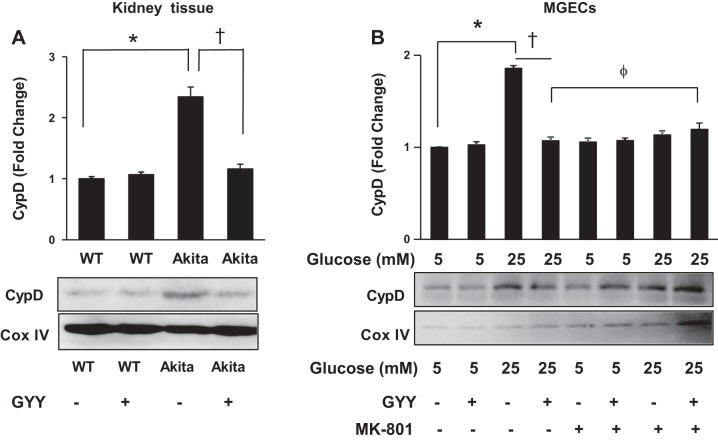
GYY mitigated CypD in diabetic conditions through NMDA-R1 pathway.
Immunoblotting analysis of CypD demonstrated a significant (more than twofold) increase in the kidney of diabetic mice compared with WT control (Fig. 5A). Following GYY treatment, the level of CypD expression was normalized in diabetic kidney. No changes of CypD expression in WT kidney were observed with or without GYY treatment (Fig. 5A). Similar to in vivo results, in vitro analysis indicated that CypD was also increased in HG conditions compared with NG conditions, and GYY treatment mitigated CypD expression in HG conditions (Fig. 5B). NMDA-R blocker MK-801 as well as the combination of GYY and MK-801 also attenuated CypD expression in HG conditions, which was comparable to the expression level of CypD in NG conditions (Fig. 5B).
Fig. 5.
Cyclophilin D (CypD) expression in mouse kidney mitochondrial extract and in mouse glomerular endothelial cells (MGECs). A: wild-type (WT) and Akita mice were supplemented with or without GYY4137 (GYY) for 8 wk, and kidney cortical tissue was analyzed by Western blotting. Bar diagram represents densitometric CypD analysis of immunoblots. B: MGECs were incubated without or with GYY and MK-801 (a specific NMDA-R blocker) in NG or HG conditions for 24 h. Isolated mitochondrial lysate was immunoblotted against CypD antibody. Bar diagram represents densitometric CypD analysis. Values are mean ± SE, n = 5 or 6/group; *,†<0.05 and ϕP > 0.05
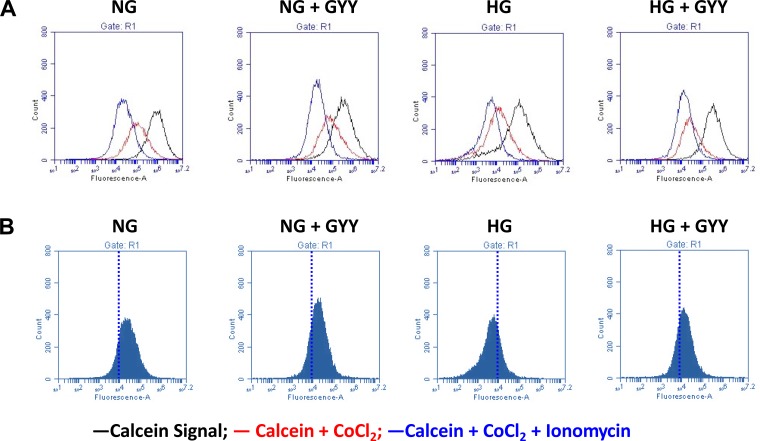
GYY prevented MPTP opening in HG conditions.
The Fig. 6, A and B histograms indicate total cellular calcein signal (cytosol + mitochondria; black line histogram), mitochondrial calcein (cytosol calcein quenched by CoCl2; red line histogram), and after addition of internal control (calcein-AM + CoCl2 + ionomycin). In NG conditions, MGECs treated without or with GYY did not show differences in total cellular calcein signal (Fig. 6, A and B). In contrast, in HG conditions, MGECs without GYY treatment demonstrated reduced calcein fluorescence in the presence of CoCl2 and ionomycin as seen by shift to the left of the blue line histogram suggesting increased MPTP opening (Fig. 6, A and B). GYY treatment to MGECs in HG conditions showed increased cellular calcein fluorescence, seen as shift to the right of the blue line histogram suggesting inhibition of MPTP opening (Fig. 6, A and B).
Fig. 6.
Mitochondrial permeability transition pore (MPTP) opening. MPTP opening was detected by using MitoProbe Transition Pore Assay Kit as described in the materials and methods. Mouse glomerular endothelial cell (MGECs) were treated with GYY4137 (GYY) (250 μM) and glucose (5 and 25 mM). A: black histogram represents fluorescent dye, calcein, in the cytosol and mitochondria; red histogram represents calcein in mitochondria only and quenching of cytosolic calcein by addition of CoCl2; blue histogram represents calcein fluorescence in cells treated with calcein-AM, CoCl2, and ionomycin, which stimulates the opening of MPTP. B: MGECs without GYY treatment showed reduced calcein fluorescence under high-glucose (HG) conditions in the presence of calcein-AM, CoCl2, and ionomycin (shifted blue histogram to the left). MGECs treated with GYY in HG conditions inhibited MPTP opening and increased calcein fluorescence (shifted blue histogram to the right). Representative images from n = 6 independent experiments. NG, normal glucose.
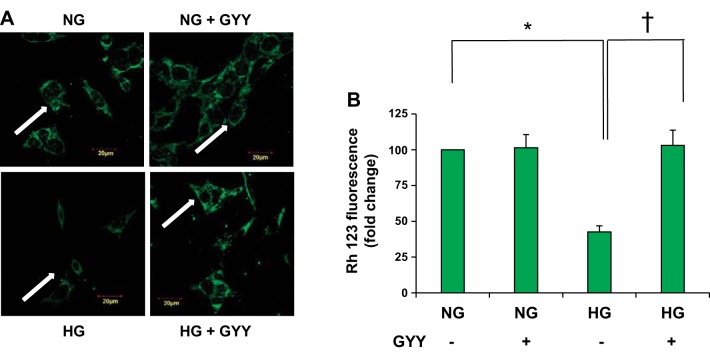
GYY treatment preserved mitochondrial membrane potential in HG conditions.
Mitochondrial membrane potential (MMP) is a key indicator of cellular heath, and opening the MPTP leads to mitochondrial swelling and cell death (32). As a result, cytochrome C releases into the cytosol, initiating apoptosis. Rh123 is a cell-permeant cationic fluorescent dye that is used to monitor mitochondrial function in living cells. If the cells are stained with Rh123 dye, the fluorescent dye distributes according to the negative membrane potential across the mitochondrial inner membrane. The loss of membrane potential therefore results in loss of fluorescence intensity. We measured MMP in MGECs treated with or without GYY in NG and HG conditions. The results indicated that fluorescence in NG cells treated with GYY remained similar to the level of untreated cells (Fig. 7, A and B). On the other hand, untreated HG cells exhibited decreased fluorescence in comparison with NG control cells. Following GYY treatment, the MMP in HG cells was restored to the normal levels with evidence of increased fluorescence (Fig. 7, A and B).
Fig. 7.
Mitochondrial membrane potential. A: Mouse glomerular endothelial cell (MGECs) were incubated with rhodamine 123 (Rh123) to detect the mitochondrial membrane potential. Less green fluorescence intensity indicates decreased membrane potential. B: bar diagram represents intensity of Rh123 fluorescence among the groups. Values are mean ± SE, n = 5/group; *,†P < 0.05. GYY, GYY4137; HG, high glucose; NG, normal glucose.
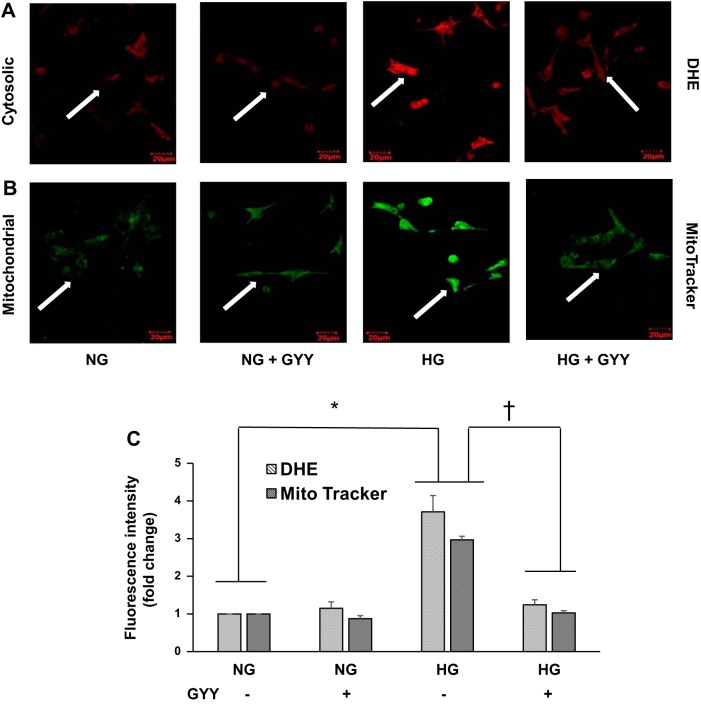
GYY mitigated cytosolic and mitochondrial ROS in HG conditions.
Cytosolic and mitochondrial ROS were measured by DHE and MitoTracker green, respectively. MGECs in NG conditions without GYY (control) showed the basal level of ROS expression, both in cytosol and mitochondria (Fig. 8, A and B). When treated with GYY, MGECs showed a similar expression pattern of cytosolic and mitochondrial ROS in NG compared with their respective control cytosolic and mitochondrial expression (Fig. 8, A and B). In HG conditions, the intensity of ROS fluorescence, in cytosol as well as in mitochondria, was elevated significantly compared with NG conditions (Fig. 8, A–C). When treated with GYY, the fluorescence level in HG conditions was significantly mitigated in cytosol as well as in mitochondria, which was toward baseline level similar to NG control (Fig. 8, A–C).
Fig. 8.
Reactive oxygen species measurement. Cytosolic (A) and mitochondrial (B) oxidative stress were detected using dihydroethidium (DHE) and MitoTracker dye, respectively. Mouse glomerular endothelial cell (MGECs) were incubated with DHE (A) or MitoTracker green (B) for 30 min, and images were captured using confocal microscopy. Bar diagram represents fold change of fluorescence intensity compared with control (C). Representative images from n = 5 independent experiments; values are mean ± SE; *,†P < 0.05. Original magnification ×60. GYY, GYY4137; HG, high glucose; NG, normal glucose.
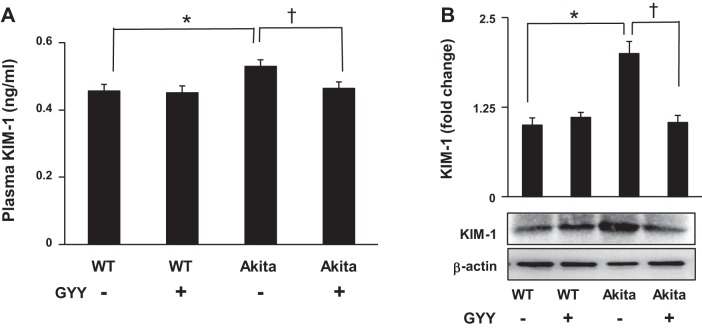
GYY mitigated KIM-1 release in the plasma and expression in the diabetic kidney.
We measured KIM-1 in the plasma and kidney tissue. The results indicated that the release and expression of KIM-1 in the plasma and kidney, respectively, increased significantly in diabetic mice compared with their normoglycemic WT control (Fig. 9, A and B). Following GYY treatment, the plasma and tissue levels of KIM-1 in diabetic kidney were mitigated, which was toward the basal level as observed in the normoglycemic conditions. The levels of KIM-1 expressed in both the WT that were either treated or nontreated with GYY remained unchanged and were at the basal levels (Fig. 9, A and B).
Fig. 9.
Plasma levels and kidney tissue expression of kidney injury molecule-1 (KIM-1). A: plasma levels of KIM-1 were measured by ELISA. Values were obtained against a standard curve. B: KIM-1 immunoblotting of tissue extract from the kidney cortex. Bar diagram represents densitometric analyses of immunoblots. Values are mean ± SE, n = 5/group; *,†P < 0.05. GYY, GYY4137; WT, wild type.
DISCUSSION
In this study, we observed significant reduction of H2S in type 1 diabetes (T1D). Our findings also demonstrated upregulation of NMDA-R1 expression associated with increased Ca2+ permeability in diabetes. Ca2+ influx induced activation of mitochondrial CypD, leading to increased MPTP opening. Constant MPTP opening led to increased oxidative stress, in part due to reduced H2S levels resulting in renal injury. These changes were evident from both in vivo and in vitro findings, and the results further suggested that treatment with GYY, an H2S donor, alleviated these effects through the NMDA-R1 pathway in diabetes conditions.
Chronic kidney disease, affecting a large number of general population, is one of the leading causes of death in patients with untreated diabetes. Both idiopathic and known causes of onset and progression of T1D include genetics, environmental factors, viruses, smoking, insulin insufficiency, oxidative stress, differential diet pattern that is associated with glucose intolerance and lifestyle (1). Studies from others and our own laboratories have underlined several molecular mechanisms in the pathophysiological renal damage and related disorders in diabetes (30, 31, 50). The precise molecular mechanism(s), however, has yet to be defined for targeted therapy.
Results from our present study demonstrated a significant decrease of H2S in diabetic kidney tissues and in MGECs exposed to HG conditions in vitro (Fig. 1, A–E). Reduction in H2S under diabetic conditions is in accordance with our and others previous findings (11, 29). GYY treatment to Akita mice for 8 wk and to MGECs in HG conditions for 24 h displayed a surge in the levels of H2S similar to their respective control groups (Fig. 1, C and D), which further supported our previous finding that reduction of H2S levels in Akita mice regained normal levels when supplemented with GYY (26, 29, 31). Together, these results suggest that GYY has therapeutic potential to restore tissue and cellular levels of H2S in diabetic conditions.
Several studies from other laboratories and from our own group under different experimental conditions have shown a marked increase in the expression of NMDA-R1, an agonist of diabetic nephropathy (30, 51, 57). Results from Western blot analysis in the kidney tissues from the present study also demonstrated a significant increase in expression of NMDA-R1 in diabetic conditions compared with WT control (Fig. 2C). A similar trend has also been reported in many in vivo animal (67, 69) and human studies (20). Interestingly, Akita mice treated with GYY for a period of 8 wk displayed a significant decrease in the levels of NMDA-R1 expression, which was comparable to WT control (Fig. 2, A–C). Furthermore, our results indicated glomerular expression of NMDA-R1 was mostly localized to the endothelium (Fig. 2A). Immunoblot results from in vitro experiment in MGECs further substantiated in vivo findings (Fig. 2D). In addition, MGECs treated with MK-801, an antagonist of NMDA-R, in HG conditions maintained basal levels of NMDA-R1 expression similar to that of NG conditions. Comparable results were also observed in MGECs treated with GYY in HG conditions (Fig. 2D). Thus, our present findings further strengthen the previous reports on NMDA-R1 expression and the possible role of H2S regulation of NMDA-R1 in renovascular remodeling under diabetic conditions (30, 31, 65).
Calcium is one of the most abundant minerals in the body and is required for many important functions including intracellular signaling (9). As a second messenger, Ca2+ modifies cellular activities, such as Ca2+-sensitive dehydrogenases activity of the Krebs cycle (19). Studies have demonstrated direct association between Ca2+ and mitochondria, in which Ca2+ accumulates in mitochondria under certain pathological conditions resulting in cellular damage and organ malfunction (38). Results from the experiments performed under in vivo and in vitro conditions in the present study showed overexpression of the voltage-dependent Ca2+ channel, Cav1.2, in kidney tissues and MGECs under diabetic conditions (Fig. 3, A and B). To our best knowledge, this is the first report indicating Cav1.2 expression in MGECs. Diabetic mice treated with GYY showed a decrease in the expression of Cav1.2, which was similar to WT treated without or with GYY, exhibiting reversible changes following H2S treatment (Fig. 3A). A similar trend was also observed in MGECs in diabetic conditions treated with or without GYY (Fig. 3B). Furthermore, we performed in vitro studies in MGECs in the absence and presence of both high glucose and MK-801 to explore the role of NMDA-R in Cav1.2 expression and thus Ca2+ regulation. Results from these experiments demonstrated an increased expression of Cav1.2 under high glucose levels (Fig. 3B), which was normalized with MK-801 treatment, suggesting the involvement of NMDA-R in Ca2+ regulation in diabetic conditions. Additional data obtained from the Fluo-4 NW experiment, a fluorescent Ca2+ indicator, showed an increase in fluorescence activity in MGECs following HG conditions compared with NG control, indicating increase in Ca2+-Fluo-4 NW binding that results from increased Ca2+ influx (Fig. 4). On the contrary, MGECs in HG conditions treated in the presence of Prb, an organic anion transporter inhibitor, showed a decreased fluorescence in comparison with HG MGECs without Prb (Fig. 4). Similar Ca2+ influx inhibition was also observed in the presence of GYY, although GYY was not as potent as Prb (Fig. 4). These results indicate Ca2+ influx in HG conditions, and GYY has a potential to mitigate Ca2+ influx.
It is worth mentioning that endothelial cells are typically non-excitable, and thus it is difficult to reconcile a small change of voltage-dependent ion changes in membrane potential. Despite this, a number of other studies support the presence of voltage-dependent Ca2+ channel in endothelial cells (17, 42, 63). However, to our best knowledge, there is virtually no report on voltage-dependent Ca2+ channel in GECs. Although we measured increased expression of Cav1.2 in kidney tissue and in MGEC in our experimental diabetic conditions, it is also possible that a nonselective cation channel that is permeable for cations including Ca2+ may have contributed in our experimental outcomes. This plausible explanation is partly supported by previous observation in which an agonist-induced activation of a nonselective ion current in GECs was reported (45).
It is also well known that NMDA-R1 independently facilitates intracellular Ca2+ influx in healthy and diabetic podocytes (27, 57) and Cav1.2 in mesangial and other renal cell types (76). Recently, Chen et al. (8) made an important observation on neuropathic pain development using spinal cord slices and human embryonic kidney 293 cells, in which they showed that a heteromeric complex of α2δd-1 (a subunit of voltage-activated Ca2+ channel) and NMDA receptor interaction is critical for neuropathic pain development. In our study, we have detected increased expression of both NMDA-R1 and Cav1.2 in diabetic conditions, which were mitigated by GYY treatment (Fig. 2, A–D, and Fig. 3, A and B, respectively). Additionally, GYY inhibited Ca2+ influx in MGECs in HG conditions, a similar effect that was also observed in cells treated with Prb, an organic anionic transporter inhibitor (Fig. 4). Although we did not measure whether a heterodimeric complex of Cav1.2 and NMDA-R1 was involved in Ca2+ influx in our study, it is highly plausible that a heterodimeric form of these molecules may have played a role, since both of these molecules were overexpressed in HG conditions and an increased Ca2+ influx was observed. To endorse or refute this possible mechanism of Ca2+ influx in diabetic kidney, future studies are warranted.
Measurement of CypD, a modulator of the MPTP, in the kidney tissues and isolated mitochondrial fraction from MGECs demonstrated a result similar to Cav1.2 expression (Fig. 5, A and B, and Fig. 3, A and B, respectively). Association between CypD and kidney disorders was demonstrated by several independent laboratories (24, 25, 28). Increased expression levels of CypD observed in the present study in diabetic conditions (Fig. 5, A and B) was in parallel with increased levels of Ca2+ (Fig. 4). Furthermore, mitochondrial fraction isolated from MGECs showed an increased expression of CypD in HG conditions that lacked GYY and MK-801 exposure (Fig. 5B). Following the treatment with GYY or with NMDA-R blocker MK-801 in HG conditions, CypD expression displayed normalization, suggesting the cytoprotective and the direct role of H2S on MPTP.
MPTP opening allows increase in the permeability of the mitochondrial membranes to molecules less than 1.5 kDa, resulting in mitochondrial swelling and cell death (32). Studies have shown that inhibition of MPTP opening benefits diabetic heart (40, 56). To evaluate whether MPTP opening ensued in HG conditions and whether GYY has any role in preventing MPTP opening, we analyzed experimental MGECs by flow cytometry following incubation with the components of the MPTP assay kit. The fluorescence shift observed between the groups, calcein + CoCl2 and calcein + CoCl2 + ionomycin, indicated the continuous opening of the MPTP and reduction of fluorescence in HG conditions (Fig. 6). Following GYY supplementation, the shift restored to normal levels, which was in concurrence with NG conditions. Studies have shown that MPTP opening is associated with mitochondrial collapse, oxidative stress, and activation of apoptotic pathway in renal injury (41), and H2S has a protective role under diseased conditions by inhibiting MPTP (36, 71, 73). In parallel with the findings from these above referenced reports, our study demonstrated increased MPTP opening in HG conditions, which was mitigated following GYY supplementation in renal GECs.
It is also important to state that increased MPTP opening is associated with loss of MMP and increased production of ROS (6). To detect the MMP in MGECs, green fluorescent dye Rh123 was used, and the results demonstrated baseline fluorescence in both the NG conditions that were supplemented with or without GYY (Fig. 7, A and B). Decreased or loss of florescence in the mitochondria of HG conditions was observed in MGECs (Fig. 7, A and B). As active mitochondria sequester the fluorescence without any cytotoxic effects, increased fluorescence observed in HG conditions following GYY treatment strongly suggest the protective role of GYY (Fig. 7, A and B). These findings are in accordance with the earlier reports from other laboratories (46, 70). Furthermore, data obtained by measuring the cytosolic (Fig. 8A) and mitochondrial (Fig. 8B) oxidative stress using DHE and MitoTracker green in MGECs suggested that GYY protected these cellular components from oxidative stress under HG conditions. This result further confirms our recent finding on diabetic models that have underscored the protective role of GYY on oxidative stress (26). Others have also reported similar observations, suggesting the beneficial role of H2S in diabetic conditions is in part due to diminished oxidative stress (33, 75).
KIM-1 is a type-1 transmembrane protein that is not normally present but expresses upon renal proximal tubular injury (5) and as such has been recognized as a biomarker of proximal tubular injury in chronic and acute renal diseases. Studies have reported increased plasma KIM-1 levels in animals and humans, suggesting a prognostic biomarker in the progression of end-stage renal disease in T1D (43, 53). Interestingly, recent studies also indicated glomerular epithelial expression of KIM-1 in diabetic nephropathy (74) and in healthy human glomerulus (16). The latter study also found that although in healthy human kidney very few KIM-1 positive cells were detected within the glomerulus, a significant increase of KIM-1 expression in podocytes and other cells was measured in diabetic kidney. More interestingly, through sequencing data of glomeruli, the same study reported 139-fold increased KIM-1 expression in cultured mice glomeruli compared with freshly isolated glomeruli (16). Thus, it is no surprise that the glomeruli of diabetic kidney also contribute to the overall plasma pool of KIM-1, in addition to tubular injury and shedding. In our present study, we measured higher plasma levels of KIM-1 in diabetic mice compared with nondiabetic mice, which was mitigated following GYY supplementation (Fig. 9A). Similar results were also observed in the Western blot analyses of kidney tissue samples (Fig. 9B). These results are consistent with the findings from others signifying clinical symptoms of kidney injury in diabetes (4, 54), which is preventable by GYY supplementation.
It is pertinent to mention that this particular manuscript is lacking renal phenotypic and functional data. However, this is an extended study of our previously published article, in which we reported amelioration of histological collagen realignment and vascular density as phenotypic improvement in Akita kidney following GYY treatment (26). Also, in another published study, we reported functional improvement in diabetic kidney following H2S treatment as evidenced from improved renal resistive index and reduction of plasma creatinine levels in Akita mice (30). Therefore, in this article, we have avoided reporting similar renal phenotypic and functional observations, although we extrapolate similar phenotypic and functional consequences may have occurred.
The major limitation of this study is attributable to the genetic T1D Akita model of C57B6/J background. Although C57BL/6J-Ins2Akita mice are severely hyperglycemic by 5–6 wk of age, there are significant strain-related differences in renal responses in these compared with DBA/2J Akita mice, which provides a more robust and uniform experimental model for model development of diabetic kidney disease (21). The widely studied C57BL/6J strain is relatively resistant to development of albuminuria despite exhibiting moderate mesangial expansion and glomerular basement membrane thickening (23, 49). Another limitation is that in our high glucose experiments, we did not use osmotic control, either l-glucose or mannitol, for the following reasons: 1) our pilot study using l-glucose as osmotic control did not show any apparent changes in their morphology or H2S content versus non-osmotic glucose control (NG) in MGECs (Fig. 1, C–E). 2) Use of mannitol as osmotic control has been highly debated because it is a polyol (sugar alcohol) and may contribute to damage as seen in diabetes by activating polyol pathways (68). In a tissue culture model of diabetes mellitus, Ozturk et al. (44) showed that mannitol can be as toxic as glucose, as both of them affected axon growth, with increased dead and apoptotic migrating cells contributing to the development of diabetic neuropathy following hyperosmolality. In addition, a study in spontaneously hypertensive rats indicated mannitol activates cardiac and renal cell death implicating heart and renal damage (72). Furthermore, clinical studies have strongly emphasized the adverse effects of mannitol in diabetic patients including nephrotoxicity (39) and acute renal failure (12, 59, 66), as well as anaphylactic reactions under certain pathological conditions (37).
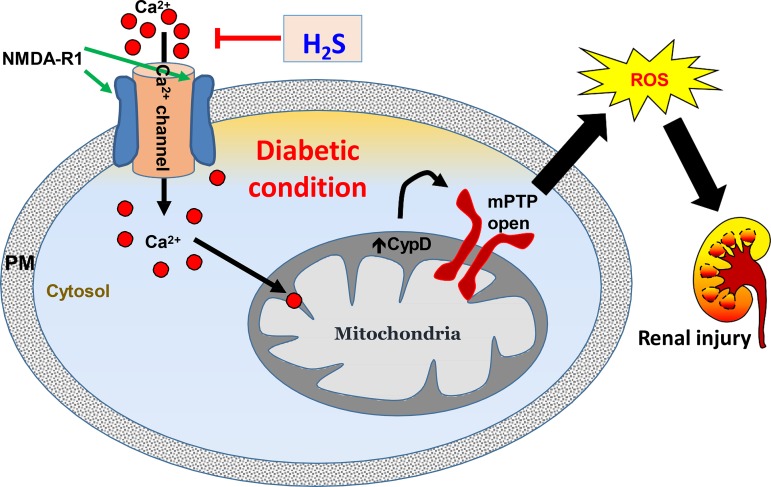
In summary, our study indicates NMDA-R1 mediates Ca2+ influx and induces activation of mitochondrial CypD, thus increasing MPTP opening and loss of MMP resulting in oxidative outburst and kidney injury in diabetic conditions (Fig. 10). These are in part due to reduced H2S production in the diabetic kidney. Further data indicated H2S normalizes these changes, suggesting that H2S has the potential to protect diabetic kidney from injury by blocking mitochondrial Ca2+ permeability through NMDA-R1 pathway (Fig. 10).
Fig. 10.
Schematic of overall findings. In diabetes, N-methyl-d-aspartate receptor 1 (NMDA-R1) mediates Ca2+ influx causing cyclophilin D (CypD) activation and mitochondrial permeability transition pore (mPTP) opening in mitochondria leading to oxidative outburst and renal endothelial injury. H2S treatment mitigates NMDA-R1 expression, possibly blocks Ca2+ channel, and thus inhibits CypD and MPTP opening and prevents renal damage. PM, plasma membrane; ROS, reactive oxygen species.
GRANTS
This work was supported in part by National Institutes of Health Grants DK-104653 and DK116591 (to U. Sen and S. C. Tyagi) and American Heart Association Scientist Development Grant 15SDG25840013 (to S. Pushpakumar).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.K. and U.S. conceived and designed research; A.S.P.J., S.K., S.P., and M.A. performed experiments; A.S.P.J. and S.K. analyzed data; S.P., S.C.T., and U.S. interpreted results of experiments; A.S.P.J., S.K., and S.P. prepared figures; A.S.P.J. and S.K. drafted manuscript; A.S.P.J., S.P., and U.S. edited and revised manuscript; U.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Aaron Tyagi for technical assistance.
Present address of S. Kundu: Department of Botany, West Bengal State University, Barasat, India.
REFERENCES
- 1.American Diabetes Association Standards of medical care in diabetes–2009. Diabetes Care 32, Suppl 1: S13–S61, 2009. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barsukova AG, Bourdette D, Forte M. Mitochondrial calcium and its regulation in neurodegeneration induced by oxidative stress. Eur J Neurosci 34: 437–447, 2011. doi: 10.1111/j.1460-9568.2011.07760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batandier C, Leverve X, Fontaine E. Opening of the mitochondrial permeability transition pore induces reactive oxygen species production at the level of the respiratory chain complex I. J Biol Chem 279: 17197–17204, 2004. doi: 10.1074/jbc.M310329200. [DOI] [PubMed] [Google Scholar]
- 4.Bayrasheva VK, Babenko AY, Dobronravov VA, Dmitriev YV, Chefu SG, Pchelin IY, Ivanova AN, Bairamov AA, Alexeyeva NP, Shatalov IS, Grineva EN. Uninephrectomized high-fat-fed nicotinamide-streptozotocin-induced diabetic rats: a model for the investigation of diabetic nephropathy in Type 2 diabetes. J Diabetes Res 2016: 8317850, 2016. doi: 10.1155/2016/8317850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonventre JV. Kidney injury molecule-1 (KIM-1): a specific and sensitive biomarker of kidney injury. Scand J Clin Lab Invest Suppl 241, suppl 241: 78–83, 2008. doi: 10.1080/00365510802145059. [DOI] [PubMed] [Google Scholar]
- 6.Briston T, Roberts M, Lewis S, Powney B, Staddon JM, Szabadkai G, Duchen MR. Mitochondrial permeability transition pore: sensitivity to opening and mechanistic dependence on substrate availability. Sci Rep 7: 10492, 2017. doi: 10.1038/s41598-017-10673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castro-Piedras I, Perez-Zoghbi JF. Hydrogen sulphide inhibits Ca2+ release through InsP3 receptors and relaxes airway smooth muscle. J Physiol 591: 5999–6015, 2013. doi: 10.1113/jphysiol.2013.257790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Li L, Chen SR, Chen H, Xie JD, Sirrieh RE, MacLean DM, Zhang Y, Zhou MH, Jayaraman V, Pan HL. The α2δ-1-NMDA receptor complex is critically involved in neuropathic pain development and gabapentin therapeutic actions. Cell Rep 22: 2307–2321, 2018. doi: 10.1016/j.celrep.2018.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clapham DE. Calcium signaling. Cell 131: 1047–1058, 2007. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 10.Devalaraja-Narashimha K, Diener AM, Padanilam BJ. Cyclophilin D deficiency prevents diet-induced obesity in mice. FEBS Lett 585: 677–682, 2011. doi: 10.1016/j.febslet.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 11.Ding T, Chen W, Li J, Ding J, Mei X, Hu H. High glucose induces mouse mesangial cell overproliferation via inhibition of hydrogen sulfide synthesis in a TLR-4-dependent manner. Cell Physiol Biochem 41: 1035–1043, 2017. doi: 10.1159/000461483. [DOI] [PubMed] [Google Scholar]
- 12.Doi K, Ogawa N, Suzuki E, Noiri E, Fujita T. Mannitol-induced acute renal failure. Am J Med 115: 593–594, 2003. doi: 10.1016/S0002-9343(03)00425-X. [DOI] [PubMed] [Google Scholar]
- 13.Dugan LL, Sensi SL, Canzoniero LM, Handran SD, Rothman SM, Lin TS, Goldberg MP, Choi DW. Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-D-aspartate. J Neurosci 15: 6377–6388, 1995. doi: 10.1523/JNEUROSCI.15-10-06377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eirin A, Li Z, Zhang X, Krier JD, Woollard JR, Zhu XY, Tang H, Herrmann SM, Lerman A, Textor SC, Lerman LO. A mitochondrial permeability transition pore inhibitor improves renal outcomes after revascularization in experimental atherosclerotic renal artery stenosis. Hypertension 60: 1242–1249, 2012. doi: 10.1161/HYPERTENSIONAHA.112.199919. [DOI] [PubMed] [Google Scholar]
- 15.Elies J, Scragg JL, Huang S, Dallas ML, Huang D, MacDougall D, Boyle JP, Gamper N, Peers C. Hydrogen sulfide inhibits Cav3.2 T-type Ca2+ channels. FASEB J 28: 5376–5387, 2014. doi: 10.1096/fj.14-257113. [DOI] [PubMed] [Google Scholar]
- 16.Endlich N, Lange T, Kuhn J, Klemm P, Kotb AM, Siegerist F, Kindt F, Lindenmeyer MT, Cohen CD, Kuss AW, Nath N, Rettig R, Lendeckel U, Zimmermann U, Amann K, Stracke S, Endlich K. BDNF: mRNA expression in urine cells of patients with chronic kidney disease and its role in kidney function. J Cell Mol Med 22: 5265–5277, 2018. doi: 10.1111/jcmm.13762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Figueroa XF, Chen CC, Campbell KP, Damon DN, Day KH, Ramos S, Duling BR. Are voltage-dependent ion channels involved in the endothelial cell control of vasomotor tone? Am J Physiol Heart Circ Physiol 293: H1371–H1383, 2007. doi: 10.1152/ajpheart.01368.2006. [DOI] [PubMed] [Google Scholar]
- 18.Furukawa H, Singh SK, Mancusso R, Gouaux E. Subunit arrangement and function in NMDA receptors. Nature 438: 185–192, 2005. doi: 10.1038/nature04089. [DOI] [PubMed] [Google Scholar]
- 19.Giorgi C, Agnoletto C, Bononi A, Bonora M, De Marchi E, Marchi S, Missiroli S, Patergnani S, Poletti F, Rimessi A, Suski JM, Wieckowski MR, Pinton P. Mitochondrial calcium homeostasis as potential target for mitochondrial medicine. Mitochondrion 12: 77–85, 2012. doi: 10.1016/j.mito.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.González-García S, González-Quevedo A, Hernandez-Diaz Z, Alvarez Camino L, Peña-Sanchez M, Cordero-Eiriz A, Brown M, Gaya JA, Betancourt-Losa M, Fernandez-Almirall I, Menendez-Sainz MC, Fernandez-Carriera R. Circulating autoantibodies against the NR2 peptide of the NMDA receptor are associated with subclinical brain damage in hypertensive patients with other pre-existing conditions for vascular risk. J Neurol Sci 375: 324–330, 2017. doi: 10.1016/j.jns.2017.02.028. [DOI] [PubMed] [Google Scholar]
- 21.Gurley SB, Clare SE, Snow KP, Hu A, Meyer TW, Coffman TM. Impact of genetic background on nephropathy in diabetic mice. Am J Physiol Renal Physiol 290: F214–F222, 2006. doi: 10.1152/ajprenal.00204.2005. [DOI] [PubMed] [Google Scholar]
- 22.Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol 46: 821–831, 2009. doi: 10.1016/j.yjmcc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 23.Hathaway CK, Gasim AM, Grant R, Chang AS, Kim HS, Madden VJ, Bagnell CR Jr, Jennette JC, Smithies O, Kakoki M. Low TGFβ1 expression prevents and high expression exacerbates diabetic nephropathy in mice. Proc Natl Acad Sci USA 112: 5815–5820, 2015. doi: 10.1073/pnas.1504777112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou W, Leong KG, Ozols E, Tesch GH, Nikolic-Paterson DJ, Ma FY. Cyclophilin D promotes tubular cell damage and the development of interstitial fibrosis in the obstructed kidney. Clin Exp Pharmacol Physiol 45: 250–260, 2018. doi: 10.1111/1440-1681.12881. [DOI] [PubMed] [Google Scholar]
- 25.Hu W, Yuan Q, Liu XH, Zhu HC, Lv SQ, Wang XH. Cyclophilin D-mediated apoptosis attributes to sorafenib-induced cytotoxicity in clear cell-renal cell carcinoma. Eur J Pharmacol 749: 142–150, 2015. doi: 10.1016/j.ejphar.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 26.John AMSP, Kundu S, Pushpakumar S, Fordham M, Weber G, Mukhopadhyay M, Sen U. GYY4137, a hydrogen sulfide donor modulates miR194-dependent collagen realignment in diabetic kidney. Sci Rep 7: 10924, 2017. doi: 10.1038/s41598-017-11256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim EY, Anderson M, Dryer SE. Sustained activation of N-methyl-D-aspartate receptors in podoctyes leads to oxidative stress, mobilization of transient receptor potential canonical 6 channels, nuclear factor of activated T cells activation, and apoptotic cell death. Mol Pharmacol 82: 728–737, 2012. doi: 10.1124/mol.112.079376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klawitter J, Pennington A, Klawitter J, Thurman JM, Christians U. Mitochondrial cyclophilin D ablation is associated with the activation of Akt/p70S6K pathway in the mouse kidney. Sci Rep 7: 10540, 2017. doi: 10.1038/s41598-017-10076-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kundu S, Pushpakumar S, Khundmiri SJ, Sen U. Hydrogen sulfide mitigates hyperglycemic remodeling via liver kinase B1-adenosine monophosphate-activated protein kinase signaling. Biochim Biophys Acta 1843: 2816–2826, 2014. doi: 10.1016/j.bbamcr.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kundu S, Pushpakumar S, Sen U. MMP-9- and NMDA receptor-mediated mechanism of diabetic renovascular remodeling and kidney dysfunction: hydrogen sulfide is a key modulator. Nitric Oxide 46: 172–185, 2015. doi: 10.1016/j.niox.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kundu S, Pushpakumar SB, Tyagi A, Coley D, Sen U. Hydrogen sulfide deficiency and diabetic renal remodeling: role of matrix metalloproteinase-9. Am J Physiol Endocrinol Metab 304: E1365–E1378, 2013. doi: 10.1152/ajpendo.00604.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwong JQ, Molkentin JD. Physiological and pathological roles of the mitochondrial permeability transition pore in the heart. Cell Metab 21: 206–214, 2015. doi: 10.1016/j.cmet.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee HJ, Lee DY, Mariappan MM, Feliers D, Ghosh-Choudhury G, Abboud HE, Gorin Y, Kasinath BS. Hydrogen sulfide inhibits high glucose-induced NADPH oxidase 4 expression and matrix increase by recruiting inducible nitric oxide synthase in kidney proximal tubular epithelial cells. J Biol Chem 292: 5665–5675, 2017. doi: 10.1074/jbc.M116.766758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta 1787: 1395–1401, 2009. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leung JC, Travis BR, Verlander JW, Sandhu SK, Yang SG, Zea AH, Weiner ID, Silverstein DM. Expression and developmental regulation of the NMDA receptor subunits in the kidney and cardiovascular system. Am J Physiol Regul Integr Comp Physiol 283: R964–R971, 2002. doi: 10.1152/ajpregu.00629.2001. [DOI] [PubMed] [Google Scholar]
- 36.Li H, Zhang C, Sun W, Li L, Wu B, Bai S, Li H, Zhong X, Wang R, Wu L, Xu C. Exogenous hydrogen sulfide restores cardioprotection of ischemic post-conditioning via inhibition of mPTP opening in the aging cardiomyocytes. Cell Biosci 5: 43, 2015. doi: 10.1186/s13578-015-0035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lightner DD, De Braganca K, Gilheeney SW, Khakoo Y, Kramer K, Balas M. A case of mannitol hypersensitivity. J Pediatr Hematol Oncol 35: e274–e275, 2013. doi: 10.1097/MPH.0b013e31828d5b3e. [DOI] [PubMed] [Google Scholar]
- 38.Ly LD, Xu S, Choi SK, Ha CM, Thoudam T, Cha SK, Wiederkehr A, Wollheim CB, Lee IK, Park KS. Oxidative stress and calcium dysregulation by palmitate in type 2 diabetes. Exp Mol Med 49: e291, 2017. doi: 10.1038/emm.2016.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsumura M. Mannitol-induced toxicity in a diabetic patient receiving losartan. Am J Med 110: 331, 2001. doi: 10.1016/S0002-9343(00)00726-9. [DOI] [PubMed] [Google Scholar]
- 40.Najafi M, Farajnia S, Mohammadi M, Badalzadeh R, Ahmadi Asl N, Baradaran B, Amani M. Inhibition of mitochondrial permeability transition pore restores the cardioprotection by postconditioning in diabetic hearts. J Diabetes Metab Disord 13: 106, 2014. doi: 10.1186/s40200-014-0106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niimi K, Yasui T, Hirose M, Hamamoto S, Itoh Y, Okada A, Kubota Y, Kojima Y, Tozawa K, Sasaki S, Hayashi Y, Kohri K. Mitochondrial permeability transition pore opening induces the initial process of renal calcium crystallization. Free Radic Biol Med 52: 1207–1217, 2012. doi: 10.1016/j.freeradbiomed.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev 81: 1415–1459, 2001. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- 43.Nowak N, Skupien J, Niewczas MA, Yamanouchi M, Major M, Croall S, Smiles A, Warram JH, Bonventre JV, Krolewski AS. Increased plasma kidney injury molecule-1 suggests early progressive renal decline in non-proteinuric patients with type 1 diabetes. Kidney Int 89: 459–467, 2016. doi: 10.1038/ki.2015.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ozturk G, Erdogan E, Ozturk M, Cengiz N, Him A. Differential analysis of effect of high glucose level in the development of neuropathy in a tissue culture model of diabetes mellitus: role of hyperosmolality. Exp Clin Endocrinol Diabetes 116: 582–591, 2008. doi: 10.1055/s-2008-1065334. [DOI] [PubMed] [Google Scholar]
- 45.Pavenstädt H, Henger A, Briner V, Fischer KG, Huber-Lang M, Schollmeyer P, Greger R. Agonist-induced activation of a non-selective ion current in glomerular endothelial cells. Kidney Int 52: 157–164, 1997. doi: 10.1038/ki.1997.315. [DOI] [PubMed] [Google Scholar]
- 46.Pouokam E, Diener M. Mechanisms of actions of hydrogen sulphide on rat distal colonic epithelium. Br J Pharmacol 162: 392–404, 2011. doi: 10.1111/j.1476-5381.2010.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Predmore BL, Lefer DJ, Gojon G. Hydrogen sulfide in biochemistry and medicine. Antioxid Redox Signal 17: 119–140, 2012. doi: 10.1089/ars.2012.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qi H, Casalena G, Shi S, Yu L, Ebefors K, Sun Y, Zhang W, D’Agati V, Schlondorff D, Haraldsson B, Böttinger E, Daehn I. Glomerular endothelial mitochondrial dysfunction is essential and characteristic of diabetic kidney disease susceptibility. Diabetes 66: 763–778, 2017. doi: 10.2337/db16-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qi Z, Fujita H, Jin J, Davis LS, Wang Y, Fogo AB, Breyer MD. Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes 54: 2628–2637, 2005. doi: 10.2337/diabetes.54.9.2628. [DOI] [PubMed] [Google Scholar]
- 50.Reidy K, Kang HM, Hostetter T, Susztak K. Molecular mechanisms of diabetic kidney disease. J Clin Invest 124: 2333–2340, 2014. doi: 10.1172/JCI72271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roshanravan H, Kim EY, Dryer SE. NMDA receptors as potential therapeutic targets in diabetic nephropathy: increased renal NMDA receptor subunit expression in akita mice and reduced nephropathy following sustained treatment with memantine or MK-801. Diabetes 65: 3139–3150, 2016. doi: 10.2337/db16-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rueda CB, Llorente-Folch I, Traba J, Amigo I, Gonzalez-Sanchez P, Contreras L, Juaristi I, Martinez-Valero P, Pardo B, Del Arco A, Satrustegui J. Glutamate excitotoxicity and Ca2+-regulation of respiration: Role of the Ca2+ activated mitochondrial transporters (CaMCs). Biochim Biophys Acta 1857: 1158–1166, 2016. doi: 10.1016/j.bbabio.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 53.Sabbisetti VS, Waikar SS, Antoine DJ, Smiles A, Wang C, Ravisankar A, Ito K, Sharma S, Ramadesikan S, Lee M, Briskin R, De Jager PL, Ngo TT, Radlinski M, Dear JW, Park KB, Betensky R, Krolewski AS, Bonventre JV. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J Am Soc Nephrol 25: 2177–2186, 2014. doi: 10.1681/ASN.2013070758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sadar S, Kaspate D, Vyawahare N. Protective effect of L-glutamine against diabetes-induced nephropathy in experimental animal: Role of KIM-1, NGAL, TGF-β1, and collagen-1. Ren Fail 38: 1483–1495, 2016. doi: 10.1080/0886022X.2016.1227918. [DOI] [PubMed] [Google Scholar]
- 55.Sanchez-Perez A, Llansola M, Cauli O, Felipo V. Modulation of NMDA receptors in the cerebellum. II. Signaling pathways and physiological modulators regulating NMDA receptor function. Cerebellum 4: 162–170, 2005. doi: 10.1080/14734220510008003. [DOI] [PubMed] [Google Scholar]
- 56.Satoh T, Saotome M, Katoh H, Nonaka D, Hasan P, Hayashi H, Maekawa Y. Intracellular renin inhibits mitochondrial permeability transition pore via activated mitochondrial extracellular signal-regulated kinase (ERK) 1/2 during ischemia in diabetic hearts. Int J Mol Sci 19: 19, 2017. doi: 10.3390/ijms19010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen J, Wang R, He Z, Huang H, He X, Zhou J, Yan Y, Shen S, Shao X, Shen X, Weng C, Lin W, Chen J. NMDA receptors participate in the progression of diabetic kidney disease by decreasing Cdc42-GTP activation in podocytes. J Pathol 240: 149–160, 2016. doi: 10.1002/path.4764. [DOI] [PubMed] [Google Scholar]
- 59.Shi J, Qian J, Li H, Luo H, Luo W, Lin Z. Renal tubular epithelial cells injury induced by mannitol and its potential mechanism. Ren Fail 40: 85–91, 2018. doi: 10.1080/0886022X.2017.1419973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strutyns’ka NA, Dorofeieva NO, Vavilova HL, Sahach VF. [Hydrogen sulfide inhibits Ca(2+)-induced mitochondrial permeability transition pore opening in spontaneously hypertensive rats]. Fiziol Zh 59: 3–10, 2013. doi: 10.15407/fz59.01.003. [DOI] [PubMed] [Google Scholar]
- 61.Strutyns’ka NA, Semenykhina OM, Chorna SV, Vavilova HL, Sahach VF. [Hydrogen sulfide inhibits Ca(2+)-induced mitochondrial permeability transition pore opening in adult and old rat heart]. Fiziol Zh 57: 3–14, 2011. [PubMed] [Google Scholar]
- 62.Tang G, Zhang L, Yang G, Wu L, Wang R. Hydrogen sulfide-induced inhibition of L-type Ca2+ channels and insulin secretion in mouse pancreatic beta cells. Diabetologia 56: 533–541, 2013. doi: 10.1007/s00125-012-2806-8. [DOI] [PubMed] [Google Scholar]
- 63.Tran QK, Ohashi K, Watanabe H. Calcium signalling in endothelial cells. Cardiovasc Res 48: 13–22, 2000. doi: 10.1016/S0008-6363(00)00172-3. [DOI] [PubMed] [Google Scholar]
- 64.Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 62: 405–496, 2010. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Velasco-Xolalpa ME, Barragán-Iglesias P, Roa-Coria JE, Godínez-Chaparro B, Flores-Murrieta FJ, Torres-López JE, Araiza-Saldaña CI, Navarrete A, Rocha-González HI. Role of hydrogen sulfide in the pain processing of non-diabetic and diabetic rats. Neuroscience 250: 786–797, 2013. doi: 10.1016/j.neuroscience.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 66.Visweswaran P, Massin EK, Dubose TD Jr. Mannitol-induced acute renal failure. J Am Soc Nephrol 8: 1028–1033, 1997. [DOI] [PubMed] [Google Scholar]
- 67.Wang J, Sun Z, Wang Y, Wang H, Guo Y. The role and mechanism of glutamic NMDA receptor in the mechanical hyperalgesia in diabetic rats. Neurol Res 39: 1006–1013, 2017. doi: 10.1080/01616412.2017.1364515. [DOI] [PubMed] [Google Scholar]
- 68.Wong TY, Phillips AO, Witowski J, Topley N. Glucose-mediated induction of TGF-beta 1 and MCP-1 in mesothelial cells in vitro is osmolality and polyol pathway dependent. Kidney Int 63: 1404–1416, 2003. doi: 10.1046/j.1523-1755.2003.00883.x. [DOI] [PubMed] [Google Scholar]
- 69.Yin H, Wang W, Yu W, Li J, Feng N, Wang L, Wang X. Changes in synaptic plasticity and glutamate receptors in Type 2 diabetic KK-Ay mice. J Alzheimers Dis 57: 1207–1220, 2017. doi: 10.3233/JAD-160858. [DOI] [PubMed] [Google Scholar]
- 70.Yin WL, He JQ, Hu B, Jiang ZS, Tang XQ. Hydrogen sulfide inhibits MPP(+)-induced apoptosis in PC12 cells. Life Sci 85: 269–275, 2009. doi: 10.1016/j.lfs.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 71.Yuan YQ, Wang YL, Yuan BS, Yuan X, Hou XO, Bian JS, Liu CF, Hu LF. Impaired CBS-H2S signaling axis contributes to MPTP-induced neurodegeneration in a mouse model of Parkinson’s disease. Brain Behav Immun 67: 77–90, 2018. doi: 10.1016/j.bbi.2017.07.159. [DOI] [PubMed] [Google Scholar]
- 72.Zhang J, Duarte CG, Ellis S. Contrast medium- and mannitol-induced apoptosis in heart and kidney of SHR rats. Toxicol Pathol 27: 427–435, 1999. doi: 10.1177/019262339902700406. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Q, Fu H, Zhang H, Xu F, Zou Z, Liu M, Wang Q, Miao M, Shi X. Hydrogen sulfide preconditioning protects rat liver against ischemia/reperfusion injury by activating Akt-GSK-3β signaling and inhibiting mitochondrial permeability transition. PLoS One 8: e74422, 2013. doi: 10.1371/journal.pone.0074422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao X, Zhang Y, Li L, Mann D, Imig JD, Emmett N, Gibbons G, Jin LM. Glomerular expression of kidney injury molecule-1 and podocytopenia in diabetic glomerulopathy. Am J Nephrol 34: 268–280, 2011. doi: 10.1159/000330187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou X, Feng Y, Zhan Z, Chen J. Hydrogen sulfide alleviates diabetic nephropathy in a streptozotocin-induced diabetic rat model. J Biol Chem 289: 28827–28834, 2014. doi: 10.1074/jbc.M114.596593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou Y, Greka A. Calcium-permeable ion channels in the kidney. Am J Physiol Renal Physiol 310: F1157–F1167, 2016. doi: 10.1152/ajprenal.00117.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zoratti M, Szabò I, De Marchi U. Mitochondrial permeability transitions: how many doors to the house? Biochim Biophys Acta 1706: 40–52, 2005. doi: 10.1016/j.bbabio.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 78.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 94: 909–950, 2014. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]