Abstract
Doxorubicin (Dox)-induced cardiac side effects are regulated through increased oxidative stress and apoptosis. However, it remains unknown whether Dox induces the specific inflammatory-mediated form of cell death called pyroptosis. The current study is undertaken to determine whether Dox induces pyroptosis in an in vitro model and to test the potential of exosomes derived from embryonic stem cells (ES-Exos) in inhibiting pyroptosis. H9c2 cells were exposed to Dox to generate pyroptosis and then subsequently treated with exosomes to investigate the protective effects of ES-Exos. Mouse embryonic fibroblast-exosomes (MEF-Exos) were used as a cell line control. We confirmed pyroptosis by analyzing the presence of Toll-like receptor 4 (TLR4)-pyrin domain containing-3 (NLRP3) inflammasome that initiates pyroptosis, which was further confirmed with pyroptotic markers caspase-1, IL-1β, caspase-11, and gasdermin-D. The presence of inflammation was confirmed for proinflammatory cytokines, TNF-α, and IL-6. Our data show that Dox exposure significantly (P < 0.05) increases expression of TLR4, NLRP3, pyroptotic markers (caspase-1, IL-1β, caspase-11, and gasdermin-D), and proinflammatory cytokines (TNF-α and IL-6) in H9c2 cells. The increased expression of inflammasome, pyroptosis, and inflammation was significantly (P < 0.05) inhibited by ES-Exos. Interestingly, our cell line control, MEF-Exos, did not show any protective effects. Furthermore, our cytokine array data suggest increased anti-inflammatory (IL-4, IL-9, and IL-13) and decreased proinflammatory cytokines (Fas ligand, IL-12, and TNF-α) in ES-Exos, suggesting that anti-inflammatory cytokines might be mediating the protective effects of ES-Exos. In conclusion, our data show that Dox induces pyroptotic cell death in the H9c2 cell culture model and is attenuated via treatment with ES-Exos.
NEW & NOTEWORTHY Doxorubicin (Dox)-induced cardiotoxicity is mediated through increased oxidative stress, apoptosis, and necrosis. We report for the first time as per the best of our knowledge that Dox initiates Toll-like receptor 4 and pyrin domain containing-3 inflammasome formation and induces caspase-1-mediated inflammatory pyroptotic cell death in H9c2 cells. Moreover, we establish that inflammation and pyroptosis is inhibited by embryonic stem cell-derived exosomes that could be used as a future therapeutic option to treat Dox-induced cardiotoxicity.
Keywords: cardiotoxicity, doxorubicin, exosomes, H9c2 cells, inflammation, pyroptosis, stem cells
INTRODUCTION
Doxorubicin (Dox) is an antineoplastic agent with activity against a wide variety of solid organ tumors and hematological malignancies, including breast cancer, lung cancer, leukemia, and lymphoma (25, 29, 82). This chemotherapeutic drug kills cancer cells primarily through DNA intercalation, oxidative stress, and inhibition of topoisomerase II (17, 55, 77, 85); however, it can cause unwanted cell death in healthy tissues such as muscle (64, 73, 79, 86). Moreover, Dox causes acute and chronic, dose-dependent cardiotoxicity and heart failure (29, 70, 97). Dox-induced cardiotoxicity (DIC) involves apoptosis and necrosis (2, 31, 43, 97); however, it remains unknown if Dox induces a new form of inflammatory-mediated cell death called pyroptosis in the heart. This study was established to determine whether Dox induces Toll-like receptor-4 (TLR4)-pyrin domain containing-3 (NLRP3) inflammasome-mediated pyroptosis in H9c2 cells.
DIC and heart failure have been attenuated with various pharmacological interventions such as antioxidants, β-adrenergic antagonists, angiotensin antagonists, β-blockers, erythropoietin, and heart transplantation (53, 81). However, these treatment options are limited because of cost, availability of donors, and risk of immune response (83, 100). Previous studies have reported that transplanted stem cells, particularly embryonic stem cells (ES cells) have the potential to treat many diseases, including cardiovascular disorders (68, 70, 71). The major restrictive side effect of ES cell therapy is teratoma formation (tumorigenesis) because of pluripotency (18, 52, 76). Recently, it has been of significant clinical interest to find a safe but effective alternative to ES cell therapy. Recent studies have introduced administration of ES cell-derived exosomes (ES-Exos) as a novel therapeutic approach (26, 54, 69). Exosomes (Exos) are 30–100-nm diameter cell-derived vesicles that contain proteins, pro- and anti-inflammatory cytokines, lipids, growth factors, and micro-RNAs (27, 79). Notably, these Exos are cell-free carriers with the ability to circumvent the major challenge of ES cells: teratoma formation (69, 74, 91). It has been reported that ES-Exos are protective in injured myocardium and play a role in cardiac regeneration (35, 92). However, whether ES-Exos inhibit Dox-induced pyroptosis in H9c2 cells remains unknown.
Therefore, in the present study we developed an in vitro cell culture model of Dox-induced pyroptosis in H9c2 cardiomyoblasts. We investigated the effect of Dox treatment on pyroptotic cell death and assessed the therapeutic ability of ES-Exos. Our data demonstrated a significant enhancement in pyroptotic cell death and inflammation following Dox administration and that ES-Exos significantly inhibit pyroptosis and inflammation in H9c2 cells.
MATERIALS AND METHODS
Exo isolation and characterization.
ES cells (CGR8, a mouse ES cell line) and mouse embryonic fibroblast (MEF) cells were purchased from the American Type Culture Collection and cultured as previously described (35, 79). In brief, ES cells were cultured in a feeder-free culture using gelatin-coated 100-mm2 tissue culture plates (Thermo Fisher Scientific) with Dulbecco’s modified Eagle’s medium (DMEM, Thermo Fisher Scientific) supplemented with 15% ES-fetal bovine serum (FBS, Gibco), leukemia inhibitory factor, glutamine, penicillin-streptomycin (P/S), sodium pyruvate (NaP), and β-mercaptoethanol. MEF cells were cultured in 100-mm2 plates and grown with DMEM supplemented with 10% FBS (Gibco), glutamine, NaP, and P/S. After 48 h, cells were ~80% confluent. ES and MEF cell culture medium was replaced with serum-free knockout DMEM (Thermo Fisher Scientific). Cells were grown in serum-free medium for an additional 48 h. Cell culture medium was collected and centrifuged at 1,500 revolutions/min for 15 min to remove any cell debris. Supernatant was transferred to a sterile tube and mixed with Exoquick-TC Exo precipitation solution (SBI) in a 5:1 ratio for 12 h at 4°C according to manufacturer’s instruction. Following centrifugation, supernatant was aspirated and the precipitated Exos pelleted to characterize Exos, and Western blot (WB) analysis was performed for Exo protein biomarkers, CD63 and heat shock protein 70 (HSP70), as previously described (101). Briefly, proteins were extracted from the precipitated pellet as well as aspirated supernatant through resuspension in radioimmunoprecipitation assay lysis buffer. The 50 µg of extracted protein were separated through 10% gel electrophoresis and then subjected to immunoblotting using CD63 (1:1,000; SBI; cat. no. EXOAB-CD63A-1) (23, 44) and HSP70 (1:1,000; SBI; cat. no. EXOAB-HSP70A-1) (23, 44) antibodies. ImageJ program was used for densitometry analysis. Data are presented as arbitrary units (AU).
H9c2 cell culture model.
H9c2 cardiomyoblasts were purchased from the American Type Culture Collection and maintained in cell culture flasks. Once ~80% confluency was reached, cells were cultured in 100-mm2 tissue culture plates (5 × 105 cells/plate; passage number 8–9) and in 8-chamber polystyrene vessel tissue culture glass slides (10,000 cells/chamber; Falcon) using DMEM supplemented with 10% FBS, glutamine, NaP, and P/S for 48 h. Cells were assigned into six different study groups: control (only medium), ES-Exos (baseline control), MEF-Exos (baseline control), Dox (2-µM concentration, 24-h incubation), Dox + ES-Exos (10-µg concentration, 24-h incubation), and Dox + MEF-Exos (10-µg concentration, 24-h incubation; used as a cell line control). Cells were treated with a 2-µM concentration of Dox (Sigma) as reported by other investigators (45, 60, 66, 75) for 24 h to induce cell death. Cells were cultured for an additional 24 h, followed by replacing medium with fresh cell culture medium and or medium containing ES-Exos or MEF-Exos in different groups (79).
Immunocytochemistry staining.
H9c2 cells cultured in eight-chamber slides were fixed with 4% paraformaldehyde (Thermo Fisher Scientific) for 20 min and 0.3% Triton X-100 (Thermo Fisher Scientific) for 15 min before blocking with 10% normal goat serum (Vector). Slides were then incubated with primary antibodies for TLR4 (1:100; cat. no. ab13556; Abcam), nucleotide-binding oligomerization domain-like receptors, NLRP3 (1:80; LSBio; cat. no. LS-B4321), caspase-1 (1:100; cat. no. ab138483; Abcam), and IL-1β (1:100; cat. no. ab2105; Abcam) overnight at 4°C. Following primary incubation, cells were washed in PBS and incubated with secondary antibody, Alexa 568 goat anti-rabbit (1:50; cat. no. A11011; Invitrogen), for 1 h at room temperature (RT). After washing was completed, mounting medium containing DAPI (Vector) was applied to stain nuclei, and slides were coverslipped. Images for presentation or quantification were taken using Keyence or Olympus fluorescent microscope. Images were merged using ImageJ software, and the percentage of pyroptotic cell death was calculated by dividing total positive cells over total DAPI multiplied by 100 [(total cells+ve/total DAPI) × 100]. Graphs were created using Sigma Plot.
SDS-PAGE and WB analysis.
WB analysis was performed as previously published (49). In brief, H9c2 cells were lysed using radioimmunoprecipitation assay cell lysis buffer, and the cell lysate was collected. Protein concentration was measured using the Bradford assay. Samples were read using a Bio-Rad plate reader. Proteins (70 µg) were loaded in 10% or 15% SDS gels (150 V for 60–90 min), and then gels were transferred to PVDF (Bio-Rad) membranes using transfer buffer and a semidry transfer machine (Bio-Rad). Membranes were blocked with 5% skim milk and incubated with primary antibodies for TLR4 (1:1,000; Abcam; cat. no. ab13556) (14, 88), NLRP3 (1:1,000; Abcam; cat. no. ab214185) (8, 24), caspase-1 (1:1,000; Abcam; cat. no. ab138483) (8, 28), IL-1β (1:1,000; Abcam; cat. no. ab2105) (19, 94), caspase-11 (1:1,000; Abcam; cat. no. ab180673) (34, 87), gasdermin-D (1:1,000; Abcam; cat. no. ab209845) (15, 57), TNF-α (1:1,000; Abcam; cat. no. ab6671) (98, 102), IL-6 (7, 102) (1:1,000; Abcam; cat. no. ab6672), and β-actin (1:1,000, Cell Signaling; cat. no. 4967L, used as loading control) (9, 65) overnight at 4°C. After being washed with 1 × Tris-buffered saline-Tween 20, membranes were incubated with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:1,000, Cell Signaling; cat. no. 7074S) (38, 78) for 1 h at RT. Following exposure with chemiluminescence ECL substrates (Thermo Fisher Scientific), membranes were developed using X-ray film. β-Actin was used as a loading control, densitometric analysis was performed using ImageJ, and graphs were created using Sigma Plot (band intensity quantities are represented as AU).
Enzyme-linked immunosorbent assay.
Inflammatory cytokine levels were measured using mouse ELISA kits for TNF-α (Ray Biotech; cat. no. ELH-TNFα-001) and IL-6 (Ray Biotech; cat. no. ELM-IL6) according to the manufacturer’s protocol. In brief, 1:2 diluted supernatant from each group was incubated in precoated wells for 2 h at RT. After being washed with 1 × wash buffer, TNF-α and IL-6 primary antibodies (1:80 dilution) were added to wells for 1 h at RT. After incubation with primary antibodies, horseradish peroxidase-conjugated secondary antibody (1:200 dilution) was added and incubated for 45 min at RT. Finally, developing substrate and stop solution were added to each well, and the plate was read at 450 nm using a Bio-Rad plate reader. Raw data were normalized with a standard curve, and quantities were expressed as AU.
Inflammation cytokine array analysis.
The cytokine expression profiles of ES-Exos and MEF-Exos were analyzed using inflammation cytokine array C1 (cat. no. AAM-INF-1–2; Ray Biotech). ES-Exos and MEF-Exos were used to perform cytokine array according to the manufacturer's instructions. ImageJ was used for densitometric analysis, and values were normalized to MEF-Exos.
Statistical analysis.
Statistical analysis performed using one-way ANOVA was followed by Tukey test and expressed as means ± SE. P < 0.05 was considered statistically significant.
RESULTS
Identification of Exos using protein markers CD63 and HSP70.
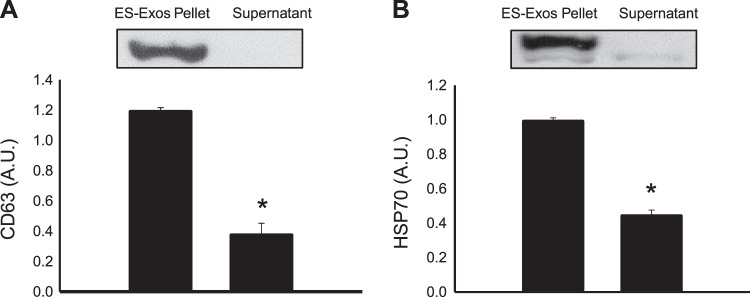
To confirm the presence of ES-Exos in the cell pellet, WB analysis was performed for the Exo markers CD63 and HSP70. Our WB data show prominent band in ES-Exos pellet for CD63 (Fig. 1A) and HSP70 (Fig. 1B) but not in the supernatant. Densitometric analysis shows a significant increase (P < 0.05) in CD63 (Fig. 1A) and HSP70 (Fig. 1B) protein expression in ES-Exos pellet as compared with the cell culture supernatant, suggesting the presence of Exos in the pellet.
Fig. 1.
Exosome protein identification. Western blot densitometric analysis and representative pictures for exosome protein biomarkers CD63 (A) and heat shock protein 70 (HSP70) (B) are shown. Statistical analysis was performed using one-way ANOVA, followed by the Tukey test. Quantities are represented as arbitrary units (AU). *P < 0.05 vs. embryonic stem cell-derived exosomes (ES-Exos) pellet.
Dox increases TLR4 activation and NLRP3 inflammasome formation in H9c2 cells.
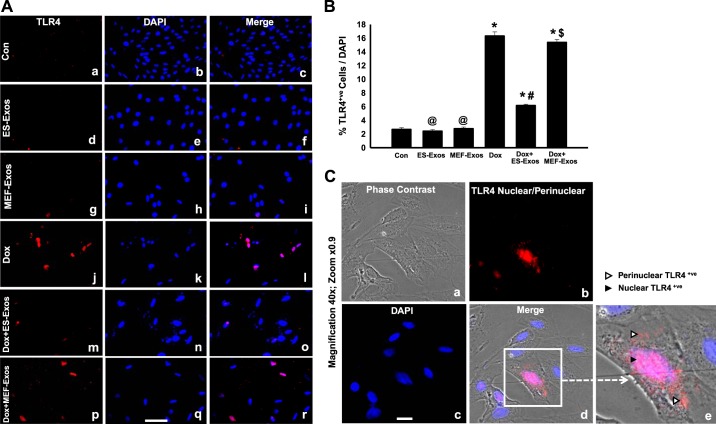
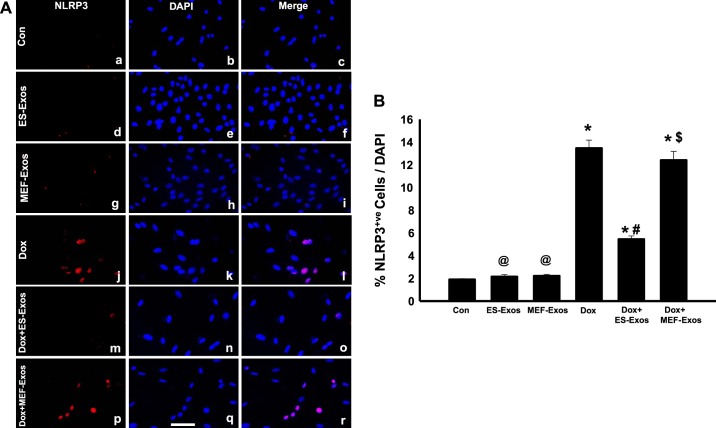
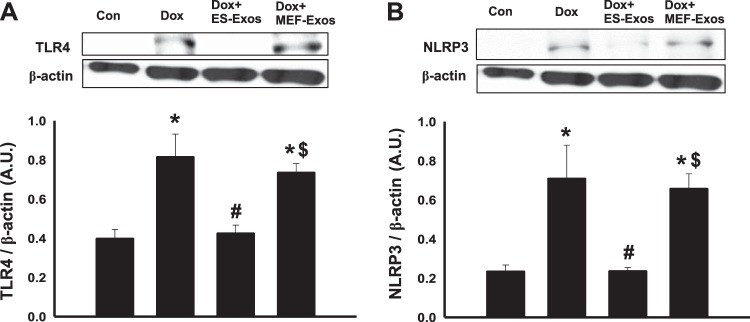
Immunofluorescent staining was performed to determine whether Dox is involved in stimulation of TLR4 and generation of the NLRP3 inflammasome in H9c2 cells. Our immunocytochemistry representative photomicrograph (Fig. 2A) indicates higher expression of TLR4 in the Dox group as compared with the cell culture and baseline controls (H9c2 cells, H9c2 cells + ES-Exos, and H9c2 cells + MEF-Exos). Next, our quantitative data show a significant increase (P < 0.05) of TLR4 in Dox-treated cells compared with controls (Fig. 2B). Furthermore, a phase contrast immunofluorescent microscopy picture of Dox-treated cells indicates a presence of H9c2 cells positive with nuclear/perinuclear staining of TLR4 (Fig. 2C), which is consistent with previously published studies on TLR4 nuclear/perinuclear localization (58, 62). Merged image and ×40 zoom shows pinkish red nuclei DAPI positive with TLR4 suffering from pyroptotic cell death. To determine whether TLR4 is able to trigger the formation of NLRP3 inflammasome, we performed NLRP3 immunofluorescent staining. Our representative staining shows increased expression of positive NLRP3 cells after Dox exposure (Fig. 3A). Upon NLRP3 quantification, we observed a significant increase (P < 0.05, Fig. 3B) in NLRP3-positive cells in Dox-treated cells versus cell culture and baseline controls. To confirm immunocytochemistry data, we performed WB analysis for TLR4 and NLRP3 markers. WB densitometric analysis showed that expression of TLR4 (Fig. 4A) and NLRP3 (Fig. 4B) was significantly increased (P < 0.05) in Dox-treated H9c2 cells compared with the control, respectively.
Fig. 2.
Treatment with embryonic stem cell-derived exosomes (ES-Exos) reduces activation of Toll-like receptor 4 (TLR4) receptor after doxorubicin (Dox) administration. TLR4 representative fluorescent imaging (A) taken with Keyence microscopy for all groups that exhibit TLR4-positive cells are in red (a, d, g, j, m, and p), DAPI in blue (b, e, h, k, n, and q), and merged images (c, f, i, l, o, and r). Bar graphs (B) show quantitative immunocytochemistry data for TLR4+ve cells over total DAPI, respectively. Phase contrast fluorescent imaging (C) of Dox-treated cells, which represents phase contrast picture (a), TLR4 nuclear/perinuclear localization (b), DAPI (c), merged image (d), and enlarged region of merged image (e). White arrowhead implies perinuclear, and black arrowhead marks nuclear TLR4+ve staining. Statistical analysis was performed using one-way ANOVA and Tukey test. *P < 0.05 vs. control, @P = nonsignificant (NS) vs. control, #P < 0.05 vs. Dox, $P = NS vs. Dox. Scale bar = 100 µm; magnification ×40, zoom ×0.9. n = 6 for all groups. Con, control; MEF-Exos, mouse embryonic fibroblast-exosomes.
Fig. 3.
Embryonic stem cell-derived exosomes (ES-Exos) inhibit generation of pyrin domain containing-3 (NLRP3) inflammasome following doxorubicin (Dox) administration in H9c2 cells. NLRP3 representative Keyence fluorescent imaging (A). NLRP3-positive cells in red (a, d, g, j, m, and p), DAPI in blue (b, e, h, k, n, and q), and merged images (c, f, i, l, o, and r). Bar graph (B) shows quantitative immunocytochemistry data for NLRP3+ve cells over total DAPI, respectively. Statistical analysis was performed using one-way ANOVA and Tukey test. *P < 0.05 vs. control, @P = nonsignificant (NS) vs. control, #P < 0.05 vs. Dox, $P = NS vs. Dox. Scale bar = 100 µm; n = 6. Con, control; MEF-Exos, mouse embryonic fibroblast-exosomes.
Fig. 4.
Embryonic stem cell-derived exosomes (ES-Exos) attenuate expression of inflammasome [Toll-like receptor 4 (TLR4) and pyrin domain containing-3 (NLRP3)] markers following doxorubicin (Dox) administration in H9c2 cells. Western blot densitometric analysis and representative pictures for TLR4 (A) and NLRP3 (B) markers are shown. Statistical analysis was performed using one-way ANOVA, which was followed by Tukey test. Quantities are represented as arbitrary units (AU). *P < 0.05 vs. control, #P < 0.05 vs. Dox, $P = nonsignificant vs. Dox. n = 4 for all groups evaluated for TLR4; n = 4, 3, 4, and 4 for NLRP3. Con, control; MEF-Exos, mouse embryonic fibroblast-exosomes.
Dox induces pyroptosis in H9c2 cells.
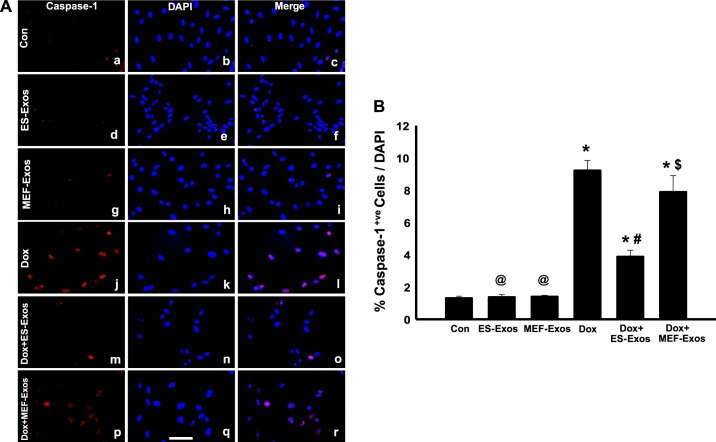
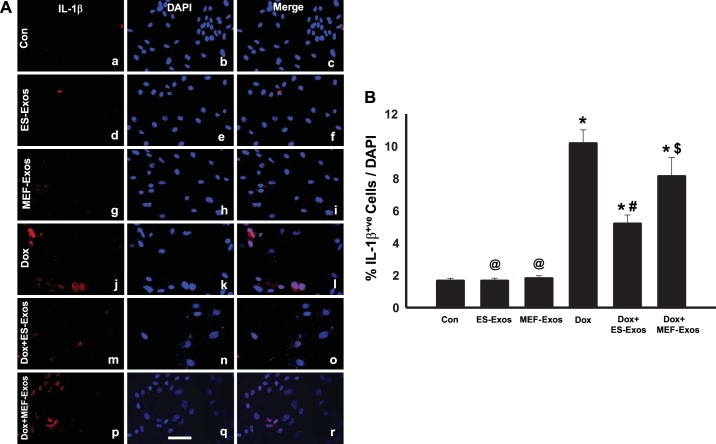
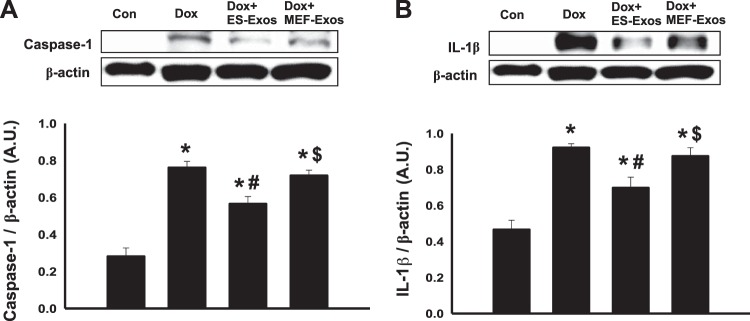
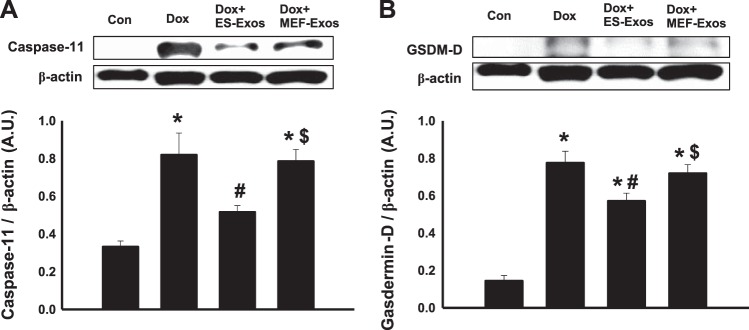
To understand if Dox induces NLRP3-mediated pyroptosis in H9c2 cells, fluorescent staining was performed for pyroptotic markers caspase-1 and IL-1β. Caspase-1 staining results revealed higher expression of caspase-1 (Fig. 5A) as well as a significant (P < 0.05) increase in percentage of caspase-1+ve cells (Fig. 5B) upon Dox exposure. Moreover, IL-1β expression was increased in the Dox treatment group (Fig. 6A), and quantification of IL-1β+ve cells in the Dox group indicates a significantly higher (P < 0.05) percentage as compared with the cell culture and baseline controls (Fig. 6B). Furthermore, WB analysis showed a significant increase (P < 0.05) in expression of caspase-1 (Fig. 7A) and IL-β (Fig. 7B) proteins following Dox administration. Moreover, to further confirm the involvement of pyroptosis, additional markers such as caspase-11 and gasdermin-D were also analyzed using WB. Densitometric analysis indicates that caspase-11 and gasdermin-D significantly increase (P < 0.05) in the Dox-treated H9c2 cells as compared with the control group (Fig. 8, A and B, respectively).
Fig. 5.
Embryonic stem cell-derived exosomes (ES-Exos) inhibit doxorubicin (Dox)-induced pyroptosis in H9c2 cells. Representative imaging of caspase-1 staining (A). Caspase-1-positive cells are in red (a, d, g, j, m, and p), DAPI in blue (b, e, h, k, n, and q), and merged images (c, f, i, l, o, and r). Bar graph (B) indicates the percentage of caspase-1+ve cells over total DAPI, respectively. One-way ANOVA and Tukey test were used for statistical analysis. *P < 0.05 vs. control, @P = nonsignificant (NS) vs. control, #P < 0.05 vs. Dox, $P = NS vs. Dox. Scale bar = 100 µm; n = 6 for all groups. Con, control; MEF-Exos, mouse embryonic fibroblast-exosomes.
Fig. 6.
Embryonic stem cell-derived exosomes (ES-Exos) treatment decreases doxorubicin (Dox)-induced IL-1β secretion in H9c2 cells. Representative Keyence microscopy imaging of IL-1β immunostaining (A). IL-1β+ve cells are in red (a, d, g, j, m, and p), DAPI in blue (b, e, h, k, n, and q), and merged images (c, f, i, l, o, and r). Bar graph (B) shows the percentage of IL-1β-positive cells over total DAPI, respectively. Statistical analysis was performed using one-way ANOVA and Tukey test. *P < 0.05 vs. control, @P = nonsignificant (NS) vs. control, #P < 0.05 vs. Dox, $P = NS vs. Dox. Scale bar = 100 µm; n = 6 for all groups. Con, control; MEF-Exos, mouse embryonic fibroblast-exosomes.
Fig. 7.
Treatment with embryonic stem cell-derived exosomes (ES-Exos) reduces expression of pyroptotic markers caspase-1 and IL-1β in doxorubicin (Dox)-exposed H9c2 cells. Western blot densitometric analysis and representative pictures for caspase-1 (A) and IL-1β (B) are shown. Statistical analysis was performed using one-way ANOVA and Tukey test, and quantities are presented as arbitrary units (AU). *P < 0.05 vs. control, #P < 0.05 vs. Dox, $P = nonsignificant vs. Dox, n = 4, 4, 4, and 3 for caspase-1; n = 4 for all groups for IL-1β. Con, control; MEF-Exos, mouse embryonic fibroblast-exosomes.
Fig. 8.
Treatment with embryonic stem cell-derived exosomes (ES-Exos) reduces pore formation in H9c2 cell membrane following doxorubicin (Dox)-induced pyroptosis. Western blot analysis and representative pictures for caspase-11 (A) and gasdermin-D (B) proteins are shown. Statistical analysis was performed using one-way ANOVA and Tukey test and then represented as arbitrary units (AU). *P < 0.05 vs. control, #P < 0.05 vs. Dox, $P = nonsignificant vs. Dox. n = 5, 4, 3, and 4 for caspase-11; n = 5, 4, 4, and 3 for gasdermin-D. Con, control; MEF-Exos, mouse embryonic fibroblast-exosomes.
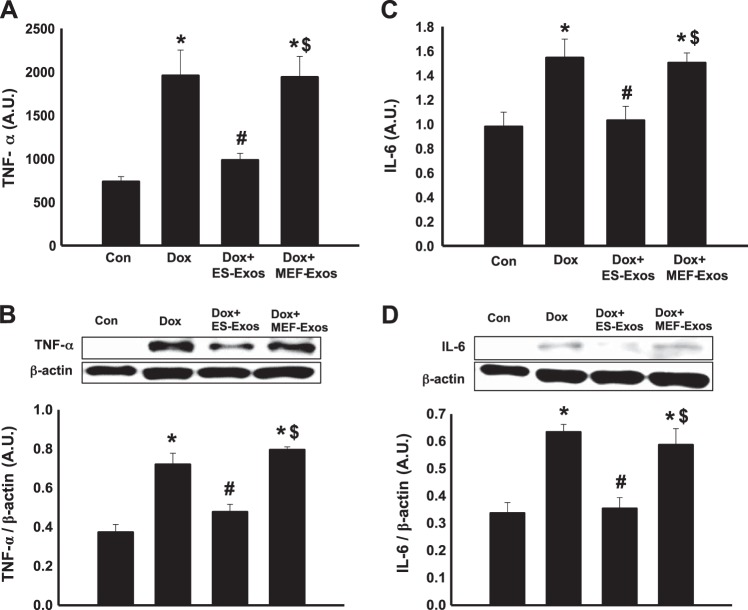
Dox increases proinflammatory cytokines TNF-α and IL-6.
To elucidate whether Dox treatment increases the secretion of proinflammatory cytokines TNF-α and IL-6, both ELISA and WB analysis were performed. Importantly, quantitative ELISA showed a significant increase (P < 0.05) in the level of secreted TNF-α (Fig. 9A) in the Dox group versus control. Moreover, the proinflammatory cytokine IL-6 was significantly increased (P < 0.05) upon Dox treatment (Fig. 9C), suggesting that Dox induced cell injury through inflammatory response. These ELISA data were further confirmed with additional WB data, which showed similar findings of increased TNF-α and IL-6 cytokine expression in Dox-treated H9c2 cells compared with control (Fig. 9, B and D).
Fig. 9.
Embryonic stem cell-derived exosomes (ES-Exos) downregulate doxorubicin (Dox)-induced proinflammatory cytokine secretion in H9c2 cells. Quantitative ELISA analysis of TNF-α (A) and IL-6 (C) are shown. Densitometric analysis and representative images for TNF-α and IL-6 markers are shown (B and D, respectively). One-way ANOVA and Tukey test were used for statistical analysis and quantities are expressed as arbitrary units (AU). *P < 0.05 vs. control, #P < 0.05 vs. Dox, $P = nonsignificant vs. Dox. n = 5 for all groups assessed via ELISA using TNF-α and IL-6 markers; n = 3–4 for TNF-α and IL-6 WB analysis. Con, control; MEF-Exos, mouse embryonic fibroblast-exosomes.
Effects of ES-Exos on TLR4 and NLRP3 inflammasome in Dox-treated H9c2 cells.
To understand the therapeutic potential of ES-Exos on inhibition of pyroptosis, H9c2 cells exposed to Dox were treated with ES-Exos. Our immunocytochemistry data indicate a significant reduction (P < 0.05) in TLR4 (Fig. 2, A and B) and NLRP3 (Fig. 3, A and B) upon ES-Exos treatment as compared with Dox. Noticeably, H9c2 cells + Dox treated with MEF-Exos did not show significant differences in comparison with Dox, suggesting that ES-Exos may contain specific protective factors compared with MEF-Exos. Furthermore, quantitative analysis of our baseline control groups, which were not exposed to Dox but treated with ES-Exos or MEF-Exos, indicated no significant changes in TLR4 (Fig. 2B) and NLRP3 (Fig. 3B) expression as compared with the control. WB analysis further corroborated that ES-Exos significantly decreased (P < 0.05) TLR4 (Fig. 4A) and NLRP3 (Fig. 4B) protein expression in the Dox group, whereas no significant change in these proteins was observed following MEF-Exos treatment.
Effects of ES-Exos on pyroptotic markers following Dox administration in H9c2 cells.
Protective effect of ES-Exos on attenuation of Dox-induced pyroptosis in H9c2 cells was investigated using immunocytochemical analysis with caspase-1 and IL-β markers, which showed a significant decrease (P < 0.05) in caspase-1+ve (Fig. 5, A and B) and IL-β+ve (Fig. 6, A and B) cells in the ES-Exos group compared with Dox. However, there was no significant reduction in pyroptotic cell death upon MEF-Exos treatment. Moreover, baseline control groups H9c2 cells + ES-Exos and H9c2 cells + MEF-Exos without Dox treatment were nonsignificant compared with cell culture control. To verify staining results, WB was performed for caspase-1 and IL-β, which indicates a significant reduction (P < 0.05) in caspase-1 (Fig. 7A) as well as IL-β (Fig. 7B) expression. Additionally, WB analysis of caspase-11 (Fig. 8A) and gasdermin-D (Fig. 8B) shows a significant decrease (P < 0.05) upon treatment with ES-Exos as compared with Dox; however, no significant changes have been reported following MEF-Exos treatment versus Dox.
Effects of ES-Exos on proinflammatory cytokine release from H9c2 cells following Dox treatment.
To clarify the protective effect of ES-Exos against inflammation, we quantified secretion of proinflammatory cytokines TNF-α and IL-6 using ELISA and WB. ELISA data show a significant downregulation (P < 0.05) in TNF-α (Fig. 9A) and IL-6 (Fig. 9C) proinflammatory cytokines upon ES-Exos administration versus Dox. Moreover, densitometric analysis of WB bands revealed a significant decrease (P < 0.05) in TNF-α (Fig. 9B) and IL-6 (Fig. 9D) expression following treatment with ES-Exos as compared with Dox. However, using both ELISA and WB analysis, H9c2 cells + Dox treatment and administration of MEF-Exos did not show any significant differences compared with the Dox group, suggesting that MEF-Exos may not have anti-inflammatory protective components.
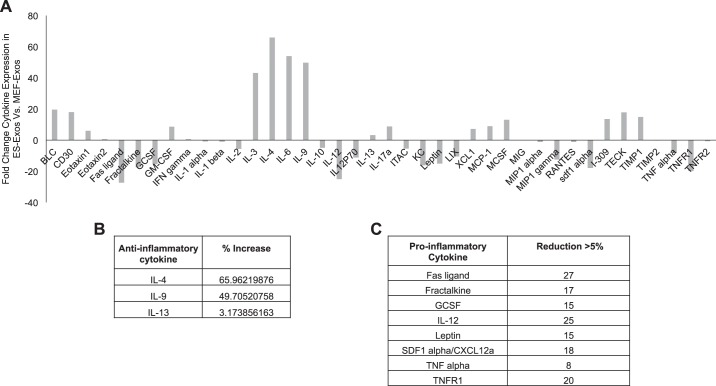
Increased anti-inflammatory and decreased proinflammatory cytokines in ES-Exos.
To understand the cell-protective components of ES-Exos in this inflammatory-induced pyroptotic cell death, we performed an inflammatory cytokine array. The cytokine arrays showed a variation in ES-Exos compared with MEF-Exos and were plotted in a histogram for detailed analysis. We observed that ES-Exos increase (3–66-fold) in anti-inflammatory cytokines (IL-4, IL-9, and IL-13; Fig. 10B) and decrease (8–27-fold) in proinflammatory cytokines (Fas ligand, Fractalkine, granulocyte colony-stimulating factor (GCSF), IL-12, leptin, stromal cell-derived factor 1 (SDF1α), TNF-α, and TNF receptor 1; Fig. 10C) as compared with MEF-Exos.
Fig. 10.
Embryonic stem cell-derived exosomes (ES-Exos) contain a higher level of anti-inflammatory and lower level of proinflammatory cytokine expression. A: densitometric analysis of inflammation cytokine array for ES-Exos that has been normalized to mouse embryonic fibroblast-exosomes (MEF-Exos). B: anti-inflammatory cytokines with >3% increase in ES-Exos vs. MEF-Exos. C: proinflammatory cytokines with >5% reduction in ES-Exos.
DISCUSSION
Cell death is a naturally occurring phenomenon; however, unwanted aberrant cell death can cause serious problems and diseases (13). Two major types of cell death that have been studied previously in the heart are apoptosis and necrosis (37, 40). Apoptosis occurs via activation of cytochrome C, formation of the apoptosome, and stimulation of caspase-3 cascade (39, 40). Characteristic apoptotic features include cell membrane blebbing, cell shrinkage, chromatin condensation, and DNA fragmentation (10). In contrast, necrosis causes damage through swelling and disruption of the cells, leading to loss of membrane integrity followed by uncontrolled release of cellular contents into the environment, promoting a strong inflammatory response in the corresponding tissue (61). Additionally, a new form of inflammatory-mediated cell death, known as pyroptosis, could play an important role in various pathological circumstances (56, 79, 99). This form of cell death is generally mediated through infection-induced inflammation as published by Yuan et al. (93). In fact, damage-associated molecular pattern molecules released through disease conditions activate the TLR4 receptor, and activation of this receptor induces formation of NLRP3 inflammasome within the cell, which results in stimulation of the caspase-1 cascade that leads to pyroptosis (3, 11, 12, 30, 47, 48, 50). Finally, activated caspase-1 triggers downstream expression of IL-1β/IL-18 cytokines and stimulation of caspase-11 and gasdermin-D proteins, which contribute in pore formation within the cell membrane, as well as secretion of proinflammatory contents from pyroptotic dead cells (3, 4, 11, 12, 21, 47, 50, 51, 56, 63).
The current study investigates for the first time whether Dox activates TLR4 and formation of the NLRP3 inflammasome, thereby activating the downstream caspase-1 cascade. This finding demonstrates initiation of pyroptosis as a new contributor in Dox-induced cell death in H9c2 cells with implications in the DIC model. Our data show that Dox-treated H9c2 cells activate the TLR4 receptor, resulting in generation of the NLRP3 inflammasome. The activation of TLR4 and NLRP3 inflammasome-mediated pyroptosis has been published in microglial cells using alcohol-fed TLR4−/− mice (in vivo) as well as in a microglial cell culture model (1). Furthermore, Lebeaupin et al. (41) reported that NLRP3 induces pyroptotic cell death in hepatocytes of ob/ob mice treated with LPS. Therefore, our data on the role of TLR4 and NLRP3 inflammasome activation in Dox-treated H9c2 cells suggest that a pyroptotic pathway is activated, which is in agreement with previously reported studies (1, 41).
Next, we also report that Dox-induced cell death in H9c2 cells involves a downstream pathway of pyroptotic markers caspase-1, IL-1β, caspase-11, and gasdermin-D. According to the study performed by Man et al. (46), caspase-1 was introduced as the major pyroptotic marker, which induces pyroptosis in response to an activated inflammasome and secrets IL-1β/IL-18 cytokines from their precursors using a caspase-1-deficient C57BL/6J mouse model. Another study performed by He et al. (20) indicates caspase-1, caspase-11, and gasdermin-D as major components involved in pyroptosis using a cell culture model. Our data on Dox-induced H9c2 pyroptotic markers corroborate previously published studies in these models (20, 46) and establishes that the observed cell death in Dox-H9c2 cells is pyroptotic in nature. This should be considered as a limitation of this study, as we were unable to determine additional evidences of pyroptosis at the ultrastructure level that determine cellular changes using electron microscopy.
Moreover, inflammasome-induced pyroptosis also contributes in secretion of proinflammatory cytokines such as TNF-α, IL-6, and IFNγ, which occur because of caspase-1-mediated cleavage of TLR4 protein (4, 33, 42). Our current study supports the previously published data (4, 33, 42) on the involvement of proinflammatory cytokines TNF-α and IL-6 in Dox-induced pyroptosis in H9c2 cells.
After establishing pyroptosis in Dox-H9c2 cells, the question arose if we can inhibit pyroptosis with ES cells, as they have been shown to inhibit apoptosis previously (68, 72). As mentioned elsewhere, ES cells pose a threat to form teratoma (52, 76); therefore, we developed a cell-free system of Exos derived from ES cells. Exos contain cell-protective components to inhibit pathophysiological alterations in the heart (80, 89, 92). Recent studies report that Exos derived from bone marrow mesenchymal stem cells and cardiac progenitor cells have shown promising cardioprotective effects by reducing oxidative stress, cardiomyocyte apoptosis, and fibrosis, as well as improving neovascularization and cardiac function (80, 89, 92). Therefore, we also tested whether ES-Exos are protective against pyroptotic cell death in Dox-H9c2 cells. Interestingly, our data show that Dox-H9c2 cells treated with ES-Exos inhibit TLR4/NLRP3 inflammasome formation, as well as pyroptotic markers and proinflammatory cytokines. To the best of our knowledge, this report is the first study to establish that ES-Exos inhibit pyroptosis in the H9c2 cell culture system. Moreover, our study is in accordance with another report that shows that ES-Exos promote cardiac regeneration and inhibit fibrosis in a myocardial infarction model (35). Our data suggest that ES-Exos are beneficial in that they are cell-free carriers of therapeutic agents (cargo transportation) which are capable of bypassing the major side effect of teratoma formation seen with ES cells (96).
Moreover, we used MEF-Exos as a cell line control in this study to confirm that MEF-Exos treatment is not protective in Dox-induced pyroptosis within H9c2 cells. This set of data further establishes that the protective effects of ES-Exos in a Dox-induced pyroptosis model are cell-specific and distinct in nature compared with MEF-Exos.
Therefore, we report for the first time that ES-Exos but not MEF-Exos contain anti-inflammatory cytokines such as IL-4 (6, 67), IL-9 (32, 59), and IL-13 (22, 90) that might be playing a significant role in cytoprotective effects as observed in ES-Exos treatment. Moreover, the additional beneficial effects observed in ES-Exos may be coming from the composition of ES-Exos, which are less inflammatory in nature, as they contain lower levels of proinflammatory cytokines such as TNF-α (5, 84), TNFR1 (84), IL-12 (16, 95), and Fas ligand (36). Furthermore, future studies are required to understand whether the presence of these anti-inflammatory cytokines have significant roles in inhibiting Dox-induced pyroptosis. It is also possible that there might be additional growth factors, micro-RNAs, phospholipids, and factors present in ES-Exos that play role in the observed cytoprotection. This study opens a new and unique area of investigation on the mechanistic role of these cell-protective components in Dox-H9c2 pyroptotic cell death.
The presence of pyroptosis in other cardiac cell types such as neonatal cardiomyocytes, endothelial cells, and vascular smooth muscle cells needs further investigation. However, we have observed the presence of pyroptosis in our in vivo (data not shown) mouse model.
In conclusion, the present study provides evidence that Dox-induced cell death in H9c2 cardiomyoblasts is pyroptotic in nature, which is mediated through the TLR4-NLRP3 inflammasome and involves inflammatory cytokine secretion. Moreover, we also establish that pyroptosis in H9c2 cells is attenuated upon ES-Exos treatment but not with MEF-Exos. We have proposed a diagram regarding pyroptosis in this model (Fig. 11). Further in vivo studies are required to determine whether DIC involves inflammatory-mediated pyroptosis in the heart.
Fig. 11.
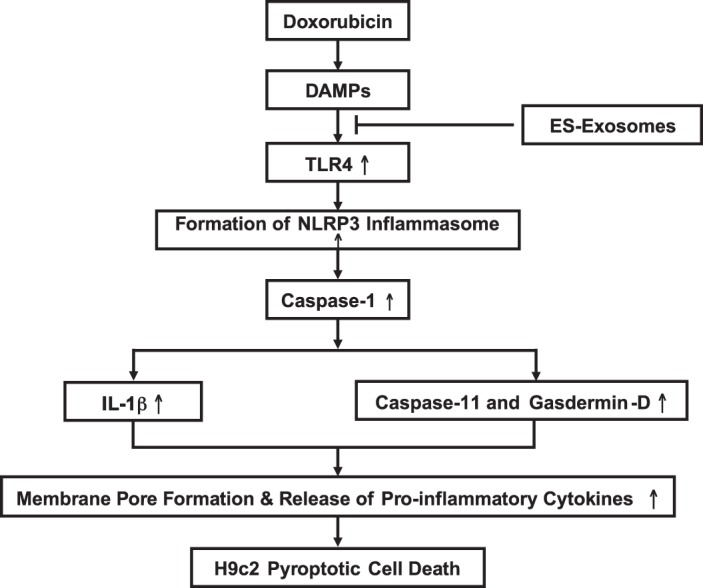
Schematic representation of doxorubicin (Dox)-induced cardiotoxicity in H9c2 cells: increased pyroptosis and inflammation. Administration of Dox activates a series of events including activation of the Toll-like receptor-4 (TLR4) receptor in response to released damage-associated molecular patterns (DAMPs), which induces generation of the nucleotide-binding oligomerization domain-like receptor pyrin domain containing-3 (NLRP3) inflammasome within the cell. The activated inflammasome stimulates the caspase-1 cascade, which triggers pore formation within the cell membrane (via activation of caspase-11 and gasdermin-D), secretion of IL-1β, and finally results in secretion of proinflammatory cytokines from the dead cell.
GRANTS
This study was supported in part by National Institutes of Health Grant R01-CA-221813 and internal support to D. K. Singla.
DISCLAIMERS
The financial support providers have no role in study design, data collection, and analysis and manuscript preparation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.K.S. conceived and designed research; Z.T.D. performed experiments; Z.T.D. analyzed data; Z.T.D. prepared figures; Z.T.D. drafted manuscript; D.K.S. interpreted results of experiments; D.K.S. edited and revised manuscript; D.K.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Kaley Garner and Jessica Hellein for proofreading the manuscript.
REFERENCES
- 1.Alfonso-Loeches S, Ureña-Peralta J, Morillo-Bargues MJ, Gómez-Pinedo U, Guerri C. Ethanol-induced TLR4/NLRP3 neuroinflammatory response in microglial cells promotes leukocyte infiltration across the BBB. Neurochem Res 41: 193–209, 2016. doi: 10.1007/s11064-015-1760-5. [DOI] [PubMed] [Google Scholar]
- 2.Arola OJ, Saraste A, Pulkki K, Kallajoki M, Parvinen M, Voipio-Pulkki LM. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res 60: 1789–1792, 2000. [PubMed] [Google Scholar]
- 3.Baldrighi M, Mallat Z, Li X. NLRP3 inflammasome pathways in atherosclerosis. Atherosclerosis 267: 127–138, 2017. doi: 10.1016/j.atherosclerosis.2017.10.027. [DOI] [PubMed] [Google Scholar]
- 4.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 7: 99–109, 2009. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charakida M, Halcox JP. Factor de necrosis tumoral alfa en la insuficiencia cardíaca: más preguntas que respuestas [Tumor necrosis factor-alpha in heart failure: more questions than answers]. Rev Esp Cardiol 58: 470–472, 2005. doi: 10.1157/13074839. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee P, Chiasson VL, Bounds KR, Mitchell BM. Regulation of the anti-inflammatory cytokines interleukin-4 and interleukin-10 during pregnancy. Front Immunol 5: 253, 2014. doi: 10.3389/fimmu.2014.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dasagrandhi D, R ASK, Muthuswamy A, Lennox AM, Jayavelu T, Devanathan V, Kesavan Swaminathan J. Ischemia/reperfusion injury in male guinea pigs: An efficient model to investigate myocardial damage in cardiovascular complications. Biomed Pharmacother 99: 469–479, 2018. doi: 10.1016/j.biopha.2018.01.087. [DOI] [PubMed] [Google Scholar]
- 8.Ding HG, Deng YY, Yang RQ, Wang QS, Jiang WQ, Han YL, Huang LQ, Wen MY, Zhong WH, Li XS, Yang F, Zeng HK. Hypercapnia induces IL-1β overproduction via activation of NLRP3 inflammasome: implication in cognitive impairment in hypoxemic adult rats. J Neuroinflammation 15: 4, 2018. doi: 10.1186/s12974-017-1051-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong B, Li D, Li R, Chen SC, Liu W, Liu W, Chen L, Chen Y, Zhang X, Tong Z, Xia Y, Xia P, Wang Y, Duan Y. A chitin-like component on sclerotic cells of Fonsecaea Pedrosoi inhibits Dectin-1-mediated murine Th17 development by masking β-glucans. PLoS One 9: e114113, 2014. [Erratum in PLoS One 10: e0119244, 2015.] doi: 10.1371/journal.pone.0114113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol 35: 495–516, 2007. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleetwood AJ, Lee MK, Singleton W, Achuthan A, Lee MC, O’Brien-Simpson NM, Cook AD, Murphy AJ, Dashper SG, Reynolds EC, Hamilton JA. Metabolic remodeling, inflammasome activation, and pyroptosis in macrophages stimulated by porphyromonas gingivalis and its outer membrane vesicles. Front Cell Infect Microbiol 7: 351, 2017. doi: 10.3389/fcimb.2017.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu Q, Wu J, Zhou XY, Ji MH, Mao QH, Li Q, Zong MM, Zhou ZQ, Yang JJ. NLRP3/Caspase-1 pathway-induced pyroptosis mediated cognitive deficits in a mouse model of sepsis-associated encephalopathy. Inflammation 42: 306–318, 2019. doi: 10.1007/s10753-018-0894-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell 147: 742–758, 2011. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao W, Li F, Zhou Z, Xu X, Wu Y, Zhou S, Yin D, Sun D, Xiong J, Jiang R, Zhang J. IL-2/Anti-IL-2 Complex attenuates inflammation and bbb disruption in mice subjected to traumatic brain injury. Front Neurol 8: 281, 2017. doi: 10.3389/fneur.2017.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ge X, Li W, Huang S, Yin Z, Xu X, Chen F, Kong X, Wang H, Zhang J, Lei P. The pathological role of NLRs and AIM2 inflammasome-mediated pyroptosis in damaged blood-brain barrier after traumatic brain injury. Brain Res 1697: 10–20, 2018. doi: 10.1016/j.brainres.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 16.George J, Patal S, Wexler D, Sharabi Y, Peleg E, Kamari Y, Grossman E, Sheps D, Keren G, Roth A. Circulating adiponectin concentrations in patients with congestive heart failure. Heart 92: 1420–1424, 2006. doi: 10.1136/hrt.2005.083345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol 57: 727–741, 1999. doi: 10.1016/S0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 18.Gordeeva O, Khaydukov S. Tumorigenic and differentiation potentials of embryonic stem cells depend on TGFβ family signaling: lessons from teratocarcinoma cells stimulated to differentiate with retinoic acid. Stem Cells Int 2017: 7284872, 2017. doi: 10.1155/2017/7284872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gui WS, Wei X, Mai CL, Murugan M, Wu LJ, Xin WJ, Zhou LJ, Liu XG. Interleukin-1β overproduction is a common cause for neuropathic pain, memory deficit, and depression following peripheral nerve injury in rodents. Mol Pain 12: 1744806916646784, 2016. doi: 10.1177/1744806916646784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ, Han J. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res 25: 1285–1298, 2015. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heilig R, Dick MS, Sborgi L, Meunier E, Hiller S, Broz P. The Gasdermin-D pore acts as a conduit for IL-1β secretion in mice. Eur J Immunol 48: 584–592, 2018. doi: 10.1002/eji.201747404. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann U, Knorr S, Vogel B, Weirather J, Frey A, Ertl G, Frantz S. Interleukin-13 deficiency aggravates healing and remodeling in male mice after experimental myocardial infarction. Circ Heart Fail 7: 822–830, 2014. doi: 10.1161/CIRCHEARTFAILURE.113.001020. [DOI] [PubMed] [Google Scholar]
- 23.Hsu YL, Hung JY, Chang WA, Lin YS, Pan YC, Tsai PH, Wu CY, Kuo PL. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene 36: 4929–4942, 2017. doi: 10.1038/onc.2017.105. [DOI] [PubMed] [Google Scholar]
- 24.Huang J, Zhou Z, Zhou M, Miao M, Li H, Hu Q. Development of benzoxazole deoxybenzoin oxime and acyloxylamine derivatives targeting innate immune sensors and xanthine oxidase for treatment of gout. Bioorg Med Chem 26: 1653–1664, 2018. doi: 10.1016/j.bmc.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Hudis CA, Schmitz N. Dose-dense chemotherapy in breast cancer and lymphoma. Semin Oncol 31, Suppl 8: 19–26, 2004. doi: 10.1053/j.seminoncol.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Ibrahim AG, Cheng K, Marbán E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports 2: 606–619, 2014. doi: 10.1016/j.stemcr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Javeed N, Mukhopadhyay D. Exosomes and their role in the micro-/macro-environment: a comprehensive review. J Biomed Res 31: 386–394, 2017. doi: 10.7555/JBR.30.20150162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang P, Guo Y, Dang R, Yang M, Liao D, Li H, Sun Z, Feng Q, Xu P. Salvianolic acid B protects against lipopolysaccharide-induced behavioral deficits and neuroinflammatory response: involvement of autophagy and NLRP3 inflammasome. J Neuroinflammation 14: 239, 2017. doi: 10.1186/s12974-017-1013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson TA, Singla DK. PTEN inhibitor VO-OHpic attenuates inflammatory M1 macrophages and cardiac remodeling in doxorubicin-induced cardiomyopathy. Am J Physiol Heart Circ Physiol 315: H1236–H1249, 2018. doi: 10.1152/ajpheart.00121.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jounai N, Kobiyama K, Takeshita F, Ishii KJ. Recognition of damage-associated molecular patterns related to nucleic acids during inflammation and vaccination. Front Cell Infect Microbiol 2: 168, 2013. doi: 10.3389/fcimb.2012.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang YJ. Molecular and cellular mechanisms of cardiotoxicity. Environ Health Perspect 109, Suppl 1: 27–34, 2001. doi: 10.1289/ehp.01109s127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karagiannis F, Wilhelm C. More is less: IL-9 in the resolution of inflammation. Immunity 47: 403–405, 2017. doi: 10.1016/j.immuni.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Kawai T, Akira S. TLR signaling. Semin Immunol 19: 24–32, 2007. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Kerur N, Fukuda S, Banerjee D, Kim Y, Fu D, Apicella I, Varshney A, Yasuma R, Fowler BJ, Baghdasaryan E, Marion KM, Huang X, Yasuma T, Hirano Y, Serbulea V, Ambati M, Ambati VL, Kajiwara Y, Ambati K, Hirahara S, Bastos-Carvalho A, Ogura Y, Terasaki H, Oshika T, Kim KB, Hinton DR, Leitinger N, Cambier JC, Buxbaum JD, Kenney MC, Jazwinski SM, Nagai H, Hara I, West AP, Fitzgerald KA, Sadda SR, Gelfand BD, Ambati J. cGAS drives noncanonical-inflammasome activation in age-related macular degeneration. Nat Med 24: 50–61, 2018. doi: 10.1038/nm.4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P, Mackie AR, Vaughan E, Garikipati VN, Benedict C, Ramirez V, Lambers E, Ito A, Gao E, Misener S, Luongo T, Elrod J, Qin G, Houser SR, Koch WJ, Kishore R. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res 117: 52–64, 2015. doi: 10.1161/CIRCRESAHA.117.305990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kondo M, Murakawa Y, Harashima N, Kobayashi S, Yamaguchi S, Harada M. Roles of proinflammatory cytokines and the Fas/Fas ligand interaction in the pathogenesis of inflammatory myopathies. Immunology 128, Suppl: e589–e599, 2009. doi: 10.1111/j.1365-2567.2008.03039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konstantinidis K, Whelan RS, Kitsis RN. Mechanisms of cell death in heart disease. Arterioscler Thromb Vasc Biol 32: 1552–1562, 2012. doi: 10.1161/ATVBAHA.111.224915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kulling PM, Olson KC, Hamele CE, Toro MF, Tan SF, Feith DJ, Loughran TP Jr. Dysregulation of the IFN-γ-STAT1 signaling pathway in a cell line model of large granular lymphocyte leukemia. PLoS One 13: e0193429, 2018. doi: 10.1371/journal.pone.0193429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar D, Kirshenbaum LA, Li T, Danelisen I, Singal PK. Apoptosis in adriamycin cardiomyopathy and its modulation by probucol. Antioxid Redox Signal 3: 135–145, 2001. doi: 10.1089/152308601750100641. [DOI] [PubMed] [Google Scholar]
- 40.Kumar D, Lou H, Singal PK. Oxidative stress and apoptosis in heart dysfunction. Herz 27: 662–668, 2002. doi: 10.1007/s00059-002-2430-3. [DOI] [PubMed] [Google Scholar]
- 41.Lebeaupin C, Proics E, de Bieville CH, Rousseau D, Bonnafous S, Patouraux S, Adam G, Lavallard VJ, Rovere C, Le Thuc O, Saint-Paul MC, Anty R, Schneck AS, Iannelli A, Gugenheim J, Tran A, Gual P, Bailly-Maitre B. ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death Dis 6: e1879, 2015. doi: 10.1038/cddis.2015.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li D, Ren W, Jiang Z, Zhu L. Regulation of the NLRP3 inflammasome and macrophage pyroptosis by the p38 MAPK signaling pathway in a mouse model of acute lung injury. Mol Med Rep 18: 4399–4409, 2018. doi: 10.3892/mmr.2018.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lim CC, Zuppinger C, Guo X, Kuster GM, Helmes M, Eppenberger HM, Suter TM, Liao R, Sawyer DB. Anthracyclines induce calpain-dependent titin proteolysis and necrosis in cardiomyocytes. J Biol Chem 279: 8290–8299, 2004. doi: 10.1074/jbc.M308033200. [DOI] [PubMed] [Google Scholar]
- 44.Loyer X, Zlatanova I, Devue C, Yin M, Howangyin KY, Klaihmon P, Guerin CL, Kheloufi M, Vilar J, Zannis K, Fleischmann BK, Hwang DW, Park J, Lee H, Menasché P, Silvestre JS, Boulanger CM. Intra-cardiac release of extracellular vesicles shapes inflammation following myocardial infarction. Circ Res 123: 100–106, 2018. doi: 10.1161/CIRCRESAHA.117.311326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maillet A, Tan K, Chai X, Sadananda SN, Mehta A, Ooi J, Hayden MR, Pouladi MA, Ghosh S, Shim W, Brunham LR. Modeling doxorubicin-induced cardiotoxicity in human pluripotent stem cell derived-cardiomyocytes. Sci Rep 6: 25333, 2016. doi: 10.1038/srep25333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Man SM, Karki R, Briard B, Burton A, Gingras S, Pelletier S, Kanneganti TD. Differential roles of caspase-1 and caspase-11 in infection and inflammation. Sci Rep 7: 45126, 2017. doi: 10.1038/srep45126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev 277: 61–75, 2017. doi: 10.1111/imr.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McKenzie BA, Mamik MK, Saito LB, Boghozian R, Monaco MC, Major EO, Lu JQ, Branton WG, Power C. Caspase-1 inhibition prevents glial inflammasome activation and pyroptosis in models of multiple sclerosis. Proc Natl Acad Sci USA 115: E6065–E6074, 2018. doi: 10.1073/pnas.1722041115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Merino H, Singla DK. Notch-1 mediated cardiac protection following embryonic and induced pluripotent stem cell transplantation in doxorubicin-induced heart failure. PLoS One 9: e101024, 2014. doi: 10.1371/journal.pone.0101024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mezzaroma E, Toldo S, Farkas D, Seropian IM, Van Tassell BW, Salloum FN, Kannan HR, Menna AC, Voelkel NF, Abbate A. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci USA 108: 19725–19730, 2011. doi: 10.1073/pnas.1108586108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev 243: 206–214, 2011. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nussbaum J, Minami E, Laflamme MA, Virag JA, Ware CB, Masino A, Muskheli V, Pabon L, Reinecke H, Murry CE. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J 21: 1345–1357, 2007. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 53.Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol 52: 1213–1225, 2012. doi: 10.1016/j.yjmcc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 54.Ong SG, Wu JC. Exosomes as potential alternatives to stem cell therapy in mediating cardiac regeneration. Circ Res 117: 7–9, 2015. doi: 10.1161/CIRCRESAHA.115.306593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qin XJ, He W, Hai CX, Liang X, Liu R. Protection of multiple antioxidants Chinese herbal medicine on the oxidative stress induced by adriamycin chemotherapy. J Appl Toxicol 28: 271–282, 2008. doi: 10.1002/jat.1276. [DOI] [PubMed] [Google Scholar]
- 56.Qiu T, Pei P, Yao X, Jiang L, Wei S, Wang Z, Bai J, Yang G, Gao N, Yang L, Qi S, Yan R, Liu X, Sun X. Taurine attenuates arsenic-induced pyroptosis and nonalcoholic steatohepatitis by inhibiting the autophagic-inflammasomal pathway. Cell Death Dis 9: 946, 2018. doi: 10.1038/s41419-018-1004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qu W, Wang Y, Wu Y, Liu Y, Chen K, Liu X, Zou Z, Huang X, Wu M. Triggering receptors expressed on myeloid cells 2 promotes corneal resistance against Pseudomonas aeruginosa by inhibiting caspase-1-dependent pyroptosis. Front Immunol 9: 1121, 2018. doi: 10.3389/fimmu.2018.01121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quintar AA, Roth FD, De Paul AL, Aoki A, Maldonado CA. Toll-like receptor 4 in rat prostate: modulation by testosterone and acute bacterial infection in epithelial and stromal cells. Biol Reprod 75: 664–672, 2006. doi: 10.1095/biolreprod.106.053967. [DOI] [PubMed] [Google Scholar]
- 59.Rauber S, Luber M, Weber S, Maul L, Soare A, Wohlfahrt T, Lin NY, Dietel K, Bozec A, Herrmann M, Kaplan MH, Weigmann B, Zaiss MM, Fearon U, Veale DJ, Cañete JD, Distler O, Rivellese F, Pitzalis C, Neurath MF, McKenzie AN, Wirtz S, Schett G, Distler JH, Ramming A. Resolution of inflammation by interleukin-9-producing type 2 innate lymphoid cells. Nat Med 23: 938–944, 2017. doi: 10.1038/nm.4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rharass T, Gbankoto A, Canal C, Kurşunluoğlu G, Bijoux A, Panáková D, Ribou AC. Oxidative stress does not play a primary role in the toxicity induced with clinical doses of doxorubicin in myocardial H9c2 cells. Mol Cell Biochem 413: 199–215, 2016. doi: 10.1007/s11010-016-2653-x. [DOI] [PubMed] [Google Scholar]
- 61.Rock KL, Kono H. The inflammatory response to cell death. Annu Rev Pathol 3: 99–126, 2008. doi: 10.1146/annurev.pathmechdis.3.121806.151456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodrigues A, Queiróz DB, Honda L, Silva EJ, Hall SH, Avellar MC. Activation of toll-like receptor 4 (TLR4) by in vivo and in vitro exposure of rat epididymis to lipopolysaccharide from Escherichia Coli. Biol Reprod 79: 1135–1147, 2008. doi: 10.1095/biolreprod.108.069930. [DOI] [PubMed] [Google Scholar]
- 63.Rühl S, Broz P. The gasdermin-D pore: Executor of pyroptotic cell death. Oncotarget 7: 57481–57482, 2016. doi: 10.18632/oncotarget.11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Satterwhite B, Zimm S. The use of scalp hypothermia in the prevention of doxorubicin-induced hair loss. Cancer 54: 34–37, 1984. doi:. [DOI] [PubMed] [Google Scholar]
- 65.Schountz T, Quackenbush S, Rovnak J, Haddock E, Black WC IV, Feldmann H, Prescott J. Differential lymphocyte and antibody responses in deer mice infected with Sin Nombre hantavirus or Andes hantavirus. J Virol 88: 8319–8331, 2014. doi: 10.1128/JVI.00004-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shin K, Klosterhoff BS, Han B. Characterization of cell-type-specific drug transport and resistance of breast cancers using tumor-microenvironment-on-chip. Mol Pharm 13: 2214–2223, 2016. doi: 10.1021/acs.molpharmaceut.6b00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shintani Y, Ito T, Fields L, Shiraishi M, Ichihara Y, Sato N, Podaru M, Kainuma S, Tanaka H, Suzuki K. IL-4 as a repurposed biological drug for myocardial infarction through augmentation of reparative cardiac macrophages: proof-of-concept data in mice. Sci Rep 7: 6877, 2017. doi: 10.1038/s41598-017-07328-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Singla DK. Akt-mTOR pathway inhibits apoptosis and fibrosis in doxorubicin-induced cardiotoxicity following embryonic stem cell transplantation. Cell Transplant 24: 1031–1042, 2015. doi: 10.3727/096368914X679200. [DOI] [PubMed] [Google Scholar]
- 69.Singla DK. Stem cells and exosomes in cardiac repair. Curr Opin Pharmacol 27: 19–23, 2016. doi: 10.1016/j.coph.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 70.Singla DK, Abdelli LS. Embryonic stem cells and released factors stimulate c-kit(+)/flk-1(+) progenitor cells and promote neovascularization in doxorubicin-induced cardiomyopathy. Cell Transplant 24: 1043–1052, 2015. doi: 10.3727/096368914X679219. [DOI] [PubMed] [Google Scholar]
- 71.Singla DK, Ahmed A, Singla R, Yan B. Embryonic stem cells improve cardiac function in Doxorubicin-induced cardiomyopathy mediated through multiple mechanisms. Cell Transplant 21: 1919–1930, 2012. doi: 10.3727/096368911X627552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singla DK, Singla RD, McDonald DE. Factors released from embryonic stem cells inhibit apoptosis in H9c2 cells through PI3K/Akt but not ERK pathway. Am J Physiol Heart Circ Physiol 295: H907–H913, 2008. doi: 10.1152/ajpheart.00279.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smuder AJ, Kavazis AN, Min K, Powers SK. Exercise protects against doxorubicin-induced markers of autophagy signaling in skeletal muscle. J Appl Physiol (1985) 111: 1190–1198, 2011. doi: 10.1152/japplphysiol.00429.2011. [DOI] [PubMed] [Google Scholar]
- 74.Song YH, Shao L, Zhang Y, Zhou J, Liu B, Pan X, Geng YJ, Yu XY, Li Y. Exosomes derived from embryonic stem cells as potential treatment for cardiovascular diseases. Adv Exp Med Biol 998: 187–206, 2017. doi: 10.1007/978-981-10-4397-0_13. [DOI] [PubMed] [Google Scholar]
- 75.Sun J, Sun G, Meng X, Wang H, Luo Y, Qin M, Ma B, Wang M, Cai D, Guo P, Sun X. Isorhamnetin protects against doxorubicin-induced cardiotoxicity in vivo and in vitro. PLoS One 8: e64526, 2013. doi: 10.1371/journal.pone.0064526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Swijnenburg RJ, Tanaka M, Vogel H, Baker J, Kofidis T, Gunawan F, Lebl DR, Caffarelli AD, de Bruin JL, Fedoseyeva EV, Robbins RC. Embryonic stem cell immunogenicity increases upon differentiation after transplantation into ischemic myocardium. Circulation 112, Suppl: I166–I172, 2005. doi: 10.1289/ehp.01109s127. [DOI] [PubMed] [Google Scholar]
- 77.Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol 65: 157–170, 2013. doi: 10.1111/j.2042-7158.2012.01567.x. [DOI] [PubMed] [Google Scholar]
- 78.Tam BT, Yu AP, Tam EW, Monks DA, Wang XP, Pei XM, Koh SP, Sin TK, Law HK, Ugwu FN, Supriya R, Yung BY, Yip SP, Wong SC, Chan LW, Lai CW, Ouyang P, Siu PM. Ablation of Bax and Bak protects skeletal muscle against pressure-induced injury. Sci Rep 8: 3689, 2018. doi: 10.1038/s41598-018-21853-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tavakoli Dargani Z, Singla R, Johnson T, Kukreja R, Singla DK. Exosomes derived from embryonic stem cells inhibit doxorubicin and inflammation-induced pyroptosis in muscle cells. Can J Physiol Pharmacol 96: 304–307, 2018. doi: 10.1139/cjpp-2017-0340. [DOI] [PubMed] [Google Scholar]
- 80.Terashvili M, Bosnjak ZJ. Stem cell therapies in cardiovascular disease. J Cardiothorac Vasc Anesth 33: 209–222, 2019. doi: 10.1053/j.jvca.2018.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thomas X, Le Q, Fiere D. Anthracycline-related toxicity requiring cardiac transplantation in long-term disease-free survivors with acute promyelocytic leukemia. Ann Hematol 81: 504–507, 2002. doi: 10.1007/s00277-002-0534-8. [DOI] [PubMed] [Google Scholar]
- 82.Thorn CF, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, Klein TE, Altman RB. Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet Genomics 21: 440–446, 2011. doi: 10.1097/FPC.0b013e32833ffb56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tonsho M, Michel S, Ahmed Z, Alessandrini A, Madsen JC. Heart transplantation: challenges facing the field. Cold Spring Harb Perspect Med 4: a015636, 2014. doi: 10.1101/cshperspect.a015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Torre-Amione G, Kapadia S, Lee J, Durand JB, Bies RD, Young JB, Mann DL. Tumor necrosis factor-alpha and tumor necrosis factor receptors in the failing human heart. Circulation 93: 704–711, 1996. doi: 10.1161/01.CIR.93.4.704. [DOI] [PubMed] [Google Scholar]
- 85.Turakhia S, Venkatakrishnan CD, Dunsmore K, Wong H, Kuppusamy P, Zweier JL, Ilangovan G. Doxorubicin-induced cardiotoxicity: direct correlation of cardiac fibroblast and H9c2 cell survival and aconitase activity with heat shock protein 27. Am J Physiol Heart Circ Physiol 293: H3111–H3121, 2007. doi: 10.1152/ajpheart.00328.2007. [DOI] [PubMed] [Google Scholar]
- 86.van Norren K, van Helvoort A, Argilés JM, van Tuijl S, Arts K, Gorselink M, Laviano A, Kegler D, Haagsman HP, van der Beek EM. Direct effects of doxorubicin on skeletal muscle contribute to fatigue. Br J Cancer 100: 311–314, 2009. doi: 10.1038/sj.bjc.6604858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang J, Sahoo M, Lantier L, Warawa J, Cordero H, Deobald K, Re F. Caspase-11-dependent pyroptosis of lung epithelial cells protects from melioidosis while caspase-1 mediates macrophage pyroptosis and production of IL-18. PLoS Pathog 14: e1007105, 2018. doi: 10.1371/journal.ppat.1007105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu XJ, Liu HM, Song XM, Zhao B, Leng Y, Wang EY, Zhan LY, Meng QT, Xia ZY. Penehyclidine hydrochloride inhibits TLR4 signaling and inflammation, and attenuates blunt chest trauma and hemorrhagic shock-induced acute lung injury in rats. Mol Med Rep 17: 6327–6336, 2018. doi: 10.3892/mmr.2018.8644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xiao J, Pan Y, Li XH, Yang XY, Feng YL, Tan HH, Jiang L, Feng J, Yu XY. Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death Dis 7: e2277, 2016. doi: 10.1038/cddis.2016.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang H, Chen Y, Gao C. Interleukin-13 reduces cardiac injury and prevents heart dysfunction in viral myocarditis via enhanced M2 macrophage polarization. Oncotarget 8: 99495–99503, 2017. doi: 10.18632/oncotarget.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci 15: 4142–4157, 2014. doi: 10.3390/ijms15034142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yuan Y, Du W, Liu J, Ma W, Zhang L, Du Z, Cai B. Stem cell-derived exosome in cardiovascular diseases: macro roles of micro particles. Front Pharmacol 9: 547, 2018. doi: 10.3389/fphar.2018.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yuan YY, Xie KX, Wang SL, Yuan LW. Inflammatory caspase-related pyroptosis: mechanism, regulation and therapeutic potential for inflammatory bowel disease. Gastroenterol Rep (Oxf) 6: 167–176, 2018. doi: 10.1093/gastro/goy011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang B, Yu Y, Aori G, Wang Q, Kong D, Yang W, Guo Z, Zhang L. Tanshinone IIA attenuates diabetic peripheral neuropathic pain in experimental rats via inhibiting inflammation. Evid Based Complement Alternat Med 2018: 2789847, 2018. doi: 10.1155/2018/2789847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang X, Starnbach MN. An excess of the proinflammatory cytokines IFN-γ and IL-12 impairs the development of the memory CD8+ T cell response to chlamydia trachomatis. J Immunol 195: 1665–1675, 2015. doi: 10.4049/jimmunol.1500457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang X, Yuan X, Shi H, Wu L, Qian H, Xu W. Exosomes in cancer: small particle, big player. J Hematol Oncol 8: 83, 2015. doi: 10.1186/s13045-015-0181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang YW, Shi J, Li YJ, Wei L. Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Arch Immunol Ther Exp (Warsz) 57: 435–445, 2009. doi: 10.1007/s00005-009-0051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao M, Tang S, Xin J, Wei Y, Liu D. Reactive oxygen species induce injury of the intestinal epithelium during hyperoxia. Int J Mol Med 41: 322–330, 2018. doi: 10.3892/ijmm.2017.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhaolin Z, Guohua L, Shiyuan W, Zuo W. Role of pyroptosis in cardiovascular disease. Cell Prolif 52: e12563, 2019. doi: 10.1111/cpr.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zheng PP, Li J, Kros JM. Breakthroughs in modern cancer therapy and elusive cardiotoxicity: Critical research-practice gaps, challenges, and insights. Med Res Rev 38: 325–376, 2018. doi: 10.1002/med.21463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhu L, Qu XH, Sun YL, Qian YM, Zhao XH. Novel method for extracting exosomes of hepatocellular carcinoma cells. World J Gastroenterol 20: 6651–6657, 2014. doi: 10.3748/wjg.v20.i21.6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhuo J, Zeng Q, Cai D, Zeng X, Chen Y, Gan H, Huang X, Yao N, Huang D, Zhang C. Evaluation of type 2 diabetic mellitus animal models via interactions between insulin and mitogen–activated protein kinase signaling pathways induced by a high fat and sugar diet and streptozotocin. Mol Med Rep 17: 5132–5142, 2018. doi: 10.3892/mmr.2018.8504. [DOI] [PMC free article] [PubMed] [Google Scholar]