Abstract
The mitochondrial unfolded protein response (UPRmt) is a cytoprotective signaling pathway triggered by mitochondrial dysfunction. UPRmt activation upregulates chaperones, proteases, antioxidants, and glycolysis at the gene level to restore proteostasis and cell energetics. Activating transcription factor 5 (ATF5) is a proposed mediator of the mammalian UPRmt. Herein, we hypothesized pharmacological UPRmt activation may protect against cardiac ischemia-reperfusion (I/R) injury in an ATF5-dependent manner. Accordingly, in vivo administration of the UPRmt inducers oligomycin or doxycycline 6 h before ex vivo I/R injury (perfused heart) was cardioprotective in wild-type but not global Atf5−/− mice. Acute ex vivo UPRmt activation was not cardioprotective, and loss of ATF5 did not impact baseline I/R injury without UPRmt induction. In vivo UPRmt induction significantly upregulated many known UPRmt-linked genes (cardiac quantitative PCR and Western blot analysis), and RNA-Seq revealed an UPRmt-induced ATF5-dependent gene set, which may contribute to cardioprotection. This is the first in vivo proof of a role for ATF5 in the mammalian UPRmt and the first demonstration that UPRmt is a cardioprotective drug target.
NEW & NOTEWORTHY Cardioprotection can be induced by drugs that activate the mitochondrial unfolded protein response (UPRmt). UPRmt protection is dependent on activating transcription factor 5 (ATF5). This is the first in vivo evidence for a role of ATF5 in the mammalian UPRmt.
Keywords: cardioprotection, chaperone, ischemia, metabolism, mitochondria, unfolded protein response
INTRODUCTION
Cardiac ischemia-reperfusion (I/R) injury is an underlying pathological mechanism of myocardial infarction, a major cause of morbidity and mortality worldwide. Mitochondria are key mediators of cardiac I/R injury and also a potential target for cardioprotective interventions (16, 24). An important concept in cytoprotection is hormesis, wherein small sublethal insults trigger signaling events that afford protection against subsequent larger injuries. Mitohormesis refers to such events at the mitochondrial level (36). An example is ischemic preconditioning (IPC), wherein small ischemic insults trigger mitochondrial reactive oxygen species (ROS) generation, signaling protection against pathological ROS during subsequent I/R (16).
The mitochondrial unfolded protein response (UPRmt) is a mitohormetic response to proteotoxic stress in the organelle (39). Similar to the endoplasmic reticulum UPR (UPRER), UPRmt signaling upregulates chaperones and proteases to restore proteostasis (20). Furthermore, the UPRmt upregulates glycolysis (23) and downregulates respiratory chain subunit expression (22) to balance cell energetics and unburden the mitochondrial translation and folding machinery.
Significant insight to the UPRmt has emerged from work in Caenorhabditis elegans, including the discovery of its central transcription factor ATFS-1 (activating transcription factor associated with stress 1) (14, 26). ATFS-1 contains both nuclear and mitochondrial target sequences, and under normal conditions is imported to mitochondria and degraded (14, 23). Mitochondrial proteotoxicity blunts ATFS-1 import, resulting in its nuclear translocation and target gene activation (22, 23).
Comparatively little is known about the mammalian UPRmt, especially in heart. Activating transcription factor 5 (ATF5) is proposed as a mammalian ATFS-1 ortholog (14) and indeed can rescue UPRmt signaling in C. elegans lacking ATFS-1 (10). However, although ATF5 has known roles in stress signaling (40), there is no in vivo evidence for its requirement in the mammalian UPRmt.
Cardiac I/R injury causes bioenergetic crisis, protein misfolding, and oxidative stress (5), whereas the UPRmt upregulates glycolysis, chaperones, and antioxidants (20). Thus, we hypothesized induction of an ATF5-dependent UPRmt may be cardioprotective against I/R injury in mammals. This hypothesis was tested using the known UPRmt inducers oligomycin and doxycycline (10, 17) in wild-type (WT) and global Atf5−/− mice (6).
METHODS
Reagents, animals, drug treatments.
All reagents were from Sigma (St. Louis, MO) unless otherwise stated. All animal experiments were approved by the University of Rochester Committee on Animal Resources (Protocol No. UCAR-2014-036) in compliance with the National Institutes of Health’s Guide for the Use and Care of Animals. Atf5-/+ mice were provided by Stavros Lomvardas on a C57BL/6(J/N) background (6) and backcrossed to WT C57BL/6J (JAX 000664) >3 generations. Mice were housed on a 12-h:12-h light-dark cycle with ad libitum food and water. Experimental mice aged 12–20 wk were of both sexes, and WT controls were age and sex matched, since high neonatal Atf5−/− mortality made using littermates infeasible. PCR genotyping (tail clip) at 21 days employed three primers, with expected amplicons at 1,006 base pairs for WT and 439 base pairs for knockout (KO): WT: 5′-TCTGATTGGATGACTGAGCGG-3′, KO: 5′-GCAGCCTCTGTTCCACATACACTTC-3′, common: 5′-TCACTTGTGTTCCAAGTCCCC-3′.
To induce UPRmt in vivo, oligomycin (500 µg/kg) or doxycycline (70 mg/kg) were injected intraperitoneally (12.5 µL/g body mass) in sterile saline with 0.1% DMSO. Controls received vehicle. Mice were returned to cages for 6 h before I/R injury. These drug doses elicited no mortality or other overt phenotypes.
I/R injury.
I/R injury was assessed using an ex vivo retrograde-perfused heart model (38). Anesthetized (tribromoethanol, 200 mg/kg ip) and heparinized (0.2 mL ip) mice were placed supine on a 37°C heat pad, the aorta cannulated (blunt 27-gauge needle), and the heart immediately transferred to the perfusion rig. Perfusion was at 4 mL/min, 37°C, with 0.22 µm filtered, 95% O2-5% CO2 gassed Krebs-Henseleit buffer comprising (in mM) 118 NaCl, 4.6 KCl, 1.2 MgSO4, 25 NaHCO3, 1.2 K2HPO4, 5 glucose, 0.1 palmitate-BSA, 0.2 pyruvate, and1.2 lactate. Left ventricular pressure was digitally recorded (DATAQ, Akron, OH) from a transducer-linked balloon. Pressure traces were analyzed using a custom MATLAB script (MathWorks, Natick, MA) to derive developed pressure, heart rate, and rate × pressure product (RPP). Following 25-min equilibration, hearts were subjected to 25 min global no-flow ischemia and then 60 min reperfusion. Post-I/R, hearts were transverse sliced (2 mm thick), stained with 1% tetrazolium chloride, formalin fixed, and imaged. Healthy (red) tissue versus infarcted (white) tissue was quantified by planimetry in MATLAB. In acute studies, doxycycline (20 µM) was dissolved in perfusion buffer with 0.25% DMSO, 0.22 µm filtered, and perfused throughout the procedure.
RNASeq, quantitative PCR, and Western blot analysis.
Following anesthesia (tribromoethanol, 200 mg/kg ip), hearts were excised, flushed with ice-cold saline, homogenized in TRIzol (Invitrogen, Carlsbad, CA), and snap frozen (liquid N2). RNA was processed and purified using RNeasy Mini-kits (Qiagen, Hilden, Germany) with on-column DNase treatment. For RNA-Seq, library preparation and analysis were performed by the University of Rochester Functional Genomics Core (oligomycin experiments) or the Memorial Sloan Kettering Integrated Genomics Operation (doxycycline experiments). Total RNA was determined spectrophotometrically (NanoDrop, Wilmington, DE), and RNA quality was assessed with the Agilent Bioanalyzer (Agilent, Santa Clara, CA). Library construction and sequencing were performed from 200 ng RNA with the TruSeq-stranded mRNA preparation kit and a HiSeq 2500 v4 platform with cBot, according to manufacturer’s protocols (Illumina, San Diego, CA). Raw reads were demultiplexed using bcl2fastq.pl v1.8.4 conversion software (Illumina). Quality filtering and adapter removal were performed using Trimmomatic 0.32 (1), and processed reads were mapped to the mouse genome with STAR 2.4.2a (7).
For quantitative PCR (qPCR), cDNA was generated using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) and used at 100 µg/well as a PCR template for target detection with iTaq universal SYBR green supermix (Bio-Rad) on a CFX connect real-time PCR system (Bio-Rad). Quantification cycle (Cq) of each RT-qPCR target was normalized to reference genes Actb or Hprt (ΔCq). Data are presented in treated versus untreated (vehicle) conditions, as relative ΔCq (i.e., ΔΔCq).
For Western blot analysis, samples of 50 µg of protein were run on 12% SDS-PAGE gels, wet-transferred to nitrocellulose, and visualized with Ponceau S. Blocking was performed with 10% milk and antibodies were incubated in 5% milk in TBS-T. Images were acquired using a KwikQuant Imager (Kindle Biosciences, Greenwich, CT) and analyzed in ImageJ. Since the enhanced chemiluinescence reagent emits blue light, densitometry and presented blot images were from the blue channel only, such that loss of background detail may have occurred due to removal of the red/green channels.
Statistics.
Comparisons between two groups employed two-tailed unpaired Student’s t-tests. Comparisons versus zero (qPCR) employed two-tailed one-sample Student’s t-tests. Univariate comparisons between greater than two groups used one-way ANOVA. Multivariate comparisons used two-way ANOVA. Differences were considered significant at P < 0.05. Data are reported as means ± SE.
RNA-Seq gene read counts were processed using R 3.4.4; Bioconductor 3.7; limma 3.34.9. One vehicle-treated Atf5−/− sample was removed due to probable failure of the KO. Genes with <50 reads in all samples were filtered yielding 14,061 genes for analysis. The voom method (19) was used with limma empirical Bayes analysis methodology (28) to assess differential expression. Gene set testing was performed using ROAST (34) to account for correlation between genes. Full data are deposited in the sequence read archive (accession no. SRP150238).
RESULTS
Effects of ATF5 ablation on cardiac function and I/R injury response.
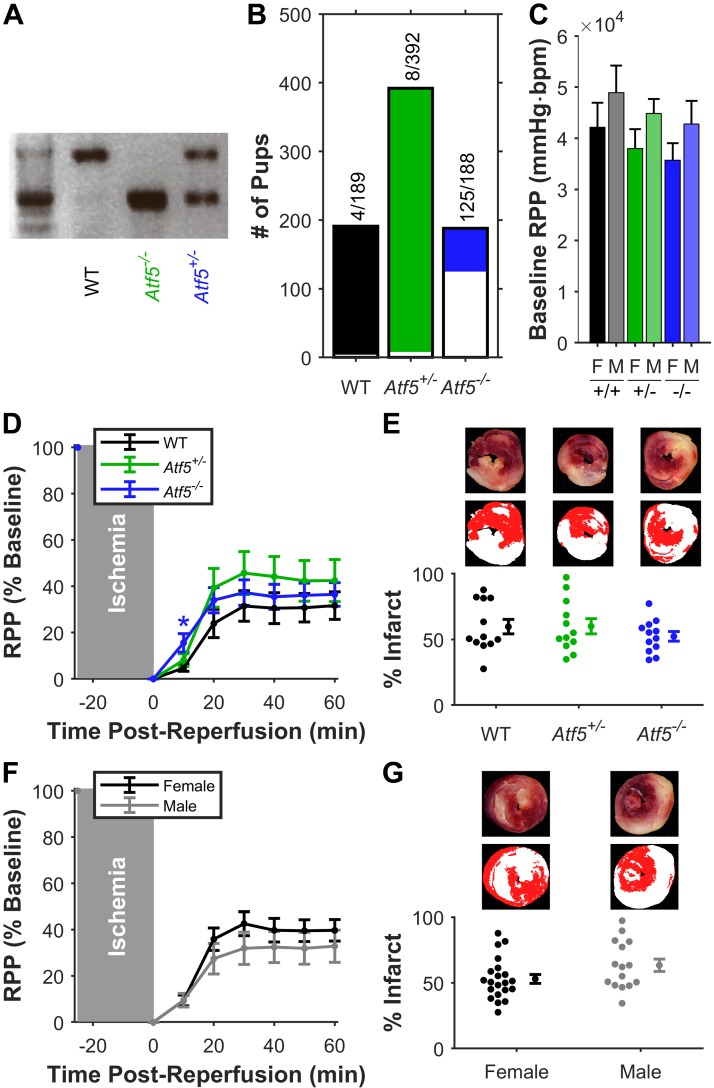
The complete data set is at DOI:10.6084/m9.figshare.7323929. Fig. 1A shows WT and Atf5−/− genotyping. Consistent with previous reports (6, 33), a confounder in these studies was high neonatal mortality in Atf5−/− mice (Fig. 1B) due to an olfactory neuron defect resulting in failure to suckle at birth. In ex vivo-perfused hearts, neither baseline function (Fig. 1C) nor post-I/R functional recovery or infarct size (Figs. 1, D–G) was different between genotypes or sexes. Further stratification by sex or genotype (not shown) did not reveal sex-specific genotype-dependent effects. Thus, for UPRmt investigations, both sexes were used, and controls were age and sex matched and not always littermates. After weaning, adult Atf5−/− mice were healthy but slightly smaller than WT and exhibited no phenotypes up to 2 yr of age.
Fig. 1.
Loss of activating transcription factor 5 (ATF5), baseline cardiac function, and ischemic-reperfusion injury. A: example PCR genotyping of Atf5, yielding amplicons at 1,006 base pair (bp) for wild type (WT), 439 bp for knockout, and both for heterozygotes. B: neonatal mortality (<21 days) for 93 litters (n = 769 mice) by genotype. Mortality is marked by white portion of bars, with fractional values above. C: baseline cardiac function [rate × pressure product (RPP)] of perfused hearts, by genotype and sex. bpm, beats/min; F, female; M, male. D: post-reperfusion functional recovery (RPP normalized to baseline) by genotype. Means ± SE, both sexes, n = 12 per genotype (WT: black, Atf5+/−: green, Atf5−/−: blue). E: infarct sizes of the hearts in D. Representative slices (E, top) and digitally parsed pseudocolor images (E, middle) show red healthy tissue and white infarct. Means ± SE shown to right of individual data. F: post-reperfusion functional recovery (data from D stratified by sex). Means ± SE, all genotypes sexes, n = 21 female (black), n = 15 male (gray). G: infarct data for hearts in F. No significant differences between any groups by 2-way ANOVA.
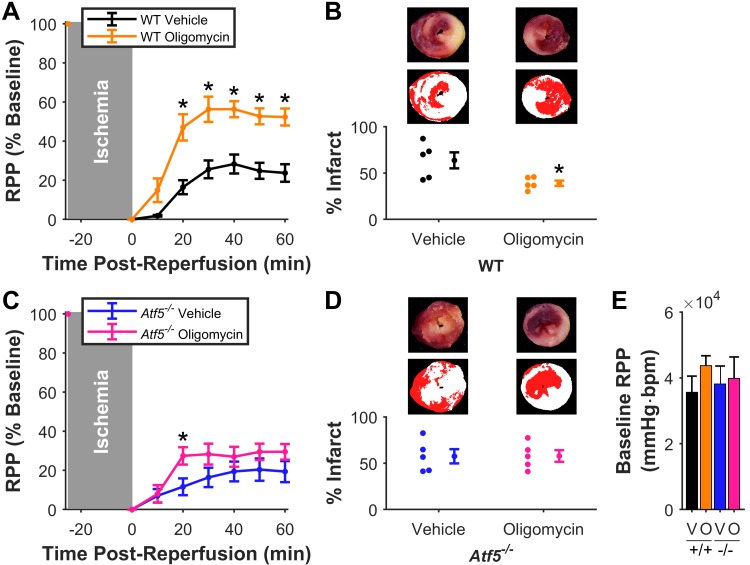
ATF5-dependent cardioprotection by oligomycin.
In vivo delivery of the UPRmt inducer oligomycin 6 h before ex vivo I/R injury elicited significant improvement in ex vivo post-I/R functional recovery (oligomycin, 52 ± 4 vs. vehicle, 24 ± 4%) and reduction in infarct size (oligomycin, 39 ± 3 vs. vehicle, 64 ± 9%) in WT mice (Fig. 2A/B). However, such protection was absent in Atf5−/− mice (functional recovery: oligomycin, 30 ± 4 vs. vehicle, 19 ± 5%; infarct; and oligomycin, 58 ± 6 vs. vehicle, 57 ± 8%) (Fig. 2, C and D). Oligomycin did not affect baseline cardiac function (Fig. 2E), consistent with previous reports of minimal effects on cardiac energetics in vivo at the same dose and in the same time frame (18).
Fig. 2.
Mitochondrial unfolded protein response induction by oligomycin is cardioprotective and requires activating transcription factor 5 (ATF5). A: post-reperfusion functional recovery [rate × pressure product (RPP), normalized to baseline] of hearts from wild-type (WT) mice given oligomycin (orange) or vehicle (black); n = 5. *P < 0.05 vs. matching time point. B: infarct sizes of hearts in A. Representative and pseudocolor images as per Fig. 1E. *P < 0.05 vs. vehicle. C: post-reperfusion functional recovery of hearts from Atf5−/− mice given oligomycin (pink) or vehicle (blue); n = 5 per group. *P < 0.05 vs. matching time point. D: infarct sizes of hearts in C. E: baseline cardiac functional data for hearts in this cohort. V, vehicle; O, oligomycin; bpm, beats/min. All data are means ± SE.
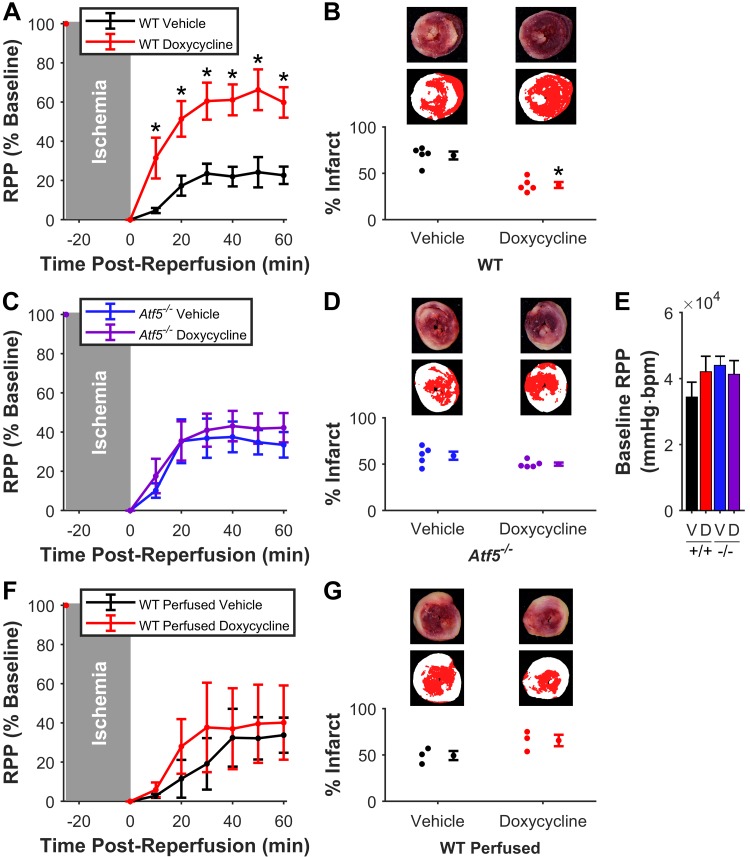
ATF5-dependent cardioprotection by doxycycline.
In vivo delivery of the UPRmt inducer doxycycline (17) 6 h before ex vivo I/R injury elicited significant improvement in ex vivo post-I/R functional recovery (doxycycline, 60 ± 8 vs. vehicle, 23 ± 4%) and reduction in infarct size (doxycycline 37 ± 3 vs. vehicle 69 ± 4%) (Fig. 3A and B). Doxycycline-induced cardioprotection was absent in Atf5−/− mice (functional recovery: doxycycline, 42 ± 7 vs. vehicle, 34 ± 7%; infarct: doxycycline, 50 ± 2 vs. vehicle, 59 ± 4%) (Fig. 3, C and D). Doxycycline did not affect baseline cardiac function (Fig. 3E).
Fig. 3.
Mitochondrial unfolded protein response induction by doxycycline is cardioprotective and requires activating transcription factor 5 (ATF5). A: post-reperfusion functional recovery [rate × pressure product (RPP), normalized to baseline] of hearts from WT mice given doxycycline (red) or vehicle (black); n = 5 per group. *P < 0.05 vs. matching time point. B: infarct sizes of hearts in A. Representative and pseudocolor images as per Fig. 1E. *P < 0.05 vs. vehicle. C: post-reperfusion functional recovery of hearts from Atf5−/− mice given doxycycline (purple) or vehicle (blue); n = 5 per group. D: infarct sizes of hearts in C. E: baseline cardiac functional data for hearts in this cohort. V, vehicle; D, doxycycline; bpm, beats/min. F: post-reperfusion functional recovery of WT hearts given acute doxycycline (red) or vehicle (black) ex vivo; n = 3. G: infarct sizes of the hearts in F. All data are means ± SE.
The ability of doxycycline to induce acute cardioprotection was also tested by direct delivery to perfused hearts, but in contrast to a previous report (30), no protection was observed (functional recovery: doxycycline, 40 ± 19 vs. vehicle, 34 ±9%; infarct: doxycycline, 66 ± 6 vs. vehicle, 49 ± 5%) (Fig. 3, F and G).
Genetic analysis of UPRmt activation.
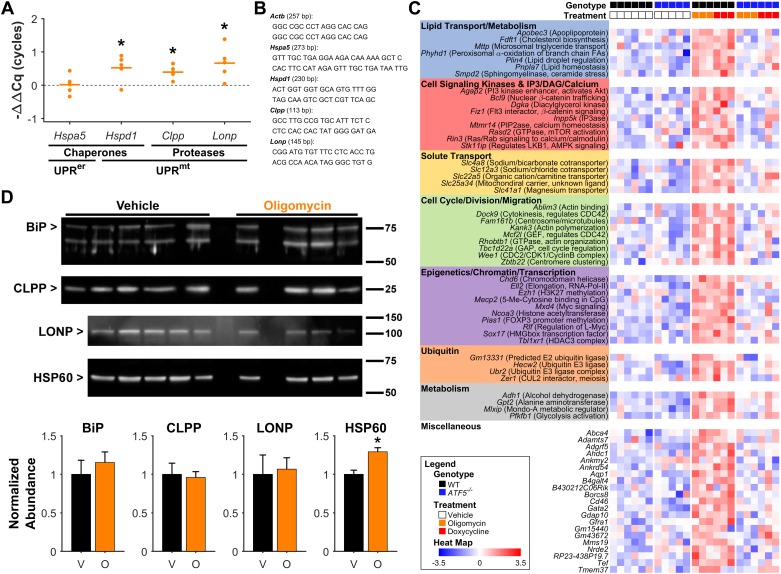
To confirm UPRmt induction, expression of known UPRmt target genes was analyzed by RT-qPCR on hearts from WT mice 6 h after oligomycin delivery. As expected, the UPRmt-associated genes Hspd1, Clpp, and Lonp (13, 39) were all significantly induced by oligomycin, whereas Hspa5 (the chaperone BiP in the endoplasmic reticulum UPR) was not induced (Fig. 4A). To confirm induction of the UPRmt at the protein level, Western blotting was performed, revealing that hearts from oligomycin-treated mice showed elevation in the level of HSP60, with no change in BiP (Fig. 4C).
Fig. 4.
Mitochondrial unfolded protein response (UPRmt) gene expression. A: relative wild-type (WT) cardiac expression of selected genes 6 h after oligomycin treatment by RT-qPCR (−ΔΔCq, normalized to Hprt1 reference gene and compared with vehicle treatment). Hspa5 (BiP) is an endoplasmic reticulum UPR chaperone, Hspd1 (HSP60) is a UPRmt chaperone, and Clpp and Lonp are UPRmt proteases. Horizontal lines represent means. *P < 0.05 vs. vehicle, n = 5. B: primers used for quantitative PCR. D: Western blot normalized to protein loading (Ponceau-stained membrane) was used to quantify relative levels of indicated proteins in hearts 6 h after vehicle or oligomycin. Values for each protein are expressed relative to abundance in vehicle-treated hearts. Representative blots are shown, with one biological replicate (animal) per lane. Note that in lane 2 of the oligomycin group, the sample was lost during preparation so the lane was left blank. *P < 0.05 compared with vehicle-treatment, n = 5/4 per group. Data are means ± SE. C: RNA-Seq heat map showing relative expression of 69 genes identified as upregulated by oligomycin/doxycycline treatment in hearts from WT mice with a blunted response in hearts from Atf5−/−; 48 of the 69 genes (left) are color-coded for annotation by functional category. V, vehicle; D, doxycycline.
To explore cardioprotective signaling by the UPRmt→ATF5 axis in more detail, RNA-Seq was performed on cardiac mRNA from WT or Atf5−/− mice treated with vehicle, oligomycin, or doxycycline. Data from both UPRmt inducers were grouped for analysis, to identify common genes induced in WT (adjusted P < 0.2), with lower or no induction in Atf5−/− (adjusted P < 0.4). Such analysis yielded 69 genes responding to UPRmt induction in an ATF5-dependent manner (Fig. 4D). Known UPRmt-induced genes (27) were poorly represented in this gene set, and unbiased pathway analysis did not yield insight to potential gene programs that may underlie cardioprotection. Nevertheless, guided annotation revealed 48 of 69 genes fell into seven broad categories with potential relevance to cardioprotection (Fig. 4D).
DISCUSSION
The key finding of this study is that pharmacological UPRmt induction is cardioprotective against I/R in an ATF5-dependent manner. This is the first in vivo demonstration that ATF5 is necessary for the mammalian UPRmt, and the first study to demonstrate a role for UPRmt in cardioprotection by doxycycline (4).
Regarding the mechanism(s) of UPRmt activation by oligomycin, its inhibition of mitochondrial ATP synthesis may impair ATP-dependent mitochondrial protein synthesis, and import (9, 25). Notably, protein turnover sits low in the hierarchy of ATP consuming processes in cells, such that mild energetic dysfunction may impair this process without affecting more important processes such as ion-homeostasis (2). Doxycycline disrupts mitochondrial ribosomes (29), such that overall both oligomycin and doxycycline likely induce a UPRmt by generating a stoichiometric imbalance between nuclear and mitochondrial DNA encoded respiratory chain subunits (17).
The significant cardioprotection observed in hearts from UPRmt-induced WT mice indicates mitochondrial proteotoxic stress is a hormetic protection mechanism, akin to other cardioprotective gene programs such as delayed preconditioning (35). UPRmt-induced cardioprotection was absent in Atf5−/− mice, yet these mice exhibited no detriment in baseline cardiac function or I/R injury, indicating loss of UPRmt-induced protection is not simply due to greater stress sensitivity in Atf5−/−.
The canonical UPRmt gene program includes chaperones, proteases, antioxidants, and glycolysis (20, 22, 23), all of which are predicted to be beneficial in I/R injury. In particular, the mitochondrial protease LONP was recently shown to play a cardioprotective role in I/R injury (31). Although qPCR showed upregulation of several UPRmt-associated genes (including LonP) in hearts from oligomycin-treated mice, and this was confirmed at the protein level for HSP60, RNA-Seq did not identify canonical UPRmt target genes induced by oligomycin or doxycycline in an ATF5-dependent manner. This discrepancy is likely due to the low relative sensitivity of RNA-Seq versus qPCR.
RNA-Seq did identify 69 UPRmt-induced ATF5-dependent genes, which fell into broad categories including metabolism, epigenetic regulation, and cell signaling (Fig. 4D). Among metabolic genes of interest, Pfkfb1 is a glycolytic activator, which could be beneficial under hypoxic conditions (11). Several modes of cardioprotection are known to require glycolytic enhancement, including ischemic preconditioning (21). The enzyme Gpt2 (alanine aminotransferase) facilitates glutamate anaplerosis to the Krebs’ cycle, which is important for fueling substrate-level phosphorylation by succinyl-CoA synthetase during ischemia (38). Stk11ip is an activator of AMP-dependent protein kinase (37), and Agap2 activates Akt (12), both established cardioprotective signals.
Although our data suggest doxycycline-induced cardioprotection requires ATF5, this tetracycline is also reported to confer cardioprotection by inhibiting matrix metalloproteinases (MMPs) (4, 32). There is no apparent connection between ATF5 and MMPs, suggesting UPRmt→ATF5 signaling and MMP inhibition are parallel independent mechanisms of doxycycline cardioprotection. Recently, cardioprotection was reported upon acute tetracycline delivery to perfused hearts 30 min. before ischemia (30). We could not reproduce this result, possibly due to low doxycycline solubility in perfusion buffer, such that the necessary microfiltration (15) may limit availability. In addition, 30 min pre-treatment may be too short to induce a gene program such as the UPRmt (3), suggesting acute tetracycline cardioprotection may be attributable to MMP-inhibition (4).
Although our data suggest ATF5 is a mammalian ortholog of C. elegans ATFS-1 in the UPRmt, it remains unclear whether other features of the nematode UPRmt are retained in mammals. Several nematode UPRmt signaling proteins have unproven mammalian orthologs, including DVE-1 (SATB2) and UBL-5 (UBL5) (13). Furthermore, the nematode UPRmt can signal cell nonautonomously, whereby a UPRmt in one cell can trigger mitochondrial protection in another (8). Since our studies used global Atf5−/− mice, we cannot exclude the possibility that pharmacological UPRmt induction in vivo may have occurred in another tissue, signaling remote cardioprotection. Further studies using tissue-specific Atf5−/− mice may therefore prove insightful.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01-HL-127891 and R01-HL-071158.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.M.H., K.N., and P.S.B. conceived and designed research; Y.T.W. and K.-T.H. performed experiments; Y.T.W., Y.L., M.N.M., K.N., and P.S.B. analyzed data; Y.T.W., Y.L., M.N.M., C.M.H., K.N., and P.S.B. interpreted results of experiments; Y.L. prepared figures; M.N.M., P.S.B. drafted manuscript; C.M.H., K.N., and P.S.B. edited and revised manuscript; and Y.T.W., Y.L., M.N.M., K.-T.H., C.M.H., K.N., and P.S.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Stavros Lomvardas (Columbia University, NY) for Atf5+/− founders.
REFERENCES
- 1.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30: 2114–2120, 2014. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buttgereit F, Brand MD. A hierarchy of ATP-consuming processes in mammalian cells. Biochem J 312: 163–167, 1995. doi: 10.1042/bj3120163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng Z, Teo G, Krueger S, Rock TM, Koh HW, Choi H, Vogel C. Differential dynamics of the mammalian mRNA and protein expression response to misfolding stress. Mol Syst Biol 12: 855, 2016. doi: 10.15252/msb.20156423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung PY, Sawicki G, Wozniak M, Wang W, Radomski MW, Schulz R. Matrix metalloproteinase-2 contributes to ischemia-reperfusion injury in the heart. Circulation 101: 1833–1839, 2000. doi: 10.1161/01.CIR.101.15.1833. [DOI] [PubMed] [Google Scholar]
- 5.Christians ES, Benjamin IJ. Proteostasis and REDOX state in the heart. Am J Physiol Heart Circ Physiol 302: H24–H37, 2012. doi: 10.1152/ajpheart.00903.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalton RP, Lyons DB, Lomvardas S. Co-opting the unfolded protein response to elicit olfactory receptor feedback. Cell 155: 321–332, 2013. doi: 10.1016/j.cell.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21, 2013. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell 144: 79–91, 2011. doi: 10.1016/j.cell.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eilers M, Oppliger W, Schatz G. Both ATP and an energized inner membrane are required to import a purified precursor protein into mitochondria [Online]. EMBO J 6: 1073–1077, 1987. doi: 10.1002/j.1460-2075.1987.tb04860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiorese CJ, Schulz AM, Lin YF, Rosin N, Pellegrino MW, Haynes CM. The transcription factor ATF5 mediates a mammalian mitochondrial UPR. Curr Biol 26: 2037–2043, 2016. doi: 10.1016/j.cub.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gohil VM, Sheth SA, Nilsson R, Wojtovich AP, Lee JH, Perocchi F, Chen W, Clish CB, Ayata C, Brookes PS, Mootha VK. Nutrient-sensitized screening for drugs that shift energy metabolism from mitochondrial respiration to glycolysis. Nat Biotechnol 28: 249–255, 2010. doi: 10.1038/nbt.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hausenloy DJ, Yellon DM. Reperfusion injury salvage kinase signalling: taking a RISK for cardioprotection. Heart Fail Rev 12: 217–234, 2007. doi: 10.1007/s10741-007-9026-1. [DOI] [PubMed] [Google Scholar]
- 13.Haynes CM, Petrova K, Benedetti C, Yang Y, Ron D, Clp P. ClpP mediates activation of a mitochondrial unfolded protein response in C. elegans. Dev Cell 13: 467–480, 2007. doi: 10.1016/j.devcel.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 14.Haynes CM, Yang Y, Blais SP, Neubert TA, Ron D. The matrix peptide exporter HAF-1 signals a mitochondrial UPR by activating the transcription factor ZC376.7 in C. elegans. Mol Cell 37: 529–540, 2010. doi: 10.1016/j.molcel.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Headrick JP, Peart J, Hack B, Flood A, Matherne GP. Functional properties and responses to ischaemia-reperfusion in Langendorff perfused mouse heart. Exp Physiol 86: 703–716, 2001. doi: 10.1111/j.1469-445X.2001.tb00035.x. [DOI] [PubMed] [Google Scholar]
- 16.Heusch G. Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res 116: 674–699, 2015. doi: 10.1161/CIRCRESAHA.116.305348. [DOI] [PubMed] [Google Scholar]
- 17.Houtkooper RH, Mouchiroud L, Ryu D, Moullan N, Katsyuba E, Knott G, Williams RW, Auwerx J. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature 497: 451–457, 2013. doi: 10.1038/nature12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramar R, Hohenegger M, Srour AN, Khanakah G. Oligomycin toxicity in intact rats. Agents Actions 15: 660–663, 1984. doi: 10.1007/BF01966788. [DOI] [PubMed] [Google Scholar]
- 19.Law CW, Chen Y, Shi W, Smyth GK. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol 15: R29, 2014. doi: 10.1186/gb-2014-15-2-r29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melber A, Haynes CM. UPRmt regulation and output: a stress response mediated by mitochondrial-nuclear communication. Cell Res 28: 281–295, 2018. doi: 10.1038/cr.2018.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nadtochiy SM, Urciuoli W, Zhang J, Schafer X, Munger J, Brookes PS. Metabolomic profiling of the heart during acute ischemic preconditioning reveals a role for SIRT1 in rapid cardioprotective metabolic adaptation. J Mol Cell Cardiol 88: 64–72, 2015. doi: 10.1016/j.yjmcc.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nargund AM, Fiorese CJ, Pellegrino MW, Deng P, Haynes CM. Mitochondrial and nuclear accumulation of the transcription factor ATFS-1 promotes OXPHOS recovery during the UPR(mt). Mol Cell 58: 123–133, 2015. doi: 10.1016/j.molcel.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nargund AM, Pellegrino MW, Fiorese CJ, Baker BM, Haynes CM. Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science 337: 587–590, 2012. doi: 10.1126/science.1223560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ong SB, Samangouei P, Kalkhoran SB, Hausenloy DJ. The mitochondrial permeability transition pore and its role in myocardial ischemia reperfusion injury. J Mol Cell Cardiol 78: 23–34, 2015. doi: 10.1016/j.yjmcc.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Otero MJ, Carrasco L. Action of oligomycin on cultured mammalian cells. Permeabilization to translation inhibitors. Mol Cell Biochem 61: 183–191, 1984. [DOI] [PubMed] [Google Scholar]
- 26.Peña S, Sherman T, Brookes PS, Nehrke K. The Mitochondrial Unfolded Protein Response Protects against Anoxia in Caenorhabditis elegans. PLoS One 11: e0159989, 2016. doi: 10.1371/journal.pone.0159989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quirós PM, Prado MA, Zamboni N, D’Amico D, Williams RW, Finley D, Gygi SP, Auwerx J. Multi-omics analysis identifies ATF4 as a key regulator of the mitochondrial stress response in mammals. J Cell Biol 216: 2027–2045, 2017. doi: 10.1083/jcb.201702058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43: e47, 2015. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suarez G, Nathans D. Inhibition of aminoacyl-sRNA binding to ribosomes by tetracycline. Biochem Biophys Res Commun 18: 743–750, 1965. doi: 10.1016/0006-291X(65)90848-X. [DOI] [Google Scholar]
- 30.Sun CL, Zhang H, Liu M, Wang W, Crowder CM. A screen for protective drugs against delayed hypoxic injury. PLoS One 12: e0176061, 2017. doi: 10.1371/journal.pone.0176061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Venkatesh S, Li M, Saito T, Tong M, Rashed E, Mareedu S, Zhai P, Bárcena C, López-Otín C, Yehia G, Sadoshima J, Suzuki CK. Mitochondrial LonP1 protects cardiomyocytes from ischemia/reperfusion injury in vivo. J Mol Cell Cardiol 128: 38–50, 2019. doi: 10.1016/j.yjmcc.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 32.Villarreal FJ, Griffin M, Omens J, Dillmann W, Nguyen J, Covell J. Early short-term treatment with doxycycline modulates postinfarction left ventricular remodeling. Circulation 108: 1487–1492, 2003. doi: 10.1161/01.CIR.0000089090.05757.34. [DOI] [PubMed] [Google Scholar]
- 33.Wang SZ, Ou J, Zhu LJ, Green MR. Transcription factor ATF5 is required for terminal differentiation and survival of olfactory sensory neurons. Proc Natl Acad Sci USA 109: 18589–18594, 2012. doi: 10.1073/pnas.1210479109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu D, Lim E, Vaillant F, Asselin-Labat ML, Visvader JE, Smyth GK. ROAST: rotation gene set tests for complex microarray experiments. Bioinformatics 26: 2176–2182, 2010. doi: 10.1093/bioinformatics/btq401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang XM, Baxter GF, Heads RJ, Yellon DM, Downey JM, Cohen MV. Infarct limitation of the second window of protection in a conscious rabbit model. Cardiovasc Res 31: 777–783, 1996. doi: 10.1016/S0008-6363(96)00026-0. [DOI] [PubMed] [Google Scholar]
- 36.Yun J, Finkel T. Mitohormesis. Cell Metab 19: 757–766, 2014. doi: 10.1016/j.cmet.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zaha VG, Young LH. AMP-activated protein kinase regulation and biological actions in the heart. Circ Res 111: 800–814, 2012. doi: 10.1161/CIRCRESAHA.111.255505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J, Wang YT, Miller JH, Day MM, Munger JC, Brookes PS. Accumulation of Succinate in Cardiac Ischemia Primarily Occurs via Canonical Krebs Cycle Activity. Cell Rep 23: 2617–2628, 2018. doi: 10.1016/j.celrep.2018.04.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Q, Wang J, Levichkin IV, Stasinopoulos S, Ryan MT, Hoogenraad NJ. A mitochondrial specific stress response in mammalian cells. EMBO J 21: 4411–4419, 2002. doi: 10.1093/emboj/cdf445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou D, Palam LR, Jiang L, Narasimhan J, Staschke KA, Wek RC. Phosphorylation of eIF2 directs ATF5 translational control in response to diverse stress conditions. J Biol Chem 283: 7064–7073, 2008. doi: 10.1074/jbc.M708530200. [DOI] [PubMed] [Google Scholar]