Abstract
Although it is known that the prevalence and severity of hypertension increases in women after menopause, the contribution of T cells to this process has not been explored. Although the immune system is both necessary and required for the development of angiotensin II (ANG II) hypertension in men, we have demonstrated that premenopausal women are protected from T cell-mediated hypertension. The goal of the current study was to test the hypotheses that 1) female protection against T cell-mediated ANG II hypertension is eliminated following progression into menopause and 2) T regulatory cells (Tregs) provide premenopausal protection against ANG II-induced hypertension. Menopause was induced in Rag-1−/− mice (via 4-vinylcyclohexene diepoxide), and all mice received a 14-day ANG II infusion. Donor CD3+ T cells were adoptively transferred 3 wk before ANG II infusion. In the absence of T cells, systolic blood pressure responses to ANG II were similar to those seen in premenopausal mice (Δ12 mmHg). After adoptive transfer of T cells, ANG II significantly increased systolic blood pressure in postmenopausal females (Δ28 mmHg). A significant increase in F4/80 positive renal macrophages, an increase in renal inflammatory gene expression, along with a reduction in renal expression of mannose receptor C-type 1, a marker for M2 macrophages, accompanied the increase in systolic blood pressure (SBP). Flow cytometric analysis identified that Tregs were significantly decreased in the spleen and kidneys of Rag-1−/− menopausal mice versus premenopausal females, following ANG II infusion. In a validation study, an anti-CD25 antibody was used to deplete Tregs in premenopausal mice, which induced a significant increase in SBP. These results demonstrate that premenopausal protection against T cell-mediated ANG II hypertension is eliminated once females enter menopause, suggesting that a change in hormonal status upregulates macrophage-induced proinflammatory and T cell-dependent responses. Furthermore, we are the first to report that the presence of Tregs are required to suppress ANG II hypertension in premenopausal females.
NEW & NOTEWORTHY Whether progression into menopause eliminated female protection against T cell-mediated hypertension was examined. Menopausal mice without T cells remained protected against angiotensin II (ANG II) hypertension; however, in the presence of T cells, blood pressure responses to ANG II increased significantly in menopause. Underlying mechanisms examined were anti-inflammatory protection provided by T regulatory cells in premenopausal females and renal inflammatory processes involving macrophage infiltration and cytokine activation.
Keywords: inflammation, kidney, macrophage, ovarian failure, T cells, VCD
INTRODUCTION
Premenopausal women are resistant to the development of hypertension compared with age-matched men (20). Experimental studies using genetic or pharmacologic approaches have demonstrated that this protection is established by elevated levels of estrogen common in premenopausal women (46). This protection is lost after menopause and contributes to the sharp increase in disease prevalence and severity. Using a novel estrogen-deplete, ovary-intact menopause model [4-vinylcyclohexene diepoxide (VCD) menopause model], we have shown that progression from perimenopause to menopause augments angiotensin II (ANG II)-induced elevations in blood pressure (BP) (22). However, the underlying mechanisms promoting these increased hypertensive responses after menopause remain ill defined and thus hinder the ability to adequately treat hypertension in postmenopausal women.
T cells of the adaptive immune system play an essential role in the genesis of hypertension. In men, T-cell activation in hypertension models leads to tissue infiltration of immune cells and systemic injury; in the kidneys, this leads to salt retention and fibrosis (5, 9, 10). Genetic ablation of the recombination-activating gene-1 (Rag-1), which leads to a complete lack of B- and T-cell formation because of the inability to form antigen receptors, attenuates ANG II- and salt-dependent hypertensive responses in males. Furthermore, T-cell adoptive transfer restored BP responses in males to wild-type levels (9, 27).
Our previous study demonstrated that premenopausal females are protected from T cell-mediated hypertension (33). Suppression of ANG II hypertension in female Rag-1−/− mice receiving exogenous T cells was associated with a significant reduction in renal T-cell infiltration, compared with male mice, and an inhibition of T cell-mediated proinflammatory cytokine expression compared with males (33).
Therefore, the current study sought to determine whether progression into menopause eliminated female protection against T cell-mediated hypertension. We hypothesized that following progression into menopause, adoptive transfer of T cells in to Rag 1−/− mice would induce a robust ANG II hypertensive response, associated with a proinflammatory shift in the kidneys, similar to our previous studies in male mice. To further test the hypothesis that an anti-inflammatory T regulatory cell (Treg) milieu contributes to the protection of premenopausal females from hypertension, we used an in vivo Treg depletion protocol and examined if loss of Tregs impacted the development of ANG II-induced hypertension in premenopausal females.
METHODS
Animals.
Menopause study mice, 8–10-wk-old female Rag-1−/−, were obtained by in-house breeding at the University of Arizona, from original breeders purchased from the Jackson Laboratory in 2014 (strain 002216, B6.129S7-Rag1tm1Mom/J). Male B6-Ly5.1[B6.SJL-PtprcaPepcb/BoyJ] mice were used as donors for the T-cell transfer experiments.
Treg knockdown studies used 8-wk-old C57BL/6J (Jackson) and 129S6 female mice (Taconic Biosciences; strain 129S6/SvEvTac).
All mice were housed in standard polypropylene cages in a temperature- and humidity- controlled facility and were maintained on a 12-h:12-h light-dark cycle with normal (0.25% NaCl: Harlan Tecklad 7013) mouse chow and water available ad libitum. All methods were approved by the University of Arizona Animal Care and Use Committee.
Experimental protocol.
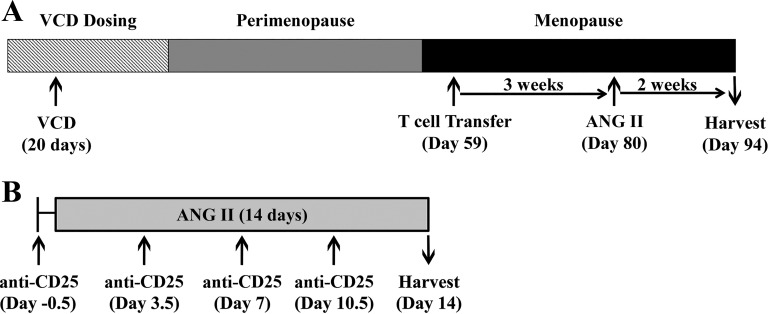
Timelines for both experimental protocols are depicted in Fig. 1. To induce ovarian failure (menopause), female Rag-1−/− mice received daily intraperitoneal injections of VCD (160 mg/kg ip, Sigma V3630) for 20 consecutive days. Vaginal cytology was measured daily to determine the onset of menopause, defined as 10 consecutive days of diestrus. The average day of onset of menopause following VCD treatment occurred on day 56 ± 0.6, similar to our previously reported studies of VCD-induced menopause in other strains of mice (22, 32). Menopausal Rag-1−/− recipient mice were separated into two groups, with one group receiving, via adoptive transfer, CD3+ T cells (~7.5 × 106 cells per recipient) 3 wk before ANG II infusion (490 ng·kg−1·min−1, A9525 Sigma; Fig. 1A), as previously described (33).
Fig. 1.
Timeline of experimental studies. A: to determine how progression of menopause impacts T cell-mediated hypertension, female Rag-1−/− mice received intraperitoneal injections of 4-vinylcyclohexene diepoxide (VCD) for 20 days to induce menopause. Following the onset of menopause (assessed via daily cytology), CD3+ T cells were adoptively transferred into the Meno/T-cell/ANG group. Three weeks post-transfer, angiotensin II (ANG II) was infused for 14 days (n = 10 per group) B: to determine if T regulatory cell depletion in vivo removed premenopausal suppression of ANG II-induced hypertension, female 129S6 and C57BL/6 mice received intraperitoneal injections of anti-CD25 antibody concurrent with 14 day ANG II infusion (n = 10 per group). ANG, angiotensin; Meno, menopause; Rag-1, recombination-activating gene-1.
To deplete Tregs in premenopausal female mice, 250 µg of anti-CD25 (PC-61, BioXCell) was injected intraperitoneally on day 0 (immediately before ANG II infusion) and every 3.5 days thereafter (Fig. 1B) in 2 different strains of mice (129S6 and C57BL/6J mice). ANG II (A9525, Sigma) in this study was infused at a rate of 800 ng·kg−1·min−1, as previously described (32). Anti-CD25 antibody alone was injected into a cohort of mice to control for the effect of antibody administration on BP.
BP and heart rate were measured in both studies, at day 0 and day 14, via noninvasive tail cuff machine (Hatteras Instruments, MC4000).
Flow cytometry.
Splenic and renal T-cell expression patterns were analyzed by flow cytometry as previously described (33). Briefly, isolated lymphocytes were stained with surface antibodies overnight at 4°C, all at 1:100 dilution, followed by a live/dead discriminator dye (1:1,000, Life Technologies) and fixation/permeabilization with the Foxp3 Staining Buffer Set (eBioscience) and intracellular staining with Foxp3 antibody (1:100). A BD Fortessa instrument (BD FACS Diva software, Becton Dickinson) was used to acquire flow cytofluorometric data. Analysis was performed using FlowJo software (Tree Star). To reduce animal use as per recommendation from the National Centre for the Replacement, Refinement and Reduction of Animals in Research (34), T-cell infiltration data obtained from a previous study (33) performed in premenopausal females was used for comparison of total T-cell infiltration in spleen and kidney with postmenopausal females.
RNA isolation and real-time quantitative PCR.
Right kidneys were excised and decapsulated (n = 5/group). RNA isolation and real-time quantitative PCR analyses were performed as previously described (33). Primer sequences were as follows: intercellular adhesion molecule (ICAM)-1 forward: CCATGCCTTAGCAGCTGAAC, reverse: AGCTTGCACGACCCTTCTAA; interleukin (IL)-2 forward: AAAGGGCTCTGACAACACATT, reverse: AGGGCTTGTTGAGATGATGC; IL-10 forward: CCCTTTGCTATGGTGTCCTT, reverse: AGTAGGGGAACCCTCTGAGC; monocyte chemoattractant protein (MCP)-1 forward: CAAGAAGGAATGGGTCCAGA, reverse: AGACCTTAGGGCAGATGCAG; transforming growth factor-β1 forward: TTGCTTCAGCTCCACAGAGA, reverse: TGGTTGTAGAGGGCAAGGAC; TNF-α forward: CTTGTTGCCTCCTCTTTTGC, reverse: ACCCGTAGGGCGATTACAGT.
Immunohistochemistry.
Immunohistochemistry was performed according to the guidelines for authors and reviewers on the use of antibodies in physiology studies (3). All antibodies were commercially obtained and specificity determined by the company source. Left kidneys were excised, decapsulated, longitudinally sectioned, fixed in 2% paraformaldehyde for 24 h, embedded in paraffin, and sectioned (5 µm). Periodic acid-Schiff staining was used to calculate glomerular area, as previously described (32). Briefly, we quantified all glomeruli in each image. To minimize the potential impact of measuring glomeruli from nonidentical planes, 15 cortical images per slide were captured at ×200 magnification, resulting in the analysis of 45–55 glomeruli per slide, ~200 glomeruli per treatment group. Each glomerular profile mean area (μm2) was measured through manually tracing the minimal convex polygon surrounding the glomerular capillary tuft, and the area calculated by computerized morphometry using ImageJ software (National Institutes of Health, Bethesda, MD) (14). All imaging was conducted in a blinded fashion.
Total macrophage infiltration was determined by F4/80+ staining, and anti-inflammatory M2-macrophage subtype was determined by mannose receptor C-type 1 (MRC1) staining. Briefly, sections were deparaffinized with xylene and rehydrated by graded ethanol solutions. Endogenous horseradish peroxidase (HRP) was blocked by incubating 15 min in 0.3% H2O2 in methanol. Antigen retrieval was performed by incubating sections in 10 mM citrate buffer for 20 min at 37°C. Nonspecific binding was blocked by 10% goat serum in 2% BSA with 0.05% Tween-20. For F4/80 staining, sections were incubated overnight at 4°C with rat anti-mouse F4/80 (1:20 dilution; Bio-Rad MCA497GA) followed by HRP-conjugated secondary antibody goat anti-rat IgG-HRP (1:200, AbD Serotec) (14). Positive staining of F4/80 was visualized with a Diaminobenzidine-Plus Substrate Kit (Life Technologies 002020) and subsequently counterstained with hematoxylin (Invitrogen, cat. no. 008011). F4/80 antibody validation included the assessment of nonspecific binding using control slides with primary and secondary antibody only. Positive staining was quantified via multistep binary algorithm using Image J software and represented as a percent of total tissue area (14).
Kidney sections were stained for MRC1 using the Opal Immunohistochemistry Kit (Perkin Elmer) following the manufacturer’s instructions and guidelines. Briefly, slides were deparaffinized and rehydrated, and heat-mediated antigen retrieval was performed. Tissue sections were incubated with anti-MRC1 rabbit polyclonal antibody (Abcam, cat. no. ab64693, 1:500) at room temperature for 1 h, followed by incubation with HRP-conjugated secondary polymer for 10 min (provided with Opal Kit, Perkin Elmer). The opal 570 fluorophore was applied for 10 min and nuclei were counterstained with 4′6-diamino-2-phenylindole (16). Antibody validation included the assessment of nonspecific binding using control slides with secondary antibody only, no primary antibody (3). Images were acquired on the Mantra Quantitative Pathology Imaging microscope at ×40 magnification. A total of 10 cortical images from each section were analyzed with Image-Prosoftware (Media Cybernetics, Bethesda, MD) to calculate the percentage of positive-stained area to total tissue area (29).
Statistics.
Statistics were evaluated according to the statistical considerations in reporting cardiovascular research (21). Data were analyzed with Graph Pad Prism Software v6 by paired and unpaired Student’s t-tests and expressed as means ± SE, with P < 0.05 considered significant.
RESULTS
T cells alone did not induce a difference in basal systolic BP and heart rate in menopausal Rag-1−/− mice.
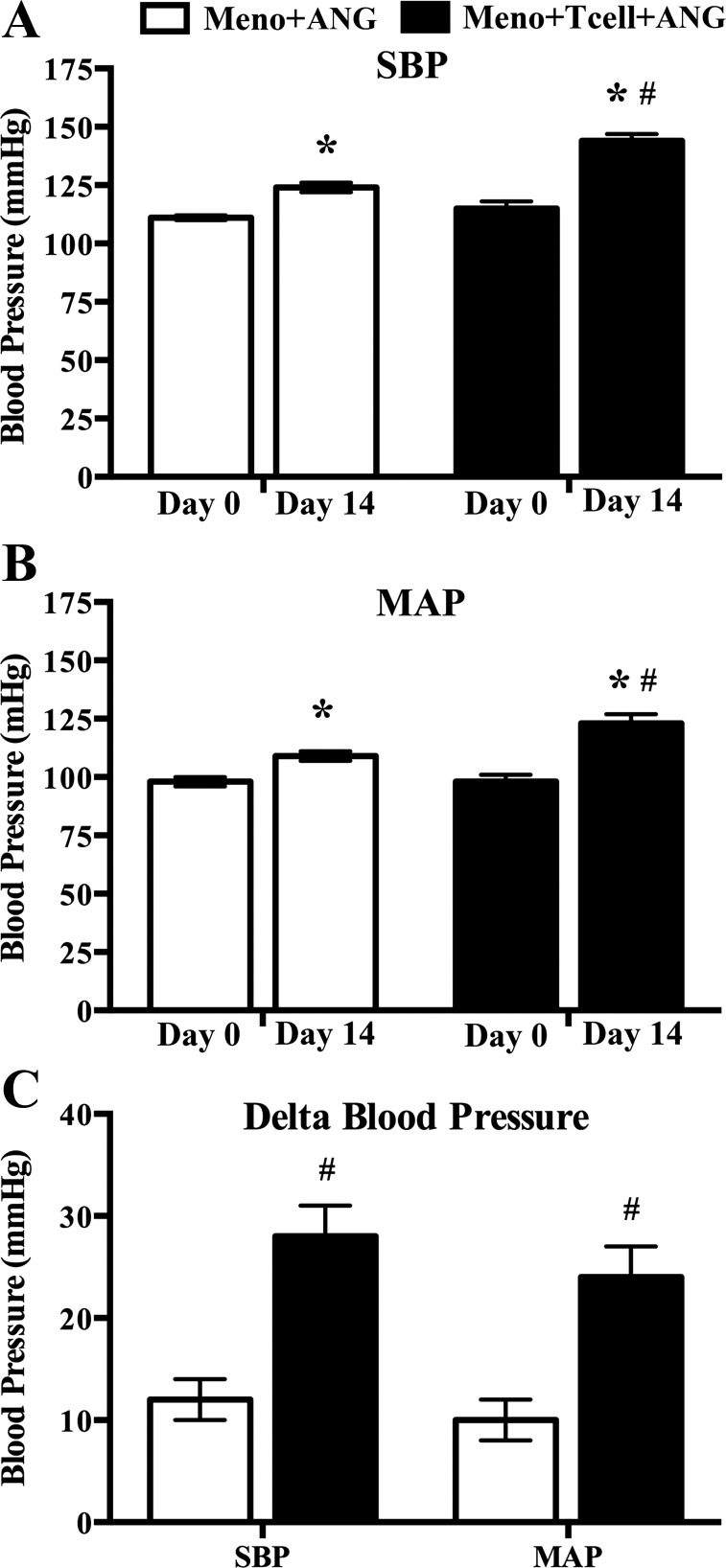
The adoptive transfer of T cells into menopausal Rag-1−/− mice did not impact baseline systolic BP (SBP) or mean arterial pressure (MAP). At day 0 before ANG II infusion, there was no significant difference between the SBP or MAP in the menopausal groups with or without T cells (Fig. 2, A and B) (SBP: Meno 111 ± 1 vs. Meno/T cell 115 ± 3 mmHg; MAP: Meno 98 ± 2 vs. Meno/T cell 98 ± 3 mmHg; not significant, n = 10 per group).
Fig. 2.
Hemodynamic responses to angiotensin II (ANG II) infusion in menopausal Rag-1−/− mice with (Meno/T-cell/ANG) or without (Meno/ANG) T-cell adoptive transfer. Systolic blood pressure (SBP) (A) and mean arterial pressure (MAP) (B) significantly increased after 14 days of ANG II infusion (490 ng·kg−1·min−1) compared with day 0. Both SBP and MAP are significantly greater in Meno/T cell/ANG compared with Meno/ANG at day 14. Changes in SBP and MAP are presented as Δ blood pressure (C) from day 0 to day 14 after onset of ANG II infusion. Results are expressed as means ± SE; n = 10 mice/group. *P < 0.05 vs. same group day 0, paired Student’s t-test; #P < 0.05 vs. Meno/ANG, unpaired Student’s t-test. ANG, angiotensin; Meno, menopause; Rag-1, recombination-activating gene-1.
ANG II infusion significantly increased hemodynamic responses in menopausal Rag-1−/− mice in the presence of T cells.
In the absence of T cells, ANG II infusion (490 ng·kg−1·min−1) into menopausal Rag-1−/− females induced a small increase of 12 mmHg in SBP and 10 mmHg in MAP (Fig. 2C; both P < 0.05 vs. Meno/ANG II) that were similar to our previous report (33). Following adoptive transfer of T cells, ANG II infusion into menopausal Rag-1−/− females induced a robust increase of 28 mmHg in SBP and 24 mmHg in MAP (Fig. 2C; both P < 0.05 vs. Meno/ANG II).
ANG II infusion increased proinflammatory renal mRNA expression in menopausal Rag-1−/− mice in the presence of T cells.
Real-time PCR was performed to assess the expression of six inflammatory markers in the kidneys of menopausal Rag-1−/− mice following ANG II infusion in the presence and absence of T cells. Expression of the proinflammatory cytokines IL-2, MCP-1, and TNF-α and the anti-inflammatory cytokine IL-10 was increased in the kidneys of ANG II-infused menopausal Rag-1−/− females following the adoptive transfer of T cells (Table 1), mirroring the responses we reported in male Rag-1−/− mice (33). Transforming growth factor β1 and ICAM-1 expression were not different.
Table 1.
T-cell adoptive transfer and ANG II infusion induced renal cytokine mRNA expression in menopausal Rag-1−/− mice
| Gene | Meno/ANG | Meno/Tcell/ANG |
|---|---|---|
| Interleukin-2 | 1.0 ± 0.2 | 5.7 ± 0.1* |
| TNF-α | 1.0 ± 0.2 | 2.1 ± 0.1* |
| MCP-1 | 1.0 ± 0.2 | 1.8 ± 0.3* |
| ICAM-1 | 1.0 ± 0.2 | 1.4 ± 0.1 |
| TGF-β1 | 1.0 ± 0.2 | 1.3 ± 0.1 |
| Interleukin-10 | 1.0 ± 0.5 | 3.7 ± 0.2* |
SYBR Green I real-time PCR assay validation was performed. The data shown are the mean relative fold changes in whole kidneys in Rag-1−/− mice. Assays for dynactin were run in parallel on each sample for subsequent normalization of the data. Results are expressed as means ± SE; n = 4 mice/group. ANG, angiotensin II; ICAM-1, intercellular adhesion molecule; MCP-1, monocyte chemoattractant protein; Meno, menopause; Rag-1, recombination-activating gene-1; TGF-β1, transforming growth factor-β; TNF-α, tumor necrosis factor-α.
P < 0.05 test vs. Meno/ANG.
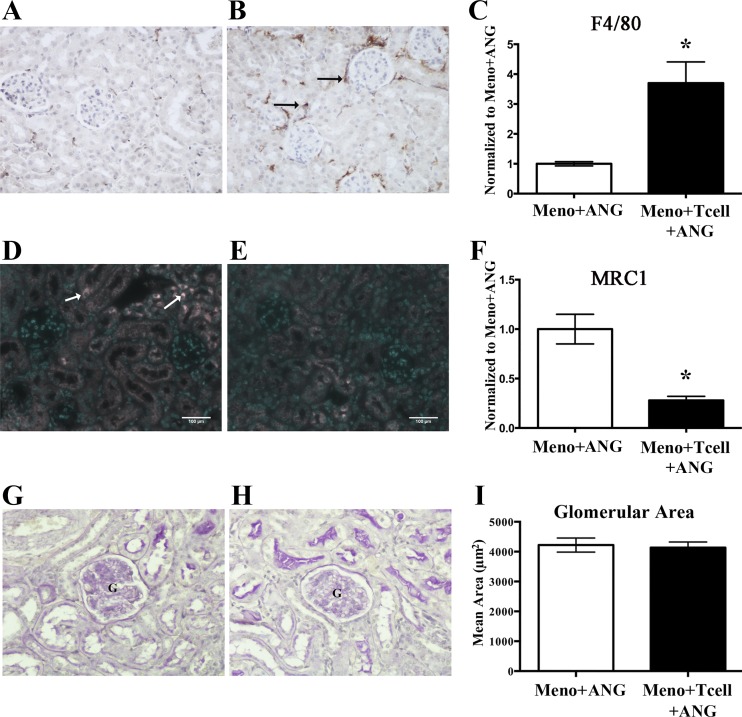
ANG II in the presence of T cells induced renal F4/80 macrophage influx, with a concomitant decline in M2 macrophages.
To determine if macrophage infiltration into the kidney increased following ANG II infusion, we used an ImageJ-based threshold analysis of F4/80 immunohistochemically stained kidney sections. Following the transfer of T cells into menopausal mice, ANG II induced a 3.7-fold increase in F4/80+ macrophages into the kidneys (Fig. 3, A–C). To assess the presence of M2 anti-inflammatory macrophages, we used an antibody against the prototypical M2 marker MRC1. In the presence of T cells, ANG II significantly reduced M2 macrophages in the kidneys of menopausal Rag-1−/− mice by 74% (Fig. 3, D–F), suggesting that T cells mediated a shift in the ratio of M1:M2 macrophages in the postmenopausal females. No change in glomerular area was seen between ANG II infusion in the presence or absence of T cells (Fig. 3, G–I), indicating that these changes occur before renal pathology. Together, these results demonstrate that T cell-dependent hypertension in menopausal Rag-1−/− mice is accompanied by the development of a proinflammatory environment within the kidney.
Fig. 3.
Effect of T-cell adoptive transfer on ANG II-induced renal macrophage infiltration and glomerular hypertrophy in menopausal Rag-1−/− mice. Representative images of F4/80 (A and B), mannose receptor C-type 1 (MRC1) (D and E), periodic acid-Schiff (G and H), stained renal sections from ANG II-infused (490 ng·kg−1·min−1) menopausal Rag-1−/− mice (Meno/ANG) (A, D, and G), or menopausal mice that received T-cell transfer (Meno/T cell/ANG) (B, E, and H). Quantification (C, F, and I) that T cells significantly increased ANG II-induced renal macrophage infiltration, identified by F4/80-positive staining (C), decreased expression of anti-inflammatory M2 macrophages (F), and had no impact on ANG II-induced glomerular hypertrophy in menopausal Rag-1−/− mice (I). Results are expressed as means ± SE for C and F (normalized to Meno/ANG); magnification ×200; n = 3 mice/group. *P < 0.05 vs. Meno/ANG, unpaired Student’s t-test. ANG, angiotensin; Meno, menopause; Rag-1, recombination-activating gene-1; G, glomerulus.
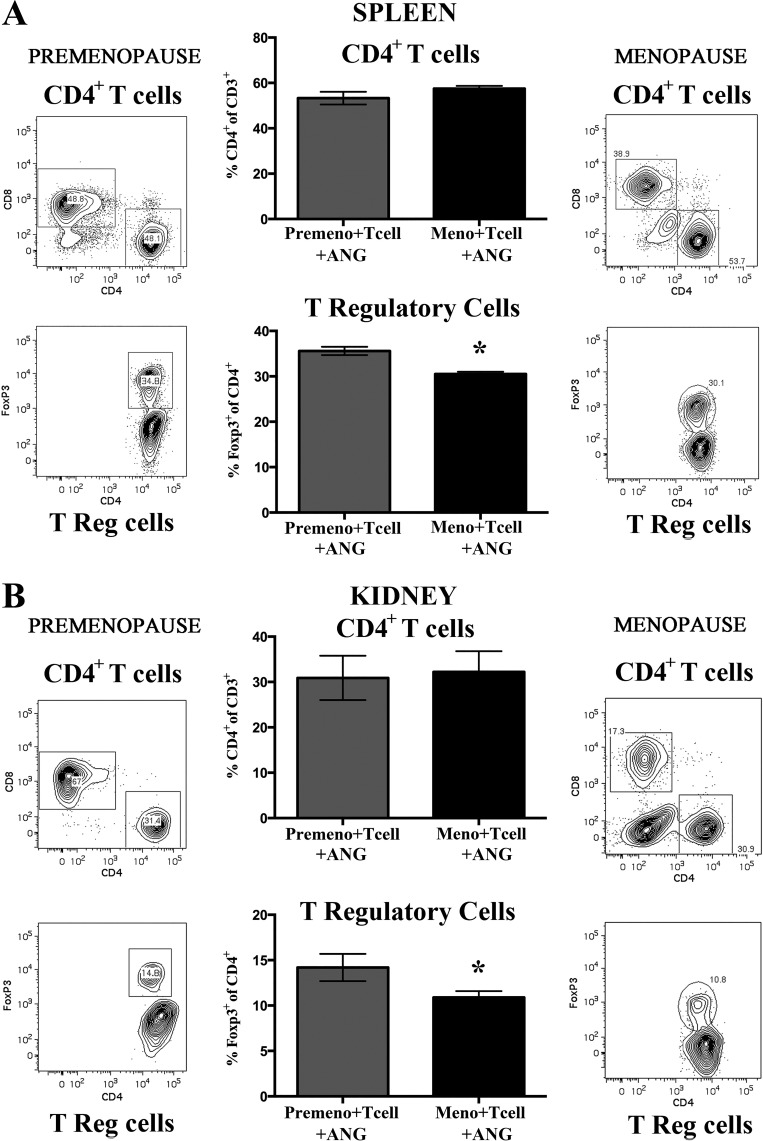
Following menopause, renal and splenic Treg frequency was significantly reduced compared with premenopausal mice.
Previous studies have suggested that robust renal T regulatory expression in the female spontaneously hypertensive rat contribute to a lower BP (42, 43). To determine if changes in T-cell expression profiles contributed to enhanced BP responses in Rag-1−/− menopausal mice, following adoptive transfer T cells, spleen and kidneys were harvested for flow cytometric analysis. Data from postmenopausal females was compared with T-cell infiltration in premenopausal female Rag-1−/− mice.
In the spleen (Fig. 4A), following T-cell adoptive transfer and ANG II infusion (490 ng·kg−1·min−1), the frequency of CD4+ T cells (as a percent of CD3+ cells) was not different between premenopausal and menopausal Rag-1−/− mice; however, the frequency of Foxp3+ Tregs (as a percent of CD4+ cells) was significantly reduced in the spleen of menopausal mice compared with premenopausal mice.
Fig. 4.
Effect of menopause on angiotensin II (ANG II)-induced splenic and renal T-cell infiltration profiles in Rag-1−/− mice. T-cell adoptive transfer was performed in both premenopausal and menopausal Rag-1−/− mice before ANG II infusion (490 ng·kg−1·min−1) for 14 days, and T-cell infiltration was measured in spleen and kidneys. A: no difference in percent of splenic CD4+ T cells (%CD4+ of CD3+ T) was seen between premenopausal ANG II-infused (Premeno/T-cell/ANG) and menopausal (Meno/T-cell/ANG) Rag-1−/− mice. However, splenic T regulatory cell expression (%Foxp3+ of CD4+) was significantly reduced in menopausal mice compared with premenopausal Rag-1−/− mice. Representative flow cytometric gating is shown for both populations of T cells from premenopausal and menopausal studies. B: no difference in percent of renal CD4+ T cells (%CD4+ of CD3+ T) was seen between premenopausal ANG II-infused (Premeno/T-cell/ANG) and menopausal (Meno/T-cell/ANG) Rag-1−/− mice. However, renal T regulatory cell expression (%Foxp3+ of CD4+) was significantly reduced in menopausal mice compared with premenopausal Rag-1−/− mice. Representative flow cytometric gating is shown for both populations of T cells from premenopausal and menopausal studies. Results are expressed as means ± SE; n = 5–6 mice/group. *P < 0.05 vs. Premeno/T cell/ANG, unpaired Student’s t-test. Rag-1, recombination-activating gene-1.
Similarly, in the kidney (Fig. 4B), following T-cell adoptive transfer and ANG II infusion, the frequency of CD4+ T cells (as a percent of CD3+ cells) was not different between premenopausal and menopausal Rag-1−/− mice; however, the frequency of Foxp3+ Tregs (as a percent of CD4+ cells) infiltrating the kidney was significantly reduced in menopausal mice compared with premenopausal animals.
Depletion of Tregs in premenopausal females removes premenopausal resistance to ANG II-induced hypertension.
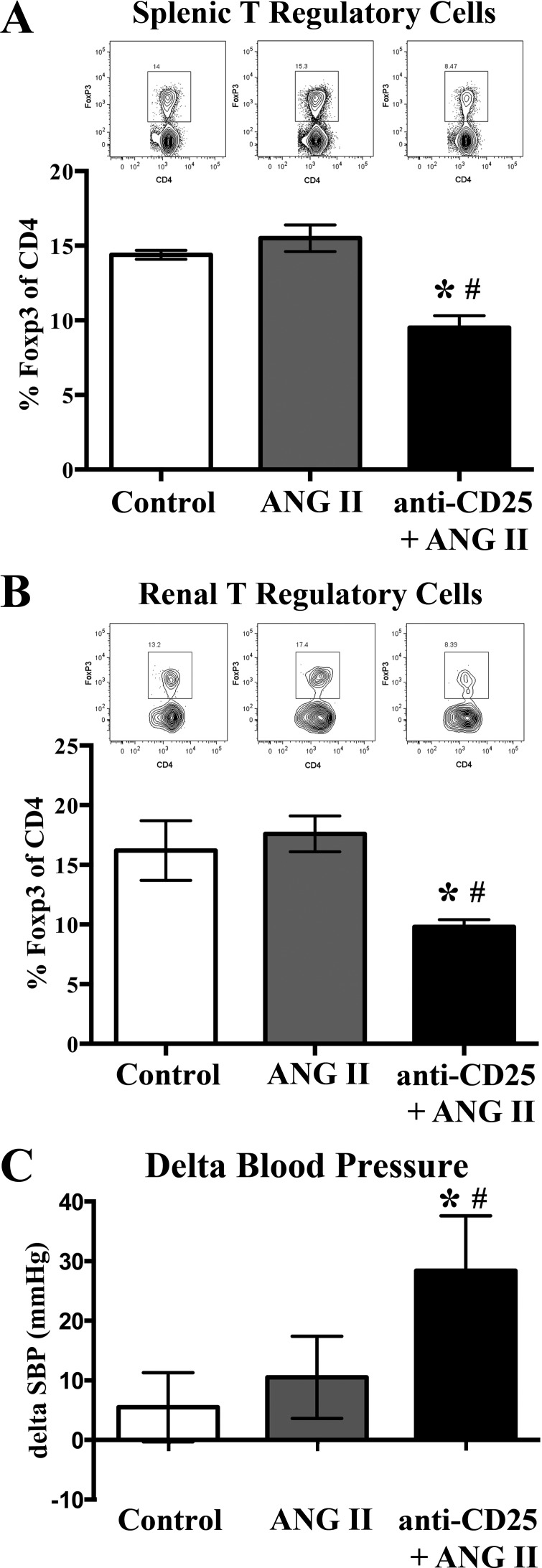
To determine if Tregs contributed to premenopausal female resistance to ANG II-induced hypertension, we administered a blocking CD25 antibody to premenopausal females concurrent with ANG II infusion (800 ng·kg−1·min−1). Noninvasive tail cuff measurements were conducted to determine the effect of Treg depletion on ANG II hypertension in premenopausal female mice. We used two strains of mice (C57BL/6J and 129S6, females) to ensure that any responses seen were relevant across strains. Using flow cytometry, we determined that anti-CD25 administration significantly reduced Foxp3+ Tregs in the spleen (Fig. 5A) and the kidneys (Fig. 5B) of premenopausal 129S6 mice. Depletion of Tregs in premenopausal females induced a significant increase in SBP following ANG II infusion (Fig. 5C), demonstrating a role for Tregs in premenopausal resistance to ANG II-induced hypertension.
Fig. 5.
T regulatory cell depletion increases hemodynamic response to angiotensin II (ANG II) infusion in 129S6 premenopausal female mice. Administration of anti-CD25 to ANG II-infused (800 ng·kg−1·min−1) premenopausal 129S6 mice (anti-CD25/ANG) significantly reduced the frequency of splenic (A) and renal (B) T regulatory cells in ANG II-infused mice compared with ANG II and control mice (n = 4/group). Representative flow cytometric gating images are shown for each corresponding group and tissue. C: premenopausal female mice administered anti-CD25 exhibited a significantly greater increase in systolic blood pressure (SBP) after 14 days of ANG II infusion. Results are expressed as means ± SE; n = 10 mice/group. *P < 0.05 vs. control; #P < 0.05 vs. ANG II, unpaired Student’s t-test.
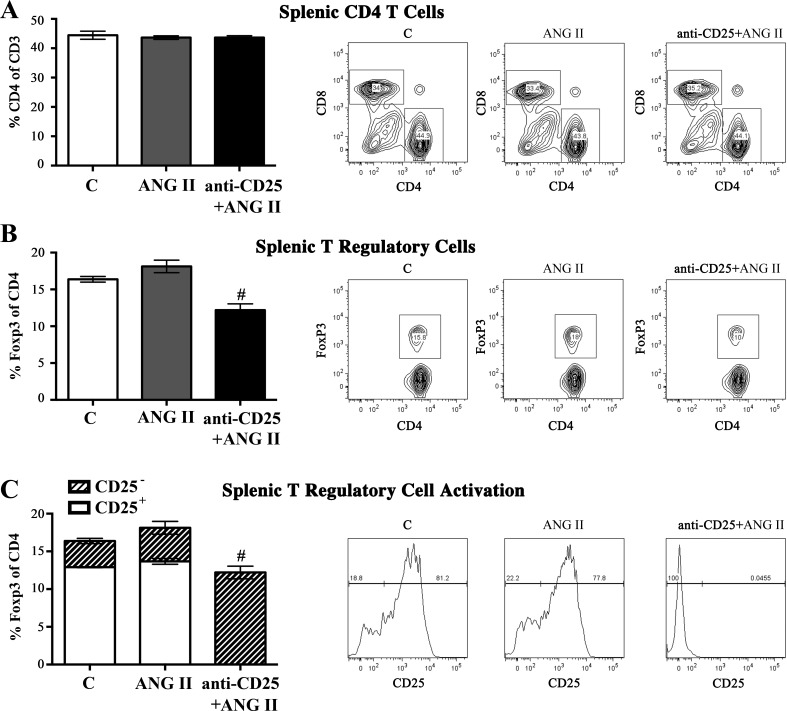
In a separate study (C57BL/6J females), anti-CD25 administration concurrent with ANG II infusion (800 ng·kg−1·min−1) significantly reduced Foxp3+ Tregs in the spleen, with no reduction in the frequency of splenic CD4+ T cells (as a percent of CD3+ cells) (Fig. 6, A and B). Upon further analysis of the Foxp3+ Tregs in the ANG II alone-infused group, 76.8% of splenic Foxp3+ Tregs coexpressed CD25, a marker of Treg activation. In contrast, 99.9% of splenic Foxp3+ Tregs from the anti-CD25/ANG-II group were CD25 negative (Fig. 6C), suggesting that anti-CD25 administration in ANG II-infused premenopausal female mice induced a significant depletion of functionally active Tregs.
Fig. 6.
Anti-CD25 administration abolishes active splenic T regulatory cells in ANG II-infused C57BL/6 premenopausal female mice. A: administration of anti-CD25 to ANG II-infused (800 ng·kg−1·min−1) premenopausal C57BL/6 mice (anti-CD25/ANG) did not significantly reduce splenic %CD4+ of CD3+ T cells compared with ANG II and control mice. B: anti-CD25 administration significantly reduced splenic T regulatory cell frequency in ANG II-infused mice compared with ANG II and control mice. C: flow cytometric analysis of CD25 expression on Foxp3+ T regulatory cells demonstrates residual Foxp3+ T regulatory cells in anti-CD25-treated mice are inactive CD25- cells. Representative flow cytometric gating images are shown for each corresponding group. Results are expressed as means ± SE; n = 4 mice/group. *P < 0.05 vs. control; #P < 0.05 vs. ANG II, unpaired Student’s t-test.
DISCUSSION
The goal of this study was to explore the impact of menopause on T cell-mediated hypertension focusing on proinflammatory versus anti-inflammatory T-cell populations in pre- and postmenopausal females. The major findings of this study were: 1) premenopausal protection against T cell-mediated hypertension is lost once females enter menopause, as ANG II induced a significant increase in blood pressure in the presence of T cells in postmenopausal Rag-1−/− ; 2) in the presence of T cells, ANG II induced a proinflammatory environment in the kidneys of postmenopausal Rag-1−/− mice, including an increase in macrophage infiltration; 3) Treg expression decreased postmenopause; and 4) in vivo depletion of Tregs resulted in a significant increase in ANG II-induced hypertension in premenopausal females. These findings reveal that upregulation of proinflammatory T cell-dependent processes promote the increase in hypertension in female mice after menopause.
Several studies have suggested that increased BP responses in ovariectomized animals leads to increases in renal macrophage infiltration, glomerular sclerosis, proteinuria, and endothelial dysfunction (11, 25, 37), and estrogen replacement in surgically induced menopause and in the VCD model of menopause reduces ovariectomy and prevents renal and vascular dysfunction (32). We have shown previously that premenopausal females are protected against T cell-mediated hypertension when we directly compared the ability of T cells to mediate ANG II hypertension in male and female Rag-1−/− mice (33). In the absence of T cells, ANG II had a minimal effect on blood pressure in either sex. Once T cells were restored, males responded to ANG II with a robust increase in blood pressure; in contrast, females remained protected from ANG II hypertension (33). Adding to this observed sex difference in T cell-mediated hypertension, the present study explicitly investigates the role of menopause in T cell-mediated hypertension.
Inflammation is prevalent in women with hypertension, and a further increase in inflammatory marker expression is seen following menopause (23, 38). Production of the proinflammatory cytokines MCP-1 and TNF-α are increased in postmenopausal women and are prevented by hormone replacement therapy (6, 44). In postmenopausal women, serum levels of MCP-1 correlate to estrogen supplementation; lower MCP-1 levels were measured in women on postmenopausal hormone replacement (40). Moreover, an increase in blood monocyte number has been demonstrated in menopause, as compared with females in the follicular phase, and monocyte counts in menopausal females decline following estrogen replacement therapy (2). Experimental induction of ovarian failure by ovariectomy in Dahl S rats produces similar increases in MCP-1 and TNF-α (36, 37). Estrogen is capable of downregulating IL-2 and TNF-α in human and mouse lymphocytes (28, 35), primarily through ERα-mediated signaling to reduce IL-2 and TNF-α release (17, 24), and estrogen has been shown to suppress Th17 differentiation and production of IL-17 (19). Decreasing estradiol (E2) concentrations can increase T-cell production of proinflammatory cytokine IFN-γ production. Similarly, estrogen suppressed lipopolysaccharide-induced MCP-1 production in vascular smooth muscle cells (15). The current study suggests that although estrogen may suppress T cell-dependent proinflammatory cytokine release during ANG II infusion in premenopausal females, the depletion of estrogen after menopause eliminates this suppressive phenotype, increasing T cell-dependent inflammation and ANG II hypertension.
In addition to the increase in renal proinflammatory markers, the current study identifies a significant increase in renal macrophage infiltration following ANG II infusion in the presence of T cells. This increase was measured via the expression of F4/80, a commonly used surface antigen for distinguishing tissue macrophages (13). The majority of renal monocyte cells are in fact macrophages (7) that contribute to renal homeostasis before and after renal injury (4, 18, 45). Two distinct phenotypes of polarized activated macrophages with distinct patterns of chemokine expression have been defined: the M1 proinflammatory phenotype and the M2 anti-inflammatory phenotype (8). Using an MRC1 antibody, a classical M2 phenotype macrophage marker (26, 47), we were able to show that an increase in renal inflammation correlates with a decrease in M2 macrophage expression in the hypertensive postmenopausal females. Although little is known about macrophage polarization in response to injury in the female kidney, the major circulating estrogen in premenopausal females, E2 does have a direct role in the modulation of innate immune function. Studies have shown that E2 attenuates production of macrophage proinflammatory cytokines, including macrophage inhibitory factor (12). In LPS-challenged human monocytes, estrogen decreased the expression of the proinflammatory chemokine CXCL8 (31), and this response was dependent on monocyte-specific expression of estrogen receptor-α (30). The activation of macrophages could mediate part or the whole of the effector inflammatory response in menopause and may also contribute to the ANG II-mediated BP increase.
Sex differences in renal T-cell profiles have identified that spontaneously hypertensive female rats have higher numbers of CD8+ and Tregs infiltrating the kidney; male rats have higher CD4+ and Th17 cells (43). In Sprague-Dawley rats, females have significantly more renal Tregs than male counterparts, and ANG II infusion further increased Treg numbers in females (48). In the estrogen high environment of pregnancy, Treg cells may play an important role in mediating maternal tolerance to the fetus. Estrogen is thought to be a regulatory factor for the peripheral development of CD4+CD25+ Tregs during the implantation period in mice (41), and depletion of Tregs causes a failure of a pregnancy (1, 39).
Building on the importance of estrogen and Tregs, the current study demonstrates that Tregs contribute to the suppression of ANG II hypertension in premenopausal females. Antibody-mediated depletion of Tregs, concurrent with ANG II infusion, eliminates premenopausal protection against ANG II hypertension. The Treg depletion was performed in two strains of mice in separate experiments. However, we did not evaluate between strains if there were differences in baseline and post-ANG II BP responses.
Combined with our data from the Rag-1−/− model, in which splenic and renal Treg expression was significantly reduced in postmenopausal mice compared with premenopausal mice, we suggest that Treg expression plays an integral role in BP regulation both before and after menopause.
In conclusion, clarifying the ways in which estrogen influences the unique function of T-cell subtypes and understanding how these regulatory events change after menopause could shed additional insight into the capacity of T cells to mediate both hypertension and will help identify potential targets for the development of a new generation of antihypertensive therapeutics with broad applications in hypertensive individuals across both sexes.
Perspectives and significance.
The present study demonstrates the important role that the hormonal environment plays on immune mechanisms of hypertension and suggests that identifying and characterizing the ways in which 17β-E2 influences T-cell expression and function may provide vital insight into novel BP regulatory pathways. These results also emphasize that sex of the animal is an important consideration to take when interpreting hypertension results and that temporal adaptations occur in prominent regulatory pathways in a sex-dependent fashion. This study suggests that targeted manipulation of T-cell subsets may prove to be an effective approach to treat hypertension.
GRANTS
This work was funded by National Institutes of Health Grants HL-131834 (to H. L. Brooks), T32-HL-007249 (to J. A. Uhlorn, D. P. Pollow, and M. A. Sylvester), and HL-075360 and HL-129823 (to M. L. Lindsey); American Heart Association Predoctoral Fellowship (to D. P. Pollow); Sarver Heart Center Research Award (to D. P. Pollow and H. L. Brooks); and the Stevie and Karl Eller Achievement Rewards for College Scientists Award (to D. P. Pollow).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.L.B. conceived and designed research; D.P.P., J.A.U., M.A.S., and M.J.R.-A. performed experiments; D.P.P., J.A.U., M.A.S., J.L.U., M.L.L., and H.L.B. analyzed data; D.P.P., J.A.U., M.A.S., M.J.R.-A., J.L.U., M.L.L., J.N.-Z., and H.L.B. interpreted results of experiments; D.P.P., M.L.L., and H.L.B. prepared figures; D.P.P., J.L.U., and H.L.B. drafted manuscript; D.P.P., J.A.U., M.A.S., M.J.R.-A., J.L.U., M.L.L., J.N.-Z., and H.L.B. edited and revised manuscript; D.P.P., J.A.U., M.A.S., M.J.R.-A., J.L.U., M.L.L., J.N.-Z., and H.L.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge excellent technical support from Elizabeth R. Flynn for the macrophage immunohistochemistry.
REFERENCES
- 1.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol 5: 266–271, 2004. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Hur H, Mor G, Insler V, Blickstein I, Amir-Zaltsman Y, Sharp A, Globerson A, Kohen F. Menopause is associated with a significant increase in blood monocyte number and a relative decrease in the expression of estrogen receptors in human peripheral monocytes. Am J Reprod Immunol 34: 363–369, 1995. doi: 10.1111/j.1600-0897.1995.tb00965.x. [DOI] [PubMed] [Google Scholar]
- 3.Brooks HL, Lindsey ML. Guidelines for authors and reviewers on antibody use in physiology studies. Am J Physiol Heart Circ Physiol 314: H724–H732, 2018. doi: 10.1152/ajpheart.00512.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao Q, Wang Y, Niu Z, Wang C, Wang R, Zhang Z, Chen T, Wang XM, Li Q, Lee VWS, Huang Q, Tan J, Guo M, Wang YM, Zheng G, Yu D, Alexander SI, Wang H, Harris DCH. Potentiating tissue-resident type 2 innate lymphoid cells by IL-33 to prevent renal ischemia-reperfusion injury. J Am Soc Nephrol 29: 961–976, 2018. doi: 10.1681/ASN.2017070774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Miguel C, Guo C, Lund H, Feng D, Mattson DL. Infiltrating T lymphocytes in the kidney increase oxidative stress and participate in the development of hypertension and renal disease. Am J Physiol Renal Physiol 300: F734–F742, 2011. doi: 10.1152/ajprenal.00454.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gameiro C, Romao F. Changes in the immune system during menopause and aging. Front Biosci (Elite Ed) E2: 1299–1303, 2010. doi: 10.2741/e190. [DOI] [PubMed] [Google Scholar]
- 7.George JF, Lever JM, Agarwal A. Mononuclear phagocyte subpopulations in the mouse kidney. Am J Physiol Renal Physiol 312: F640–F646, 2017. doi: 10.1152/ajprenal.00369.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 5: 953–964, 2005. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 9.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension 57: 132–140, 2011. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hinojosa-Laborde C, Craig T, Zheng W, Ji H, Haywood JR, Sandberg K. Ovariectomy augments hypertension in aging female Dahl salt-sensitive rats. Hypertension 44: 405–409, 2004. doi: 10.1161/01.HYP.0000142893.08655.96. [DOI] [PubMed] [Google Scholar]
- 12.Hsieh YC, Frink M, Hsieh CH, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Downregulation of migration inhibitory factor is critical for estrogen-mediated attenuation of lung tissue damage following trauma-hemorrhage. Am J Physiol Lung Cell Mol Physiol 292: L1227–L1232, 2007. doi: 10.1152/ajplung.00479.2006. [DOI] [PubMed] [Google Scholar]
- 13.Hume DA, Perry VH, Gordon S. Immunohistochemical localization of a macrophage-specific antigen in developing mouse retina: phagocytosis of dying neurons and differentiation of microglial cells to form a regular array in the plexiform layers. J Cell Biol 97: 253–257, 1983. doi: 10.1083/jcb.97.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irsik DL, Romero-Aleshire MJ, Chavez EM, Fallet RW, Brooks HL, Carmines PK, Lane PH. Renoprotective impact of estrogen receptor-α and its splice variants in female mice with type 1 diabetes. Am J Physiol Renal Physiol 315: F512–F520, 2018. doi: 10.1152/ajprenal.00231.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang P, Xu J, Zheng S, Huang J, Xiang Q, Fu X, Wang T. 17beta-estradiol down-regulates lipopolysaccharide-induced MCP-1 production and cell migration in vascular smooth muscle cells. J Mol Endocrinol 45: 87–97, 2010. doi: 10.1677/JME-09-0166. [DOI] [PubMed] [Google Scholar]
- 16.Jung M, Ma Y, Iyer RP, DeLeon-Pennell KY, Yabluchanskiy A, Garrett MR, Lindsey ML. IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic Res Cardiol 112: 33, 2017. doi: 10.1007/s00395-017-0622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kassi E, Moutsatsou P. Estrogen receptor signaling and its relationship to cytokines in systemic lupus erythematosus. J Biomed Biotechnol 2010: 317452, 2010. doi: 10.1155/2010/317452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22: 317–326, 2011. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lélu K, Laffont S, Delpy L, Paulet PE, Périnat T, Tschanz SA, Pelletier L, Engelhardt B, Guéry JC. Estrogen receptor α signaling in T lymphocytes is required for estradiol-mediated inhibition of Th1 and Th17 cell differentiation and protection against experimental autoimmune encephalomyelitis. J Immunol 187: 2386–2393, 2011. doi: 10.4049/jimmunol.1101578. [DOI] [PubMed] [Google Scholar]
- 20.Lima R, Wofford M, Reckelhoff JF. Hypertension in postmenopausal women. Curr Hypertens Rep 14: 254–260, 2012. doi: 10.1007/s11906-012-0260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindsey ML, Gray GA, Wood SK, Curran-Everett D. Statistical considerations in reporting cardiovascular research. Am J Physiol Heart Circ Physiol 315: H303–H313, 2018. doi: 10.1152/ajpheart.00309.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lohff JC, Christian PJ, Marion SL, Hoyer PB. Effect of duration of dosing on onset of ovarian failure in a chemical-induced mouse model of perimenopause. Menopause 13: 482–488, 2006. doi: 10.1097/01.gme.0000191883.59799.2e. [DOI] [PubMed] [Google Scholar]
- 23.Maas AH, Franke HR. Women’s health in menopause with a focus on hypertension. Neth Heart J 17: 68–72, 2009. doi: 10.1007/BF03086220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maret A, Coudert JD, Garidou L, Foucras G, Gourdy P, Krust A, Dupont S, Chambon P, Druet P, Bayard F, Guéry JC. Estradiol enhances primary antigen-specific CD4 T cell responses and Th1 development in vivo. Essential role of estrogen receptor alpha expression in hematopoietic cells. Eur J Immunol 33: 512–521, 2003. doi: 10.1002/immu.200310027. [DOI] [PubMed] [Google Scholar]
- 25.Maric C, Sandberg K, Hinojosa-Laborde C. Glomerulosclerosis and tubulointerstitial fibrosis are attenuated with 17beta-estradiol in the aging Dahl salt sensitive rat. J Am Soc Nephrol 15: 1546–1556, 2004. doi: 10.1097/01.ASN.0000128219.65330.EA. [DOI] [PubMed] [Google Scholar]
- 26.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6: 13, 2014. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattson DL, Lund H, Guo C, Rudemiller N, Geurts AM, Jacob H. Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. Am J Physiol Regul Integr Comp Physiol 304: R407–R414, 2013. doi: 10.1152/ajpregu.00304.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moulton VR, Holcomb DR, Zajdel MC, Tsokos GC. Estrogen upregulates cyclic AMP response element modulator α expression and downregulates interleukin-2 production by human T lymphocytes. Mol Med 18: 370–378, 2012. doi: 10.2119/molmed.2011.00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mouton AJ, DeLeon-Pennell KY, Rivera Gonzalez OJ, Flynn ER, Freeman TC, Saucerman JJ, Garrett MR, Ma Y, Harmancey R, Lindsey ML. Mapping macrophage polarization over the myocardial infarction time continuum. Basic Res Cardiol 113: 26, 2018. doi: 10.1007/s00395-018-0686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy AJ, Guyre PM, Wira CR, Pioli PA. Estradiol regulates expression of estrogen receptor ERalpha46 in human macrophages. PLoS One 4: e5539, 2009. doi: 10.1371/journal.pone.0005539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pioli PA, Jensen AL, Weaver LK, Amiel E, Shen Z, Shen L, Wira CR, Guyre PM. Estradiol attenuates lipopolysaccharide-induced CXC chemokine ligand 8 production by human peripheral blood monocytes. J Immunol 179: 6284–6290, 2007. doi: 10.4049/jimmunol.179.9.6284. [DOI] [PubMed] [Google Scholar]
- 32.Pollow DP Jr, Romero-Aleshire MJ, Sanchez JN, Konhilas JP, Brooks HL. ANG II-induced hypertension in the VCD mouse model of menopause is prevented by estrogen replacement during perimenopause. Am J Physiol Regul Integr Comp Physiol 309: R1546–R1552, 2015. doi: 10.1152/ajpregu.00170.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollow DP, Uhrlaub J, Romero-Aleshire M, Sandberg K, Nikolich-Zugich J, Brooks HL, Hay M. Sex differences in T-lymphocyte tissue infiltration and development of angiotensin II hypertension. Hypertension 64: 384–390, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prescott MJ, Lidster K. Improving quality of science through better animal welfare: the NC3Rs strategy. Lab Anim (NY) 46: 152–156, 2017. doi: 10.1038/laban.1217. [DOI] [PubMed] [Google Scholar]
- 35.Roggia C, Tamone C, Cenci S, Pacifici R, Isaia GC. Role of TNF-alpha producing T-cells in bone loss induced by estrogen deficiency. Minerva Med 95: 125–132, 2004. [PubMed] [Google Scholar]
- 36.Sandberg K, Ji H, Einstein G, Au A, Hay M. Is immune system-related hypertension associated with ovarian hormone deficiency? Exp Physiol 101: 368–374, 2016. doi: 10.1113/EP085149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sartori-Valinotti JC, Venegas-Pont MR, Lamarca BB, Romero DG, Yanes LL, Racusen LC, Jones AV, Ryan MJ, Reckelhoff JF. Rosiglitazone reduces blood pressure in female Dahl salt-sensitive rats. Steroids 75: 794–799, 2010. doi: 10.1016/j.steroids.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solak Y, Afsar B, Vaziri ND, Aslan G, Yalcin CE, Covic A, Kanbay M. Hypertension as an autoimmune and inflammatory disease. Hypertens Res 39: 567–573, 2016. doi: 10.1038/hr.2016.35. [DOI] [PubMed] [Google Scholar]
- 39.Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology 112: 38–43, 2004. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Störk S, Baumann K, von Schacky C, Angerer P. The effect of 17 beta-estradiol on MCP-1 serum levels in postmenopausal women. Cardiovasc Res 53: 642–649, 2002. doi: 10.1016/S0008-6363(01)00461-8. [DOI] [PubMed] [Google Scholar]
- 41.Tai P, Wang J, Jin H, Song X, Yan J, Kang Y, Zhao L, An X, Du X, Chen X, Wang S, Xia G, Wang B. Induction of regulatory T cells by physiological level estrogen. J Cell Physiol 214: 456–464, 2008. doi: 10.1002/jcp.21221. [DOI] [PubMed] [Google Scholar]
- 42.Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have a compensatory increase in renal regulatory T cells in response to elevations in blood pressure. Hypertension 64: 557–564, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have greater renal anti-inflammatory T lymphocyte infiltration than males. Am J Physiol Regul Integr Comp Physiol 303: R359–R367, 2012. doi: 10.1152/ajpregu.00246.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vural P, Akgul C, Canbaz M. Effects of hormone replacement therapy on plasma pro-inflammatory and anti-inflammatory cytokines and some bone turnover markers in postmenopausal women. Pharmacol Res 54: 298–302, 2006. doi: 10.1016/j.phrs.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 45.Xavier S, Sahu RK, Landes SG, Yu J, Taylor RP, Ayyadevara S, Megyesi J, Stallcup WB, Duffield JS, Reis ES, Lambris JD, Portilla D. Pericytes and immune cells contribute to complement activation in tubulointerstitial fibrosis. Am J Physiol Renal Physiol 312: F516–F532, 2017. doi: 10.1152/ajprenal.00604.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xue B, Johnson AK, Hay M. Sex differences in angiotensin II- induced hypertension. Braz J Med Biol Res 40: 727–734, 2007. doi: 10.1590/S0100-879X2007000500018. [DOI] [PubMed] [Google Scholar]
- 47.Yabluchanskiy A, Ma Y, DeLeon-Pennell KY, Altara R, Halade GV, Voorhees AP, Nguyen NT, Jin YF, Winniford MD, Hall ME, Han HC, Lindsey ML. Myocardial Infarction Superimposed on Aging: MMP-9 Deletion Promotes M2 Macrophage Polarization. J Gerontol A Biol Sci Med Sci 71: 475–483, 2016. doi: 10.1093/gerona/glv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zimmerman MA, Baban B, Tipton AJ, O’Connor PM, Sullivan JC. Chronic ANG II infusion induces sex-specific increases in renal T cells in Sprague-Dawley rats. Am J Physiol Renal Physiol 308: F706–F712, 2015. doi: 10.1152/ajprenal.00446.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]