Abstract
Despite significant decreases in cardiovascular disease (CVD) mortality in the past three decades, it still remains the leading cause of death in women. Following menopause and the accompanying loss of estrogen, women experience a unique, accelerated rise in CVD risk factors. Dysfunction of the endothelium represents an important antecedent to CVD development, with rapid declines in endothelial vasodilator function reportedly taking place across the menopause transition. Importantly, the decline in endothelial function is independent of chronological age and is associated with estrogen deficiency. Estrogen-mediated effects, including increasing nitric oxide bioavailability and attenuating oxidative stress and inflammation, contribute to preserving endothelial health. This review will discuss studies that have probed the role of estrogen on endothelial vasodilator function in women at discrete stages of the menopause transition and the effects of estradiol supplementation in postmenopausal women. Estrogen receptor signaling is also an important aspect of endothelial function in women, and studies suggest that expression is reduced with both acute and prolonged estrogen deficiency. Changes in regulatory mechanisms of estrogen receptor-α expression as well as sensitivity to estrogen may underlie the differential effects of estrogen therapy in early (≤5 yr past final menstrual period) and late postmenopausal women (>5 yr past final menstrual period). Lastly, this review presents potential therapeutic targets that include increasing l-arginine bioavailability and estrogen receptor activation to prevent endothelial dysfunction in postmenopausal women as a strategy for decreasing CVD mortality in this high-risk population.
Keywords: dysfunction, endothelial, estradiol, menopause, vascular aging
INTRODUCTION
Cardiovascular disease (CVD) remains the leading cause of mortality in both men and women, and importantly, sex differences in the progression of disease have been widely observed (6, 24, 87). Because of the decline in ovarian function and subsequent changes in sex hormone production, the menopause transition is marked by an augmented lipid profile, fat distribution, insulin sensitivity, and increases in blood pressure resulting in accelerated risk for CVD development (4, 50, 89). Before menopause, incidence of CVD is lower in women in comparison with age-matched men (14, 24). However, after menopause and the accompanying loss of endogenous estradiol as well as changes in other reproductive hormones, these sex differences narrow, and rates of CVD escalate in the postmenopausal years (50).
Vascular aging, which refers to the progressive stiffening of arteries and decline in vasodilatory capacity, underlies the development of CVD and appears to progress differently in men and women (6, 40, 87). The onset of menopause coincides with accelerated vascular aging, a phenomenon that provides an optimal setting for vascular disease such as hypertension and atherosclerosis and appears to be distinct from the gradual loss of vascular function that takes place with chronological aging (40). Endothelial dysfunction is a primary antecedent to the development of both obstructive and nonobstructive coronary artery disease, and studies suggest that dysfunction of the endothelium in the absence of obstructive coronary artery disease and other metabolic complications may be of greater relevance to CVD development in women than in men (73, 84). Given the disparate progression of CVD in men and women, understanding sex-specific mechanisms of disease development is imperative to identify intervention strategies that target CVD risk reduction for women during the peri- and postmenopausal years (6, 24).
This review will discuss studies that have probed the role of estrogen, specifically in relation to endothelium-dependent vasodilator function. Studies examined in this paper are ones that have assessed endothelial function in healthy women at discrete stages of the menopause transition as well as in postmenopausal women who were administered estrogen therapy. This review will highlight mechanisms of estrogen, as well as the potentially even more critical role of estrogen receptor (ER) signaling, on endothelial vasodilator function in aging women. Potential therapeutic targets and strategies to improve vasodilator function in aging women will also be addressed.
THE VASCULAR ENDOTHELIUM
The endothelium plays a pivotal role in maintaining vascular homeostasis by synthesizing and secreting substances involved in vasodilation and vasoconstriction, serving as a barometer for vascular health (1, 86). Progressive dysfunction of the endothelial cell layer of the vascular wall, resulting in a distortion of the balance of endothelium-derived substances, is one aspect of vascular aging and has been identified as a key initiating step in the pathogenesis of atherosclerotic CVD (75). Agonists such as acetylcholine, as well as mechanical forces such as arterial shear stress, can trigger endothelium-dependent vasodilation (EDV) mediated by the diffusion of endothelium-derived nitric oxide (NO) into vascular smooth muscle cells, resulting in relaxation. Reduced NO bioavailability because of increased oxidative stress, elevated proinflammatory cytokines, and decreased NO synthesis all contribute to impaired EDV, a hallmark feature of endothelial dysfunction (29, 78, 79).
Sex differences in endothelial function.
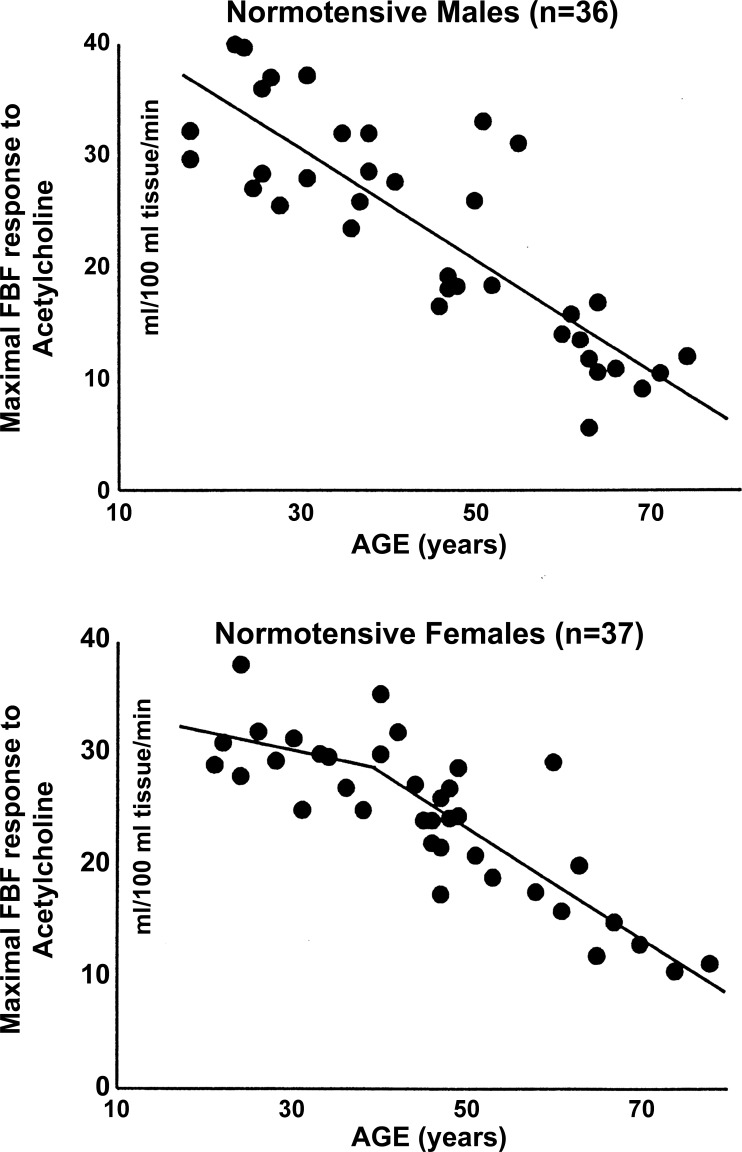
An early cross-sectional analysis revealed differences in forearm blood flow responses to infusion of intrabrachial acetylcholine, an endothelium-dependent vasodilator, in men and women. Specifically, normotensive premenopausal women exhibited a slight decrease in acetylcholine-mediated vasodilation (0.5% decline per yr); however, impairments to EDV only became evident after menopause, in which the response to acetylcholine declined more rapidly (2.1% per yr). In contrast, normotensive men exhibited a constant linear decrease in vasodilation to acetylcholine with age (1.8% per yr) (87). No sex differences in smooth muscle function, as assessed by response to sodium-nitroprusside administration, were observed, reinforcing that menopause affects endothelium-dependent and not endothelium-independent function (87) (Fig. 1). These findings provide further evidence to support the protective effects of ovarian hormones, including estrogen, on preserving endothelial function and also highlight the distinction between chronological and reproductive aging.
Fig. 1.
Relation between age and maximal forearm blood flow (FBF) response to acetylcholine in normotensive men and women (87). Used with permission.
It also appears that there are sex differences with respect to the effects of moderate obesity on endothelial function, independent of other metabolic complications. Evaluation of both microvascular and conduit macrovascular endothelial function were performed in a group of middle-aged moderately obese and nonobese men and women without diabetes. Brachial artery flow-mediated dilation (FMD) was used to evaluate conduit artery endothelial function, which represents a validated marker of NO-mediated EDV in response to shear stress, and is a prognostic marker for prediction of future hypertension development and CVD events (2, 9, 32, 74, 75, 95). Microvascular function was evaluated in vitro using adipose arterioles obtained by biopsy from participants. EDV to increasing concentrations of acetylcholine was measured by videomicroscopy, and responses were previously found to be ∼95% dependent on endothelium-derived NO synthase (12). Findings from this study demonstrated that women who were moderately obese (body mass index ≥ 30 kg/m2) had significantly lower conduit and microvascular endothelial function than women who were nonobese. When further stratified by menstrual status, it was found that differences in endothelial function among the two groups were driven by postmenopausal women with moderate obesity. No significant differences in endothelial function were found between moderately obese and nonobese men (84). These observations suggest that postmenopausal women with moderate obesity may be more susceptible to impairments in endothelial function than men and postmenopausal women without moderate obesity, independent of diabetes status.
Estrogen and endothelial function.
The association between estrogen and generation of endothelium-derived essential vasodilator and signaling compounds, including NO, have been described previously (7, 8, 55). Estrogen-mediated generation of NO diffuses into vascular smooth muscle and activates guanylate cyclase, leading to the production of cGMP, which contributes to smooth muscle cell relaxation, resulting in vasodilation. Estrogen is also associated with lowering endothelin-1, a potent vasoconstrictor and proinflammatory peptide secreted by the endothelium (7, 42, 76). In women, the functional changes that are reflective of impaired endothelial function are unique in that they coincide with marked changes in ovarian hormones occurring during the menopause transition (6, 55, 87). Although increases in follicle-stimulating hormone are also implicated in contributing to endothelial dysfunction, it is clear that the loss of estrogen during the menopause transition plays a key role (56). However, our understanding of the relative contribution of estrogen deficiency placed onto the backdrop of chronological aging is incomplete.
Evidence suggests that the onset of menopause and accompanying hormonal changes precedes endothelial dysfunction in women. For instance, severe decrements in EDV were observed within 1 wk of bilateral ovariectomy in women (63a). Furthermore, 1 wk of raloxifene treatment, a selective ER modulator, was able to restore brachial artery (macrovascular) endothelial vasodilator function (88). Ovariectomy also impairs microvascular function in women, and vasodilation in response to acetylcholine infusion is restored to baseline following 3 mo of exogenous estrogen supplementation (17β-estradiol by transdermal patches) (68). To draw a cause-and-effect relationship is challenging, as the role of other ovarian hormones changes, such as testosterone, which is lowered by more than 40% with ovariectomy (41). However, these observations bring light to the importance of further studying estrogen and its association with accelerated impairments to endothelial vasodilator function that occur with menopause.
THE MENOPAUSE TRANSITION
The Study of Women’s Health Across the Nation (SWAN) was a prospective study of the menopause transition in minority (African American, Hispanic, Japanese, and Chinese) and Caucasian women who were not on hormone therapy (50). In a sample of 1,054 women, the authors found that within the 1-yr interval before and following the final menstrual period, there was a significant rise in total cholesterol, low-density lipoprotein cholesterol, and apolipoprotein-B in women, markers that are linked to endothelial dysfunction and contribute to atherosclerosis (22, 35). Although outcome measures specific to endothelial function were not performed, this large prospective trial specifically identified the adverse changes to the lipid profile that take place at the time of the final menstrual period and demonstrated that these changes are unique from the linear model of chronological aging (50).
Endothelial function across menopause.
The functional changes that take place over the course of the menopause transition that are reflective of endothelial dysfunction are understudied. In a cross-sectional study, Moreau and colleagues (55) investigated macrovascular endothelial vasodilator function across the stages of the menopause transition in healthy women, including premenopausal, perimenopausal, and postmenopausal women. Both perimenopausal and postmenopausal women were further categorized into early or late according to the Stages of Reproductive Aging Workshop (STRAW) criteria (82), which is largely based on self-reported bleeding. Consistent with their initial hypothesis, the investigators found that brachial artery FMD was progressively reduced across the menopause transition and was correlated with reduced estradiol concentrations. Interestingly, the impairment in EDV was greatest from the early to late perimenopausal stage, even when controlling for age and adjusting for other CVD risk factors (~17 vs. 35% impairment compared with premenopausal women, respectively). The authors posit that ovarian hormone levels are likely sufficient during the early perimenopausal period to provide adequate protection to the endothelium. However, the precipitous decline in FMD in the late perimenopausal period suggests that circulating estrogen levels are no longer sufficient and/or ER signaling is compromised (19). They speculate that reduced estrogen-mediated generation of NO likely underlies the progressive decline in EDV (23). Elevations in the vasoconstrictors endothelin-1 and norepinephrine were also observed; however, the inverse association between FMD and menopause stage was still present after statistically controlling for these factors.
Recent work from our laboratory demonstrates that changes to conduit arterial shear patterns, an important determinant of endothelial function, take place across the menopause transition, independent of chronological age (81). Arterial blood flow naturally engenders a frictional force per unit area on the endothelium of the luminal wall that is described as shear stress. Disturbed patterns of shear, characterized by high oscillatory (bidirectional) and retrograde (backward) blood flow, provide a weak stimulus for endothelial release of vasoactive substances and can promote endothelial inflammation and leukocyte endothelial adhesion (90, 100). Specifically, this proatherogenic profile described can, over time, lead to increased production of superoxide radicals and upregulate cell adhesion molecules such as vascular adhesion molecule-1 and intracellular adhesion molecule-1 (ICAM-1), contributing to endothelial dysfunction (25, 30, 64). In a cross-sectional investigation, we found that retrograde and oscillatory shear increased in both the brachial and common femoral artery with advancing menopausal stage (81). Although we did not measure NO directly, findings from Padilla and colleagues (66) showed that NO synthase inhibition in the forearm circulation of young men and women increased resting retrograde and oscillatory shear to values that were similar to those observed in older men and women. Their findings suggest that changes in conduit artery shear in older individuals are associated with reduced NO bioavailability with chronological aging, which may also be relevant to reproductive aging in women; however, definitive evidence is needed to explore this potential association.
REDUCED NO BIOAVAILABILITY ACROSS THE MENOPAUSE TRANSITION AND POTENTIAL THERAPEUTIC TARGETS
Reduced NO synthesis.
One possible explanation for the decline in EDV that is observed across the menopause transition is a reduced enzymatic synthesis of NO. Using the same cross-sectional analysis described earlier (56), Moreau and colleagues (36) reported a relative l-arginine deficiency in women that was progressive across the menopausal stages, which is suggestive of an underlying mechanism for the decline in EDV across menopause. l-arginine represents the precursor for endothelial-derived NO, which is catalyzed primarily by endothelial NO synthase (eNOS) in conjunction with the cofactor tetrahydrobiopterin (BH4) and molecular oxygen (8). Stability of eNOS can be disturbed in the face of either a deficiency in the substrate l-arginine and/or BH4, resulting in uncoupling of eNOS and, subsequently, increased superoxide production. The authors found several markers indicative of augmented l-arginine metabolism and eNOS inhibition by methylarginine, contributing to a relative l-arginine deficiency that became more prominent with the menopausal stage.
Reductions in bioavailability of the important cofactor BH4 can also lead to eNOS uncoupling and generation of reactive oxygen species (ROS) (77). BH4 bioavailability is reduced in postmenopausal women who are estrogen deficient (57), which may contribute to increased oxidative stress and endothelial dysfunction (10). In women who were given transdermal estradiol for 2 days, acute supplementation with BH4 did not further increase brachial artery FMD, although it did in estrogen-deficient postmenopausal women (57). This suggests that estrogen plays a role in maintenance of the cofactor BH4 and may represent a therapeutic target (77). Declines in BH4 across the menopause transition have not been studied and may provide insight into the time course of changes in BH4 bioavailability as well as its relative contribution to endothelial dysfunction.
Increased inactivation of NO.
An additional explanation for reduced endothelial function across the menopause transition is inactivation of NO. Increased oxidative stress and proinflammatory cytokines can inactivate NO and perpetuate the decline in NO synthesis by contributing to eNOS uncoupling (7, 29). The direct antioxidant properties of estrogens such as 17β-estradiol, estrone, and estriol are well established (8, 58). Estrogen has been shown to directly inhibit scavenging of NO by ROS because of hydrogen-donating of its phenolic structure (29). Estrogen can also lower ROS concentrations indirectly by increasing antioxidant enzyme expression and/or decreasing oxidative proteins via interaction with its estrogenic nuclear receptors (62). Estrogen-deficient postmenopausal women (45–65 yr) who were given an acute systemic infusion of the antioxidant ascorbic acid exhibited increases in brachial artery FMD; however, ascorbate did not further improve FMD in women who were receiving estrogen therapy (59). Similar findings were also observed during tumor necrosis factor (TNF)-α blockade in postmenopausal women (54). TNF-α, an inflammatory cytokine, has pleiotropic effects, causing severe impairments in endothelial function (3, 7, 55). Although acute supplementation with the TNF-α blocker etanercept increased brachial artery FMD in postmenopausal women who were estrogen deficient, TNFα blockade had no effect in premenopausal women or postmenopausal women supplemented with transdermal estradiol for 2 days (54).
In a cross-sectional analysis, biomarkers of vascular function were evaluated in premenopausal (48 ± 1 yr) and recent postmenopausal (51 ± 1 yr) women. Women in both categories were of similar age; however, recent postmenopausal women had higher concentrations of soluble ICAM-1, a proinflammatory marker associated with atherosclerosis and cardiovascular events (63). In postmenopausal women, supplementation with transdermal estradiol for 12 mo decreased serum levels of cell adhesion molecules (ICAM-1, vascular adhesion molecule-1, and E-selectin) that are induced by inflammatory cytokines to facilitate leukocyte attachment and migration across endothelial cells (85). These studies help to corroborate the underlying antioxidant and anti-inflammatory properties of estrogen (63, 85). Furthermore, they collectively suggest that estrogen deficiency likely attenuates vascular endothelial function through an upregulation of systemic inflammation and/or oxidative stress.
EFFECTS OF ESTROGEN SUPPLEMENTATION ON ENDOTHELIAL VASODILATOR FUNCTION IN POSTMENOPAUSAL WOMEN
Research findings from numerous investigations have revealed the antioxidant (59), anti-inflammatory (54), l-arginine (36), and BH4-associated (57) properties of estrogen. Given the accumulating evidence suggesting that estrogen affords protection to the vascular endothelium in premenopausal women, one would be inclined to ask: why would postmenopausal women not chronically supplement with estrogen? Researchers have attempted to investigate this question with a series of longitudinal prospective clinical trials involving supplementation with conjugated equine estrogens and estradiol. With respect to studies that have evaluated the effects of estradiol supplementation alone on vascular function in women without CVD risk factors, the 5-yr Kronos Early Estrogen Prevention Study (KEEPS) is one that aimed to deliver low dose transdermal estradiol in women who were within 36 mo of their final menstrual period (28). Researchers found a trend toward less progression of coronary artery calcium (a marker of atherosclerotic plaque) in groups administered estradiol; however, their findings were not statistically significant. Investigators of this trial attempted to probe endothelial function using digital tonometry, which provided a reactive hyperemia index. They found no significant changes to the reactive hyperemia index following 4 yr of estrogen therapy (37). The authors concluded that the utility of digital tonometry in measuring endothelial function is questionable, and there is marked variability in the results that have been reported (37, 53). Although shorter in duration, a double-blind crossover trial performed in postmenopausal women (55 ± 7 yr) involving supplementation with 2 separate doses of oral 17β-estradiol (1 mg/d, 2 mg/d) and a placebo over 9 wk for each treatment phase demonstrated improvements in brachial artery FMD, a validated measure of macrovascular endothelial function, following estradiol treatments (46).
The timing of estradiol supplementation following menopause may be an important factor to consider. One study demonstrated that time from menopause is a predictor of impaired endothelial vasodilator function as well as its improvement following acute and chronic estradiol administration (93). Brachial artery FMD was assessed within 1 h of acute administration of estrogen and following 3 mo of oral estrogen therapy (1 mg/day). They found that EDV improved in all women following estrogen therapy; however, the improvement was greater in women who were within 5 yr since menopause versus after 5 yr menopause. It has been suggested that ER function and expression may decline in late postmenopausal women (19, 55). Interestingly, in women who were >5 yr since menopause, estrogen therapy was more effective in increasing FMD in those who had received hormone therapy previously. Vitale and colleagues (93) suggest that the late postmenopausal women who were previous users of hormone therapy maintained some sensitivity of endothelial ERs, which may be why they demonstrated greater improvements in endothelial vasodilator function following the intervention than naïve late postmenopausal users. To follow up on this suggestion, the modulatory influence of estrogen on ER function in endothelial cells will be discussed.
ERα: A KEY DETERMINANT IN MAINTAINING ENDOTHELIAL VASODILATOR FUNCTION
Beyond the effects of estrogen deficiency, another key component that is associated with the progression of endothelial dysfunction in postmenopausal women is the influence of estrogen status on ER function (5). ER function may be equally as important as serum concentrations of estradiol and likely changes with prolonged deficiency of estrogen. ER sensitivity was mentioned previously as potentially underlying the low efficacy of estrogen therapy in improving endothelial function in late postmenopausal women (67). The impact of estrogen, mediated via genomic and nongenomic acute signaling pathways, is dependent on ER subtype (27). ERα receptor expression is of particular interest, as it is expressed abundantly in endothelial cells (67). Research from cell culture and ERα knockout animal models demonstrate the importance of ERα modulation of eNOS, the enzyme primarily involved in endogenous endothelial-derived production of NO (27, 48). With respect to nongenomic actions, estrogen bound to plasma membrane-localized ERα can result in activation of several kinase signaling pathways that directly activate eNOS by phosphorylation and take place within minutes of hormone application to cultured endothelial cells (8, 31, 48, 51). Specifically, the molecular pathways include phosphorylation of eNOS by a signaling cascade involving ERα/phosphatidylinositol 3-kinase/Akt and MAPK signaling (51, 60). Additionally, ERα can interact with G proteins, specifically, formation of the ERα-Gαι complex can contribute to the acute signaling pathways described (51).
Activation of ERα can also alter gene expression by either directly binding to the estrogen response element on DNA or indirectly via activation of other transcription factors leading to upregulation of eNOS, as well as superoxide dismutase 2 expression, which can lower ROS generation. Phosphorylation of ERα can also take place in the absence of estrogen via activation of kinases by growth factors, triggering binding to the estrogen response element as well as resulting in protein-protein interactions (91). Ligand-independent effects play a role in maintaining endothelial health and, importantly, are not dependent on estrogen binding (27). There also exist splice variants of ERα such as ER46, which are expressed preferentially on the membrane in estrogen-deprived endothelial cells and can more efficiently modulate eNOS phosphorylation (44, 60).
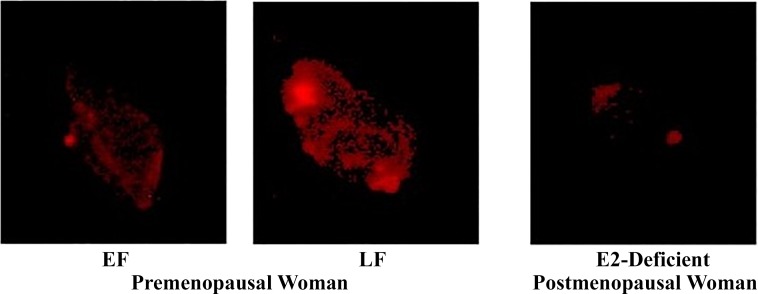
Importantly, there appears to be plasticity in ERα expression. Evidence in ovariectomized rats suggests that prolonged deprivation of estrogen can lower endothelial ERα expression and disrupt ERα/eNOS signaling. Both mRNA and protein expression of ERα as well as phosphorylated eNOS in aortic tissues were significantly lower in rats following 8 mo versus 1 mo of bilateral ovariectomy (67). Evidence of ERα plasticity also exists in women at varying points of the menstrual cycle and following menopause. Gavin and colleagues (19) assessed ERα expression in healthy premenopausal women during their early follicular (EF) and late follicular (LF) phase as well as in postmenopausal women who had been estrogen deficient for 0.6–10 yr (Fig. 2). Endothelial cells obtained from peripheral veins were harvested, and expression was evaluated using quantitative immunofluorescence. It was found that ERα expression differed based on circulating estrogen across the menstrual cycle, such that ERα expression in women during their EF phase was 30% lower than in their LF phase. In postmenopausal women, ERα expression was 33% lower than in premenopausal women in their LF phase, whereas it was similar to women in their EF phase. The positive association found between circulating estrogen and ERα receptor expression suggests that there is a modulatory role of estrogen on ER function in endothelial cells (67).
Fig. 2.
Estrogen receptor-α protein expression in endothelial cells collected during the early follicular (EF) and late follicular (LF) phases of the menstrual cycle in premenopausal women and in estrogen-deficient postmenopausal women. (19). Used with permission.
Brachial artery FMD and eNOS expression were also determined in a subset of the women studied that included both pre- and postmenopausal women. Associations were found between reduced ERα expression (67) and impaired EDV, as well as a positive relation between ERα expression and eNOS signaling. Despite this observed association, as well as the finding that ERα expression is acutely regulated in premenopausal women, a recent study demonstrated that endothelial function remains stable across the menstrual cycle (80). The authors speculate that eNOS activation via genomic and nongenomic mechanisms of ER activation may be regulated in such a way to maintain endothelial function, despite fluctuations in estrogen across the menstrual cycle. Described earlier, the ER46 isoform expressed in estrogen-deprived endothelial cells may help to explain maintenance of endothelial function in premenopausal women when estrogen is acutely lowered, as well as restoration of endothelial function with estrogen therapy in early postmenopausal women. Age-associated increases in methylation of ER promoter regions, namely the ERα CpG island, as well as removal of acetyl groups from histones that condense chromatin structure, are thought to contribute to placing the ERα gene in a transcriptionally inactive state, suggesting a senescence of regulatory mechanisms over time (51, 69, 71). Additionally, changes in posttranslational modifications that influence ERα membrane localization and activity could influence acute signaling pathways and may negate the effects of estrogen therapy on endothelial function in late postmenopausal women (52).
Investigating the time course of changes in expression of both ERα and variants of ERα, eNOS signaling, as well as changes in ERα sensitivity to estrogen during the menopause transition, may help to illuminate potential targets to preserve endothelial function in aging women.
RESTORING ENDOTHELIAL FUNCTION AND RESILIENCY IN POSTMENOPAUSAL WOMEN
Novel approaches to restore endothelial vasodilator function in older women are needed. The therapeutic role of citrulline in boosting l-arginine bioavailability may represent one strategy to increase NO synthesis in women (16), given the relative deficiency reported across the menopause transition (36). l-arginine can be generated by de novo synthesis from citrulline and accounts for roughly 5%–15% of circulating arginine concentrations (36, 70). Supplementation with l-citrulline is better able to increase plasma levels of l-arginine, as it is not catabolized by arginase and bypasses liver metabolism (18, 70). Short-term l-citrulline supplementation (8 wk, 6 g/day) was shown to increase plasma NO metabolites in prehypertensive and hypertensive postmenopausal women (96). In another study of the same duration but lower dosage (8 wk, 800 mg/day), supplementation improved brachial artery FMD in older men and women (41–64 yr) with vasospastic angina; however, menopausal status was not reported (61). The Modulating Oxidative Stress and Inflammation in Elders (MOXIE) trial proposes to explore the effects of dietary l-citrulline (~1 g/day) in the form of 100% watermelon juice on vascular function and markers of oxidative stress and inflammation in heathy African American and European American postmenopausal women aged 55–59 yr (16). Findings from this study may help to inform the use of dietary l-citrulline as a possible nutritional strategy to preserve endothelial function in healthy, aging women.
Supplementation with the polyphenolic compound resveratrol (trans-3,5,40-trihydroxystilbene) may also represent a potential strategy to improve endothelial function. Evidence in human umbilical vein endothelial cells points to resveratrol having estrogenic effects because of its ability to bind to ERα and activate similar acute signaling pathways, resulting in increased eNOS activity (38). Additionally, resveratrol is implicated in preventing eNOS uncoupling by lowering NADPH oxidase activity and expression via distinct signaling pathways (38, 43, 99). Recently demonstrated by Fabricio and colleagues (17), 90 days of resveratrol treatment (10 mg/kg body wt daily) in ovariectomized rats prevented endothelial dysfunction induced by estrogen deficiency. Clinical studies performed in healthy older men and postmenopausal women who were obese and overweight, evaluating the acute (30, 90, and 270 mg) and chronic (6 wk, 75 mg/day) effects of resveratrol on endothelial function have reported time- and dose-dependent improvements in brachial artery FMD (97, 98), whereas a recent study involving 4 wk of resveratrol supplementation (150 mg/day) in a similar population did not (92). Importantly, these studies did not report participant data by sex and represents a limitation to this work. As such, clinical investigations of resveratrol supplementation in women alone are warranted to determine if treatment can preserve ERα signaling and promote increased NO bioavailability in postmenopausal women. Findings from a current clinical trial (http://clinicaltrials.gov, NCT02256540) investigating the acute effect of resveratrol (250 mg) combined with a single exercise session on endothelial function in postmenopausal women will be informative and may help direct therapeutic strategies in aging women.
Other phytoestrogens, such as isoflavones and lignans found in plants, appear to bind with low affinity to ERs (10−4 to 10−2 of estradiol) and have shown mixed findings in the literature with respect to their effects on endothelial function (33). A population-based, cross-sectional study of 301 postmenopausal women showed that dietary intake of phytoestrogens had no association with endothelial function (39), and in a 12-mo randomized control trial, supplementation with soy isoflavones (100 mg/day) had no effect on endothelial function in postmenopausal women (72). Conversely, supplementation with the isoflavone genistein, a component of soy protein, caused acute NO-dependent vasodilation of the forearm vasculature in healthy men (20–51 yr) and premenopausal women (29–33 yr) (94), as well as improvements to brachial artery FMD in postmenopausal women after 1 yr of supplementation (54 mg/day), with similar potency to estradiol (83). The reported effects of genistein on endothelial function are suggested to be mediated in part by estrogenic receptors; however, it can also activate other distinct signaling pathways that promote NO bioavailability (20, 26). Whether supplementation with genistein alone can preserve endothelial function across menopause has yet to be determined.
Assessing endothelial resiliency in aging women is an area that also warrants further study. The endothelium is sensitive to ischemia-reperfusion (I/R) injury, particularly during reperfusion, and the health of the endothelium determines recovery from injury. Previous investigations in humans have shown that agents that upregulate NO bioavailability, such as sildenafil (21), organic nitrates (13), and rosuvastatin (47), as well as habitual exercise training, (11) can provide protection to the endothelium against I/R injury that is induced in the peripheral vasculature. Luca and colleagues (49) found an inverse association between serum estradiol concentration and endothelial dysfunction induced by forearm I/R injury. They showed that women in their EF phase exhibit blunted FMD in response to forearm-induced I/R injury, whereas FMD was preserved in the LF phase. No differences in baseline FMD were observed across the menstrual cycle (49). These findings highlight the importance of assessing the effects of therapeutic interventions not only on resting endothelial function but also on the attenuation of I/R injury in women, particularly during the menopause transition.
CONCLUSIONS AND CONSIDERATIONS
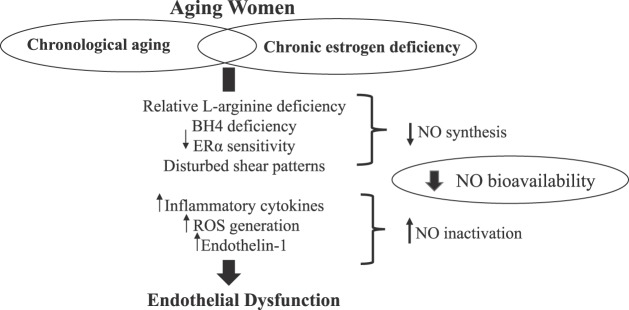
It is clear that estrogen has pleiotropic effects and is crucial for the maintenance of endothelial function. Estrogen increases NO bioavailability by promoting NO synthesis and has direct and indirect antioxidant properties and anti-inflammatory effects (Fig. 3). It is evident that declines in endothelial vasodilator function across the menopause transition are rapid, even when adjusting for age and CVD risk factors. In addition to the loss of estrogen, ER function and signaling are also an important aspect of endothelial health and reduced expression and sensitivity of ERα to estrogen may underlie the null effects of estrogen therapy in late postmenopausal women. Further attempts to probe the relative contribution or redundancy of factors contributing to endothelial dysfunction in aging women may offer insight into therapeutic targets. Although much of the focus of this review is on the role of estrogen and ERα on endothelial function in women, it is important to note that other hormones likely contribute to declines in endothelial function. For example, recent evidence suggests that follicle-stimulating hormone may exert independent adverse effects on the vasculature (45), and this hormone precipitously rises across the menopause transition. Although the modulatory role of androgens on CVD risk in women is controversial, a population-based study in women at discrete stages of menopause suggests they may be protective against CVD development (34). Another important relationship to consider in women is the feedback regulatory processes of the pituitary-ovarian axis. Estrogen is known to promote prolactin secretion from the pituitary, which can result in inhibition of eNOS (15). Therefore, the integrated actions of estrogen as well as other ovarian hormones on eNOS should also be explored across the lifespan.
Fig. 3.
Summary of potential therapeutic targets and contributing mediators of reduced NO bioavailability and endothelial dysfunction during the menopause transition. BH4, tetrahydrobiopterin; ER, estrogen receptor; NO, nitric oxide; ROS, reactive oxygen species.
Given that postmenopausal women represent a population at heightened risk for CVD development, exploring effective strategies that increase NO bioavailability and preserve endothelial function as well as endothelial resiliency is important.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Y.B.S. drafted manuscript; Y.B.S., J.A.P., M.J.D.S., P.M.K.-E., and D.N.P. edited and revised manuscript; Y.B.S., J.A.P., M.J.D.S., P.M.K.-E., and D.N.P. approved final version of manuscript.
REFERENCES
- 1.Anderson TJ, Charbonneau F, Title LM, Buithieu J, Rose MS, Conradson H, Hildebrand K, Fung M, Verma S, Lonn EM. Microvascular function predicts cardiovascular events in primary prevention: long-term results from the Firefighters and Their Endothelium (FATE) study. Circulation 123: 163–169, 2011. doi: 10.1161/CIRCULATIONAHA.110.953653. [DOI] [PubMed] [Google Scholar]
- 2.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC, Selwyn AP. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol 26: 1235–1241, 1995. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 3.Arenas IA, Armstrong SJ, Xu Y, Davidge ST. Chronic tumor necrosis factor-alpha inhibition enhances NO modulation of vascular function in estrogen-deficient rats. Hypertension 46: 76–81, 2005. doi: 10.1161/01.HYP.0000168925.98963.ef. [DOI] [PubMed] [Google Scholar]
- 4.Barton M, Meyer MR. Postmenopausal hypertension: mechanisms and therapy. Hypertension 54: 11–18, 2009. doi: 10.1161/HYPERTENSIONAHA.108.120022. [DOI] [PubMed] [Google Scholar]
- 5.Barton M, Meyer MR, Haas E. Hormone replacement therapy and atherosclerosis in postmenopausal women: does aging limit therapeutic benefits? Arterioscler Thromb Vasc Biol 27: 1669–1672, 2007. doi: 10.1161/ATVBAHA.106.130260. [DOI] [PubMed] [Google Scholar]
- 6.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 7.Chakrabarti S, Lekontseva O, Davidge ST. Estrogen is a modulator of vascular inflammation. IUBMB Life 60: 376–382, 2008. doi: 10.1002/iub.48. [DOI] [PubMed] [Google Scholar]
- 8.Chakrabarti S, Morton JS, Davidge ST. Mechanisms of estrogen effects on the endothelium: an overview. Can J Cardiol 30: 705–712, 2014. doi: 10.1016/j.cjca.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R; International Brachial Artery Reactivity Task Force . Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39: 257–265, 2002. doi: 10.1016/S0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 10.Delp MD, Behnke BJ, Spier SA, Wu G, Muller-Delp JM. Ageing diminishes endothelium-dependent vasodilatation and tetrahydrobiopterin content in rat skeletal muscle arterioles. J Physiol 586: 1161–1168, 2008. doi: 10.1113/jphysiol.2007.147686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeVan AE, Umpierre D, Harrison ML, Lin HF, Tarumi T, Renzi CP, Dhindsa M, Hunter SD, Tanaka H. Endothelial ischemia-reperfusion injury in humans: association with age and habitual exercise. Am J Physiol Heart Circ Physiol 300: H813–H819, 2011. doi: 10.1152/ajpheart.00845.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dharmashankar K, Welsh A, Wang J, Kizhakekuttu TJ, Ying R, Gutterman DD, Widlansky ME. Nitric oxide synthase-dependent vasodilation of human subcutaneous arterioles correlates with noninvasive measurements of endothelial function. Am J Hypertens 25: 528–534, 2012. doi: 10.1038/ajh.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dragoni S, Gori T, Lisi M, Di Stolfo G, Pautz A, Kleinert H, Parker JD. Pentaerythrityl tetranitrate and nitroglycerin, but not isosorbide mononitrate, prevent endothelial dysfunction induced by ischemia and reperfusion. Arterioscler Thromb Vasc Biol 27: 1955–1959, 2007. doi: 10.1161/ATVBAHA.107.149278. [DOI] [PubMed] [Google Scholar]
- 14.Dubey RK, Imthurn B, Barton M, Jackson EK. Vascular consequences of menopause and hormone therapy: importance of timing of treatment and type of estrogen. Cardiovasc Res 66: 295–306, 2005. doi: 10.1016/j.cardiores.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Duckles SP, Miller VM. Hormonal modulation of endothelial NO production. Pflugers Arch 459: 841–851, 2010. doi: 10.1007/s00424-010-0797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellis AC, Dudenbostel T, Locher JL, Crowe-White K. Modulating oxidative stress and inflammation in elders: The MOXIE Study. J Nutr Gerontol Geriatr 35: 219–242, 2016. doi: 10.1080/21551197.2016.1250693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fabricio V, Oishi JC, Biffe BG, Ruffoni LD, Silva KA, Nonaka KO, Rodrigues GJ. Resveratrol treatment normalizes the endothelial function and blood pressure in ovariectomized rats. Arq Bras Cardiol 108: 116–121, 2017. doi: 10.5935/abc.20170012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Figueroa A, Wong A, Jaime SJ, Gonzales JU. Influence of L-citrulline and watermelon supplementation on vascular function and exercise performance. Curr Opin Clin Nutr Metab Care 20: 92–98, 2017. doi: 10.1097/MCO.0000000000000340. [DOI] [PubMed] [Google Scholar]
- 19.Gavin KM, Seals DR, Silver AE, Moreau KL. Vascular endothelial estrogen receptor alpha is modulated by estrogen status and related to endothelial function and endothelial nitric oxide synthase in healthy women. J Clin Endocrinol Metab 94: 3513–3520, 2009. doi: 10.1210/jc.2009-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gencel VB, Benjamin MM, Bahou SN, Khalil RA. Vascular effects of phytoestrogens and alternative menopausal hormone therapy in cardiovascular disease. Mini Rev Med Chem 12: 149–174, 2012. doi: 10.2174/138955712798995020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gori T, Sicuro S, Dragoni S, Donati G, Forconi S, Parker JD. Sildenafil prevents endothelial dysfunction induced by ischemia and reperfusion via opening of adenosine triphosphate-sensitive potassium channels: a human in vivo study. Circulation 111: 742–746, 2005. doi: 10.1161/01.CIR.0000155252.23933.2D. [DOI] [PubMed] [Google Scholar]
- 22.Gradinaru D, Borsa C, Ionescu C, Prada GI. Oxidized LDL and NO synthesis–Biomarkers of endothelial dysfunction and ageing. Mech Ageing Dev 151: 101–113, 2015. doi: 10.1016/j.mad.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Green DJ, Dawson EA, Groenewoud HM, Jones H, Thijssen DH. Is flow-mediated dilation nitric oxide mediated? A meta-analysis. Hypertension 63: 376–382, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02044. [DOI] [PubMed] [Google Scholar]
- 24.Green DJ, Hopkins ND, Jones H, Thijssen DH, Eijsvogels TM, Yeap BB. Sex differences in vascular endothelial function and health in humans: impacts of exercise. Exp Physiol 101: 230–242, 2016. doi: 10.1113/EP085367. [DOI] [PubMed] [Google Scholar]
- 25.Green DJ, Hopman MT, Padilla J, Laughlin MH, Thijssen DH. Vascular adaptation to exercise in humans: role of hemodynamic stimuli. Physiol Rev 97: 495–528, 2017. doi: 10.1152/physrev.00014.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grossini E, Molinari C, Mary DA, Uberti F, Caimmi PP, Surico N, Vacca G. Intracoronary genistein acutely increases coronary blood flow in anesthetized pigs through beta-adrenergic mediated nitric oxide release and estrogenic receptors. Endocrinology 149: 2678–2687, 2008. doi: 10.1210/en.2007-1361. [DOI] [PubMed] [Google Scholar]
- 27.Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem 276: 36869–36872, 2001. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- 28.Harman SM, Brinton EA, Cedars M, Lobo R, Manson JE, Merriam GR, Miller VM, Naftolin F, Santoro N. KEEPS: The Kronos Early Estrogen Prevention Study. Climacteric 8: 3–12, 2005. doi: 10.1080/13697130500042417. [DOI] [PubMed] [Google Scholar]
- 29.Hildreth KL, Kohrt WM, Moreau KL. Oxidative stress contributes to large elastic arterial stiffening across the stages of the menopausal transition. Menopause 21: 624–632, 2014. doi: 10.1097/GME.0000000000000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hwang J, Saha A, Boo YC, Sorescu GP, McNally JS, Holland SM, Dikalov S, Giddens DP, Griendling KK, Harrison DG, Jo H. Oscillatory shear stress stimulates endothelial production of O2- from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J Biol Chem 278: 47291–47298, 2003. doi: 10.1074/jbc.M305150200. [DOI] [PubMed] [Google Scholar]
- 31.Ihionkhan CE, Chambliss KL, Gibson LL, Hahner LD, Mendelsohn ME, Shaul PW. Estrogen causes dynamic alterations in endothelial estrogen receptor expression. Circ Res 91: 814–820, 2002. doi: 10.1161/01.RES.0000038304.62046.4C. [DOI] [PubMed] [Google Scholar]
- 32.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 26: 631–640, 2010. doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
- 33.Khalil RA. Estrogen, vascular estrogen receptor and hormone therapy in postmenopausal vascular disease. Biochem Pharmacol 86: 1627–1642, 2013. doi: 10.1016/j.bcp.2013.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khatibi A, Agardh CD, Shakir YA, Nerbrand C, Nyberg P, Lidfeldt J, Samsioe G. Could androgens protect middle-aged women from cardiovascular events? A population-based study of Swedish women: The Women’s Health in the Lund Area (WHILA) Study. Climacteric 10: 386–392, 2007. doi: 10.1080/13697130701377265. [DOI] [PubMed] [Google Scholar]
- 35.Kim JA, Montagnani M, Chandrasekran S, Quon MJ. Role of lipotoxicity in endothelial dysfunction. Heart Fail Clin 8: 589–607, 2012. doi: 10.1016/j.hfc.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klawitter J, Hildreth KL, Christians U, Kohrt WM, Moreau KL. A relative l-arginine deficiency contributes to endothelial dysfunction across the stages of the menopausal transition. Physiol Rep 5: e13409, 2017. doi: 10.14814/phy2.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kling JM, Lahr BA, Bailey KR, Harman SM, Miller VM, Mulvagh SL. Endothelial function in women of the Kronos Early Estrogen Prevention Study. Climacteric 18: 187–197, 2015. doi: 10.3109/13697137.2014.986719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klinge CM, Wickramasinghe NS, Ivanova MM, Dougherty SM. Resveratrol stimulates nitric oxide production by increasing estrogen receptor α-Src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J 22: 2185–2197, 2008. doi: 10.1096/fj.07-103366. [DOI] [PubMed] [Google Scholar]
- 39.Kreijkamp-Kaspers S, Kok L, Bots ML, Grobbee DE, van der Schouw YT. Dietary phytoestrogens and vascular function in postmenopausal women: a cross-sectional study. J Hypertens 22: 1381–1388, 2004. doi: 10.1097/01.hjh.0000125435.28861.d2. [DOI] [PubMed] [Google Scholar]
- 40.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003. doi: 10.1161/01.CIR.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 41.Laughlin GA, Barrett-Connor E, Kritz-Silverstein D, von Mühlen D. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: the Rancho Bernardo Study. J Clin Endocrinol Metab 85: 645–651, 2000. doi: 10.1210/jcem.85.2.6405. [DOI] [PubMed] [Google Scholar]
- 42.Lekontseva O, Chakrabarti S, Davidge ST. Endothelin in the female vasculature: a role in aging? Am J Physiol Regul Integr Comp Physiol 298: R509–R516, 2010. doi: 10.1152/ajpregu.00656.2009. [DOI] [PubMed] [Google Scholar]
- 43.Li H, Förstermann U. Resveratrol: a multifunctional compound improving endothelial function. Editorial to: “Resveratrol supplementation gender independently improves endothelial reactivity and suppresses superoxide production in healthy rats” by S. Soylemez et al. Cardiovasc Drugs Ther 23: 425–429, 2009. doi: 10.1007/s10557-009-6209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li L, Haynes MP, Bender JR. Plasma membrane localization and function of the estrogen receptor α variant (ER46) in human endothelial cells. Proc Natl Acad Sci USA 100: 4807–4812, 2003. doi: 10.1073/pnas.0831079100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, Chen W, Li P, Wei J, Cheng Y, Liu P, Yan Q, Xu X, Cui Y, Gu Z, Simoncini T, Fu X. Follicular stimulating hormone accelerates atherogenesis by increasing endothelial VCAM-1 expression. Theranostics 7: 4671–4688, 2017. doi: 10.7150/thno.21216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lieberman EH, Gerhard MD, Uehata A, Walsh BW, Selwyn AP, Ganz P, Yeung AC, Creager MA. Estrogen improves endothelium-dependent, flow-mediated vasodilation in postmenopausal women. Ann Intern Med 121: 936–941, 1994. doi: 10.7326/0003-4819-121-12-199412150-00005. [DOI] [PubMed] [Google Scholar]
- 47.Liuni A, Luca MC, Gori T, Parker JD. Rosuvastatin prevents conduit artery endothelial dysfunction induced by ischemia and reperfusion by a cyclooxygenase-2-dependent mechanism. J Am Coll Cardiol 55: 1002–1006, 2010. doi: 10.1016/j.jacc.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 48.Lu Q, Schnitzler GR, Ueda K, Iyer LK, Diomede OI, Andrade T, Karas RH. ER α rapid signaling is required for estrogen induced proliferation and migration of vascular endothelial cells. PLoS One 11: e0152807, 2016. doi: 10.1371/journal.pone.0152807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luca MC, Liuni A, Harvey P, Mak S, Parker JD. Effects of estradiol on measurements of conduit artery endothelial function after ischemia and reperfusion in premenopausal women. Can J Physiol Pharmacol 94: 1304–1308, 2016. doi: 10.1139/cjpp-2015-0589. [DOI] [PubMed] [Google Scholar]
- 50.Matthews KA, Crawford SL, Chae CU, Everson-Rose SA, Sowers MF, Sternfeld B, Sutton-Tyrrell K. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol 54: 2366–2373, 2009. doi: 10.1016/j.jacc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Menazza S, Murphy E. The expanding complexity of estrogen receptor signaling in the cardiovascular system. Circ Res 118: 994–1007, 2016. doi: 10.1161/CIRCRESAHA.115.305376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller VM, Mulvagh SL. Sex steroids and endothelial function: translating basic science to clinical practice. Trends Pharmacol Sci 28: 263–270, 2007. doi: 10.1016/j.tips.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 53.Moerland M, Kales AJ, Schrier L, van Dongen MG, Bradnock D, Burggraaf J. Evaluation of the EndoPAT as a tool to assess endothelial function. Int J Vasc Med 2012: 904141, 2012. doi: 10.1155/2012/904141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moreau KL, Deane KD, Meditz AL, Kohrt WM. Tumor necrosis factor-α inhibition improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Atherosclerosis 230: 390–396, 2013. doi: 10.1016/j.atherosclerosis.2013.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moreau KL, Hildreth KL. Vascular aging across the menopause transition in healthy women. Adv Vasc Med 2014: 204390, 2014. doi: 10.1155/2014/204390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab 97: 4692–4700, 2012. doi: 10.1210/jc.2012-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moreau KL, Meditz A, Deane KD, Kohrt WM. Tetrahydrobiopterin improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Am J Physiol Heart Circ Physiol 302: H1211–H1218, 2012. doi: 10.1152/ajpheart.01065.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moreau KL, Ozemek C. Vascular adaptations to habitual exercise in older adults: time for the sex talk. Exerc Sport Sci Rev 45: 116–123, 2017. doi: 10.1249/JES.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moreau KL, Stauffer BL, Kohrt WM, Seals DR. Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J Clin Endocrinol Metab 98: 4507–4515, 2013. doi: 10.1210/jc.2013-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moriarty K, Kim KH, Bender JR. Minireview: estrogen receptor-mediated rapid signaling. Endocrinology 147: 5557–5563, 2006. doi: 10.1210/en.2006-0729. [DOI] [PubMed] [Google Scholar]
- 61.Morita M, Sakurada M, Watanabe F, Yamasaki T, Doi H, Ezaki H, Morishita K, Miyake T. Effects of oral l-citrulline supplementation on lipoprotein oxidation and endothelial dysfunction in humans with vasospastic angina. Immunol Endocr Metab Agents Med Chem 13: 214–220, 2013. doi: 10.2174/18715222113139990008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Novella S, Dantas AP, Segarra G, Medina P, Hermenegildo C. Vascular aging in women: is estrogen the fountain of youth? Front Physiol 3: 165, 2012. doi: 10.3389/fphys.2012.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nyberg M, Seidelin K, Andersen TR, Overby NN, Hellsten Y, Bangsbo J. Biomarkers of vascular function in premenopausal and recent postmenopausal women of similar age: effect of exercise training. Am J Physiol Regul Integr Comp Physiol 306: R510–R517, 2014. doi: 10.1152/ajpregu.00539.2013. [DOI] [PubMed] [Google Scholar]
- 63a.Ohmichi M, Kanda Y, Hisamoto K, Morishige K, Takahashi K, Sawada K, Minekawa R, Tasaka K, Murata Y. Rapid changes of flow-mediated dilatation after surgical menopause. Maturitas 44: 125–131, 2003. doi: 10.1016/S0378-5122(02)00320-1. [DOI] [PubMed] [Google Scholar]
- 64.O’Keeffe LM, Muir G, Piterina AV, McGloughlin T. Vascular cell adhesion molecule-1 expression in endothelial cells exposed to physiological coronary wall shear stresses. J Biomech Eng 131: 081003, 2009. doi: 10.1115/1.3148191. [DOI] [PubMed] [Google Scholar]
- 66.Padilla J, Simmons GH, Fadel PJ, Laughlin MH, Joyner MJ, Casey DP. Impact of aging on conduit artery retrograde and oscillatory shear at rest and during exercise: role of nitric oxide. Hypertension 57: 484–489, 2011. doi: 10.1161/HYPERTENSIONAHA.110.165365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pinna C, Cignarella A, Sanvito P, Pelosi V, Bolego C. Prolonged ovarian hormone deprivation impairs the protective vascular actions of estrogen receptor alpha agonists. Hypertension 51: 1210–1217, 2008. doi: 10.1161/HYPERTENSIONAHA.107.106807. [DOI] [PubMed] [Google Scholar]
- 68.Pinto S, Virdis A, Ghiadoni L, Bernini G, Lombardo M, Petraglia F, Genazzani AR, Taddei S, Salvetti A. Endogenous estrogen and acetylcholine-induced vasodilation in normotensive women. Hypertension 29: 268–273, 1997. doi: 10.1161/01.HYP.29.1.268. [DOI] [PubMed] [Google Scholar]
- 69.Pinzone JJ, Stevenson H, Strobl JS, Berg PE. Molecular and cellular determinants of estrogen receptor alpha expression. Mol Cell Biol 24: 4605–4612, 2004. doi: 10.1128/MCB.24.11.4605-4612.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pons A, Bescós R, Sureda A, Tur JA. Metabolic precursors of l-arginine supplementation in sports: a focus on l-citrulline and l-ornithine BT. In: l-Arginine in Clinical Nutrition, edited by Patel VB, Preedy VR, Rajendram R. Cham, Switzerland: Humana, 2017, p. 311–318. [Google Scholar]
- 71.Post WS, Goldschmidt-Clermont PJ, Wilhide CC, Heldman AW, Sussman MS, Ouyang P, Milliken EE, Issa JP. Methylation of the estrogen receptor gene is associated with aging and atherosclerosis in the cardiovascular system. Cardiovasc Res 43: 985–991, 1999. doi: 10.1016/S0008-6363(99)00153-4. [DOI] [PubMed] [Google Scholar]
- 72.Pusparini DR, Dharma R, Suyatna FD, Mansyur M, Hidajat A. Effect of soy isoflavone supplementation on vascular endothelial function and oxidative stress in postmenopausal women: a community randomized controlled trial. Asia Pac J Clin Nutr 22: 357–364, 2013. doi: 10.6133/apjcn.2013.22.3.13. [DOI] [PubMed] [Google Scholar]
- 73.Reis SE, Holubkov R, Conrad Smith AJ, Kelsey SF, Sharaf BL, Reichek N, Rogers WJ, Merz CN, Sopko G, Pepine CJ; WISE Investigators . Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J 141: 735–741, 2001. doi: 10.1067/mhj.2001.114198. [DOI] [PubMed] [Google Scholar]
- 74.Rossi R, Chiurlia E, Nuzzo A, Cioni E, Origliani G, Modena MG. Flow-mediated vasodilation and the risk of developing hypertension in healthy postmenopausal women. J Am Coll Cardiol 44: 1636–1640, 2004. doi: 10.1016/j.jacc.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 75.Rossi R, Nuzzo A, Origliani G, Modena MG. Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. J Am Coll Cardiol 51: 997–1002, 2008. doi: 10.1016/j.jacc.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 76.Saitta A, Altavilla D, Cucinotta D, Morabito N, Frisina N, Corrado F, D’Anna R, Lasco A, Squadrito G, Gaudio A, Cancellieri F, Arcoraci V, Squadrito F. Randomized, double-blind, placebo-controlled study on effects of raloxifene and hormone replacement therapy on plasma no concentrations, endothelin-1 levels, and endothelium-dependent vasodilation in postmenopausal women. Arterioscler Thromb Vasc Biol 21: 1512–1519, 2001. doi: 10.1161/hq0901.095565. [DOI] [PubMed] [Google Scholar]
- 77.Schmidt TS, Alp NJ. Mechanisms for the role of tetrahydrobiopterin in endothelial function and vascular disease. Clin Sci (Lond) 113: 47–63, 2007. doi: 10.1042/CS20070108. [DOI] [PubMed] [Google Scholar]
- 78.Seals DR, Kaplon RE, Gioscia-Ryan RA, LaRocca TJ. You’re only as old as your arteries: translational strategies for preserving vascular endothelial function with aging. Physiology (Bethesda) 29: 250–264, 2014. doi: 10.1152/physiol.00059.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seals DR, Moreau KL, Gates PE, Eskurza I. Modulatory influences on ageing of the vasculature in healthy humans. Exp Gerontol 41: 501–507, 2006. doi: 10.1016/j.exger.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 80.Shenouda N, Priest SE, Rizzuto VI, MacDonald MJ. Brachial artery endothelial function is stable across a menstrual and oral contraceptive pill cycle but lower in premenopausal women than in age-matched men. Am J Physiol Heart Circ Physiol 315: H366–H374, 2018. doi: 10.1152/ajpheart.00102.2018. [DOI] [PubMed] [Google Scholar]
- 81.Somani YB, Moore DJ, Kim DJ, Gonzales JU, Barlow MA, Elavsky S, Proctor DN. Retrograde and oscillatory shear increase across the menopause transition. Physiol Rep 7: e13965, 2019. doi: 10.14814/phy2.13965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, Woods N. Stages of Reproductive Aging Workshop (STRAW). J Womens Health Gend Based Med 10: 843–848, 2001. doi: 10.1089/152460901753285732. [DOI] [PubMed] [Google Scholar]
- 83.Squadrito F, Altavilla D, Crisafulli A, Saitta A, Cucinotta D, Morabito N, D’Anna R, Corrado F, Ruggeri P, Frisina N, Squadrito G. Effect of genistein on endothelial function in postmenopausal women: a randomized, double-blind, controlled study. Am J Med 114: 470–476, 2003. doi: 10.1016/S0002-9343(03)00059-7. [DOI] [PubMed] [Google Scholar]
- 84.Suboc TM, Dharmashankar K, Wang J, Ying R, Couillard A, Tanner MJ, Widlansky ME. Moderate obesity and endothelial dysfunction in humans: influence of gender and systemic inflammation. Physiol Rep 1: e00058, 2013. doi: 10.1002/phy2.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sumino H, Ichikawa S, Kasama S, Takahashi T, Kumakura H, Takayama Y, Kanda T, Kurabayashi M. Different effects of oral conjugated estrogen and transdermal estradiol on arterial stiffness and vascular inflammatory markers in postmenopausal women. Atherosclerosis 189: 436–442, 2006. doi: 10.1016/j.atherosclerosis.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 86.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 101: 948–954, 2000. doi: 10.1161/01.CIR.101.9.948. [DOI] [PubMed] [Google Scholar]
- 87.Taddei S, Virdis A, Ghiadoni L, Mattei P, Sudano I, Bernini G, Pinto S, Salvetti A. Menopause is associated with endothelial dysfunction in women. Hypertension 28: 576–582, 1996. doi: 10.1161/01.HYP.28.4.576. [DOI] [PubMed] [Google Scholar]
- 88.Takahashi K, Mori-Abe A, Takata K, Ohta T, Kawagoe J, Tsutsumi S, Ohmichi M, Kurachi H. Raloxifene improves the ovariectomy-induced impairment in endothelium-dependent vasodilation. Menopause 14: 656–661, 2007. doi: 10.1097/01.gme.0000248704.30204.33. [DOI] [PubMed] [Google Scholar]
- 89.Tchernof A, Calles-Escandon J, Sites CK, Poehlman ET. Menopause, central body fatness, and insulin resistance: effects of hormone-replacement therapy. Coron Artery Dis 9: 503–512, 1998. doi: 10.1097/00019501-199809080-00006. [DOI] [PubMed] [Google Scholar]
- 90.Thijssen DH, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension 53: 986–992, 2009. doi: 10.1161/HYPERTENSIONAHA.109.131508. [DOI] [PubMed] [Google Scholar]
- 91.Thomas RS, Sarwar N, Phoenix F, Coombes RC, Ali S. Phosphorylation at serines 104 and 106 by Erk1/2 MAPK is important for estrogen receptor-alpha activity. J Mol Endocrinol 40: 173–184, 2008. doi: 10.1677/JME-07-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van der Made SM, Plat J, Mensink RP. Trans-resveratrol supplementation and endothelial function during the fasting and postprandial phase: a randomized placebo-controlled trial in overweight and slightly obese participants. Nutrients 9: 9, 2017. doi: 10.3390/nu9060596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vitale C, Mercuro G, Cerquetani E, Marazzi G, Patrizi R, Pelliccia F, Volterrani M, Fini M, Collins P, Rosano GM. Time since menopause influences the acute and chronic effect of estrogens on endothelial function. Arterioscler Thromb Vasc Biol 28: 348–352, 2008. doi: 10.1161/ATVBAHA.107.158634. [DOI] [PubMed] [Google Scholar]
- 94.Walker HA, Dean TS, Sanders TA, Jackson G, Ritter JM, Chowienczyk PJ. The phytoestrogen genistein produces acute nitric oxide-dependent dilation of human forearm vasculature with similar potency to 17beta-estradiol. Circulation 103: 258–262, 2001. doi: 10.1161/01.CIR.103.2.258. [DOI] [PubMed] [Google Scholar]
- 95.Welsch MA, Allen JD, Geaghan JP. Stability and reproducibility of brachial artery flow-mediated dilation. Med Sci Sports Exerc 34: 960–965, 2002. doi: 10.1097/00005768-200206000-00009. [DOI] [PubMed] [Google Scholar]
- 96.Wong A, Alvarez-Alvarado S, Jaime SJ, Kinsey AW, Spicer MT, Madzima TA, Figueroa A. Combined whole-body vibration training and L-citrulline supplementation improves pressure wave reflection in obese postmenopausal women. Appl Physiol Nutr Metab 41: 292–297, 2016. doi: 10.1139/apnm-2015-0465. [DOI] [PubMed] [Google Scholar]
- 97.Wong RH, Berry NM, Coates AM, Buckley JD, Bryan J, Kunz I, Howe PR. Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults. J Hypertens 31: 1819–1827, 2013. doi: 10.1097/HJH.0b013e328362b9d6. [DOI] [PubMed] [Google Scholar]
- 98.Wong RH, Howe PR, Buckley JD, Coates AM, Kunz I, Berry NM. Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr Metab Cardiovasc Dis 21: 851–856, 2011. doi: 10.1016/j.numecd.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 99.Xia N, Daiber A, Förstermann U, Li H. Antioxidant effects of resveratrol in the cardiovascular system. Br J Pharmacol 174: 1633–1646, 2017. doi: 10.1111/bph.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Young CN, Deo SH, Padilla J, Laughlin MH, Fadel PJ. Pro-atherogenic shear rate patterns in the femoral artery of healthy older adults. Atherosclerosis 211: 390–392, 2010. doi: 10.1016/j.atherosclerosis.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]