Abstract
Patients with type-2 diabetes mellitus (T2DM) have exaggerated sympathetic activity and blood pressure responses to exercise. However, the underlying mechanisms for these responses, as well as how these responses change throughout disease progression, are not completely understood. For this study, we examined the effect of the progression of T2DM on the exercise pressor reflex, a critical neurocardiovascular mechanism that functions to increase sympathetic activity and blood pressure during exercise. We also aimed to examine the effect of T2DM on reflexive cardiovascular responses to static contraction, as well as those responses to tendon stretch when an exaggerated exercise pressor reflex was present. We evoked the exercise pressor reflex and mechanoreflex by statically contracting the hindlimb muscles and stretching the Achilles tendon, respectively, for 30 s. We then compared pressor and cardioaccelerator responses in unanesthetized, decerebrated University of California Davis (UCD)-T2DM rats at 21 and 31 wk following the onset of T2DM to responses in healthy nondiabetic rats. We found that the pressor response to static contraction was greater in the 31-wk T2DM [change in mean arterial pressure (∆MAP) = 39 ± 5 mmHg] but not in the 21-wk T2DM (∆MAP = 24 ± 5 mmHg) rats compared with nondiabetic rats (∆MAP = 18 ± 2 mmHg; P < 0.05). Similarly, the pressor and the cardioaccelerator responses to tendon stretch were significantly greater in the 31-wk T2DM rats [∆MAP = 69 ± 6 mmHg; change in heart rate (∆HR) = 28 ± 4 beats/min] compared with nondiabetic rats (∆MAP = 14 ± 2 mmHg; ∆HR = 5 ± 3 beats/min; P < 0.05). These findings suggest that the exercise pressor reflex changes as T2DM progresses and that a sensitized mechanoreflex may play a role in exaggerating these cardiovascular responses.
NEW & NOTEWORTHY This is the first study to provide evidence that as type-2 diabetes mellitus (T2DM) progresses, the exercise pressor reflex becomes exaggerated, an effect that may be due to a sensitized mechanoreflex. Moreover, these findings provide compelling evidence suggesting that impairments in the reflexive control of circulation contribute to exaggerated blood pressure responses to exercise in T2DM.
Keywords: central command, exercise pressor reflex, mesencephalic locomotor region, sympathetic nerve activity, type-2 diabetes
INTRODUCTION
The onset of exercise evokes increases in heart rate (HR), myocardial contractility, and arterial vasoconstriction due to increasing sympathetic and decreasing parasympathetic activity. This, in turn, redistributes blood flow (BF) to working skeletal muscles and results in an intensity-dependent increase in blood pressure. Two main mechanisms are responsible for increasing sympathetic activity during exercise: namely, central command (10, 14) and the exercise pressor reflex (1, 35). Furthermore, the arterial and cardiopulmonary baroreflexes play a modulatory role in these changes (11, 24). Both metabolic and mechanical stimuli are produced during muscle contraction. Metabolic stimuli evoke the metaboreflex by stimulating primarily group IV afferent endings, whereas mechanical stimuli evoke the mechanoreflex by stimulating primarily group III afferent endings (26, 35). Previous studies have shown that the exercise pressor reflex is exaggerated in cardiovascular-related diseases such as heart failure (36, 50), peripheral artery disease (3, 47), hypertension (2, 32) and type-1 diabetes (16). Type-2 diabetes mellitus (T2DM) is also considered a cardiovascular-related disease, as individuals with T2DM are two- to fourfold more likely to die from a cardiovascular-related event than those without T2DM, an effect that, in turn, is associated with disease duration and severity (17, 51). However, the effect of T2DM on the exercise pressor reflex remains incompletely understood.
Individuals with T2DM have exaggerated blood pressure responses to isometric and dynamic exercise, as well as attenuated vasodilatory responses to dynamic exercise. Several studies have shown that increases in blood pressure to both moderate and maximal intensity dynamic exercise are exaggerated in adolescents (41), middle-aged adults (43, 44), and older adults (38) with T2DM. Although these findings are categorized by age, the time of disease onset is difficult to pinpoint in humans and at this point remains a limitation in all T2DM studies examining cardiovascular changes throughout the progression of the disease. Other studies have shown that T2DM impairs vasodilatory capacity, which in turn leads to a reduced BF response to exercise (29, 31, 34). Muscle ischemia is a potent stimulus of group III and IV muscle afferents (13). Thus, a reduced BF response to exercise could increase the activation of the exercise pressor reflex. Although these altered hemodynamic responses are evident in T2DM, the effects of T2DM on the reflexive control of circulation during exercise are not completely understood. Currently, the effects of T2DM on baroreflex control are inconclusive (8, 21). However, a recent study reported that patients with T2DM had increased muscle sympathetic nerve activity (MSNA) and augmented blood pressure responses to isometric handgrip exercise, a response which was maintained during post-exercise occlusion, suggesting that the metaboreflex was exaggerated in patients with T2DM (20). Although these studies suggest that the metabolic component of the exercise pressor reflex (i.e., metaboreflex) is altered in T2DM, it is not known whether the complete exercise pressor reflex (i.e., mechano- and metaboreflex activation together independent of central command) is altered in T2DM. Furthermore, studies have not examined the mechanoreflex in isolation in T2DM.
T2DM is a progressive disease where risks of complications are strongly associated with the severity and duration of hyperglycemia. Although there are many therapies aimed at glycemic control, none of these entirely prevents the progression of the disease (12). Therefore, disparities often exhibited in human studies, (e.g., differences in age, disease severity, duration, complications, and medications) make it difficult to isolate the effects of T2DM on the reflexive control of the circulation. Furthermore, data are lacking on the effects of the progression of T2DM on the neural control of circulation. Diabetic peripheral neuropathy (DPN) is one of the most common complications in diabetes, increasing in severity as T2DM progresses (49). DPN affects as many as 50% of patients with T2DM and is associated with significant decrements in quality of life and increased comorbidity (42, 49). A common symptom of DPN is mechanical allodynia, which is characterized as sensitization of peripheral nerve endings, such as those on thinly myelinated group III (A-δ fibers) afferents (27) and unmyelinated group IV (C-fibers) afferents (39), causing pain to normally nonpainful mechanical stimulus. As previously mentioned, group III and IV muscle afferents are also responsible for evoking the exercise pressor reflex. Thus, it is likely that the effects of T2DM on these afferents may also alter the exercise pressor reflex throughout the progression of the disease.
The overall purpose of this study was to determine the effects of T2DM on the exercise pressor reflex at two different time points during the disease. We also sought to determine whether the mechanoreflex was exaggerated in the T2DM rats when an exaggerated exercise pressor reflex was present. We hypothesized that T2DM rats would have an exaggerated exercise pressor reflex compared with healthy nondiabetic rats and that this response would be further enhanced with the progression of the disease. Furthermore, we hypothesized that compared with nondiabetic rats, the mechanoreflex would also be exaggerated in T2DM rats when an exaggerated exercise pressor reflex was present.
MATERIALS AND METHODS
All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of The University of Texas at Austin. Adult male University of California Davis (UCD)-T2DM rats (n = 16; body weight: 526 ± 15 g) provided from the breeding colony of UCD-T2DM rats in the Department of Nutrition at UCD, and adult male Sprague-Dawley rats (n = 23; body wt, 464 ± 18 g; ~5–7 mo old) from Charles River (Wilmington, MA) were used in these experiments. The UCD-T2DM rat model combines polygenic adult-onset obesity and insulin resistance with inadequate islet function/β-cell compensation leading to the development of overt hyperglycemia/diabetes over time with pathophysiology more similar to that in humans than other rodent models of T2DM (7, 30). The rats were housed in a temperature-controlled room (24 ± 1°C) with a 12-h:12-h light-dark cycle and fed a standard diet and tap water ad libitum. These rats did not receive any other treatment. Pressor responses were measured in UCD-T2DM rats at 21 (n = 5) and 31 (n = 5) wk following the onset of T2DM. It is important to note that we began the study with n = 21 UCD-T2DM rats, and through the 21–31-wk-long progression of T2DM, we yielded ~50% success rate with the pressor reflex studies. Furthermore, one rat did not develop hyperglycemia and was therefore excluded from the study. Biomarkers were measured on the same rats, as well as additional rats, like those used in the pressor reflex experiments.
Surgical preparation.
On the day of the experiment, nonfasted rats were anesthetized with isoflurane gas (2–5%) in 100% oxygen. Body weight, blood glucose (Nova Biomedical, Waltham, MA), and HbA1c (PTS diagnostics, Indianapolis, IN) were assessed. Rats were considered diabetic if the nonfasted blood glucose level was >300 mg/dL and/or the HbA1c level was >6.5%. The trachea was cannulated, and the lungs mechanically ventilated (Harvard Apparatus, Holliston, MA). The right jugular vein and both common carotid arteries were cannulated (PE-50) for fluid and drug delivery and blood pressure measurement, respectively. Although both carotid arteries were cannulated, the aortic baroreflex was undisturbed and remained fully functional. One carotid arterial catheter was connected to a pressure transducer (CWE DTX-1, Ardmore, PA). HR was calculated beat by beat from pulsatile blood pressure using Spike2 software (CED, Cambridge, UK). In all rats, the sciatic nerve was surgically isolated and a stimulating electrode was placed underneath it for evoking a muscle contraction. The hindlimb muscles of the left leg were exposed. The calcaneal bone of the same leg was severed, and the Achilles tendon was attached to a force transducer (model FT-03, Grass Instruments, West Warwick, RI) that was connected to a rack and pinion. For some rats, an ultrasonic flow probe (Transonic Systems, Ithaca, NY) was placed around the left popliteal artery, which supplies the triceps surae muscles, to measure BF. Arterial Pco2 and pH were measured using an automated blood-gas analyzer (Nova Biomedical, Waltham, MA) and were maintained within normal range by either adjusting ventilation or by intravenous injection of sodium bicarbonate (8.5%). Body temperature was measured using a rectal temperature probe and maintained between 36.5 and 38°C with a heating plate and lamp.
Each rat was placed in a Kopf stereotaxic frame, and spinal clamps were placed on the rostral lumbar vertebrae. The pelvis was stabilized using customized metal spikes clamped below the iliac crest. Dexamethasone (2 mg/mL; 0.2 mL) was injected intravenously to prevent excessive swelling in the brain, and a precollicular decerebration was performed. All neural tissue rostral to the section was removed. Bleeding was controlled, and the cranial vault was filled with gauze. Immediately after decerebration, gas anesthesia was discontinued, and the rat was allowed to stabilize for at least 1 h before any experimental protocol began (45).
Experimental protocol.
Before contraction, the Achilles tendon was stretched so that baseline tension was set between 90 and 100 g for at least 30 s. Static contraction of the hindlimb muscles was evoked by electrically stimulating (40 Hz; 0.01 ms; ≤2 times motor threshold; for 30 s) the isolated sciatic nerve. After statically contracting the hindlimb muscles, 0.5 mL pancuronium bromide was injected intravenously to paralyze the rat, and the sciatic nerve was again stimulated using the same parameters as those used to contract the hindlimb muscles. This was done to ensure that the pressor response seen during static contraction was not a result of direct electrical activation of the axons of thin fiber afferents in the sciatic nerve. If no pressor response was seen when stimulating after paralyzing the rat, it was concluded that the pressor response was solely evoked from the muscle contraction, and only then were the data included. After control measures were taken (~10 min), the Achilles tendon was stretched so that baseline tension was set between 90 and 100 g for at least 30 s. To test the contribution of the mechanoreflex, a static tendon stretch was performed by rapidly turning the rack and pinion and stretching the triceps surae muscles for 30 s (54).
Blood sampling and analysis.
Following the experiments, whole blood was drawn from the carotid artery into a serum separator vacutainer tube. Blood was allowed to clot at room temperature for 30 min, centrifuged for 15 min at 1,000 g, and then the serum was aliquoted into tubes and frozen at −80°C for subsequent batch analyses. A rat multiplex kit (RADPKMAG, EMD Millipore, Burlington, MA) was used to determine insulin and leptin in the aliquoted samples. Standards, controls, and samples were added, in duplicate, to 96-well plates and analyzed according to the manufacturer's recommendations. Plates were then read using a Luminex 200 (Luminex, Austin, TX) to determine mean fluorescent intensity for each well. Five-parameter logistic regression was used to construct a standard curve, which was then used to calculate control and sample unknown concentrations. R2 for the standard curves ranged from 0.999 to 1, all controls were within the range supplied by the manufacturer, and coefficient of variation between sample duplicates averaged <5%. According to the manufacturer, there is no cross-reactivity between the antibodies for insulin and leptin, which were supplied in the same kit. The minimum detectable concentration for insulin is 14 pg/mL and leptin 3.1 pg/mL. The intra-assay precision (%CV) for insulin and leptin is <10%. The inter-assay precision (%CV) for insulin and leptin is <15%.
Data analysis.
Mean arterial blood pressure (MAP) and HR are presented as means ± SE. MAP (mmHg), HR (beats/min), popliteal BF (mL/min) and muscle tension (g) were recorded continuously with a Spike2 data acquisition system and stored on a computer hard drive (Dell). Vascular conductance was calculated by dividing popliteal arterial BF by MAP. Mean BF and vascular conductance were calculated by averaging second-by-second percent change from baseline, normalized to baseline, during 30 s of muscle contraction. The tension-time index (TTI, kg·s) was calculated by integrating the area between the tension trace and the baseline level. All variables were compared among the three groups. Data were analyzed using one-way analysis of variance (ANOVA), as only one independent variable (group) was used for each dependent measurement. Holm-Sidak’s post hoc tests were used for all pairwise comparisons when a significant interaction was detected. Changes in MAP in response to stretch, plotted over time, were analyzed using a two-way ANOVA with Holm-Sidak’s post hoc test. Baseline MAP, HR, and BF were analyzed using one-way ANOVAs to detect differences among groups. GraphPad Prism 7 Software (La Jolla, CA) was used for statistical analyses. The criterion level of significance was P ≤ 0.05.
RESULTS
Biomarkers.
All T2DM rats developed diabetes between 13 and 17 wk of age based on their nonfasted blood glucose levels. Plasma insulin concentrations (nonfasted) were significantly greater in nondiabetic rats (1.15 ± 0.17 ng/mL, n = 10) than in the 21-wk (0.18 ± 0.02 ng/mL, n = 7) and the 31-wk (0.47 ± 0.09 ng/mL, n = 6) T2DM rats, suggesting β-cell decompensation led to impaired insulin secretion in UCD-T2DM rats compared with nondiabetic control rats (P < 0.01). Likewise, plasma leptin concentrations were significantly greater in the nondiabetic rats (2.76 ± 0.16 ng/mL, n = 9) compared with the 21-wk (0.66 ± 0.23 ng/mL, n = 7) and the 31-wk (0.64 ± 0.15 ng/mL, n = 6) T2DM rats, suggesting that T2DM rats produced significantly less leptin than nondiabetic rats (P < 0.01). This has previously been shown in UCD-T2DM rats with disease progression and is most likely because insulin is a major regulator of leptin production by adipocytes (7, 19).
Baseline measurements.
Body weight, blood glucose, and HbA1c for all rats included in the reflex experiments are shown in Table 1. Body weight was similar between groups (P > 0.05). As expected, both T2DM groups had significantly elevated blood glucose and HbA1c levels compared with the nondiabetic rats (P < 0.05). Baseline MAP, HR, and BF before muscle contraction and baseline MAP and HR before tendon stretch are reported in Table 2. Before static contraction, baseline MAP was similar in the 21- and 31-wk T2DM rats compared with nondiabetic rats (P > 0.05). Baseline HR, however, was significantly lower in both groups of T2DM rats compared with nondiabetic rats, and lower in the 21- compared with 31-wk T2DM rats (P < 0.05). Before statically stretching the Achilles tendon, baseline MAP and baseline HR were similar in the 31-wk T2DM rats compared with nondiabetic rats (P > 0.05). Before static contraction, baseline BF was significantly greater in the 21-wk (P < 0.05) but not in the 31-wk (P > 0.05) T2DM rats compared with nondiabetic rats. Similarly, baseline vascular conductance was significantly greater in the 21-wk (P < 0.05) but not in the 31-wk (P > 0.05) T2DM rats compared with nondiabetic rats.
Table 1.
Descriptive characteristics of nondiabetic and T2DM rats
| Nondiabetic | 21-wk T2DM | 31-wk T2DM | |
|---|---|---|---|
| n | 8 | 5 | 5 |
| Weight, g | 513 ± 25 | 537 ± 18 | 546 ± 26 |
| Glucose, mg/dL | 197 ± 20 | 579 ± 14* | 549 ± 28* |
| HbA1c, % | 4.9 ± 0.05 | 12.8 ± 0.2* | 12.8 ± 0.2* |
Values are means ± SE; n, number of rats. T2DM, type-2 diabetes mellitus.
P < 0.05, significantly different from nondiabetic rats.
Table 2.
Baseline cardiovascular values for contraction and stretch experiments
| Nondiabetic | 21-wk T2DM | 31-wk T2DM | |
|---|---|---|---|
| Contraction | |||
| MAP, mmHg | 77 ± 5 | 66 ± 5 | 73 ± 7 |
| HR, beats/min | 383 ± 14 | 276 ± 8* | 336 ± 8*# |
| BF, mL/min | 0.30 ± 0.06 | 1.06 ± 0.20* | 0.35 ± 0.05 |
| Stretch | |||
| MAP, mmHg | 88 ± 10 | – | 78 ± 10 |
| HR, beats/min | 406 ± 23 | – | 353 ± 12 |
Values are means ± SE. T2DM, type-2 diabetes mellitus; MAP, mean arterial pressure; HR, heart rate; BF, blood flow.
P < 0.05, significantly different from nondiabetic rats.
P < 0.05, significantly different from 21-wk T2DM rats.
Static muscle contraction.
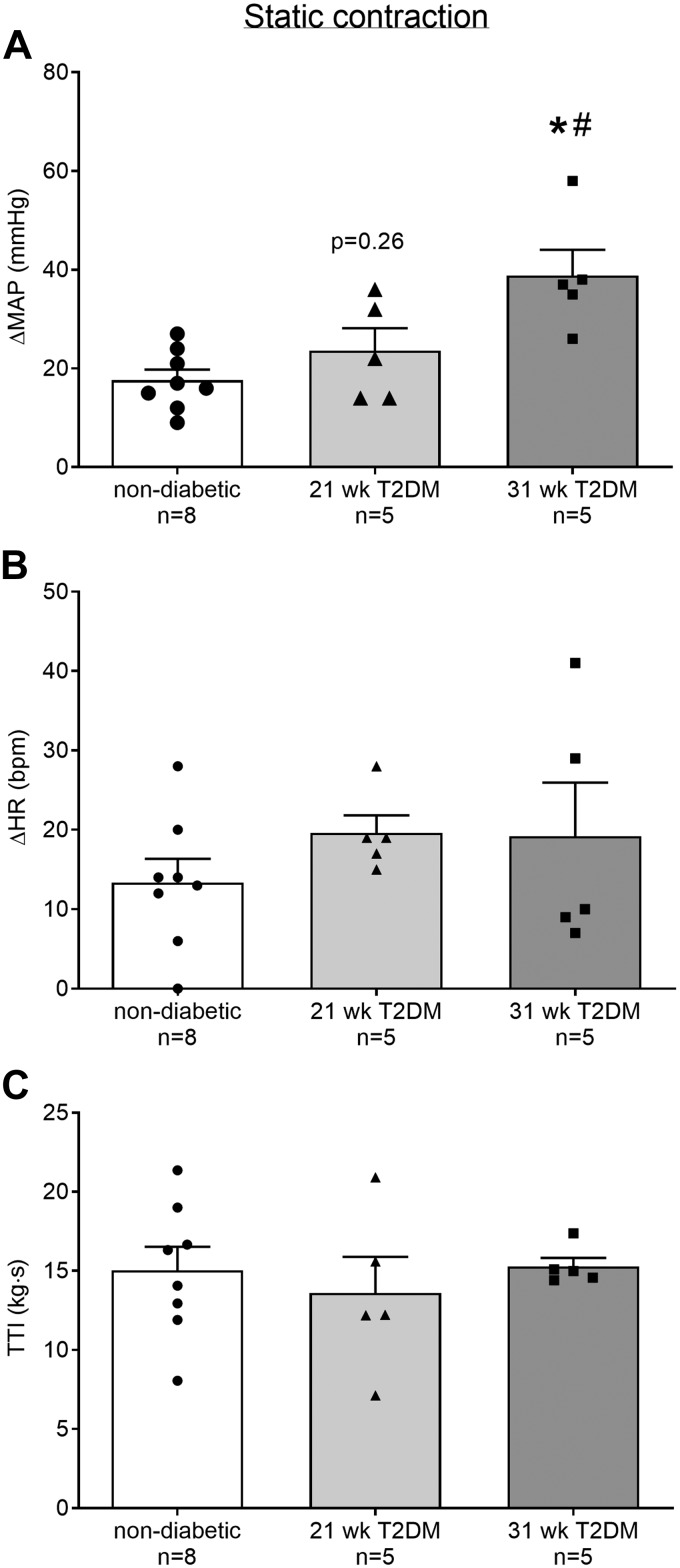
The peak pressor response to static contraction was significantly greater in the 31-wk (n = 5) but not in the 21-wk (n = 5) T2DM rats compared with the nondiabetic rats (n = 8; Fig. 1A, P < 0.05). The pressor response to static contraction was significantly greater in the 31- compared with the 21-wk T2DM rats (P < 0.05). The peak cardioaccelerator response to static contraction was not significantly different in either 21- or 31-wk T2DM rats compared with nondiabetic rats, or between 21- and 31-wk T2DM groups (Fig. 1B, P > 0.05). Developed tension during static contraction was similar between groups (Fig. 1C, P > 0.05). In all experiments, injecting pancuronium bromide into the jugular vein abolished the pressor response to electrical stimulation of the sciatic nerve using the same parameters as those before paralyzing the rat. This confirms that the pressor responses were reflex in origin and were evoked by the contraction of the hindlimb muscles.
Fig. 1.
Means ± SE (n, number of rats) and individual data showing that statically contracting the hindlimb muscles evoked an exaggerated peak pressor response in the 31-wk compared with the responses in 21-wk T2DM and nondiabetic rats (A). Peak changes in heart rate were not different among the groups (B). Developed tensions [tension-time index (TTI)] were similar among groups (C). *P < 0.05 (1-way ANOVA) indicates statistically greater response compared with nondiabetic rats. #P < 0.05 (1-way ANOVA) indicates statistically greater pressor response compared with 21-wk type-2 diabetes mellitus (T2DM) rats. MAP, mean arterial pressure; HR, heart rate; bpm, beats/min,
Static tendon stretch.
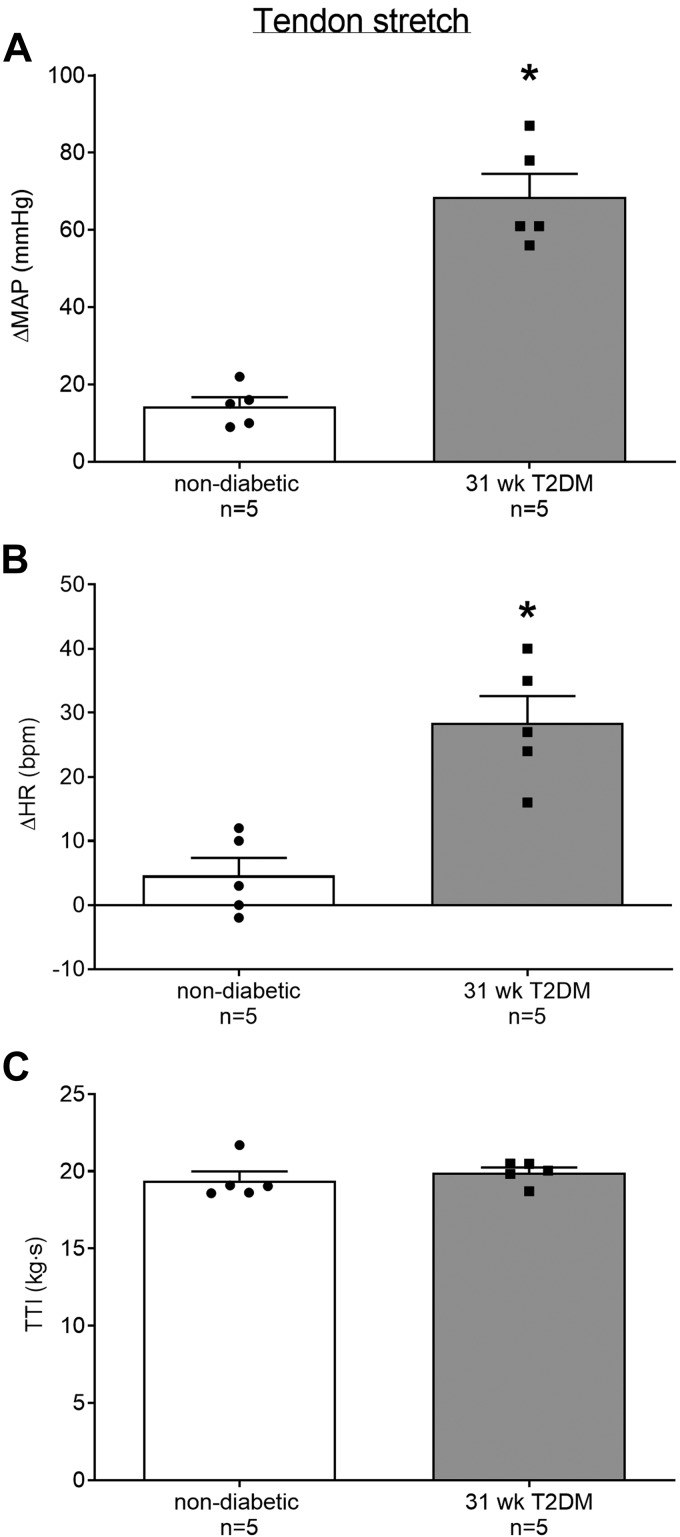
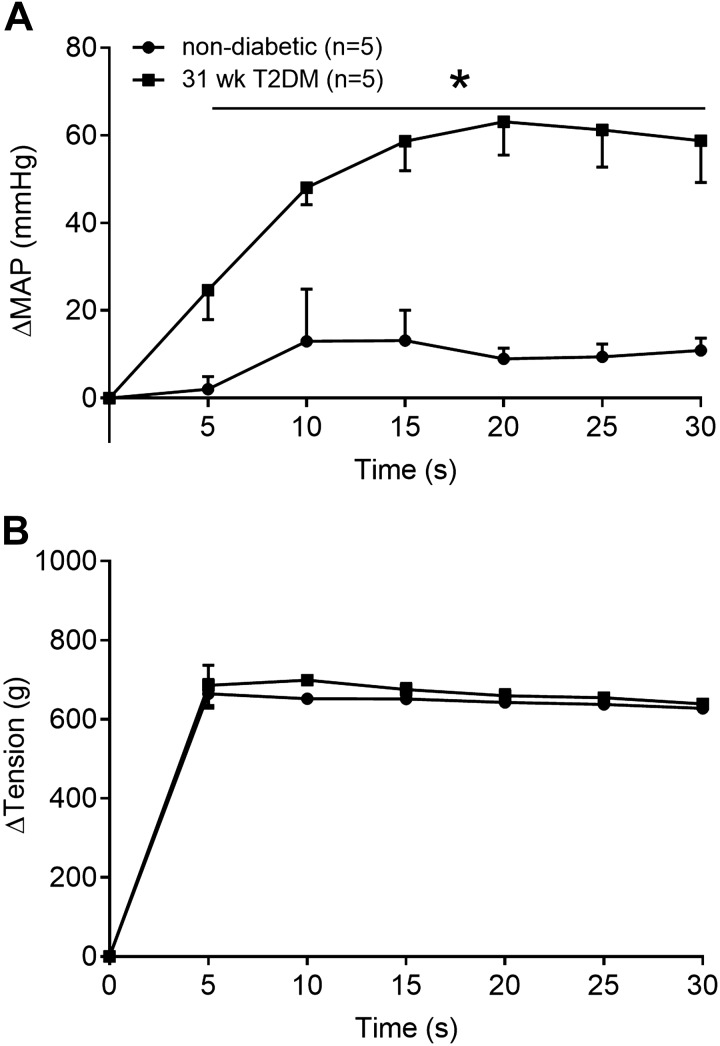
The peak pressor response to tendon stretch was significantly greater in the 31-wk T2DM rats (n = 5) compared with the nondiabetic rats (n = 5; Fig. 2A, P < 0.05). Furthermore, the peak cardioaccelerator response to static tendon stretch was significantly greater in the 31-wk T2DM rats compared with the nondiabetic rats (Fig. 2B, P < 0.05). Developed tension during static contraction was not significantly different between groups (Fig. 2C, P > 0.05). We analyzed changes in blood pressure in 5-s intervals during the 30 s of tendon stretch (Fig. 3, A and B) and found a significant interaction between group and time (P < 0.05). Specifically, the pressor response to tendon stretch was significantly greater in the 31-wk T2DM rats beginning at second 5 (Fig. 3A). No significant interaction was found for the cardioaccelerator response over time (data are not shown); however, a main effect of treatment was detected (P < 0.05). Developed tension was similar between groups (Fig. 3B, P > 0.05).
Fig. 2.
Means ± SE (n, number of rats) and individual data showing that stretching the Achilles tendon evoked exaggerated peak pressor (A) and cardioaccelerator (B) responses in the 31-wk type-2 diabetes mellitus (T2DM) rats compared with nondiabetic rats. Developed tensions [tension-time index TTI)] were similar among groups (C). *P < 0.05 (Student’s t-test) indicates statistically greater response compared with nondiabetic rats.
Fig. 3.
The pressor response (A) over 30 s of static stretch in 31-wk type-2 diabetes mellitus (T2DM) (black square, n = 5) and nondiabetic (white circle, n = 5) rats. There was a significant interaction between time and group for change in mean arterial pressure (ΔMAP; P < 0.05). Horizontal black line and *P < 0.05 (2-way ANOVA) indicate significantly greater response compared with nondiabetic rats at each time point. Developed tension (B) was not different between groups at any time point (P > 0.05).
Blood flow.
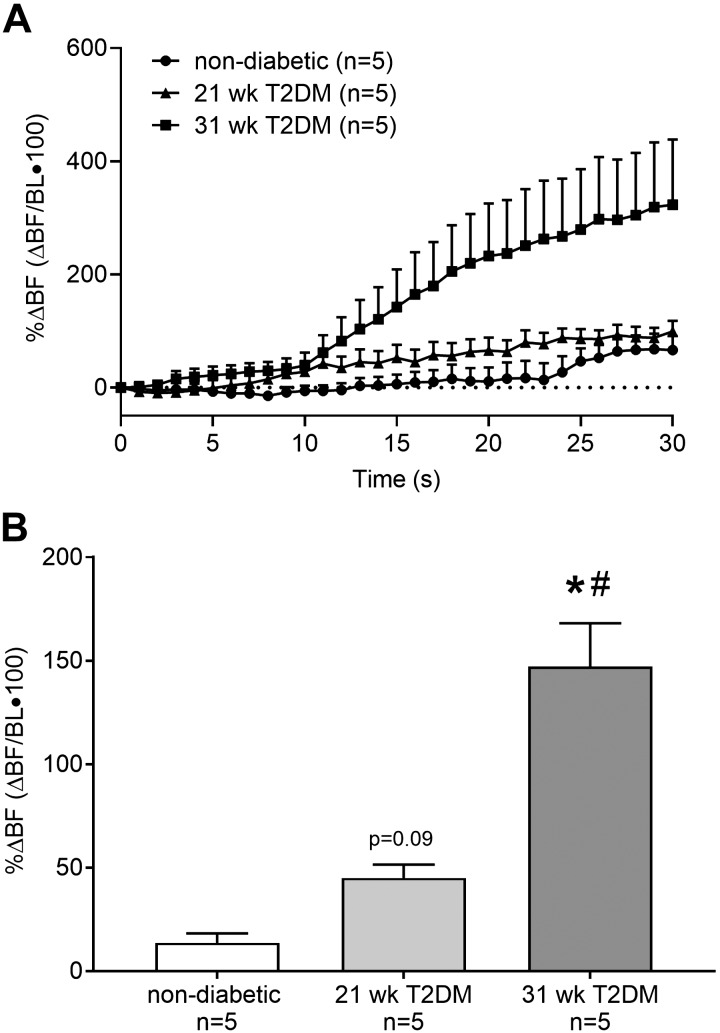
The percent change in BF from baseline during 30 s of contraction is plotted second by second for 21-wk T2DM (n = 5), 31-wk T2DM (n = 5), and nondiabetic (n = 5) rats to show the temporal changes in BF for each group (Fig. 4A). To determine whether BF was, on average, different between T2DM and nondiabetic rats, the data were averaged and then compared. We found that statically contracting the hindlimb muscles evoked a significant increase in mean BF (Fig. 4B) in the 31- but not in the 21-wk T2DM rats compared with nondiabetic rats (P < 0.05). Moreover, the increase in mean BF in the 31-wk T2DM rat was significantly greater than that in the 21-wk T2DM rat (P < 0.05). Likewise, statically contracting the hindlimb muscles evoked a significant increase in vascular conductance in the 31-wk (75 ± 12%) but not in the 21-wk (18 ± 4%, P = 0.4) T2DM rats compared with nondiabetic rats (8 ± 4%); P < 0.05. Moreover, the increase in vascular conductance in the 31-wk T2DM rats was significantly greater than that in the 21-wk T2DM rats (P < 0.05).
Fig. 4.
The percent change in popliteal arterial blood flow (%ΔBF) from baseline (BL) was averaged second by second to show the temporal changes that occur during 30 s of static contraction (A). Percent change in BF was then averaged over the 30 s of contraction to determine whether mean BF was different between type-2 diabetes mellitus (T2DM) rats and nondiabetic rats (B). Mean BF was greater in the 31-wk (n = 5) compared with 21-wk (n = 5) T2DM rats and nondiabetic rats (n = 5), while it was trending toward being greater in the 21-wk T2DM rats compared with nondiabetic rats. *P < 0.05 (1-way ANOVA) indicates statistically greater percent change in blood flow compared with nondiabetic rats. #P < 0.05 (1-way ANOVA) indicate statistically greater percent change in blood flow compared with 21-wk T2DM rats.
DISCUSSION
The primary aim of this study was to determine the effects of T2DM and the role of disease progression on the exercise pressor reflex. We also aimed to determine the role of the mechanoreflex in causing these effects. We found that the expression of the exercise pressor reflex changed with the progression of T2DM. Specifically, 31-wk T2DM rats had an exaggerated exercise pressor reflex compared with 21-wk T2DM and nondiabetic rats. In contrast, the 21-wk T2DM rats did not have an exaggerated exercise pressor reflex compared with nondiabetic rats. A secondary aim was to determine whether the mechanoreflex was exaggerated in the T2DM rats when an exaggerated exercise pressor reflex was present. Consistent with our hypothesis, we found that the mechanoreflex is exaggerated in the 31-wk T2DM rats, suggesting that the mechanoreflex may play a significant role in the exaggerated exercise pressor reflex. We also determined changes in BF to the working skeletal muscle and, surprisingly, found that mean BF to the working muscle was greater in the 31- but not in the 21-wk T2DM rats compared with nondiabetic rats. Collectively, these findings suggest that the expression of the exercise pressor reflex changes as T2DM progresses. Furthermore, a sensitized mechanoreflex may play a role in exaggerating the exercise pressor reflex as T2DM progresses.
The exaggerated exercise pressor reflex found in T2DM rats is similar to findings from previous studies where patients with T2DM had an augmented blood pressure response to exercise (25, 40, 43, 44). However, the underlying mechanisms for this exaggerated response are not fully understood. We do know that patients with T2DM have a higher level of MSNA at rest, with and without the presence of essential hypertension (22). These individuals are also at an increased risk of cardiovascular disease and mortality (4). However, given the well-documented exaggerated blood pressure response to exercise and the elevated MSNA at rest, there are surprisingly few studies investigating potential underlying mechanisms for these alterations. The recent study by Holwerda et al. (20) provided critical insight to potential underlying neurovascular changes. The study found that patients with T2DM had an augmented metaboreflex, suggesting that the metabolic component of the exercise pressor reflex was sensitized and contributes to exaggerated MSNA and pressor response to isometric handgrip exercise. Interestingly, the MSNA response during metaboreflex activation was also correlated with plasma glucose, HbA1c levels, and insulin resistance (HOMA-IR), suggesting that the expression of these responses may change as a result of disease severity (20). The current study supports these findings in that the exercise pressor reflex was exaggerated only after plasma glucose and HbA1c levels were high and sustained for an extended period of time.
Recently, Kim et al. (28) determined that the exercise pressor reflex was exaggerated in high-fat diet, low-dose streptozotocin-induced T2DM rats. Although different T2DM rat models were used, findings from Kim et al. are in agreement with those from the current study. We also expanded these findings by determining that the mechanoreflex was also exaggerated in T2DM. Unlike Kim et al., we stimulated the sciatic nerve to evoke muscle contraction, whereas they stimulated L4 and L5 ventral roots, which could explain the smaller developed tension and pressor responses to static contraction in the current study. Furthermore, we were able to track and follow the development and progression of T2DM. We determined disease onset and related changes in the exercise pressor reflex, at two different time points, to how long the rats were diabetic. We know that T2DM progresses over time, and our findings suggest that the progression of T2DM, and not necessarily the onset or early stages of T2DM, leads to an exaggerated spike in blood pressure during physical activity that could result in adverse cardiovascular events.
We found that the mechanoreflex was exaggerated in T2DM rats, which is consistent with other reports showing an altered mechanoreflex in cardiovascular-related diseases such as hypertension (32), heart failure (23, 46), peripheral artery disease (33), and type-1 diabetes (15). Although we did not measure sympathetic activity in this study, it is likely that the exaggerated pressor and cardioaccelerator responses to tendon stretch are primarily mediated through an increase in sympathetic activity to the heart and vasculature (9, 53). Furthermore, we did not attempt to elucidate any underlying mechanisms contributing to the sensitization of the mechanoreflex. Future studies are warranted to elucidate potential underlying mechanisms to further explain the role of the mechanoreflex and metaboreflex in T2DM.
Group III and IV thin fiber afferents are polymodal sensory afferents that respond to both metabolic and mechanical stimuli produced during muscle contraction. Previous studies have suggested that group III and IV afferents play a significant role in the development of mechanical allodynia (18) as well as the exercise pressor reflex (26). For example, in patients with neuropathic pain, a peripheral nerve block (lidocaine) successfully attenuated pain sensation by attenuating group III and IV thin fiber afferent activity (18). Other studies have shown that DPN, which also affects group III and IV afferents, develops in stages. Similar to the current study’s findings, these symptoms generally worsen as the disease progresses (52). In another study, using a mouse model of T2DM, nerve growth factor, which mediates nociception through group III and IV thin fiber afferents, increased with nerve sensitization and the manifestation of mechanical allodynia (5). Similarly, nerve growth factor has been found to play a role in mediating the exaggerated exercise pressor reflex in peripheral artery disease (33). Both peripheral artery disease and hypertension are prevalent in individuals with T2DM, and this prevalence increases with age and disease severity. Therefore, it may also be reasonable to consider that overlap in the pathology of these diseases may be responsible for the exaggerated cardiovascular responses to exercise in T2DM, although peripheral artery disease was not determined in the current study.
We were also interested in whether the exaggerated exercise pressor reflex seen in T2DM rats was due to a decrease in BF, causing an ischemic state during contraction and potentially stimulating group III and IV muscle afferents. Based on several studies showing an attenuated BF response to exercise in T2DM (29, 31, 34), we expected BF to the contracting muscle to be impaired in T2DM rats compared with healthy nondiabetic rats. Surprisingly, we found that mean BF responses to isometric contraction were greater in the 31-wk rats compared with that in the nondiabetic rats. This finding suggests that impaired macrovascular BF during exercise was not responsible for the exaggerated reflex. However, we then determined whether the pressor response in 31-wk T2DM rats during static contraction was driving the increase in BF. To do this, we calculated vascular conductance and found that the exaggerated pressor response in the 31-wk T2DM rats was not the sole stimulus for increased BF.
Although the BF response in the 31-wk T2DM rats was somewhat surprising, no studies at this time have reported changes in popliteal BF during static contraction in the later stages of T2DM. We can only speculate possible explanations for increased BF, which include impaired vascular tone (37, 48), loss of sympathetic control (48), and impaired vasoconstriction via endothelin 1 (37). Another explanation for these findings is that this study determined changes in BF during muscle contraction in untreated T2DM rats, while most human studies investigating similar effects use treated patients with T2DM. Although our findings were different from those found in human studies, they do align with that of Copp et al. (6), who found a greater BF response, specifically to fast-twitch glycolytic fibers, in T2DM rats compared with healthy controls. Future studies are warranted to determine the mechanisms behind increased BF to the contracting skeletal muscle in T2DM.
The current study measured insulin and leptin concentrations to validate the UCD-T2DM rat model and ensure that these rats developed T2DM as expected. We found that both insulin and leptin concentrations were decreased in both groups of T2DM rats compared with nondiabetic controls. This suggests that the progression of the disease led not only to impaired insulin action but also to β-cell deficiency. Insulin concentrations were measured in nonfasted rats, which could explain some of the variability within these measurements. These findings are similar to those shown in the study by Cummings et al. (7), where circulating insulin levels increased at the onset of diabetes but decreased as the disease progressed. Leptin production, which is positively regulated by insulin-mediated glucose metabolism in adipocytes (19), was decreased along with insulin in the T2DM rats compared with nondiabetic control rats. This finding is similar to that reported by Cummings et al. (7) where leptin concentrations were normal before rats became diabetic and then decreased over time as rats progressed with the disease.
It should be noted that small sample sizes were used in this study. The current study was limited in the number of rats that reached 21 and 31 wk following the onset of T2DM. Although the pressor responses to static contraction in the 31-wk T2DM rats were largely greater than that in both the nondiabetic and 21-wk T2DM rats, it is important to note that a larger sample size in the 21-wk T2DM rats may have resulted in a statistically greater pressor response to static contraction than that in nondiabetic rats. However, with the assumption that the mean pressor response would be similar, 21-wk T2DM rats would still only have ~6 mmHg greater response to contraction than the nondiabetic rats. More importantly, even if greater responses were found, the exaggerated pressor response evoked in the 31-wk T2DM rats (~39 mmHg) would still likely be substantially greater than the response seen in the 21-wk T2DM rats. Therefore it is not likely that a larger sample size would better emphasize the temporal effects of T2DM on the exercise pressor reflex as it progresses over time.
We conclude that the expression of the exercise pressor reflex changes with the progression of T2DM. Although HbA1c measurements were not different between groups of T2DM rats, findings from this study suggest that the duration of the disease (21 vs. 31 wk) played a role in exaggerating the reflexive cardiovascular responses to exercise. Furthermore, this alteration is likely due, in part, to a sensitized mechanoreflex, as evidenced by the exaggerated pressor and cardioaccelerator responses to tendon stretch in the same group of rats that had an exaggerated exercise pressor reflex. Contrary to our hypothesis, we did not observe impaired BF to the contracting skeletal muscle in T2DM rats, suggesting that the exaggerated pressor responses to muscle contraction were not due to the lack of macrovascular perfusion. These findings strongly suggest that the exercise pressor reflex is indeed partly responsible for evoking the exaggerated blood pressure responses seen in T2DM, and the mechanoreflex may be an important mediator of this response. Unlike studies performed in humans, the current study was able to identify the time of disease onset and isolate the exercise pressor reflex in the absence of central command. Moreover, these findings are clinically relevant as they provide new insight into neural mechanisms that contribute to the adverse cardiovascular responses in T2DM to physical activity.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-144723.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.J.S. conceived and designed research; A.-K.G., C.K.C., Y.H., K.M.Y., M.L.H., and A.J.S. performed experiments; A.-K.G., C.K.C., M.L.H., and A.J.S. analyzed data; A.-K.G., C.K.C., Y.H., M.L.H., P.J.H., P.J.F., and A.J.S. interpreted results of experiments; A.-K.G. and A.J.S. prepared figures; A.-K.G. and A.J.S. drafted manuscript; A.-K.G., C.K.C., Y.H., K.M.Y., M.L.H., P.J.H., P.J.F., and A.J.S. edited and revised manuscript; A.-K.G., C.K.C., Y.H., K.M.Y., M.L.H., J.L.G., K.L.S., P.J.H., P.J.F., and A.J.S. approved final version of manuscript.
REFERENCES
- 1.Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89: 372–383, 1937. doi: 10.1113/jphysiol.1937.sp003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoki K, Sato K, Kondo S, Pyon CB, Yamamoto M. Increased response of blood pressure to rest and handgrip in subjects with essential hypertension. Jpn Circ J 47: 802–809, 1983. doi: 10.1253/jcj.47.802. [DOI] [PubMed] [Google Scholar]
- 3.Baccelli G, Reggiani P, Mattioli A, Corbellini E, Garducci S, Catalano M. The exercise pressor reflex and changes in radial arterial pressure and heart rate during walking in patients with arteriosclerosis obliterans. Angiology 50: 361–374, 1999. doi: 10.1177/000331979905000502. [DOI] [PubMed] [Google Scholar]
- 4.Barretto AC, Santos AC, Munhoz R, Rondon MU, Franco FG, Trombetta IC, Roveda F, de Matos LN, Braga AM, Middlekauff HR, Negrão CE. Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Int J Cardiol 135: 302–307, 2009. doi: 10.1016/j.ijcard.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 5.Cheng HT, Dauch JR, Hayes JM, Hong Y, Feldman EL. Nerve growth factor mediates mechanical allodynia in a mouse model of type 2 diabetes. J Neuropathol Exp Neurol 68: 1229–1243, 2009. doi: 10.1097/NEN.0b013e3181bef710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Copp SW, Hageman KS, Behnke BJ, Poole DC, Musch TI. Effects of type II diabetes on exercising skeletal muscle blood flow in the rat. J Appl Physiol (1985) 109: 1347–1353, 2010. doi: 10.1152/japplphysiol.00668.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummings BP, Digitale EK, Stanhope KL, Graham JL, Baskin DG, Reed BJ, Sweet IR, Griffen SC, Havel PJ. Development and characterization of a novel rat model of type 2 diabetes mellitus: the UC Davis type 2 diabetes mellitus UCD-T2DM rat. Am J Physiol Regul Integr Comp Physiol 295: R1782–R1793, 2008. doi: 10.1152/ajpregu.90635.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Moura-Tonello SC, Porta A, Marchi A, de Almeida Fagundes A, Francisco CO, Rehder-Santos P, Milan-Mattos JC, Simões RP, Gois MO, Catai AM. Cardiovascular Variability Analysis and Baroreflex Estimation in Patients with Type 2 Diabetes in Absence of Any Manifest Neuropathy. PLoS One 11: e0148903, 2016. doi: 10.1371/journal.pone.0148903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drew RC. Baroreflex and neurovascular responses to skeletal muscle mechanoreflex activation in humans: an exercise in integrative physiology. Am J Physiol Regul Integr Comp Physiol 313: R654–R659, 2017. doi: 10.1152/ajpregu.00242.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eldridge FL, Millhorn DE, Kiley JP, Waldrop TG. Stimulation by central command of locomotion, respiration and circulation during exercise. Respir Physiol 59: 313–337, 1985. doi: 10.1016/0034-5687(85)90136-7. [DOI] [PubMed] [Google Scholar]
- 11.Fisher JP, Young CN, Fadel PJ. Autonomic adjustments to exercise in humans. Compr Physiol 5: 475–512, 2015. doi: 10.1002/cphy.c140022. [DOI] [PubMed] [Google Scholar]
- 12.Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care 32, Suppl 2: S151–S156, 2009. doi: 10.2337/dc09-S301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freund PR, Rowell LB, Murphy TM, Hobbs SF, Butler SH. Blockade of the pressor response to muscle ischemia by sensory nerve block in man. Am J Physiol 237: H433–H439, 1979. doi: 10.1152/ajpheart.1979.237.4.H433. [DOI] [PubMed] [Google Scholar]
- 14.Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol 226: 173–190, 1972. doi: 10.1113/jphysiol.1972.sp009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grotle AK, Garcia EA, Harrison ML, Huo Y, Crawford CK, Ybarbo KM, Stone AJ. Exaggerated mechanoreflex in early-stage type 1 diabetic rats: role of Piezo channels. Am J Physiol Regul Integr Comp Physiol 316: R417–R426, 2019. doi: 10.1152/ajpregu.00294.2018. [DOI] [PubMed] [Google Scholar]
- 16.Grotle AK, Garcia EA, Huo Y, Stone AJ. Temporal changes in the exercise pressor reflex in type 1 diabetic rats. Am J Physiol Heart Circ Physiol 313: H708–H714, 2017. doi: 10.1152/ajpheart.00399.2017. [DOI] [PubMed] [Google Scholar]
- 17.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 339: 229–234, 1998. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 18.Haroutounian S, Nikolajsen L, Bendtsen TF, Finnerup NB, Kristensen AD, Hasselstrøm JB, Jensen TS. Primary afferent input critical for maintaining spontaneous pain in peripheral neuropathy. Pain 155: 1272–1279, 2014. doi: 10.1016/j.pain.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 19.Havel PJ. Update on adipocyte hormones: regulation of energy balance and carbohydrate/lipid metabolism. Diabetes 53, Suppl 1: S143–S151, 2004. doi: 10.2337/diabetes.53.2007.S143. [DOI] [PubMed] [Google Scholar]
- 20.Holwerda SW, Restaino RM, Manrique C, Lastra G, Fisher JP, Fadel PJ. Augmented pressor and sympathetic responses to skeletal muscle metaboreflex activation in type 2 diabetes patients. Am J Physiol Heart Circ Physiol 310: H300–H309, 2016. doi: 10.1152/ajpheart.00636.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holwerda SW, Vianna LC, Restaino RM, Chaudhary K, Young CN, Fadel PJ. Arterial baroreflex control of sympathetic nerve activity and heart rate in patients with type 2 diabetes. Am J Physiol Heart Circ Physiol 311: H1170–H1179, 2016. doi: 10.1152/ajpheart.00384.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huggett RJ, Scott EM, Gilbey SG, Stoker JB, Mackintosh AF, Mary DA. Impact of type 2 diabetes mellitus on sympathetic neural mechanisms in hypertension. Circulation 108: 3097–3101, 2003. doi: 10.1161/01.CIR.0000103123.66264.FE. [DOI] [PubMed] [Google Scholar]
- 23.Ives SJ, Amann M, Venturelli M, Witman MA, Groot HJ, Wray DW, Morgan DE, Stehlik J, Richardson RS. The Mechanoreflex and Hemodynamic Response to Passive Leg Movement in Heart Failure. Med Sci Sports Exerc 48: 368–376, 2016. doi: 10.1249/MSS.0000000000000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joyner MJ. Baroreceptor function during exercise: resetting the record. Exp Physiol 91: 27–36, 2006. doi: 10.1113/expphysiol.2005.032102. [DOI] [PubMed] [Google Scholar]
- 25.Karavelioglu Y, Karapinar H, Gul İ, Kucukdurmaz Z, Yilmaz A, Akpek M, Kaya MG. Blood pressure response to exercise is exaggerated in normotensive diabetic patients. Blood Press 22: 21–26, 2013. doi: 10.3109/08037051.2012.701045. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- 27.Khan GM, Chen SR, Pan HL. Role of primary afferent nerves in allodynia caused by diabetic neuropathy in rats. Neuroscience 114: 291–299, 2002. doi: 10.1016/S0306-4522(02)00372-X. [DOI] [PubMed] [Google Scholar]
- 28.Kim HK, Hotta N, Ishizawa R, Iwamoto GA, Vongpatanasin W, Mitchell JH, Smith SA, Mizuno M. Exaggerated pressor and sympathetic responses to stimulation of the mesencephalic locomotor region and exercise pressor reflex in type II diabetic rats. Am J Physiol Regul Integr Comp Physiol 317: R270–R279, 2019. doi: 10.1152/ajpregu.00061.2019. [DOI] [PubMed] [Google Scholar]
- 29.Kingwell BA, Formosa M, Muhlmann M, Bradley SJ, McConell GK. Type 2 diabetic individuals have impaired leg blood flow responses to exercise: role of endothelium-dependent vasodilation. Diabetes Care 26: 899–904, 2003. doi: 10.2337/diacare.26.3.899. [DOI] [PubMed] [Google Scholar]
- 30.Kleinert M, Clemmensen C, Hofmann SM, Moore MC, Renner S, Woods SC, Huypens P, Beckers J, de Angelis MH, Schürmann A, Bakhti M, Klingenspor M, Heiman M, Cherrington AD, Ristow M, Lickert H, Wolf E, Havel PJ, Müller TD, Tschöp MH. Animal models of obesity and diabetes mellitus. Nat Rev Endocrinol 14: 140–162, 2018. doi: 10.1038/nrendo.2017.161. [DOI] [PubMed] [Google Scholar]
- 31.Lalande S, Gusso S, Hofman PL, Baldi JC. Reduced leg blood flow during submaximal exercise in type 2 diabetes. Med Sci Sports Exerc 40: 612–617, 2008. doi: 10.1249/MSS.0b013e318161aa99. [DOI] [PubMed] [Google Scholar]
- 32.Leal AK, Williams MA, Garry MG, Mitchell JH, Smith SA. Evidence for functional alterations in the skeletal muscle mechanoreflex and metaboreflex in hypertensive rats. Am J Physiol Heart Circ Physiol 295: H1429–H1438, 2008. doi: 10.1152/ajpheart.01365.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu J, Xing J, Li J. Role for NGF in augmented sympathetic nerve response to activation of mechanically and metabolically sensitive muscle afferents in rats with femoral artery occlusion. J Appl Physiol (1985) 113: 1311–1322, 2012. doi: 10.1152/japplphysiol.00617.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacAnaney O, Reilly H, O’Shea D, Egaña M, Green S. Effect of type 2 diabetes on the dynamic response characteristics of leg vascular conductance during exercise. Diab Vasc Dis Res 8: 12–21, 2011. doi: 10.1177/1479164110389625. [DOI] [PubMed] [Google Scholar]
- 35.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Negrão CE, Rondon MU, Tinucci T, Alves MJ, Roveda F, Braga AM, Reis SF, Nastari L, Barretto AC, Krieger EM, Middlekauff HR. Abnormal neurovascular control during exercise is linked to heart failure severity. Am J Physiol Heart Circ Physiol 280: H1286–H1292, 2001. doi: 10.1152/ajpheart.2001.280.3.H1286. [DOI] [PubMed] [Google Scholar]
- 37.Nugent AG, McGurk C, Hayes JR, Johnston GD. Impaired vasoconstriction to endothelin 1 in patients with NIDDM. Diabetes 45: 105–107, 1996. doi: 10.2337/diab.45.1.105. [DOI] [PubMed] [Google Scholar]
- 38.O’Connor E, Green S, Kiely C, O’Shea D, Egaña M. Differential effects of age and type 2 diabetes on dynamic vs. peak response of pulmonary oxygen uptake during exercise. J Appl Physiol (1985) 118: 1031–1039, 2015. doi: 10.1152/japplphysiol.01040.2014. [DOI] [PubMed] [Google Scholar]
- 39.Ørstavik K, Namer B, Schmidt R, Schmelz M, Hilliges M, Weidner C, Carr RW, Handwerker H, Jørum E, Torebjörk HE. Abnormal function of C-fibers in patients with diabetic neuropathy. J Neurosci 26: 11287–11294, 2006. doi: 10.1523/JNEUROSCI.2659-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petrofsky JS, Stewart B, Patterson C, Cole M, Al Malty A, Lee S. Cardiovascular responses and endurance during isometric exercise in patients with Type 2 diabetes compared to control subjects. Med Sci Monit 11: CR470–CR477, 2005. [PubMed] [Google Scholar]
- 41.Pinto TE, Gusso S, Hofman PL, Derraik JG, Hornung TS, Cutfield WS, Baldi JC. Systolic and diastolic abnormalities reduce the cardiac response to exercise in adolescents with type 2 diabetes. Diabetes Care 37: 1439–1446, 2014. doi: 10.2337/dc13-2031. [DOI] [PubMed] [Google Scholar]
- 42.Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, Sosenko JM, Ziegler D. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care 40: 136–154, 2017. doi: 10.2337/dc16-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Regensteiner JG, Bauer TA, Reusch JE, Quaife RA, Chen MY, Smith SC, Miller TM, Groves BM, Wolfel EE. Cardiac dysfunction during exercise in uncomplicated type 2 diabetes. Med Sci Sports Exerc 41: 977–984, 2009. doi: 10.1249/MSS.0b013e3181942051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scott JA, Coombes JS, Prins JB, Leano RL, Marwick TH, Sharman JE. Patients with type 2 diabetes have exaggerated brachial and central exercise blood pressure: relation to left ventricular relative wall thickness. Am J Hypertens 21: 715–721, 2008. doi: 10.1038/ajh.2008.166. [DOI] [PubMed] [Google Scholar]
- 45.Smith SA, Mitchell JH, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol 537: 961–970, 2001. doi: 10.1113/jphysiol.2001.012918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith SA, Mitchell JH, Naseem RH, Garry MG. Mechanoreflex mediates the exaggerated exercise pressor reflex in heart failure. Circulation 112: 2293–2300, 2005. doi: 10.1161/CIRCULATIONAHA.105.566745. [DOI] [PubMed] [Google Scholar]
- 47.Stone AJ, Kaufman MP. The exercise pressor reflex and peripheral artery disease. Auton Neurosci 188: 69–73, 2015. doi: 10.1016/j.autneu.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takahashi T, Nishizawa Y, Emoto M, Kawagishi T, Matsumoto N, Ishimura E, Inaba M, Okuno Y, Shimada H, Morii H. Sympathetic function test of vasoconstrictor changes in foot arteries in diabetic patients. Diabetes Care 21: 1495–1501, 1998. doi: 10.2337/diacare.21.9.1495. [DOI] [PubMed] [Google Scholar]
- 49.Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab Res Rev 28, Suppl 1: 8–14, 2012. doi: 10.1002/dmrr.2239. [DOI] [PubMed] [Google Scholar]
- 50.Wang HJ, Li YL, Gao L, Zucker IH, Wang W. Alteration in skeletal muscle afferents in rats with chronic heart failure. J Physiol 588: 5033–5047, 2010. doi: 10.1113/jphysiol.2010.199562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei M, Gaskill SP, Haffner SM, Stern MP. Effects of diabetes and level of glycemia on all-cause and cardiovascular mortality. The San Antonio Heart Study. Diabetes Care 21: 1167–1172, 1998. doi: 10.2337/diacare.21.7.1167. [DOI] [PubMed] [Google Scholar]
- 52.Yagihashi S. Pathology and pathogenetic mechanisms of diabetic neuropathy. Diabetes Metab Rev 11: 193–225, 1995. doi: 10.1002/dmr.5610110304. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto K, Kawada T, Kamiya A, Takaki H, Sugimachi M, Sunagawa K. Static interaction between muscle mechanoreflex and arterial baroreflex in determining efferent sympathetic nerve activity. Am J Physiol Heart Circ Physiol 289: H1604–H1609, 2005. doi: 10.1152/ajpheart.00053.2005. [DOI] [PubMed] [Google Scholar]
- 54.Yamauchi K, Kim JS, Stone AJ, Ruiz-Velasco V, Kaufman MP. Endoperoxide 4 receptors play a role in evoking the exercise pressor reflex in rats with simulated peripheral artery disease. J Physiol 591: 2949–2962, 2013. doi: 10.1113/jphysiol.2012.247973. [DOI] [PMC free article] [PubMed] [Google Scholar]