Abstract
Intracoronary cardiosphere-derived cells (icCDCs) infused into the infarct-related artery reduce scar volume but do not improve left ventricular (LV) ejection fraction (LVEF). We tested the hypothesis that this reflects the inability of regional delivery to prevent myocyte death or promote myocyte proliferation in viable myocardium remote from the infarct. Swine (n = 23) pretreated with oral cyclosporine (200 mg/day) underwent a 1-h left anterior descending coronary artery (LAD) occlusion, which reduced LVEF from 61.6 ± 1.0 to 45.3 ± 1.5% 30 min after reperfusion. At that time, animals received global infusion of allogeneic icCDCs (n = 8), regional infusion of icCDCs restricted to the LAD using the stop-flow technique (n = 8), or vehicle (n = 7). After 1 mo, global icCDCs increased LVEF from 44.8 ± 1.9 to 60.8 ± 3.8% (P < 0.05) with no significant change after LAD stop-flow icCDCs (44.8 ± 3.6 to 50.9 ± 3.1%) or vehicle (46.5 ± 2.5 to 47.7 ± 2.6%). In contrast, global icCDCs did not alter infarct volume (%LV mass) assessed at 2 days (11.2 ± 2.3 vs. 12.6 ± 2.3%), whereas it was reduced after LAD stop-flow icCDCs (7.1 ± 1.1%, P < 0.05). Histopathological analysis of remote myocardium after global icCDCs demonstrated a significant increase in myocyte proliferation (147 ± 32 vs. 14 ± 10 nuclei/106 myocytes, P < 0.05) and a reduction in myocyte apoptosis (15 ± 9 vs. 46 ± 10 nuclei/106 myocytes, P < 0.05) that increased myocyte nuclear density (1,264 ± 39 vs. 1,157 ± 33 nuclei/mm2, P < 0.05) and decreased myocyte diameter (13.2 ± 0.2 vs. 14.5 ± 0.3 μm, P < 0.05) compared with vehicle-treated controls. In contrast, remote zone changes after regional LAD icCDCs were no different from vehicle. These data indicate that changes in global LVEF after icCDCs are dependent upon preventing myocyte loss and hypertrophy in myocardium remote from the infarct. These arise from stimulating myocyte proliferation and reducing myocyte apoptosis indicating the importance of directing cell therapy to viable remote regions.
NEW & NOTEWORTHY Administration of allogeneic cardiosphere-derived cells to the entire heart via global intracoronary infusion shortly after myocardial infarction favorably influenced left ventricular ejection fraction by preventing myocyte death and promoting myocyte proliferation in remote, noninfarcted myocardium in swine. In contrast, regional intracoronary cell infusion did not significantly affect remote zone myocyte remodeling. Global cell administration targeting viable myocardium remote from the infarct may be an effective approach to prevent adverse ventricular remodeling after myocardial infarction.
Keywords: acute myocardial infarction, cardiac regeneration, cardiosphere-derived cells, cell-based therapy, ischemic heart disease
INTRODUCTION
Cell-based regenerative therapy has emerged as a promising approach to repair the failing heart by stimulating myocyte proliferation and improving left ventricular (LV) function (10, 14, 31). Clinical trials administering intracoronary cardiosphere-derived cells (icCDCs) into the infarct-related artery using the stop-flow technique have shown beneficial effects on improving regional function and reducing scar volume (21). Nevertheless, although cardiosphere-derived cells (CDCs) attenuated the post-infarction decline in LV ejection fraction (EF) as compared with untreated controls, they did not produce absolute improvements in global systolic function (21). One possible explanation for this may be the fact that, like nearly all cell-based therapeutic approaches, treatment has been directed at replacing infarct scar with myocytes as opposed to preventing myocyte loss from chronic remodeling of viable myocardium remote from the infarct (27).
Previous studies have demonstrated a progressive deterioration in LV function following large infarctions that is associated with chronic myocyte loss throughout the heart due to apoptosis (2, 27). Over time, the total number of myocytes lost from remote zone remodeling approaches that which occurs during the acute ischemic event (1, 26). This is accompanied by myocyte cellular hypertrophy, LV dilatation, and a decline in overall EF that presage the development of heart failure. Since rapid reperfusion with contemporary cardiovascular care has substantially reduced myocardial infarct size, the average EF in patients presenting with a first ST elevation myocardial infarction (MI) now averages ~50% and is only mildly depressed below normal. Whether remote zone remodeling with myocyte loss and cellular hypertrophy impacts LV dysfunction in this large patient population remains uncertain, as they have been excluded from clinical trials including those of cell-based therapy. Likewise, there is little objective data quantifying remote zone myocyte loss after cell therapy in large animal models of MI.
We previously demonstrated that global intracoronary infusion of autologous and allogeneic mesenchymal stem cells (MSCs) and CDCs could stimulate endogenous myocyte proliferation in ischemic as well as normally perfused remote regions of the heart in swine with chronic hibernating myocardium in the absence of infarction (36). Importantly, these favorable changes occurred in a circumstance in which LVEF was relatively preserved. We conducted the present study to determine if a similar global delivery approach could be implemented at the time of reperfusion to prevent post-infarction remodeling and improve global LVEF by promoting myocyte proliferation and/or attenuating apoptotic myocyte loss in remote, noninfarcted myocardium. We employed blinded methodology to study closed-chest swine with a relatively modest acute infarct (10–15% of LV mass) and an initial depression in LVEF that is similar to what is encountered in unselected humans with primary percutaneous coronary intervention for ST-segment elevation MI (EF ~45%). Since it would not be clinically feasible to have autologous cells available for patients, we evaluated allogeneic icCDCs that could be stored for administration at the time of study. Animals were pretreated with oral cyclosporine to attenuate allogeneic CDC rejection and were randomized to receive global icCDCs versus vehicle administered 30 min after reperfusion. They were compared with a third group of swine receiving icCDCs administered only into the infarct region using the widely employed stop-flow technique (17). Our results demonstrate that a one-time global intracoronary infusion of allogeneic CDCs administered shortly after reperfusion favorably influences global LV function through a combination of cytoprotective and regenerative mechanisms that increase myocyte number and prevent myocyte cellular hypertrophy in viable myocardium remote from the site of infarction.
METHODS
All procedures and protocols conformed to institutional guidelines for the care and use of animals in research and were approved by the University at Buffalo Institutional Animal Care and Use Committee. In the experiments evaluating global administration of CDCs, all personnel involved in data collection and analysis [including echocardiography, multidetector computed tomography (MDCT) imaging, and histopathological measurements] were blinded to the treatment status of each animal. The nature of using regional administration using the stop-flow technique precluded complete blinding (including imaging) of this subset of experiments. Sample size was empirically determined based upon our prior experience with the pig model. Only female swine with male donors for CDCs were employed in the studies to use sex-mismatched approaches to assess CDC fate. A power analysis was not prospectively performed.
Experimental design.
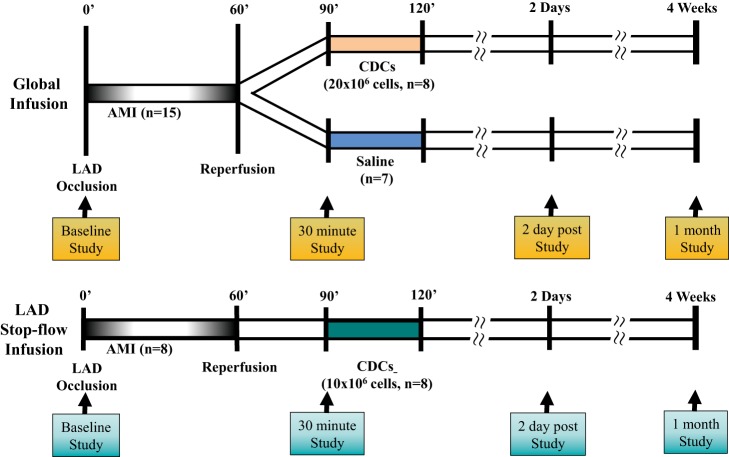
Female Yorkshire swine (n = 28, 36.2 ± 0.6 kg) were studied in the closed-chest state using the protocols summarized in Fig. 1 and described in detail below. All animals were treated with cyclosporine A beginning 3 days before the initial study and continuing throughout terminal experiments (200 mg/day orally; Watson Pharma) as we have used in prior studies of allogeneic cell therapy (36, 39). Acute, reperfused MI was induced using a percutaneous, closed-chest procedure based on previously described methodology (38). Following initial sedation with a Telazol (100 mg/ml)-xylazine (100 mg/ml) mixture (0.04 ml/kg im), each animal was brought to the laboratory and maintained on a continuous infusion of propofol (5–10 mg·kg−1·h−1) through an 18-gauge angiocath placed in an ear vein. Animals were intubated and mechanically ventilated with oxygen at a respiratory rate of ~15 breaths/min throughout the study. A 5-Fr Millar Mikro-Tip pressure catheter was inserted into the LV apex for continuous pressure measurement. After equilibration, hemodynamic measurements were performed and LV function was assessed via transthoracic 2-D echocardiography (GE Vivid 7) using a right parasternal approach and quantified using American Society of Echocardiography criteria as previously described (25). Regional wall thickening was determined manually from off axis M mode imaging with a cursor placed in the left anterior descending coronary artery (LAD) and remote regions. A 6-Fr guiding catheter (Cordis, H-Stick) was placed in the left coronary artery through which an appropriately sized balloon angioplasty catheter (Boston Scientific, Maverick, 2.5–3.0 mm × 15 mm) was advanced into the LAD. The balloon was inflated distal to the second diagonal branch for 60 min, and angiography was used to confirm complete cessation of flow during occlusion as well as full reperfusion after balloon deflation. To minimize the likelihood of ventricular fibrillation, all animals were pretreated with amiodarone (5 mg/kg bolus and 0.04 mg·kg−1·min−1 iv) and lidocaine (1.5 mg/kg bolus and 0.05 mg·kg−1·min−1 iv). Both drugs were infused throughout coronary occlusion and 10 min into the reperfusion period. If ventricular fibrillation developed, biphasic defibrillation was performed.
Fig. 1.
Experimental protocol. Following baseline measurements, swine were subjected to a 60-min occlusion of the left anterior descending coronary artery (LAD) in the closed-chest state. In global infusion of CDCs (top), animals were randomized to receive either allogeneic CDCs (total amount of 20 × 106 with ~7 × 106 in each of the 3 major coronary arteries) or saline infused into each of the 3 coronary arteries, with study personnel blinded to all aspects of the protocol. In stop-flow regional infusion of CDCs (bottom), animals received allogeneic CDCs (total amount of 10 × 106) in the LAD region during the inflation of an over-the-wire balloon catheter. At 2 days and 1 mo after reperfusion, multidetector computed tomography imaging of infarct size, echocardiography, and hemodynamic analysis were repeated. Hearts were excised for postmortem assessment of infarct size, myocyte morphometry, myocyte proliferation, and myocyte apoptosis. See text for additional details. AMI, acute myocardial infarction; CDCs, cardiosphere-derived cells.
Thirty minutes after reperfusion, hemodynamics and echocardiographic evaluation were repeated. In the initial protocol, animals were randomized to receive either saline or allogeneic icCDCs administered via intracoronary infusion in a blinded fashion (35, 39). Animals randomized to global icCDCs received a total of 20 × 106 CDCs suspended in 30 ml saline with 100 U/ml of heparin. This dose was chosen to provide approximately the same number of CDCs/g of myocardial tissue as in our prior studies of global CDCs in hibernating myocardium in somewhat larger swine. Approximately 10 ml of the icCDC suspension or vehicle was infused into each of the 3 major coronary arteries (~7 × 106 CDCs per vessel) at a rate of 1 ml/min without occluding coronary flow (39). In a third group, we restricted infusion of icCDCs to the infarct-related artery using the stop-flow technique (17). An over-the-wire balloon catheter was advanced through a 6-Fr guiding into the mid-LAD. Allogeneic CDCs (~10 × 106) were administered through the balloon lumen during brief occlusion. The latter dose was selected to compare with prior results based on the upper dose limit used in a previous dose-escalating study of CDCs in acute MI by Kanazawa et al. (16). In these experiments, the balloon was inflated for 3 min by use of low pressure to stop coronary flow and repeated in three cycles. During the inflation, one-third of the CDC solution was infused distally through the central port of the catheter. Between each inflation, the balloon was deflated for 3 min to allow for reperfusion.
After completing the infusion protocols, catheters were removed, hemostasis was achieved, and animals were returned to the animal facility. Hemodynamic measurements, echocardiography, and MDCT imaging to assess changes in infarct size over time were repeated at 2 days and 1 mo. After the final study, animals were deeply anesthetized (isoflurane, 4–5%) and euthanized with a potassium chloride bolus. Hearts were rapidly excised and rinsed with saline, and the left ventricle was weighed and sectioned for postmortem pathological analyses.
Allogeneic cardiosphere-derived cell preparation.
Myocardial tissue specimens were collected from a healthy male pig via needle biopsy of the LV free wall, and CDCs were isolated as previously described (34, 36, 39). Briefly, tissue fragments were plated on culture dishes coated with fibronectin. After 1 wk, the cells surrounding the explants were harvested by gentle enzymatic digestion and plated on ultra-low attachment dishes to form cardiospheres. After 4–6 days in culture, the cardiospheres were harvested and plated on fibronectin-coated plates to form a monolayer of CDCs. After 1–3 passages, CDCs were isolated and stored at −80°C. Seven days before injection, they were thawed and cultured for an additional 1–3 passages until sufficient numbers were available. Approximately 20 × 106 allogeneic CDCs (98% viability) were prepared for injection.
Cardiac MDCT imaging of infarct size.
Serial contrast-enhanced MDCT imaging was performed with a 64-slice MDCT scanner (Discovery PET/CT 690 VCT, GE Healthcare) to assess infarct volume 2 days and 1 mo post-MI. After scout acquisition to localize the heart, arterial phase ECG-gated computed tomography (CT) was performed during intravenous administration of iohexol (Omnipaque, 350 mg I/ml, 2 ml/kg at 4 ml/s) via a femoral vein, followed by delayed-contrast CT 5 min later. First-pass image acquisitions were reconstructed in 10% phases from 5 to 95% throughout the entire R-R interval and reformatted at 6-mm slice thickness in the short axis. Delayed-contrast CT images were reconstructed at end-diastole (95% of the R-R interval) and reformatted to contiguous, 8-mm thick short-axis slices for manual measurement of infarct size by 2 independent blinded investigators using ImageJ software (National Institutes of Health). The following parameters were used for each scan: gantry rotation time 400 ms, temporal resolution 175 ms, slice thickness 0.625 mm, spatial resolution 0.97 × 0.97 mm (voxel size 0.97 × 0.97 × 0.625 mm), helical pitch variable depending on heart rate (range 0.20–0.26), tube voltage 120 kV, tube current 600 mA. Infarct size was quantified as absolute infarct volume (g) and as a percentage of LV mass at each time point (%). The entire LV was analyzed in all cases, with an average of 11 ± 1 slices evaluated in animals studied at 48 h and 12 ± 1 slices at 4 wk to account for increases in LV mass over time.
Myocardial histopathology.
Postmortem histopathological analyses were performed as previously described (36, 39). Immediately following euthanasia, hearts were excised, rinsed with saline, weighed, and sectioned into alternating thin (0.3 cm) and thick (1 cm) rings parallel to the atrioventricular groove. The thin concentric rings were incubated in 1% 2,3,5-triphenyltetrazolium chloride (TTC) for 30 min for assessment of myocardial infarct size. A 1-cm midventricular ring was analyzed for histopathology. Samples corresponding to the infarct border (grossly normal myocardium immediately adjacent to infarct) and normal remote region (adjacent to the posterior descending artery) were fixed in formalin and paraffin-embedded for histopathologic analyses. Connective tissue was quantified using Masson Trichrome-stained sections (5 μm) using ImageJ (National Institutes of Health). Periodic acid-Schiff glycogen-stained sections (5 μm) were used to quantify myocyte diameter and myocyte nuclear density in each region as previously described (36, 39). Paraffin-fixed tissue sections from each region were incubated with Factor VIII-related antigen for assessment of capillary density (Biocare Medical). Samples were post-treated with Alexa Fluor 488-conjugated anti-mouse antibody (Invitrogen). Nuclei were stained with DAPI. Image acquisition was performed with a Zeiss Axio Imager fluorescence microscope at ×200 magnification. The number of capillaries were quantified by ImageJ software using the analyze particle feature. Ten random fields were selected, and data were expressed as capillary number per tissue area (mm2) (35, 39).
Quantification of myocyte proliferation and apoptosis.
Myocyte proliferation was quantified with Ki67 and mitosis with phospho-Histone H3 (pHH3) as we have previously described (36, 39). Briefly, paraffin-fixed tissue sections (5 μm) were incubated with either anti-Ki67 (mouse monoclonal antibody, clone MIB-1, Dako) or anti-pHH3 (rabbit polyclonal antibody, Upstate Biotech). Myocyte nuclei were identified with cardiac troponin I (cTnI; Santa Cruz, 1:100) and DAPI (Vectashield). Myocyte apoptosis was quantified using the In Situ Cell Death Detection Kit, Fluorescein (Roche) according to manufacturer’s guidelines. Apoptotic cells were detected by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) and epifluorescence with an FITC filter. Samples were costained with cTnI antibody, and colocalization of TUNEL+ and cTnI+ signals was used to quantify apoptotic cardiomyocytes. TUNEL+ nuclei that could not be definitively confirmed to be of myocyte origin were excluded. Images were acquired with a laser confocal microscope (Zeiss LSM 700) and Axio Imager equipped with ApoTome (Zeiss). We evaluated an average of 111 ± 4 fields at ×200 magnification in each histological section. The numbers of proliferating (Ki67 and pHH3) and apoptotic (TUNEL) myocytes were expressed as the number of positive cells per million myocytes as previously described (5).
Quantification of donor-derived cells.
Cells arising from allogeneic male donor-derived CDCs were quantified in tissue samples from CDC-treated female swine (stop-flow CDCs, n = 6; global CDCs, n = 8) using a real-time PCR-based approach adapted from previously published protocols (12). Genomic DNA was prepared from male porcine CDCs, allogeneic CDC-treated porcine female heart tissue, and untreated porcine female heart tissue (Qiagen). DNA samples were quantified using a NanoDrop 2000 spectrophotometer. For the detection of allogeneic CDCs, PCR primers were designed against porcine Y-chromosome-specific SRY (forward 5′ CTCACAGCCCATGAACATAACC 3′, reverse 5′ GAAAGTCCCGGCTGTAAACC 3′). A standard curve for CDC quantification was generated by serially diluting male CDC genomic DNA from 60,000 to 60 pg, where SRY was the target. All standard curve reactions contained a background of 100 ng untreated female heart genomic DNA. DNA (100 ng) from allogeneic CDC-treated heart tissue was run per PCR reaction. Five reactions were run per sample for SRY detection. Porcine GPI (glucose-6-phosphate isomerase, forward 5′ ATTCAGGACGTTCAACTCAATAGG 3′, reverse 5′ GGGACTATGACTGTCAGGTAAGG 3′) served as a positive control for the presence of porcine DNA. Targets were amplified using SsoFast EvaGreen Supermix (Bio-Rad) and CFX Real-Time PCR Detection System (Bio-Rad). Data were processed using Bio-Rad CFX Manager software and included melt-curve analysis for the verification of single PCR products for each reaction.
Statistical analysis.
Data are expressed as mean ± standard error. Differences between treatment groups in serial physiological measurements were assessed by two-way ANOVA with repeated measures to account for treatment effect (global CDCs vs. vehicle vs. LAD stop-flow CDCs) and time point (2 days post-MI vs. 1 mo post-MI). Postmortem histological analyses were compared with a two-way ANOVA to account for treatment and region. When significant differences were detected, the Holm-Sidak test was used for all pairwise comparisons (SigmaStat 4.0). For data that were not normally distributed, square root and logarithmic transformations were performed (SigmaStat 4.0).
RESULTS
All animals were in good health throughout the study. Three pigs assigned to global infusion of CDCs or vehicle were excluded before randomization: two developed refractory ventricular fibrillation during occlusion and a third was excluded because the angioplasty balloon was displaced from the LAD during defibrillation. None of the animals died after randomization, and 15 completed the entire study protocol. In the stop-flow subgroup, two pigs died from refractory ventricular fibrillation during the infusion procedure, and the remaining eight animals completed the study protocol.
Effects of icCDCs on LV function after acute MI.
Fig. 2 summarizes the effects of each treatment on echocardiographic measurements of global and regional function. Resting hemodynamic parameters at selected time points are summarized in Table 1, and serial echocardiographic measurements of LV function are summarized in Table 2. There were expected reductions in contractility immediately after infarction but no differences in hemodynamic parameters among treatment groups at any time point. Prior to infarction, the LVEF averaged 61.6 ± 1.0 and decreased to 45.3 ± 1.5% 30 min after reperfusion following a 1-h total LAD occlusion (P < 0.01). There were reductions in regional wall thickening in the infarcted LAD region (58.0 ± 3.4 to 10.9 ± 3.2%, P < 0.01) as well as in remote myocardium (114.5 ± 8.1 to 76.3 ± 5.5%, P < 0.01). There were no significant differences in global or regional function before or immediately after infarction among treatment groups.
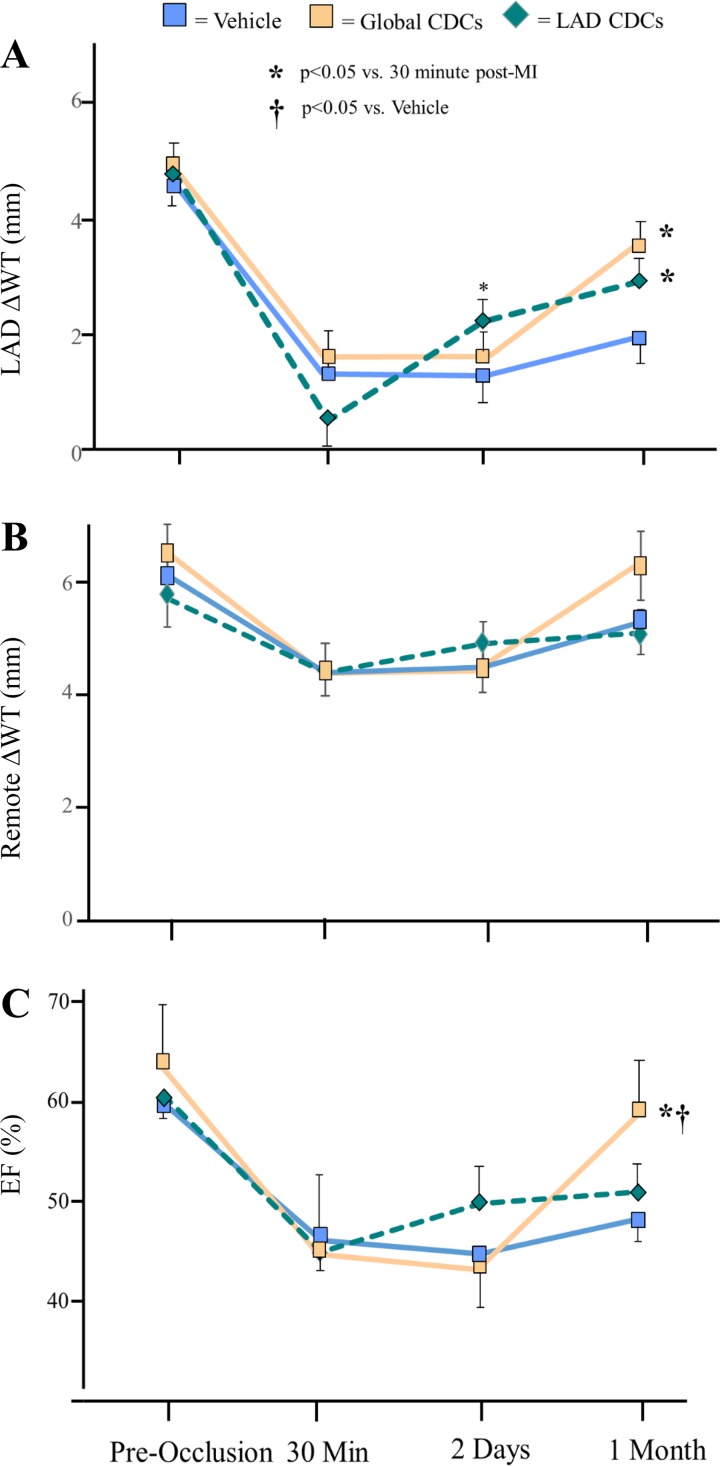
Fig. 2.
Functional improvement from 30 min to 1 mo following global icCDCs assessed with echocardiography. Data represent the effect of icCDCs on regional wall thickening (A and B) and left ventricular ejection fraction (LVEF; C). Although LVEF remained depressed (from 47 ± 3 at 30 min post-MI to 45 ± 2% at 2 days post-MI, P = not significant) in vehicle-treated animals, stop-flow infusion of CDCs improved wall thickening in the infarcted LAD region as early as 2 days post-MI. Regional improvement was maintained until 1 mo post-MI with no change in LVEF. In global icCDC-treated animals, LVEF remained depressed at 2 days but subsequently increased 1 mo later. Global icCDC treatment increased echocardiographic systolic wall thickening in the infarcted LAD region of the LV. CDC, cardiosphere-derived cells; EF, ejection fraction; icCDC, intracoronary cardiosphere-derived cells; LAD, left anterior descending coronary artery; LV, left ventricle; MI, myocardial infarction; WT, wall thickening.
Table 1.
Effect of icCDCs on resting hemodynamic parameters
| n | Mean Aortic Pressure, mmHg | LV Systolic Pressure, mmHg | Heart Rate, beats/min | LV dP/dtmax, mmHg/s | LV dP/dtmin, mmHg/s | |
|---|---|---|---|---|---|---|
| Preocclusion | ||||||
| Vehicle | 7 | 99 ± 4 | 132 ± 5 | 86 ± 8 | 2,471 ± 120 | −2,369 ± 180 |
| Global CDCs | 8 | 108 ± 4 | 139 ± 4 | 80 ± 5 | 2,448 ± 163 | −2,654 ± 131 |
| Stop-flow CDCs | 8 | 112 ± 3 | 139 ± 3 | 74 ± 8 | 2,005 ± 142 | −2,362 ± 91 |
| 30 Min post-MI | ||||||
| Vehicle | 7 | 64 ± 8 | 100 ± 3 | 63 ± 3 | 1,146 ± 59 | −1,326 ± 158 |
| Global CDCs | 8 | 67 ± 2 | 94 ± 4 | 61 ± 3 | 1,109 ± 72 | −1,144 ± 96 |
| Stop-flow CDCs | 8 | 76 ± 4 | 105 ± 5 | 63 ± 5 | 1,045 ± 43 | −1,490 ± 123 |
| 1 Mo post-MI | ||||||
| Vehicle | 7 | 100 ± 3 | 123 ± 4 | 89 ± 7 | 1,826 ± 88* | −1,972 ± 110* |
| Global CDCs | 8 | 104 ± 5 | 129 ± 6 | 90 ± 8 | 2,264 ± 180* | −2,093 ± 108* |
| Stop-flow CDCs | 8 | 108 ± 3 | 134 ± 4 | 72 ± 7 | 1,603 ± 129*† | −1,912 ± 123* |
Values are means ± SE; n = no. of swine. CDC, cardiosphere-derived cells; dP/dtmax, maximum rate of change of left ventricular pressure; dP/dtmin, minimum rate of change of left ventricular pressure; icCDCs, intracoronary cardiosphere-derived cells; LV, left ventricle; MI, myocardial infarction.
P < 0.05 vs. 30-min post-MI;
P < 0.05 vs. global CDCs.
Table 2.
Effect of icCDCs on echocardiographic measurements of left ventricular function
| LAD | Remote | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | EDWT, mm | ΔWT, mm | WT, % | EDWT, mm | ΔWT, mm | WT, % | FS, % | EF, % | |
| Preocclusion | |||||||||
| Vehicle | 7 | 8.9 ± 0.4 | 4.7 ± 0.4 | 52.1 ± 2.7 | 5.3 ± 0.5 | 6.4 ± 0.3 | 127.6 ± 12.4 | 31.6 ± 0.9 | 59.9 ± 1.2 |
| Global CDCs | 8 | 7.8 ± 0.3 | 4.8 ± 0.2 | 61.5 ± 3.0 | 5.8 ± 0.4 | 6.3 ± 0.5 | 115.4 ± 16.8 | 34.5 ± 1.9 | 64.0 ± 2.4 |
| Stop-flow CDCs | 8 | 8.5 ± 0.5 | 4.8 v 0.5 | 59.5 ± 9.0 | 5.9 ± 0.4 | 5.7 ± 0.5 | 102.1 ± 12.4 | 32.0 ± 0.7 | 60.6 ± 1.1 |
| 30 Min post-MI | |||||||||
| Vehicle | 7 | 12.2 ± 1.0 | 1.3 ± 0.8 | 12.9 ± 8.6 | 5.6 ± 0.4 | 4.5 ± 0.5 | 85.6 ± 13.0 | 22.9 ± 1.4 | 46.5 ± 2.5 |
| Global CDCs | 8 | 10.0 ± 0.7 | 1.5 ± 0.3 | 14.7 ± 2.8 | 6.0 ± 0.4 | 4.4 ± 0.3 | 76.6 ± 8.2 | 21.9 ± 1.1 | 44.8 ± 1.9 |
| Stop-flow CDCs | 8 | 10.5 ± 0.3 | 0.5 ± 0.5 | 5.3 ± 4.9 | 6.8 ± 0.5 | 4.4 ± 0.3 | 67.9 ± 7.6 | 22.0 ± 2.0 | 44.8 ± 3.6 |
| 2 Days post-MI | |||||||||
| Vehicle | 7 | 9.5 ± 0.3 | 1.3 ± 0.4 | 14.3 ± 4.9 | 6.0 ± 0.4 | 4.5 ± 0.3 | 77.4 ± 6.7 | 22.0 ± 1.1 | 44.8 ± 2.0 |
| Global CDCs | 8 | 9.2 ± 0.6 | 1.5 ± 0.5 | 16.2 ± 4.5 | 5.8 ± 0.4 | 4.5 ± 0.4 | 81.6 ± 9.6 | 21.0 ± 2.9 | 43.4 ± 4.3 |
| Stop-flow CDCs | 8 | 10.6 ± 1.0 | 2.2 ± 0.4* | 21.3 ± 3.7* | 6.2 ± 0.5 | 4.9 ± 0.4 | 86.3 ± 11.5 | 25.2 ± 2.6 | 49.7 ± 4.1 |
| 1 Mo post-MI | |||||||||
| Vehicle | 7 | 7.7 ± 0.5 | 2.0 ± 0.4 | 25.7 ± 3.5 | 6.4 ± 0.3 | 5.5 ± 0.3 | 87.9 ± 7.1 | 21.9 ± 1.3 | 47.7 ± 2.6 |
| Global CDCs | 8 | 7.6 ± 0.4 | 3.2 ± 0.5* | 41.8 ± 7.0* | 6.2 ± 0.3 | 6.1 ± 0.5 | 98.9 ± 7.3 | 29.7 ± 3.2*† | 60.8 ± 3.4*† |
| Stop-flow CDCs | 8 | 8.4 ± 0.9 | 2.9 ± 0.4* | 35.2 ± 2.5* | 6.9 ± 0.6 | 5.1 ± 0.4 | 80.7 ± 12.6 | 25.7 ± 1.8 | 50.9 ± 3.1 |
Values are means ± SE; n = number of swine. CDC, cardiosphere-derived cells; EDWT, end-diastolic wall thickening; EF, ejection fraction; FS, fractional shortening; icCDCs, intracoronary cardiosphere-derived cells; LAD, left anterior descending coronary artery; MI, myocardial infarction; WT, wall thickening.
P < 0.05 vs. 30-min post-MI;
P < 0.05 vs. vehicle.
After 2 days, global function remained depressed with no significant change in LVEF, which averaged 46.0 ± 2.2% with no difference among treatment groups. Regional LAD and remote zone function also remained depressed. Values of wall thickening were unchanged versus those present immediately after reperfusion, with the exception of a significant increase in LAD wall thickening in animals receiving CDCs using the stop-flow technique (5.3 ± 4.9 30 min after reperfusion to 21 ± 4% at 2 days post-MI, P < 0.05). There were small, insignificant increases in LAD wall thickening after global CDC infusion as well as in vehicle-treated controls. As a result, differences in regional wall thickening among groups were not significant (ANOVA).
However, significant differences in global and regional function among treatment groups were apparent after 1 mo. Although EF tended to increase by a small extent in each group, only animals receiving global icCDC infusion achieved a statistically significant increase in LVEF (from 43.4 ± 4.5 to 60.8 ± 3.8%; P < 0.05). This was also significantly higher than vehicle-control treated animals (Fig. 2, P < 0.05), which did not change over time (44.8 ± 2.0 to 47.7 ± 2.6%, P = not significant). Although animals treated with regional LAD CDC infusion and the stop-flow technique increased LVEF from 44.8 ± 3.6 to 50.9 ± 3.1%, the difference was not significant (P = 0.25). Evaluation of regional function after 1 mo demonstrated that LAD wall thickening tended to increase after global CDCs (41.8 ± 7.0 vs. 25.7 ± 3.5% in vehicle-treated controls, P = 0.07), whereas increases in the stop-flow group (35.2 ± 2.5%) did not reach significance. Collectively, these results demonstrate a delayed improvement in LVEF following the global administration of CDCs. In contrast, the functional changes following stop-flow administration into the LAD were restricted to an early improvement in regional LAD wall thickening.
Effects of CDCs on myocardial infarct size.
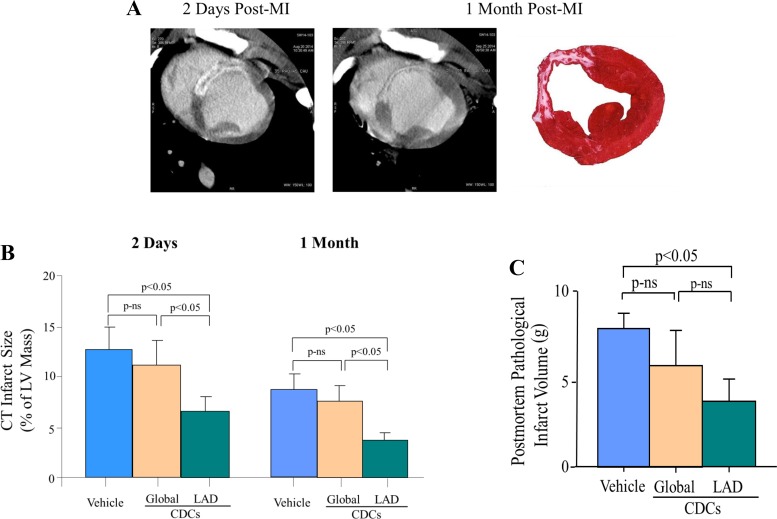
We used cardiac MDCT to assess changes in infarct volume beginning 2 days after reperfusion as summarized in Fig. 3. When assessed after 2 days, myocardial infarct volume after stop-flow CDCs was lower than vehicle-treated controls (7.1 ± 1.1 vs. 12.6 ± 2.3% of LV, P < 0.05). In contrast, although infarct volume tended to be lower in animals receiving global icCDCs, the difference did not reach significance (11.2 ± 2.3% of LV, P = not significant vs. vehicle-treated controls). As expected, with resolution of acute edema and contraction of the scar after 1 mo, infarct volume declined in all groups with a persistent reduction after stop-flow CDCs (3.8 ± 1.0 vs. 8.6 ± 1.6% of LV in vehicle-treated controls, P < 0.05) but no significant difference after global CDCs (7.6 ± 1.5% of LV, P = not significant). Between 2 days and 1 mo post-MI, vehicle-treated animals exhibited a reduction in infarct size of 2.5 ± 0.7 g (4.0 ± 0.9% of LV). Similar reductions in infarct size were observed in global CDC-treated animals (2.4 ± 1.5 g, P = 0.92 vs. vehicle; 3.5 ± 1.7% of LV, P = 0.82 vs. vehicle) and stop-flow regional CDC-treated animals (3.2 ± 0.4 g, P = 0.39 vs. vehicle; 3.3 ± 0.4% of LV, P = 0.45 vs. vehicle). Postmortem TTC staining at 1 mo confirmed similar differences in infarct size among treatment groups (Fig. 3C). These results indicate a differential effect of stop-flow CDC administration into the infarct-related artery versus global CDC infusion on early as well as chronic myocardial infarct size.
Fig. 3.
Serial infarct size assessed with delayed contrast-enhanced MDCT. A: infarct volume using delayed contrast enhancement on the left (shown as white and high intensity) vs. postmortem TTC on the right (pale area) in a vehicle-treated animal. B: as expected, CT infarct volume contracted over the initial month in each treatment group. There were no differences in infarct size between global icCDC-treated and vehicle-treated control animals at 2 days or 1 mo after reperfusion. In contrast, animals receiving stop-flow icCDC treatment restricted to the LAD had a smaller infarct vs. vehicle-treated at 2 days, which was maintained at 1 mo. C: postmortem TTC demonstrated similar changes. Blue bars represent vehicle-treated, gold bars are global CDC infusion, and green bars are LAD stop-flow CDC infusion. Values are mean ± SE. CDC, cardiosphere-derived cells; CT, computed tomography; icCDC, intracoronary cardiosphere-derived cells; LAD, left anterior descending coronary artery; LV, left ventricle; MDCT, multidetector computed tomography; MI, myocardial infarction; ns, not significant; TTC, triphenyltetrazolium chloride.
Effect of CDCs on myocyte proliferation (Ki67), mitosis (pHH3), and apoptosis (TUNEL) in remote myocardium.
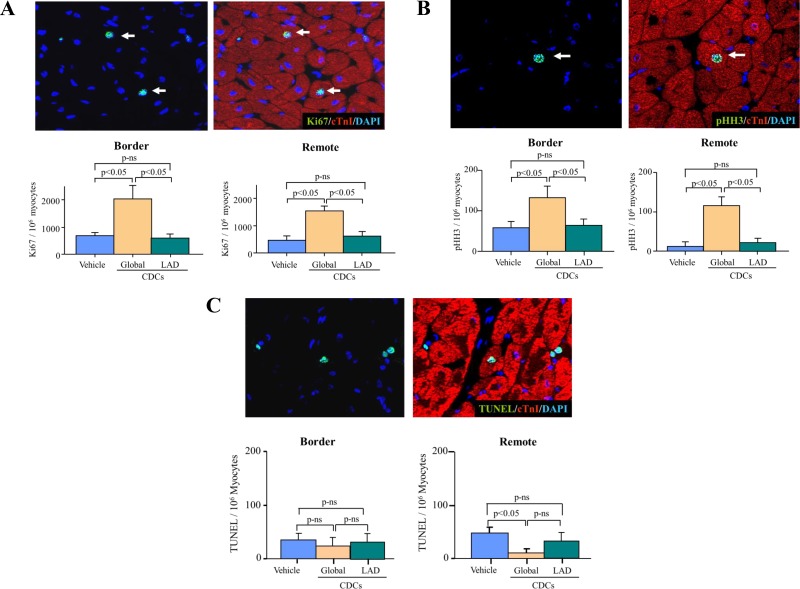
The effects of CDCs on myocyte proliferation were quantified from myocyte nuclei expressing Ki67 and pHH3 and are summarized in Fig. 4, A and B. After global CDCs, Ki67-positive myocytes increased in noninfarcted remote myocardium (global CDCs 1,424 ± 145 nuclei/106 myocytes vs. 510 ± 123 nuclei/106 myocytes in vehicle-treated controls, P < 0.05). Similarly, global CDCs increased the number of pHH3-positive myocytes in remote myocardium (global CDCs 147 ± 32 nuclei/106 myocytes vs. 14 ± 10 nuclei/106 myocytes in vehicle, P < 0.05). In contrast, regional LAD administration of CDCs using the stop-flow technique did not increase Ki67 positive myocytes (stop-flow CDCs 570 ± 79 nuclei/106 myocytes vs. 510 ± 123 nuclei/106 myocytes in vehicle, P = not significant) nor pHH3-positive myocytes in remote myocardium (stop-flow CDCs 12 ± 8 nuclei/106 myocytes vs. 14 ± 10 nuclei/106 myocytes in vehicle, P = not significant vs. vehicle). Similar changes were found in the infarct border zone with no significant differences versus remote myocardium (Fig. 3, A and B). Thus, global CDC infusion is required to stimulate myocyte proliferation in viable myocardium outside of the risk region.
Fig. 4.
Reciprocal changes in Ki67 and phospho-Histone-H3 (pHH3) vs. myocyte apoptosis (TUNEL) in border and remote myocardium. A: four weeks after global icCDCs, Ki67-positive myocytes were significantly increased in the infarct border and remote regions of the LV vs. vehicle-treated controls with no change after LAD stop-flow icCDCs. B: cardiac myocytes in the mitotic phase (pHH3) were also increased in border and remote regions vs. vehicle-treated animals, but there was no change in remote myocardium from animals treated with LAD stop-flow icCDCs. C: global icCDCs decreased TUNEL-positive myocyte nuclei (green nuclei colocalized to myocytes with red cTnI staining) in the remote region but not in the border zone. Stop-flow icCDCs did not alter TUNEL-positive nuclei in the border or remote regions of the LV. CDC, cardiosphere-derived cells; icCDC, intracoronary cardiosphere-derived cells; LAD, left anterior descending coronary artery; LV, left ventricle; ns, not significant; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling.
Fig. 4C summarizes the effects of CDCs on myocyte apoptosis at 1 mo. The number of TUNEL-positive myocytes in the noninfarcted remote regions decreased after global CDC treatment (global CDCs 15 ± 9 nuclei/106 myocytes vs. 46 ±10 nuclei/106 myocytes in vehicle-treated controls, P < 0.05). In contrast, stop-flow CDCs did not significantly decrease TUNEL-positive myocytes in noninfarcted remote myocardium (stop-flow CDCs 31 ± 10 nuclei/106 myocytes, P = not significant vs. vehicle-treated controls). The changes in apoptosis in border regions adjacent to the infarct were similar to remote myocardium in all groups. Thus, global CDC infusion is required to attenuate remote zone apoptosis following MI.
Effects of CDCs on connective tissue and myocyte morphometry in viable myocardium.
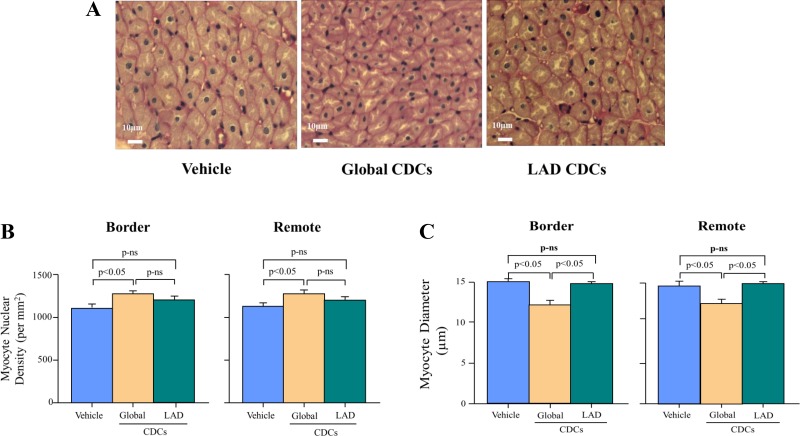
The net effect of increased myocyte proliferation and reduced apoptosis was quantified by assessing myocyte nuclear density and myocyte diameter (Fig. 5). Interstitial connective tissue area was increased in the infarct border (global CDCs 6.6 ± 2.4, stop-flow CDCs 6.3 ± 1.1, and vehicle 8.6 ± 1.2%, P = not significant) as compared with the remote region (global CDCs 0.8 ± 0.2, stop-flow CDCs 0.9 ± 0.1, and vehicle 0.9 ± 0.1%, P = not significant), but there were no differences among treatment groups. Myocyte nuclear density in remote noninfarcted myocardium was higher after global CDCs (1,264 ± 39 nuclei/mm2 vs. 1,157 ± 33 nuclei/mm2 in vehicle-treated controls, P < 0.05) but was unchanged after stop-flow CDCs (1,189 ±327 nuclei/mm2, P = not significant vs. vehicle-treated controls). Similar changes to remote myocardium were seen in border zones adjacent to the infarct in all treatment groups.
Fig. 5.
Effects of icCDCs on myocyte nuclear density and myocyte diameter. A: top images summarize images of cardiomyocytes from vehicle-treated (left), global icCDC-treated (middle), and stop-flow icCDC-treated (right) animals. B: after global icCDCs, myocyte nuclear density increased. In contrast, myocyte nuclear density was no different after LAD stop-flow vs. vehicle control. C: increase in myocyte nuclear density was associated with a reduction in myocyte size after global icCDCs as compared with vehicle-treated animals. In contrast, stop-flow icCDCs did not alter myocyte nuclear density or myocyte size. Changes in border regions were similar to remote regions of the LV. CDC, cardiosphere-derived cells; icCDC, intracoronary cardiosphere-derived cells; LAD, left anterior descending coronary artery; LV, left ventricle; ns, not significant.
Fig. 5C summarizes the effects of treatment on myocyte diameter. Remote zone myocyte diameter decreased after global CDCs (13.2 ± 0.2 μm vs. 14.5 ± 0.3 μm in vehicle-treated controls, P < 0.05), but there was no change after stop-flow CDCs (15.0 ± 0.1 μm, P = not significant vs. the vehicle-treated group). Similar changes were seen after each treatment in border zone myocardium. Neither global nor stop-flow CDCs altered capillary density in the remote region (global CDCs 1,322 ± 85/mm2, stop-flow CDCs 1,210 ± 29/mm2, vehicle 1,237 ± 52/mm2, P = not significant) or infarct border zones (global CDCs 1,125 ± 108/mm2, stop-flow CDCs 1,099 ± 25/mm2, vehicle 1,087 ± 118/mm2, P = not significant).
Transplanted allogeneic CDCs are detectable 1 mo after global intracoronary infusion.
One month after global CDC infusion, quantitative PCR analysis demonstrated Y-chromosomes in female recipient hearts treated with male donor CDCs. Although variable, the number of Y-positive cells within the infarct and surrounding border area (587 ± 221 positive cells/g) tended to be lower than in remote regions (3,790 ± 2,480 positive cells/g, P = 0.28). When the latter value was multiplied by the viable LV mass, the estimated number of Y-positive cells (i.e., those arising from donor CDCs) present at 1 mo post-MI averaged 267,450 ± 179,679 cells, representing ~1.3 ± 1.0% of the originally injected dose. One month after stop-flow CDC infusion, Y-positive cells were exclusively distributed in the LAD region except for one animal who had a detectable Y-positive signal in the remote region.
DISCUSSION
The results of the present study afford several new and important insights into the determinants of improved global myocardial function after the intracoronary administration of CDCs. Consistent with prior studies, restricting icCDCs to the LAD region resulted in modest increases in regional wall thickening and reduced myocardial infarct size. Nevertheless, these changes were not sufficient to result in an improvement in LVEF. In contrast, when icCDCs were administered to the entire left ventricle without stop-flow, there was no significant reduction in infarct size, yet LVEF improved. Histological studies demonstrated that this arose in association with increases in myocyte nuclear density and reductions in myocyte size in regions outside of the infarct zone. The preservation of remote zone cardiomyocytes was secondary to both a reduction in myocyte apoptosis as well as an increase in myocyte proliferation. These beneficial effects were absent when CDC infusion was restricted to the infarct-related artery. Our findings indicate an important role of remote zone cellular remodeling in determining the physiological response to intracoronary cell therapy.
Dissociation between the effects of CDCs on infarct size versus global LV function.
The initial rationale for using cell-based therapy in patients with ischemic cardiomyopathy focused on regenerating new myocardium within an infarct and replacing myocytes lost because of ischemic injury. Regenerated myocytes would replace the fibrotic scar, reduce infarct size, and attenuate post-infarction LV remodeling. Based on this premise, a variety of cell types have been studied following intracoronary or intramyocardial administration restricted to the infarct-related artery. Unfortunately, basic and clinical research in this area has largely focused on the minority of subjects with large infarctions and severely reduced LVEF. Most completed studies have demonstrated only small and inconsistent benefits of cell therapy on LV structure and function (29). Furthermore, most clinical trials have used autologous cell preparations, which precludes cell administration to patients with acute MI because of the delay in isolating and expanding the cell preparation. Although autologous preparations circumvent immune responses, they may have compromised reparative efficacy due to aging and other comorbidities that impact regenerative capacity (7).
To address some of these limitations, attention has shifted toward developing allogeneic cell-based therapeutic approaches using “immune-tolerant” cell populations, such as CDCs and MSCs, that can be produced in advance, stored until use, and safely administered immediately after reperfusion. Limitations in the efficacy of autologous cells from patients with disease can be overcome by using cells isolated from healthy young subjects (33). In preclinical studies, our laboratory (36, 39) and others (24, 43) have recently shown that allogeneic CDCs and MSCs are safe and improve LV function when given via intracoronary infusion in large animal models of chronic infarction and hibernating myocardium. Nevertheless, translation of these encouraging preclinical results has yet to be realized using any form of cell therapy, with a host of negative results accumulating from randomized clinical trials. For example, in terms of CDCs, the ALLSTAR trial (Allogeneic Heart Stem Cells to Achieve Myocardial Regeneration, NCT01458405), which was designed to assess the safety and efficacy of allogeneic CDCs administered to the infarct-related artery between 30 days and 1 yr following MI, was recently terminated because of a low probability of achieving its primary efficacy end point (a reduction in infarct size) (11). These negative results in the face of promising preclinical data in rodent and large animal models suggest the immediate need to reevaluate and challenge many aspects of the cell therapy paradigm. Some of these issues relate to scientific rigor and blinding in preclinical research, whereas others relate to whether altering myocardial fibrosis and infarct size is actually the correct target.
In the present study, we challenged the wisdom of restricting cell therapy to the infarct-related artery as well as the necessity of using the “stop-flow” technique. CDCs were administered very early (30 min) after reperfusion of ischemic myocardium. We used allogeneic cells that could be immediately available and infused at a time when acute myocardial salvage may still be possible via cellular postconditioning (15, 16). Like these prior preclinical studies, we found that regional administration of CDCs using the widely employed stop-flow technique reduced infarct size but did not result in a significant increase in LVEF. In contrast, when CDCs were administered via global intracoronary infusion to the entire heart using a nonocclusive delivery approach, global LVEF improved without a significant reduction in infarct size as compared with vehicle-treated animals. The global infusion of cells under continuous flow was based upon the feasibility of clinical translation and our prior observations that global CDC infusion in animals with hibernating myocardium stimulated myocyte proliferation in ischemic as well as remote regions of the heart (36, 39).
Although there is a general correlation between infarct size and global LV function, there is considerable variability. Following infarction, EF can clearly improve with pharmacological therapies to reduce neurohormonal activation (beta blockers, angiotensin inhibition, and aldosterone antagonists) in ischemic and nonischemic cardiomyopathy and presumably do so without a change in infarct volume (42). EF can also decline with disease progression without a change in infarct volume. Our results further dissociate changes in healed infarct volume from global LV function by demonstrating functional improvement without a change in infarct size after global CDC infusion. In addition, like prior studies, we demonstrated a reduction in infarct volume with stop-flow CDCs restricted to the LAD without an increase in EF. These discordant results indicate that the effects of CDCs on viable myocardium may be a more important determinant of their effect on global LVEF than their ability to reduce myocardial scar volume. Whether a hybrid approach with LAD stop-flow CDC infusion along with CDC infusion under continuous flow to remote myocardium could improve function more than global CDCs without stop flow will require further study.
Modifying myocyte cell death and promoting cell proliferation in remote myocardium.
In the post-infarction period, apoptosis in remote myocardium increases and post-MI alterations in noninfarcted remote myocardium play a substantial role in mediating chronic LV remodeling. In addition to myocyte apoptosis, remote zone changes include inflammatory cell infiltration, extracellular matrix remodeling, myocyte cellular hypertrophy, and interstitial fibrosis (3, 4, 19, 30). These changes begin in the acute phase of infarction and persist to varying degrees to contribute to progressive adverse LV remodeling over the subsequent weeks and months (3, 28, 41). Although the rate of remote zone myocyte death is much lower than that found acutely in the infarct zone, the chronicity over many weeks can lead to a degree of myocyte loss approaching that which occurs in the original infarct (1, 26). In swine with chronic hibernating myocardium where background apoptosis is absent, we previously demonstrated that global administration of MSCs as well as CDCs increased indices of myocyte proliferation (Ki67 and pHH3), increased myocyte nuclear density, and reduced myocyte size (36, 39). These changes resulted in improved function that was independent of alterations of myocardial perfusion.
Based on this, we hypothesized that treating remote myocardium along with the infarct-related artery with icCDCs would more effectively improve global function by preventing apoptotic myocyte loss as well as promoting myocyte proliferation. Our results support this hypothesis, as global icCDC-treated animals exhibited a higher myocyte nuclear density and reduced myocyte diameter as well as a significant increase in LVEF 1 mo after infarction. In contrast, animals receiving CDCs restricted to the LAD infarct zone using the stop-flow technique exhibited rates of remote zone apoptosis that were not significantly different than vehicle-treated animals. In addition, there was no increase in myocyte proliferation, and myocyte nuclear density and size were similar to vehicle-treated controls. Although we cannot ascertain the effects of global CDCs on remote zone myocyte remodeling at earlier time points, our histopathologic analyses 1 mo after treatment support the importance of administering cell therapy to the entire heart.
Many prior studies have emphasized the effects of inhibiting apoptosis and promoting myocyte proliferation in the border zone surrounding an MI. Studies that also report remote zone changes do not usually identify any effects of cell therapy, but most studies restrict administration of cells to the infarct-related artery. Our findings demonstrate that the effects in the infarct border zone in swine with reperfused MI are quite similar to those observed in the remote region. Thus, by administering CDCs to viable remote regions, we were able to extend their salubrious effects to the entire diseased heart. It is important to note that this is not necessarily a generalized effect of CDCs under all circumstances, as we have previously demonstrated that there is no effect of icCDCs on myocyte proliferation in the normal heart (36). Thus, substrate factors in the diseased heart are critical in order for CDCs to effect proliferation and likely attenuate apoptosis. Although speculative, intermittent preload elevation and myocyte stretch following MI may play a role in promoting apoptosis in nonischemic remote myocardium (40) as well as alter myocardial paracrine factors that modulate the effects of CDCs (20). Further studies will be required to identify the critical determinants of this response in the diseased heart.
Contribution of inhibiting apoptosis versus myocyte proliferation on myocyte number.
The precise contribution of inhibiting myocyte cell death versus promoting proliferation on total myocyte number 1 mo after infarction is difficult to resolve unambiguously in large animal models. Although we demonstrated that global icCDC infusion could increase myocyte nuclear number, we cannot conclusively determine the relative magnitude to which this reflects inhibiting apoptosis versus promoting endogenous myocyte cell division, since our immunofluorescence data indicate that both processes are ongoing. The similarity in function among groups at 2 days after CDCs argues against an early effect on apoptosis. At 1 mo, the higher numbers of Ki67 and pHH3 myocytes versus TUNEL-positive myocytes suggests that proliferation may exceed apoptosis-induced myocyte loss at this time point. That said, we are confident that very few exogenous myocardial cells arose from the injected icCDCs based upon qPCR. A recent study of intramyocardial cortical bone stem cell administration after reperfusion in swine used the thymidine analog 5-ethynyl-2′-deoxyuridine to demonstrate that only a small percentage of cells from the total myocyte pool arose via new myocyte formation (32). This supports the notion that the increases in myocyte number we observed after icCDCs may primarily be mediated by preventing myocyte cell death in remote myocardium. The importance of preventing myocyte apoptosis in the initial hours and days after MI is underscored by a few studies demonstrating favorable remote zone myocyte remodeling after regional administration of mesenchymal precursor cells (13) and CDCs (15) versus others showing no effect when cells are administered several weeks after MI (24, 43). Interestingly, remote zone myocyte remodeling after cell therapy can also be effected in viable dysfunctional or “hibernating” myocardium from a chronic coronary stenosis. In this circumstance, there is residual perfusion of the dysfunctional region and no significant scar. Although myocyte apoptosis was absent in remote myocardium at the time of cell therapy, global icCDC and icMSC infusion stimulated endogenous myocyte proliferation (35, 36, 39). Although we did not quantify myocardial cKit+ cells in this study, we previously demonstrated that CDCs as well as MSCs increase cKit+ CD45− cells throughout the heart following a similar global intracoronary delivery approach (35, 36, 39). The contribution of this population to endogenous myocyte regeneration continues to be an area of considerable controversy. Thus, the ability of CDCs delivered immediately after reperfusion to attenuate myocyte death versus stimulate myocyte regeneration is likely to be time and context dependent.
Mechanisms underlying intracoronary CDC-mediated repair.
Our study was primarily directed at assessing the physiological effects of CDCs and determining how regional versus global delivery impacts remote zone myocyte loss and infarct size. Although our ability to address molecular mechanisms in large animal experiments is limited, the beneficial effects despite minimal chronic CDC retention is consistent with many other studies. This supports the notion that indirect paracrine effects, rather than direct differentiation of CDCs into myocytes, are the primary mechanism responsible for the therapeutic benefits of cardiac cell therapy (9, 22). A growing body of evidence suggests a key role for extracellular vesicles (e.g., exosomes) secreted by injected cells that transport cardioprotective and/or regenerative microRNAs to host cells to favorably influence endogenous repair processes (18). The role of exosome release by CDCs has been particularly well studied. For example, de Couto et al. (6) demonstrated that exosomal transfer of microRNA-181b from CDCs to macrophages plays a key role in conferring CDC-mediated cardioprotection in rats. Although this has fueled interest in the use of CDC-derived exosomes as a cell-free therapeutic product, it was recently shown that intracoronary exosome infusion is an ineffective delivery approach, likely because of low first-pass retention with this administration technique (8). Thus, it appears that transient cell retention of a sufficient duration to allow exosome release is necessary to achieve the therapeutic benefits of CDC treatment. To this issue, it is notable that we found evidence of CDC engraftment, albeit at a low rate (~1% of the injected dose), 1 mo after global intracoronary infusion. This contrasts with previously published studies in which retention of allogeneic CDCs was completely absent 2 mo after injection (24). This may be explained by the concomitant administration of cyclosporine in the present study and tracking sex-mismatched allogeneic cells as the end point. Although the safety of allogeneic CDC and MSC transplantation without immunosuppression has been established in preclinical studies (23) and humans (11), they can elicit a relatively mild immune response. It is probable that this impacts their viability over time and accelerates donor cell clearance. Although speculative, it is possible that the therapeutic potential of icCDCs in our study was enhanced by concomitant oral cyclosporine immunosuppression. This may have extended the duration of CDC retention and amplified antiapoptotic and/or proliferative actions by increasing the time available for exosomal transfer to target cells. Future studies directly comparing allogeneic icCDC therapy with and without cyclosporine immunosuppression will be necessary to test this intriguing hypothesis. In addition, if immunosuppression is beneficial, an approach to administer therapeutically effective doses of cyclosporine at the time of reperfusion will be required.
Limitations.
Our observations on infarct size are limited by the fact that we did not assess the ischemic area at risk. In a recent study using a similar experimental protocol and mid-LAD occlusion in swine, we demonstrated that this averages ~20% of LV mass (37). Although LV mass as a percentage of the LV is a better determinant of LV remodeling, our observation that regional CDC administration reduces infarct size needs to be confirmed when normalized to the area at risk. Second, our results were obtained in animals without any background medical therapy routinely administered to patients with MI. Although beta blockers and angiotensin inhibition therapy are not known to effect myocyte proliferation, they attenuate myocyte apoptosis. Thus, in the presence of background pharmacological therapy, the antiapoptotic effects of icCDC therapy may be less significant. The final considerations relate to the dose of CDCs we employed and the use of cyclosporine immunosuppression. We chose CDC doses for global infusion (~7 million/vessel for a total of 20 million CDCs) that provided a similar delivery of CDCs per gram of tissue as in our prior studies in hibernating myocardium (39). The dose of CDCs used for LAD stop-flow experiments (10 million) was chosen to be comparable to prior stop-flow studies by others (16). Nevertheless, although the regional LAD dose was higher in stop-flow experiments, the total CDC dose administered was 50% lower than with global infusion. As a result, if the total rather than regional dose was most important, we could have underestimated the effects of LAD stop-flow administration. Although we previously demonstrated that cyclosporine had no impact on myocyte proliferation in the absence of CDCs (39), we cannot determine whether it is required for these therapeutic effects. It is plausible that prolonging the in vivo viability of CDCs may have contributed to favorable effects via prolongation of any paracrine mediator release. Further studies directly comparing icCDCs with and without cyclosporine will be required to address this issue.
Conclusions.
In summary, our data demonstrate favorable effects of global icCDC infusion in a setting in which LV systolic function is moderately impaired and similar to the average patient undergoing primary coronary intervention after MI. This suggests that preventing myocyte loss by attenuating apoptosis as well as promoting endogenous myocyte proliferation in remote myocardium by global CDC administration may be a more viable strategy than restricting cells to the infarct-related artery. The fact that favorable effects can be seen with allogeneic CDC preparations indicates that the approach we employed is clinically feasible. Moreover, it may benefit a much broader patient population than current strategies using stop-flow administration into the infarct-related artery, which focus on replacing scar. Successful translation of this therapeutic paradigm could produce a novel adjunctive therapy to prevent LV remodeling in patients following percutaneous coronary intervention for acute ST-segment elevation MI.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-55324, HL-61610, and F32-HL-114335; American Heart Association Grant 17SDG33660200; New York State Department of Health Grant NYSTEM CO24351; and the Albert and Elizabeth Rekate Fund in Cardiovascular Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.S., R.F.Y., and J.M.C. conceived and designed research; G.S., B.R.W., and R.F.Y. performed experiments; G.S., B.R.W., J.A.F., and J.M.C. analyzed data; G.S., B.R.W., J.A.F., and J.M.C. interpreted results of experiments; G.S. and B.R.W. prepared figures; G.S. and J.M.C. drafted manuscript; G.S., B.R.W., and J.M.C. edited and revised manuscript; G.S., B.R.W., R.F.Y., J.A.F., and J.M.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Elaine Granica and Beth Palka for assistance in completing this study.
REFERENCES
- 1.Anversa P, Olivetti G, Leri A, Liu Y, Kajstura J. Myocyte cell death and ventricular remodeling. Curr Opin Nephrol Hypertens 6: 169–176, 1997. doi: 10.1097/00041552-199703000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Beltrami CA, Finato N, Rocco M, Feruglio GA, Puricelli C, Cigola E, Quaini F, Sonnenblick EH, Olivetti G, Anversa P. Structural basis of end-stage failure in ischemic cardiomyopathy in humans. Circulation 89: 151–163, 1994. doi: 10.1161/01.CIR.89.1.151. [DOI] [PubMed] [Google Scholar]
- 3.Carrick D, Haig C, Rauhalammi S, Ahmed N, Mordi I, McEntegart M, Petrie MC, Eteiba H, Lindsay M, Watkins S, Hood S, Davie A, Mahrous A, Sattar N, Welsh P, Tzemos N, Radjenovic A, Ford I, Oldroyd KG, Berry C. Pathophysiology of LV remodeling in survivors of STEMI: inflammation, remote myocardium, and prognosis. JACC Cardiovasc Imaging 8: 779–789, 2015. doi: 10.1016/j.jcmg.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan W, Duffy SJ, White DA, Gao XM, Du XJ, Ellims AH, Dart AM, Taylor AJ. Acute left ventricular remodeling following myocardial infarction: coupling of regional healing with remote extracellular matrix expansion. JACC Cardiovasc Imaging 5: 884–893, 2012. doi: 10.1016/j.jcmg.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 5.Dawn B, Stein AB, Urbanek K, Rota M, Whang B, Rastaldo R, Torella D, Tang XL, Rezazadeh A, Kajstura J, Leri A, Hunt G, Varma J, Prabhu SD, Anversa P, Bolli R. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci USA 102: 3766–3771, 2005. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Couto G, Liu W, Tseliou E, Sun B, Makkar N, Kanazawa H, Arditi M, Marbán E. Macrophages mediate cardioprotective cellular postconditioning in acute myocardial infarction. J Clin Invest 125: 3147–3162, 2015. doi: 10.1172/JCI81321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dimmeler S, Leri A. Aging and disease as modifiers of efficacy of cell therapy. Circ Res 102: 1319–1330, 2008. doi: 10.1161/CIRCRESAHA.108.175943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallet R, Dawkins J, Valle J, Simsolo E, de Couto G, Middleton R, Tseliou E, Luthringer D, Kreke M, Smith RR, Marbán L, Ghaleh B, Marbán E. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J 38: 201–211, 2017. doi: 10.1093/eurheartj/ehw240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med 11: 367–368, 2005. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 10.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res 107: 913–922, 2010. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henry TD, Kereiakes DJ, Kowalchuk GJ, Aguirre FV, Malliaras K, DeMaria AN, Francis GS, Povsic TJ, Schatz RA, Traverse JH, Chakravarty T, Pogoda J, Williams P, Rudy J, Smith RD, Marban L, Marban E, Ascheim DD, Makkar RR. 6-Month Results of ALLogeneic Heart STem Cells to Achieve Myocardial Regeneration (ALLSTAR) trial: a randomized, placebo-controlled, double-blind study. Circulation 136: e463, 2017. [Google Scholar]
- 12.Hong KU, Li QH, Guo Y, Patton NS, Moktar A, Bhatnagar A, Bolli R. A highly sensitive and accurate method to quantify absolute numbers of c-kit+ cardiac stem cells following transplantation in mice. Basic Res Cardiol 108: 346, 2013. doi: 10.1007/s00395-013-0346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houtgraaf JH, de Jong R, Kazemi K, de Groot D, van der Spoel TI, Arslan F, Hoefer I, Pasterkamp G, Itescu S, Zijlstra F, Geleijnse ML, Serruys PW, Duckers HJ. Intracoronary infusion of allogeneic mesenchymal precursor cells directly after experimental acute myocardial infarction reduces infarct size, abrogates adverse remodeling, and improves cardiac function. Circ Res 113: 153–166, 2013. doi: 10.1161/CIRCRESAHA.112.300730. [DOI] [PubMed] [Google Scholar]
- 14.Johnston PV, Sasano T, Mills K, Evers R, Lee ST, Smith RR, Lardo AC, Lai S, Steenbergen C, Gerstenblith G, Lange R, Marbán E. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation 120: 1075–1083, 2009. doi: 10.1161/CIRCULATIONAHA.108.816058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanazawa H, Tseliou E, Dawkins JF, De Couto G, Gallet R, Malliaras K, Yee K, Kreke M, Valle I, Smith RR, Middleton RC, Ho CS, Dharmakumar R, Li D, Makkar RR, Fukuda K, Marbán L, Marbán E. Durable benefits of cellular postconditioning: long-term effects of allogeneic cardiosphere-derived cells infused after reperfusion in pigs with acute myocardial infarction. J Am Heart Assoc 5: 1–16, 2016. doi: 10.1161/JAHA.115.002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanazawa H, Tseliou E, Malliaras K, Yee K, Dawkins JF, De Couto G, Smith RR, Kreke M, Seinfeld J, Middleton RC, Gallet R, Cheng K, Luthringer D, Valle I, Chowdhury S, Fukuda K, Makkar RR, Marbán L, Marbán E. Cellular postconditioning: allogeneic cardiosphere-derived cells reduce infarct size and attenuate microvascular obstruction when administered after reperfusion in pigs with acute myocardial infarction. Circ Heart Fail 8: 322–332, 2015. doi: 10.1161/CIRCHEARTFAILURE.114.001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keith MC, Tokita Y, Tang XL, Ghafghazi S, Moore JB IV, Hong KU, Elmore JB, Amraotkar AR, Guo H, Ganzel BL, Grubb KJ, Flaherty MP, Vajravelu BN, Wysoczynski M, Bolli R. Effect of the stop-flow technique on cardiac retention of c-kit positive human cardiac stem cells after intracoronary infusion in a porcine model of chronic ischemic cardiomyopathy. Basic Res Cardiol 110: 46, 2015. doi: 10.1007/s00395-015-0503-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kishore R, Khan M. More than tiny sacks: stem cell exosomes as cell-free modality for cardiac repair. Circ Res 118: 330–343, 2016. doi: 10.1161/CIRCRESAHA.115.307654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee WW, Marinelli B, van der Laan AM, Sena BF, Gorbatov R, Leuschner F, Dutta P, Iwamoto Y, Ueno T, Begieneman MP, Niessen HW, Piek JJ, Vinegoni C, Pittet MJ, Swirski FK, Tawakol A, Di Carli M, Weissleder R, Nahrendorf M. PET/MRI of inflammation in myocardial infarction. J Am Coll Cardiol 59: 153–163, 2012. doi: 10.1016/j.jacc.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Hampton T, Morgan JP, Simons M. Stretch-induced VEGF expression in the heart. J Clin Invest 100: 18–24, 1997. doi: 10.1172/JCI119510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marbán L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marbán E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 379: 895–904, 2012. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malliaras K, Ibrahim A, Tseliou E, Liu W, Sun B, Middleton RC, Seinfeld J, Wang L, Sharifi BG, Marbán E. Stimulation of endogenous cardioblasts by exogenous cell therapy after myocardial infarction. EMBO Mol Med 6: 760–777, 2014. doi: 10.1002/emmm.201303626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malliaras K, Li TS, Luthringer D, Terrovitis J, Cheng K, Chakravarty T, Galang G, Zhang Y, Schoenhoff F, Van Eyk J, Marbán L, Marbán E. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation 125: 100–112, 2012. doi: 10.1161/CIRCULATIONAHA.111.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malliaras K, Smith RR, Kanazawa H, Yee K, Seinfeld J, Tseliou E, Dawkins JF, Kreke M, Cheng K, Luthringer D, Ho CS, Blusztajn A, Valle I, Chowdhury S, Makkar RR, Dharmakumar R, Li D, Marbán L, Marbán E. Validation of contrast-enhanced magnetic resonance imaging to monitor regenerative efficacy after cell therapy in a porcine model of convalescent myocardial infarction. Circulation 128: 2764–2775, 2013. doi: 10.1161/CIRCULATIONAHA.113.002863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malm BJ, Suzuki G, Canty JM Jr, Fallavollita JA. Variability of contractile reserve in hibernating myocardium: dependence on the method of inotropic stimulation. Cardiovasc Res 56: 422–432, 2002. doi: 10.1016/S0008-6363(02)00599-0. [DOI] [PubMed] [Google Scholar]
- 26.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski S, Reed JC, Anversa P. Apoptosis in the failing human heart. N Engl J Med 336: 1131–1141, 1997. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 27.Olivetti G, Quaini F, Sala R, Lagrasta C, Corradi D, Bonacina E, Gambert SR, Cigola E, Anversa P. Acute myocardial infarction in humans is associated with activation of programmed myocyte cell death in the surviving portion of the heart. J Mol Cell Cardiol 28: 2005–2016, 1996. doi: 10.1006/jmcc.1996.0193. [DOI] [PubMed] [Google Scholar]
- 28.Reinstadler SJ, Stiermaier T, Liebetrau J, Fuernau G, Eitel C, de Waha S, Desch S, Reil JC, Poss J, Metzler B, Lucke C, Gutberlet M, Schuler G, Thiele H, Eitel I. Prognostic significance of remote myocardium alterations assessed by quantitative noncontrast T1 mapping in ST-segment elevation myocardial infarction. JACC Cardiovasc Imaging, 2017. doi: 10.1016/j.jcmg.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 29.Rosen MR, Myerburg RJ, Francis DP, Cole GD, Marbán E. Translating stem cell research to cardiac disease therapies: pitfalls and prospects for improvement. J Am Coll Cardiol 64: 922–937, 2014. doi: 10.1016/j.jacc.2014.06.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruparelia N, Digby JE, Jefferson A, Medway DJ, Neubauer S, Lygate CA, Choudhury RP. Myocardial infarction causes inflammation and leukocyte recruitment at remote sites in the myocardium and in the renal glomerulus. Inflamm Res 62: 515–525, 2013. doi: 10.1007/s00011-013-0605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J, Kellner J, Zviman MM, Hatzistergos KE, Detrick B, Conte JV, McNiece I, Steenbergen C, Lardo AC, Hare JM. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J 30: 2722–2732, 2009. doi: 10.1093/eurheartj/ehp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharp TE III, Schena GJ, Hobby AR, Starosta T, Berretta RM, Wallner M, Borghetti G, Gross P, Yu D, Johnson J, Feldsott E, Trappanese DM, Toib A, Rabinowitz JE, George JC, Kubo H, Mohsin S, Houser SR. Cortical bone stem cell therapy preserves cardiac structure and function after myocardial infarction. Circ Res 121: 1263–1278, 2017. doi: 10.1161/CIRCRESAHA.117.311174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simpson DL, Mishra R, Sharma S, Goh SK, Deshmukh S, Kaushal S. A strong regenerative ability of cardiac stem cells derived from neonatal hearts. Circulation 126, Suppl 1: S46–S53, 2012. doi: 10.1161/CIRCULATIONAHA.111.084699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marbán E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation 115: 896–908, 2007. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki G, Iyer V, Lee TC, Canty JM Jr. Autologous mesenchymal stem cells mobilize cKit+ and CD133+ bone marrow progenitor cells and improve regional function in hibernating myocardium. Circ Res 109: 1044–1054, 2011. doi: 10.1161/CIRCRESAHA.111.245969. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki G, Weil BR, Leiker MM, Ribbeck AE, Young RF, Cimato TR, Canty JM Jr. Global intracoronary infusion of allogeneic cardiosphere-derived cells improves ventricular function and stimulates endogenous myocyte regeneration throughout the heart in swine with hibernating myocardium. PLoS One 9: e113009, 2014. doi: 10.1371/journal.pone.0113009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Techiryan G, Weil BR, Palka BA, Canty JM Jr. Effect of intracoronary metformin on myocardial infarct size in swine. Circ Res 123: 986–995, 2018. doi: 10.1161/CIRCRESAHA.118.313341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weil BR, Konecny F, Suzuki G, Iyer V, Canty JM Jr. Comparative hemodynamic effects of contemporary percutaneous mechanical circulatory support devices in a porcine model of acute myocardial infarction. JACC Cardiovasc Interv 9: 2292–2303, 2016. doi: 10.1016/j.jcin.2016.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weil BR, Suzuki G, Leiker MM, Fallavollita JA, Canty JM Jr. Comparative efficacy of intracoronary allogeneic mesenchymal stem cells and cardiosphere-derived cells in swine with hibernating myocardium. Circ Res 117: 634–644, 2015. doi: 10.1161/CIRCRESAHA.115.306850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weil BR, Suzuki G, Young RF, Iyer V, Canty JM Jr. Troponin release and reversible left ventricular dysfunction after transient pressure overload. J Am Coll Cardiol 71: 2906–2916, 2018. doi: 10.1016/j.jacc.2018.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Westman PC, Lipinski MJ, Luger D, Waksman R, Bonow RO, Wu E, Epstein SE. Inflammation as a driver of adverse left ventricular remodeling after acute myocardial infarction. J Am Coll Cardiol 67: 2050–2060, 2016. doi: 10.1016/j.jacc.2016.01.073. [DOI] [PubMed] [Google Scholar]
- 42.Wilcox JE, Fonarow GC, Ardehali H, Bonow RO, Butler J, Sauer AJ, Epstein SE, Khan SS, Kim RJ, Sabbah HN, Díez J, Gheorghiade M. “Targeting the heart” in heart failure: myocardial recovery in heart failure with reduced ejection fraction. JACC Heart Fail 3: 661–669, 2015. doi: 10.1016/j.jchf.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 43.Yee K, Malliaras K, Kanazawa H, Tseliou E, Cheng K, Luthringer DJ, Ho CS, Takayama K, Minamino N, Dawkins JF, Chowdhury S, Duong DT, Seinfeld J, Middleton RC, Dharmakumar R, Li D, Marbán L, Makkar RR, Marbán E. Allogeneic cardiospheres delivered via percutaneous transendocardial injection increase viable myocardium, decrease scar size, and attenuate cardiac dilatation in porcine ischemic cardiomyopathy. PLoS One 9: e113805, 2014. doi: 10.1371/journal.pone.0113805. [DOI] [PMC free article] [PubMed] [Google Scholar]