Abstract
Introduction
Irisin is a newly identified cytokine that has gained increasing attention because of its potential therapeutic applications in metabolic diseases and human cancers. Recently, accumulating evidence indicates that irisin plays an important role in the development and metastasis of various tumors. The aim of this study was to evaluate the effects and underlying mechanisms of irisin on malignant growth of pancreatic cancer cells.
Materials and methods
The anti-proliferative effect of irisin was examined using the CCK-8 assay. Irisin-induced apoptosis was determined by the annexin V-FITC/PI staining assay. The effects of irisin on cell migration and invasion were assessed using the scratch-induced wound healing assay and transwell invasion assay, respectively. The expression and phosphorylation of signaling proteins were detected by Western blot analysis.
Results
Our results showed that irisin inhibited cell proliferation and induced apoptosis of pancreatic cancer cells in a dose-dependent manner. In addition, irisin decreased the migration and invasion of pancreatic cancer cells. Finally, Western blot analysis revealed that irisin downregulated the PI3K/AKT signaling pathway.
Conclusion
Our findings suggest that irisin is a novel therapeutic agent for pancreatic cancer.
Keywords: irisin, pancreatic cancer, cell growth, migration, apoptosis
Introduction
Pancreatic cancer is one of the most malignant digestive tract tumors. The prognosis of pancreatic cancer is extremely poor due to its rapid progression, late-stage diagnosis, and early metastasis. Although surgical resection is the only choice for the current therapy, only 20% of patients are capable of radical excision.1,2 The 5-year survival rate among patients with pancreatic cancer is <7%, which is the lowest one among all cancer types.3,4 Therefore, it is of utmost importance to develop novel and effective therapeutic strategies, which may help to improve overall outcomes of pancreatic cancer patients.
Irisin, a recently identified myokine by Bostrom et al (2013), is secreted from fibronectin type III domain containing 5 (FNDC5) expressing cells during exercise.5,6 In addition to skeletal muscle, irisin is widely expressed in brain, heart, stomach, pancreas, liver, testis, and adipose, suggesting that irisin is involved in many biological functions.7–9 Irisin dissipates the energy in the form of heat by inducing the white fat tissue into the brown adipose tissue that contains a large number of uncoupling protein-1 (UCP-1) in mitochondria.5,10 Therefore, several studies have shown that irisin regulates glucose homeostasis, improves insulin resistance, and ameliorates cognitive function.11–13 Recently, irisin has been implicated in the development and metastasis of tumors, including breast and colorectal cancers, and proposed as a novel diagnostic marker and even therapeutic target.14,15 In this study, however, we unexpectedly found irisin to exhibit anticancer activities in the pancreatic cancer cells and explored its underlying mechanism.
Materials and methods
Cell lines and culture conditions
Human pancreatic cancer cell lines, PANC-1 and BxPC-3, were obtained from the Cell Bank of Chinese Academy of Sciences (Shanghai, China) and routinely maintained in DMEM medium (Hyclone, UT, USA) supplemented with 10% fetal bovine serum, 100 U/mL penicillin and 100 µg/mL streptomycin (Hyclone, UT, USA) in 25 cm2 culture flasks. The cells were maintained at 37 °C in a humidified atmosphere of 5% CO2. PANC-1 and BxPC-3 are epithelioid carcinoma cell lines, isolated from pancreatic carcinoma of ductal cell origin.
Cell counting kit-8 (CCK-8) assay
The cells were grown in 96-well plates at a density of 5×103 cells/well overnight. Following day, cells were treated with 0 nM, 10 nM, 20 nM, and 50 nM of irisin (Cayman Chemicals, MI, USA) for different periods (24 h, 48 h, and 72 h) at 37 °C. The effect of irisin on cell proliferation was assayed using the CCK-8 (7Sea Biotech, Shanghai, China) according to the manufacturer’s instructions. The CCK-8 solution was added and incubated for 1 h at 37 °C and the absorbance was measured using a microplate reader (Bio-Tek, VT, USA) at 450 nm.
The annexin-V-FITC/PI assay
The cells were seeded at a density of 2×105 cells/well in 6-well plates and treated with different concentrations of irisin for 24 h. Next, cells were collected, washed twice with PBS, gently re-suspended and incubated with the binding buffer of the annexin V-FITC/PI staining kit (7Sea Biotech, Shanghai, China). Cells were subsequently stained with 5 μl annexin V-FITC and 10 μl PI in the dark for 15 min and analyzed by using an Accuri C6 Flow Cytometer (BD Biosciences, CA, USA).
Scratch-induced wound healing assay
Cells were seeded in 6-well plates containing regular growth medium and allowed to grow till confluence. A single scratch wound was generated in the confluent monolayer cells using a pipette tip and was rinsed with PBS. The scratched cells were then cultured in DMEM treated with or without 50 nM irisin and incubated for 24 h. Images were acquired at 0 h and 24 h at the same position (scratched area) using an inverted microscope (Olympus, Tokyo, Japan). The percentage of wound closure was calculated by measuring the healed area relative to the initial wound area.
Transwell invasion assay
The invasion potential of the pancreatic cancer cells treated with irisin was assessed using transwell invasion chambers (8-μm pore size; Corning Incorporated, NY, USA) coated with 30 µl of Matrigel (BD Biosciences, NJ, USA). The cell suspensions (5 x 104) were added to the upper chamber containing serum-free DMEM medium. The lower chamber was filled with 600 μL DMEM supplemented with 10% fetal bovine serum. After 24 h of incubation at 37 °C, the non-invaded cells in the upper surface of the filter were removed with cotton swabs. The cells that had invaded the underside of the membrane were fixed with 4% paraformaldehyde at room temperature for 20 min and stained with 0.1% crystal violet. The stained cells from at least 3 random fields were counted under a microscope at high magnification.
Western blot analysis
The cells cultured in 6-well plates were lysed with the RIPA buffer. The concentration of total protein was measured using the BCA protein assay kit (Beyotime, Shanghai, China). Proteins of equal amounts from different cell lysates were separated on an SDS-PAGE and then transferred to PVDF membrane (Beyotime, Shanghai, China). The membranes were blocked in 5% fat-free milk at room temperature and incubated with primary antibodies overnight at 4 °C. Primary antibodies against AKT, p-AKT, Bax, Bcl-2, and β-actin were purchased from Cell Signaling Technology, Inc. (MA, USA). The membranes were then washed with TBST and incubated with HRP-conjugated secondary antibodies for 2 h at room temperature. The immune complexes were detected using the ECL method (Millipore, MA, USA).
Statistical analysis
Experiments were carried out in triplicates and presented as mean ± standard deviation (SD). Statistical analysis was performed using GraphPad Prism 7 software (GraphPad Software Inc., CA, USA). The experimental data was tested for normality. The comparison between two groups was analyzed by Student’s t-test. Comparisons among multiple groups were analyzed with one-way analysis of variance (ANOVA) followed by Dunnett’s test. P<0.05 was considered statistically significant.
Results
Irisin decreased the proliferation of pancreatic cancer PANC-1 and BxPC-3 cells
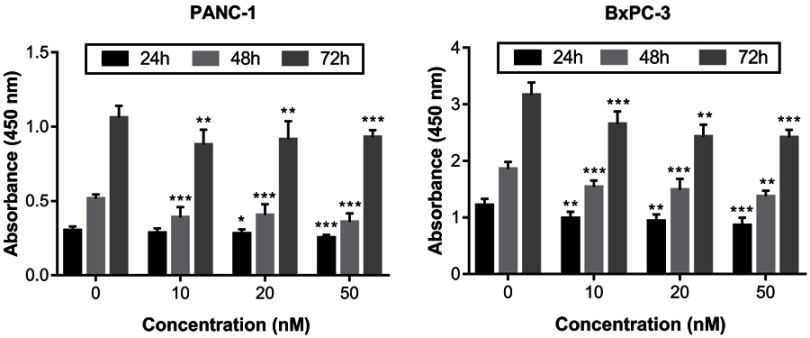
We used the CCK-8 assay to examine the effects of different concentrations of irisin on the proliferation of PANC-1 and BxPC-3 cells. Our results showed that irisin was able to reduce proliferation of both PANC-1 and BxPC-3 cells in a dose-dependent manner (Figure 1). Thus, BxPC-3 cells were used for subsequent experiments in this study.
Figure 1.
Irisin inhibited the growth of pancreatic cancer cells.
Notes: PANC-1 and BxPC-3 cells were treated with irisin (0 nM, 10 nM, 20 nM, and 50 nM) for indicated periods (24 h, 48 h, and 72 h) and then subjected to the CCK-8 assay. *P<0.05, **P<0.01, and ***P<0.001 compared with control.
Irisin induced apoptosis of the pancreatic cancer BxPC-3 cells
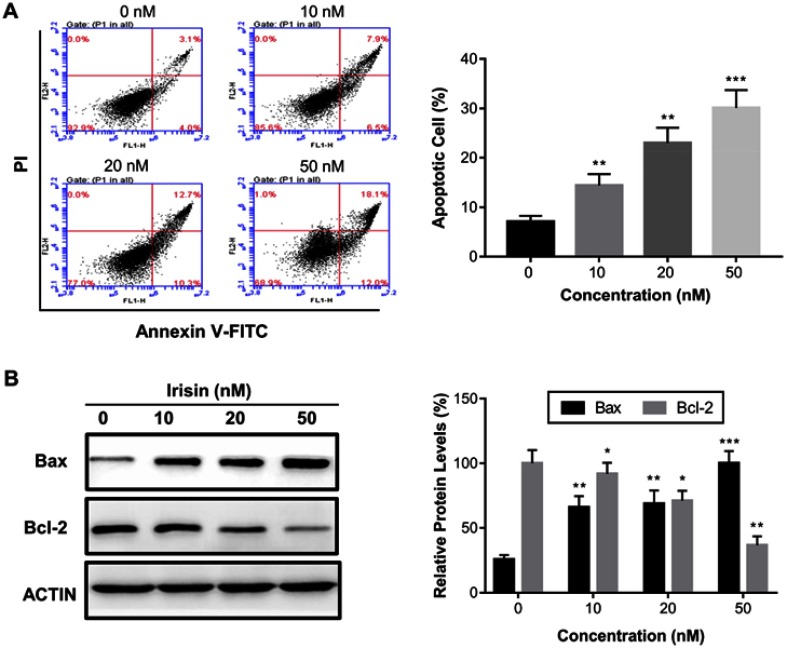
To investigate the mechanism by which irisin inhibited cell proliferation, we performed the annexin V-FITC/PI double staining assay to test whether irisin induces apoptosis of these cells treated with different concentrations of irisin for 24 h. We found that irisin significantly increased the apoptosis of treated cells in a dose-dependent manner (Figure 2A). To further examine the underlying mechanism of apoptosis induced by irisin, the levels of mitochondrial apoptotic pathway proteins such as Bax and Bcl-2 were evaluated by Western blot analysis. The results showed that the level of pro-apoptotic protein Bax was increased while anti-apoptotic protein Bcl-2 decreased following treatment with irisin (Figure 2B).
Figure 2.
Irisin induced apoptosis in pancreatic cancer cells. BxPC-3 cells were treated with irisin (0 nM, 10 nM, 20 nM and 50 nM) for 24 h. (A) Apoptosis was analyzed using the annexin V-FITC/PI double staining assay. (B) The effect of irisin on the levels of Bax and Bcl-2 was determined by Western blotting. *p<0.05, **p<0.01, and ***p<0.001 compared with control.
Irisin attenuated migration and invasion of pancreatic cancer BxPC-3 cells
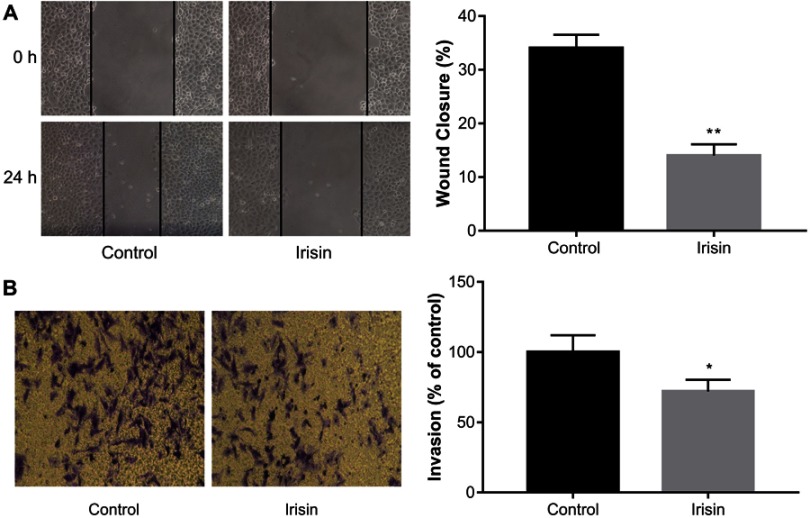
The effects of irisin on BxPC-3 cell migration was examined by the scratch-induced wound-healing assay. The result showed that irisin significantly inhibited wound closure in BxPC3 cells compared with that in the control cells (Figure 3A).
Figure 3.
Irisin inhibited migration and invasion of pancreatic cancer cells.
Notes: (A) After treatment with indicated concentrations of irisin, the scratch-induced wound healing assay was used to measure cell migration at 24 h after scratch wounding confluent cell layer. (B) Transwell assay was used to determine the effect of irisin on the invasion of pancreatic cancer cells. Images of cells were captured using an inverted microscope following staining with crystal violet. *P<0.05 and **P<0.01 compared with control cells.
Transwell assay was performed to investigate the effect of irisin on the invasion ability of BxPC-3 cells. After treatment with irisin, the number of cells that invaded through the Matrigel-coated membrane was significantly reduced compared with the control group. These results indicate that irisin has potential in inhibiting the invasive ability of BxPC-3 cells (Figure 3B).
Irisin downregulated the activity of the PI3K/AKT signaling pathway
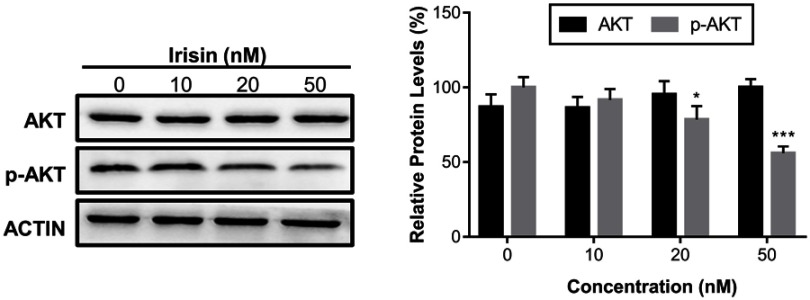
The PI3K/AKT signaling pathway is important in the development and progression of pancreatic cancer. Further, our data revealed that irisin downregulated the levels of the p-AKT in a dose-dependent manner without affecting the levels of total AKT protein (Figure 4).
Figure 4.
Irisin suppressed the protein phosphorylation of the PI3K/AKT signaling pathway.
Notes: BxPC-3 cells were treated with irisin (0 nM, 10 nM, 20 nM, and 50 nM) for 24 h. The levels of total AKT, p-AKT, and Actin were analyzed by Western blot. *P< 0.05 and ***P<0.001 compared with control.
Discussion
In recent years, accumulating evidence indicates that irisin is involved in the occurrence and development of a variety of tumors. The positive immunoreactivity of irisin in cancer tissues, such as pancreatic, gastric, and breast cancers is significantly increased compared with that in normal tissues.16,17 However, serum levels of irisin in breast and colorectal cancer patients were significantly reduced compared with healthy controls, suggesting that serum irisin may serve as a novel diagnostic biomarker of cancer.14,15 Irisin has been reported to possess an antitumor effect in different tumors such as breast, lung, and prostate cancers as well as osteosarcoma.18–21 In this study, we investigated the anti-tumor activity of irisin in pancreatic cancer cells and explored the potential mechanisms. We found that irisin can inhibit the growth of pancreatic cancer cells in a dose-dependent manner.
In addition, we employed the annexin V-FITC/PI staining assay to evaluate the effect of irisin on the apoptosis of BxPC-3 cells. The results showed that irisin increased the apoptotic cell proportions in a dose-dependent manner. Induction of apoptosis in tumor cells is an important cellular process to eliminate malignant cells while preventing tumor development. Bcl-2 protein family members play an essential role in mitochondria-mediated apoptosis pathway via regulating the anti-apoptotic protein Bcl-2 and the pro-apoptotic protein Bax. Here, we found that irisin elevated the levels of Bax protein and reduced Bcl-2 protein levels in BxPC-3 cells, suggesting that irisin induces the apoptosis of BxPC-3 cells presumably via the mitochondrial apoptosis pathway. Invasion and metastasis are the major biological cellular activities of malignant tumors, which often are the main cause of the most deaths in cancer patients. Therefore, it is crucial to find new therapeutic agents to prevent cell invasion and metastasis for the treatment and prevention of cancer recurrence, which might be useful in prolonging the survival of the patients. In our study, at 24 h after irisin treatment, the migration and the invasive abilities of BxPC-3 cells were remarkably reduced.
The PI3K/AKT signaling pathway is involved in several cellular processes, including cell proliferation, differentiation, survival, and motility.22 It has been reported that the expression and activity of the PI3K and AKT are upregulated in pancreatic cancer cells, which is associated with poor prognosis.23,24 It is well known that the PI3K/AKT signaling pathway is a key regulatory pathway not only in pancreatic tumorigenesis but also in the treatment of other cancers.25 It has been reported that irisin reduces the migration and invasion of lung cancer cells via inhibiting the PI3K/AKT signaling pathway.20 Irisin has also been demonstrated to increase the number and function of endothelial progenitor cells via the PI3K/AKT pathway.26 In the present study, we observed that irisin inhibited the levels of the p-AKT protein, suggesting that irisin attenuated the activity of the PI3K/AKT signaling pathway in BxPC-3 cells. While preparing this manuscript, a related work was published, which showed the potential of irisin in suppressing pancreatic cancer cell growth via the AMPK-mTOR signaling pathway.27 However, they did not evaluate the effect of irisin on apoptosis of pancreatic cancer cells and its mechanism of action. Induction of tumor cell apoptosis is an important cellular activity through which anti-tumor drugs exert their therapeutic effects. Here, we further illustrate the effect of irisin on the induction of apoptosis in pancreatic cancer cells, which was associated with alteration of levels between Bax and Bcl-2 proteins. In addition, irisin attenuated the activity of the PI3K/AKT signaling pathway, which was also involved in cancer cell apoptosis via interactions with the Bcl-2 family.
Conclusion
In conclusion, our findings suggest that irisin could inhibit cell proliferation and induce the apoptosis of pancreatic cancer cells. In addition, irisin was able to downregulate the activation of the PI3K/AKT signaling pathway in pancreatic cancer cells. These findings provide strong evidence to suggest that irisin may be further developed as a novel potential therapeutic agent for the treatment of pancreatic cancer. Further studies are needed to understand the precise role of irisin in pancreatic cancer in vivo.
Acknowledgment
This work was supported by the Shandong Province Key Research and Development Project (No.2018GSF118057), National Natural Science Foundation of China (No.81601617), Qingdao Livelihood Science and Technology Project (No.17-3-3-40-nsh), and Qingdao Science and Technology Development Project (No.2016-3-017-YY).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362(17):1605–1617. doi: 10.1056/NEJMra0901557 [DOI] [PubMed] [Google Scholar]
- 2.Li J, Wientjes MG, Au JL. Pancreatic cancer: pathobiology, treatment options, and drug delivery. Aaps J. 2010;12(2):223–232. doi: 10.1208/s12248-010-9181-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nipp R, Tramontano AC, Kong CY, et al. Disparities in cancer outcomes across age, sex, and race/ethnicity among patients with pancreatic cancer. Cancer Med. 2018;7(2):525–535. doi: 10.1002/cam4.1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 5.Bostrom P, Wu J, Jedrychowski MP, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2013;481(4):463–468. doi: 10.1038/nature10777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahgoub MO, D’Souza C, Al Darmaki R, Baniyas M, Adeghate E. An update on the role of irisin in the regulation of endocrine and metabolic functions. Peptides. 2018;104:15–23. doi: 10.1016/j.peptides.2018.03.018 [DOI] [PubMed] [Google Scholar]
- 7.Huh JY, Panagiotou G, Mougios V, et al. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. 2012;61(12):1725–1738. doi: 10.1016/j.metabol.2012.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aydin S, Kuloglu T, Aydin S, et al. A comprehensive immunohistochemical examination of the distribution of the fat-burning protein irisin in biological tissues. Peptides. 2014;61:130–136. doi: 10.1016/j.peptides.2014.09.014 [DOI] [PubMed] [Google Scholar]
- 9.Lv J, Pan Y, Li X, et al. Study on the distribution and elimination of the new hormone irisin in vivo: new discoveries regarding irisin. Horm Metab Res. 2015;47(8):591–595. doi: 10.1055/s-0035-1547261 [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Li R, Meng Y, et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes. 2014;63(2):514–525. doi: 10.2337/db13-1106 [DOI] [PubMed] [Google Scholar]
- 11.Wrann CD, White JP, Salogiannnis J, et al. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013;18(5):649–659. doi: 10.1016/j.cmet.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hojlund K, Bostrom P. Irisin in obesity and type 2 diabetes. J Diabetes Complications. 2013;27(4):303–304. doi: 10.1016/j.jdiacomp.2013.04.002 [DOI] [PubMed] [Google Scholar]
- 13.Yang Z, Chen X, Chen Y, Zhao Q. Decreased irisin secretion contributes to muscle insulin resistance in high-fat diet mice. Int J Clin Exp Pathol. 2015;8(6):6490–6497. [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu H, Liu M, Zhang N, et al. Serum and adipose tissue mRNA levels of ATF3 and FNDC5/Irisin in colorectal cancer patients with or without obesity. Front Physiol. 2018;9:1125. doi: 10.3389/fphys.2018.01125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Provatopoulou X, Georgiou GP, Kalogera E, et al. Serum irisin levels are lower in patients with breast cancer: association with disease diagnosis and tumor characteristics. BMC Cancer. 2015;15(1):898. doi: 10.1186/s12885-015-1584-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aydin S, Kuloglu T, Ozercan MR, et al. Irisin immunohistochemistry in gastrointestinal system cancers. Biotech Histochem. 2016;91(4):242–250. doi: 10.3109/10520295.2015.1136988 [DOI] [PubMed] [Google Scholar]
- 17.Kuloglu T, Celik O, Aydin S, et al. Irisin immunostaining characteristics of breast and ovarian cancer cells. Cell Mol Biol (noisy-le-grand). 2016;62(8):40–44. [PubMed] [Google Scholar]
- 18.Tekin S, Erden Y, Sandal S, Yilmaz B. Is irisin an anticarcinogenic peptide. Med-Sci. 2015;4(2):2172–2180. doi: 10.5455/medscience.2014.03.8210 [DOI] [Google Scholar]
- 19.Gannon NP, Vaughan RA, Garcia-Smith R, Bisoffi M, Trujillo KA. Effects of the exercise-inducible myokine irisin on malignant and non-malignant breast epithelial cell behavior in vitro. Int J Cancer. 2015;136(4):E197–E202. doi: 10.1002/ijc.29142 [DOI] [PubMed] [Google Scholar]
- 20.Shao L, Li H, Chen J, et al. Irisin suppresses the migration, proliferation, and invasion of lung cancer cells via inhibition of epithelial-to-mesenchymal transition. Biochem Biophys Res Commun. 2017;485(3):598–605. doi: 10.1016/j.bbrc.2016.12.084 [DOI] [PubMed] [Google Scholar]
- 21.Kong G, Jiang Y, Sun X, et al. Irisin reverses the IL-6 induced epithelial-mesenchymal transition in osteosarcoma cell migration and invasion through the STAT3/Snail signaling pathway. Oncol Rep. 2017;38(5):2647–2656. doi: 10.3892/or.2017.5973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27(41):5497–5510. doi: 10.1038/onc.2008.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reichert M, Saur D, Hamacher R, Schmid RM, Schneider G. Phosphoinositide-3-kinase signaling controls S-phase kinase-associated protein 2 transcription via E2F1 in pancreatic ductal adenocarcinoma cells. Cancer Res. 2007;67(9):4149–4156. doi: 10.1158/0008-5472.CAN-06-4484 [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto S, Tomita Y, Hoshida Y, et al. Prognostic significance of activated Akt expression in pancreatic ductal adenocarcinoma. Clin Cancer Res. 2004;10(8):2846–2850. [DOI] [PubMed] [Google Scholar]
- 25.Ng SSW, Tsao MS, Chow S, Hedley DW. Inhibition of phosphatidylinositide 3-kinase enhances gemcitabine-induced apoptosis in human pancreatic cancer cells. Cancer Res. 2000;60(19):5451–5455. [PubMed] [Google Scholar]
- 26.Zhu G, Wang J, Song M, et al. Irisin increased the number and improved the function of endothelial progenitor cells in diabetes mellitus mice. J Cardiovasc Pharmacol. 2016;68(1):67–73. doi: 10.1097/FJC.0000000000000386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, Song N, Huang Y, Chen Y. Irisin inhibits pancreatic cancer cell growth via the AMPK-mTOR pathway. Sci Rep. 2018;8(1):15247. doi: 10.1038/s41598-018-33229-w [DOI] [PMC free article] [PubMed] [Google Scholar]