Abstract
Atypical hemolytic uremic syndrome (aHUS) is a chronic life threatening condition that arises from genetic abnormalities resulting in uncontrolled complement amplifying activity. The introduction of eculizumab, the humanized monoclonal antibody, has brought about a paradigm shift in the management of aHUS. However, there are many knowledge gaps, diagnostic issues, access and cost issues, and patient or physician challenges associated with the use of this agent. Limited data on the natural history of aHUS along with the underlying genetic mutations make it difficult to predict the relapses and thereby raising concerns about the appropriate duration and monitoring of treatment. In this review, we discuss the safety and efficacy of eculizumab in patients with aHUS and its associated challenges.
Keywords: atypical hemolytic uremic syndrome, eculizumab, challenges, thrombotic microangiopathy
Introduction
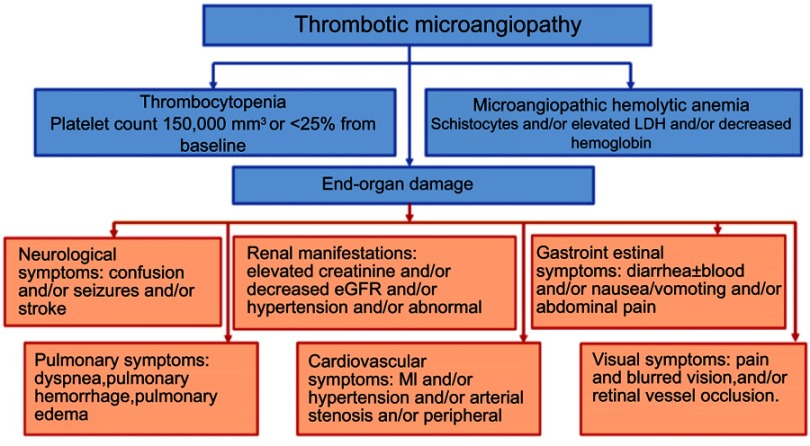
Thrombotic microangiopathies (TMA) are life-threatening pathologies characterized by the formation of microthrombi in small blood vessels.1,2 They present with microangiopathic hemolytic anemia (MAHA), thrombocytopenia and end-organ damage (Figure 1)3–16 with kidneys being the most commonly affected leading to acute kidney injury (AKI). MAHA is the destruction of red blood cells (RBCs) due to shearing in the small vessel walls. Two main types of TMA include thrombotic thrombocytopenic purpura and hemolytic uremic syndrome (HUS).1,2
Figure 1.
Manifestations of thrombotic microangiopathy and the clinical presentations of end-organ damage. Data from references.3–16
Abbreviations: GI, gastrointestinal; MI, myocardial infarction, eGFR, estimated glomerular filtration rate; LDH, lactate dehydrogenase.
HUS is broadly classified as 1) infection-associated HUS: Shiga toxin producing Escherichia coli, Streptococcus pneumonia, influenza A, H1N1 2) HUS secondary to organ transplantation (e.g., renal transplant), systemic malignancies, autoimmune conditions (e.g., systemic lupus erythematosus, antiphospholipid antibody syndrome, scleroderma), drugs (e.g., quinine, calcineurin inhibitors, chemotherapeutic agents like gemcitabine), malignant hypertension, 3) HUS due to cobalamin C disorder, and 4) atypical HUS (aHUS) due to alternative complement pathway dysregulation and mutations in diacylglycerol kinase ε (DGKE) gene.16,17
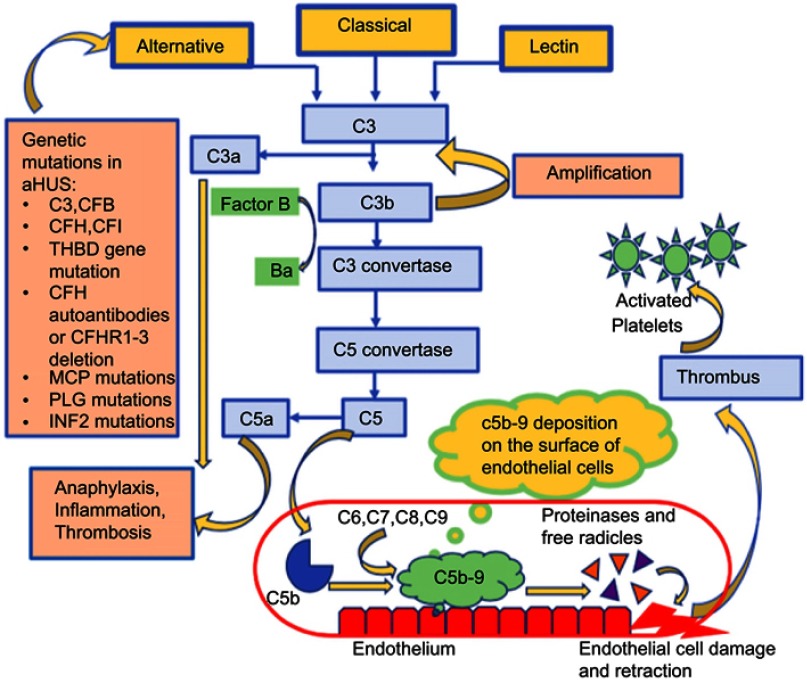
The complement system is a part of the body’s extracellular innate immunity contributing to the first line of host-defense against pathogenic microorganisms. The complement pathway proteins act as anaphylatoxins promoting inflammation, mediate opsonization amplifying the capacity of antibodies and phagocytic cells to destroy pathogens and cause direct cell lysis. Three pathways that activate the complement include classical, alternative, and lectin. The alternative complement pathway is continuously active at low levels in the body. This event is due to an unstable thioester bond in C3 which causes its spontaneous hydrolysis in an aqueous environment and triggers the formation of the fluid phase C3b like molecule called C3(H2O). In contrast, when C3 directly comes in contact with the microbe’s surface and gets activated, it forms the surface-bound C3b. The generation of C3b (fluid phase or surface-bound) allows the alternative pathway to mount an amplified immune response. The activated C3b combines with factor B to create a C3 convertase. C3 convertase eventually leads to the formation of the C5 convertase, which activates C5b and helps form the C6-C9 complex, also known as the membrane attack complex (MAC). This complex forms a channel on the cell membrane and leads to cell lysis (Figure 2).3,16,18–25
Figure 2.
The alternative complement pathway in atypical hemolytic uremic syndrome and the associated genetic mutations.
Note: Data from references 3, 16, 18-25.
Abbreviations: CFB, complement factor B; CFD, complement factor D; CFI, complement factor I; CFH, complement factor H; MCP, membrane cofactor protein; THBD, thrombomodulin; MAC, membrane attack complex; CFHR, complement factor receptor; PLG, plasminogen; INF2, Inverted Formin 2; aHUS, atypical hemolytic uremic syndrome.
The alternative complement pathway is tightly regulated by complement regulatory proteins to prevent its uncontrolled activation. The complement regulatory proteins include factor H (FH), factor I (FI), membrane cofactor protein (MCP), complement 3 (C3), factor B). FI is a serine protease that causes the breakdown of C3b into fragments by using FH as a cofactor, preventing the formation of C3 convertase. MCP, a membrane-bound regulator of complement is also a cofactor for the breakdown of C3b and C4b by FI. Thrombomodulin helps in degrading C3a and C5a anaphylatoxins and enhances the CFI-mediated degradation of C3b.20 Uncontrolled alternative pathway activation causes neutrophils, macrophages, and platelets to continuously accumulate on the endothelial cell surface inducing a prothrombotic state.26 This formation results in deposition of microthrombi in the renal vessels and shearing of RBCs, resulting in TMA. The dysregulation of the complement in aHUS can be either genetic or acquired. The genetic mutations lead to either a decrease in FH, FI, C3, MCP, and thrombomodulin or an upregulation in complement factor H (CFH) autoantibodies.16 Mutations in DGKE lead to a form of aHUS with no obvious complement abnormality. DGKE is a lipid kinase present in the endothelium, platelets, and podocytes and it phosphorylates diacylglycerol (DAG) to phosphatidic acid. Conversion to phosphatidic acid blocks the DAG-induced activation of protein kinase C, thereby inhibiting thrombosis. DGKE gene mutations cause uncontrolled activation of protein kinase C leading to a prothrombotic state.27
Historically, aHUS had a poor prognosis with about 50% of all patients progressing to end-stage renal disease (ESRD).3 Prior to the introduction of eculizumab, plasma infusion or plasma exchange (PI/PE) was the standard of care by supplying the normal/functional complement inhibitory molecules, but they do not address the underlying pathology. The long- and short-term outcome of patients treated with PE/PI depends on the underlying genetic mutations. Noris et al analyzed clinical outcomes in 273 patients of aHUS treated with PE/PI, and found that nearly 70% of patients with genetic mutations either died or became dialysis dependent during the first episode or within 3 years from first manifestation.23 PE/PI also has associated adverse effects, including hypotension, symptomatic hypocalcemia, allergic reaction, and catheter-related thrombosis especially in patients of pediatric age group.28 Eculizumab, a humanized monoclonal antibody, was approved by the FDA in 2011.29 With clinical trials favoring eculizumab, it has brought about a paradigm shift in the management of aHUS.30 The effectiveness of treatment in aHUS is measured by the duration free of TMA events. A TMA event-free status in 68–88% of patients treated with eculizumab has been reported in various studies.23,31,32 Use of eculizumab, though preferred over PE/PI, is associated with limitations and challenges. By inhibiting the formation of MAC, patients become more susceptible to infections with encapsulated organisms. Others include deciding the adequate duration of treatment, monitoring of the effectiveness of eculizumab therapy, and the current cost of the medication.33 In this review, we discuss the use of eculizumab to treat aHUS and the associated challenges.
Pharmacokinetics
Eculizumab is a humanized monoclonal IgG2/4κ antibody produced by recombinant DNA technology. It has an inhibitory action on complement factor C5, thereby blocking its breakdown, resulting in the downstream inhibition of complement cascade and inhibiting the formation of the MAC. The inhibition of the terminal complement activity inhibits cell lysis and the clinical manifestations seen in aHUS.34,35
Eculizumab has a predominant blood plasma distribution, and its distribution in other tissues has not been studied. In the population pharmacokinetic studies done in aHUS patients, clearance of eculizumab was found to be 14.6 mL/h with a volume of distribution of 6.91 L and elimination half-life of 12.1 days in an average 70 kg patient.36 Plasma exchange or infusion leads to an increase in the clearance and decreased half-life of eculizumab, hence additional doses may be required37 Other factors which affect pharmacokinetics are the age and weight of the patient, and C5 and soluble C5b-9 levels since eculizumab binds to these factors, and their increased plasma levels will result in decreased concentration of the drug.36,37
The dosing of eculizumab is weight based for patients aged 18 years or younger. This dosing has been extrapolated from data from adult pharmacokinetic studies.36 Table 1 shows dosing in adult patients and patients aged 18 years or younger.36 If PE/PI is done, then an additional dose of 300–600 mg is required.
Table 1.
Dosing regimen for eculizumab in patients with atypical hemolytic uremic syndrome
| Induction dose | Maintenance dose | ||
|---|---|---|---|
| Dose in patients 18 years or older | 900 mg once a week for 4 weeks | 1200 mg in week 5 after induction dose is completed. Followed by 1200 mg once every 2 weeks | |
| Dose in patients <18 years | Weight >40 kg | 900 mg once a week for 4 weeks | 1200 mg in week 5 after induction dose is completed. Followed by 1200 mg every 2 weeks. |
| 30–40 kg | 600 mg once a week for 2 weeks | 900 mg in week 3 after induction dose is completed. Followed by 900 mg every 2 weeks. | |
| 20–30 kg | 600 mg once a week for 2 weeks | 600 mg in week 3 after induction dose is completed. Followed by 600 mg every 2 weeks. | |
| 10–20 kg | 600 mg in week 1 | 300 mg in week 2. 300 mg every 3 weeks. | |
| 5–10 kg | 300 mg in week 1 | 300 mg in week 2. 300 mg every 3 weeks. | |
Note: Data from Soliris (Eculizumab) highlights of prescribing information. US Food and Drug Administration. 2007. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125166s172lbl.pdf.36
Since eculizumab is a complement inhibitor, its chief adverse effect is life-threatening infections by encapsulated organisms, such as Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae.16,36 Patients receiving eculizumab should be vaccinated against these organisms to prevent bacterial sepsis at least 2 weeks before initiating therapy. Hypertension (5%), renal impairment (5%), headache, diarrhea, upper respiratory tract infection, nausea, vomiting, and cough are additional adverse events that have been reported. As with all protein products, administration of eculizumab may result in infusion reactions, including anaphylaxis or other hypersensitivity reactions.36
Clinical trials on eculizumab
Since the approval of eculizumab by US-FDA, many clinical trials and studies have been conducted and have demonstrated the advantages of eculizumab over the conventional treatment of aHUS using PE/PI prior. Palma et al conducted a critical appraisal of eculizumab in aHUS and reviewed the data from clinical trials, case series, and case reports of aHUS and concluded that “aHUS may be controlled in overwhelming majority of patients and likely at a higher frequency with eculizumab than with PE alone by historical database comparisons”.29
Genetic mutation testing and its association with the use of eculizumab have also been reported in many case reports and clinical studies. Raina et al conducted a meta-analysis of case reports of aHUS over the duration of 10 years and analyzed the impact of eculizumab treatment and plasma exchange on the duration of resolution of clinical features and mortality in aHUS. A statistically significant difference in the mortality rate between the eculizumab group compared to non-eculizumab group (P=0.045) was noted; however, there was no change in the mortality rate with the use of plasma exchange therapy as compared to non-plasma exchange group (P=0.76). No significant difference was observed in the time to resolution of symptoms and serum creatinine or platelet count normalization in the two groups (P=0.17, P=0.36, P=0.83), and between plasma exchange vs non-plasma exchange group (P=0.15, P=0.14, P=0.78). The authors also showed satisfactory evidence favoring initiation of therapy with eculizumab once the diagnosis of aHUS was confirmed by genetic testing.38
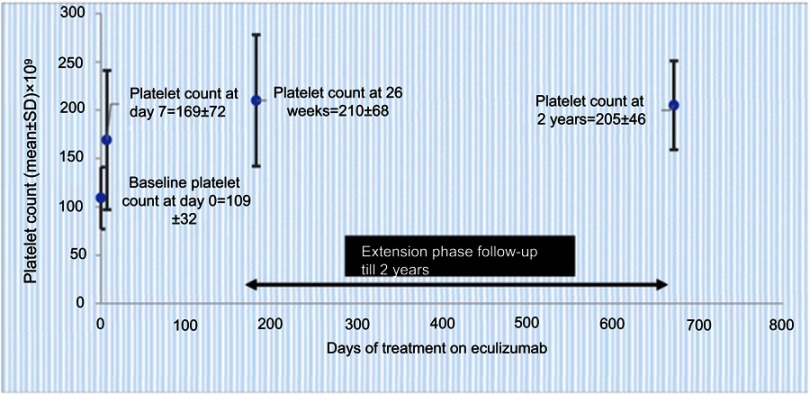
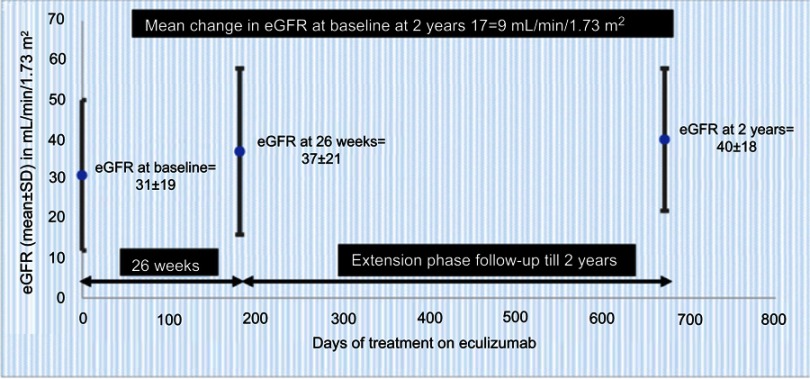
Legendre et al published the results of the two prospective trials in aHUS. Patients of both trials received eculizumab for 26 weeks and during long-term extension phases. For trial 1, a complete TMA response was achieved in 65% of patients at 26 weeks and 76% patients at 2 years. Nearly 50% of patients had a normal platelet count after 1 week, and by the end of the study, platelets and lactate dehydrogenase (LDH) levels were normal in 90% (Figure 3). Patients had an improved renal function, and 80% discontinued dialysis throughout the 26 weeks. For trial 2, 80% of the patients had a TMA event-free status by the end of the study. Patients in trial 2 were able to stop plasma exchange therapy, discontinue dialysis, and have an improved kidney function (Figure 4). For both trials 1 and 2, it was seen that earlier the eculizumab was given greater the improvements in estimated glomerular filtration rate (eGFR). The authors also reported clinically significant improvements in the quality of life in more than 70% of patients in both the trials.23
Figure 3.
Sustained increase in platelet count during ongoing eculizumab treatment in trial 1 data (from Soliris (Eculizumab) highlights of prescribing information. US Food and Drug Administration. 2007. Available from: https://www.access data.fda.gov/drugsatfda_docs/label/2011/125166s172lbl.pdf36) (bars represent SD); normalization of platelet count was defined as count >150±109/L).
Figure 4.
Improved renal function through 2 years with ongoing eculizumab treatment in trial 2 data (from Soliris (Eculizumab) highlights of prescribing information. US Food and Drug Administration. 2007. Available from: https://www.access data.fda.gov/drugsatfda_docs/label/2011/125166s172lbl.pdf36) (bars represent SD); normalization of platelet count was defined as count >150±109/L).
Abbreviation: aHUS, atypical hemolytic uremic syndrome.
A systematic review, which included the above trials, showed that eculizumab was effective in the management of aHUS, although there were limitations of sample size, lack of control groups, and use of surrogate markers.39 Licht et al conducted a follow-up to Legendre’s publication from 26 weeks and 1 year and assessed outcomes after 2 years. Both studies showed that eculizumab inhibited terminal complement activity. In trial 1, patients had an improved platelet count and eGFR compared to baseline and 1 year; 15 of 17 patients had hematologic normalization after 1 and 2years. In trial 2, 20 patients were followed to assess primary endpoints; 8 patients had improved eGFR by 2 years, 19 patients had TMA event-free status by year 2, and 18 patients had hematologic remission. Overall, the study confirmed that clinical benefits achieved by eculizumab treatment of aHUS were maintained at 2 years follow-up.40
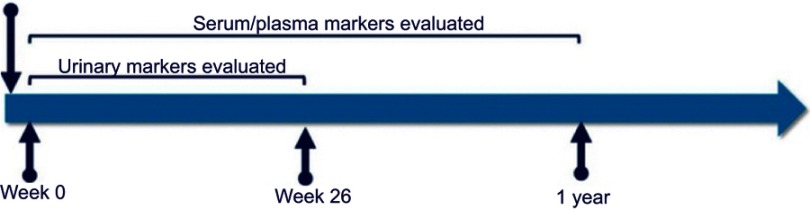
Cofiell et al evaluated 41 adult patients with aHUS before and after eculizumab therapy and measured blood and urinary levels of biological markers associated with aHUS at baseline and at 26 weeks, followed by an optional extension phase of 1 year (Figure 5) and compared them to healthy volunteers. Patients with aHUS had significantly elevated markers of terminal complement activation and MAC formation, i.e., urinary C5a (U-C5a) and urinary soluble C5b-9 (UsC5b-9). A rapid decrease in the levels of terminal complement components was noted following administration of eculizumab, and this reduction was sustained with ongoing therapy to levels comparable to those measured in healthy volunteers. The mean reduction in urinary levels of U-C5a and UsC5b-9 at week 26 was 90±7.5% and 98±1.4%, respectively. A reduction in markers of endothelial cell activation and damage, i.e., soluble vascular cell adhesion molecule-1 and thrombomodulin, respectively, was noted, but the levels remained elevated as opposed to healthy volunteers. Serum levels of Ba, a marker of proximal complement activation, were also elevated as compared to healthy volunteers while on treatment with eculizumab reflecting a proximal complement dysregulation. The authors concluded that although treatment with eculizumab blocks terminal complement activity and reduces biomarkers of renal injury, inflammation and endothelial damage, individuals with genetic predisposition continue to have ongoing proximal complement activity and activation of endothelium promoting a proinflammatory state.41
Figure 5.
Markers evaluated at baseline in patients with aHUS41
Abbreviation: eGFR, estimated glomerular filtration rate.
Patients in Japan diagnosed with TMA caused by aHUS participated in a study evaluating patient outcomes and safety at 6 months, 12 months, and annually thereafter, after beginning eculizumab for an interim analysis of a post-marketing surveillance mandated by Japanese regulations. Thirty-three patients with aHUS and 27 patients with secondary TMA were enrolled for 24 weeks. Among the 29 aHUS patients with available baseline data, platelet count, LDH, and serum creatinine improved in 1 month after beginning eculizumab. Nineteen patients were TMA event-free, 5 patients had complete TMA response, 13 patients had platelet normalization, and 16 patients had a decrease in serum creatinine. This interim analysis confirmed the acceptable safety profile and effectiveness of eculizumab for Japanese adult aHUS patients.31
There is extensive evidence advocating the superiority of eculizumab in addition to supportive management over other forms of treatment (Table 2).23,31,40–48 However, therapy with this agent has many challenges and larger clinical trials are required to address these limitations.
Table 2.
Clinical trials and studies on eculizumab
| Study | Number of patients | Inclusion criteria (IC) and primary end-points for patients (EP) | Number of patients with genetic mutations | Patients who received dialysis before eculizumab initiation | Patients who received PE/PI before Eculizumab initiation | Patients with history of renal transplant | Duration of study | Outcome |
|---|---|---|---|---|---|---|---|---|
| Legendre et al (2013) Prospective study23 |
37 17 in trial 1 20 in trial 2 ≥12 years old |
IC – trial 1: low platelet counts and renal damage Trial 2: renal damage, but no decrease in platelet count of more than 25% for at least 8 weeks during PE/PI EP – trial 1: change in platelet count Trial 2: TMA event-free status (no decrease in platelet count of >25%, no PE/PI, and no dialysis) |
13 in trial 1 and 14 in trial 2 | 5 in trial 1 and 2 in trial 2 | 16 in trial 1 and 20 in trial 2 | 7 in trial 1 and 8 in trial 2 | 26 weeks Optional extension phase: 1 year |
-Trial 1: 50% of patients had a normal platelet count after 1 week and by the end of the study, platelets and LDH levels were normal in 90% of patients. -80% were able to discontinue dialysis throughout the 26 weeks -Trial 2: 80% of the patients had TMA event-free status. -Patients discontinued PE/PI and dialysis, and had improved kidney function -Patients in both trials where eculizumab was started earlier had better improvement in renal function (P=0.007 in trial 1 and P<0.001 in trial 2) |
| Licht et al (2015)40 Two-year analysis for Legendre et al's study |
Same as noted above | Same as noted above | Same as noted above | Same as noted above | Same as noted above | Same as noted above | 2 years | -Data were evaluated at three time points, i.e., 26 weeks, 1 year, and 2 years. -In trial 1, statistically significant increase in platelet counts was noted at all three time points. -14/17 patients attained normal platelet count at 26 weeks, and 15/17 attained the same at 1- and 2-year time points -In trial 2, 16/20 patients achieved TMA free event status at week 26 and nearly all patients (19/20) had TMA event-free event status by 2 years. |
| Cofiell et al (2015)41 Open-label, nonrandomized, single-group, multi-center trial |
41 patients ≥18 years old |
IC – platelet counts <150×103/L, hemoglobin levels ≤ LLN, LDH levels 1.5× ULN, SCr ≥ULN at screening, ADAMTS13 activity ≥5% or higher and no positive Shiga toxin-producing E. coli test EP – serum, plasma and urine biomarker levels at baseline, 26 and 52 weeks. |
20 | 24 | 35 | 9 | 26 weeks Optional extension phase 1 year |
-After 1 year, patients had reduced U-C5a, U-sC5b-9 levels, renal injury markers (clustering cystatin-c, b2-M, L-FABP-1), markers of inflammation (sTNFR1), markers of coagulation (prothrombin F112 and D-dimer), and endothelial damage (thrombomodulin). -TCA and renal markers were reduced to levels seen in healthy volunteers, but other biomarkers were only reduced to levels compared to pre-treatment. |
| Cavero et al (2017)42 Retrospective analysis |
29 patients | IC – patients with secondary aHUS, worsening renal function and persistent TMA despite plasmapheresis. EP – normalization of platelet count and hemoglobin, disappearance of all MAHA markers, improved renal function with a ≥25% reduction of SCr baseline |
8 patients were detected with genetic mutations but only 3 were considered pathogenic. 2 patients had Anti FH auto antibodies |
14 | 24 | 1 | 2–30 weeks Discontinued at 8 weeks on average |
−68% of patients experienced a rapid resolution of the TMA -Only four patients needed dialysis at last follow-up -12/15 patients with drug-induced aHUS, and all patients with postpartum, cancer-related, acute humoral rejection and intestinal lymphangiectasis, responded to eculizumab. -Patients with aHUS secondary to systemic diseases formed a larger part of the cohort that did not respond to treatment with only 2 of 8 patients responding. -Six patients had hematological resolution but no improvement in renal function -Three patients had persistent hematological and renal abnormalities despite eculizumab treatment |
| Walle et al (2017)43 Post-hoc analysis of four Phase II prospective studies |
97 Age 1 month-80 years |
IC – documented set of TMA onset symptoms, baseline eGFR of <90 mL/min/1.73 m2 EP – proportion of patients achieving sustained eGFR increase (defined: C15 mL/min/1.73 m2 for C28 days) and platelet count normalization evaluated 1-year post-treatment. |
57 | 43 | 71 | 26 | 1 year | -Patients who received eculizumab ≤7 days after initial presentation of the aHUS showed a significantly (P<0.05) greater improvement in mean eGFR after 1 month -17/21 patients in the group that received eculizumab in ≤7days after presentation had a sustained increase in eGFR after 3 months which remained stable throughout 1 year. -In the group that received eculizumab after >7days, 36/76 had sustained increase in eGFR at1 year. |
| Greenbaum et al (2016)44 First prospective trial conducted exclusively in aHUS patients <18 years |
22 19 completed 26 weeks Ages 5 months–17 years |
IC – LDH ≥1.5× ULN, hemoglobin ≤ LLN, fragmented RBCs with a negative Coombs test EP – complete TMA response by 26 weeks |
11 | 11 | 10 | 2 | 26 weeks | −14 patients had achieved a complete TMA response by 26 weeks. -18 patients achieved hematological normalization, and 16 had 25% or better serum creatinine after a median of 55 and 21 days, respectively. -9/11 patients discontinued dialysis -PE/PI was discontinued in all patients. |
| Kato et al (2019)31 Interim analysis of the post-marketing surveillance |
33 patients with aHUS 27 patients with secondary TMA |
IC – patients with aHUS diagnosis based on the Japanese diagnostic guide and received at least 1 dose of eculizumab EP – TMA event-free status, complete TMA response, hematologic outcomes, and renal outcomes |
11 of the 18 aHUS patients tested for genetic mutations | 17 | 18 | None | 24 weeks | -Among 29 aHUS patients with available baseline data, platelet count, LDH, and SCr improved in 1 month after beginning eculizumab. -19 patients were TMA event-free, 5 patients had a complete TMA response, 13 patients had platelet normalization, and 16 patients had a decrease in serum creatinine. |
| Kumar et al45 Retrospective cohort study |
14 Median age 64 months |
IC –platelet count ≥150×109/L LDH levels <ULN ≥25% decrease in SCr level from baseline on two consecutive measurements obtained ≥4 weeks apart |
NA | 6 | 6 | NA | Patients were followed from January 2012 to January 2018 | −9 days after patients had received eculizumab treatment, 14 had improved hematological response and 13 had improved TMA response. -6 patients, who had required PE/PI, none continued to require further transfusions after eculizumab treatment. - 6 patients who had previously required dialysis, only 1 remained on dialysis after treatment. |
| Fakhouri et al46 (2016) Open-label single-arm phase 2 trial | 41 patients Age 18 years or older |
IC – platelet count <150×10(3)/μL, hemoglobin ≤ LLNLDH≥1.5×ULN, and SCr≥ULN EP – complete TMA response |
21 | 24 | 35 | 9 | 26 weeks | -Platelet counts and eGFR increased from baseline in 40 and 22 patients, respectively (P<0.001). -35 patients receiving PE/PI at the beginning, discontinued by week 26. -OF 24 dialysis- dependent patients, five recovered kidney function before starting therapy with eculizumab and 15 of the remaining 19 discontinued dialysis during eculizumab treatment. |
| Merill et al47 (2017) Single center, retrospective review |
17 Median patient age at presentation was 46 years, with 76% females |
IC – patients fulfilling aHUS criteria, testing negative for Shiga toxin, with ADAMTS13 levels above 10%, and receiving eculizumab EP – dialysis independence at last follow-up, TMA-event-free status |
11 | 9 | 12 | None | Median duration of eculizumab therapy was 90.5 days Follow-up after cessation of eculizumab was a median 308.5 days |
−94% of all patients had TMA event-free status -82% patients were dialysis-free after last follow-up -Two patients died during eculizumab treatment |
| Huerta et al48 (2018) Retrospective study | 22 | IC – women with pregnancy-associated aHUS | 9 | 9 | NA | NA | Median duration of eculizumab treatment – 10 months | −17 patients received PE/PI, but only 3 showed improvement in renal function. -Ten patients received eculizumab and all of them had resolution of TMA - Amongst the remaining 12 patients, six patients needed RRT during follow- up and later-on received renal transplants. |
Abbreviations: TMA, thrombotic microangiopathy; TCA, total complement activity; LDH, lactate dehydrogenase; PE/PI, plasma exchange/plasma infusion; eGFR, estimated glomerular filtration rate; SCr, serum creatinine; ULN, upper limit of normal; LLN, lower limit of normal; NA, data unavailable; sTNFR1, soluble tumor necrosis factor receptor-1; MAHA, microangiopathic hemolytic anemia; aHUS, atypical hemolytic uremic syndrome; FH, factor H.
Challenges of eculizumab
Patient’s perspective
The aHUS Alliance, an umbrella group of aHUS advocates and patient groups in over 30 nations, launched its aHUS Global Patients’ Research Agenda49 on Rare Disease Day 2019. An international project developed over 4 years, the aHUS Global Patients’ Research Agenda lists 15 central questions grouped into 5 main categories: Causes and Precautions, Diagnosis, Treatment, Impact: Clinical/Psychological, and Impact: Socio-Economic. Patient access to eculizumab, as well as drug costs, affects all five categories either tangentially or directly. Early and accurate, diagnosis drives aHUS patient outcomes, but rare disease policies and health care options among nations vary widely and determine each nation’s degree of access to eculizumab, based largely on the drug’s high cost. The corporate site for Alexion Pharmaceuticals (developer and manufacturer of eculizumab under the trade name Soliris) currently notes that it has “operations in place to serve patients in nearly 50 countries”, which is roughly one-quarter of nations worldwide.
In its 2016, aHUS Global Poll,50 the aHUS Alliance survey of aHUS patients and caregivers in 23 nations (N=233) included questions about patient experiences, research interests, treatment issues, and eculizumab access. When poll data included the high response rates from the USA (N=100), a nation where eculizumab is available to any aHUS clinical subtypes, 77% of the respondents could access this therapeutic option. An additional 14% of respondents from the 2016, aHUS Global Polls noted conditional availability in their nation, with the most common exclusion being aHUS patients on dialysis, which in effect blocked some from renal transplantation lists. The aHUS Alliance analyzed the 2016, poll data and released a white paper on eculizumab access, noting that the availability of the medication dropped from 77% (responses from all 23 countries) to 37% for aHUS patients in nations outside the US and EU. Restricted access to eculizumab rose from 14% (all 23 nations) to 37% in nations outside of the US and EU.51 Addressing issues regarding eculizumab as one of the four expensive drugs used by pediatric nephrologists, Diana Karpman noted:
Access to and costs of orphan drugs have most profound implications for patients, but also for their physicians, hospitals, insurance policies, and society at large, particularly from financial and ethical standpoints.52
Various aHUS Alliance projects for Rare Disease Day and its annual aHUS Awareness Day campaign (24 September) have helped to illustrate the needs and concerns of individual patients and of national aHUS patient organizations. Treatment issues often mentioned include affordable access to eculizumab, drug delivery concerns, questions about tailored treatment to suit individual patient needs, distinguishing possible side effects apart from multi-organ involvement, what alternatives may be in the pipeline for alternatives to eculizumab, and under what parameters it might be safe to discontinue eculizumab. Clinician-researchers are often the first line not only in diagnostic and therapeutic care, but they also are deeply engaged in the central, human interaction of provider-patient dialogue which can provide insights for all stakeholders. Alexion Pharmaceuticals states that it serves patients in almost 50 nations, but it then follows that lack of access to eculizumab is an issue for patients, and the physicians who treat them, in almost three-quarters worldwide. South Africa is prompting the well-supported rationale for a Global Review Panel Model for aHUS Drug Access.53
Fragmented information flow and variants in terminology in fields related to aHUS research impact not only physician education initiatives and professional conferences, but the ease and ability of aHUS patient organizations and other stakeholders to keep current. Individuals and groups within the aHUS Alliance have been active partners in collaborations that include discussion of eculizumab and aHUS treatment, such as the aHUS Global Patient Registry,54 the KDIGO Controversies Conference on aHUS treatment,55 and the ISN working group on increasing access to integrated ESKD and global kidney health56 Original content about eculizumab access and its challenges regularly appear on the aHUS Alliance website, through videos and content such the “aHUS Perspectives“ articles and “The Reluctant Advocate” series on how aHUSUK families advocated in partnership with physicians for access to eculizumab to change the UK government policy (NICE approval).
Diagnosis and case management for aHUS patients is complex, with factors such as extra-renal involvement and secondary aHUS issues further complicating treatment. While decline in kidney function is usually most common aHUS manifestation at presentation, an estimated 10–48% of aHUS patients may experience difficulties with cardiovascular, pulmonary, gastrointestinal, or varied loss of functions in other organs or systems.16 Challenges for patients include accurate and timely diagnosis, complex care necessitating multiple specialists, and lack of a clear treatment path especially regarding availability and duration of eculizumab therapy. Differentiating aHUS from alternate types of TMA or other medical conditions is difficult, and “diagnosis by exclusion” is not familiar to patients and their families. The aHUS Alliance partnered with Boston area physicians to create an innovative symposium on “aHUS through the Lens of Thrombotic Microangiopathy” (TMA Boston), which also featured aHUS patient speakers from three nations to precede each clinical presentation.57 Positive patient outcomes and negative impacts from the lack of access to eculizumab were addressed by aHUS patients of varied subtypes, each followed by clinical presentations illustrating the effectiveness of coordinated care from an aHUS and TMA multi-disciplinary team approach.58
A growing field of case studies and clinical trials illustrates the broad and diverse spectrum of aHUS clinical presentations, among them duration of aHUS activity (acute episodes or chronic illness), extra-renal involvement, disease penetrance in familial genetics, and triggering events. Longitudinal studies related to patient surveillance and monitoring are topics in need of further study, particularly for aHUS patients who have exhibited extended periods absent from aHUS activity. The current trend in personalized medicine is a challenge for patients and physician-researchers alike, with several multi-center studies or research efforts underway on such topics as individualized therapy, restrictive use,33 and optimal duration or withdrawal from eculizumab to include: CUREiHUS, NTR5988 (NL); STOPECU, NCT02574403 (FR); and SETSaHUS, ISRCTN17503205 (UK). Noting eculizumab use and shifting paradigms, Gema Ariceta stated:
Originally approved for long-life treatment, clinical experience from highly expert centers supports moving from a fixed treatment schedule to a personalized dosing, at least in selected patients.59
Eculizumab challenges are not only confined to the drug itself, but broaden to include the management of different aHUS subtypes, improved education and information flow, collaboration across geographic borders and market segments, integration of patients as partners across all stages of initiatives, and recognition of and response to physician concerns. Many of the knowledge gaps, diagnosis issues, access and cost issues, and patient or physician challenges will affect other stakeholders and efforts as new aHUS therapeutic options reach future markets.60
Cost and availability
Once labeled as the most expensive drug worldwide by Forbes, the cost of a single 30 mL/300 mg vial of eculizumab is around $6830 or £3150 with an annual cost of treatment being £350,000 or $465,635 approximately.61 The high price and limited availability of the drug limits its use, especially in the developing world.52,62 Reimbursement limitations, even in developed countries like UK, pose challenges to both patients and physicians and restrict the access to this life-saving drug.63 The biweekly hospitalization for the administration of eculizumab during the maintenance phase of treatment further adds to the rising health care-associated financial burden. “A balance between pharmaceutical companies’ incentives and societal budgetary constraints” must be maintained to ensure the timely and regular availability of orphan drugs like eculizumab.64
Infections
Due to its terminal complement blocking activity, patients treated with eculizumab have a high risk of contracting infections with encapsulated organisms especially Neisseria meningitidis. The risk is estimated to be 1000-fold to 2000-fold higher in these patients rather than the general population.65 All patients receiving eculizumab should be vaccinated with both the MenACWY conjugate vaccine and the MenB vaccine with a booster of MenACWY every 5 years as per CDC recommendations.66 However, conflicting reports raising concerns about the protectiveness of vaccination-induced antibodies have been described where despite appropriate vaccination, patients developed disseminated meningococcal infection.67,68 The efficacy of vaccination in a setting of AKI, chronic kidney disease, immunosuppressive agents, and during complement blockade is also questionable.
In cases where urgent eculizumab therapy is warranted, antibiotic prophylaxis for a minimum of 2 weeks after vaccination is recommended.69 But in countries like France and the UK, it is recommended that antibiotic prophylaxis be continued providing the patient is receiving eculizumab and up to 60 days after stopping the drug.55
In light of the reported incidences of meningococcal infections among vaccinated patients, there should be a high index of suspicion amongst physicians for signs and symptoms of meningococcal infection. In addition, awareness in patients receiving eculizumab therapy for clinical features of meningitis or meningococcemia can help in rapid diagnosis and prompt management. The health care professionals must provide a patient safety information card to all patients receiving eculizumab and should also discuss the importance and proper use of the card.36
When to start eculizumab therapy
Early initiation of eculizumab has been found to be associated with better treatment outcomes and greater improvements in eGFR in patients treated with eculizumab24 In conditions where eculizumab is unavailable, PE/PI should be started immediately. In adult patients, it is recommended to start initial therapy with PE/PI for 5–7 days while secondary causes of TMA like organ transplantation, malignancy, autoimmune diseases, drugs, malignant hypertension, and pre-existing nephropathy are ruled out.17,33
For children with aHUS, the consensus clinical practice recommendations generated by HUS International Group recommend early initiation of eculizumab therapy (i.e., within 24–48 hrs of admission). Walle et al, in a post-hoc analysis of four Phase II, open-label, single-arm, prospective clinical studies of eculizumab for patients with aHUS, demonstrated that the mean eGFR change from baseline to 1 year was significantly higher in patients treated with eculizumab in ≤7 days than ≥7 days (57 vs 23 mL/min/1.73 m2, p=0.0098) with improvements in eGFR being higher in younger patients.43
In a significant proportion of school-age children with aHUS, between the ages of 5 and 15 years, across the world,70,71 anti-FH antibodies are significantly elevated (1000–20,000 AU/mL). The aim of therapy in these cases is a reduction of antibody titers. The American Society for Apheresis assigns level I category to anti-FH associated HUS, implying that PE is a primary therapeutic intervention. Guidelines from expert groups72 recommend combined therapy with PE and immunosuppressive agents for patients with anti-FH antibodies. Prompt initiation and continued PE for at least 3–5 weeks are thus advised. Immunosuppression (oral steroids and IV cyclophosphamide) inhibits further production of antibodies, especially following PE, with improved short- and medium-term outcomes.
Many centers in the developed world initiate empiric therapy of aHUS with eculizumab. Once a high titer of anti-FH antibodies is found, there is a discussion whether eculizumab therapy be continued, or replaced by PE and immunosuppression. Complement blockade by eculizumab is effective in inducing hematological remission but has a limited impact on antibody titers. The use of eculizumab was reported in 18 patients with anti-FH-associated HUS, either after failing PE or as first-line therapy.73 Prospective studies are required to determine the comparative efficacy of eculizumab to PE and immunosuppression in these patients. Therapy with eculizumab is definitely indicated in patients who are refractory to PE, show life-threatening features, or have concomitant defect(s) in complement regulation.
Dosing interval and monitoring
The current dosing regimen for eculizumab includes an induction dose followed by twice-weekly maintenance doses. The regular administration of eculizumab at biweekly intervals can be questioned in the light of many small cohort studies demonstrating adequate complement blockade while increasing the maintenance dosing intervals to more than 2 weeks.74 Gatault et al predicted that the dosing interval for maintenance treatment could be increased to 4 weeks in patients <90 kg and 6 weeks in patients <70 kg while maintaining an adequate complement blockade by evaluating the pharmacokinetics of eculizumab. The study was conducted in a one compartment model with nine patients receiving eculizumab and authors observed mean trough eculizumab concentrations ranging from 55±12 to 733±164 mg/mL with 55% of the patients having trough levels >300 mg/mL.75 Ardissino et al evaluated 38 patients of aHUS, where 22 patients received eculizumab every 4 weeks and 16 every 3 weeks during the maintenance phase of treatment. With a target classical complement pathway activity of <30%, no relapses were noticed after a cumulative observation period of 1208 months.76 Cugno et al studied 18 patients with aHUS receiving eculizumab therapy and followed them for a period of 160 months with complement activity measured regularly. They demonstrated that complete complement blockade was maintained at 3 weeks and partial blockade at 4 weeks after eculizumab infusion.77 Data from the above studies certainly support increasing the time between maintenance doses, but it requires regular monitoring to promptly identify the relapses. The total complement activity (CH50) and alternative pathway complement activity (AH50) are the most commonly used tests to assess the complement pathway.78 More specific tests like eculizumab trough levels, ex-vivo serum-induced endothelial C5b-9 deposits, and soluble C5b-C9 levels (sC5b-C9) have also been introduced (Table 3)33,43,69,78–81 but are available only in limited laboratories. Hence, standardized tests which are uniformly available and can efficiently determine the level of complement inhibition are a pre-requisite to consider increasing the maintenance dosing intervals to more than 2 weeks.
Table 3.
Monitoring of eculizumab therapy and complement activity in atypical hemolytic uremic syndrome (aHUS)
| Mechanism | Recommended levels | Limitations | Advantages | |
|---|---|---|---|---|
| CH50 (total complement activity) | Detects the ability of serum complement to lyse 50% of sheep RBCs in a reaction mixture | <10% of normal | -Normal range depends on the type of assay used -CH50 levels will be low in congenital complement deficiency |
Easy availability |
| AH50 (alternative pathway hemolytic activity) | Tests the ability of alternate or terminal pathway complement components to lyse 50% of rabbit erythrocytes | <10% of normal | -Will be low in congenital deficiency of C3, CFI, CFB, properdin, CFH, and CFD -Normal range depends on the type of assay used |
Easy availability |
| Eculizumab trough levels | ELISA-based assay using C5 coated plates, patient sera, and an anti-human IgG | 50–100 mg/mL | -Assays detect both the bound and unbound fraction43 -Recommended trough levels are based on a meta-analysis of patients with paroxysmal nocturnal hemoglobinuria (PNH) treated with eculizumab33 |
Not affected by complement deficiencies |
| Ex vivo serum-induced endothelial C5b-9 deposits | Patient serum is added to activated endothelial cells and C5b-C9 deposition is assessed | -limited availability79 | ||
| Soluble C5b-C9 levels (sC5b-C9) | Abnormal activation of complement leads to elevated levels of sC5b-C9 and levels should decrease during treatment | Variable results in different studies80,81 | -Longer half-life, detects terminal complement activation as opposed to other markers (C3, C5a) of early complement activity78 |
Optimal duration of therapy
The use of eculizumab has dramatically improved the outcomes and prognosis of patients with aHUS. But it becomes immensely difficult to predict the time between the relapses in patients with aHUS due to the natural history of aHUS depending on the underlying genetic abnormality. One of the most inordinate questions associated with eculizumab therapy is the optimal duration of treatment. Current recommendations for considering eculizumab cessation emphasize on a case by case analysis in patients who have received the treatment for a minimum of 6–12 months and have had a normal renal function for at least 3 months except in recipients of renal transplant, patients with recurrent relapses, and children until they are 3–5 years old.55,69 Following discontinuation of eculizumab, patients with aHUS should be monitored for signs and symptoms of TMA complications for at least 12 weeks. Wjisma et al recently proposed a protocol for eculizumab therapy in patients with native kidneys having aHUS, who are in remission after the first episode. The protocol advocates standard treatment for 3 months with eculizumab during the induction phase followed by five proposed scenarios for either discontinuation or tapering of eculizumab dose with monitoring for recurrence of TMA at regular intervals.33
Data supporting eculizumab cessation are limited to only small cohort studies and case reports. Macia et al analyzed the data from case studies of patients with aHUS who discontinued eculizumab and identified that 30% patients who received multiple doses of eculizumab and 80% who received one dose relapsed after treatment discontinuation.82 One of the drawbacks of these studies is their small duration of follow-up. A longer follow-up period is required to look for the long-term outcomes of treatment discontinuation and to identify the factors associated with higher risks of relapses. The discontinuation of eculizumab as per physician’s discretion introduces a selection bias and may trivialize these results necessitating a need for larger scale studies with a longer duration of follow-up and set protocols to ensure proper randomization.
Underlying genetic mutations with CFH were noted to be most predominant in the majority of the patients who relapsed after stopping treatment (Table 4).47,82–86 Fakhouri et al demonstrated that 72% patients with CFH variants, 50% with MCP variants, and none of 16 patients with no rare identified variant relapsed after discontinuation of eculizumab.83 In conclusion, patients with underlying genetic mutations in complement genes seem to have a greater risk of relapse after eculizumab discontinuation. However, the limited access and lack of timely availability of the results of genetic analysis present a hurdle in using them consistently.
Table 4.
Studies describing the outcome of eculizumab regimen discontinuation in aHUS patients
| Study group | Number of patients | Median duration of eculizumab treatment | Number of patients who discontinued treatment with eculizumab | Median duration of follow-up after stopping treatment | Number of patients who relapsed after discontinuation | Proportion of relapsed patients with mutations and type of mutations | Outcome |
|---|---|---|---|---|---|---|---|
| Ardissino et al84 (2015) | 16 | 4.3 (0.5–14.4) months | 16 | 0.7–40 months | 5/16 (31.2%) | 5/5 (CFH-4, CFI-1) | All patients who relapsed were restarted on eculizumab and had rapid improvement of renal function. |
| Fakhouri et al83(2017) | 108 | 17.5 months | 38 | 22 months | 12/38 (31.5%) | 12/12 (CFH-8, MCP-4) | All patients had rapid remission of TMA after restarting eculizumab with no long-term sequelae. |
| Meril et al47(2017) | 17 | 3 months | 15 | 10.2 months | 3/15 (20%) | 3/3 (CFH-2, ADAMTS 13–1) | 2 of 3 patients restarted eculizumab and renal function returned to baseline. |
| Macia et al82(data from clinical trials) (2017) | 130 | 6.3 months | 61 | 6.3 months | 12/61 (19.6) | 7/12 (CHF-5, others-2) | 3 patients progressed to ESRD with one patient requiring dialysis despite restarting eculizumab |
| Wijnsma et al85 (2017) | 20 | 3.8 months | 17 | 27.4 months | 5/17 (29.4%) | 5/5 (CFH-4, C3-1) | No chronic sequelae after restart of eculizumab were noted. |
| Sheerin et al86 (2016) | 43 | 6 months | 14 | 12 months | 3/14(21%) | 2/3 (CFH-1, CD46-1) | Complete recovery was noted in all three patients after reintroducing eculizumab. |
Ahus and kidney transplant
Patients with aHUS need dialysis or kidney transplant if they develop an ESRD secondary to aHUS. A significant proportion of patients who develop ESRD have underlying genetic mutations (Table 5).3–5,23,47,80,87–89,91–97 Caprioli et al performed genetic analysis in 156 patients with non-Shiga toxin HUS. They demonstrated that patients with FH and IF mutations had unfavorable outcomes post-kidney transplant due to the recurrence of the disease.4
Table 5.
Frequency of patients with mutations and ESRD in aHUS
| IDENTIFIED MUTATIONS | Frequency in patients with aHUS (%)3-5,23,47,88–96 | ESRD or death within 3–10 years of diagnosis (%)3,5,23,96,97 | |
|---|---|---|---|
| CFH mutations | 20–52 | 66–80 | |
| CFH autoantibodies and/or CFHR1-3 deletions | 5–10 | 30–63 | |
| CFI mutations | 4–10 | 50–72 | |
| THBD mutations | 3-10 | 54–60 | |
| C3 mutations | 2–10 | 56–67 | |
| CFB mutations | 1–4 | 70 | |
| Isolated MCP mutations | 5–15 | 6–38 | |
| Combined MCP mutations | 2 | 47 | |
| DGKE mutations | ~27 | 46 | |
| NO IDENTIFIED MUTATIONS | 30–50 | 32–50 | |
Abbreviations: CFB, complement factor B; CFD, complement factor D; CFI, complement factor I; CFH, complement factor H; MCP, membrane cofactor protein; THBD, thrombomodulin gene; MAC, membrane attack complex; CFHR, complement factor receptor; DGKE, diacylglycerol kinase ε; aHUS, atypical hemolytic uremic syndrome.
Quintrec et al demonstrated a 5-year graft survival rate of 51% which was associated with a recurrence of the disease in the graft.87 Legendre et al performed a post-hoc analysis of four prospective clinical trials of eculizumab in aHUS. Amongst 100 patients, 26 patients had transplanted kidneys and had received totaling 38 grafts. After eculizumab, no patient was reported to have graft loss and an improvement in eGFR was noted from the baseline in 25 of the 26 patients.88 Siedlecki et al89 in an observational study, described 188 from the Global aHUS registry who received kidney transplant and received eculizumab. The patients were divided into two groups and followed postoperatively for a minimum of 1 year. Group 1 (n=88) included patients who had received eculizumab before and during their most recent transplant while group 2 (n=100) included patients who received eculizumab after transplant. They observed a significantly better graft function within 6 months of transplantation in group 1 compared with group 2.
Despite data suggesting the efficacy of eculizumab in prevention of recurrence of aHUS in allograft, there are concerns regarding the dosing and duration of treatment.
Conclusion
Eculizumab, though has revolutionized the treatment of aHUS, also has limitations. Financial constraints, due to the high cost and need for hospitalization, limit the use of eculizumab in the developed world. Paucity of data on the natural history of aHUS, especially in relation to underlying genetic mutations, makes it difficult to predict relapses and raises concerns about appropriate duration of treatment. Large-scale prospective trials of longer duration are needed to improve treatment strategies for the long-term management of patients with aHUS.
Acknowledgment
The authors would like to thank Jennifer Clark for her contribution in editing and reviewing the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371:654–666. doi: 10.1056/NEJMoa1410490 [DOI] [PubMed] [Google Scholar]
- 2.Amorosi E, Ultmann J. Thrombotic thrombocytopenic purpura: report of 16 cases and review of the literature. Medicine. 1966;45:139–159. doi: 10.1097/00005792-196603000-00003 [DOI] [Google Scholar]
- 3.Noris M, Remuzzi G. Atypical hemolytic–uremic syndrome. N Engl J Med. 2009;361:1676–1687. doi: 10.1056/NEJMra0902814 [DOI] [PubMed] [Google Scholar]
- 4.Caprioli J, Noris M, Brioschi S, et al. Genetics of HUS: the impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood. 2006;108:1267–1279. doi: 10.1182/blood-2006-03-013334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sellier-Leclerc AL, Fremeaux-Bacchi V, Dragon-Durey MA, et al. Differential impact of complement mutations on clinical characteristics in atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2007;18(8):2392–2400. Epub 2007 June 28. doi: 10.1681/ASN.2006080811 [DOI] [PubMed] [Google Scholar]
- 6.Laurence J. Atypical hemolytic uremic syndrome (aHUS): making the diagnosis. Clin Adv Hematol Oncol. 2012;10(10 S17):1–12. [PubMed] [Google Scholar]
- 7.Noris M, Bucchioni S, Galbusera M, et al. Complement factor H mutation in familial thrombotic thrombocytopenic purpura with ADAMTS13 deficiency and renal involvement. J Am Soc Nephrol. 2005;16(5):1177–1183. doi: 10.1681/ASN.2005010086 [DOI] [PubMed] [Google Scholar]
- 8.Ohanian M, Cable C, Halka K. Eculizumab safely reverses neurologic impairment and eliminates need for dialysis in severe atypical hemolytic uremic syndrome. Clin Pharmacol. 2011;3:5–12. Epub 2011 May 24. doi: 10.2147/CPAA.S17904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dragon-Durey MA, Sethi SK, Bagga A. Clinical features of anti-factor H autoantibody-associated hemolytic uremic syndrome. J Am Soc Nephrol. 2010;21(12):2180–2187. Epub 2010 Nov 4. doi: 10.1681/ASN.2010030315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Akash SI, Almond PS, Savell VH Jr, Gharaybeh SI, Hogue C. Eculizumab induces long-term remission in recurrent post-transplant HUS associated with C3 gene mutation. Pediatr Nephrol. 2011;26(4):613–619. Epub 2010 Dec 2. doi: 10.1007/s00467-010-1708-6 [DOI] [PubMed] [Google Scholar]
- 11.Sallée M, Daniel L, Piercecchi MD, et al. Myocardial infarction is a complication of factor H-associated atypical HUS. Nephrol Dial Transplant. 2010;25(6):2028–2032. Epub 2010 Mar 19. doi: 10.1093/ndt/gfq160 [DOI] [PubMed] [Google Scholar]
- 12.Noris M, Remuzzi G. Cardiovascular complications in atypical hemolytic uremic syndrome. Nat Rev Nephrol. 2014;10:174. doi: 10.1038/nrneph.2013.280 [DOI] [PubMed] [Google Scholar]
- 13.Loirat C, Noris M, Fremeaux-Bacchi V. Complement and the atypical hemolytic uremic syndrome in children. Pediatr Nephrol. 2008;23(11):1957–1972. Epub 2008 Jul 2. doi: 10.1007/s00467-008-0872-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larakeb A1, Leroy S, Frémeaux-Bacchi V, et al. Ocular involvement in hemolytic uremic syndrome due to factor H deficiency--are there therapeutic consequences? Pediatr Nephrol. 2007;22(11):1967–1970. Epub 2007 Jul 10. doi: 10.1007/s00467-007-0540-0 [DOI] [PubMed] [Google Scholar]
- 15.Zheng X, Gorovoy IR, Mao J, Jin J, Chen X, Cui QN. Recurrent ocular involvement in pediatric atypical hemolytic uremic syndrome. J Pediatr Ophthalmol Strabismus. 2014;51:e62–5. doi: 10.3928/01913913-20140923-03 [DOI] [PubMed] [Google Scholar]
- 16.Raina R, Krishnappa V, Blaha T, et al. Atypical hemolytic uremic syndrome: an update on pathophysiology, diagnosis, and treatment. Ther Apher Dial. 2019;23(1):4–21. doi: 10.1111/1744-9987.12763 [DOI] [PubMed] [Google Scholar]
- 17.Fakhouri F, Zuber J, Frémeaux-Bacchi V, Loirat C. Haemolytic uraemic syndrome. Lancet. 2017;38:817–824. [DOI] [PubMed] [Google Scholar]
- 18.Moskovich O, Fishelson Z. Live cell imaging of outward and inward vesiculation induced by the complement C5b‐9 complex. J Biol Chem. 2007;282(41):29977–29986. doi: 10.1074/jbc.M703742200 [DOI] [PubMed] [Google Scholar]
- 19.Walport MJ. Complement. First of Two Parts. N Engl J Med. 2001;344(14):1058–1066. doi: 10.1056/NEJM200104053441406 [DOI] [PubMed] [Google Scholar]
- 20.Noris M, Remuzzi G. Overview of complement activation and regulation. Semin Nephrol. 2013;33(6):479–492. doi: 10.1016/j.semnephrol.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Licht C, Kirsch M. The role of complement in disease In: Geary D, Schaefer F, editors. Pediatric Kidney Disease. Berlin (Heidelberg): Springer; 2016:583–596. [Google Scholar]
- 22.Vaisbich MH. Hemolytic-uremic syndrome in childhood. J Bras Nefrol. 2014;36(2):208–220. [DOI] [PubMed] [Google Scholar]
- 23.Noris M, Caprioli J, Bresin E, et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010;5(10):1844–1859. doi: 10.2215/CJN.02210310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor Eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368:2169–2181. doi: 10.1056/NEJMoa1208981 [DOI] [PubMed] [Google Scholar]
- 25.Krisinger MJ, Goebeler V, Lu Z. Thrombin generates previously unidentified C5 products that support the terminal complement activation pathway. Blood. 2012;120(8):1717–1725. Epub 2012 July 16. doi: 10.1182/blood-2012-02-412080 [DOI] [PubMed] [Google Scholar]
- 26.Stuhlinger W, Kourilsky O, Kanfer A, Sraer JD. Letter: haemolytic-uraemic syndrome: evidence for intravascular C3 activation. Lancet. 1974;2:788–789. doi: 10.1016/S0140-6736(74)90991-X [DOI] [PubMed] [Google Scholar]
- 27.Lemaire M, Frémeaux-Bacchi V, Schaefer F, et al. Recessive mutations in DGKE cause atypical hemolytic-uremic syndrome. Nat Genet. 2013;45(5):531–536. Epub 2013 Mar 31. doi: 10.1038/ng.2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michon B, Moghrabi A, Winikoff R, et al. Complications of apheresis in children. Transfusion. 2007;47(10):1837–1842. doi: 10.1111/j.1537-2995.2007.01405.x [DOI] [PubMed] [Google Scholar]
- 29.Palma LM, Langman CB. Critical appraisal of Eculizumab for atypical hemolytic uremic syndrome. J Blood Med. 2016;7:39–72. doi: 10.2147/JBM.S36249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao M, Leite BN, Ferreiro T, et al. Eculizumab modifies outcomes in adults with atypical hemolytic uremic syndrome with acute kidney injury. Am J Nephrol. 2018;48(3):225–233. Epub 2018 Sep 11. doi: 10.1159/000492865 [DOI] [PubMed] [Google Scholar]
- 31.Kato H, Miyakawa Y, Hidaka Y, et al. Safety and effectiveness of Eculizumab for adult patients with atypical hemolytic-uremic syndrome in Japan: interim analysis of post-marketing surveillance. Clin Exp Nephrol. 2018;23(1):65–75. doi: 10.1007/s10157-018-1609-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenbaum L, Legendre CM, Babu S, et al. Eculizumab (ECU) in Atypical Hemolytic Uremic Syndrome (aHUS) Patients with Progressing Thrombotic Microangiopathy (TMA): 2-year data. Blood. 2012;120(21):2084. [Google Scholar]
- 33.Wijnsma KL, Duineveld C, Wetzels JFM, et al. Eculizumab in atypical hemolytic uremic syndrome: strategies toward restrictive use. Pediatr Nephrol. 2018. doi: 10.1007/s00467-018-4091-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidtko J, Peine S, El-Housseini Y, Pascual M, Meier P. Treatment of atypical hemolytic uremic syndrome and thrombotic microangiopathies: a focus on Eculizumab. Am J Kidney Dis. 2013;61(2):289–299. Epub 2012 Nov 7. doi: 10.1053/j.ajkd.2012.07.028 [DOI] [PubMed] [Google Scholar]
- 35.Harder MJ, Kuhn N, Schrezenmeier H, et al. Incomplete inhibition by Eculizumab: mechanistic evidence for residual C5 activity during strong complement activation. Blood. 2017;129(8):970–980. doi: 10.1182/blood-2016-08-732800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soliris (Eculizumab) highlights of prescribing information.US Food and Drug Administration. 2007. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125166s172lbl.pdf Accessed February28, 2019.
- 37.Wijnsma KL, Ter Heine R, Moes DJAR, et al. Pharmacology, pharmacokinetics and pharmacodynamics of eculizumab, and possibilities for an individualized approach to eculizumab. Clin Pharmacokinet. 2019;58(7):859–874.. doi: 10.1007/s40262-019-00742-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krishnappa V, Gupta M, Shah H, et al. Atypical hemolytic uremic syndrome: a meta-analysis of case reports confirms the prevalence of genetic mutations and the shift of treatment regimens. Ther Apher Dial. 2018;22:178–188. doi: 10.1111/1744-9987.12641 [DOI] [PubMed] [Google Scholar]
- 39.Rathbone J, Kaltenthaler E, Richards A, Tappenden P, Bessey A, Cantrell A. A systematic review of eculizumab for atypical haemolytic uraemic syndrome (aHUS). BMJ Open. 2013;3:e003573. doi: 10.1136/bmjopen-2013-003573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Licht C, Greenbaum LA, Muus P, et al. Efficacy and safety of Eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int. 2015;87:1061–1073. doi: 10.1038/ki.2014.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cofiell R, Kukreja A, Bedard K, et al. Eculizumab reduces complement activation, inflammation, endothelial damage, thrombosis, and renal injury markers in aHUS. Blood. 2015;125:3253–3262. doi: 10.1182/blood-2014-09-600411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cavero T, Rabasco C, Lopez A, et al. Eculizumab in secondary atypical hemolytic uraemic syndrome. Nephrol Dial Transplant. 2017;32:466–474. doi: 10.1093/ndt/gfw453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walle JV, Delmas Y, Ardissino G, Wang J, Kincaid JF, Haller H. Improved renal recovery in patients with atypical hemolytic uremic syndrome following rapid initiation of Eculizumab treatment. J Nephrol. 2017;30:127–134. doi: 10.1007/s40620-016-0288-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greenbaum LA, Fila M, Ardissino G, et al. Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney Int. 2016;89:701–711. doi: 10.1016/j.kint.2015.11.026 [DOI] [PubMed] [Google Scholar]
- 45.Kumar G, Al-Masri O, Alismaili Z, et al. Eculizumab in pediatric atypical hemolytic uremic syndrome: lessons learned from a single center experience in the United Arab Emirates. J Paediatr Child Health. 2019. doi: 10.1111/jpc.14390 [DOI] [PubMed] [Google Scholar]
- 46.Fakhouri F, Hourmant M, Campistol JM, et al. Terminal complement inhibitor eculizumab in adult patients with atypical hemolytic uremic syndrome: a single-arm, open-label trial. Am J Kidney Dis. 2016;68(1):84–93. doi: 10.1053/j.ajkd.2015.12.034 [DOI] [PubMed] [Google Scholar]
- 47.Merrill SA, Brittingham ZD, Yuan X, Moliterno AR, Sperati CJ, Brodsky RA. Eculizumab cessation in atypical hemolytic uremic syndrome. Blood. 2017;130:368–372. doi: 10.1182/blood-2017-02-770214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huerta A, Arjona E, Portoles J, et al. A retrospective study of pregnancy-associated atypical hemolytic uremic syndrome. Kidney Int. 2018;93:450–459. doi: 10.1016/j.kint.2017.06.022 [DOI] [PubMed] [Google Scholar]
- 49.aHUS Global Patients’ Research Agenda. aHUS Alliance, consensus document released. February 28, 2019. Available from: https://bit.ly/2UH5nZc. Accessed July23, 2019.
- 50.Burke L, Woodward L, Shah KD. Graphs & Insights - Results from the 2016 aHUS Global Poll. aHUS Alliance. July 2016. Article Available from: https://bit.ly/2FXtFpW. Accessed July23, 2019.
- 51.Access to aHUS Treatment: 2016 aHUS Global Poll White Paper. Orphan drug access – are rare disease treatments like eculizumab equally available worldwide? Available from: https://bit.ly/2VtqgnZ. Accessed July23, 2019.
- 52.Karpman D, Höglund P. Orphan drug policies and use in pediatric nephrology. Pediatr Nephrol. 2017;32:1–6. doi: 10.1007/s00467-016-3520-4 [DOI] [PubMed] [Google Scholar]
- 53.Gottlich E, McCulloch M South African paediatric nephrology perspective on eculizumab therapy for atypical HUS: a global review panel model for aHUS drug access. aHUS Alliance article. Available from: https://bit.ly/2Vw58h6. Accessed July23, 2019.
- 54.Woodward L, Johnson S, Walle JV, et al. An innovative and collaborative partnership between patients with rare disease and industry-supported registries: the Global aHUS Registry. Orphanet J Rare Dis. 2016;11:154. doi: 10.1186/s13023-016-0537-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goodship TH, Cook HT, Fakhouri F, et al. Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a “Kidney Disease: improving Global Outcomes” (KDIGO) controversies conference. Kidney Int. 2017;91(3):539–551. Epub 2016 Dec 16. doi: 10.1016/j.kint.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 56.Harris DCH, Davies SJ, Finkelstein FO, et al. Increasing access to integrated ESKD care as part of universal health coverage. Kidney Int. 2019;95(4S):S1–S33. doi: 10.1016/j.kint.2018.12.005 [DOI] [PubMed] [Google Scholar]
- 57.TMA Boston, link to article w. agenda Available from: https://bit.ly/2uPBo2G. Accessed July23, 2019.
- 58.Gordon CE, Chitalia VC, Sloan JM, et al. Thrombotic microangiopathy: a multidisciplinary team approach. Am J Kidney Dis. 2017;70(5):715–721. doi: 10.1053/j.ajkd.2017.05.017 [DOI] [PubMed] [Google Scholar]
- 59.Ariceta G. Optimal duration of treatment with Eculizumab in atypical hemolytic uremic syndrome (aHUS)—a question to be addressed in a scientific way. Pediatr Nephrol. 2019;34(5):943–949. [DOI] [PubMed] [Google Scholar]
- 60.Burke L Atypical HUS therapeutic drug pipeline in 2018: drug discovery and market factors within the aHUS Arena. aHUS Alliance article. May 2018. Available from: https://bit.ly/2OSHQ2h. Accessed July23, 2019.
- 61. The rising cost of orphan drugs. Lancet Haematol. 2015;2(11):e456. doi: 10.1016/S2352-3026(15)00229-X [DOI] [PubMed] [Google Scholar]
- 62.Sethi SK, Rohatgi S, Dragon-Durey MA, et al. Eculizumab for atypical hemolytic-uremic syndrome in India: first report from India and the challenges faced. Indian J Nephrol. 2017;27(1):58–61. doi: 10.4103/0971-4065.179369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.NICE (National Institute for Health and Care Excellence). Eculizumab for treating atypical haemolytic uraemic syndrome. Available from: https://www.nice.org.uk/guidance/hst1/resources/Eculizumab-fortreating-atypical-haemolytic-uraemic-syndrome-pdf. Published January 2015. Accessed February28, 2019.
- 64.Rodriguez-Monguio R, Spargo T, Seoane-Vazquez E. Ethical imperatives of timely access to orphan drugs: ispossible to reconcile economic incentives and patients’ health needs? Orphanet J Rare Dis. 2017;12(1):1. doi: 10.1186/s13023-016-0551-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Food and Drug Administration. Alexion briefing information for the November 18, 2014, meeting of the Drug Safety and Risk Management Advisory Committee. Available from: https://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DrugSafetyandRiskManagementAdvisoryCommittee/ucm423029.htm. Accessed July23, 2019.
- 66.Managing the risk of meningococcal disease among patients who receive eculizumab therapy. Available from: https://www.cdc.gov/meningococcal/clinical/eculizumab.html Accessed March3, 2019.
- 67.Cullinan N, Gorman KM, Riordan M, Waldron M, Goodship TH, Awan A. Case report: benefits and challenges of long-term Eculizumab in atypical hemolytic uremic syndrome. Pediatrics. 2015;135:e1506–e1509. doi: 10.1542/peds.2014-3503 [DOI] [PubMed] [Google Scholar]
- 68.Struijk GH, Bouts AH, Rijkers GT, Kuin EA, Ten Berge IJ, Bemelman FJ. Meningococcal sepsis complicating Eculizumab treatment despite prior vaccination. Am J Transplant. 2013;13:819–820. doi: 10.1111/ajt.12032 [DOI] [PubMed] [Google Scholar]
- 69.Loirat C, Fakhouri F, Ariceta G, et al. An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatr Nephrol. 2016;31:15–39. doi: 10.1007/s00467-015-3076-8 [DOI] [PubMed] [Google Scholar]
- 70.Loirat C, Fremeaux-Bacchi V. Anti-factor H autoantibody- associated hemolytic uremic syndrome: the earlier diagnosed and treated, the better. Kidney Int. 2014;85:1019–1022. doi: 10.1038/ki.2013.447 [DOI] [PubMed] [Google Scholar]
- 71.Schaefer F, Ardissino G, Ariceta G. Clinical and genetic predictors of atypical hemolytic uremic syndrome phenotype and outcome. Kidney Int. 2018;94(2):408–418. Epub 2018 Jun 19. doi: 10.1016/j.kint.2018.02.029 [DOI] [PubMed] [Google Scholar]
- 72.Bagga A, Khandelwal P, Mishra K, et al. Hemolytic uremic syndrome in a developing country: consensus guidelines. Pediatr Nephrol. 2019;34:1465–1482. Epub ahead of print. doi: 10.1007/s00467-019-04233-7 [DOI] [PubMed] [Google Scholar]
- 73.Fakhouri F, Loirat C. Anticomplement treatment in atypical and typical hemolytic uremic syndrome. Semin Hematol. 2018;55:150–158. doi: 10.1053/j.seminhematol.2018.04.009 [DOI] [PubMed] [Google Scholar]
- 74.Volokhina E, Wijnsma K, van der Molen R, et al. Eculizumab dosing regimen in atypical HUS: possibilities for individualized treatment. Clin Pharmacol Ther. 2017;102:671–678. doi: 10.1002/cpt.686 [DOI] [PubMed] [Google Scholar]
- 75.Gatault P, Brachet G, Ternant D, et al. Therapeutic drug monitoring of Eculizumab: rationale for an individualized dosing schedule. mAbs. 2015;7(6):1205–1211. doi: 10.1080/19420862.2015.1086049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ardissino G, Tel F, Sgarbanti M, et al. Complement functional tests for monitoring Eculizumab treatment in patients with atypical hemolytic uremic syndrome: an update. Pediatr Nephrol. 2018;33(3):457–461. Epub 2017 Oct 18. doi: 10.1007/s00467-017-3813-2 [DOI] [PubMed] [Google Scholar]
- 77.Cugno M, Gaultierotti R, Possenti I, et al. Complement functional tests for monitoring Eculizumab treatment in patients with atypical hemolytic uremic syndrome. J Thromb Haemost. 2014;12(9):1440–1448. Epub 2014 Jul 16. doi: 10.1111/jth.12615 [DOI] [PubMed] [Google Scholar]
- 78.Ricklin D, Barratt-Due A, Mollnes TE. Complement in clinical medicine: clinical trials, case reports and therapy monitoring. Mol Immunol. 2017;89:10–21. doi: 10.1016/j.molimm [DOI] [PubMed] [Google Scholar]
- 79.Noris M, Galbusera M, Gastoldi S, et al. Dynamics of complement activation in aHUS and how to monitor Eculizumab therapy. Blood. 2014;124(11):1715–1726. Epub 2014 Jul 18. doi: 10.1182/blood-2014-02-558296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wehling C, Amon O, Bommer M, et al. Monitoring of complement activation biomarkers and Eculizumab in complement mediated renal disorders. ClinExp Immunol. 2017;187:304–315. doi: 10.1111/cei.12890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Puissant-Lubrano B, Puissochet S, Congy-Jolivet N, et al. Alternative complement pathway hemolytic assays reveal incomplete complement blockade in patients treated with Eculizumab. Clin Immunol. 2017;183:1–7. doi: 10.1016/j.clim.2017.06.007 [DOI] [PubMed] [Google Scholar]
- 82.Macia M, de Alvaro Moreno F, Dutt T, et al. Current evidence on the discontinuation of Eculizumab in patients with atypical haemolytic uraemic syndrome. Clin Kidney J. 2017;10:310–319. doi: 10.1093/ckj/sfw115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fakhouri F, Fila M, Provôt F, et al. Pathogenic variants in complement genes and risk of atypical hemolytic uremic syndrome relapse after Eculizumab discontinuation. Clin J Am Soc Nephrol. 2017;12:50–59. doi: 10.2215/cjn.06440616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ardissino G, Possenti I, Tel F, Testa S, Salardi S, Ladisa V. Discontinuation of Eculizumab treatment in atypical hemolytic uremic syndrome: an update. Am J Kidney Dis. 2015;66:172–173. doi: 10.1053/j.ajkd.2015.04.010 [DOI] [PubMed] [Google Scholar]
- 85.Wijnsma KL, Duineveld C, Volokhina EB, van Den Heuvel LP, van de Kar N, Wetzels JFM. Safety and effectiveness of restrictive Eculizumab treatment in atypical haemolytic uremic syndrome. Nephrol Dial Transplant. 2018;33(4):635–645. doi: 10.1093/ndt/gfx196 [DOI] [PubMed] [Google Scholar]
- 86.Sheerin NS, Kavanagh D, Goodship TH, Johnson S. A national specialized service in England for atypical haemolytic uraemic syndrome-the first years’ experience. QJM. 2016;109:27–33. doi: 10.1093/qjmed/hcv082 [DOI] [PubMed] [Google Scholar]
- 87.Le Quintrec M, Zuber J, Moulin B, et al. Complement genes strongly predict recurrence and graft outcome in adult renal transplant recipients with atypical hemolytic and uremic syndrome. Am J Transplant. 2013;13:663–675. doi: 10.1111/ajt.12077 [DOI] [PubMed] [Google Scholar]
- 88.Legendre CM, Campistol JM, Feldkamp T, et al. Outcomes of patients with atypical haemolytic uraemic syndrome with native and transplanted kidneys treated with Eculizumab: a pooled post hoc analysis. Transpl Int. 2017;30:1275–1283. doi: 10.1111/tri.13022 [DOI] [PubMed] [Google Scholar]
- 89.Siedlecki AM, Isbel N, Vande Walle J, et al. Eculizumab use for kidney transplantation in patients with a diagnosis of atypical hemolytic uremic syndrome. Kidney Int Rep. 2019;4(3):434–446. doi: 10.1016/j.ekir.2018.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fremeaux-Bacchi V, Fakhouri F, Garnier A, et al. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol. 2013;8(4):554–562. Epub 2013 Jan 10. doi: 10.2215/CJN.04760512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Noris M, Remuzzi G. Managing and preventing atypical hemolytic uremic syndrome recurrence after kidney transplantation. Curr Opin Nephrol Hypertens. 2013;22(6):704–712. doi: 10.1097/MNH.0b013e328365b3fe [DOI] [PubMed] [Google Scholar]
- 92.Loirat C, Frémeaux-Bacchi V. Atypical hemolytic uremic syndrome. Orphanet J Rare Dis. 2011;6:60. doi: 10.1186/1750-1172-6-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pickering MC, de Jorge EG, Martinez-Barricarte R, et al. Spontaneous hemolytic uremic syndrome triggered by complement factor H lacking surface recognition domains. J Exp Med. 2007;204(6):1249–1256. Epub 2007 May 21. doi: 10.1084/jem.20070301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Esparza-Gordillo J, Goicoechea de Jorge E, Buil A, et al. Predisposition to atypical hemolytic uremic syndrome involves the concurrence of different susceptibility alleles in the regulators of complement activation gene cluster in 1q32. Hum Mol Genet. 2005;14(5):703–712. Epub 2005 Jan 20. doi: 10.1093/hmg/ddi066 [DOI] [PubMed] [Google Scholar]
- 95.Kavanagh D, Goodship TH, Richards A. Atypical haemolytic uraemic syndrome. Br Med Bull. 2006;77-78:5–22. Epub 2006 Sep 11. doi: 10.1093/bmb/ldl004 [DOI] [PubMed] [Google Scholar]
- 96.Kavanagh D, Goodship TH. Atypical hemolytic uremic syndrome, genetic basis, and clinical manifestations. Hematol Am Soc Hematol Educ Program. 2011;2011:15–20. doi: 10.1182/asheducation-2011.1.15 [DOI] [PubMed] [Google Scholar]
- 97.Bresin E, Rurali E, Caprioli J, et al. Combined complement gene mutations in atypical hemolytic uremic syndrome influence clinical phenotype. J Am Soc Nephrol. 2013;24(3):475–486. Epub 2013 Feb 21. doi: 10.1681/ASN.2012090884 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Soliris (Eculizumab) highlights of prescribing information.US Food and Drug Administration. 2007. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125166s172lbl.pdf Accessed February28, 2019.