Abstract
Pulmonary hypertension (PH) is a common complication of chronic obstructive pulmonary disease (COPD) and is associated with increased morbidity and mortality. Reference standard method to diagnose PH is right heart catheterization. Several non-invasive imaging techniques have been employed in the detection of PH. Among them, computed tomography (CT) is the most commonly used for phenotyping and detecting complications of COPD. Several CT findings have also been described in patients with severe PH. Nevertheless, CT analysis is currently based on visual findings which can lead to reproducibility failure. Therefore, there is a need for quantification in order to assess objective criteria. In this review, progresses in automated analyses of CT parameters and their values in predicting PH and COPD outcomes are presented.
Keywords: computed tomography, pulmonary hypertension, COPD, prediction
Introduction
Chronic obstructive pulmonary disease (COPD) is a common, preventable and treatable disease that is characterized by persistent respiratory symptoms (ie, dyspnea, cough and/or sputum production) and airflow limitation that is due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases.1 COPD has an increasing prevalence worldwide and is mainly caused by tobacco smoke exposure. Diagnosis is based on spirometry with a post-bronchodilator FEV1/FVC ratio lower than 0.70.2 The level of COPD severity is now assessed using both functional (ie, FEV1 in percentage of predicted values) and clinical data (ie, symptoms scores (CAT and/or mMRC) and the number of exacerbations within the previous 12 months).2
COPD is frequently associated with comorbidities. Among them, pulmonary hypertension (PH) is causally related to COPD. PH is defined by a mean pulmonary arterial pressure (mPAP) equal or higher than 25 mmHg at rest, measured using the reference method right heart catheterization (RHC).3–6 The prevalence of PH and the underlying pathophysiologic processes in patients suffering from COPD remain unclear. Indeed, prevalence increases with COPD severity, and its rate has been reported varying from 20% to 90%.7–11 An increase of mPAP is associated with an increased number of hospitalizations, morbidity and mortality in COPD.12–15 The few patients with severe PH secondary to COPD, defined at RHC as a mPAP ≥35 mmHg or mPAP ≥25 with cardiac index (CI) <2 l/min/m2, are considered as a subgroup with potentially serious and high complication rate.5 So far, only a few studies have evaluated the effects of vasodilator in patients with severe PH secondary to COPD, meaning that this subpopulation might benefit from a specific care.16–18
There are no specific clinical symptoms of PH, and this complication starts insidiously, the most common symptom being dyspnea.8 PH in COPD patients has been shown to be associated with an increased exacerbation rate,8 worsen exercise capacity8 and a poorer prognosis.8,13,15 For instance in a study from Andersen et al,19 the survival rate was 63% for COPD patients without PH vs 37% in those with PH. The most reliable index of PH is the mPAP, a parameter so far measured only by RHC, an invasive technique which remains the reference method for the diagnosis of PH.3,4 This unique test enables a direct assessment of various pulmonary arterial pressure indices, systolic, diastolic, mean and capillary pressures, as well as resistance, cardiac output and therefore enables to differentiate pre- and post-capillary PH,20,21 a major way for assessing PH etiology. However, RHC can be responsible for the occurrence of adverse events, related to complication to venous access (such as hematoma, pneumothorax), but also those due to arrhythmias, hypotensive episodes due to vagal reaction or pulmonary vasoreactivity testing. Whereas RHC serious adverse events remain rare in experienced centers (ie, 1.1% and mortality is extremely rare),22 RHC is not totally safe, especially in unstable patients. Thus, noninvasive diagnosis techniques able to reduce the delay in diagnosing PH and to avoid side effects of RHC are suitable. Echocardiography has been proposed to evaluate systolic pulmonary arterial pressure (sPAP), which is correlated with mPAP, but its specificity is low.23 However, echocardiography remains the primary tool for patients screening, especially in others PH’s etiologies.4 Nevertheless, echocardiography may be technically difficult to perform, especially in patients with an inflated thorax and/or emphysema. Computed tomography (CT) is a common tool to investigate COPD and its role is crucial in the diagnosis and characterization of emphysema, airway disease, and advocated in various clinical situations such as follow-up, exacerbations, pre-operative surgery and lung transplantation.2,24–27 CT is a useful tool to assess in vivo observation; nevertheless, radiation exposure risk is not null;28 in addition, large inappropriate indications of this examination might lead to overrun radiology departments. Moreover, iodine contrast injection and repetitive CT acquisitions are needed to assess hemodynamic alterations related to PH which could increase risk of radiation and adverse reactions to contrast injection. Other interesting technics could be used to study PH, such as Dual energy CT,29 or magnetic resonance imaging (MRI). MRI is much less employed in lung diseases due to the low signal intensity of the lung but recent improvements have been published30–34 and MRI is currently the best tool for evaluating heart morphology and function,35 or estimating pulmonary arterial pressure using phase contrast MRI.36
CT is currently performed for screening PH etiology, notably in the assessment of chronic thromboembolic PH, lung disease, pulmonary arterial hypertension and pulmonary veno-occlusive disease.37–40 CT is also used as a prognostic marker in patients with PH.41
This review is focused on the role of CT in detecting PH in COPD patients. We will first describe the technical characteristics of CT and then we will discuss the morphological assessment of the parenchymal, bronchial and vascular compartments in COPD and their relationships with PH with a special interest in quantitative characterization.
COPD a multi-compartment disease
COPD is defined by functional irreversible airflow limitation but characteristic morphological changes are present in lung parenchyma, airways and pulmonary vasculature.1,42 Airflow limitation can be, at least partially, explained by parenchymal destruction due to emphysema.1 Emphysema is defined histologically by alveolar wall destruction and can be assessed visually using CT.43–45 The severity of emphysema can also be measured by quantitative CT indices reflecting the low attenuation area due to lung destruction. These quantitative indices have been shown to correlate with macroscopic and microscopic changes histologically.46,47
Proximal bronchial wall thickening is a common feature in COPD.48,49 Histological characteristics of proximal airways include increased infiltration of CD8(+) T lymphocytes, CD3+ T lymphocytes, macrophages.48–50 This thickening appeared to be more important in a particular COPD-phenotype characterized by less severe respiratory disease, older subjects and higher rates of obesity and cardiovascular comorbidities.51 Such a proximal thickening can be directly assessed by CT using 2D52,53 and 3D reconstruction.54–60
Bronchial wall thickening is however predominant in distal airways.61–65 At histological level, it associates luminal exudates, inflammatory cell infiltration and airway remodeling including peribronchial fibrosis, epithelial metaplasia, mucous gland hypertrophy and increased bronchial smooth muscle mass of the small airways.62,66 The higher the severity of COPD based on decreased FEV1, the higher the histological abnormalities.66 Moreover, inflammatory cell infiltration is related with tobacco smoking.67 A loss of small airways has been observed using microCT, a high-resolution imaging technique dedicated to small samples or animal models, contributing to airflow limitation.62 In humans, the small airway disease can be assessed two-folds morphologically: directly by showing centrilobular opacities and indirectly by using air trapping.67–69 Such an air trapping has been shown to correlate with the number of inflammatory cells (ie, neutrophils and mast cells) infiltrating the bronchial smooth muscle layer.67
It has been shown that the toxicity of tobacco smoke affects bronchi and parenchyma, but can also directly alter pulmonary vessels both in animals70 and humans.6,71 At histological level, pulmonary arterial remodeling appeared to be more important in COPD than in control subjects, and is particularly important in upper lobe and in small muscular pulmonary arteries.72 Those vessel alterations are multiples and can happen at initial stage of COPD71 with endothelial dysfunction, inflammation, suggesting that the history of PH in COPD might be related with cigarette-smoke-induced endothelial alterations.73,74 The quantitative amount of emphysema in COPD was often negatively correlated to pulmonary microvasculature abnormalities measured using CT,75–79 except for Wrobel et al,72 which did not find any significant correlation.
CT technical considerations
CT scan is the most reliable imaging modality for imaging pulmonary diseases, and it is available and commonly used in routine practice. The new generation of multidetector CT scanners allows acquisition of the whole lung in one breath hold with a submillimetric slice thickness and isotropic voxels using adequate matrix.
The COPD Gene study provides recommendations to standardize CT acquisition parameters in order to obtain high signal-to-noise ratio and accordingly accurate assessment of the images.80 Thin sections with non-contrast-enhanced volumetric acquisition is a standard technique for COPD imaging.81 However, intravenous iodinated contrast material is necessary in case of exacerbations or suspected pulmonary embolism. Standard reconstruction algorithm (smooth filter) is required for quantitative automatic analysis.82 Spirometric gated acquisition have been proposed to improve quantitative assessment, but is only used in dedicated research programs due to its complexity and low availability.83 Although COPD patients are older and radiation risks are thus minimized, balance between radiation dose and image quality should be considered. Misevaluation of quantitative parameters because of noisy images leads to standard tube parameters (kVp and mAs).84 However, low-dose CT acquisition with new iterative reconstruction algorithms allows noise reduction with acceptable quantitative measurements.83,85
Lung parenchyma
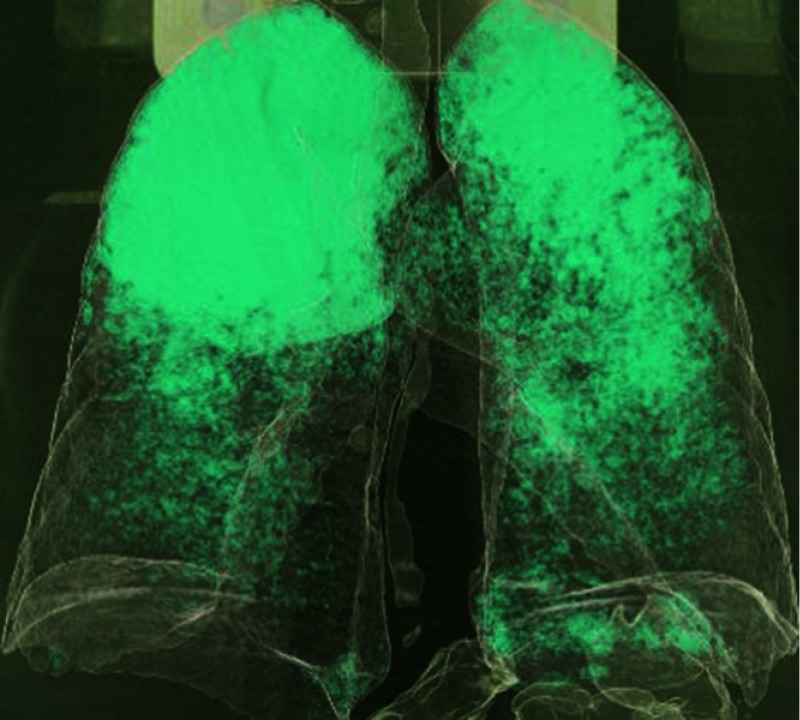
Historically, parenchymal alteration, and more precisely, emphysema, has been the first lung component investigated in order to explain PH in COPD. Indeed, emphysema is a major characteristic of COPD that could be quantified in patients when CT is performed. At CT, emphysema can be quantitatively evaluated, using low attenuation area percent (LAA%) derived from the voxel frequency distribution histogram. Several thresholds have been proposed but the most commonly employed is the value of –950 Hounsfield units (HU)45,46,86–89 (see Figure 1). It has been initially hypothesized that emphysema was correlated with mPAP. However, surprisingly, no relationship between PH and emphysema has been demonstrated neither in human nor in animal models.7,44,45,90,91 In addition, no significant difference in LAA% between COPD patients with or without PH was observed.45,92,93 Nonetheless, one single study observed that among COPD patients with PH, the LAA% measured using automated CT was correlated with right ventricular (RV) dysfunction.94 Emphysema can also be reflected using CT scan with the threshold of −960 HU, the evaluation of the first percentile,95 or in longitudinal studies, the 15th percentile is preferred to follow-up emphysema changes.96
Figure 1.
Reconstructed chest CT scan in COPD patient. This image was acquired with high-spatial-frequency algorithm reconstruction using fully automated Pulmo3D software (Siemens, Munich, Germany). Low attenuation area (LAA%) was derived from the voxel frequency distribution histogram and represented the percentage of lung voxels less than −950 HU. In this COPD patient, LAA value was 23%.
Abbreviations: COPD, chronic obstructive pulmonary disease; CT, computed tomography.
Proximal bronchi
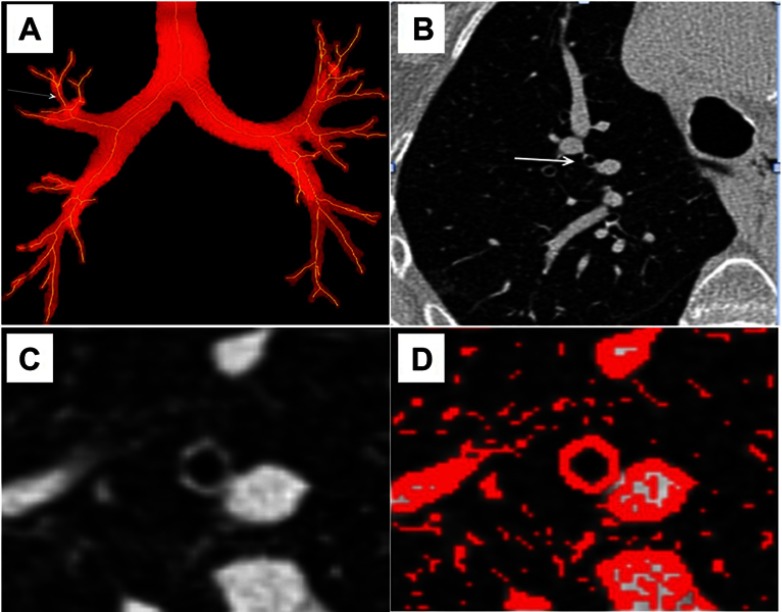
Bronchi can be studied using CT with 2D52,53 or 3D54–60 acquisitions. Various methods of bronchial segmentation and various parameters have been proposed, with so far no consensus (see Figure 2). Lumen area, wall area, wall area %, wall thickness and -Pi10, that reflects the normalized wall area of a theoretical 10-mm bronchi section area, have been proposed as indices for quantitating the severity of bronchial wall changes.45,52,86,89,97–101 We have demonstrated that bronchial thickness assessed by CT was increased in COPD patients with PH as compared to that of COPD patients without PH, whereas demographic, clinical and functional data (except PaO2 and 6 mins walk test distance, not surprisingly) remain unchanged.45 Moreover, a correlation between bronchial wall thickness and mPAP has been reported both in PH45 and in severe PH secondary to COPD.86,102 In addition, bronchial wall thickness measured using CT was correlated to histological measurements of airway remodeling,52 to exacerbation frequency,103 respiratory symptoms89 and to FEV1%.104
Figure 2.
Bronchi segmentation. (A) Frontal view of a propagation algorithm to obtain a skeleton binary volume based on bi-thresholding. Arrow shows bronchi in which measurements were assessed. (B) Peripheral bronchus is designated (arrow) on a native transverse thin-section CT. (C) Thin-section CT scan used to obtain measurements. (D) A Laplacian of Gaussian algorithm was assessed to segment the designed airway and measure bronchial thickness.
Abbreviation: CT, computed tomography.
Small airways
Since obstruction of small airways, defined as airways with a diameter under 2 mm, has been shown to be the main determinant of obstruction in COPD,62–65 some imaging technics have intended to characterize small airway disease in COPD. Mosaic attenuation at inspiratory and air trapping at expiratory CT reflect small airway disease. Both are related to a decreased lung attenuation and therefore cannot be easily distinguished from emphysema.105 However, we have found that air trapping was correlated with inflammatory cell infiltration and might reflect peripheral airway obstruction in patients exposed to cigarette smoking.67 Galbán et al have assessed both expiratory and inspiratory CT using parametric response map in order to estimate functional small airway disease in COPD patients.106 Parametric response map was able to differentiate COPD phenotypes. In addition, Matsuoka et al calculated relative volume change (expiratory minus inspiratory relative volume) using CT with the threshold of −860 HU that was correlated with airway dysfunction (ie, assessed at PFTs) in COPD regardless of the degree of emphysema.68 To the best of our knowledge, there is no study dedicated to the small airways of COPD patients with PH using CT.
Central vessels
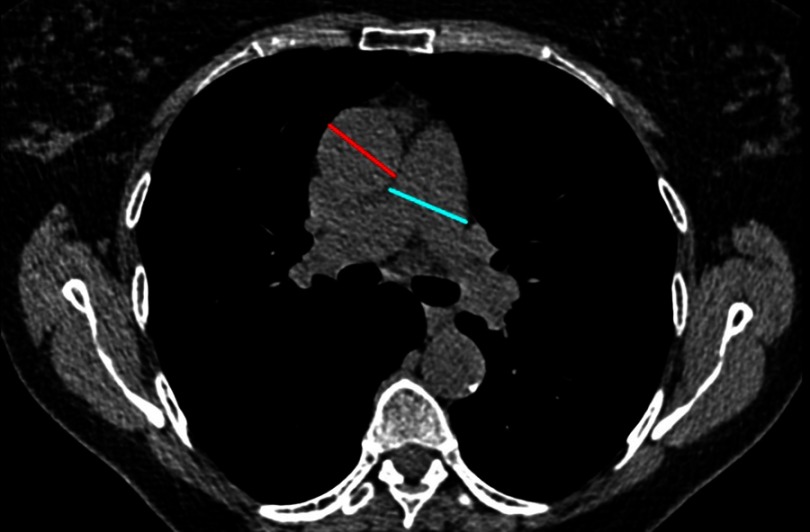
In COPD, a correlation has been shown between mPAP and enlargement of the main pulmonary artery truncus (MAP) diameter, and this increase is observed when MAP is normalized by ascending aorta diameter (MAP/AO).86,107–110 Usually, normalization is made on ascending aorta107,108,111,112 (see Figure 3) but it has also been done on descending aorta.21 Usually, MAP widest dimension is measured on axial CT images, on inspiratory acquisition, at bifurcation level, and, AO is measured on the same image.107,108,111,112 Descending aorta is also measured at the same level.112 Thresholds values to detect PH in COPD patients are: MAP ≥29 mm21,113,114 and MAP/AO>1.107,110,111,115,116
Figure 3.
Pulmonary artery and aorta measurements. Ratio measurement obtained in a COPD patient in a transverse CT section.
Abbreviations: COPD, chronic obstructive pulmonary disease; CT, computed tomography.
Increased MAP/AO ratio, a very simple index measured on a routine CT acquisition, has been shown linked to an increased risk of exacerbation in COPD108,111,117 and a lower 6-mins walk distance.118 Mortality in COPD population was also correlated to MAP/AO ratio in some reports115,119 but not in others.108,110 Interestingly, this ratio was not correlated to mortality in the general population.119 However, in healthy children, the ratio MAP/AO is higher than one, so the threshold of 1 cannot be applied in children.112
Pulmonary artery distensibility, that reflects elasticity using volume variations of the MAP between diastole and systole, is decreased in PH.3,4,120 The increase of stiffness is also observed in group 3 of PH121 and in PH related to COPD.122 Cardiac magnetic resonance (CMR) or ECG-gated CT can be also used to evaluate PA stiffness.3,4,120,121
In addition, dynamic-contrast-enhanced CT can be used to discriminate patients with or without PH from different etiology.123 A delayed flow of contrast and a reduced bolus propagation speed were observed in PH in comparison to control patients. Regions of interests were placed in the MAP and in the left and right pulmonary arteries. However, this study presented some limitations since bolus propagation also reflects the left heart function, the respiratory function and the speed of injection.123
Small vessels
The segmental pulmonary artery-to-bronchus ratio, semi-quantitatively evaluated for apical and posterior segments that run perpendicularly to the axial CT-scan, is positively correlated with mPAP.124 Ratio>1 in three or more lobes is specific of the presence of PH113,124 in group 3 of PH classification that also comprise COPD.125 In the same way, the size of the segmental arterial diameters is also positively correlated with mPAP124 in group 3 of PH.125
Regarding small pulmonary arteries, several of their characteristics have been studied, notably, number, volumes and areas. CT is an efficient tool in order to evaluate in vivo alterations of vessels, follow-up of illness or evaluating drug effects.
Some tools have been developed using 2D35,79,86,102 (see Figure 4) or 3D78,118,126 reconstructions.
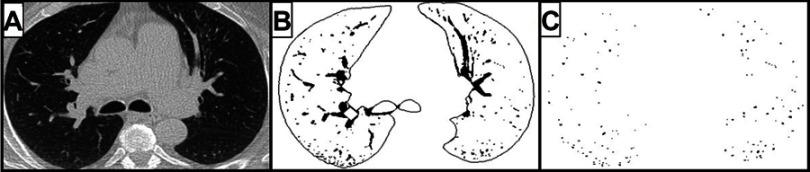
Figure 4.
Measurement of cross-sectional areas (CSA) of small pulmonary vessels using Image J free-software. (A) CT image segmented within the threshold values from −500 to −1024 HU of lung field. (B) Segmented image segmented into binary images. (C) Mask image for the particle analysis after setting circularity within [0.9–1.0] and vessel size within [0–5] mm2.
Abbreviation: CT, computed tomography.
Using 2D reconstruction on CT images, an automated measurement has been assessed using a free software Image J. These automated measurements allowed the visualization of small vessels alterations first on 3 CT slices44,127,128 and then on the whole lung.86,102 Software enables calculation of the percentage of cross-sectional areas (CSA%) of small pulmonary vessels under 5 mm2 (%CSA<5) and between 5 and 10 mm2 (CSA5–10), normalized by lung area, measuring subsubsegmental and subsegmental levels, respectively. Thus, in COPD patients without severe PH, the correlation between %CSA<5 and mPAP was negative44,86 whereas in COPD with severe PH correlation was positive.86,102
The 3D reconstruction technique allowed to measure volumes of small vessels.78,118,129 In both studies,78,118 index using 3D for assessment of pulmonary vessels was negatively correlated with MAP. For Ma et al,78 3D and 2D techniques were correlated. Studies using 2D and 3D are in the same line and showed a negative correlation between small pulmonary vessels and mPAP in COPD patients with most of the time moderate PH. As mentioned by others, all those vascular impairments support a vascular etiology of smoking-induced lung disease.6,130 CT metrics, such as %CSA<5, also allowed evaluation of the effect of pulmonary vasodilators in patients with PH related to COPD.79
In the automated measurements previously reported, pulmonary arteries were never separated from veins and this may affect the sensitivity of the technique. Tools have been developed in 3D in order to circumvent this limitation but not employed, to the best of our knowledge, for evaluating PH in COPD patients.126,129,131,132 A summary of vessels analyses is presented in Table 1.
Table 1.
Summary of vessels analyzes in COPD with PH
| Publication year | First author | Journal | CT findings | Qualitative /quantitative /Automated /manual | No. of patients | Screened population | Search for |
|---|---|---|---|---|---|---|---|
| 2014 | Iyer et al107 | Chest | MAP/AO>1 better than echocardiography to evaluate PH in COPD patients | Quantitative manual | 60 | Severe COPD | mPAP ≥ 25 mmHg |
| 2017 | Iliaz S et al108 | Clin Respir J | MAP/AO correlated with PH, number of exacerbation, not with mortality | Quantitative manual | 156 | COPD exacerbation | Number of COPD exacerbation 1 year after the first one |
| 2017 | Cuttica MJ et al109 | Int J Chron Obstruct Pulmon Dis | MAP/AO associated with pulmonary hemodynamics and right heart structure and function changes | Semi-quantitative manual | 88 | Mil-to-moderately severe COPD | mPAP ≥ 25 mmHg |
| 2016 | Ortaç Ersoy E et al110 | J Crit Care | MAP/AO≥1 associated with pulmonary hypertension, but not with mortality | Quantitative manual | 106 | COPD | mPAP ≥ 25 mmHg |
| 2016 | Coste F et al86 | Thorax | % cross-sectional area of small pulmonary vessels <5 mm2 (%CSA<5) is negatively correlated to mPAP for moderated PH (25–35 mmHg), and positively correlated to mPAP for severe PH (>35 mmHg) | Quantitative automated | 105 | COPD | mPAP ≥ 25 mmHg 35 mmHg |
| 2012 | Wells JM et al111 | NEJM | MAP/AO>1 associated with severe COPD exacerbation | Quantitative manual | 3464 | Smokers with COPD | COPD exacerbation |
| 2015 | Compton GL et al112 | AJR | Description of technical measurements of MAP/AO in children, threshold is greater than 1, closer to 1.09 | Quantitative manual | 400 | General children population | Threshold of MAP/AO in children |
| 2011 | Chan AL et al21 | BMC Med Imaging | Significant predictors of PH: MAP≥ 29 mm, MAP/AO≥ 0.84, MAP/descending aorta≥ 1.29 mm | Quantitative manual | 101 | Heterogenous diagnoses | mPAP ≥ 25 mmHg |
| 1998 | Tan RT et al113 | Chest | MAP≥ 29 mm. Artery to bronchus ratio ≥1 in 3 or 4 lobes | Quantitative manual | 28 | Parenchymal lung disease | mPAP ≥ 20 mmHg |
| 2012 | Truong QA et al114 | Circ Cardiovasc Imaging | MAP≥ 29 mm in men, MAP≥ 27 mm in women, MAP/AO≥ 0.9 | Quantitative manual | 3171 | Asymptomatic community-based population | 90th percentile |
| 2014 | Shin S et al115 | Repir Med | MAP/AO>1 associated with PH in patients with COPD, independent predictor of mortality | Quantitative manual | 65 | Advanced COPD | mPAP ≥ 25 mmHg survival |
| 2016 | Mohamed Hoesein FA et al116 | Lung | MAP≥ 30 mm and MAP/AO> 1 associated with PH | Quantitative manual | 92 | COPD | mPAP ≥ 25 mmHg |
| 2016 | Wells JM et al117 | Chest | MAP/AO>1 predict exacerbation of COPD | Quantitative manual | 134 | Patient with acute exacerbation of COPD | Predict clinical outcome with MAP/AO |
| 2015 | Wells JM et al118 | Circ Cardiovasc Imaging | Intraparenchymal pulmonary blood vessel volume and the volume of distal vessels with cross-sectional area (CSA) of <5 mm2 negatively correlated with MAP/AO. MAP/AO, MAP negatively correlated with 6MWT | Quantitative automated | 24 | Non severe COPD | MAP/AO>1 |
| 2017 | Terzikhan N et al119 | Eur Respir J | In general population, MAP/AO >1 not associated with mortality. In COPD population, MAP/AO >1 associated with mortality | Quantitative manual | 2197 | General and COPD populations | Pronostic information of MAP/AO |
| 2009 | Revel MP et al120 | Radiology | Decreased pulmonary arterial distensibility in PH, ECG-gated CT | Quantitative manual | 45 | Different group 1, 2 etiology of PH | mPAP ≥ 25 mmHg |
| 2014 | Pienn M et al123 | Eur Radiol | Reduced bolus propagation speed and time differences between contrast material peaks in PH patients, using dynamic-contrast-enhanced CT | Quantitative semi-automated | 33 | Different potential etiology of PH, notably lung disease, and control patients | mPAP ≥ 25 mmHg |
| 2010 | Devaraj A et al124 | Radiology | MAP/AO and Artery to bronchus ratio correlated with mPAP | Quantitative manual | 77 | Spectrum of disease associated with PH (Groups 1, 3, 4,5) | mPAP ≥ 25 mmHg 34 mmHg |
| Size of segmental arterial diameter correlated positively with mPAP | |||||||
| 2010 | Matsuoka S et al44 | Am J Respir Crit Care Med | % cross-sectional area of small pulmonary vessels <5 mm2 (%CSA<5) is negatively correlated to mPAP for moderated PH, in severe emphysema population | Quantitative automated | 79 | COPD | Correlation with mPAP |
| 2015 | Ando K et al79 | Lung | %CSA<5 used to assess pulmonary vasodilators longitudinal evaluation | Quantitative automated | 42 | COPD | Effect of pulmonary vasodilators in COPD-PH patients |
| 2017 | Ma J et al78 | J Xray Sci Technol | 3D tool able to detect the mean lumen area that was negatively correlated with MAP, and mean number of vessels negatively correlated with emphysema (LAA%, −950 HU) | Quantitative automated | 102 | Heavy smokers | Computerized scheme to detect pulmonary vessels |
| 2011 | Matsuoka S et al76 | Acad. Radiol. | Negative correlation between %CSA<5 and LAA% | Quantitative automated | 191 | Smoking history | Evaluate correlation small vessels- emphysema |
| 2011 | Uejima I et al128 | Jpn J Radiol | Positive correlation between %CSA<5 and FEV1/FVC | Quantitative automated | 30 | Non smokers | Evaluate correlation small vessels- PFTs |
| 2013 | Park S et al129 | Med Phys | Assessing volumetric technic allowing pulmonary artery/vein separation | Quantitative automated | 10 | COPD | Evaluate volumetric technic allowing pulmonary artery/vein separation |
| 2016 | Iyer S et al130 | Am J Respir Crit Care Med | After sildenafil arterial CSA and perfused blood volume decreased only in centriacinar emphysema patients, using dual-energy CT perfused blood volume | Quantitative automated | 17 | Current smokers | Effect of sildenafil on vascular perfusion |
| 2016 | Payer C et al131 | Med Image Anal | Assessing 3D technic allowing pulmonary artery/vein separation | Quantitative automated | 25 | Lung vascular disease | Evaluate 3D technic allowing pulmonary artery/vein separation |
| 2016 | Charbonnier JP et al132 | IEEE Trans Med Imaging | Automatic separation and classification of pulmonary arteries and veins using CT with an accuracy of 0.89 | Quantitative automated | 55 | Lung cancer | Evaluate separation and classification of pulmonary arteries/veins |
| 2019 | Coste F et al102 | Int J Chron Obstruct Pulmon Dis | % cross-sectional area of small pulmonary vessels <5 mm2 (%CSA<5) is positively correlated to mPAP for severe PH (>35 mmHg) | Quantitative automated | 24 | COPD | mPAP ≥35 mmHg |
Abbreviations: COPD, chronic obstructive pulmonary disease; CT, computed tomography; PH, pulmonary hypertension.
Scores
As mentioned above, several parameters reflecting various components in COPD are modified in COPD patients with PH. Building a score involving these parameters can be a strategy to improve the detection and the severity assessment of PH in COPD.
Tan et al have associated MAP ≥29 mm with artery-to-bronchus ratio >1 in 3 or 4 lobes and have reached a specificity of 100% for detecting PH in patients suffering from parenchymal lung diseases.113
Another index was composed of MAP/AO and right ventricular systolic pressure (RVSP) derived from echocardiography and was correlated with mPAP (R2=0.55)124 for group 1, 3, 4 and 5 of PH.3,4
Johns et al mentioned many RV parameters manually measured that are correlated with PH evolution in COPD patients.133 They tried to identify the best combination able to predict PH. First combination “cardiac magnetic resonance-RV” (“CMR-RV”) included RV mass and septal angle,134 second “CMR PA/RV” combined RV mass, septal angle and MAP measurements, and the third “α-index” encompass RV ejection fraction (RVEF) and minimal MAP size.135 The second model was the best model able to predict mPAP ≥25 mmHg. The same team also confirmed the interest of those results in another recent study using a validation cohort.136
We have built a “paw score” in order to predict severe PH in COPD patients combining CT automated measurements of bronchial wall thickness and pulmonary small vessels areas (%CSA<5) with PaO286,102
The various scores and their sensitivity are reported in Table 2.
Table 2.
Summary of scores built in order to diagnose PH or severe PH in lung disease or COPD
| Publication year | First author | Journal | Score | CT metrics | No. of patients | Screened population | Search for | Sens. Spe. | PPV NPV |
|---|---|---|---|---|---|---|---|---|---|
| 1998 | Tan RT et al113 | Chest | MAP ≥29 mm + artery to bronchus ratio ≥1 in 3 or 4 lobes | MAP+A/B | 28 | Parenchymal lung disease | mPAP≥ 20 mmHg | NA 1.00 | NA |
| 2010 | Devaraj A et al124 | Radiology | MAP/AO + Right ventricular systolic pressure (echocardiography) | MAP/AO | 77 | Spectrum of disease associated with PH (Groups 1, 3, 4, 5) | mPAP≥ 25 mmHg 34 mmHg |
0.96 0.59 0.73 0.88 |
NA |
| 2016 | Coste F et al86 | Thorax | “Paw score”: %cross-sectional area of small pulmonary vessels <5 mm2+ bronchial wall thickening + PaO2 | %CSA<5+ WT | 105 | COPD | mPAP≥ 35 mmHg |
0.75 0.80 |
0.36 0.96 |
| 2019 | Coste F et al102 | Int J Chron Obstruct Pulmon Dis | “Paw score”: %cross-sectional area of small pulmonary vessels <5 mm2+ bronchial wall thickening + PaO2 | %CSA<5+ WT | 24 | COPD | mPAP≥ 35 mmHg |
0.88 NA |
NA NA |
| 2018 | Johns CS et al133 | Eur. Radio. | “CMR-RV”: RV mass + septal angle | 102 | COPD | mPAP≥ 25 mmHg |
0.90 0.79 |
0.96 0.58 |
|
| “CMR-PA/RV”: RV mass + septal angle + MAP measurement | MAP measurement | 0.92 0.80 |
0.96 0.63 |
||||||
| “α-index”: RV ejection fraction + minimal MAP size | Minimal MAP size | 1.00 0.13 |
0.87 1.00 |
||||||
Abbreviations: COPD, chronic obstructive pulmonary disease; CT, computed tomography; NA, not attributed; NPV, negative predictive value; PPV, predictive positive value; PH, pulmonary hypertension.
Conclusion
PH secondary to COPD is a serious complication that is important to predict with non-invasive tools. Integration of imaging into standard clinical practice is an asset for patient’s care. Automated measurements of markers reflecting pulmonary small vessels, bronchial wall and emphysema changes are emerging and might be used routinely in the future for predicting PH or severe PH. All these CT markers could be used in morpho-phenotyping studies. Further studies are required to assess their prognostic impact and follow-up under treatment.
Acknowledgment
This study has received funding from the Laboratory of Excellence TRAIL, ANR-10-LABX-57.
Abbreviations
AO, aorta; COPD, chronic obstructive pulmonary disease; CMR, cardiac magnetic resonance; CT, computed tomography; FEV1, forced expiratory volume in 1 second; FVC, forced volume capacity; HU, Hounsfield units; LAA%, low-attenuation area percentage; m, s, dPAP, mean, systolic, diastolic pulmonary arterial pressure; MAP, Main Pulmonary Artery truncus; MRI, magnetic resonance imaging; NA, not attributed; NPV, negative predictive value; PA, pulmonary artery; PaO2, arterial partial pressure of oxygen (mmHg); PFT: pulmonary Function test; PH, pulmonary hypertension; PPV, positive predictive value; RHC, right heart catheterization; RV, right ventricle; WA, bronchial Wall Area (mm); WT, mean bronchial Wall Thickness (mm); 6MWT, 6 mins walk tests; %CSA<5, percentage of total cross-sectional area of vessels less than 5 mm2 normalized by lung area.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Professor Laurent reports personal fees from Boehringer-Ingelheim, Roche, and Chiesi, outside the submitted work. Professor Pierre-Olivier Girodet reports personal fees, non-financial support from Novartis, personal fees, non-financial support from Chiesi, personal fees, non-financial support from Boehringer-Ingelheim, personal fees, non-financial support from AstraZeneca, personal fees, non-financial support from ALK, outside the submitted work. Professor Patrick Berger reports grants from Novartis, personal fees, non-financial support from AstraZeneca, personal fees, non-financial support from Menarini, personal fees, non-financial support from Circassia, personal fees, non-financial support from Sanofi, personal fees from Teva, outside the submitted work; in addition, Prof Berger has a patent Geometric characterization of airways using MRI. 22605-FR pending. The authors report no other conflicts of interest in this work.
References
- 1.From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD); 2018. Available from: http://www.goldcopd.org/. Accessed July31, 2009.
- 2.From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD); 2017. Available from: https://goldcopd.org. Accessed July31, 2009.
- 3.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Rev Esp Cardiol. 2016;69(2):177. doi: 10.1016/j.recesp.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 4.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67–119. doi: 10.1093/eurheartj/ehv317 [DOI] [PubMed] [Google Scholar]
- 5.Seeger W, Adir Y, Barbera JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62(25 Suppl):D109–D116. doi: 10.1016/j.jacc.2013.10.036 [DOI] [PubMed] [Google Scholar]
- 6.Barbera JA, Peinado VI, Santos S. Pulmonary hypertension in chronic obstructive pulmonary disease. Eur Respir J. 2003;21(5):892–905. [DOI] [PubMed] [Google Scholar]
- 7.Scharf SM, Iqbal M, Keller C, et al. Hemodynamic characterization of patients with severe emphysema. Am J Respir Crit Care Med. 2002;166(3):314–322. doi: 10.1164/rccm.2107027 [DOI] [PubMed] [Google Scholar]
- 8.Chaouat A, Naeije R, Weitzenblum E. Pulmonary hypertension in COPD. Eur Respir J. 2008;32(5):1371–1385. doi: 10.1183/09031936.00015608 [DOI] [PubMed] [Google Scholar]
- 9.Blanco I, Piccari L, Barbera JA. Pulmonary vasculature in COPD: the silent component. Respirology. 2016;21(6):984–994. doi: 10.1111/resp.12772 [DOI] [PubMed] [Google Scholar]
- 10.Thabut G, Dauriat G, Stern JB, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest. 2005;127(5):1531–1536. doi: 10.1378/chest.127.5.1531 [DOI] [PubMed] [Google Scholar]
- 11.Hoeper MM, Humbert M, Souza R, et al. A global view of pulmonary hypertension. Lancet Respir Med. 2016;4(4):306–322. doi: 10.1016/S2213-2600(15)00543-3 [DOI] [PubMed] [Google Scholar]
- 12.Kessler R, Faller M, Fourgaut G, Mennecier B, Weitzenblum E. Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159(1):158–164. doi: 10.1164/ajrccm.159.1.9803117 [DOI] [PubMed] [Google Scholar]
- 13.Weitzenblum E, Hirth C, Ducolone A, Mirhom R, Rasaholinjanahary J, Ehrhart M. Prognostic value of pulmonary artery pressure in chronic obstructive pulmonary disease. Thorax. 1981;36(10):752–758. doi: 10.1136/thx.36.10.752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper R, Ghali J, Simmons BE, Castaner A. Elevated pulmonary artery pressure. An independent predictor of mortality. Chest. 1991;99(1):112–120. doi: 10.1378/chest.99.1.112 [DOI] [PubMed] [Google Scholar]
- 15.Oswald-Mammosser M, Weitzenblum E, Quoix E, et al. Prognostic factors in COPD patients receiving long-term oxygen therapy. Importance of pulmonary artery pressure. Chest. 1995;107(5):1193–1198. doi: 10.1378/chest.107.5.1193 [DOI] [PubMed] [Google Scholar]
- 16.Barbera JA, Blanco I. Management of pulmonary hypertension in patients with chronic lung disease. Curr Hypertens Rep. 2015;17(8):62. doi: 10.1007/s11906-015-0574-9 [DOI] [PubMed] [Google Scholar]
- 17.Tanabe N, Taniguchi H, Tsujino I, et al. Multi-institutional retrospective cohort study of patients with severe pulmonary hypertension associated with respiratory diseases. Respirology. 2015;20(5):805–812. doi: 10.1111/resp.12530 [DOI] [PubMed] [Google Scholar]
- 18.Vitulo P, Stanziola A, Confalonieri M, et al. Sildenafil in severe pulmonary hypertension associated with chronic obstructive pulmonary disease: a randomized controlled multicenter clinical trial. J Heart Lung Transplant. 2017;36(2):166–174. doi: 10.1016/j.healun.2016.04.010 [DOI] [PubMed] [Google Scholar]
- 19.Andersen KH, Iversen M, Kjaergaard J, et al. Prevalence, predictors, and survival in pulmonary hypertension related to end-stage chronic obstructive pulmonary disease. J Heart Lung Transplant. 2012;31(4):373–380. doi: 10.1016/j.healun.2011.11.020 [DOI] [PubMed] [Google Scholar]
- 20.Tuder RM, Archer SL, Dorfmuller P, et al. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D4–D12. doi: 10.1016/j.jacc.2013.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan AL, Juarez MM, Shelton DK, et al. Novel computed tomographic chest metrics to detect pulmonary hypertension. BMC Med Imaging. 2011;11:7. doi: 10.1186/1471-2342-11-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoeper MM, Lee SH, Voswinckel R, et al. Complications of right heart catheterization procedures in patients with pulmonary hypertension in experienced centers. J Am Coll Cardiol. 2006;48(12):2546–2552. doi: 10.1016/j.jacc.2006.07.061 [DOI] [PubMed] [Google Scholar]
- 23.Arcasoy SM, Christie JD, Ferrari VA, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med. 2003;167(5):735–740. doi: 10.1164/rccm.200210-1130OC [DOI] [PubMed] [Google Scholar]
- 24.O’Brien C, Guest PJ, Hill SL, Stockley RA. Physiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary care. Thorax. 2000;55(8):635–642. doi: 10.1136/thorax.55.8.635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348(21):2059–2073. doi: 10.1056/NEJMoa030287 [DOI] [PubMed] [Google Scholar]
- 26.Klooster K, ten Hacken NH, Hartman JE, Kerstjens HA, van Rikxoort EM, Slebos DJ. Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med. 2015;373(24):2325–2335. doi: 10.1056/NEJMoa1507807 [DOI] [PubMed] [Google Scholar]
- 27.Reilly J. Using computed tomographic scanning to advance understanding of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(5):450–455. doi: 10.1513/pats.200604-101AW [DOI] [PubMed] [Google Scholar]
- 28.Leuraud K, Richardson DB, Cardis E, et al. Ionising radiation and risk of death from leukaemia and lymphoma in radiation-monitored workers (INWORKS): an international cohort study. Lancet Haematol. 2015;2(7):e276–e281. doi: 10.1016/S2352-3026(15)00094-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dournes G, Verdier D, Montaudon M, et al. Dual-energy CT perfusion and angiography in chronic thromboembolic pulmonary hypertension: diagnostic accuracy and concordance with radionuclide scintigraphy. Eur Radiol. 2014;24(1):42–51. doi: 10.1007/s00330-013-2975-y [DOI] [PubMed] [Google Scholar]
- 30.Dournes G, Grodzki D, Macey J, et al. Quiet submillimeter MR imaging of the lung is feasible with a PETRA Sequence at 1.5 T. Radiology. 2015;276(1):258–265. doi: 10.1148/radiol.15141655 [DOI] [PubMed] [Google Scholar]
- 31.Dournes G, Menut F, Macey J, et al. Lung morphology assessment of cystic fibrosis using MRI with ultra-short echo time at submillimeter spatial resolution. Eur Radiol. 2016;26(11):3811–3820. doi: 10.1007/s00330-016-4218-5 [DOI] [PubMed] [Google Scholar]
- 32.Dournes G, Berger P, Refait J, et al. Allergic bronchopulmonary aspergillosis in cystic fibrosis: MR imaging of airway mucus contrasts as a tool for diagnosis. Radiology. 2017;285(1):261–269. doi: 10.1148/radiol.2017162350 [DOI] [PubMed] [Google Scholar]
- 33.Dournes G, Yazbek J, Benhassen W, et al. 3D ultrashort echo time MRI of the lung using stack-of-spirals and spherical k-Space coverages: evaluation in healthy volunteers and parenchymal diseases. J Magn Reson Imaging. 2018;48(6):1489–1497. doi: 10.1002/jmri.26212 [DOI] [PubMed] [Google Scholar]
- 34.Benlala I, Berger P, Girodet PO, Dromer C, Macey J, Laurent F. Automated Volumetric Quantification of Emphysema Severity by Using Ultrashort Echo Time MRI: Validation in Participants with Chronic Obstructive Pulmonary Disease.. Radiology. 2019. Jul;292(1):216–225. doi: 10.1148/radiol.2019190052 (in press). [DOI] [PubMed] [Google Scholar]
- 35.Vonk-Noordegraaf A, Haddad F, Chin KM, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol. 2013;62(25 Suppl):D22–D33. doi: 10.1016/j.jacc.2013.10.027 [DOI] [PubMed] [Google Scholar]
- 36.Reiter U, Reiter G, Fuchsjager M. MR phase-contrast imaging in pulmonary hypertension. Br J Radiol. 2016;89(1063):20150995. doi: 10.1259/bjr.20150995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reichelt A, Hoeper MM, Galanski M, Keberle M. Chronic thromboembolic pulmonary hypertension: evaluation with 64-detector row CT versus digital substraction angiography. Eur J Radiol. 2009;71(1):49–54. doi: 10.1016/j.ejrad.2008.03.016 [DOI] [PubMed] [Google Scholar]
- 38.Tamura M, Yamada Y, Kawakami T, et al. Diagnostic accuracy of lung subtraction iodine mapping CT for the evaluation of pulmonary perfusion in patients with chronic thromboembolic pulmonary hypertension: correlation with perfusion SPECT/CT. Int J Cardiol. 2017;243:538–543. doi: 10.1016/j.ijcard.2017.05.006 [DOI] [PubMed] [Google Scholar]
- 39.Resten A, Maitre S, Capron F, Simonneau G, Musset D. [Pulmonary hypertension: CT findings in pulmonary veno-occlusive disease]. J Radiol. 2003;84(11 Pt 1):1739–1745. [PubMed] [Google Scholar]
- 40.Mineo G, Attina D, Mughetti M, et al. Pulmonary veno-occlusive disease: the role of CT. Radiol Med. 2014;119(9):667–673. doi: 10.1007/s11547-013-0363-y [DOI] [PubMed] [Google Scholar]
- 41.Rajaram S, Swift AJ, Condliffe R, et al. CT features of pulmonary arterial hypertension and its major subtypes: a systematic CT evaluation of 292 patients from the ASPIRE registry. Thorax. 2015;70(4):382–387. doi: 10.1136/thoraxjnl-2014-206088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol. 2009;4:435–459. doi: 10.1146/annurev.pathol.4.110807.092145 [DOI] [PubMed] [Google Scholar]
- 43.Coxson HO, Dirksen A, Edwards LD, et al. The presence and progression of emphysema in COPD as determined by CT scanning and biomarker expression: a prospective analysis from the ECLIPSE study. Lancet Respir Med. 2013;1(2):129–136. doi: 10.1016/S2213-2600(13)70006-7 [DOI] [PubMed] [Google Scholar]
- 44.Matsuoka S, Washko GR, Yamashiro T, et al. Pulmonary hypertension and computed tomography measurement of small pulmonary vessels in severe emphysema. Am J Respir Crit Care Med. 2010;181(3):218–225. doi: 10.1164/rccm.200908-1189OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dournes G, Laurent F, Coste F, et al. Computed tomographic measurement of airway remodeling and emphysema in advanced chronic obstructive pulmonary disease. Correlation with pulmonary hypertension. Am J Respir Crit Care Med. 2015;191(1):63–70. doi: 10.1164/rccm.201408-1423OC [DOI] [PubMed] [Google Scholar]
- 46.Bankier AA, De Maertelaer V, Keyzer C, Gevenois PA. Pulmonary emphysema: subjective visual grading versus objective quantification with macroscopic morphometry and thin-section CT densitometry. Radiology. 1999;211(3):851–858. doi: 10.1148/radiology.211.3.r99jn05851 [DOI] [PubMed] [Google Scholar]
- 47.Madani A, Zanen J, de Maertelaer V, Gevenois PA. Pulmonary emphysema: objective quantification at multi-detector row CT–comparison with macroscopic and microscopic morphometry. Radiology. 2006;238(3):1036–1043. doi: 10.1148/radiol.2382042196 [DOI] [PubMed] [Google Scholar]
- 48.Di Stefano A, Turato G, Maestrelli P, et al. Airflow limitation in chronic bronchitis is associated with T-lymphocyte and macrophage infiltration of the bronchial mucosa. Am J Respir Crit Care Med. 1996;153(2):629–632. doi: 10.1164/ajrccm.153.2.8564109 [DOI] [PubMed] [Google Scholar]
- 49.O’Shaughnessy TC, Ansari TW, Barnes NC, Jeffery PK. Inflammation in bronchial biopsies of subjects with chronic bronchitis: inverse relationship of CD8+ T lymphocytes with FEV1. Am J Respir Crit Care Med. 1997;155(3):852–857. doi: 10.1164/ajrccm.155.3.9117016 [DOI] [PubMed] [Google Scholar]
- 50.Saetta M. Airway inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(5 Pt 2):S17–S20. doi: 10.1164/ajrccm.160.supplement_1.6 [DOI] [PubMed] [Google Scholar]
- 51.Burgel PR, Paillasseur JL, Peene B, et al. Two distinct chronic obstructive pulmonary disease (COPD) phenotypes are associated with high risk of mortality. PLoS One. 2012;7(12):e51048. doi: 10.1371/journal.pone.0051048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakano Y, Wong JC, de Jong PA, et al. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med. 2005;171(2):142–146. doi: 10.1164/rccm.200407-874OC [DOI] [PubMed] [Google Scholar]
- 53.Berger P, Perot V, Desbarats P, Tunon-de-Lara JM, Marthan R, Laurent F. Airway wall thickness in cigarette smokers: quantitative thin-section CT assessment. Radiology. 2005;235(3):1055–1064. doi: 10.1148/radiol.2353040121 [DOI] [PubMed] [Google Scholar]
- 54.Montaudon M, Berger P, de Dietrich G, et al. Assessment of airways with three-dimensional quantitative thin-section CT: in vitro and in vivo validation. Radiology. 2007;242(2):563–572. doi: 10.1148/radiol.2422060029 [DOI] [PubMed] [Google Scholar]
- 55.Meng Q, Kitasaka T, Nimura Y, Oda M, Ueno J, Mori K. Automatic segmentation of airway tree based on local intensity filter and machine learning technique in 3D chest CT volume. Int J Comput Assist Radiol Surg. 2017;12(2):245–261. doi: 10.1007/s11548-016-1492-2 [DOI] [PubMed] [Google Scholar]
- 56.Lo P, van Ginneken B, Reinhardt JM, et al. Extraction of airways from CT (EXACT’09). IEEE Trans Med Imaging. 2012;31(11):2093–2107. doi: 10.1109/TMI.2012.2209674 [DOI] [PubMed] [Google Scholar]
- 57.Aykac D, Hoffman EA, McLennan G, Reinhardt JM. Segmentation and analysis of the human airway tree from three-dimensional X-ray CT images. IEEE Trans Med Imaging. 2003;22(8):940–950. doi: 10.1109/TMI.2003.815905 [DOI] [PubMed] [Google Scholar]
- 58.Tschirren J, Hoffman EA, McLennan G, Sonka M. Intrathoracic airway trees: segmentation and airway morphology analysis from low-dose CT scans. IEEE Trans Med Imaging. 2005;24(12):1529–1539. doi: 10.1109/TMI.2005.857654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lo P, Sporring J, Ashraf H, Pedersen JJ, de Bruijne M. Vessel-guided airway tree segmentation: a voxel classification approach. Med Image Anal. 2010;14(4):527–538. doi: 10.1016/j.media.2010.03.004 [DOI] [PubMed] [Google Scholar]
- 60.Patyk M, Obojski A, Gojny L, Panaszek B, Zaleska-Dorobisz U. Airway evaluation with multidetector computed tomography post-processing methods in asthmatic patients. Adv Exp Med Biol. 2016;934:41–47. doi: 10.1007/5584_2016_23 [DOI] [PubMed] [Google Scholar]
- 61.Monzon J, Liu L, Brill H, et al. CDKN2A mutations in multiple primary melanomas. N Engl J Med. 1998;338(13):879–887. doi: 10.1056/NEJM199803263381305 [DOI] [PubMed] [Google Scholar]
- 62.McDonough JE, Yuan R, Suzuki M, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365(17):1567–1575. doi: 10.1056/NEJMoa1106955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med. 1968;278(25):1355–1360. doi: 10.1056/NEJM196806202782501 [DOI] [PubMed] [Google Scholar]
- 64.Van Brabandt H, Cauberghs M, Verbeken E, Moerman P, Lauweryns JM, Van de Woestijne KP. Partitioning of pulmonary impedance in excised human and canine lungs. J Appl Physiol. 1983;55(6):1733–1742. doi: 10.1152/jappl.1983.55.6.1733 [DOI] [PubMed] [Google Scholar]
- 65.Yanai M, Sekizawa K, Ohrui T, Sasaki H, Takishima T. Site of airway obstruction in pulmonary disease: direct measurement of intrabronchial pressure. J Appl Physiol. 1992;72(3):1016–1023. doi: 10.1152/jappl.1992.72.3.1016 [DOI] [PubMed] [Google Scholar]
- 66.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–2653. doi: 10.1056/NEJMoa032158 [DOI] [PubMed] [Google Scholar]
- 67.Berger P, Laurent F, Begueret H, et al. Structure and function of small airways in smokers: relationship between air trapping at CT and airway inflammation. Radiology. 2003;228(1):85–94. doi: 10.1148/radiol.2281020187 [DOI] [PubMed] [Google Scholar]
- 68.Matsuoka S, Kurihara Y, Yagihashi K, Hoshino M, Watanabe N, Nakajima Y. Quantitative assessment of air trapping in chronic obstructive pulmonary disease using inspiratory and expiratory volumetric MDCT. AJR Am J Roentgenol. 2008;190(3):762–769. doi: 10.2214/AJR.07.2820 [DOI] [PubMed] [Google Scholar]
- 69.Gruden JF, Webb WR, Warnock M. Centrilobular opacities in the lung on high-resolution CT: diagnostic considerations and pathologic correlation. AJR Am J Roentgenol. 1994;162(3):569–574. doi: 10.2214/ajr.162.3.8109498 [DOI] [PubMed] [Google Scholar]
- 70.Xing AP, Hu XY, Shi YW, Du YC. Implication of PDGF signaling in cigarette smoke-induced pulmonary arterial hypertension in rat. Inhal Toxicol. 2012;24(8):468–475. doi: 10.3109/08958378.2012.688885 [DOI] [PubMed] [Google Scholar]
- 71.Peinado VI, Barbera JA, Ramirez J, et al. Endothelial dysfunction in pulmonary arteries of patients with mild COPD. Am J Physiol. 1998;274(6 Pt 1):L908–L913. doi: 10.1152/ajplung.1998.274.6.L908 [DOI] [PubMed] [Google Scholar]
- 72.Wrobel JP, McLean CA, Thompson BR, et al. Pulmonary arterial remodeling in chronic obstructive pulmonary disease is lobe dependent. Pulm Circ. 2013;3(3):665–674. doi: 10.1086/674339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barbera JA, Peinado VI, Santos S, Ramirez J, Roca J, Rodriguez-Roisin R. Reduced expression of endothelial nitric oxide synthase in pulmonary arteries of smokers. Am J Respir Crit Care Med. 2001;164(4):709–713. doi: 10.1164/ajrccm.164.4.2101023 [DOI] [PubMed] [Google Scholar]
- 74.Peinado VI, Barbera JA, Abate P, et al. Inflammatory reaction in pulmonary muscular arteries of patients with mild chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159(5 Pt 1):1605–1611. doi: 10.1164/ajrccm.159.5.9807059 [DOI] [PubMed] [Google Scholar]
- 75.Yoshimura K, Suzuki Y, Uto T, Sato J, Imokawa S, Suda T. Morphological changes in small pulmonary vessels are associated with severe acute exacerbation in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2016;11:1435–1445. doi: 10.2147/COPD.S107424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Matsuoka S, Yamashiro T, Diaz A, et al. The relationship between small pulmonary vascular alteration and aortic atherosclerosis in chronic obstructive pulmonary disease: quantitative CT analysis. Acad Radiol. 2011;18(1):40–46. doi: 10.1016/j.acra.2010.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Estepar RS, Kinney GL, Black-Shinn JL, et al. Computed tomographic measures of pulmonary vascular morphology in smokers and their clinical implications. Am J Respir Crit Care Med. 2013;188(2):231–239. doi: 10.1164/rccm.201301-0162OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ma J, Yu N, Shen C, Wang Z, He T, Guo YM. A three-dimensional approach for identifying small pulmonary vessels in smokers. J Xray Sci Technol. 2017;25(3):391–402. doi: 10.3233/XST-16216 [DOI] [PubMed] [Google Scholar]
- 79.Ando K, Kuraishi H, Nagaoka T, et al. Potential role of CT metrics in chronic obstructive pulmonary disease with pulmonary hypertension. Lung. 2015;193(6):911–918. doi: 10.1007/s00408-015-9813-8 [DOI] [PubMed] [Google Scholar]
- 80.Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. Copd. 2010;7(1):32–43. doi: 10.3109/15412550903499522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mayo JR. CT evaluation of diffuse infiltrative lung disease: dose considerations and optimal technique. J Thorac Imaging. 2009;24(4):252–259. doi: 10.1097/RTI.0b013e3181c227b2 [DOI] [PubMed] [Google Scholar]
- 82.Hackx M, Bankier AA, Gevenois PA. Chronic obstructive pulmonary disease: CT quantification of airways disease. Radiology. 2012;265(1):34–48. doi: 10.1148/radiol.12111270 [DOI] [PubMed] [Google Scholar]
- 83.Madani A, Van Muylem A, Gevenois PA. Pulmonary emphysema: effect of lung volume on objective quantification at thin-section CT. Radiology. 2010;257(1):260–268. doi: 10.1148/radiol.10091446 [DOI] [PubMed] [Google Scholar]
- 84.Madani A, De Maertelaer V, Zanen J, Gevenois PA. Pulmonary emphysema: radiation dose and section thickness at multidetector CT quantification–comparison with macroscopic and microscopic morphometry. Radiology. 2007;243(1):250–257. doi: 10.1148/radiol.2431060194 [DOI] [PubMed] [Google Scholar]
- 85.Mets OM, Willemink MJ, de Kort FP, et al. The effect of iterative reconstruction on computed tomography assessment of emphysema, air trapping and airway dimensions. Eur Radiol. 2012;22(10):2103–2109. doi: 10.1007/s00330-012-2489-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Coste F, Dournes G, Dromer C, et al. CT evaluation of small pulmonary vessels area in patients with COPD with severe pulmonary hypertension. Thorax. 2016;71(9):830–837. doi: 10.1136/thoraxjnl-2015-207696 [DOI] [PubMed] [Google Scholar]
- 87.Matsuoka S, Yamashiro T, Washko GR, Kurihara Y, Nakajima Y, Hatabu H. Quantitative CT assessment of chronic obstructive pulmonary disease. Radiographics. 2010;30(1):55–66. doi: 10.1148/rg.301095110 [DOI] [PubMed] [Google Scholar]
- 88.Gevenois PA, De Vuyst P, de Maertelaer V, et al. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1996;154(1):187–192. doi: 10.1164/ajrccm.154.1.8680679 [DOI] [PubMed] [Google Scholar]
- 89.Grydeland TB, Dirksen A, Coxson HO, et al. Quantitative computed tomography measures of emphysema and airway wall thickness are related to respiratory symptoms. Am J Respir Crit Care Med. 2010;181(4):353–359. doi: 10.1164/rccm.200907-1008OC [DOI] [PubMed] [Google Scholar]
- 90.Biernacki W, Gould GA, Whyte KF, Flenley DC. Pulmonary hemodynamics, gas exchange, and the severity of emphysema as assessed by quantitative CT scan in chronic bronchitis and emphysema. Am Rev Respir Dis. 1989;139(6):1509–1515. doi: 10.1164/ajrccm/139.6.1509 [DOI] [PubMed] [Google Scholar]
- 91.Criner GJ, Scharf SM, Falk JA, et al. Effect of lung volume reduction surgery on resting pulmonary hemodynamics in severe emphysema. Am J Respir Crit Care Med. 2007;176(3):253–260. doi: 10.1164/rccm.200608-1114OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chaouat A, Savale L, Chouaid C, et al. Role for interleukin-6 in COPD-related pulmonary hypertension. Chest. 2009;136(3):678–687. doi: 10.1378/chest.08-2420 [DOI] [PubMed] [Google Scholar]
- 93.Hurdman J, Condliffe R, Elliot CA, et al. Pulmonary hypertension in COPD: results from the ASPIRE registry. Eur Respir J. 2013;41(6):1292–1301. doi: 10.1183/09031936.00079512 [DOI] [PubMed] [Google Scholar]
- 94.Huang YS, Hsu HH, Chen JY, Tai MH, Jaw FS, Chang YC. Quantitative computed tomography of pulmonary emphysema and ventricular function in chronic obstructive pulmonary disease patients with pulmonary hypertension. Korean J Radiol. 2014;15(6):871–877. doi: 10.3348/kjr.2014.15.6.871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Madani A, Van Muylem A, de Maertelaer V, Zanen J, Gevenois PA. Pulmonary emphysema: size distribution of emphysematous spaces on multidetector CT images – comparison with macroscopic and microscopic morphometry. Radiology. 2008;248(3):1036–1041. doi: 10.1148/radiol.2483071434 [DOI] [PubMed] [Google Scholar]
- 96.Diaz AA, Strand M, Coxson HO, et al. Disease severity dependence of the longitudinal association between CT lung density and lung function in smokers. Chest. 2018;153(3):638–645. doi: 10.1016/j.chest.2017.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Johannessen A, Skorge TD, Bottai M, et al. Mortality by level of emphysema and airway wall thickness. Am J Respir Crit Care Med. 2013;187(6):602–608. doi: 10.1164/rccm.201209-1722OC [DOI] [PubMed] [Google Scholar]
- 98.Ostridge K, Williams NP, Kim V, et al. Relationship of CT-quantified emphysema, small airways disease and bronchial wall dimensions with physiological, inflammatory and infective measures in COPD. Respir Res. 2018;19(1):31. doi: 10.1186/s12931-018-0734-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grydeland TB, Thorsen E, Dirksen A, et al. Quantitative CT measures of emphysema and airway wall thickness are related to D(L)CO. Respir Med. 2011;105(3):343–351. doi: 10.1016/j.rmed.2010.10.018 [DOI] [PubMed] [Google Scholar]
- 100.Nakano Y, Muro S, Sakai H, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med. 2000;162(3 Pt 1):1102–1108. doi: 10.1164/ajrccm.162.3.9907120 [DOI] [PubMed] [Google Scholar]
- 101.Lee YK, Oh YM, Lee JH, et al. Quantitative assessment of emphysema, air trapping, and airway thickening on computed tomography. Lung. 2008;186(3):157–165. doi: 10.1007/s00408-008-9071-0 [DOI] [PubMed] [Google Scholar]
- 102.Coste F, Benlala I, Dournes G, et al. Quantitative CT assessment of bronchial and vascular alterations in severe precapillary pulmonary hypertension. Int J Chron Obstruct Pulmon Dis. 2019;14:381–389. doi: 10.2147/COPD.S177638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Han MK, Kazerooni EA, Lynch DA, et al. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology. 2011;261(1):274–282. doi: 10.1148/radiol.11110173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Washko GR, Dransfield MT, Estepar RS, et al. Airway wall attenuation: a biomarker of airway disease in subjects with COPD. J Appl Physiol. 2009;107(1):185–191. doi: 10.1152/japplphysiol.00216.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Burgel PR, Bourdin A, Chanez P, et al. Update on the roles of distal airways in COPD. Eur Respir Rev. 2011;20(119):7–22. doi: 10.1183/09059180.10010610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Galban CJ, Han MK, Boes JL, et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med. 2012;18(11):1711–1715. doi: 10.1038/nm.2971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Iyer AS, Wells JM, Vishin S, Bhatt SP, Wille KM, Dransfield MT. CT scan-measured pulmonary artery to aorta ratio and echocardiography for detecting pulmonary hypertension in severe COPD. Chest. 2014;145(4):824–832. doi: 10.1378/chest.13-1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Iliaz S, Tanriverdio E, Chousein EGU, et al. Importance of pulmonary artery to ascending aorta ratio in chronic obstructive pulmonary disease. Clin Respir J. 2018;12(3):961–965. doi: 10.1111/crj.12612 [DOI] [PubMed] [Google Scholar]
- 109.Cuttica MJ, Bhatt SP, Rosenberg SR, et al. Pulmonary artery to aorta ratio is associated with cardiac structure and functional changes in mild-to-moderate COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:1439–1446. doi: 10.2147/COPD.S131413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ortac Ersoy E, Durusu Tanriover M, Ocal S, Gulsun Akpinar M, Topeli A. Measurement of pulmonary artery to aorta ratio in computed tomography is correlated with pulmonary artery pressure in critically ill chronic obstructive pulmonary disease patients. J Crit Care. 2016;33:42–46. doi: 10.1016/j.jcrc.2016.01.020 [DOI] [PubMed] [Google Scholar]
- 111.Wells JM, Washko GR, Han MK, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med. 2012;367(10):913–921. doi: 10.1056/NEJMoa1203830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Compton GL, Florence J, MacDonald C, Yoo SJ, Humpl T, Manson D. Main pulmonary artery-to-ascending aorta diameter ratio in healthy children on MDCT. AJR Am J Roentgenol. 2015;205(6):1322–1325. doi: 10.2214/AJR.15.14301 [DOI] [PubMed] [Google Scholar]
- 113.Tan RT, Kuzo R, Goodman LR, Siegel R, Haasler GB, Presberg KW. Utility of CT scan evaluation for predicting pulmonary hypertension in patients with parenchymal lung disease. Medical college of wisconsin lung transplant group. Chest. 1998;113(5):1250–1256. doi: 10.1378/chest.113.5.1250 [DOI] [PubMed] [Google Scholar]
- 114.Truong QA, Massaro JM, Rogers IS, et al. Reference values for normal pulmonary artery dimensions by noncontrast cardiac computed tomography: the Framingham Heart Study. Circ Cardiovasc Imaging. 2012;5(1):147–154. doi: 10.1161/CIRCIMAGING.111.968610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shin S, King CS, Brown AW, et al. Pulmonary artery size as a predictor of pulmonary hypertension and outcomes in patients with chronic obstructive pulmonary disease. Respir Med. 2014;108(11):1626–1632. doi: 10.1016/j.rmed.2014.08.009 [DOI] [PubMed] [Google Scholar]
- 116.Mohamed Hoesein FA, Besselink T, Pompe E, et al. Accuracy of CT pulmonary artery diameter for pulmonary hypertension in end-stage COPD. Lung. 2016;194(5):813–819. doi: 10.1007/s00408-016-9926-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wells JM, Morrison JB, Bhatt SP, Nath H, Dransfield MT. Pulmonary artery enlargement is associated with cardiac injury during severe exacerbations of COPD. Chest. 2016;149(5):1197–1204. doi: 10.1378/chest.15-1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wells JM, Iyer AS, Rahaghi FN, et al. Pulmonary artery enlargement is associated with right ventricular dysfunction and loss of blood volume in small pulmonary vessels in chronic obstructive pulmonary disease. Circ Cardiovasc Imaging. 2015;8(4):e002546. doi: 10.1161/CIRCIMAGING.114.002546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Terzikhan N, Bos D, Lahousse L, et al. Pulmonary artery to aorta ratio and risk of all-cause mortality in the general population: the Rotterdam Study. Eur Respir J. 2017;49(6):1602168. doi: 10.1183/13993003.02168-2016 [DOI] [PubMed] [Google Scholar]
- 120.Revel MP, Faivre JB, Remy-Jardin M, Delannoy-Deken V, Duhamel A, Remy J. Pulmonary hypertension: ECG-gated 64-section CT angiographic evaluation of new functional parameters as diagnostic criteria. Radiology. 2009;250(2):558–566. doi: 10.1148/radiol.2502080315 [DOI] [PubMed] [Google Scholar]
- 121.Sanz J, Kariisa M, Dellegrottaglie S, et al. Evaluation of pulmonary artery stiffness in pulmonary hypertension with cardiac magnetic resonance. JACC Cardiovasc Imaging. 2009;2(3):286–295. doi: 10.1016/j.jcmg.2008.08.007 [DOI] [PubMed] [Google Scholar]
- 122.Ertan C, Tarakci N, Ozeke O, Demir AD. Pulmonary artery distensibility in chronic obstructive pulmonary disease. Echocardiography. 2013;30(8):940–944. doi: 10.1111/echo.12170 [DOI] [PubMed] [Google Scholar]
- 123.Pienn M, Kovacs G, Tscherner M, et al. Non-invasive determination of pulmonary hypertension with dynamic contrast-enhanced computed tomography: a pilot study. Eur Radiol. 2014;24(3):668–676. doi: 10.1007/s00330-013-3067-8 [DOI] [PubMed] [Google Scholar]
- 124.Devaraj A, Wells AU, Meister MG, Corte TJ, Wort SJ, Hansell DM. Detection of pulmonary hypertension with multidetector CT and echocardiography alone and in combination. Radiology. 2010;254(2):609–616. doi: 10.1148/radiol.09090548 [DOI] [PubMed] [Google Scholar]
- 125.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D34–D41. doi: 10.1016/j.jacc.2013.10.029 [DOI] [PubMed] [Google Scholar]
- 126.Washko GR, Nardelli P, Ash SY, et al. Arterial vascular pruning, right ventricular size and clinical outcomes in COPD. Am J Respir Crit Care Med. 2019. doi: 10.1164/rccm.201811-2063OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Matsuoka S, Washko GR, Dransfield MT, et al. Quantitative CT measurement of cross-sectional area of small pulmonary vessel in COPD: correlations with emphysema and airflow limitation. Acad Radiol. 2010;17(1):93–99. doi: 10.1016/j.acra.2009.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Uejima I, Matsuoka S, Yamashiro T, Yagihashi K, Kurihara Y, Nakajima Y. Quantitative computed tomographic measurement of a cross-sectional area of a small pulmonary vessel in nonsmokers without airflow limitation. Jpn J Radiol. 2011;29(4):251–255. doi: 10.1007/s11604-010-0551-9 [DOI] [PubMed] [Google Scholar]
- 129.Park S, Lee SM, Kim N, Seo JB, Shin H. Automatic reconstruction of the arterial and venous trees on volumetric chest CT. Med Phys. 2013;40(7):071906. doi: 10.1118/1.4771960 [DOI] [PubMed] [Google Scholar]
- 130.Iyer KS, Newell JD Jr, Jin D, et al. Quantitative dual-energy computed tomography supports a vascular etiology of smoking-induced inflammatory lung disease. Am J Respir Crit Care Med. 2016;193(6):652–661. doi: 10.1164/rccm.201506-1196OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Payer C, Pienn M, Balint Z, et al. Automated integer programming based separation of arteries and veins from thoracic CT images. Med Image Anal. 2016;34:109–122. doi: 10.1016/j.media.2016.05.002 [DOI] [PubMed] [Google Scholar]
- 132.Charbonnier JP, Brink M, Ciompi F, Scholten ET, Schaefer-Prokop CM, van Rikxoort EM. Automatic pulmonary artery-vein separation and classification in computed tomography using tree partitioning and peripheral vessel matching. IEEE Trans Med Imaging. 2016;35(3):882–892. doi: 10.1109/TMI.2015.2500279 [DOI] [PubMed] [Google Scholar]
- 133.Johns CS, Rajaram S, Capener DA, et al. Non-invasive methods for estimating mPAP in COPD using cardiovascular magnetic resonance imaging. Eur Radiol. 2018;28(4):1438–1448. doi: 10.1007/s00330-017-5143-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Swift AJ, Rajaram S, Hurdman J, et al. Noninvasive estimation of PA pressure, flow, and resistance with CMR imaging: derivation and prospective validation study from the ASPIRE registry. JACC Cardiovasc Imaging. 2013;6(10):1036–1047. doi: 10.1016/j.jcmg.2013.01.013 [DOI] [PubMed] [Google Scholar]
- 135.Moral S, Fernandez-Friera L, Stevens G, et al. New index alpha improves detection of pulmonary hypertension in comparison with other cardiac magnetic resonance indices. Int J Cardiol. 2012;161(1):25–30. doi: 10.1016/j.ijcard.2011.04.024 [DOI] [PubMed] [Google Scholar]
- 136.Johns CS, Kiely DG, Rajaram S, et al. Diagnosis of pulmonary hypertension with cardiac MRI: derivation and validation of regression models. Radiology. 2019;290(1):61–68. doi: 10.1148/radiol.2018180603 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD); 2018. Available from: http://www.goldcopd.org/. Accessed July31, 2009.