Abstract
Objective
To assess clinical outcomes of intolerant, relapsed or refractory patients who could not be treated with new tyrosine kinase inhibitors or experimental therapies.
Methods
A retrospective cohort of 90 chronic myeloid leukemia patients in all phases of the disease treated with imatinib mesylate as their first TKI therapy, and with dasatinib or nilotinib as the next line of therapy. We evaluated clinical outcomes of these patients, with special focus on the group that needed more than two therapy lines.
Results
Thirty-nine percent of patients were refractory or intolerant to imatinib. An 8-year overall survival rate of the patients who went through three or more lines of treatment was significantly lower, compared to those who were able to maintain imatinib as their first-line therapy (83% and 22%, respectively p < 0.01). Decreased overall survival was associated with advanced-phase disease (p < 0.01), failure to achieve major molecular response in first-line treatment (p < 0.01) and interruption of first-line treatment due to any reason (p = 0.023). Failure in achieving complete cytogenetic response and major molecular response and treatment interruption were associated with the progression to the third-line treatment.
Conclusion
The critical outcome observed in relapsed, intolerant or refractory chronic phase CML patients reflects the unmet need for this group of patients without an alternative therapy, such as new drugs or experimental therapies in clinical trials. Broader access to newer treatment possibilities is a crucial asset to improve survival among CML patients, especially those refractory or intolerant to first-line therapies.
Keywords: Chronic myeloid leukemia, Imatinib mesylate, Dasatinib, Nilotinib, Survival analysis
Introduction
Chronic myeloid leukemia (CML) is a well-identified clonal myeloproliferative disorder, for which imatinib was the first of the approved tyrosine kinase inhibitors (TKI). Despite its effectiveness, it is estimated that approximately 20% of patients initially treated with this drug may be intolerant or may become primary or secondary resistant.1, 2 Patients in the chronic phase who are judged to have experienced treatment failure with imatinib, either as a consequence of intolerance or resistance, are routinely offered treatment with dasatinib or nilotinib, known as second-generation TKIs. Another therapy option is the allogeneic hematopoietic stem cell transplant (HSCT), effective but associated with morbidity.3 In the absence of an alternative treatment, in our setting, the multi-TKI failure chronic-phase CML patients, without clinical performance or on a waiting list for HSCT, may benefit from therapy with interferon. In the long term, however, interferon is associated with a worse clinical outcome and multiple adverse events.4
New CML treatment drugs, tested in clinical trials, can be offered to rescue-refractory TKIs patients, especially those not candidates for an HSCT. In Brazil, multinational companies were conducting approximately 80% of new medication studies. Even though cancer is currently the national research field with the highest number of active studies, none of CML refractory TKIs clinical trials were available in our region over the last years.5
Objective
The main interest of this study was to assess the CML patient characteristics and outcome, especially the patient treated with more than two TKI lines (imatinib, dasatinib or nilotinib), without access to new TKIs or experimental therapies.
Methods
Medical records were reviewed, from a retrospective cohort of 90 CML patients, diagnosed from January 2009, when imatinib was released as first-line treatment in Brazil, to October 2017. All the patients were treated at Hospital de Clínicas de Porto Alegre (HCPA), Brazil.
The analysis included patients in chronic phase (CP), accelerated phase (AP) and blast crisis (BC), followed for at least 12 months, who received imatinib mesylate as their first-line TKI therapy, and dasatinib or nilotinib as the next line.
Pediatric patients (under 18 years old) and those treated with HSCT as second-line treatment were excluded from the analysis. The data was analyzed for demographic, clinical and laboratory data, Sokal score, treatment modality, molecular response (MR), cytogenetic response (CR) and overall survival (OS). Primary resistance was defined as response failure to the TKI, and secondary resistance was defined as further loss of response, according to the European Leukemia Net criteria, ELN, version 2009 for patients diagnosed until December 2013 and the 2013 version from January 2014.6, 7 Treatment adherence was evaluated through medical or pharmaceutical records at the patient appointments. Any interruption of more than 7 days due to any cause, such as nonadherence and hematologic or non-hematological adverse effect, was considered.
The data was analyzed using the SPSS Statistics 22.0 and presented either as absolute or relative frequency, as mean or median standard error, or as minimum/maximum values, considering the normality of data distribution. The normality of variable distribution was assessed with the Shapiro–Wilk's test. The Mann–Whitney test was used to compare medians. Parametric data comparison between groups was made using the ANOVA. Non-parametric data between groups was compared using the Kruskal–Wallis test. Categorical variables were analyzed by using chi-square tests. For comparison of treatment responses between groups, the McNemar's test was used for paired samples. Overall survival distribution was calculated using the Kaplan–Meier test. The log-rank test was utilized to compare the curves. Differences were considered significant if p < 0.05.
This study was reviewed and approved by the research ethics committee at Hospital de Clínicas de Porto Alegre (CAE 52377216.3.0000.5327). The study was conducted in accordance with the provisions of the Declaration of Helsinki and all experiments described herein comply with the current Brazilian legislation.
Results
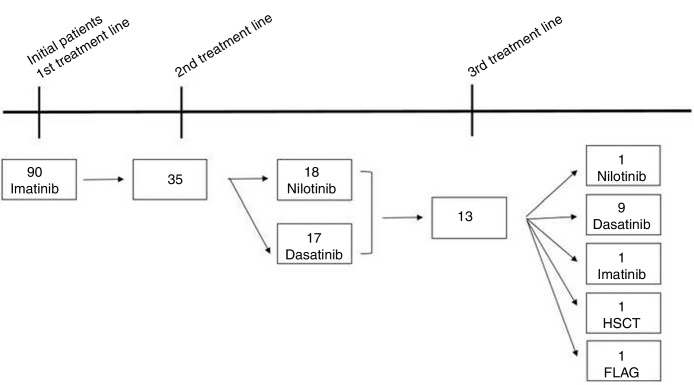
A total of 90 CML patients received imatinib as first-line TKI therapy and the populational baseline characteristics are described in Figure 1 and Table 1. The median time from diagnosis to the introduction of the first-line TKI was 41 days (14–90 days) and no difference in OS was observed, comparing the patient population that started treatment before or after 41 days from the diagnosis (88% vs 90%, respectively, p = 0.64). Thirty-five (38.8%) patients relapsed or were refractory or intolerant to imatinib, and 13 (14.5%) progressed to 3 or more lines of treatment. Fifty patients met the criteria for optimal response, according to the ELN 20096 recommendations, and five patients, according to the ELN 2013.7 As for the second-line treatment, the proportions of patients treated with dasatinib or nilotinib were similar (Figure 1). The median time to treatment change to a second-line TKI was 290 (63–2139) days. Populational characteristics, according to lines of treatment, are described in Table 2, including CML patients in all disease phases. Not progressing beyond the second line of treatment was associated with higher rates of complete cytogenetic response (p = 0.043), with a deeper MR (p = 0.052) and no treatment interruption (p = 0.012). Patients in BC had extremely high mortality.
Figure 1.
Chronic myelogenous leukemia patient therapeutic evolution.
Table 1.
Baseline characteristics of the population.
| Variables | Results |
|---|---|
| Gender (%) | |
| Female | 34 (37.8) |
| Male | 56 (62.2) |
| Age at diagnosis in years (median – minimum and maximum) | 54.5 (18–93) |
| Diagnosis phase (%) | |
| Chronic | 81 (90.0) |
| Accelerated | 6 (6.7) |
| Blast crisis | 3 (3.3) |
Table 2.
Overall populational characteristics, according to the number of treatments needed.
| Lines of treatment | 1 n = 55 |
2 n = 22 |
3 or more n = 13 |
|---|---|---|---|
| Age (years: median – minimum – maximum) | 57.5 (16–99) | 56.5 (29–84) | 55.5 (28 –83) |
| Gender (%) | |||
| Female | 21 (38.2) | 8 (36.4) | 5 (38.5) |
| Male | 34 (88.8) | 14 (63.6) | 8 (61.5) |
| Diagnosis phase (%) | |||
| Chronic | 50 (90.9) | 19 (86.4) | 12 (92.3) |
| Accelerated | 5 (9.1) | – | 1 (7.7) |
| Blast crisis | – | 3 (13.6) | – |
| Sokal (%) | n = 52 | n = 21 | n = 13 |
| Low-risk | 21 (40.4) | 8 (38.1) | 5 (38.5) |
| Intermediate-risk | 18 (48.6) | 12 (57.1) | 7 (53.8) |
| High-risk | 13 (25) | 1 (4.8) | 1 (7.7) |
| Complete cytogenetic response – first line (%)b | n = 51 | n = 20 | n = 13 |
| Yes | 51 (100) | 10 (50) | 6 (46.2) |
| No | – | 10 (50) | 7 (53.8) |
| Molecular response – first line (%)b | n = 50 | n = 13 | n = 9 |
| ≥MMR | 49 (98) | 5 (38.5) | 1 (11.1) |
| <MMR | 1 (2) | 8 (61.5) | 8 (88.9) |
| Treatment interruption – first line (%)b | n = 54 | n = 22 | n = 13 |
| Yes | 17 (31.5) | 13 (59.a) | 6 (46.2) |
| No | 37 (68.5) | 9 (40.9) | 7 (53.8) |
| Interruption's reason – first line (%)b | n = 17 | n = 13 | n = 6 |
| Hematological toxicity | 4 (23.5) | 2 (15.4) | 4 (66.7) |
| Non-hematological toxicity | 5 (29.4) | 8 (61.5) | 1 (16.7) |
| Othera | 8 (47.1) | 3 (23.1) | 1 (16.7) |
MMR: major molecular response.
Other reasons for interruption refer to lack of medication, patient forgetfulness, pregnancy and surgical procedure. The interruption was transitory.
% of patients that achieved response with the first-line treatment.
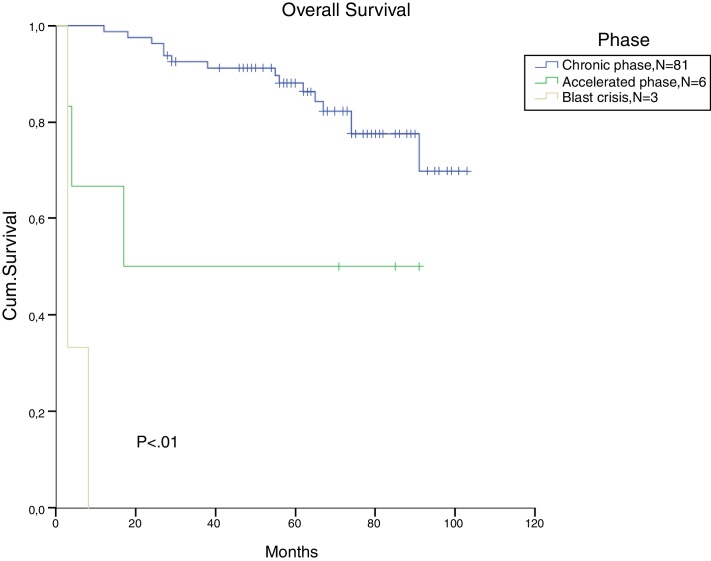
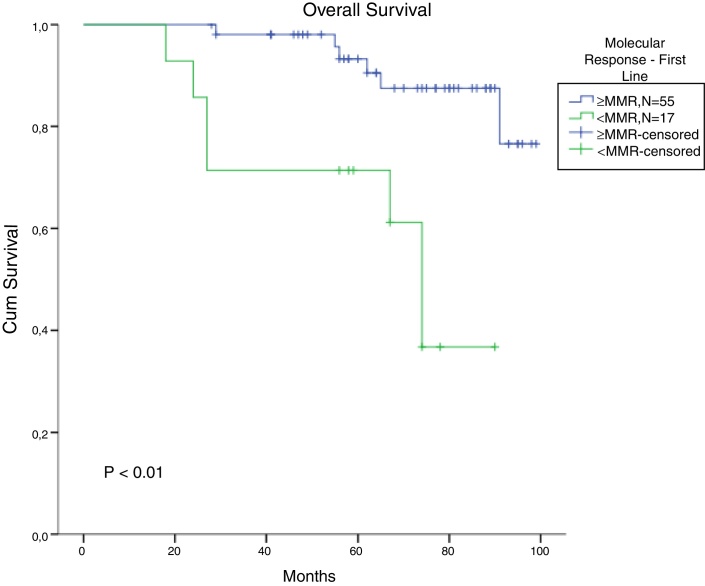
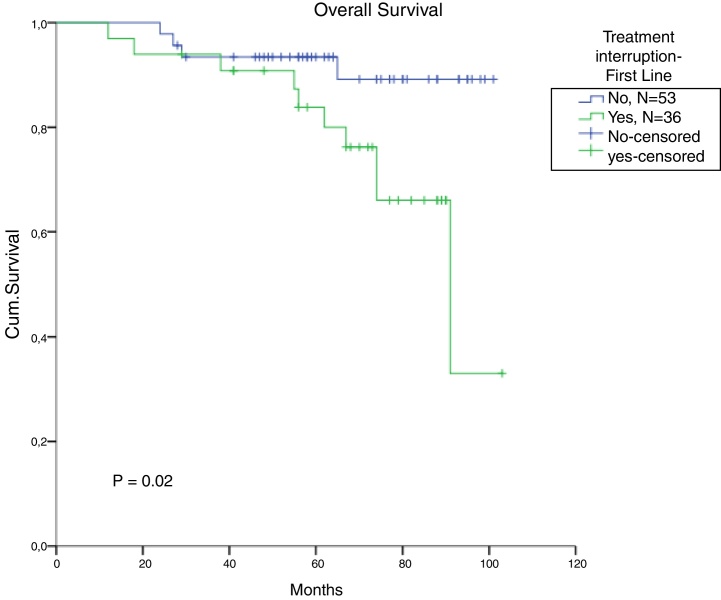
The total populational overall survival (OS) was 83% in 5 years. The median OS for CP, AP and BC is illustrated in Figure 2 (p < 0.01). Considering solely the CP patients, excluding the deaths not related to CML, the overall survival rate was 94.5%. The longest survival rate was observed in the group of CP CML patients that did not need to change therapy, totalling 86% in 5 years. The attainment of DNA mismatch repair (MMR) during the first-line treatment and no treatment interruption were associated with a significant increase in OS (p < 0.01 and p = 0.02, respectively) (Figure 3, Figure 4). There were only 6 patients in the AP patient group.
Figure 2.
Cumulative survival, according to the disease stage at diagnosis.
Figure 3.
Cumulative survival for patients who achieved MMR with imatinib in chronic-phase CML patients (missing = 15).
Figure 4.
Comparison of survival, according to interruption in the first line of treatment for chronic-phase CML patients (missing = 2).
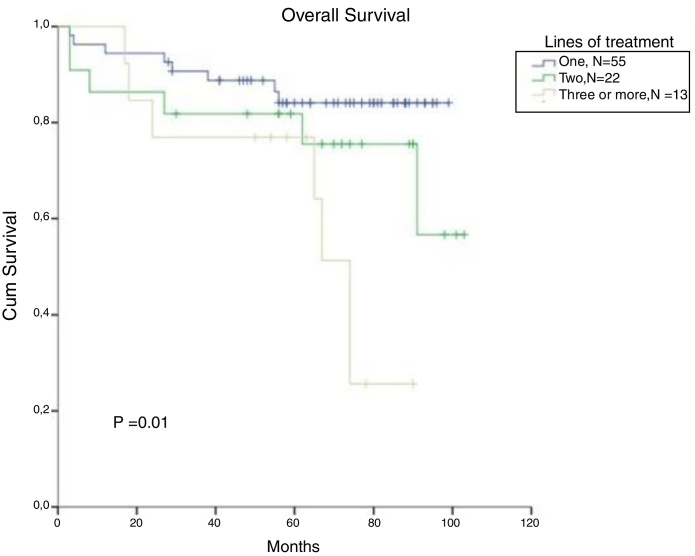
In CML, for patients treated with a second line and a third line and those beyond TKI, the OS in 5 years decreased to 82% and 77%, respectively (p = 0.01). There was no significant difference in OS between patients treated with one or two lines of therapy (Figure 5). From the total population, 13 patients in CP and AP went through the three available TKIs due to intolerance, relapse or lack of response. In the total population, bone marrow transplantation was feasible just in 2 patients, and one died from a CML blast crisis.
Figure 5.
Cumulative survival in CML patients, stratified according to the number of treatment lines.
Mutation analyses were performed for 17 patients. Eleven tests were negative and 6 patients presented abnormalities (2 patients with G250E and an additional mutation: V370I in one and M351T in the other. The last 4 patients demonstrated an isolated mutation: T949C, F359C, V299C and F317C). All of these were resistant to first- or second-line treatment and the choice of a new drug was based on the mutation analysis.
Discussion
In Brazil, imatinib was approved as a second line after interferon failure or disease progression in 2003 and in the first line only in 2008. The FDA (Food and Drug Agency) had already approved the medication in first-line treatment since May 2001 and the EMA (European Medicines Agency), in November 2001. Dasatinib was available in 2009 and nilotinib in 2010. All the results shown in our study should be interpreted with this bias.
Molecular monitoring of CML patients in complete cytogenetic response and the search for mutation analysis in special situations are recommended by the International Guidelines (NCCN and ELN2013)7 and the Brazilian Health Ministry. However, these molecular tests are not available at most public health care centers due to lack of government funding. In this case, molecular monitoring relies on pharmaceutical companies (Novartis® and Bristol®) sponsoring. In our real world scenario, we are not always able to provide molecular monitoring in the recommended timing. Therefore, this might be another bias to be considered, such as the late introduction of second generation TKIs in the second-line treatment.
In this context, the study aims to analyze the clinical characteristics, treatment response and OS in CML, especially in patients treated after the second line of treatment. Ribeiro et al. (2015) described 25 consecutive adult CML patients who were resistant or intolerant to two prior TKIs and were switched to a third TKI, and the OS was comparable to our results.8 These results may be relevant to other health care centers that might face the same hurdles in access to clinical trials and newer drugs.9, 10
Complete cytogenetic response, major molecular response and no treatment interruption were the most impacting factors in predicting whether patients would be successful in their first-line treatment. Patients in the CP who required more than two treatment lines, regardless of the reason: relapse, intolerance or resistance, had a significantly worse prognosis. Given the impossibility of including these patients in clinical trials or submitting them to HSCT, many patients are left solely with the possibility of palliative care. Ibrahim et al.11 published a large series of cases of CP CML patients in third-line TKI therapy, showing that those patients had a failure rate of 42.3% and a mortality rate 34.6% during the follow-up and all but one patient died due to a cause not related to CML.
Even though approximately half of the patients refractory to TKI do not carry a BCR/ABL additional mutation, and for most of them there is not much data regarding the mechanism of resistance, testing for the mutation is recommended in all cases that do not meet treatment response milestones.10, 12 In this study, only 17 patients were tested for mutations, of whom four were positive. Just like the PCR test for BCR/ABL, the mutational test is not widely available in the public health institutions in Brazil, which even further prevents the possibility of providing adequate treatment for these patients.
Although HSCT is currently the only possible manner of providing the cure for CML, donor availability, ideal referral timing, in addition to the morbidity and the mortality associated with the procedure, are strong limiting factors to this therapeutic option.13 A Brazilian study shows that, on a long-term basis, these patients tend to have higher mortality due to chronic graft-versus-host disease, when compared to patients treated solely with medication therapy.14 In our study, only two patients underwent HSCT.
Conclusion
The study demonstrated the decrease in the OS of CML patients, refractory or intolerant to more than two different TKIs and without access to newer therapies. Presently there are third-generation TKIs, as well as more incipient therapies involving cellular therapy with natural killer cells,15 that may prove to be useful tools to improve the clinical outcomes of these patients. This scenario may be modified in the future, with new on-going clinical trials. However, it is also equally important that healthcare professionals and public authorities enrolled in the caring for these patients develop strategies to improve their treatment, minimizing interruptions and providing adequate response to treatment monitoring.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Schiffer C.A. BCR-ABL tyrosine kinase inhibitors for chronic myelogenous leukemia. N Engl J Med. 2007;19(3573):258–265. doi: 10.1056/NEJMct071828. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien S.G., Guilhot F., Larson R.A., Gathmann I., Baccarani M., Cervantes F. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 3.Carvalho F., Zuckermann J., Paz A., Fischer G., Daudt L.E., Rigoni L.D. Characterization of patients with chronic myeloid leukemia unresponsive to tyrosine kinase inhibitors who underwent allogeneic hematopoietic stem cell transplantation. Int J Hematol Stem Cell Res. 2016;11:30–36. [PMC free article] [PubMed] [Google Scholar]
- 4.Gale R.P., Hehlmann R., Zhang M.J., Hasford J., Goldman J.M., Heimpel H. Survival with bone marrow transplantation versus hydroxyurea or interferon for chronic myelogenous leukemia. Blood. 1998;91:1810–1819. [PubMed] [Google Scholar]
- 5.Zucchetti C., Morrone F.B. Perfil da pesquisa clínica no brasil. Rev HCPA. 2012;32(3):340–347. [Google Scholar]
- 6.Baccarani M., Cortes J., Pane F., Niederwieser D., Saglio G., Apperley J. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27(35):6041–6051. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baccarani M., Deininger M.W., Rosti G. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–884. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ribeiro B.F., Miranda E.C., Albuquerque D.M., Delamain M.T., Oliveira-Duarte G., Almeida M.H. Treatment with dasatinib or nilotinib in chronic myeloid leukemia patients who failed to respond to two previously administered tyrosine kinase inhibitors – a single center experience. Clinics (Sao Paulo) 2015;70(8):550–555. doi: 10.6061/clinics/2015(08)04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hochhaus A., O’Brien S.G., Guilhot F., Druker B.J., Branford S., Foroni L. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009;23:1054–1061. doi: 10.1038/leu.2009.38. [DOI] [PubMed] [Google Scholar]
- 10.Kantarjian H., Brien S.O., Jabbour E., Garcia-manero G., Shan J., Rios M.B. Improved survival in chronic myeloid leukemia since the introduction of imatinib therapy: a single-institution historical experience. Blood. 2012;119:1981–1987. doi: 10.1182/blood-2011-08-358135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibrahim A.R., Paliompeis C., Bua M., Milojkovic D., Szydlo R., Khorashad J.S. Efficacy of tyrosine kinase inhibitors (TKI) as third line therapy in patients with chronic myeloid leukemia in chronic phase who have failed two prior TKI. Blood. 2010;116:5497–5501. doi: 10.1182/blood-2010-06-291922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quintás-Cardama A., Jabbour E.J. Considerations for early switch to nilotinib or dasatinib in patients with chronic myeloid leukemia with inadequate response to first-line imatinib. Leuk Res. 2013;37:487–495. doi: 10.1016/j.leukres.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Pavlú J., Szydlo R.M., Goldman J.M., Apperley J.F. Three decades of transplantation for chronic myeloid leukemia: what have we learned? Blood. 2011;117:755–763. doi: 10.1182/blood-2010-08-301341. [DOI] [PubMed] [Google Scholar]
- 14.Goldman J.M., Majhail N.S., Klein J.P., Wang Z., Sobocinski K.A., Arora M. Relapse and late mortality in 5-year survivors of myeloablative allogeneic hematopoietic cell transplantation for chronic myeloid leukemia in first chronic phase. J Clin Oncol. 2010;28:1888–1895. doi: 10.1200/JCO.2009.26.7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silla L.M., Pincus S.M., Locker J.D., Glover J., Elder E.M., Donnenberg A.D. Generation of activated natural killer (A-NK) cells in patients with chronic myelogenous leukaemia and their role in the in vitro disappearance of BCR/abl-positive targets. Br J Haematol. 1996;93:375–385. doi: 10.1046/j.1365-2141.1996.4991043.x. [DOI] [PubMed] [Google Scholar]