Abstract
Integrin-linked kinase (ILK) is a critical intracellular signaling node for integrin receptors. Its role in liver development is complex, as ILK deletion at E10.5 (before hepatocyte differentiation) results in biochemical and morphological differences that resolve as mice age. Nevertheless, mice with ILK depleted specifically in hepatocytes are protected from the hepatic insulin resistance during obesity. Despite the potential importance of hepatocyte ILK to metabolic health, it is unknown how ILK controls hepatic metabolism or glucoregulation. The present study tested the role of ILK in hepatic metabolism and glucoregulation by deleting it specifically in hepatocytes, using a cre-lox system that begins expression at E15.5 (after initiation of hepatocyte differentiation). These mice develop the most severe morphological and glucoregulatory abnormalities at 6 wk, but these gradually resolve with age. After identifying when the deletion of ILK caused a severe metabolic phenotype, in depth studies were performed at this time point to define the metabolic programs that coordinate control of glucoregulation that are regulated by ILK. We show that 6-wk-old ILK-deficient mice have higher glucose tolerance and decreased net glycogen synthesis. Additionally, ILK was shown to be necessary for transcription of mitochondrial-related genes, oxidative metabolism, and maintenance of cellular energy status. Thus, ILK is required for maintaining hepatic transcriptional and metabolic programs that sustain oxidative metabolism, which are required for hepatic maintenance of glucose homeostasis.
Keywords: glucose homeostasis, glycogen metabolism, hepatocyte signaling, in vivo physiology, integrin signaling, liver metabolism
INTRODUCTION
The liver is critical for a range of metabolic processes, including glucoregulation, absorption of nutrients, lipid synthesis, and detoxification (57). These functions are carried out primarily by hepatocytes, which are organized into plates separated by sinusoids. Hepatocytes adhere to an atypical basement membrane via integrins, which are the principal receptors for extracellular matrix. Integrin receptors consist of an α- and β-subunit and act as both adhesion and signaling molecules. They are critical for the organization and tissue structure of the liver (35), which determines its metabolic functions.
The ubiquitously expressed integrin β1-subunit interacts with intracellular signaling proteins, including integrin-linked kinase (ILK) (23, 34, 63). ILK acts as a central integrin signaling relay through its interactions with integrin receptors, the actin cytoskeleton, and an array of intracellular proteins, including those central to insulin signaling (28, 34, 35, 50, 63, 65). Through these interactions, ILK couples integrin signaling to cellular processes, including cytoskeletal organization, survival, and proliferation (42). Despite upregulation of ILK expression and signaling in pathological conditions, including certain cancers (20, 42) and fibrosis (7, 67), understanding of ILK with regard to metabolism remains largely unknown.
ILK is necessary for normal liver development. Deletion of ILK at approximately embryonic day (E)10.5, before hepatocyte differentiation (32), causes increased hepatocyte apoptosis, fibrosis, biliary hyperplasia, and mitosis at 6 wk of age (18). However, by 30 wk of age these biochemical and histological alterations were resolved, but a doubling of the liver-to-body weight ratio occurred. This was hypothesized to be a result of compensatory proliferation due to loss of contact inhibition. Even after morphological phenotypes are resolved, exposure of these mice to hepatic resection or toxicity results in an enhanced regenerative response (3, 6, 12, 13, 18).
Importantly, we have shown that ILK contributes to hepatic insulin resistance during diet-induced obesity (64). This was shown in mice where ILK was deleted at E15.5, after initiation of hepatocyte differentiation (48). In this model, the cellular mechanisms whereby hepatocyte ILK regulates metabolic health, hepatic metabolism, and glucoregulation were not studied. Therefore, the aim of the present studies was to define the metabolic and glucoregulatory role of ILK under uncompensated and unprovoked conditions. This was accomplished by identifying when the deletion of ILK caused a severe metabolic phenotype and then performing in depth studies at this time point to define the metabolic programs that coordinate control of glucoregulation determined by ILK.
MATERIALS AND METHODS
Animal models.
The Vanderbilt University Animal Care and Use Committee approved all procedures. Mice were maintained in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Male C57BL/6J mice with loxP sites flanking the ILK gene (ILKlox/lox) (54) were crossed to transgenic mice heterozygous for expression of Cre recombinase under control of the albumin (Alb) promoter [Alb-cre, Tg(Alb-cre)21Mgn; The Jackson Laboratory, Bar Harbor, ME] to generate ILKlox/loxAlbcre+/− mice and ILKlox/lox littermates (64). Additionally, male C57BL/6J mice with loxP sites flanking the Itgβ1 gene (Itgβ1lox/lox) (9) were crossed to transgenic mice heterozygous for expression of Cre recombinase under control of the Alb promoter to generate Itgβ1lox/loxAlbcre+/− mice [hepItgβ1-knockout (KO)] and Itgβ1lox/lox littermates. Mice were provided free access to chow (PicoLab Laboratory Rodent Diet 5L0D; Purina, Richmond, IN) and water. Mice were housed in a temperature- and humidity-controlled room on a 12:12-h light-dark cycle. Body composition was determined using an mq10 nuclear magnetic resonance analyzer (Bruker, Billerica, MA).
Liver histology and immunohistochemistry.
Liver tissue was fixed in 10% zinc-buffered formalin (Thermo Fisher Scientific, Waltham, MA), paraffin-embedded, and sectioned (5 μm). Liver sections were stained with hematoxylin and eosin (H & E) for assessment of gross liver histology as well as Masson’s trichrome stain for evaluation of fibrosis. For immunohistochemistry, anti-cytokeratin 19 (cat. no. 14965-1-AP, RRID:AB 2133324; Proteintech Group, Rosemont, IL) was used to assess biliary hyperplasia, and anti-Ki-67 (cat. no. 12202S RRID:AB_2620142; Cell Signaling Technology, Danvers, MA), was used to assess proliferation in paraffin-embedded tissue using the Leica Bond Max.
Oral glucose tolerance tests.
Mice were placed in bedded containers without food or water. After 5 h of fasting, tails of mice were nicked and baseline samples taken for glucose (2 µl) and insulin (20 µl). Samples for measurement of glucose concentrations were taken at t = 5, 10, 15, 20, 30, 45, 60, 90, and 120 min after a glucose gavage (2 g/kg whole body mass in ILKlox/lox and hepILK-KO; 2 g/kg lean mass in Itgβ1lox/lox and hepItgβ1-KO). Normalization to lean mass resulted in an ∼35% lower dose of glucose for oral glucose tolerance tests (OGTTs) in Itgβ1lox/lox and hepItgβ1-KO compared with OGTTs in ILKlox/lox and hepILK-KO mice. Because comparisons were between Albcre−/− and Albcre+/− littermates from the ILKlox/lox or Itgβ1lox/lox backgrounds, this difference in glucose dosage results in lower overall OGTT AUCs for experiments performed in Itgβ1lox/lox and hepItgβ1-KO mice. Plasma samples for insulin measurements were also taken at t = 10 min after the glucose gavage in Itgβ1lox/lox and hepItgβ1-KO mice. At the conclusion of the study, mice were returned to their home cages.
Hepatic RNA isolation, RT-PCR, and RNA sequencing analysis.
RNA was extracted by homogenization in TRIzol (Thermo Fisher Scientific), followed by purification on RNeasy columns (Qiagen, Hilden, Germany). Columns for RNA sequencing included an on-column DNase (Qiagen). For quantitative PCR (qPCR) analyses, RNA was reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). qPCR was performed using TaqMan (Applied Biosystems, Foster City, CA) Universal PCR Master Mix as well as TaqMan probes for glucose-6-phosphatase catalytic subunit (G6pc; cat. no. Mm00839363_m1), phosphoenolpyruvate carboxykinase (Pck1; cat. no. Mm00440636), mitochondrial dynamin-like GTPase (Opa1; cat. no. Mm01349707_g1), mitofusin 2 (Mfn2; cat. no. Mm00500120_m1), dynamin-1-like protein, (Dnm1l; cat. no. Mm01342903_m1), and fibroblast growth factor 21 (Fgf21; Mm00840165_g1).
Total RNA quality was assessed using the 2100 Bioanalyzer (Agilent, Santa Clara, CA). Two-hundred nanograms of DNase-treated total RNA with an RNA integrity number >7 was used to generate polyA-enriched mRNA libraries using KAPA Stranded mRNA sample kits with indexed adaptors (Roche, Basel, Switzerland). Library quality was assessed using the 2100 Bioanalyzer (Agilent), and libraries were quantitated using KAPA Library Quantification Kits (Roche). Pooled libraries were subjected to 75-bp paired-end sequencing according to the manufacturer’s protocol (HiSeq3000; Illumina, San Diego, CA). Bcl2fastq2 Conversion Software (Illumina) was used to generate de-multiplexed Fastq files. Fastq data files were uploaded to Galaxy (1) and converted to fastqsanger files. Low-quality reads were removed from fastqsanger files using trimmomatic version 0.36.3 (8). Reads were aligned to the mm10 mouse reference genome with Hisat version 1.1.2 (33). Counts of overlapping reads and gene exons from mm10 were determined using the counts function of HTseq version 0.6.1p1 (2). Count expression levels were filtered and assessed for differential expression using edgeR v (52). Enrichment of gene ontology terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were determined for genes significantly increased or decreased in hepILK-KO livers compared with control livers using the limma version 3.36.2 goana and kegga functions (51).
Mouse hepatocyte isolation and high-resolution respirometry.
Hepatocytes were isolated as previously described (16). Briefly, livers were perfused via the inferior vena cava with wash buffer, followed by digestion buffer with collagenase from Clostridium histolyticum (Sigma-Aldrich; St. Louis, MO). Cell counts and viability were obtained via trypan blue exclusion method. Hepatocyte O2 consumption was evaluated using an Oxygraph-2k (Oroboros Instruments, Innsbruck, Austria) at 37°C. Routine respiration was measured in the suspension media. Oligomycin (2.5 mM) was used to assess futile (LEAK) respiration. Maximal respiration by the electron transfer system (ETS) was determined via stepwise titrations (0.5 μM) of carbonyl cyanide p-trifluoromethoxyphenylhydrazone.
Whole body energy balance.
Between 5 and 6 wk of age, mice were individually housed in a Promethion Metabolic Analyzer (Sable Systems, North Las Vegas, NV) at an ambient temperature (20–22°C). Cages contained Pure-O’ Cel bedding (The Andersons, Maumee, OH), and mice had ad libitum access to chow and water throughout the study. O2 and CO2 were continuously monitored for assessment of energy expenditure (EE) and the respiratory quotient (RQ; V̇co2/V̇o2). EE was calculated by the Weir formula (62). Analysis of covariance (ANCOVA) was performed to assess interactions between lean body mass and genotypes in relation to EE (29–31). Energy intake (EI) was calculated using the equation {food intake (g) × energy content of rodent chow (cal/g)}. Mouse cage behavior, including roaming (XYZ beam breaks), feeding, and drinking, was monitored continuously for 9 days.
Surgical procedures.
Catheter implantation procedures (4, 5) were performed on 5-wk-old mice in preparation of metabolic flux experiments using stable isotopes. Carotid artery and jugular vein catheters were implanted for sampling and infusions, respectively. The free ends of the catheters were exteriorized behind the neck, flushed with 5 mg/ml ampicillin and 200 U/ml heparinized saline, and sealed with stainless-steel plugs. Following surgery, the mice were housed individually for 7 days before experiments.
Stable isotopes to measure metabolic fluxes in the liver.
On the day of the study, mice were placed in bedded containers without food or water and fasted for 3 h. Upon connection to sampling and infusion lines, an 80-μl arterial blood sample was drawn to determine the natural isotopic abundance of circulating glucose. Venous infusions were performed as previously described (25). After the baseline sample, a bolus of [2H2]water (99.9%) was delivered over 25 min to enrich total body water to 4.5%. A [6,6-2H2]glucose prime (440 μmol/kg) was dissolved in the bolus. Following the prime, a continuous infusion (4.4 μmol·kg−1·min−1) of [6,6-2H2]glucose was administered for the duration of the study. At t = 120 min after the [2H2]water bolus sodium [U-13C3]propionate (i.e., [U-13C3]propionate) was delivered as a continuous infusion. All infusates were prepared in a 4.5% [2H2]water-saline solution. All stable isotopes were from Cambridge Isotope Laboratories (Tewksbury, MA). Blood glucose was monitored (AccuCheck; Roche Diagnostics, Indianapolis, IN), and donor erythrocytes were infused to maintain hematocrit throughout the study. At t = 90 min after the onset of the [U-13C3]propionate infusion, blood samples (100 μl each) were collected at 10-min intervals over 30 min. Samples were centrifuged in EDTA-coated tubes for plasma isolation. Plasma was stored at −80°C before glucose derivatization and gas chromatography-mass spectrometry analysis. The duration between the [2H2]water bolus/[6,6-2H2]glucose prime and steady-state isotopic sampling was 3.5 to 4 h. Mice were then euthanized for liver extraction and analyses.
2H/13C metabolic flux analysis.
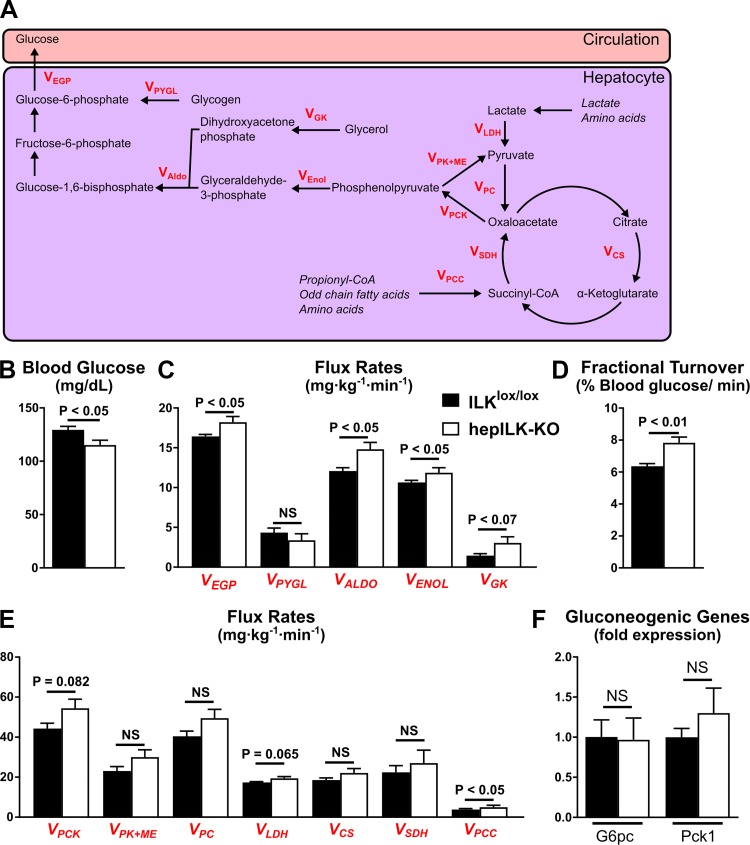
Metabolic flux analyses MFAs were conducted using samples from 120 min before as well as 90, 100, and 110 min following the [U-13C3]propionate bolus, as previously described (25, 27). Fragment ion ranges used for determining mass isotopomer distributions were aldonitrile, m/z 173–178, 259–264, 284–289, and 370–376; methyloxime, m/z 145–149; and di-O-isopropylidene, m/z 301–311. Flux estimates in the network for each sample underwent 50 iterations, beginning with random initial values to determine the best fit. Goodness of fit was accepted according to a chi-square test (P < 0.05). By taking multiple samples over time, an isotopic steady state is confirmed over the interval of 90 to 120 min following the beginning of the [U-13C3]propionate infusion. Flux values for each mouse are an average of estimates at steady state (90, 100, and 110 minutes following the [U-13C3]propionate bolus) and were normalized to body weight. Metabolic fluxes from MFA analysis are denoted as a V followed by a subscript shorthand for the metabolic flux or enzyme process described (Fig 8, C and E). The fluxes represented in our data are defined as: VCS = flux from oxaloacetate and acetyl-coA to citrate, VEGP = endogenous glucose production, VEnol = flux from phosphoenolpyruvate to 1,3-bisphosphoglycerate, VGK = flux from glycerol to dihydroxyacetone phosphate, VLDH = unlabeled nonphosphoenolpyruvate-derived sources of anaplerosis to pyruvate, VPC = Flux from pyruvate to oxaloacetate, VPCC = flux from propionyl-CoA to succinyl-CoA, VPCK = flux from oxaloacetate to phosphoenolpyruvate, VPK + ME = contribution of pyruvate kinase (PK) and malic enzyme (ME) to pyruvate, VPYGL = flux from glycogen to glucose-6-phosphate, VSDH = flux from succinyl-CoA to oxaloacetate. The fractional turnover of the glucose pool was defined as steady state glucose production (mg·kg−1·min−1)/(steady state average blood glucose [mg/ml]/200 [ml/kg]) and expressed as a percentage.
Fig. 8.
A: Graphic model of metabolic fluxes contributing to hepatic glucose output obtained from metabolic flux analysis (MFA). B: average blood glucose concentration during steady state of MFA for integrin-linked kinase (ILK)lox/lox (n = 8) and hepILK-knockout (KO) (n = 7) mice. C: rates of fluxes contributing to hepatic glucose production in ILKlox/lox (n = 8) and hepILK-KO (n = 7) mice modeled by MFA. D: fractional turnover of the circulating glucose pool determined by dividing VEGP by the blood glucose concentration at each time point during MFA in ILKlox/lox (n = 8) and hepILK-KO (n = 7) mice. E: rates of fluxes contributing to hepatic glucose production as well as the TCA cycling in ILKlox/lox (n = 8) and hepILK-KO (n = 7) mice modeled by MFA. F: gene expression of gluconeogenic genes [glucose-6-phosphatase catalytic subunit (G6PC) and phosphoenolpyruvate carboxykinase 1 (PEPCK)] from livers of ILKlox/lox (n = 8) and hepILK-KO (n = 7) mice after undergoing MFA. GAPDH was used as the control gene. Data are means ± SE. NS, not significant. Metabolic fluxes from MFA analysis are denoted as a V followed by a subscript shorthand for the metabolic flux or enzyme process described: VALDO, flux from dihydroxyacetone phosphate and glyceraldhyde-3-phosphate; VCS, flux from oxaloacetate and acetyl-coA to citrate; VEGP, endogenous glucose production; VEnol, flux from phosphoenolpyruvate to 1,3-bisphosphoglycerate; VGK, flux from glycerol to dihydroxyacetone phosphate; VLDH, unlabeled nonphosphoenolpyruvate-derived sources of anaplerosis to pyruvate; VPC, flux from pyruvate to oxaloacetate, VPCC, flux from propionyl-CoA to succinyl-CoA, VPCK, flux from oxaloacetate to phosphoenolpyruvate; VPK + ME, contribution of pyruvate kinase (PK) and malic enzyme (ME) to pyruvate; VPYGL, flux from glycogen to glucose-6-phosphate; VSDH, flux from succinyl-CoA to oxaloacetate.
Blood, plasma, and tissue analyses.
Blood glucose was measured using an Accu-Chek glucometer (Roche Diagnostics). Plasma insulin was measured by radioimmunoassay (43). Plasma glucagon (Mercodia, Winston Salem, NC) and FGF21 (Abcam, Cambridge, MA) were assessed by ELISA. Liver glycogen content was assessed using the method of Chan and Exton (11). Hepatic adenine nucleotide levels were quantified as previously described (24). Energy charge was calculated using the equation {[ATP] + (0.5 × [ADP])}/[TAN]. Lipids were extracted using the method of Folch et al. (15). The extracts were filtered and lipids recovered in the chloroform phase. Individual lipid classes were separated by thin-layer chromatography using Silica Gel 60 A plates developed in petroleum ether, ethyl ether, and acetic acid (80:20:1) and visualized by rhodamine 6G. Diglycerides, triglycerides, and cholesteryl esters were scraped from the plates and methylated using BF3-methanol, as described by Morrison and Smith (44). Determination of total tissue cholesterol was adapted from Rudel et al. (53). Plasma amino acids were quantified in the Vanderbilt Hormone Assay and Analytical Services Core by ion exchange HPLC with lithium citrate buffer system and post-column ninhydrin quantification (Biochrom US, Holliston, MA). Lactate levels in plasma and tissue were determined using an l-Lactate assay kit (cat. no. ab65331; Abcam). Plasma β-hydroxybutyrate levels were quantified using a colorimetric assay kit (cat. no. 700190; Cayman Chemical, Ann Arbor, MI). Activity of liver hexokinases was assessed using the method described by Tiedge et al. (56).
Immunoblotting.
Liver tissue or isolated hepatocytes were lysed as previously described (27). Liver (15–25 μg) proteins were denatured and reduced at 70°C, separated on a NuPAGE 4–12% Bis-Tris gel (Invitrogen, Carlsbad, CA), and transferred to a PVDF membrane. Membranes were probed with the antibodies obtained from Cell Signaling Technology for ILK (cat. no. 3856, RRID:AB_2233861), glycogen synthase kinase 3β (GSK3β; cat. no. 9315, RRID:AB_490890), GSK3β phosphorylated at Ser9 (cat. no. 9323, RRID:AB_2115201), protein kinase B (Akt, cat. no. 9271, RRID:AB_329825), Akt phosphorylated at Ser473 (cat. no. 9272, RRID:AB_329827), glycogen synthase (GS; cat. no. 3893, RRID:AB_2279563), GS phosphorylated at Ser641 (cat. no. 3891, RRID:AB_2116390), 5′AMP-activated protein kinase (AMPK; cat. no. 2532, RRID:AB_330331), AMPK phosphorylated at Thr172 (cat. no. 2535, RRID:AB_331250), acetyl-CoA carboxylase (ACC, cat. no. 3662, RRID:AB_2219400), ACC phosphorylated at Ser79 (cat. no. 3661, RRID:AB_330337), sequestosome 1 (p62, cat. no. 5114, RRID:AB_10624872), and BCL2/adenovirus E1B 19 kDa protein-interacting protein 3 (Bnip3, cat. no. 3769, RRID:AB_2259284). Mitochondrial complexes were detected using the total OXPHOS rodent Western blot antibody cocktail (abcam, cat. no. MS604-300). Membranes were exposed to a chemiluminescent horseradish peroxidase (HRP) substrate (MilliporeSigma, Billerica, MA) after incubation with a HRP-conjugated secondary antibody or quantification on a Li-Cor imaging system (Li-Cor Biotechnology, Lincoln, NE) after incubation with IRDye 800CW secondary antibody (Li-Cor). ImageJ software was used for densitometry measurements.
Statistical analyses.
Student’s t-tests with Welch’s correction were used to detect statistical differences (P < 0.05). All data are reported as means ± SE.
RESULTS
Absence of hepatocyte ILK causes hepatocellular damage, fibrosis, inflammation, and biliary hyperplasia.
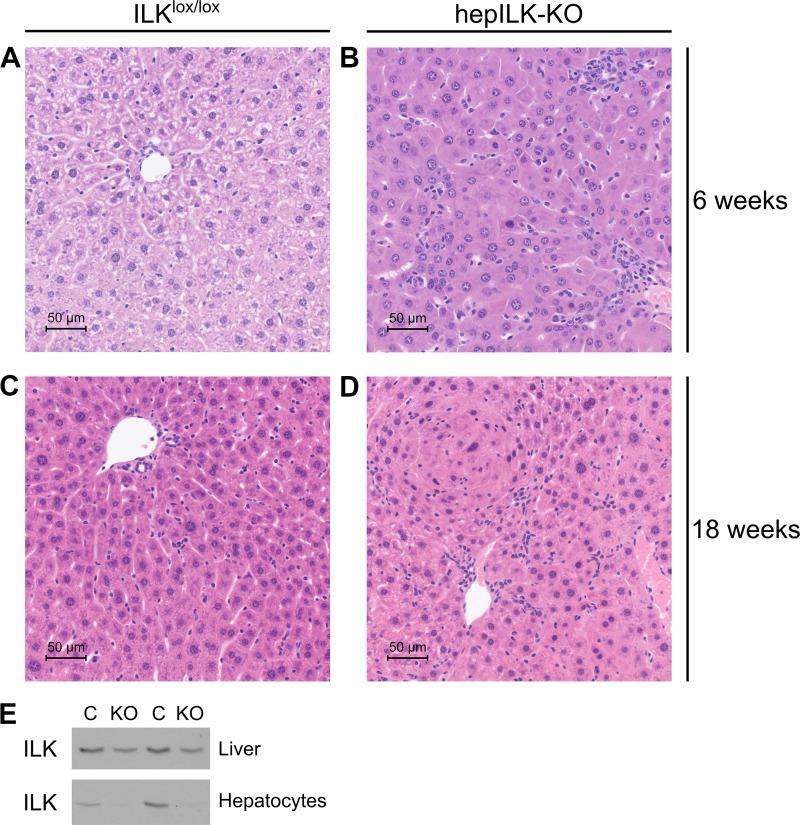
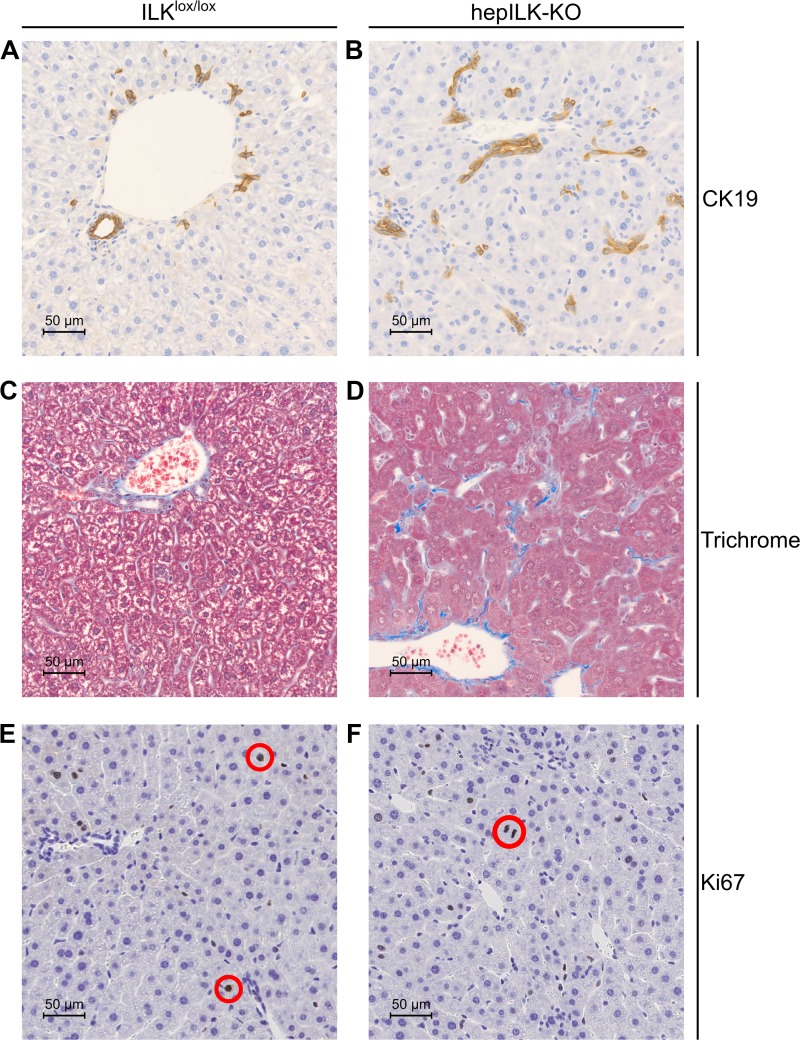
We previously demonstrated hepatocyte-specific knockout of ILK in this strain (64), and this was confirmed (Fig. 1E). Histological evaluation of livers from hepILK-KO and ILKlox/lox were performed at 6 and 18 wk of age. Livers from 6-wk-old ILKlox/lox and hepILK-KO mice stained with hematoxylin and eosin (H & E) (Fig. 1, A and B) were evaluated by a veterinary pathologist (K. L. Boyd). The presence of hepatocellular degeneration, inflammation, fibrosis, and biliary hyperplasia was evident. Fibrosis was confirmed with Masson’s trichrome staining, and biliary hyperplasia was demonstrated with immunohistochemistry (IHC) for cytokeratin 19 (Fig. 2, A–D). At 6 wk, hepILK-KO mice lack any significant differences in hepatocyte proliferation index according to pathologist evaluation and IHC for nuclear Ki-67 (Fig. 2, E and F). There are also no changes in liver to body weight ratios at 32 wk of age (0.045 ± 0.001 in controls vs. 0.044 ± 0.002 in hepILK-KOs; internal data not shown), which contrasts with the larger liver to body weight ratio in 32-wk-old mice using alf-alb-cre promoter system (18). Evaluation of H & E sections from 18-wk-old ILKlox/lox and hepILK-KO mice (Fig. 1, C and D) showed biliary hyperplasia and inflammation in hepILK-KO livers. Fibrosis was resolved by 18 wk. Hepatocyte hyperplastic responses were evident by 18 wk of age and resulted in adenoma formation in three of eight livers examined (Fig. 1D). Overall, histology of the 6-wk-old mouse liver reflected greater cellular disorganization and fibrosis than in livers from 18-wk-old mice. Results were consistent with previously reported liver histology in mice lacking hepatocyte ILK (18).
Fig. 1.
A: representative image of hematoxylin and eosin (H & E)-stained liver section at ×40 magnification from 6-wk-old integrin-linked kinase (ILK)lox/lox mice. B: representative image of H & E-stained liver section at ×40 magnification from 6-wk-old hepILK-knockout (KO) mice. C: representative image of H & E-stained liver section at ×40 magnification from 18-wk-old ILKlox/lox mice. D: representative image of H & E-stained liver section at ×40 magnification from 18-wk-old hepILK-KO mice. E: Western blots of ILK protein content in cells from whole liver and enriched for hepatocytes from ILKlox/lox [control (C); n = 2] or hepILK-KO (n = 2) mice at 24 wk of age.
Fig. 2.
A: representative image of immunohistochemical (IHC) staining for CK19 in liver section at ×40 magnification from 6-wk-old integrin-linked kinase (ILK)lox/lox mice. B: representative image of IHC staining for CK19 in liver section at ×40 magnification from 6-wk-old hepILK-knockout (KO) mice. C: representative image of Masson’s trichrome staining in liver section at ×40 magnification from 6-wk-old ILKlox/lox mice. D: representative image of Masson’s trichrome staining in liver section at ×40 magnification from 6-wk-old hepILK-KO mice. E: representative image of IHC staining for Ki-67 in liver section at ×40 magnification from 6-wk-old ILKlox/lox mice. F: representative image of IHC staining for Ki-67 in liver section at ×40 magnification from 6-wk-old hepILK-KO mice. Red circles denote nuclei stained positively for Ki-67 in E and F.
Hepatocyte ILK is required for glucose homeostasis in young mice.
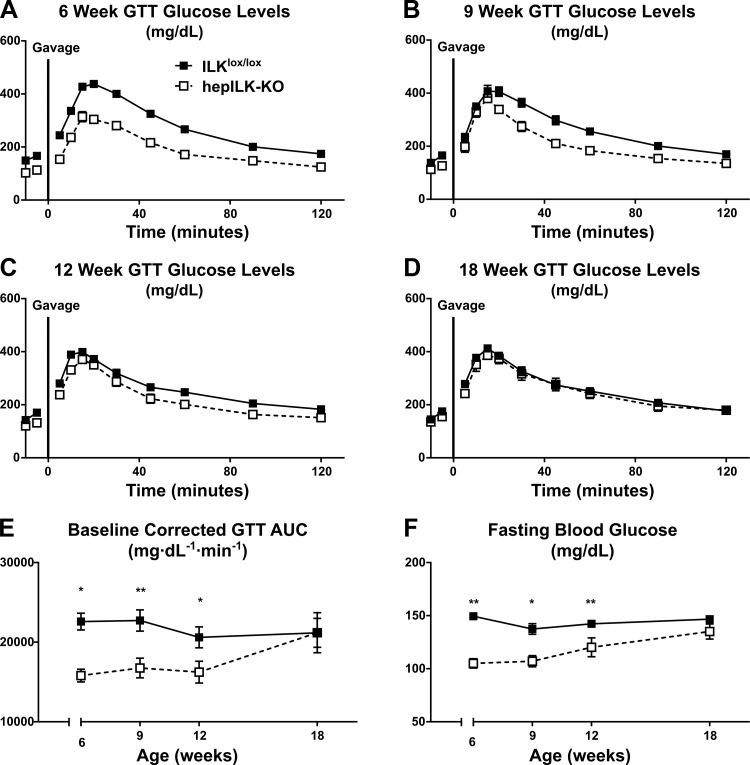
Blood glucose levels after a 5 h fast were decreased by ∼30% in hepILK-KO mice at 6 wk of age (Fig. 3A). This effect was accompanied by lower fasting insulin (Table 1). OGTTs revealed increased glucose tolerance in hepILK-KO mice at 6 wk as the baseline-corrected area under the glucose curve (AUC) was decreased compared with ILKlox/lox mice (Fig. 3, A and E). Fasting blood glucose and insulin rose, whereas glucose tolerance decreased progressively in hepILK-KO mice so that by 18 wk of age these variables were normalized to values in ILKlox/lox mice (Table 1 and Fig. 3, A–F). HepILK-KO mice were ∼15% smaller with 3.2% higher lean mass than ILKlox/lox counterparts at 6 wk, but this phenotype was normalized by 9 wk. A small difference in body composition (2.2% higher lean mass) was observed only in 12-wk-old hepILK-KO mice (Table 1). Differences in blood glucose and glucose tolerance were progressively resolved by 18 wk of age.
Fig. 3.
A: blood glucose levels of 6-wk-old integrin-linked kinase (ILK)lox/lox (n = 30) and hepILK-knockout (KO) (n = 20) mice during oral glucose tolerance tests (GTT). B: blood glucose levels of 9-wk-old ILKlox/lox (n = 17) and hepILK- KO (n = 7) mice during oral GTT. C: blood glucose levels of 12-wk-old ILKlox/lox (n = 15) and hepILK- KO (n = 10) mice during oral GTT. D: blood glucose levels of 18-wk-old ILKlox/lox (n = 15) and hepILK-KO (n = 7) mice during oral GTT. E: baseline corrected area under the glucose curves (AUC) in A–D. F: fasting blood glucose concentrations in A–D. Data are means ± SE *P < 0.05; **P < 0.01.
Table 1.
Basic metabolic characteristics of ILKlox/lox and hepILK-KO mice at multiple ages
| Gentoype | ILKlox/lox (n = 13–28) | hepILK-KO (n = 11–19) | ILKlox/lox (n = 16–20) | hepILK-KO (n = 6–9) | ILKlox/lox (n = 13–14) | hepILK-KO (n = 7–9) | ILKlox/lox (n = 10–15) | hepILK-KO (n = 6–7) |
|---|---|---|---|---|---|---|---|---|
| Weight, g | 20.9 ± 0.4 | 18.0 ± 0.6* | 24.7 ± 0.5 | 23.4 ± 0.6 | 26.3 ± 0.4 | 25.6 ± 0.6 | 30.4 ± 0.7 | 30.0 ± 0.8 |
| Fat mass, % | 4.8 ± 0.4 | 5.2 ± 0.9 | 5.1 ± 0.5 | 4.6 ± 0.3 | 6.0 ± 0.5 | 5.4 ± 0.5 | 6.7 ± 0.8 | 5.6 ± 0.7 |
| Lean mass, % | 67.6 ± 0.4 | 70.8 ± 1.0** | 68.3 ± 1.2 | 68.1 ± 0.4 | 67.1 ± 0.4 | 69.3 ± 0.4* | 66.0 ± 0.7 | 67.6 ± 1.0 |
| Study age, wk | 6 | 6 | 9 | 9 | 12 | 12 | 18 | 18 |
| FBG, mg/dl | 150 ± 3 | 103 ± 5# | 137 ± 5 | 107 ± 5 | 144 ± 3 | 120 ± 9** | 147 ± 3 | 135 ± 7 |
| FPI, ng/ml | 0.5 ± 0.03 | 0.3 ± 0.04* | 0.6 ± 0.04 | 0.6 ± 0.08 | 0.6 ± 0.06 | 0.5 ± 0.06 | 0.6 ± 0.06 | 0.7 ± 0.14 |
Data are means ± SE. n = No. of mice. FBG, fasting blood glucose; FPI, fasting plasma insulin; ILK, integrin-linked kinase; KO, knockout. P values calculated between groups on the same diet. FBG and FPI are fasting blood glucose and fasting plasma insulin, respectively.
P < 0.05;
P < 0.01;
P < 0.001.
Hepatocyte ILK determines the transcriptional program of the liver.
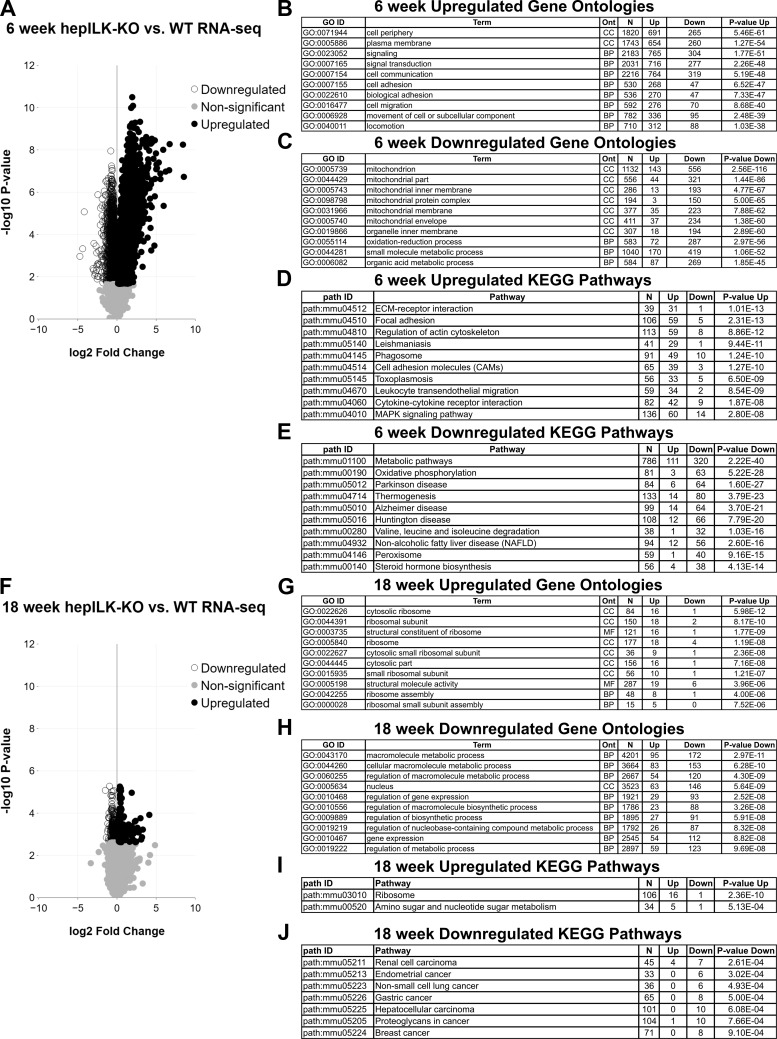
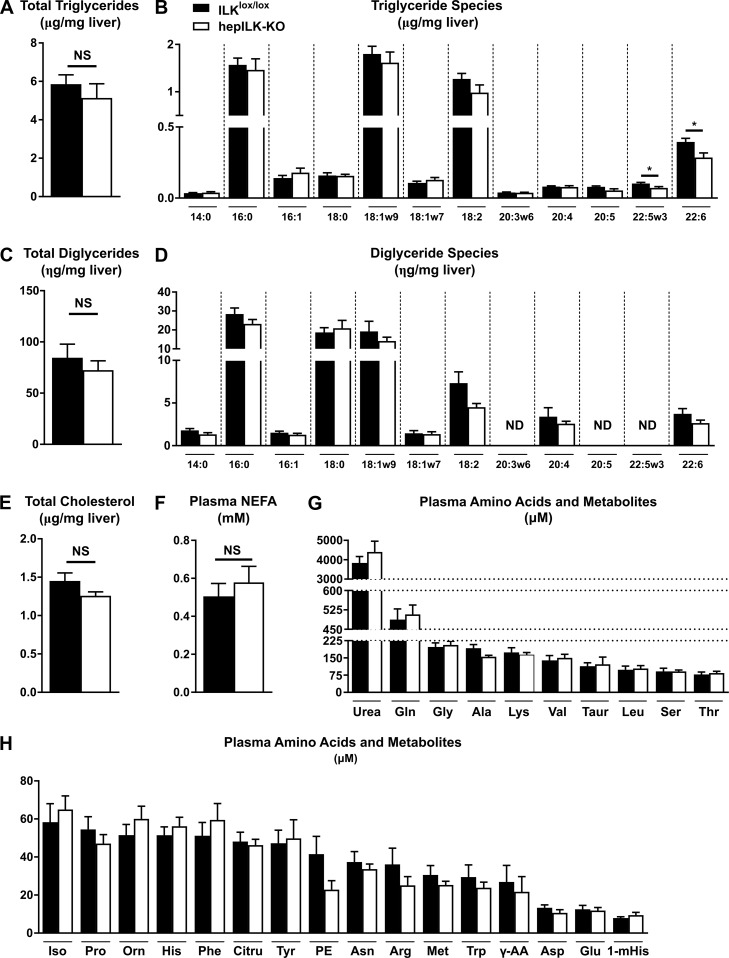
Given the difference in glucose homeostasis of 6 and 18-wk-old hepILK-KO mice, we hypothesized specific differences in hepatic transcriptional profiles relating to metabolic functions at these ages. RNA sequencing from whole livers of 5-h-fasted, 6-wk-old ILKlox/lox and hepILK-KO mice revealed a large number (n = 3925) of differentially expressed genes in hepILK-KO livers (Fig. 4A), representing an alteration in transcriptional profiles. Conversely, RNA sequencing of livers from 5-h-fasted, 18-wk-old ILKlox/lox, and hepILK-KO mice showed far fewer differentially expressed genes (n = 423), and the magnitude of differential expression was considerably less (Fig. 4F). Gene ontology terms overrepresented by genes significantly increased in 6-wk-old hepILK-KO livers (Fig. 4B) primarily indicated aspects of cell interactions, organization, or signaling (functions related to ILK). This is likely a compensatory response for cells to re-establish focal adhesion signaling and organization in the absence of ILK. Genes with decreased expression in 6-wk-old hepILK-KO livers were overrepresented by gene ontologies relating to mitochondria structure and oxidative metabolism (Fig. 4C). KEGG pathway analysis demonstrated upregulation of pathways related to integrin interactions and signaling (Fig. 4D) and decreases in a number of metabolic pathways and pathologies, including oxidative phosphorylation and nonalcoholic fatty liver disease (Fig. 4E). Despite this finding, no major differences in hepatic lipid profiles, circulating nonesterified fatty acids, circulating amino acids, or related metabolites were observed in hepILK-KO mice (Fig. 5). Gene ontologies and KEGG pathways enriched for significantly increased or decreased genes present in 6-wk-old hepILK-KO mice were largely resolved in 18-wk-old hepILK-KO livers (Fig. 4, G–J).
Fig. 4.
A: volcano plot of genes obtained from RNA sequencing (RNA-seq) of whole livers from 6-wk-old, 5-h-fasted integrin-linked kinase (ILK)lox/lox (n = 5) and hepILK-knockout (KO) (n = 5) mice. ○, Genes significantly decreased in hepILK-KO livers; ●, genes significantly increased in hepILK-KO livers. Gray circles, genes that were nonsignificantly altered in hepILK-KO livers. B: 10 Gene Ontology terms with highest P values for representation of significantly increased genes in 6-wk-old, 5-h-fasted hepILK-KO livers. C: 10 Gene Ontology terms with highest P values for representation of significantly decreased genes in 6-wk-old, 5-h-fasted hepILK-KO livers. D: 10 KEGG pathways with highest P values for representation of significantly decreased genes in 6-wk-old, 5-h-fasted hepILK-KO livers. E: 10 KEGG pathways with highest P values for representation of significantly increased genes in 6-wk-old, 5-h-fasted hepILK-KO livers. F: volcano plot of genes obtained from RNA-seq of whole livers from 18-wk-old, 5-h-fasted ILKlox/lox (n = 5) and hepILK-KO (n = 5) mice. Circles are as indicated above. G: 10 Gene Ontology terms with highest P values for representation of significantly increased genes in 18-wk-old, 5-h-fasted hepILK-KO livers. H: 10 Gene Ontology terms with highest P values for representation of significantly decreased genes in 18-wk-old, 5-h-fasted hepILK-KO livers. I: KEGG pathways with P < 0.001 for representation of significantly increased genes in 18-wk-old, 5-h-fasted hepILK-KO livers. J: KEGG pathways with P < 0.001 for representation of significantly decreased genes in 18-wk-old, 5-h-fasted hepILK-KO livers. WT, wild type.
Fig. 5.
A: total triglyceride content of livers from 5-h-fasted, 6-wk-old integrin-linked kinase (ILK)lox/lox (n = 7) and hepILK-knockout (KO) (n = 8) mice. B: quantification of triglyceride side chain species from A. C: total diglyceride content of livers from 5-h-fasted, 6-wk-old ILKlox/lox (n = 7) and hepILK-KO (n = 8) mice. D: quantification of diglyceride side chain species from D. E: total cholesterol content of livers from 5-h-fasted, 6-wk-old ILKlox/lox (n = 7) and hepILK-KO (n = 8) mice. F: concentration of nonesterified fatty acids (NEFA) in plasma samples taken at t = 100 during metabolic flux analysis (MFA) studies of 6-wk-old ILKlox/lox (n = 8) and hepILK-KO (n = 7) mice. G: concentration of higher level plasma amino acids and related metabolites in plasma samples taken at t = 120 during MFA studies of 6-wk-old ILKlox/lox (n = 8) and hepILK-KO (n = 7) mice. H: concentration of lower level plasma amino acids and related metabolites in plasma samples taken at t = 120 during MFA studies of 6-wk-old ILKlox/lox (n = 8) and hepILK-KO (n = 7) mice. All amino acids are depicted with standard 3-letter abbreviations. The remaining metabolites are as follows: γ-AA, γ-aminobutyric acid; Citru, citrulline; 1-mHis, 1-methyl-histidine; Orn, ornithine; PE, phosphoethanolamine; Taur, taurine. Data are means ± SE. *P < 0.05. ND, not detectable; NS, not significant.
Hepatocyte ILK is necessary for hepatocyte oxidative metabolism.
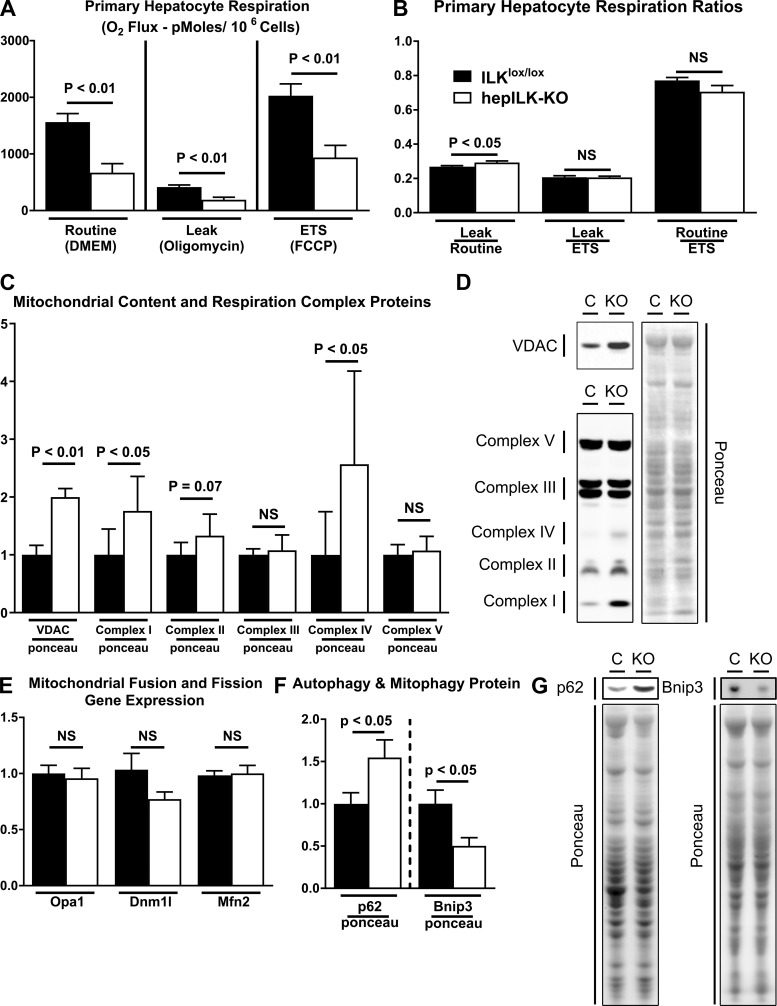
Hepatic compensation from 6 to 18 wk was so effective that differences in fibrosis, glucoregulation, and the transcriptional profile were diminished by 18 wk of age. Thus, we focused on metabolic mechanisms underlying altered regulation of circulating glucose in hepILK-KO mice at 6 wk of age. First, we assessed whether decreased expression of genes involved in mitochondrial structure and oxidative processes was linked to functional capacity at the cellular level. O2 consumption in isolated primary hepatocytes without treatment (routine), treated with oligomycin (leak), or FCCP (ETS) was lower in hepILK-KO hepatocytes than in ILKlox/lox hepatocytes (Fig. 6A). In hepILK-KO hepatocytes, the ratio of O2 consumption during oligomycin treatment to O2 consumption in cells without treatment was higher. This reflects a greater fraction of O2 consumption uncoupled from ATP production (Fig. 6B). With apparent deficiencies in mitochondrial function of hepILK-KO hepatocytes, protein content of mitochondrial oxidative phosphorylation complexes and voltage-dependent anion channel (VDAC) were assessed as indicators of mitochondrial content and function. Interestingly, mitochondrial complexes were either unchanged (complexes II, III, and V) or increased (complex I and IV) (Fig. 6, C and D). VDAC protein was also significantly increased (∼2-fold) in hepILK-KO cells (Fig. 6, C and D), indicating that hepILK-KO hepatocytes possess no deficiencies in the number of mitochondria despite decreased functionality.
Fig. 6.
A: respiration of hepatocytes isolated from integrin-linked kinase (ILK)lox/lox (n = 6) or hepILK-knockout (KO) (n = 7) in basal media (routine), in the presence of oligomycin (leak), and in the presence of FCCP [electron transfer system (ETS)]. B: ratios of respiration rates in the 3 conditions represented in A. C: quantification of voltage-dependent anion channel (VDAC) and mitochondrial complex protein levels relative to Ponceau total protein stain from samples used in A. D: representative images of VDAC, mitochondrial complexes, and Ponceau total protein stain in C. E: gene expression of optic atrophy 1 (opa1), dynamin 1 like protein (dnm1l), and mitofusin 2 (mfn2) in samples used in A. GAPDH was used as a control (C) gene. F: quantification of p62 and Bnip3 protein relative to Ponceau total protein stain in livers of ILKlox/lox (n = 8) and hepILK-KO (n = 7), which underwent metabolic flux analysis. G: representative Western blots of p62 and Bnip3 protein as well as Ponceau staining quantified in F. Data are means ± SE. NS, not significant.
Mitochondrial function can be affected by differential management of the mitochondrial network. Mitochondrial fission and fusion markers were assessed to evaluate these processes. Transcript of the fission gene dynamin 1-like (Dnm1l) and the fusion genes optic atrophy 1/mitochondrial dynamin-like GTPase (Opa1) and mitofusin 2 (Mfn2) was not different between hepILK-KO and ILKlox/lox mice (Fig. 6E). Protein content of autophagy (p62) and mitophagy (Bnip3) markers demonstrated an increase in p62 and a decrease in Bnip3 content in these livers (Fig. 6, F and G). These p62 and Bnip3 levels reflect decreases in autophagy and mitophagy, which can decrease mitochondrial function (37, 58).
Overall, these data reflect poorly functioning hepatocyte mitochondria in hepILK-KO livers. This impaired hepatic mitochondrial function is related to an altered transcriptional program and impaired autophagy/mitophagy.
Loss of hepatocyte ILK reduces whole body EE and increases carbohydrate oxidation.
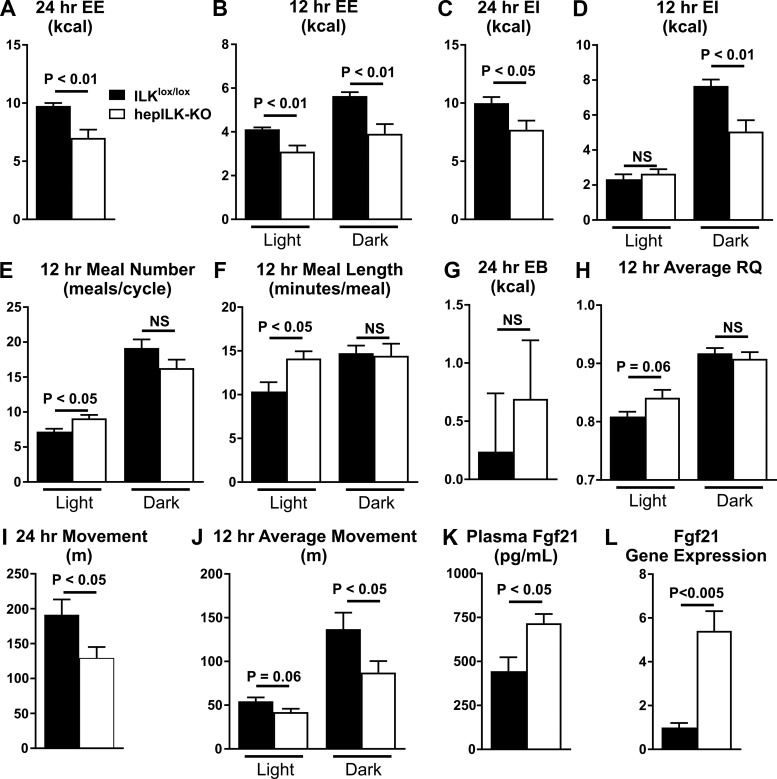
Deficient mitochondrial function in the liver has the capacity to alter whole body metabolism. Substrate utilization at the whole body level was assessed using indirect calorimetry. EE (Fig. 7A) was decreased in hepILK-KO mice at 6 wk of age during both the light and dark phases (Fig. 7B). EE remained significantly lower in hepILK-KO after for differences in lean mass by ANCOVA were accounted for (24 h: P < 0.05; light cycle: P < 0.001; dark cycle: P < 0.01). Therefore, EE in hepILK-KO mice was not due to lower lean mass and was driven by a genotype effect. Consistent with decreased EE, hepILK-KO mice consume less food and have lower EI overall (Fig. 7C). EI matched controls during the light cycle and remained significantly decreased during the dark cycle (Fig. 7D). This reflects more active feeding behavior during the light cycle, as quantified by number and length of meals (Fig. 7, E and F). Overall, hepILK-KO mice maintain a positive energy balance (EB; the difference between EI and EE), which is no different from control animals (Fig. 7G). Whereas the RQ during the dark cycle was no different between ILKlox/lox and hepILK-KO mice, light cycle RQ was significantly elevated in hepILK-KO mice, indicating increased utilization of carbohydrates as fuel (Fig. 7H). The decrease in energy expenditure is related to a decrease in the activity of hepILK-KO mice (Fig. 7, I and J). FGF21, which is secreted by the liver and is known to promote glucose utilization, was measured to gain insight into the possible mechanism by which hepILK-KO increased carbohydrate oxidation. Hepatic FGF21 transcript levels were increased (∼5-fold), and plasma FGF21 was increased (∼2-fold) in 6-wk-old hepILK-KO mice (Fig. 7, K and L). The increase in circulating FGF21 could explain the preference for utilization of carbohydrates in hepILK-KO mice as FGF21 stimulates carbohydrate utilization on extrahepatic tissues.
Fig. 7.
A: average 24-h energy expenditure (EE) of integrin-linked kinase (ILK)lox/lox (n = 8) and hepILK-knockout (KO) (n = 7) mice measured over a 7-day period. B: EE during 12-h light and dark phase, ILKlox/lox (n = 8), and hepILK-KO (n = 7). C: 24-h energy intake (EI) of ILKlox/lox (n = 8) and hepILK-KO (n = 7) mice. D: EI during 12-h light and dark phase, ILKlox/lox (n = 8), and hepILK-KO (n = 7). E: 24-h energy balance (EB = EI − EE) in (ILKlox/lox (n = 8) and hepILK-KO (n = 7) mice. F: average no. of meals/day during the light and dark phase, ILKlox/lox (n = 8), and hepILK-KO (n = 7). Twenty-four-hour EI of ILKlox/lox (n = 8) and hepILK-KO (n = 7) mice. G: average time of each meal period during the light and dark phase, ILKlox/lox (n = 8), and hepILK-KO (n = 7). H: average respiratory quotient during 12-h light and dark phase, ILKlox/lox (n = 8), and hepILK-KO (n = 7). I: average 24 h movement of ILKlox/lox (n = 8) and hepILK-KO (n = 7) mice measured over a 7-day period. J: movement during 12-h light and dark phase, ILKlox/lox (n = 8), and hepILK-KO (n = 7). K: concentration of fibroblast growth factor 21 (FGF21) in plasma from ILKlox/lox (n = 5) and hepILK-KO mice (n = 7) after a 5-h fast. L: gene expression of FGF21 from livers of ILKlox/lox (n = 6) and hepILK-KO (n = 7) mice after a 5-h fast. GAPDH was used as the control gene. Data are means ± SE.
Hepatocyte ILK is necessary for hepatic glucoregulation, glycogen storage, and energy status.
Given an increased preference for carbohydrates, deficient hepatic mitochondrial function, and altered glucose homeostasis of hepILK-KO mice at 6 wk of age, endogenous glucose production (EGP) and metabolic processes contributing to EGP were quantified using stable isotopes (Fig. 8A). Based on reduced blood glucose after a 5-h fast and impaired mitochondrial function, we hypothesized that EGP would be decreased. Arterial glucose was decreased in the hepILK-KO mice, consistent with previous fasting glucose data (Fig. 8B). However, a paradoxical increase in EGP due to accelerated gluconeogenic pathway flux was observed (Fig. 8C). As blood glucose was in a steady state, glucose utilization was increased equivalently. Increased glucose utilization from a reduced circulating glucose pool requires a marked increase in fractional glucose turnover (Fig. 8D). No differences in glycogenolytic rates between genotypes were evident (Fig. 8C). Increases in flux rates associated with the tricarboxylic acid cycle (TCA) did not reach significance (Fig. 8E). Increases in gluconeogenic fluxes were not coupled to changes in the expression of the gluconeogenic genes Pck1 and G6pc (Fig. 8F).
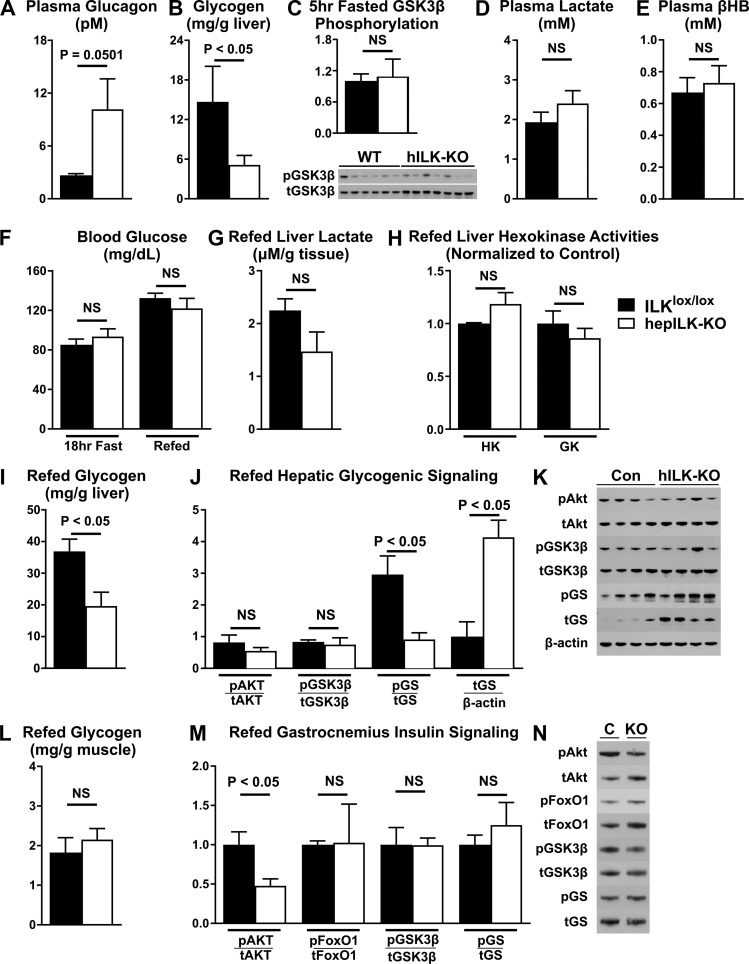
The absence of an increase in hepatic glycogenolysis occurred despite increased plasma glucagon (P = 0.0501; Fig. 9A) and decreased fasting plasma insulin in hepILK-KO mice (Table 1). This paradox led us to evaluate net glycogen storage in hepILK-KO mice. Glycogen content in 5-h-fasted hepILK-KO mice was decreased to the point of near depletion (Fig. 9B). Phosphorylation of glycogen synthase kinase-3β (GSK3β) was unchanged (Fig. 9C). No changes in plasma lactate (Fig. 9D) or β-hydroxybutyrate (Fig. 9E) were observed despite lower hepatic glycogen. To determine the effectiveness by which hepILK-KO mice stored glycogen, fasting/refeeding studies were conducted. After 18 h of fasting, blood glucose was reduced in ILKlox/lox mice to levels in hepILK-KO mice (Fig. 9F). After 6 h of refeeding, blood glucose rose similarly in both groups (Fig. 9F). However, net hepatic glycogen storage was ∼50% lower in refed hepILK-KO mice (Fig. 9I). Hepatic lactate content (Fig. 9G), as well as glucokinase and hexokinase activities (Fig. 9H), was no different between hepILK-KO and ILKlox/lox livers after refeeding. To examine whether there was a deficit in the insulin-stimulated glycogen synthesis signaling cascade, levels of total and phosphorylated proteins (Akt, GSK3β, and GS) were assessed. Phosphorylation of Akt (Ser473) was similar in ILKlox/lox and hepILK-KO mice (Fig. 9, G and H). Additionally, the inhibitory Akt-dependent phosphorylation of GSK3β (Ser9) was similar in the two genotypes (Fig. 9, J and K). The ratio of GS phosphorylated at Ser641 to total GS was decreased in hepILK-KO mice (Fig. 9, J and K). This GS phosphorylation site is GSK3β-dependent site and is inhibitory for GS activity. The decrease in phosphorylation of GS (Ser641) would thereby favor glycogen synthesis. The altered ratio of phosphorylated to total GS was due primarily to an approximately fourfold increase in total GS protein in hepILK-KO livers (Fig. 9, J and K). A signal associated with low hepatic glycogen storage may feed back to upregulate GS protein expression.
Fig. 9.
A: plasma glucagon concentrations in integrin-linked kinase (ILK)lox/lox (n = 7) and hepILK-knockout (KO) (n = 9) mice during metabolic flux analysis experiments. B: hepatic glycogen content of ILKlox/lox (n = 6) and hepILK-KO (n = 6) mice after a 5-h fast. C: quantification of the ratio of GSK3β phosphorylated at Ser9 (p-GSK3β) to total GSK3β (t-GSK3β) protein in livers of ILKlox/lox (n = 6) and hepILK-KO (n = 7) mice after a 5-h fast. D: plasma lactate concentration of ILKlox/lox (n = 5) and hepILK-KO (n = 5) mice after a 5-h fast. E: plasma β-hydroxybutyrate concentration of ILKlox/lox (n = 5) and hepILK-KO (n = 5) mice after a 5-h fast. F: blood glucose concentration of ILKlox/lox (n = 4) and hepILK-KO (n = 4) mice after an 18-h fast and after 6 h of ad libitum food access following the 18-h fast. G: liver lactate content of ILKlox/lox (n = 4) and hepILK-KO (n = 4) mice following the 6-h refeeding period. H) Enzymatic activities of glucokinase (GK) and all other hexokinases (HK) in livers of ILKlox/lox (n = 4) and hepILK-KO (n = 4) mice following the 6-h refeed period. I: hepatic glycogen content of ILKlox/lox (n = 4) and hepILK-KO (n = 4) mice following the 6-h refeeding period. J: quantification of densitometric ratios for designated phosphorylated (p) and total (t) proteins (K). Ratio of total glycogen synthase (t-GS) protein to β-actin is also shown. K: images of blots for Akt phosphorylated at Ser473 (p-Akt) total Akt (t-Akt), p-GSK3β, t-GSK3β, glycogen synthase (GS) phosphorylated (p-GS) at Ser641, total GS (t-GS), and β-actin in livers from ILKlox/lox (n = 4) and hepILK-KO (n = 4) mice following the 6-h refeeding period. L: gastrocnemius muscle glycogen content of ILKlox/lox (n = 4) and hepILK-KO (n = 4) mice following the 6-h refeeding period. M: quantification of densitometric ratios for designated phosphorylated and total proteins (N). N: representative images of blots for Akt phosphorylated at Ser473 (p-Akt), total Akt (t-Akt), FoxO1 phosphorylated at Ser256 (p-FoxO1) total FoxO1 (t-FoxO1), p-GSK3β, t-GSK3β, p-GS, and t-GS in livers from ILKlox/lox (n = 3) and hepILK-KO (n = 4) mice following the 6-h refeeding period. Data are means ± SE.
Because skeletal muscle can account for a significant portion of glucose disposal, we assessed the glycogen content and related signaling in gastrocnemius muscle in hepILK-KO and ILKlox/lox mice following refeeding. No differences were observed in glycogen content (Fig. 9L) or phosphorylation of FoxO1, GSK3β, and GS in muscle (Fig. 9, M and N). However, a significant decrease in muscle Akt phosphorylation was observed in these mice (Fig. 9, M and N). Based on results from OGTTs, this may be a result of a reduced insulin response to feeding. Overall the hepatic signaling profile favors glycogen synthesis, although net glycogen accumulation is reduced compared with ILKlox/lox mice. The net accumulation of glycogen could be attenuated due to simultaneous liver glycogen breakdown. In support of this possibility, the fractional glycogen turnover (the ratio of glycogenolysis to liver glycogen) was increased in hepILK-KO mice.
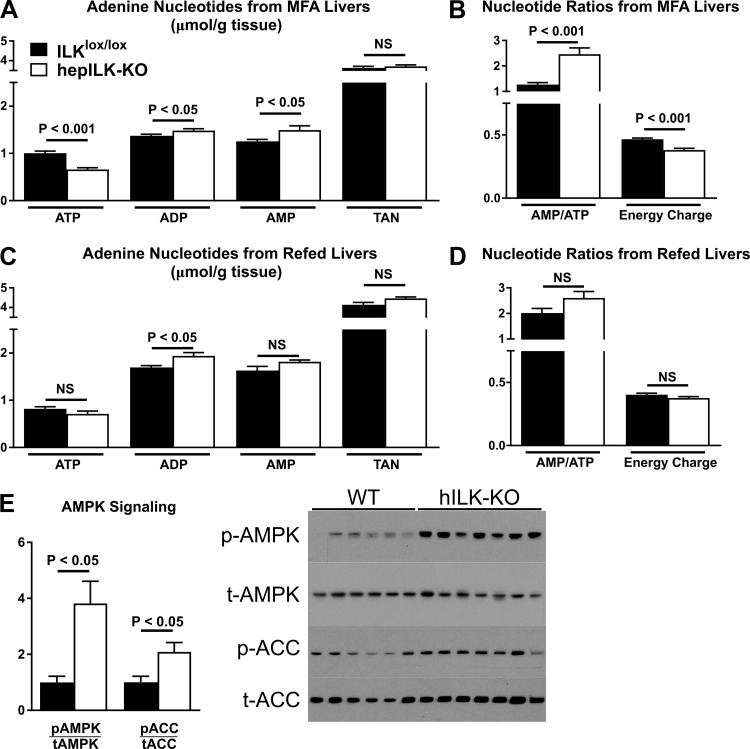
Deficient oxidative function coupled with gluconeogenesis decreased energetic content of the adenine nucleotide pool. In line with this, ATP content was significantly reduced in livers of hepILK-KO mice, with a concurrent increase in ADP and AMP (Fig. 10A). Total adenine nucleotides were the same in both hepILK-KO and ILKlox/lox mice (Fig. 10A). Increased AMP/ATP and decreased energy charge were observed in the liver of hepILK-KO mice (Fig. 10B). Upon refeeding, AMP and ATP in hepILK-KO mouse livers returned to concentrations in ILKlox/lox mice, whereas ADP was elevated (Fig. 10C). Energy charge of refed hepILK-KO livers was restored to levels in ILKlox/lox mice (Fig. 10D). Decreased energy charge in hepILK-KO mice caused a fourfold increase in AMPK phosphorylation after a 5-h fast (Fig. 10, E and F). Phosphorylation of the downstream AMPK target protein acetyl-CoA carboxylase (ACC) was also increased (Fig. 10, E and F), confirming that AMPK phosphorylation leads to functional activation.
Fig. 10.
A: adenine nucleotide levels (ATP, ADP, and AMP) in livers from integrin-linked kinase (ILK)lox/lox (n = 8) and hepILK-KO (n = 9) mice, which underwent metabolic flux analysis (MFA). B: total adenine nucleotide (TAN) levels (sum of ATP, ADP, and AMP levels) in livers from ILKlox/lox (n = 8) and hepILK-KO (n = 9) mice, which underwent MFA. Ratio of AMP to ATP and calculated energy charge from the same liver samples. C: adenine nucleotide levels (ATP, ADP, and AMP) in livers from ILKlox/lox (n = 4) and hepILK-KO (n = 4) mice, which underwent 18-h fast and 6-h refeed. D: TAN levels (sum of ATP, ADP, and AMP levels) in livers from ILKlox/lox (n = 4) and hepILK-KO (n = 4) mice, which underwent 18-h fast and 6-h refeed. Ratio of AMP to ATP and calculated energy charge from the same liver samples. E: quantification of AMPK phosphorylated at Thr172 (p-AMPK) and ACC phosphorylated at Ser79 (p-ACC) relative to their respective total proteins (t-AMPK and t-ACC) from livers of 5-h-fasted ILKlox/lox (n = 6) and hepILK-KO (n = 7). Data are means ± SE.
Integrin β1 signals upstream of ILK to determine hepatic glucose control.
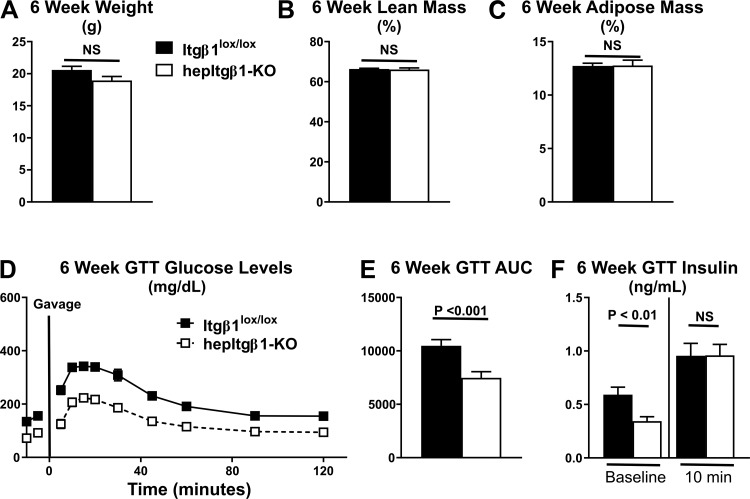
Integrin β1 (Itgβ1) is a ubiquitously expressed integrin receptor subunit that directly binds to and is an upstream signal input for ILK (23, 34). To determine whether the effects of hepILK-KO on glucose homeostasis were due to an inability to transduce upstream integrin extracellular matrix (ECM) signaling, a genetic hepatocyte knockout model of Itgβ1 was studied. Similarly to hepILK-KO mice, hepItgβ1-KO demonstrated lower body weights compared with Itgβ1lox/lox littermates. However, this difference did not reach statistical significance (Fig. 11A). As was the case with hepILK-KO mice, hepItgβ1-KO did not affect percent lean or percent fat masses (Fig. 11, B and C). Fasting blood glucose was decreased in hepItgβ1-KO mice, and the glucose tolerance was increased (Fig. 11, D and E) similarly to hepILK-KO mice (Fig. 11E). Fasting plasma insulin in hepItgβ1-KO mice was reduced compared with Itgβ1lox/lox littermates. This decrease was similar to the decrease in fasting plasma insulin in hepILK-KO mice. (Fig. 11F). Overall, the effects of hepItgβ1-KO on fasting glucose, fasting insulin, and glucose tolerance are nearly identical to the phenotype of hepILK-KO mice. Findings in the hepItgβ1-KO show that the results seen in the hepILK-KO mice on hepatic metabolism and glucose homeostasis are a result of disruption of ECM signals transmitted through the Itgβ1 subunit of integrin receptors.
Fig. 11.
A: body weight of Itgβ1lox/lox (n = 17) and hepItgβ1-knockout (KO) (n = 14) mice at 6 wk of age. B: lean mass body composition of Itgβ1lox/lox (n = 17) and hepItgβ1-KO (n = 14) mice at 6 wk of age. C: adipose mass body composition of Itgβ1lox/lox (n = 17) and hepItgβ1-KO (n = 14) mice at 6 wk of age. D: blood glucose levels of 6-wk-old Itgβ1lox/lox (n = 17) and hepItgβ1-KO (n = 14) mice during oral glucose tolerance tests (GTT). E: baseline corrected area under the glucose curves (AUC) of 6-wk-old Itgβ1lox/lox (n = 17) and hepItgβ1-KO (n = 14) mice during oral GTT. F: plasma insulin concentrations of 6-wk-old Itgβ1lox/lox (baseline; n = 17, 10 min; n = 17) and hepItgβ1-KO (baseline; n = 11, 10 min; n = 13) mice during oral GTT. Data are means ± SE. NS, not significant.
DISCUSSION
Liver ILK signaling has been proposed as a therapeutic target for several disease states, including insulin resistance (20, 22, 36, 64), yet there is little known about the physiology or metabolism of ILK signaling. These experiments demonstrate that hepatocyte ILK is required for glucose homeostasis through transcriptional and metabolic programming of the liver. Gene ontology analysis of RNAseq data showed that liver-specific deletion of ILK caused an upregulation of genes involved in classic integrin functions such as ECM-receptor interactions, focal adhesion, and actin cytoskeleton regulation. On the other hand, these analyses revealed decreased expression of genes encoding proteins involved in mitochondrial structure and function. Differential transcriptional profiles in hepILK-KO mice were resolved by 18 wk of age. ILK deletion resulted in functional defects characterized by reduced fasting blood glucose, plasma insulin, and more rapid disposal of an oral glucose load at 6 wk of age. As with differential gene expression at 6 wk, differences in the glucoregulatory variables were also resolved by 18 wk. The liver was, for the most part, histologically, transcriptionally, and metabolically reconstituted over time. The results of these studies and the temporal organization of hepatocyte-specific knockout in the alb-cre system directed us to focus on metabolic regulation in 6-wk-old mice.
Consistent with the genetic reprogamming in 6-wk-old mice, hepatocyte mitochondrial O2 consumption and whole body EE were diminished. Mitochondrial function was decreased, although protein content indicative of mitchondrial content was either unchanged (mitochondrial complexes II, III, and V) or increased (mitochondrial complexes I and IV and VDAC) in hepatocytes of hepILK-KO mice. Moreover, hepatic TCA cycle fluxes were not different in the hepILK-KO livers. Results indicate a buildup of poorly functioning mitochondria that contributes to decreased respiration. Deficits in autophagy and mitophagy were reflected by increased p62 and decreased Bnip3 protein in hepILK-KO livers. Impairments in autophagy and mitophagy can contribute to mitochondrial dysfunction, as it could underlie ineffective “pruning” of the mitochondrial network (37, 58).
Impaired mitochondrial function resulted in decreased energy charge. The increased AMP and decreased ATP, as observed in these studies, stimulate glycolysis by allosteric mechanisms and underlie a striking fourfold increase in AMPK activation. This signal alters the fate of glucose by inhibiting glycogen synthase (10) and activating glycolytic flux (39, 40). In this regard, the impaired capacity to store liver glycogen shown in the present study suggests that glucose is directed to glycolysis. AMPK activation also stimulates pathways for substrate oxidation to counter the decreased energy state of the cell (17). However, in the presence of impaired mitochondrial function, even a fourfold increase in AMPK was unable to fully compensate for impaired mitochondrial function in hepILK-KO livers. Althouugh AMPK activation stimulates lipid oxidation, comprehensive analyses revealed no reduction in circulating free fatty acids, total liver triglycerides, diglycerides, or cholesterol.
To confirm that the effects of hepILK-KO on glucose homeostasis were due to an inability to transduce ECM alterations, baseline glucose values were obtained and OGTTs performed on Itgβ1lox/lox and hepItgβ1-KO mice. Fasting blood glucose was decreased in hepItgβ1-KO mice by ∼50% and the glucose tolerance (AUC) reduced by ∼30%. These changes were similar to the effects in hepILK-KO mice. Fasting arterial insulin in hepItgβ1-KO mice was reduced by ∼40% compared with Itgβ1lox/lox littermates. This reduction was also comparable with that seen in hepILK-KO mice. Overall, the effects of hepItgβ1-KO on fasting glucose, fasting insulin, and glucose tolerance are nearly identical to the phenotype of hepILK-KO mice. The same findings in the hepItgβ1-KO as the hepILK-KO mice demonstrate that effects on glucose homeostasis result from a disruption of ECM signals transmitted through the Itgβ1-subunit of integrin receptors.
Glucose fluxes and the hepatic metabolic pathways contributing to it were assessed using 2H/13C metabolic flux analysis in conscious 5-h-fasted mice. Despite ILK deletion from the primary glucose-producing organ, the reduced blood glucose in hepILK-KO mice was not due to an impairment in EGP but rather an increase in the removal of glucose, as measured by absolute and fractional glucose turnover rate. This finding was consistent with the greater glucose tolerance and elevated the light cycle RQ in hepILK-KO mice. Although blood glucose was reduced, overt hypoglycemia was prevented by accelerated gluconeogenic flux rates upstream of the pyruvate cycle (e.g., through enolase) in hepILK-KO mice. A reduction in glycogen stores and a decreased capacity to store glycogen are consistent with impaired mitochondrial function and a greater reliance on glycolysis. The liver is simultaneously a glucose-consuming and glucose-producing organ even in the fasted state (61). We speculate that the liver may be the site of both increased glucose utilization and production. Analysis of skeletal muscle insulin signaling shows that it is not increased, making it an unlikely site for the added glucose disposal. Glucagon was elevated (P = 0.0501) and insulin decreased in hepILK-KO mice in response to the reduced blood glucose. Increased glucagon-to-insulin ratio in hepILK-KO mice can potently stimulate gluconeogenesis even in the presence of a reduced hepatic energy state (60).
FGF21 hepatic gene expression and circulating levels were elevated in hepILK-KO mice. FGF21 increases EE (49, 55). However, the effects of FGF21 on EE were offset in the hepILK-KO mice by decreased physical activity. Energy balance in hepILK-KO mice was in steady state, as EE and EI were equal at 6 wk. The elevated FGF21 in the light phase is consistent with the relative preference for carbohydrate as a fuel, as described above. This is consistent with increased glucose turnover and the actions of FGF21. Feeding behavior was also altered in hepILK-KO mice. Increased number of meals and length of meals during the light cycle were increased in hepILK-KO mice. The increase in light cycle feeding is consistent with the elevated circulating FGF21 in this phase (47).
Although blood glucose was reduced in hepILK-KO mice after a 5-h fast, blood glucose was equal in ILKlox/lox and hepILK-KO mice after an 18-h fast and rose equally during subsequent refeeding. Despite equal glucose loads, net hepatic glycogen storage was reduced by 50% in hepILK-KO mice. Impaired net glycogen storage occurred despite comparable glucokinase activities, metabolite levels, and a signaling profile that favors glycogen synthesis. Remarkably, GS protein was elevated nearly fourfold in hepILK-KO livers. Net hepatic glycogen storage is the difference between the rate of glucosyl unit incorporation into glycogen and the rate of glycogenolysis. Reduced net hepatic glycogen storage in the presence of a signaling environment conducive to glycogen synthesis suggests increased glycogen breakdown. Using MFA, we show that glycogenolysis is equal in ILKlox/lox and hepILK-KO mice despite the fact that glycogen stores are reduced by ∼70% in hepILK-KO mice, indicating that the fractional glycogen turnover (e.g., glycogenolysis per glycogen mass) is increased in hepILK-KO mice. This supports the concept that impaired net liver glycogen storage is a result of concurrent glycogen synthesis and breakdown.
Genetic knockout models may be complicated by many issues, including developmental effects of the deletion and compensation for the deleted gene. Previous studies in liver ILK KO mice had been conducted using the alf-alb-cre system, which becomes active early in development (32), resulting in genetic KO before differentiation of biliary epithelial cells from the bipotential hepatoblast progenitor population (∼E13.5) (12, 17). Influence of this phenomenon on previous alf-alb-cre ILK-KO models has been acknowledged as a potential contributor to observed phenotypes (7). We attempted to minimize developmental effects of gene deletion by utilizing the alb-cre system for hepatocyte-specific deletion. The alb-cre system deletion does not commence cre expression until differentiation of the albumin expressing hepatocyte population is complete (16, 19). Cre expression occurring after differentiation combined with dynamic perinatal and postnatal changes to the hepatocyte population result in later-onset and completion of hepatocyte-specific KO (between 3 and 6 wk of age) (16). Benefits of the cre system are 1) an ability to mitigate in utero and developmental consequences of earlier gene KO and 2) a hepatocyte-specific cre expression profile. It is notable that, in contrast to hepatic ILK KO using the alf-alb-cre system (18), neither increased hepatocyte proliferation nor increased liver-to-body weight ratio using the alb-cre system was observed. Evidence of a transient, unprovoked (i.e., absent of external stimuli or stressors) phenotype was previously observed in studies using the alf-alb-cre system. The present study using the alb-cre system shows many of the same transient effects and shows that this transience extends to glucose homeostasis as well. Despite the transience of the unprovoked phenotype, mature hepILK-KO mice possess accelerated recovery from chemical injury (6), regeneration (3), and improved insulin resistance resulting from overnutrition (64), regardless of which of the two cre systems are used. Thus, although the effects of gene deletion may become masked to conditions for which they have adapted, provocative stimuli for which they have not adapted expose the role of the deleted gene.
Fibrosis at 6 wk of age results from hepILK-KO regardless of the hepatic/hepatocyte cre system (18, 19). Effects of hepatic damage, fibrosis, and cirrhosis to altered glucose homeostasis have been well characterized (41, 45, 46). However, these conditions are typically associated with impaired glucose tolerance, hyperinsulinemia, and impaired insulin sensitivity. Results of our studies in 6-wk-old hepILK-KO mice reveal improved glucose tolerance and decreased insulin in addition to previously documented protection from insulin resistance during overnutrition in mature mice (64). In the absence of acute signals from the fibrotic environment, hepatocyte oxidative mitochondrial function is still impaired. The liver is also a central hub for amino acid and urea metabolism. Distinct changes in circulating amino acids and related metabolites have been linked to fibrogenic severity (14, 26). Plasma profiles of amino acids and these other key metabolites were equivalent in hepILK-KO mice. The ability to sustain gluconeogenesis at an accelerated rate also indicates that liver damage is not severe. It may be that hepILK-KO limits the hepatocyte interpretive capacity of the fibrotic environment by limiting aspects of integrin sensing and signaling related to the extracellular matrix. Although speculative, it may explain certain metabolic protections observed in hepILK-KO mice.
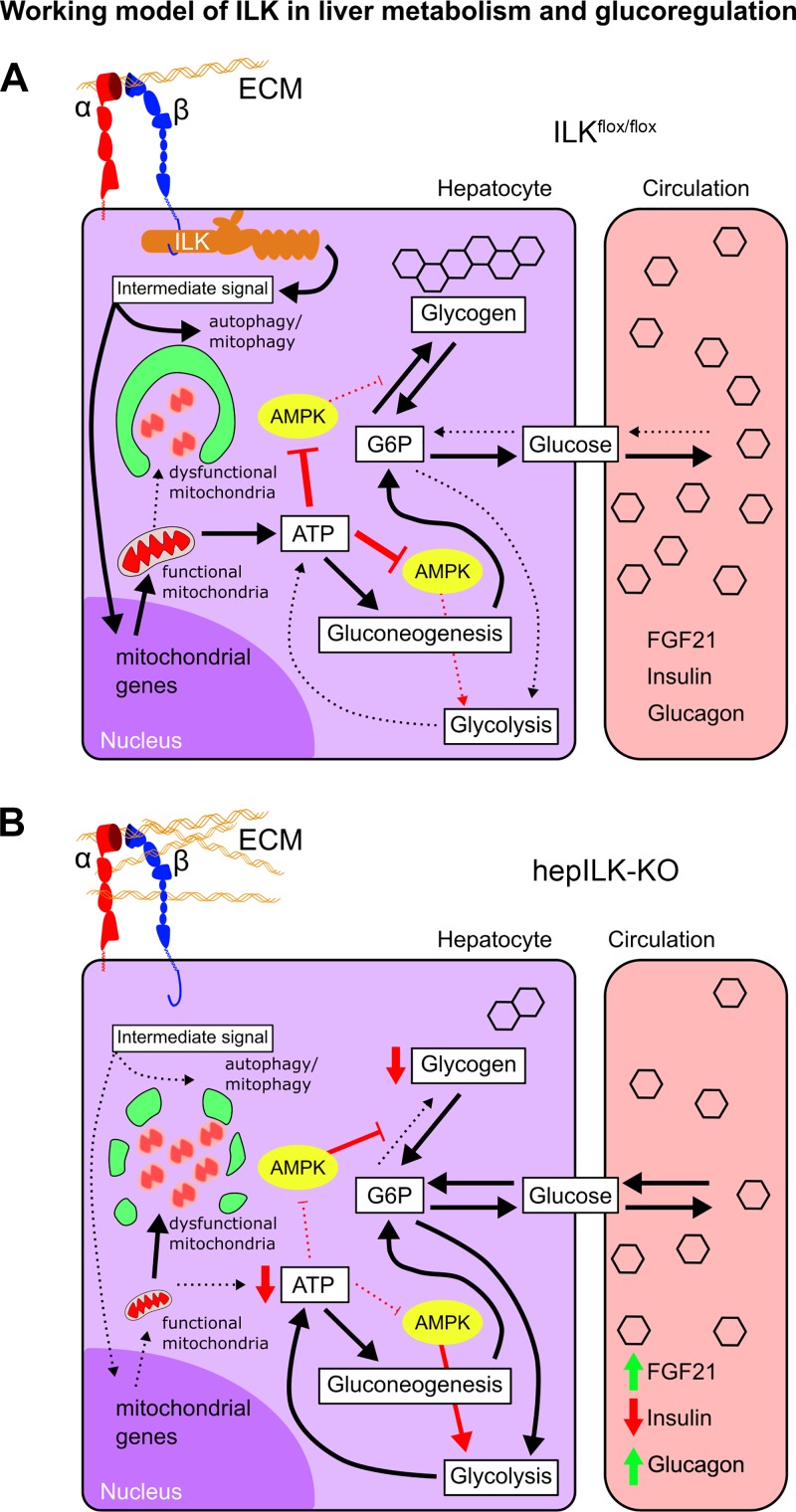
Previous studies have implicated inhibition of ILK signaling in treatment of a number of conditions, including insulin resistance (64), cancer (20, 22, 42, 66), and fibrosis (7, 67). This study defines the requirement for ILK in fundamental metabolic processes of the liver. The proposed role of ILK and the effects of hepatic-specific ILK KO are summarized in Fig. 12. In the present studies, we show that, although ILK deletion accelerates recovery of the liver from stress, ILK deletion compromises hepatic metabolism in healthy, young mice. Specifically, 6-wk-old hepILK-KO mice exhibit differential gene expression and functional changes in hepatic metabolism that lead to increased glucose removal, impaired net glycogen storage, impaired mitochondrial function, and increased gluconeogenesis.
Fig. 12.
A: proposed model of extracellular matrix (ECM)-integrin-integrin-linked kinase (ILK) signaling and contributions to hepatic metabolism and glucoregulatory functions. B: model of altered hepatocyte metabolism and glucoregulatory functions upon disruption of integrin signaling via hepILK-knockout (KO). FGF21, fibroblast growth factor 21.
In conclusion, our study establishes a role for ILK in maintaining liver metabolism and glucoregulatory functions. These effects likely contribute to the role of ILK in mediating insulin resistance during diet-induced obesity. Furthermore, these findings inform our understanding of pathological conditions affiliated with altered ECM and metabolism, including nonalcoholic fatty liver disease. As such, it is important to consider the physiological and metabolic effects of ILK when targeting the inhibition of integrin-mediated signaling pathways and potentially invoking this metabolic influence for therapeutic potential in the future.
GRANTS
This work was supported by funding from the National Institutes of Health (Molecular Endocrinology Training Program, DK-007563, and an individual NRSA fellowship, DK-112553, to E. Trefts; DK-069221 and DK-083187 to R. Zent; and CA-162433 and DK-095761 to A. Pozzi; as well as DK-050277 and DK-054902 to D. H. Wasserman). This work was also made possible by Veterans Affairs Merit Reviews 1I01BX002025-01 to A. Pozzi and 1I01BX002196-01 to R. Zent. This work was also supported by The Diabetes Research and Training Center (DK-20593).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.T. and D.H.W. conceived and designed research; E.T. and C.C.H. performed experiments; E.T., C.C.H., L.L., and K.L.B. analyzed data; E.T., L.L., D.S.L., K.L.B., and D.H.W. interpreted results of experiments; E.T. and K.L.B. prepared figures; E.T. drafted manuscript; E.T., C.C.H., L.L., K.L.B., A.P., R.Z., and D.H.W. edited and revised manuscript; E.T., C.C.H., L.L., A.P., R.Z., and D.H.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Reinhard Fässler (Max Planck Institute of Biochemistry, Martinsried, Germany) for generously providing the ILK and Itgβ1 floxed mice. We also thank Dr. Dale S. Edgerton, Dr. Larry L. Swift, Susan Hajizadeh, E. Patrick Donahue, Carla Harris, and Merrygay James (Vanderbilt Mouse Metabolic Phenotyping Center; DK-059637) for providing expertise and technical services.
REFERENCES
- 1.Afgan E, Baker D, Batut B, van den Beek M, Bouvier D, Cech M, Chilton J, Clements D, Coraor N, Grüning BA, Guerler A, Hillman-Jackson J, Hiltemann S, Jalili V, Rasche H, Soranzo N, Goecks J, Taylor J, Nekrutenko A, Blankenberg D. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res 46: W537–W544, 2018. doi: 10.1093/nar/gky379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anders S, Pyl PT, Huber W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31: 166–169, 2015. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apte U, Gkretsi V, Bowen WC, Mars WM, Luo JH, Donthamsetty S, Orr A, Monga SPS, Wu C, Michalopoulos GK. Enhanced liver regeneration following changes induced by hepatocyte-specific genetic ablation of integrin-linked kinase. Hepatology 50: 844–851, 2009. doi: 10.1002/hep.23059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayala JE, Bracy DP, Malabanan C, James FD, Ansari T, Fueger PT, McGuinness OP, Wasserman DH. Hyperinsulinemic-euglycemic clamps in conscious, unrestrained mice. J Vis Exp: 3188, 2011. doi: 10.3791/3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse Diabetes 55: 390–397, 2006. doi: 10.2337/diabetes.55.02.06.db05-0686. [DOI] [PubMed] [Google Scholar]
- 6.Bhushan B, Edwards G, Desai A, Michalopoulos GK, Apte U. Liver-specific deletion of integrin-linked kinase in mice attenuates hepatotoxicity and improves liver regeneration after acetaminophen overdose. Gene Expr 17: 35–45, 2016. doi: 10.3727/105221616X691578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blattner SM, Kretzler M. Integrin-linked kinase in renal disease: connecting cell-matrix interaction to the cytoskeleton. Curr Opin Nephrol Hypertens 14: 404–410, 2005. doi: 10.1097/01.mnh.0000172730.67746.5b. [DOI] [PubMed] [Google Scholar]
- 8.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30: 2114–2120, 2014. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brakebusch C, Grose R, Quondamatteo F, Ramirez A, Jorcano JL, Pirro A, Svensson M, Herken R, Sasaki T, Timpl R, Werner S, Fässler R. Skin and hair follicle integrity is crucially dependent on β 1 integrin expression on keratinocytes. EMBO J 19: 3990–4003, 2000. doi: 10.1093/emboj/19.15.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bultot L, Guigas B, Von Wilamowitz-Moellendorff A, Maisin L, Vertommen D, Hussain N, Beullens M, Guinovart JJ, Foretz M, Viollet B, Sakamoto K, Hue L, Rider MH. AMP-activated protein kinase phosphorylates and inactivates liver glycogen synthase. Biochem J 443: 193–203, 2012. doi: 10.1042/BJ20112026. [DOI] [PubMed] [Google Scholar]
- 11.Chan TM, Exton JH. A rapid method for the determination of glycogen content and radioactivity in small quantities of tissue or isolated hepatocytes. Anal Biochem 71: 96–105, 1976. doi: 10.1016/0003-2697(76)90014-2. [DOI] [PubMed] [Google Scholar]
- 12.Donthamsetty S, Bowen W, Mars W, Bhave V, Luo JH, Wu C, Hurd J, Orr A, Bell A, Michalopoulos G. Liver-specific ablation of integrin-linked kinase in mice results in enhanced and prolonged cell proliferation and hepatomegaly after phenobarbital administration. Toxicol Sci 113: 358–366, 2010. doi: 10.1093/toxsci/kfp281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donthamsetty S, Mars WM, Orr A, Wu C, Michalopoulos GK. Protection against Fas-induced fulminant hepatic failure in liver specific integrin linked kinase knockout mice. Comp Hepatol 10: 11, 2011. doi: 10.1186/1476-5926-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enomoto H, Sakai Y, Aizawa N, Iwata Y, Tanaka H, Ikeda N, Hasegawa K, Yoh K, Ishii A, Takashima T, Iwata K, Saito M, Imanishi H, Iijima H, Nishiguchi S. Association of amino acid imbalance with the severity of liver fibrosis and esophageal varices. Ann Hepatol 12: 471–478, 2013. [PubMed] [Google Scholar]
- 15.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957. [PubMed] [Google Scholar]
- 16.Foretz M, Hébrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, Sakamoto K, Andreelli F, Viollet B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest 120: 2355–2369, 2010. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia D, Shaw RJ. AMPK: Mechanisms of cellular energy sensing and restoration of metabolic balance. Mol Cell 66: 789–800, 2017. doi: 10.1016/j.molcel.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gkretsi V, Apte U, Mars WM, Bowen WC, Luo JH, Yang Y, Yu YP, Orr A, St-Arnaud R, Dedhar S, Kaestner KH, Wu C, Michalopoulos GK. Liver-specific ablation of integrin-linked kinase in mice results in abnormal histology, enhanced cell proliferation, and hepatomegaly. Hepatology 48: 1932–1941, 2008. doi: 10.1002/hep.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gkretsi V, Mars WM, Bowen WC, Barua L, Yang Y, Guo L, St-Arnaud R, Dedhar S, Wu C, Michalopoulos GK. Loss of integrin linked kinase from mouse hepatocytes in vitro and in vivo results in apoptosis and hepatitis. Hepatology 45: 1025–1034, 2007. doi: 10.1002/hep.21540. [DOI] [PubMed] [Google Scholar]
- 20.Han KS, Li N, Raven PA, Fazli L, Ettinger S, Hong SJ, Gleave ME, So AI. Targeting integrin-linked kinase suppresses invasion and metastasis through downregulation of epithelial-to-mesenchymal transition in renal cell carcinoma. Mol Cancer Ther 14: 1024–1034, 2015. doi: 10.1158/1535-7163.MCT-14-0771. [DOI] [PubMed] [Google Scholar]
- 22.Hannigan G, Troussard AA, Dedhar S. Integrin-linked kinase: a cancer therapeutic target unique among its ILK. Nat Rev Cancer 5: 51–63, 2005. doi: 10.1038/nrc1524. [DOI] [PubMed] [Google Scholar]
- 23.Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC, Dedhar S. Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature 379: 91–96, 1996. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- 24.Hasenour CM, Ridley DE, Hughey CC, James FD, Donahue EP, Shearer J, Viollet B, Foretz M, Wasserman DH. 5-Aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR) effect on glucose production, but not energy metabolism, is independent of hepatic AMPK in vivo. J Biol Chem 289: 5950–5959, 2014. doi: 10.1074/jbc.M113.528232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasenour CM, Wall ML, Ridley DE, Hughey CC, James FD, Wasserman DH, Young JD. Mass spectrometry-based microassay of (2)H and (13)C plasma glucose labeling to quantify liver metabolic fluxes in vivo. Am J Physiol Endocrinol Metab 309: E191–E203, 2015. doi: 10.1152/ajpendo.00003.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holecek M. Ammonia and amino acid profiles in liver cirrhosis: effects of variables leading to hepatic encephalopathy. Nutrition 31: 14–20, 2015. doi: 10.1016/j.nut.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 27.Hughey CC, James FD, Bracy DP, Donahue EP, Young JD, Viollet B, Foretz M, Wasserman DH. Loss of hepatic AMP-activated protein kinase impedes the rate of glycogenolysis but not gluconeogenic fluxes in exercising mice. J Biol Chem 292: 20125–20140, 2017. doi: 10.1074/jbc.M117.811547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang X, Wang J, Zhang K, Tang S, Ren C, Chen Y. The role of CD29-ILK-Akt signaling-mediated epithelial-mesenchymal transition of liver epithelial cells and chemoresistance and radioresistance in hepatocellular carcinoma cells. Med Oncol 32: 141, 2015. doi: 10.1007/s12032-015-0595-x. [DOI] [PubMed] [Google Scholar]
- 29.Kaiyala KJ. Mathematical model for the contribution of individual organs to non-zero y-intercepts in single and multi-compartment linear models of whole-body energy expenditure. PLoS One 9: e103301, 2014. doi: 10.1371/journal.pone.0103301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaiyala KJ, Morton GJ, Leroux BG, Ogimoto K, Wisse B, Schwartz MW. Identification of body fat mass as a major determinant of metabolic rate in mice. Diabetes 59: 1657–1666, 2010. doi: 10.2337/db09-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaiyala KJ, Schwartz MW. Toward a more complete (and less controversial) understanding of energy expenditure and its role in obesity pathogenesis. Diabetes 60: 17–23, 2011. doi: 10.2337/db10-0909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kellendonk C, Opherk C, Anlag K, Schütz G, Tronche F. Hepatocyte-specific expression of Cre recombinase. Genesis 26: 151–153, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 33.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12: 357–360, 2015. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Legate KR, Montañez E, Kudlacek O, Füssler R. ILK, PINCH and parvin: the tIPP of integrin signalling. Nat Rev Mol Cell Biol 7: 20–31, 2006. doi: 10.1038/nrm1789. [DOI] [PubMed] [Google Scholar]
- 35.Legate KR, Wickström SA, Fässler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev 23: 397–418, 2009. doi: 10.1101/gad.1758709. [DOI] [PubMed] [Google Scholar]
- 36.Lu H, Fedak PWM, Dai X, Du C, Zhou YQ, Henkelman M, Mongroo PS, Lau A, Yamabi H, Hinek A, Husain M, Hannigan G, Coles JG. Integrin-linked kinase expression is elevated in human cardiac hypertrophy and induces hypertrophy in transgenic mice. Circulation 114: 2271–2279, 2006. doi: 10.1161/CIRCULATIONAHA.106.642330. [DOI] [PubMed] [Google Scholar]
- 37.Mansouri A, Gattolliat C-H, Asselah T. Mitochondrial dysfunction and signaling in chronic liver diseases. Gastroenterology 155: 629–647, 2018. doi: 10.1053/j.gastro.2018.06.083. [DOI] [PubMed] [Google Scholar]
- 39.Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, Van den Berghe G, Carling D, Hue L. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol 10: 1247–1255, 2000. doi: 10.1016/S0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- 40.Marsin AS, Bouzin C, Bertrand L, Hue L. The stimulation of glycolysis by hypoxia in activated monocytes is mediated by AMP-activated protein kinase and inducible 6-phosphofructo-2-kinase. J Biol Chem 277: 30778–30783, 2002. doi: 10.1074/jbc.M205213200. [DOI] [PubMed] [Google Scholar]
- 41.Mavrogiannaki A, Karamanos B, Manesis EK, Papatheodoridis GV, Koskinas J, Archimandritis AJ. Prevalence of glucose intolerance in patients with chronic hepatitis B or C: a prospective case-control study. J Viral Hepat 16: 430–436, 2009. doi: 10.1111/j.1365-2893.2009.01077.x. [DOI] [PubMed] [Google Scholar]
- 42.McDonald PC, Fielding AB, Dedhar S. Integrin-linked kinase-essential roles in physiology and cancer biology. J Cell Sci 121: 3121–3132, 2008. doi: 10.1242/jcs.017996. [DOI] [PubMed] [Google Scholar]
- 43.Morgan CR, Lazarow A. Immunoassay of pancreatic and plasma insulin following alloxan injection of rats. Diabetes 14: 669–671, 1965. doi: 10.2337/diab.14.10.669. [DOI] [PubMed] [Google Scholar]
- 44.Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride–methanol. J Lipid Res 5: 600–608, 1964. [PubMed] [Google Scholar]
- 45.Nishida T. Diagnosis and Clinical Implications of Diabetes in Liver Cirrhosis: A Focus on the Oral Glucose Tolerance Test. J Endocr Soc 1: 886–896, 2017. doi: 10.1210/js.2017-00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ochi T, Kawaguchi T, Nakahara T, Ono M, Noguchi S, Koshiyama Y, Munekage K, Murakami E, Hiramatsu A, Ogasawara M, Hirose A, Mizuta H, Masuda K, Okamoto N, Suganuma N, Chayama K, Yamaguchi M, Torimura T, Saibara T. Differences in characteristics of glucose intolerance between patients with NAFLD and chronic hepatitis C as determined by CGMS. Sci Rep 7: 10146, 2017. doi: 10.1038/s41598-017-09256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Owen BM, Ding X, Morgan DA, Coate KC, Bookout AL, Rahmouni K, Kliewer SA, Mangelsdorf DJ. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab 20: 670–677, 2014. doi: 10.1016/j.cmet.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Postic C, Magnuson MA. DNA excision in liver by an albumin-Cre transgene occurs progressively with age. Genesis 26: 149–150, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 49.Potthoff MJ, Finck BN. Head over hepatocytes for FGF21. Diabetes 63: 4013–4015, 2014. doi: 10.2337/db14-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qin J, Wu C. ILK: a pseudokinase in the center stage of cell-matrix adhesion and signaling. Curr Opin Cell Biol 24: 607–613, 2012. doi: 10.1016/j.ceb.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43: e47, 2015. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140, 2010. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rudel LL, Kelley K, Sawyer JK, Shah R, Wilson MD. Dietary monounsaturated fatty acids promote aortic atherosclerosis in LDL receptor-null, human ApoB100-overexpressing transgenic mice. Arterioscler Thromb Vasc Biol 18: 1818–1827, 1998. doi: 10.1161/01.ATV.18.11.1818. [DOI] [PubMed] [Google Scholar]
- 54.Sakai T, Li S, Docheva D, Grashoff C, Sakai K, Kostka G, Braun A, Pfeifer A, Yurchenco PD, Fässler R. Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. Genes Dev 17: 926–940, 2003. doi: 10.1101/gad.255603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sarruf DA, Thaler JP, Morton GJ, German J, Fischer JD, Ogimoto K, Schwartz MW. Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes 59: 1817–1824, 2010. doi: 10.2337/db09-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tiedge M, Richter T, Lenzen S. Importance of cysteine residues for the stability and catalytic activity of human pancreatic beta cell glucokinase. Arch Biochem Biophys 375: 251–260, 2000. doi: 10.1006/abbi.1999.1666. [DOI] [PubMed] [Google Scholar]
- 57.Trefts E, Gannon M, Wasserman DH. The liver. Curr Biol 27: R1147–R1151, 2017. doi: 10.1016/j.cub.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ueno T, Komatsu M. Autophagy in the liver: functions in health and disease. Nat Rev Gastroenterol Hepatol 14: 170–184, 2017. doi: 10.1038/nrgastro.2016.185. [DOI] [PubMed] [Google Scholar]
- 60.Wasserman DH. Four grams of glucose. Am J Physiol Endocrinol Metab 296: E11–E21, 2009. doi: 10.1152/ajpendo.90563.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wasserman DH, Lacy DB, Green DR, Williams PE, Cherrington AD. Dynamics of hepatic lactate and glucose balances during prolonged exercise and recovery in the dog. J Appl Physiol (1985) 63: 2411–2417, 1987. doi: 10.1152/jappl.1987.63.6.2411. [DOI] [PubMed] [Google Scholar]
- 62.Weir JBV. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109: 1–9, 1949. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wickström SA, Lange A, Montanez E, Fässler R. The ILK/PINCH/parvin complex: the kinase is dead, long live the pseudokinase! EMBO J 29: 281–291, 2010. doi: 10.1038/emboj.2009.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams AS, Trefts E, Lantier L, Grueter CA, Bracy DP, James FD, Pozzi A, Zent R, Wasserman DH. Integrin-linked kinase is necessary for the development of diet-induced hepatic insulin resistance. Diabetes 66: 325–334, 2017. doi: 10.2337/db16-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Winograd-Katz SE, Fässler R, Geiger B, Legate KR. The integrin adhesome: from genes and proteins to human disease. Nat Rev Mol Cell Biol 15: 273–288, 2014. doi: 10.1038/nrm3769. [DOI] [PubMed] [Google Scholar]
- 66.Yoganathan TN, Costello P, Chen X, Jabali M, Yan J, Leung D, Zhang Z, Yee A, Dedhar S, Sanghera J. Integrin-linked kinase (ILK): a “hot” therapeutic target. Biochem Pharmacol 60: 1115–1119, 2000. doi: 10.1016/S0006-2952(00)00444-5. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y, Ikegami T, Honda A, Miyazaki T, Bouscarel B, Rojkind M, Hyodo I, Matsuzaki Y. Involvement of integrin-linked kinase in carbon tetrachloride-induced hepatic fibrosis in rats. Hepatology 44: 612–622, 2006. doi: 10.1002/hep.21315. [DOI] [PubMed] [Google Scholar]