Abstract
Fetal hypoxemia is associated with pregnancy conditions that cause an early activation of fetal glucose production. However, the independent role of hypoxemia to activate this pathway is not well understood. We hypothesized that fetal hypoxemia would activate fetal glucose production by decreasing umbilical glucose uptake and increasing counter-regulatory hormone concentrations. We induced hypoxemia for 9 days with maternal tracheal N2 gas insufflation to reduce maternal and fetal arterial Po2 by ~20% (HOX) compared with fetuses from ewes receiving intratracheal compressed air (CON). At 0.9 of gestation, fetal metabolic studies were performed (n = 7 CON, 11 HOX). Umbilical blood flow rates, net fetal oxygen and glucose uptake rates, and fetal arterial plasma glucose concentrations were not different between the two groups. Fetal glucose utilization rates were lower in HOX versus CON fetuses but not different from umbilical glucose uptake rates, demonstrating the absence of endogenous glucose production. In liver tissue, mRNA expression of gluconeogenic genes G6PC (P < 0.01) and PCK1 (P = 0.06) were six- and threefold greater in HOX fetuses versus CON fetuses. Increased fetal norepinephrine and cortisol concentrations and hepatic G6PC and PCK1 expression were inversely related to fetal arterial Po2. These findings support a role for fetal hypoxemia to act with counter-regulatory hormones to potentiate fetal hepatic gluconeogenic gene expression. However, in the absence of decreased net fetal glucose uptake rates and plasma glucose concentrations, hypoxemia-induced gluconeogenic gene activation is not sufficient to activate fetal glucose production.
Keywords: fetus, glucose, hypoxemia, liver, oxygen
INTRODUCTION
Fetal hypoxemia from decreased oxygen supply is a feature of pregnancy conditions, including placental insufficiency, preeclampsia, high-altitude (>2,500 m) exposure, umbilical cord compression, and maternal obesity (1, 2, 33, 34, 41, 42). We have demonstrated previously that fetuses with placental insufficiency-induced intrauterine growth restriction (PI-IUGR) have an early activation of hepatic glucose production and increased hepatic gluconeogenic gene expression, specifically glucose-6-phosphatase (G6PC) and phosphoenolpyruvate carboxykinase [PCK1 (cytosolic) and PCK2 (mitochondrial)] (25, 36, 37). Under normal conditions, fetal endogenous glucose production is absent because glucose supply by the mother and transfer across the placenta are sufficient to meet fetal glucose requirements (11, 12, 18). Just before parturition, fetal cortisol and norepinephrine secretion and plasma concentrations increase and the insulin to glucagon ratio decreases, which together initiate activation of the fetal gluconeogenic pathway (11, 12, 21). Fetuses with PI-IUGR experience chronic and progressive reductions in both glucose and oxygen supply coupled with decreased plasma insulin concentrations and increased plasma norepinephrine, cortisol, and glucagon concentrations (7, 24, 25, 37, 38). Collectively, this hypoglycemia, hypoxemia, and endocrine milieu is likely responsible for the early activation of glucose production in the PI-IUGR fetus. Indeed, experimental conditions with reduced fetal glucose supply and resulting hypoglycemia activate fetal glucose production (19, 20, 28, 31, 39). Some of these conditions, such as PI-IUGR, are also confounded by reduced fetal oxygen supply and concentrations; however, the independent effects of chronic hypoxemia on fetal glucose supply and activation of endocrine signals that initiate the induction of endogenous hepatic glucose production are not well understood.
Previous studies indicate that hypoxemia in late gestation may activate fetal hepatic glucose production. Acute hypoxemia for 30 min increases fetal hepatic glucose output; however, this is likely the result of an acute stress response and increased glycogenolysis rather than gluconeogenesis (5, 33). Sustained hypoxemia for 1–2 wk increases norepinephrine concentrations in the fetus, providing an endocrine signal that could activate hepatic glucose production, but the gluconeogenic pathway was not investigated (23). Anemic hypoxemia over 9 days increases fetal cortisol and glucagon concentrations, increases hepatic PCK1 expression, and decreases fetal hepatic glycogen content (9). Importantly, neither of these studies (9, 23) measured rates of endogenous fetal glucose production. Furthermore, the role of endocrine signals and the contribution of glycogenolysis versus gluconeogenesis to total hepatic glucose production during sustained hypoxemia is unclear.
We produced a model with sustained hypoxemia by maternal tracheal N2 insufflation to subsequently reduce fetal arterial Po2 for 9 days at late gestation. This model allowed us to precisely target the fetal hypoxemia observed in late-gestation PI-IUGR fetuses and test the specific role of hypoxemia on activation of fetal glucose production. We hypothesized that sustained (9 days) fetal hypoxemia would increase fetal counter-regulatory hormones (cortisol, glucagon, and norepinephrine) and decrease insulin concentrations that collectively would increase hepatic gluconeogenic gene expression and activate fetal glucose production by gluconeogenesis. Furthermore, we hypothesized that sustained fetal hypoxemia would reduce net fetal glucose uptake rates from the placenta and fetal plasma glucose concentrations that also would activate glucose production in the fetus.
MATERIALS AND METHODS
Ethics statement.
All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Colorado School of Medicine. Animals were supplied from Nebeker Ranch (Lancaster, CA) and studied at the Perinatal Research Center (Aurora, CO). The Perinatal Research Center is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care and is compliant with the Animal Welfare Act and Public Health Service Policy. Experimental details are reported according to the Animal Research: Reporting of In Vivo Experiments guidelines (22).
Animal procedures.
Pregnant Columbia-Rambouillet ewes (n = 31, 58.5 ± 1.69 kg, 1–3 yr) carrying singletons were used. Ewes were fed ad libitum alfalfa pellets (Standlee Hay, Kimberly, ID) and had free access to water. Feed and water intake logs and medical records were maintained daily. Ewes were housed in individual carts during the duration of experimental procedures.
Surgical procedures and instrumentation.
Surgery was performed at ∼119 days of gestation. Ewes were fasted for 24 h before surgery. A maternal jugular catheter was placed for administration of diazepam (0.2 mg/kg) and ketamine (17.5 mg/kg), and ewes were then maintained on isoflurane inhalation anesthesia (2–5%) for the remainder of the surgical procedure. At surgery, procaine penicillin G (600,000 U im) and ampicillin (500 mg, intra-amniotically) were administered before uterine and abdominal incisions were closed. Flunixin meglumine analgesic (Banamine, 2.2 mg/kg im) and probiotics (10 g, oral; Probios) were administered for 72 h postoperatively to the ewe.
During surgery, the uterus was exteriorized with a midline incision to surgically place indwelling polyvinyl catheters (20 G) in the maternal and fetal vasculature, as previously described (18, 19). Maternal catheters were placed in the femoral artery and femoral vein via a groin incision. Fetal catheters were placed in the common umbilical vein, fetal artery (advanced into the abdominal aorta), and femoral vein (advanced into the inferior vena cava). Catheters were filled with 5% heparinized saline and subcutaneously tunneled to the ewe’s flank, exteriorized through the skin, and kept in a plastic pouch sutured to the skin.
A tracheotomy was performed to place a non-occlusive catheter (Formulation ND-100-65; 13 G) in the maternal trachea (13, 15, 43). A vertical skin incision was made below the endotracheal tube cuff, and the trachea was cauterized between two cartilaginous rings. The catheter was advanced through the tracheotomy and anchored to the surrounding tissue using suture, and the skin incision was sutured closed. The tracheal catheter was subcutaneously tunneled to the ewe’s shoulder and kept in a plastic pouch sutured to the skin. Ewes were allowed to recover for ≥5 days before experimental procedures began.
Experimental design.
Ewes were randomly assigned at a ratio of 1 to 4 to a control (CON; n = 7) or hypoxemia (HOX; n = 24) treatment group. Beginning on day 125 ± 0.3 of gestation, maternal hypoxemia was induced using tracheal insufflation of humidified nitrogen gas (100% N2) to reduce maternal and subsequently fetal partial pressure of oxygen (Po2) for ∼9 days. Control ewes received humidified, compressed air gas (21% O2, 78% N2, and 1% other trace gasses) by tracheal insufflation for the same duration. Daily whole blood samples were collected from the maternal and fetal artery to monitor fetal Po2, and the rate of N2 gas was subsequently adjusted to target and maintain a fetal Po2 between 12 and 16 mmHg for the study duration. This fetal Po2 range was chosen to mimic fetal hypoxemia in age-matched PI-IUGR fetuses (7, 36). The duration of tracheal gas insufflation was ∼9 days in each group (see Table 2), and thus “day 9” is used to represent data measured on final day of study. On days 0 (before gas insufflation), 1, 4, and 9 of study, whole blood was simultaneously sampled from the maternal artery, umbilical vein, and fetal artery, and plasma was separated by centrifugation.
Table 2.
Maternal and fetal whole blood gasses and oximetry and plasma metabolites from ewes receiving compressed air (CON) or nitrogen (HOX) gas at late gestation
| CON (n = 7), Day of Treatment |
HOX (n = 13), Day of Treatment |
P value* |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 4 | 9 | 0 | 1 | 4 | 9 | TRT | DAY | INT | ||
| Whole blood gasses and oximetry | ||||||||||||
| O2 content, mM | ||||||||||||
| Maternal artery | 5.30 ± 0.26a,b | 5.37 ± 0.27a,b | 5.38 ± 0.23a,b | 4.93 ± 0.21b,c | 5.58 ± 0.20a | 5.05 ± 0.15b | 4.54 ± 0.14c,d | 4.32 ± 0.21d | 0.17 | <0.0001 | 0.0005 | |
| Umbilical vein | 5.03 ± 0.11 | 4.64 ± 0.27 | 5.05 ± 0.25 | 4.44 ± 0.19 | 4.90 ± 0.19 | 4.15 ± 0.18 | 4.01 ± 0.34 | 3.39 ± 0.22 | 0.009 | <0.0001 | 0.07 | |
| Fetal artery | 3.34 ± 0.08 | 2.94 ± 0.18 | 2.52 ± 0.21 | 2.45 ± 0.16 | 2.93 ± 0.20 | 2.22 ± 0.18 | 1.65 ± 0.13 | 1.55 ± 0.17 | 0.001 | <0.0001 | 0.37 | |
| So2, % | ||||||||||||
| Maternal artery | 92.85 ± 1.02a,b | 94.77 ± 1.02a,b | 95.32 ± 1.60a,b | 93.75 ± 1.02a,b | 95.91 ± 0.69a | 89.24 ± 1.87b | 81.58 ± 3.08c | 75.98 ± 2.91d | 0.0004 | <0.0001 | <0.0001 | |
| Umbilical vein | 79.14 ± 3.01a | 76.84 ± 5.52a | 74.73 ± 4.27a | 67.15 ± 4.99a,b | 76.48 ± 3.02a | 64.45 ± 3.15b,c | 59.23 ± 3.92c,d | 53.16 ± 3.06d | 0.006 | <0.0001 | 0.04 | |
| Fetal artery | 51.76 ± 2.32 | 47.03 ± 2.58 | 36.27 ± 3.91 | 43.42 ± 5.85 | 43.35 ± 3.74 | 34.78 ± 3.18 | 24.73 ± 1.92 | 23.46 ± 2.38 | 0.002 | <0.0001 | 0.20 | |
| Hematocrit, % | ||||||||||||
| Maternal artery | 29.83 ± 1.47 | 29.36 ± 1.49 | 29.30 ± 1.65 | 27.19 ± 1.34 | 30.16 ± 1.10 | 29.41 ± 0.84 | 28.98 ± 1.01 | 29.24 ± 1.05 | 0.69 | 0.002 | 0.28 | |
| Umbilical vein | 32.95 ± 1.38 | 31.30 ± 1.50 | 34.52 ± 0.80 | 31.90 ± 1.36 | 32.92 ± 0.87 | 33.03 ± 0.89 | 34.34 ± 1.42 | 32.74 ± 1.70 | 0.83 | 0.10 | 0.96 | |
| Fetal artery | 33.14 ± 1.43 | 31.77 ± 1.27 | 35.10 ± 0.91 | 32.19 ± 1.33 | 33.06 ± 0.90 | 32.68 ± 0.88 | 33.93 ± 1.17 | 32.96 ± 1.22 | 0.85 | 0.06 | 0.70 | |
| pCO2, mmHg | ||||||||||||
| Maternal artery | 36.17 ± 0.91 | 36.59 ± 1.38 | 34.62 ± 0.96 | 35.06 ± 1.15 | 33.71 ± 1.22 | 31.45 ± 0.96 | 30.08 ± 1.04 | 31.52 ± 0.73 | 0.01 | 0.004 | 0.17 | |
| Umbilical vein | 43.58 ± 1.25a | 44.40 ± 1.55a | 44.68 ± 0.74a | 43.33 ± 1.38a,b | 44.07 ± 0.98a | 41.30 ± 0.80b,c | 40.17 ± 1.22c | 40.61 ± 1.36c | 0.03 | 0.05 | 0.003 | |
| Fetal artery | 50.71 ± 1.89a,b | 52.04 ± 1.19a | 54.17 ± 1.23a,b | 51.46 ± 1.74a,b | 51.39 ± 1.32a,b | 48.65 ± 1.15b,c | 47.23 ± 1.32c,d | 45.48 ± 1.31d | 0.02 | 0.07 | 0.003 | |
| Bicarbonate, mM | ||||||||||||
| Maternal artery | 26.30 ± 1.03 | 26.11 ± 1.31 | 25.50 ± 1.08 | 24.70 ± 0.87 | 24.92 ± 0.77 | 23.27 ± 0.52 | 22.27 ± 0.69 | 21.56 ± 0.59 | 0.01 | 0.0001 | 0.08 | |
| Umbilical vein | 25.97 ± 0.44a | 26.20 ± 0.46a | 25.98 ± 0.65a | 25.43 ± 0.54a,b | 26.65 ± 0.44a | 25.65 ± 0.52a | 23.84 ± 0.79b | 22.26 ± 1.22c | 0.05 | <0.0001 | 0.002 | |
| Fetal artery | 27.41 ± 0.60a,b,c | 27.93 ± 0.39a,b | 27.83 ± 0.74a,b | 26.63 ± 0.37a,b,c | 28.05 ± 0.43a | 26.22 ± 0.91b,c | 25.42 ± 0.62c | 23.78 ± 0.89d | 0.03 | 0.003 | 0.052 | |
| pH | ||||||||||||
| Maternal artery | 7.49 ± 0.02 | 7.48 ± 0.01 | 7.50 ± 0.01 | 7.48 ± 0.01 | 7.49 ± 0.01 | 7.49 ± 0.01 | 7.48 ± 0.01 | 7.46 ± 0.01 | 0.65 | 0.14 | 0.32 | |
| Umbilical vein | 7.40 ± 0.01 | 7.42 ± 0.02 | 7.39 ± 0.01 | 7.40 ± 0.01 | 7.41 ± 0.01 | 7.42 ± 0.01 | 7.39 ± 0.02 | 7.38 ± 0.02 | 0.64 | 0.04 | 0.89 | |
| Fetal artery | 7.37 ± 0.01 | 7.37 ± 0.01 | 7.35 ± 0.01 | 7.35 ± 0.01 | 7.38 ± 0.01 | 7.38 ± 0.01 | 7.37 ± 0.01 | 7.36 ± 0.02 | 0.20 | 0.09 | 0.76 | |
| Plasma metabolites | ||||||||||||
| Glucose, mM | ||||||||||||
| Maternal artery | 3.34 ± 0.15 | 3.67 ± 0.20 | 3.56 ± 0.13 | 3.78 ± 0.11 | 3.38 ± 0.12 | 3.53 ± 0.07 | 3.54 ± 0.08 | 3.75 ± 0.13 | 0.76 | 0.006 | 0.86 | |
| Umbilical vein | 1.18 ± 0.08 | 1.23 ± 0.16 | 1.35 ± 0.07 | 1.18 ± 0.09 | 1.24 ± 0.06 | 1.3 ± 0.05 | 1.37 ± 0.06 | 1.39 ± 0.11 | 0.21 | 0.31 | 0.70 | |
| Fetal artery | 0.98 ± 0.06 | 1.04 ± 0.09 | 1.09 ± 0.06 | 0.98 ± 0.09 | 1.05 ± 0.05 | 1.07 ± 0.04 | 1.12 ± 0.05 | 1.17 ± 0.08 | 0.19 | 0.56 | 0.55 | |
| Lactate, mM | ||||||||||||
| Maternal artery | 0.64 ± 0.03 | 0.77 ± 0.10 | 0.77 ± 0.05 | 0.83 ± 0.10 | 0.68 ± 0.05 | 0.69 ± 0.06 | 0.85 ± 0.08 | 0.89 ± 0.08 | 0.69 | 0.03 | 0.54 | |
| Umbilical vein | 2.05 ± 0.15 | 1.82 ± 0.12 | 2.93 ± 0.44 | 2.29 ± 0.15 | 2.47 ± 0.34 | 4.06 ± 0.61 | 5.94 ± 1.03 | 7.83 ± 2.33 | 0.002 | 0.02 | 0.06 | |
| Fetal artery | 1.92 ± 0.17 | 1.93 ± 0.20 | 2.74 ± 0.43 | 2.11 ± 0.14 | 2.31 ± 0.34 | 3.53 ± 0.50 | 5.34 ± 0.84 | 7.04 ± 1.87 | 0.008 | 0.02 | 0.08 | |
Data are means ± SE. CON, control; DAY, day of treatment; HOX, hypoxemia; INT, interaction between DAY and treatment group; TRT, treatment group.
Data were analyzed by 2-way ANOVA for the fixed effects of TRT, DAY, INT.
a,
b,
c,
Within a row, means with different superscripted letters are different (P ≤ 0.05).
Fetal metabolic study and tissue collection.
Fetuses were studied on day 9 to measure umbilical blood flow and glucose metabolism (17, 26). A 3-ml bolus of 3H2O and [U-13C]glucose was infused, followed by a continuous infusion at 3 ml/h (15 μCi/ml 3H2O, 30 mg/ml [U-13C] glucose), as previously described (6, 26, 27). After isotopic steady state was reached (∼90 min), blood samples were simultaneously sampled from the umbilical vein and fetal artery four times at 20-min intervals to characterize the steady-state period. Fetal blood was replaced isovolumetrically with heparinized maternal arterial blood (15 ml/h) throughout the steady-state period.
Immediately following the metabolic study, ewes were anesthetized with diazepam (0.2 mg/kg) and ketamine (17.5 mg/kg) intravenously (iv) to deliver the fetus via maternal laparotomy and hysterotomy. A liver biopsy was obtained from the anesthetized fetus and immediately frozen in liquid nitrogen. Subsequently, a lethal dose of pentobarbital sodium (390 mg/ml, Fatal Plus; Vortech Pharmaceuticals, Dearborn, MI) was administered iv to euthanize the ewe and fetus. Fetal weight was recorded, and organs were dissected, weighed, and snap-frozen in liquid nitrogen.
A total of seven CON and 24 HOX ewes were assigned to experimental treatments. Eleven HOX ewes experienced a fetal demise during the experimental hypoxemia (HOX + DEMISE). Fetal demise was declared by lack of fetal blood collection due to blood clotting, lack of fetal blood gas chemistry detection, or abortion. Blood gas and metabolite variables were similar in the HOX and HOX + DEMISE ewes and fetuses (see Supplemental Tables S1 and S2, respectively; all Supplemental material is available at https://doi.org/10.6084/m9.figshare.7905350.v1). The HOX + DEMISE fetuses were removed from the final analysis. Additionally, tracheal catheters were dislodged from the tracheal lumen of two HOX ewes. Data for these ewes and their fetuses are included from the time until the catheter failed and re-oxygenation occurred (days 4 and 9 of study). Herein, in vivo data are reported for seven CON and 13 HOX, unless otherwise noted. Due to umbilical venous catheter failures, final metabolic studies on day 9 were completed on seven CON and eight HOX animals.
Biochemical analyses.
Whole blood Po2, O2 content, hemoglobin O2 saturation (So2), partial pressure of carbon dioxide (Pco2), hematocrit, pH, and bicarbonate were measured with the ABL 800 Flex blood gas analyzer (Radiometer, Copenhagen, Denmark). Plasma glucose and lactate concentrations were measured using the Yellow Springs Instrument model 2900 Select Biochemistry Analyzer (Yellow Springs Instruments, Yellow Springs, OH). Fetal arterial plasma insulin and cortisol concentrations were measured using ovine-specific ELISA assays (Alpco Diagnostics, Windham, NH) and norepinephrine by HPLC (model no. 2475; Waters, Milford, MA). Fetal arterial plasma glucagon concentrations were measured by radioimmunoassay (Millipore, Billerica, MA). All assay specifications were reported previously (4).
Glucose tracer enrichments.
Glucose tracer enrichments [molar percent excess (MPE)] were measured in the fetal artery and umbilical vein plasma samples, as previously described (6, 20). Briefly, glucose was converted to the aldonitrile peracetate derivative for GC/MS analysis. Glucose [U-13C] enrichment was monitored at m/z of 334/328 ratio. Glucose MPE was calculated as the difference in peak area ratios between unenriched (baseline) and enriched samples.
Calculations.
Nutrient-oxygen metabolic quotients were calculated for the substrates glucose and lactate (3, 16, 29, 32). Umbilical substrate-oxygen quotients were calculated by dividing the whole blood umbilical vein-fetal artery difference in substrate concentration by the umbilical vein-fetal artery difference in whole blood O2 content multiplied by the number of oxygen molecules required to oxidize one molecule of substrate. Umbilical blood flow was determined by steady-state diffusion (26). Umbilical (net fetal) uptake rates of oxygen and glucose were calculated using the Fick principle (19, 26, 27). Fetal oxygen extraction was calculated as the umbilical vein-fetal artery difference in O2 content divided by the O2 content input (umbilical vein) to the fetus and expressed as a percentage (9). Fetal oxygen delivery rate was calculated by multiplying umbilical venous O2 content by umbilical blood flow. Fetal glucose utilization rate was calculated as previously described (18, 19, 25, 36). Fetal glucose production rate was calculated as the difference between fetal glucose utilization rate and net fetal glucose uptake rate (19).
Hepatic glycogen content.
Hepatic glycogen contents were measured as described previously (25). Data are expressed as milligrams of glycogen per gram wet tissue weight.
Real-time quantitative PCR.
RNA was isolated from the fetal liver biopsy (left lobe) and reverse-transcribed to cDNA, and quantitative PCR (qPCR) was performed as described previously (37). Primers were used as previously reported for ribosomal protein S15 (S15), G6PC, fructose-1,6- bisphosphatase 1 (FBP1), PCK1, PCK2, and pyruvate carboxylase (PC) (7, 25, 37, 39). The reference gene S15 was not different between groups and used to normalize qPCR results. Data are expressed as a fold change relative to the mean in the CON group.
Western blotting.
Whole cell lysates were prepared from the fetal liver biopsy (left lobe), and Western blotting was performed as previously described (36, 37). Antibodies for G6Pase, PEPCK-C, and β-actin were previously validated (36, 39). Specificity of antibodies was verified by the presence of a single band at the expected molecular weight. Protein bands were visualized using IR-Dye IgG secondary antibody (LI-COR, Lincoln, NE), and protein expression was quantified with Image Studio (LI-COR). Samples were run on two blots (9 samples each plus a reference sample). One representative blot is shown. Equality of protein loading was verified with β-actin expression. Target band densities were normalized to the reference sample on each blot and expressed relative to β-actin. Data are presented as a fold change relative to the mean of the CON group.
Statistical analysis.
Data collected at multiple time points were analyzed using the mixed procedure of SAS version 9.4 (SAS Institute, Cary, NC). Fetal sex was included in the model as a fixed effect for fetal variables, and no differences were found, so fetal sex was not included in the final analyses. The statistical model was a 2 × 4 factorial arrangement of treatments with fixed effects of treatment group (TRT: CON, HOX) and day of treatment (days 0, 1, 4, and 9) and the interaction (INT). A random effect of animal was included to account for multiple time points measured within an individual sheep. For dependent variables analyzed with more than four time points (insufflation rate, maternal arterial Po2, and fetal arterial Po2), a repeated statement was included, and covariate structures were selected based on the lowest Akaike Iteration Criterion value (toeplitz or autoregressive). Pairwise comparisons were made using the PDIFF option. Data collected at one time point were analyzed by Student’s t-test or Mann-Whitney U-test, and linear regression analyses were performed using GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA). The fetal glucose production rate was tested against a theoretical mean of zero by a one-sample t-test and Wilcoxon signed-rank test. Data are presented as means ± SE. Statistical differences are declared at P ≤ 0.05, and tendencies are discussed at 0.05 > P < 0.1 when biologically meaningful.
RESULTS
Development of fetal hypoxemia with maternal tracheal N2 insufflation.
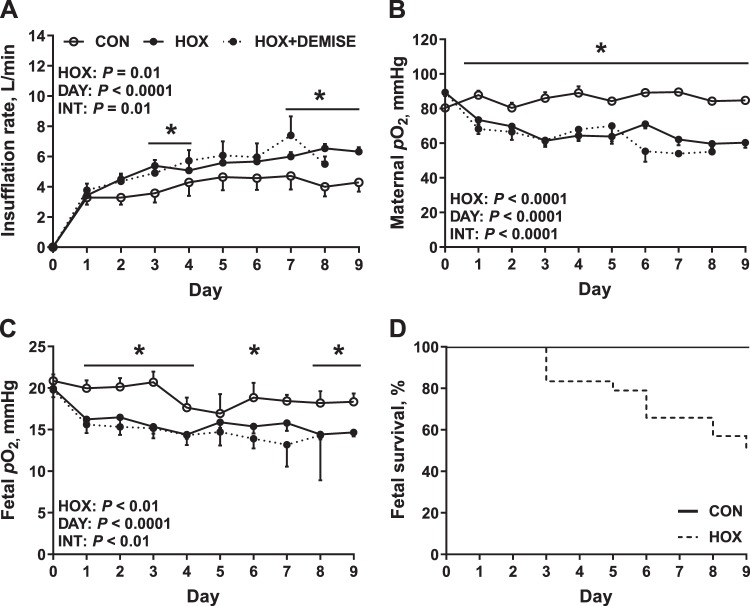
Nitrogen gas insufflation reduced maternal arterial Po2 by 16% in HOX compared with CON ewes on day 1 (Fig. 1, A and B). Maternal arterial Po2 remained lower in HOX ewes compared with CON for the duration of study, resulting in an average 26% reduction in maternal Po2 from days 2 to 9 and producing a 30% reduction in maternal Po2 on day 9 when the final metabolic study was conducted (Fig. 1, A and B). Fetal arterial Po2 was decreased by 15% on day 1 (Fig. 1C). Fetal arterial Po2 remained lower in HOX (average of 18% from days 2 to 9) compared with CON fetuses for the duration of study, resulting in a 20% reduction in fetal Po2 on day 9 when the final metabolic study was conducted (Fig. 1, A and B). The duration of treatment, gestational age of fetuses, and fetal body weight were similar between CON and HOX groups (Table 1). Fetal liver weights were similar in HOX compared with CON fetuses and remained similar when expressed as a percent of fetal weight (Table 1).
Fig. 1.
Experimental model of sustained fetal hypoxemia. A: pregnant ewes received a tracheal insufflation of compressed air [control (CON)] or nitrogen [hypoxemia (HOX)] gas during late gestation. B and C: maternal arterial Po2 (B) and fetal arterial Po2 (C) responses were measured daily. D: survival rate in CON and HOX fetuses during the treatment period; n = 7 CON ewes (2 male and 5 female fetuses), 13 HOX ewes (8 male and 5 female fetuses), and 11 HOX + DEMISE ewes (ewes that experienced a fetal demise during the experimental hypoxemia; fetal sex not recorded). Data are presented as means ± SE. Data were analyzed by 2-way ANOVA using repeated measures with fixed effects of treatment (HOX), day (DAY), and the interaction (INT). *Within a day of study, CON and HOX are different (P < 0.05). HOX + DEMISE are not included in the statistical comparison because of incomplete data collection.
Table 1.
Descriptive details and fetal characteristics at final metabolic study and necropsy
| Variable | CON | HOX | P Value |
|---|---|---|---|
| Study details | |||
| Days of treatment | 9 ± 0.5 | 9 ± 0.2 | 0.64 |
| Maternal feed intake, kg/day | 1.9 ± 0.2 | 1.8 ± 0.2 | 0.62 |
| Maternal water intake, l/day | 9.9 ± 1.3 | 7.0 ± 0.8 | 0.07 |
| Fetal characteristics | |||
| Gestational age, days | 134 ± 0.3 | 133 ± 0.4 | 0.71 |
| Fetal sex (males/females) | 2/5 | 6/5 | |
| Body weight, g | 3,049.1 ± 142.8 | 2,953.3 ± 181.5 | 0.71 |
| Liver weight, g | 79.4 ± 5.6 | 80.9 ± 7.1 | 0.88 |
| Liver: body weight, % | 2.6 ± 0.1 | 2.7 ± 0.1 | 0.58 |
Data are means ± SE.
All fetuses from CON ewes survived the study, whereas 46% of fetuses (n = 11 of 24) from HOX ewes died between days 3 and 9 (HOX + DEMISE) (Fig. 1D). The nitrogen insufflation rate (Fig. 1A) and the magnitude of reductions in maternal and fetal Po2 were similar between the HOX and HOX + DEMISE groups (Fig. 1, B and C). Other maternal and fetal blood gas parameters, oximetry, and metabolite data measured were similar between HOX and HOX + DEMISE (see Supplemental Figures S1 and S2, respectively).
Maternal response to sustained hypoxemia.
Maternal arterial blood chemistry and metabolites were measured at baseline (day 0) and on days 1, 4, and 9 of treatment. Maternal arterial O2 content and So2 were lower on days 4 and 9 in HOX compared with CON ewes (Table 2). Maternal arterial Pco2 and bicarbonate were lower in HOX compared with CON ewes throughout the study (Table 2). There were no differences in maternal arterial pH and hematocrit between treatment groups; however, the maternal hematocrit decreased as gestation advanced (Table 2).
Fetal response to sustained hypoxemia.
Umbilical vein and fetal artery samples, which represent the input and output across the umbilical circulation to the fetus, were measured for blood chemistry and metabolites at baseline (day 0) and on days 1, 4, and 9 (Table 2). Umbilical venous O2 content and So2 were lower, by 14 and 15%, respectively, in HOX compared with CON fetuses throughout the study (Table 2). Fetal arterial O2 content and So2 were 25 and 30% lower, respectively, in HOX compared with CON fetuses throughout the study (Table 2). Umbilical vein and fetal arterial Pco2 and bicarbonate concentration decreased throughout the study period in HOX compared with CON fetuses (Table 2). There were no differences in the umbilical vein or fetal arterial hematocrit or pH between treatment groups (Table 2).
Maternal and fetal glucose and lactate metabolism in response to sustained hypoxemia.
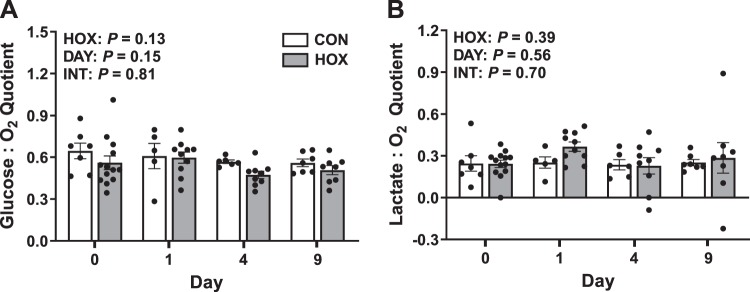
Maternal and fetal glucose and lactate concentrations (Table 2) were measured and used to calculate umbilical (net fetal) glucose and lactate metabolic quotients at baseline (day 0) and on days 1, 4, and 9 of study (Fig. 2). Maternal arterial plasma glucose and lactate concentrations were not different in HOX compared with CON; however, the concentration of both glucose and lactate increased as gestation advanced (Table 2). There were no differences in the umbilical vein or fetal arterial plasma glucose concentrations in HOX compared with CON fetuses (Table 2). Umbilical vein and fetal arterial plasma lactate concentrations were more than threefold higher in HOX compared with CON fetuses (Table 2). The umbilical (net fetal) glucose-oxygen and lactate-oxygen metabolic quotients were not different in HOX compared with CON fetuses (Fig. 2). There were no differences in maternal feed intake and water intake between treatment groups, consistent with maintenance of fetal glucose supply (Table 1).
Fig. 2.
Effect of sustained hypoxemia (HOX) on fetal nutrient-oxygen quotients. Nutrient-oxygen quotients were calculated for glucose (A) and lactate (B) on days 0, 1, 4, and 9 of the study. Data were analyzed by 2-way ANOVA with fixed effects of treatment (HOX), day (DAY), and the interaction (INT). Individual data points with group means ± SE are shown (n = 2 male and 5 female CON fetuses and 8 male and 5 female HOX fetuses).
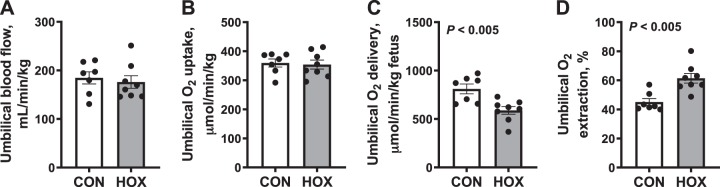
Umbilical (net fetal) metabolic studies.
Umbilical blood flow, oxygen uptake, and glucose flux rates were measured on day 9 of the study and expressed relative to fetal weight. Umbilical blood flow rate was similar in HOX compared with CON fetuses (Fig. 3A). Umbilical net oxygen uptake rate was similar in HOX compared with CON fetuses (Fig. 3B). The rate of umbilical oxygen delivery was 30% lower in HOX compared with CON fetuses (P < 0.01; Fig. 3C). However, oxygen extraction was 35% greater in HOX compared with CON fetuses (P < 0.01; Fig. 3D).
Fig. 3.
Effects of sustained hypoxemia (HOX) on umbilical blood flow and oxygen uptake rates. Umbilical blood flow (A) was measured and used to calculate umbilical (net fetal) oxygen uptake (B), delivery (C), and extraction (D). All rates are expressed relative to fetal weight. Data were analyzed by Student’s t-test. Individual data points with group means ± SE are shown [n = 2 male and 5 female control (CON) fetuses and 3 male and 5 female HOX fetuses].
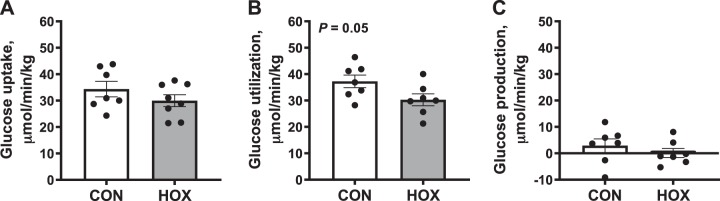
Umbilical (net fetal) glucose uptake rates were similar in HOX compared with CON fetuses (Fig. 4A). Fetal glucose utilization rates were lower (P = 0.05) in HOX fetuses compared with CON (Fig. 4B). The fetal glucose production rate was similar and not different from zero in HOX and CON fetuses (Fig. 4C).
Fig. 4.
Effects of sustained hypoxemia (HOX) on fetal glucose metabolism. Umbilical (net fetal) glucose uptake (A) and glucose utilization rates (B) were measured and used to calculate fetal glucose production rate (C). All rates are expressed relative to fetal weight. Data were analyzed by Student’s t-test. Individual data points with group means ± SE are shown [n = 2 male and 5 female control (CON) fetuses and 3 male and 5 female HOX fetuses].
Fetal hepatic gluconeogenic gene and protein responses to sustained hypoxemia.
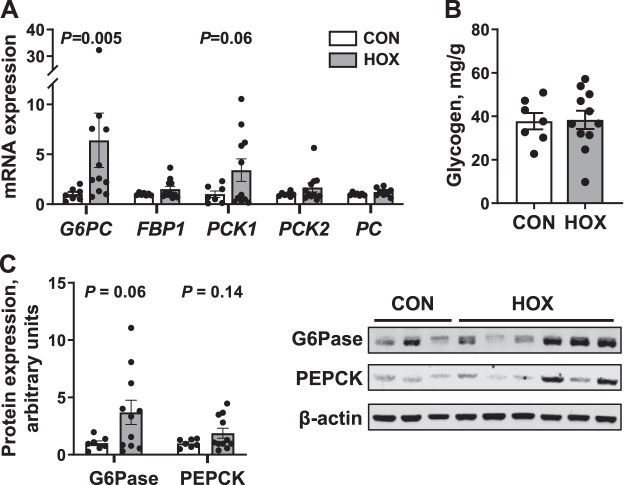
Hepatic mRNA expression of G6PC was sixfold greater (P < 0.01), and PCK1 tended to be threefold greater (P = 0.06) in HOX compared with CON fetuses (Fig. 5A). Hepatic mRNA expression of FBP1, PCK2, and PC were not different between groups (Fig. 5A). There was no difference in hepatic glycogen contents in HOX compared with CON fetuses (Fig. 5B). Hepatic protein expression of G6Pase trended (P = 0.06) to be 3.6-fold greater in HOX compared with CON fetuses; however, PEPCK protein expression was not different between groups (Fig. 5C).
Fig. 5.
Effects of sustained hypoxemia (HOX) on fetal liver. A: expression of genes for gluconeogenesis in the fetal liver was measured. B: fetal hepatic glycogen content was measured. C: protein expression of glucose-6-phosphatse catalytic subunit (G6Pase), phosphoenolpyruvate carboxykinase (PEPCK), and β-actin was quantified in whole cell liver lysates. A representative blot of each protein is shown. Data were analyzed by Student’s t-test or Mann-Whitney U-test. Individual data points with group means ± SE are shown [n = 2 male and 5 female control (CON) fetuses and 6 male and 5 female HOX fetuses]. FBP1, fructose-1,6- bisphosphatase 1; PC, pyruvate carboxylase; PCK1, phosphoenolpyruvate carboxykinase 1; PCK2, phosphoenolpyruvate carboxykinase 2.
Fetal endocrine responses to sustained hypoxemia.
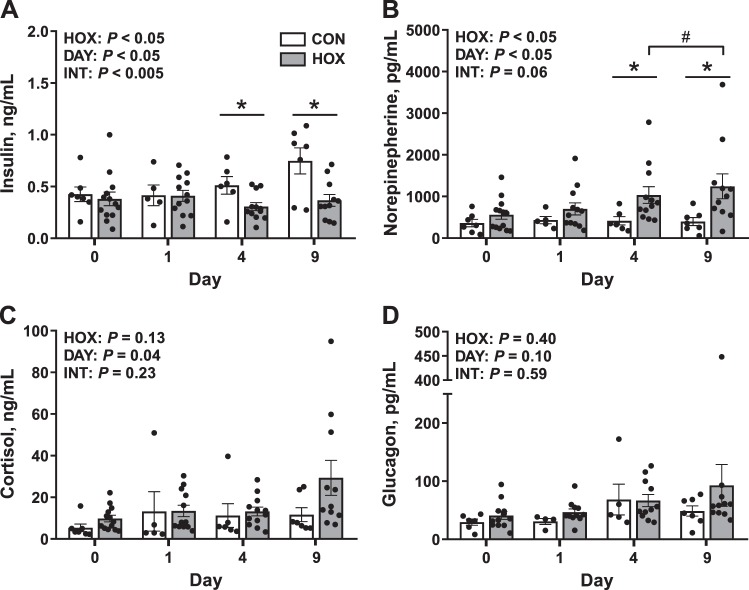
Fetal arterial plasma insulin concentrations increased as gestation advanced in CON but not HOX fetuses (Fig. 6A). On day 9, insulin concentrations were 49% lower in HOX versus CON fetuses (P < 0.05; Fig. 6A). Fetal arterial plasma norepinephrine concentrations were greater by 2.5-fold in HOX versus CON fetuses on day 4 and were threefold greater on day 9 in HOX compared with CON fetuses (P < 0.05; Fig. 6B). Within HOX fetuses, arterial plasma norepinephrine concentrations increased between days 4 and 9 (P < 0.05; Fig. 6B). Fetal arterial plasma cortisol concentrations were not different between CON and HOX, yet they increased as gestation advanced (Fig. 6C). Fetal arterial plasma glucagon concentrations were not different between CON and HOX or as gestation advanced (Fig. 6D).
Fig. 6.
Effect of sustained hypoxemia (HOX) on fetal endocrine milieu. Fetal arterial plasma insulin (A), norepinephrine (B), cortisol (C), and glucagon (D) concentrations were measured. Data were analyzed by 2-way ANOVA with fixed effects of treatment (HOX), day (DAY), and the interaction (INT). For post hoc analysis: *differences between control (CON) and HOX within a day of study; #HOX means are different between days 4 and 9. Individual data points with group means ± SE are shown (n = 2 male and 5 female CON fetuses and 8 male, 5 female HOX fetuses).
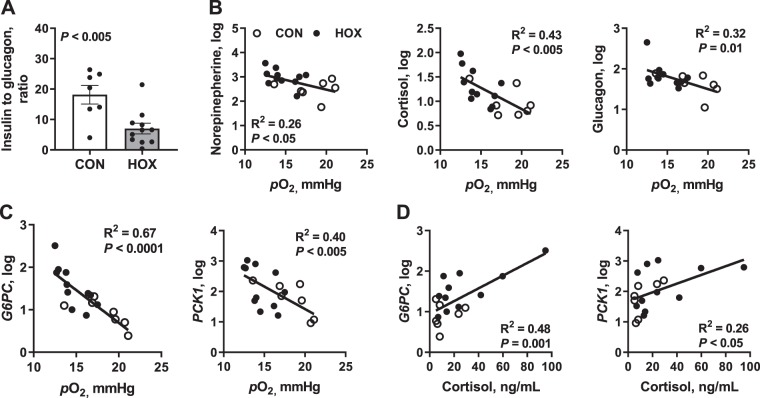
The relationship between fetal arterial Po2 with hormone concentrations or gluconeogenic gene expression was analyzed on day 9 of study among all fetuses. The fetal insulin to glucagon ratio was 61% lower in HOX versus CON fetuses (Fig. 7A). Fetal arterial Po2 was inversely correlated with plasma concentrations of all three counter-regulatory hormones (norepinephrine: r2 = 0.26, cortisol: r2 = 0.43, and glucagon: r2 = 0.32) that activate hepatic glucose production (Fig. 7B). Fetal hepatic G6PC and PCK1 gene expressions were inversely correlated to fetal arterial Po2, with the greatest expression observed at the lowest fetal oxygenation status (r2 = 0.67 and 0.40, respectively; Fig. 7C). Increased hepatic PCK1 and G6PC expression was positively correlated with increased plasma cortisol concentrations (r2 = 0.48 and 0.26, respectively; Fig. 7D).
Fig. 7.
Relationship between fetal arterial Po2 and gluconeogenic factors after 9 days of study. A: plasma insulin to glucagon ratio in control (CON) and hypoxemia (HOX) fetuses. B: correlation analyses were performed to test the relationship of fetal arterial Po2 and counter-regulatory hormone concentrations. C and D: correlation analyses were also performed for the relationship of fetal arterial Po2 (C) and plasma cortisol (D) concentrations with glucose-6-phosphatase (G6PC) mRNA and phosphoenolpyruvate carboxykinase 1 (PCK1) mRNA expression. Data were log transformed, and individual data points are shown (n = 2 male and 5 female CON fetuses and 6 male and 5 female HOX fetuses).
DISCUSSION
The goal of our study was to determine whether fetal hypoxemia induces the early activation of fetal hepatic glucose production. To accomplish this, we developed a model of sustained (9 days) hypoxemia in late gestation fetal sheep and measured fetal glucose metabolism rates (uptake, utilization, and production) and factors that activate fetal glucose production, including umbilical glucose supply, counter-regulatory hormone concentrations, and gluconeogenic gene expression. Our results demonstrate that sustained fetal hypoxemia does not result in a detectable rate of endogenous glucose production in the fetus, yet it potentiates fetal hepatic expression of the gluconeogenic genes G6PC and PCK1 and increases G6Pase protein. Importantly, activation at the molecular level occurred in the absence of changes in fetal glucose concentrations or umbilical (net fetal) glucose uptake and in the presence of a modest reduction in fetal glucose utilization rates. These results support a unique role for hypoxemia and the associated endocrine response to prime the molecular pathways for gluconeogenic gene expression, but hypoxemia itself is not sufficient to activate gluconeogenesis and net endogenous glucose production.
The expression of both G6PC and PCK1 was greatest in fetuses with the most severe hypoxemia (lowest arterial Po2) and greatest cortisol concentrations. In support of these observations, hypoxia exposure (3% O2) increases PCK1 expression in isolated primary fetal sheep hepatocytes (9). These results are also consistent with previous literature demonstrating increased fetal hepatic G6PC and PCK1 expression during 9 days of anemic hypoxemia (9). However, anemic hypoxemia decreased fetal hepatic glycogen content (9), whereas hepatic glycogen content was preserved in our model. In the absence of active hepatic glucose production in either model, the relative contribution of gluconeogenesis versus glycogenolysis remains unknown.
The modest expression of gluconeogenic genes and lack of glucose production observed in hypoxemic fetuses may be explained by glycemia-dependent effects on these pathways. For example, in other sheep models of PI-IUGR and hypoglycemia with active endogenous glucose production rates, the fetuses have reduced glucose uptake and arterial glucose concentrations (20, 25, 31, 36, 39). The PI-IUGR and hypoglycemic fetuses also have increased expression of additional genes in the gluconeogenic pathway (FBP1, PCK2, and PC) that were not increased in livers from hypoxemic fetuses (31, 36, 39). Thus, a key difference in hypoxemic fetuses that do not have endogenous glucose production is euglycemia and normal rates of glucose uptake. It is likely that concurrent decreases in fetal glucose concentrations and glucose uptake are necessary requirements to fully activate fetal glucose production and that hypoxemia alone is not sufficient. This supports synergistic or additive roles of fetal hypoxemia and hypoglycemia on the early activation of hepatic glucose production observed during PI-IUGR.
Sustained hypoxemia produced an endocrine milieu that likely primes the fetal liver for activation of hepatic gluconeogenic gene activation. We observed an increase in fetal norepinephrine concentrations after 4 days of hypoxemia, which was maintained throughout the study. We also observed a concurrent decrease in fetal insulin concentrations and the insulin-to-glucagon ratio. Cortisol had the strongest correlation with fetal oxygen levels, with the most hypoxemic fetuses exhibiting the highest cortisol concentrations (see Fig. 7D). Thus, the modest gluconeogenic gene activation and lack of fetal glucose production may be explained by the variable increase in cortisol concentrations (P = 0.13; see Fig. 6C) that did not reach a threshold necessary to activate glucose production (10, 11). Although we cannot separate out the specific role of counter-regulatory hormones from hypoxemia on gluconeogenic gene expression, we have previously reported that chronic anemic hypoxemia increases hepatic G6PC and PCK1 mRNA, but without an increase in fetal norepinephrine or decrease in fetal insulin concentrations (9). Instead, anemic hypoxemia raised glucagon concentrations (9). Furthermore, at euglycemia, hypoxemia decreases plasma insulin concentrations in adrenal-demedullated fetuses (43). This demonstrates a physiological effect of hypoxemia independent of norepinephrine to lower fetal insulin concentrations. Additional studies support the need for reduced fetal glucose uptake in combination with increased gluconeogenic counter-regulatory hormone concentrations to activate net hepatic glucose production. First, the induction of fetal glucose production in response to maternal fasting and subsequent fetal hypoglycemia is prevented in adrenalectomized fetuses, supporting the essential role of the adrenal hormones, cortisol, and norepinephrine (10). Second, fetal dexamethasone infusions did not activate hepatic glucose output nor decrease fetal glucose uptake rates (40). Third, fetal hepatic glucose output was observed during glucagon-somatostatin infusions that concurrently decreased umbilical glucose uptake (35). Together, these data support the need for reduced fetal glucose supply and increased counter-regulatory hormone concentrations to completely activate fetal hepatic glucose production. We speculate that in our model of sustained hypoxemia, the resulting combination of increased norepinephrine concentrations, decreased insulin concentrations, variably increased cortisol concentrations, and maintained fetal glycemia allow for the expression of fetal hepatic gluconeogenic gene expression in the absence of glucose production.
Fetal oxygen uptake rates were maintained during sustained hypoxemia. The maintenance of oxygen uptake in the hypoxemic fetus, despite lower umbilical oxygen delivery, is explained by an increase in oxygen extraction. This indicates preservation of metabolic rate and growth in the HOX fetus. Interestingly, sustained hypoxemia marginally reduces fetal glucose utilization after 9 days. Given the maintenance of oxygen utilization rates, one explanation for the match between lower glucose uptake and lower glucose utilization without an activation of endogenous glucose production by the HOX fetus is that alternative substrates such as lactate or amino acids are oxidized instead of glucose. It is also possible that the reduced insulin concentrations may contribute to reductions in fetal glucose utilization. Additional studies are needed to determine the effect of sustained hypoxemia on glucose oxidation, lactate production, and amino acid metabolism in the whole fetus and within different tissues/organs. Studies also are needed to determine the placental versus umbilical contribution to fetal fuel availability during sustained hypoxemia.
Perspectives and Significance
Our data demonstrate that sustained fetal hypoxemia potentiates hepatic G6PC and PCK1 gene expression, decreases insulin concentrations, and increases counter-regulatory hormone concentrations but does not result in endogenous fetal glucose production. Notably, fetal glucose uptake and plasma glucose were maintained, and cortisol was only variably increased with hypoxemia. Therefore, these observations demonstrate the capacity for hypoxemia to produce an endocrine milieu that only primes the molecular pathways (genes) for activation of fetal glucose production and that hypoxemia alone is not sufficient to activate fetal glucose production. Our findings prompt future studies to determine the combined effects of hypoxemia and hypoglycemia on the regulation fetal hepatic glucose production. Fetal hypoxemia is a pathological condition found in many pregnancy disorders, including PI-IUGR. Thus, our findings are important for understanding the mechanisms that contribute to activation of fetal glucose production in such conditions with relevance for improving neonatal glucose homeostasis in affected infants and for reducing later life disease risk in offspring exposed to intrauterine hypoxemia (8, 14, 30).
GRANTS
This work was supported by the following National Institute of Health Grants: R01-DK-108910 (S. R. Weslowski), F32-DK-120070 (A. K. Jones); T32-HD-007186, K12-HD-068372 (W. W. Hay, Jr.); R01-DK-088139, R01-HD-093701 (P. J. Rozance); R01-HD-079404 (L. D. Brown); and R01-DK084842 (S. W. Limesand).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.K.J. and S.R.W. performed experiments; A.K.J., D.A.G., and S.R.W. analyzed data; A.K.J., P.J.R., L.D.B., W.W.H.J., S.W.L., and S.R.W. interpreted results of experiments; A.K.J. and S.R.W. prepared figures; A.K.J. and S.R.W. drafted manuscript; A.K.J., P.J.R., L.D.B., D.A.G., W.W.H.J., S.W.L., and S.R.W. edited and revised manuscript; A.K.J., P.J.R., L.D.B., D.A.G., W.W.H.J., S.W.L., and S.R.W. approved final version of manuscript; P.J.R., L.D.B., W.W.H.J., S.W.L., and S.R.W. conceived and designed research.
ACKNOWLEDGMENTS
We thank Vince Abushaban, David Caprio, Leanna Nguyen, Gates Roe, Karen Trembler, Larry Toft, and Dong Wang from the University of Colorado for their assistance with these studies.
REFERENCES
- 1.Ananth CV. Ischemic placental disease: a unifying concept for preeclampsia, intrauterine growth restriction, and placental abruption. Semin Perinatol 38: 131–132, 2014. doi: 10.1053/j.semperi.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Ananth CV, Friedman AM. Ischemic placental disease and risks of perinatal mortality and morbidity and neurodevelopmental outcomes. Semin Perinatol 38: 151–158, 2014. doi: 10.1053/j.semperi.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Battaglia FC, Meschia G. Fetal and placental metabolism: part1 oxygen and carbohydrates. In: An Introduction to Fetal Physiology. Orlando, FL: Academic Press, 1986, p. 49–98. [Google Scholar]
- 4.Benjamin JS, Culpepper CB, Brown LD, Wesolowski SR, Jonker SS, Davis MA, Limesand SW, Wilkening RB, Hay WW Jr, Rozance PJ. Chronic anemic hypoxemia attenuates glucose-stimulated insulin secretion in fetal sheep. Am J Physiol Regul Integr Comp Physiol 312: R492–R500, 2017. doi: 10.1152/ajpregu.00484.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bristow J, Rudolph AM, Itskovitz J, Barnes R. Hepatic oxygen and glucose metabolism in the fetal lamb. Response to hypoxia. J Clin Invest 71: 1047–1061, 1983. doi: 10.1172/JCI110855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown LD, Kohn JR, Rozance PJ, Hay WW Jr, Wesolowski SR. Exogenous amino acids suppress glucose oxidation and potentiate hepatic glucose production in late gestation fetal sheep. Am J Physiol Regul Integr Comp Physiol 312: R654–R663, 2017. doi: 10.1152/ajpregu.00502.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown LD, Rozance PJ, Bruce JL, Friedman JE, Hay WW Jr, Wesolowski SR. Limited capacity for glucose oxidation in fetal sheep with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol 309: R920–R928, 2015. doi: 10.1152/ajpregu.00197.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowett RM, Oh W, Schwartz R. Persistent glucose production during glucose infusion in the neonate. J Clin Invest 71: 467–475, 1983. doi: 10.1172/JCI110791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Culpepper C, Wesolowski SR, Benjamin J, Bruce JL, Brown LD, Jonker SS, Wilkening RB, Hay WW Jr, Rozance PJ. Chronic anemic hypoxemia increases plasma glucagon and hepatic PCK1 mRNA in late-gestation fetal sheep. Am J Physiol Regul Integr Comp Physiol 311: R200–R208, 2016. doi: 10.1152/ajpregu.00037.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fowden AL, Forhead AJ. Adrenal glands are essential for activation of glucogenesis during undernutrition in fetal sheep near term. Am J Physiol Endocrinol Metab 300: E94–E102, 2011. doi: 10.1152/ajpendo.00205.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fowden AL, Mundy L, Silver M. Developmental regulation of glucogenesis in the sheep fetus during late gestation. J Physiol 508: 937–947, 1998. doi: 10.1111/j.1469-7793.1998.937bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girard J. Gluconeogenesis in late fetal and early neonatal life. Biol Neonate 50: 237–258, 1986. doi: 10.1159/000242605. [DOI] [PubMed] [Google Scholar]
- 13.Gleed RD, Poore ER, Figueroa JP, Nathanielsz PW. Modification of maternal and fetal oxygenation with the use of tracheal gas infusion. Am J Obstet Gynecol 155: 429–435, 1986. doi: 10.1016/0002-9378(86)90846-X. [DOI] [PubMed] [Google Scholar]
- 14.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. 1992. Int J Epidemiol 42: 1215–1222, 2013. doi: 10.1093/ije/dyt133. [DOI] [PubMed] [Google Scholar]
- 15.Harvey LM, Gilbert RD, Longo LD, Ducsay CA. Changes in ovine fetal adrenocortical responsiveness after long-term hypoxemia. Am J Physiol Endocrinol Metab 264: E741–E747, 1993. doi: 10.1152/ajpendo.1993.264.5.E741. [DOI] [PubMed] [Google Scholar]
- 16.Hay WW Jr, Myers SA, Sparks JW, Wilkening RB, Meschia G, Battaglia FC. Glucose and lactate oxidation rates in the fetal lamb. Proc Soc Exp Biol Med 173: 553–563, 1983. doi: 10.3181/00379727-173-41686. [DOI] [PubMed] [Google Scholar]
- 17.Hay WW Jr, Meznarich HK. The effect of hyperinsulinaemia on glucose utilization and oxidation and on oxygen consumption in the fetal lamb. Q J Exp Physiol 71: 689–698, 1986. doi: 10.1113/expphysiol.1986.sp003027. [DOI] [PubMed] [Google Scholar]
- 18.Hay WW Jr, Sparks JW, Battaglia FC, Meschia G. Maternal-fetal glucose exchange: necessity of a three-pool model. Am J Physiol 246: E528–E534, 1984. doi: 10.1152/ajpendo.1984.246.6.E528. [DOI] [PubMed] [Google Scholar]
- 19.Hay WW Jr, Sparks JW, Quissell BJ, Battaglia FC, Meschia G. Simultaneous measurements of umbilical uptake, fetal utilization rate, and fetal turnover rate of glucose. Am J Physiol 240: E662–E668, 1981. doi: 10.1152/ajpendo.1981.240.6.E662. [DOI] [PubMed] [Google Scholar]
- 20.Houin SS, Rozance PJ, Brown LD, Hay WW Jr, Wilkening RB, Thorn SR. Coordinated changes in hepatic amino acid metabolism and endocrine signals support hepatic glucose production during fetal hypoglycemia. Am J Physiol Endocrinol Metab 308: E306–E314, 2015. doi: 10.1152/ajpendo.00396.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalhan S, Parimi P. Gluconeogenesis in the fetus and neonate. Semin Perinatol 24: 94–106, 2000. doi: 10.1053/sp.2000.6360. [DOI] [PubMed] [Google Scholar]
- 22.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8: e1000412, 2010. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitanaka T, Alonso JG, Gilbert RD, Siu BL, Clemons GK, Longo LD. Fetal responses to long-term hypoxemia in sheep. Am J Physiol Regul Integr Comp Physiol 256: R1348–R1354, 1989. doi: 10.1152/ajpregu.1989.256.6.R1348. [DOI] [PubMed] [Google Scholar]
- 24.Limesand SW, Camacho LE, Kelly AC, Antolic AT. Impact of thermal stress on placental function and fetal physiology. Anim Reprod 15, Suppl 1: 886–898, 2018. doi: 10.21451/1984-3143-AR2018-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Limesand SW, Rozance PJ, Smith D, Hay WW Jr. Increased insulin sensitivity and maintenance of glucose utilization rates in fetal sheep with placental insufficiency and intrauterine growth restriction. Am J Physiol Endocrinol Metab 293: E1716–E1725, 2007. doi: 10.1152/ajpendo.00459.2007. [DOI] [PubMed] [Google Scholar]
- 26.Meschia G, Cotter JR, Makowski EL, Barron D. Simultaneous measurement of uterine and umbilical blood flows and oxygen uptakes. Q J Exp Physiol 52: 1–18, 1966. [Google Scholar]
- 27.Molina RD, Meschia G, Battaglia FC, Hay WW Jr. Gestational maturation of placental glucose transfer capacity in sheep. Am J Physiol Regul Integr Comp Physiol 261: R697–R704, 1991. doi: 10.1152/ajpregu.1991.261.3.R697. [DOI] [PubMed] [Google Scholar]
- 28.Narkewicz MR, Carver TD, Hay WW Jr. Induction of cytosolic phosphoenolpyruvate carboxykinase in the ovine fetal liver by chronic fetal hypoglycemia and hypoinsulinemia. Pediatr Res 33: 493–496, 1993. doi: 10.1203/00006450-199305000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Regnault TRH, de Vrijer B, Galan HL, Wilkening RB, Battaglia FC, Meschia G. Umbilical uptakes and transplacental concentration ratios of amino acids in severe fetal growth restriction. Pediatr Res 73: 602–611, 2013. doi: 10.1038/pr.2013.30. [DOI] [PubMed] [Google Scholar]
- 30.Rozance PJ, Hay WW Jr. New approaches to management of neonatal hypoglycemia. Matern Health Neonatol Perinatol 2: 3, 2016. doi: 10.1186/s40748-016-0031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rozance PJ, Limesand SW, Barry JS, Brown LD, Thorn SR, LoTurco D, Regnault TR, Friedman JE, Hay WW Jr. Chronic late-gestation hypoglycemia upregulates hepatic PEPCK associated with increased PGC1alpha mRNA and phosphorylated CREB in fetal sheep. Am J Physiol Endocrinol Metab 294: E365–E370, 2008. doi: 10.1152/ajpendo.00639.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rozance PJ, Zastoupil L, Wesolowski SR, Goldstrohm DA, Strahan B, Cree-Green M, Sheffield-Moore M, Meschia G, Hay WW Jr, Wilkening RB, Brown LD. Skeletal muscle protein accretion rates and hindlimb growth are reduced in late gestation intrauterine growth-restricted fetal sheep. J Physiol 596: 67–82, 2018. doi: 10.1113/JP275230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudolph CD, Roman C, Rudolph AM. Effect of acute umbilical cord compression on hepatic carbohydrate metabolism in the fetal lamb. Pediatr Res 25: 228–233, 1989. doi: 10.1203/00006450-198903000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Soria R, Julian CG, Vargas E, Moore LG, Giussani DA. Graduated effects of high-altitude hypoxia and highland ancestry on birth size. Pediatr Res 74: 633–638, 2013. doi: 10.1038/pr.2013.150. [DOI] [PubMed] [Google Scholar]
- 35.Teng C, Battaglia FC, Meschia G, Narkewicz MR, Wilkening RB. Fetal hepatic and umbilical uptakes of glucogenic substrates during a glucagon-somatostatin infusion. Am J Physiol Endocrinol Metab 282: E542–E550, 2002. doi: 10.1152/ajpendo.00248.2001. [DOI] [PubMed] [Google Scholar]
- 36.Thorn SR, Brown LD, Rozance PJ, Hay WW Jr, Friedman JE. Increased hepatic glucose production in fetal sheep with intrauterine growth restriction is not suppressed by insulin. Diabetes 62: 65–73, 2013. doi: 10.2337/db11-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thorn SR, Regnault TRH, Brown LD, Rozance PJ, Keng J, Roper M, Wilkening RB, Hay WW Jr, Friedman JE. Intrauterine growth restriction increases fetal hepatic gluconeogenic capacity and reduces messenger ribonucleic acid translation initiation and nutrient sensing in fetal liver and skeletal muscle. Endocrinology 150: 3021–3030, 2009. doi: 10.1210/en.2008-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thorn SR, Rozance PJ, Brown LD, Hay WW Jr. The intrauterine growth restriction phenotype: fetal adaptations and potential implications for later life insulin resistance and diabetes. Semin Reprod Med 29: 225–236, 2011. doi: 10.1055/s-0031-1275516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thorn SR, Sekar SM, Lavezzi JR, O’Meara MC, Brown LD, Hay WW Jr, Rozance PJ. A physiological increase in insulin suppresses gluconeogenic gene activation in fetal sheep with sustained hypoglycemia. Am J Physiol Regul Integr Comp Physiol 303: R861–R869, 2012. doi: 10.1152/ajpregu.00331.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Timmerman M, Teng C, Wilkening RB, Fennessey P, Battaglia FC, Meschia G. Effect of dexamethasone on fetal hepatic glutamine-glutamate exchange. Am J Physiol Endocrinol Metab 278: E839–E845, 2000. doi: 10.1152/ajpendo.2000.278.5.E839. [DOI] [PubMed] [Google Scholar]
- 41.Wesolowski SR, Hay WW Jr. Role of placental insufficiency and intrauterine growth restriction on the activation of fetal hepatic glucose production. Mol Cell Endocrinol 435: 61–68, 2016. doi: 10.1016/j.mce.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wesolowski SR, Mulligan CM, Janssen RC, Baker PR II, Bergman BC, D’Alessandro A, Nemkov T, Maclean KN, Jiang H, Dean TA, Takahashi DL, Kievit P, McCurdy CE, Aagaard KM, Friedman JE. Switching obese mothers to a healthy diet improves fetal hypoxemia, hepatic metabolites, and lipotoxicity in non-human primates. Mol Metab 18: 25–41, 2018. doi: 10.1016/j.molmet.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yates DT, Macko AR, Chen X, Green AS, Kelly AC, Anderson MJ, Fowden AL, Limesand SW. Hypoxaemia-induced catecholamine secretion from adrenal chromaffin cells inhibits glucose-stimulated hyperinsulinaemia in fetal sheep. J Physiol 590: 5439–5447, 2012. doi: 10.1113/jphysiol.2012.237347. [DOI] [PMC free article] [PubMed] [Google Scholar]