Abstract
Ultrasonic-assisted extraction of quercetin from Dendrobium officinale was optimized by response surface methodology (RSM) using high-performance liquid chromatography as a separative method. Based on single-factor experiments and two-level factorial analysis, the ethanol concentration, solid-to-liquid ratio and ultrasonic power were selected as significant response factors. The amount of quercetin that we extracted from Dendrobium officinale was 2.506–2.594 μg/g under the extraction conditions, which showed that optimization could improve the extration rate of quercetin from Dendrobium officinale. Quercetin was extracted and detected within 12 consecutive months after the germination of Dendrobium officinale by optimizing the extraction process to analyze the accumulation of quercetin. The UV-B exposure experiments showed that the Dendrobium officinale leaves have different responses to low- and high-dose UV light. The results showed that the quercetin content in Dendrobium officinale could be changed by UV-B radiation, and the response of distinct tissue parts to varying intensities of UV-B radiation was different.
Keywords: Theoretical chemistry, Pharmaceutical chemistry, Plant biology, Biotechnology, Metabolite, Dendrobium officinale, Quercetin, RSM, HPLC, UV-B
1. Introduction
Dendrobium officinale, a plant that grows on rocky cliffs, is mainly distributed in Southwestern Anhui, Eastern Zhejiang, Western Fujian, Northwestern Guangxi, Sichuan and Southeastern Yunnan of China [1, 2]. Dendrobium officinalehas anti-tumor, anti-fatigue and anti-oxidation properties [3, 4, 5]. Modern studies show that Dendrobium officinalecontains a variety of chemical constituents, and the types of compounds identified so far include alkaloids, carbohydrates, bibenzyls, steroidal saponins, and flavonoids [6, 7].
Flavonoids, widely exist in medicinal plants, have extensive pharmacological activities. According to the oxidation degree of the intermediate tricarbon chain and the position of phenyl linkage, they can be divided into flavonoids, flavonols, dihydroflavones, isoflavones and other compounds [8]. As a flavonol, quercetin has many pharmacological effects and attracted increasing attention [9, 10]. In the recent years, quercetin have been found and extracted from different matrices [11, 12, 13] Hot water, alkali liquor and ultrasonic-assistedcan be used to extract quercetin from natural plants, ultrasonic-assisted extraction is widely used since its high extraction efficiency, short extraction time and simple operation [14, 15, 16].
Response surface methodology (RSM) is a method to optimize complex processes by using multivariate methods [17]. RSM is a time- and labor-saving method when compare with other optimization methods, as it needs fewer experiments to evaluate multiple factors and their interactions. It is widely used in the fields of biology and chemistry, as well as the food industry and other areas. RSM was used to optimize the extraction process of polyphenols, anthocyanins and phenolic compounds from raspberry, Eucalyptus robusta and lemon [18, 19, 20].
UV-B is ultraviolet radiation with a wavelength range of 280–320 nm. Surface UV-B radiation has become an important ecological factor affecting plant growth [21]. Studies show that UV-B radiation reduces plant height and slows down the photosynthesis rate. Plants need their own defense mode to cope with radiation stress to adapt to the living environment. The induction of flavonoid synthesis and accumulation is considered as one of the main ways for plants to tolerate UV-B radiation [22]. Enhanced UV-B radiation can increase the content of flavonoids in leaf epidermal cells, because flavonoids can reduce the net flux of UV-B radiation into plant cells by absorbing and shielding UV-B radiation. Liu et al. [23] found that a certain dose of UV-B radiation could effectively maintain fruit firmness and promote the accumulation of total phenols and flavonoids in tomato during the ature-green tomato fruit.
At present, research on flavonoids is still in its infancy, and the flavonoids were mainly used as the standard to control the quality of Dendrobium officinale, and carry out preliminary physiological and biochemical determinations of total flavonoids in Dendrobium officinale. There is very little research on one given kind of Dendrobium officinale flavonoids. Based on single-factor and two-level factorial analysis of experimental data, the extraction process of quercetin from Dendrobium officinale was optimized by RSM using high-performance liquid chromatography as a detection method. Thus, an ideal extraction process was established. It was studied that UV-B, as an abiotic elicitor, affect the quercetin content at different irradiation intensities and times as well as the application fields of Dendrobium officinale flavonoids.
2. Main text
2.1. Herbal samples
Tissue-cultured Dendrobium was provided by the medicinal plant research group of the school of life sciences, Anhui Agricultural University. The aseptic seeds of Dendrobium officinale were evenly sown on MS medium and cultured in a sterile tissue culture room at a constant temperature (12 h/12 h light/darkness cycle, 25 °C). After 60 days of sowing, the seedlings were transferred to the medium for further cultivation, and the medium was replaced every 30 days. During the experimental period, some of the plants were collected every 30 days and dried to a constant weight in a 60 °C oven to detect the change of quercetin content in Dendrobium officinale during360 days of growth. In the first 30 days, the whole seedlings were used to detect quercetin content, because the seedlings were too small to distinguish the stem and leaf. After that, when the Dendrobium officinale plants were approximately 5 cm high, they were taken out from the medium and dried in a 60 °C oven to a steady weight. The dried samples were ground into powder and used in RSM experiments.
After 300 days of growth, Dendrobium officinale tissue culture seedlings were divided into three groups and cultured in a temperature-controlled tissue culture room for culturing (12 h/12 h light/darkness cycle, 25 °C). Using an ultraviolet lamp tube (313 nm, 20 W) as a UV-B light source to irradiate the seedlings, the irradiation intensity of control group was 0 kJ/m2, low-dose group was 1.6 kJ/m2 and high-dose group was 4.8 kJ/m2. Irradiation time was four hours a day (10 a.m.–2 p.m.) for 14 days. During the irradiation course, the leaves and stems of Dendrobium seedlings were washed with clean water and then dried in a 60 °C oven. The dried samples were ground into powder to measured quercetin content.
2.2. Chemicals and solvents
Quercetin standard was purchased from Aladdin Polytron Technologies Inc. (Shanghai, China, Lot K1607044). The reagents and solvents used in the experiments were of HPLC grade. Methanol and acetonitrile were purchased from Anhui Tiandi High Purity Solvent Co., Ltd. (Anhui, China). Ethanol was purchased from Tianjin Four Friends Fine Chemicals Co., Ltd. (Tianjin, China). Formic acid was purchased from Aladdin Biochemical Polytron Technologies Inc., (Shanghai, China). Purified water was purchased from Hangzhou Wahaha Group (Zhejiang, China).
2.3. Extraction procedure
A certain amount of ethanol solution (2 mL–6 mL) was added to the sample powder of Dendrobium officinale (100 mg) in a 10 mL centrifuge tube. The powder was placed into an NC ultrasonic cleaner (Kunshan Ultrasound Instrument Co., Ltd.) and extracted by ultrasound at a certain power and temperature for a predetermined period of time. After ultrasonic-assisted extraction, the mixture was centrifuged at 5000 rpm for 10 min. The supernatant was withdrawn, and the solvent in the solution was removed by nitrogen gas. The extract after solvent removal was concentrated and redissolved in methanol in a 1/10 of supernatant volume. All experiments were done in triplicate.
2.4. Reverse-phase HPLC analysis
Ultimate 3000 high-performance liquid chromatography equipped with a diode array detector (DAD) was used for chromatographic analysis. Quercetin was separated on a Thermo Hypersil Gold C18 reverse-phase column (4.6 mm × 250 mm) with a 5-micron particle size. The column temperature was 25 °C, the injection volume was 10 μL, the flow rate was 1.0 mL/min, and acetonitrile (A) and 0.02% (v/v) formic acid aqueous solution (B) were used as mobile phases. The gradient elution was performed from 0 min to 40 min, during which the proportion of organic phase in the elution solvent increased from 5% to 80%, and then the proportion of organic phase decreased to 5% for 10 min to rebalance the column. The detection wavelength was 368 nm. The standard curve was calculated using seven standard quercetin solutions with different concentrations from 0.39 to 25 μg/mL. The standard curve equation was linear in the examined range and the relative equation was y = 0.6289x-0.3117, with R2 = 0.9995, where y was the quercetin concentration (μg/mL), and x was the peak area.
2.5. Experimental design
RSM uses multiple response factors and central composite design (CCD) to derive the optimal level of independent variables. This experimental design specifies 20 experimental combinations, including six repetitions of central points. Before designing the response surface experiment, it is necessary to carry out single-factor experiments on the selected influencing factors to determine the optimal range of each influencing factor [24]. In this experiment, ethanol concentration (10–90%), solid-to-liquid ratio (1:20–1:60 g/mL), ultrasound power (120–200 W), ultrasound time (5–60 min), and temperature (30–70 °C) were selected as the factors influencing quercetin extraction, and single-factor experiments were carried out.
After that, the data obtained from single-factor experiments were analyzed by two-level factorial analysis. Through the two-level factorial analysis experiment, several influencing factors, which have the most significant effect on response value, can be selected [25]. In the present study, five influencing factors were screened, and 32 experimental combinations were designed.
Finally, RSM was used to design the response surface experiment scheme by using the selected significant influencing factors and central composite design (CCD), to explore the influencing factors on quercetin extraction. The data obtained were analyzed by Design Expert software (8.0.6 Stat-Ease, Minneapolis, MN, USA), and the multivariate regression equation model was established. The data were analyzed by the multivariate regression equation using the least-square method. All experimentsin the model were done in triplicate.
2.6. Single-factor experiment on quercetin extraction
2.6.1. Effect of the solid-to-liquid ratio on quercetin extraction from Dendrobium officinale
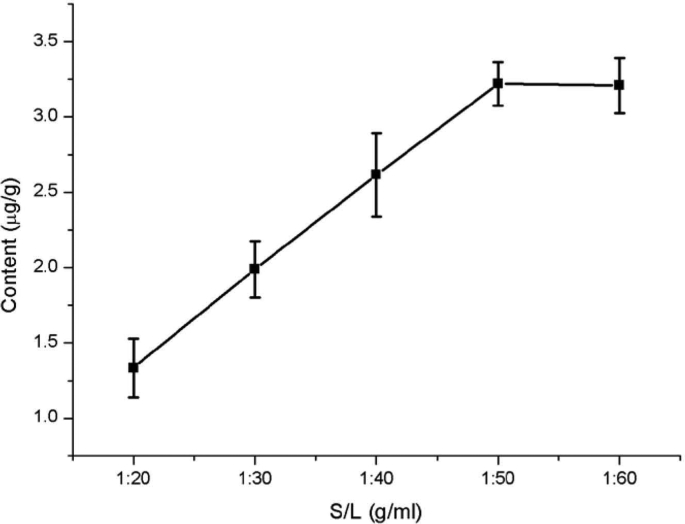
The extraction efficiency of quercetin was significantly affected by the solid-to-liquid ratio. The results are shown in Fig. 1. Quercetin extraction from Dendrobium officinale increased gradually with the increasing of solid-to-liquid ratio. When the solid-to-liquid ratio was 1:50 g/mL, the extracted amount of quercetin (3.2 μg/g) from Dendrobium officinale was the highest. When the solid-to-liquid ratio was more than 1:50 g/mL, the extracted amount of quercetin was no longer significantly increased, butexhibit a decreasing trend. This phenomenon may be due to an increase in soluble polysaccharides and chlorophyll, which may bind to quercetin, to precipitate or adsorb it, resulting in a decrease in the quercetin extraction rate [26]. Therefore, the solid-to-liquid ratio in subsequent experiments was optimized between 1:40 g/mL and 1:60 g/mL.
Fig. 1.
Effect of the solid-to-liquid ratio on quercetin extraction from Dendrobium officinale.
2.6.2. Effect of temperature on quercetin extraction from Dendrobium officinale
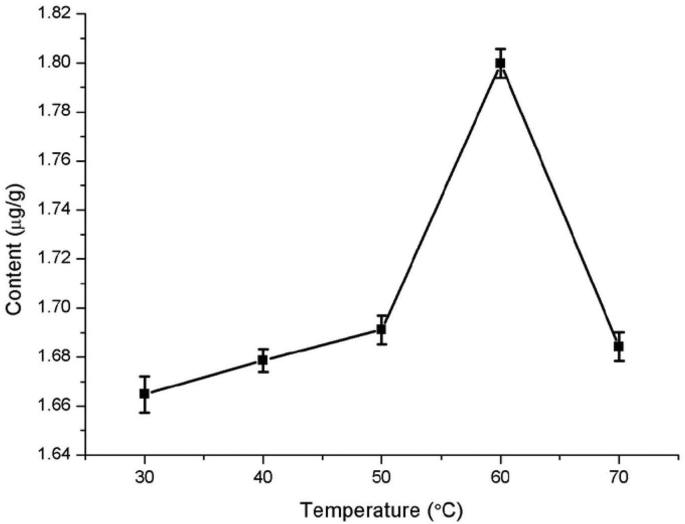
Temperature has a certain effect on quercetin extraction from Dendrobium officinale (Fig. 2). The extracted amount of quercetin increased with increasing temperature. When the temperature was 60 °C, the extracted amount reached the maximum (1.8 μg/g). Subsequently, quercetin extraction ratio decreased with increasing temperature. This result may be due to an increase in temperature within a certain range promotes the dissolution of quercetin, but excessive temperature leading to the destruction or volatilization of quercetin in Dendrobium officinale [27]. Therefore, the temperature in subsequent experiments was optimized between 50 °C and 70 °C.
Fig. 2.
Effect of the extraction temperature on quercetin extraction from Dendrobium officinale.
2.6.3. Effect of ultrasonic power on quercetin extraction from Dendrobium officinale
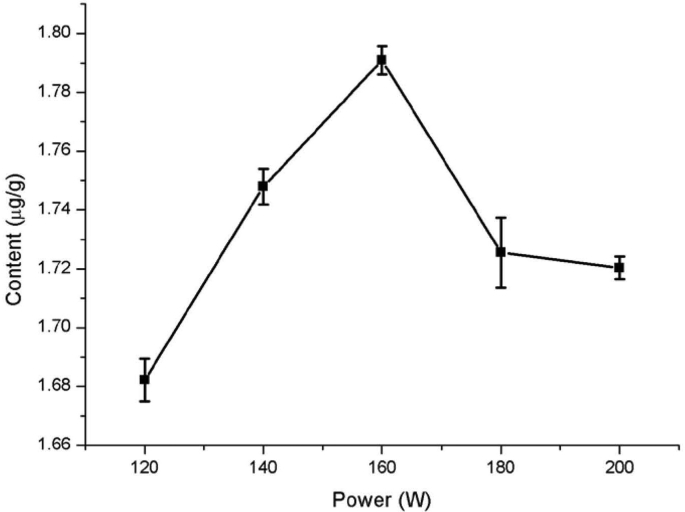
The ultrasound power had a significant impact on quercetin extraction from Dendrobium officinale (Fig. 3). When the ultrasonic power was between 120 W and 160 W, the extracted amount of quercetin increased with increasing ultrasonic power, but it decreased with the increase in ultrasonic power when the power continued to increase. The reason for this phenomenon may be that increasing ultrasonic power produces a greater effect on the activity of cavitation bubbles. the flow speed of solvents is accelerated withthe further increases in ultrasonic power, the residence time of extracts in the ultrasonic field is reduced, the wall-breaking effect of the ultrasound is weakened, leadingto reduced extraction rate of quercetin [28]. At the same time, the thermal effect of higher ultrasonic power may give rise to damaged quercetin structure, contribute to a lower extraction rate [29]. Therefore, the ultrasonic power in subsequent experiments was optimized between 140 W and 180 W.
Fig. 3.
Effect of ultrasonic power on quercetin extraction from Dendrobium officinale.
2.6.4. Effect of ethanol concentration on quercetin extraction from Dendrobium officinale
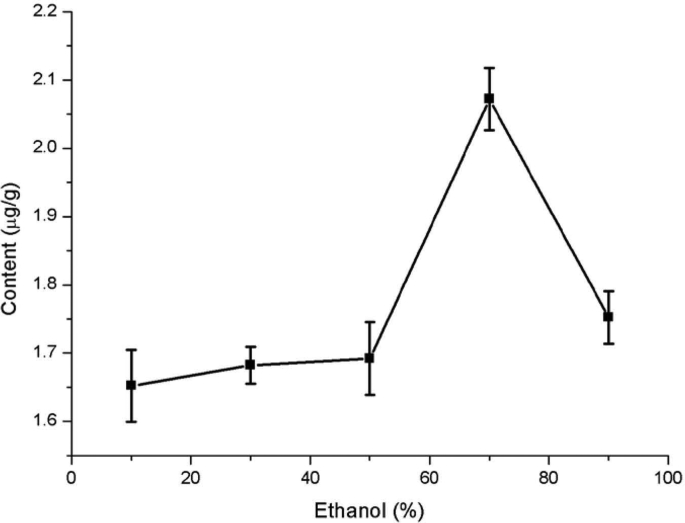
The ethanol concentration significantly affected quercetin extraction from Dendrobium officinale (Fig. 4). The extracted amount of quercetin firstly increased with increasing ethanol concentration, but then decreased with further increases in the ethanol concentration. The cause of this phenomenon may be related to quercetin polarity [30]. Quercetin contains - OH, has a certain polarity, and quercetin is almost insoluble in water, can be dissolved in high concentration of ethanol, so the amount of quercetin extraction may be related to the solubility of quercetin to ethanol. Therefore, the ethanol concentration in subsequent experiments was optimized between 50% and 90%.
Fig. 4.
Effect of the ethanol concentration on quercetin extraction from Dendrobium officinale.
2.6.5. Effect of time on quercetin extraction from Dendrobium officinale
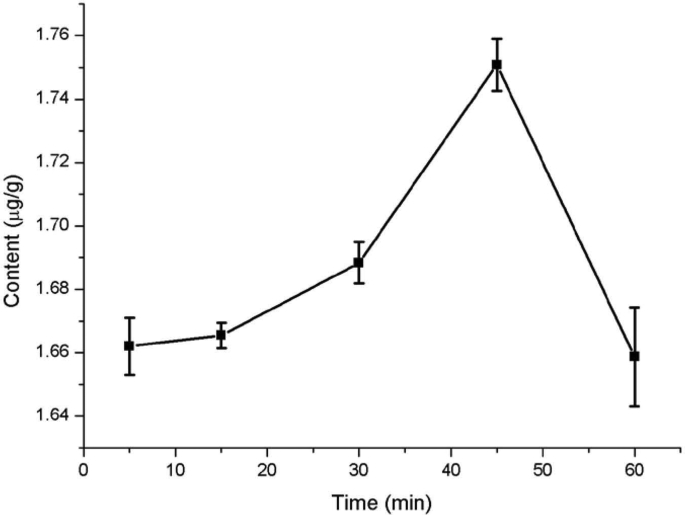
The extraction time is another important factor during quercetin extraction from Dendrobium officinale (Fig. 5). The extracted amount of quercetin increased continuously with increasing extraction time and reached the maximum at 45 min. Upon further increases in extraction time, the extracted amount of quercetin decreased rapidly. Because ultrasonic extraction may destroy the extracted quercetin, for example, long-term ultrasound heating may oxidize quercetin. Therefore, the extraction time in subsequent experiments was optimized between 30 min and 60 min.
Fig. 5.
Effect of time on quercetin extraction from Dendrobium officinale.
2.7. Screening of quercetin extraction factors by the two-level factorial design
Based on single-factor experiments, the above five factors were selected for mixed factor screening experiments. The two-level factorial analysis was designed, and the results were shown in Table 1.
Table 1.
Two-level factorial design and results.
| Run | Ethanol (%) | Temperature (°C) | Time (min) | Power (W) | Liquid Ratio (%) | Quercetin (μg/g) |
|---|---|---|---|---|---|---|
| 1 | 90 | 70 | 60 | 180 | 60 | 4.0283 |
| 2 | 50 | 50 | 60 | 140 | 60 | 4.2728 |
| 3 | 50 | 50 | 30 | 140 | 60 | 3.9518 |
| 4 | 50 | 50 | 30 | 180 | 60 | 3.8855 |
| 5 | 50 | 50 | 30 | 140 | 40 | 2.5772 |
| 6 | 90 | 70 | 60 | 180 | 40 | 2.7356 |
| 7 | 50 | 50 | 30 | 180 | 40 | 2.7316 |
| 8 | 50 | 70 | 60 | 180 | 40 | 2.5752 |
| 9 | 50 | 50 | 60 | 140 | 40 | 2.7216 |
| 10 | 90 | 70 | 30 | 180 | 40 | 2.9232 |
| 11 | 50 | 70 | 30 | 180 | 60 | 3.7777 |
| 12 | 90 | 50 | 60 | 140 | 40 | 2.8444 |
| 13 | 90 | 70 | 60 | 140 | 40 | 2.9480 |
| 14 | 90 | 50 | 30 | 180 | 40 | 2.9076 |
| 15 | 50 | 50 | 60 | 180 | 60 | 3.9451 |
| 16 | 50 | 70 | 30 | 180 | 40 | 2.6568 |
| 17 | 90 | 50 | 30 | 140 | 60 | 4.0861 |
| 18 | 50 | 70 | 60 | 140 | 60 | 4.0650 |
| 19 | 50 | 70 | 60 | 140 | 40 | 2.8132 |
| 20 | 50 | 70 | 30 | 140 | 40 | 2.6696 |
| 21 | 90 | 50 | 60 | 180 | 60 | 3.9680 |
| 22 | 50 | 70 | 60 | 180 | 60 | 3.9433 |
| 23 | 90 | 70 | 60 | 140 | 60 | 4.1090 |
| 24 | 90 | 70 | 30 | 180 | 60 | 4.0663 |
| 25 | 90 | 50 | 30 | 140 | 40 | 2.9244 |
| 26 | 90 | 50 | 30 | 180 | 60 | 4.2873 |
| 27 | 50 | 50 | 60 | 180 | 40 | 2.6480 |
| 28 | 90 | 70 | 30 | 140 | 40 | 2.9668 |
| 29 | 50 | 70 | 30 | 140 | 60 | 3.9662 |
| 30 | 90 | 70 | 30 | 140 | 60 | 4.0120 |
| 31 | 90 | 50 | 60 | 140 | 60 | 4.3385 |
| 32 | 90 | 50 | 60 | 180 | 40 | 2.7552 |
According to the two-level factorial experimental results shown in Table 2, the concentration of ethanol, ultrasonic power and solid-to-liquid ratio were significant influencing factors, with P values less than 0.05.
Table 2.
ANOVA of the two-level factorial design.
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 13.3700 | 9 | 1.4900 | 280.32 | <0.0001 |
| Ethanol | 0.2300 | 1 | 0.2300 | 42.98 | <0.0001 |
| Temperature | 0.0110 | 1 | 0.0110 | 2.05 | 0.1667 |
| Time | 0.0032 | 1 | 0.0032 | 0.61 | 0.4435 |
| Power | 0.0640 | 1 | 0.0640 | 12.09 | 0.0021 |
| Liquid Ratio | 12.8800 | 1 | 12.8800 | 243.78 | <0.0001 |
2.8. Optimization of quercetin extraction by RSM
2.8.1. Analysis of the response surface model
According to the experimental results of two-level factorial analysis, the response surface experiment was designed with the quercetin extraction amount as the dependent variable. The coding values of the three factors and the corresponding actual values were designed. The experimental results are shown in Table 3.
Table 3.
Central composite design and results.
| Run | Ethanol (%,A) | Power (W,B) | Liquid Ratio (%,C) | Quercetin (μg/g) |
|---|---|---|---|---|
| 1 | 90 (1) | 140 (-1) | 40 (-1) | 2.5818 |
| 2 | 70 (0) | 160 (0) | 33.18 (-1.68) | 2.5070 |
| 3 | 50 (-1) | 140 (-1) | 40 (-1) | 2.3590 |
| 4 | 70 (0) | 160 (0) | 50 (0) | 2.5661 |
| 5 | 50 (-1) | 140 (-1) | 60 (1) | 2.3644 |
| 6 | 70 (0) | 160 (0) | 50 (0) | 2.6033 |
| 7 | 90 (1) | 180 (1) | 60 (1) | 2.3880 |
| 8 | 70 (0) | 126.36 (-1.68) | 50 (0) | 2.3731 |
| 9 | 50 (-1) | 180 (1) | 40 (-1) | 2.4710 |
| 10 | 70 (0) | 160 (0) | 50 (0) | 2.5772 |
| 11 | 90 (1) | 140 (-1) | 60 (1) | 2.4206 |
| 12 | 70 (0) | 160 (0) | 66.82 (1.68) | 2.4871 |
| 13 | 103.64 (1.68) | 160 (0) | 50 (0) | 2.4652 |
| 14 | 70 (0) | 160 (0) | 50 (0) | 2.5619 |
| 15 | 50 (-1) | 180 (1) | 60 (1) | 2.4925 |
| 16 | 36.36 (-1.68) | 160 (0) | 50 (0) | 2.3438 |
| 17 | 70 (0) | 160 (0) | 50 (0) | 2.5570 |
| 18 | 70 (0) | 160 (0) | 50 (0) | 2.5355 |
| 19 | 90 (1) | 180 (1) | 40 (-1) | 2.4703 |
| 20 | 70 (0) | 193.64 (1.68) | 50 (0) | 2.4909 |
Design-Expert 8.0.6.1 software was used to fit the response value of quercetin extraction by multiple linear regression. The quadratic polynomial regression equations for the ethanol concentration (A), solid-liquid ratio (B), ultrasonic power (C) and quercetin extraction amount from Dendrobium officinale were obtained. The results for the model coefficient of significance and variance analysis were shown in Table 4. The regression model produced the following equation of the quercetin extraction amount as a function of the ethanol concentration (A), solid-to-liquid ratio (B) and ultrasonic power (C):
| Y = 2.57 + 0.028A-0.018B + 0.022C-0.034AB-0.048AC+0.012BC-0.056A2-0.023B2-0.046C2 |
Table 4.
ANOVA of the central composite design.
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 0.1200 | 9 | 0.0140 | 19.1800 | <0.0001 | ** |
| A | 0.0100 | 1 | 0.0100 | 14.7400 | 0.0033 | ** |
| B | 0.0046 | 1 | 0.0046 | 6.4500 | 0.0294 | * |
| C | 0.0063 | 1 | 0.0063 | 8.9200 | 0.0137 | * |
| AB | 0.0091 | 1 | 0.0091 | 12.8700 | 0.0049 | ** |
| AC | 0.0180 | 1 | 0.0180 | 26.0300 | 0.0005 | ** |
| BC | 0.0012 | 1 | 0.0012 | 1.5900 | 0.2357 | |
| A2 | 0.0450 | 1 | 0.0450 | 63.8300 | <0.0001 | ** |
| B2 | 0.0078 | 1 | 0.0078 | 11.0500 | 0.0077 | ** |
| C2 | 0.0310 | 1 | 0.0310 | 43.6200 | <0.0001 | ** |
| Residual | 0.0071 | 10 | 0.0007 | |||
| Lack of Fit | 0.0046 | 5 | 0.0009 | 1.79 | 0.2685 | |
| Pure Error | 0.0026 | 5 | 0.0005 | |||
| Cor Total | 0.1300 | 19 | ||||
| R2 | 0.9452 | |||||
| R2Adj | 0.8960 |
The data in Table 4 show that R2 = 0.9452 adjusts the complex correlation coefficient R2Adj = 0.8960 and the model misfit term P > 0.05. This shows that the model has high reliability. The quadratic regression equation obtained can predict the response value well and has a good fit. At the same time, the first term A, the interaction terms AB and AC, and the second terms A2, B2, and C2 showed extremely significant performance, while the first terms B and C also showed significant performance.
2.8.2. Interaction effects of different experimental factors on response variables
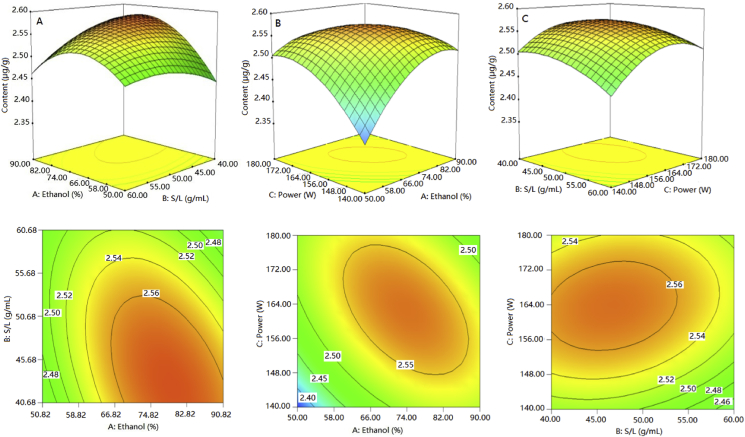
Response surface images of three-dimensional surface maps consisting of the ethanol concentration (A), solid-to-liquid ratio (B) and ultrasonic power (C) illustrate the pairwise interactions between the factors [31]. The highest center of the surface diagram showed the extreme value for pairwise interactions. As shown in Fig. 6 (A–C), the surfaces in Fig. 6 (A) and (B) were steep, indicating that the interactions between the ethanol concentration (A) and solid-to-liquid ratio (B), as well as the ethanol concentration (A) and ultrasonic power (C) were very significant. The surface in Fig. 6 (C) was relatively smooth, indicating that the interaction between the solid-to-liquid ratio (B) and ultrasonic power (C) was significant. From the trends in Fig. 6 (A), (B) and (C), it can be concluded that ethanol concentration (A) had the greatest influence on the response value, while the solid-to-liquid ratio (B) and ultrasonic power (C) had relatively weak influences on the response value. The above results are consistent with the results of the variance analysis.
Fig. 6.
Response surface plots of the effect of factor interactions on quercetin extraction yield. (A) Effect of the interaction between ethanol concentration and the solid-to-liquid ratio; (B) Effect of the interaction between ultrasonic power and ethanol concentration; (C) Effect of the interaction between the solid-to-liquid ratio and ultrasonic power.
2.8.3. Validation of optimal conditions
The optimum extraction condition of quercetin from Dendrobium officinale was obtained by analysis with Design-Expertsoftware, the ethanol concentration was 81.6%, the solid-to-liquid ratio was 1:41.4 g/mL, the ultrasonic power was 156.36 W, the extraction time was 30 min, and the temperature was 60 °C. Under these conditions, the theoretical extraction ratio of quercetin from Dendrobium officinale was 2.58 μg/g. In practical operations, the extraction process was adjusted to an ethanol concentration of 81.6%, a solid-to-liquid ratio of 1:41.4 g/mL, a power of 160 W, an extraction time of 30 min, and a temperature of 60 °C. Under these conditions, the actual value of quercetin extracted in six parallel experiments was 2.55 ± 0.044 μg/g, as shown in Table 5. The deviation between the theoretical value and the actual value of the model prediction was [Deviation=(Theoretical value-Actual value)/Theoretical value * 100%], which was less than 10%. This shows that the regression model obtained by the response surface method is reliable, and the optimized extraction process of quercetin from Dendrobium officinale has application value [32].
Table 5.
Verification of the experimental results.
| Category | Run | Yield (μg/g) |
STDEV (%) | |
|---|---|---|---|---|
| Predictive | Experimental | |||
| Quercetin | 1 | 2.58 | 2.59 | 0.0084 |
| 2 | 2.58 | 2.57 | ||
| 3 | 2.58 | 2.47 | ||
| 4 | 2.58 | 2.58 | ||
| 5 | 2.58 | 2.58 | ||
| 6 | 2.58 | 2.56 | ||
2.9. Analysis of quercetin in Dendrobium officinale seedlings
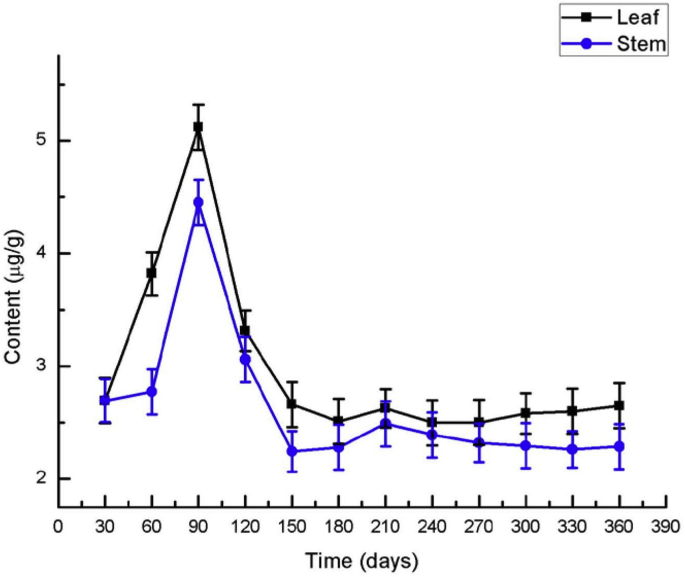
The contents of accumulated quercetinin Dendrobium officinale Seedlingsof tissue culture were determined by the above method for optimizing the extraction of quercetin (Fig. 7). The results showed that there were some differences in quercetin accumulation in different parts of Dendrobium officinale within 360 days after germination. In general, the quercetin content in the leaves was higher than that in the stems, and the quercetin content in both parts increased first and then decreased. The quercetin content in leaves reached the peak in the 90 days, then decreased and reached a relatively stable low level after the 150 days. The increased rate of quercetin in the stems was slower during first 60 days and then faster, but the increased rate of quercetin in the leaves was relatively stable. The content of quercetin in leaves of 90 days was 5.119 μg/g, almost twice as much as that in seedlings of 30 days (2.695 μg/g). This may be due to the fact that the cuticle of the plant is insufficient to resist the external environment at the early stage of growth. It is necessary to accumulate flavonoid secondary metabolites such as quercetin in order to adapt to the external environment [33, 34].
Fig. 7.
Quercetin content in the leaves and stems of Dendrobium officinale at different culture times.
2.10. Effects of different UV-B intensities on quercetin content in Dendrobium officinaleseedlings
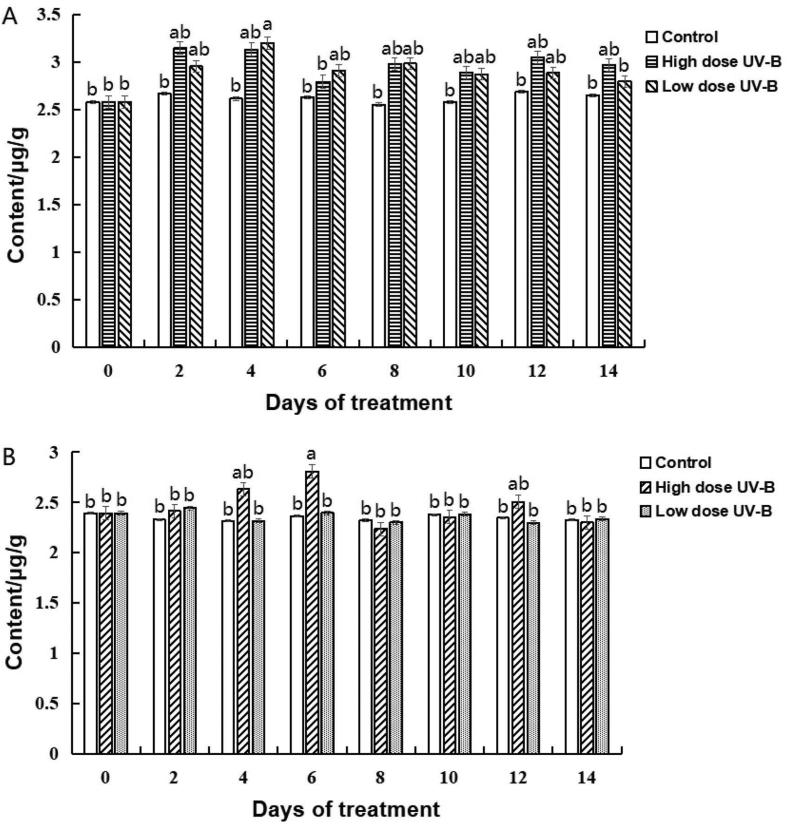
The quercetin content in leaves and stems in the control group was basically stable during the experimental period. The quercetin content in the leaves of the irradiated group increased at the beginning of the experiment compared with that of the control group (Fig. 8 A). The quercetin content in the leaves of the irradiated high-dose and low-dose groups reached the maximum on the second and fourth days, respectively. After that, the quercetin content gradually decreased and stabilized, but it was still higher than that of the control group. These experimental results are similar to those obtained by Mosadegh et al. [35] for the UV-B effect on sweet basil. Compared with leaves, stems were less sensitive to UV-B radiation (Fig. 8 B). The quercetin content in stems increased at a high irradiation dose and reached its maximum on the sixth day, then, it gradually decreased with the increase in time. The final quercetin content in stems was similar to that in the control group. The quercetin content in the stems of the low-dose irradiation group was almost the same as that of the control group during the whole experimental period. At the same time, the quercetin content in leaves and stems was similar to that in the high-dose irradiation group on the sixth day. At other times, the quercetin content in leaves was higher than that in stems.
Fig. 8.
Effects of different irradiation intensities on quercetin content in Dendrobium officinale, (A), quercetin content in leaves; (B), quercetin content in stems.
3. Conclusions
Based on single-factor experiments, two-level factorial analysis was used to screen the significant factors affecting quercetin extraction from Dendrobium officinale. Finally, the optimal extraction process of quercetin was determined by central composite design response surface methodology (CCD). The optimum conditions were as follows: ethanol concentration, 81.6%; solid-to-liquid ratio, 1:41.4 g/mL; ultrasonic power, 160 W; extraction time, 30 min; and temperature 60 °C. The actual amount of quercetin extracted from Dendrobium officinale was 2.506–2.594 μg/g under these conditions. The content of quercetin in Dendrobium officinale was higher in the early growth stage after optimizing the extraction process for one year after germination. The content of quercetin in the leaves was higher than that in the stems, as the plant grows, the content of quercetin decreases in both parts.
In the UV-B radiation experiment, the response of distinct Dendrobium officinale tissues to different radiation intensities varied. The response of the leaves of Dendrobium officinale to UV-B radiation was more stronger than that of the stems. Quercetin content in the leaves of Dendrobium officinale increased under high-dose and low-dose UV-B irradiation conditions, and the peak times of quercetin content increases caused by irradiation intensities were different, while the quercetin content in the stems changed only under high-dose irradiation conditions.
Declarations
Author contribution statement
Yongping Cai, Qing Jin: conceived and designed the experiments; contributed reagents, materials, analysis tools or data.
Lei Zhang, Jinfeng Tong: performed the experiments.
Chunyan Jiao, Yan Chang, Weina Sun: analyzed and interpreted the data; contributed reagents, materials, analysis tools or data.
Yingpeng Zhu, Jiangliu Yu: performed the experiments; analyzed and interpreted the data; wrote the paper.
Funding statement
This work was supported by The Universities Natural Science Research Project of Anhui, China (Project number KJ2016A224) and the Major Science and Technology Project in Anhui Province (Project number 706138977056)
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We extend our thanks to the reviewers and editors for their careful reading and helpful comments on this manuscript.
Contributor Information
Qing Jin, Email: jinqing169@126.com.
Yongping Cai, Email: ypcaiah@163.com.
References
- 1.Feng S., Zhao H., Lu J., Liu J., Shen B., Wang H. Preliminary genetic linkage maps of Chinese herb Dendrobium nobile and;D-moniliforme. Jpn. J. Genet. 2013;92(2):205–212. [PubMed] [Google Scholar]
- 2.Jin Q., Yao Y., Cai Y., Lin Y. Molecular cloning and sequence analysis of a phenylalanine ammonia-lyase gene from Dendrobium. J. Plos One. 2013;8(4) doi: 10.1371/journal.pone.0062352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei W., Li Z.P., Zhu T., Fung H.Y., Wong T.L., Wen X., Ma D.L., Leung C.H., Han B. Anti-fatigue effects of the unique polysaccharide marker of Dendrobium officinale on BALB/c mice. J. Molecules. 2017;22(1) doi: 10.3390/molecules22010155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao X., Dou M., Zhang Z., Zhang D., Huang C. Protective effect of Dendrobium officinale polysaccharides on H2O2-induced injury in H9c2 cardiomyocytes. J. Biomed. Pharmacother. 2018;94:72–78. doi: 10.1016/j.biopha.2017.07.096. [DOI] [PubMed] [Google Scholar]
- 5.Wei Y., Wang L., Wang D., Wang D., Wen C., Han B., Ouyang Z. Characterization and anti-tumor activity of a polysaccharide isolated from Dendrobium officinale grown in the Huoshan County. J. Chin. Med. 2018;13:47. doi: 10.1186/s13020-018-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lv G.Y., Yan M.Q., Chen S.H. Review of pharmacological activities of Dendrobium officinale based on traditional functions. China J. Chin. Mater. Med. 2013;38(4):489–493. [PubMed] [Google Scholar]
- 7.Huang X., Nie S., Cai H., Zhang G., Cui Steve W., Xie M., Phillips Glyn O. Study on Dendrobium officinale, O-acetyl-glucomannan (Dendronan): Part IV. Immunomodulatory activity in vivo. J. Funct. Foods. 2015;15:525–532. [Google Scholar]
- 8.Gao J., Inagaki Y., Liu Y. Research progress on flavonoids isolated from traditional Chinese medicine in treatment of Alzheimer's disease. J. Intractable Rare Dis. Res. 2013;2(2):3–10. doi: 10.5582/irdr.2013.v2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andres S., Pevny S., Ziegenhagen R., Bakhiya N., Schafer B., Hirsch-Ernst K.I., Lampen A. Safety aspects of the use of quercetin as a dietary supplement. J. Mol. Nutr. Food Res. 2018;62(1) doi: 10.1002/mnfr.201700447. [DOI] [PubMed] [Google Scholar]
- 10.D'Andrea G. Quercetin: a flavonol with multifaceted therapeutic applications? J. Fitoterapia. 2015;106:256–271. doi: 10.1016/j.fitote.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Cheng Y., Nie J., Li J., Liu H., Yan Z., Kuang L. Synthesis and characterization of core-shell magnetic molecularly imprinted polymers for selective recognition and determination of quercetin in apple samples. J. Food Chem. 2019;287:100–106. doi: 10.1016/j.foodchem.2019.02.069. [DOI] [PubMed] [Google Scholar]
- 12.Buiarelli F., Bernardini F., Di Filippo P., Riccardi C., Pomata D., Simonetti G., Risoluti R. Extraction, purification, and determination by HPLC of quercetin in some Italian wines. J. Food Anal. Methods. 2018;11(12):3558–3562. [Google Scholar]
- 13.Asma’a A.R., Ahmad A., Amani A., Zeid A. AL Othman. Analysis of quercetin and Kaempferol in an alcoholic extract of Convolvulus pilosellifolius using HPLC. J. Commun. Soil Sci. Plant Anal. 2015;46(11):1411–1418. [Google Scholar]
- 14.Liu Y., Gao Z.X., Li L., Deng T., Luo L.J., Wang H.D. Study on the effect of different methods on the extraction of rutin and quercetin in Rosa Laevigata Michx. J. Medicinal Plant. 2011;2(2):50–52. [Google Scholar]
- 15.Chekalina N.I., Shut S.V., Trybra t T.A., Burmak Y.H., Petrov Y.Y., Manusha Y.I., Kazakov Y.M. Effect of quercetin on parameters of central hemodynamics and myocardial ischemia in patients with stable coronary heart disease. J. Wiad Lek. 2017;70(4):707–711. [PubMed] [Google Scholar]
- 16.Chen L., Teng H., Xie Z., Cao H., Cheang W.S., Skalicka-Woniak K., Georgiev M.I., Xiao J. Modifications of dietary flavonoids towards improved bioactivity: an update on structure-activity relationship. J. Crit. Rev. Food Sci. Nutr. 2018;58(4):513–527. doi: 10.1080/10408398.2016.1196334. [DOI] [PubMed] [Google Scholar]
- 17.Mushtaq M., Sultana B., Bhatti H.N., Asghar M. RSM based optimized enzyme-assisted extraction of antioxidant phenolics from underutilized watermelon (Citrullus lanatus Thunb.) rind. J. Food Sci. Technol. 2015;52(8):5048–5056. doi: 10.1007/s13197-014-1562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teng H., Lee W., Choi Y. Optimization of ultrasonic-assisted extraction of polyphenols, anthocyanins, and antioxidants from raspberry ( rubus coreanus miq.) using response surface methodology. J. Food Anal. Meth. 2014;7(7):1536–1545. doi: 10.1002/jssc.201300303. [DOI] [PubMed] [Google Scholar]
- 19.Papoutsis K., Pristijono P., Golding J.B., Stathopoulos C.E., Bowyer M.C., Scarlett C.J., Vuong Q.V. Optimizing a sustainable ultrasound-assisted extraction method for the recovery of polyphenols from lemon by-products: comparison with hot water and organic solvent extractions. J. Eur. Food Res. Technol. 2018;244(8):1353–1365. [Google Scholar]
- 20.Bhuyan D.J., Quan Van V., Chalmers A.C., van Altena I.A., Bowyer M.C., Scarlett C.J. Microwave-assisted extraction of Eucalyptus robusta leaf for the optimal yield of total phenolic compounds. J. Industrial Crops and Products. 2015;69:290–299. [Google Scholar]
- 21.Santin M., Lucini L., Castagna A., Chiodelli G., Hauser M.T., Ranieri A. Post-harvest UV-B radiation modulates metabolite profile in peach fruit. J. Postharvest Biol. Technol. 2018;139:127–134. [Google Scholar]
- 22.Mo Y.C., Zeng L.J., Huang H., Feng H.Y., Ye J.W., Li J.B., Zhou C.R. Effects of UV-B radiation on photosynthetic Pigments,Flavonoids and PAL activity in Dendrobium officinale. J. Guizhou Agric. Sci. 2015;43(7):34–37. [Google Scholar]
- 23.Liu C., Han X., Cai L., Lu X., Ying T., Jiang Z. Postharvest UV-B radiation maintains sensory qualities and enhances antioxidant capacity in tomato fruit during storage. J. Postharvest Biol. Technol. 2011;59(3):232–237. [Google Scholar]
- 24.Mazarei F., Jooyandeh H., Noshad M., Hojjati M. Polysaccharide of caper (Capparis spinosa L.) Leaf: extraction optimization, antioxidant potential and antimicrobial activity. J. Int. J. Biol. Macromol. 2017;95:224–231. doi: 10.1016/j.ijbiomac.2016.11.049. [DOI] [PubMed] [Google Scholar]
- 25.Kala H.K., Mehta R., Sen K.K., Tandey R., Mandal V. Critical analysis of research trends and issues in microwave assisted extraction of phenolics: have we really done enough. J. Trac Trends Anal. Chem. 2016;85:140–152. [Google Scholar]
- 26.Rodrigues S., Pinto G.A., Fernandes F.A. Optimization of ultrasound extraction of phenolic compounds from coconut (Cocos nucifera) shell powder by response surface methodology. J. Ultrason. Sonochem. 2008;15(1):95–100. doi: 10.1016/j.ultsonch.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Chang J., Ma X., Hu Z. Optimization for ultrasound assisted extraction condition of total flavone from licorice by uniform experiment design. J. Guangdong Chem. Ind. 2014;4:7–8. [Google Scholar]
- 28.Huang Q., Chen W., Tian Y., Huang Y., Yu D. Ultrasound extraction and antioxidant activity of total flavones from Bauhinia championii Benth. J. Food Sci. Technol. 2013;38:224–227. [Google Scholar]
- 29.Zhang S., Cao L. Study on optimization the ultrasonic extraction of millet bran flavone. J. Farm Prod. Process. 2016;6:36–39. [Google Scholar]
- 30.Zhang Q.A., Fan X.H., Zhang Z.Q. Ultrasound-assisted extraction technology for flavones from Semen Astragali Complanati optimized by response surface methodology. J. Sci. Technol. Food Ind. 2012;33(19):275–2880. [Google Scholar]
- 31.Lee W.C., Yusof S., Hamid N., Baharin B. Optimizing conditions for hot water extraction of banana juice using response surface methodology (RSM) J. Food Eng. 2006;75(4):473–479. [Google Scholar]
- 32.Bimakr M., Rahman R.A., Taip F.S., Adzahan N.M., Sarker M.Z., Ganjloo A. Supercritical carbon dioxide extraction of seed oil from winter melon (Benincasa hispida) and its antioxidant activity and fatty acid composition. J. Molecules. 2013;18(1):997–1014. doi: 10.3390/molecules18010997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Müller V., Albert A., Winkler J.B., Lankes C., Noga G., Hunsche M. Ecologically relevant UV-B dose combined with high PAR intensity distinctly affect plant growth and accumulation of secondary metabolites in leaves of Centella asiatica L. Urban. J. Photochem. Photobiol. B Biol. 2013;5(127):161–169. doi: 10.1016/j.jphotobiol.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 34.Ruhland C.T., Day T.A. Changes in UV-B radiation screening effectiveness with leaf age in Rhododendron maximum. J. Plant Cell Environ. 1996;19:740–745. [Google Scholar]
- 35.Mosadegh H., Trivellini A., Ferrante A., Lucchesini M., Vernieri P., Mensuali A. Applications of UV- lighting to enhance phenolic accumulation of sweet basil. J. Sci. Hortic. 2018;229:107–116. [Google Scholar]