Highlights
-
•
The term “sebaceous cyst” has fallen into disuse, the current term is an epidermoid cyst.
-
•
Other common synonyms include infundibular cyst, epidermal cyst, epidermal inclusion cyst, and epidermoid inclusion cyst.
-
•
These cysts are slow-growing masses that elevate the skin and often have a central punctum.
-
•
On radiology, they have round to oval structure, well-circumscribed, avascular mass; restricted diffusion is typical.
-
•
Removal may be accomplished by simple excision or incision.
Abbreviations: ADC, apparent diffusion coefficient; CT, computed tomography scan; DWI, diffusion weighted images; MRI, magnetic resonance images; PDW, proton-density weighted; T1W, T1-weighted; T2W, T2-weighted
Keywords: Cutaneous cyst, Epidermal, Epidermoid, Keratin, Sebaceous, Skin lesion
Abstract
Epidermoid cysts are slow-growing, painless masses that elevate the skin and often have a central punctum that represents the plugged orifice of the pilosebaceous follicle. On ultrasound, they have a round to oval structure, well-circumscribed, avascular mass located in subcutaneous tissue along with phenomena of dorsal acoustic amplification and lateral shadowing. On MRI, they have slightly hypointense signal intensity on T1-weighted and intermediate to high signal on T2-weighted. Restricted diffusion is typical of epidermoid cysts. These signs are useful in the differentiation of epidermal cysts from neoplastic lesions. They need early treatment as they can cause cosmetic and functional impairment.
1. Introduction
Various types of cutaneous cysts containing fluid or semi-solid material and showing variable histopathologic features and clinical significance have been defined. Some cysts are surrounded by an epithelial cell wall, which is either stratified squamous or unstratified squamous epithelium. These are generally called true cysts. On the other hand, a subset of cysts, namely pseudocysts, is not lined by epithelium but instead is surrounded by connective or granulation tissue. Generally, cutaneous cysts are round, dome-shaped, protruding, or deeply located dermal or subcutaneous papules or nodules seen at different locations. Confirmation of the diagnosis is mostly via histopathologic examination. However, some clinical and imaging features, including the location of the cyst, may serve as diagnostic clues leading to a presumptive diagnosis [1].
Cutaneous cysts can be classified into three main types based on their morphology and specific pattern of differentiation include : (1) stratified squamous epithelium, (2) non-stratified squamous epithelium, (3) absence of epithelium; in it, epidermoid cysts are folded into the group the first (Table A1) [2]. Also, a milium is considered epidermoid cyst occurring transient [1].
In addition to occurring in many visceral organs, epidermoid cysts may also appear on the skin and mucous membranes. An epidermoid cyst is a common type of cutaneous cyst with an epidermis-like epithelial lining (wall). The lining of the cyst produces keratin. The term sebaceous cyst, which has formerly been used as a synonym for epidermoid cyst, is inappropriate because of the absence of sebaceous glands within the cyst lining. It is the result of the proliferation of surface epidermoid cells within the dermis. Other common synonyms include infundibular cyst, epidermal cyst, epidermal inclusion cyst and epidermoid inclusion cyst [1,3].
The term "dermoid" also has been used extensively to describe dermoid and epidermoid cysts; however, they are different entities. Both are cystic choristomas filled with keratin, cholesterol clefts, or degenerated blood components, and produced by keratinizing squamous epithelium; but whereas true dermoid cysts have skin appendages on their walls, epidermoid cysts wall do not have these appendages [4]. Their clinical behaviors also differ. Dermoid cysts are common, diagnosed during infancy or early childhood, usually located superficially or in the anterior orbit, commonly mold bone, and rarely induce bone lysis. Conversely, epidermoid cysts can develop in any part of the body and are usually diagnosed later in life [5]. They typically develop relating with bone if they are in the orbit. Although these cysts are recognized as benign lesions, rare malignancy can arise. These cysts may progress slowly for years [6,7].
2. Etiology
Most lesions are sporadic but familial inheritance is also possible, especially in individuals with multiple lesions. Multiple epidermoid cysts may be encountered as irregularly distributed lesions on the whole body or localized grouped lesions which is common in the retroauricular area [1]. Early-onset and atypical localization of epidermoid cysts such as limb involvement may be signs of autosomal dominant Gardner syndrome, a disease associated with colorectal polyps that have a high risk for the development of malignancy. Multiple epidermoid cysts on the trunk and extremities may also be seen in patients with Gorlin syndrome (basal cell nevus syndrome). In Favre-Racouchot syndrome (nodular elastosis with cysts and comedones), epidermoid cysts may result from chronic sun damage in elderly patients [3]. Patients with BRAF inhibitors can develop epidermoid cysts on the face. Imiquimod and cyclosporine have been noted to be able to cause epidermal inclusion cysts. Epidermoid cysts occurring before puberty in unusual locations and quantity raise the suspicion of a congenital syndrome [8,9].
3. Epidemiology
The epidermoid cyst can occur at any age, but it is more frequent in adulthood [9]. Zito et al reported that it occurs in a wide age range, from birth to 72 years but most typically arise in the third and fourth decades of life [3]. Approximately 1% of epidermoid cysts have been noted to have a malignant transformation to squamous cell carcinoma and basal cell carcinoma [3,9]. The face, neck, periauricular area, and upper trunk are more commonly involved, but any part of the body including sites such as the nipple, genitalia, and palmoplantar area may be involved. Janarthanam and Mahadevan described 7% of these cysts occur in the head and neck, oral cavity representing only 1.6% [8]. Lesions may be solitary or multiple [1].
It is rare to find these cysts before puberty. They are predominantly found in males. In the neonatal period, small epidermal cysts referred to as milium are common. Although, milium is epidermoid cyst occurring at all ages of life. Transient milia of the face is a very common problem, arising in approximately half of the newborns. This very common variant of a cutaneous cyst may be idiopathic (so-called primary milia) or may occur on sites of trauma such as burns and in the course of some dermatoses (so-called secondary milia). Moreover, milia may appear as a feature of some genodermatoses. Bullous dermatoses involving the dermo-epidermal junction, such as variants of inherited epidermolysis bullosa, acquired epidermolysis bullosa, bullous pemphigoid, porphyria cutanea tarda, and sunburn may cause milia at different ages on sites of the healing bullae. Milia may also appear following cosmetic procedures such as dermabrasion or after cryotherapy of skin tumors. In some instances, milia may occur following treatment of cutaneous leishmaniasis and lupus vulgaris. Multiple milia appearing at a young age may be seen in the setting of Bazex-Dupré- Christol syndrome, Rombo syndrome, Gorlin syndrome, hereditary tricho-dysplasia (Maria-Unna hypotrichosis), oral-facial digital syndrome type I, and Brooke-Spiegler syndrome.
4. Pathophysiology
Epidermoid cysts are a fluid-filled protrusion originating from the follicular infundibulum and lying just under the surface of the skin. Lesions usually occur spontaneously. However, implantation of the epithelium as a result of injury is considered an etiologic factor. Therefore, it may also be called epidermal inclusion cyst. Besides, due to surgery or skin disorders cyst, epidermal cells penetrate deep into the skin and multiply. The cyst wall is lined with stratified squamous epithelium, therefore, peeling of keratin layers will accumulate inside the cyst. Generally, plugging of the follicular orifice can cause an epidermoid.
A noticeable characteristic is that the cyst can communicate with the skin surface through a keratin-filled orifice so-called punctum. Epidermoid cysts may also occur as a result of the obstruction of the follicular orifice as seen in patients with acne vulgaris. Disruption of the follicle is important in the pathogenesis as those with acne vulgaris may have multiple epidermoid cysts originating from comedones. This punctum is the clinical sign indicating a connection between the cyst surface and the cyst lining. The central punctum is more prominent on large lesions. Rarely, more than one punctum may be noticed. Telangiectasia may also be seen on the surface of some lesions (Fig. 5A). Giant comedones with a black keratinous plug are also epidermoid cysts and are usually localized on the back. An epidermoid cyst may be confused with other cutaneous tumors such as lipoma, cystic basal cell carcinoma, and other variants of cutaneous cysts. A single lesion of an epidermoid cyst is more protruding and firm than a lipoma. The cystic lesion of a basal cell carcinoma may also have telangiectases on the surface but does not have a marked central punctum. A trichilemmal cyst is usually found on the scalp, is mildly alopecic and lacks a central punctum. Protrusion of cheese-like, malodorous material seen spontaneously or by compression is a helpful clue in the diagnosis of an epidermoid cyst (Fig. 1C).
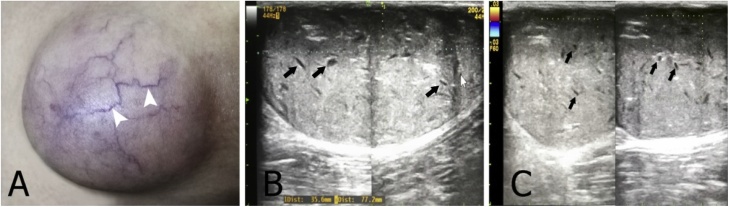
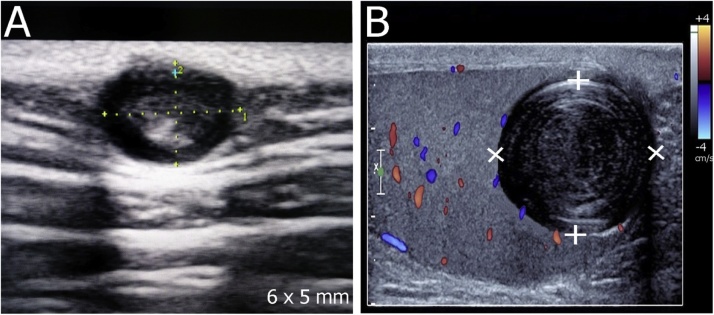
Fig. 5.
(A) A 40-year-old male with a large epidermoid cyst on the right thigh with telangiectases on the surface (arrowheads). (B) Grayscale ultrasound image shows a mildly echogenic mass with scattered internal dark clefts (arrows). (C) Color Doppler ultrasound image shows no internal blood vessels.
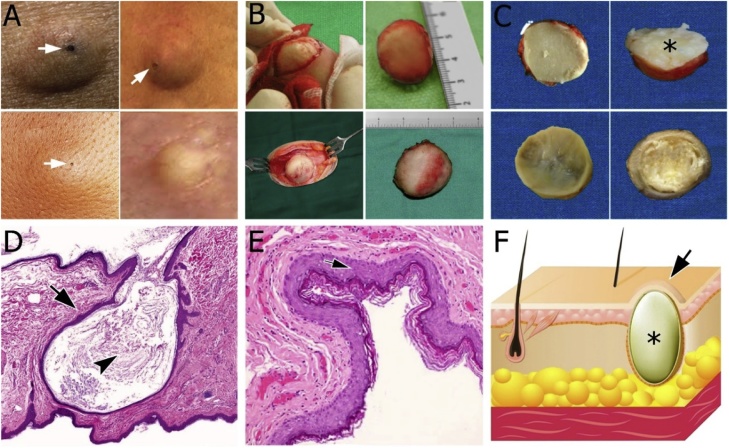
Fig. 1.
(A) Clinical variability of epidermoid cysts. Puncta of epidermoid cysts (arrows) that dark color keratin plug overlying cyst cavity tether the cyst to the overlying epidermis. (B) Intraoperative view and surgical specimen. (C) Photograph of the sectioned surgical specimen shows masses with a fibrous capsule that was composed of a laminated white-yellow paste-like material, typical of keratin. (D) Photomicrograph of pathology shows a solitary epidermoid cyst is appreciated in the dermis. Note the thin squamous epithelium image (arrow) and abundant keratin contents image (arrowhead); Original magnification, ×10; Hematoxylin and Eosin staining. (E) Photomicrograph of pathology shows the contents of the epidermoid cyst consist of laminated orthokeratotic material. Cyst wall lined by keratinizing stratified squamous epithelium image similar to the epidermis (arrow); Original magnification, ×100; Hematoxylin and Eosin staining. (F) Schematic illustration of a segment of skin with epidermoid cyst (asterisk). Note the epidermoid cyst that does bulging skin surface (arrow) (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
5. Histopathology
Histologically, an epidermoid cyst is lined by an epithelial cell wall (Fig. 2B). This epithelium is stratified squamous epithelium resembling epidermis and includes a granular layer and keratin lamellae in the lumen (Fig. 1). In contrast, “true” dermoid cysts when skin adnexa such as hair follicles, sebaceous glands, and sweat glands are present and teratoid cysts when tissues from all three germ layers, such as cartilage, bone, muscle, and respiratory or gastrointestinal epithelium are present [7]. If the cyst has been infected, chronic inflammatory cell infiltration may be present outside the cyst wall. If the lining of an epidermal cyst is ruptured and the keratin squames contained within the cyst are spilled out into the surrounding soft tissue, then an acute foreign body granulomatous reaction will develop in response to the keratin squames [3,10]. Clinically, this may initially be characterized by local tenderness. Inferiorly, the focal collection of chronic inflammatory cells outside the cyst wall is the result of a previous infection [10,11]. A granular layer is present that is filled with keratohyalin granules [4]. Additionally, epidermoid cysts in Gardner syndrome may show uncommon histologic features such as hybrid cysts or pilomatrixoma-like changes [1].
Fig. 2.
A 34-year-old male with a subcutaneous epidermoid cyst on the left buttock. (A) Ultrasound shows a circumscribed mildly echogenic mass. (B) Histologic examination shows a cystic cavity filled with laminated keratin lined by a stratified squamous epithelium that includes a granular layer.
6. History and physical
Epidermoid cysts can be found anywhere but are commonly found on the face, neck, chest, upper back, scrotum, and genitals. They can also be found on the buttocks, palms, and plantar side of feet if due to penetrating trauma. If occurring on the distal portion of the fingers, changes to the nail plate may occur [10,12]. Physical examination generally reveals several millimeters to several centimeters of non-fluctuant, compressible mass. A central, dark comedone opening (punctum) is often described (Figs. 1A and 3 A). Although epidermoid cysts usually remain asymptomatic, they may become inflamed as a result of the rupture of the cyst lining. A foul-smelling yellowish cheese-like material discharged from the lump may be described. Erythema, swelling, tenderness, palpation, pain, and fluctuation may occur suddenly, caused by inflammation. Inflamed cysts may be mistaken for a furuncle or carbuncle [3,4]. Spontaneous or surgical drainage will facilitate the healing process [9]. Scrotal epidermoid cysts may be calcified, leading to calcinosis cutis [1].
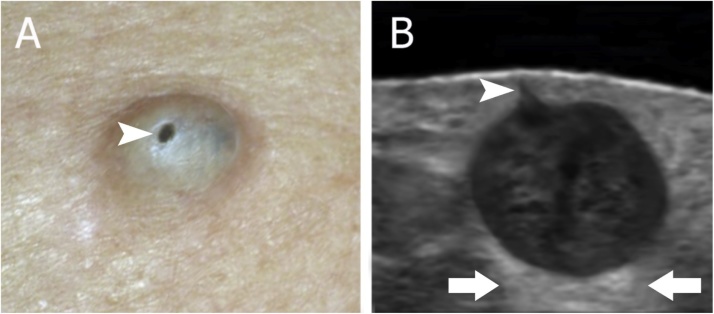
Fig. 3.
(A) A 42-year-old female with an epidermoid cyst on the back with a punctum on the surface (arrowhead). (B) Grayscale ultrasound image shows an oval-shaped hypoechoic structure located in the upper hypodermis and dermis with a central hypoechoic band. Notice the connecting punctum (arrowhead) to the subepidermal region. In spite of the dense and irregular appearance, the posterior acoustic enhancement is conserved (arrows).
Some patients may describe an event of a fall on their back or someone slapping their back causing cystic rupture and painful swelling lesion. Taking a good history can aid in determining if the cyst is an isolated case, caused by medications, or if it is part of a genetic syndrome. The cyst wall tends to become thicker after that what requires complete surgical excision. Malignant transformation of an epidermoid cyst is a rare event but possible [2,3].
A milium is a minute epidermoid cyst occurring at all ages of life. Transient milia of the face is a very common problem, arising in approximately half of the newborns. A typical papule of milium is white or cream-colored, firm, asymptomatic, and 1–2 mm in size. Idiopathic lesions mostly occur on the face, especially on the cheeks, nose, periorbital areas, and ears. They may sometimes be numerous. Lesions developing in association with dermatoses or as a result of skin injury may be seen at any site [1]. Most lesions are discrete papules, but the secondary lesions may be grouped. Multiple milia within an erythematous, edematous base are called milia en plaque. The etiology is not clear. Most patients are middle-aged women. These lesions are mostly located on the face, especially the retroauricular area. Multiple eruptive milia are defined as the sudden onset of numerous extensive milia. General, clinical features are mostly diagnostic of milium. The history of an associated disease or other triggering factors may also support the diagnosis. Histopathologic examination reveals small epidermoid cysts in the superficial dermis [1,3,13].
7. Diagnostic imaging
7.1. Ultrasound
Ultrasound is increasingly being used for the initial evaluation of soft-tissue masses. Certain clinical and imaging findings allow diagnosis of selected soft-tissue masses. Epidermoid cysts are quite typical on ultrasound; however, it sometimes has a nonspecific appearance and requires further imaging. Anatomic location and relationship to surrounding structures are ideally evaluated with ultrasound and can be reliably visualized features such as echogenicity, size, and margination [14]. In combination with an appropriate clinical history and certain sonographic findings, a specific diagnosis can sometimes be made. Ultrasound is being increasingly used by ordering providers as the initial imaging modality for evaluation of palpable soft-tissue masses. Because of the dynamic nature of the examination, sonographic evaluation should ideally be performed personally by a radiologist to yield the most information. Emphasis on proper technique and recognition of artifact are essential for accurate characterization of masses [14,15].
Typical, epidermoid cysts have the typical characteristics of cysts; however, they can show a cystic to solid. They have a round to oval structure, well-circumscribed, avascular mass located in the dermis and subcutaneous tissue along with the phenomena of dorsal acoustic amplification and lateral shadowing (Figs. 3B, 4 A and 8 B) [16]. Lee HS et al reported that epidermoid cysts have ovoid or spherical in 71%, lobulated in 21%, and tubular in 8%. The longest diameter ranged from 1 to 6 cm (mean 3.1 cm). About 96% were associated with posterior sound enhancement [17]. Denison et al described eight cases of epidermoid cysts in the breast, all of which appeared on ultrasound as circumscribed hypoechoic masses with through transmission [18]. Yasumoto et al reported on the sonographic appearance of epidermoid cysts in the head and neck; they were mostly hyperechoic with slight or no posterior sound enhancement [19].
Fig. 4.
(A) Epidermoid cyst with internal echogenicities in connection with the skin. The outlet of the sebaceous cyst is not visible in this ultrasound image. (B) Ultrasound of the testicular shows a round, well-defined lesion with multiple concentric hyperintense internal layers, resembling an onion skin. Pathology findings confirm an intratesticular epidermoid cyst.
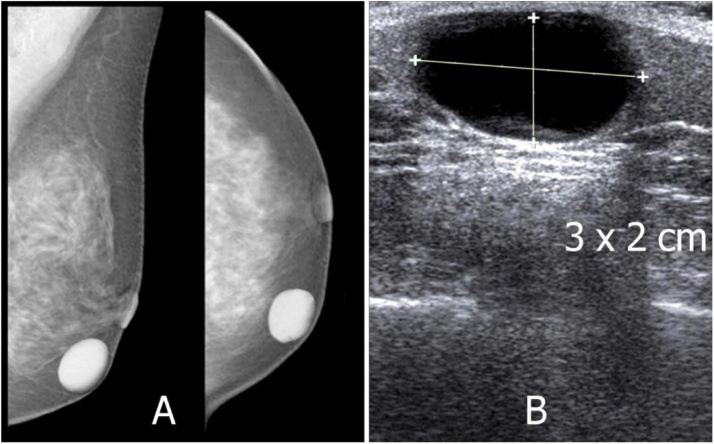
Fig. 8.
A 57-year-old female with a palpable left breast nodule. (A) Mammograms show a rounded nodule in the left breast that is well-defined and adherent to the skin. (B) Ultrasound shows a cystic appearance, with some inner echogenicities debris. Histopathology confirms as an epidermoid cyst.
Also, these cysts usually present inner echogenicities (debris) (Fig. 8B) and sometimes show a pseudocyesis pattern appearance (brighter inner echoes and anechoic filiform areas) as the result of highly compacted deposits of keratin and cholesterol. These findings are reported with previous studies; Dogra et al described that increased echogenicity in epidermoid cysts results from high acoustic impedance of the keratin debris (Fig. 8B) [20]. The onion-like appearance is very suggestive of an epidermoid cyst, especially in intratesticular lesions (Fig. 4B). Commonly, a connecting anechoic tract to the epidermis (punctum) can be detected on ultrasound (Fig. 3B). They also show central echoes of non-anechoic intensity owing to their contents as well as partial indentation to the dermis when they are subcutaneous (Fig. 4A) [9,10]. Kuwano Yoshihiro et al demonstrated epidermoid cysts have sensitivity after ultrasonography (65.9%) higher than that after palpation (43.2%). Also, the specificity after ultrasonography (99.3%) was significantly higher than after palpation (93.5%) as well. These data strongly support the usefulness of ultrasonography for the preoperative diagnosis of subcutaneous benign lesions [21].
If a rupture occurs, the keratin is spread into the surrounding tissue, leading to a reactive inflammation which causes a surrounding hypoechoic fluid collection. A big change in the morphology will result in an ill-defined hypoechoic structure (Fig. 6). The posterior acoustic enhancement, a classic artifact of cystic lesions, is usually conserved during all the phases [12]. Epidermoid cysts most often appeared sonographically as a hypoechoic mass containing variable echogenic foci without color Doppler signals (Figs. 2A and 5 C). However, they may demonstrate twinkling artifact. Increased blood flow in the periphery of the cysts during the phases of inflammation and rupture, frequently low flows, can be noted (Fig. 6) [10,11]. According to Lee et al, Color Doppler signals were absent in 83.3% cases, but some vascularity was noted in 16.7% ruptured epidermoid cysts, in areas of granulation tissue. They also may show color Doppler signals mimicking a solid mass [17].
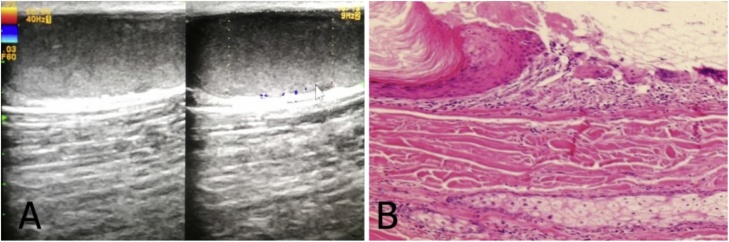
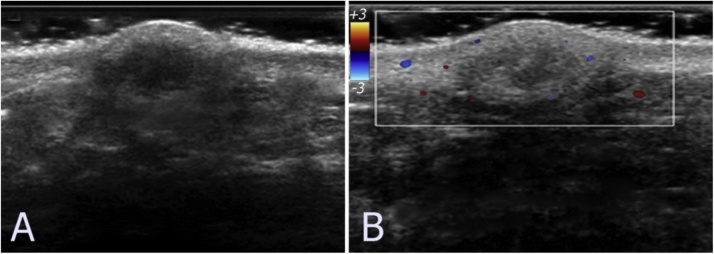
Fig. 6.
A 50-year-old woman with a subcutaneous epidermoid cyst. (A) Grayscale ultrasound image shows well-defined hyperechoic lesion with the hypoechoic central area. (B) Color Doppler ultrasound image shows some peripherals vascularity. The lesion was diagnosed as an epidermoid cyst with inflamed.
Ultrasound with a frequency higher than 15 MHz can be considered high frequency and is acceptable for an examination of the skin or skin lesions. Ultrasound with a lower frequency not as precise, but many cases are still exactly diagnosed. In conclusion, ultrasonography of epidermoid cysts greatly increases the reliability of preoperative diagnosis and is useful for preoperative examination [17,22]. Hung Esther et al reported that sensitivity and specificity of the first ultrasound diagnosis were 80.0% and 95.4% for epidermoid cyst and the accuracy of ultrasound examination for assessing superficial soft-tissue masses was 79.0% when all differential diagnoses were considered [15].
7.2. Radiograph
Epidermoid cysts are benign slow-growing lesions that may be congenital or acquired. Clinically, they manifest as non-tender, slowly expanding masses enlarging over years or decades [1,13]. A radiograph is not very valuable for evaluating epidermoid cysts. However, it can assess bone-related cases or calcified within the soft tissues (Figs. 7B and C, 8 A). On a radiograph, epidermoid cysts appear as well-demarcated intradiploic osteolytic lesions with smooth sclerotic margins (Fig. 7B and C). These lesions often cause remodeling and expansion of the cortical bone. Also on radiography can show soft tissue masses with high density (Figs. 7A and 8 A) [23].
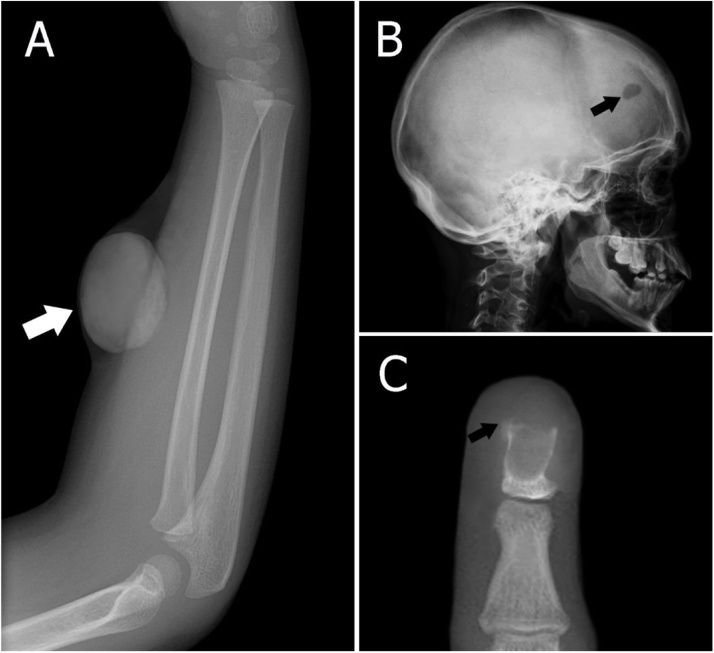
Fig. 7.
Radiographs of epidermoid cysts. (A) Radiograph shows a soft tissue mass in the forearm of a 5-year-old male (arrow). (B) Sagittal skull radiograph shows a lytic lesion in the frontal skull of a 50-year-old female (arrow). (C) The anteroposterior radiograph of finger shows the osteolytic lesion (arrow). Histopathology confirms as an intraosseous epidermoid cyst.
7.3. CT
Both CT and MRI play an essential role in diagnosing epidermoid cysts and determining the surgical excision strategy. CT is useful in confirming the diagnosis of a large epidermoid cyst; however, if the epidermoid cysts are small in size, CT is not effective.
CT demonstrates a well-encapsulated mass of heterogeneous densities that represent a mixture of fat and keratin. They can calcify inside (Fig. 9C and F) [18]. An unruptured epidermoid cyst is demonstrated as a noninfiltrating, fluid density mass with a thin sclerotic wall. The wall can enhance on contrast-enhanced images [11]. CT is fast and cost-effective compared with contrast-enhanced MRI studies, but MRI may be superior in delineating soft-tissue and vascular involvement for surgical planning (Fig. 10). CT guided fine-needle aspiration is also beneficial for diagnoses.
Fig. 9.
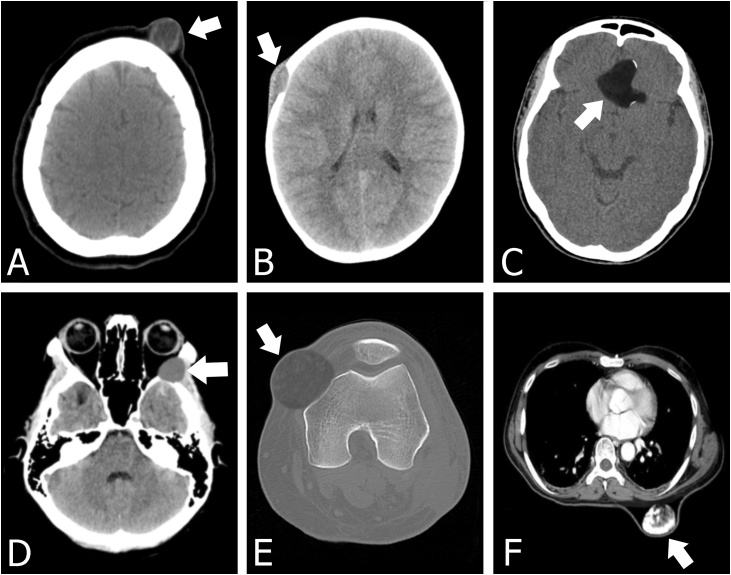
(A) Epidermoid cyst in a subcutaneous frontal region with a lower density. (B) Axial head CT image in the soft tissue window showing an osseous destructive lesion in the right frontal calvarium with beveled edges and a soft tissue component. Pathology proved epidermoid cyst in a 6-year-old boy. (C) Axial CT scan showing suprasellar epidermoid cyst with the calcified wall. (D) A bone-destroying epidermoid cyst with sclerotic borders is detected on the lateral wall of the left orbit on axial CT scan image. The lesion mainly located on the greater wing of the sphenoid extends into the zygomatic bone, temporalis fossa, and orbit. (E) A 40-year-old male with an epidermoid cyst in the left knee. Axial CT image in the bone window reveals a mass with the homogeneous density of soft tissue extending into the femur bone. Notice the lesion that causes the femur to have sclerosis. (F) Axial CT image obtained through the chest demonstrates a complex subcutaneous cystic masses of the posterior aspect of the upper back. The mass has coarse regions of calcifications.
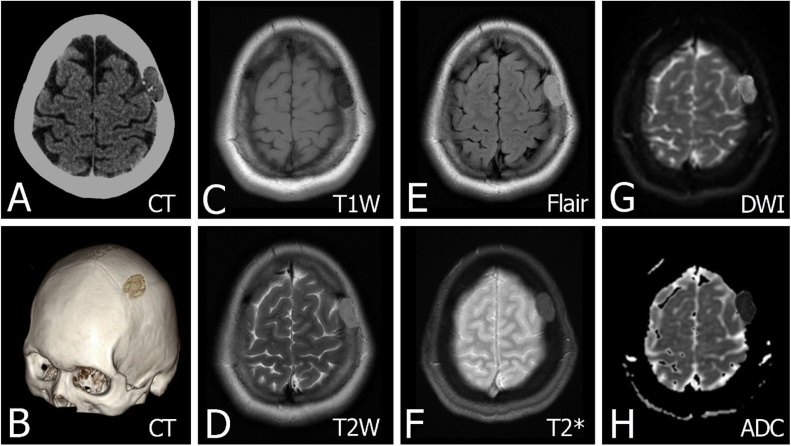
Fig. 10.
An epidermoid cyst has been confirmed on pathological anatomy in a 55-year-old female. (A) Axial head CT image in the soft tissue window with expansile soft tissue mass in the skull vault. The lesion is the intradiploic location with well-demarcated lysis expanding both the inner and outer tables of the skull and well-defined borders. (B) Sagittal oblique CT 3D reformat image shows scalloped bone margin. (C) Axial non-contrast T1W image shows the lesion with a decreased signal. (D–F) Axial T2W, Flair and T2 Gradient images show the mass with an increased signal. (G–H) DWI shows evidence of restricted diffusion along with the ADC map.
Gonzalo Blanco et al described the confusion of mass in the bony orbit and extending to the cranial cavity and temporalis fossa. Initially, it was diagnosed as a dermoid cyst, but the pathology confirmed that it was an epidermoid cyst. This is an exceedingly rare occurrence of an epidermoid cyst located in the bony orbit. Therefore Its clinical presentation, imaging characteristics, and management rationale are also stressed. Epidermoid cyst must be considered in the differential diagnosis of benign bone-destroying lesions affecting the orbit (Fig. 9D).
Epidermoid cysts are is very well evaluated on CT if it is located near the bone. Three types of secondary bone changes have been described: sclerosis, erosion, and fossa formation with bone attenuation. Characteristic bone destruction with sclerotic borders also can reveal (Fig. 9E).
In general, epidermoid cysts are an ovoid structure with a noninfiltrating rim. The superficial margin of the cyst usually touches the deep skin line, and the cyst is filled with mucoid attenuation material and scattered dystrophic calcifications. Uncommonly, they may become infected or rupture, causing a foreign body reaction [6].
7.4. MRI
MRI findings of epidermal inclusion cysts are dependent on the maturation of the cyst, its compactness and the amount of keratin it contains (Fig. 13). Previous studies have described diverse MRI findings; Tsunehiro Shibata et al reported that the size of the lesion ranged from 2 to 10 cm with an average of 5.2 cm. On T1W MRI, the tumor had a slightly high signal intensity in three out of five cases and iso-signal intensity in the remaining two cases. On T2W images, they all showed high signal intensity. Irregular low signal intensity areas were noted in the tumors on both T1W and T2W images. No enhancement was observed inside the tumors [24].
Fig. 13.
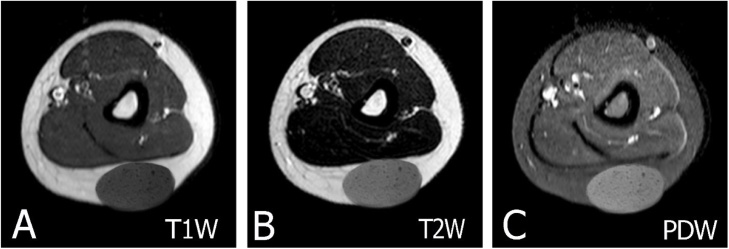
MRI in a 45-year-old male with a medium-size epidermal cyst at the arm. (A) Axial T1W image shows a well-defined oval-shaped mass with a rather monotonous signal that was hyperintense relative to muscle. (B–C) The lesion shows high signal intensity on axial T2W image without and with fat suppression. Intracyst debris of keratinized material is apparent on MRI.
The MRI features of unruptured epidermoid cysts have been described in previous studies as a well-defined mass with high T2 signal background with internal low T2, high T1 signal foci (Fig. 10, Fig. 11, Fig. 12, Fig. 13) [25]. Compared with muscular signals on MRI, uncomplicated epidermoid cysts show hypo or isointense signals on T1W images and marked hyperintense signal characteristics on T2W images. T1W images can reveal some slightly hyperintense foci. Contrast-enhanced T1W images demonstrate a mass with central nonenhancement and peripheral thin rim enhancement [10,11].
Fig. 11.
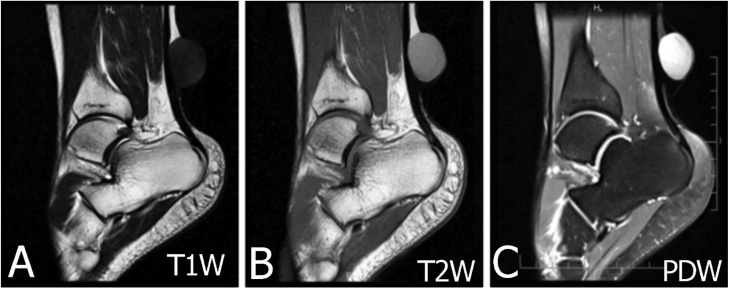
MRI in a 28-year-old male with a subcutaneous epidermal cyst at behind the leg posterior of the leg. (A) On T1W image displays slightly hypointense signal intensity. (B–C) On T2W and PDW images display intermediate to high signal.
Fig. 12.
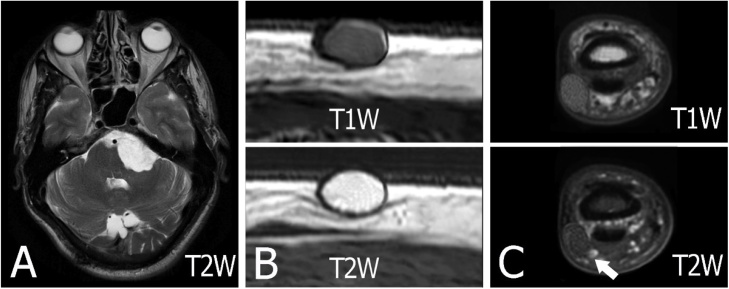
MRI of epidermoid cysts. Note that the signal of epidermoid cyst in the brain may be different from a subcutaneous. (A) The characteristic appearance of an epidermoid cyst in the cerebellopontine angle. The lesion has a similar signal intensity to cerebrospinal fluid on T2-weighted sequences. The diagnosis is confirmed on DWI which demonstrates very bright signals with intermediate ADC values similar to the brain parenchyma (DWI and ADC are not presented here). (B) Axial T1 and T2-weighted images of the lesion. There is a well-circumscribed, ovoid epidermoid cyst in the subcutaneous location. Its signals are low on T1-weighted and high signal on T2-weighted. (C) MRI in a 15-year-old female with a small size epidermal cyst in the finger. MRI shows a well-defined oval-shaped lesion with slightly high signal intensity on a T1W image and low signal intensity on a T2W image (arrow).
In studies of intratesticular epidermoid cysts, a concentric ring on ultrasound and a bulls-eye or target appearance on MRI have been described as important diagnostic features; these were shown to be caused by dense-layered debris in the center of the cyst [26]. In a study of Hee Kyung Kim et al, they found two subcutaneous epidermal cysts with a concentric ring or target appearance (one case on ultrasound and the other case on MRI); both lesions were located in the distal tip of the finger [22].
DWI show that the signal intensities of the subcutaneous epidermoid cyst are rated high while the ADC values are low (Fig. 10, Fig. 12A). An interesting note is that the ADC values of subcutaneous epidermoid cysts are significantly lower than those of intracranial epidermoid cysts (Fig. 10, Fig. 11, Fig. 12, Fig. 13). A ruptured cyst may have septa with thick and irregular rim enhancement accompanied by a blurry indistinct enhancement of surrounding tissues [27].
In summary, the characteristic MRI findings of epidermoid cysts include a well-circumscribed mass confined to the subcutaneous layer, with high T2 signal and possibly with low-signal-intensity debris, and thin rim enhancement on contrast-enhanced T1W images. Despite its limited ability to define bone changes, MRI also has a role in these lesions. Moreover, MRI may demonstrate the invasive into surrounding soft tissues and differential diagnosis with other injuries [[24], [25], [26], [27], [28]].
8. Treatment
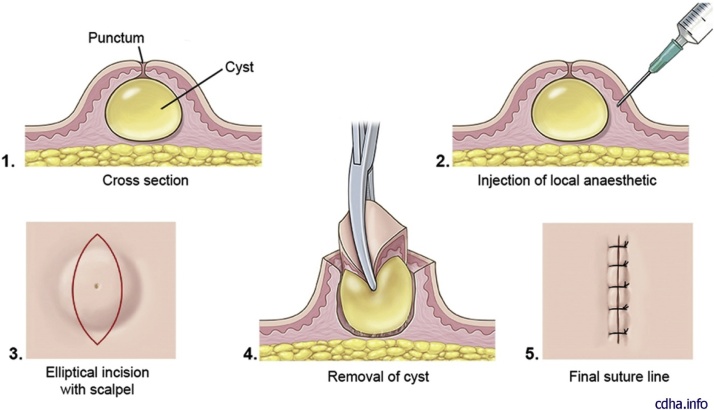
Small uncomplicated cysts usually do not need treatment. Removal may be accomplished by simple complete surgical excision of the cyst with the cyst wall intact (Fig. 14, Fig. 15). In these cases, an initial incision and drainage may be indicated with a potential for reoccurrence in the future. A local anesthetic with epinephrine is preferred to minimize bleeding. The anesthetic should be injected around the cyst, with avoidance of direct injection into the cyst [11]. A small diameter elliptical incision with the inclusion of the central core, or punctum can be utilized. For optimal cosmetic results, maintaining the incision in the minimal skin tension lines is important. A multiple-layered subcuticular and epidermal closure will yield an optimal outcome (Fig. 3, Fig. 4) [29,30].
Fig. 14.
Illustration step-by-step of resection of an epidermoid cyst. Note that if the cyst is not inflamed or scarred and does not have a residual punctum on the skin, do a linear incision over the middle. If a punctum or scar is present, a small elliptical incision is advisable.
Fig. 15.
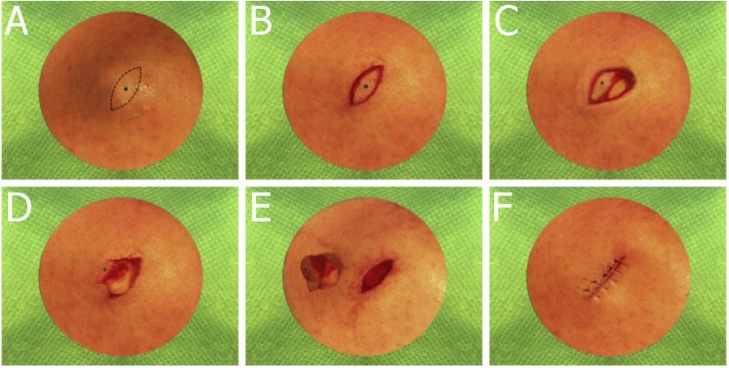
Intra operative images of step-by-step of resection of an epidermoid cyst (sebaceouscyst).
The complete excision should be delayed if an active infection is present as the planes of dissection will be difficult. It is best to avoid surgery while the cyst is actively inflamed due to a higher risk of infection, wound dehiscence and cyst recurrence. Following the resolution of the infection, the lesion is excised. An alternative surgical approach can also be done with a punch biopsy and expulsion of the intact cyst through the small defect or standard excision. If there is surrounding inflammation, intralesional triamcinolone may be used to help decrease inflammation in addition to a delay in surgical removal. If the entire cyst wall is not removed, the cyst may recur. Cysts are more difficult to remove once they have ruptured [29].
While small cysts may be treated by CO2- or erbium-YAG-laser, larger cysts need a surgical approach with cold steel. Since skin sagging is a possible outcome after removal of larger cysts, a small sheet of the epidermis above the cyst is excised. This allows an individualized adaption of the surgical margins. All epidermoid cysts removed surgically should be subjected to histopathological confirmation, to ensure complete excision and avoid misdiagnosis. Possible malignant transformation, although not seen in our cases, is another important argument for regular histopathologic analysis [4].
The method of complete excision includes sterile preparation, draping, local anesthesia, excision by a combination of sharp and blunt dissection, and closure of the wound. The key is to remove the cyst sac intact with minimal or no leakage of sebum. An alternate method involves a smaller incision into the sac, manual expression of the sebum, and removal of the empty sac using forceps and scissors. Although the scar is smaller, the wound will have a higher rate of inflammation and drainage. Some physicians using the microincision technique have treated the empty sac with the chemical phenol. Choose the appropriate skin incision as outlined. If the cyst is not inflamed or scarred and does not have a residual punctum on the skin, do a linear incision over the middle. If a punctum or scar is present, a small elliptical incision is advisable. If the cyst has had recurrent inflammation or scar tissue formation, more radical excision is indicated [3,29].
Some small subcutaneous benign lesions may be removed in an in-office procedure. However, when the diagnosis is uncertain, patients with very small lesions are typically referred to as a hospital for surgery. The improvement of diagnostic ability with ultrasonography may lead to an increase in the number of patients who require only an in-office procedure [21].
As mentioned, milium is a transient lesion. The course of milium is unpredictable. Whereas some lesions heal spontaneously in several months, others are persistent. Multiple lesions in newborns generally resolve spontaneously in a few weeks without scar formation. Topical retinoids may be used for multiple facial lesions in adults or in patients with milia en plaque but have a limited effect. Incision of the overlying epidermis with a sterile needle or scalpel followed by manual compression and evacuation of the cyst content is an easy method for the removal of a milium. A small excision may be used for a relatively larger lesion. Electrocautery and laser ablation are also effective in management but maybe complicated with minute scars [1,16,30].
9. Differential diagnosis
The differential diagnoses of epidermoid cysts include the following: pilar cyst (isthmus-catagen cyst, trichilemmal, wen), lipoma, melanoma, dermoid cyst, furuncle, branchial cleft cyst, milia, a pilonidal cyst, calcinosis cutis, pachyonychia congenital, steatocyst simplex, steatocystoma, and cutaneous findings of Gardner syndrome [3,7].
10. Complications and prognosis
Complications of rupture include erythema, swelling, and pain. Complications of surgical removal include bleeding, infection, and scaring. Epidermal inclusion cysts are recognized as benign cysts.
However, rare malignancy can occur. Squamous cell carcinoma is the most common malignancy followed by basal cell carcinoma [3].
11. Conclusions
Epidermoid cysts are common benign intradermal or subcutaneous tumors. For an accurate diagnosis, general knowledge about etiology, epidemiology, pathophysiology, histopathology, clinical, and radiology is needed. Epidermoid cysts need early treatment as they can cause cosmetic and functional impairment.
Declaration of Competing Interest
The authors and author's institutions have no conflicts of interest.
Appendix A
Table A1.
Cutaneous cysts classification.
| Stratified squamous epithelium | Non-stratified squamous epithelium | Absence of epithelium (pseudocysts) |
|---|---|---|
|
-Epidermoid (infundibular) cyst -Milium -Trichilemmal cyst -Proliferating trichilemmal cyst Proliferating epidermoid (infundibular) cyst -Vellus hair cyst -Steatocystoma -Cutaneous keratocyst -Pigmented follicular cyst -Dermoid cyst -Verrucous cyst -Ear pit cyst -Pilonidal cyst |
-Hydrocystoma (eccrine, apocrine) -Bronchogenic cyst -Thyroglossal duct cyst -Branchial cleft cyst -Cutaneous ciliated cyst -Ciliated cyst of the vulva -Median raphe cyst -Omphalomesenteric duct cyst |
-Mucocele -Digital mucous cyst (pseudocyst) -Ganglion -Preudocyst of the auricle -Cutaneous metaplastic synovial cyst (pseudocyst) |
References
- 1.Baykal C., Yazganoğlu K. Clinical atlas of skin tumors. Springer Sci. Bus. Media. 2014 [Google Scholar]
- 2.Bolognia J., Schaffer J., Cerroni L. 4th ed. Elsevier; 2017. Dermatology.https://www.elsevier.com/books/dermatology-2-volume-set/bolognia/978-0-7020-6275-9 [Google Scholar]
- 3.Zito P., Schar F.Cyst. StatPearls Publishing; 2019. Epidermoid (Sebaceous Cyst). Treasure Island (FL)https://www.ncbi.nlm.nih.gov/books/NBK499974/ StatPearls [Internet] [Google Scholar]
- 4.Wollina U., Langner D., Tchernev G., França K., Lotti T. Epidermoid cysts - A wide spectrum of clinical presentation and successful treatment by surgery: a retrospective 10-year analysis and literature review. Open Access Maced. J. Med. Sci. 2018;6(1):28–30. doi: 10.3889/oamjms.2018.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanco G., Esteban R., Galarreta D., Saornil M.A. Orbital intradiploic giant epidermoid cyst. JAMA Ophthalmol. 2001;119(5):771–773. doi: 10.1001/archopht.119.5.771. [DOI] [PubMed] [Google Scholar]
- 6.Curtin H., Som P. 4th ed. Mosby; 2002. Head and Neck Imaging; pp. 2173–2183. [Google Scholar]
- 7.Dutta M., Saha J., Biswas G.S.C., Sen I., Sinha R. Epidermoid cysts in head and neck: our experiences, with review of literature. Indian J. Otolaryngol. Head Neck Surg. 2013;65(2013):14–21. doi: 10.1007/s12070-011-0363-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janarthanam J., Mahadevan S. Epidermoid cyst of submandibular region. J. Oral Maxillofac. Pathol. 2012;16(3):435–437. doi: 10.4103/0973-029X.102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendonca de J., Jardim E., Dos Santos C., Masocatto D., Quadros de D., Oliveira M. Epidermoid cyst: clinical and surgical case report. Ann. Maxillofac. Surg. 2017;7(1):151–154. doi: 10.4103/ams.ams_68_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pires-Gonçalves L., Silva C.T., Costa-Dias S., Sousa-Mendes V. Testicular epidermoid cyst - Ultrasound and MR typical findings with macroscopy correlation. Int. Braz J Urol. 2011;37(4):534–535. doi: 10.1590/s1677-55382011000400014. [DOI] [PubMed] [Google Scholar]
- 11.Bin Manie M., Al-Qahtani K., Al Ammar A., Islam T., Otaibi F. Epidermoid cyst of the suprasternal region: a rare case report. Braz. J. Otorhinolaryngol. 2016:1–3. doi: 10.1016/j.bjorl.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lincoski C., Bush D., Millon S. Epidermoid cysts in the hand. J Hand Surg Eur. 2009;34(6):792–796. doi: 10.1177/1753193409347509. [DOI] [PubMed] [Google Scholar]
- 13.Takemura N., Fujii N., Tanaka T. Epidermal cysts: the best surgical method can be determined by ultrasonographic imaging. Clin. Exp. Dermatol. 2007;32(4):445–447. doi: 10.1111/j.1365-2230.2007.02408.x. [DOI] [PubMed] [Google Scholar]
- 14.Carra B., Bui-Mansfield L., O’Brien S., Chen D. Sonography of musculoskeletal soft-tissue masses: techniques, pearls, and pitfalls. Am. J. Roentgenol. 2014;202(6):1281–1290. doi: 10.2214/AJR.13.11564. [DOI] [PubMed] [Google Scholar]
- 15.Hung E., Griffith J., Hung N., Lee R., Lau D., Leung J. Ultrasound of musculoskeletal soft-tissue tumors superficial to the investing fascia. Am. J. Roentgenol. 2014;202(6):W532–W540. doi: 10.2214/AJR.13.11457. [DOI] [PubMed] [Google Scholar]
- 16.Jones E. Proliferating epidermoid cysts. JAMA Dermatol. 1966;94(1):11–19. [PubMed] [Google Scholar]
- 17.Lee H., Joo K., Song H., Kim Y., Park D., Park C. Relationship between sonographic and pathologic findings in epidermal inclusion cysts. J. Clin. Ultrasound. 2001;29(7):374–383. doi: 10.1002/jcu.1052. https://www.ncbi.nlm.nih.gov/pubmed/11579399 [DOI] [PubMed] [Google Scholar]
- 18.Denison C., Ward V., Lester S., DiPiro P., Smith D., Meyer J. Epidermal inclusion cysts of the breast: three lesions with calcifications. Radiology. 1997;204(2):493–496. doi: 10.1148/radiology.204.2.9240542. [DOI] [PubMed] [Google Scholar]
- 19.Yasumoto M., Shibuya H., Gomi N., Kasuga T. Ultrasonographic appearance of dermoid and epidermoid cysts in the head and neck. J. Clin. Ultrasound. 1991;19(8):455–461. doi: 10.1002/jcu.1870190802. https://www.ncbi.nlm.nih.gov/pubmed/1658076 [DOI] [PubMed] [Google Scholar]
- 20.Dogra V. Testicular epidermoid cysts. AJR Am. J. Roentgenol. 2002;179(4):1075. doi: 10.2214/ajr.179.4.1791075a. author reply 1075-6. [DOI] [PubMed] [Google Scholar]
- 21.Kuwano Y., Ishizaki K., Watanabe R., Nanko H. Efficacy of diagnostic ultrasonography of lipomas, epidermal cysts, and ganglions. Arch. Dermatol. 2009;145(7):761–764. doi: 10.1001/archdermatol.2009.61. [DOI] [PubMed] [Google Scholar]
- 22.Kim H., Kim S., Lee S., Racadio J., Shin M. Subcutaneous epidermal inclusion cysts: ultrasound (US) and MR imaging findings. Skeletal Radiol. 2011;40(11):1415–1419. doi: 10.1007/s00256-010-1072-4. [DOI] [PubMed] [Google Scholar]
- 23.Gomez C., Schiffman S., Bhatt A. Radiological review of skull lesions. Insights Imaging. 2018;9(5):857–882. doi: 10.1007/s13244-018-0643-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibata T., Hatori M., Satoh T., Ehara T., Kokubun S. Magnetic resonance imaging features of epidermoid cyst in the extremities. Arch. Orthop. Trauma Surg. 2003;(123):239–241. doi: 10.1007/s00402-003-0509-9. 2003. [DOI] [PubMed] [Google Scholar]
- 25.Yang D., Yoon M., Kim H., Oh Y., Ha S., Oh J. Presacral epidermoid cyst: imaging findings with histopathologic correlation. Abdom. Imaging. 2001;26(1):79–82. doi: 10.1007/s002610000118. https://www.ncbi.nlm.nih.gov/pubmed/11116367 [DOI] [PubMed] [Google Scholar]
- 26.Cho J., Chang J., Park B., Lee J., Son C. Sonographic and MR imaging findings of testicular epidermoid cysts. AJR Am. J. Roentgenol. 2002;178(3):743–748. doi: 10.2214/ajr.178.3.1780743. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki C., Maeda M., Matsumine A., Matsubara T., Taki W., Maier S. Apparent diffusion coefficient of subcutaneous epidermal cysts in the head and neck comparison with intracranial epidermoid cysts. Acad. Radiol. 2007;14(9):1020–1028. doi: 10.1016/j.acra.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 28.Hong S., Chung H., Choi J., Koh Y., Choi J., Kang H. MRI findings of subcutaneous epidermal cysts: emphasis on the presence of rupture. AJR Am. J. Roentgenol. 2006;186(4):961–966. doi: 10.2214/AJR.05.0044. [DOI] [PubMed] [Google Scholar]
- 29.Sempowski I. Sebaceous cysts. Ten tips for easier excision. Can. Fam. Physician. 2006;52(3):315–317. https://www.ncbi.nlm.nih.gov/pubmed/16572575 [PMC free article] [PubMed] [Google Scholar]
- 30.Shah S., Wain R., Syed S. Step-by-Step sebaceous cyst excision: a pictorial guide. Internet J. Plast. Surg. 2009;7(1):1–5. http://ispub.com/IJPS/7/1/4654 [Google Scholar]