Abstract
The importance of a precisely coordinated neuroendocrine, autonomic, and behavioral stress response was a primary theme at the Stress Neurobiology Workshop 2018, held in the beautiful setting of Banff Provincial Park in Alberta, Canada. Much of the research featured at this meeting reinforced the importance of appropriately responding to stress in order to avoid various neuropsychiatric pathologies, including Post-Traumatic Stress Disorder (PTSD), depression, and addiction. Corticotropin-Releasing Factor (CRF) neurons in the paraventricular nucleus of the hypothalamus (PVN) are central players in the stress response, integrating both external and visceral stress-relevant information, then directing neuroendocrine, autonomic and behavioral adaptations via endocrine and neural outputs of the PVN. The PVN contains a densely packed array of neuron types that respond to stress, including CRF neurons that activate the Hypothalamic-Pituitary-Adrenal (HPA) axis. Recently, identification of a new population of neurons in the PVN that express CRF Receptor 1 (CRFR1) has suggested that CRF release in the PVN signals to neighboring CRF responsive neurons, potentially functioning in HPA axis feedback, neuroendocrine coordination, and autonomic signaling. Here, we review our recent work characterizing an intra-PVN microcircuit in which locally released CRF release activates CRFR1+ neurons that make recurrent inhibitory GABAergic synapses onto CRF neurons to dampen excitability , therebylimiting HPA axis hyperactivity in response to stress and promoting stress recovery, which we presented in a poster session at the conference. We then discuss questions that have arisen following publication of our initial characterization of the microcircuit, regarding specific features of intra-PVN CRF signaling and its potential role in coordinating neuroendocrine, autonomic, and behavioral outputs of the PVN. Our presented work, as well as many of the presentations at the Stress Neurobiology Workshop 2018 together establish intra-PVN signaling as an important regulatory node in stress response pathways, which are central to the pathogenesis of neuropsychiatric disorders.
Keywords: Stress, HPA axis, PVN, CRF, CRH, CRFR1, CRHR1, Negative feedback
1. Introduction
Threats to survival rapidly activate a generalized endocrine, autonomic, and behavioral stress response, which together increase the ability of an organism to withstand the physiologic and psychological threats encountered in life (Mason, 1971; McEwen, 2000; Selye, 1970; Ulrich-Lai and Herman, 2009). Neural circuits activated by stress converge on the paraventricular nucleus of the hypothalamus (PVN), a key hypothalamic nucleus that receives multiple sources of afferent information about external threats and internal physiologic status, which are interpreted by the PVN and translated into outputs that control hormone release, adjust autonomic tone, and influence behaviors that together improve the likelihood of an organism's survival (Cullinan et al., 1996; Daviu, 2018; Ferguson et al., 2008; Füzesi et al., 2016; Wang et al., 2018). During initiation of the stress response, Corticotropin-Releasing Factor (CRF, also referred to as Corticotropin-Releasing Hormone or CRH; see Hauger et al., 2003 for the International of Phamacology official nomenclature for the complete CRF receptor/ligand family) neurons in the PVN become highly active and release CRF peptide at the median eminence, which transits the portal system and signals pituitary corticotropes to release adrenocorticotropic hormone (ACTH) into the circulation (Vale et al., 1981). ACTH, in turn, acts on adrenal cortical cells to elevate corticosteroid production (Cort; cortisol in humans and corticosterone in rodents), dramatically increasing the concentration of circulating Cort (in the range of 5–20 fold), the primary effector hormone of the Hypothalamic-Pituitary-Adrenal (HPA) axis. Elevated Cort impacts the function of nearly every cell and tissue in the body by activating glucocorticoid receptors (GR) and mineralocorticoid receptors (MR) that modify gene expression programs via direct binding to specific DNA sequences and recruitment of transcriptional complexes (Arriza et al., 1987; de Kloet, 2000; Joëls and de Kloet, 1992; Weinberger et al., 1985; Whirledge and DeFranco, 2018). Cort signaling provides negative feedback to the HPA axis, in part, by downregulating CRF and ACTH expression, thereby decreasing HPA axis activity to prevent the deleterious effects of sustained Cort signaling (Raubenheimer et al., 2006; Sapolsky and McEwen, 1985; Smith and Vale, 2006; Tsigos and Chrousos, 2002). Negative feedback mechanisms also function to promote the process of stress recovery in which HPA axis activity and circulating Cort concentrations are normalized, and other physiological systems recruited during the stress response, relax, returning the body to a state of homeostasis (Herman and Cullinan, 1997; Ostrander et al., 2006; Yehuda and LeDoux, 2007).
2. Synaptic negative feedback mechanisms function rapidly
Many mechanisms have been described in which the end product of the HPA axis, Cort, feeds back to limit HPA axis activity (Raubenheimer et al., 2006; Sapolsky and McEwen, 1985; Smith and Vale, 2006; Tsigos and Chrousos, 2002). However, Cort based negative feedback mechanisms act via GR or MR to modify gene expression at the timescale of transcriptional regulation, which functions in the hours to days following exposure to the stressor (Sapolsky et al., 2000). The stress response must include mechanisms capable of faster behavioral and physiological adjustments, which are required to avoid an active threat. For example during a predatory event, rapidly changing autonomic and behavioral states capable of quickly shifting during periods of hiding or running would improve the chances of successful escape from a predator (Kloet et al., 2005; Yuan et al., 2019). Faster timescale stress responses would be predicted to occur via synaptic mechanisms that act rapidly as physiologic demands change. Synaptic modulation of CRF neuron excitability has been postulated as a mechanism with the potential to impact both behavioral and endocrine features of the stress response rapidly Herman et al., 2002, Herman and Tasker, 2016, Mikics et al., 2008, Pariante and Lightman, 2008, Tasker and Herman, 2011, Thrivikraman et al., 2000) . Recurrent CRF-mediated synaptic modulation is one putative mechanism positioned to function rapidly to modulate CRF neuron excitability. Such mechanisms have been proposed because they are positioned to control CRF neuron excitability to regulate HPA axis hyperactivity, while concurrently adjusting behaviors and autonomic functions mediated by central actions of CRF neurons rapidly (J. P. Herman and Cullinan, 1997; Keller-Wood and Dallman, 1984). However, the existence and nature of CRF-mediated, fast-acting synaptic feedback mechanisms that modulate CRF neuron excitability have remained ambiguous.
3. Identification of CRFR1 expressing neurons in the PVN
One potential reason that few CRF mediated synaptic feedback mechanisms have been characterized is due to a problem that the field of CRF research has faced for years: the accurate identification of neurons that respond to CRF. As of 2019, almost 30 years after the first CRF receptor, CRFR1 was cloned (Perrin et al., 1993), antibodies that selectively recognize either of the two CRF receptors, CRFR1 or CRFR2 (Kishimoto et al., 1995; Lovenberg et al., 1995; Perrin et al., 1995, 1993), have not been successfully generated. To address the inability to easily identify CRF responsive neurons, we (N.J.), as a member of the Vale lab, generated a BAC transgenic mouse line that expresses GFP in neurons that express CRFR1 (Justice et al., 2008). GFP expression in CRFR1 expressing neurons allows for the identification of CRF responsive cells with high sensitivity and resolution (Justice et al., 2008). In addition, CRFR1-GFP mice have made available many standard techniques used to characterize neurons, allowing for the characterization of molecular, electrophysiological, and functional properties of multiple CRFR1+ cellular populations (Graham et al., 2011; Herman et al., 2013; Huising et al., 2010; Kuperman et al., 2016; Rosinger et al., 2019a, 2019b, 2017).
One neural population we noticed in our first observations of CRFR1-GFP brain tissue was a population of GFP + neurons located within the PVN, amongst CRF neurons, which were perhaps overlooked previously because of relatively low CRFR1 expression in the PVN detected by in situ hybridization (Justice et al., 2008, Van Pett et al., 2000). The proximity of CRFR1-GFP + neurons to CRF neurons immediately suggested that CRF signaling occurs within the PVN, potentially functioning in the regulation of HPA axis activity. PVN CRFR1-GFP + cells do not express peptides or hormones that define other well-characterized PVN neural populations, including vasopressin (VP) or oxytocin (OT) in magnocellular neurons, or thyrotropin-releasing hormone (TRH) or CRF in parvocellular neurons. In addition, they do not label with systemically delivered fluorogold that identifies peripherally projecting cells that release peptides or hormones into the circulation (Ramot et al., 2017). We have yet to find any marker that positively labels CRFR1-GFP PVN neurons (with the exception of Gad, and vGat which are expressed by all GABAergic neurons), suggesting that we discovered a previously uncharacterized population of neurons in the PVN that express CRFR1, allowing them to respond to CRF (Justice et al., 2008. Ramot et al., 2017; Jiang et al., 2018).
4. CRFR1 expression is regulated by Cort
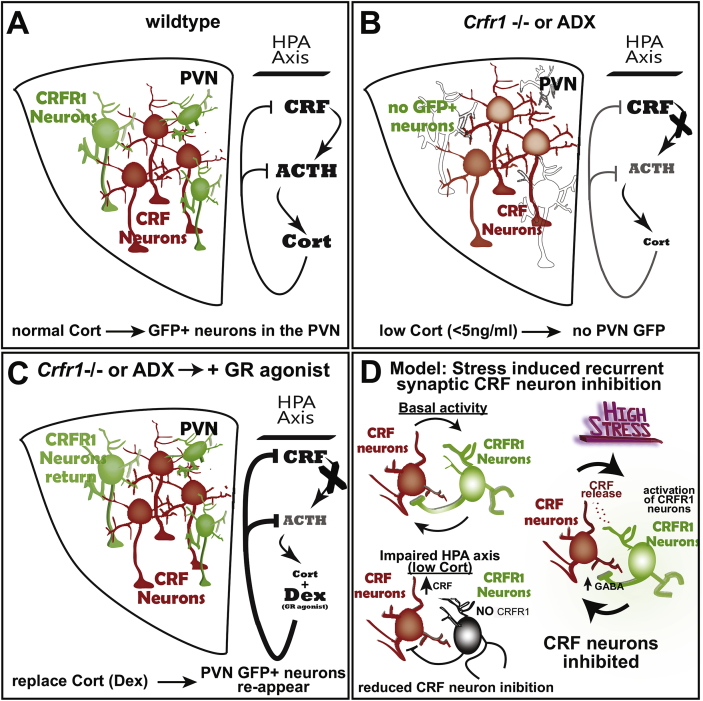
The functional relevance of PVN CRFR1+ neurons in HPA axis regulation was first suggested when CRFR1-GFP transgenic expression was examined in mice mutant for Crfr1 (Fig. 1). In Crfr1 knockouts carrying the CRFR1-GFP transgene (CRFR1-gfp; Crfr1 −/−), GFP + neurons normally visible in the PVN were absent, suggesting that either the neurons were not present, or that they had stopped expressing CRFR1-GFP (Fig. 1B; the global pattern of CRFR1-GFP expression in Crfr1 mutants was largely unchanged). To test whether the absence of GFP + neurons in the PVN resulted from very low circulating Cort levels caused by Crfr1 loss-of-function, we injected dexamethasone (Dex), a Glucocorticoid Receptor agonist, then sacrificed animals 24 h later. After Dex treatment, GFP + cells were again visible in the PVN, indicating that Cort signaling drives CRFR1 expression in these neurons (Fig. 1C; Ramot et al., 2017). In adrenalectomized CRFR1- GFP mice, GFP expression was similarly absent in the PVN, and returned when animals were treated with Dex (Fig. 1B, C;Ramot et al., 2017). Together these observations indicate that CRFR1 expression is positively regulated by Cort in PVN CRFR1+ neurons, in contrast to the role of Cort to downregulate genes that drive HPA axis activity in many well characterized negative feedback mechanisms (Keller-Wood and Dallman, 1984; Kovács et al., 2000; McClennen et al., 1998; Plotsky and Vale, 1984; Sawchenko, 1987; Young et al., 1995). Following the logic of homeostatic feedback where the end product of a pathway inhibits its own production, as Cort inhibits CRF and ACTH expression to limit HPA axis activity (Keller-Wood and Dallman, 1984; Kovacs and Mezey, 1987), positive CRFR1 regulation by Cort implies that PVN CRFR1 functions to inhibit HPA axis activity. We therefore hypothesized that PVN CRFR1 neurons are activated by local CRF release and make recurrent inhibitory synapses onto CRF neurons. In certain stress contexts, perhaps during persistent or intense stress exposure, hyperactive CRF neurons release CRF locally, activating surrounding CRFR1+ neurons and thereby increasing inhibitory tone onto CRF neurons. This mechanism of local CRF release and recurrent inhibition would function to prevent CRF neuron hyperexcitability and corresponding hyperactivity of the HPA axis (Fig. 2; Jiang et al., 2018; Ramot et al., 2017).
Fig. 1.
An intra-PVN CRF signaling microcircuit: CRFR1 expression is positively regulated by Cort. (A) Under non-stressed conditions, CRFR1 expressing neurons neighbor CRF neurons in the PVN that activate the HPA axis, which controls adrenal Corticosteroids (Cort) via pituitary ACTH release. (B) In Crfr1 mutant or adrenalectomized animals lacking Cort, PVN CRFR1 neurons stop expressing CRFR1 and GFP + neurons are absent in the PVN, suggesting that Cort positively regulates CRFR1 expression. (C) Restoring Cort signaling using the Glucocorticoid Receptor agonist, Dexamethasone (Dex) causes GFP + neurons to reappear in the PVN, indicating the re-expression of CRFR1-GFP. (D) Our working model suggests that CRF signals to CRFR1 neurons in a negative feedback microcircuit via intra-PVN CRF release, which activates recurrent inhibition by surrounding GABAergic CRFR1+ neurons. In basal stress contexts, the importance of this microcircuit in HPA axis regulation remains unclear. However, when Cort is experimentally removed (Crfr1 −/−; or adx), PVN CRFR1 neurons stop expressing CRFR1, inactivating this mechanism limiting HPA axis activity, and interpreted as the suppression of one of many mechanisms invoked in low Cort conditions meant to restore HPA axis activity (e.g. CRF expression is dramatically increased in adx and Crfr1 −/− mice). By contrast, in conditions of high stress and excessive CRF neuron activity, CRF is released within the PVN to activate CRF responsive GABAergic neurons (CRFR1+), elevating inhibitory tone onto CRF neurons and decreasing excitability. This feedback mechanism capable of adjusting CRF neuron activity functions to prevent excessive HPA axis activity acutely during responses to stress as well as during stress recovery. It also likely influences autonomic and behavioral stress response by controlling CRF neuron excitability.
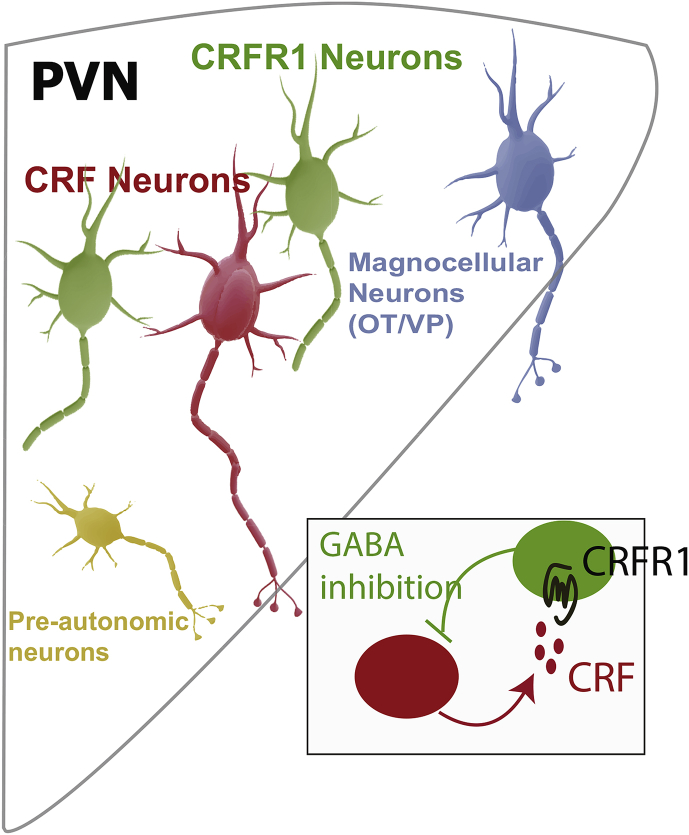
Fig. 2.
Intra-PVN CRF signaling mediates local inhibition of CRF neurons. CRF neurons (red) when active release CRF to initiate HPA axis activity. In certain contexts, CRF is also released within the PVN where it excites PVN CRFR1+ neurons (green) which send recurrent GABAergic synapses back onto CRF neurons thereby providing enhanced inhibitory tone in response to local CRF release (inset). PVN CRFR1 neurons also make bidirectional connections with other cell types in the PVN including magnocellular Oxytocin (OT) and Vasopressin (VP) neurons (blue), and pre-autonomic neurons (yellow), potentially functioning to coordinate signaling between distinct neuroendocrine and autonomic PVN outputs. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
5. CRF release activates CRFR1 neurons
Direct evidence for the existence and function of an intra-PVN CRF-mediated inhibitory microcircuit became possible with the invention of modern techniques that allow for the manipulation of genetically defined cell types. We generated a new CRFR1-Cre BAC transgenic mouse line and used viral delivery of probes to selectively manipulate CRFR1+ PVN neurons to demonstrate the existence of a CRF-mediated, intra-PVN inhibitory feedback microcircuit that regulates HPA axis activity (presented as a poster at the 2018 Neurobiology of Stress conference; Jiang et al., 2018). Evidence for this circuit relies on three independent experimental approaches: slice electrophysiology, neural circuit tracing, and loss-of-function manipulations. In a series of experiments using slice preparations from mice expressing fluorescent protein markers and optogenetic probes, we characterized the connectivity of the circuit. Using laser stimulated ChR2 activation of CRF neurons (10 ms pulses, <1 mW) while recording from GFP + neurons in slice (Crf- IRES-Cre (Huang); CRFR1-GFP transgenic mice injected in the PVN with virus encoding Cre-dependent ChR2), 1–20 Hz stimulation resulted in the cell-autonomous elevation of recorded CRFR1-GFP + neuron activity, that was blocked by the CRFR1 selective antagonist, antalarmin. This demonstrates that CRF neurons release CRF in the PVN that signals to and activates neighboring CRFR1+ neuron, to our knowledge the first published demonstration of local CRF release by PVN CRF neurons (Fig. 2; Jiang et al., 2018). Interestingly, CRFR1 neurons do not increase activity within seconds of CRF neuron activation, but become depolarized and increase action potential firing frequency only after minutes of CRF neuron laser stimulation (Jiang et al., 2018). Furthermore, action potentials in CRFR1 neurons were not phase locked with laser pulses, suggesting that CRF neurons do not signal to CRFR1 neurons via fast synaptic neurotransmission (Jiang et al., 2018). Of the 80 CRFR1 neurons recorded from in the PVN, only one displayed synaptic currents within milliseconds of a laser pulses that would indicate the presence of a monosynaptic connection. Given studies that demonstrate PVN CRF neuron signaling in nearby nuclei via excitatory glutamatergic synapses (Fuzesi et al., 2016), we were surprised that CRF released from CRF neurons is the signal that increases the activity of PVN CRFR1 neurons, in the absence of excitatory synaptic neurotransmission (Jiang et al., 2018). Our interpretation of the current data is that CRF is released (either by axons or dendrites, or both) into the interstitial space of the PVN, close enough to diffuse and engage CRFR1 receptors within seconds to minutes. The nature of the site of cellular CRF release and the optimal optogenetic stimulus that evokes release have yet to be determined (see Remaining question a. below).”
6. CRFR1 neurons make GABAergic synapses onto CRF neurons
CRF dependent synaptic negative feedback on the HPA axis requires that CRFR1 neurons inhibit CRF neurons. In the most straightforward mechanism, this would be achieved by recurrent GABAergic synapses. To demonstrate the presence of this synapse, we optogenetically stimulated PVN CRFR1 neurons while recording from neighboring neurons in slice (Crfr1-cre mice PVN injected with Cre-dependent ChR2 expressing AAV). In this experiment, we did not have the tools to genetically define recorded post-synaptic neurons, so we used their electrophsiological properties to classify each neuron as parvocellular, magnocellular or pre-autonomic (Luther et al., 2002; Luther and Tasker, 2000; Stern, 2001; Tasker and Dudek, 1991). Laser activation of CRFR1 neurons caused GABAergic currents ~2 ms after each laser pulse in individually recorded neighboring neurons. Currents were blocked by the GABAergic antagonist, bicuculline, providing evidence that CRFR1+ PVN neurons make GABAergic synapses with their neighbors (Jiang et al., 2018). Outward (inhibitory) currents in postsynaptic neurons were blocked by Tetrodotoxin (TTX, a voltage-gated sodium channel antagonist that blocks action potentials as well as synaptic release events). TTX blocked synaptic events were rescued by 4-AP (a non-selective voltage-dependent potassium channel antagonist which rescues Chr2 mediated monosynaptic events in the presence of TTX), demonstrating CRFR1 neurons make direct, monosynaptic GABAergic synapses onto neighboring neurons (Jiang et al., 2018). The most common neurons that received laser-evoked GABAergic synapses were parvocellular (~50%), of which, we presume, the majority were CRF neurons (Jiang et al., 2018). However, we observed direct synapses with neighboring magnocellular neurons (~15%) and pre-autonomic neurons (~35%), indicating that CRFR1 neurons make synapses with other PVN cell types (Fig. 2; Jiang et al., 2018). Thus, the hypothesized microcircuit architecture was verified: activated CRF neurons release CRF in the PVN which excites nearby CRFR1 neurons, resulting in increased inhibitory synaptic signaling back onto CRF neurons (Jiang et al., 2018).
7. CRFR1+ PVN neurons are required to limit acute HPA axis activation and promote stress recovery
Demonstration of inhibitory feedback on HPA axis activity via intra-PVN CRF signaling was perhaps the most difficult aspect of the hypothesis to support with experimental evidence. In initial attempts to establish the functional relevance of intra-PVN CRF signaling in HPA axis regulation we mutated Crfr1 selectively in PVN neurons (Sim1-Cre; Crfr1 fl/fl) and assayed stress-induced Cort release. HPA axis phenotypes were identified, however they were present only after chronic stress (chronic social defeat), and not at baseline or during acute stress exposure (Ramot et al., 2017). To reveal the inhibitory function of CRFR1 neurons on HPA axis activity during acute stress, we deleted not only the Crfr1 gene, but ablated the entire population of PVN CRFR1 neurons using Cre-Dependent expression of Diptheria Toxin Receptors (DTR; CRFR1-Cre mice PVN injected with AAV-DIO-DTR-GFP) followed by Diptheria toxin injection. In animals that lacked PVN CRFR1 neurons, we observed exaggerated HPA axis responses to acute stress exposure (Jiang et al., 2018). PVN CRFR1 neuron ablated animals displayed higher peak Cort levels at the 60 min timepoint of a 120 min immobilization stress. Moreover, 2 h after release from immobilization, Cort remained higher in CRFR1 neuron ablated animals, indicating a slower declination in circulating Cort concentration following release from immobilization (Jiang et al. 2018). Thus, CRFR1+ neurons are required to limit HPA axis activation during acute stress exposure, and also function to decrease HPA axis activity during stress recovery. This strongly supports the functional importance of intra-PVN CRF signaling. However, we have yet to determine the precise stress context in which CRF mediated inhibitory synaptic feedback is most relevant. We continue to search for the precise excitability state required to cause intra-PVN CRF release to invoke local inhibition by neighboring CRFR1 neurons.
8. Remaining questions
8.1. What structure releases CRF onto CRFR1 neurons?
Electrophysiology and tracing experiments independently demonstrate connections and signaling between CRF and CRFR1 neurons in the PVN. However, when interpreted together these data present conflicting interpretations, hinting that exciting and atypical features may exist in this circuit. To clarify, in electrophysiologic experiments, optogenetic stimulation of CRF neurons activate surrounding CRFR1 neurons (via CRFR1) in the absence of fast synaptic transmission, demonstrating that CRF is the relevant signaling molecule. However, this data also indicates that fast synapses from CRF neurons onto CRFR1 neurons are extremely rare, or are not functional. In direct contrast to this finding, pseudotyped rabies viral tracing of CRFR1 neurons reveal abundant synaptic projections from CRF to CRFR1 neurons (Jiang et al., 2018). The infectious properties of rabies virus have been extensively characterized; rabies virus only transynaptically infects neurons at sites of synaptic contact, from the post-synaptic to the pre-synaptic neuron (retrograde direction; Ugolini, 1995). Thus rabies virus in CRFR1 neurons infects CRF neurons at synaptic contacts that we do not detect in electrophysiological recordings. To explain this discrepancy, it is tempting to speculate that a synaptic structure allowing transsynaptic rabies infection is present, functioning as a “neuropeptide” synapse that does not release fast acting neurotransmitters. However, extensive examination by many independent groups using electron microscopy have never found clusters of large dense core vesicles containing CRF or other neuropeptides at sites of apposition in the PVN that would indicate the existence of a “neuropeptide synapse” (A Van der Pol, pers. comm). Another possible explanation is that fast synapses between CRF and CRFR1 neurons do exist, but are not activated by the optogenetic stimuli we used, or require additional signals (e.g. norepinephrine) to become active. Preliminary experiments testing this hypothesis have not supported the presence of a “silent” synapse between CRF and CRFR1 neurons. We are eager to find an explanation that solves this puzzle, with the hope that new and perhaps unprecedented facets of synaptic organization in the PVN will be revealed.
8.2. Do CRFR1+ neurons function to integrate PVN activity?
In electrophysiological experiments, we observed other PVN cell types post-synaptic to CRFR1 neurons. Moreover, trans-synaptic tracing experiments indicate that CRFR1 neurons receive projections from most other cell types in the PVN. The largest percentage of PVN neurons that made mononsynpatic projections onto CRFR1 neurons were CRF neurons (~25% of traced PVN neurons). Vasopressin neurons (~20%), and Oxytocin neurons (~10%) were also traced indicative of direct synapses onto PVN CRFR1 neurons. The presence of bi-directional connections with multiple cell types suggests that CRFR1+ PVN neurons signal with PVN neurons that control distinct PVN outputs, potentially relaying information regarding CRF neuron activity status to hormonal axes distinct from the HPA axis, rapidly relaying information regarding stress conditions to potentially impact hormonal release. We have also observed Oxytocin neurons expressing CRFR1 in certain reproductive contexts (N.J. unpublished observations), suggesting direct CRF signaling occurs between PVN resident neuroendocrine axes A clear direction forward in our research is the identification of specific requirements for intra-PVN CRF signaling to coordinate hormonal, autonomic and behavioral output of the PVN in both adaptive and maladaptive contexts.
8.3. Are CRFR1+ PVN neurons a homogenous neural population?
PVN CRFR1 neurons inhibit CRF neuron activity thereby limiting excitability to prevent HPA axis hyperactivity and drive stress recovery (Jiang et al., 2018). However, in experiments characterizing synaptic targets of CRFR1 neurons, we observed a substantial number of projection sites outside of the PVN (Jiang et al., 2018). These include well characterized PVN targets such as the Nucleus of the Solitary Tract (NTS) and Parabrachial Nucleus (PBn), both members of the stress responsive “Central Autonomic System” and well known mediators of autonomic control exerted by the PVN (Saper, 2002). In addition, we observed projections to nuclei that were not known previously to receive PVN projections. One interesting example is an area in the septum/medial forebrain, where dense fields of presynaptic terminals originating from infected PVN CRFR1 neurons clustered in specific regions, labeled by expression of the presynaptic protein synaptophysin fused with RFP. We and other groups have now found that CRF and other PVN neuron subclasses project to forebrain targets where they directly modulate behavior (Hunt et al., 2018). Interestingly, PVN CRFR1 neurons within the PVN and in forebrain structures make GABAergic synapses (Jiang et al., 2018), while PVN CRFR1+ projections in the nucleus of solitary tract (NTS) are exclusively glutamatergic (Jiang et al., 2018), suggesting multiple independent populations of neurons. How additional functions for various sub-populations of PVN CRFR1 neurons relate to the role of CRF in organizing the stress response remains an open question.
9. Concluding remarks
Discovery and proof that CRFR1 bearing neurons exist in the PVN, and play functional roles in the regulation of the HPA axis, integration of PVN activity, and coordinating PVN output has developed as an idea over the course of the last 10 years, when we first observed these neurons in the CRFR1-GFP mouse (Justice et al., 2008). In the course of these studies it has become clear that intra-PVN signaling via CRF has the potential to function in many roles of the PVN. In the following decade, we hope to define some of these functions, including, 1) the potentiation of autonomic signaling by projections of CRFR1 neurons to brainstem targets, 2) the behavioral relevance of newly discovered forebrain projections emanating from both CRFR1 and CRF neurons in the PVN, and 3) integration of CRF/stress signaling with distinct neuroendocrine axes (new findings indicate that the Oxytocin pathway is a unique target of CRF in postpartum females(N.J. unpublished data). Experiments are underway to interrogate each of these functions, with the hope that another decade does not pass before further functional capacities of CRF signaling in the PVN are defined.
Funding
Our work presented at the meeting and described in this minireview was supported primarily by funding from the NIMH R56MH114032 (N.J.), and R01MH112768 (N.J.), and by additional funding sources: R21AA026022, the University of Texas Health Sciences Center at Houston, and a travel award from the Stress Neurobiology Workshop 2018 (Z.J.).
Acknowledgements
We thank the chair, Dr. Matthew Hill and the co-chair, Dr. Jaideep Bains and the many other organizing committee members for hosting the Stress Neurobiology Workshop 2018.
References
- Arriza J.L., Weinberger C., Cerelli G., Glaser T.M., Handelin B.L., Housman D.E., Evans R.M. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987;237:268–275. doi: 10.1126/science.3037703. [DOI] [PubMed] [Google Scholar]
- Cullinan W.E., Helmreich D.L., Watson S.J. Fos expression in forebrain afferents to the hypothalamic paraventricular nucleus following swim stress. J. Comp. Neurol. 1996;368:88–99. doi: 10.1002/(SICI)1096-9861(19960422)368:1<88::AID-CNE6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Daviu N. 2018. Behavior Control of Survival Instincts. [Google Scholar]
- de Kloet E.R. Stress in the brain. Eur. J. Pharmacol. 2000;405:187–198. doi: 10.1016/s0014-2999(00)00552-5. [DOI] [PubMed] [Google Scholar]
- Ferguson A.V., Latchford K.J., Samson W.K. The paraventricular nucleus of the hypothalamus - a potential target for integrative treatment of autonomic dysfunction. Expert Opin. Ther. Targets. 2008;12:717–727. doi: 10.1517/14728222.12.6.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Füzesi T., Daviu N., Wamsteeker Cusulin J.I., Bonin R.P., Bains J.S. Hypothalamic CRH neurons orchestrate complex behaviours after stress. Nat. Commun. 2016;7:11937. doi: 10.1038/ncomms11937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham C.E., Basappa J., Turcan S., Vetter D.E. The cochlear CRF signaling systems and their mechanisms of action in modulating cochlear sensitivity and protection against trauma. Mol. Neurobiol. 2011;44:383–406. doi: 10.1007/s12035-011-8203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger R.L., Grigoriadis D.E., Dallman M.F., Plotsky P.M., Vale W.W., Dautzenberg F.M. International union of pharmacology. XXXVI. Current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol. Rev. 2003;55:21–26. doi: 10.1124/pr.55.1.3. [DOI] [PubMed] [Google Scholar]
- Herman J.P., Cullinan W.E. Neurocircuitry of stress: central control of the hypothalamo–pituitary– adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herman J.P., Tasker J.G. Paraventricular hypothalamic mechanisms of chronic stress adaptation. Front. Endocrinol. 2016 doi: 10.3389/fendo.2016.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J.P., Tasker J.G., Ziegler D.R., Cullinan W.E. Local circuit regulation of paraventricular nucleus stress integration. Pharmacol. Biochem. Behav. 2002 doi: 10.1016/s0091-%203057(01)00681-5. [DOI] [PubMed] [Google Scholar]
- Herman M.A., Contet C., Justice N.J., Vale W., Roberto M. Novel subunit-specific tonic GABA currents and differential effects of ethanol in the central amygdala of CRF receptor-1 reporter mice. J. Neurosci. 2013;33:3284–3298. doi: 10.1523/JNEUROSCI.2490-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huising M.O., van der Meulen T., Vaughan J.M., Matsumoto M., Donaldson C.J., Park H., Billestrup N., Vale W.W. CRFR1 is expressed on pancreatic beta cells, promotes beta cell proliferation, and potentiates insulin secretion in a glucose-dependent manner. Proc. Natl. Acad. Sci. U.S.A. 2010;107:912–917. doi: 10.1073/pnas.0913610107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt A.J., Jr., Dasgupta R., Rajamanickam S., Jiang Z., Beierlein M., Chan C.S., Justice N.J. Paraventricular hypothalamic and amygdalar CRF neurons synapse in the external globus pallidus. Brain Struct. Funct. 2018;223:2685–2698. doi: 10.1007/s00429-018-1652-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z., Rajamanickam S., Justice N.J. Local corticotropin-releasing factor signaling in the hypothalamic paraventricular nucleus. J. Neurosci. 2018;38:1874–1890. doi: 10.1523/JNEUROSCI.1492-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M., de Kloet E.R. Control of neuronal excitability by corticosteroid hormones. Trends. Neurosci. 1992;15:25–30. doi: 10.1016/0166-2236(92)90345-9. [DOI] [PubMed] [Google Scholar]
- Justice N.J., Yuan Z.F., Sawchenko P.E., Vale W. Type 1 corticotropin-releasing factor receptor expression reported in BAC transgenic mice: implications for reconciling ligand-receptor mismatch in the central corticotropin-releasing factor system. J. Comp. Neurol. 2008;511:479–496. doi: 10.1002/cne.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller-Wood M.E., Dallman M.F. Corticosteroid inhibition of ACTH secretion. Endocr. Rev. 1984;5:1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Pearse R.V., 2nd, Lin C.R., Rosenfeld M.G. A sauvagine/corticotropin-releasing factor receptor expressed in heart and skeletal muscle. Proc. Natl. Acad. Sci. U.S.A. 1995;92:1108–1112. doi: 10.1073/pnas.92.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloet E.R. de, de Kloet E.R., Joëls M., Holsboer F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 2005 doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Kovács K.J., Földes A., Sawchenko P.E. Glucocorticoid negative feedback selectively targets vasopressin transcription in parvocellular neurosecretory neurons. J. Neurosci. 2000;20:3843–3852. doi: 10.1523/JNEUROSCI.20-10-03843.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperman Y., Weiss M., Dine J., Staikin K., Golani O., Ramot A., Nahum T., Kühne C., Shemesh Y., Wurst W., Harmelin A., Deussing J.M., Eder M., Chen A. CRFR1 in AgRP neurons modulates sympathetic nervous system Activity to adapt to cold stress and fasting. Cell Metabol. 2016;23:1185–1199. doi: 10.1016/j.cmet.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovenberg T.W., Liaw C.W., Grigoriadis D.E., Clevenger W., Chalmers D.T., De Souza E.B., Oltersdorf T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc. Natl. Acad. Sci. U.S.A. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther J.A., Daftary S.S., Boudaba C., Gould G.C., Halmos K.C., Tasker J.G. Neurosecretory and non-neurosecretory parvocellular neurones of the hypothalamic paraventricular nucleus express distinct electrophysiological properties. J. Neuroendocrinol. 2002;14:929–932. doi: 10.1046/j.1365-2826.2002.00867.x. [DOI] [PubMed] [Google Scholar]
- Luther J.A., Tasker J.G. Voltage-gated currents distinguish parvocellular from magnocellular neurones in the rat hypothalamic paraventricular nucleus. J. Physiol. 2000;523(Pt 1):193–209. doi: 10.1111/j.1469-7793.2000.t01-1-00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason J.W. A re-evaluation of the concept of “non-specificity” in stress theory. J. Psychiatr. Res. 1971;8:323–333. doi: 10.1016/0022-3956(71)90028-8. [DOI] [PubMed] [Google Scholar]
- McClennen S.J., Cortright D.N., Seasholtz A.F. Regulation of pituitary corticotropin-releasing hormone-binding protein messenger ribonucleic acid levels by restraint stress and adrenalectomy. Endocrinology. 1998;139:4435–4441. doi: 10.1210/endo.139.11.6311. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- Mikics E., Halasz J., Toth M., Kruk M.R., Haller J. PsycEXTRA Dataset; 2008. Neural Mechanisms Underlying Hypoarousal- Related Abnormal Aggression in a Laboratory Model. [DOI] [Google Scholar]
- Ostrander M.M., Ulrich-Lai Y.M., Choi D.C., Richtand N.M., Herman J.P. Hypoactivity of the hypothalamo-pituitary-adrenocortical axis during recovery from chronic variable stress. Endocrinology. 2006;147:2008–2017. doi: 10.1210/en.2005-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante C.M., Lightman S.L. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Perrin M., Donaldson C., Chen R., Blount A., Berggren T., Bilezikjian L., Sawchenko P., Vale W. Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Proc. Natl. Acad. Sci. U.S.A. 1995;92:2969–2973. doi: 10.1073/pnas.92.7.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin M.H., Donaldson C.J., Chen R., Lewis K.A., Vale W.W. Cloning and functional expression of a rat brain corticotropin releasing factor (CRF) receptor. Endocrinology. 1993;133:3058–3061. doi: 10.1210/endo.133.6.8243338. [DOI] [PubMed] [Google Scholar]
- Plotsky P.M., Vale W. Hemorrhage-induced secretion of corticotropin-releasing factor-like immunoreactivity into the rat hypophysial portal circulation and its inhibition by glucocorticoids. Endocrinology. 1984;114:164–169. doi: 10.1210/endo-114-1-164. [DOI] [PubMed] [Google Scholar]
- Ramot A., Jiang Z., Tian J.-B., Nahum T., Kuperman Y., Justice N., Chen A. Hypothalamic CRFR1 is essential for HPA axis regulation following chronic stress. Nat. Neurosci. 2017;20:385–388. doi: 10.1038/nn.4491. [DOI] [PubMed] [Google Scholar]
- Raubenheimer P.J., Young E.A., Andrew R., Seckl J.R. The role of corticosterone in human hypothalamic-pituitary-adrenal axis feedback. Clin. Endocrinol. 2006;65:22–26. doi: 10.1111/j.1365-2265.2006.02540.x. [DOI] [PubMed] [Google Scholar]
- Rosinger Z.J., Jacobskind J.S., Bulanchuk N., Malone M., Fico D., Justice N.J., Zuloaga D.G. Characterization and gonadal hormone regulation of a sexually dimorphic corticotropin-releasing factor receptor 1 cell group. J. Comp. Neurol. 2019;527:1056–1069. doi: 10.1002/cne.24588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosinger Z.J., Jacobskind J.S., De Guzman R.M., Justice N.J., Zuloaga D.G. A sexually dimorphic distribution of corticotropin-releasing factor receptor 1 in the paraventricular hypothalamus. Neuroscience. 2019;409:195–203. doi: 10.1016/j.neuroscience.2019.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosinger Z.J., Jacobskind J.S., Park S.G., Justice N.J., Zuloaga D.G. Distribution of corticotropin- releasing factor receptor 1 in the developing mouse forebrain: a novel sex difference revealed in the rostral periventricular hypothalamus. Neuroscience. 2017;361:167–178. doi: 10.1016/j.neuroscience.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper C.B. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu. Rev. Neurosci. 2002;25:433–469. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M., McEwen B.S. Down-regulation of neural corticosterone receptors by corticosterone and dexamethasone. Brain Res. 1985;339:161–165. doi: 10.1016/0006-8993(85)90638-9. [DOI] [PubMed] [Google Scholar]
- Sawchenko P.E. Evidence for a local site of action for glucocorticoids in inhibiting CRF and vasopressin expression in the paraventricular nucleus. Brain Res. 1987;403:213–223. doi: 10.1016/0006-8993(87)90058-8. [DOI] [PubMed] [Google Scholar]
- Selye H. The evolution of the stress concept. Am. J. Cardiol. 1970;26:289–299. doi: 10.1016/0002-9149(70)90796-4. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Vale W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006;8:383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern J.E. Electrophysiological and morphological properties of pre-autonomic neurones in the rat hypothalamic paraventricular nucleus. J. Physiol. 2001;537:161–177. doi: 10.1111/j.1469-7793.2001.0161k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasker J.G., Dudek F.E. Electrophysiological properties of neurones in the region of the paraventricular nucleus in slices of rat hypothalamus. J. Physiol. 1991;434:271–293. doi: 10.1113/jphysiol.1991.sp018469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasker J.G., Herman J.P. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic–pituitary–adrenal axis. Stress. 2011. [DOI] [PMC free article] [PubMed]
- Thrivikraman K.V., Nemeroff C.B., Plotsky P.M. Sensitivity to glucocorticoid-mediated fast- feedback regulation of the hypothalamic–pituitary–adrenal axis is dependent upon stressor specific neurocircuitry. Brain Res. 2000 doi: 10.1016/s0006-8993(00)02405-7. [DOI] [PubMed] [Google Scholar]
- Tsigos C., Chrousos G.P. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Ugolini G. Specificity of rabies virus as a transneuronal tracer of motor networks: transfer from hypoglossal motoneurons to connected second-order and higher order central nervous system cell groups. J. Comp. Neurol. 1995;356:457–480. doi: 10.1002/cne.903560312. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai Y.M., Herman J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale W., Spiess J., Rivier C., Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Wang L.A., Nguyen D.H., Mifflin S.W. Corticotropin-releasing hormone projections from the paraventricular nucleus of the hypothalamus to the nucleus of the solitary tract increases blood pressure. J. Neurophysiol. 2018 doi: 10.1152/jn.00623.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger C., Hollenberg S., Ong E., Harmon J., Brower S., Cidlowski J., Thompson E., Rosenfeld M., Evans R. Identification of human glucocortticoid receptor complementary DNA clones by epitope selection. Science. 1985 doi: 10.1126/science.2581314. [DOI] [PubMed] [Google Scholar]
- Whirledge S., DeFranco D.B. Glucocorticoid signaling in Health and disease: insights from tissue-specific GR knockout mice. Endocrinology. 2018;159:46–64. doi: 10.1210/en.2017-00728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R., LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Young E.A., Kwak S.P., Kottak J. Negative feedback regulation following administration of chronic exogenous corticosterone. J. Neuroendocrinol. 1995;7:37–45. doi: 10.1111/j.1365-2826.1995.tb00665.x. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Wu W., Chen M., Cai F., Fan C., Shen W., Sun W., Hu J. Reward inhibits paraventricular. CRH neurons to relieve stress. Curr. Biol. 2019;29:1243–1251. doi: 10.1016/j.cub.2019.02.048. e4. [DOI] [PubMed] [Google Scholar]