Abstract
During human gastric carcinogenesis, intestinal metaplasia is frequently seen in the atrophic stomach. In mice, a distinct type of metaplasia known as spasmolytic polypeptide-expressing metaplasia (SPEM) is found in several inflammatory and genetically engineered models. Given the diversity of long- and short-term models of mouse SPEM, it remains unclear whether all models have a shared or distinct molecular mechanism. The origin of SPEM in mice is presently under debate. It is postulated that stem or progenitor cells acquire genetic alterations that then supply metaplastic cell clones, whereas the possibility of transdifferentiation or dedifferentiation from mature gastric chief cells has also been suggested. In this study, we report that loss of chief cells was sufficient to induce short-term regenerative SPEM-like lesions that originated from chief cell precursors in the gastric neck region. Furthermore, Lgr5+ mature chief cells failed to contribute to both short- and long-term metaplasia, whereas isthmus stem and progenitor cells efficiently contributed to long-term metaplasia. Interestingly, multiple administrations of high-dose pulsed tamoxifen induced expansion of Lgr5 expression and Lgr5-CreERT recombination within the isthmus progenitors apart from basal chief cells. Thus we conclude that short-term SPEM represents a regenerative process arising from neck progenitors following chief cell loss, whereas true long-term SPEM originates from isthmus progenitors. Mature gastric chief cells may be dispensable for SPEM development.
NEW & NOTEWORTHY Recently, dedifferentiation ability in gastric chief cells during metaplasia development has been proposed. Our findings reveal that lesions that were thought to be acute metaplasia in fact represent normal regeneration supplied from neck lineage and that isthmus stem/progenitors are more responsible for sustained metaplastic changes. Cellular plasticity in gastric chief cells may be more limited than recently highlighted.
Keywords: gastric chief cell, Lgr5, metaplasia, stem cell
INTRODUCTION
Gastric cancer arises in the setting of chronic Helicobacter pylori (H. pylori) infection through a multistep histopathological cascade known as the Correa pathway. This pathway consists of sequential stages of gastritis, atrophy, intestinal metaplasia, dysplasia, and eventually cancer (7, 8). In mouse models, Helicobacter infection leads to a distinct type of metaplasia known as spasmolytic polypeptide-expressing metaplasia (SPEM) (41, 48, 49). Transgenic expression of mutant KrasG12D in the stomach leads to the stable development of metaplasia, characterized by the presence of Trefoil factor 2 (TFF2; formerly spasmolytic polypeptide)-expressing cells in the metaplastic glands (6, 13, 26, 28, 36). SPEM lesions in these models are stable over time and generally irreversible and thus have been termed long-term metaplasia. Drug-induced models of SPEM, in which DMP-777, L-635, or high-dose tamoxifen (HDT) is administered to induce metaplasia for a few days, have also been described (19, 32, 35). All of these drugs induce an acute loss of parietal cells, mimicking temporarily gastric atrophy, although the loss of parietal cells alone is insufficient to induce any type of metaplasia (5). This suggests that the development of metaplasia requires a trigger in addition to parietal cell loss. Metaplastic lesions in these models are reversible in 2–3 wk after discontinuation of drug treatment and have thus been designated as short-term metaplasia. Both short- and long-term metaplasia express the gastric neck cell markers TFF2 and Griffonia simplicifolia lectin II (GSII). In addition, in the short-term models, there are proliferating cells that express both chief and neck cell markers in the metaplastic lesions. However, it remains unclear how such proliferating cells contribute to the development of long-term metaplasia and cancer, given the reversible nature of short-term metaplasia (14, 15).
Despite these diverse mouse models, the mechanisms of gastric metaplasia have yet to be fully elucidated (10), and the cell of origin remains unresolved. Several lines of evidence support the notion that long-lived stem or progenitor cells in the isthmus are the origin of metaplasia in long-term chronic Helicobacter infection-type models (14, 15, 18), including the fact that 1) metaplastic lesions are located below the isthmus in the center of the glands rather than at the very base (49); 2) metaplastic lesions expand in a clonal fashion through gland fission over a long period (12); and 3) metaplastic lesions persist even after the eradication of the bacteria (24, 25). Contrary to this, on the basis of models of short-term drug-induced metaplasia, others have proposed that metaplastic lesions originate from mature chief cells through a process of transdifferentiation (30, 32, 39). This notion is primarily based on the expression of several chief cell markers in metaplasia, as well as on lineage-tracing experiments using Mist1-CreERT mice, which genetically tag mature chief cells. However, Mist1-CreERT has also been found to be a marker for isthmus stem cells (13). Mature basal chief cells expressing Lgr5, found to be a specific marker for corpus chief cells, failed to supply short-term or Helicobacter-induced long-term SPEM cells in Lgr5-EGFP-IRES-CreERT mice, which suggests that Lgr5+ chief cells are not the origin of metaplasia (34). In contrast, a recent report suggested that HDT treatment causes dedifferentiation of Lgr5+ chief cells into long-lived stem-like cells that give rise to long-term metaplasia (26).
Gastric chief cells (also known as zymogenic cells) are cells that stain basophilic on hematoxylin and eosin (H and E) staining because of enriched endoplasmic reticulum in their cytoplasm (37). They are located in the deep portion of the corpus glands and secrete digestive enzymes, such as pepsinogen and lipase. Whereas gastric intrinsic factor (GIF) is expressed in parietal cells in humans, it is expressed in chief cells in rodents (9). Like other mature cell types, chief cells arise from gastric stem cells at the isthmus. However, rather than directly giving rise to chief cells, stem cells first give rise to neck cells just below the isthmus, which gradually migrate down and give rise to intermediate chief cell precursors through a gradual process of differentiation in the deep neck region (4, 21). Thus the transdifferentiation proposed in the chief cell-derived SPEM model appears to be equivalent but the reverse of the normal differentiation process by which chief cells arise from the neck lineage, representing a backward flow of differentiation against normal cellular production from the isthmus and neck region. Nevertheless, the molecular mechanisms of this dedifferentiation, as well as recently reported reserve stem cell, are unclear (26).
Because multiple conclusions have been drawn from different models in this field, we aimed to investigate the detailed cellular kinetics of metaplasia development in multiple mouse models. Surprisingly, we find that loss of chief cells is an essential mechanism for the development of short-term metaplasia, and this metaplasia represents a compensatory regenerative process from chief cell precursors for chief cell loss. In addition, Lgr5+ mature chief cells do not contribute to metaplasia development, whereas HDT pulse treatment induces Lgr5 expression and Cre recombination in the isthmus region that contributes to lineage tracing and metaplasia development.
MATERIALS AND METHODS
Animals.
Animal protocol was reviewed and approved by the Ethics Committee of The University of Tokyo, Institute for Adult Diseases, Asahi Life Foundation, and Columbia University. Mice were bred under specific pathogen-free conditions. Rosa26-lox-stop-lox EYFP mice (R26-EYFP mice), Rosa26-lox-stop-lox tdTomato mice (R26-Tomato mice), Rosa26-lox-stop-lox DTA mice (R26-DTA mice), Lox-stop-lox KrasG12D mice (LSL-KrasG12D mice), Mist1-CreERT mice, K19-CreERT mice, and Lgr5-EGFP-IRES-CreERT mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Lgr5-DTR-EGFP mice were obtained from Genentech (South San Francisco, CA). Cre recombinase was activated by oral administration of tamoxifen (2–6 mg/0.2 ml corn oil, as indicated). For Lgr5+ cell ablation, diphtheria toxin (DT) was administered intraperitoneally at a dose of 1 μg/20-g mouse. At least two mice per time point were analyzed in each experiment. Tissue decolorization and 3D reconstitution were performed as described previously (40).
H. pylori infection model.
The H. pylori PMSS1 were obtained from Anne Muller at the University of Zurich (1). As described previously (16, 17), the H. pylori strains were grown on plates of Brucella broth (Becton Dickinson, Franklin Lakes, NJ) under microaerobic conditions at 37°C for 24 h. Colonies were harvested and grown on Brucella broth under microaerobic conditions at 37°C for 48 h. Mice were inoculated intragastrically with a 0.1 ml bacterial suspension containing ~1 × 108 CFU/ml. Animals were euthanized at 4 mo after infection.
Histological and immunohistochemical analysis.
Sections stained with H and E were used for histological analysis. Alcian blue at pH 2.5 (Sigma-Aldrich, St. Louis, MO) was used according to the manufacturer’s instructions. Immunohistochemical analysis was performed as described previously (23, 41). The antibodies and reagents used in this study included the following: rabbit polyclonal anti-phospho-ERK1/2 (1:100; Cell Signaling Technology, Danvers, MA), rabbit monoclonal anti-Ki67 (1:100; Thermo Fisher Scientific, Waltham, MA), rat monoclonal anti-Ki67 (1:100; Biolegend, San Diego, CA), rabbit polyclonal anti-intrinsic factor (1:2,000; a gift from Dr. D. Alpers, Washington University, St. Louis, MO), rat monoclonal anti-CD44v6 (1:100; AbD Serotec, Oxford, United Kingdom), mouse monoclonal anti-MIST1 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA), lectin GSII (1:2,000; Vector Laboratories, Peterborough, MA), rabbit monoclonal anti-green fluorescent protein (GFP) (1:200; Thermo Fisher Scientific), chicken monoclonal anti-GFP (1:200; Abcam, Cambridge, MA), and rabbit or goat monoclonal anti-red fluorescent protein (1:200; Rockland Immunochemicals, Limerick, PA). In situ hybridization was performed on paraffin-embedded sections using RNAscope 2.5 High-Definition Brown Assay (Advanced Cell Diagnostics, Newark, CA) according to the manufacturer’s instructions. For the quantification of immunohistochemical staining, representative images were captured at high magnification (×200); the number and position of the cell of interest were then recorded in at least 20 glands for each group. Metaplasia score in Helicobacter infection model is quantified as described previously (41).
RESULTS
Defining mature chief cells in the mouse stomach.
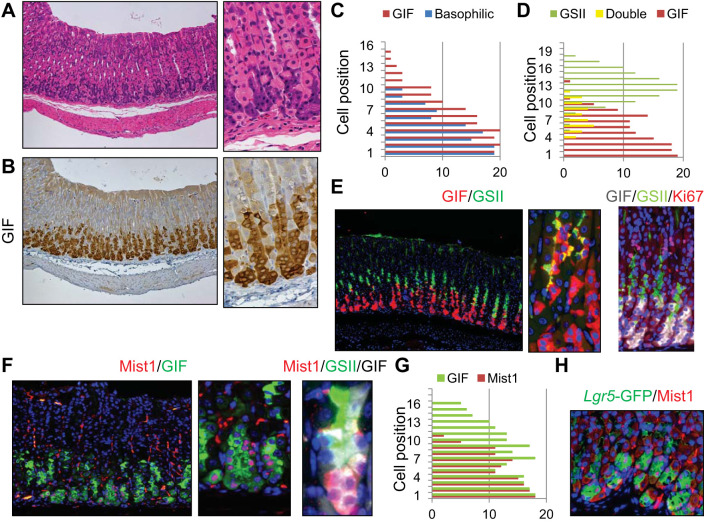
Gastric chief cells are often recognized on routine histological sections as basophilic cells that stain purple on H and E. Such cells were observed in well-oriented mouse gastric glands at positions +1 to +10 (Fig. 1, A and C). As expected, these cells strongly expressed GIF. However, detailed quantification revealed that the region encompassed by GIF+ staining was wider than the H and E-defined chief cell region and included cells at additional superior positions (e.g., +15) whose appearance was similar to the neck cell structure (Fig. 1, B and C). Costaining with the neck cell marker GSII revealed that nonbasophilic GIF+ cells seemed to represent GIF+GSII+ intermediate chief cell precursors, as previously reported (50) (Fig. 1, D and E). There was an average of three GIF+GSII+ cells per gland, consisting of ~15% of the total GIF+ cells. GIF+ cells, including GIF+GSII+ prechief cells, were mostly nonproliferative (Fig. 1E). Mist1 staining revealed that Mist1 protein expression was confined to GIF single positive cells in wild-type mice, whereas GIF+GSII+ prechief cells were mostly negative for Mist1 protein (Fig. 1, F and G). The recently described chief cell marker Lgr5 was efficiently detected as GFP expression in Lgr5-DTR-GFP mice (45). In these mice, GFP expression almost completely overlapped with Mist1 protein expression (Fig. 1H). Thus mature chief cells should be defined as Mist1 protein-expressing basophilic cells near the glandular base that are not simply characterized by GIF positivity because the GIF+ population also includes GIF+GSII+ chief cell precursors.
Fig. 1.
Defining mature chief cells in the mouse stomach. A–C: hematoxylin and eosin (H and E) (A) and gastric intrinsic factor (GIF) (B) staining of wild-type mouse gastric corpus. Sequential sections were stained with the same region. Basophilic cell position on H and E-stained and GIF+ cells are quantified in C. D and E: simultaneous staining for GIF (red) and Griffonia simplicifolia lectin II (GSII) (green) of wild-type mouse corpus (E, left and middle) and cell position quantification of GSII+, GIF+GSII+, and GIF+ cells (D). E, right is triple immunofluorescence for GIF (gray), GSII (green), and Ki67 (red). F, left and middle: immunofluorescence for Mist1 (red) and GIF (green) of wild-type mouse corpus. F, right: immunofluorescence of Mist1 (red), GSII (green), and GIF (gray). G: cell position quantification of GIF+ and Mist1+ cells. H: green fluorescent protein (GFP) (green) and Mist1 (red) staining of Lgr5-DTR-EGFP mice.
Loss of chief cells induces reversible short-term regenerative metaplasia.
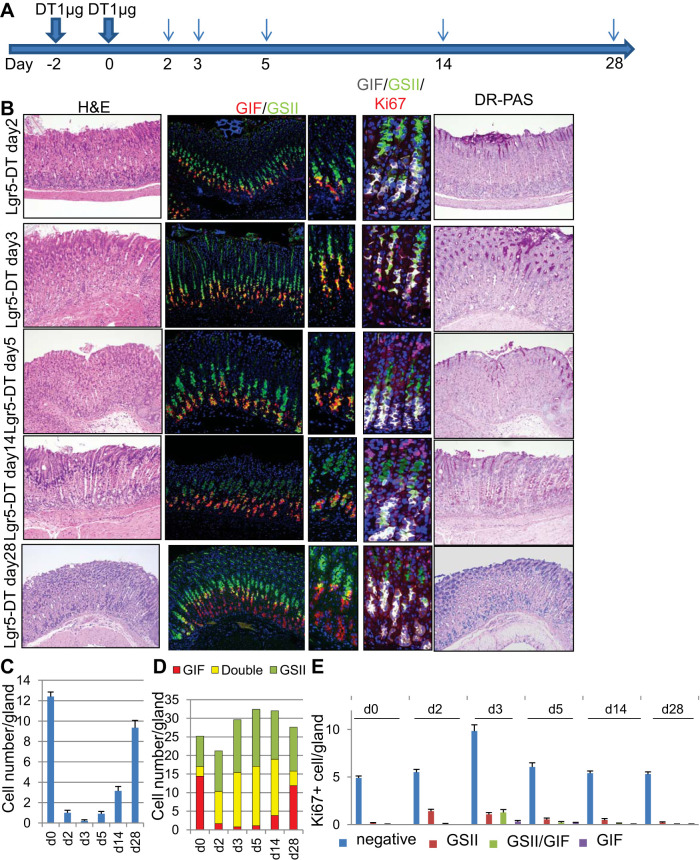
It was recently suggested that gastric chief cells act as a reserve stem cell population and contribute to mucosal homeostasis (26, 43). To study the role of chief cells in epithelial homeostasis, we ablated Lgr5+ mature chief cells by giving two doses of 1 μg DT to Lgr5-DTR-GFP mice (Fig. 2A). As expected, H and E staining revealed almost complete basophilic chief cell ablation 2–3 days after DT administration (Fig. 2, B and C). Loss of mature chief cells was sustained for 5–7 days, and infiltration of the basal glandular region by inflammatory cells was observed at these time points. After 10 days, chief cells were gradually regenerated, with almost complete repair by 1 mo. It is interesting that, at around 14 days after DT administration, we observed less basophilic and more eosinophilic chief-like cells near the base of the glands (Fig. 2B). Diastase-resistant Periodic acid Schiff (DR-PAS) staining revealed that both these lesions and hyperplastic neck cells stained red, similar to previous reports of short-term SPEM lesions induced by DMP-777 (11, 35) (Fig. 2B). Throughout the time course following DT ablation of Lgr5+ cells, there was no change in the numbers of parietal or tuft cells in the corpus as well as gastrin-expressing G cells in the antrum; thus these cells likely did not affect the development of the SPEM-like lesions (not shown). On immunostaining, DT ablation almost completely diminished GIF+ mature chief cells within 2–3 days, whereas the number of GIF+GSII+ chief cell precursors increased, likely to compensate for chief cell loss (Fig. 2, B and D). An increase in GSII+ DR-PAS-positive chief cell precursors and neck cells was maintained for 2 wk, likely representing the expansion and regeneration of the chief cell population from these GIF+GSII+ SPEM-like lesions. In fact, increased cell proliferation shown by Ki67 staining was observed primarily in isthmus progenitors and later in GSII+ neck cells and GIF+GSII+ chief cell precursors but was rarely observed in GIF+ chief cells at any of the early time points (days 2–5; Fig. 2, B and E). The majority of proliferation (>90%) was restricted in GIF−GSII− isthmus progenitors, and Ki67 positivity in the neck-chief region was relatively rare. It is also notable that GIF+GSII+ cells quickly ceased proliferation after day 5; thus GIF+GSII+ chief cell precursors have only short-term proliferative ability (Fig. 2E).
Fig. 2.
Loss of chief cells induces regenerative metaplasia. A: protocol of diphtheria toxin (DT) administration. B: hematoxylin and eosin (H and E), gastric intrinsic factor (GIF) (red), and Griffonia simplicifolia lectin II (GSII) (green), GIF (gray)/GSII (green)/Ki67 (red), and diastase-resistant Periodic acid Schiff (DR-PAS) staining of Lgr5-DTR mouse corpus after DT treatment at the indicated time points. C: average numbers of basophilic chief cells on H and E staining per gland at the indicated time points after Lgr5 ablation. D: numbers of GSII+, GIF+GSII+, and GIF+ cells per gland at the indicated time points after Lgr5 ablation. E: average numbers of Ki67+ cells in GSII+, GIF+GSII+, GIF+, and double-negative cells per gland at the indicated time points after Lgr5 ablation.
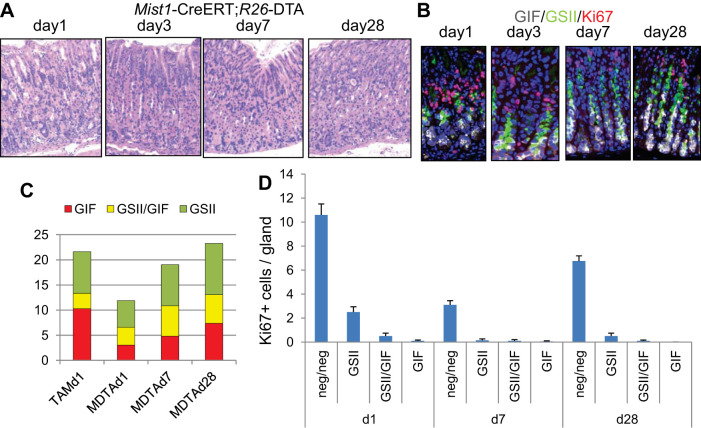
To confirm these findings, we analyzed another model of chief cell ablation using Mist1-CreERT; R26-DTA mice. After the administration of tamoxifen, Mist1-expressing cells begin to express DT, leading to immediate apoptosis. When a low dose (2 mg) of tamoxifen was given to these mice, Mist1-expressing GIF+ chief cells were quickly ablated by day 3 (Fig. 3, A–C). Loss of chief cells was maintained for >1 wk, with a decrease in proliferation in all cell types, including isthmus progenitors (Fig. 3, A–D). This is likely because Mist1-expressing isthmus stem cells were also ablated in these mice (13), and thus regeneration was delayed compared with the Lgr5-DTR ablation model. Nonetheless, chief cells and gland structure reverted to normal by day 28 with the appearance of GIF+GSII+ SPEM-like lesions (Fig. 3, A–C). Taken together, these results suggest that the loss of chief cells is sufficient to induce the short-term expansion of GIF+GSII+ SPEM-like lesions, which indeed suggests that the process of chief cell regeneration derives from GIF+GSII+ normal neck progenitors.
Fig. 3.
Regenerative metaplasia in Mist1-CreERT; R26-DTA mice. A and B: hematoxylin and eosin (H and E) (A) and gastric intrinsic factor (GIF) (gray)/Griffonia simplicifolia lectin II (GSII) (green)/Ki67 (red; B) staining of Mist1-CreERT; R26-DTA mouse corpus at the indicated time points after the administration of 2 mg tamoxifen. C: numbers of GSII+, GIF+GSII+, and GIF+ cells per gland in Mist1-CreERT; R26-DTA mice at the indicated time points after the administration of tamoxifen. D: average numbers of Ki67+ cells in GSII+, GIF+GSII+, GIF+, and double-negative cells per gland in Mist1-CreERT; R26-DTA mice.
Lgr5+ chief cells are dispensable for the development of metaplasia.
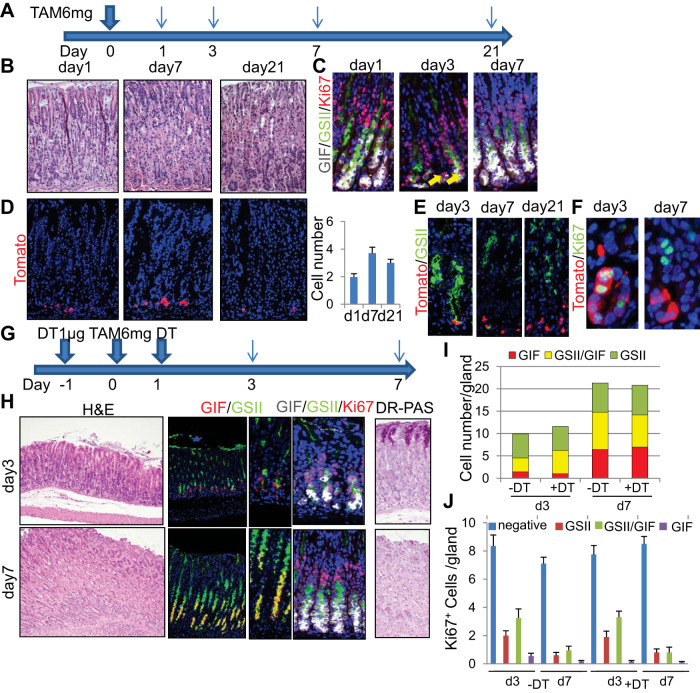
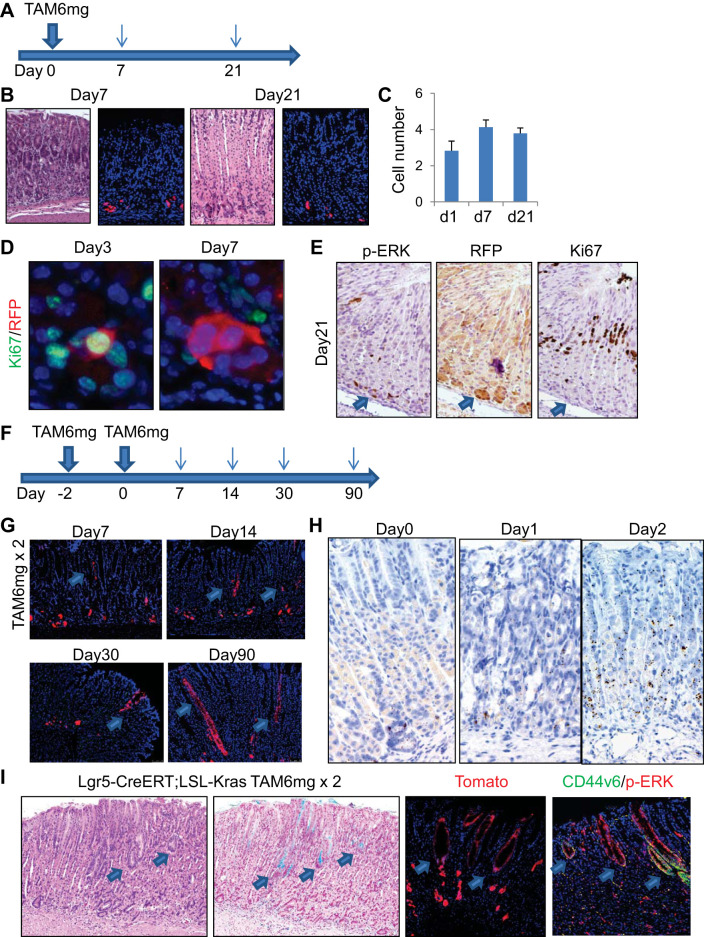
A prior study reported that Lgr5+ chief cells do not give rise to metaplasia in DMP-777, L-635, and Helicobacter infection models in Lgr5-EGFP-IRES-CreERT mice (34). However, on the basis of a separate Lgr5-2A-CreERT mouse line, it was more recently suggested that Lgr5+ chief cells can dedifferentiate to long-lived multipotent stem-like cells and give rise to metaplasia after HDT-induced injury (26). We performed an equivalent HDT protocol in Lgr5-EGFP-IRES-CreERT mice (Fig. 4A). After the administration of 6 mg tamoxifen, the gastric epithelium was rapidly injured with a massive loss of parietal cells (Fig. 4B). As reported previously, rare Ki67+ proliferating cells could be observed near the basal region 2–3 days after HDT treatment (19) (Fig. 4C). By this time point, GIF+GSII+ chief cell precursors and GSII+ neck cells appeared to expand and migrate down to the base, resulting in more widely disseminated proliferation (Fig. 4, C, I, and J; –DT group), whereas GIF+ mature chief cell decreased dramatically. Proliferation in the isthmus region was greatly increased compared with the normal state (Fig. 4J). On day 7 after HDT treatment, proliferation of the basal cells mostly ceased, whereas the number of GSII+GIF+ precursors remained greater than on day 3 (Fig. 4, C, I, and J). These changes in marker expression including GIF, GSII, and Ki67 appear to be quite similar to the chief cell ablation models shown in Figs. 2 and 3, which suggests that HDT injury may cause substantial chief cell loss, in addition to parietal cell injury, thus leading to subsequent regeneration. When Lgr5-EGFP-IRES-CreERT mice were crossed with R26-TdTomato mice and treated with the same protocol, TdTomato expression was induced in Lgr5+ basal chief cells at 24 h, in a mosaic fashion, as reported previously (2, 34) (Fig. 4D). During regeneration after HDT treatment, TdTomato+ (Lgr5+) cells were slightly increased in number on day 7 but remained stable in number and location at the base throughout the remainder of the time course. They did not further expand or lineage trace the corpus glands. After the administration of tamoxifen, a subset of Tomato+ cells transiently expressed GSII and Ki67, which have been thought to be surrogate markers of dedifferentiation into proliferating SPEM or stem-like cells, but no GSII+ or Ki67+ cells were traced by Tomato clones after day 7 (Fig. 4, E and F). This suggests that Lgr5+ cells in these mice are not a major source of epithelial regeneration in the HDT model regardless of their temporal expression of GSII and Ki67.
Fig. 4.
Lgr5+ chief cells are dispensable for the development of metaplasia. A: high-dose tamoxifen (HDT) administration protocol. B and C: hematoxylin and eosin (H and E) (B) and gastric intrinsic factor (GIF) (gray)/Griffonia simplicifolia lectin II (GSII) (green)/Ki67 (red; C) staining of mouse corpus at the indicated time points after HDT administration. Arrows indicate Ki67+ cells at the base. D: TdTomato expression (left) and numbers of TdTomato+ cells (right) in Lgr5-EGFP-IRES-CreERT; R26-TdTomato mouse corpus. E and F: GSII (E, green) and Ki67 (F, green) staining of Lgr5-EGFP-IRES-CreERT; R26-TdTomato mouse corpus after HDT treatment. G: HDT + diphtheria toxin (DT) administration protocol. H: H and E (left), GIF (red)/GSII (green; middle, left), GIF (gray)/GSII (green)/Ki67 (red; middle, right), and diastase-resistant Periodic acid Schiff (DR-PAS) (right) staining of HDT + DT-treated Lgr5-DTR mouse corpus at the indicated time points. I: average numbers of GSII+, GIF+GSII+, and GIF+ cells per gland at the indicated time points after HDT treatment with or without Lgr5 ablation. J: average numbers of Ki67+ cells in GSII+, GIF+GSII+, GIF+, and double-negative cells per gland at the indicated time points after HDT treatment with or without Lgr5 ablation.
To further corroborate the contribution of Lgr5+ cells to regeneration after HDT-induced injury, we treated Lgr5-DTR mice with HDT in the setting of chief cell ablation (Fig. 4G). After the administration of two doses of 1 μg DT and one dose of 6 mg tamoxifen, parietal cells and GIF+ chief cells decreased dramatically by day 3. Indeed, there were more GSII+GIF+ cells in mice treated with DT + HDT at day 3 than in mice treated with HDT alone, suggesting that more complete chief cell ablation with DT triggered a more robust regenerative response (Fig. 4, H and I). Proliferation was increased in isthmus progenitors as well as in the neck lineage in both groups (Fig. 4, H and J). Seven days after HDT treatment, both parietal cells and chief cells were regenerated, but the newly regenerated chief cells were positive for GSII and DR-PAS, similar to chief cell ablation models (Fig. 4H). These GIF+GSII+ cells were nonproliferative at this time point (Fig. 4, H and J). Overall, the cellular kinetics and marker expression were quite comparable between the DT + HDT group and the HDT alone group, which again suggests that Lgr5+ chief cells do not contribute to acute SPEM development and tissue regeneration.
Given that acute reversible SPEM may represent the expansion and differentiation of chief cell precursors rather than a true metaplasia, we examined the effects of Lgr5-DT ablation in a model of H. pylori–induced chronic metaplasia. DT was administered two times before H. pylori infection, and then once a week after H. pylori infection (Fig. 5A). After 4 mo, H. pylori-infected mouse stomachs developed a mucous-rich metaplasia positive for Alcian blue staining and GIF/GSII double immunofluorescence (Fig. 5B). It is interesting that similar metaplastic lesions were observed in the Lgr5-ablated, H. pylori-infected stomach (Fig. 5, B and C). Taken together, these data suggest that Lgr5+ chief cells are dispensable for the development of both short-term and long-term metaplasia.
Fig. 5.
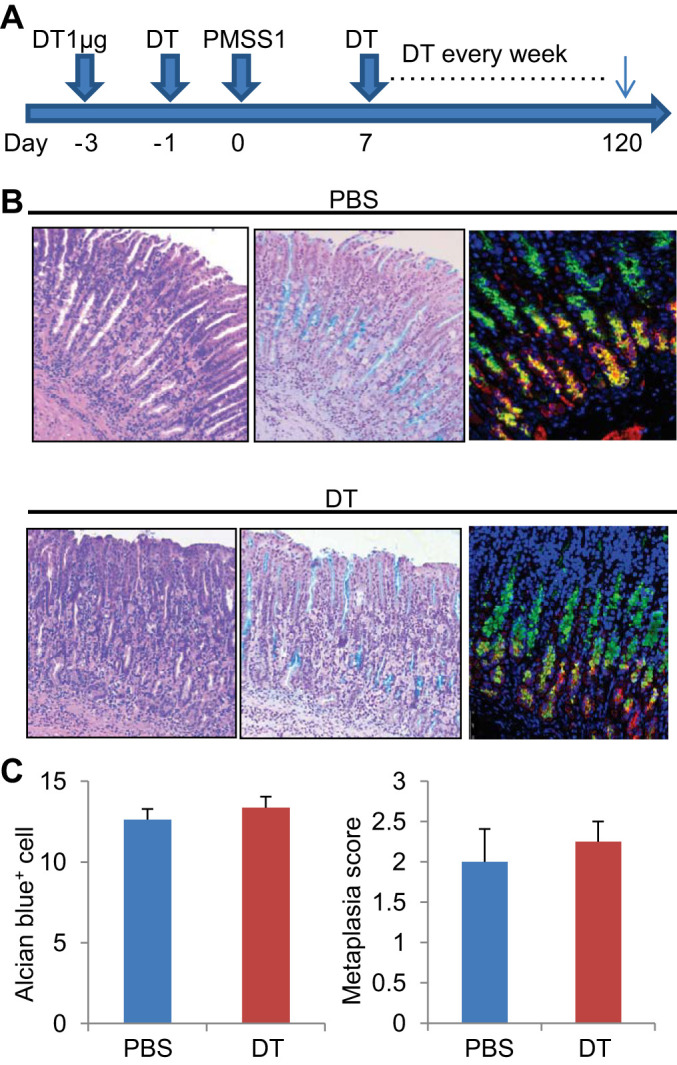
Lgr5+ chief cells are dispensable for Helicobacter-induced metaplasia. A: protocol for PMSS1 infection and diphtheria toxin (DT) ablation. B: Helicobacter pylori infection-induced metaplastic glands shown by hematoxylin and eosin (H and E), Alcian blue, and gastric intrinsic factor (GIF) (red)/Griffonia simplicifolia lectin II (GSII) (green) staining with (bottom) or without (top) Lgr5 ablation. C: average numbers of Alcian blue-stained metaplastic cells per gland and metaplastic score in Helicobacter pylori-infected Lgr5-DTR mouse corpus with or without Lgr5-DT ablation. Mice were analyzed 4 mo after Helicobacter pylori infection.
Chief cells are not responsible for Kras-induced metaplasia.
HDT treatment causes long-term metaplasia in a recently established Lgr5-2A-CreERT mouse crossed with LSL-KrasG12D (26). We investigated whether HDT treatment causes such metaplasia in Lgr5-EGFP-IRES-CreERT; LSL-KrasG12D; R26-TdTomato mice (Fig. 6A). Unlike the Lgr5-2A-CreERT line (26), the Lgr5-EGFP-IRES-CreERT mice failed to generate Kras-induced metaplasia even after HDT (6 mg tamoxifen) treatment, showing no expansion of the Kras-activated, Tomato+ Lgr5 lineage (Fig. 6, B and C). Recombined Tomato+ cells in this model showed some proliferation at early time points; however, these cells later become nonproliferative despite the continued presence of Tomato and phosphorylated ERK expression (downstream of activated Kras; Fig. 6, D and E), suggesting that transient proliferation and Kras activation in Lgr5+ chief cells are not sufficient to account for Kras-induced metaplasia.
Fig. 6.
Chief cells are not responsible for Kras-induced metaplasia. A: protocol. B: hematoxylin and eosin (H and E) staining and TdTomato expression in high-dose tamoxifen (HDT)-treated Lgr5-EGFP-IRES-CreERT; R26-TdTomato mouse corpus. C: average numbers of TdTomato+ cells per gland at the indicated time points. D: Ki67 (green)/red fluorescent protein (RFP) (red) staining of HDT-treated Lgr5-EGFP-IRES-CreERT; R26-TdTomato mouse corpus. E: phosphorylated ERK (p-ERK, left), RFP (middle), and Ki67 staining on Lgr5-EGFP-IRES-CreERT; R26-TdTomato mouse corpus 28 days after HDT treatment. Sequential sections were used for staining, with the same region shown each time. F: protocol for tamoxifen pulse treatment. G: Lgr5-EGFP-IRES-CreERT; R26-TdTomato lineage tracing after tamoxifen pulse treatment. Arrows indicate isthmus-derived clones. H: in situ hybridization for Lgr5 using RNA scope with or without HDT treatment. I: H and E, Alcian blue, TdTomato, and CD44v6 (green)/p-ERK (red) staining on Lgr5-EGFP-IRES-CreERT; LSL-KrasG12D; R26-TdTomato mouse corpus 28 days after the tamoxifen pulse protocol. Arrows indicate isthmus-derived metaplastic clusters.
To elucidate whether any Lgr5+ cells can expand and lineage trace in Lgr5-EGFP-IRES-CreERT mouse corpus, we treated Lgr5-EGFP-IRES-CreERT; R26-TdTomato mice with two doses of 6 mg tamoxifen pulse (Fig. 6F). Even in this setting, most recombined Lgr5+ cells did not show lineage tracing from the chief cell region. However, we were surprised to observe rare Tomato+ cells near the upper isthmus region by 7 days after the last dose of HDT; these cells were a population distinct from the basal chief cells (Fig. 6G). The isthmus Tomato+ cells located at this higher position persisted for at least 3 mo, with a gradual expansion and lineage tracing of the entire gland (Fig. 6G). In contrast, there was a complete absence of upward lineage tracing from basal Tomato+ chief cells. To test whether Lgr5 expression exists outside of the basal chief cell region in the high-dose tamoxifen pulse regimen, we performed in situ hybridization. Before HDT treatment, Lgr5 mRNA expression was restricted to basal chief cells, as expected (Fig. 6H). However, 24 h after treatment with 6 mg tamoxifen, Lgr5 expression even decreased at the glandular base, consistent with a loss of Lgr5+ chief cells attributable to HDT-induced acute injury. Nevertheless, Lgr5 expression increased dramatically and expanded widely 48 h after treatment, reaching the upper isthmus region (Fig. 6H). Thus additional tamoxifen treatment at this point may have resulted in recombination in a broader range of cells, including isthmus progenitors, in the tamoxifen pulse protocol. The same tamoxifen pulse protocol induced mutant Kras recombination in Lgr5-EGFP-IRES-CreERT; LSL-KrasG12D; R26-TdTomato mice, which led to the development of metaplasia from the isthmus region, with expression of metaplasia markers such as Alcian blue and CD44v6 (41) (Fig. 6I). Therefore, our data suggest that induction of Kras activation in Lgr5+ chief cells does not generate metaplasia, whereas HDT pulse treatment induces Lgr5 expression in isthmus progenitors that may contribute to isthmus-derived metaplasia development.
Isthmus progenitors are responsible for Kras-induced metaplasia.
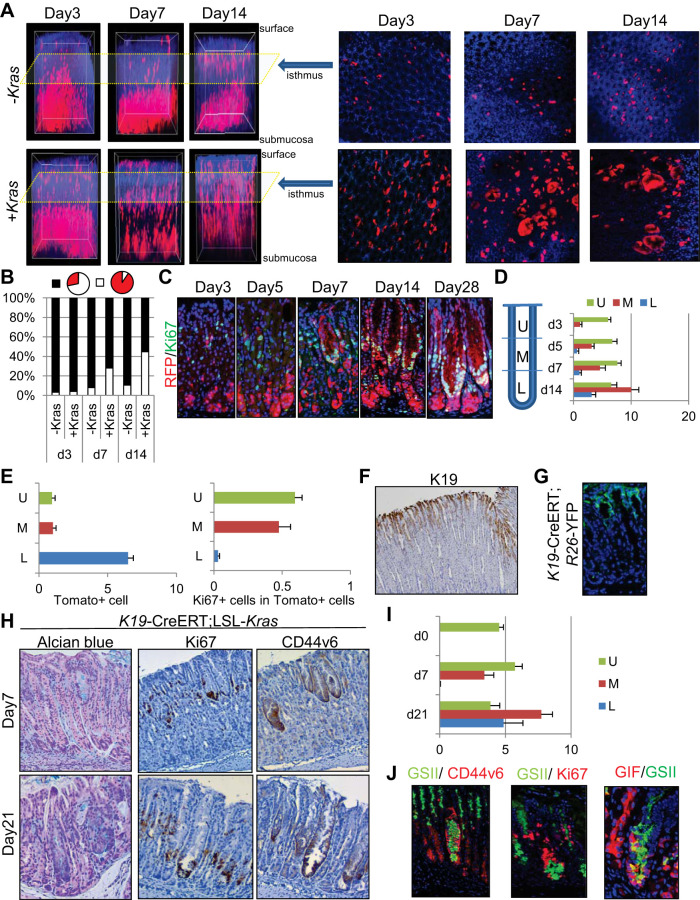
We previously reported that Mist1 is expressed in both chief cells and isthmus stem cells and that Mist1 isthmus cells efficiently generate long-term metaplasia when mutant Kras is induced (13). Given that Mist1+ isthmus stem cells are much rarer than basal chief cells, detection and visualization of isthmus Mist1+ cells is difficult in standard 5-μm sections. Therefore, we performed full layer scanning of the Mist1-CreERT; R26-TdTomato mouse stomach after tissue decolorization and 3D reconstitution (40). We observed Tomato+ isthmus stem cells in the superior part of the glands, apart and distinct from basal chief cells, shortly after the induction with tamoxifen (Fig. 7A). Most Tomato+ cells resided as single cells or doublets for a period of 2 wk in cross-sectional images (Fig. 7A, right), while while 3D reconstructed images (Fig. 7A, left) revealed gradually expanded longitudinal red stripes toward the top and base of glands (Fig. 7, A and B). Thus Mist1+ isthmus stem cells appeared to first supply daughter cells longitudinally, which later expand toward cross-sectional direction. In contrast, when the Kras mutation was induced in Mist1+ cells, Tomato-expressing Mist1+ isthmus cells divided and expanded more rapidly both longitudinally and cross sectionally (Fig. 7, A and B). Rapid expansion of Tomato+ cells from the isthmus was observed even 3 days after induction, and Kras-mutated isthmus Tomato+ clones migrated downward toward the basal region, while also supplying red hyperplastic pit cell clones upward toward the mucosal surface around days 7–14 (Fig. 7A). On cross-sectional analysis of the isthmus, we observed a rapid increase in monoclonally traced glands in Kras-mutated mice, which suggests a clonal increase in Kras-mutated Mist1+ cells within the isthmus, as reported previously in intestinal crypts (47). Furthermore, lateral expansion of monoclonal glands via crypt fission at the isthmus level was seen in Kras-mutated mice 14 days after induction with tamoxifen (Fig. 7, A and B), with Tomato+ cells occupying a much wider area than in control mice. Proliferation in Kras-mutated mice was confined to the upper middle part of the glands at day 3, but the proliferative zone then expanded and migrated downward over the remainder of the time course (Fig. 7, C and D). Initial recombination was much greater in the basal region than in the isthmus region, which suggests at least a 20-fold greater proliferative ability of isthmus cells compared with chief cells in response to the Kras mutation (Fig. 7E).
Fig. 7.
Isthmus progenitors are responsible for Kras-induced metaplasia. A: 3D reconstituted (left) and cross-sectional isthmal (right) images of Mist1-CreERT; R26-TdTomato (top) and Mist1-CreERT; LSL-KrasG12D; R26-TdTomato mice at the indicated time points after treatment with 3 mg tamoxifen. B: proportion of glands in which TdTomato+ cells are >50% (white) or <50% (black) at the isthmus level in cross-sectional analysis. C: red fluorescent protein (RFP) (red)/Ki67 (green) staining on Mist1-CreERT; LSL-KrasG12D; R26-TdTomato mouse corpus after treatment with 3 mg tamoxifen. D: average numbers of Ki67+ cells per gland in the upper (U), middle (M), and lower (L) portion of Mist1-CreERT; LSL-KrasG12D; R26-TdTomato mouse corpus glands at the indicated time points. E, left: average numbers of TdTomato+ cells per gland at each portion of Mist1-CreERT; LSL-KrasG12D; R26-TdTomato corpus glands 3 days after treatment with 3 mg tamoxifen. E, right: average numbers of Ki67+ cells in TdTomato+ cells at each portion of Mist1-CreERT; LSL-KrasG12D; R26-TdTomato corpus glands 7 days after tamoxifen treatment. F: K19 immunostaining of mouse corpus. G: green fluorescent protein (GFP) staining (green) on K19-CreERT; R26-YFP mouse corpus 1 day after the administration of 3 mg tamoxifen. H: Alcian blue, Ki67, and CD44v6 staining of K19-CreERT; R26-YFP mouse corpus 7 and 21 days after treatment with 3 mg tamoxifen. I: average numbers of Ki67+ cells per gland in each portion of K19-CreERT; LSL-KrasG12D mouse corpus glands. J, left: GSII (green)/CD44v6 (red). J, middle: GSII (green)/Ki67 (red). J, right: gastric intrinsic factor (GIF) (red)/GSII (green) staining of K19-CreERT; LSL-KrasG12D mouse corpus 21 days after tamoxifen treatment.
We next used a K19-CreERT knockin mouse (31) to test whether cells residing in the upper sections of the glands, but not at the base, can give rise to Kras-induced metaplasia. K19 was expressed specifically in the surface pit cells in the isthmus region, and recombination in K19-CreERT occurred at the same site (Fig. 7, F and G). In K19-CreERT; LSL-KrasG12D mice, proliferating cell clusters that were positive for Alcian blue and CD44v6 staining first appeared in the upper part of the glands and then migrated downward, reaching the glandular base within 3 wk (Fig. 7, H and I). It is important to note that, when Kras-induced cell clusters reached the gland base, they expressed the neck cell marker GSII and chief cell marker GIF on immunostaining (Fig. 7J). K19+ cells above the isthmus normally did not express these markers (not shown). Therefore, isthmus progenitors, rather than basal chief cells, are the major source of Kras-induced metaplasia. These cells rapidly expand and migrate down to the gland base and occupy the chief cell region while expressing neck and chief cell markers.
Distinct marker expression in acute and chronic metaplasia.
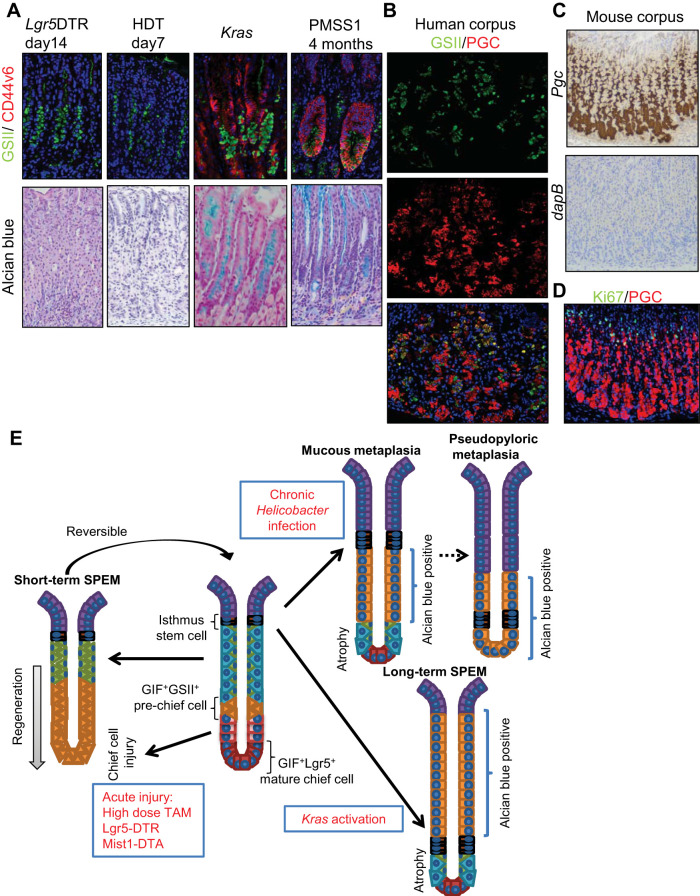
Finally, given the complexity and diversity of available metaplasia models, we compared the expression of metaplasia markers in short- and long-term models. Markers that are normally expressed in mucous neck cells, including GSII and DR-PAS+ mucin, were expressed in both short- and long-term SPEM lesions (Fig. 8A). However, CD44v6 and Alcian blue+ mucin, which are not expressed in normal neck cells, were positive in long-term SPEM lesions in Kras-activated or Helicobacter-infected mice but not in the short-term lesions seen in Lgr5-DTR ablation or HDT models. Thus these lesions appear to be molecularly distinct despite sharing several expression markers. Recently, it was reported that SPEM cells, which express GSII and pepsinogen C (PGC), can be found in human gastric cancer specimens (39). Indeed, we confirmed abundant GSII+PGC+ double-positive cells at the transitional zone between neck cells and chief cells, even in normal human corpus glands, suggesting that these cells are chief cell precursors rather than true SPEM (Fig. 8B). It should be noted that PGC expression in mice is quite broader and reaches the isthmus in normal corpus, both at mRNA and protein levels (Fig. 8, C and D), indicating that PGC-targeting approach would not be useful when arguing chief cell-derived origin in mice.
Fig. 8.
Distinct marker expression in acute and chronic metaplasia. A: Griffonia simplicifolia lectin II (GSII) (green)/CD44v6 (red; top) and Alcian blue (bottom) staining of Lgr5-DTR mice 14 days after diphtheria toxin (DT) treatment, wild-type mice 7 days after high-dose tamoxifen (HDT) treatment, Mist1-CreERT; LSL-KrasG12D mice 14 days after tamoxifen treatment, and wild-type mice 4 mo after PMSS1 Helicobacter pylori strain infection. B: pepsinogen C (PGC)/GSII staining in normal human corpus. C: in situ hybridization for Pgc and dapB (negative control) in mouse corpus. D: Ki67 (green)/PGC (red) staining in mouse corpus. E: schematic model of metaplasia development in the mouse corpus gland. SPEM, spasmolytic polypeptide-expressing metaplasia.
DISCUSSION
In the hierarchical model, a limited stem cell population is capable of giving rise to both differentiated cell types and cancer (3, 27). Nevertheless, recent studies have suggested a much greater degree of cellular plasticity, whereby mature cell types can undergo dedifferentiation and thus function as reserve progenitors following the loss of stem cells by injury (20, 26, 43, 44, 46, 51–53). In the stomach, chief cells have been suggested to be reserve stem cells, as well as a source of metaplastic cells (26, 33). However, our present findings in conjunction with previous work (13) strongly suggest a predominant role for chief cell precursors in the neck region in short-term gastric regeneration and for isthmus stem or progenitor cells in long-term metaplasia development.
Work from other groups, as well as our own analysis, has demonstrated that a subset of chief cells expressing Lgr5 or GIF have transient proliferative potential, as demonstrated by Ki67 immunostaining shortly after epithelial injury (26, 33). This proliferation does not extend the life of these cells, which quickly return to a quiescent state and fail to show lineage tracing. This transient proliferation might contribute in a small way to local regeneration; however, proliferation is a characteristic of short-lived progenitors and thus does not necessarily reflect stemness or multipotentiality. Demonstrating that such proliferating cells give rise to entire glands, in the manner of a tissue-restricted stem cell, would require detailed genetic lineage-tracing observation. Our detailed quantification at various time points indicated that isthmus progenitors, as well as intermediate chief cell precursors, are the major suppliers of regenerated cells, rather than mature basal chief cells.
A recent study using Lgr5-2A-CreERT mice concluded that HDT treatment interconverts Lgr5+ chief cells into multipotent, long-lived stem cells that can be a source of Kras-induced metaplasia. When we treated a distinct Lgr5-tagged CreERT mouse line (Lgr5-EGFP-IRES-CreERT) with the same HDT protocol, we failed to observe similar interconversion from Lgr5+ chief cells. In fact, a previous study using the same Lgr5-EGFP-IRES-CreERT mice also showed that Lgr5+ chief cells do not contribute to normal lineage tracing or metaplasia development (34). One possibility for this discrepancy is the difference between mouse lines, as the original knock-in (Lgr5-EGFP-IRES-CreERT) mouse line is haploinsufficient for the Lgr5 gene, shows decreased recombination efficacy and a mosaic pattern of expression, and marks a more limited chief cell population. Indeed, the newer Lgr5-2A-CreERT construct is more efficiently expressed and may mark a broader chief cell population, including subpopulations that can behave differently from the mature chief cells at the glandular base.
Nonetheless, we propose yet another possibility, which is that Lgr5 expression can be induced in isthmus progenitor cells after HDT-induced injury, as shown in our in situ hybridization and lineage-tracing experiments. Although the expression of Wnt ligands in the corpus was not increased after HDT treatment in our analysis (not shown), it has been suggested that Lgr5 expression is upregulated during tissue regeneration, possibly in response to induced niche factors, such as R-spondin (22, 29, 42). Given that, even in the less sensitive Lgr5-EGFP-IRES-CreERT mouse, recombination can be induced in upper isthmus lesions after pulsed HDT, the possibility that the Lgr5-2A-CreERT transgene can be activated outside of the chief cell region, including the isthmus, should be carefully investigated. A more detailed examination of recombination events at earlier time points in Lgr5-2A-CreERT mice will be needed in future studies. Because tamoxifen itself can induce both epithelial injury and Cre recombination, it would be ideal to use a tamoxifen-independent system to analyze such cellular kinetics.
Another major concern in the field of gastric metaplasia research is the origin and mechanism of SPEM. Some researchers define SPEM by the temporal expression of normal neck cell and proliferation markers in conjunction with chief cell markers (33, 39, 50). However, given that metaplasia is defined as cells not found in normal epithelial lineages (38), true SPEM requires additional marker expression that is distinct from that found in normal neck and chief cell precursors. Given the short-term, reversible nature of injury-induced SPEM lesions and the lack of Alcian blue+ mucin and CD44v6 expression in these models, we do not believe that temporally proliferating GSII+GIF+ cells represent true SPEM. Moreover, our data suggest that these cells derive from GSII+GIF+ chief cell precursors that are normally nonproliferative but can quickly migrate and expand downward after injury. In our view, the proliferation and dedifferentiation ability of chief cells has been overestimated. The majority of proliferation occurs in the isthmus and neck region after injury, whereas chief cell proliferation is quite rare and occurs within a very defined time period. A recent study that utilized fluorouracil, which can block proliferation in all gastric cell types, reported that HDT-induced SPEM can develop even in the absence of proliferation and concluded that SPEM did not arise from stem cells (39). The conclusions from this study regarding stem cells appear to lack foundation. Furthermore, the notion that proliferating cells during HDT-induced injury are not the origin of SPEM would seem to require more support, including genetic depletion of intermediate prechief and upper isthmus cells. In our opinion, acute SPEM-like lesions are unlikely to represent true metaplasia, but rather a hyperplasia of chief cell precursors and/or newly regenerated chief cells that maintain both neck and chief cell marker expression, reflecting their neck cell origin.
It was thought that the key trigger of short-term SPEM development was acute gastric atrophy, representing a loss of parietal cells. Drugs used in acute injury models, such as DMP-777, L-635, and HDT, likely act as protonophores that induce rapid apoptosis in parietal cells. Nevertheless, a recent study demonstrated that loss of parietal cells is dispensable for acute short-term SPEM development (5) although it remains unclear whether parietal cell loss is directly related to long-term SPEM and cancer development. In this study, we showed that chief cell ablation induces the rapid expansion of GSII+GIF+ cells that are very similar to acute short-term SPEM cells induced in acute injury drug models. Therefore, the key event for short-term SPEM development, which we believe occurs via expansion of chief cell precursors rather than metaplasia, is the loss of chief cells, which is another pathological definition of gastric atrophy. We do not exclude the possibility that the drugs DMP-777, L-635, and HDT may induce dedifferentiation of chief cells; however, an exact molecular mechanism explaining the selective activation of chief cells independent of other cell types is required.
Kras activation in the stomach leads to marked hyperplasia and metaplasia. Questions remain as to the range of cell types that can give rise to metaplasia when Kras is induced. Our findings suggest that isthmus progenitor cells are the predominant source of metaplasia, whereas mature chief cells lack the ability to transform even after Kras activation. Thus, to generate metaplasia, it would seem necessary to target cells that continuously produce daughter cells, such as long-lived stem cells or progenitors. Given that chief cell precursors normally serve as a readily available and immediate source of mature chief cells, these intermediate cells may account for the first wave of Kras-induced metaplasia, particularly when broader CreERT lines such as eR1-CreERT (18, 28) or Lgr5-2A-CreERT are used. Nonetheless, because isthmus-derived metaplasia eventually gives rise to chief cell precursors that migrate to the base and express both neck and chief cell markers, it is difficult at late time points to distinguish isthmus-derived SPEM from SPEM arising from chief cell precursors.
In the present study, Lgr5+ cell ablation did not influence the development of HDT-induced short-term SPEM and H. pylori-induced long-term SPEM. We previously showed that Lgr5+ cell ablation did not affect Kras-induced metaplasia from Mist1+ isthmus cells (13). Thus it is clear that Lgr5+ chief cells are largely dispensable for any SPEM development, which appears logical given that metaplasia and cancer arise from the atrophic stomach, where chief cells are often reduced or absent following long-term Helicobacter infection in humans. Greater attention and focus should be given to studies of the role of stem and stem-like progenitors in the pathogenesis of gastric cancer.
GRANTS
This work was supported by the KAKENHI Grants-in Aid for Scientific Research (H. Kinoshita, 17K15927; Y. Hayakawa, 16H06749 and 17H05081; and Y. Hirata, 17K09346), the Project for Cancer Research and Therapeutic Evolution (P-CREATE) from the Japan Agency of Medical Research and Development, AMED, the Takeda Science Foundation Visionary Research Grant, the Mitsubishi Foundation, Natural Sciences, the research grant of Astellas Foundation for Research on Metabolic Disorders, and the Advanced Research and Development Programs for Medical Innovation (PRIME) (Y. Hayakawa). T. C. Wang received the National Institutes of Health Grants R35CA210088 and 5U01DK103155-04.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.K. and Y. Hayakawa conceived and designed research; H.K., Y. Hayakawa, Z.N., M.K., M.H., M.T., Y. Hayata, Y. Hikiba, and S.I. performed experiments; H.K., Y. Hayakawa, Z.N., Y. Hikiba, and S.I. analyzed data; H.K., Y. Hayakawa, Y. Hikiba, H.N., and Y. Hirata interpreted results of experiments; H.K. and Y. Hayakawa prepared figures; H.K. and Y. Hayakawa drafted manuscript; H.K., Y. Hayakawa, Z.N., S.I., H.N., Y. Hirata, and T.C.W. edited and revised manuscript; H.K., Y. Hayakawa, Z.N., M.K., M.H., M.T., Y. Hayata, Y. Hikiba, S.I., H.N., Y. Hirata, T.C.W., and K.K. approved final version of manuscript.
ACKNOWLEDGMENTS
Tissue decolorization buffer LUCID was kindly provided from Dr. Hiroshi Onodera, University of Tokyo, Tokyo, Japan.
REFERENCES
- 1.Arnold IC, Lee JY, Amieva MR, Roers A, Flavell RA, Sparwasser T, Müller A. Tolerance rather than immunity protects from Helicobacter pylori-induced gastric preneoplasia. Gastroenterology 140: 199–209, 2011. doi: 10.1053/j.gastro.2010.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R, Clevers H. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6: 25–36, 2010. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457: 608–611, 2009. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 4.Bredemeyer AJ, Geahlen JH, Weis VG, Huh WJ, Zinselmeyer BH, Srivatsan S, Miller MJ, Shaw AS, Mills JC. The gastric epithelial progenitor cell niche and differentiation of the zymogenic (chief) cell lineage. Dev Biol 325: 211–224, 2009. doi: 10.1016/j.ydbio.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burclaff J, Osaki LH, Liu D, Goldenring JR, Mills JC. Targeted apoptosis of parietal cells is insufficient to induce metaplasia in stomach. Gastroenterology 152: 762-766, 2016. doi: 10.1053/j.gastro.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi E, Hendley AM, Bailey JM, Leach SD, and Goldenring JR. Expression of activated Ras in gastric chief cells of mice leads to the full spectrum of metaplastic lineage transitions. Gastroenterology 150: 918–930, 2016. doi: 10.1053/j.gastro.2015.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process-First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 52: 6735–6740, 1992. [PubMed] [Google Scholar]
- 8.Correa P, Shiao YH. Phenotypic and genotypic events in gastric carcinogenesis. Cancer Res 54, Suppl: 1941s–1943s, 1994. [PubMed] [Google Scholar]
- 9.Dieckgraefe BK, Seetharam B, Alpers DH. Developmental regulation of rat intrinsic factor mRNA. Am J Physiol Gastrointest Liver Physiol 254: G913–G919, 1988. [DOI] [PubMed] [Google Scholar]
- 10.Giroux V, Rustgi AK. Metaplasia: tissue injury adaptation and a precursor to the dysplasia-cancer sequence. Nat Rev Cancer 17: 594–604, 2017. doi: 10.1038/nrc.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldenring JR, Ray GS, Coffey RJ Jr, Meunier PC, Haley PJ, Barnes TB, Car BD. Reversible drug-induced oxyntic atrophy in rats. Gastroenterology 118: 1080–1093, 2000. doi: 10.1016/S0016-5085(00)70361-1. [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez-Gonzalez L, Graham TA, Rodriguez-Justo M, Leedham SJ, Novelli MR, Gay LJ, Ventayol-Garcia T, Green A, Mitchell I, Stoker DL, Preston SL, Bamba S, Yamada E, Kishi Y, Harrison R, Jankowski JA, Wright NA, and McDonald SA. The clonal origins of dysplasia from intestinal metaplasia in the human stomach. Gastroenterology 140: 1251–1260, 2011. doi: 10.1053/j.gastro.2010.12.051. [DOI] [PubMed] [Google Scholar]
- 13.Hayakawa Y, Ariyama H, Stancikova J, Sakitani K, Asfaha S, Renz BW, Dubeykovskaya ZA, Shibata W, Wang H, Westphalen CB, Chen X, Takemoto Y, Kim W, Khurana SS, Tailor Y, Nagar K, Tomita H, Hara A, Sepulveda AR, Setlik W, Gershon MD, Saha S, Ding L, Shen Z, Fox JG, Friedman RA, Konieczny SF, Worthley DL, Korinek V, Wang TC. Mist1 expressing gastric stem cells maintain the normal and neoplastic gastric epithelium and are supported by a perivascular stem cell niche. Cancer Cell 28: 800–814, 2015. doi: 10.1016/j.ccell.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayakawa Y, Fox JG, Wang TC. Isthmus stem cells are the origins of metaplasia in the gastric corpus. Cell Mol Gastroenterol Hepatol 4: 89–94, 2017. doi: 10.1016/j.jcmgh.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayakawa Y, Fox JG, Wang TC. The origins of gastric cancer from gastric stem cells: lessons from mouse models. Cell Mol Gastroenterol Hepatol 3: 331–338, 2017. doi: 10.1016/j.jcmgh.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayakawa Y, Hirata Y, Kinoshita H, Sakitani K, Nakagawa H, Nakata W, Takahashi R, Sakamoto K, Maeda S, Koike K. Differential roles of ASK1 and TAK1 in Helicobacter pylori-induced cellular responses. Infect Immun 81: 4551–4560, 2013. doi: 10.1128/IAI.00914-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayakawa Y, Hirata Y, Nakagawa H, Sakamoto K, Hikiba Y, Kinoshita H, Nakata W, Takahashi R, Tateishi K, Tada M, Akanuma M, Yoshida H, Takeda K, Ichijo H, Omata M, Maeda S, Koike K. Apoptosis signal-regulating kinase 1 and cyclin D1 compose a positive feedback loop contributing to tumor growth in gastric cancer. Proc Natl Acad Sci USA 108: 780–785, 2011. doi: 10.1073/pnas.1011418108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayakawa Y, Wang TC. Isthmus progenitors, not chief cells, are the likely origin of metaplasia in eR1-CreERT; LSL-KrasG12Dmice. Gastroenterology 152: 2078–2079, 2017. doi: 10.1053/j.gastro.2017.02.043. [DOI] [PubMed] [Google Scholar]
- 19.Huh WJ, Khurana SS, Geahlen JH, Kohli K, Waller RA, and Mills JC. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology 142: 21–24, 2012. doi: 10.1053/j.gastro.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jadhav U, Saxena M, O'Neill NK, Saadatpour A, Yuan GC, Herbert Z, Murata K, and Shivdasani RA. Dynamic reorganization of chromatin accessibility signatures during dedifferentiation of secretory precursors into Lgr5+ intestinal stem cells. Cell Stem Cell 21: 65–77, 2017. doi: 10.1016/j.stem.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. III. Inward migration of neck cells followed by progressive transformation into zymogenic cells. Anat Rec 236: 297–313, 1993. doi: 10.1002/ar.1092360204. [DOI] [PubMed] [Google Scholar]
- 22.Kim HS, Lee C, Kim WH, Maeng YH, Jang BG. Expression profile of intestinal stem cell markers in colitis-associated carcinogenesis. Sci Rep 7: 6533, 2017. doi: 10.1038/s41598-017-06900-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinoshita H, Hirata Y, Nakagawa H, Sakamoto K, Hayakawa Y, Takahashi R, Nakata W, Sakitani K, Serizawa T, Hikiba Y, Akanuma M, Shibata W, Maeda S, Koike K. Interleukin-6 mediates epithelial-stromal interactions and promotes gastric tumorigenesis. PLoS One 8: e60914, 2013. doi: 10.1371/journal.pone.0060914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kodama M, Murakami K, Okimoto T, Sato R, Uchida M, Abe T, Shiota S, Nakagawa Y, Mizukami K, Fujioka T. Ten-year prospective follow-up of histological changes at five points on the gastric mucosa as recommended by the updated Sydney system after Helicobacter pylori eradication. J Gastroenterol 47: 394–403, 2012. doi: 10.1007/s00535-011-0504-9. [DOI] [PubMed] [Google Scholar]
- 25.Lee CW, Rickman B, Rogers AB, Ge Z, Wang TC, Fox JG. Helicobacter pylori eradication prevents progression of gastric cancer in hypergastrinemic INS-GAS mice. Cancer Res 68: 3540–3548, 2008. doi: 10.1158/0008-5472.CAN-07-6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leushacke M, Tan SH, Wong A, Swathi Y, Hajamohideen A, Tan LT, Goh J, Wong E, Denil SL, Murakami K, Barker N. Lgr5-expressing chief cells drive epithelial regeneration and cancer in the oxyntic stomach. Nat Cell Biol 19: 774–786, 2017. doi: 10.1038/ncb3541. [DOI] [PubMed] [Google Scholar]
- 27.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science 327: 542–545, 2010. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuo J, Kimura S, Yamamura A, Koh CP, Hossain MZ, Heng DL, Kohu K, Chih-Cheng Voon D, Hiai H, Unno M, Yan So JB, Zhu F, Srivastava S, Meng T, Yeoh KG, Osato M, Ito Y. Identification of stem cells in the epithelium of the stomach corpus and antrum of mice. Gastroenterology 152: 218-231, 2016. doi: 10.1053/j.gastro.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 29.Metcalfe C, Kljavin NM, Ybarra R, de Sauvage FJ. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell 14: 149–159, 2014. doi: 10.1016/j.stem.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Mills JC, Goldenring JR. Metaplasia in the stomach arises from gastric chief cells. Cell Mol Gastroenterol Hepatol 4: 85–88, 2017. doi: 10.1016/j.jcmgh.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakagawa H, Suzuki N, Hirata Y, Hikiba Y, Hayakawa Y, Kinoshita H, Ihara S, Uchino K, Nishikawa Y, Ijichi H, Otsuka M, Arita J, Sakamoto Y, Hasegawa K, Kokudo N, Tateishi K, Koike K. Biliary epithelial injury-induced regenerative response by IL-33 promotes cholangiocarcinogenesis from peribiliary glands. Proc Natl Acad Sci USA 114: E3806–E3815, 2017. doi: 10.1073/pnas.1619416114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nam KT, Lee HJ, Sousa JF, Weis VG, O'Neal RL, Finke PE, Romero-Gallo J, Shi G, Mills JC, Peek RM, Jr., Konieczny SF, Goldenring JR. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology 139: 2028–2037, 2010. doi: 10.1053/j.gastro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nam KT, Lee HJ, Sousa JF, Weis VG, O’Neal RL, Finke PE, Romero-Gallo J, Shi G, Mills JC, Peek RM Jr, Konieczny SF, Goldenring JR. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology 139: 2028–2037.e9, 2010. doi: 10.1053/j.gastro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nam KT, O’Neal RL, Coffey RJ, Finke PE, Barker N, Goldenring JR. Spasmolytic polypeptide-expressing metaplasia (SPEM) in the gastric oxyntic mucosa does not arise from Lgr5-expressing cells. Gut 61: 1678–1685, 2012. doi: 10.1136/gutjnl-2011-301193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nomura S, Yamaguchi H, Ogawa M, Wang TC, Lee JR, Goldenring JR. Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild-type and gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol 288: G362–G375, 2005. doi: 10.1152/ajpgi.00160.2004. [DOI] [PubMed] [Google Scholar]
- 36.Okumura T, Ericksen RE, Takaishi S, Wang SS, Dubeykovskiy Z, Shibata W, Betz KS, Muthupalani S, Rogers AB, Fox JG, Rustgi AK, Wang TC. K-ras mutation targeted to gastric tissue progenitor cells results in chronic inflammation, an altered microenvironment, and progression to intraepithelial neoplasia. Cancer Res 70: 8435–8445, 2010. doi: 10.1158/0008-5472.CAN-10-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Owen DA. Normal histology of the stomach. Am J Surg Pathol 10: 48–61, 1986. doi: 10.1097/00000478-198601000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Petersen CP, Mills JC, Goldenring JR. Murine models of gastric corpus preneoplasia. Cell Mol Gastroenterol Hepatol 3: 11–26, 2016. doi: 10.1016/j.jcmgh.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radyk MD, Burclaff J, Willet SG, Mills JC. Metaplastic cells in the stomach arise, independently of stem cells, via dedifferentiation or transdifferentiation of chief cells. Gastroenterology 154: 839-843, 2018. doi: 10.1053/j.gastro.2017.11.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sekitani T, Yokota T, Kuribara K, Kaltenbrunner M, Fukushima T, Inoue Y, Sekino M, Isoyama T, Abe Y, Onodera H, Someya T. Ultraflexible organic amplifier with biocompatible gel electrodes. Nat Commun 7: 11425, 2016. doi: 10.1038/ncomms11425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serizawa T, Hirata Y, Hayakawa Y, Suzuki N, Sakitani K, Hikiba Y, Ihara S, Kinoshita H, Nakagawa H, Tateishi K, Koike K. Gastric metaplasia induced by Helicobacter pylori is associated with enhanced SOX9 expression via interleukin-1 signaling. Infect Immun 84: 562–572, 2015. doi: 10.1128/IAI.01437-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sigal M, Logan CY, Kapalczynska M, Mollenkopf HJ, Berger H, Wiedenmann B, Nusse R, Amieva MR, Meyer TF. Stromal R-spondin orchestrates gastric epithelial stem cells and gland homeostasis. Nature 548: 451–455, 2017. doi: 10.1038/nature23642. [DOI] [PubMed] [Google Scholar]
- 43.Stange DE, Koo BK, Huch M, Sibbel G, Basak O, Lyubimova A, Kujala P, Bartfeld S, Koster J, Geahlen JH, Peters PJ, van Es JH, van de Wetering M, Mills JC, Clevers H. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell 155: 357–368, 2013. doi: 10.1016/j.cell.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tetteh PW, Basak O, Farin HF, Wiebrands K, Kretzschmar K, Begthel H, van den Born M, Korving J, de Sauvage F, van Es JH, van Oudenaarden A, Clevers H. Replacement of lost Lgr5-positive stem cells through plasticity of their enterocyte-lineage daughters. Cell Stem Cell 18: 203–213, 2016. doi: 10.1016/j.stem.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 45.Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478: 255–259, 2011. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Es JH, Sato T, van de Wetering M, Lyubimova A, Yee Nee AN, Gregorieff A, Sasaki N, Zeinstra L, van den Born M, Korving J, Martens ACM, Barker N, van Oudenaarden A, Clevers H. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol 14: 1099–1104, 2012. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vermeulen L, Morrissey E, van der Heijden M, Nicholson AM, Sottoriva A, Buczacki S, Kemp R, Tavaré S, Winton DJ. Defining stem cell dynamics in models of intestinal tumor initiation. Science 342: 995–998, 2013. doi: 10.1126/science.1243148. [DOI] [PubMed] [Google Scholar]
- 48.Wang TC, Dangler CA, Chen D, Goldenring JR, Koh T, Raychowdhury R, Coffey RJ, Ito S, Varro A, Dockray GJ, Fox JG. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology 118: 36–47, 2000. doi: 10.1016/S0016-5085(00)70412-4. [DOI] [PubMed] [Google Scholar]
- 49.Wang TC, Goldenring JR, Dangler C, Ito S, Mueller A, Jeon WK, Koh TJ, Fox JG. Mice lacking secretory phospholipase A2 show altered apoptosis and differentiation with Helicobacter felis infection. Gastroenterology 114: 675–689, 1998. doi: 10.1016/S0016-5085(98)70581-5. [DOI] [PubMed] [Google Scholar]
- 50.Weis VG, Petersen CP, Weis JA, Meyer AR, Choi E, Mills JC, and Goldenring JR. Maturity and age influence chief cell ability to transdifferentiate into metaplasia. Am J Physiol Gastrointest Liver Physiol 312: G67–G76, 2017. doi: 10.1152/ajpgi.00326.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Westphalen CB, Asfaha S, Hayakawa Y, Takemoto Y, Lukin DJ, Nuber AH, Brandtner A, Setlik W, Remotti H, Muley A, Chen X, May R, Houchen CW, Fox JG, Gershon MD, Quante M, Wang TC. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J Clin Invest 124: 1283–1295, 2014. doi: 10.1172/JCI73434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, Su N, Luo Y, Heilshorn SC, Amieva MR, Sangiorgi E, Capecchi MR, Kuo CJ. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci USA 109: 466–471, 2012. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yan KS, Gevaert O, Zheng GX, Anchang B, Probert CS, Larkin KA, Davies PS, Cheng ZF, Kaddis JS, Han A, Roelf K, Calderon RI, Cynn E, Hu X, Mandleywala K, Wilhelmy J, Grimes SM, Corney DC, Boutet SC, Terry JM, Belgrader P, Ziraldo SB, Mikkelsen TS, Wang F, von Furstenberg RJ, Smith NR, Chandrakesan P, May R, Chrissy MAS, Jain R, Cartwright CA, Niland JC, Hong YK, Carrington J, Breault DT, Epstein J, Houchen CW, Lynch JP, Martin MG, Plevritis SK, Curtis C, Ji HP, Li L, Henning SJ, Wong MH, Kuo CJ. Intestinal enteroendocrine lineage cells possess homeostatic and injury-inducible stem cell activity. Cell Stem Cell 21: 78–90, 2017. doi: 10.1016/j.stem.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]