Abstract
Studies of Dahl salt-sensitive (SS) rats have shown that renal CD3+ T cells and ED-1+ macrophages are involved in the development of salt-sensitive hypertension and renal damage. The present study demonstrated that the increase in renal immune cells, which accompanies renal hypertrophy and albuminuria in high-salt diet-fed Dahl SS rats, is absent in Sprague-Dawley and SSBN13 rats that are protected from the SS disease phenotype. Flow cytometric analysis demonstrated that >70% of the immune cells in the SS kidney are M1 macrophages. PCR profiling of renal myeloid cells showed a salt-induced upregulation in 9 of 84 genes related to Toll-like receptor signaling, with notable upregulation of the Toll-like receptor 4/CD14/MD2 complex. Because of the prominent increase in macrophages in the SS kidney, we used liposome-encapsulated clodronate (Clod) to deplete macrophages and assess their contribution to salt-sensitive hypertension and renal damage. Dahl SS animals were administered either Clod-containing liposomes (Clod-Lipo), Clod, or PBS-containing liposomes as a vehicle control. Clod-Lipo treatment depleted circulating and splenic macrophages by ∼50%; however, contrary to our hypothesis, Clod-Lipo-treated animals developed an exacerbated salt-sensitive response with respect to blood pressure and albuminuria, which was accompanied by increased renal T and B cells. Interestingly, those treated with Clod also demonstrated an exacerbated phenotype, but it was less severe than Clod-Lipo-treated animals and independent of changes to the number of renal immune cells. Here, we have shown that renal macrophages in Dahl SS animals sustain a M1 proinflammatory phenotype in response to increased dietary salt and highlighted potential adverse effects of Clod-Lipo macrophage depletion.
Keywords: clodronate, hypertension, liposome, macrophage, salt sensitive
INTRODUCTION
Recent reports have indicated that hypertension is a primary, modifiable risk factor for cardiovascular, cerebrovascular, and renal disease (35) and is the largest individual contributing factor to disease and mortality in the world (38, 62). Experimental and human data indicate that immune mechanisms play a key role in the development of hypertension and kidney damage (31, 47, 65), as individuals with hypertension have a greater degree of glomerulosclerosis and renal fibrosis as well as increased numbers of macrophages and T lymphocytes in the renal interstitium (29) compared with normotensive individuals. Patients whose arterial blood pressure changes in response to alterations in dietary salt comprise 30–50% of individuals with hypertension (34, 36). This salt-sensitive population experiences more cardiovascular or cardiovascular-related events (54), has greater mortality than patients with salt-resistant hypertension (75), and exhibits renal damage when hypertensive due to elevated dietary salt (3, 8, 10, 19). A greater understanding of the mechanisms mediating inflammation in the kidney in salt-sensitive hypertension may be beneficial in the development of more effective therapies.
A preclinical model of salt-sensitive hypertension and associated renal end-organ damage is the Dahl salt-sensitive (SS) rat, which has been used extensively to study cardiovascular and renal disease. It is well documented that there is a prominent role of the immune system to potentiate the progression of hypertension in male animals (22, 64), and a number of studies have modulated the immune system to address the relative impact of specific immune cell types. Many studies have also investigated a sexual dimorphism that exists in the role that the immune system plays in this disease progression (30, 60, 61, 71). We have shown in male animals that T cells, B cells, and macrophages are increased in the renal interstitial space in male Dahl SS rats developing hypertension and renal damage when fed a 4.0% NaCl diet (47). Nonspecific pharmacological suppression using mycophenolate mofetil attenuated the salt-sensitive phenotype seen in the Dahl SS rat (49), and we saw similar results when T cells (67) or T and B cells (51) were genetically deleted. These genetic studies implicated the role of the adaptive immune system in salt-sensitive hypertension but did not address the role of innate immunity. Recent studies by others have attempted to investigate the role of the innate immune system in the initial recruitment and activation of immune processes in experimental models of hypertension (5, 23, 24), but the role of innate immunity in Dahl SS hypertension and renal damage is still unclear.
The macrophage is a myeloid-derived cell type of the innate immune system with various functions, including phagocytic removal of debris, antigen presentation, and production of inflammatory interleukins and cytokines. Alongside dendritic cells, the macrophage provides the crucial role of antigen presentation in the transition to an adaptive immune response. After activation by pattern recognition receptors, macrophages can elicit either a classical M1 proinflammatory or an alternative M2 anti-inflammatory phenotype, which can modulate how the cell responds (18, 46, 55). In states of kidney damage, resident or infiltrating macrophages in the kidney usually shift toward a M2 phenotype to promote tissue repair (72); however, a M1 phenotype predominates (20) in some disease models (37, 74). Because of the renal fibrosis seen in the Dahl SS rat (11) accompanied by continual and worsening disease progression, we hypothesized that the M1 phenotype will dominate macrophages present in the kidney.
The present study was performed to test the hypothesis that the increases in macrophages and myeloid-derived cells in the renal interstitial space play a role to mediate the development of salt-sensitive hypertension and renal end-organ damage in the Dahl SS rat. The initial experiments compared the influence of elevated salt intake on the number of immune cells in the kidneys of Dahl SS rats and two control strains, the Sprague-Dawley (SD) rat and the consomic SSBN13 rat, which do not demonstrate or have an attenuation of Na+-dependent hypertension and renal damage. A subsequent assessment of renal M1/M2 macrophages by flow cytometry was used, as previously described by Rubio-Navarro et al. (66). A further analysis of these renal myeloid-derived cells then used a PCR profiler array of Toll-like receptor (TLR) signaling to assess one of the primary activation pathways of these cells (32). Finally, functional experiments were performed to deplete macrophages using clodronate (Clod)-encapsulated liposomes to test the effect of macrophage depletion on the development of the Dahl SS disease phenotype.
METHODS
Animals
All experiments were performed on age-matched male inbred Dahl SS rats (SS/JrHSDMcwi, referred to as SS), a consomic line of inbred Dahl SS rats with an introgressed chromosome 13 from the Brown Norway background (SS-Chr 13BN/Mcwi, referred to as SSBN13), and outbred SD rats obtained from a commercial vendor (Envigo). The SD rat does not demonstrate salt sensitivity of blood pressure or renal damage, and the SSBN13 strain shows a drastic attenuation in this phenotype relative to the intact SS background (11). SS or SSBN13 rats were born from breeding colonies at the Medical College of Wisconsin, weaned, and maintained on a 0.4% NaCl diet (AIN-76A, Dyets) until 9 wk of age, at which point half of the rats were switched to a 4.0% NaCl diet (AIN-76A, Dyets), while the other half was maintained on the 0.4% NaCl diet. All SD animals were switched to the same 0.4% NaCl diet upon arrival at our facilities during the third week of age. One half was switched to the 4.0% NaCl diet at the ninth week of age, whereas the other half was maintained on the 0.4% NaCl diet. All experimental animal procedures were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee.
Urinalysis
Overnight urine collections were performed on days −1, 7, 14, and 21 of increased dietary salt. Urine electrolytes were measured by flame photometry (model 410, Corning). Urine creatinine values were measured by an autoanalyzer with an assay based on the Jaffé reaction (ACE, Alfa Wasserman, Fairfield, NJ). Urine albumin was quantified with a fluorescent assay that used albumin blue 580 dye (Sigma-Aldrich, St. Louis, MO) and a fluorescent plate reader (FL-600, BioTek, Winooski, VT).
Tissue Harvest
At the conclusion of the study period, rats were anesthetized with isoflurane gas inhalation. Aortic blood was collected, and the kidneys flushed with heparinized saline. The right kidney and part of the spleen were extracted for histological analysis, and the left kidney was extracted for flow cytometry analysis.
Immune Cell Isolation
Circulating immune cells.
Peripheral blood mononuclear cells were isolated as previously described (1, 2) using Histopaque-1083 in a 15-ml conical tube. After centrifugation (400 g for 30 min at room temperature), the mononuclear cell layer was washed in Dulbecco-PBS containing 2% heat-inactivated FBS and 2 mM EDTA; this solution will further be referred to as “wash buffer.”
Renal immune cells.
Immune cells in the kidney were isolated as previously described (1, 2). Briefly, the left kidney was minced and incubated in RPMI-1640 media containing l-glutamine, HEPES, collagenase type IV, and DNase. The solution was then passed through a series of 100-, 70-, and 40-µm filters. Mononuclear cells were separated by Percoll density gradient centrifugation (400 g for 30 min at room temperature) and washed. Cells from the kidney as well as the circulation were then pelleted and resuspended in the wash buffer solution, and the concentration of cells was determined by counting on a hemocytometer.
Flow Cytometry
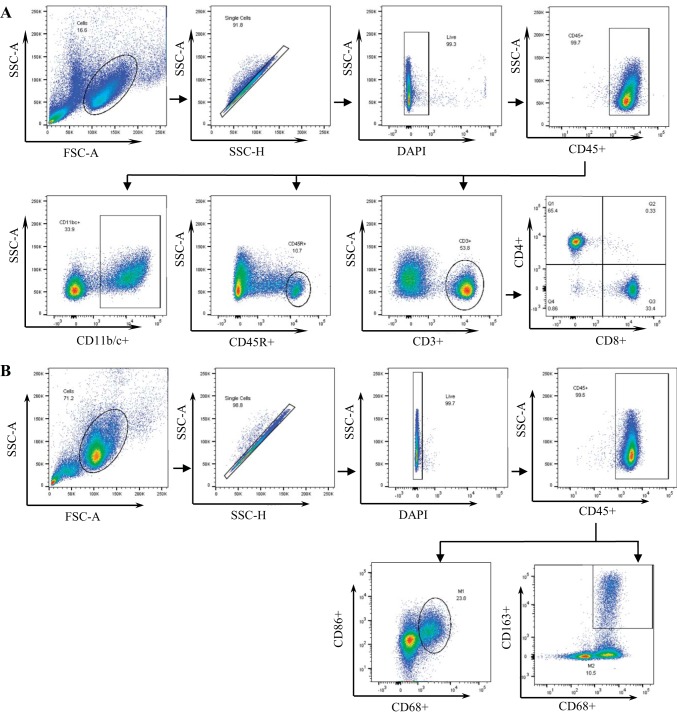
General characterization of immune cell types was performed as previously described (1, 2). Mononuclear cells were incubated with CD32 for 5 min followed by an incubation for 30 min in a solution containing antibodies for the following extracellular markers: anti-CD3 (eBioscience) for T cells, anti-CD4 (BioLegend) for T helper cells, anti-CD8 (BioLegend) for cytotoxic T cells, anti-CD45R (BD Bioscience) for B cells, and anti-CD11b/c (eBioscience) for mononuclear myeloid-derived cells. Additional M1/M2 phenotyping was performed as previously described by Rubio-Navarro et al. (66). Briefly, after mononuclear cell isolation, cells were incubated for 30 min in a solution containing antibodies for the extracellular markers anti-CD45 (BioLegend) and anti-CD163 (Bio-Rad) along with Live/Dead Fixable viability dye (ThermoFisher). After being washed, cells were incubated in a series of solutions containing anti-CD68 (Bio-Rad) for 50 min and anti-CD86 (BioLegend) for 20 min. All cells were then analyzed by flow cytometry (LSRII, Becton-Dickinson) with FACSDIVA software (Becton-Dickinson) and FlowJo Software (TreeStar). The gating strategies are shown in Fig. 1.
Fig. 1.
Flow cytometry gating strategies. A: after gating for the general cell population using forward scatter (FSC) and side scatter (SSC), those that were singlets, and those negative for DAPI staining, CD45+ cells were used to gate for total leukocytes. From this population, gates were placed for CD11b/c+ myeloid cells, CD45R+ B cells, and CD3+ T cells along with their CD4+ helper and CD8+ cytotoxic subsets. B: like in A, CD45+ total leukocytes were gated, from which gates were placed for CD68+/CD86+ M1 macrophages and CD68+/CD163+ M2 macrophages.
TLR Expression Profile in Myeloid-Derived Cells
Renal immune cells were isolated as described above from Dahl SS rats fed either a 0.4% or 4.0% NaCl diet for 21 days. Mononuclear cells were incubated in RPMI-1640 medium for 3 h at 37°C and 5% CO2. The adherent cells were then lifted with 1× trypsin (Life Technologies) and washed, and an aliquot was pelleted and flash frozen. Another aliquot underwent flow cytometric analysis, where isolated cells from 0.4% NaCl diet-fed animals were 93.4 ± 1.9% and 4.0% NaCl diet-fed animals were 91.4 ± 1.8% CD11b/c+. RNA was then extracted by the TRIzol (Life Technologies) method. The TRIzol method was used to maximize the RNA yield from the small number of cells available for analysis. Expression of genes related to TLR signaling was assessed using a Qiagen RT2 PCR Profiler Array (PARN-018Z) on a Quantstudio 6 Flex (ThermoFisher) according to the manufacturer’s instructions.
Surgical Procedure and Blood Pressure Monitoring for Macrophage Depletion Experiments
At 7 wk of age, SS rats underwent carotid telemeter implantation surgery, as previously described (1, 67). Briefly, rats were deeply anesthetized under isoflurane gas inhalation. Using an aseptic technique, the carotid artery was exposed, and a telemetry catheter was inserted into the artery. Telemetry units (HDS10, Data Sciences, St. Paul, MN) were placed under the skin at the nape of the neck. Analgesics (0.3 mg/kg Buprenorphine SR) and antibiotics (25 mg/kg Cefazolin) were administered postsurgically. After a recovery period, blood pressure was monitored for 5 days, at which point rats were again deeply anesthetized under isoflurane gas inhalation and received a tail vein injection of either PBS-containing liposomes (PBS-Lipo), Clod (50 mg/kg), or Clod-containing liposomes (50 mg/kg, FormuMax Scientific). Liposomes, spherical vesicles made from a phospholipid bilayer, serve as a delivery system to target drugs to macrophages (7). Because of the macrophages’ innate phagocytic capacity, liposomes containing a drug of interest are readily phagocytosed and digested by intracellular phagosomes, thus releasing the drug directly to macrophages. Therefore, this study used liposomes to deliver Clod to deplete macrophages from the circulation. After drug administration, an additional 2 days of baseline blood pressure was recorded while rats were fed the 0.4% NaCl diet, at which point an overnight baseline urine collection in metabolic cages was performed. Afterward, all animals were switched to the 4.0% NaCl diet. Booster injections of PBS-Lipo, Clod, or Clod-Lipo were administered in the same manner every 7 days after the primary injection at a reduced concentration (25 mg/kg), and overnight urine collections were repeated every 7 days after the primary collection. The Clod-Lipo dose was chosen based on work by other investigators (28, 73). Animals were maintained on the 4.0% NaCl diet for 21 days.
Histological Analysis
After extraction, the right kidney and the spleen were fixed in 10% neutral buffered formalin, paraffin embedded, cut in 4-µm sections, and mounted. Slices were stained with periodic acid-Schiff stain or anti-CD68. For CD68 staining, all slides were deparaffinized, antigen retrieved, and stained on the Leica BondRx automated staining platform. Antigen was retrieved using Retrieval Citrate Buffer Solution (AR9661, Leica) and blocked with peroxidase (S200389, DAKO), and an avidin and biotin blocking kit was applied (SP-2001, Vector Laboratories). After protein block (X090930-2, DAKO), primary anti-CD68 (1:200, sc70760, Santa Cruz Biotechnology) and secondary donkey anti-mouse biotin (1:500) were applied. Streptavidin-horseradish peroxidase and 3,3-diaminobenzidine were added to each section, and slides were counterstained with 15% aniline blue. Slides were dehydrated, cleared, and mounted with synthetic mounting media. Stained kidney and spleen images were then taken using Nikon Coolscan or Nikon E400. The outer medullary cast percentage in the kidney as well as percentage of CD68 staining in the spleen were determined by color inclusion via MetaMorph software. Glomerular injury was determined by assigning >50 glomeruli/kidney categorical scores, as previously described (1, 2, 50), on a scale of 1 (best) to 4 (worst) based on the degree of glomerulosclerosis and mesangial expansion. All histological analysis was performed in a blinded manner.
Statistical Analysis
One-way ANOVA with a Holm-Sidak post hoc test, two-way ANOVA with a Holm-Sidak post hoc test, two-way repeated-measures ANOVA with a Holm-Sidak post hoc test, or Student’s t-test was used where appropriate. Data are expressed as means ± SE. Values of P < 0.05 were considered statistically significant. SigmaPlot 12.5 software (Systat Software) was used for all statistical analysis.
RESULTS
Dahl SS Animals Develop Salt-Sensitive Renal Damage
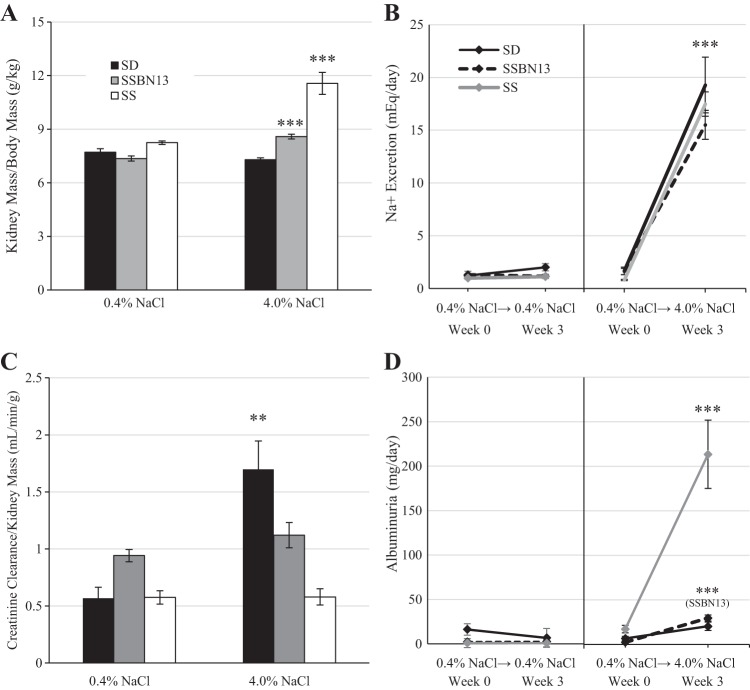
Similar to previous data (48, 50, 52, 58), when SS animals were switched from a 0.4% to 4.0% NaCl diet, they developed a significant increase in renal mass (P < 0.001; Fig. 2A) and albuminuria (P < 0.001; Fig. 2D). Figure 2, B and D, shows urinary excretions of animals maintained on the 0.4%NaCl diet (Fig. 2, B and D, left) and those switched to the 4.0%NaCl diet (Fig. 2, B and D, right). Despite equal excretion of Na+ (Fig. 2B), this salt-sensitive response was not seen in SD animals (renal mass and albuminuria: P > 0.05). SSBN13 rats displayed slight increases in renal mass (P < 0.001) and albuminuria (P < 0.001) when placed on the 4.0% NaCl diet; however, this increase was much lower than the increases observed in SS rats fed the 4.0% diet (renal weight and albuminuria: P < 0.001, SS vs. SSBN13 rats). In addition, the SD strain showed increased creatinine clearance relative to kidney mass when fed the 4.0% NaCl diet (P < 0.01), which was attenuated in SSBN13 animals (P > 0.05) and absent in SS animals (P > 0.05; Fig. 2C). These results are consistent with previous work in the SS rat (12), which has an impaired hemodynamic response to elevated dietary salt.
Fig. 2.
A: kidney mass-to-body mass ratio in male age-matched Sprague-Dawley (SD), Dahl salt-sensitive (SS), and SSBN13 rats maintained on a 0.4% NaCl diet or fed a 4.0% NaCl diet for 3 wk. B: Na+ excretion. C: creatinine clearance relative to kidney mass. D: albuminuria. n = 6 SD, 6 SSBN13, and 6 SS rats on each diet. B–D: urinary excretions of rats maintained on the 0.4%NaCl diet (left) and those switched to the 4.0% NaCl diet (right). Two-way ANOVA or two-way repeated-measures ANOVA with a Holm-Sidak post hoc test was used. ***P < 0.001 and **P < 0.01 vs. the 0.4% NaCl diet within strain.
Dahl SS Animals Demonstrate a Salt-Sensitive Increase in Renal Immune Cells Not Observed in Normotensive Strains
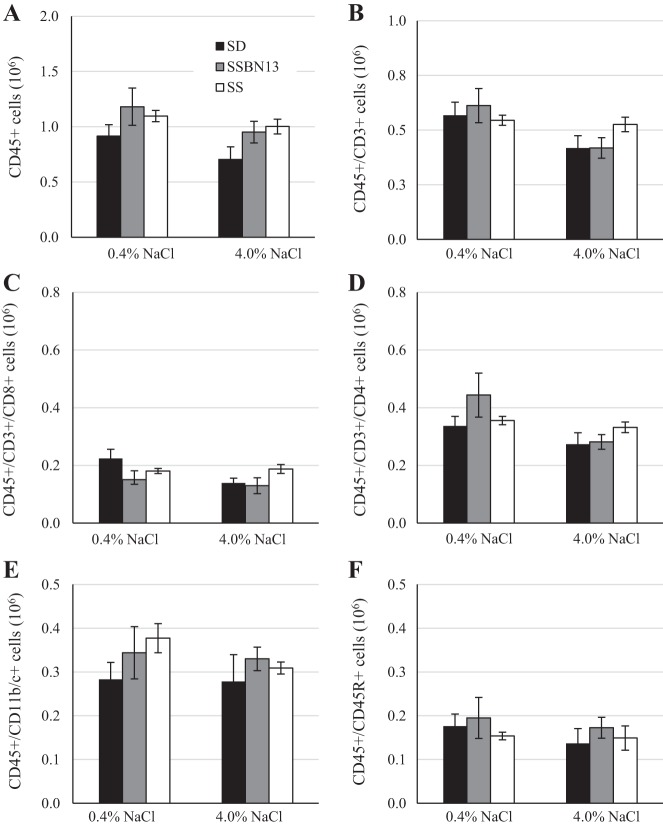
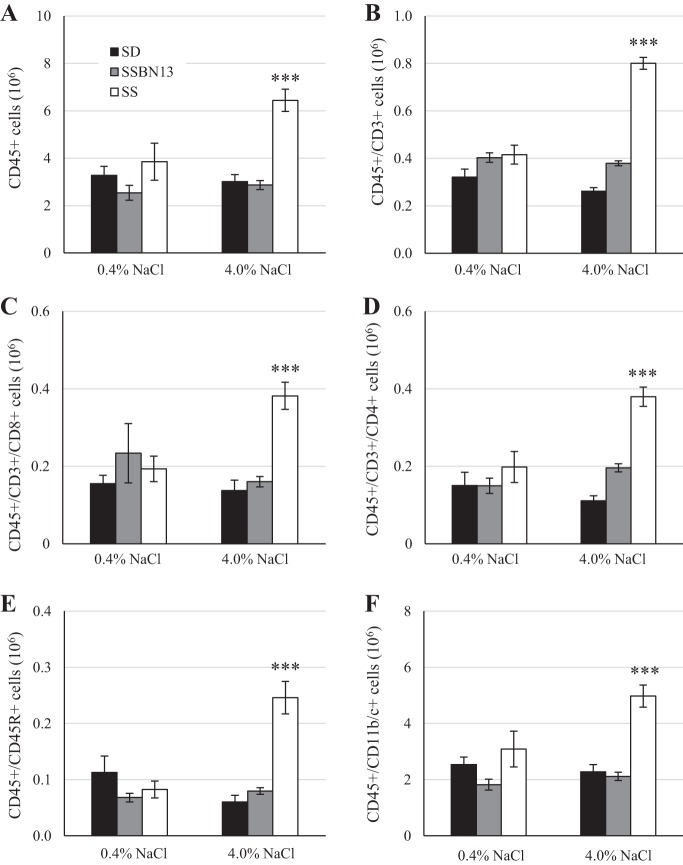
After 3 wk of either the 0.4 or 4.0% NaCl diet, renal immune cells were assessed by flow cytometry. The change in diet did not affect the numbers of circulating immune cells (Fig. 3). Compared with SS animals fed 0.4% NaCl, those fed the 4.0% NaCl diet for 3 wk showed a 67% greater number of CD45+ leukocytes in the kidney, including CD3+ T cells (93% increase), CD4+ T helper cells (92% increase), CD8+ cytotoxic T cells (98% increase), CD45R+ B cells (198% increase), and CD11b/c+ myeloid cells (61% increase; Fig. 4). This increase in renal immune cells in 4.0% NaCl diet-fed animals was not observed in SD or SSBN13 strains.
Fig. 3.
Circulating immune cells of male age-matched Sprague-Dawley (SD), Dahl salt-sensitive (SS), and SSBN13 rats maintained on a 0.4% NaCl diet or fed a 4.0% NaCl diet for 3 wk. A: CD45+ leukocytes. B: CD45+/CD3+ T cells. C: CD45+/CD3+/CD4+ T helper cells. D: CD45+/CD3+/CD8+ cytotoxic T cells. E: CD45+/CD45R+ B cells. F: CD45+/CD11b/c+ monocytes/myeloid cells. n = 5 SD, 6 SSBN13, and 6 SS rats on each diet. No statistical differences were observed using two-way ANOVA with a Holm-Sidak post hoc test.
Fig. 4.
Total immune cells per kidney of male age-matched Sprague-Dawley (SD), Dahl salt-sensitive (SS), and SSBN13 rats maintained on a 0.4% NaCl diet or fed a 4.0% NaCl diet for 3 wk. A: CD45+ leukocytes. B: CD45+/CD3+ T cells. C: CD45+/CD3+/CD4+ T helper cells. D: CD45+/CD3+/CD8+ cytotoxic T cells. E: CD45+/CD45R+ B cells. F: CD45+/CD11b/c+ myeloid cells. n = 6 SD, 6 SSBN13, and 6 SS rats on each diet. Two-way ANOVA with a Holm-Sidak post hoc test was used. ***P < 0.001 vs. the 0.4% NaCl diet within strain.
M1 Phenotype Predominates Macrophages in the Kidneys of SS Rats Fed the 4.0% NaCl Diet
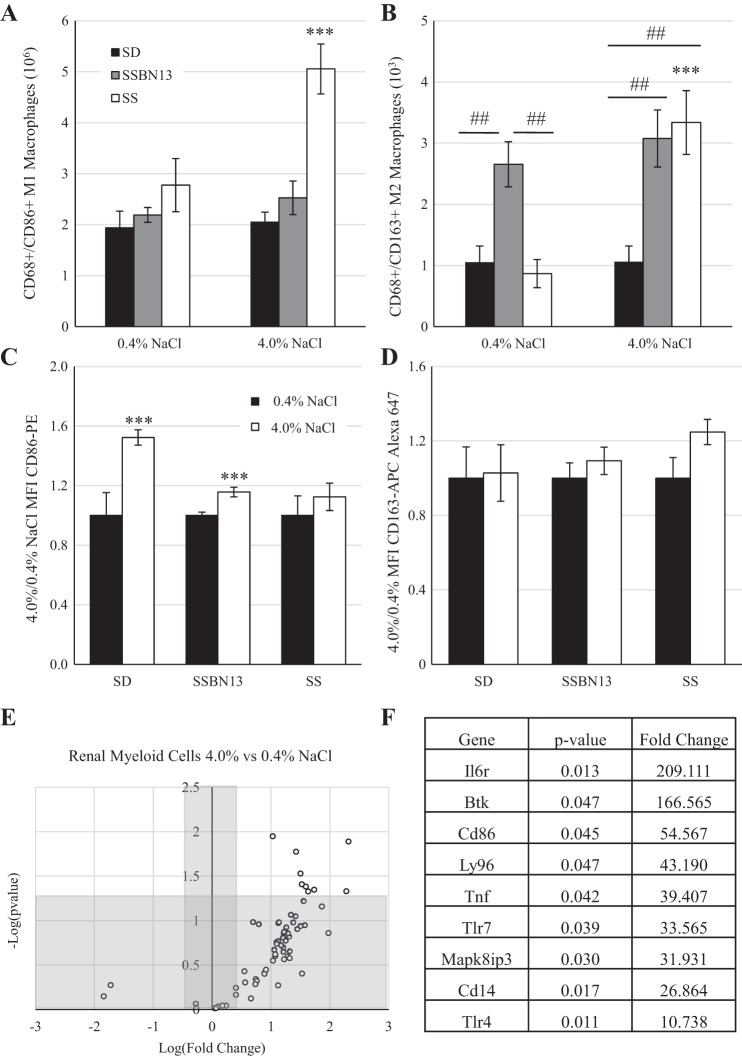
Because the increased number of myeloid cells in the kidney was much greater than that of the lymphocyte populations, further examination of the M1/M2 polarization was performed. Figure 5, A and B, shows that in response to the salt challenge the SS rat strain demonstrated an increased presence of both CD86+ M1 macrophages (2.8 × 106 ± 0.5 × 106 vs. 5.1 × 106 ± 0.5 × 106 cells/kidney, 0.4% vs. 4.0% NaCl diet; Fig. 4A) and CD163+ M2 macrophages (868.6 ± 229.9 vs. 3,336.9 ± 520.9 cells/kidney; Fig. 4B); however, the magnitude of M1 macrophages was far greater (millions) than that of M2 macrophages (hundreds to thousands). This difference between diets was not observed in SD or SSBN13 animals. Additionally, while on the 0.4% NaCl diet, SSBN13 animals showed a greater number of M2 macrophages in the kidney than either SD or SS animals (Fig. 5B). The mean fluorescent intensity of CD86 (Fig. 5C) showed that SD and SSBN13 strains demonstrated an elevation in the CD86 signal in response to salt challenge, whereas the SS strain did not. In addition, no difference in the CD163 signal was detectable in any strain between the two diets (Fig. 5D).
Fig. 5.
A and B: number of macrophages per kidney of male age-matched Sprague-Dawley (SD), Dahl salt-sensitive (SS), and SSBN13 rats maintained on a 0.4% NaCl diet or fed a 4.0% NaCl diet for 3 wk showing a M1 proinflammatory phenotype (A) or a M2 anti-inflammatory phenotype (B). C and D: ratio of mean fluorescent intensity (MFI) of CD86-PE (C) and CD163-APC (D). E: PCR profiler array of genes relating to Toll-like receptor (TLR) signaling comparing isolated renal myeloid cells from age-matched SS rats fed either a 0.4% or 4.0% NaCl diet for 3 wk as a volcano plot. F: list of genes that were significantly upregulated in 4.0% NaCl diet-fed animals. n = 6 SD, 6 SSBN13, and 6 SS rats on each diet in A–D and n = 6 for each diet in E and F. Two-way ANOVA with a Holm-Sidak post hoc test was used in A–D. ***P < 0.001 vs. the 0.4% NaCl diet within strain; ##P < 0.01 between strains. A Student’s t-test was used in E and F, with a P value cutoff of 0.05 and a fold change cutoff of 2.
Isolated Renal Myeloid Cells From SS Rats Fed the 4.0% NaCl Diet Show Enhanced TLR Signaling
To further assess the activation of myeloid cells in the kidneys of SS animals, the expression of genes related to TLR signaling was performed using a commercially available PCR array. Of the 84 genes evaluated, expression of 71 genes was detectable. When we compared myeloid cells isolated from kidneys of rats fed the 4.0% NaCl diet with those isolated from rats fed the 0.4% NaCl diet, 68 genes were upregulated, and 9 genes were significantly upregulated (Fig. 5E). Figure 5F shows a list of these genes, including three genes [TLR4, CD14, and lymphocyte antigen 9 (Ly96)] that all encode proteins in the TLR4 complex, which is activated by lipopolysaccharide and induces MyD88/TIR domain-containing adapter-inducing interferon-β pathway activation.
Administration of Clod or Clod-Lipo Resulted in Renal Hypertrophy in SS Rats
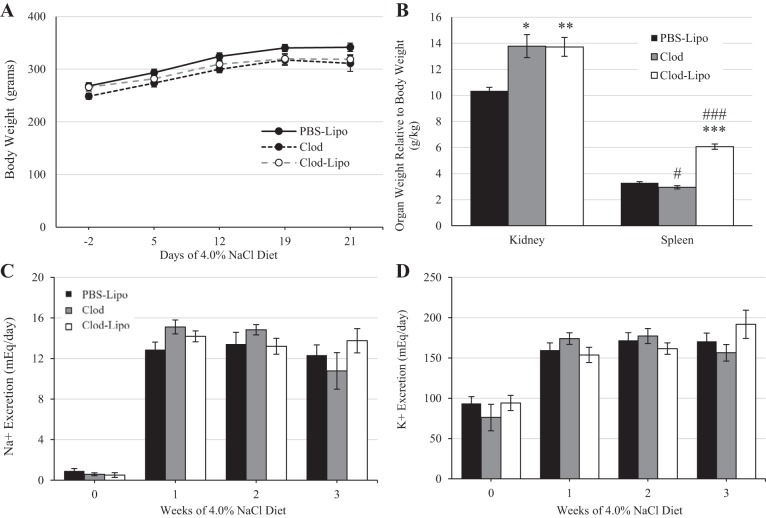
Body weight did not differ throughout the duration of the study between any of the groups treated with PBS-Lipo, Clod, or Clod-Lipo on days −3, 4, 11, and 18 of the 21-day 4.0% NaCl diet protocol (Fig. 6A). By the end of the study, animals that received Clod or Clod-Lipo developed greater renal hypertrophy compared with PBS-Lipo-treated control animals (P < 0.01 vs. Clod and P < 0.001 vs. Clod-Lipo). In addition, animals that received Clod-Lipo developed a larger spleen compared with PBS-Lipo-treated control rats (P < 0.001), whereas those that received only Clod had smaller spleens (P < 0.05; Fig. 6B). The excretion of the electrolytes Na+ and K+ did not differ between any of the groups throughout the course of the study (Fig. 6, C and D).
Fig. 6.
A: body weight of Dahl salt-sensitive (SS) animals that received either PBS-containing liposomes (PBS-Lipo), clodronate (Clod) alone, or Clod-containing liposomes (Clod-Lipo) on days −3, 4, 11, and 18 of the 21-day 4.0% NaCl chow protocol. B: relative total kidney weight and spleen weight to body weight at the end of the experiment. C: Na+ excretion when rats were fed a 0.4% NaCl diet and weekly during the 4.0% NaCl period. D: K+ excretion when rats were fed the 0.4% NaCl diet and weekly during the 4.0% NaCl period. n = 11 for PBS-Lipo treatment, 5 for Clod treatmen, and 8 for Clod-Lipo treatment. Two-way repeated-measures ANOVA with a Holm-Sidak post hoc test was used for A, C, and D. One-way ANOVA was used in B. ***P < 0.001, **P < 0.01, and *P < 0.05 vs. PBS Lipo; ###P < 0.001 vs. Clod.
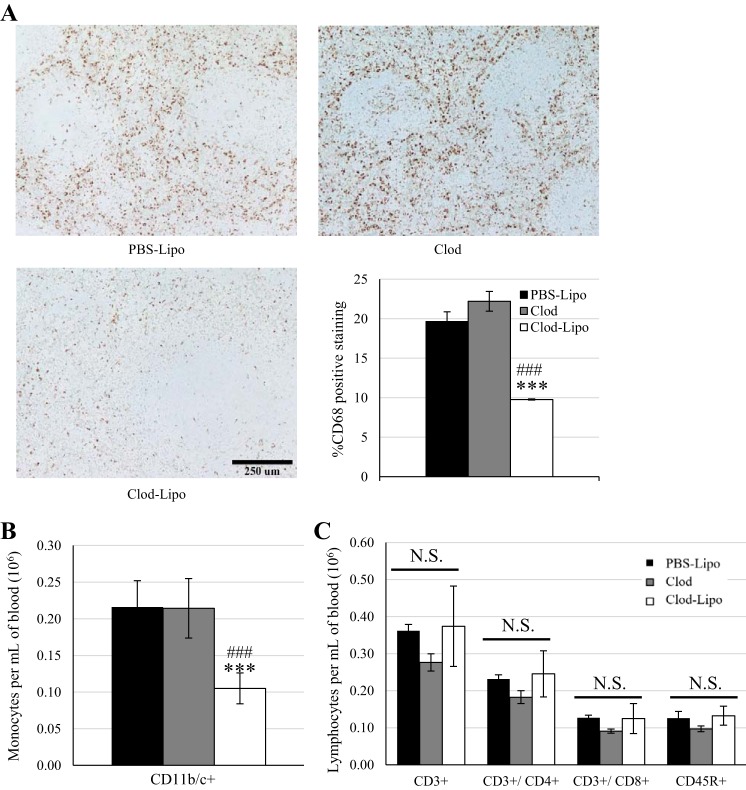
Clod-Lipo Decreases Circulating Monocytes and Splenic Macrophages in SS Rats
Although the number of circulating monocytes per milliter of blood was similar between PBS-Lipo- and Clod-treated animals, there was a significant depletion in those treated with the Clod-Lipo (P < 0.001; Fig. 7B). The number of CD3+ T cells and CD45R+ B cells per milliter of blood was not significantly different between animals treated with PBS-Lipo, Clod, and Clod-Lipo (Fig. 7C). Depletion of macrophages was confirmed with anti-CD68 staining in the spleen, where PBS-Lipo- and Clod-treated animals showed similar percentages of splenic tissue staining for CD68, but Clod-Lipo treated animals showed a reduction (P < 0.001; Fig. 7A).
Fig. 7.
A: CD68 staining for macrophages in the spleen. n = 6 for PBS-containing liposome (PBS-Lipo) treatment, 5 for clodronate (Clod) treatment, and 3 for Clod-containing liposome (Clod-Lipo) treatment. B: circulating CD11b/c+ myeloid cells. C: circulating CD3+ T cells and CD3+/CD4+ T helper cells, CD3+/CD8+ cytotoxic T cells, and CD45R+ B cells. All measurements were made at the end of the protocol to Dahl salt-sensitive (SS) rats that received PBS-Lipo (n = 11), Clod (n = 5), or Clod-Lipo (n = 7) on days −3, 4, 11, and 18 of the 21-day 4.0% NaCl chow feeding. One-way ANOVA with a Holm-Sidak post hoc test was used in B and C. ***P < 0.001 vs. PBS-Lipo; ###P < 0.05 vs. Clod. NS, not significant.
Liposome Delivery Enhances Clod-Dependent Exacerbation of Salt-Sensitive Hypertension and Renal Damage
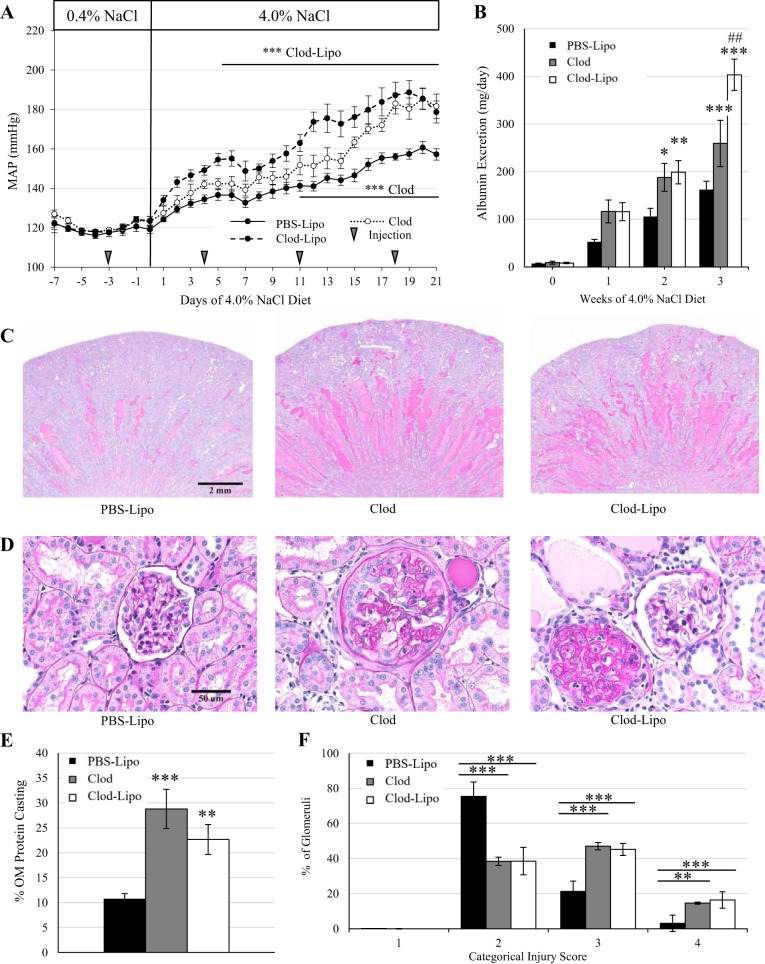
During baseline blood pressure recording, mean arterial pressures were comparable among groups while on the 0.4% NaCl diet, either before or after the first injection on day −3. However, starting on day 5 of the 4.0% NaCl diet in the animals that received Clod-Lipo and on day 11 in the animals that received Clod alone, there was an exacerbation in the salt-sensitive increase in blood pressure compared with PBS-Lipo-treated control animals. By the end of the study, Clod- and Clod-Lipo-treated animals developed a similar exacerbation of the salt-sensitive increase in blood pressure of ∼20 mmHg compared with PBS-Lipo-treated control animals (P < 0.001; Fig. 8A). It is important to note that the elevation in blood pressure seen in the animals that received Clod-Lipo was more rapid than those that received Clod alone.
Fig. 8.
A: mean arterial pressure over 7 days of the 0.4% NaCl diet and 21 days of the 4.0% NaCl diet. B: weekly albumin excretion. C: ×1 original magnification of periodic acid Schiff staining. D: ×40 representative images of glomeruli. E: quantification of protein casting in the renal outer medulla. F: glomerular injury assessed by assigning >50 glomeruli/kidney a categorical score of 0–4. n = 11 for PBS-containing liposome (PBS-Lipo) treatment, 5 for clodronate (Clod) treatment, and 8 for Clod-containing liposome (Clod-Lipo) treatment. Two-way repeated-measures ANOVA with a Holm-Sidak post hoc test was used for A, B, and F. One-way ANOVA with a Holm-Sidak post hoc test was used in E. ***P < 0.001, **P < 0.01, and *P < 0.05 vs. PBS-Lipo; ##P < 0.01 vs. Clod.
Albuminuria was similar across all groups during the 0.4% NaCl period as well as after 1 wk of the 4.0% NaCl diet. However, by the second week, both Clod- and Clod-Lipo-treated animals developed greater albuminuria compared with PBS-Lipo-treated control animals (P < 0.05, PBS-Lipo vs. Clod, and P < 0.01, PBS-Lipo vs. Clod-Lipo). By the third week of the 4.0% NaCl diet, Clod-treated animals sustained an elevated albuminuria (P < 0.001, PBS-Lipo vs. Clod); however, Clod-Lipo-treated animals showed a further elevation that was significantly greater than both Clod-treated animals (P < 0.01) and PBS-Lipo-treated control animals (P < 0.001; Fig. 8B). At the end of the study, renal damage was assessed histologically using periodic acid-Schiff staining to identify medullary protein casting. Both Clod- and Clod-Lipo-treated animals had significantly greater outer medullary protein casting (P < 0.001, Clod vs. PBS-Lipo, and P < 0.01, Clod-Lipo vs. PBS-Lipo) than PBS-Lipo-treated control animals (Fig. 8, C and E). In addition, categorical scoring of glomerular injury demonstrated that both Clod- and Clod-Lipo-treated animals had more glomeruli with scores of 3 and 4 than PBS-Lipo-treated animals (P < 0.001), indicating enhanced glomerular injury with these treatments (Fig. 8, D and F).
Liposome Delivery of Clodronate Reduces Renal Myeloid Cells and Enhances T and B Cell Presence in the Kidney
Clod treatment did not alter the number of renal CD3+ T cells, CD45R+ B cells, or CD11b/c+ myeloid cells compared with PBS-Lipo controls (P > 0.05). However, when delivered via liposome, there was a significant reduction in myeloid cells (P < 0.001 vs. Clod and PBS-Lipo) and a significant increase in renal T and B cells compared with PBS-Lipo controls (P < 0.001) or Clod treatment alone (P < 0.05; Fig. 9).
Fig. 9.
A: renal CD11b/c+ myeloid cells. B: renal CD3+ T cells, CD3+/CD4+ T helper cells, CD3+/CD8+ cytotoxic T cells, and CD45R+ B cells. All measurements were made at the end of the protocol to Dahl salt-sensitive (SS) rats that received PBS-containing liposomes (PBS-Lipo; n = 11), clodronate (Clod; n = 5), or Clod-containing liposomes (Clod-Lipo; n = 7) on days −3, 4, 11, and 18 of the 21-day 4.0% NaCl chow feeding. One-way ANOVA with a Holm-Sidak post hoc test was used. ***P < 0.001 vs. PBS-Lipo; #P < 0.05 vs. Clod.
DISCUSSION
The initial experiments confirmed that the SS strain develops renal damage, assessed by albuminuria, when switched to the 4.0% NaCl diet. This was present but attenuated in the SSBN13 strain and absent in SD animals. The SD strain demonstrated an elevated creatinine clearance on the 4.0% NaCl diet, which is known to occur when dietary salt is increased (14, 68). This increase was not observed in the SSBN13 or SS strains, confirming the impaired hemodynamic response of the Dahl rat (12), which is also observed in states of renal damage (4, 21).
The present study has demonstrated that there is a direct relationship between increases in renal immune cells and salt sensitivity of renal damage. As previously shown, the increase in dietary salt amplified renal immune cell numbers in SS animals (13). This is the first study to demonstrate that the salt-resistant phenotypes of the SD and SSBN13 strains are concordant with resistance to dietary salt-induced increases in renal immune cells. It is notable that, of the cell types assessed, the predominant immune cell subset that increased in the kidney was CD45+/CD11b/c+, indicative of cells of the myeloid lineage.
To assess functional differences of these cells, we analyzed the presence of M1 proinflammatory and M2 anti-inflammatory macrophages in the kidney. The increased dietary salt caused an increase in both M1 (CD86+) and M2 (CD163+) macrophages into SS kidneys that was not seen in SD or SSBN13 animals. This pattern of increased M1 macrophages is consistent with the renal injury induced by aldosterone plus salt treatment in Wistar rats (45). Although the immune system provides an inflammatory brake with M2 macrophages, it appears that in this disease progression they do not play a significant role in controlling the extent of renal damage during the malignant phase of hypertension seen in the SS rat. Interestingly, SSBN13 animals showed an elevation in the presence of these M2 cells while still on the 0.4%NaCl diet but again did not change when dietary salt was increased. Further studies will look at earlier stages of this pathology to clarify whether M2 cells play a role in contributing to the attenuation of the SS phenotypes seen in SSBN13 animals. It is unlikely that M2 activation is solely responsible for salt resistance, as salt-resistant SD and SS animals have a similar number of these cells while on the 0.4% NaCl diet. Although no difference in CD163 intensity was observed across any strain, an increase in CD86 mean fluorescent intensity, a proxy for surface expression (9, 33, 39, 44, 53), was observed in the salt-resistant SD and SSBN13 strains during high-salt feeding. Interestingly, the SS strain, which showed an increase in the number of CD68+/CD86+ M1 cells in the kidney when fed high salt, demonstrated no change in the mean fluorescent intensity ratio, consistent with other models of chronic kidney disease (17, 44). Further studies are necessary to investigate whether CD86+ pathway activation plays a role in determining the salt resistance seen in SD and SSBN13 strains.
To assess general activation of myeloid cells in SS rats, PCR profiling revealed an overall upregulation of TLR signaling, one of the primary paths for macrophage activation. Consistent with a previous report (67), the gene encoding the receptor for IL-6 was significantly upregulated in response to increased dietary salt, reinforcing the potential role of that pathway. In addition, three genes of interest that we have shown to be upregulated are CD14, TLR4, and Ly96 (MD2). These genes all encode proteins that work in tandem to respond to bacterial infection, whereby the partner molecule CD14 facilitates docking of the canonical ligand lipopolysaccharide to the TLR4/MD2 complex. Studies have specifically implicated a clinical significance for CD14 in the context of hypertensive and kidney pathology, as mutations in the CD14 gene or alterations in CD14 expression are associated with primary hypertension in children (40), IgA nephropathy (69), and cardiovascular disease (63). This complex provides a targetable pathway for further investigations into macrophage activation.
It is of note that the presented experiments were performed in male animals. Important recent work has demonstrated a fundamental difference between male and female animals in the development of hypertension and a role the immune system may or may not have. Seminal work by Sullivan et al. (71) demonstrated that female spontaneously hypertensive rats exhibit a severe attenuation in the age-dependent hypertension seen in their male counterparts, which was accompanied by a protection from renal damage and renal immune cell accumulation. This protection was lessened by ovariectomy, demonstrating a distinct role for female sex hormones in disease progression in this animal model. Further work by Ji et al. (30) used an elegant adoptive transfer technique to show that transfer of male T cells into RAG−/− mice exacerbated ANG II-induced hypertension, whereas transfer of female T cells had no effect. Furthermore, transfer of male cells resulted in an increase in renal CD4+ and CD8+ T cells, whereas transfer of female cells did not have this effect. Other work by Pollow et al. (61) has also shown in a similar experimental design that transfer of male T cells into male RAG−/− mice exacerbated ANG II-induced hypertension, whereas male T cells transferred into female RAG−/− mice had no effect. In either study, femaleness of either T cell donors or recipients conferred protection from the full development of hypertension seen in male animals. Most recently, a new model of ovary-intact 4-vinylcyclohexene diepoxide-induced menopause, such as the work by Sullivan et al. (71), demonstrated that loss of female sex hormones removes the protection from T cell-exacerbated ANG II-induced hypertension and renal macrophage accumulation (60). In addition, this study pointed to a vital role of T regulatory cells in modulating the hypertensive phenotype, similar to what has been shown in male mice. The hypertensive model discussed in the presented work uses the Dahl SS model, which exhibits a similar protective role of female sex hormones (6, 25, 26, 41) from the full extent of salt-induced hypertension but not on baseline blood pressures (57). Although other models show an increase in renal macrophages in the female Dahl SS rat (15), there are currently no studies documenting the interaction between female sex hormones and renal immune cells, including the macrophage, in the Dahl SS rat.
Although the nuances of macrophage activation are a field in and of themselves, this study also looked to gauge the general contribution of the macrophage to the development of salt-sensitive hypertension and renal damage through macrophage depletion. Mixed results by various investigators have indicated that Clod-Lipo treatment had the potential to deplete macrophages and attenuate ANG II-induced, Dahl SS, and DOCA-salt hypertension (28, 73). However, alternative studies have demonstrated that this treatment instead induced salt sensitivity in the SD rat (43) or had no effect on clinical parameters of aldosterone plus salt treatment (45). In the present study, we demonstrated that Clod-Lipo treatment depleted macrophages in the spleen, blood, and kidneys of SS rats fed a 4.0% NaCl diet. Most interestingly, depletion of myeloid cells in Dahl SS animals resulted in an increase in adaptive immune cells in the kidney and an amplification of salt-sensitive hypertension and renal damage. To investigate potential mechanisms, we observed that treatment with Clod alone, in the absence of liposomes (to facilitate uptake into macrophages), also led to an amplification of salt-sensitive hypertension and renal damage in the absence of macrophage depletion. This surprising finding is not explained but indicates that effects of Clod, a bisphosphonate, amplify the disease phenotype independently of macrophage depletion. One potential explanation is the reported renal toxicity associated with bisphosphonate compounds (27, 59). Specifically, Clod, as well as other bisphosphonates, has been shown to have cytotoxic effects on cultured rat (NRK-52E) and human (HK-2) kidney epithelial cells (42), indicating that possible leak/release of this drug into the renal interstitium may induce unintended tissue damage. There is distinct evidence that Clod itself can induce renal ischemia in humans (56) due to drug-induced vasculitis and may have detrimental ocular effects (16). Additional evidence shows that liposome encapsulation of another bisphosphonate, zoledronic acid, exacerbated toxicity and increased mortality in a mouse tumor model (70). Therefore, this liposome delivery of Clod method has not yet reached the specificity needed to target the macrophage exclusively without adverse effects.
A limitation of this study design is that the difference between infiltrating cells and expansion of tissue resident cells was not directly investigated, although we believe that the magnitude of renal immune cell expansion is far too great to solely be resident cell expansion. A second limitation of this study is that we did not assess changes to the immune cell profile during the early phase of this disease progression, which may provide additional information related to the initial recruitment and expansion of renal immune cells.
In summary, this study has shown that 1) salt sensitivity is associated with increases in all renal immune cell subsets assessed, predominantly CD11bc+ myeloid cells; 2) M1 polarization dominates the macrophage inflammatory spectrum with a scarce presence of M2 macrophages; 3) Clod-Lipo can deplete circulating, splenic, and renal myeloid cells in SS rats after increasing dietary salt that results in exacerbated renal lymphocyte infiltration, tissue injury, and SS hypertension; and 4) Clod by itself appears to induce enhanced renal damage and SS hypertension.
GRANTS
This work was supported by National Institutes of Health Grants HL-116264, HL-137748, 18-PRE-3400038, and 1-F31-HL-144084-01.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.J.F. and D.L.M. conceived and designed research; D.J.F., J.M.A.-B., J.H.D., and H.L. performed experiments; D.J.F. analyzed data; D.J.F. and D.L.M. interpreted results of experiments; D.J.F. prepared figures; D.J.F. drafted manuscript; D.J.F., J.M.A.-B., J.H.D., H.L., and D.L.M. edited and revised manuscript; D.J.F., J.M.A.-B., J.H.D., H.L., and D.L.M. approved final version of manuscript.
REFERENCES
- 1.Abais-Battad JM, Lund H, Fehrenbach DJ, Dasinger JH, Alsheikh AJ, Mattson DL. Parental dietary protein source and the role of CMKLR1 in determining the severity of Dahl salt-sensitive hypertension. Hypertension 73: 440–448, 2019. doi: 10.1161/HYPERTENSIONAHA.118.11994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abais-Battad JM, Lund H, Fehrenbach DJ, Dasinger JH, Mattson DL. Rag1-null Dahl SS rats reveal that adaptive immune mechanisms exacerbate high protein-induced hypertension and renal injury. Am J Physiol Regul Integr Comp Physiol 315: R28–R35, 2018. doi: 10.1152/ajpregu.00201.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bigazzi R, Bianchi S, Baldari D, Sgherri G, Baldari G, Campese VM. Microalbuminuria in salt-sensitive patients. A marker for renal and cardiovascular risk factors. Hypertension 23: 195–199, 1994. doi: 10.1161/01.HYP.23.2.195. [DOI] [PubMed] [Google Scholar]
- 4.Boesen EI, Pollock JS, Pollock DM. Contrasting effects of intervention with ETA and ETB receptor antagonists in hypertension induced by angiotensin II and high-salt diet. Can J Physiol Pharmacol 88: 802–807, 2010. doi: 10.1139/Y10-051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bomfim GF, Rodrigues FL, Carneiro FS. Are the innate and adaptive immune systems setting hypertension on fire? Pharmacol Res 117: 377–393, 2017. doi: 10.1016/j.phrs.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Brinson KN, Rafikova O, Sullivan JC. Female sex hormones protect against salt-sensitive hypertension but not essential hypertension. Am J Physiol Regul Integr Comp Physiol 307: R149–R157, 2014. doi: 10.1152/ajpregu.00061.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buiting AM, Van Rooijen N. Liposome mediated depletion of macrophages: an approach for fundamental studies. J Drug Target 2: 357–362, 1994. doi: 10.3109/10611869408996810. [DOI] [PubMed] [Google Scholar]
- 8.Campese VM. Salt sensitivity in hypertension. Renal and cardiovascular implications. Hypertension 23: 531–550, 1994. doi: 10.1161/01.HYP.23.4.531. [DOI] [PubMed] [Google Scholar]
- 9.Corsini E, Galbiati V, Esser PR, Pinto A, Racchi M, Marinovich M, Martin SF, Galli CL. Role of PKC-β in chemical allergen-induced CD86 expression and IL-8 release in THP-1 cells. Arch Toxicol 88: 415–424, 2014. doi: 10.1007/s00204-013-1144-z. [DOI] [PubMed] [Google Scholar]
- 10.Cowley AW Jr, Roman RJ. The role of the kidney in hypertension. JAMA 275: 1581–1589, 1996. doi: 10.1001/jama.1996.03530440061038. [DOI] [PubMed] [Google Scholar]
- 11.Cowley AW Jr, Roman RJ, Kaldunski ML, Dumas P, Dickhout JG, Greene AS, Jacob HJ. Brown Norway chromosome 13 confers protection from high salt to consomic Dahl S rat. Hypertension 37: 456–461, 2001. doi: 10.1161/01.HYP.37.2.456. [DOI] [PubMed] [Google Scholar]
- 12.Cowley AW Jr, Ryan RP, Kurth T, Skelton MM, Schock-Kusch D, Gretz N. Progression of glomerular filtration rate reduction determined in conscious Dahl salt-sensitive hypertensive rats. Hypertension 62: 85–90, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Miguel C, Das S, Lund H, Mattson DL. T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 298: R1136–R1142, 2010. doi: 10.1152/ajpregu.00298.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunn SR, Qi Z, Bottinger EP, Breyer MD, Sharma K. Utility of endogenous creatinine clearance as a measure of renal function in mice. Kidney Int 65: 1959–1967, 2004. doi: 10.1111/j.1523-1755.2004.00600.x. [DOI] [PubMed] [Google Scholar]
- 15.Fernandes R, Garver H, Harkema JR, Galligan JJ, Fink GD, Xu H. Sex Differences in Renal Inflammation and Injury in High-Fat Diet-Fed Dahl Salt-Sensitive Rats. Hypertension 72: e43–e52, 2018. doi: 10.1161/HYPERTENSIONAHA.118.11485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fietta P, Manganelli P, Lodigiani L. Clodronate induced uveitis. Ann Rheum Dis 62: 378, 2003. doi: 10.1136/ard.62.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girndt M, Sester M, Sester U, Kaul H, Köhler H. Defective expression of B7-2 (CD86) on monocytes of dialysis patients correlates to the uremia-associated immune defect. Kidney Int 59: 1382–1389, 2001. doi: 10.1046/j.1523-1755.2001.0590041382.x. [DOI] [PubMed] [Google Scholar]
- 18.Gordon S. Alternative activation of macrophages. Nat Rev Immunol 3: 23–35, 2003. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 19.Grim CE, Wilson TW, Nicholson GD, Hassell TA, Fraser HS, Grim CM, Wilson DM. Blood pressure in blacks. Twin studies in Barbados. Hypertension 15: 803–809, 1990. doi: 10.1161/01.HYP.15.6.803. [DOI] [PubMed] [Google Scholar]
- 20.Guiteras R, Flaquer M, Cruzado JM. Macrophage in chronic kidney disease. Clin Kidney J 9: 765–771, 2016. doi: 10.1093/ckj/sfw096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haller ST, Kumarasamy S, Folt DA, Wuescher LM, Stepkowski S, Karamchandani M, Waghulde H, Mell B, Chaudhry M, Maxwell K, Upadhyaya S, Drummond CA, Tian J, Filipiak WE, Saunders TL, Shapiro JI, Joe B, Cooper CJ. Targeted disruption of Cd40 in a genetically hypertensive rat model attenuates renal fibrosis and proteinuria, independent of blood pressure. Kidney Int 91: 365–374, 2017. doi: 10.1016/j.kint.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrison DG. The immune system in hypertension. Trans Am Clin Climatol Assoc 125: 130–138, 2014. [PMC free article] [PubMed] [Google Scholar]
- 23.Harwani SC. Macrophages under pressure: the role of macrophage polarization in hypertension. Transl Res 191: 45–63, 2018. doi: 10.1016/j.trsl.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higaki A, Caillon A, Paradis P, Schiffrin EL. Innate and innate-like immune system in hypertension and vascular injury. Curr Hypertens Rep 21: 4, 2019. doi: 10.1007/s11906-019-0907-1. [DOI] [PubMed] [Google Scholar]
- 25.Hinojosa-Laborde C, Craig T, Zheng W, Ji H, Haywood JR, Sandberg K. Ovariectomy augments hypertension in aging female Dahl salt-sensitive rats. Hypertension 44: 405–409, 2004. doi: 10.1161/01.HYP.0000142893.08655.96. [DOI] [PubMed] [Google Scholar]
- 26.Hinojosa-Laborde C, Lange DL, Haywood JR. Role of female sex hormones in the development and reversal of dahl hypertension. Hypertension 35: 484–489, 2000. doi: 10.1161/01.HYP.35.1.484. [DOI] [PubMed] [Google Scholar]
- 27.Hirschberg R. Renal complications from bisphosphonate treatment. Curr Opin Support Palliat Care 6: 342–347, 2012. doi: 10.1097/SPC.0b013e328356062e. [DOI] [PubMed] [Google Scholar]
- 28.Huang L, Wang A, Hao Y, Li W, Liu C, Yang Z, Zheng F, Zhou MS. Macrophage depletion lowered blood pressure and attenuated hypertensive renal injury and fibrosis. Front Physiol 9: 473, 2018. doi: 10.3389/fphys.2018.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughson MD, Gobe GC, Hoy WE, Manning RD Jr, Douglas-Denton R, Bertram JF. Associations of glomerular number and birth weight with clinicopathological features of African Americans and whites. Am J Kidney Dis 52: 18–28, 2008. doi: 10.1053/j.ajkd.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 30.Ji H, Zheng W, Li X, Liu J, Wu X, Zhang MA, Umans JG, Hay M, Speth RC, Dunn SE, Sandberg K. Sex-specific T-cell regulation of angiotensin II-dependent hypertension. Hypertension 64: 573–582, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson RJ, Rodriguez-Iturbe B, Nakagawa T, Kang DH, Feig DI, Herrera-Acosta J. Subtle renal injury is likely a common mechanism for salt-sensitive essential hypertension. Hypertension 45: 326–330, 2005. doi: 10.1161/01.HYP.0000154784.14018.5f. [DOI] [PubMed] [Google Scholar]
- 32.Juhas U, Ryba-Stanisławowska M, Szargiej P, Myśliwska J. Different pathways of macrophage activation and polarization. Postepy Hig Med Dosw 69: 496–502, 2015. doi: 10.5604/17322693.1150133. [DOI] [PubMed] [Google Scholar]
- 33.Kawarabayashi R, Motoyama K, Nakamura M, Yamazaki Y, Morioka T, Mori K, Fukumoto S, Imanishi Y, Shioi A, Shoji T, Emoto M, Inaba M. The association between monocyte surface CD163 and insulin resistance in patients with type 2 diabetes. J Diabetes Res 2017: 1–8, 2017. doi: 10.1155/2017/6549242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawasaki T, Delea CS, Bartter FC, Smith H. The effect of high-sodium and low-sodium intakes on blood pressure and other related variables in human subjects with idiopathic hypertension. Am J Med 64: 193–198, 1978. doi: 10.1016/0002-9343(78)90045-1. [DOI] [PubMed] [Google Scholar]
- 35.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 365: 217–223, 2005. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 36.Kotchen TA, Cowley AW Jr, Frohlich ED. Salt in health and disease−a delicate balance. N Engl J Med 368: 1229–1237, 2013. doi: 10.1056/NEJMra1212606. [DOI] [PubMed] [Google Scholar]
- 37.Li L, Wei W, Li Z, Chen H, Li Y, Jiang W, Chen W, Kong G, Yang J, Li Z. The spleen promotes the secretion of CCL2 and supports an M1 dominant phenotype in hepatic macrophages during liver fibrosis. Cell Physiol Biochem 51: 557–574, 2018. doi: 10.1159/000495276. [DOI] [PubMed] [Google Scholar]
- 38.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, , et al. . A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2224–2260, 2012. [Erratum in Lancet 381: 1276, 2013.] doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lisi L, Ciotti GM, Braun D, Kalinin S, Currò D, Dello Russo C, Coli A, Mangiola A, Anile C, Feinstein DL, Navarra P. Expression of iNOS, CD163 and ARG-1 taken as M1 and M2 markers of microglial polarization in human glioblastoma and the surrounding normal parenchyma. Neurosci Lett 645: 106–112, 2017. doi: 10.1016/j.neulet.2017.02.076. [DOI] [PubMed] [Google Scholar]
- 40.Litwin M, Michałkiewicz J, Trojanek J, Niemirska A, Wierzbicka A, Szalecki M. Altered genes profile of renin-angiotensin system, immune system, and adipokines receptors in leukocytes of children with primary hypertension. Hypertension 61: 431–436, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00181. [DOI] [PubMed] [Google Scholar]
- 41.Ludvigsen S, Mancusi C, Kildal S, de Simone G, Gerdts E, Ytrehus K. Cardiac adaptation to hypertension in adult female Dahl salt-sensitive rats is dependent on ovarian function, but loss of ovarian function does not predict early maladaptation. Physiol Rep 6: e13593, 2018. doi: 10.14814/phy2.13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lühe A, Künkele KP, Haiker M, Schad K, Zihlmann C, Bauss F, Suter L, Pfister T. Preclinical evidence for nitrogen-containing bisphosphonate inhibition of farnesyl diphosphate (FPP) synthase in the kidney: implications for renal safety. Toxicol In Vitro 22: 899–909, 2008. doi: 10.1016/j.tiv.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Machnik A, Dahlmann A, Kopp C, Goss J, Wagner H, van Rooijen N, Eckardt KU, Müller DN, Park JK, Luft FC, Kerjaschki D, Titze J. Mononuclear phagocyte system depletion blocks interstitial tonicity-responsive enhancer binding protein/vascular endothelial growth factor C expression and induces salt-sensitive hypertension in rats. Hypertension 55: 755–761, 2010. doi: 10.1161/HYPERTENSIONAHA.109.143339. [DOI] [PubMed] [Google Scholar]
- 44.Makidon PE, Smith DM, Groom Ii JV, Cao Z, Landers JJ, Baker JR Jr. Effect of chronic uremia on the cell surface expression of B7 family costimulatory molecules in an HLA-A2 transgenic mouse model of chronic kidney disease. Comp Med 65: 308–314, 2015. [PMC free article] [PubMed] [Google Scholar]
- 45.Martín-Fernández B, Rubio-Navarro A, Cortegano I, Ballesteros S, Alía M, Cannata-Ortiz P, Olivares-Álvaro E, Egido J, de Andrés B, Gaspar ML, de Las Heras N, Lahera V, Moreno JA. Aldosterone Induces Renal Fibrosis and Inflammatory M1-Macrophage Subtype via Mineralocorticoid Receptor in Rats. PLoS One 11: e0145946, 2016. doi: 10.1371/journal.pone.0145946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6: 13, 2014. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mattson DL. Infiltrating immune cells in the kidney in salt-sensitive hypertension and renal injury. Am J Physiol Renal Physiol 307: F499–F508, 2014. doi: 10.1152/ajprenal.00258.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mattson DL, Dwinell MR, Greene AS, Kwitek AE, Roman RJ, Jacob HJ, Cowley AW Jr. Chromosome substitution reveals the genetic basis of Dahl salt-sensitive hypertension and renal disease. Am J Physiol Renal Physiol 295: F837–F842, 2008. doi: 10.1152/ajprenal.90341.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 48: 149–156, 2006. doi: 10.1161/01.HYP.0000228320.23697.29. [DOI] [PubMed] [Google Scholar]
- 50.Mattson DL, Kunert MP, Kaldunski ML, Greene AS, Roman RJ, Jacob HJ, Cowley AW Jr. Influence of diet and genetics on hypertension and renal disease in Dahl salt-sensitive rats. Physiol Genomics 16: 194–203, 2004. doi: 10.1152/physiolgenomics.00151.2003. [DOI] [PubMed] [Google Scholar]
- 51.Mattson DL, Lund H, Guo C, Rudemiller N, Geurts AM, Jacob H. Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. Am J Physiol Regul Integr Comp Physiol 304: R407–R414, 2013. doi: 10.1152/ajpregu.00304.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCormick CP, Rauch AL, Buckalew VM Jr. Differential effect of dietary salt on renal growth in Dahl salt-sensitive and salt-resistant rats. Hypertension 13: 122–127, 1989. doi: 10.1161/01.HYP.13.2.122. [DOI] [PubMed] [Google Scholar]
- 53.Mitachi T, Mezaki M, Yamashita K, Itagaki H. Acidic conditions induce the suppression of CD86 and CD54 expression in THP-1 cells. J Toxicol Sci 43: 299–309, 2018. doi: 10.2131/jts.43.299. [DOI] [PubMed] [Google Scholar]
- 54.Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S, Inenaga T, Kimura G. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet 350: 1734–1737, 1997. doi: 10.1016/S0140-6736(97)05189-1. [DOI] [PubMed] [Google Scholar]
- 55.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8: 958–969, 2008. [Erratum in Nat Rev Immunol 10: 460, 2010.] doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Notarnicola A, Maccagnano G, Casalino A, Moretti L, Piazzolla A, Moretti B. Bilateral renal ischemia after kyphoplasty and clodronate treatment: a case report. J Med Case Reports 8: 76, 2014. doi: 10.1186/1752-1947-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pai AV, West CA, de Souza AM, Cheng X, West DA Jr, Ji H, Wu X, Baylis C, Sandberg K. Salt-sensitive (Rapp) rats from Envigo spontaneously develop accelerated hypertension independent of ovariectomy on a low-sodium diet. Am J Physiol Regul Integr Comp Physiol 315: R915–R924, 2018. doi: 10.1152/ajpregu.00449.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pechman KR, Basile DP, Lund H, Mattson DL. Immune suppression blocks sodium-sensitive hypertension following recovery from ischemic acute renal failure. Am J Physiol Regul Integr Comp Physiol 294: R1234–R1239, 2008. doi: 10.1152/ajpregu.00821.2007. [DOI] [PubMed] [Google Scholar]
- 59.Perazella MA, Markowitz GS. Bisphosphonate nephrotoxicity. Kidney Int 74: 1385–1393, 2008. doi: 10.1038/ki.2008.356. [DOI] [PubMed] [Google Scholar]
- 60.Pollow DP, Uhlorn JA, Sylvester MA, Romero-Aleshire MJ, Uhrlaub JL, Lindsey ML, Nikolich-Zugich J, Brooks HL. Menopause and FOXP3+ Treg cell depletion eliminate female protection against T cell-mediated angiotensin II hypertension. Am J Physiol Heart Circ Physiol. In press. doi: 10.1152/ajpheart.00792.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pollow DP, Uhrlaub J, Romero-Aleshire M, Sandberg K, Nikolich-Zugich J, Brooks HL, Hay M. Sex differences in T-lymphocyte tissue infiltration and development of angiotensin II hypertension. Hypertension 64: 384–390, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poulter NR, Prabhakaran D, Caulfield M. Hypertension. Lancet 386: 801–812, 2015. doi: 10.1016/S0140-6736(14)61468-9. [DOI] [PubMed] [Google Scholar]
- 63.Reiner AP, Lange EM, Jenny NS, Chaves PH, Ellis J, Li J, Walston J, Lange LA, Cushman M, Tracy RP. Soluble CD14: genomewide association analysis and relationship to cardiovascular risk and mortality in older adults. Arterioscler Thromb Vasc Biol 33: 158–164, 2013. doi: 10.1161/ATVBAHA.112.300421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodríguez-Iturbe B, Pons H, Quiroz Y, Johnson RJ. The immunological basis of hypertension. Am J Hypertens 27: 1327–1337, 2014. doi: 10.1093/ajh/hpu142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodríguez-Iturbe B, Pons H, Quiroz Y, Lanaspa MA, Johnson RJ. Autoimmunity in the pathogenesis of hypertension. Nat Rev Nephrol 10: 56–62, 2014. doi: 10.1038/nrneph.2013.248. [DOI] [PubMed] [Google Scholar]
- 66.Rubio-Navarro A, Guerrero-Hue M, Martín-Fernandez B, Cortegano I, Olivares-Alvaro E, de Las Heras N, Alía M, de Andrés B, Gaspar ML, Egido J, Moreno JA. Phenotypic characterization of macrophages from rat kidney by flow cytometry. J Vis Exp (116): 2016. doi: 10.3791/54599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rudemiller N, Lund H, Jacob HJ, Geurts AM, Mattson DL; PhysGen Knockout Program . CD247 modulates blood pressure by altering T-lymphocyte infiltration in the kidney. Hypertension 63: 559–564, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ruta LA, Dickinson H, Thomas MC, Denton KM, Anderson WP, Kett MM. High-salt diet reveals the hypertensive and renal effects of reduced nephron endowment. Am J Physiol Renal Physiol 298: F1384–F1392, 2010. doi: 10.1152/ajprenal.00049.2010. [DOI] [PubMed] [Google Scholar]
- 69.Shinzawa M, Yamamoto R, Nagasawa Y, Shoji T, Obi Y, Namba T, Kitamura H, Kaneko T, Okada N, Iwatani H, Yamauchi A, Tsubakihara Y, Imai E, Isaka Y, Rakugi H. Gene polymorphisms contributing to hypertension in immunoglobulin A nephropathy. Clin Exp Nephrol 16: 250–258, 2012. doi: 10.1007/s10157-011-0553-7. [DOI] [PubMed] [Google Scholar]
- 70.Shmeeda H, Amitay Y, Tzemach D, Gorin J, Gabizon A. Liposome encapsulation of zoledronic acid results in major changes in tissue distribution and increase in toxicity. J Control Release 167: 265–275, 2013. doi: 10.1016/j.jconrel.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 71.Sullivan JC, Semprun-Prieto L, Boesen EI, Pollock DM, Pollock JS. Sex and sex hormones influence the development of albuminuria and renal macrophage infiltration in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 293: R1573–R1579, 2007. doi: 10.1152/ajpregu.00429.2007. [DOI] [PubMed] [Google Scholar]
- 72.Tang PM, Nikolic-Paterson DJ, Lan HY. Macrophages: versatile players in renal inflammation and fibrosis. Nat Rev Nephrol 15: 144–158, 2019. doi: 10.1038/s41581-019-0110-2. [DOI] [PubMed] [Google Scholar]
- 73.Thang LV, Demel SL, Crawford R, Kaminski NE, Swain GM, Van Rooijen N, Galligan JJ. Macrophage depletion lowers blood pressure and restores sympathetic nerve α2-adrenergic receptor function in mesenteric arteries of DOCA-salt hypertensive rats. Am J Physiol Heart Circ Physiol 309: H1186–H1197, 2015. doi: 10.1152/ajpheart.00283.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tian S, Zhang L, Tang J, Guo X, Dong K, Chen SY. HMGB1 exacerbates renal tubulointerstitial fibrosis through facilitating M1 macrophage phenotype at the early stage of obstructive injury. Am J Physiol Renal Physiol 308: F69–F75, 2015. doi: 10.1152/ajprenal.00484.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 37: 429–432, 2001. doi: 10.1161/01.HYP.37.2.429. [DOI] [PubMed] [Google Scholar]