Abstract
Background
Mutations in the transcription factor hepatocyte nuclear factor 1B (HNF1B) are the most common inherited cause of renal malformations, yet also associated with renal tubular dysfunction, most prominently magnesium wasting with hypomagnesemia. The presence of hypomagnesemia has been proposed to help select appropriate patients for genetic testing. Yet, in a large cohort, hypomagnesemia was discriminatory only in adult, but not in pediatric patients. We therefore investigated whether hypomagnesemia and other biochemical changes develop with age.
Methods
We performed a retrospective analysis of clinical, biochemical, and genetic results of pediatric patients with renal malformations tested for HNF1B mutations, separated into 4 age groups. Values were excluded if concurrent estimated glomerular filtration rate (eGFR) was <30 ml/min per 1.73 m2, or after transplantation.
Results
A total of 199 patients underwent HNF1B genetic testing and mutations were identified in 52 (mut+). The eGFRs were comparable between mut+ and mut− in any age group. Although median plasma magnesium concentrations differed significantly between mut+ and mut− patients in all age groups, overt hypomagnesemia was not present until the second half of childhood in the mut+ group. There was also a significant difference in median potassium concentrations in late childhood with lower values in the mut+ cohort.
Conclusions
The abnormal tubular electrolyte handling associated with HNF1B mutations develops with age and is not restricted to magnesium, but consistent with a more generalized dysfunction of the distal convoluted tubule, reminiscent of Gitelman syndrome. The absence of these abnormalities in early childhood should not preclude HNF1B mutations from diagnostic considerations.
Keywords: alkalosis, children, HNF1B, hypokalemia, hypomagnesemia, renal tubular function
HNF1B is a transcription factor highly expressed in the developing kidney, genital tract, pancreas, and liver.1 Heterozygote mutations in the encoding gene lead to autosomal dominant tubulointerstitial kidney disease–HNF1B,2 and besides cystic kidney disease the clinical spectrum can include renal malformations, diabetes, genital tract abnormalities, exocrine pancreatic insufficency, and gout.3, 4 The high clinical variability, a spontaneous mutation rate of approximately 50%, and variable penetrance hamper clinical diagnosis.5
Previously, we reported hypomagnesemia as part of the clinical spectrum, suggesting a role for HNF1B not only in morphological renal development, but also in the maintenance of tubular function.6 To rationalize patient selection for genetic testing, a clinical tool had subsequently been proposed that predicts the presence of HNF1B mutations based on a score derived from several clinical features, including hypomagnesemia.7 Yet, when this score was applied to a large cohort in the United Kingdom, which included patients investigated here, hypomagnesemia was found to be discriminatory only in adult patients, not in children.8 In another predominantly pediatric cohort, hypomagnesemia was present in only a quarter of patients with HNF1B mutations.9
HNF1B-associated hypomagnesemia is associated with altered transactivation of the gamma-subunit of the Na+-K+-ATPase in the distal convoluted tubule (DCT), which regulates epithelial ion transport.6, 10 Impaired general transport activity in the DCT is usually associated with a Gitelman-like tubulopathy, consisting of hypokalemic hypochloraemic alkalosis with hypocalciuria, in addition to hypomagnesemia.11 We prevously reported hypocalciuria in children with HNF1B mutations, but had not investigated hypokalemia, hypochloremia, or alkalosis, although hypokalemia has previously been reported in adult patients.6, 12
We hypothesized that the electrolyte abnormalities associated with HNF1B mutations develop during childhood and therefore the application of the score in younger children may wrongly predict the absence of a mutation. We thus decided to assess this in our cohort of children with renal malformations with and without identified HNF1B mutations.
Methods
Patients
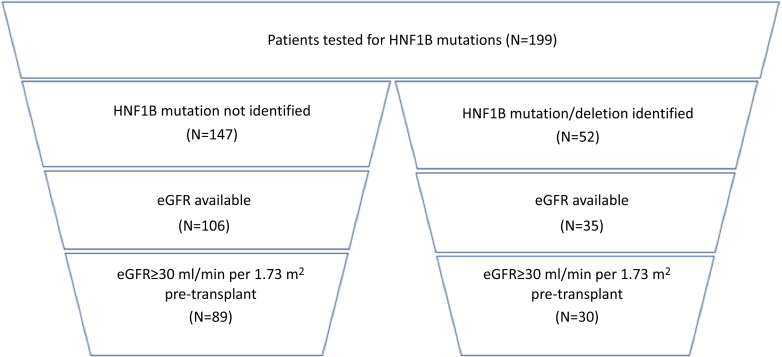
We performed a retrospective analysis of clinical, biochemical, and genetic results of patients tested for HNF1B mutations seen at Great Ormond Street Hospital with chronic kidney disease stage 1 to 3 between 2000 and 2017. Mutation analysis had been performed at the discretion of the individual treating physician and patients’ leucocyte DNA was screened for HNF1B mutations as described previously.5, 6, 13, 14 An overview of patient and data selection is given in Figure 1.
Figure 1.
Funnel diagram of patient identification. Shown is the number (n) of patients included in the analysis. A total of 199 patients with renal malformations were identified that had testing for HNF1B performed. After exclusion of those without an available glomerular filtration rate (GFR; measured or estimated) and those with a GFR of <30 ml/min per 1.73 m2, 30 mut+ and 89 mut− patients remained with biochemical values suitable for analysis. eGFR, estimated glomerular filtration rate.
Biochemical Data
Biochemical and clinical data were retrieved from relevant hospital databases. Results were anonymized and analyzed.
Plasma and urine biochemistries were obtained and compared between those with confirmed HNF1B mutations (mut+) and those without (mut−).
Formal measured glomerular filtration rates were used if available. Clinical parameters were otherwise used to calculate eGFR using the Schwartz-Haycock formula with the factor k specifically adapted to our hospital laboratory, as described previously.15 Results with a concurrent measured or eGFR below 30 ml/min per 1.73 m2 and all results post transplant were excluded.
All available results for the following biochemical parameters were obtained: plasma concentrations of sodium, potassium, magnesium, chloride, calcium, phosphate, and bicarbonate (measured as total CO2), as well as urine calcium/creatinine ratios. As the normal range for urine calcium/creatinine ratio changes with age, ratios were normalized to the respective upper limit of the normal range, as described previously.16
Results were then separated into 4 age groups: 0 to <4.5, 4.5 to <9, 9 to <13.5, and 13.5 to 18 years of age.
If within 1 age group more than 1 value per biochemical parameter was availble for an individual patient, we calculated median values to exclude bias from overrepresentation of patients with more available results.
HNF1B Score
We identified those patients who had been included in a previous study of the HNF1B score8 and who were included in this study because of available biochemistries. The previously calculated HNF1B score was retrieved and adjusted using the latest plasma magnesium concentration.
Statistical Analysis
Statistical analysis was performed using R (Vienna, Austria).17 Nonparametric Wilcoxon and Fisher’s exact tests were implemented for statistical analysis.
We used Fisher’s exact test (2-tailed) to assess the siginificance of the difference in number of patients with abnormal results, that is, below (Mg, K, and Cl) or above (bicarbonate) the respective reference range between the mut+ and mut− groups.
We used the Wilcoxon test to compare all the numerical values in the respective groups to determine the signficance of the difference in the medians.
Results
Patients
A total of 199 children had genetic testing for HNF1B performed, 72 of whom had also been included in our initial report of the association of hypomagnesemia with HNF1B.6 In 52 patients (26%), mutations were identified, constituting the mut+ cohort, most (n = 33) being whole gene deletions. As in our previous review, no difference in electrolyte patterns could be seen between patients with intragenic mutations versus whole gene deletions (data not shown).6
The remaining 147 patients constituted the mut− cohort. There was no significant (P = 0.3–0.7) difference between the 2 cohorts (mut+ vs. mut−) with respect to glomerular filtration rates in any age group (Table 1).
Table 1.
Plasma and urine biochemistry values
| Parameters by age, yr | n (mut+) | mut+ Median (range) |
mut− Median (range) |
Wilcoxon |
|---|---|---|---|---|
| GFR | ||||
| 0–4.5 | 76 (20) | 61 (43–91) | 70 (32–117) | 0.4 |
| 4.5–9.0 | 51 (11) | 69 (31–95) | 60 (30–115) | 0.4 |
| 9.0–13.5 | 43 (11) | 71 (43–101) | 62 (30–106) | 0.3 |
| 13.5–18.0 | 26 (5) | 59 (36–78) | 62 (32–109) | 0.7 |
| Magnesium | Normal >0.66 (<9 yr) or 0.7 (>9 yr) mmol/l | |||
| 0–4.5 | 71 (18) | 0.76 (0.53–0.88) | 0.83 (0.61–1.11) | 0.004a |
| 4.5–9.0 | 49 (10) | 0.69 (0.52–0.77) | 0.77 (0.52–0.97) | 0.005 |
| 9.0–13.5 | 40 (10) | 0.57 (0.45–0.77) | 0.81 (0.64–0.96) | 0.00002a |
| 13.5–18.0 | 24 (5) | 0.53 (0.5–0.64) | 0.84 (0.61–1.01) | 0.001a |
| Potassium | Normal >3.5 mmol/l | |||
| 0–4.5 | 73 (20) | 4.2 (3.8–5.3) | 4.2 (3.8–5.3) | 0.4 |
| 4.5–9.0 | 51 (11) | 4.1 (3.7–4.6) | 4.1 (2.8–5.6) | 0.09 |
| 9.0–13.5 | 43 (11) | 3.9 (3.3–5.1) | 4.2 (3.3–5.0) | 0.09 |
| 13.5–18.0 | 26 (5) | 3.6 (3.4–4.0) | 4.2 (3.4–4.9) | 0.02a |
| Chloride | Normal >100 mmol/l | |||
| 0–4.5 | 14 (3) | 106 (101–106) | 105 (94–108) | 1 |
| 4.5–9.0 | 14 (4) | 104 (101–107) | 106 (101–112) | 0.9 |
| 9.0–13.5 | 16 (4) | 101 (101–109) | 104 (97–109) | 0.26 |
| 13.5–18.0 | 6 (3) | 100 (99–100) | 103 (101–109) | 0.14 |
| Bicarbonate | Normal <30 mmol/l | |||
| 0–4.5 | 71 (20) | 24 (16–27) | 22 (15–34) | 0.11 |
| 4.5–9.0 | 48 (11) | 25 (22–29) | 25 (18–29) | 0.09 |
| 9.0–13.5 | 41 (11) | 28 (24–31) | 23 (18–30) | 0.0002a |
| 13.5–18.0 | 23 (5) | 27 (25–31) | 24 (18–28) | 0.0007a |
| Normalized UCCR | Normal <1 | |||
| 0–4.5 | 7 (2) | 0.07 (0.07–0.07) | 0.5 (0.3–1.1) | 0.3 |
| 4.5–9.0 | 5 (3) | 0.1 (0.07–0.1) | 0.9 (0.1–1.8) | 0.7 |
| 9.0–13.5 | 1 (0) | No results | 0.5 | n/a |
| 13.5–18.0 | 9 (3) | 0.07(0.04–0.2) | 0.7 (0.2–1.1) | 0.07 |
| All ages | 22 (8) | 0.07 (0.04–0.2) | 0.5 (0.1–1.8) | 0.0005a |
GFR, glomerular filtration rate; n/a, not applicable; UCCR, urine calcium/creatinine ratio.
Shown are pertinent plasma and urine biochemistries separated according to HNF1B mutations status (mut+ vs. mut−) and according to the 4 age groups. The respective lower or upper limit of normal is indicated for each electrolyte concentration. The number of patients with available data “n” according to mutation status is provided in the second column, with the number of mut+ patients in parentheses. Note that patients can be represented in more than 1 age group, if data were available.
The Wilcoxon test compares the median values between the mut+ and mut− groups. The UCCR is normalized to the respective upper limit of normal for age to allow comparison across the age groups. There were too few measurements for this parameter to allow robust statistical comparison in the individual age groups.
Note that median magnesium values are significantly different between mut+ and mut− patients in all age groups. Median bicarbonate and potassium values were also significantly different but only in late childhood.
Significant (P < 0.05) values.
Age
Both cohorts were comparable with respect to median age at first (2.19 years, range 0.15–15.9 [mut+] vs. 2.8 years, range 0.02–17.1 [mut−]) and last available blood test (8.9 years, range 0.21–17.3 [mut+] vs. 7.3 years, range 1.1–17.4 [mut− ]).
Magnesium
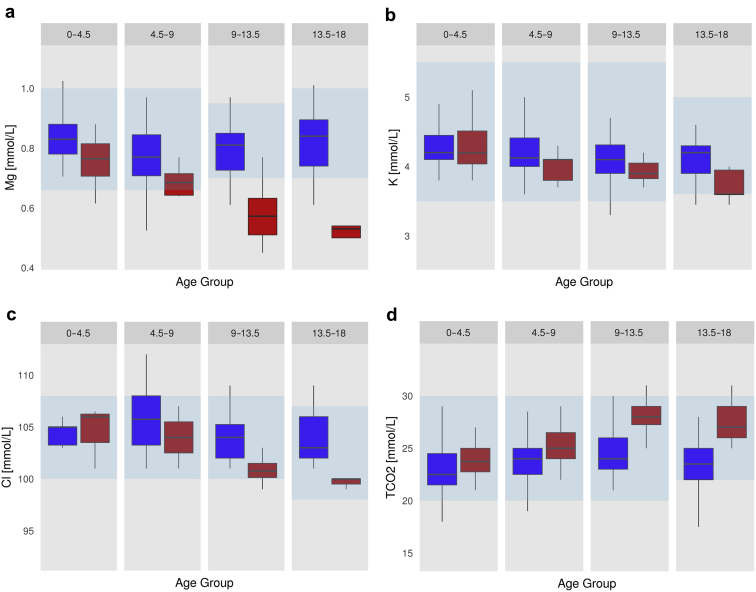
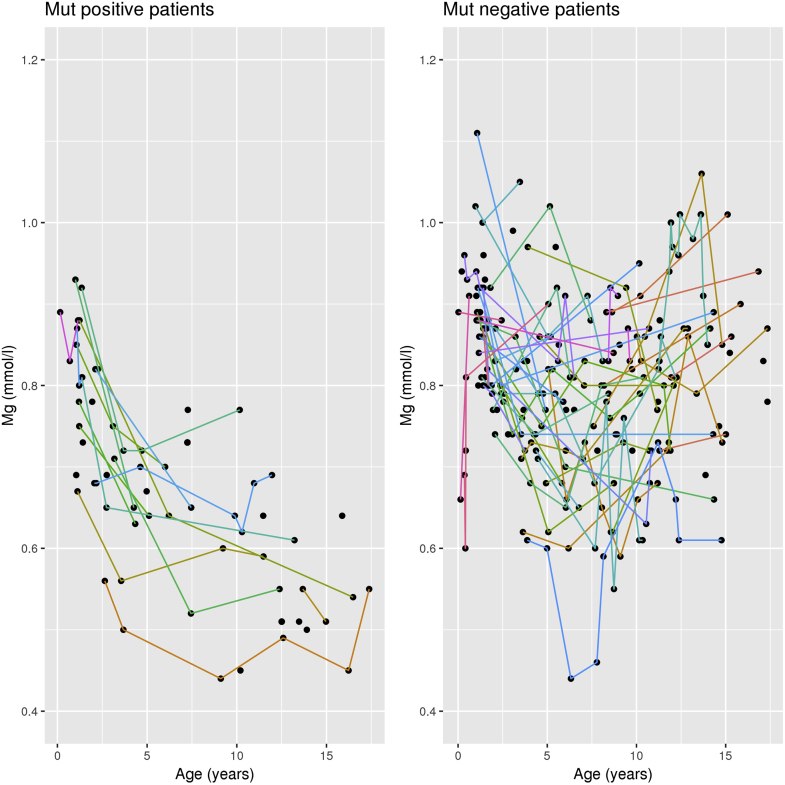
Table 1 summarizes the analysis of median biochemical concentrations assessed by mutation status and age group and they are graphically represented in Figure 2. Individual plasma magnesium measurements are plotted in Figure 3. Of note, although median plasma magnesium values differed significantly between mut+ and mut− in every age group, the number of patients with overt hypomagnesemia (median magnesium concentration below 0.7 mmol/l) became significantly different between the mutation groups only in the second half of childhood (Table 2). The median age at which hypomagnesemia was first noted in mut+ patients was 10.0 years (1.05–17.4 years).
Figure 2.
Plasma electrolyte abnormalities in mut+ patients develop over time. Shown are box plots for the plasma concentrations of (a) magnesium (Mg), (b) potassium (K), (c) chloride (Cl), and (d) bicarbonate (TCO2) according to the 4 age groups. Box plot graphs represent the median and interquartile range (IQR); the upper and lower whiskers include data points within 1.5 × IQR. Outliers are plotted individually. The blue boxes represent the mut− group, and the red boxes represent mut+. The respective normal range is represented by the transparent blue boxes. Note the development with increasing age of a Gitelman-like tubulopathy with hypomagnesemia and hypokalemic, hypochloremic metabolic alkalosis in the mut+ group. For number of patients for each plot and results of statistical comparisons, see Table 1.
Figure 3.
Magnesium levels in individual patients over time. Plotted are all plasma magnesium measurements included in the analysis with individual patients represented by colored lines, if more than 1 measurement was available. Note the decreasing plasma magnesium levels in the mut+ group, whereas no such trend is noticeable in the mut− group.
Table 2.
Comparison of hypomagnesemia in HNF1B mut+ and mut− groups
| Age (yr) | HNF1B mutation | Hypomagnesemia patients, n (%) | Normomagnesemia patients, n (%) | Fisher’s exact comparison |
|---|---|---|---|---|
| 0–4.5 | + | 4 (22) | 14 (78) | P = 0.26 |
| − | 2 (4) | 51 (96) | ||
| 4.5–9.0 | + | 5 (50) | 5 (50) | P = 0.18 |
| − | 9 (23) | 30 (77) | ||
| 9.0–13.5 | + | 9 (90) | 1 (10) | P = 0.0001a |
| − | 5 (17) | 25 (83) | ||
| 13.5–18.0 | + | 5 (100) | 0 (0) | P = 0.02a |
| − | 3 (16) | 16 (84) |
Shown are the number (n) and percentage (%) of patients with hypomagnesemia by age group and HNF1B mutation status. The Fisher exact test compares the number of patients with hypomagnesemia across the mutation groups. Note that the frequency of hypomagnesemia increases with age and the difference between mutation groups becomes significant in the second half of childhood. Also note that individual patients may be represented in more than 1 age group, if their follow-up extended beyond this age group.
Significant (P < 0.05) values.
Table 3 highlights the predictive values of a low plasma magnesium level in the different age quartiles and shows that absence of hypomagnesemia in the second half of childhood is highly predictive of not having a HNF1B mutation in children with renal tract malformations.
Table 3.
Predictive values of hypomagnesemia for HNF1B mutation
| Age (yr) | Positive predictive value | Negative predictive value |
|---|---|---|
| 0–4.5 | 0.6 | <0.5 |
| 4.5–9.0 | 0.5 | <0.5 |
| 9.0–13.5 | 0.7 | 0.9 |
| 13.5–18.0 | 0.7 | 0.9 |
Shown are the positive and negative predictive values for hypomagnesemia and HNF1B mutation. Note, absence of hypomagnesemia in the second half of childhood is highly predictive of not having an HNF1B mutation in patients with renal tract malformations.
Potassium, Chloride, and Bicarbonate
HNF1B mutations were associated with lower plasma potassium concentrations, but this was significant (P < 0.05) only in the oldest age group.
Similarly, plasma chloride concentrations trended lower with increasing age in mut+ patients, whereas bicarbonate concentrations increased with age in the mut+ group (Figure 2). However, the difference between the cohorts was not statistically significant and values outside the reference range were rare in both cohorts.
The cohorts were comparable with respect to plasma sodium and phosphate concentrations across the length of follow-up (data not shown).
Urine Calcium/Creatinine Ratios
Values were available for only 22 patients and thus were too few for meaningful comparison across age and mutation status (Table 1).
HNF1B Score
A total of 92 patients from this study had their HNF1B score calculated in the previous UK study.8 The median scores after adjustment for the latest available magnesium concentration was only slightly higher in patients older than 9 years compared with the younger patients, but the percentage of patients with a score ≥8, which had previously been suggested as a discriminator between mut+ and mut− patients, increased to more than 90% in the older age group (Table 4).7
Table 4.
HNF1B score according to age
| Age | <9 yr | >9 yr | ||
|---|---|---|---|---|
| HNF1B mutation | + | − | + | − |
| Median score (n) | 11 (13) | 8 (37) | 12 (13) | 7.5 (28) |
| Score ≥8, % | 77 | 51 | 92 | 50 |
Shown are the median HNF1B scores, as calculated previously,8 but adjusted for the latest available plasma magnesium concentration. Median scores are higher in the mut+ group, yet similar across the age groups. Note that the percentage of patients with a score ≥8 increases in the mut+ group with age, consistent with better discrimination between mut+ and mut− when using the suggested score cutoff of 8. For more details see text.
Discussion
Our study provides important insights into the nature of the tubular dysfunction associated with HNF1B mutations and informs selection of pediatric patients for mutation analysis.
Most important, we show that hypomagnesemia develops with increasing age. Although there was a significant difference in median magnesium concentrations between mut+ and mut− patients across all age groups, overt hypomagnesemia was not apparent until age group 9.0 to 13.5 years. Moreover, the median age at which hypomagnesemia was first noted was 10.0 years (range 1.0–17.4 years). Thus, the absence of hypomagnesemia in younger children should not be used as an argument against testing for HNF1B, as the negative predictive value is low.
Next, we show that HNF1B mutations are not only associated with hypomagnesemia, but with a trend for a more complex pattern of electrolyte abnormalities comparable to Gitelman syndrome. In some patients, this can be quite dramatic, as in the boy reported previously, who presented marked electrolyte abnormalities with the typical pattern of Gitelman syndrome (K: 3.2, Cl: 97, and bicarbonate: 33 mmol/l) and consequently received this as his clinical diagnosis, yet on genetic testing was found to have an HNF1B mutation.13
Interestingly, as with magnesium, these Gitelman-like electrolyte abnormalities become apparent only with increasing age, so that the difference in potassium concentration between mut+ and mut− patients in our cohort became significant only after age 13.5 years. This may explain why in our initial review of children with HNF1B mutations, hypokalemia was not noted as a specific feature, whereas in a review of adult patients, approximately half were noted to have hypokalemia with renal potassium wasting, even despite worsening eGFR.6, 12 Hypomagnesemia may contribute to hypokalemia, as lack of intracellular magnesium unblocks the secretory potassium channel KCNJ1.18 However, in familial hypomagnesemia with secondary hypocalcemia due to mutations in TRPM6, hypokalemia has not been reported, arguing against a substantial contribution of hypomagnesemia to decreased potassium levels.19, 20
Our study has several limitations, including a relatively small sample size and the retrospective design with inconsistent availability of the various laboratory values. Individual patients may be represented in more than one age group, if data were available. Although this may bias the results toward those patients with multiple measurements, it also allows the tracking of the development of electrolyte abnormalities in individual patients, as shown for magnesium (Figure 3).
Differences in plasma chloride and bicarbonate concentrations and urine calcium were not significant in our study, and this likely reflects the small number of patients with available measurements (Table 1). Nevertheless, the trend for hypochloremia and alkalosis with increasing age is apparent in our data (Figure 2). Clinical symptoms potentially associated with the electrolyte abnormalities were not captured in this review. Autosomal dominant tubulointerstitial kidney disease–HNF1B is rare and larger cohort studies, ideally based on national or international registries, will be needed to overcome these limitations.
The DCT is the key nephron segment for magnesium regulation.21 HNF1B has been shown to regulate expression of FXYD2, which in turn regulates the activity of the basolateral Na+-K+-ATPase, the overall “engine” for all transport activity in this segment.6, 10 Thus, the biochemical phenotype is expected to be similar to that associated with mutations in FXYD2. Patients with mutations in this gene are exceedingly rare and only 1 mutation has so far been described. Initially, FXYD2 disease was described as a cause of isolated hypomagnesemia, yet subsequent data on newly discovered patients also show a trend to a Gitelman-like tubulopathy.22, 23 Interestingly, in a recent report of mutations in ATP1A1, encoding the alpha subunit of the Na+-K+-ATPase, hypomagnesemia was the predominant electrolyte abnormality, with hypokalemic alkalosis much less noticable.24 These data suggest that impaired activity of the Na+-K+-ATPase in DCT appears to primarily affect magnesium reabsorption. This also fits with the observation that impaired energy provision from mitochondria can also predominantly affect plasma magnesium levels.25
The finding of a slow evolution of the electrolyte abnormalities throughout childhood fits with clinical observations in other disorders of the DCT and raises interesting questions about the development of the role of this nephron segment. The archetypical disorder of impaired salt reabsorption in DCT is Gitelman syndrome and affected patients typically present during school age or even adulthood.26, 27, 28 A similar slow development of electrolyte abnormalities has been reported in a family with EAST/SeSAME syndrome.29 Gordon syndrome, the mirror image of Gitelman syndrome, also typically manifests later in life.30 Perhaps even more interesting is the clinical observation that patients with mutations in CLCNKB (Bartter syndrome type 3) often initially present with classical Bartter syndrome, but later in childhood may revert to a Gitelman-like phenotype.31 This chloride channel is expressed both in the thick ascending limb of Henle and in DCT, and the phenotypic switch to DCT-typical electrolyte abnormalities may represent the developing and increasing importance of salt reabsorption in this segment during childhood.11 Our clinical observations raise the question of whether HNF1B may actually be a transcriptional driver of this developmental change in apparent DCT activity. Yet, although there are several studies demonstrating the critical importance of HNF1B for kidney and especially also for tubular development, there are no data yet available to investigate the role of HNF1B in postnatal tubular maintenance and transport activity.32, 33, 34, 35, 36
Conclusion
Our analysis of clinical data shows that the renal tubular dysfunction associated with mutations in HNF1B extends beyond isolated renal magnesium loss toward a Gitelman-like phenotype. Importantly, the electrolyte abnormalities associated with this tubulopathy develop during childhood and become most apparent in adolescence. The absence of these abnormalities in younger children with other suggestive findings thus does not argue against a potential underlying HNF1B mutation.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The study was supported by the National Institute for Health Research Biomedical Research Centre at Great Ormond Street Hospital (GOSH)/Institute of Child Health, Kids Kidney Research/Kidney Research UK, and by St. Peter’s Trust for Kidney, Bladder and Prostate Research. This work was facilitated by the GOSH Digital Research Environment. SE is a Wellcome Trust Senior Investigator. ASW is supported by Kidney Research UK project grant JFS/RP/008/20160916.
SA and WNH contributed equally.
References
- 1.Coffinier C., Thepot D., Babinet C. Essential role for the homeoprotein vHNF1/HNF1beta in visceral endoderm differentiation. Development. 1999;126:4785–4794. doi: 10.1242/dev.126.21.4785. [DOI] [PubMed] [Google Scholar]
- 2.Eckardt K.U., Alper S.L., Antignac C. Autosomal dominant tubulointerstitial kidney disease: diagnosis, classification, and management—a KDIGO consensus report. Kidney Int. 2015;88:676–683. doi: 10.1038/ki.2015.28. [DOI] [PubMed] [Google Scholar]
- 3.Bingham C., Bulman M.P., Ellard S. Mutations in the hepatocyte nuclear factor-1beta gene are associated with familial hypoplastic glomerulocystic kidney disease. Am J Hum Genet. 2001;68:219–224. doi: 10.1086/316945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bockenhauer D., Jaureguiberry G. HNF1B-associated clinical phenotypes:the kidney and beyond. Pediatr Nephrol. 2016;31:707–714. doi: 10.1007/s00467-015-3142-2. [DOI] [PubMed] [Google Scholar]
- 5.Clissold R.L., Hamilton A.J., Hattersley A.T. HNF1B-associated renal and extra-renal disease-an expanding clinical spectrum. Nat Rev Nephrol. 2015;11:102–112. doi: 10.1038/nrneph.2014.232. [DOI] [PubMed] [Google Scholar]
- 6.Adalat S., Woolf A.S., Johnstone K.A. HNF1B mutations associate with hypomagnesemia and renal magnesium wasting. J Am Soc Nephrol. 2009;20:1123–1131. doi: 10.1681/ASN.2008060633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faguer S., Chassaing N., Bandin F. The HNF1B score is a simple tool to select patients for HNF1B gene analysis. Kidney Int. 2014;86:1007–1015. doi: 10.1038/ki.2014.202. [DOI] [PubMed] [Google Scholar]
- 8.Clissold R., Shields B., Ellard S. Assessment of the HNF1B score as a tool to select patients for HNF1B genetic testing. Nephron. 2015;130:134–140. doi: 10.1159/000398819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raaijmakers A., Corveleyn A., Devriendt K. Criteria for HNF1B analysis in patients with congenital abnormalities of kidney and urinary tract. Nephrol Dial Transplant. 2015;30:835–842. doi: 10.1093/ndt/gfu370. [DOI] [PubMed] [Google Scholar]
- 10.Ferre S., Veenstra G.J., Bouwmeester R. HNF-1B specifically regulates the transcription of the gamma-subunit of the Na+/K+-ATPase. Biochem Biophys Res Commun. 2011;404:284–290. doi: 10.1016/j.bbrc.2010.11.108. [DOI] [PubMed] [Google Scholar]
- 11.Kleta R., Bockenhauer D. Salt-losing tubulopathies in children:what's new, what's controversial? J Am Soc Nephrol. 2018;29:727–739. doi: 10.1681/ASN.2017060600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faguer S., Decramer S., Chassaing N. Diagnosis, management, and prognosis of HNF1B nephropathy in adulthood. Kidney Int. 2011;80:768–776. doi: 10.1038/ki.2011.225. [DOI] [PubMed] [Google Scholar]
- 13.Ashton E.J., Legrand A., Benoit V. Simultaneous sequencing of 37 genes identified causative mutations in the majority of children with renal tubulopathies. Kidney Int. 2018;93:961–967. doi: 10.1016/j.kint.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Kasperaviciute D., Catarino C.B., Chinthapalli K. Uncovering genomic causes of co-morbidity in epilepsy:gene-driven phenotypic characterization of rare microdeletions. PLoS One. 2011;6 doi: 10.1371/journal.pone.0023182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez Celedon C., Bitsori M., Tullus K. Progression of chronic renal failure in children with dysplastic kidneys. Pediatr Nephrol. 2007;22:1014–1020. doi: 10.1007/s00467-007-0459-5. [DOI] [PubMed] [Google Scholar]
- 16.Bockenhauer D., Bokenkamp A., van't Hoff W. Renal phenotype in Lowe Syndrome: a selective proximal tubular dysfunction. Clin J Am Soc Nephrol. 2008;3:1430–1436. doi: 10.2215/CJN.00520108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.team Rc. R Foundation for Statistical Computing; Vienna, Austria: 2013. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 18.Huang C.L., Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol. 2007;18:2649–2652. doi: 10.1681/ASN.2007070792. [DOI] [PubMed] [Google Scholar]
- 19.Schlingmann K.P., Weber S., Peters M. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet. 2002;31:166–170. doi: 10.1038/ng889. [DOI] [PubMed] [Google Scholar]
- 20.Walder R.Y., Landau D., Meyer P. Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Genet. 2002;31:171–174. doi: 10.1038/ng901. [DOI] [PubMed] [Google Scholar]
- 21.Viering D., de Baaij J.H.F., Walsh S.B. Genetic causes of hypomagnesemia, a clinical overview. Pediatr Nephrol. 2017;32:1123–1135. doi: 10.1007/s00467-016-3416-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meij I.C., Koenderink J.B., van Bokhoven H. Dominant isolated renal magnesium loss is caused by misrouting of the Na(+),K(+)-ATPase gamma-subunit. Nat Genet. 2000;26:265–266. doi: 10.1038/81543. [DOI] [PubMed] [Google Scholar]
- 23.de Baaij J.H., Dorresteijn E.M., Hennekam E.A. Recurrent FXYD2 p.Gly41Arg mutation in patients with isolated dominant hypomagnesaemia. Nephrol Dial Transplant. 2015;30:952–957. doi: 10.1093/ndt/gfv014. [DOI] [PubMed] [Google Scholar]
- 24.Schlingmann K.P., Bandulik S., Mammen C. Germline de novo mutations in ATP1A1 cause renal hypomagnesemia, refractory seizures, and intellectual disability. Am J Hum Genet. 2018;103:808–816. doi: 10.1016/j.ajhg.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson F.H., Hariri A., Farhi A. A cluster of metabolic defects caused by mutation in a mitochondrial tRNA. Science. 2004;306:1190–1194. doi: 10.1126/science.1102521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bettinelli A., Bianchetti M.G., Girardin E. Use of calcium excretion values to distinguish two forms of primary renal tubular hypokalemic alkalosis: Bartter and Gitelman syndromes. J Pediatr. 1992;120:38–43. doi: 10.1016/s0022-3476(05)80594-3. [DOI] [PubMed] [Google Scholar]
- 27.Peters M., Jeck N., Reinalter S. Clinical presentation of genetically defined patients with hypokalemic salt-losing tubulopathies. Am J Med. 2002;112:183–190. doi: 10.1016/s0002-9343(01)01086-5. [DOI] [PubMed] [Google Scholar]
- 28.Vargas-Poussou R., Dahan K., Kahila D. Spectrum of mutations in Gitelman syndrome. J Am Soc Nephrol. 2011;22:693–703. doi: 10.1681/ASN.2010090907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scholl U.I., Dave H.B., Lu M. SeSAME/EAST syndrome—phenotypic variability and delayed activity of the distal convoluted tubule. Pediatr Nephrol. 2012;27:2081–2090. doi: 10.1007/s00467-012-2219-4. [DOI] [PubMed] [Google Scholar]
- 30.Achard J.M., Disse-Nicodeme S., Fiquet-Kempf B. Phenotypic and genetic heterogeneity of familial hyperkalaemic hypertension (Gordon syndrome) Clin Exp Pharmacol Physiol. 2001;28:1048–1052. doi: 10.1046/j.1440-1681.2001.03575.x. [DOI] [PubMed] [Google Scholar]
- 31.Jeck N., Konrad M., Peters M. Mutations in the chloride channel gene, CLCNKB, leading to a mixed Bartter-Gitelman phenotype. Pediatr Res. 2000;48:754–758. doi: 10.1203/00006450-200012000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Desgrange A., Heliot C., Skovorodkin I. HNF1B controls epithelial organization and cell polarity during ureteric bud branching and collecting duct morphogenesis. Development. 2017;144:4704–4719. doi: 10.1242/dev.154336. [DOI] [PubMed] [Google Scholar]
- 33.Heliot C., Desgrange A., Buisson I. HNF1B controls proximal-intermediate nephron segment identity in vertebrates by regulating Notch signalling components and Irx1/2. Development. 2013;140:873–885. doi: 10.1242/dev.086538. [DOI] [PubMed] [Google Scholar]
- 34.Dudziak K., Mottalebi N., Senkel S. Transcription factor HNF1beta and novel partners affect nephrogenesis. Kidney Int. 2008;74:210–217. doi: 10.1038/ki.2008.149. [DOI] [PubMed] [Google Scholar]
- 35.Naylor R.W., Przepiorski A., Ren Q. HNF1beta is essential for nephron segmentation during nephrogenesis. J Am Soc Nephrol. 2013;24:77–87. doi: 10.1681/ASN.2012070756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferre S., Igarashi P. New insights into the role of HNF-1β in kidney (patho)physiology. Pediatr Nephrol. 2019;34:1325–1335. doi: 10.1007/s00467-018-3990-7. [DOI] [PMC free article] [PubMed] [Google Scholar]