Abstract
Background
Further advances have been achieved in the field of intravenous ultrasound (IVUS) guided drug eluting stent (DES) implantation and hence there was a need to rejuvenate the evidence. Hence, we performed a cumulative meta-analysis with trial sequential analysis (TSA) of randomized controlled trials (RCTs) comparing IVUS versus angiogram guided DES implantation.
Methodology
We searched PubMed/Medline and Cochrane database for relevant articles using predefined inclusion and exclusion criteria. Outcomes of interest were cardiovascular mortality, myocardial infarction (MI), target lesion revascularisation (TLR), stent thrombosis (ST). We used Mantel-Haenszel method with random error model to calculate odds ratio (OR) with 95% confidence interval (CI). We also performed TSA to accommodate for possible type I error.
Results
A total of 11 RCTs with 5352 patients were included in the final analysis. Follow up duration of included studies varied from 12 to 24 months. IVUS use was associated with significantly reduced incidence of cardiovascular mortality [OR: 0.45, CI: 0.25–0.80, p value = 0.007, I2 = 0%, χ2p-value = 0.98], TLR [OR: 0.56, CI: 0.41–0.77, p value = 0.0004, I2 = 0%, χ2p-value = 0.95] and ST [OR: 0.47, CI: 0.24–0.94, p value = 0.03, I2 = 0%, χ2p-value = 0.75]. IVUS use had no effect on incidence of MI on follow up. The cumulative z curve crosses the TSA boundary indicating sufficient evidence without type I error for reduced incidence of cardiovascular mortality and TLR with the use IVUS.
Conclusion
IVUS-guided DES implantation should be the standard of care as it significantly reduced cardiovascular mortality and TLR.
Keywords: IVUS, DES, Meta-analysis, Trial sequential analysis, TLR, Stent thrombosis
1. Introduction
Drug eluting stent (DES) implantation has emerged as a standard treatment in patients undergoing percutaneous coronary intervention for acute coronary syndrome [1]. However, use of DES is associated with target lesion revascularisation (TLR) and stent thrombosis (ST), which persists as a substantial concern [2]. Trials are under way to procure approaches engendering improved outcomes with DES implantation. Several randomized control trials (RCTs) have documented better long-term clinical outcomes associated with the use of intravascular ultrasound (IVUS) as compared to angiogram-guided DES implantation [3,4]. The current recommendations for the use of IVUS-guided DES implantation are based on observational studies [5]. Previous meta-analysis of RCTs studying the role IVUS guided DES implantation have not analysed or commented regarding the power of their evidence. Also, further advances have been achieved in the field of IVUS guided DES implantation, and hence, there was a need to rejuvenate the evidence. Hence, we performed an updated meta-analysis with trial sequential analysis of RCTs comparing IVUS versus angiogram guided DES implantation.
2. Methodology
The meta-analysis was performed according to the PRISMA (preferred reporting items for systemic review) and AHA (American heart association) guidelines [6,7]. Medline/PubMed and Cochrane databases were used to search relevant articles from inception to April 2019. The search terms used included “intravascular ultrasound”, “IVUS”, “angiogram”, “Drug eluting stent”, “DES”, “Percutaneous coronary intervention”, “PCI”. The identified citations were imported to Mendeley reference manager. A manual search for relevant citations form bibliographies of articles and reviews was performed. We checked for any duplicates among the citations included using the Mendeley reference manager.
Articles were eligible for inclusion if they met the following inclusion criteria, 1) RCTs studying IVUS versus angiogram guided DES (Both first and second generation) implantation, from inception to April 2019, with no restriction on sample size or language of the published article. 2) Manuscripts reporting either one of the following four outcomes: a) Cardiovascular Mortality, b) Myocardial infarction (MI), c) TLR or d) ST.
Two reviewers AK and MS independently performed data extraction. Any disparity was resolved by mutual consensus and in consultation with the senior author RD. The data was extracted as per a predesigned data extraction form. The following data was extracted from each article; author's first name/Trial name, year of publication, study design, duration of study, mean age of the population, percentage male, number randomized, mean of minimum luminal diameter and percentage diameter stenosis between the two groups, number of the following events in the two groups a) Cardiovascular mortality, b) MI, c) TLR and d) definite/probable ST.
The analysis was carried out using RevMan Version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). For statistical analysis we used Mantel-Haenszel method with random error model to calculate odds ratio (OR) with 95% confidence interval (CI). Heterogeneity was assessed using the I2 test >60% or χ2 p-value <0.05. We used the funnel plot to assess for publication bias visually. We refrained from using Egger's test to statistically report asymmetry in the funnel plot because of small number of included studies.
We performed trial sequential analysis (TSA) on outcomes, to accommodate for increased risk of type I error resulting from repeated significance testing because of the addition of new trials in a meta-analysis. Ergo trails sequential analysis helps in eliminating the possibility of type I error from the final estimate [8]. TSA for MI was not generated as the results were not in favour of either arm.
3. Results
We included 11 RCTs comparing IVUS versus angiogram guided DES implantation [3,4,[9], [10], [11], [12], [13], [14], [15], [16], [17]]. The PRISMA flow diagram is shown in Supplementary Fig. 1. The baseline characteristics of included studies are mentioned in Supplementary Table 1. Pooled estimates were calculated for cardiovascular mortality, MI, TLR and ST. The analysis included a total of 5,352 patients. The follow up period varied from 12 to 24 months among RCTs. The weighed mean follow up was 13.68 months.
3.1. Cardiovascular mortality
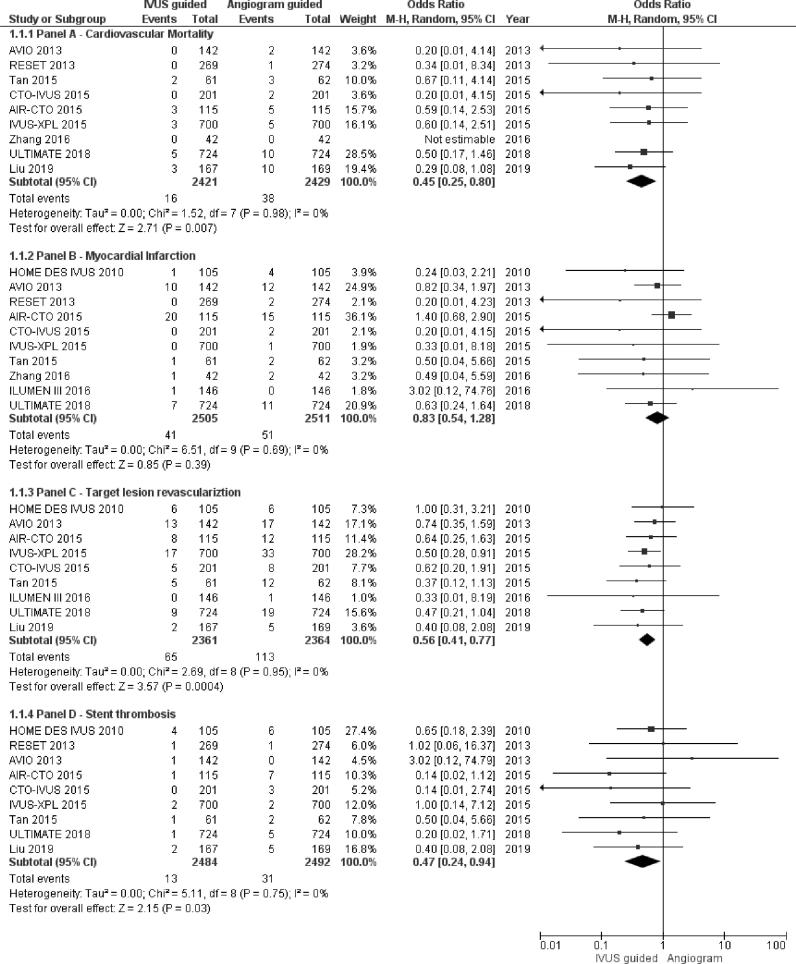
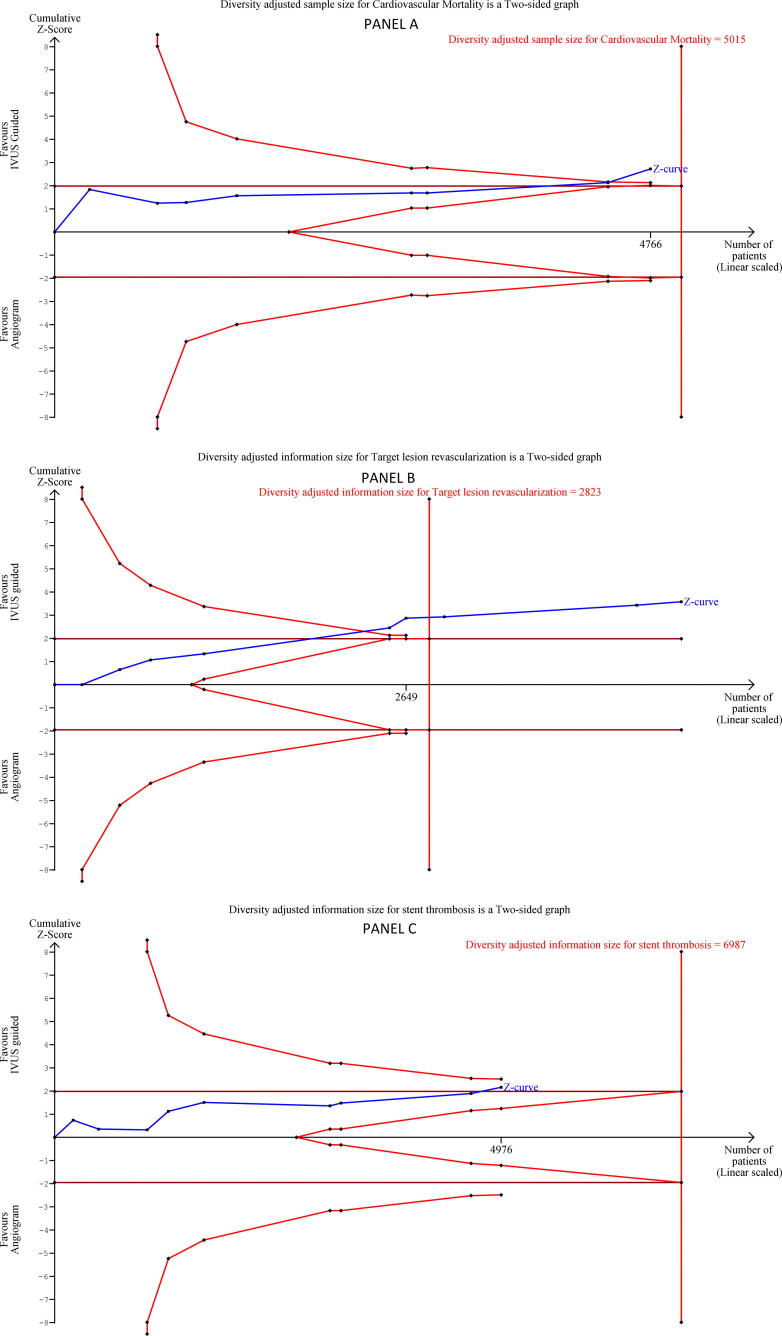
IVUS-guided DES implantation resulted in significantly reduced incidence of cardiovascular mortality [OR: 0.45, CI: 0.25–0.80, p value = 0.007, I2 = 0%, χ2 p-value = 0.98] (Fig. 1, Panel A). There was no heterogeneity associated with the pooled estimation as suggested by I2 = 0% and χ2 p-value = 0.89. Supplementary Fig. 2 represents the funnel plot for assessing publication bias for cardiovascular mortality outcome, indicates publication bias. TSA adjusted confidence interval for alpha error of 5% and beta error of 20% was 0.24–0.84. (Fig. 2, Panel A). The cumulative z curve crosses the TSA boundary indicating sufficient evidence without significant type I error for reduced incidence of cardiovascular mortality.
Fig. 1.
Forest plot for a) Panel A- cardiovascular mortality, b) Panel B- myocardial infarction, c) Panel C– target lesion revascularisation, d) Panel D- stent thrombosis. IVUS- intravascular ultrasound, OR- odds ratio, CI- confidence interval.
Fig. 2.
TSA plot for a) Panel A- cardiovascular mortality, b) Panel B – target lesion revascularisation, c) Panel C – stent thrombosis. IVUS- intravascular ultrasound.
3.2. Myocardial infarction
The incidence of MI was lower following IVUS-guided DES implantation, however, the association was not statistically significant.[OR: 0.83, CI: 0.54–1.28, p value = 0.39, I2 = 0%, χ2 p-value = 0.69] (Fig. 1, Panel B). Supplementary Fig. 3 demonstrates the funnel plot, assessing publication bias for reporting of MI outcome, denotes minimal publication bias.
3.3. Target lesion revascularisation
IVUS-guided DES implantation was associated with a lower incidence of TLR on follow up [OR: 0.56, CI: 0.41–0.77, p value = 0.0004, I2 = 0%, χ2 p-value = 0.95] (Fig. 1, Panel C). Supplementary Fig. 4 represents the funnel plot, denoting publication bias more than minimal. TSA adjusted confidence interval for alpha error of 5% and beta error of 20% was 0.40–0.79. (Fig. 2, Panel B). As evident from the figure that the cumulative z curve crosses TSA boundary indicating the outcome is associated with an acceptable level of type I error.
3.4. Stent thrombosis
IVUS-guided DES implantation significantly reduced the incidence of ST [OR: 0.47, CI: 0.24–0.94, p value = 0.03, I2 = 0%, χ2 p-value = 0.75] (Fig. 1, Panel D). Supplementary Fig. 5 represents the funnel plot for ST outcome, indicates possibility of minimal publication bias. TSA adjusted confidence interval for alpha error of 5% and beta error of 20% was 0.20–1.13. (Fig. 2, Panel C). The decreased incidence of ST is therefore associated with an increased risk of type I error as evident from TSA plot and TSA adjusted confidence interval.
4. Discussion
The results of this meta-analysis concluded that IVUS as compared to angiogram guided DES implantation resulted in a lower incidence of cardiovascular mortality, TLR and ST. IVUS-guided DES implantation, although resulted in a lower incidence of MI, the association was not statistically significant. A recently published meta-analysis compared IVUS versus angiogram guided DES implantation [18]. The present meta-analysis augments the evidence in the field as compared to the previous meta-analysis by the addition of one more randomized control trial [15], reporting no statistically significant drop in incidence of MI with IVUS-guided DES implantation and TSA of outcomes. The discovery of no statistically significant lowering in the incidence of MI with DES implantation in the present meta-analysis is also contrary to a previously published patient-level meta-analysis of 2345 procedures [19]. Furthermore, this meta-analysis included TSA of outcomes not depicted in earlier meta-analyses. This meta-analysis demonstrated that the strength of evidence for outcomes a) Cardiovascular mortality and b) TLR were sufficient without the risk of type I error. However, ST was associated with a risk of type I error as evident from TSA.
IVUS helps in overcoming many limitations of angiogram, which produces a two dimensional lumenogram of a three dimensional structure. IVUS helps to determine plaque burden, calcium and eccentricity, and stent expansion [20]. IVUS during PCI can also be used to determine stent size, identify optimal proximal and distal stent edge landing zones. [21]. The AVIO trial which studied the use of IVUS as compared to angiogram for DES implantation for complex coronary lesions, a benefit was observed with IVUS in complex lesion in post procedural minimum lumen diameter, which is one of the important determinant of late complications [13]. Two recent RCTs also demonstrated that IVUS-guided implantation resulted in increased acute gain and late lumen loss when compared with angiography guided implantation [13] [15]. Clinical benefit with these improvements was further confirmed in the ULTIMATE trial [16]. IVUS-guided stent implantation has the additional advantage of being associated with minimal complications in patients with renal disease, as it does not require the administration of contrast [22]. However, IVUS-guided stent placement has a steep learning curve and requires a certain level of clinical expertise. As per a study using the national inpatients sample (NIS) the number of IVUS guided PCI procedures did not change significantly from ~5% in 2007 to ~6.5% in 2013. They also reported a heterogeneity in IVUS utilization across institutions in the united states with more IVUS guided procedures in urban teaching and urban non-teaching hospitals [23]. Training program directors may consider intravascular imaging (either IVUS or optical coherence tomography) to be a part of a fellows' curriculum. At present there is class IIb evidence for the use of IVUS for coronary stent implantation [20]. Hopefully, this along with other recently published meta-analyses can help change the guidelines.
There are several limitations in our meta-analysis. The results of this meta-analysis should be considered with caution, keeping in mind the possibility of publication bias. Each included RCT is associated with different study design and biases, which has not been addressed in this analysis. Type and site of the lesion were not determined, and hence, these results cannot be generalized. Few larger trials may have influenced the results of this study. Non-adherence to anti-platelet therapy is also one of the most important reasons for ST. This information was not available as with any meta-analysis. Definitions of outcomes varied in different RCTs. Future studies considering patient level meta-analysis would help augment the present available evidence. The randomized trials included in this analysis had a heterogeneous patient sample, for example; all comes; patient with chronic total occlusion, STEMI, NSTEMI etc. Furthermore, the analysis didn't account for different period of follow up among studies during pooled estimation.
In conclusion, IVUS-guided DES implantation significantly reduces cardiovascular mortality and TLR without type I error. It also reduced ST, albeit with type I error and requires more studies to comfirm it. IVUS-guided DES implantation should be the standard of care, especially for complex coronary lesions. Hopefully, this study will guide interventionalist to practice in the right direction as indicated by the current evidences.
Declaration of competing interest
The authors have nothing to disclose.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2019.100419.
Appendix A. Supplementary data
Supplementary material
References
- 1.Piccolo R., Bonaa K.H., Efthimiou O., Varenne O., Baldo A., Urban P., Kaiser C., Remkes W., Räber L., de Belder A., van't Hof A.W.J., Stankovic G., Lemos P.A., Wilsgaard T., Reifart J., Rodriguez A.E., Ribeiro E.E., Serruys P.W.J.C., Abizaid A., Sabaté M., Byrne R.A., de la Torre Hernandez J.M., Wijns W., Jüni P., Windecker S., Valgimigli M., Piccolo R., Bonaa K.H., Efthimiou O., Varenne O., Baldo A., Urban P., Kaiser C., Remkes W., Räber L., de Belder A., van't Hof A.W.J., Stankovic G., Lemos P.A., Wilsgaard T., Reifart J., Rodriguez A.E., Ribeiro E.E., Serruys P.W.J.C., Abizaid A., Sabaté M., Byrne R.A., de la Torre Hernandez J.M., Wijns W., Jüni P., Windecker S., Valgimigli M. Drug-eluting or bare-metal stents for percutaneous coronary intervention: a systematic review and individual patient data meta-analysis of randomised clinical trials. Lancet. 2019 doi: 10.1016/S0140-6736(19)30474-X. [DOI] [PubMed] [Google Scholar]
- 2.Palmerini T., Biondi-Zoccai G., Della Riva D., Mariani A., Genereux P., Branzi A., Stone G.W. Stent thrombosis with drug-eluting stents. J. Am. Coll. Cardiol. 2013;62:1915 LP–1921. doi: 10.1016/j.jacc.2013.08.725. [DOI] [PubMed] [Google Scholar]
- 3.Hong S.-J., Kim B.-K., Shin D.-H., Nam C.-M., Kim J.-S., Ko Y.-G., Choi D., Kang T.-S., Kang W.-C., Her A.-Y., Kim Y.H., Hur S.-H., Hong B.-K., Kwon H., Jang Y., Hong M.-K., for the I.-X. Investigators Effect of intravascular ultrasound–guided vs angiography-guided everolimus-eluting stent implantation: the IVUS-XPL randomized clinical trial. JAMA. 2015;314:2155–2163. doi: 10.1001/jama.2015.15454. [DOI] [PubMed] [Google Scholar]
- 4.Byeong-Keuk K., Dong-Ho S., Myeong-Ki H., Sik P.H., Seung-Woon R., S M.G., Jung-Sun K., Sang K.J., Seung-Jin L., Hee-Yeol K., Bum-Kee H., Woong-Chol K., Jin-Ho C., Yangsoo J. Clinical impact of intravascular ultrasound–guided chronic total occlusion intervention with zotarolimus-eluting versus biolimus-eluting stent implantation. Circ. Cardiovasc. Interv. 2015;8:e002592. doi: 10.1161/CIRCINTERVENTIONS.115.002592. [DOI] [PubMed] [Google Scholar]
- 5.Levine G.N., Bates E., C B.J., R B.S., A B.J., Bojan C., E C.C., G E.S., A G.R., M H.S., N K.U., A L.R., Laura M., Roxana M., D M.I., Debabrata M., K N.B., H. T.H. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. Circulation. 2011;124:e574–e651. doi: 10.1161/CIR.0b013e31823ba622. [DOI] [PubMed] [Google Scholar]
- 6.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goutham R., Francisco L.-J., Jack B., Frank D., H D.N., A H.M., George H., Katherine K., Christopher M., M P.-W.T., E S.A., P. W.C., Jennifer W. Methodological standards for meta-analyses and qualitative systematic reviews of cardiac prevention and treatment studies: a scientific statement from the American Heart Association. Circulation. 2017;136:e172–e194. doi: 10.1161/CIR.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 8.Wetterslev J., Jakobsen J.C., Gluud C. Trial sequential analysis in systematic reviews with meta-analysis. BMC Med. Res. Methodol. 2017;17:39. doi: 10.1186/s12874-017-0315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhanga J.-Q., Shib R., Pangc W., Guob Q., Xub Y., Zhangb J., Yangb Q., Lib Y., Meib J.-P., Jiangb T.-M., Lib Y.-M. Application of intravascular ultrasound in stent implantation for small coronary arteries. J. Clin. Invasive Cardiol. 2016;3:2–8. [Google Scholar]
- 10.N.-L. Tian, S.-K. Gami, F. Ye, J.-J. Zhang, Z.-Z. Liu, S. Lin, Z. Ge, S.-J. Shan, W. You, L. Chen, Y.-J. Zhang, G.S. Mintz, S.-L. Chen, Angiographic and clinical comparisons of intravascular ultrasound- versus angiography-guided drug-eluting stent implantation for patients with chronic total occlusion lesions: two-year results from a randomised AIR-CTO study, EuroIntervention. 10 (20AD) 1409–1417. https://eurointervention.pcronline.com/article/angiographic-and-clinical-comparisons-of-intravascular-ultrasound-versus-angiography-guided-drug-eluting-stent-implantation-for-patients-with-chronic-total-occlusion-lesions-two-year-results-from-a-randomised. [DOI] [PubMed]
- 11.Tan Q., Wang Q., Liu D., Zhang S., Zhang Y., Li Y. Intravascular ultrasound-guided unprotected left main coronary artery stenting in the elderly. Saudi Med. J. 2015;36:549–553. doi: 10.15537/smj.2015.5.11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J.-S., Kang T.-S., Mintz G.S., Park B.-E., Shin D.-H., Kim B.-K., Ko Y.-G., Choi D., Jang Y., Hong M.-K. Randomized comparison of clinical outcomes between intravascular ultrasound and angiography-guided drug-eluting stent implantation for long coronary artery stenoses. JACC Cardiovasc. Interv. 2013;6:369–376. doi: 10.1016/j.jcin.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Chieffo A., Latib A., Caussin C., Presbitero P., Galli S., Menozzi A., Varbella F., Mauri F., Valgimigli M., Arampatzis C., Sabate M., Erglis A., Reimers B., Airoldi F., Laine M., Palop R.L., Mikhail G., MacCarthy P., Romeo F., Colombo A. A prospective, randomized trial of intravascular-ultrasound guided compared to angiography guided stent implantation in complex coronary lesions: the AVIO trial. Am. Heart J. 2013;165:65–72. doi: 10.1016/j.ahj.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Jakabčin J., Špaček R., Bystroň M., Kvašňák M., Jager J., Veselka J., Kala P., Červinka P. Long-term health outcome and mortality evaluation after invasive coronary treatment using drug eluting stents with or without the IVUS guidance. Randomized control trial. HOME DES IVUS. Catheter. Cardiovasc. Interv. 2010;75:578–583. doi: 10.1002/ccd.22244. [DOI] [PubMed] [Google Scholar]
- 15.Ali Z.A., Maehara A., Généreux P., Shlofmitz R.A., Fabbiocchi F., Nazif T.M., Guagliumi G., Meraj P.M., Alfonso F., Samady H., Akasaka T., Carlson E.B., Leesar M.A., Matsumura M., Ozan M.O., Mintz G.S., Ben-Yehuda O., Stone G.W. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet. 2016;388:2618–2628. doi: 10.1016/S0140-6736(16)31922-5. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J., Gao X., Kan J., Ge Z., Han L., Lu S., Tian N., Lin S., Lu Q., Wu X., Li Q., Liu Z., Chen Y., Qian X., Wang Juan, Chai D., Chen C., Li X., Gogas B.D., Pan T., Shan S., Ye F., Chen S.-L. Intravascular ultrasound-guided versus angiography-guided implantation of drug-eluting stent in all-comers: the ULTIMATE trial. J. Am. Coll. Cardiol. 2018:25553. doi: 10.1016/j.jacc.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Liu X.M., Yang Z.M., Liu X.K., Zhang Q., Liu C.Q., Han Q. Le, Sun J.H. Intravascular ultrasound-guided drug-eluting stent implantation for patients with unprotected left main coronary artery lesions: a single-center randomized trial. Anatol. J. Cardiol. 2019;21:83–90. doi: 10.14744/AnatolJCardiol.2018.21447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elgendy I.Y., Mahmoud A.N., Elgendy A.Y., Mintz G.S. Intravascular ultrasound-guidance is associated with lower cardiovascular mortality and myocardial infarction for drug-eluting stent implantation ― insights from an updated meta-analysis of randomized trials. Circ. J. 2019 doi: 10.1253/circj.CJ-19-0209. (advpub) [DOI] [PubMed] [Google Scholar]
- 19.Shin D.-H., Hong S.-J., Mintz G.S., Kim J.-S., Kim B.-K., Ko Y.-G., Choi D., Jang Y., Hong M.-K. Effects of intravascular ultrasound–guided versus angiography-guided new-generation drug-eluting stent implantation: meta-analysis with individual patient–level data from 2,345 randomized patients. JACC Cardiovasc. Interv. 2016;9:2232–2239. doi: 10.1016/j.jcin.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 20.E. Shlofmitz, N. Khalid, Intravascular ultrasound (IVUS), in: StatPearls [Internet], n.d. https://www.ncbi.nlm.nih.gov/books/NBK537019/.
- 21.Das P., Meredith I. Role of intravascular ultrasound in unprotected left main percutaneous coronary intervention. Expert. Rev. Cardiovasc. Ther. 2007;5:81–89. doi: 10.1586/14779072.5.1.81. [DOI] [PubMed] [Google Scholar]
- 22.Funatsu A., Hano Y., Kobayashi T., Nakamura S. TCT-348 efficacy of intravascular ultrasound guided minimum contrast percutaneous coronary intervention for chronic kidney disease patients. J. Am. Coll. Cardiol. 2014;64:B101. [Google Scholar]
- 23.Elgendy I.Y., Ha L.D., Elbadawi A., Ogunbayo G.O., Olorunfemi O., Mahmoud A.N., Mojadidi M.K., Abuzaid A., Anderson R.D., Bavry A.A. Temporal trends in inpatient use of intravascular imaging among patients undergoing percutaneous coronary intervention in the United States. JACC Cardiovasc. Interv. 2018;11:913 LP–915. doi: 10.1016/j.jcin.2018.01.254. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material