Introduction
Recently there has been interest in the role of CTLA-4 Ig therapy for nephrotic syndrome due to minimal change disease (MCD) and focal segmental glomerulosclerosis (FSGS).1, 2 Although the effects of CTLA-4 Ig for FSGS have been mixed,1, 2, 3 there is very limited information on the effect of CTLA-4 Ig (abatacept) in MCD.1, 4 There has been one report of a child with relapsing MCD and high levels of urinary CD80 (also known as B7.1) in which administration of abatacept resulted in rapid remission but subsequent relapse.1 Here we report a case of a young man with severe, relapsing MCD who has demonstrated a several-year period of sustained disease remission, in response to ongoing CTLA-4 Ig therapy.
Case Presentation
A 23-year-old Caucasian man of Armenian descent, with past medical history of hypothyroidism, alopecia, nail dystrophy, azoospermia, and a remote serum sickness–like syndrome, presented in 2006 with massive proteinuria, hypoalbuminemia, and edema, consistent with nephrotic syndrome. There was no family history of renal disease. Extensive serologic testing (e.g., antinuclear antibody, complement levels, double-stranded DNA) was normal. A kidney biopsy was performed. Thirty-seven glomeruli were examined under light microscopy. None of the glomeruli were globally sclerotic. There was no increase in mesangial matrix or cellularity. Capillary loops were patent, with no evidence of double contours. None of the glomeruli showed evidence of segmental sclerosis. Immunofluorescence was weakly positive for IgM deposition. On electron microscopy, there were rare paramesangial electron-dense deposits. There was extensive, near global effacement of glomerular foot processes. A diagnosis of MCD (IgM variant) was made. Proteinuria remitted within a few days of starting prednisone (60 mg/d), although he suffered 2 relapses during a 4-month prednisone taper. Given his pattern of steroid dependence, mycophenolate mofetil was started as steroid-sparing agent. This was eventually switched to tacrolimus, due to lack of therapeutic benefit. He responded well to tacrolimus (3 mg twice a day), with eventual tapering off of prednisone, and ongoing remission. Efforts at lowering the tacrolimus dose resulted in disease relapse.
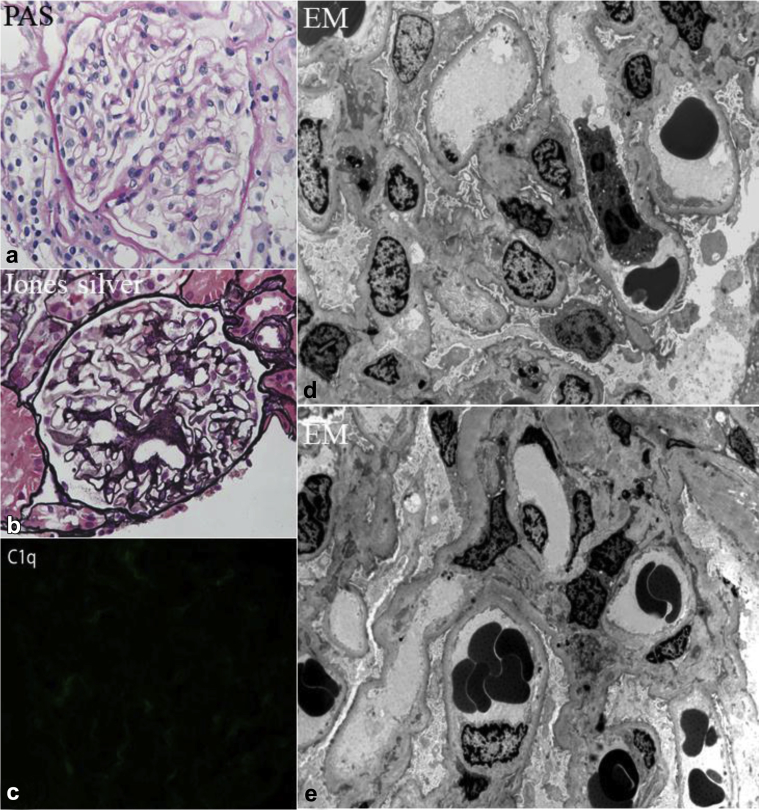
Five years after onset of disease, in August 2011, he transitioned care to our institution. By then, he had remained on tacrolimus 3 mg twice a day and lisinopril 5 mg/d, the latter for control of both proteinuria and mild essential hypertension. He relapsed again in September 2011. After transient remission of proteinuria on prednisone 60 mg/d, within weeks he again developed a full relapse with 10 g/d proteinuria, severe hypoalbuminemia (1 g/dl), and anasarca, complicated by transient spontaneous acute kidney injury (AKI) (peak creatinine 1.5 mg/dl) and hyperkalemia (6.8 mEq/l). Total cholesterol was 477 mg/dl, low-density lipoprotein 352 mg/dl, high-density lipoprotein 94 mg/dl, and triglycerides 213 mg/dl (not on lipid-lowering therapy). He was hospitalized and converted to i.v. methylprednisolone 125 mg/d, due to concerns for gut edema and possible malabsorption of medications. He was given standard medical treatment for acute hyperkalemia. Tacrolimus and his angiotensin-converting enzyme inhibitor were temporarily discontinued. His AKI and hyperkalemia improved and he was discharged, although still heavily nephrotic, with unmeasurable serum albumin <1.0 g/dl, and urine protein-to-creatinine of 12.8. He was restarted on tacrolimus (target trough 6–9 ng/ml) and prednisone 60 mg daily. A repeat biopsy was performed in February 2012 (Figure 1), due to slow and incomplete remission of his nephrosis that had relapsed in September 2011, in spite of 3.5 months of ongoing prednisone, which had been reduced to 40 mg/d, and repeatedly stable trough tacrolimus levels. Specifically, the purpose of this second biopsy was to rule out potentially missed FSGS on his first biopsy. At the time of this second biopsy, serum creatinine was 0.8 mg/dl, albumin was 2.2 g/dl, and urine protein-to-creatinine ratio 2.5. This biopsy showed no evidence of segmental sclerosis on light microscopy, although only 4 glomeruli were sampled. The glomeruli were otherwise all normal in appearance (Figure 1a and b). There was approximately 10% interstitial fibrosis and tubular atrophy. On immunofluorescence, there was trace codominant staining for C1q (Figure 1c). Electron microscopy showed patchy and incomplete foot process effacement, microvillus transformation, along with scattered electron-dense deposits in the mesangial areas (Figure 1d and e). Although staining for C1q was weak, because of the presence of electron-dense deposits on electron microscopy, a diagnosis of MCD, C1q variant, was favored. FSGS could not be excluded, due to the small number of sampled glomeruli. Re-biopsy was considered, but not performed. By August 2012, he achieved remission while still on prednisone 30 mg/d and ongoing tacrolimus. Serum albumin normalized to 3.7 g/dl, and urine protein to creatinine ratio fell to 0.1.
Figure 1.
Glomerular changes consistent with minimal change disease during partial remission. (a) Normal glomerulus. The glomerulus is slightly enlarged, but shows normal cellularity. All of the capillary loops are patent, and the capillary walls are of normal thickness. All 4 glomeruli in this biopsy showed this appearance (periodic acid–Schiff [PAS], original magnification ×300). (b) Normal glomerulus on Jones silver stain. The vascular pole is sectioned so that it appears in the center of the glomerulus, but the glomerulus is of normal size and cellularity. All of the capillary loops are patent, and the capillary walls are of normal thickness (original magnification ×300). (c) Immunofluorescence showed 1+ staining for C1q. This is of uncertain significance, but, in the context of visible immune complex deposits on electron microscopy, raises consideration for C1q nephropathy. (d,e) The ultrastructural appearance was nearly normal by electron microscopy (EM). The glomerular basement membranes demonstrate normal thickness and texture. There is no increase in mesangial matrix. The endothelial fenestrations are normal. Partial foot process effacement is present, and there are microvillus changes in the podocyte cytoplasm. These changes are less diffuse than typically seen in the setting of untreated minimal change nephrotic syndrome.
Between August and September 2012, prednisone was slowly tapered and while on 20 mg/d, he again relapsed, but responded to dose increase to 40 mg/d with full remission. His prednisone was again tapered, and this time by late October, while on 20 mg/d, and ongoing tacrolimus 2 mg twice a day, he was still in remission.
Alternative steroid-sparing options were discussed to maintain remission, including a possible trial of oral cyclophosphamide, but the patient was reluctant to consider this drug, because of his preexisting diagnosis of infertility. Recently, anti-CD80 therapy has been suggested as alternative for patients with difficult-to-treat MCD and high urinary CD80 levels.1 We hypothesized that if urinary CD80 levels were indeed found to be elevated in our patient, then potentially a trial of the specific CD80 blocker, CTLA-4 IgG1 (abatacept), might offer therapeutic benefit.
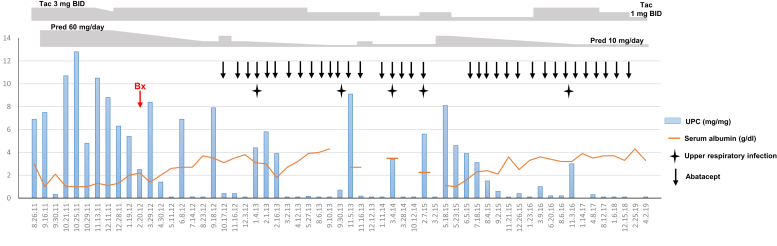
We had been able to measure urine CD80 during partial remission in May 2012. His level was mildly elevated (97 ng/g creatinine) compared with our historical published controls.5 A decision to start abatacept, as a potential steroid/calcineurin inhibitor sparing therapy, was made. He received 750 mg on infusion day number 1 (November 2, 2012), then 750 mg 2 weeks later, 750 mg again 2 weeks later, then 750 mg at once-a-month intervals. Since the initiation of abatacept, the patient has had only 1 full relapse in May 2015. Just before this isolated relapse, he had missed his monthly abatacept dose, due to a change in his insurance. Reinitiation of abatacept the following month, along with prednisone increase back to 30 mg/d, led to reinduction of remission over a several-week period. Since then, the patient has remained free of full relapses despite gradual dose reduction of his prednisone and tacrolimus, to 10 mg/d and 1 mg twice a day, respectively. Over the past 4 years, since reinitiation of abatacept, he has had only had transient, self-limiting episodes of mild proteinuria, in every instance triggered by an upper respiratory infection, not requiring repeat dose escalation of prednisone (Figure 2). Apart from the occurrence of mild upper respiratory infections at a rate of approximately 1 to 2 per year (under combined immunosuppression with abatacept, tacrolimus, and prednisone), abatacept has been well tolerated now for a cumulative 6+ year history, with no serious opportunistic infections, or other side effects. When last checked in January 2018 (while in remission), total cholesterol (without lipid-lowering therapy) was normal at 150 mg/dl, low-density lipoprotein 87 mg/dl, high-density lipoprotein 49 mg/dl, and triglycerides 71 mg/dl.
Figure 2.
Chronological representation of urine protein-to-creatinine ratio (UPC), with doses of coadministered tacrolimus (Tac) and prednisone (Pred), timing of abatacept administration (and interruption), and dates of upper respiratory infections accompanied by proteinuria flares. BID, twice a day; Bx, renal biopsy.
Discussion
We report here the case of a young gentleman with relapsing, steroid-dependent MCD in whom the number and severity of disease relapses has been dramatically reduced after the initiation of abatacept therapy. The clinical course of this patient falls into the spectrum of MCD as opposed to FSGS. Although FSGS could not be ruled out on the second biopsy performed in 2012, due to the small number of sampled glomeruli, the patient’s first biopsy, from 2006, when he was initially floridly nephrotic, was quite adequate, with 37 glomeruli sampled, none with evidence of segmental sclerosis. Hence, between the patient’s clinical presentation with rapid onset and offset of symptoms, rapid induction of remission with steroids, still normal estimated glomerular filtration rate in spite of a 13+ year history since initial disease presentation (serum creatinine 0.87 mg/dl in April 2019 = glomerular filtration rate 111 ml/min), mildly elevated urinary CD80 obtained during partial remission, and lack of segmental sclerosis on 2 biopsies, we feel that this patient’s disease presentation is more consistent with MCD than FSGS.
MCD is a rare but often devastating disease due to its relapsing nature and its potential for comorbidities and complications. In spite of the advances in podocyte biology, the mechanisms of proteinuria in MCD remain incompletely understood. This is in part because of the heterogeneity of the disease, lack of animal models, and the poor understanding of the underlying molecular signature. Not surprisingly, current therapies for patients with MCD are nonspecific as well as associated with significant side effects.
CD80, a costimulatory molecule expressed by antigen-presenting cells, and its natural inhibitor, CTLA-4, have been thought to play a role in MCD.6 Urinary CD80 levels have been proposed as a surrogate biomarker of disease activity in MCD.1, 2, 5, 7 Although there is an ongoing debate about the cellular source of CD80 found in urine of these patients with MCD,8 urinary CD80 levels have been consistently shown to help to distinguish patients with MCD and FSGS during relapse.1, 2, 5, 7 The patient was selected for use of abatacept based on his complicated course (steroid and calcineurin dependence at high doses and multiple relapses over a several-year period) and the presence of high urine CD80, suggestive of an underlying dysregulation of the CD80/CTLA-4 axis. In addition, there have been reports of abatacept use in patients with FSGS, although with conflicting results.1, 2, 3 In our experience, abatacept does not reduce proteinuria in patients with FSGS patients.1 Of note, these patients with FSGS had lower or normal urinary CD80 than those seen in MCD.1, 2, 5, 7 There are no reports of high urine CD80 levels in patients with FSGS to date. Recently, Ling et al.7 also found that high urine CD80 is a predictor of good outcome, measured as response to steroid and progression of renal dysfunction, in children after 5-year follow-up.
In this case report, the role of prednisone and tacrolimus to help maintain remission cannot be excluded. However, the patient has experienced a striking reduction in the number and severity of relapses during a 6-year follow-up period since initiation of abatacept. Furthermore, this prolonged period free of relapse has been maintained, for the first time in this patient, despite tapering prednisone and tacrolimus to lower doses. The mechanism(s) by which abatacept induces and/or maintains remission in patients with MCD with high urinary CD80 is unclear. Yu et al.2 proposed that abatacept may stabilize β1-integrin activation in podocytes in proteinuric patients. Our group found that CTLA-4 IgG1 prevents nephrin dephosphorylation in cultured podocytes treated with sera from patients in relapse (Cara-Fuentes G, Garin EH. Nephrin phosphorylation in minimal change disease [abstract]. Presented at: American Society of Nephrology Kidney Week 2014. November 11–16, 2014; Philadelphia, PA). Lately, researchers have been interested on the role of B cells in idiopathic nephrotic syndrome. This has been supported by the improved clinical outcome noted in some patients after rituximab therapy.9 Whether abatacept may play a regulatory role on circulating B cells or in glomerular cells is still unclear.
In conclusion, our case represents the longest reported successful use of abatacept for treatment of a patient with relapsing, steroid-dependent MCD. Concomitant use of abatacept has allowed dose decrease of both prednisone and tacrolimus, with ongoing disease remission. The fact that the patient’s only significant relapse of disease since abatacept initiation, in the autumn of 2012, occurred after transient dose interruption of his monthly abatacept in the spring of 2015, with reinduction of remission following reinitiation of abatacept, further underscores our hypothesis that abatacept has been playing a fundamental role in maintaining disease remission in our patient. The remarkable change in the relapse pattern observed in this patient after abatacept therapy, along with the growing clinical evidence supporting urinary CD80 as diagnostic and prognostic biomarker, highlight the need for further basic, translational, and clinical research to fully understand the role of CD80 in patients with MCD who may potentially benefit from targeted therapy. Distinct from historical nonspecific immunosuppressive regimens that have been offered to patients with MCD, abatacept, via its molecular mechanism of action, may therefore represent the first available, truly mechanistic approach to treatment in patients with MCD and elevated urinary CD80 levels (Table 1).
Table 1.
Teaching points
|
|
|
|
|
|
Disclosure
RJJ has patents or patents pending in fructose and uric acid metabolism, equity with Colorado Research Partners LLC, equity with XORT Therapeutics, and honoraria from Danone Research Foundation, Astra Zeneca, Horizon Pharma, and Eli Lilly. All the other authors declared no competing interests.
References
- 1.Garin E.H., Reiser J., Cara-Fuentes G. Case series: CTLA4-IgG1 therapy in minimal change disease and focal segmental glomerulosclerosis. Pediatr Nephrol. 2015;30:469–477. doi: 10.1007/s00467-014-2957-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu C.C., Fornoni A., Weins A. Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med. 2013;369:2416–2423. doi: 10.1056/NEJMoa1304572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delville M., Baye E., Durrbach A. B7–1 Blockade Does not improve post-transplant nephrotic syndrome caused by recurrent FSGS. J Am Soc Nephrol. 2016;27:2520–2527. doi: 10.1681/ASN.2015091002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dado D., Parikh S., Ayoub I. Abatacept efficacy in steroid-resistant minimal-change disease revealed by the speed of proteinuria reduction after the start of abatacept. Clin Nephrol. 2018;89:376–380. doi: 10.5414/CN109290. [DOI] [PubMed] [Google Scholar]
- 5.Cara-Fuentes G., Wei C., Segarra A. CD80 and suPAR in patients with minimal change disease and focal segmental glomerulosclerosis: diagnostic and pathogenic significance. Pediatr Nephrol. 2014;29:1363–1371. doi: 10.1007/s00467-013-2679-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cara-Fuentes G., Wasserfall C.H., Wang H. Minimal change disease: a dysregulation of the podocyte CD80-CTLA-4 axis? Pediatr Nephrol. 2014;29:2333–2340. doi: 10.1007/s00467-014-2874-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ling C., Liu X., Shen Y. Urinary CD80 excretion is a predictor of good outcome in children with primary nephrotic syndrome. Pediatr Nephrol. 2018;33:1183–1187. doi: 10.1007/s00467-018-3885-7. [DOI] [PubMed] [Google Scholar]
- 8.Novelli R., Gagliardini E., Ruggiero B. Any value of podocyte B7–1 as a biomarker in human MCD and FSGS? Am J Physiol Renal Physiol. 2016;310:F335–F341. doi: 10.1152/ajprenal.00510.2015. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia D., Sinha A., Hari P. Rituximab modulates T- and B-lymphocyte subsets and urinary CD80 excretion in patients with steroid-dependent nephrotic syndrome. Pediatr Res. 2018;84:520–526. doi: 10.1038/s41390-018-0088-7. [DOI] [PubMed] [Google Scholar]