Abstract
Persons with acquired solitary kidney, including those who have had a unilateral nephrectomy for living kidney donation, renal malignancies, or trauma, have decreased renal mass that leads to increased intraglomerular pressure and glomerular hyperfiltration. These physiologic adaptations of solitary kidney may exacerbate other preexisting and genetic conditions that could create a predisposition to or worsen glomerular pathologies, leading to unfavorable renal outcomes. Hence, these persons may benefit from special care and lifestyle modifications, including nutritional interventions. There is a lack of consensus and evidence for proper surveillance and management after nephrectomy, and misconceptions in both directions of having a “normal” versus “abnormal” kidney status may cause confusion among patients and healthcare providers pertaining to long-term kidney health monitoring and management. We have reviewed available data on the impact of lifestyle modifications, particularly nutritional measures, and pharmacologic interventions, on short- and long-term outcomes after nephrectomy. We recommend avoidance of excessively high dietary protein intake (>1 g/kg per day) and high dietary sodium intake (>4 grams/d), adequate dietary fiber intake from plant-based foods, a target body mass index of <30 kg/m2 (in non-athletes and non-bodybuilders), and judicious management of risk factors of progressive chronic kidney disease (CKD), and future studies should help to better determine optimal care practices for these persons.
Keywords: chronic kidney disease, dietary management, living donor renal transplantation, nephrectomy, proteinuria, solitary kidney
CKD, which exists in over 10% of the adults with 2 kidneys, can develop in persons with a solitary kidney and may progress to end-stage renal disease (ESRD) resulting in high physical and psychological burdens in addition to extraordinary healthcare costs. Whereas in the past, nephrectomy for living kidney donation was considered to be safe without a higher likelihood of CKD,1 more recent data suggest that there is a 3–5 times higher relative risk of ESRD after a unilateral nephrectomy, while the absolute risk remains small.2, 3, 4 The pathogenesis of CKD and ESRD in kidney donors with a solitary kidney may be different from that in those CKD patients without nephrectomy. Glomerulonephritis appears to be the most common renal disease, leading to early ESRD in living kidney donors, and underlying genetic predispositions may contribute to faster progression of CKD to ESRD in some groups of living kidney donors.5 Misconceptions in both directions of having a “normal” versus “abnormal” kidney status cause confusion among patients and healthcare providers pertaining to long-term management.
According to the conventional definition and staging of CKD, persons with only one kidney from congenital or acquired causes, such as donor nephrectomy, are classified as CKD patients. Physiological adaptation in solitary kidney leads to higher glomerular filtration rates (GFRs) relative to units of nephron, which can initially increase GFR, known as glomerular hyperfiltration, but in the long-term, it may lead to a gradual decline in kidney function, and this trend can happen even in living kidney donors. The progression to ESRD may be related to unrevealed intrinsic risks of kidney diseases such as genetic aberrations.6 The resultant burden on kidney health, particularly if aggravated by other causes of glomerular hyperfiltration, such as high dietary protein intake, may lead to de novo glomerular diseases such as secondary focal segmental glomerulosclerosis (FSGS), and may accelerate other preexisting glomerular pathologies. Similar to most other causes of CKD, clinical manifestation of solitary kidney is silent. Therefore, initial screening for signs of worsening renal function and accurately determining renal function are warranted. In addition to usual approaches for CKD management, lifestyle modification including nutritional and dietary interventions can be considered for persons with a solitary kidney, and may be complemented by certain pharmacologic interventions, as reviewed in this article.
Epidemiology of Solitary Kidney
Congenital solidary kidney, also known as unilateral renal agenesis, occurs in a ratio of about 1:1000, often on the left, with a male-to-female ratio of 1.8:1.7
Acquired solitary kidney after a unilateral radical nephrectomy in adults is mainly due to living kidney donation, renal tumor, and trauma. Over the last 30 years, rate of living kidney donation had gradually increased from 1800 donations in 1998 to 6600 donations in 2004. However, it has decreased since 2011 and has been stable around 5650 kidney donations per year.8 The most common age range of living kidney donors is 35 to 49 years.9
The incidence of renal cell carcinoma is 63,990 cases each year. The risk for developing renal cancer significantly increases in individuals aged >60 years, and males are at an almost 2 times greater risk than females.10 There were 10,123 and 4299 radical nephrectomies performed during 1991–2002 and 1992–2007, respectively, due to renal cell carcinoma.11
The incidence of renal trauma is varied. One study reported 757 radical nephrectomies among 9002 renal trauma patients from 2002–2007.12 Young adult males are the most commonly affected population.
Pathophysiological Changes in Patients With a Solitary Kidney
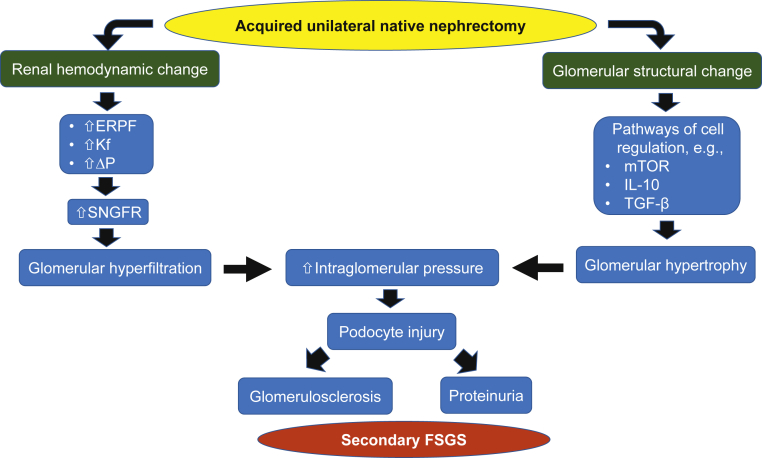
GFR is correlated with the number of nephrons, and it may vary by age, gender, and body habitus. Loss of nephrons is usually not a cause of decreased GFR, owing to compensatory mechanisms, although these do not provide full compensation and GFR increases to 65%–70% of pre-donation GFR in healthy donors aged <60 years.13 Since the excretory function of kidney is needed to maintain fluid, electrolyte, and mineral balances, physiological adaptation occurs immediately after nephrectomy, with increases in effective renal plasma flow, glomerular ultrafiltration coefficient (Kf), and transcapillary hydraulic pressure gradient (ΔP), leading to increased single-nephron GFR, glomerular hyperfiltration, and overall increased GFR.14, 15
In addition to renal hemodynamic change after nephrectomy, structural nephron alteration in the form of both hypertrophy and hyperplasia may occur.16 This compensatory glomerular hypertrophy is involved in several pathways including activation in mammalian target of rapamycin complex (mTOR), interleukin 10, and transforming growth factor-β.17 However, this compensatory mechanism postnephrectomy in living kidney donor differs from patients after nephrectomy from other reasons.18
The compensatory glomerular hyperfiltration can cause damage to the solitary kidney in the long term, especially if there are other factors that would aggravate glomerular hyperfiltration, such as high dietary protein intake resulting in afferent arteriole dilation and leading to intraglomerular hypertension, or high dietary sodium intake resulting in increases in systemic hypertension and volume retention.19, 20 Intraglomerular hypertension causes podocyte injury and loss of perm-selectivity of the filtrating function of the slit diaphragm between foot processes, causing proteinuria. In addition, endothelial-mesangial hyperplasia and glomerulomegaly mediated by increased transforming growth factor-β1 and angiotensin II cause podocyte detachment from glomerular basement membrane and subsequently glomerulosclerosis.21 These ultimately lead to pathological changes similar to those seen in FSGS and albuminuria, a decline in GFR, and CKD progression (Figure 1).
Figure 1.
Pathophysiological changes after unilateral native nephrectomy. ERPF, effective renal plasma flow; FSGS, focal segmental glomerulosclerosis; GFR, glomerular filtration rate; IL-10, interleukin-10; Kf; glomerular ultrafiltration coefficient; mTOR, mammalian target of rapamycin; SNGFR, single-nephron glomerular filtration rate; TGF-β, transforming growth factor-beta; ΔP; transcapillary hydraulic pressure gradient.
Consequence of Acquired Solitary Kidney After Radical Nephrectomy
As discussed above, native nephrectomy causes glomerular hypertension and hypertrophy and pathological change with FSGS, which are associated with poor renal outcomes. Similar to common glomerular diseases in patients with both kidneys such as minimal change disease (MCD), FSGS, and diabetic nephropathy, glomerulomegaly and enlarged kidney size are associated with progression of CKD.22 In addition, glomerular hypertrophy secondary to nephronopenic change in long-surviving transplant renal allograft is associated with FSGS.23 In acquired solitary kidney patients, histologic changes also lead to poorer renal and patient outcomes.
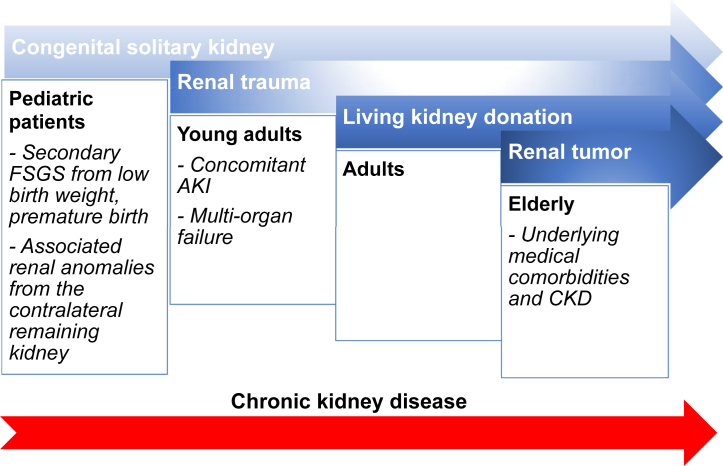
The severity of progressive renal dysfunction and long-term renal outcomes are determined by the remaining nephron masses immediately after nephrectomy, the duration of having a solitary kidney, concomitant comorbidities, and lifestyle, including dietary habits (Figure 2). Pediatric patients with congenital solitary kidney, even with subclinical defects, carry a greater risk for developing ESRD compared with those who have unilateral or bilateral renal hypodysplasia, or multicystic or horseshoe kidney.24 Unilateral nephrectomy due to renal trauma and from living kidney donation is common in young and middle-aged persons, respectively, whereas kidney malignancies are more common in older age groups, who are more likely to have underlying comorbidities, which increases the risk of CKD. In this review, we discuss the outcomes of acquired solitary kidney.
Figure 2.
Etiologies of solitary kidney across age groups and factors determining long-term renal function, including nephron mass at the initial onset of having a solitary kidney and concomitant underlying renal anomalies or comorbidities. Duration of having a solitary kidney, depending on age at the onset of solitary kidney, also leads to possible cumulative lifetime risk for developing progressive chronic kidney disease (CKD). AKI, acute kidney injury; FSGS, focal segmental glomerulosclerosis.
Living Kidney Donation
One cohort showed no increased risk of developing ESRD or mortality among donors.1 However, more recent studies have consistently demonstrated increased long-term risk of ESRD2, 3, 4 and higher mortalities.2 Table 11, 2, 3, 4 shows long-term outcomes in living kidney donors from 4 recent clinical studies. However, these epidemiologic studies followed living kidney donors for up to only 2 decades. Recent clinical investigations to understand the long-term pathophysiologic change of the remaining kidney in living kidney donors with a median follow-up of 6.3 years demonstrated that adaptive glomerular hyperfiltration and hypertrophy, but not glomerular hypertension, persists after donor nephrectomy, due to increased renal plasma flow and heightened Kf.25 This glomerular hyperfiltration in living kidney donors is different from the glomerular hyperfiltration in diabetic kidney disease that results from increased glomerular hydraulic pressure (PGC).26 In addition, a recent retrospective study with long-term follow-up of living kidney donors showed that postdonation GFR steadily increased for several years until donors reached 70 years of age.6 Indirect evidence of early signs of CKD was a higher level of serum intact fibroblast growth factor 23, and renal tubular fractional excretion of inorganic phosphorus, as well as lower serum phosphorus and calcitriol levels in living kidney donors compared with nondonor controls.27 Kasiske et al.28 reported laboratory changes in living donors that were consistent with the findings in patients with mild CKD, including higher levels of serum parathyroid hormone, uric acid, homocysteine, and potassium, but a lower level of hemoglobin compared with a control group.28 Hence, there is consistent evidence to suggest that unilateral radical nephrectomy for living kidney donation may increase the overall risk of progressive CKD, even though the absolute risk is likely small.
Table 1.
Summary of long-term outcomes in living kidney donors from 4 recent clinical studies
| Reference | Study design | Study population | Duration of follow-up | Results |
||||
|---|---|---|---|---|---|---|---|---|
| ESRD | Mortality | Proteinuria | Hypertension | Additional outcomes | ||||
| Ibrahim et al. 20091 | Single center; living kidney donation 1963 through 2007; study period 2003– 2007 | 3698 living kidney donors vs. matched (1:1) controls based on age, sex, and race or ethnic group | Mean (±SD) of 12.2 ± 9.2 yr after donation | Development of ESRD in 11 living kidney donors (180 cases/million persons per year) vs. 268 cases/million per year in general population; GFR of ≥60 ml/min per 1.73 m2 of BSA in 85.5% of donor subgroupa |
Death in 268 donors; donor survival appeared to be similar to that of controls in general population | Albuminuria 12.7% of donor subgroupa; a longer time since donation was associated with albuminuria | Hypertension in 32.1% of donor subgroupa | Older age and higher BMI were associated with a GFR of <60 ml/min per 1.73 m2 and hypertension |
| Mjoen et al. 20142 | Single center; living kidney donation 1963 through 2007 | 1901 living kidney donors vs. 32,621 controls who would have been eligible for donation |
A median follow-up of 15.1 yr (living kidney donors) and 24.9 yr (control group) | Development of ESRD in 9 living kidney donors (302 cases/million) vs. 22 controls; HR for ESRD 11.38 (95% CI, 4.37–29.6) |
HR for all-cause mortality 1.30 (95% CI, 1.11–1.52) and for cardiovascular death 1.40 (95% CI, 1.03–1.91), for donors compared with controls | NA | NA | NA |
| Muzaale et al. 20143 | Population-based study | 96,217 living kidney donors in the US, per OPTN between April 1994 and November 2011 vs. 20,024 matched healthy participants of the NHANES III between 1988 and 1994 |
Maximum follow-up of 15.0 yr; median follow-up of 7.6 yr (IQR: 3.9–11.5 yr) for kidney donors vs. 15.0 yr (IQR 13.7–15.0 yr) for matched healthy nondonors | Development of ESRD in 99 donors in a mean (SD) of 8.6 (3.6) yr after donation vs. 36 matched healthy nondonors in 10.7 (3.2) yr; estimated risk of ESRD at 15 yr after donation was 30.8 per 10,000 (95% CI, 24.3–38.5) in kidney donors and 3.9 per 10,000 (95% CI, 0.8–8.9) in their matched healthy nondonors (P < .001); estimated lifetime risk of ESRD was 90 per 10,000 donors vs. 326 per 10,000 unscreened nondonors (general population) vs. 14 per 10,000 healthy nondonors |
An estimated risk of ESRD was highest in black donors and lowest in white nondonors | |||
| Grams et al. 20164 | A meta-analysis of 7 general-population cohorts, calibrated to the population-level incidence of ESRD and mortality in the US | 52,998 living kidney donors in the US vs. 4,933,314 nondonors from 7 cohorts | Median of 4 to 16 yr | Projected 15-yr observed risk of ESRD 3.5 to 5.3 times greater in living kidney donors compared to age-matched nondonors | Projected 15-yr risk of ESRD in nondonors depending on race and sex; (highest in black men and lowest in white women) | |||
BMI, body mass index; BSA, body surface area; CI, confidence interval; ESRD, end-stage renal disease; HR, hazard ratio; IQR, interquartile range; NA, not available; NHANES III, Third National Health and Nutrition Examination Survey; OPTN, Organ Procurement and Transplantation Network.
Glomerular filtration rate (GFR) and urinary albumin excretion and were measured in a total of 255 donors from 2003 through 2007, and the prevalence of hypertension was examined.
Common causes of advanced CKD and ESRD in living kidney donors include diabetes mellitus, hypertension, and glomerulonephritis and are similar to those in the non-nephrectomized population; however, the time of ESRD onset may be different. A study of 125,427 living kidney donors with a median follow-up of 11 years showed that glomerulonephritis was the most common cause of ESRD during the first 10 years after kidney donation, whereas diabetes and hypertension were more common thereafter.5 The pathogenesis of ESRD in living kidney donors involves not only glomerular hyperfiltration but also intrinsic factors, which may explain an earlier onset of ESRDs such as genetic kidney diseases including apolipoprotein L1–related disease; the 2017 Kidney Disease Improving Global Outcomes guidelines have suggested that apolipoprotein L1 genotyping be offered in potential living kidney donors with sub-Saharan African ancestors.29 Although the most recent large retrospective cohort studies demonstrated an 8–11 times greater risk for ESRD in living donors, absolute risk was very low. Residual confounders were non-perfectly matched controls for donors in both studies.2, 3 Two small studies utilized siblings of the donors to achieve better-matched controls and showed no increased risk for ESRD in living donors.30, 31 From the aforementioned study, the second “hit” to living donors from immunologic- or environmental-related renal diseases, not CKD progression or FSGS due to hyperfiltration, is likely the major reason of ESRD.1, 2, 5
The baseline functioning nephrons that everyone has at birth, also known as “nephron endowment,” may determine the risk for CKD and may need to be taken into consideration during living-donor evaluation.32
Several studies have demonstrated a positive correlation between the number of nephrons and birth weight. Infants with low birth weight, either preterm infants with appropriate weight for gestational age, or term infants with small for gestational age, have a decreased number of nephrons and an increased risk of hypertension, cardiovascular diseases (CVDs), and progressive CKD leading to ESRD. In adults, several studies revealed an association between low birth weight and higher blood pressure (BP), as well as between low birth weight and microalbuminuria and proteinuria. A meta-analysis showed that low birth weight is associated with a 70% increased risk of CKD defined as albuminuria, low estimated GFR (eGFR; <60 ml/min per 1.73 m2 or <10th percentile for age/sex), or ESRD.32 Hence, it is biologically plausible that solitary kidney, by virtue of having 50% lower nephron endowment, results in a higher risk of progressive CKD. However, there is a U-shape relationship between birthweight and CKD in men, and high birth weight (≥4500 mg) was found to be associated with CKD, possibly from maternal and future insulin resistance in men.33
A higher ratio of donor kidney weight to recipient body weight is associated with lower levels of proteinuria in deceased donor recipients and better 3-year outcomes in living donor recipients.34 Kidney transplant recipients with higher transplant kidney cross-sectional area divided by recipient body weight had higher 5-year renal allograft function.35 Given that history of low birth weight, age, and gender are associated with nephron number, information on these factors may be useful when evaluating potential living kidney donors, informing renal prognosis after living kidney donation, and appropriately selecting potential living kidney donors to match recipient demand.
Based on the original CKD definition, a person with structural damage to a kidney, including partial or radical nephrectomy, has CKD, even if GFR is normal without albuminuria (<30 mg/g of creatinine). However, the justifiability of labeling a living kidney donor or even cancer nephrectomized patient as having CKD remains debatable given that such a “diagnosis” may have a wide range of psychosocial implications and may affect employment and health insurable eligibility. Nevertheless, we believe that periodic assessment of “kidney function of donor” is a critical responsibility of healthcare providers, given the higher than normal risk of developing ESRD in living kidney donors, and notwithstanding the fact that the absolute risk remains small.
Unilateral Native Nephrectomy for Renal Cancer
Patients with kidney cancer who undergo unilateral nephrectomy are likely to encounter a long-term higher risk for CKD and subsequently higher mortality, compared with the general population. However, a significant proportion of these patients may have preexisting CKD. A retrospective cohort of 4633 patients diagnosed with renal cell carcinoma showed that 11% of patients undergoing partial nephrectomy developed CKD; up to 20% of patients with radical nephrectomy had CKD; and the incidence of ESRD was 2% and 4% after partial and radical nephrectomy, respectively.36 In another study of 7073 US veterans who had a partial or radical nephrectomy between 2004 and 2013, mostly for kidney cancer, worse postnephrectomy kidney function and higher mortality were observed with radical nephrectomy, and a low presurgical eGFR and a greater decrease in eGFR postsurgery were associated with higher mortality, irrespective of the type of nephrectomy.37
Unilateral Native Nephrectomy After Renal Trauma
Renal trauma patients requiring unilateral nephrectomy is another group with a solitary kidney whose contralateral renal function determines a long-term renal outcome. The incidence of acute renal failure after nephrectomy for renal trauma is 5%–10%.38 Several factors increase the risk of renal failure, including the patient’s age, other organ injury, and multi-organ failure. The prevalence of CKD or CKD progression in renal trauma patients with unilateral nephrectomy is unclear, but subgroups of these patients who are at higher risk for developing CKD are likely to be those who had acute kidney injury or worsening underlying CKD from trauma or after nephrectomy.
Cardiovascular Risk in Solitary Kidney
As with the general population, CVD is an important cause of morbidity in living kidney donors. Even in relatively older living kidney donors (≥55 years old), their combined mortality and CVD did not differ from matched healthy older individuals.39 However, a decreased GFR in living kidney donors may lead to higher cardiovascular risk. Moody et al.40 demonstrated that living kidney donors had significant increases in left ventricular mass-to-volume ratio but decreases in aortic distensibility and global circumferential strain compared with healthy controls at 12-months postdonation. In that study, mean changes in GFR during a 12-month follow-up were –28 ± 11 and 3 ± 11 ml/min per 1.73 m2 in donor and control groups, respectively.
Care for Persons with a Solitary Kidney
To prevent CKD or slow progression of a pre-existing CKD in a person with a solitary kidney, nonpharmacologic interventions should be utilized, although concurrent pharmacologic interventions may be used in selected patients.
Nonpharmacologic Interventions
Low-Protein Diet
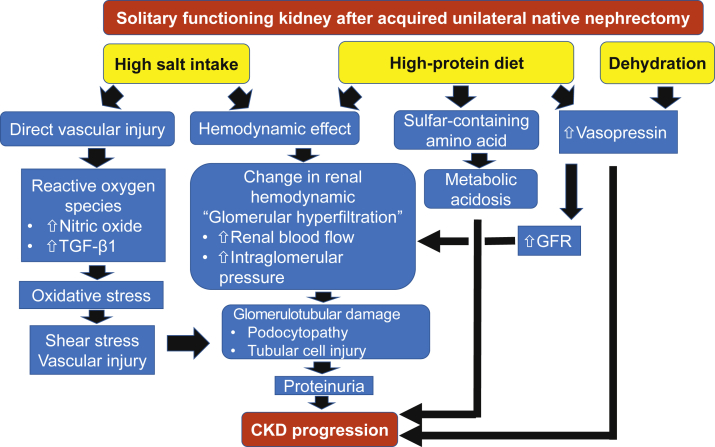
Evidence suggests that high dietary protein intake is associated with higher risk for CKD or faster CKD progression, because it leads to afferent arteriolar vasodilatation which in turn increases intraglomerular pressure.20 Whereas a high-protein diet leads to an initial increase in GFR, in the long-term, increased intraglomerular pressure can lead to glomerular hyperfiltration and loss of kidney function (Figure 3).19 A population-based study involving 1522 middle-age persons with a mean eGFR of 84.0 ± 11.4 ml/min per 1.73 m2 showed a positive cross-sectional correlation between protein intake and GFR, but after a mean follow-up of 12 years, every 1 g/d increase in protein intake was associated with a 4.1 ml/min per 1.73 m2 decline in eGFR (95% confidence interval −5.1, −3.1) and a 78% higher risk for incidence of CKD defined as eGFR <60 ml/min per 1.73 m2 (95% confidence interval 1.15, 2.78).41
Figure 3.
Mechanism of renal injury from high-protein diet, high salt intake, and dehydration. CKD, chronic kidney disease; GFR, glomerular filtration rate; TGF-β1, transforming growth factor-beta 1.
Several experimental studies and clinical trials demonstrated a beneficial effect of low dietary protein intake on slowing CKD progression, whereas a high protein intake increases risk of renal failure. A cross-sectional study in more than 4000 persons showed an association between a high-protein diet and increased GFR, with a sigmoid relationship between GFR and overnight urinary urea nitrogen, with the threshold around the recommended daily allowance for protein intake of 0.8 g/kg per day.42 Moreover, there is evidence of a linear relationship between the amount of protein intake and a decrease in eGFR.19
The quality of the protein may also play a role. Several epidemiologic studies reveal the benefit of plant-based proteins over an animal-based diet.43 Data from an 11-year follow-up among female participants with an eGFR >55 to <80 ml/min per 1.73 m2 showed a significant decline in eGFR of 1.21 ml/min per 1.73 m2 per 10-g increase in nondairy animal protein intake.44 Another prospective cohort study of 11,952 patients with an eGFR >60 ml/min per 1.73 m2 showed an association between an increased risk of CKD and red and processed meat intake, but a decreased risk of CKD is associated with a diet with nuts, low-fat dairy products, and legumes.45
The diet should include adequate fiber from plant-based sources, as does the Dietary Approaches to Stop Hypertension (DASH) diet and other diets listed in Table 2, and the amount of daily protein should be adjusted by level of renal function and proteinuria.19 It is often recommended that patients with hypertension follow an energy-controlled DASH diet, which is high in complex carbohydrates including fruits, vegetables, and whole grains, as well as legumes, and low in animal-based protein such as meat, saturated fat, refined grains, sweets, and processed food. These dietary strategies may help with weight maintenance or reduction in weight gain in obesity.
Table 2.
The contemporary “healthy” diets in Western societies and suggestions for persons with a solitary kidneya
| Diet type | Features | Relevance to solitary kidney care |
|---|---|---|
| DASH diet (Dietary Approaches to Stop Hypertension) | Mix of fruits, vegetables, whole grains, lean protein, and low-fat dairy | A preferred diet for persons with solitary kidney |
| Mediterranean diet | High in fruits and vegetables, as well as healthy fatty foods like fish, nuts, and olive oil | Mediterranean diet is favorable as long as excessive protein intake of >1 g/kg per day and high sodium intake of >4 g/d are avoided |
| MIND diet | A mix of DASH and the Mediterranean diet | Acceptable diet regimen |
| Flexitarian diet | A blend of the words flexible and vegetarian; eat vegetarian most of the time for better health | Acceptable diet for persons with solitary kidney as long as high salt intake of >4 g/d is avoided |
| Weight Watchers | The PointsPlus system encourages consumption of fruit, vegetables, and fiber-rich foods, and discourages consumption of high-fat and energy-dense foods | Excessive protein intake of >1 g/kg per day to be avoided |
| TLC diet (Therapeutic Lifestyle Changes) | To lower high cholesterol | Excessive protein intake of >1 g/kg per day and high sodium intake of >4 g/d to be avoided |
| Volumetrics | To pay attention to the energy density in foods | Excessive protein intake of >1 g/kg per day and high sodium intake of >4 g/d to be avoided |
Note that in addition to the listed diet, vegetarian or vegan diet can also be recommended.
No human study shows the benefits of a low-protein diet in protecting renal outcomes after nephrectomy, and a typically low dietary protein intake, such as 0.6–0.8 g/kg per day, which is recommended to patients with an eGFR <45 ml/min per 1.73 m2 or proteinuria >0.3 g/d, may not be needed for healthy kidney donors.19 However, we believe that it is wise to avoid a high protein intake of greater than 1 g/kg per day, except in the case of professional athletes or bodybuilders with well thought-out calculations on the needed protein amounts for anabolic goals. Currently, there are no data to corroborate these suggestions.
Low Dietary Sodium Intake
High sodium intake can cause direct vascular injury even without hypertension and indirect renal damage from hemodynamic mechanisms mediated by elevated BP and proteinuria. Similar to a high-protein diet, a high-sodium diet leads to increased intraglomerular pressure, causing glomerular hyperfiltration and subsequently renal injury (Figure 3).19 A recent meta-analysis demonstrated no preventive benefit for CKD progression from reducing sodium intake in the long term,46 and one longitudinal study using serial 24-hour urine collections in 3939 CKD patients suggested that the highest versus lowest quartile of urinary sodium excretion (≥4.5 vs. <2.7 g/d) was associated with 45% higher mortality and 54% greater risk of disease progression.47 Incrementally worse cardiovascular outcomes were observed when dietary sodium intake exceeded 4 g/day.48 However, recent observations in the general population suggest that there is a J-shaped association in which both higher and lower dietary sodium intake (>5 and <3 g/d) were associated with higher risk of CVD and death.49 A prospective study in 95,676 participants without CVD demonstrated that a mean sodium intake of >5 g/d was associated with stroke but not cardiovascular events.50
In a recent prospective cohort study of 3106 hypertensive and 4871 nonhypertensive persons, in whom 28% of hypertensive and 17% of nonhypertensive patients developed CKD (eGFR <60 ml/min per 1.73 m2) over a 10- to 11-year follow-up, respectively, hypertensive patients with sodium intake of <2.1 and >4 g/d had a significantly higher incidence of CKD than those with sodium intake between 2.9 and 4 g/d.51 We recommend avoiding a diet with >4 g/d of sodium in individuals at higher risk of developing future CKD, including those with a solitary kidney.19 Studies are needed to further delineate this area in persons with a solitary kidney.
Weight Control
Even in living kidney donors who are determined to be healthy individuals, obese donors had a 1.86 times higher risk of ESRD over 20 years following nephrectomy compared to nonobese donors, and overweight donors exhibited incrementally a 7% higher risk of ESRD52 for every 1 kg/m2 higher body mass index above 27 kg/m2. Nevertheless, in persons with more advanced CKD, including dialysis patients, a paradoxically greater longevity has been reported with larger body size, known as the obesity paradox or reverse epidemiology, possibly reflecting a more resilient nutritional profile and muscle mass.53 Hence, any unintentional edema-free weight loss warrants timely work-up, and dietary interventions may be considered in those with more advanced CKD. We recommend a target body mass index of <30 kg/m2 in non-athletes and non–bodybuilders with a solitary kidney.
Adequate Hydration
Both animal and human studies demonstrate the inverse relationship between fluid intake and the long-term trajectory of GFR and risk of proteinuria. The mechanism of an increased GFR with low fluid intake may be related to increased secretion of vasopressin.54 Higher vasopressin also leads to increased urinary albumin excretion,55 and dietary protein also increases vasopressin secretion in humans (Figure 3).56 One study demonstrated a beneficial effect of increased fluid intake in preventing CKD or its progression at earlier stages.57 However, few studies suggested the opposite results.58 We suggest adequate to generous fluid intake (>2.5 L/day) in persons with a solitary kidney and eGFR >60 ml/min per 1.73 m2, as long as there is no material risk of hyponatremia.
Smoking Cessation
Smoking is a known risk for many pathologies including CKD, based on some but not all studies, and may worsen CKD progression.59 Smokers have 76% higher 15-year projections of ESRD risk in the absence of kidney donation compared with nonsmokers. The risk is attenuated but still as high as 45% in former smokers.4 Living kidney donors who smoked do not appear to have increased surgical mortality risk compared with nonsmokers; however, they had 5.3 times greater adjusted mortality risk over 4 years.60 Smoking cessation may slow the rate of renal function deterioration in CKD patients.61 Persons with solitary kidney should be advised routinely to avoid smoking.
Physical Activity After Nephrectomy
After nephrectomy, physical and functional, as well as mental, changes from postoperative recovery, including pain, are associated with outcomes. Evidence showed that exercise improves some side effects for cancer, quality of life, and survival, via alteration in neuro-hormones, cell growth regulatory pathway, gene expression, and tumor immunity.62 Trinh et al.63 reported an association between physical activity and quality of life in kidney cancer survivors; however, 56% of those in the study population were completely sedentary.63 Therefore, medical providers for cancer patients still face this challenge but have the opportunity to improve their patient’s outcomes. For living kidney donors, physical activity improves not only their health and weight control, but also their mental health. Longer time to return to daily activities after donor nephrectomy is associated with lower satisfaction with life (SWL) as assessed by the SWL scale.64
Pharmacologic Interventions
Blood Pressure Control
An increased night-to-day systolic BP ratio, and a decreased dipper pattern in CKD patients before, compared with after, a unilateral nephrectomy have been reported in patients with renal and/or ureteral cancer.65 However, the level of BP by 24-hour BP monitoring, and the circulating renin–angiotensin system, including plasma renin activity and angiotensin II levels, are not altered. In living kidney donors, BP does not appear to be elevated in the short term,66 and similar data are reported in long-term follow-up studies (up to 5 years) and even in donors with pre-donation hypertension.66
Landmark studies comparing BP targets for diabetes67, 68 and nondiabetes,69 as well as the recent guidelines70, 71, 72 for target BP are shown in Table 3. According to the 2017 American College of Cardiology/American Heart Association guidelines, a target BP of <130/80 mm Hg, and a BP threshold to initiate antihypertensive therapy of ≥140/90 mm Hg, are recommended for patients with no clinical CVD and a 10-year atherosclerotic cardiovascular disease risk of <10%; for patients with the latter risk of ≥10%, antihypertensive medications should be initiated when BP ≥ 130/80 mm Hg.72 There is no evidence for recommending any specific BP target in living kidney donors. However, since most living kidney donors are healthy and have no significant comorbidity, a target BP of <130/80 mm Hg may be appropriate. Until there is strong evidence demonstrating that outcomes of living kidney donors are related to BP, individualized BP control appears to be appropriate.
Table 3.
BP control and renal outcomes in major clinical trials and guidelines for target BP that may be relevant to solitary kidney conditions
| BP | Studies | Population | Compared groups | Main results |
|---|---|---|---|---|
| Clinical trials for BP | UKPDS67 | Type 2 DM | Intensive vs. standard BP control | No significant difference in proteinuria, change in serum creatinine, new-onset microalbuminuria, except lower macroalbuminuria level in tight BP compared with standard BP control (6.6% vs. 87%; P <0.000 in ACCORD trial) |
| ACCORD68 | Type 2 DM | Intensive vs. standard BP control | ||
| SPRINT69 | Non-DM | Intensive (SBP <120 mm Hg) vs. standard (SBP <140 mm Hg) BP control | A significant decrease in all cardiovascular outcomes and all-cause mortality in intensive group; a composite of renal outcomes including the first occurrence of a reduction in the estimated GFR of ≥50%, long-term dialysis, or kidney transplantation of 1% with no difference between 2 groups | |
| Target BP | Threshold BP | |||
| Guidelines for BP targets | 2017 ADA70 | <140/90 mm Hg | <130/80 mm Hg (patients with no undue treatment burden) | |
| JNC 871 | <140/90 mm Hg | |||
| 2017 ACC/AHA72 | <130/80 (10-yr ASCVD < or ≥10%) | ≥140/90 (10-yr ASCVD <10%) ≥130/80 (10-yr ASCVD ≥10%) |
ACC/AHA, American College of Cardiology/American Heart Association; ACCORD, Action to Control Cardiovascular Risk in Diabetes; ADA, American Diabetes Association; ASCVD, atherosclerotic cardiovascular disease; BP, blood pressure; DM, diabetes mellitus; GFR, glomerular filtration rate; JNC, Joint National Committee; SBP, systolic blood pressure; SPRINT, Systolic Blood Pressure Intervention Trial; UKPDS, United Kingdom Prospective Diabetes Study.
Proteinuria Management in Solitary Kidney
Angiotensin pathway modulators including angiotensin converting–enzyme inhibitors and angiotensin receptor blockers are often used to improve proteinuria and slow progression of renal disease. Renin–angiotensin system blockade provides renoprotective and antiproteinuric effects in animals with nephrectomy, including solitary kidney;73 however, there is limited evidence of these effects of renin–angiotensin system blockade in humans with a solitary kidney. Angiotensin converting–enzyme inhibitors and angiotensin receptor blockers lower intraglomerular pressure by dilating efferent arterioles more than afferent arterioles, especially in patients with glomerular diseases, because there is afferent arteriolar dilatation at baseline to maintain GFR. As with a low-protein and low-sodium diet, decreased intraglomerular pressure will mitigate glomerular hyperfiltration and may slow progression of renal function decline.
Antiplatelet Agents
Evidence suggests that aspirin lowers cardiovascular events in CKD and it may also delay CKD progression.74 An epidemiologic study showed that compared with nonregular aspirin users, CKD patients using aspirin regularly had an 0.80 ml/min per 1.73 m2/yr (95% confidence interval 0.1, 1.5) slower decline in GFR.75 However, a case–control study showed paradoxically a 2.5-times greater risk of CKD in patients regularly using low-dose aspirin (≥ twice a week for 2 months) compared with the nonaspirin users.76 We currently have no recommendation for or against intake of aspirin or other antiplatelet agents in persons with an acquired solitary kidney.
Evaluation and Follow-up for Renal Function in Solitary Kidney
After unilateral nephrectomy, patients often have concerns pertaining to the risk of permanent loss of renal function and the likelihood of development of ESRD. Therefore, accurate estimated renal function is critical to determine and monitor in these patients.
GFR Estimation and Monitoring
Several equations have been developed to estimate GFR (Table 4).77, 78, 79 Endogenous and exogenous determinants may cause deviations in variables in the equations; therefore, an “estimated” GFR (eGFR), be it based on serum creatinine or cystatin C, is not an accurate method for assessing true GFR and may lead to inaccuracies.77, 78 The Kidney Disease Improving Global Outcomes 2012 Clinical Practice Guideline for the Evaluation and Management of CKD,80 and the 2017 Kidney Disease Improving Global Outcomes Clinical Practice Guideline on the Evaluation and Care of Living Kidney Donors29 recommend that an initial assessment for kidney function be conducted using serum creatinine and the eGFR equation, and that additional tests, e.g., serum cystatin C or a clearance measurement, be used as confirmatory tests when eGFR based on serum creatinine is less accurate. A major limitation of the creatinine-based GFR equations is related to creatinine production, renal creatinine secretion, extrarenal creatinine excretion, and technical issues pertaining to creatinine measurement. Several studies have demonstrated that creatinine-based eGFR inaccurately estimates GFR in patients with a solitary kidney, including pediatric and adult populations with congenital renal diseases or after nephrectomy due to acquired renal diseases, as well as adult living kidney donors (Table 5).81, 82, 83, 84, 85, 86
Table 4.
Characteristics of estimated and measured GFR in persons with a solitary kidney
| GFR | Clinical use | Limitations | Clinical utilities for patients with a solitary kidney |
|---|---|---|---|
| Estimated | |||
| Cockcroft-Gault equation | When serum creatinine is stable; drug dosinga | Determined by muscle mass in steady state - Age - Gender - Race 10%–40% overestimate creatinine clearance Estimate creatinine clearance, not GFR Imprecise in CKD |
Follow-up for stable renal function |
| MDRD Study equation | Accurately estimate77 eGFR <60 ml/min per 1.73 m2; accurate in nonhospitalized CKD | Not precise77 when eGFR >60 ml/min per 1.73 m2 Leading to overestimate CKD prevalence Require steady state or stable renal function |
Follow-up for stable renal function |
| CKD-EPI equation | Can be used77 to estimate eGFRs >60 ml/min per 1.73 m2 Compared to MDRD equation, CKD-EPI is better for higher levels of GFR, diabetes, transplant status, elderly, and at higher body mass index |
Precision remains depending on creatinine measurement | Renal cell carcinoma with comorbidity, e.g., elderly, diabetes, obesity Living kidney donor whose renal functions are generally normal |
| Cystatin C–based GFR | Less affected by race and muscle wasting Conditions causing variation of serum creatinine Diabetes with high GFR High-protein diet Asian Pregnant Unusual muscle mass, body habitus, weight (e.g., morbid obesity, amputees) |
May lower precision compared with creatinine-based GFR | Confirm the result from creatinine-based GFR Consider in solitary kidney patients who have hyperfiltration |
| Creatinine-cystatin C–based GFR | More precision and accuracy than creatinine-based or cystatin-based equations | Not widely available | Confirm the result from creatinine-based GFR |
| KeGFR | Can be used during acute change of renal function | Need subsequent serum creatinine measurement to follow up the trend eGFR | Monitor renal function during early postnephrectomy |
| Measured | |||
| Creatinine clearance | Commonly used in clinical practice | Affected by creatinine secretion, production, measurement | Confirm eGFR |
| Iothalamate clearance | Radioactive of nonradioactive labels78 | Iodine allergy Tubular secretion causes bias in measuring GFR (compared to urinary inulin clearance)79 |
Confirm eGFR Consider using in living kidney donors |
| Iohexol clearance | Nonradioactive radiographic contrast agent78 | Tubular reabsorption underestimates GFR from iohexol plasma clearance compared to urinary inulin clearance78 Iodine allergy |
Confirm eGFR |
| 51Cr-EDTA | Underestimates inulin clearance by 5% to 15% | Confirm eGFR | |
| DTPA | Short half-life (6 h) that minimizes radiation exposure, high counting efficiency of 99mTc | GFR underestimation from dissociation of 99mTc from DTPA and plasma protein binding78 | Confirm eGFR |
| Gadolinium-DTPA or gadolinium-DOTA | A highly sensitive, immunoassay technique | Rare complication of systemic nephrogenic fibrosis | |
CKD, chronic kidney disease; CKD-EPI, Chronic Kidney Disease Epidemiology collaboration; 51Cr-EDTA, chromium-51 labeled ethylenediamine tetraacetic acid; DTPA, diethylenetriamine pentaacetic acid; eGFR, estimated glomerular filtration rate; gadolinium-DOTA, 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid; gadolinium-DTPA, gadolinium-diethylenetriamine pentaacetic acid; KeGFR, kinetic eGFR; MDRD, Modification of Diet in Renal Disease; mGFR, measured glomerular filtration rate
The most accurate method for drug dosing, e.g., MDRD, CKD-EPI equations.
Table 5.
Comparing methods of glomerular filtration rate estimation in different populations with a solitary kidney
| Reference | n | Age of study population (yr) | Causes of a solitary kidney | Reference method of GFR measurement | eGFR | Misclassification of CKD stage |
|---|---|---|---|---|---|---|
| Pierrat et al. 200381 | 176 | Children 3–19 (mean: 13.2 ± 0.36) Adults 20–75 (mean: 46 ± 1.23) |
Children: 30 patients with SK and 30 patients with KT Adult: 28 patients with SK and 88 patients with KT |
Corrected Cin CrClm |
CrClCG Schwartz82 GFR (Sch) MDRD GFR |
Children: Means of Sch and MDRD GFR overestimated mean of Cin Adult: Mean of CrClCG and of MDRD GFR were not different from Cin. In SK, mean MDRD GFR underestimated Cin CrCl overestimated Cin |
| Tan et al. 201083 | 64 | 21–70 (median: 49) | Living kidney donation with median time after donation of 13 months | iGFR | Urinary CrCl CrClm 4-variable MDRD estimating equation (eGFR) CKD-EPI GFR estimating equation |
CrCl overestimates iGFR Both Cr-based estimating equations underestimated and were poorly correlated with iGFR Misclassification was greater in donors aged ≥ 55 yr |
| Ferreira-Filho et al. 201184 | 36 | Mean: 50.7 ± 10.6 | Living kidney donation 28 patients Renal stones with hydronephrosis, 8 patients Mean duration after unilateral nephrectomy: 11.6 ± 9 (2 mon to 38 yr) |
CrClm |
|
CrClCG had a better correlation with CrClm than MDRD GFR (r2 0.64 vs. 0.34, respectively). At CrClm of >90 ml/min per m2, CrClCG and MDRD GFR underestimate CrClm At CrClm <90 ml/min per m2, CrClCG and MDRD GFR overestimate CrClm |
| Westland et al. 201385 | 77 | 1.5–19.8 | Congenital, 26 patients (34%) Acquired, 51 patients (66%) |
GFR-inulin | 2 Cr-based (eGFR [eGFR]-Schwartz,82 urinary CrCl); 2 cystatin C–based (eGFR-Zappitelli1,86 eGFR-CKiD 1); and 2 cystatin C/Cr–based (eGFR-Zappitelli2,86 eGFR-CKiD2) | eGFR-CKiD2 (height, sex, serum creatinine, cystatin C, and BUN) Mean bias 20.9 ml/min per 1.73 m2 95% of values within ±30% of GFR-inulin 54% of values within ±10% of GFR-inulin |
BUN, blood urea nitrogen; Cin, inulin clearance; CKD, chronic kidney disease; CKD-EPI, Chronic Kidney Disease-Epidemiology Collaboration; CKiD, Chronic Kidney Disease in Children; CrCl, creatinine clearance; CrClCG, creatinine clearance by Cockroft-Gault equation; CrClm, creatinine clearance from a 24-hour urine collection; eGFR, estimated glomerular filtration rate; GFR, glomerular filtration rate; iGFR, urinary iothalamate clearance; MDRD, Modification of Diet in Renal Disease; Sch, estimated glomerular filtration rate by Schwartz (Schwartz = K × height (cm)/Pcr (mg/dl) where K = 0.55 for children aged 2 to 12 years, K = 0.55 for girls 13 to 21 years, K = 0.70 for boys 13 to 21 years, and Pcr = serum creatinine [mg/dl])82; SK, single kidney; KT, kidney transplantation.
Cystatin C is an endogenous kidney filtration marker that may overcome some of the limitations of creatinine. Cystatin C is freely filtrated and not reabsorbed; however, it is metabolized by renal tubular cells. The combined cystatin C and creatinine-based eGFR formulas (eGFR-CKiD [chronic kidney disease in children 2]) that include height, sex, serum creatinine, cystatin C, and blood urea nitrogen may provide a better estimated GFR than creatinine-based or cystatin-based GFR in children with a solitary functioning kidney.85
As discussed above, persons with a solitary kidney often develop glomerular hyperfiltration after nephrectomy, which occurs as early as the first week postoperatively and can continue for longer than 10 years.87 Creatinine is not an ideal marker of GFR given that it is not a perfect filtration marker and is secreted and reabsorbed by renal tubules. Hyperfiltration is defined as a high filtration fraction of >18% in pediatric patients with various renal diseases, decreased effective renal plasma flow, and subsequently, lowered creatinine in the peritubular capillary. Decreased creatinine in the peritubular capillary lowers renal tubular creatinine secretion88 and has a slight effect of increasing renal tubular creatinine reabsorption.89 These lead to underestimated GFR. Tan et al.83 reported that the Modification of Diet in Renal Disease and the Chronic Kidney Disease Epidemiology Collaboration GFR estimating equations underestimated GFR, especially in living kidney donors ≥55 years old. Different from creatinine-based eGFR, cystatin C–based eGFR is not affected by a high filtration fraction or hyperfiltration;88 however, cystatin-based or creatinine–cystatin C–based GFR have less accuracy in identifying living kidney donors with measured GFR <60 ml/min per 1.73 m2; therefore, creatinine-based eGFR remains the preferred method to follow up on renal function after living kidney donation.90
Given the limitations of eGFR, measured GFR should be used, particularly when a more accurate GFR assessment is necessary for clinical decision making (Table 4).78 For instance, a patient with renal cell carcinoma and solitary kidney may receive renally toxic chemotherapy, in which case more accurate assessment and monitoring of GFR is prudent. A measured GFR can be performed by 24-hour urine collections or by assessing filtration markers that can be either endogenous or exogenous. A 24-hour urine test for creatinine clearance is a classic approach; however, it is inconvenient and can overestimate the true GFR due to renal tubular creatinine secretion. Two commonly used filtration markers are iothalamate and iohexol clearances. In addition, there are other filtration markers whose advantages and limitations are summarized in Table 4.
Albuminuria Measurement
In the setting of postnephrectomy for living kidney donation or any other reason, proteinuria may emerge and worsen over time. A meta-analysis including 48 studies with a total of 5048 donors revealed that an average 24-hour urine protein was 154 mg/d, and the average GFR was 86 ml/min over an average 7 years postdonation.91 The 2017 Kidney Disease Improving Global Outcomes guidelines recommend checking assessment and monitoring of albuminuria in living kidney donors at least once a year for early detection of proteinuria.29
Conclusions
Persons with a normal functioning solitary kidney are likely at higher risk of developing CKD and of progression to ESRD, whereas the absolute risk remains small compared to that in the general population. Consistent data suggest an association between higher dietary protein intake and glomerular hyperfiltration and risk of CKD incidence and progression in those with less nephron endowment, including those with a solitary kidney. Additionally, data support the role of nutrition and dietary management to mitigate any future risk for CKD progression; however, there is no strong evidence demonstrating benefits from several interventions in patients with a solitary kidney. Recommendations pertaining to lifestyle modifications and nutrition for patients who have undergone or will undergo nephrectomy are warranted to achieve the goal of maintaining longevity and health similar to that in the general population.
Disclosure
KKZ has received honoraria and/or grants from Abbott, Abbvie, Alexion, Amgen, DaVita, Fresenius, Genzyme, Keryx, Otsuka, Shire, Rockwell, and Vifor, the manufacturers of drugs or devices and/or providers of services for CKD patients. KKZ serves as a physician in a US Department of Veterans Affairs medical center. Opinions expressed in this paper are those of the authors and do not represent official opinions of the US Department of Veterans Affairs. All the other authors declared no competing interests.
Acknowledgments
This work was supported by research grants from the National Institute of Diabetes, Digestive and Kidney Disease of the National Institutes of Health K24-DK091419 and philanthropic grants from Mr. Louis Chang and Dr. Joseph Lee.
References
- 1.Ibrahim H.N., Foley R., Tan L. Long-term consequences of kidney donation. N Engl J Med. 2009;360:459–469. doi: 10.1056/NEJMoa0804883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mjoen G., Hallan S., Hartmann A. Long-term risks for kidney donors. Kidney Int. 2014;86:162–167. doi: 10.1038/ki.2013.460. [DOI] [PubMed] [Google Scholar]
- 3.Muzaale A.D., Massie A.B., Wang M.C. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311:579–586. doi: 10.1001/jama.2013.285141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grams M.E., Sang Y., Levey A.S. Kidney-failure risk projection for the living kidney-donor candidate. N Engl J Med. 2016;374:411–421. doi: 10.1056/NEJMoa1510491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anjum S., Muzaale A.D., Massie A.B. Patterns of end-stage renal disease caused by diabetes, hypertension, and glomerulonephritis in live kidney donors. Am J Transplant. 2016;16:3540–3547. doi: 10.1111/ajt.13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matas A.J., Vock D.M., Ibrahim H.N. GFR </=25 years postdonation in living kidney donors with (vs. without) a first-degree relative with ESRD. Am J Transplant. 2018;18:625–631. doi: 10.1111/ajt.14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shapiro E., Goldfarb D.A., Ritchey M.L. The congenital and acquired solitary kidney. Rev Urol. 2003;5:2–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Hart A., Smith J.M., Skeans M.A. OPTN/SRTR 2017 Annual Data Report: Kidney. Am J Transplant. 2019;19(suppl 2):19–123. doi: 10.1111/ajt.15274. [DOI] [PubMed] [Google Scholar]
- 9.Himmelmann A., Hansson L., Hansson B.G. ACE inhibition preserves renal function better than beta-blockade in the treatment of essential hypertension. Blood Press. 1995;4:85–90. doi: 10.3109/08037059509077575. [DOI] [PubMed] [Google Scholar]
- 10.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 11.Shuch B., Hanley J., Lai J. Overall survival advantage with partial nephrectomy: a bias of observational data? Cancer. 2013;119:2981–2989. doi: 10.1002/cncr.28141. [DOI] [PubMed] [Google Scholar]
- 12.McClung C.D., Hotaling J.M., Wang J. Contemporary trends in the immediate surgical management of renal trauma using a national database. J Trauma Acute Care Surg. 2013;75:602–606. doi: 10.1097/TA.0b013e3182a53ac2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delanaye P., Weekers L., Dubois B.E. Outcome of the living kidney donor. Nephrol Dial Transplant. 2012;27:41–50. doi: 10.1093/ndt/gfr669. [DOI] [PubMed] [Google Scholar]
- 14.ter Wee P.M., Tegzess A.M., Donker A.J. Pair-tested renal reserve filtration capacity in kidney recipients and their donors. J Am Soc Nephrol. 1994;4:1798–1808. doi: 10.1681/ASN.V4101798. [DOI] [PubMed] [Google Scholar]
- 15.Saxena A.B., Myers B.D., Derby G. Adaptive hyperfiltration in the aging kidney after contralateral nephrectomy. Am J Physiol Renal Physiol. 2006;291:F629–F634. doi: 10.1152/ajprenal.00329.2005. [DOI] [PubMed] [Google Scholar]
- 16.Hayslett J.P. Functional adaptation to reduction in renal mass. Physiol Rev. 1979;59:137–164. doi: 10.1152/physrev.1979.59.1.137. [DOI] [PubMed] [Google Scholar]
- 17.Kasinath B.S., Feliers D., Sataranatarajan K. Regulation of mRNA translation in renal physiology and disease. Am J Physiol Renal Physiol. 2009;297:F1153–F1165. doi: 10.1152/ajprenal.90748.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauser P., Kainz A., Perco P. Transcriptional response in the unaffected kidney after contralateral hydronephrosis or nephrectomy. Kidney Int. 2005;68:2497–2507. doi: 10.1111/j.1523-1755.2005.00725.x. [DOI] [PubMed] [Google Scholar]
- 19.Kalantar-Zadeh K., Fouque D. Nutritional management of chronic kidney disease. N Engl J Med. 2017;377:1765–1776. doi: 10.1056/NEJMra1700312. [DOI] [PubMed] [Google Scholar]
- 20.Ko G.J., Obi Y., Tortorici A.R. Dietary protein intake and chronic kidney disease. Curr Opin Clin Nutr Metab Care. 2017;20:77–85. doi: 10.1097/MCO.0000000000000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riser B.L., Ladson-Wofford S., Sharba A. TGF-beta receptor expression and binding in rat mesangial cells: modulation by glucose and cyclic mechanical strain. Kidney Int. 1999;56:428–439. doi: 10.1046/j.1523-1755.1999.00600.x. [DOI] [PubMed] [Google Scholar]
- 22.Fogo A., Hawkins E.P., Berry P.L. Glomerular hypertrophy in minimal change disease predicts subsequent progression to focal glomerular sclerosis. Kidney Int. 1990;38:115–123. doi: 10.1038/ki.1990.175. [DOI] [PubMed] [Google Scholar]
- 23.Bhathena D.B. Glomerular size and the association of focal glomerulosclerosis in long-surviving human renal allografts. J Am Soc Nephrol. 1993;4:1316–1326. doi: 10.1681/ASN.V461316. [DOI] [PubMed] [Google Scholar]
- 24.Sanna-Cherchi S., Ravani P., Corbani V. Renal outcome in patients with congenital anomalies of the kidney and urinary tract. Kidney Int. 2009;76:528–533. doi: 10.1038/ki.2009.220. [DOI] [PubMed] [Google Scholar]
- 25.Lenihan C.R., Busque S., Derby G. Longitudinal study of living kidney donor glomerular dynamics after nephrectomy. J Clin Invest. 2015;125:1311–1318. doi: 10.1172/JCI78885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tonneijck L., Muskiet M.H., Smits M.M. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J Am Soc Nephrol. 2017;28:1023–1039. doi: 10.1681/ASN.2016060666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young A., Hodsman A.B., Boudville N. Bone and mineral metabolism and fibroblast growth factor 23 levels after kidney donation. Am J Kidney Dis. 2012;59:761–769. doi: 10.1053/j.ajkd.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 28.Kasiske B.L., Anderson-Haag T., Israni A.K. A prospective controlled study of living kidney donors: three-year follow-up. Am J Kidney Dis. 2015;66:114–124. doi: 10.1053/j.ajkd.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lentine K.L., Kasiske B.L., Levey A.S. KDIGO Clinical Practice Guideline on the Evaluation and Care of Living Kidney Donors. Transplantation. 2017;101(suppl 1):S1–S109. doi: 10.1097/TP.0000000000001769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams S.L., Oler J., Jorkasky D.K. Long-term renal function in kidney donors: a comparison of donors and their siblings. Ann Intern Med. 1986;105:1–8. doi: 10.7326/0003-4819-105-1-1. [DOI] [PubMed] [Google Scholar]
- 31.Najarian J.S., Chavers B.M., McHugh L.E. 20 years or more of follow-up of living kidney donors. Lancet. 1992;340:807–810. doi: 10.1016/0140-6736(92)92683-7. [DOI] [PubMed] [Google Scholar]
- 32.White S.L., Perkovic V., Cass A. Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am J Kidney Dis. 2009;54:248–261. doi: 10.1053/j.ajkd.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 33.Li S., Chen S.C., Shlipak M. Low birth weight is associated with chronic kidney disease only in men. Kidney Int. 2008;73:637–642. doi: 10.1038/sj.ki.5002747. [DOI] [PubMed] [Google Scholar]
- 34.Seun Kim Y., Soo Kim M., Suk Han D. Evidence that the ratio of donor kidney weight to recipient body weight, donor age, and episodes of acute rejection correlate independently with live-donor graft function. Transplantation. 2002;74:280–283. doi: 10.1097/00007890-200207270-00021. [DOI] [PubMed] [Google Scholar]
- 35.Nicholson M.L., Windmill D.C., Horsburgh T. Influence of allograft size to recipient body-weight ratio on the long-term outcome of renal transplantation. Br J Surg. 2000;87:314–319. doi: 10.1046/j.1365-2168.2000.01390.x. [DOI] [PubMed] [Google Scholar]
- 36.Sun M., Bianchi M., Hansen J. Chronic kidney disease after nephrectomy in patients with small renal masses: a retrospective observational analysis. Eur Urol. 2012;62:696–703. doi: 10.1016/j.eururo.2012.03.051. [DOI] [PubMed] [Google Scholar]
- 37.Streja E., Kalantar-Zadeh K., Molnar M.Z. Radical versus partial nephrectomy, chronic kidney disease progression and mortality in US veterans. Nephrol Dial Transplant. 2018;33:95–101. doi: 10.1093/ndt/gfw358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Velmahos G.C., Constantinou C., Gkiokas G. Does nephrectomy for trauma increase the risk of renal failure? World J Surg. 2005;29:1472–1475. doi: 10.1007/s00268-005-7874-1. [DOI] [PubMed] [Google Scholar]
- 39.Reese P.P., Bloom R.D., Feldman H.I. Mortality and cardiovascular disease among older live kidney donors. Am J Transplant. 2014;14:1853–1861. doi: 10.1111/ajt.12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moody W.E., Ferro C.J., Edwards N.C. Cardiovascular effects of unilateral nephrectomy in living kidney donors. Hypertension. 2016;67:368–377. doi: 10.1161/HYPERTENSIONAHA.115.06608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cirillo M., Lombardi C., Chiricone D. Protein intake and kidney function in the middle-age population: contrast between cross-sectional and longitudinal data. Nephrol Dial Transplant. 2014;29:1733–1740. doi: 10.1093/ndt/gfu056. [DOI] [PubMed] [Google Scholar]
- 42.Cirillo M., Zingone F., Lombardi C. Population-based dose-response curve of glomerular filtration rate to dietary protein intake. Nephrol Dial Transplant. 2015;30:1156–1162. doi: 10.1093/ndt/gfv026. [DOI] [PubMed] [Google Scholar]
- 43.Joshi S., Shah S., Kalantar-Zadeh K. Adequacy of plant-based proteins in chronic kidney disease. J Ren Nutr. 2019;29:112–117. doi: 10.1053/j.jrn.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 44.Knight E.L., Stampfer M.J., Hankinson S.E. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med. 2003;138:460–467. doi: 10.7326/0003-4819-138-6-200303180-00009. [DOI] [PubMed] [Google Scholar]
- 45.Haring B., Selvin E., Liang M. Dietary protein sources and risk for incident chronic kidney disease: results from the Atherosclerosis Risk in Communities (ARIC) Study. J Ren Nutr. 2017;27:233–242. doi: 10.1053/j.jrn.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nomura K., Asayama K., Jacobs L. Renal function in relation to sodium intake: a quantitative review of the literature. Kidney Int. 2017;92:67–78. doi: 10.1016/j.kint.2016.11.032. [DOI] [PubMed] [Google Scholar]
- 47.He J., Mills K.T., Appel L.J. Urinary sodium and potassium excretion and CKD progression. J Am Soc Nephrol. 2016;27:1202–1212. doi: 10.1681/ASN.2015010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mills K.T., Chen J., Yang W. Sodium excretion and the risk of cardiovascular disease in patients with chronic kidney disease. JAMA. 2016;315:2200–2210. doi: 10.1001/jama.2016.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stolarz-Skrzypek K., Kuznetsova T., Thijs L. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA. 2011;305:1777–1785. doi: 10.1001/jama.2011.574. [DOI] [PubMed] [Google Scholar]
- 50.Mente A., O'Donnell M., Rangarajan S. Urinary sodium excretion, blood pressure, cardiovascular disease, and mortality: a community-level prospective epidemiological cohort study. Lancet. 2018;392:496–506. doi: 10.1016/S0140-6736(18)31376-X. [DOI] [PubMed] [Google Scholar]
- 51.Yoon C.Y., Noh J., Lee J. High and low sodium intakes are associated with incident chronic kidney disease in patients with normal renal function and hypertension. Kidney Int. 2018;93:921–931. doi: 10.1016/j.kint.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 52.Locke J.E., Reed R.D., Massie A. Obesity increases the risk of end-stage renal disease among living kidney donors. Kidney Int. 2017;91:699–703. doi: 10.1016/j.kint.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kleine C.E., Moradi H., Streja E. Racial and ethnic disparities in the obesity paradox. Am J Kidney Dis. 2018;72:S26–S32. doi: 10.1053/j.ajkd.2018.06.024. [DOI] [PubMed] [Google Scholar]
- 54.Anastasio P., Cirillo M., Spitali L. Level of hydration and renal function in healthy humans. Kidney Int. 2001;60:748–756. doi: 10.1046/j.1523-1755.2001.060002748.x. [DOI] [PubMed] [Google Scholar]
- 55.Bardoux P., Bichet D.G., Martin H. Vasopressin increases urinary albumin excretion in rats and humans: involvement of V2 receptors and the renin-angiotensin system. Nephrol Dial Transplant. 2003;18:497–506. doi: 10.1093/ndt/18.3.497. [DOI] [PubMed] [Google Scholar]
- 56.Edwards R.M., Trizna W., Kinter L.B. Renal microvascular effects of vasopressin and vasopressin antagonists. Am J Physiol. 1989;256:F274–F278. doi: 10.1152/ajprenal.1989.256.2.F274. [DOI] [PubMed] [Google Scholar]
- 57.Clark W.F., Sontrop J.M., Macnab J.J. Urine volume and change in estimated GFR in a community-based cohort study. Clin J Am Soc Nephrol. 2011;6:2634–2641. doi: 10.2215/CJN.01990211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hebert L.A., Greene T., Levey A. High urine volume and low urine osmolality are risk factors for faster progression of renal disease. Am J Kidney Dis. 2003;41:962–971. doi: 10.1016/s0272-6386(03)00193-8. [DOI] [PubMed] [Google Scholar]
- 59.Pinto-Sietsma S.J., Mulder J., Janssen W.M. Smoking is related to albuminuria and abnormal renal function in nondiabetic persons. Ann Intern Med. 2000;133:585–591. doi: 10.7326/0003-4819-133-8-200010170-00008. [DOI] [PubMed] [Google Scholar]
- 60.Segev D.L., Muzaale A.D., Caffo B.S. Perioperative mortality and long-term survival following live kidney donation. JAMA. 2010;303:959–966. doi: 10.1001/jama.2010.237. [DOI] [PubMed] [Google Scholar]
- 61.Orth S.R. Effects of smoking on systemic and intrarenal hemodynamics: influence on renal function. J Am Soc Nephrol. 2004;15(suppl 1):S58–S63. doi: 10.1097/01.asn.0000093461.36097.d5. [DOI] [PubMed] [Google Scholar]
- 62.Thomas R.J., Holm M., Al-Adhami A. Physical activity after cancer: an evidence review of the international literature. Br J Med Pract. 2014;7:a708. [Google Scholar]
- 63.Trinh L., Plotnikoff R.C., Rhodes R.E. Associations between physical activity and quality of life in a population-based sample of kidney cancer survivors. Cancer Epidemiol Biomarkers Prev. 2011;20:859–868. doi: 10.1158/1055-9965.EPI-10-1319. [DOI] [PubMed] [Google Scholar]
- 64.Messersmith E.E., Gross C.R., Beil C.A. Satisfaction with life among living kidney donors: a RELIVE study of long-term donor outcomes. Transplantation. 2014;98:1294–1300. doi: 10.1097/TP.0000000000000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ohashi N., Isobe S., Ishigaki S. The effects of unilateral nephrectomy on blood pressure and its circadian rhythm. Intern Med. 2016;55:3427–3433. doi: 10.2169/internalmedicine.55.7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tent H., Sanders J.S., Rook M. Effects of preexistent hypertension on blood pressure and residual renal function after donor nephrectomy. Transplantation. 2012;93:412–417. doi: 10.1097/TP.0b013e318240e9b9. [DOI] [PubMed] [Google Scholar]
- 67.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 68.Cushman W.C., Evans G.W., Byington R.P. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Group S.R., Wright J.T., Jr., Williamson J.D. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.American Diabetes Association 9. Cardiovascular Disease and Risk Management. Diabetes Care. 2017;40(suppl 1):S75–S87. doi: 10.2337/dc17-S012. [DOI] [PubMed] [Google Scholar]
- 71.James P.A., Oparil S., Carter B.L. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 72.Whelton P.K., Carey R.M., Aronow W.S. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269–1324. doi: 10.1161/HYP.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 73.Singh R.R., Lankadeva Y.R., Denton K.M. Improvement in renal hemodynamics following combined angiotensin II infusion and AT1R blockade in aged female sheep following fetal unilateral nephrectomy. PLoS One. 2013;8:e68036. doi: 10.1371/journal.pone.0068036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jardine M.J., Ninomiya T., Perkovic V. Aspirin is beneficial in hypertensive patients with chronic kidney disease: a post-hoc subgroup analysis of a randomized controlled trial. J Am Coll Cardiol. 2010;56:956–965. doi: 10.1016/j.jacc.2010.02.068. [DOI] [PubMed] [Google Scholar]
- 75.Evans M., Fored C.M., Bellocco R. Acetaminophen, aspirin and progression of advanced chronic kidney disease. Nephrol Dial Transplant. 2009;24:1908–1918. doi: 10.1093/ndt/gfn745. [DOI] [PubMed] [Google Scholar]
- 76.Fored C.M., Ejerblad E., Lindblad P. Acetaminophen, aspirin, and chronic renal failure. N Engl J Med. 2001;345:1801–1808. doi: 10.1056/NEJMoa010323. [DOI] [PubMed] [Google Scholar]
- 77.Levey A.S., Coresh J. Chronic kidney disease. Lancet. 2012;379:165–180. doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- 78.Stevens L.A., Levey A.S. Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol. 2009;20:2305–2313. doi: 10.1681/ASN.2009020171. [DOI] [PubMed] [Google Scholar]
- 79.Petri M., Bockenstedt L., Colman J. Serial assessment of glomerular filtration rate in lupus nephropathy. Kidney Int. 1988;34:832–839. doi: 10.1038/ki.1988.257. [DOI] [PubMed] [Google Scholar]
- 80.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 81.Pierrat A., Gravier E., Saunders C. Predicting GFR in children and adults: a comparison of the Cockcroft-Gault, Schwartz, and modification of diet in renal disease formulas. Kidney Int. 2003;64:1425–1436. doi: 10.1046/j.1523-1755.2003.00208.x. [DOI] [PubMed] [Google Scholar]
- 82.Schwartz G.J., Haycock G.B., Edelmann C.M., Jr. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263. [PubMed] [Google Scholar]
- 83.Tan J.C., Ho B., Busque S. Imprecision of creatinine-based GFR estimates in uninephric kidney donors. Clin J Am Soc Nephrol. 2010;5:497–502. doi: 10.2215/CJN.05280709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ferreira-Filho S.R., Cardoso C.C., de Castro L.A. Comparison of measured creatinine clearance and clearances estimated by Cockcroft-Gault and MDRD formulas in patients with a single kidney. Int J Nephrol. 2011;2011:626178. doi: 10.4061/2011/626178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Westland R., Abraham Y., Bokenkamp A. Precision of estimating equations for GFR in children with a solitary functioning kidney: the KIMONO study. Clin J Am Soc Nephrol. 2013;8:764–772. doi: 10.2215/CJN.07870812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zappitelli M., Parvex P., Joseph L. Derivation and validation of cystatin C-based prediction equations for GFR in children. Am J Kidney Dis. 2006;48:221–230. doi: 10.1053/j.ajkd.2006.04.085. [DOI] [PubMed] [Google Scholar]
- 87.Anderson R.G., Bueschen A.J., Lloyd L.K. Short-term and long-term changes in renal function after donor nephrectomy. J Urol. 1991;145:11–13. doi: 10.1016/s0022-5347(17)38232-0. [DOI] [PubMed] [Google Scholar]
- 88.Huang S.H., Sharma A.P., Yasin A. Hyperfiltration affects accuracy of creatinine eGFR measurement. Clin J Am Soc Nephrol. 2011;6:274–280. doi: 10.2215/CJN.02760310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Perrone R.D., Madias N.E., Levey A.S. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38:1933–1953. [PubMed] [Google Scholar]
- 90.Issa N., Kukla A., Jackson S. Comparison of cystatin C and creatinine-based equations for GFR estimation after living kidney donation. Transplantation. 2014;98:871–877. doi: 10.1097/TP.0000000000000129. [DOI] [PubMed] [Google Scholar]
- 91.Garg A.X., Muirhead N., Knoll G. Proteinuria and reduced kidney function in living kidney donors: a systematic review, meta-analysis, and meta-regression. Kidney Int. 2006;70:1801–1810. doi: 10.1038/sj.ki.5001819. [DOI] [PubMed] [Google Scholar]