INTRODUCTION
Mutations of the BRCA1 or BRCA2 gene (BRCA1/2) confer an increased lifetime risk of developing breast, ovarian, pancreatic, and prostate cancers, among others.1,2 BRCA1/2-deficient cancer cells from germline BRCA1/2 mutation carriers often lose the second BRCA1/2 allele through deletion of all or part of chromosome 17q or 13q, respectively, or inactivating point mutations or small insertions or deletions.3-6 Loss of both alleles leads to impaired homologous recombination of double-strand DNA breaks and increased sensitivity to radiation, platinum-based chemotherapy, and poly (ADP-ribose) polymerase (PARP) inhibitors.7,8
PARP inhibitors target PARP1 and PARP2 enzymes that bind single-strand DNA breaks and catalyze post-translational modification of DNA repair proteins.9 In the absence of functional BRCA1 or BRCA2 protein, PARP1/2 inhibition compromises DNA repair and leads to cell-cycle arrest and apoptosis.10 PARP inhibitors are US Food and Drug Administration approved for the treatment of ovarian and breast cancers with germline BRCA1 and BRCA2 mutations, but they also have antitumor activity in castration-resistant prostate cancer (CRPC) carrying germline or somatic mutations in genes involved with DNA repair, such as BRCA1, BRCA2, ATM, PALB2, FANCA, CHEK2, and CDK12.11 In a cohort of 16 patients with metastatic CRPC (mCRPC) carrying mutations in DNA repair genes, PARP inhibitor olaparib achieved response rates as high as 88%.11 These results fostered ongoing clinical trials of PARP inhibitors in mCRPC and supported breakthrough therapy designation of olaparib by the US Food and Drug Administration for the treatment of BRCA1/2- and ATM-mutated mCRPC in January 2016.
In germline BRCA1/2 mutation carriers treated with platinum-based chemotherapy or PARP inhibitors, resistance eventually develops through several mechanisms, including acquisition of somatic BRCA1/2 mutations that restore the open reading frame (ie, BRCA reversion mutations) of the germline allele, which in turn restores production of functional BRCA1/2 protein.12-18 BRCA reversion mutations have been reported in BRCA-mutated ovarian, breast, and pancreatic cancer cell lines with acquired resistance to platinum compounds or PARP inhibitors.13,15-18
Here we report a case of acquired resistance to PARP inhibitor olaparib in BRCA2-mutant mCRPC resulting from multiple acquired reversion mutations detected by circulating tumor DNA (ctDNA) analysis that restored both the BRCA2 germline mutation and the somatic second-hit loss-of-function mutation on the second allele. We also report the prevalence of BRCA2 reversion mutations among a large cohort of 1,534 patients with mCRPC who underwent ctDNA testing.
METHODS
Blood for cell-free DNA (cfDNA) analysis was drawn during the patient’s regularly schedule clinic visit. The cfDNA next-generation sequencing (NGS) analysis was performed at Guardant Health (Guardant360; Redwood City, CA), a Clinical Laboratory Improvement Amendments–certified, College of American Pathologists–accredited, New York State Department of Health–approved laboratory. Barcoded sequencing libraries were generated from 5 to 30 ng of plasma cfDNA. The exons of 73 cancer genes were captured using biotinylated custom bait oligonucleotides (Agilent, Santa Clara, CA), resulting in a capture footprint of 148,000 base pairs (78 kb). The mean cfDNA loaded into each sequencing reaction was 22 ng (range, 5 to 30 ng). Samples were paired-end sequenced on an Illumina HiSeq 2500 (San Diego, CA), followed by algorithmic reconstruction of the digitized sequencing signals. The coverage depth across all coding sequences in all samples averaged approximately 15,000×. Illumina sequencing reads were mapped to the hg19/GRCh37 human reference sequence, and genomic alterations in cfDNA were identified from Illumina sequencing data by proprietary bioinformatic algorithms. These algorithms quantify the absolute number of unique DNA fragments at a given nucleotide position, thereby enabling ctDNA to be quantitatively measured as a fraction of total cfDNA. The Guardant360 assay detects single-nucleotide variants, indels, fusions, and copy-number alterations in cfDNA with a reportable range of ≥ 0.04%, ≥ 0.02%, ≥ 0.04%, and ≥ 2.12 copies, respectively.19,20 This research was approved by the Quorum institutional review board for the generation of deidentified data sets for research purposes (Guardant protocol) and the Northwestern University institutional review board (protocol STU00205723).
CASE REPORT
The patient was a 63-year-old white male of Ashkenazi Jewish ancestry who underwent a radical prostatectomy revealing Gleason 5 + 4 = 9 adenocarcinoma,21 with involvement of seminal vesicles, perineural invasion, and negative margins. He received adjuvant androgen-deprivation therapy and radiation therapy (70 Gy in 35 fractions) and developed biochemical recurrence 1 year later, when he was treated with bicalutamide. Two years later, prostate-specific antigen (PSA) rose to 218 ng/mL. Computed tomography scan showed retroperitoneal and pelvic lymphadenopathy and a vertebral body metastasis. He received seven cycles of docetaxel followed by prolonged control of disease with 13 cycles of cabazitaxel before a new liver metastasis was identified on scans. Liver biopsy confirmed prostate adenocarcinoma, and NGS (Foundation Medicine, Cambridge, MA) of the liver biopsy identified two mutations in BRCA2: c.5946delT (p.Ser1982fs*, also known as 6174delT) and c.5754_5755delTA (p.His1918fs*5). BRCA2 allelic loss was not reported. Tissue NGS also revealed CDKN2a (p16INK4a H83Y; p14ARF A97V), as well as losses of PTEN and FAS and 12 variants of unknown significance. Germline testing confirmed a heterozygous BRCA2 c.5946delT mutation in the patient, which was inherited from his father, who had died as a result of colon cancer at age 81 years.
After liver biopsy, the patient was treated with olaparib (400 mg twice per day), resulting in rapid reduction of PSA from 821 to 300 ng/mL and improvement of lymphadenopathy and liver lesions. One year after starting olaparib, PSA rose to 779 ng/mL. Computed tomography scans showed stable adenopathy and liver lesions, but bone scan demonstrated marked progression of disease. ctDNA analysis was performed at the time of disease progression during olaparib treatment. The patient died 3 months later.
RESULTS
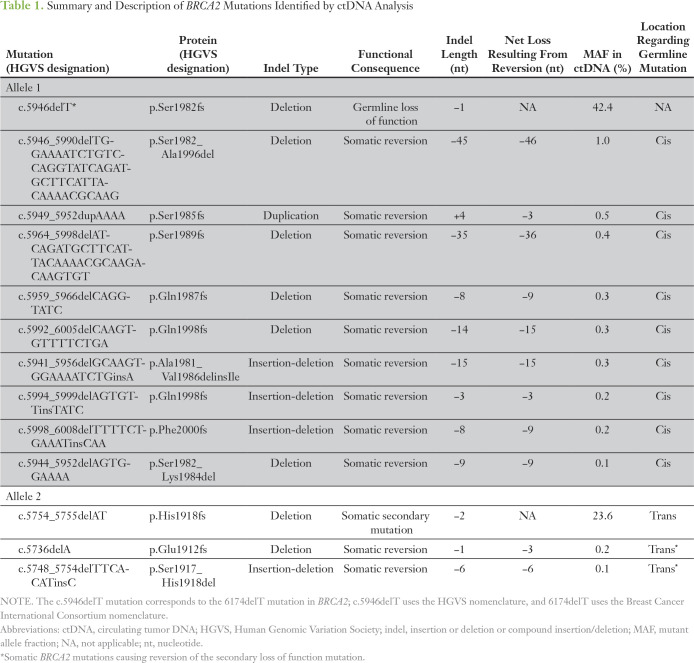
Analysis of ctDNA identified the BRCA2 c.5946delT (p.Ser1982fs*) mutation at a mutant allele fraction (MAF) of 42.4%, consistent with germline origin, and the c.5982_5983delTA (p.His1918fs) mutation at 23.6%, consistent with secondary somatic mutation. ctDNA also detected 11 additional somatic BRCA2 mutations not identified in the pre-PARP liver biopsy specimen (Table 1), all of which occurred at low MAFs (range, 0.1% to 1.0%), consistent with subclonal somatic origin. Nine of these 11 somatic BRCA2 mutations occurred in cis with the germline mutation, and three overlapped with the original germline mutation (Fig 1). All occurred within zero to 52 nucleotides of the c.5946delT germline mutation and restored the BRCA2 open reading frame. Interestingly, the two remaining somatic BRCA2 mutations (c.5736delA and c.5749_5754delTCACAT) occurred in close proximity to the putative somatic second hit: c.5754_5755delTA. Both were in cis to the c.5754_5755delTA mutation, and both were predicted to restore the open reading frame, suggesting that these acquired somatic variants occur on the alternate allele relative to the germline BRCA2 c.5946delT mutation. In addition to multiple BRCA2 mutations, ctDNA analyses revealed the following alterations: TP53 F113fs; GATA3 D336D; ARID1A S1755T; MYC P72A; and amplification of MYC, KRAS, CCND2, and BRAF.
Table 1.
Summary and Description of BRCA2 Mutations Identified by ctDNA Analysis
Fig 1.
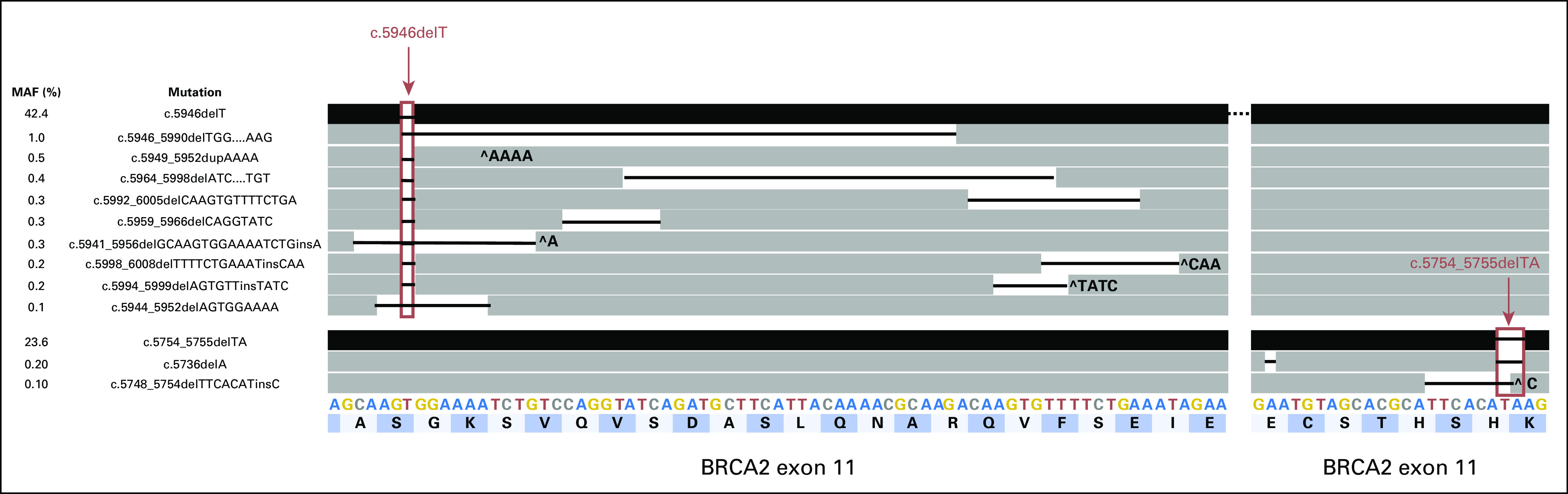
Schematic representation of the germline c.5946delT and secondary c.5754_5755delTA loss-of-function mutations (black bars; top row and third row from bottom) in relation to the acquired somatic reversion mutations (dark gray bars). Black lines between bars represent the nucleotides deleted. ^ indicates an insertion. Letters across the bottom two rows represent the nucleotide (A, C, G, T) and amino acid (dark and light blue bars) sequences of wild-type BRCA2 exon 11.
Because the biallelic reversion of both germline and truncal somatic BRCA1/2 alterations contrasted the generally accepted model of monoallelic reversion of germline BRCA1/2 mutations, we attempted to estimate the relative prevalence of germline versus somatic BRCA1/2 reversion events in patients with mCRPC using a large genomic database including comprehensive ctDNA results from more than 40,000 patients with a variety of solid tumors. Between October 5, 2015, and April 25, 2017, 1,765 samples from 1,534 unique patients with mCRPC underwent ctDNA testing (Guardant Health), which included complete sequencing of all BRCA1 and BRCA2 exons and exon-intron borders. Of these, 24 patients (1.6%) had a deleterious BRCA2 mutation falling within the germline MAF (40% to 80%). There were no putative germline mutations in the BRCA1 gene in this mCRPC cohort. Five of these 24 patients were receiving either a PARP inhibitor or platinum-based chemotherapy at the time of the blood draw. Two of the five patients, one receiving olaparib and one carboplatin, had BRCA2 reversion mutations detected by the ctDNA analysis. Therefore, in this germline mutation–positive, platinum- or PARP-exposed cohort, the frequency of BRCA2 reversion was 40% (n = 2 of 5). A third case of reversion was identified, but the patient had no previous exposure to platinum-based chemotherapy or PARP inhibitors.
DISCUSSION
We report a case of acquired resistance to olaparib in BRCA2 germline–positive mCRPC resulting from multiple acquired BRCA2 reversion mutations of both the germline mutation and a second-hit somatic mutation on the opposite allele. This case is similar to one recently reported by Goodall et al,22 in which acquired reversion mutations restored the open reading frame of not only the primary germline mutation but also the secondary loss-of-function mutation. Although previous studies in ovarian cancer have established that reversion of the germline allele is necessary and sufficient to restore normal BRCA protein function, this case suggests functional comparability of the variants despite their origin (ie, somatic or germline). This observation challenges the established model of BRCA1/2 reversion as restricted to germline mutations and suggests that the germline or somatic origin of the allele may not play a critical biologic role in this mechanism of resistance.
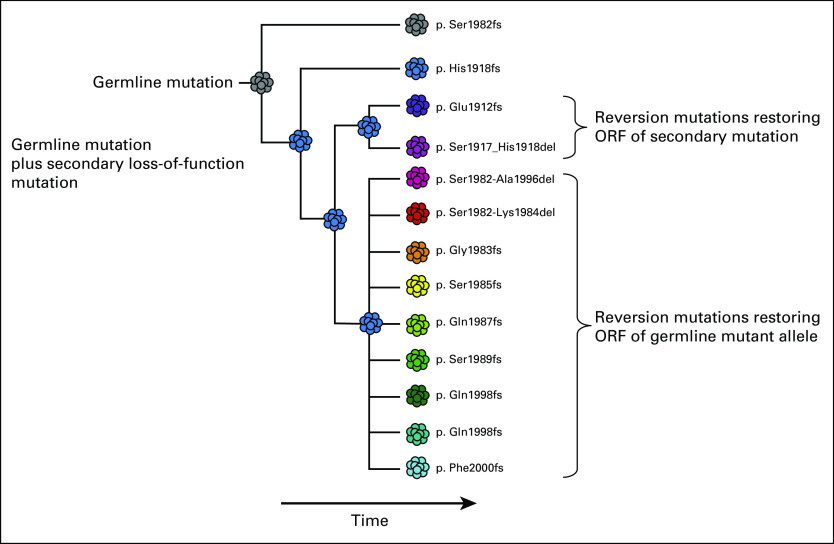
Furthermore, this case is a powerful illustration of convergent evolution of multiple BRCA2 reversion mutations arising in different clones of the metastatic lesion or within multiple metastases (Fig 2), as has been described.23 Other studies of acquired resistance have compared ctDNA with tissue-based testing on biopsies from multiple metastatic lesions in the same patient. These studies have shown that a single tissue biopsy often does not capture the full spectrum of acquired resistance mutations, whereas ctDNA may provide a more global summary of tumor heterogeneity, as seen in this case.24,25 ctDNA analyses also enable monitoring and early detection of mutations driving treatment resistance to PARP inhibitors, with meaningful clinical implications.
Fig 2.
Circulating tumor DNA profiling of a patient experiencing disease progression during treatment with a poly (ADP-ribose) polymerase inhibitor, showing a known germline frameshift mutation and somatic second-hit frameshift mutation, as well as 11 additional frameshift mutations. Phasing the mutation using a Dollo parsimony model allows a presumptive evolutionary history of the tumor population to be inferred. Both somatic and germline lineages contain multiple independent revertant subclones. ORF, open reading frame.
Once a BRCA1/2 mutation is detected, longitudinal monitoring with ctDNA can be relevant for early detection of reversion BRCA1/2 mutations to predict resistance to PARP inhibitors, as illustrated by the case presented here. In women with platinum-resistant ovarian cancer, presence of BRCA reversion mutations was a more accurate predictor of response to subsequent platinum or PARP inhibitor therapy than duration of response to previous lines of platinum therapy.26 Another study identified reversion of germline BRCA1/2 mutations in high-grade serous ovarian carcinoma using ctDNA and was able to predict treatment responses.27 There are limited data on the prevalence of BRCA reversion mutations and rates of resistance to platinum or PARP inhibitors in mCRPC. Estimates of BRCA1/2 reversion rates in women with platinum-resistant ovarian cancer range from 25% to 70%, but these are based on small series.18,26 Analysis of genomic data from large databases may be one way to overcome this limitation. Our ctDNA NGS study estimates a frequency of 40% among patients with mCRPC carrying BRCA2 germline mutations exposed to platinum or PARP therapy. However, caution should be used when interpreting the reversion frequency reported here, because it is based on a small series of platinum- or PARP inhibitor–exposed patients. Larger prospective studies are needed to determine the true frequency of reversion mutations in a platinum- or PARP-exposed cohort.
There are several limitations to our study. Although genomic testing on tissue before PARP inhibitor therapy was performed for the index case, this information was not available for the additional patient cases showing evidence of reversion mutation. All patients underwent cfDNA analysis at the time of clinical progression, suggesting that they had developed platinum or PARP inhibitor resistance, but the duration of their response during therapy or presence of reversion mutations before exposure is unknown. With regard to the retrospective cohort analysis, our BRCA2 germline mutation rate was lower than that previously described in the literature.28 Possible explanations for this include exclusion of putative germline missense and nonsense mutations in the analysis and overly restrictive germline MAF thresholds resulting in exclusion of putative germline mutations in patients with more severe allele imbalance. Lastly, one patient case with evidence of a reversion mutation had no prior exposure to PARP inhibitors or platinum. Review of the patient’s treatment history revealed treatment with taxane-based chemotherapy, radium-223, and mitoxantrone. The latter is a DNA intercalating agent used in the treatment of breast cancer, prostate cancer, and acute myeloid leukemia. Interestingly, Ikeda et al29 reported a patient with Fanconi anemia with biallelic BRCA2 mutations and previous exposure to mitoxantrone for acute myeloid leukemia. At the time of relapse, a bone marrow biopsy was performed, and a patient-derived cell line showed loss of the Fanconi anemia phenotype because of monoallelic reversion of the BRCA2 mutation and restoration of wild-type BRCA2 function. The authors suggest that DNA intercalating agents such as mitoxantrone may have the ability to induce reversion mutations and lead to resistance.
Compared with biopsy, cfDNA analyses allow easier monitoring and potentially earlier detection of mutations that result in treatment resistance. cfDNA analysis, which allows detection of both somatic and germline mutations in a single test, is well suited to distinguish whether a somatic BRCA mutation represents a second-hit loss of function or a reversion of the germline BRCA mutation. To make the distinction, the exact location of the mutations must be known, because to restore the reading frame, a revert must be located near the inactivating mutation (ie, before the end of the same exon).27 Furthermore, cfDNA may provide a more global summary of tumor heterogeneity and the full spectrum of acquired resistance mutations than a single tissue biopsy.24,25 The case presented here illustrates convergent evolution of multiple BRCA2 reversion mutations arising in different clones of the metastatic lesion or within multiple metastases (Fig 2), as has been described elsewhere.23 Incorporation of routine cfDNA analyses into standard of care of BRCA1/2-mutated cancers treated with PARP inhibitors or platinum-based chemotherapy requires validation of the germline calls from cfDNA but may allow early detection of treatment resistance and subsequent change in therapy before significant disease progression.
Footnotes
See accompanying articles doi:https://doi.org/10.1200/PO.17.00169, https://doi.org/10.1200/PO.17.00044 and https://doi.org/10.1200/PO.18.00001
AUTHOR CONTRIBUTIONS
Conception and design: Benedito A. Carneiro, Katharina Ann Collier, Rebecca J. Nagy, Stephen Fairclough, Richard B. Lanman, Timothy M. Kuzel, Massimo Cristofanilli, Sarki A. Abdulkadir, Francis J. Giles
Financial support: Sarki A. Abdulkadir, Francis J. Giles
Administrative support: Benedito A. Carneiro, Sarki A. Abdulkadir, Francis J. Giles
Provision of study material or patients: Benedito A. Carneiro, Rebecca J. Nagy, Stephen Fairclough, Justin Odegaard, Timothy M. Kuzel, Alice Fan, Massimo Cristofanilli, Francis J. Giles
Collection and assembly of data: Rebecca J. Nagy, Sahithi Pamarthy, Vinay Sagar, Stephen Fairclough, Timothy M. Kuzel, Alice Fan, Francis J. Giles
Data analysis and interpretation: Benedito A. Carneiro, Rebecca J. Nagy, Sahithi Pamarthy, Stephen Fairclough, Justin Odegaard, Richard B. Lanman, Ricardo Costa, Timothy Taxter, Timothy M. Kuzel, Young Kwang Chae, Massimo Cristofanilli, Maha H. Hussain, Sarki A. Abdulkadir, Francis J. Giles
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Benedito A. Carneiro
Consulting or Advisory Role: Bristol-Myers Squibb, Bayer HealthCare Pharmaceuticals
Katharine Ann Collier
No relationship to disclose
Rebecca J. Nagy
Employment: Guardant Health
Stock and Other Ownership Interests: Guardant Health
Sahithi Pamarthy
No relationship to disclose
Vinay Sagar
No relationship to disclose
Stephen Fairclough
Employment: Guardant Health
Stock and Other Ownership Interests: Guardant Health
Research Funding: Guardant Health
Patents, Royalties, Other Intellectual Property: Guardant Health
Travel, Accommodations, Expenses: Guardant Health
Justin Odegaard
Employment: Guardant Health
Stock and Other Ownership Interests: Guardant Health
Richard B. Lanman
Employment: Guardant Health, Veracyte
Leadership: Guardant Health
Stock and Other Ownership Interests: Guardant Health
Research Funding: Guardant Health
Ricardo Costa
No relationship to disclose
Timothy Taxter
Employment: Tempus
Stock and Other Ownership Interests: Tempus
Timothy M. Kuzel
Honoraria: Genentech/Roche, Celgene, Eisai, Argos Therapeutics, Amgen, Astellas Pharma, Bristol-Myers Squibb, Medivation, Exelixis, AbbVie, Merck
Consulting or Advisory Role: CVS, Kyowa Hakko Kirin, Stemline Therapeutics
Speakers’ Bureau: Celgene, Genentech/Roche, Astellas Pharma, Medivation
Research Funding: Genentech/Roche (Inst), Eisai (Inst), Bristol-Myers Squibb (Inst)
Travel, Accommodations, Expenses: Genentech, Celgene, Astellas Pharma, Argos Therapeutics, Medivation, Amgen, Kyowa Hakko Kirin, Merck, Stemline Therapeutics
Alice Fan
Stock and Other Ownership Interests: Molecular Decisions
Consulting or Advisory Role: Verily
Research Funding: Calithera Biosciences
Patents, Royalties, Other Intellectual Property: Stanford Patent has been licensed
Young Kwang Chae
Consulting or Advisory Role: Foundation Medicine, Boehringer Ingelheim, Biodesix, Counsyl, AstraZeneca, Guardant Health,
Speakers’ Bureau: Merck, Genentech/Roche
Travel, Accommodations, Expenses: Hanmi
Massimo Cristofanilli
Honoraria: Dompé Farmaceutici, Pfizer
Consulting or Advisory Role: Dompé Farmaceutici, Newomics, Vortex Biosciences
Maha H. Hussain
Honoraria: Onclive, Sanofi
Research Funding: Genentech (Inst), Pfizer (Inst), PCCTC (Inst), AstraZeneca (Inst)
Patents, Royalties, Other Intellectual Property: Title: Systems and methods for tissue imaging, 3676 our file, serial No. UM-14437/US-1/PRO 60/923,385UM-14437/US-2/ORD 12/101,753US 8,185,186 (US patent No.), Systems and methods for tissue imaging (issued patent) EP 08745653.9 (EP application No.), Systems and methods for tissue imaging (pending) CA 2683805 (Canadian application No.), Systems and methods for tissue imaging (pending) US 13/362,500 (US application No.), Systems and methods for tissue imaging (continuation application of US 8,185,186); Method of treating cancer, docket No., serial No. 224990/10-016P2/311733 61/481/671, application filed on February 5, 2011; Dual inhibition of MET and VEGF for the treatment of castration resistant prostate cancer and osteoblastic bone metastases, applicant/proprietor: Exelexis, application No./patent No. 11764665.4- 1464, application No./patent No. 11764656.2-1464, application filed on September 26, 2011
Travel, Accommodations, Expenses: Sanofi
Sarki A. Abdulkadir
Stock and Other Ownership Interests: Vortex Therapeutics
Patents, Royalties, Other Intellectual Property: Patent pending through Northwestern University for new MYC inhibitors
Francis J. Giles
Employment: Actuate
Leadership: Actuate
Honoraria: Novartis
Consulting or Advisory Role: Novartis
Travel, Accommodations, Expenses: MedImmune, Novartis, Foundation Medicine
REFERENCES
- 1.Steichen-Gersdorf E, Gallion HH, Ford D, et al. : Familial site-specific ovarian cancer is linked to BRCA1 on 17q12-21. Am J Hum Genet 55:870-875, 1994 [PMC free article] [PubMed] [Google Scholar]
- 2.Wooster R, Bignell G, Lancaster J, et al. : Identification of the breast cancer susceptibility gene BRCA2. Nature 378:789-792, 1995 [Erratum: Nature 379:749, 1996] [DOI] [PubMed] [Google Scholar]
- 3.Cleton-Jansen AM, Collins N, Lakhani SR, et al. : Loss of heterozygosity in sporadic breast tumours at the BRCA2 locus on chromosome 13q12-q13. Br J Cancer 72:1241-1244, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gudmundsson J, Johannesdottir G, Bergthorsson JT, et al. : Different tumor types from BRCA2 carriers show wild-type chromosome deletions on 13q12-q13. Cancer Res 55:4830-4832, 1995 [PubMed] [Google Scholar]
- 5.Neuhausen SL, Marshall CJ: Loss of heterozygosity in familial tumors from three BRCA1-linked kindreds. Cancer Res 54:6069-6072, 1994 [PubMed] [Google Scholar]
- 6.Collins N, McManus R, Wooster R, et al. : Consistent loss of the wild type allele in breast cancers from a family linked to the BRCA2 gene on chromosome 13q12-13. Oncogene 10:1673-1675, 1995 [PubMed] [Google Scholar]
- 7.Farmer H, McCabe N, Lord CJ, et al. : Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434:917-921, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Tutt AN, Lord CJ, McCabe N, et al. : Exploiting the DNA repair defect in BRCA mutant cells in the design of new therapeutic strategies for cancer. Cold Spring Harb Symp Quant Biol 70:139-148, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Durkacz BW, Omidiji O, Gray DA, et al. : (ADP-ribose)n participates in DNA excision repair. Nature 283:593-596, 1980 [DOI] [PubMed] [Google Scholar]
- 10.Bryant HE, Schultz N, Thomas HD, et al. : Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434:913-917, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Mateo J, Carreira S, Sandhu S, et al. : DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med 373:1697-1708, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouwman P, Jonkers J: Molecular pathways: How can BRCA-mutated tumors become resistant to PARP inhibitors? Clin Cancer Res 20:540-547, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Dhillon KK, Swisher EM, Taniguchi T: Secondary mutations of BRCA1/2 and drug resistance. Cancer Sci 102:663-669, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lord CJ, Ashworth A: PARP inhibitors: Synthetic lethality in the clinic. Science 355:1152-1158, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barber LJ, Sandhu S, Chen L, et al. : Secondary mutations in BRCA2 associated with clinical resistance to a PARP inhibitor. J Pathol 229:422-429, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Edwards SL, Brough R, Lord CJ, et al. : Resistance to therapy caused by intragenic deletion in BRCA2. Nature 451:1111-1115, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Sakai W, Swisher EM, Jacquemont C, et al. : Functional restoration of BRCA2 protein by secondary BRCA2 mutations in BRCA2-mutated ovarian carcinoma. Cancer Res 69:6381-6386, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakai W, Swisher EM, Karlan BY, et al. : Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature 451:1116-1120, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanman RB, Mortimer SA, Zill OA, et al. : Analytical and clinical validation of a digital sequencing panel for quantitative, highly accurate evaluation of cell-free circulating tumor DNA. PLoS One 10:e0140712, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guardant Health: Guardant360. http://www.guardanthealth.com.
- 21.Epstein JI, Egevad L, Amin MB, et al. : The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: Definition of grading patterns and proposal for a new grading system. Am J Surg Pathol 40:244-252, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Goodall J, Mateo J, Yuan W, et al. : Circulating cell-free DNA to guide prostate cancer treatment with PARP inhibition. Cancer Discov 7:1006-1017, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quigley D, Alumkal JJ, Wyatt AW, et al. : Analysis of circulating cell-free DNA identifies multiclonal heterogeneity of BRCA2 reversion mutations associated with resistance to PARP inhibitors. Cancer Discov 7:999-1005, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Mattos-Arruda L, Weigelt B, Cortes J, et al. : Capturing intra-tumor genetic heterogeneity by de novo mutation profiling of circulating cell-free tumor DNA: A proof-of-principle. Ann Oncol 25:1729-1735, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russo M, Siravegna G, Blaszkowsky LS, et al. : Tumor heterogeneity and lesion-specific response to targeted therapy in colorectal cancer. Cancer Discov 6:147-153, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norquist B, Wurz KA, Pennil CC, et al. : Secondary somatic mutations restoring BRCA1/2 predict chemotherapy resistance in hereditary ovarian carcinomas. J Clin Oncol 29:3008-3015, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christie EL, Fereday S, Doig K, et al. : Reversion of BRCA1/2 germline mutations detected in circulating tumor DNA from patients with high-grade serous ovarian cancer. J Clin Oncol 35:1274-1280, 2017 [DOI] [PubMed] [Google Scholar]
- 28.Pritchard CC, Offit K, Nelson PS: DNA-repair gene mutations in metastatic prostate cancer. N Engl J Med 375:1804-1805, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Ikeda H, Matsushita M, Waisfisz Q, et al. : Genetic reversion in an acute myelogenous leukemia cell line from a Fanconi anemia patient with biallelic mutations in BRCA2. Cancer Res 63:2688-2694, 2003 [PubMed] [Google Scholar]