Abstract
Purpose of Review
Breast cancer disparities that exist between high-income countries (HIC) and low- and middle-income countries (LMICs) are also reflected within population subgroups throughout the United States (US). Here we examine three case studies of US populations “left behind” in breast cancer outcomes/equity.
Recent Findings
African Americans in Chicago, non-Latina White women in Appalachia, and Latinas in the Yakima Valley of Washington State all experience a myriad of factors that contribute to lower rates of breast cancer detection and appropriate treatment as well as poorer survival. These factors, related to the social determinants of health, including geographic isolation, lack of availability of care, and personal constraints, can be addressed with interventions at multiple levels.
Summary
Although HICs have reduced mortality of breast cancer compared to LMICs, there remain inequities in the US healthcare system. Concerted efforts are needed to ensure that all women have access to equitable screening, detection, treatment, and survivorship resources.
Keywords: Breast cancer, Underserved populations, High-income countries, Socio-ecological model
Introduction
Breast cancer is the most common cancer diagnosed among women, as well as the leading cause of cancer death among women worldwide [1–4]. High-income countries (HICs), including much of Western Europe and North America, demonstrate higher breast cancer incidence rates (71.7 per 100,000), compared to low- and middle-income countries (LMICs) (29.3 per 100,000) [1, 5, 6]. Since the 1990s, incidence rates have decreased or remained stable in HICs—with the exception of some Asian countries—while mortality rates have decreased [5]. Five-year survival among women diagnosed with breast cancer has also increased in most developed nations [7, 8]. Conversely, in LMICs, both incidence and mortality have increased steadily [5]. Five-year relative survival rates in LMICs for women diagnosed with breast cancer at any stage lag behind those of HICs [7], ranging from 12% in West Africa to nearly 90% in the United States (US), Australia, Canada, and parts of Europe [6].
Breast cancer disparities that exist between HICs and LMICs—and their causes—are also reflected within population subgroups throughout the US. Although breast cancer incidence rates have remained relatively stable since 1990 and mortality rates have declined approximately 2% per year in the same period in the US [9], these improvements have not been experienced equitably throughout racial/ethnic groups and geographic locations. Women of color, socially and economically disadvantaged women, and women who live in rural areas suffer from increased mortality after a breast cancer diagnosis.
Breast Cancer Incidence and Mortality Across Racial/Ethnic Groups and Geographic Locations
The incidence of breast cancer among African American women in the US was lower than that of non-Latina white (NLW) women until 2012, when the rates converged; furthermore, in some southern states, African American women demonstrate higher breast cancer incidence rates and poorer stage-specific survival compared to NLW women [10, 11]. African American in the US are more than twice as likely, and Hispanics are 1.2 times as likely to be diagnosed with metastatic disease than NLWs [12]. Both groups are more likely to be diagnosed with more aggressive sub-types for which 5-year survival is much lower [10, 11, 13]. Among Latina subgroups, Mexican women are nearly twice as likely to present with Stage IV disease than NLWs [14]. Women of all racial/ethnic backgrounds who live in rural areas also experience higher breast cancer mortality than women living in urban areas [15], despite demonstrating lower incidence [16].
Poverty and Access to Care: Factors That Influence Late-Stage Diagnosis
Women who receive earlier-stage diagnoses demonstrate longer survival from breast cancer than those who are diagnosed at distant stages. However, in the US, women of all racial and ethnic groups who live in poverty and those who live in rural areas are at increased risk of late-stage diagnosis [14, 15, 17]. Although women with lower incomes are less likely to be diagnosed with breast cancer, lower income is associated with an increased risk of a late-stage breast cancer diagnosis. Specifically, women whose household income is less than $12,500 per year are nearly 2.5 times as likely to be diagnosed with distant stage breast cancer than those whose household income is over $50,000, and those living at or below 100% of the federal poverty level are more than three times as likely to receive a late-stage diagnosis [12].
Mammography capacity is also associated with screening uptake. In the US, mammography facilities are distributed unequally across geographic areas. Mammography capacity has declined across the US as a whole [18] while the percent of women living in low-capacity areas is increasing [19]. Over one quarter of the counties in the US have no mammography facilities at all, and 42 of the 50 states have at least one county without a facility. The counties without mammography facilities are more often rural, have lower population density, and a have high proportion of residents living in poverty [18].
Affluent NLW women living in urban settings have historically benefited from screening mammography to detect breast cancer at earlier stages and timely access to effective loco-regional and systemic therapies [20]. However, these strategies may not be reaching underserved groups whose awareness and understanding of breast cancer, access to care, and cultural factors differ from those of NLW women. As well, many underserved groups may not obtain such early detection or treatment because it is not available to them geographically, not offered to them, or not covered by insurance.
The barriers to effective early detection and treatment of breast cancer experienced by marginalized groups in the US that contribute to poor breast health outcomes include the social determinants of health, access to care, and cultural factors. These were outlined in a theoretical framework expounded by Warnecke et al. [21] As shown in Fig. 1, there are multiple levels that contribute to, and are involved in, reducing health disparities. The framework demonstrates that health disparities are affected by a number of levels, including biological antecedents, individual risk behaviors and demographics, the social and physical context of behavior, and social conditions and policies influencing detection and diagnosis. Further, reducing health disparities requires a focus on the numerous levels responsible for the health disparities.
Fig. 1.
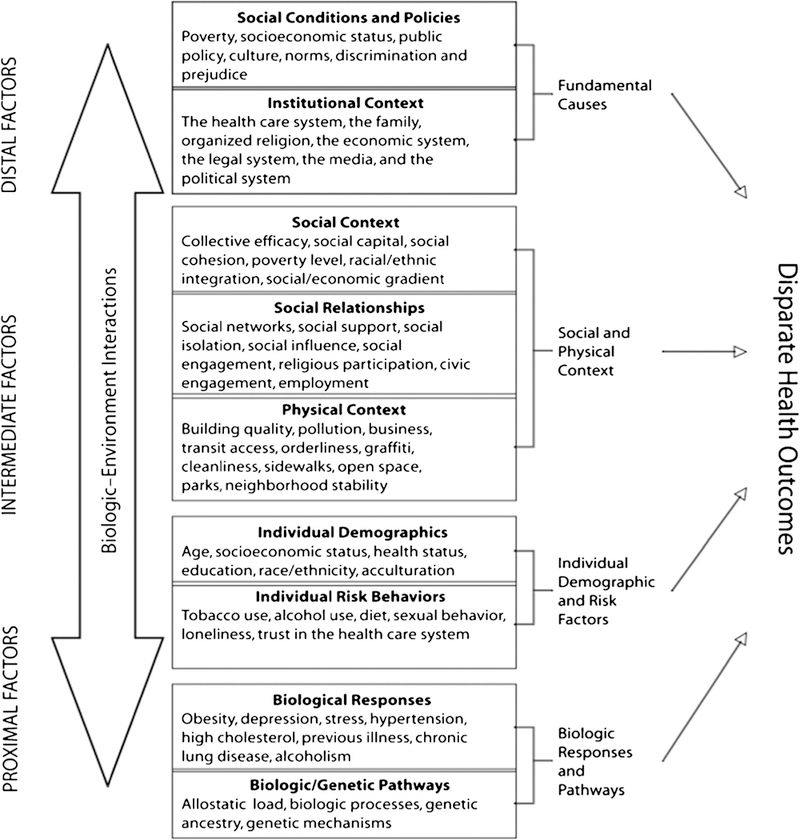
Warnecke Transdisciplinary Model of Population Health and Health. Figure 1 used with permission. Originally printed within Approach health disparities from a population perspective: the National Institutes of Health Centers for Population health and health Disparities?, RB Warnecke. American Journal of Public Health, September 2008; 98 (9): 1608 to 1615
We present three case studies of groups in the US that experience disparities more commonly associated with LMICs, and note how researchers and communities have partnered together to address the unequal burden of breast cancer in those populations. All three case studies are examined in the context of the socio-ecological model as described by Warnecke and colleagues [21], and all use a multilevel approach in seeking remedies to the inequities of breast cancer. Table 1 offers a comparison of breast cancer characteristics as well as a snapshot of factors that influence of breast cancer incidence and mortality across the three groups.
Table 1.
Incidence, Mortality, and determinants of breast cancer among women in Chicago, Appalachian Ohio, and Yakima, Washington
| U.S. population | African American women in Chicago, Illinois |
White women in Appalachian Ohio |
Latinas in Yakima County |
|
|---|---|---|---|---|
| Breast cancer incidence, per 100,000, age adjusted | 123.61 | 170.53 | 134.85 | 125.2*6 |
| Breast cancer mortality, per 100,000, age adjusted | 21.21 | 20.13 | 21.05 | 19.3*6 |
| Percent of women ≥ 40 who had a mammogram in the last 2 years | 65.31 | ~ 80.04 | 67–845 | Data not available |
| Percent living below of FPL | 12.702 | 30.02 | 17.82 | 28.02 |
| Percent uninsured | 8.82 | 10.02 | 7.72 | 41.02 |
| Total area population size (millions) | 325.72 | 2.72 | 2.02 | 0.12 |
| Percent with high school education | 90.02 | 85.02 | 83.62 | 54.82 |
Source: Surveillance Epidemiology and End Results (SEER) Registry, 2010–2014 Cancer Incidence Data
Source: U.S. Census
Source: Illinois Department of Public Health
Source: Susan G. Komen Foundation Chicagoland Area
Source: Ohio Department of Health
Source: Washington State Department of Health
These rates are for the general population in Yakima Valley, as statistics for Latina women were not available
African American Women in Urban Chicago, Illinois
Setting and Population
Chicago, IL, represents a large Midwestern city that has 77 community areas and 2.7 million residents [22]. Chicago has one of the largest urban African American communities in the US, with 31% of residents identifying as African American [22]. With regard to demographics, approximately 15% have not completed a high school education, over 30% are living in poverty, and 10% have no health insurance [23]. African Americans have largely lived in the same South and West Chicago neighborhoods due to societal and community forces [24].
Breast Cancer Characteristics
Similar to national patterns, breast cancer incidence rates have begun to converge between African American and NLW women living in Chicago and surrounding areas [25]. African American women in Chicago also appear to be more likely to be diagnosed with ER/PR negative tumors relative to NLW counterparts, in line with national findings [26, 27]. Late-stage breast cancer diagnoses represented approximately a third of all cases among African American and NLW women living in Chicago during 2005–2014 [25]. West African ancestry has also been linked to a greater risk for late stage at diagnosis among Chicago-based African American breast cancer patients [26].
Disparities in breast cancer mortality rates have been well-documented by Chicago-based working groups; 17 of the 20 community areas with the highest breast cancer mortality in Chicago are predominantly African American [28]. These studies have reported dynamic changes in mortality disparities between 1990 and 2018 [29–36], with increased disparity in outcomes during the early 1990s and 2000s, and a shift in the mid-2000s towards a reduction in these disparities. Recent studies have suggested a significant decline in breast cancer mortality among Chicago-based African Americans, with an annual decrease of 1.5% per year between 1999 and 2013. A decline in breast cancer disparities can also be seen, wherein the disparity between African American and NLW women in Chicago was approximately 1.5 between 1995 and 2005 and was 1.4 between 2006 and 2013 [36]. While this trend is promising, more nuanced analyses have suggested heterogeneity in this reduction both by age and specific community areas/neighborhoods [28, 37]. For example, the NLW: African American breast cancer mortality rate ratio for women younger than 40 years old in Chicago is 2.57, whereas the NLW: African breast cancer mortality rate for women who are older than 65 is 1.19 [37], suggesting greater disparities for younger African American women.
Screening mammography and its effects on Chicago-based racial disparities in late-stage diagnoses and mortality represent a complex picture. Similar to national trends, Chicago-specific data from the Behavioral Risk Factor Surveillance System (BRFSS) suggests that African American and NLW women have relatively similar rates of screening mammography uptake (78–80%) [38], and research suggests that Chicago-based African American women are less likely to overestimate mammography history relative to other ethnic minorities in Chicago [39]. In Chicago, recent efforts have focused on quality of screening mammography and other breast cancer care, which may drive local disparities [40–48]. These studies collectively suggest disparities in late-stage diagnoses and mortality may persist, even when there are no differences in breast cancer care uptake, if quality in care differs.
Breast Cancer Barriers
Barriers to high-quality care for Chicago-based African American women exist at intrapersonal, interpersonal, and contextual levels, as shown in the socio-ecological model (Fig. 1) [47]. At the intrapersonal level, cultural misconceptions, lack of knowledge, and cancer fatalism/fear have been identified as factors contributing to decisions to not seek care/receipt of a late-stage diagnosis [49–52]. At the interpersonal level, trust in and accessible communication with regular doctors and cancer care specialists have been identified as important [49, 50, 53]. Relative to other areas, a substantial amount of work has focused on the relationship between late-stage diagnosis and the adverse effects of neighborhood social environment and distance to primary care clinics [54–57].
Efforts to Intervene
A substantial amount of transdisciplinary work has been dedicated to eliminating the disproportionate breast cancer burden among Chicago-based African American women. Of these, the Metropolitan Chicago Breast Cancer Task Force is the best known and was developed by academic, community, and clinical partners to address breast cancer inequities through political advocacy, direct services, and public health surveillance [58, 59]. Local researchers have characterized disparities through observational studies [21, 27, 44–46, 51, 59] and have implemented intervention studies largely focused on patient navigation [40, 47, 60–64] and those committed to community education [65–70]. Most efficacious are patient navigation efforts which take women through the process of both screening and treatment. Local grassroots organizations have implemented continuous support and resources for local African American women (e.g., Women on Top of Their Game, Sisters Working It Out). The majority of the research described above has been a result of the National Institutes of Health’s commitment to cancer equity through Centers for Population Health and Health Disparities (CPHHD) Initaitive [21], including one CPHHD Center that lasted 10 years in Chicago. Chicago is also a pilot city for the Susan G. Komen’s African American Health Equity Initiative, launched in 2016, which has the goal of reducing breast cancer mortality disparities by 25% within 5 years of program implementation (no data available at this time). Program implementation includes building trust and a trust presence in the African American community beyond traditional events (e.g., Race for the Cure), building infrastructure for breast cancer care access and education, and funding of genetic counselors and navigators.
Non-Latina White Women in Rural Appalachia Ohio
Setting and Population
Appalachia is a federally designated geographic region, with a population of approx. 25 million; it was identified in the 1960s in response to the region’s higher than average poverty and unemployment rates. It spans 420 counties in 13 states along the Appalachian mountain range, is mostly rural, and is divided into five areas [71]. This case study focuses on a region spanning 32 counties in Ohio in the south and east of Appalachia, with a population of 2,023,656 residents. Compared to the US as a whole, the residents of Appalachia Ohio, US, are mainly white (91.4 vs 62.3%), more likely to live in poverty (17.8 vs 15.5%), older (41.3 median years vs 37.6 median years), with lower educational achievement (83.6% with a high school diploma vs 86.7%) [72].
Breast Cancer Characteristics
In 2010–2014, the average annual breast cancer incidence rate in Appalachia Ohio was lower than that for non-Appalachia Ohio (134.8 per 100,000 women vs 153.7 per 100,000 women) [73]. Mortality rates are high in certain Appalachian counties in Ohio (Adams, Harrison, Mahoning, Monroe, Washington), compared to other similar Ohio counties. The prevalence of mammography screening in the past 2 years among women aged 50 to 74 years varies according to annual household income and educational attainment (both of which, as mentioned above, are lower in Appalachia Ohio, compared to non-Appalachia Ohio), ranging from 67 to 84% [74]. The percentage of women diagnosed with late-stage disease in Appalachia Ohio is 28.5%, compared to 28.9% in non-Appalachia Ohio; however, slightly more women in Appalachia Ohio were diagnosed with an unstaged breast cancer (3.0 versus 2.1%) [74].
Breast Cancer Barriers
Barriers to receiving prompt and quality breast cancer services in Appalachia Ohio are centered at multiple levels as defined in the socio-ecological model (Fig. 1). First, individuals often have fatalistic views about cancer, undermining the ability of early detection tests to be effective [75, 76]. Secondly, socioeconomic factors (both at the individual and community levels) impact the ability of residents to have insurance coverage for mammography and treatment [76, 77]. Thirdly, access to screening and treatment is limited in this region: six of the 32 counties have no mammography facilities and 12 have only one in the entire county. In addition, treatment facilities are sparse [77]. Geographic distances make travel to medical facilities difficult especially with the lack of public transportation and road conditions being subject to adverse weather.
Efforts to Intervene
There are few efforts to address the low mammography rates in Ohio Appalachia. The Ohio State University Comprehensive Cancer Center (OSUCCC) has received funding from Susan G. Komen, Columbus to address the lack of mammography services in Appalachia Ohio. Using a continuum of care patient navigation model with community partnerships, our community health workers (CHWs) identify women in need of screening, educate them about mammography, and link them to patient navigation. Next, the patient navigators (PN) identify how to pay for each woman’s mammogram (i.e., insurance, state Breast and Cervical Cancer Early Detection Program, or Komen grant funds) and schedule the woman for a convenient appointment at either a local facility (if available) or at a mobile mammography screening unit sponsored by the OSUCCC hospital. The PN also identifies and addresses barriers to screening, calls to remind women of the test, and follow-up with women regarding test results, including resolution of abnormalities and assurance of treatment, as needed. Community partners including local community cancer coalitions, medical facilities, and Ohio University College of Osteopathic Health that provides a companion screening van to perform clinical breast exams and Pap tests at select mobile van screening days. To date, 80 events have been held with 1750 women screened. Even with the success of the mobile screenings and patient navigation, there is still a need to address barriers to diagnostic follow-up and treatment for these women with limited services close to home.
Latina Women in Rural Washington State
Setting and Population
The Lower Yakima Valley is a rural area comprising many small agricultural communities in south central Washington State, including those in Yakima, Benton, and Franklin counties. The region has the largest population of Latinos in the state; approximately half of the residents are Latino, 90% of whom are from Mexico [78]. Franklin county in particular demonstrated the fastest growing population in the state between 2000 and 2010, with the proportion of Latinos increasing in that county by 71% [78]. Compared to the state of Washington, residents of Yakima and Franklin counties are more likely to live below the federal poverty level (27.8 vs. 12.7%), be Latino (69.0 vs. 12.1%), and are less likely to have health insurance (41.4 vs. 90.2%). Compared to NLW, Latino community members in the Lower Yakima Valley often work in agriculture, have few years of formal education (45.2% have less than a high school diploma vs. 6.2% for NLW), and lower household income (less than $35,444 per year compared to $51,940 for NLW) [78, 79]. They are also more likely to be uninsured than the general population and, consequently, have limited access to health care.
Breast Cancer Characteristics
Latina women in the US overall are less likely to be diagnosed with any breast cancer (91.1/100,000) compared to NLWs (127.3/100,000) and African American women (118.4/100,000) [10]. However, it remains the most common cancer among this ethnic group [10], and compared to NLWs, Latinas demonstrate a higher breast cancer incidence to mortality ratio [11]. Latinas also experience high rates of diagnosis with aggressive sub-types of breast cancer for which fewer treatment options exist and lower 5-year survival rates are observed [13]. Furthermore, Latina women born in the US who are more acculturated are up to six times more likely to be diagnosed with breast cancer than foreign-born, less acculturated Latinas [20, 80]. The burden of breast cancer borne by Latinas in the Lower Yakima Valley of Washington State likely corresponds to that which exists in the US at large. However, similar to the challenges encountered in LMICs, issues of data quality plague reporting for racial/ethnic minorities in the US, particularly those living in hard-to-reach rural areas like the Yakima Valley. Studies have found that Latinos are undercounted in cancer registries, lending to under-classification of their burden of disease [81–83], and the counties in the Yakima Valley are not included at all in the national Surveillance, Epidemiology, and End Results (SEER) registry. Other studies have found that death rates according to ethnic origin are understated in national registries, including the National Center for Health Statistics [84] and the Current Population Survey [85].
Latinas are less likely than NLWs to obtain routine screening mammography, resulting in later stages of diagnosis, worse prognosis, and shorter survival [86]. In addition to inadequate screening, once diagnosed, Latinas are also more likely to experience delays in initial treatment as well as low levels of initiation and adherence to adjuvant therapy, factors associated with increased breast cancer mortality [87–92].
Breast Cancer Barriers
Latinas in the Lower Yakima Valley experience unique and multilevel, socially and structurally determined barriers to breast cancer screening and treatment that contribute to poor breast health outcomes. Although data available indicates that breast cancer incidence and mortality in Franklin, Benton, and Yakima counties are lower than the national average, it is likely that the burden of breast cancer is higher than the statistics indicate for the reasons discussed above. Latinas here suffer disproportionately from determinants that impact breast health, such as poverty, low levels of educational attainment, insurance status and language barriers as discussed above [22]. These factors contribute to lack of understanding about where and how often to obtain screening mammography, a problem often exacerbated by communication barriers and a lack of culturally and language-appropriate materials available about breast cancer screening and treatment. Indeed, living in poverty, low levels of education, and lack of health insurance are associated with lower rates of screening and shorter cancer-specific survival [93–95]. In addition to unique communication needs [96], fear and mistrust of the medical system, often related to documentation status, also influence this population’s adherence to breast cancer screening, treatment, and follow-up care [96]. Finally, in this rural area, access to care may be constrained by lack of transport to distant care facilities.
Efforts to Intervene
The Fred Hutchinson Cancer Research Center has partnered with community organizations across Washington State, including those in the Lower Yakima Valley, to address factors that contribute to disparate breast cancer outcomes among Latinas using culturally informed interventions. Through a community-based participatory approach, in which the community members are involved in each phase of research [97], researchers train promotores, bilingual and bicultural lay health workers from the community, to educate Latinas in the Yakima Valley about breast cancer. Promotores travel to study participants’ homes, where they facilitate home health parties (HHPs), engaging women in discussions about breast cancer screening and treatment. The HHPs often involve Latina women’s larger social and familial networks, and are designed to match women’s language, dispel myths, and build on their current understanding of breast cancer. The promotora-led HHPs have resulted in improved knowledge about breast cancer, increased report of discussions with doctors regarding mammograms, and increased intention to obtain a mammogram [98]. In addition, the HHPs demonstrated significant increases in knowledge as well as increased interactions with friends and family regarding breast cancer screening [99]. In attempts to address disparities further along the breast cancer care continuum, researchers and community members have partnered to facilitate survivor support groups to address adherence to adjuvant treatment and quality of life among Latina survivors. Support groups offer educational components as well as interventions to bolster social support and quality of life—which have been shown to be lacking among Latina breast cancer patients and survivors [100–102]—with a long-term goal of reducing rates of recurrence and mortality [103].
Discussion
The case studies presented here characterize disparate pockets of women in the US who experience difficulties in screening, detection, treatment, and survival of breast cancer. As shown in Fig. 1, these specific health disparities are consequences of factors at multiple levels. Although all are residents of an HIC, they lag behind the average screening, detection, treatment, and survival rates of the average NLW women in the US regardless of the facilities and treatment available in the HIC, they experience many of the same outcomes as women residing in LMICs. That such areas exist in the US, an HIC with widely available screening through mammography and state-of-the-art treatment, is a travesty. Yet, it is obvious from our case studies that race, ethnicity, culture, rural residence, insurance status, and intrapersonal and interpersonal barriers contribute to these disparities.
The multilevel interventions across the three communities described here have yielded some success in bolstering breast cancer awareness and education, increasing screening rates, and potentially reducing disparities in breast cancer. [36, 37, 98, 99] However, much work remains to reduce the burden of breast cancer among these and other underserved women throughout the US. Across all three cases, poverty, lack of education and awareness about breast cancer, and lack of access to high-quality care represent persistent challenges to closing the gap in breast health disparities. In Chicago, the apparent disparities between NLW and African American women under 50 in Chicago who are diagnosed with breast cancer suggests that more attention should be given to screening guidelines for women of African descent. Additionally, the city continues to be afflicted by adverse effects of neighborhood factors, such as segregation, that impact breast health disparities. In Appalachia, mobile mammography vans have helped bolster screening rates, but the low mammography capacity in the region remains, contributing to increased late-stage diagnoses among women there. In the Yakima Valley, language barriers, culturally held stigma, poverty status, and access to care impact women’s treatment initiation and adherence to adjuvant therapies. These factors, all of which are represented in Warnecke’s model, represent areas for future work.
In terms of addressing disparities, all three case studies note the importance of working with communities at a variety of levels of influence. Consistent with a community-based participatory research approach, the case studies appear to recognize that working with people is the best way to address the problem of disparities. Whether it is educating individuals, assisting with logistics including finances, or arranging treatment, working with a community’s resources makes it feasible to identify potential solutions to breast cancer disparities. Changing the disparities is not a single-level strategy; rather, it takes participation at the community, clinic, cultural, and societal level to achieve equity.
From the three case studies, it is clear that unconventional approaches are necessary to reach such underserved women. Rather than focusing on office reminder systems and general media blitzes on mammography, these three studies used a “meeting the women where they are” strategy; that is, the researchers went to the communities to determine how best to reach the underserved populations. For example, Chicago-based academic, community, and clinic partners, as well as national partners, worked to address the disproportionate rates of breast cancer mortality that local African American women face by increasing access to high-quality locally available mammography, ensuring mammography quality surveillance occurs regularly, and addressing the multilevel barriers African American women face through patient navigation.
In Appalachia, rural conditions and limited access to screening facilities make breast screening inaccessible to many residents of the Appalachian counties. Combined with the fatalistic views of many women, it is understandable that few take advantage of screening for early detection. Further, poverty and the associated lack of insurance coverage makes it clearer why women suffer disparities of screening, detection, treatment, and survival. Through a Susan G. Komen Foundation grant, researchers work with communities and clinics to identify women in need of mammography and link them to patient navigation and appointments at either a mammography facility or a hospital-sponsored mammovan to obtain breast cancer screening. The patient navigators ensure that women with a positive screen seek appropriate treatment.
In the Lower Yakima Valley, community input resulted in the identification and/or training of promotores, or lay health workers, to address disparities in screening and treatment. The home health party model used by the promotores is successful in increasing women’s knowledge of breast cancer and intention to be screened. The promotores also act as patient navigators, helping women find resources (for example, through the Breast, Cervical, and Colon Health Services) to pay for the screening. Researchers in the Lower Valley also have conducted survivor support groups to assist women who are breast cancer survivors. In this way, the entire continuum of screening, detection, treatment, and survivorship is covered.
Education can help women overcome the intrapersonal and interpersonal barriers to screening; it can also help women understand and mitigate some of the cultural barriers. Mammovans and assistance in traveling to screening facilities can reduce some of the geographic barriers. One remaining barrier is financing for screening. In terms of the future, policy interventions can be useful in removing this barrier. Studies from the effects of the Affordable Care Act indicate that when screening is covered by insurance, all groups of individuals, from the very poor to those suffering low education, take advantage of the screening services [104–107]. Policies to provide such coverage should continue. Patient navigation and resources to provide such navigation appear to reduce barriers and therefore should also be employed. Patient-centered medical homes are taking advantage of this strategy with some success [108, 109].
Conclusion
Although HICs have reduced mortality of breast cancer compared to LMICs, there remain significant inequities in the US system. These inequities, as described in these case studies, can be addressed, but concerted efforts are needed to ensure that all have access to equitable systems of screening, detection, treatment, and survivorship. Future research is needed to identify other strategies that will alleviate the inequities.
Acknowledgments
Sarah D. Hohl was supported by a grant from the National Institutes of Health, National Cancer Institute (T32CA092508).
Electra D. Paskett has received a grant from Merck and is a stockholder of Pfizer.
Footnotes
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Compliance with Ethical Standards
Conflict of Interest
Beti Thompson, Yamile Molina, James L. Fisher, Ryan D. Baltic, and Chasity M. Washington declare no conflict of interest.
References
- 1.Coughlin SS, Ekwueme DU. Breast cancer as a global health concern. Cancer Epidemiol. 2009;33(5):315–8. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–917. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1893–907. [DOI] [PubMed] [Google Scholar]
- 4.Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27. [DOI] [PubMed] [Google Scholar]
- 5.DeSantis CE, Bray F, Ferlay J, Lortet-Tieulent J, Anderson BO, Jemal A. International variation in female breast cancer incidence and mortality rates. Cancer Epidemiol Biomarkers Prev. 2015;24(10):1495–506. [DOI] [PubMed] [Google Scholar]
- 6.Youlden DR, Cramb SM, Dunn NA, Muller JM, Pyke CM, Baade PD. The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality Cancer Epidemiol. 2012;36(3):237–48. [DOI] [PubMed] [Google Scholar]
- 7.Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2015;385(9972):977–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allemani C, Sant M, Weir HK, Richardson LC, Baili P, Storm H, et al. Breast cancer survival in the US and Europe: a CONCORD high-resolution study Int J Cancer. 2013;132(5):1170–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 10.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64(1):52–62. [DOI] [PubMed] [Google Scholar]
- 11.DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: convergence of incidence rates between black and white women. CA Cancer J Clin. 2015 [DOI] [PubMed] [Google Scholar]
- 12.Clegg LX, Reichman ME, Miller BA, Hankey BF, Singh GK, Lin YD, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control. 2009;20(4):417–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, Li CI. Racial disparities in breast cancer diagnosis and treatment by hormone receptor and HER2 status. Cancer Epidemiol Biomarkers Prev. 2015;24(11): 1666–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ooi SL, Martinez ME, Li CI. Disparities in breast cancer characteristics and outcomes by race/ethnicity. Breast Cancer Res Treat. 2011;127(3):729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh G, HJemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950–2014: over six decades of changing patterns and widening inequalities. J Environ Public Health. 2017;19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zahnd WE, James AS, Jenkins WD, Izadi SR, Fogleman AJ, Steward DE, et al. Rural-urban differences in cancer incidence and trends in the United States. Cancer Epidemiol Biomarkers Prev. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78–93. [DOI] [PubMed] [Google Scholar]
- 18.Peipins LA, Miller J, Richards TB, Bobo JK, Liu T, White MC, et al. Characteristics of US counties with no mammography capacity. J Community Health. 2012;37(6):1239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eberth JM, Eschbach K, Morris JS, Nguyen HT, Hossain MM, Elting LS. Geographic disparities in mammography capacity in the south: a longitudinal assessment of supply and demand. Health Serv Res. 2014;49(1):171–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porter P “Westernizing” women’s risks? Breast cancer in lower-income countries. N Engl J Med. 2008;358(3):213–6. [DOI] [PubMed] [Google Scholar]
- 21.Warnecke RB, Oh A, Breen N, Gehlert S, Paskett E, Tucker KL, et al. Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health. 2008;98(9):1608–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.U.S. Bureau of the Census. American Community Survey, 2012–2016. 2018; https://www.census.gov/programs-surveys/acs/. Accessed 25 Mar 2018.
- 23.Chicago Department of Public Health Healthy Chicago 2.0: Partnering to Improve Health Equity. Chicago, IL; 2016. [Google Scholar]
- 24.Sampson RJ. Great American city: Chicago and the enduring neighborhood effect. University of Chicago Press; 2012. [Google Scholar]
- 25.Illinois Department of Public Health. Illinois State Cancer Registry. 2014; http://www.dph.illinois.gov/data-statistics/epidemiology/cancer-registry. Accessed 25 Mar 2018.
- 26.Al-Alem U, Rauscher G, Shah E, et al. Association of genetic ancestry with breast cancer in ethnically diverse women from Chicago. PLoS One. 2014;9(11):e112916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rauscher GH, Kresovich JK, Poulin M, Yan L, Macias V, Mahmoud AM, et al. Exploring DNA methylation changes in promoter, intragenic, and intergenic regions as early and late events in breast cancer formation. BMC Cancer. 2015;15(1):816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Francois-Blue T, Tossas-Milligan T, Murphy AM. How far have we come? Improving access to and quality of breast health services in Chicago. Chicago, IL; 2014. [Google Scholar]
- 29.Hirschman J, Whitman S, Ansell D. The black: white disparity in breast cancer mortality: the example of Chicago. Cancer Causes Control. 2007;18(3):323–33. [DOI] [PubMed] [Google Scholar]
- 30.Hunt B, Whitman S. Black: white health disparities in the United States and Chicago: 1990–2010. J Racial Ethn Health Disparities. 2015;2(1):93–100. [DOI] [PubMed] [Google Scholar]
- 31.Margellos H, Silva A, Whitman S. Comparison of health status indicators in Chicago: are black-white disparities worsening? Am J Public Health. 2004;94(1):116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunt BR, Whitman S, Hurlbert MS. Increasing black: white disparities in breast cancer mortality in the 50 largest cities in the United States. Cancer Epidemiol. 2014;38(2):118–23. [DOI] [PubMed] [Google Scholar]
- 33.Hunt BR, Hurlbert MS. Black: white disparities in breast cancer mortality in the 50 largest cities in the United States, 2005–2014. Cancer Epidemiol. 2016;45:169–73. [DOI] [PubMed] [Google Scholar]
- 34.Orsi JM, Margellos-Anast H, Whitman S. Black–white health disparities in the United States and Chicago: a 15-year progress analysis. Am J Public Health. 2010;100(2):349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitman S, Ansell D, Orsi J, Francois T. The racial disparity in breast cancer mortality. J Community Health. 2011;36(4):588–96. [DOI] [PubMed] [Google Scholar]
- 36.Sighoko D, Murphy AM, Irizarry B, Rauscher G, Ferrans C, Ansell D. Changes in the racial disparity in breast cancer mortality in the ten US cities with the largest African American populations from 1999 to 2013: the reduction in breast cancer mortality disparity in Chicago. Cancer Causes Control. 2017;28(6):563–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sighoko D, Hunt BR, Irizarry B, Watson K, Ansell D, Murphy AM. Disparity in breast cancer mortality by age and geography in 10 racially diverse US cities. Cancer Epidemiol. 2018;53:178–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Susan G Komen Foundation Chicagoland Area Community Profile Report. Chicago, IL; 2015. [Google Scholar]
- 39.Allgood KL, Rauscher GH, Whitman S, Vasquez-Jones G, Shah AM. Validating self-reported mammography use in vulnerable communities: findings and recommendations. Cancer Epidemiology and Prevention Biomarkers. 2014:cebp 1253.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molina Y, Kim S, Berrios N, Calhoun EA. Medical mistrust and patient satisfaction with mammography: the mediating effects of perceived self-efficacy among navigated African American women. Health Expect. 2015;18(6):2941–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dookeran KA, Silva A, Warnecke RB, Rauscher GH. Race/ethnicity and disparities in mastectomy practice in the breast Cancer Care in Chicago study. Ann Surg Oncol. 2015;22(1):66–74. [DOI] [PubMed] [Google Scholar]
- 42.Mortel M, Rauscher GH, Murphy AM, Hoskins K, Warnecke RB. Racial and ethnic disparity in symptomatic breast cancer awareness despite a recent screen: the role of tumor biology and mammography facility characteristics. Cancer Epidemiol Biomarkers Prev. 2015;24(10):1599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rauscher GH, Murphy AM, Orsi JM, Dupuy DM, Grabler PM, Weldon CB. Beyond the mammography quality standards act: measuring the quality of breast cancer screening programs. Am J Roentgenol. 2014;202(1):145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rauscher GH, Allgood KL, Whitman S, Conant E. Disparities in screening mammography services by race/ethnicity and health insurance. J Women’s Health. 2012;21(2):154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rauscher GH, Conant EF, Khan JA, Berbaum ML. Mammogram image quality as a potential contributor to disparities in breastcancer stage at diagnosis: an observational study. BMC Cancer. 2013;13(1):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rauscher GH, Khan JA, Berbaum ML, Conant EF. Potentially missed detection with screening mammography: does the quality of radiologist’s interpretation vary by patient socioeconomic advantage/disadvantage? Ann Epidemiol. 2013;23(4):210–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tejeda S, Darnell JS, Cho YI, Stolley MR, Markossian TW, Calhoun EA. Patient barriers to follow-up care for breast and cervical cancer abnormalities. J Women’s Health. 2013;22(6):507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silva A, Rauscher GH, Hoskins K, Rao R, Ferrans CE. Assessing racial/ethnic disparities in chemotherapy treatment among breast cancer patients in context of changing treatment guidelines. Breast Cancer Res Treat. 2013;142(3):667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaiser K, Cameron KA, Curry G, Stolley M. Black women’s awareness of breast cancer disparity and perceptions of the causes of disparity. J Community Health. 2013;38(4):766–72. [DOI] [PubMed] [Google Scholar]
- 50.Peek ME, Sayad JV, Markwardt R. Fear, fatalism and breast cancer screening in low-income African-American women: the role of clinicians and the health care system. J Gen Intern Med. 2008;23(11):1847–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rauscher GH, Ferrans CE, Kaiser K, Campbell RT, Calhoun EE, Warnecke RB. Misconceptions about breast lumps and delayed medical presentation in urban breast cancer patients. Cancer Epidemiol Biomarkers Prev. 2010;19(3):640–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferrans C, Rauscher G, Akpan B, et al. Cultural beliefs contributing to disparities in later-stage breast cancer among newly diagnosed African-American, Latina, and Caucasian women. Paper presented at: Oncology Nursing Forum; 2007. [Google Scholar]
- 53.Simon MA, Ragas DM, Nonzee NJ, Phisuthikul AM, Luu TH, Dong X. Perceptions of patient-provider communication in breast and cervical cancer-related care: a qualitative study of low-income English-and Spanish-speaking women. J Community Health. 2013;38(4):707–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barrett RE, Cho YI, Weaver KE, Ryu K, Campbell RT, Dolecek TA, et al. Neighborhood change and distant metastasis at diagnosis of breast cancer. Ann Epidemiol. 2008;18(1):43–7. [DOI] [PubMed] [Google Scholar]
- 55.Tarlov E, Zenk SN, Campbell RT, Warnecke RB, Block R. Characteristics of mammography facility locations and stage of breast cancer at diagnosis in Chicago. J Urban Health. 2009;86(2): 196–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zenk SN, Tarlov E, Sun J. Spatial equity in facilities providing low-or no-fee screening mammography in Chicago neighborhoods. J Urban Health. 2006;83(2):195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim S, Chukwudozie B, Calhoun E. Sociodemographic characteristics, distance to the clinic, and breast cancer screening results. J Health Dispar Res Pract. 2013;6(1):70. [PMC free article] [PubMed] [Google Scholar]
- 58.Ansell D, Grabler P, Whitman S, Ferrans C, Burgess-Bishop J, Murray LR, et al. A community effort to reduce the black/white breast cancer mortality disparity in Chicago. Cancer Causes Control. 2009;20(9): 1681–8. [DOI] [PubMed] [Google Scholar]
- 59.Thompson B, Molina Y, Viswanath K, Warnecke R, Prelip ML. Strategies to empower communities to reduce health disparities. Health Aff 2016;35(8):1424–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Markossian TW, Calhoun EA. Are breast cancer navigation programs cost-effective? Evidence from the Chicago Cancer Navigation Project. Health Policy. 2011;99(1):52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Markossian TW, Darnell JS, Calhoun EA. Follow-up and timeliness after an abnormal cancer screening among underserved, urban women in a patient navigation program. AACR; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Molina Y, Kim SJ, Berrios N, Glassgow AE, San Miguel Y, Darnell JS, et al. Patient navigation improves subsequent breast cancer screening after a noncancerous result: evidence from the patient navigation in medically underserved areas study. J Women’s Health. 2018;27(3):317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Molina Y, Glassgow AE, Kim SJ, Berrios NM, Pauls H, Watson KS, et al. Patient navigation in medically underserved areas study design: a trial with implications for efficacy, effect modification, and full continuum assessment. Contemp Clin Trials. 2017;53:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim S, Molina Y, Glassgow AE, Berrios N, Guadamuz J, Calhoun E. The effects of navigation and types of neighborhoods on timely follow-up of abnormal mammogram among black women. Med Res Arch. 2015;2015(3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Allgood KL, Hunt B, Kanoon JM, Simon MA. Evaluation of mammogram parties as an effective community navigation method. J Cancer Educ. 2017:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hunt BR, Allgood KL, Kanoon JM, Benjamins MR. Keys to the successful implementation of community-based outreach and navigation: lessons from a breast health navigation program. J Cancer Educ. 2017;32(1):175–82. [DOI] [PubMed] [Google Scholar]
- 67.Hunt BR, Allgood K, Sproles C, Whitman S. Metrics for the systematic evaluation of community-based outreach. J Cancer Educ. 2013;28(4):633–8. [DOI] [PubMed] [Google Scholar]
- 68.Matthews AK, Berrios N, Darnell JS, Calhoun E. A qualitative evaluation of a faith-based breast and cervical cancer screening intervention for African American women. Health Educ Behav. 2006;33(5):643–63. [DOI] [PubMed] [Google Scholar]
- 69.Gehlert S, Coleman R. Using community-based participatory research to ameliorate cancer disparities. Health Soc Work. 2010;35(4):302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shapiro LD, Thompson D, Calhoun E. Sustaining a safety net breast and cervical cancer detection program. J Health Care Poor Underserved. 2006;17(2):20–30. [DOI] [PubMed] [Google Scholar]
- 71.President’s Appalachian Regional Commission. Appalachia: a report by the President’s Appalachian Regional Commission, 1964. Washington, DC: US Department of Commerce: 1966. [Google Scholar]
- 72.Pollard K, Jacobsen LA. The Appalachian Region: a data overview from the 2011–2015 American Community Survey Appalachian Regional Commission;2017. [Google Scholar]
- 73.Ohio Department of Health. Ohio Cancer Incidence Surveillance System. 2018; https://www.odh.ohio.gov/health/cancer/ocisshs/reporting1.aspx. Accessed 25 Mar 2018.
- 74.Ohio Department of Health, The Ohio State University. Breast cancer in Ohio, 2010–2014. 2017; https://www.odh.ohio.gov/health/cancer/ocisshs/reporting1.aspx. Accessed 25 Mar 2018.
- 75.Vanderpool RC, Huang B. Cancer risk perceptions, beliefs, and physician avoidance in Appalachia: results from the 2008 HINTS Survey. J Health Commun. 2010;15(sup3):78–91. [DOI] [PubMed] [Google Scholar]
- 76.Schoenberg NE, Kruger TM, Bardach S, Howell BM. Appalachian women’s perspectives on breast and cervical cancer screening. Rural Remote Health. 2013;13(3):2452. [PMC free article] [PubMed] [Google Scholar]
- 77.Paskett ED, Fisher JL, Lengerich EJ, Schoenberg NE, Kennedy SK, Conn ME, et al. Disparities in underserved white populations: the case of cancer-related disparities in Appalachia. Oncologist. 2011;16(8):1072–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.U.S. Bureau of the Census. U.S. Census Bureau. American FactFinder. 2016; https://www.census.gov/quickfacts/. Accessed March 20, 2018.
- 79.Diebel J, Norda J, Kretchmer O. Statistical Atlas-Yakima County. 2009–2013; https://statisticalatlas.com/county/Washington/Yakima-County/Household-Income. Accessed 8 Apr 2018.
- 80.John EM, Phipps AI, Davis A, Koo J. Migration history, acculturation, and breast cancer risk in Hispanic women. Cancer Epidemiol Biomarkers Prev. 2005;14(12):2905–13. [DOI] [PubMed] [Google Scholar]
- 81.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12(8):703–11. [DOI] [PubMed] [Google Scholar]
- 82.Clegg LX, Reichman ME, Hankey BF, Miller BA, Lin YD, Johnson NJ, et al. Quality of race, Hispanic ethnicity, and immigrant status in population-based cancer registry data: implications for health disparity studies. Cancer Causes Control. 2007;18(2):177–87. [DOI] [PubMed] [Google Scholar]
- 83.Gomez SL, Glaser SL. Misclassification of race/ethnicity in a population-based Cancer registry (United States). Cancer Causes Control. 2006;17(6):771–81. [DOI] [PubMed] [Google Scholar]
- 84.Rosenberg HM, Maurer JD, Sorlie PD, et al. Quality of death rates by race and Hispanic origin: a summary of current research, 1999. Vital and health statistics Series 2, Data evaluation and methods research. 1999(128):1–13. [PubMed] [Google Scholar]
- 85.Williams DR. The health of US racial and ethnic populations. J Gerontol Ser B Psychol Sci Soc Sci. 2005;60(Special_Issue_2): S53–62. [DOI] [PubMed] [Google Scholar]
- 86.Smith-Bindman R, Miglioretti DL, Lurie N, Abraham L, Barbash RB, Strzelczyk J, et al. Does utilization of screening mammography explain racial and ethnic differences in breast cancer? Ann Intern Med. 2006;144(8):541–53. [DOI] [PubMed] [Google Scholar]
- 87.Smith EC, Ziogas A, Anton-Culver H. Delay in surgical treatment and survival after breast cancer diagnosis in young women by race/ethnicity. JAMA Surg. 2013;148(6):516–23. [DOI] [PubMed] [Google Scholar]
- 88.Fedewa SA, Edge SB, Stewart AK, Halpern MT, Marlow NM, Ward EM. Race and ethnicity are associated with delays in breast cancer treatment (2003–2006). J Health Care Poor Underserved. 2011;22(1):128–41. [DOI] [PubMed] [Google Scholar]
- 89.Fedewa SA, Ward EM, Stewart AK, Edge SB. Delays in adjuvant chemotherapy treatment among patients with breast cancer are more likely in African American and Hispanic populations: a national cohort study 2004–2006. J Clin Oncol. 2010;28(27):4135–41. [DOI] [PubMed] [Google Scholar]
- 90.Hershman DL, Shao T, Kushi LH, Buono D, Tsai WY, Fehrenbacher L, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126(2):529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bickell NA, Wang JJ, Oluwole S, Schrag D, Godfrey H, Hiotis K, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24(9):1357–62. [DOI] [PubMed] [Google Scholar]
- 92.Livaudais JC, Hershman DL, Habel L, Kushi L, Gomez SL, Li CI, et al. Racial/ethnic differences in initiation of adjuvant hormonal therapy among women with hormone receptor-positive breast cancer. Breast Cancer Res Treat. 2012;131(2):607–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sprague BL, Trentham-Dietz A, Gangnon RE, Ramchandani R, Hampton JM, Robert SA, et al. Socioeconomic status and survival after an invasive breast cancer diagnosis. Cancer. 2011;117(7):1542–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Halpern MT, Bian J, Ward EM, Schrag NM, Chen AY. Insurance status and stage of cancer at diagnosis among women with breast cancer. Cancer. 2007;110(2):403–11. [DOI] [PubMed] [Google Scholar]
- 95.Harper S, Lynch J, Meersman SC, Breen N, Davis WW, Reichman MC. Trends in area-socioeconomic and race-ethnic disparities in breast cancer incidence, stage at diagnosis, screening, mortality, and survival among women ages 50 years and over (1987–2005). Cancer Epidemiol Biomarkers Prev. 2009;18(1):121–31. [DOI] [PubMed] [Google Scholar]
- 96.Molina Y, Hohl SD, Ko LK, Rodriguez EA, Thompson B, Beresford SAA. Understanding the patient-provider communication needs and experiences of Latina and non-Latina white women following an abnormal mammogram. J Cancer Educ. 2014;29(4):781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Israel BA, Schulz AJ, Parker EA, Becker AB. Review of community-based research: assessing partnership approaches to improve public health. Annu Rev Public Health. 1998;19(1): 173–202. [DOI] [PubMed] [Google Scholar]
- 98.Livaudais JC, Coronado GD, Espinoza N, Islas I, Ibarra G, Thompson B. Educating Hispanic women about breast cancer prevention: evaluation of a home-based promotora-led intervention. J Women’s Health. 2010;19(11):2049–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Scheel JR, Molina Y, Briant KJ, Ibarra G, Lehman CD, Thompson B. Latinas’ mammography intention following a home-based promotores-led intervention. J Community Health. 2015;40(6):1185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Molina Y, Hohl S, Nguyen M, et al. Ethnic differences in social support after initial receipt of an abnormal mammogram. Cult Divers Ethn Minor Psychol. 2016;22:588–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alferi SM, Carver CS, Antoni MH, Weiss S, Durán RE. An exploratory study of social support, distress, and life disruption among low-income Hispanic women under treatment for early stage breast cancer. Health Psychol. 2001;20(1):41–6. [DOI] [PubMed] [Google Scholar]
- 102.Lopez-Class M, Perret-Gentil M, Kreling B, Caicedo L, Mandelblatt J, Graves KD. Quality of life among immigrant Latina breast cancer survivors: realities of culture and enhancing cancer care. J Cancer Educ. 2011;26(4):724–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ceballos RM, Molina Y, Malen RC, Ibarra G, Escareno M, Marchello N. Design, development, and feasibility of a Spanish-language cancer survivor support group. Support Care Cancer. 2015;23(7):2145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cooper GS, Kou TD, Dor A, Koroukian SM, Schluchter MD. Cancer preventive services, socioeconomic status, and the Affordable Care Act. Cancer. 2017;123(9):1585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cooper GS, Kou TD, Schluchter MD, Dor A, Koroukian SM. Changes in receipt of cancer screening in Medicare beneficiaries following the Affordable Care Act. J Natl Cancer Inst. 2016;108(5):djv374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ku L, Bysshe T, Steinmetz E, Bruen BK. Health reform, medicaid expansions, and women’s cancer screening. Women’s Health Issues. 2016;26(3):256–61. [DOI] [PubMed] [Google Scholar]
- 107.Ward E, Halpem M, Schrag N, Cokkinides V, DeSantis C, Bandi P, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58(1):9–31. [DOI] [PubMed] [Google Scholar]
- 108.Battaglia TA, Bak SM, Heeren T, et al. Boston patient navigation research program: the impact of navigation on time to diagnostic resolution after abnormal cancer screening. AACR; 2012; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Phillips CE, Rothstein JD, Beaver K, Sherman BJ, Freund KM, Battaglia TA. Patient navigation to increase mammography screening among inner city women. J Gen Intern Med. 2011;26(2):123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


