Abstract
47,XYY syndrome (XYY) is one of the common forms of sex chromosome aneuploidy in males. XYY males tend to have tall stature, early speech, motor delays, social and behavioral challenges, and a high rate of language impairment. Recent studies indicate that 20-40% of males with XYY meet diagnostic criteria for autism spectrum disorder (rate in the general population is 1-2%). Although many studies have examined the neural correlates of language impairment in autism spectrum disorder (ASD), few similar studies have been conducted in individuals with XYY syndrome. Studies using magnetoencephalography (MEG) in idiopathic ASD have demonstrated delayed neurophysiological responses to changes in the auditory stream revealed in the mismatch negativity (MMN) or its magnetic counterpart the mismatch field (MMF). This study investigated whether similar findings are observed in XYY-associated ASD and whether delayed processing is also present in individuals with XYY without ASD. MEG measured MMFs arising from left and right superior temporal gyrus during an auditory oddball paradigm with vowel stimuli (/a/ and /u/) in children/adolescents with XYY both with and without a diagnosis of ASD, as well as idiopathic ASD (ASD-I) and typically developing (TD) controls. Ninety male participants (6 to 17 years) were included in the final analyses (TD, n = 38, 11.50 ± 2.88 years; ASD-I; n = 21, 13.83 ± 3.25 years; XYY without ASD, n = 15, 12.65 ± 3.91 years; XYY with ASD, n = 16, 12.62 ± 3.19 years). Groups did not differ on age (p > .05). There was a main effect of group on MMF latency (p < .001). Delayed MMF latencies were found in participants with 47, XYY syndrome both with and without an ASD diagnosis, as well as in the ASD-I group compared to the TD group (ps < .001). Furthermore, participants with 47, XYY (with and without ASD) showed a longer MMF latency compared to the ASD-I group (ps < .001). There was, however, no significant difference in MMF latency between individuals with 47, XYY with versus without ASD. Delayed MMF latencies were associated with severity of language impairment. Findings suggest that auditory MMF latency delays are pronounced in this specific Y chromosome aneuploidy disorder, both with and without ASD diagnosis and thus may implicate the genes of the Y chromosome in mediating atypical MMF activity.
Keywords: XYY syndrome, autism spectrum disorder, magnetoencephalography, vowel mismatch fields, language impairment
Introduction
47, XYY syndrome (hereafter, XYY) is one of the common sex chromosome aneuploidy (SCA) / sex chromosome trisomies (SCTs) and occurs in 1 in 1000 males [1–3]. The cognitive phenotype in boys with XYY typically includes normal to mildly diminished general intelligence [4,5], with verbal IQ commonly more impaired than performance IQ [6–8]. Most boys with XYY are diagnosed postnatally in the first decade of life because of developmental delays, behavioral issues, tall stature [5], autistic behaviors [9,10] and language impairments [11–14].
In a neuroanatomical study in boys with XYY syndrome, Bryant et al. [15] assessed global and regional brain matter variations associated with XYY syndrome. The authors suggested that the genetic variations associated with XYY syndrome were associated with increased brain volume, a finding putatively related to the increased frequency of Autism Spectrum Disorder (ASD) in individuals with XYY, with rates of ASD in XYY of up to 20–40% [8,9,13,14].
Brain functional studies (i.e., electroencephalography; EEG, magnetoencephalography; MEG) have reported abnormal auditory language discrimination processing (i.e., delayed latency) in ASD [16,17,18], assessed by the mismatch negativity (MMN) potential or its magnetic counterpart, the mismatch field (MMF). Such findings have been reported in infancy, childhood and adults with ASD [17,19], therefore suggesting the persistence of the processing deficit. The MMN/MMF reflects the ability of an individual to detect changes in the auditory stream and thus is thought to index neural discrimination processing [16–24]. The ability to detect an auditory change (e.g., pitch, frequency, and syllable) is an important neural processing feature and compromise thereof likely contributes to downstream language and communication skills in individuals with developmental disorders such as ASD [16,17].
In an early MMN/MMF ASD study, Tecchio et al. [22] reported that moderately to severely impaired verbal individuals with ASD (8 to 32 years) demonstrated a weak or absent MMF response compared with TD (tone burst oddball paradigm). They suggested that impaired auditory discrimination ability in preconscious cortical processing stages may hinder the development of more complex connections in ASD. Oram Cardy et al. [18] reported MMF elicited by changes in streams of vowels or spectrally matched tones in children and adolescents with ASD and TD (8 to 17 years) to explore whether impaired sound discrimination may contribute to language impairments in ASD. Although the MMF was observed in both groups, the children with ASD demonstrated a delayed MMF latency compared with TD. The authors suggested that difficulty parsing transient differences in sounds may lead to impaired acoustic or phonological representations and subsequent language impairment in ASD. Berman et al. [23] showed an association between MMF latency delay and language impairment in children with ASD (8 to 12 years) as well as a corresponding relationship with microstructural diffusion tensor MRI of the arcuate fasciculus, supporting a white-matter connectivity disruption hypothesis for the biological basis of MMF delays. While these studies discuss the latency of the MMF component in ASD, a recent study by Matsuzaki et al. [19] reported atypical hemispheric bias in MMF amplitude (tending rightward in adults with ASD, compared to leftward in typically developing controls). The functional significance of this asymmetry is yet to be determined. Although many MMF studies have been conducted on ASD populations [21, 22], investigations of the neural correlates of auditory and language processing in children and adolescents with XYY are very limited [25].
To contribute to the knowledge of the neurophysiological mechanisms of auditory language discrimination processes in children and adolescents with XYY, MEG was used to measure cortical responses to an auditory oddball paradigm with vowel stimuli (/a/ and /u/) identical to those used in Roberts et al. [16], and compared with age-matched children with idiopathic (no known genetic etiology) ASD (ASD-I) as well as with TD. Hypotheses were: 1) MMF latencies arising from superior temporal gyrus (STG) would be delayed in children and adolescents with XYY syndrome with and without ASD compared to idiopathic ASD (ASD-I) and TD, 2) MMF latency delays may be exacerbated in participants with XYY syndrome with ASD compared to XYY without ASD, 3) Delayed MMFs latencies would be associated with language ability, and 4) atypical rightward lateralization of MMF amplitudes would be evidenced in both children and adolescents within ASD-I and both XYY groups.
Materials and Methods
Participants
Recruitment and Inclusion / Exclusion Criteria
Males with karyotypic diagnosis of non-mosaic XYY syndrome (6-17years) were recruited through the eXtraordinarY Kids Clinics (for children with sex chromosome variations), at Nemours/Alfred I. duPont Hospital for Children. Age- and gender-matched (male) individuals with ASD as well as typically developing (TD) controls were recruited from the Children’s Hospital of Philadelphia (CHOP), via CHOP’s Recruitment Enhancement Core, which enables recruitment of children seen anywhere within the CHOP care system (e.g., primary and specialty care centers and specialty practices). XY status (in either ASD-I or TD cohorts) was not confirmed by genetic testing; with a 1/1000 XYY prevalence, the expected number of XYY subjects inadvertently included in our XY sub-cohort totals of 59 is 0.06 and thus considered unlikely. Participants in the TD group had no current or past history of DSM-5 Axis I disorders according to parent report during initial screening and had no symptoms of ASD (i.e. were below diagnostic cut-offs), as measured by direct observation on the Autism Diagnostic Observation Schedule 2nd edition [ADOS-2; 26], Social Communication Questionnaire [SCQ; 27] and Social Responsiveness Scale-2 [SRS-2; 28]. Children in the ASD-I group had a prior diagnosis of ASD, made by autism specialists at CHOP or other regional hospitals and practices, according to DSM-5 criteria and with utilization of autism diagnostic tools. The current diagnostic battery confirmed the original diagnosis in the ASD-I group via the ADOS-2, SCQ, and SRS-2. Given the complexity of establishing ASD diagnosis in the XYY cohort, diagnosis in the XYY+ASD group was established by expert clinical consensus of two clinical psychologists (JM and LB) who reviewed all available diagnostic information and case conferenced each participant to arrive at a consensus diagnosis. Dimensional symptom severity indices were obtained from the ADOS-2 Calibrated Severity Score metric [29] and from the SRS-2 [28]. A measure of full scale IQ (FSIQ) was obtained for all participants. The specific measure of FSIQ utilized varied depending on group and included tboth he DAS-II [30] and WISC-V [31]. Language function was assessed with the Clinical Evaluation of Language Fundamentals-5th Edition [CELF-5; 32]. The following inclusion/exclusion criteria were used for all participants: 1) no history of traumatic brain injury or other significant medical or neurological abnormality, or other genetic syndrome, 2) no active psychosis, 3) no MRI contraindications, 4) no significant sensory impairments (e.g., vision or hearing impairment), 5) English as a first language, and 6) no known drug or alcohol use prior to any study procedure. Additionally, all participants were required to score at or above the 2nd percentile (SS > 70) on an index of nonverbal IQ via the WISC-V or DAS -II. Nine children were prescribed stimulant medications at the time of participation. Data from these participants did not show evidence of forming an outlier cluster in terms of MMF responses and so these data were retained. Data from some TD control participants (n =18) have in part been previously submitted for publication and are included herein to provide context for the 47, XYY responses.
Auditory stimuli
The auditory vowel stimuli (/a/ and /u/) were presented using Eprime v1.1 experimental software (Psychology Software Tools Inc., Pittsburgh, PA). Auditory stimuli were delivered via a sound pressure transducer and sound conduction tubing to the subject’s peripheral auditory canal via eartip inserts (ER3A, Etymotic Research, Illinois) at 45dB sensation level. 300 ms stimuli (vowels /a/ and /u/) were played with each token as the standard (85%) or deviant (15%) stimulus and with a stimulus onset asynchrony (SOA) of 700ms [16]. Two runs with the vowels alternating as standard/deviant were conducted to allow matched token subtraction (i.e., deviant /a/-standard /a/ and deviant /u/–standard /u/).
MEG recording
MEG data were recorded in a magnetically shielded room (MSR) using a 275-channel whole-cortex CTF magnetometer (CTF MEG, Coquitlam, BC, Canada). At the start of the session, three head-position indicator coils were attached to the scalp to provide continuous specification of the position and orientation of the head relative to the MEG sensor array [16]. Foam wedges were inserted inside of the MEG dewar by the side of each subject’s head to increase subject comfort and ensure that the head remained in the same location within the dewar across recording sessions. To minimize fatigue and encourage an awake state, subjects viewed (but did not listen to) a movie projected on to a screen positioned at a comfortable viewing distance. To identify eye-blink activity, the electro-oculogram (EOG, bipolar oblique, upper right and lower left sites) was collected. Electrodes were also attached to the left and right collarbone for electrocardiogram (ECG) recording.
Data analysis
Analyses were performed blind to participant group. As previously described [19], after a band-pass filter (0.03 - 150 Hz), EOG, ECG, and MEG signals were downsampled to 500 Hz. Synthetic third order gradiometer environmental noise reduction was implemented for the MEG data. Epochs of 600ms duration (100 msec pre-stimulus to 500 msec post-stimulus) were defined from the continuous recording. To remove eye-blink related artifact, a typical eye blink was manually identified in the raw data (including EOG) for each participant. The pattern search function in BESA Research 6.1 (BESA GmbH, Germany) was then used to scan the raw data to identify other similar blink events and to compute an eye-blink average. An eye blink was modeled by its first component topography from principal component analysis (PCA), typically accounting for more than 99% of the variance in the eye-blink average. In addition to eye-blink activity, a heartbeat average was obtained and activity was modeled by the first two PCA component topographies of a heartbeat average, typically accounting for more than 85% of the variance in the heartbeat average. Reviewing eye blink and heartbeat-corrected raw data, epochs with artifacts other than blinks and heartbeat were rejected by amplitude and gradient criteria (amplitude > 300 fT, gradients > 25 fT/cm).
Using all 275 channels of MEG data, determination of the latency and amplitude of sources of the auditory evoked field in bilateral STG was accomplished by applying a standard source model which transformed each individual’s raw MEG recordings into brain space (MEG data coregistered to the Montreal Neurologic Institute (MNI-152) averaged brain) using a model with multiple sources [33–35]. Specifically, the standard source model applied to each subject was constructed by including (1) bilateral STG dipole sources (placed at Heschl’s gyrus), and (2) nine fixed regional sources that modeled brain background activity and served as probe sources for additional electrophysiological activity. The eye-blink and heartbeat source vectors derived for each participant were also included in each participant’s source model to account for eye-blink and heartbeat activity [36,37]. The final source model served as a montage for the raw MEG data [38,39]. This spatial filter thus disentangled the source activities of the different brain regions that had overlapped at the sensor level. For the source analysis, a 1 Hz (12 dB/octave, zero phase) to 55 Hz (48 dB/octave, zero-phase) band-pass filter and powerline notch filter at 60 Hz (width 5Hz) were applied.
MMF peaks were defined from the difference wave obtained by subtraction of the response to the standard token from the response deviant for each token, with the MMF peak identified as the first peak following the residual 100 ms response in the subtracted waveform (and occurring ~150-350 ms post stimulus onset). The difference wave was obtained separately for standard and deviant occurrences of each token from source-space waveforms. MMF responses were thus defined for each hemisphere and each token separately. To evaluate hemispheric laterality; laterality indices (LIs) were computed for each token using the formula,
where LH and RH represent the amplitude of the MMF in the left and right hemispheres, respectively.
Statistics
Potential effects of group (TD, ASD-I, XYY-ASD, XYY+ASD) on age and neuropsychological assessments were evaluated with analysis of variance. Potential effects of group (TD, ASD-I, XYY-ASD, XYY+ASD), hemisphere (LH, RH) and token (/a/ and /u/) on MMF latency and amplitude were evaluated with full factorial linear mixed models (LMMs) using these factors as fixed effects and age as a covariate with subject as a random effect. LIs of MMF amplitudes were assessed using a linear mixed model with fixed effects of group and stimulus token, with age as a covariate and with subject as a random effect. Hierarchical regression assessed the influence of language and general cognitive ability above and beyond effects of age, hemisphere and token on MMF latency and amplitude. For the LIs, hierarchical regression also assessed the influence of language and cognitive ability above and beyond effects of age and token. Bonferroni correction was applied for multiple comparisons. All statistical analyses were performed with SPSS Statistics Version 25 (IBM, Armonk, USA).
Results
Demographics
Twenty-three participants who did not meet neuropsychological assessment criteria for inclusion, or who did not complete all measurements or who did not have analyzable MEG were excluded from analysis. As shown in Table 1, ninety male participants (aged 6 to 17 years) remained and were included in the final analyses (TD: n = 38, 11.50 ± 2.88 years; ASD - I: n = 21, 13.83 ± 3.25 years; XYY without ASD “XYY-ASD”: n = 15, 12.65 ± 3.91 years; XYY with ASD, “XYY+ASD”: n = 16, 12.62 ± 3.19 years). Groups did not differ on age (p > .05). There were statistically significant main effects of group on SCQ [F (3,83) = 50.81, p < . 001], SRS-2 [F (3,84) =48.91, p < . 001], CELF core language score [F (3, 81) = 16.29, p <.001] and GAI / FSIQ [F (3, 83) = 15.07, p < .001). Post-hoc tests revealed differences between TD and all groups, as well as between ASD-I and both XYY groups on SCQ (ps < .05). There were group differences between TD versus the other three groups on SRS-2 (ps < .05). There were differences between TD and both XYY groups as well as between ASD-I and both XYY groups on FSIQ (ps < .05). There were differences between TD and both XYY groups as well as between ASD-I and XYY+ASD (but not XYY-ASD) on CELF core language index (ps < .05). There were no differences between XYY-ASD and XYY+ASD on SCQ, SRS-2, FSIQ or CELF.
Table 1.
Characteristics of study participants.
| TD (Mean ± SD) | ASD-I (Mean ± SD) | XYY-ASD (Mean ± SD) | XYY+ASD (Mean ± SD) | |
|---|---|---|---|---|
| Number of participants | 38 | 21 | 15 | 16 |
| Handedness (R:L:A) | 31:7:0 | 21:0:0 | 15:0:0 | 13:2:1 |
| Age | 11.50 ± 2.88 | 13.83 ± 3.25 | 12.65 ± 3.91 | 12.62 ± 3.19 |
| SCQ | 2.36±2.40 | 18.20±5.97 | 9.87±6.37 | 13.56±5.87 |
| SRS | 41.51±7.85 | 73.10±11.45 | 70.47±16.80 | 76.31±16.16 |
| GAI / Estimated FSIQ / GCA | 113.58±14.41 | 106.50±17.48 | 92.00±11.03 | 87.00±16.10 |
| CELF Core language index | 107.46±11.78 | 97.95±17.47 | 90.20±15.58 | 78.07±13.40 |
TD: typically developing children. ASD: autism spectrum disorder. ASD-I: idiopathic-ASD. XYY-ASD: XYY syndrome without ASD. XYY+ASD: XYY syndrome with ASD
SCQ: Social Communication Questionnaire.
SRS: Social Responsives Scale.
GAI/Estimated FSIQ/GCA: General Ability Index / Estimated Full Scale Intellectual Quotient/General Conceptual Ability score. [GAI and Estimated FSIQ (Wechsler Intelligence Scale – fourth edition; WISC-IV) and fifth edition (WISC-V). GCA (Differential Ability Scale - II; DAS-II)]. CELF core language index: Clinical Evaluation of Language Fundamentals – fourth and fifth edition.
MMFs latencies and amplitude
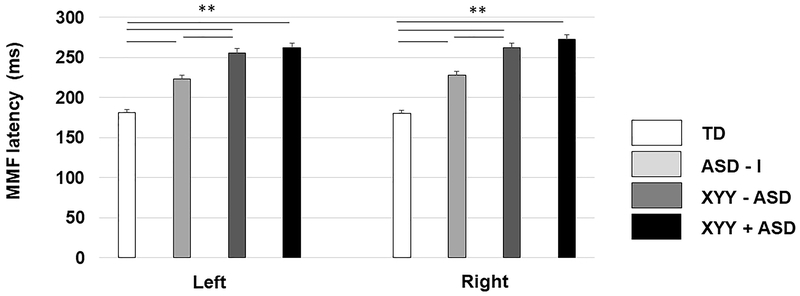
Example MMF waveforms were shown in Figure 1. A linear mixed model (LMM) with fixed effects of group, hemisphere and token and age (and with subject as a random effect) showed a significant effect of group on MMF latency [TD = 180.75±3.57ms; ASD - I = 225.62±4.83ms; XYY - ASD = 258.82±5.84ms; XYY + ASD = 267.80±5.24ms; F (3,76) = 82.85, p < .001], hemisphere [LH: 230.61±2.58 ms; RH: 235.73±2.58ms; F (1,228) = 11.12, p < .001] and a small effect of token [/a/: 231.59±2.58ms; /u/: 234.76±2.58 ms; F (1,228) = 4.24, p < .05] and no interactions [p’s > .05]. Delayed MMF latencies were found in both hemispheres in participants with XYY with and without ASD, as well as in the ASD - I group compared to the TD group (p’s < .001, Figure 2). Delays in the XYY (with and without ASD) group were pronounced compared to ASD - I group (p < .001). However, there were no group differences between individuals with XYY with and without ASD (p > 0.05). There was no significant effect of age and no age x group interactions.
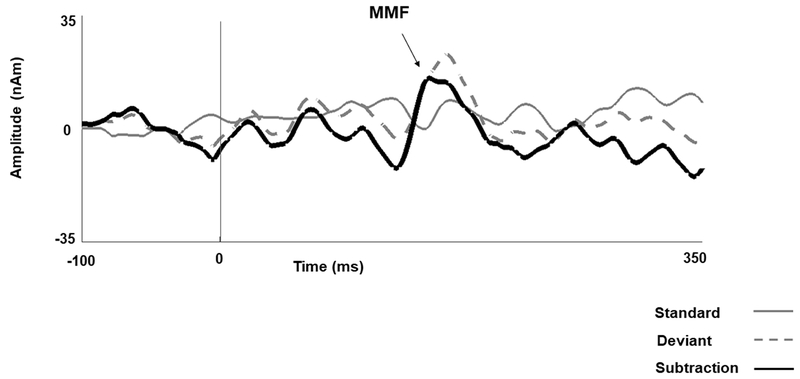
Figure 1.
Source response waveforms from superior temporal gyrus. Vertical lines on the waveform indicate stimulus onset (zero ms). Arrows indicate MMF latency in a representative TD child (168 ms). The solid gray line indicates the standard response, the dashed gray line indicates the deviant response and the solid black line indicates their subtraction to yield the difference wave.
Figure 2.
Estimated marginal mean MMF latencies by group and hemisphere. Error bars represent one standard error of the marginal means. There was a significant main effect of group on MMF latency (p < .0001), which was evidenced in both hemispheres. Post-hoc t-tests revealed significant differences between pairwise comparisons of all groups (except XYY+ASD vs XYY-ASD), in both hemispheres (all p’s<0.001), with XYY+ASD and XYY-ASD>ASD-I > TD.
Hierarchical regression was used to investigate the association between MMF latency and language ability across the whole cohort: when entered first (after age, hemisphere and token had accounted for R2 = 0.05), FSIQ accounted for significant variance (ΔR2 =0.24, p<0.001), and CELF core language score accounted for additional significant variance (ΔR2 =0.07, p<0.001) in MMF latency. When the order was reversed, CELF score (ΔR2 = 0.30, p<0.001) and FSIQ (ΔR2 = 0.017, p<0.01) again both accounted for significant variance in MMF latency suggesting unique contributions from both FSIQ and CELF to MMF latency, with FSIQ and CELF themselves correlated (R = 0.74, p <0.001).
A linear mixed model (LMM) with fixed effects of group, hemisphere and token and age (and with subject as a random effect) showed a significant effect of group on MMF amplitudes [TD = 13.05±2.03 nAm; ASD-I = 19.44±2.59 nAm; XYY - ASD = 8.34±3.35 nAm; XYY+ASD = 10.14±2.93 nAm F (3,72) = 2.99, p < .05],with no effect of hemisphere [LH: 13.40±1.61 nAm; RH: 12.08±1.61 nAm; F (1,216) =0.63, p > .05] or token [/a/: 13.62 ± 1.61 nAm ; /u/: 11.87±1.61 nAm ; F (1,216) = 0.29, p > .05] and no interactions [ps> .05]. ASD-I tended to show an increased MMF amplitude compared to TD, but there were no group differences with post-hoc t-tests (p > .05). There are no associations between MMF amplitude, FSIQ and CELF core language score.
Laterality Indices
A linear mixed model (LMM) with fixed effects of group and token and age (and with subject as a random effect) showed a significant effect of group on LIs [TD = + 0.06±0.04; ASD-I = −0.09±0.06; XYY-ASD = −0.23±0.07; XYY+ASD = −0.15±0.06 F (3,144) = 3.96, p < .01],with no effect on token [/a/: −0.12±0.04; /u/: −0.06 ± 0.04; F (1,144) = 0.81, p > .05] and no interactions [p> .05]. While TD showed leftward lateralization, ASD-I and both XYY groups showed atypical rightward lateralization. Post- hoc tests revealed group differences between TD vs both XYY groups (TD vs XYY- ASD; p < .05, TD vs XYY+ASD; p = 0.059). Differences between TD and ASD-I did not reach significance (p> 0.05). Regarding association between LIs and FSIQ/CELF, when entered first (after age and token had accounted for R2 = 0.09), FSIQ (ΔR2 = 0.026, p < 0.05) and then (when entered second) CELF core language score (ΔR2 = 0.023, p<0.05) both accounted for significant variance in LI. When the order was reversed, whereas CELF did not account for significant variance (ΔR2 = 0.000, p > 0.05), when entered next FSIQ accounted for significant variance in LI (ΔR2 = 0.05, p < 0.01), suggesting the laterality index is sensitive to general cognitive ability and not language ability in this cohort.
Discussion
The main findings of this study were: 1) delayed MMF latencies in XYY children with and without ASD compared with children with ASD or TD, 2) no differences in the extent of the delay in XYY+ASD versus XYY-ASD, 3) delayed latencies were associated with poorer performance on language and IQ tests, and 4) a lack of neurotypical left-hemisphere lateralization of MMF amplitude in XYY children (with and without ASD).
Previous studies have reported prolonged MMF latencies in higher-functioning children and adults with ASD [16, 19, 40]. For example, using a similar oddball paradigm with vowel stimuli, Roberts et al. [16] reported that auditory MMF abnormalities are frequently observed in children with ASD age 6-15 years, and that MMF abnormalities may have sequelae in terms of clinical language impairment. In particular, MMF delays were most pronounced (~50 msec) in children with ASD with concomitant language impairments, suggesting a neurobiological basis as well as a clinical biomarker for language impairment in ASD. In an adult ASD cohort using a similar auditory vowel MMF task, Matsuzaki et al. [19] found that the MMF was delayed in adults with ASD compared to the TD participants (~15 ms). Furthermore, whereas TD participants showed a leftward lateralization of MMF amplitude, participants with ASD showed a rightward lateralization. Findings suggest that adults with ASD have hemispherically- and temporally- abnormal auditory discrimination processing.
The present study found delayed MMF latency in ASD-I compared to TD children and adolescents (~45 ms), a finding consistent with previous ASD studies. Furthermore, individuals with XYY (with and without a diagnosis of ASD) showed a delayed MMF latency even compared to ASD-I (~30-40ms). Previous studies have suggested that MMF / mismatch negativity (MMN) latency decreases as individuals mature [41, 42]. In this study, XYY participants had normal to mildly diminished general intelligence as well as language ability (XYY-ASD: FSIQ: 92.00 ± 11.03, CELF: 90.20 ± 15.58, XYY+ASD: FSIQ: 87.00 ± 16.10, CELF: 78.07 ± 13.40). We speculate that XYY children (with and without ASD) may have delayed white matter myelination and atypical connectivity for both bottom-up and top-down attentional/inhibitory control networks compared to the relatively higher-functioning children with ASD (ASD-I: FSIQ:106.50 ± 17.48, CELF: 97.95 ± 17.47) and TD.
Findings of atypical rightward MMF amplitude lateralization are consistent with MEG studies in children and adults with ASD [19, 43]. Hemisphere differences in MMF amplitude may be interpreted in light of differences in the developmental trajectories of each hemisphere, and in ASD the reversed lateralization possibly associated with dysfunction of interhemispheric inhibition and/or connectivity. Lin et al. [44] reported on individuals with sex chromosome aneuploidy which suggested that the X and Y chromosomes may have opposing effects on cortical development and cortical thickness asymmetry [45, 46]. Further, they suggested these patterns might be related to reported differences in cognitive profile and interhemispheric connectivity in sex differences [47]. Atypical laterality may also be considered in terms of the potential effects of early testosterone exposure as discussed by Geschwind et al. [48].
Interhemispheric inhibition is also dependent on the microstructure of the corpus callosum (CC) which connects homologous cortical areas of the two cerebral hemispheres. In several ASD studies, using diffusion-weighted MRI, decreased fractional anisotropy and increased mean diffusivity in white-matter tracts spanning the CC have been reported [49, 50], suggesting impaired interhemispheric connectivity. Whereas in the present study the children and adolescents with XYY syndrome did not show differences in laterality compared to the ASD-I group, the abnormalities relative to TD may be related to both autistic features and/or cognitive ability. Further studies are needed to examine associations between laterality index and fractional anisotropy and mean diffusivity in white-matter tracts spanning the CC in XYY and ASD cohorts.
Additionally, previous MEG research has also revealed abnormal gamma-band oscillatory activity during task-evoked auditory processing in ASD [51–54]. Wilson et al. (2007) examined integrity of local circuitry by focusing on gamma band activity of auditory magnetic steady-state responses (SSR) and the study reported the production and/or maintenance of left hemispheric gamma oscillations appeared abnormal in children and adolescents with ASD, suggesting a lack of local inhibitory interneurons may be the neural mechanism underlying this impairment due to the known role of such cells in generating high-frequency activity [51]. Edgar et al. (2015) assessed activity in STG auditory areas in children with ASD and reported decreased early evoked gamma activity and inter - trial coherence in children with ASD [53]. Port et al. (2016), reported perturbed auditory cortex neural activity during sinusoidal tones, as evident by M100 latency delays as well as reduced transient gamma-band activity in children with ASD [54].
It has been proposed that ASD may be associated, at least in a subset of the population, with imbalances in excitatory and inhibitory neurotransmission (E/I imbalance) [55]; gamma-band activity is thought to be one neural process related to E/I balance, with support coming from observed correlations between gamma and underlying GABA concentrations (e.g. [56]). This hypothesis would be attractive in that it implicates a biochemical target for intervention, and it might represent an objective marker of ASD. It has thus motivated concomitant investigation of GABA, primarily using magnetic resonance spectroscopy (MRS), as a part of a multimodal characterization of ASD, work that could readily be extended to 47, XYY. In general, it is of clear and immediate interest to observe whether the range of electrophysiological (and neurochemical) anomalies that have been reported in children and adolescents with ASD (including both evoked responses and oscillatory activity) are recapitulated in 47,XYY syndrome (as is shown for the MMF findings reported in the present study).
Finally, the observation of delayed MMF in XYY with ASD and XYY without ASD may point to a role of the genes of the Y chromosome in mediating brain responses. It is of note that in the general population the prevalence of ASD is reported as being 4x greater in males than in females (i.e. approx. 2.5%) [57]. The rate of ASD in XYY is reported as being as high as 20-40% [8,9,13,14]., and those individuals with XYY who do not meet strict diagnostic criteria for ASD may still manifest behavioral and clinical symptoms associate with ASD [9,10] (note the elevated SRS scores and diminished FSIQ and language function in this cohort). If we interpret the MMF delay as an indicator of diminished capacity in general cognitive ability or domain-specific language ability, it is not surprising that the XYY-ASD cohort exhibited a delayed MMF response (consistent with their clinical/behavioral phenotype) despite not meeting diagnostic criteria for ASD per se.
A potential study limitation is that there were no individuals recruited with other SCA/SCT conditions (i.e., XXY / Klinefelter syndrome, and XXYY). Further, precise relation to the literature findings in ASD-I is tempered by our inclusion of only male subjects (to gender match to the XYY cohort), while many other studies in ASD-I include at least some female participants. Also, the apparent association between MMF latency and language ability could be consistent with theories that auditory language discrimination and acquisition of vowel stimuli are related to either basic sensory functions or higher-level language processes. To examine the dependencies between lower-order sensory perception and higher-order linguistics skills, other tasks, using word, pseudoword or sentence listening may provide more detailed information regarding MMF and language impairment. Additionally, we did not assess other modalities (for example, cortical myelin content, diffusion tensor imaging and GABA magnetic resonance spectroscopy) and so cannot address structural or neurochemical underpinnings of the observed MMF response delays.
Conclusion
This study demonstrated delayed MMF in children and adolescents with XYY syndrome and showed that delayed MMF responses were associated with greater language impairment and lower IQ. Although MMF latencies were also delayed in ASD-I, MMF latency delays were more profound in XYY. There was no additional delay in XYY individuals who met diagnostic criteria for ASD versus those who did not, perhaps suggesting a XYY “ceiling effect”. Findings support a neurophysiological signature of language impairment in XYY similar to that seen in ASD, and thus provides some shared evidence for considering XYY as a human genetic model of at least a subset of ASD.
Acknowledgements:
Dr Roberts and authors gratefully acknowledge all participants, their families and the Oberkircher family for the Oberkircher Family Chair in Pediatric Radiology. Excellent assistance was provided by John Dell, Rachel Golembski, Peter Lam and MEG lab team, Department of Radiology at CHOP.
Funding: This study was supported in part by NIH_R21MH109158 (TR), NIH R01-DC008871 (TR), R01-HD073258 (DE), U54-HD086984 (TR) as well as a grant from the Department of Defense AR140927 (TR).
Footnotes
Statement of Ethics: The study was approved by the Children’s Hospital of Philadelphia Internal Review Board, and by the Human Studies Committee at Nemours DuPont Hospital for Children. Written informed consent and assent (when age-appropriate) was obtained from all participating families, in accordance with the principles of the Declaration of Helsinki.
Disclosure Statement: Dr Roberts declares consulting/advisory board relationships with Prism Clinical Imaging, CTF, Ricoh and Spago Nanomedical and Avexis. Additionally, he and Dr Edgar disclose intellectual property related to MEG as a biomarker for pharmaceutical therapy, under licensing negotiation.
References
- 1.Jacobs PA, Melville M, Ratcliffe S, Keay AJ, Syme J. A cytogenetic survey of 11,680 newborn infants. Ann Hum Genet. 1974. 37(4): 359–76. DOI: 10.1111/j.1469-1809.1974.tb01843.x. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen J, Sillesen I. Incidence of chromosome aberrations among 11 148 newborn children. Humangenetik. 1975. 30(1):1–12. DOI: 10.1007/BF00273626. [DOI] [PubMed] [Google Scholar]
- 3.Leggett V, Jacobs P, Nation K, Scerif G, Bishop DVM Neurocognitive outcomes of individuals with a sex chromosome trisomy: XXX, XYY, or XXY: A systematic review. Dev Med Child Neurol. 2010. DOI: 10.1111/j.1469-8749.2009.03545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender BG, Puck MH, Salbenblatt JM, Robinson A The developmental of four unselected 47, XYY boys. Clin Genet. 1984. 25(5):435–45. DOI: 10.1111/j.1399-0004.1984.tb02013.x. [DOI] [PubMed] [Google Scholar]
- 5.Robinson A, Bender BG, Puck MH, Salbenblatt JA. Growth and Development of Children With a 47,XYY Karyotype. New York, NY: Alan R. Liss, Inc; 1985. [Google Scholar]
- 6.Christensen AL, & Nielsen J Psychological studies of ten patients with the XYY syndrome. Br J Psychiatry. 1973. 123(573):219–21. DOI: 10.1192/bjp.123.2.219. [DOI] [PubMed] [Google Scholar]
- 7.Ratcliffe SG and Field MAS Emotional disorder in XYY children: Four case reports. J. Child Psychol. Psychiatry. 1982. 23(4):401-4-6 DOI : 10.1111/j.1469-7610.1982.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 8.Ross JL, Roeltgen DP, Kushner H, Zinn AR, Reiss A, Bardsley MZ, et al. Behavioral and Social Phenotypes in Boys With 47,XYY Syndrome or 47,XXY Klinefelter Syndrome. PEDIATRICS. 2012. 129(4):769–78. DOI: 10.1542/peds.2011-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bishop DV, Jacobs PA, Lachlan K, Wellesley D, Barnicoat A, Boyd PA, et al. Autism, language and communication in children with sex chromosome trisomies. Arch Dis Child. 2011. 96(10):954–959. DOI: 10.1136/adc.2009.179747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition. American Psychiatric Association. Washington, DC: American Psychiatric Publishing, Inc; 947 2013. [Google Scholar]
- 11.Lehrke RG Birth defects original article series. Ann Intern Med. 1971;74(1):157 DOI: 10.7326/0003-4819-74-1-157_8 [DOI] [PubMed] [Google Scholar]
- 12.Merhar SL, Manning-Courtney P Two boys with 47, XXY and autism. J. Autism Dev. Disord. 200737(5):840–6. DOI: 10.1007/s10803-006-0211-1. [DOI] [PubMed] [Google Scholar]
- 13.Tartaglia NR, Wilson R, Miller JS, Rafalko J, Cordeiro L, Davis S, et al. Autism Spectrum Disorder in Males with Sex Chromosome Aneuploidy: XXY/Klinefelter Syndrome, XYY, and XXYY. J Dev Behav Pediatr. 2017. 38(3):197–207. DOI: 10.1097/DBP.0000000000000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Rijn S A review of neurocognitive functioning and risk for psychopathology in sex chromosome trisomy (47,XXY, 47,XXX, 47, XYY). Curr Opin Psychiatry. 2019. 32 (2) 79–84. DOI: 10.1097/YCO.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bryant DM, Oeft FH, Lai S, Lackey J, Roeltgen D, Ross J, et al. Sex chromosomes and the brain: a study of neuroanatomy in XYY syndrome. Dev Med Child Neurol. 2012. 54: 1149–1156. DOI: 10.1111/j.1469-8749.2012.04418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts TPL, Cannon KM, Tavabi K, Blaskey L, Khan SY, Monroe JF, et al. Auditory magnetic mismatch field latency: A biomarker for language impairment in autism. Biol Psychiatry. 2011. 70(3):263–9. DOI: 10.1016/j.biopsych.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz S, Shinn-Cunningham B, Tager-Flusberg H Meta-analysis and systematic review of the literature characterizing auditory mismatch negativity in individuals with autism. Neurosci. Biobehav. Rev. 2018 87: 106–117. 10.1016/j.neubiorev.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oram Cardy JE, Flagg EJ, Roberts W, & Roberts TPL Delayed mismatch field for speech and non-speech sounds in children with autism. Neuroreport. 2005. 16(5):521–5. DOI: 10.1097/00001756-200504040-00021. [DOI] [PubMed] [Google Scholar]
- 19.Matsuzaki J, Ku M, Berman JI, Blaskey L, Bloy L, Chen YH, et al. Abnormal auditory mismatch fields in adults with autism spectrum disorder. Neurosci Lett. 2019 698:140–145. DOI: 10.1016/j.neulet.2018.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Näätänen R, Gaillard AWK, Mantysalo S, Gaillard WK Early selective-attention effect on evoked potential reinterpreted. Acta Psychol. 198742(4):313–329. DOI: 10.1016/0001-6918(78)90006-9. [DOI] [PubMed] [Google Scholar]
- 21.Näätänen R, Paavilainen P, Rinne T, Alho K The mismatch negativity (MMN) in basic research of central auditory processing: A review. Clin Neurophysiol. 2007. 118(12):2544–90. DOI: 10.1016/j.clinph.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 22.Tecchio F, Benassi F, Zappasodi F, Gialloreti LE, Palermo M, Seri S, et al. Auditory sensory processing in autism: A magnetoencephalographic study. Biol Psychiatry. 2003. 54(6):647–54. DOI: 10.1016/S0006-3223(03)00295-6. [DOI] [PubMed] [Google Scholar]
- 23.Berman JI, Edgar JC, Blaskey L, Kuschner ES, Levy SE, Ku M, et al. Multimodal Diffusion-MRI and MEG Assessment of Auditory and Language System Development in Autism Spectrum Disorder. Front Neuroanat. 2016 10: 30 DOI: 10.3389/fnana.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshimura Y, Kikuchi M, Hayashi N, Hiraishi H, Hasegawa C, Takahashi T, et al. Altered human voice processing in the frontal cortex and a developmental language delay in 3- to 5-year-old children with autism spectrum disorder. Sci Rep. 2017. 7:17116, DOI: 10.1038/s41598-017-17058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bloy L, Ku M, Edger JC, Miller JS, Blaskey L, Ross J, et al. Auditory Evoked Response Delays in Children with 47,XYY syndrome. Neuroreport. 2019. 30(7):504–509. DOI: 10.1097/WNR.0000000000001233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop SL Autism Diagnostic Observation Schedule - 2nd ed., Torrance (CA): Western Psychological Services; 2012. [Google Scholar]
- 27.Rutter M, Bailey A, Lord C The social communication questionnaire: Manual, Los Angeles, CA, Western Psychological Services; 2003. [Google Scholar]
- 28.Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, et al. Validation of a Brief Quantitative Measure of Autistic Traits: Comparison of the Social Responsiveness Scale with the Autism Diagnostic Interview-Revised. J Autism Dev Disord. 2003. 33(4): 427–433. DOI: 10.1023/A:1025014929212. [DOI] [PubMed] [Google Scholar]
- 29.Gotham K, Pickles A, Lord C Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J Autism Dev Disord. 2009. 39(5):693–705. DOI: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elliott CD Differential Ability Scales-second edition. San Antonio, TX: Harcourt Assessment; 2007. [Google Scholar]
- 31.Wechsler D Wechsler Intelligence Scale for Children - Fifth Edition, Pearson, San Antonio, TX: 2014. [Google Scholar]
- 32.Wiig EH, Semel E, & Secord WA Clinical Evaluation of Language Fundamentals - Fifth Edition (CELF-5). Pearson, San Antonio, TX: 2013. [Google Scholar]
- 33.Scherg M, Von Cramon, D. A new interpretation of the generators of BAEP waves I-V: results of a spatio-temporal dipole model. Electroencephalogr Clin Neurophysiol. 1985. 62(4):290–9. DOI: 10.1016/0168-5597(85)90006-1. [DOI] [PubMed] [Google Scholar]
- 34.Scherg M Fundamentals of dipole source potential analysis. Adv audiol., (6th ed). Basel, Switzerland: Karger; 1990. pp. 40–69. [Google Scholar]
- 35.Scherg M, Berg P. New concepts of brain source imaging and localization. Electroencephalogr Clin Neurophysiol Suppl. 1996. 46: 127–137. [PubMed] [Google Scholar]
- 36.Lins OG, Picton TW, Berg P, Scherg M Ocular artifacts in recording EEGs and event-related potentials. II: source dipoles and source components. Brain Topogr. 1993. 6:65–78. DOI: 10.1007/BF01234128. [DOI] [PubMed] [Google Scholar]
- 37.Berg P, Scherg M A multiple source approach to the correction of eye artifacts. Electroencephalogr Clin Neurophysiol. 1994. 90:229–241. DOI: 10.1016/0013-4694(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 38.Scherg M, Ebersole JS Brain source imaging of focal and multifocal epileptiform EEG activity. Clin. Neurophysiol. 1994. 24:51–60. DOI: 10.1016/S0987-7053(05)80405-8. [DOI] [PubMed] [Google Scholar]
- 39.Scherg M, Ille N, Bornfleth H, Berg P Advanced tools for digital EEG review: virtual source montages, whole-head mapping, correlation, and phase analysis. J. Clin. Neurophysiol. 2002. 19: 91–112. DOI: 10.1097/00004691-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Roberts TPL, Heiken K, Kahn SY, Qasmieh S, Blaskey L, Solot C, et al. Delayed magnetic mismatch negativity field, but not auditory M100 response, in specific language impairment. Neuroreport.2012. 23(8): 463–468. DOI: 10.1097/WNR.0b013e32835202b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glass E, Sachse S, & Von Suchodoletz W Auditory sensory memory in 2-year-old children: An event-related potential study. Neuroreport. 2008. 19(5):569–73. DOI: 10.1097/WNR.0b013e3282f97867. [DOI] [PubMed] [Google Scholar]
- 42.Morr ML, Shafer VL, Kreuzer JA, Kurtzberg D (2002). Maturation of Mismatch Negativity in Typically Developing Infants and Preschool Children. Ear Hear, 23(2):118–136. DOI: 10.1097/00003446-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Flagg EJ, Cardy JEO, Roberts W, Roberts TPL Language lateralization development in children with autism: Insights from the late field magnetoencephalogram. Neurosci Lett. 2005 386: 82–87. DOI: 10.1016/j.neulet.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 44.Lin A, Clasen L, Lee NR, Wallace GL, Lalonde F, Blumenthal J,et al. Mapping the Stability of Human Brain Asymmetry across Five Sex-Chromosome Aneuploidies. J Neurosci. 2015. 35(1): 140–145. DOI: 10.1523/JNEUROSCI.3489-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herbert MR, Harris GJ, Adrien KT, Ziegler DA, Makris N, Kennedy DN, et al. Abnormal asymmetry in language association cortex in autism. Ann Neurol. 2002. 52:588–596, DOI: 10.1002/ana.10349. [DOI] [PubMed] [Google Scholar]
- 46.Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008. 28:3586–3594, DOI: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, et al. Sex differences in the structural connectome of the human brain. Proc. Natl. Acad. Sci. U.S.A. 2014. 111 (2) :823–828, DOI: 10.1073/pnas.1316909110, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geschwind N, Galaburda A,Cerebral M Lateralization Biological Mechanisms, Associations, and Pathology: I. A Hypothesis and a Program for Research. Arch Neurol. 1985. 42(5) :428–459. DOI: 10.1001/archneur.1985.04060050026008 [DOI] [PubMed] [Google Scholar]
- 49.Travers BG, Adluru N, Ennis C, Tromp DPM, Destiche D, Doran S, et al. Diffusion Tensor Imaging in Autism Spectrum Disorder: A Review. Autism Res, 2012. 5: 289–313. DOI: 10.1002/aur.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giuliano A, Saviozzi I, Brambilla P, Muratori F, Retico A, Calderoni S The effect of age, sex and clinical features on the volume of Corpus Callosum in pre-schoolers with Autism Spectrum Disorder: a case-control study. Eur J Neurosci. 2018. 47:568–578. DOI: 10.1111/ejn.13527. [DOI] [PubMed] [Google Scholar]
- 51.Wilson TW, Rojas DC, Reite ML, Teale PD, Rogers SJ Children and Adolescents with Autism Exhibit Reduced MEG Steady-State Gamma Responses. Biol Psychiatry.2007 62:192–197, DOI: 10.1016/j.biopsych.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rojas DC, Wilson LB Gamma-band abnormalities as markers of autism spectrum disorders. Biomark Med. 8:3, 2014. DOI: 10.2217/bmm.14.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edgar JC, Fisk Iv CL, Berman JI, Chudnovskaya D, Liu S, Pandey J, et al. , Auditory encoding abnormalities in children with autism spectrum disorder suggest delayed development of auditory cortex. Mol. Autism. 2015. 6(69), DOI: 10.1186/s13229-015-0065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Port RG, Edgar JC, Ku M, Bloy L, Murray R, Blaskey L et al. , Maturation of auditory neural processes in autism spectrum disorder - A longitudinal MEG study. Neuroimage: Clin. 2016 11:566–577, DOI: 10.1016/j.nicl.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rubenstein JL and Merzenich M EM Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes, Brain Behav. 2003. 2(5):255–67, DOI: 10.1034/j.1601-183X.2003.00037.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Port RG, Gaetz W, Bloy L, Wang D-J, Blaskey L, Kuschner E, et al. , Exploring the relationship between cortical GABA concentrations, auditory gamma‐band responses and development in ASD: Evidence for an altered maturational trajectory in ASD. Aut. Res. 2017. 10(4):593–607. DOI: 10.1002/aur.1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, et al. Recurrence Risk for Autism Spectrum Disorders: A Baby Siblings Research Consortium Study. Pediatrics. 2011. 128(3): e488–e459. DOI: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]