Abstract
Background:
In the past few years, the issue of ‘microdosing’ psychedelics has been openly discussed in the public arena where claims have been made about their positive effect on mood state and cognitive processes such as concentration. However, there are very few scientific studies that have specifically addressed this issue, and there is no agreed scientific consensus on what microdosing is.
Aim:
This critique paper is designed to address questions that need to be answered by future scientific studies and to offer guidelines for these studies.
Approach:
Owing to its proximity for a possible approval in clinical use and short-lasting pharmacokinetics, our focus is predominantly on psilocybin. Psilocybin is allegedly, next to lysergic acid diethylamide (LSD), one of the two most frequently used psychedelics to microdose. Where relevant and available, data for other psychedelic drugs are also mentioned.
Conclusion:
It is concluded that while most anecdotal reports focus on the positive experiences with microdosing, future research should also focus on potential risks of (multiple) administrations of a psychedelic in low doses. To that end, (pre)clinical studies including biological (e.g. heart rate, receptor turnover and occupancy) as well as cognitive (e.g. memory, attention) parameters have to be conducted and will shed light on the potential negative consequences microdosing could have.
Keywords: Psychedelics, microdosing, psychoactive substances
Background
Psychedelics are a class of psychoactive substances that induce complex behavioural, psychological and physiological effects primarily through activation of serotonin 5-HT2A receptors. In the past few years, the issue of ‘microdosing’ psychedelics has been openly discussed in the public arena with several books (Cruz, 2017; Kumar, 2016; Waldman, 2017) claiming value to the authors who tried this concept. However, there are very few scientific studies that have specifically addressed this issue, and there is no agreed scientific consensus on what microdosing entails (Cameron et al., 2019; Horsley et al., 2018). This paper is designed to address questions that need to be answered by future scientific studies and to offer guidelines for these studies. Although a number of classic psychedelics exist, two of them, lysergic acid diethylamide (LSD) and psilocybin, are allegedly most frequently used to microdose. The following review focuses predominantly on psilocybin due to its proximity for a possible approval in clinical use and short-lasting pharmacokinetics (Passie et al., 2002) in comparison with LSD (Dolder et al., 2017). However, where relevant and available, data for other psychedelic drugs are also mentioned.
As early as the 16th century, low doses of psilocybin, ‘teonanacatl’ or sacred mushroom, were used medically (Schultes, 1940). Bernardino de Sahagún, a Franciscan friar during the period of the Spanish conquest of the Americas (1519–1521), reported that, ‘teonanacatl were … medicinal for fevers and for rheumatism. Only two or three need to be eaten. Those who eat them see visions and feel a faintness of the heart. And they provoke lust to those who eat a number, or even a few, of them’. However, by 1640, 94% of the Aztec population was wiped out and alongside them, the traditions involving ‘teonanacatl’. Of note, the mentioning of visions here suggests this ancient ‘low-dose’ use does not refer to what is currently seen as microdosing, something that will be addressed below.
Psychedelic studies underwent a significant expansion following the discovery of the mind-altering properties of LSD by Albert Hofmann in 1943 (Hofmann, 1970). The subsequent growth of psychedelic use allegedly had a profound effect on innovation in science and technology. A popular example is that of Francis Crick, one of the co-discoverers of the double-helix structure of DNA, who used LSD, though this use was never confirmed nor denied by him (Roberts, 2008). Furthermore, Kary Mullis, who discovered a means to automate the polymerase chain reaction, claimed that the idea came to him after using LSD (Doyle, 2002). These discoveries greatly advanced the field of genetic research (Luke, 2006). In this atmosphere of innovation, Frederick Terman was appointed as Provost of Stanford, 1955–1965. During his tenure, Terman ‘set out to create a community of technical scholars in Silicon Valley’ (Leslie and Kargon, 1996). This community developed alongside the psychedelic capital of the world, San Francisco, and over time technology and psychedelics began to merge. By 2005, the founder of Apple and one of the most influential figures in Silicon Valley, Steve Jobs, highlighted that LSD had played a pivotal and transformative role in his life (Dormehl, 2012).
Although there was accumulating evidence to suggest that the intake of psychedelics led not only to hallucinations but also to an improvement of cognition and creativity, scientific progress in the field was prohibited by government agencies on account of the growing political concern over the recreational use of psychedelics (Belouin and Henningfield, 2018). Thus, the only study investigating psychedelics in problem solving was ended by the US Food and Drug administration (FDA) in 1966 (Harman et al., 1966). However, James Fadiman, a young researcher in this study, continued his research after the UN Convention on Psychotropic Substances of 1971 banned psychoactive substances and bundled his knowledge into a book, which now acts as a guide for those interested in microdosing. His book The Psychedelic Explorer’s Guide: Safe, Therapeutic, and Sacred Journeys (Fadiman, 2011) published in 2011, is often referred to as a protocol for those practising microdosing. Of note, no study to date has revealed statistically significant effects of microdosing on creativity under placebo-controlled circumstances (Passie, 2019).
Although microdosing became prominent due to the belief it improved cognition, a growing number of individuals began to microdose psychedelics to improve conditions of pain (Johnstad, 2018), cluster headache or migraine (Andersson et al., 2017). It seems that the efficacy of microdosing may derive from its non-psychedelic dose range, which provides treatment without affecting cognition. Individuals also reported relief of pain with a long-term psychedelic microdosing regimen (Johnstad, 2018). Thus, psychedelic microdosing might constitute a different paradigm to single psychedelic therapeutic sessions with macrodoses where the nature and content of the experience plays a key role in predicting therapeutic outcome (Roseman et al., 2018; Schenberg, 2018). However, many questions remain about the definition, safety, potential mechanism and future research involving microdosing.
Question 1: What does microdosing mean?
The term microdosing is not a uniquely psychedelic term. In pharmacology, microdosing is a process used in drug development (Lappin and Garner, 2008) and drug selection (Lappin et al., 2006) where a minute dose of a substance is used to assess the pharmacokinetics of a drug. A microdose, in this regulatory arena, has been defined by a position paper from the European Medicines Agency 2004 (EMEA, 2003), guidelines from the U.S. Food and Drug Administration in 2006 (FDA, 2006) and the Ministery of Health, Labour and Welfare in Japan in 2008 (MHLW, 2008), and the current definitive international guideline in 2009 (ICH, 2009) as being a dose of drug that is 1% of the pharmacologically active dose, up to a maximum of 100 µg. Thus, psychedelic microdosing (‘5–10 µg of LSD’ (Fadiman, 2011)) would be 5–10% of a usual psychoactive dose and lie between a full pharmacological dose (100%) and a ‘pharmacological microdose’.
Microdosing psychedelics has been described in a similar manner by different individuals. Fadiman describes it as a practice ‘to use sub-threshold doses of psychedelic drugs in an attempt to enhance cognitive tasks, to boost physical energy levels, to promote emotional balance, and to treat anxiety, depression and addiction’ resulting in typically subtle though noticeable effects (Fadiman, 2011). Similarly, Aylet Waldman in her book (Waldman, 2017) states the same intention for microdosing but describes the process as ‘the act of integrating sub-perceptual doses of psychedelic drugs, in your weekly routine’. In addition, Johnstad emphasizes that ‘to microdose with a psychedelic drug means to take a dose small enough to provide no intoxication or significant alteration of consciousness’ (Johnstad, 2018).
Thus, the term ‘microdosing’ appears to consist of three components:
The use of a low dose below the perceptual threshold that does not impair ‘normal’ functioning of an individual.
A procedure that includes multiple dosing sessions.
The intention to improve well-being and enhance cognitive and/or emotional processes.
Existing dosing categories for psychedelics when used in research are very low dose, low dose, medium dose, and high dose (Table 1). A microdose has been defined as approximately one-tenth to one-twentieth of a recreational dose, varying within and between substances, so it can be seen as being somewhat below a very low dose. Although microdosing of psychedelics does not have an agreed scientific definition, we have decided to continue to use the term because of its prevalent societal use. Hopefully, this paper will help to facilitate research towards establishing it as a scientific construct.
Table 1.
Varying doses of psychedelic compounds used in preclinical and clinical studies.
| Substance | Subjects/participants (animal/human) | Route of administration | Microdose | Very low dose | Low dose | Medium dose | High dose |
|---|---|---|---|---|---|---|---|
| Psilocin (Hasler et al., 2004; Wackermann et al., 2008) | Human (both studies) | Oral | <1 mg | 3.15 mg | 8 mg | 15 mg | 22 mg |
| LSD (Dandiya et al., 1969) | Rats | Intraperitoneally | 10–25 μg | 30– 40 μg | 60–110 μg | 150 μg | 200+ μg |
| Ibogaine HCl (Glick et al., 2000; Lotsof and Wachtel, 2002; Schechter and Gordon, 1993)a | Rats Humansa |
Intraperitoneally Orala |
200 mg | 300–400 mg | 700 mg | 1400 mga | 2800 mg |
| DMT (Shulgin, 1976) | Humans | Intramuscular injection | 6 mg | 10 mg | 20 mg | 30 mg | 50–70 mgb |
Per kilogram dose values have been converted to values for a 70-kg person. These doses are approximate values.
Study conducted in humans using a single oral dose of 1400 mg.
When inhaled, 30 mg would be considered a high dose.
The most widely distributed species of psychedelic mushrooms are Psilocybe cubensis and those of the genus Copelandia, which consists of 12 species (Guzmán et al., 1998). The psilocin (the active metabolite of psilocybin) and psilocybin content in the whole body of these mushrooms when dried was estimated to be in the range of 0.14–0.42% (psilocin) and 0.37–1.30% (psilocybin) for P. cubensis and 0.43–0.76% (psilocin) and 0.08–0.22% (psilocybin) for Copelandia, respectively. Thus, the former is more psilocybin-rich than the latter, and the latter contains more psilocin compared to the former (Tsujikawa et al., 2003). The Psilocybe semilanceata is the most common British species. This mushroom only contains psilocybin, in the range from 0.17 to 1.96%, as shown by one Norwegian analysis (Christiansen et al., 1981; Rumack and Spoerke, 1994). These data show that the psilocybin concentration varies between and within species but is also dependent on the time of collection, the preservation of the material and growth conditions. User reported recreational doses depend on the species and experience of the user (Rumack and Spoerke, 1994).
A hallucinogenic dose of dried P. cubensis, for example, is between 3 and 5 g (Rumack and Spoerke, 1994). These values equate to a recreational dosing range of 8.6 to 14.7 mg of psilocin per dose. Thus, a microdose would range from 0.43 to 0.73 mg of psilocin per dose because a microdose of psilocybin is generally one-tenth of a full dose (Fadiman, 2011). That positions a recreational dose of psilocin between a low and medium dose and a microdose below a very low dose. However, variations in psilocin content between doses of dried mushroom may be seen due to variations between individual fungi within a species. A microdose of LSD ranges between 10 and 20 μg with 20 μg being the upper limit that might already produce perceptual changes in some. A microdose of ibogaine hydrochloride is approximately 25 mg (Kroupa and Wells, 2005), and when smoked, that of N,N-dimethyltryptamine (DMT) is approximately 6 mg (May, 2018).
Question 2: What microdosing schedules have been used?
The data presented here were collected using a search of microdosing protocols that included books, online fora and surveys. The keywords of this search included microdosing, microdosing protocols, microdosing approaches and psilocybin microdose. In this search, it was found that users mainly followed three approaches. The most popular of these was the Fadiman approach, outlined in his book (Fadiman, 2011), which involves two consecutive dosing days followed by two non-dosing days. Another popular approach involves ‘weekday’ dosing, i.e. from Monday to Friday and not dosing on Saturday and Sunday. Additionally, some users indicated that they followed a balanced low/microdose approach, which involved dosing every other day. Dosing periods ranged from 1 week to 2 years. This variation in microdosing schedules was confirmed by a recent survey which demonstrated that half of the respondents who microdosed came up with their own schedule (Hutten et al., 2019).
Question 3: What controlled studies have been done so far?
The first placebo-controlled LSD microdosing study was published recently (Yanakieva et al., 2018). Findings showed a delay of time perception in the absence of self-rated effects on perception, mentation and concentration after administration of single doses of 5, 10, and 20 μg LSD. To our knowledge there has been only one published study designed specifically to measure the effects of psilocybin microdosing per se (Prochazkova et al., 2018) where the effects of psychedelic mushrooms were explored in a recreational setting. This study suffers from a number of methodological issues, particularly the lack of a placebo control as well as uncertainty over dose taken. However, there have been several more controlled studies where a low dose of psilocybin has been used as a control for a regular dose; these are presented below.
For example, Hasler and colleagues (2004) compared four doses of psilocybin in healthy humans in a placebo-controlled experimental design and found slight physiological and psychological differences between single administration of placebo and a very low dose (VLD) (Hasler et al., 2004). A VLD was defined as 45 μg/kg p.o., equating to approximately 2.3 mg of psilocin for an average 70-kg human. VLD was compared with a low dose (LD), a medium dose (MD) and a high dose (HD) defined as 115, 215 and 315 μg/kg p.o., respectively. Although most physiological measures were similar between the VLD dose and placebo, a significant decrease was seen in maximum heart rate at the 6-hour point after VLD administration. Acute self-rated/self-reported psychological responses of VLD included slight drowsiness, increased sensitivity and intensification of pre-existing mood states; an increase in introversion compared to placebo was only shown for the MD and HD at peak drug effect, 95 minutes post-administration.
Building on that, Griffiths and colleagues (2011) investigated the effects of psilocybin in varying doses where each participant received five dosing sessions, spread across 1-month intervals (Griffiths et al., 2011). The doses used were 0, 5, 10, 20 and 30 mg/70 kg. Using a Monitor Rating Questionnaire with a 5-point scale, they found that a dose of 5 mg/70 kg increased stimulation, distance from ordinary reality and sense of peace. Intensity, somaesthesia, affect, perception, cognition and volition measured on the Hallucinogen Rating Scale all increased after administration of a 5 mg/70 kg dose. In other words, they did not find a dose without psychological effects. Interestingly, when using an 11 mg/70 kg and 15 mg/70 kg dose of psilocybin, Lewis and colleagues (2017) found a significant decrease in global cerebral blood flow in the frontal, parietal, temporal, limbic, cingulate and occipital cortex, insula, caudate, putamen, pallidum, amygdala, hippocampus and thalamus (Lewis et al., 2017). This may relate to the psychological effects seen with lower doses.
Psychological effects of microdosing have been regularly reported by users after multiple administrations of psilocybin. Independent accounts from online fora and surveys (Fadiman and Korb, 2019; www.thethirdwave.co; www.dmt-nexus.me, 2018; www.reddit.com, 2018) reveal that users report improvements in energy, mood, cognition, concentration, management of stress, creativity, spiritual awareness, productivity, language capabilities, relationships and visual capabilities. Further, users also reported reduced anxiety, depression and addiction and pain relief. In a recent survey by Anderson and colleagues (2018), users also noted drawbacks such as illegality, stigma, physical discomfort, anxiety, overstimulation, cognitive interference, emotional difficulty and uncertainty of effect (Anderson et al., 2018). All of these reports are confounded by the lack of certainty relating to the actual dose used, or indeed the provenance of the active ingredient, and the absence of placebo conditions. For a recent review of past research with psychedelic microdosing, please see Passie (2019).
Question 4: Are there any relevant preclinical studies?
We found only two preclinical studies involving microdosing (Cameron et al., 2019; Horsley et al., 2018). Horsley and colleagues (2018) investigated the effect of microdosing on anxiety using an elevated plus-maze and observation of ecological behaviours. They defined a microdose of psilocin as 0.05 mg/kg, which equates to 3.5 mg for an average 70-kg human. Rats received three dosing sessions over 6 days with the last dosing session on the 6th day. Anxiety profiles were measured in Wistar rats 2 days after the final dosing session. Ethological behaviours including rears, head dips and stretch-attend were also measured during this period. Psilocin at 0.05 mg/kg significantly reduced entries into open arms, suggesting that microdosing may have an anxiogenic effect. This effect was not replicated in the ethological measures. Although the authors conclude that these results might have implications for future therapeutic applications, as they produce counter-productive behaviour, one obvious limitation is the interspecies scaling issue (Sharma and McNeill, 2009). It is questionable whether doses administered to animals translate to humans and the authors also acknowledged that the translational value of their results needs to be determined in a therapeutic context.
Cameron and colleagues (2019) tested the effect of repeated low doses of DMT in rats. They gave a dose for 2 months every third day and assessed behaviour with a broad range of tests. In a cued fear extinction learning test, they showed that animals froze significantly less than a control group, suggesting that DMT facilitates fear extinction memory. In the forced swim test, an antidepressant-like effect was observed. No change was observed in dendritic spine density in the layer V pyramidal neurons, and no changes were observed in gene expression (EGR1, EGR2, ARC, FOS, BDNF and 5HT2A). However, an impact on metabolism was observed in male rats; the weight increased by 182%, compared to 165% with vehicle (Cameron et al., 2019). Comparable to the Horsley et al. (2018) study, the interspecies scaling is a point of discussion together with the question of whether a short-acting substance such as DMT would show beneficial effects in humans without administration of a monoamine oxidase (MAO) inhibitor. Lastly, it should be emphasized that there is a need to conduct more research on long-term effects in order to assess the long-term safety of repeat doses.
Question 5: What is the pharmacology of psychedelics when used in microdoses?
The pharmacology of psilocybin and psilocin is still unclear due to the rapid decline in psychedelic drug research following their being made Schedule 1 drugs in 1968 (Rucker et al., 2018). This decline predated the growth of modern neuropharmacology. Thus, more research is required to build a more complete pharmacological profile of psilocybin and psilocin.
Psilocybin (3[2-(dimethylamino)ethyl]indol-4-ol dihydrogen phosphate ester; O-phosphoryl-4-hydroxy-N,N-dimethyltryptamine) belongs to the indolealkylamine class of psychoactive compounds (Table 2). It is an indole prodrug characterised by a 4-substituent, a phosphate group (Repke et al., 1977), six hydrogen bond acceptors and low lipophilicity (Geiger et al., 2018). Low lipophilicity may contribute to the notion that psilocybin does not cross the blood–brain barrier (Rautio et al., 2008).
Table 2.
The physical and chemical properties of psilocybin.
| Name | Psilocybin |
|---|---|
| IUPAC name | 3[2-(dimethylamino)ethyl]indol-4-ol dihydrogen phosphate |
| Other common name | 4-Phosphoryl-N,N-dimethyltryptamine |
| Chemical formula | C12H17N2O4P |
| Molar mass | 204.27 g/mol |
| Melting point | 173 to 176°C (343 to 349°F) |
| Physical form | Solid |
| Soluble in | Water Saline |
In vivo, however, the majority of the prodrug psilocybin is rapidly converted to psilocin by alkaline phosphatases present in the blood and tissues. Psilocin has fewer hydrogen bond acceptors in its structure, which increases lipophilicity. In addition, NMR spectral studies have implicated an intramolecular hydrogen bond in psilocin that reduces the basicity of psilocin, increases its lipophilicity and also may render it stable to the action of MAO (Migliaccio et al., 1981). Following systemic circulation, psilocin is metabolized by either phase I or phase II metabolism (Figure 1). The former involves an oxidation reaction to form 4-hydroxyindole-3-acetaldehyde followed by either an oxidation to 4-hydroxyindole-3-acetic acid or a reduction to 4-hydroxytryptophole. It is believed that none of these metabolites are biologically active. The latter pathway involves the formation of a psilocin O-glucuronide conjugate through small intestine and liver enzymes UGT1A10 and UGT1A9, respectively. There is evidence that to some extent, glucuronidated psilocin can be converted back to psilocin (Brown et al., 2017). Although more than 80% of psilocin undergoes phase II metabolism, both phase I and II metabolites are ultimately eliminated through renal excretion.
Figure 1.
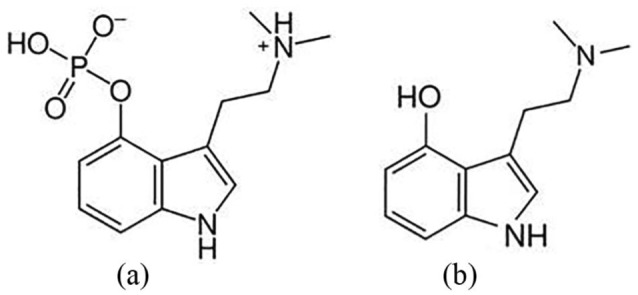
The structure of (a) psilocybin and (b) psilocin.
Depending on body weight, the minimum active oral dose of psilocybin is approximately 4 to 10 mg in humans (van Amsterdam et al., 2011). Onset of action as defined by the first appearance of acute psychological symptoms begins 20 to 60 minutes following oral ingestion and 10 to 40 minutes following buccal administration (Geiger et al., 2018) and almost immediately following i.v. injection (Carhart-Harris et al., 2012).
Psilocin begins to appear in the plasma approximately 25 minutes after oral dosage, with peak levels reached after approximately 105 ± 37 minutes (Brown et al., 2017). A typical user responds to a full active dose for approximately 4 to 7 hours. Even a VLD can produce responses for up to 6 hours after dose administration (Hasler et al., 2004).
Question 6: Is microdosing safe?
Preclinical studies to assess the safety of repeated doses of psilocybin in rodents have not been conducted. That may be due to several factors, including the historical background of psilocybin as an ingredient in magic mushrooms that had been used in many cultures without apparent harm. Evidence from these accounts demonstrates a lack of serious adverse events resulting from psilocybin administration. There are, however, several non-clinical investigations of psilocybin’s safety profile. The risk of an adverse cardiovascular event due to hERG (human ether-a-go-go-related protein) potassium channel blockade is low, with hERG assay results demonstrating minimal effect of psilocybin at concentrations up to 1000 μM (nominal) and completely without effect at 100 μM. That means that unwanted cardiac chronotropic effects with microdosing are very unlikely as the maximum plasma concentration of psilocybin produced by a 25-mg dose would not reach 160 nM (Brown et al., 2017).
Other potential and serious adverse events are cardiac valvulopathies due to repeated activation of serotonin 5-HT2B receptors, which psilocin activates along with many other serotonin receptors. Several drugs have recently been pulled from the market due to this concern. The first example of this was the diet medication Phen/Fen, which had an unacceptably high fatality rate due to its effects on 5-HT2B receptors in the heart (Connolly et al., 1997). Another example is methysergide, an ergot-derived prescription drug that is still being used today as a prophylaxis in difficult to treat migraine and cluster headache (MacGregor and Evers, 2017). It has a known risk of increasing cardiac valve dysfunction (Joseph et al., 2003). In early reports it was shown that although aortic insufficiencies disappeared in most cases after arrest of the methysergide therapy, the mitral insufficiencies remained unchanged (Graham, 1967). It remains to be seen whether repeated low-dose psilocybin administration in preclinical studies might produce valvular hyperplasia, and whether or not this would translate to the human user population. This concern is discussed more in the next section. So far psilocybin testing in preclinical studies has not revealed any signals of valvulopathy.
A different psychedelic that is more often used for microdosing, LSD, has been examined in rodents after repeated dosing schedules similar to microdosing. Comparatively low doses of LSD administered every other day for several months were shown to produce persistent negative behavioural changes that lasted for at least several weeks to months after LSD administration was discontinued (Marona-Lewicka et al., 2011). These changes included increased aggression, scruffy appearance, anhedonia and hyper-reactivity. Analysis of gene expression in key cortical regions like the medial prefrontal cortex indicated that LSD produced alterations in genes enriched for schizophrenia and bipolar depression that lasted long after the drug was discontinued (Martin et al., 2014). Of note, here the interspecies scaling question arises, and it is disputable whether the (low) doses used in animals are comparable to those used by humans (Sharma and McNeill, 2009). Related to these preclinical findings, another primary safety concern for 5-HT2A agonists is the potential for adverse psychological response in humans (Carhart-Harris et al., 2016; Johnston et al., 2010; Vollenweider et al., 1998).
The lethal dose of psilocybin in a single administration in 50% of animals tested (the LD50) ranges from 280 mg/kg in rats and mice to 12.5 mg/kg in rabbits (Usdin and Efron, 1972; Williams, 2013). Animals receiving a very HD of psilocybin (10 mg/kg) exhibit sympathetic system effects such as irregularities in heart and breathing rate as well as mydriasis, piloerection, hyperglycaemia and hypertonia (Cerletti, 1958). Similar central excitatory effects were seen after the administration of 2–4 mg/kg intraperitoneal psilocybin in rhesus monkeys (Horibe, 1974).
Question 7: What receptors will be involved in the activity of microdosed psilocybin?
Psilocin predominantly binds to serotonin receptors: 5-HT1A, 5-HT1D, 5-HT1E, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT5, 5-HT6 and 5-HT7 (Table 3) (McKenna et al., 1990) and the serotonin transporter and partially to the norepinephrine transporter, similar to MDMA (Rickli et al., 2016). Hill slope values demonstrate that psilocin binds independently at all 5-HT receptors except 5-HT2B where cooperative binding is exhibited (McKenna et al., 1990).
Table 3.
An overview of 5-HT receptors that are stimulated by psilocin. Ki values are based on displacement of an antagonist radioligand.
| Receptor type | G-protein | Distribution | Physiological response | Behavioural response | Agonist | Antagonist | Drug classes that act on this receptor | Psilocin binding affinity: Ki (nM) (Halberstadt and Geyer, 2011) |
|---|---|---|---|---|---|---|---|---|
| 5-HT1A | Gi | Cerebral cortex, hippocampus, septum, amygdala and raphe nucleus in high densities. Low amounts also exist in the basal ganglia and thalamus (Beliveau et al., 2017) | I. Hypotension II. Increase DA release in the medial prefrontal cortex, striatum and hippocampus |
I. Decreased anxiety and depression (Campos and Guimarães,
2008) II. Impairment of declarative and non-declarative memory III. Decreased aggression and impulsivity IV. Inhibition of drug-seeking behaviour |
8-OH DPAT, buspirone, 5-CT (Barnes and Sharp, 1999), psilocybin (Mckenna et al., 1990) |
WAY 100135 (methiothepin nonselective) (Barnes and Sharp, 1999) | I Analgesics (agonists) II Antidepressants (post-synaptic receptor agonists and pre-synaptic autoreceptor antagonists) III Anxiolytics (antagonists) |
567.4 |
| 5-HT1D | Gi | Trigeminal sensory neurones including peripheral and central projections to dural blood vessels and to the medulla (Longmore et al., 1997) | Inhibition of adenylyl cyclase | I. Modulates locomotion and anxiety II. Migraine prophylaxis (Longmore et al., 1997) |
Dextromethorphan, sumatriptan, L694247, 5-CT (Barnes and Sharp, 1999), psilocybin (McKenna et al., 1990) | Sumatriptan, GR 127935 (metergoline, methiothepin nonselective) (Barnes and Sharp, 1999) | I. Triptans (agonists used for migraine) | 36.4 |
| 5-HT1E | Gi | High levels in olfactory bulb glomeruli and molecular layer of dentate gyrus. Low amounts in the adventitial layer of cerebral arteries (Klein and Teitler, 2012) | Inhibition of adenylyl cyclase | 5-HT (Barnes and Sharp, 1999), psilocybin (McKenna et al., 1990) | None (methiothepin weak) (Barnes and Sharp, 1999) | N/A | ||
| 5-HT2A | Gq/11 | High concentrations on the apical dendrites of pyramidal cells in layer V, neocortex (mainly prefrontal, parietal and somatosensory cortex) and the olfactory tubercle, as well as cardiovascular system (Beliveau et al., 2017) | I. Vasoconstriction II. Platelet aggregation III. Bronchoconstriction IV. Anti-inflammatory |
I. Modulates addiction II. Increased anxiety III. Increased appetite IV. Improved cognition (learning and memory) V. Decreased sleep VI. Modulates sexual behaviour |
Alpha-methyl-5-HT, DOI (Barnes and Sharp, 1999), psilocybin (McKenna et al., 1990) | Ketanserin, pimvanserin, pirenperone (Barnes and Sharp, 1999) | I. Atypical antipsychotics
(antagonists) II. Antidepressants and anxiolytics (antagonists) |
107.2 |
| 5-HT2B | Gq/11 | Predominantly peripheral, widespread tissue distribution including liver and kidneys (Julius et al., 1990) | Vasoconstriction | I. Regulates sleep (Qian et al.,
2017) II. Increased GI motility, especially small intestine III. Increased cardiac hypertrophy in mice |
Alpha-methyl-5-HT, DOI (Barnes and Sharp, 1999), psilocybin (Mckenna et al., 1990) | SB 200646 (also 5-HT2C antagonist) | 4.6 | |
| 5-HT2C | Gq/11 | Mainly in choroid plexus, high concentrations in hippocampus, anterior olfactory nucleus, substantia nigra, amygdala, subthalamic nucleus and lateral habenula (Julius et al., 1990) | I. Vasoconstriction II. Increase phosphoinositide turnover |
I. Increased anxiety II. Increased GI motility III. Modulates locomotion IV. Modulates mood and sexual behaviour |
Alpha-methyl-5-HT, DOI, psilocybin (McKenna et al., 1990) | Mesulergine (also 5-HT2A antagonist) | I. Antidepressant (antagonists) II. Orexigenic (antagonists) III. Anorectic (agonists) IV. Antipsychotic (agonists) |
97.3 |
| 5HT5A | Gi/Go | High concentrations in olfactory bulb and medial habenula of wild-type mice. Lower densities in neocortex, hippocampus and trigeminal nucleus | N/A | I. Modulates locomotion II. Increases sleep |
5-CT, valerenic acid (partial agonist) | Methiothepin, ritanserin, asenapine, psilocybin (McKenna et al., 1990) | N/A | 83.7 |
| 5-HT6 | Gs | Predominantly in the caudate nucleus, with lower concentrations in hippocampus and amygdala. Very low levels of expression in the thalamus, subthalamic nucleus and substantial nigra (Yoshioka et al., 1998) | Activation of adenylyl cyclase (HEK 293 cells) | I. Increased anxiety II. Reduced cognition and memory III. Negative effect on mood |
EDMT, EMD-386,088 | Amitriptyline aripiprazole, MS-245, psilocybin (McKenna et al., 1990) | I. Antidepressants (antagonists) II. Anxiolytics (antagonists) III. Nootropics (antagonists) IV. Anorectics (antagonists) |
57.0 |
| 5-HT7 | Gs | Predominantly the caudate and putamen nuclei, the pyramidal
layer of the CA2 field of the hippocampus, the centromedial
thalamic nucleus and the dorsal raphe nucleus (Ruat et al., 1993) |
I. Activation of adenylyl cyclase (HeLa cells and COS
cells) II. Vasoconstriction |
I. Increased anxiety II. Decreased mood III. Reduced working and reference memory |
5-CT, 8-OH-DPAT, aripiprazole, AS-19, psilocybin (Glennon, 2003) | Methiothepin, mianserin, SB-269,270 | I. Antidepressants (antagonists) II. Anxiolytics (antagonists) III. Nootropics (antagonists) |
3.5 |
Cerebral 5-HT receptors that can be stimulated by psilocin are highly distributed among different regions (Table 3). Many behavioural and neuropsychological effects claimed to be elicited by microdosing are known to be modulated by these receptors (Anderson et al., 2018).
Psilocin acts as a partial agonist at the 5-HT2A receptor with 46% (+/−2.4) response compared with the response produced by serotonin for signalling through the phospholipase C (PLC) pathway (Kurrasch-Orbaugh et al., 2003). It has a lower binding affinity to the 5-HT2A receptor compared to LSD (Rickli et al., 2016). Currently there is only one study of the in vivo cerebral 5-HT2A receptor occupancy produced by the psilocybin metabolite psilocin in humans. That was done by the Copenhagen group led by Knudsen who used the PET tracer [11C]Cimbi-36. This tracer is an agonist of the 5-HT2A receptor and therefore particularly sensitive to displacement by another agonist, psilocin. Having performed a dose-finding study of psilocybin that ranged from 3 to 30 mg p.o. per person, they found that the plasma concentration that produced a 50% occupancy of the 5-HT2A receptor was 1.95 (range 1.16–3.15) μg psilocin/L (Madsen et al., 2019). They also found that plasma psilocin was positively correlated with subjective intensity ratings and that doses producing less than 20% occupancy (i.e. probably less than 0.028 mg/kg body weight) were not detectable either by psychological or physiological measurements (Madsen et al., 2019), suggesting that this concentration might represent the threshold for microdosing, based on brain 5-HT2A receptor occupancy.
At this dose level, several 5-HT receptors other than the 5-HT2A receptor may also be affected. This could include antagonist activity at the 5-HT6 and 5-HT7 receptors that may improve mood and cognition (Ballaz et al., 2007; Mnie-Filali et al., 2009). The 5-HT7 receptor is also implicated in the regulation of circadian rhythms (Lovenberg et al., 1993). Upon assessing binding affinity of LSD and DMT at 5-HT7 receptors, similarly high Ki values of 9.5 nM (Ruat et al., 1993) were found. Additionally, it has been found that 5-HT7 receptor activation reduces secondary hypersensitization in response to capsaicin in mice (Brenchat et al., 2009). Thus, psilocybin agonist activity at 5-HT7 may relate to the ancient use of ‘teonanacatl’ to ease rheumatism.
Further, psilocin also binds with relatively high affinities to 5-HT1D (Ki = 36.4 nM) and 5-HT2B (Ki = 4.6 nM) receptors. 5-HT1D is predominantly expressed in the trigeminal system, which may account for the recent reports of self-medication using microdoses of psychedelics to produce migraine prophylaxis (May, 2018). With regard to the 5-HT2B there are concerns of the development of cardiac valvulopathy associated with agonism at 5-HT2B (Elangbam et al., 2005). It is mostly the use of intermittent high-dose psilocybin intake that has been discredited. However, even with repeated microdosing there is a possibility that 5-HT2B receptors might be stimulated enough to lead to tissue overgrowth. A potential mitigation against this risk is the suggestion that the efficacy of psilocin (EC50 > 20 μM) (May, 2018) is lower than that of 5-HT. Nonetheless, because psilocin also has a higher affinity for the 5-HT2B receptor than 5-HT, further investigation is needed to understand better the risks associated with microdosing.
Some stimulation at 5-HT1A receptors may also occur. Such activity has been implicated in the mechanism of action of antidepressant medications including SSRIs (Celeda et al., 2013). Activation of these receptors by psilocin could conceivably be involved in reduction in anxiety and increased mood swings (Carhart-Harris and Nutt, 2017) due to dense distribution of the receptor in the midbrain, limbic and cortical regions that regulate stress and emotion.
Question 8: Are the claims of the benefits of microdosing biologically plausible?
There have been only a few studies on the basic neurobiology of psychedelics at the 5-HT2A receptor. Recent work has shown that psychedelics like DOI and LSD directly produce transcriptional activation of Immediate Early Genes (IEGs) like cfos in only about 5% of neurons within key brain structures, and that these activated Trigger Population neurons express significantly higher levels of receptor than the non-activated neurons (Martin and Nichols, 2016). Transcriptional activation of IEGs within neurons is generally accepted to be a reliable marker for neural activity (Joo et al., 2016). Further, psychedelics also act on subsets of inhibitory neurons, and non-neuronal cells like glia and astrocytes (Martin and Nichols, 2016). Together, these data indicate that within specific brain regions, psychedelics trigger complex patterns of excitatory and inhibitory neurons in small subsets of cells, and that how these cells are activated differs between brain regions (Martin and Nichols, 2016).
Genes acutely activated by LSD in the brain are predominantly involved in synaptic plasticity (Nichols and Sanders-Bush, 2002; Nichols and Sanders-Bush, 2004). Accordingly, activation of 5-HT2A receptors in brain slice culture modulates aspects of long-term plasticity, and expression of brain-derived neurotrophic factor (BDNF) (Vollenweider and Kometer, 2010). BDNF expression is also observed to increase in primary cultures of cortical neurons 24 hours following the application of psychedelics (DOI, DMT, LSD) (Ly et al., 2018). Blockade of the receptor for BDNF, Trk-B, prevents increased spinogenesis and synaptogenesis in cortical neurons that have been treated with psychedelics. Interestingly, the mammalian target of the rapamycin (mTOR) pathway is activated downstream of psychedelics in cortical neuron cultures similarly to ketamine, and likely mechanistically underlies the synaptogenesis (Ly et al., 2018). None of these preclinical studies, however, utilized psilocybin, and it remains to be seen if it produces the same effects, and if so, at what dose?
Psychedelics are known to induce behavioural tolerance, an absence of behavioural effects after repeated intake of a substance. Previously it was shown that behavioural effects were for example diminished after repeated doses of LSD (Abramson et al., 1956); in addition, another study showed these effects to be associated with reduced cortical 5-HT2A receptor binding (Gresch et al., 2005). Serotonin syndrome-related symptoms, skin jerks, shaking behaviour and hyperthermia, induced by a single dose of the 5-HT2A agonist DOI in rats were absent after repeated low dosing, suggesting behavioural tolerance (Pranzatelli and Pluchino, 1991). Although speculative, this downregulation of the 5-HT2A receptor might be a mechanism of action underlying some of its putative therapeutic effects. An example is obsessive-compulsive disorder (OCD), a psychiatric condition that is characterized by increased 5-HT2A binding (Adams et al., 2005). Preliminary data have shown that administration of low to high doses of psilocybin lead to symptom reduction in patients with OCD (Moreno et al., 2006). It was previously suggested that a re-balance between 5-HT1A and 5-HT2A receptors might be responsible for observed therapeutic actions (Buchborn et al., 2014), but this remains to be investigated.
In peripheral tissues, very low doses of psychedelics have profound anti-inflammatory effects (Yu et al., 2008). In general, psychedelics in the phenethylamine class such as DOI have more potency than those in the ergoline class such as LSD. In a rodent model of asthma, for example, levels of the R stereoisomer (R)-DOI that are 30 times lower than the behavioural threshold can have profound effects to prevent inflammation, T-Helper Cell Type 2 (Th2) cell recruitment, eosinophilia and mucus production in the lung, resulting in animals that can breathe normally after exposure to an allergen (Nau et al., 2015). In another animal model of inflammatory bowel disease, levels of the psychedelic (R)-DOI 30 times lower than the behavioural threshold nearly completely prevented intestinal inflammation (Nau et al., 2013). It remains to be seen whether very low levels of psychedelics are also anti-inflammatory in humans, and if the anti-inflammatory activity also occurs in the brain, but if these findings do translate then levels typically used in microdosing regimes for some psychedelic compounds would be predicted to have significant and beneficial anti-inflammatory effects. Interestingly, although LSD is one of the most powerful and potent mind-altering psychedelics, it is comparatively among the least potent anti-inflammatories tested (Yu et al., 2008).
Question 9: What is the legal position of microdosing?
The answer to this question is complex due to differences in national regulations. In general, under the UN Conventions, LSD and related compounds, psilocybin and DMT are controlled as Schedule 1 drugs – i.e. are defined as being the most harmful and as having no medicinal value. In other words, they are subject to the most extreme restrictions and penalties for unapproved possession. These constraints apply to any dose of the drug, even a sub-psychoactive or microdose level. Research can be carried out with the right ethical regulatory and institutional approvals, but dosing would have to be conducted in a secure environment like a hospital or research ward. For repeated microdosing, this adds significant costs and complexity to any study, which is likely why none have yet been reported.
However, this situation is easing for psilocybin as a result of several successful clinical trials in recent years, and both the European and US regulators have given approval for studies (NIH, 2018) with psychedelic doses of synthetic psilocybin made to GMP standards. That means that microdosing trials of similarly sourced product for clinical therapy are likely also to be approved, though as yet we do not believe any have been submitted.
Question 10: What are the regulatory issues?
Unfortunately, due to their long history of anecdotal use in recreational settings, none of the psychedelics has ever followed the conventional drug research and development path expected by contemporary standards. Thus, at best, doses have been selected based on published data in a variety of indications but mostly to provide an indication for an upper safety limit. In a pooled analysis of psilocybin Studerus et al. (2011) classified active oral doses within a vast range of about one order of magnitude difference, between 0.045 and 0.315 mg/kg, which translates into 3.15 to 22.05 mg for a 70-kg human (Studerus et al., 2011). Such a range is quite surprising for active principles with the pharmacological potency of psychedelics. Given such underdetermination, regulatory standards will most likely require dedicated dose-finding studies (more than one) to provide a rationale explaining the known individual differences that have been reported in the clinical response to treatment and most importantly, the dose chosen for late development. In this context, the information provided from oral dosing, resulting plasma psilocin levels and corresponding brain 5-HT2A receptor occupancy will turn out to be informative.
Parallel fixed dose designs are usually recommended (ICH, 1994). In some cases, four arm range studies could be necessary; under these circumstances microdoses could be used to test pseudo-placebo properties or alternatively a peculiar pharmacological activity.
Another issue pertains to the limited pharmacokinetic data available in order to evaluate a dose-concentration–response relationship for psychedelics. Updated ADME studies are not available for psychedelics, although the characterization of their metabolites and their role in the active principle efficacy or safety profile might prove relevant to interpret and predict their clinical effect. Once pharmacokinetics of the parent compound and its metabolite(s) are established, variation of clearance if any, its prediction by body weight and the concentration–response relationship for the claimed clinical effect must be presented. If possible, biomarker(s) (e.g. single-nucleotide polymorphisms (SNPs)) at the 5-HT receptor subtypes where they have affinity should be linked to the risk/benefit profile and thus to the therapeutic effect, and they could be used to enrich/stratify the population of interest. Because psychedelics have been reported by some to possess a large inter-individual sensitivity, the definition of a precise concentration–response relationship may be difficult to demonstrate, especially once a microdose range is reached.
Question 11: What are the future research needs?
Microdosing is generally accepted as the use of a functionally low dose of a psychedelic compound over multiple dosing sessions with the intention of improving mental and physical well-being, cognition or creativity (Fadiman, 2011; Johnstad, 2018). A systematic study of microdosing psychedelics investigated by means of observation changes in psychological variables of microdosers. Small changes in a sub-set of variables were found, i.e. decreased depression and stress, decreased mind wandering, increased absorption and increased neuroticism. Interestingly, these variables were not those that participants most expected to change, suggesting that long-term changes may be due to biological changes and not only expectations (Polito and Stevenson, 2019). Nonetheless, the possible effects and implications of microdosing remain largely unknown. Although there is a large database of reported effects of ‘microdosing’ on online fora, the true amount of active substance in these is unknown as are the peak plasma psilocin concentrations achieved during ‘intoxication’. Further, while in these anecdotal reports the user deliberately ingests a substance for a reason, expecting positive effects, it is difficult to distinguish between expectation ‘placebo’ effects and the effect of a microdose. These non-pharmacological effects, described as set and setting, are also known to be of influence when taking a full dose of a psychedelic (Hartogsohn, 2017). Another unknown is whether effects are noticeable after only one microdose or that a certain ‘build-up’ is needed, supported by underlying neurobiological changes, before effects occur.
Therefore, rigorous placebo-controlled clinical studies need to be conducted with different low doses of the drug to determine whether there is any evidence for the claims being made by microdosers. The types of cognitive testing performed should include several different validated psychological instruments and preferably cover the concepts mentioned in the Research Domain Criteria (Cuthbert and Kozak, 2013), and not simply rely on anecdotal accounts or simple tests. Generated knowledge in healthy volunteers will provide clear information on which cognitive aspects can be enhanced with microdosing. This knowledge will provide a first hint as to whether microdosing can be of value in the treatment of specific symptoms in psychiatric populations. Anecdotal reports suggest, for example, that microdosing might help in combatting attention deficit hyperactivity disorder (ADHD) symptoms; studies including measures related to symptom domains like executive functioning, attention and temporal processing will help to decode the potential of microdosing as a therapeutic agent. In terms of biological mechanism of action, more (pre)clinical work needs to be performed to understand fully the complex interaction of different cell types, and their responses to psychedelics at the molecular level such as elucidating peripheral or central signalling pathways involved, if any, in the process of microdosing (Kuypers, 2019). Resulting findings can provide theoretical grounds for why microdosing could work in alleviating cluster headache in patients suffering from it (Anderson et al., 2018; Johnstad, 2018).
Whereas most anecdotal reports focus on the positive experiences with microdosing, future research should investigate the molecular mechanisms behind low-dose psilocybin behavioural effects as well as address potential risks of (multiple) administrations of a psychedelic in low doses. Although extensive toxicology has been conducted on a single active dose of psilocybin and has been proven to be safe (Brown et al., 2017; Johnson et al., 2018), further research is required to understand better the possible health risks incurred by microdosing, especially in relation to cardiac and lung tissue. These studies would involve (pre)clinical safety and tolerability tests of multiple low/microdoses of psilocybin over an extended period of time. To that end, continuous monitoring of physiological parameters including heart functioning in addition to assessment of receptor turnover at low/microdoses as well as receptor occupancy will shed light on the potential negative consequences microdosing could have.
Acknowledgments
Livia Ng was a paid intern and Anaïs Soula an employee for COMPASS Pathways.
Footnotes
Declaration of conflicting interest: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: David Nutt is a scientific adviser and Luca Pani is a consultant for COMPASSPathways. The other authors declare that there is no conflict of interest.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Kim PC Kuypers  https://orcid.org/0000-0001-7634-3809
https://orcid.org/0000-0001-7634-3809
References
- Abramson HA, Jarvik ME, Gorin MH, et al. (1956) Lysergic acid diethylamide (LSD-25): XVII. tolerance development and its relationship to a theory of psychosis. J Psychol 41: 81–105. [Google Scholar]
- Adams KH, Hansen ES, Pinborg LH, et al. (2005) Patients with obsessive-compulsive disorder have increased 5-HT2A receptor binding in the caudate nuclei. Int J Neuropsychopharmacol 8: 391–401. [DOI] [PubMed] [Google Scholar]
- Anderson T, Petranker R, Dinh-Williams LA. (2018) Demography of microdosing community survey. Available at: osf.io/g5cwy (accessed 25 June 2019). [Google Scholar]
- Andersson M, Persson M, Kjellgren A. (2017) Psychoactive substances as a last resort: A qualitative study of self-treatment of migraine and cluster headaches. Harm Reduct J 14: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballaz SJ, Akil H, Watson SJ. (2007) Analysis of 5-HT6 and 5-HT7 receptor gene expression in rats showing differences in novelty-seeking behavior. Neuroscience 147: 428–438. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. (1999) A review of central 5-HT receptors and their function. Neuropharmacology 38: 1083–1152. [DOI] [PubMed] [Google Scholar]
- Beliveau V, Ganz M, Feng L, et al. (2017) A high-resolution in vivo atlas of the human brain’s serotonin system. J Neurosci 37: 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belouin SJ, Henningfield JE. (2018) Psychedelics: Where we are now, why we got here, what we must do. Neuropharmacology 142: 7–19. [DOI] [PubMed] [Google Scholar]
- Brenchat A, Romero L, Garcia M, et al. (2009) 5-HT7 receptor activation inhibits mechanical hypersensitivity secondary to capsaicin sensitization in mice. Pain 141: 239–247. [DOI] [PubMed] [Google Scholar]
- Brown RT, Nicholas CR, Cozzi NV, et al. (2017) Pharmacokinetics of escalating doses of oral psilocybin in healthy adults. Clin Pharmacokinet 56: 1543–1554. [DOI] [PubMed] [Google Scholar]
- Buchborn T, Schröder H, Höllt V, et al. (2014) Repeated lysergic acid diethylamide in an animal model of depression: Normalisation of learning behaviour and hippocampal serotonin 5-HT2 signalling. J Psychopharmacol 28: 545–552. [DOI] [PubMed] [Google Scholar]
- Cameron LP, Benson CJ, Defelice BC, et al. (2019) Chronic, intermittent microdoses of the psychedelic N,N-Dimethyltryptamine (DMT) produce positive effects on mood and anxiety in rodents. ACS Chem Neurosci. Available at: 10.1021/acschemneuro.8b00692 (accessed 25 June 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Erritzoe D, Williams T, et al. (2012) Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci 109: 2138–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Kaelen M, Bolstridge M, et al. (2016) The paradoxical psychological effects of lysergic acid diethylamide (LSD). Psychol Med 46: 1379–1390. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Nutt DJ. (2017) Serotonin and brain function: A tale of two receptors. J Psychopharmacol 31: 1091–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerletti A. (1958) Etude Pharmacologique de la Psilocybine. In: Heim R, Wasson RG. (eds) Les Champignons Hallucinogenes du Mexique. Paris: Musée de historie naturelle. [Google Scholar]
- Christiansen A, Rasmussen K, Tønnesen F. (1981) Determination of psilocybin in Psilocybe semilanceata using high-performance liquid chromatography on a silica column. J Chromatogr A, 210: 163–167. [Google Scholar]
- Connolly HM, Crary JL, McGoon MD, et al. (1997) Valvular heart disease associated with fenfluramine–phentermine. N Engl J Med 337: 581–588. [DOI] [PubMed] [Google Scholar]
- Cruz RW. (2017) Microdosing LSD: The Definitive Guide to Increased Creativity and Productivity. Independently Published. [Google Scholar]
- Cuthbert BN, Kozak MJ. (2013) Constructing constructs for psychopathology: The NIMH research domain criteria. J Abnorm Psychol 122: 928–937. [DOI] [PubMed] [Google Scholar]
- Dandiya PC, Gupta BD, Gupta ML, et al. (1969) Effects of LSD on open field performance in rats. Psychopharmacologia 15: 333–340. [DOI] [PubMed] [Google Scholar]
- Dolder PC, Schmid Y, Steuer AE, et al. (2017) Pharmacokinetics and pharmacodynamics of lysergic acid diethylamide in healthy subjects. Clin Pharmacokinet 56: 1219–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormehl L. (2012) The Apple Revolution: Steve Jobs, the Counter Culture and how the Crazy Ones Took Over the World. London: Virgin Books. [Google Scholar]
- Doyle R. (2002) LSDNA: Rhetoric, consciousness expansion, and the emergence of biotechnology. Philosop Rhetoric 35: 153–174. [Google Scholar]
- Elangbam CS, Lightfoot RM, Yoon LW, et al. (2005) 5-Hydroxytryptamine (5HT) receptors in the heart valves of cynomolgus monkeys and Sprague-Dawley rats. J Histochem Cytochem 53: 671–677. [DOI] [PubMed] [Google Scholar]
- EMEA (2003) EMEA Position Paper on Non-clinical Safety Studies to Support Clinical Trials with a Single Microdose. London: European Agency for the Evaluation of Medicinal Products. [Google Scholar]
- Fadiman J. (2011) The Psychedelic Explorer’s Guide: Safe, Therapeutic, and Sacred Journeys. South Paris, ME: Park Street Press. [Google Scholar]
- Fadiman J, Korb S. (2019) Might microdosing psychedelics be safe and beneficial? An initial exploration. J Psychoactive Drugs 51: 118–122. [DOI] [PubMed] [Google Scholar]
- FDA (2006) Guidance for Industry, Investigators, and Reviewers Exploratory IND Studies. Rockville, MD: U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). [Google Scholar]
- Geiger HA, Wurst MG, Daniels RN. (2018) DARK classics in chemical neuroscience: Psilocybin. ACS Chem Neurosci 9: 2438–2447. [DOI] [PubMed] [Google Scholar]
- Glennon RA. (2003) Higher-end serotonin receptors: 5-HT5, 5-HT6, and 5-HT7. J Med Chem 46: 2795–2812. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Szumlinski KK. (2000) 18-Methoxycoronaridine (18-MC) and ibogaine: Comparison of antiaddictive efficacy, toxicity, and mechanisms of action. Ann N Y Acad Sci 914: 369–386. [DOI] [PubMed] [Google Scholar]
- Graham JR. (1967) Cardiac and pulmonary fibrosis during methysergide therapy for headache. Trans Am Clin Climatol Assoc 78: 79–92. [PMC free article] [PubMed] [Google Scholar]
- Gresch PJ, Smith RL, Barrett RJ, et al. (2005) Behavioral tolerance to lysergic acid diethylamide is associated with reduced serotonin-2A receptor signaling in rat cortex. Neuropsychopharmacology 30: 1693. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Richards WA, et al. (2011) Psilocybin occasioned mystical-type experiences: Immediate and persisting dose-related effects. Psychopharmacology 218: 649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán G, Allen JW, Gartz J. (1998) A worldwide geographical distribution of the neurotropic fungi, an analysis and discussion. Annali del Museo Civico di Rovereto 14: 189–280. [Google Scholar]
- Halberstadt AL, Geyer MA. (2011) Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology 61: 364–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman WW, Mckim RH, Mogar RE, et al. (1966) Psychedelic agents in creative problem-solving: A pilot study. Psychol Rep 19: 211–227. [DOI] [PubMed] [Google Scholar]
- Hartogsohn I. (2017) Constructing drug effects: A history of set and setting. Drug Sci Pol Law 3: 2050324516683325. [Google Scholar]
- Hasler F, Grimberg U, Benz MA, et al. (2004) Acute psychological and physiological effects of psilocybin in healthy humans: A double-blind, placebo-controlled dose-effect study. Psychopharmacology (Berl) 172: 145–156. [DOI] [PubMed] [Google Scholar]
- Hofmann A. (1970) The discovery of LSD and subsequent investigations on naturally occurring hallucinogens. In: Ayd FJ, Blackwell B. (eds) Discoveries in Biological Psychiatry. Philadelphia: Lippincott, pp. 91–106. [Google Scholar]
- Horibe M. (1974) The effects of psilocybin on EEG and behaviour in monkeys. Act Nerv Super (Praha) 16: 40–42. [PubMed] [Google Scholar]
- Horsley RR, Palenicek T, Kolin J, et al. (2018) Psilocin and ketamine microdosing: Effects of subchronic intermittent microdoses in the elevated plus-maze in male Wistar rats. Behav Pharmacol 29: 530–536. [DOI] [PubMed] [Google Scholar]
- Hutten NRPW, Mason NL, Dolder PC, et al. (2019) Motives and side-effects of microdosing with psychedelics among users. Int J Neuropsychopharmacol 22: 5426–5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICH (1994) Topic E4 Dose Response Information to Support Drug Registration. London: European Agency for the Evaluation of Medicinal Products. [Google Scholar]
- ICH (2009) Note for Guidance on Non-Clinical Safety Pharmacology Studies for Human Pharmaceuticals M3(R2). London: European Medicines Agency. [Google Scholar]
- Johnson MW, Griffiths RR, Hendricks PS, et al. (2018) The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act. Neuropharmacology 142: 143–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstad PG. (2018) Powerful substances in tiny amounts: An interview study of psychedelic microdosing. Nord Stud Alcohol Dr 35: 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, et al. (2010) Monitoring the Future: National Survey Results on Drug Use, 1975–2009. Volume I: Secondary School Students. NIH Publication No. 10–7584. National Institute on Drug Abuse (NIDA). [Google Scholar]
- Joseph T, Tam SKC, Kamat BR, et al. (2003) Successful repair of aortic and mitral incompetence induced by methylsergide maleate: Confirmation by intraoperative transesophageal echocardiography. Echocardiography 20: 283–287. [DOI] [PubMed] [Google Scholar]
- Julius D, Huang KN, Livelli TJ, et al. (1990) The 5HT2 receptor defines a family of structurally distinct but functionally conserved serotonin receptors. Proc Natl Acad Sci U S A 87: 928–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein MT, Teitler M. (2012) Distribution of 5-HT(1E) receptors in the mammalian brain and cerebral vasculature: An immunohistochemical and pharmacological study. Br J Pharmacol 166: 1290–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroupa PK, Wells H. (2005) Ibogaine in the 21st century: Boosters, Tune-ups and Maintenance. Multidisciplinary Association for Psychedelic Studies(MAPS) Bulletin XV: 21–24. [Google Scholar]
- Kumar N. (2016) Microdosing: Enhance Your Vitality, Temperament and Efficiency with Hallucinogenic. Scotts Valley, CA: CreateSpace Independent Publishing Platform. [Google Scholar]
- Kurrasch-Orbaugh DM, Watts VJ, Barker EL, et al. (2003) Serotonin 5-hydroxytryptamine 2A receptor-coupled phospholipase C and phospholipase A2 signaling pathways have different receptor reserves. J Pharmacol Exp Ther 304: 229–237. [DOI] [PubMed] [Google Scholar]
- Kuypers KPC. (2019) Psychedelic medicine: The biology underlying the persisting psychedelic effects. Med Hypotheses 125: 21–24. [DOI] [PubMed] [Google Scholar]
- Lappin G, Garner RC. (2008) The utility of microdosing over the past 5 years. Expert Opin Drug Metab Toxicol 4: 1499–1506. [DOI] [PubMed] [Google Scholar]
- Lappin G, Kuhnz W, Jochemsen R, et al. (2006) Use of microdosing to predict pharmacokinetics at the therapeutic dose: Experience with 5 drugs. Clin Pharmacol Ther 80: 203–215. [DOI] [PubMed] [Google Scholar]
- Leslie SW, Kargon RH. (1996) Selling Silicon Valley: Frederick Terman’s model for regional advantage. Bus Hist Rev 70: 435–472. [Google Scholar]
- Lewis CR, Preller KH, Kraehenmann R, et al. (2017) Two dose investigation of the 5-HT-agonist psilocybin on relative and global cerebral blood flow. Neuroimage 159: 70–78. [DOI] [PubMed] [Google Scholar]
- Longmore J, Shaw D, Smith D, et al. (1997) Differential distribution of 5Ht1D-and 5HT1B-immunoreactivity within the human trigemino-cerebrovascular system: Implications for the discovery of new antimigraine drugs. Cephalalgia 17: 833–842. [DOI] [PubMed] [Google Scholar]
- Lotsof HS, Wachtel B. (2002) Manual for Ibogaine Therapy: Screening, Safety, Monitoring & Aftercare. Ibogaine Dossier. [Google Scholar]
- Lovenberg TW, Baron BM, De Lecea L, et al. (1993) A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron 11: 449–458. [DOI] [PubMed] [Google Scholar]
- Luke D. (2006) A Tribute to Albert Hofmann on his 100th Birthday: The Mysterious Discovery of LSD and the Impact of Psychedelics on Parapsychology. The Paranormal Review 37: 1–8. [Google Scholar]
- Ly C, Greb AC, Cameron LP, et al. (2018) Psychedelics promote structural and functional neural plasticity. Cell Rep 23: 3170–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor EA, Evers S. (2017) The role of methysergide in migraine and cluster headache treatment worldwide: A survey in members of the International Headache Society. Cephalalgia 37: 1106–1108. [DOI] [PubMed] [Google Scholar]
- Madsen MK, Fisher PM, Burmester D, et al. (2019) Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels. Neuropsychopharmacology 44: 1328–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marona-Lewicka D, Nichols CD, Nichols DE. (2011) An animal model of schizophrenia based on chronic LSD administration: Old idea, new results. Neuropharmacology 61: 503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DA, Marona-Lewicka D, Nichols DE. (2014) Chronic LSD alters gene expression profiles in the mPFC relevant to schizophrenia. Neuropharmacology 83: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DA, Nichols CD. (2016) Psychedelics recruit multiple cellular types and produce complex transcriptional responses within the brain. EBioMedicine 11: 262–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May J. (2018) The Ibogaine Conversation Part 9: How Safe is Ibogaine? [Online]. Available at: https://www.psymposia.com/magazine/how-safe-is-ibogaine-we-asked-clare-wilkins-who-has-facilitated-over-700-treatments/.
- McKenna DJ, Repke DB, Lo L, et al. (1990) Differential interactions of indolealkylamines with 5-hydroxytryptamine receptor subtypes. Neuropharmacology 29: 193–198. [DOI] [PubMed] [Google Scholar]
- Migliaccio GP, Shieh TL, Byrn SR, et al. (1981) Comparison of solution conformational preferences for the hallucinogens bufotenin and psilocin using 360-MHz proton NMR spectroscopy. J Med Chem 24: 206–209. [DOI] [PubMed] [Google Scholar]
- Ministery of Health, Labour and Welfare (2008) Microdose clinical studies. In: Pharmaceutical and Medical Safety Bureau, M. O. H. L. A. W. (ed.) English Regulatory Information Task Force Japan Pharmaceutical Manufacturers Association. Tokyo, Japan: MHLW. [Google Scholar]
- Mnie-Filali O, Lambas-Senas L, Scarna H, et al. (2009) Therapeutic potential of 5-HT7 receptors in mood disorders. Curr Drug Targets 10: 1109–1117. [DOI] [PubMed] [Google Scholar]
- Moreno FA, Wiegand CB, Taitano EK, et al. (2006) Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J Clin Psychiatry 67: 1735–1740. [DOI] [PubMed] [Google Scholar]
- Nau F, Jr., Miller J, Saravia J, et al. (2015) Serotonin 5-HT(2) receptor activation prevents allergic asthma in a mouse model. Am J Physiol Lung Cell Mol Physiol 308: L191–L198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nau F, Jr, Yu B, Martin D, et al. (2013) Serotonin 5-HT2A receptor activation blocks TNF-alpha mediated inflammation in vivo. PLoS One 8: e75426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols CD, Sanders-Bush E. (2002) A single dose of lysergic acid diethylamide influences gene expression patterns within the mammalian brain. Neuropsychopharmacology 26: 634–642. [DOI] [PubMed] [Google Scholar]
- Nichols CD, Sanders-Bush E. (2004) Molecular genetic responses to lysergic acid diethylamide include transcriptional activation of MAP kinase phosphatase-1, C/EBP-beta and ILAD-1, a novel gene with homology to arrestins. J Neurochem 90: 576–584. [DOI] [PubMed] [Google Scholar]
- NIH (2018) Trials with ‘Psilocybin’. NIH US National Libary of Medicine. ClinicalTrials.gov.
- Passie T. (2019) Science of Microdosing Psychedelics. London: Psychedelic Press. [Google Scholar]
- Passie T, Seifert J, Schneider U, et al. (2002) The pharmacology of psilocybin. Addict Biol 7: 357–364. [DOI] [PubMed] [Google Scholar]
- Polito V, Stevenson RJ. (2019) A systematic study of microdosing psychedelics. PLoS One 14: e0211023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranzatelli MR, Pluchino RS. (1991) The relation of central 5-HT1A and 5-HT2 receptors: Low dose agonist-induced selective tolerance in the rat. Pharmacol Biochem Behav 39: 407–413. [DOI] [PubMed] [Google Scholar]
- Prochazkova L, Lippelt DP, Colzato LS, et al. (2018) Exploring the effect of microdosing psychedelics on creativity in an open-label natural setting. Psychopharmacology 235: 3401–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Cao Y, Deng B, et al. (2017) Sleep homeostasis regulated by 5HT2b receptor in a small subset of neurons in the dorsal fan-shaped body of Drosophila. Elife 6: e26519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautio J, Laine K, Gynther M, et al. (2008) Prodrug approaches for CNS delivery. AAPS J 10: 92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repke DB, Leslie DT, Mandell DM, et al. (1977) GLC-mass spectral analysis of psilocin and psilocybin. J Pharm Sci 66: 743–744. [DOI] [PubMed] [Google Scholar]
- Rickli A, Moning OD, Hoener MC, et al. (2016) Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. Eur Neuropsychopharmacol 26: 1327–1337. [DOI] [PubMed] [Google Scholar]
- Roberts A. (2008) Albion Dreaming: A Popular History of LSD in Britain (Revised Edition with a New Foreword by Dr. Sue Blackmore). Singapore: Marshall Cavendish International (Asia) Ptd Limited. [Google Scholar]
- Roseman L, Nutt DJ, Carhart-Harris RL. (2018) Quality of acute psychedelic experience predicts therapeutic efficacy of psilocybin for treatment-resistant depression. Front Pharmacol 8: 974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruat M, Traiffort E, Leurs R, et al. (1993) Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc Natl Acad Sci U S A 90: 8547–8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker JJH, J Iliff, DJ Nutt. (2018) Psychiatry & the psychedelic drugs. Past, present & future. Neuropharmacology 142: 200–218. [DOI] [PubMed] [Google Scholar]
- Rumack BH, Spoerke DG. (1994) Handbook of Mushroom Poisoning: Diagnosis and Treatment. Boca Raton, FL: Taylor & Francis. [Google Scholar]
- Schechter MD, Gordon TL. (1993) Comparison of the behavioral effects of ibogaine from three sources: Mediation of discriminative activity. Eur J Pharmacol 249: 79–84. [DOI] [PubMed] [Google Scholar]
- Schenberg EE. (2018) Psychedelic-assisted psychotherapy: A paradigm shift in psychiatric research and development. Front Pharmacol 9: 733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultes RE. (1940) Teonanacatl: The narcotic mushroom of the Aztecs. Am Anthropol 42: 429–443. [Google Scholar]
- Sharma V, McNeill JH. (2009) To scale or not to scale: The principles of dose extrapolation. Br J Pharmacol 157: 907–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulgin AT. (1976) DMT & TMA-2. J Psychedelic Drugs 8: 167–169. [Google Scholar]
- Studerus E, Kometer M, Hasler F, et al. (2011) Acute, subacute and long-term subjective effects of psilocybin in healthy humans: A pooled analysis of experimental studies. J Psychopharmacol 25: 1434–1452. [DOI] [PubMed] [Google Scholar]
- Tsujikawa K, Kanamori T, Iwata Y, et al. (2003) Morphological and chemical analysis of magic mushrooms in Japan. Forensic Sci Int 138: 85–90. [DOI] [PubMed] [Google Scholar]
- Usdin E, Efron DH. (1972) Psychotropic Drugs and Related Compounds. Washington, DC: National Institute of Mental Health. [Google Scholar]
- van Amsterdam J, Opperhuizen A, Van Den Brink W. (2011) Harm potential of magic mushroom use: A review. Regul Toxicol Pharmacol 59: 423–429. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Kometer M. (2010) The neurobiology of psychedelic drugs: Implications for the treatment of mood disorders. Nat Rev Neurosci 11: 642–651. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, et al. (1998) Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 9: 3897–3902. [DOI] [PubMed] [Google Scholar]
- Wackermann J, Wittmann M, Hasler F, et al. (2008) Effects of varied doses of psilocybin on time interval reproduction in human subjects. Neurosci Lett 435: 51–55. [DOI] [PubMed] [Google Scholar]
- Waldman A. (2017) A Really Good Day: How Microdosing Made a Mega Difference in My Mood, My Marriage, and My Life. New York: Knopf. [Google Scholar]
- Williams M. (ed.) (2013) The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals. 15th ed. Cambridge: Royal Society of Chemistry. [Google Scholar]
- www.dmt-nexus.me (2018) Microdosing [Online] (accessed 28 August 2018).
- www.reddit.com (2018) Psilocybin, Microdose Providing more Energy and Motivation: Microdosing [Online] (accessed 28 August 2018).
- Yanakieva S, Polychroni N, Family N, et al. (2018) The effects of microdose LSD on time perception: A randomised, double-blind, placebo-controlled trial. Psychopharmacology (Berl). Epub ahead of print 26 November 2018. DOI: 10.1007/s00213-018-5119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka M, Matsumoto M, Togashi H, et al. (1998) Central distribution and function of 5-HT6 receptor subtype in the rat brain. Life Sci 62: 1473–1477. [DOI] [PubMed] [Google Scholar]
- Yu B, Becnel J, Zerfaoui M, et al. (2008) Serotonin 5-hydroxytryptamine(2A) receptor activation suppresses tumor necrosis factor-alpha-induced inflammation with extraordinary potency. J Pharmacol Exp Ther 327: 316–323. [DOI] [PubMed] [Google Scholar]


