Abstract
Treatment planning is an essential step of the radiotherapy workflow. It has become more sophisticated over the past couple of decades with the help of computer science, enabling planners to design highly complex radiotherapy plans to minimize the normal tissue damage while persevering sufficient tumor control. As a result, treatment planning has become more labor intensive, requiring hours or even days of planner effort to optimize an individual patient case in a trial-and-error fashion. More recently, artificial intelligence has been utilized to automate and improve various aspects of medical science. For radiotherapy treatment planning, many algorithms have been developed to better support planners. These algorithms focus on automating the planning process and/or optimizing dosimetric trade-offs, and they have already made great impact on improving treatment planning efficiency and plan quality consistency. In this review, the smart planning tools in current clinical use are summarized in 3 main categories: automated rule implementation and reasoning, modeling of prior knowledge in clinical practice, and multicriteria optimization. Novel artificial intelligence–based treatment planning applications, such as deep learning–based algorithms and emerging research directions, are also reviewed. Finally, the challenges of artificial intelligence–based treatment planning are discussed for future works.
Keywords: artificial intelligence machine learning radiotherapy treatment planning automation
Introduction
Artificial intelligence (AI) has recently become one of the most popular words in both industry and academia. Properly known as a modern technology term, AI was perceived as a powerful entity that could “think and act humanly without losing rationality.”1 In computer science fields, AI is defined as the study of algorithms and devices that perceive information from the environment and take action to maximize the chance of achieving specific goals.2 Due to the rapid increases in computational power as well as in data collection and sharing capabilities, a large number of AI techniques, particularly deep learning theories and algorithms, have been published in recent several years. Following this burst of techniques, AI has permeated nearly every aspect of our lives and is rapidly revolutionizing how we live. In the field of radiation oncology, the AI revolution has also been grounded in the automated support of various parts of the radiotherapy clinical workflow: target and tissue segmentation, treatment planning, radiotherapy delivery, and treatment response assessment. This article reviews automatic treatment planning (ATP) tools in radiotherapy treatment planning, which have evolved from simple automation execution to the development of AI as a future replacement of current day manual treatment planning process. Artificial intelligence in radiotherapy treatment planning, particularly deep learning–based investigations, would be the focus of this article. Artificial intelligence applications in other aspects of radiotherapy such as autosegmentation, image processing, or QA can be found in other reviews.3,4
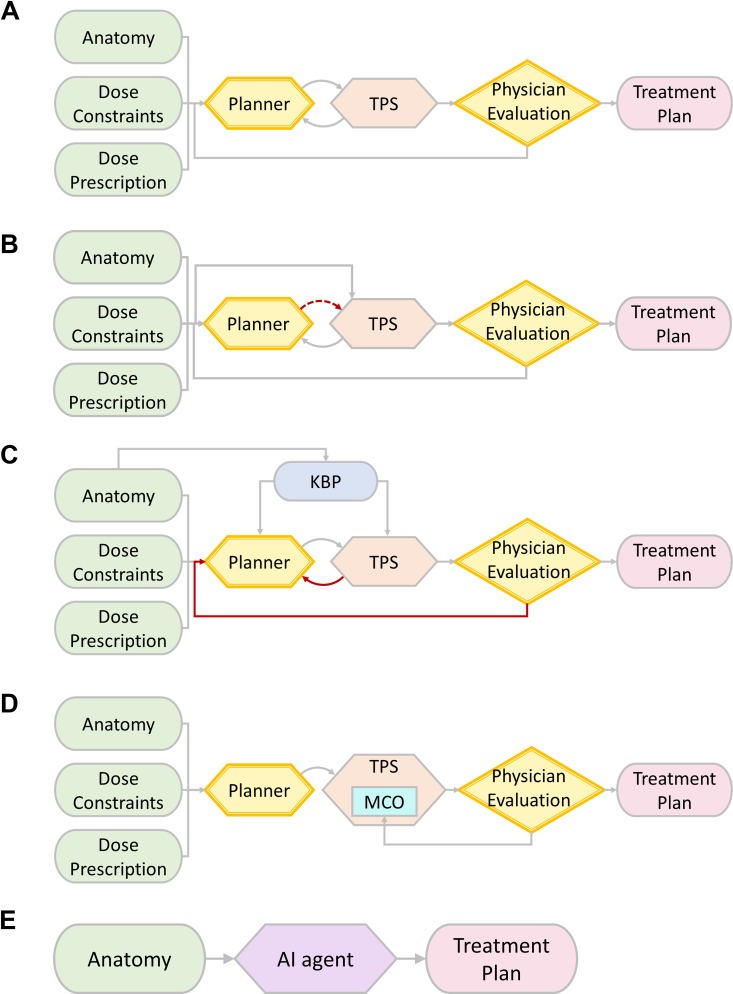
Radiotherapy treatment planning, especially inverse treatment planning, is a laborious process taking hours or even days to complete. Figure 1A shows a brief workflow of manual treatment planning process in the current clinic practice. A workflow starts with a list of dosimetric requirements, including target coverage and organ-at-risk (OAR) constraints. Based on the specific requirements of each case, a human planner makes decisions about basic planning parameters, including beam energy, number, angles, and so on. While generating a minimally acceptable plan may be quick, improving a plan is much less straightforward and often requires many iterations between planners treatment planning system (TPS). In addition, physicians may need to interact with human planners back and forth for plan improvement based on intermediate plan results. The iterative nature of these interactions leads to tremendous human efforts and time commitment.
Figure 1.
A, A brief workflow of manual treatment planning. B, A brief workflow of ARIR in treatment planning. C, A brief workflow of KBP in treatment planning. D, A brief workflow of MCO in treatment planning. E, A brief workflow of AI use in future treatment planning. AI indicates artificial intelligence; ARIR, automated rule implementation and reasoning; KBP, knowledge-based planning; MCO, multicriteria optimization.
Because of the automatic nature, ATP has successfully reduced plan generation time, especially human interactions (mostly repetitive operations) with TPSs.5,6 Thus, human planners are able to devote more time to explore the optimal dosimetry for individually optimized treatment planning. Furthermore, the improved efficiency could also enable clinical paradigm changes, including novel treatment planning strategies,7 treatment course monitoring methods,8 and treatment delivery workflows.9,10
Besides efficiency, ATP has also improved plan quality consistency and error rate. Because conventional treatment planning is a trial-and-error process exploring dosimetric and clinical trade-offs, the final plan quality is dependent on both planner experience and time available for planning. Many ATP studies across various disease sites have reported a more consistent treatment plan quality compared to manual plans.11-16 As such, ATP can reduce health-care disparities by making advanced treatment planning expertise broadly available.17
Unlike efficiency and consistency, plan quality has not been improved by current ATP techniques. While studies have reported that machine-generated plans are clinically acceptable, others have indicated that ATP requires essential human tuning or manipulation to ensure acceptable quality and safety.18,19 While humans should remain the center of treatment planning for plan safety and quality, an important goal of AI-based treatment planning algorithms is to augment treatment plan quality. Many new approaches are currently being explored in this area. In the following sections, we will review the past efforts of ATP applications in current research directions, as well as future research topics and challenges.
Current ATP Techniques
This section summarizes the current ATP techniques in the past decade. The literature was searched using National Library of Medicine PubMed search engine, and key works regarding technical developments and clinical investigations were selected based on our clinic practice experience and our professional society consensus. Based on the impact of clinic practice workflow, the current ATP solutions are organized into 3 categories for discussion20:
automated rule implementation and reasoning (ARIR);
modeling of prior knowledge in clinical practice; and
multicriteria optimization (MCO).
Automated Rule Implementation and Reasoning
In Figure 1A, human planners need to decide basic treatment planning parameters. These decisions are usually determined based on institutional guidelines as well as the planner’s individual preferences, that is, rules with straightforward criteria. For example, in upper esophagus planning, static beams are often arranged in 2 “bouquets” avoiding lateral lung to reduce lung V5 Gy. To explore the dose trade-offs, for example, OAR dose constraints and target coverage, experienced human planners usually need a few trials with adjustments of planning strategies by experienced planners.21 These adjustments are typically simple combinations of basic operations in the TPS, but the reasoning process may require complex, extensive human knowledge, modeled by “if-then” binary actions.
To implement simple clinical guidelines with possible iterative adjustments following binary logic, an automated computer program with hard-coded rules and “if-then” structures would be an ideal solution. Figure 1B shows a brief illustration workflow of ARIR. As presented, the TPS directly analyzes patient anatomy and dosimetric requirements and mimics the reasoning process in manual treatment planning. Following a well-defined logic reasoning scheme from human definitions, ARIR can reduce the requirement of human operations in plan generation (as presented in red arrow), particularly repetitive operations.
Vendors of modern TPS have provided ARIR solutions to enable scripting functions by users’ inputs. For example, Varian Eclipse system (Varian Medical System, Palo Alto, California) has a scripting application programming interface function. This function, ESAPI, allows the user to build specific automatic programs for research and clinic use. Built in a C# environment, ESAPI has function libraries that can simulate most actions in the treatment planning process, including contour manipulation, beam setup, inverse dose–volume histogram (DVH) optimization, dose calculation, and plan statistics calculation. Users dynamically adjust DVH constraints to iteratively achieve a specific dosimetric goal after evaluation of ongoing optimization results (ie, reasoning).22
Another available solution in enhanced integration is AutoPlanning in the Pinnacle TPS (Philips Radiation Oncology Systems, Fitchburg, Wisconsin). Unlike ESAPI, AutoPlanning is a fully developed product as an optional function in the Pinnacle TPS. It does not involve computer program scripting; instead, a planning template is required to list specific requirements in target prescription and OAR sparing.19 According to the input template, AutoPlanning automatically generates auxiliary planning structures for spatial dose distribution manipulation. Then the program starts the optimization and follows the iterative process as in manual planning to fine-tune the plan based on the input template. Some underlying settings, such as cold/hot spot control, can be adjusted through the Pinnacle platform (Philips Radiation Oncology Systems, Fitchburg, Wisconsin).
Much work has been focused on realizing ATP through the rule-based implementation of reasoning with vendor-specific automation capabilities. While early efforts started in prostate intensity-modulated radiotherapy (IMRT) planning,23 researchers have since focused on IMRT and volumetric modulated arc therapy (VMAT) planning of head and neck cancer due to its complex trade-off relationships in manual planning.6,12,24-26 Automation of rule-based implementation reasoning is frequently used with other machine learning algorithms, with recent examples including rapid breast and pelvis treatment planning.27,28
Modeling of Prior Knowledge in Clinical Practice
In human practice, a natural way to improve manual treatment planning efficiency and quality is to review similar prior “good” cases. Specifically, planning parameters in previous cases, such as beam arrangements and DVH objectives in inverse planning, can be introduced directly to the planning process or used as decision references for a current case. Following this idea, researchers have developed statistical models to extract certain features from prior “good” cases using best clinical judgment and knowledge. When using corresponding features from a current case as inputs, these models can predict planning parameters with possible distribution intervals, which can be used for treatment planning with improved efficiency. This approach, also known as knowledge-based planning (KBP), has gained popularity. Figure 1C projects a workflow of KBP utilization. Information extracted from KBP can assist human planners during initial decision-making. Such information can be used as TPS input for certain automated processes. In general, the use of KBP can potentially reduce the iterative plan adjustments before reaching a satisfied treatment plan.
In DVH-based inverse optimization, DVH constraints are important to high-quality plans; optimal constraints can quickly lead to convergence with the balanced dosimetric outcome. Thus, many studies have been developed for DVH-based knowledge modeling. Specifically, a number of previous acceptable or superior clinical cases of a given anatomical site are used. The required case number (typically a few 10s) varies widely in different clinical applications, which depend on treatment sites, delivery techniques, and clinical evaluation standards.16 Characteristic relationships between these cases’ DVH results and anatomical/geometrical features of target(s)/OARs are established during the modeling process. For a new patient with the same anatomical site, a set of achievable DVH curves, including both target(s) and OARs, can be predicted by the model. The predicted DVH curves can be used as references for the human to select DVH constraints during the manual planning process or as the inputs for an automated treatment planning workflow.
A representative DVH-based knowledge modeling method was published by Yuan et al.29 In their work, models for prostate and head and neck IMRT treatments were built. The geometry of an OAR relative to the planning target volume (PTV) was represented by the distance-to-target histogram (DTH), and characteristic geometry and dosimetric features were derived from DTH and DVH by principal component analysis (PCA), respectively. Results showed good OAR dose prediction results in both modeled sites. This DVH-based knowledge modeling has been commercially developed as RapidPlan by Varian (Varian Medical System) as an optional function in the Eclipse TPS. Besides RapidPlan, a number of research studies in DVH-based knowledge modeling methods have been reported for a variety of treatment sites, including prostate,30-33 brain,34 head-and-neck,35-37 lung,38-40 liver,41 and pelvis.42
The key limitation of DVH-based approach is the lack of spatial information, and the planners may need extra work to deal with a case with uncommon OAR/target geometry. Thus, in addition to the DVH-based approach, knowledge-based modeling has been reported for voxel-based prediction, in which dose values of individual voxels are predicted with preserved spatial information.43-45 Knowledge-based modeling has also been reported for beam angle arrangement for lung IMRT treatment.46,47
Multicriteria Optimization
In the DVH-based inverse optimization of most commercially available TPS, a cost function has to be defined for the minimization problem. This cost function combines information from all volumes of interest as a weighted sum of the penalty from each dosimetric criterion from DVH constraints. The trade-off between the target(s) and different OARs is represented by the weighting coefficient of each criterion. The drawback of this approach is the requirement of reoptimization if the dosimetric preference of humans is changed during plan evaluation. Thus, finding the optimal trade-off may become time-consuming. To overcome this issue, MCO was proposed to generate multiple “anchor” plans simultaneously instead of a single plan during the inverse planning process. In each anchor plan, a single DVH criterion of OAR is optimized for best sparing without compromising tumor target dosimetric criteria.48,49 These plans will form a hypersurface in the N-dimension space, where N is the number of independent (ie, competing) OAR dosimetric criteria. Referred to as the Pareto surface, this hypersurface contains the optimal plans following different dosimetric criteria. Figure 1D shows a workflow using MCO in treatment planning. Clinicians can interactively work with TPS: Specifically, one can navigate through a series of Pareto-optimal plans on different Pareto surfaces with linear interpolations to choose an optimal plan based on the clinicians’ evaluation of all dosimetric criteria.50 Thus, if one changes the dosimetric criteria in plan evaluation, the optimal plan can be found in a very short time without reoptimizations from human planners.
In theory, MCO requires the generation of many plans to form the Pareto surface, which may become very time-consuming even with automation. Craft and Bortfeld analyzed head and neck IMRT plans and proved that only a small number of plans are needed to form the Pareto database of feasible plans through objective correlation matrices and PCA of the beamlet solutions.51 Specifically, if N-independent dosimetric criteria are defined, N + 1 plans can form a feasible Pareto surface. This finding led to the clinically feasible application of MCO, which was first implemented in the RayStation TPS (RaySearch, Stockholm, Sweden). The MCO has also become available in the recent version of the Eclipse TPS. In these TPSs, users select the optimal plan by adjusting the combination of dosimetric criteria through interactive sliding bars (ie, a posteriori interaction).
The MCO can also be implemented in the a priori approach with a set of defined dosimetric preferences before the inverse optimization. Only a single optimal plan is generated with full automation, and thus, it requires no need for human interactions. This approach was proposed by Breedveld et al in their work of IMRT Cycle (iCycle).52,53 In iCycle, the generation of the optimal plan is governed by a “wish list,” which contains the desired dosimetric criteria with assigned priorities in order. During the optimization, these criteria are sequentially minimized according to the ascribed priorities to reach the desired plan on the Pareto surface. In addition, iCycle allows beam arrangement optimization, including beam number and beam angle. Full Pareto-optimal plans for each number of beam directions can be used as outputs for human’s a posteriori interaction. So far, iCycle and its derived platform have been demonstrated in IMRT planning of head and neck,53 spine,54 prostate,55 pelvis,56 and gastric cancers.57
Novel AI Applications in ATP
Future treatment planning process using a powerful AI agent can be effective and efficient with minimum human intervention. Figure 1E demonstrates the role of AI in future treatment planning workflow. We envision that AI can implement all human operations and reasoning logics based on the comprehensive analysis of patient anatomy. Parameter such as treatment prescription and delivery technique can be specified as human inputs from physicians, but these parameters can be incorporated into AI decision-making. The future workflow involves minimum human efforts from human planners and physicians; the saved human efforts can be used for other human-centered clinic care tasks.
Currently, a few emerging research topics have been reported focusing on the ATP. While there is also active research ongoing along the directions discussed in the abovementioned section, this section focuses on the novel AI algorithms in ATP, primarily deep learning–based approaches with deep architecture and compositionality.58 In this section, we discuss recent pioneering efforts of novel AI applications in ATP and introduce a few emerging research directions.
Recent Progresses of AI in ATP
A knowledge-based modeling approach was one of the earliest breakthroughs in ATP. Boutilier et al evaluated the clinical applicability of the simultaneous prediction of optimization objective weights for prostate IMRT. Using optimal weights of objectives in previous cases, multinomial logistic regression and weighted K-nearest neighbor algorithms were deployed in the training of weight prediction.59 Results showed that both methodologies could produce good predictions for clinical plans, although no significant performance improvements were found in comparison with the model using logistic regression. Ma et al proposed knowledge-based modeling using support vector regression (SVR).60 In their work, a PTV-only optimization in the absence of OAR considerations was used as the model input in addition to anatomical/geometrical features, and DVH prediction was implemented by SVR as a robust supervised learning technique. In a comparison study, this model was more accurate than the RapidPlan model in bladder and rectum DVH prediction.60
Another major research area in ATP is the prediction of spatial dose distribution. While DVH-based prediction is prevalent, its lack of spatial information may not reveal certain dosimetric end points, such as dose conformity and gradient measurements. Accurate spatial dose distribution prediction can provide guidance for humans in decision-making during the manual treatment planning process for potentially improved quality and efficiency. In addition, the predicted dose distribution can be used for a fully automated ATP workflow without the need for DVH-based inverse optimization. Campbell and Miften developed an artificial neural network dose models for spatial dose distribution prediction of pancreatic stereotactic body radiation therapy (SBRT).61 The network was trained by clinical plans with plan parameters voxel-based geometric parameters. Results showed promising accuracy of 3D dose distribution. Nguyen et al used a modification on the U-net architecture for coplanar prostate IMRT dose distribution prediction.62 Based upon fully convolutional networks, U-net was proposed for image segmentation with transposed convolution operations to maintain original image dimension.63 U-net allows direct image input which avoids the feature extractions (handcrafted feature selections). This could reduce the requirement of data interpretation during the classic modeling process (eg, knowledge-based modeling process). In 2D-based prediction, the average values of absolute dose difference were found to be around 2% in PTV and under 5% of the prescription dose in OARs.62 Similarly, Kearney et al proposed a fully convolutional volumetric dose prediction neural network (DoseNet) for 3D dose distribution prediction of prostate SBRT with possible noncoplanar treatment regimen.64 Compared to U-net, DoseNet was claimed to have reduced network redundancy as a result of the inclusion of residual blocks. Chen et al adopted a published convolutional neural network (CNN) model, ResNet,65 for the dose distribution prediction of nasopharynx cancer in simultaneous integrated boost radiotherapy.66 This prediction model was used to predict a coarse dose map of each patient with reduced intensity content, and a full dose map was recovered from the coarse dose map by a Gaussian regularized low-pass filter. Based on a combined architecture of DenseNet and U-net,67 Barragan-Montero et al incorporated beam angle variation in lung IMRT and developed a model that can predict spatial dose distribution with varying beam arrangement.68
Successful prediction of dosimetric parameters has to be converted to clinical treatment plans. A few studies have explored the feasibility of automatic plan generation that can lead to deliverable plans. Long et al proposed a framework of threshold-driven optimization for reference-based auto-planning, which automatically generates a treatment plan from a predicted reference DVH set derived from voxel-based dose distribution.69 Mahmood et al proposed a KBP workflow for plan generation.70 For oropharyngeal IMRT plan, 3D dose distribution was predicted, and the actual plan was generated forwardly by using DVH-based optimization and a set of constraints at predetermined dose–volume coordinates. In a recent study reported by Fan et al,71 3D dose distribution in head and neck cancer was first predicted by a ResNet-based framework. For plan generation, instead of DVH-based inverse optimization, the inverse problem was solved by an L2-norm problem between the predicted dose distribution and the actual dose distribution. An open-source software of fluence map optimization was utilized for this L2-norm problem, and clinically acceptable plans were generated.72
Emerging Research Directions of AI in ATP
The CNN-based algorithms, particularly deep CNN algorithms with large numbers of hidden layers, have been recently extensively studied in medical imaging, making image-based AI applications a dominant topic in ATP research. Along this research direction, dose distribution prediction to further improve the dose prediction accuracy and efficiency remains the focus. This may lead to a paradigm shift in plan generation: Instead of DVH-based optimization, a plan can be generated as an image reconstruction problem when using predicted dose distribution as the reference data. Direct prediction of plan parameters could become another potential research area. If certain plan parameters can be converted to 2D/3D space object(s) (“equivalent images”), CNN-based algorithms may be utilized for the prediction, which can lead to automatic plan generation. Potentially, 2D fluence maps of static IMRT beams, 2D aperture series of step-and-shoot delivery, and dynamic multi-leaf collimator sequences (2D + time) of VMAT may be candidates for such predictions.
While most recent AI studies in radiation oncology focused on predictions, few have simulated the reasoning process in treatment planning. In the manual treatment planning process, the decision-making strategy when solving a specific dosimetric trade-off problem varies among different planners; one with more experience may make effective actions more efficiently than another qualified planner with less experience.73 Decision-making strategy will be central to the implementation of ATP with fully automated workflow without human intervention.
In manual treatment planning, each decision regarding dose trade-offs leads to one or more actions in series; this feature is well suited for reinforcement learning, which led to the success of AlphaGo, the famous AI success in the board game Go that had been challenging for conventional computer algorithms.74 Reinforcement learning has 2 distinct features: a trial-and-error search and a delayed reward.75 With a defined reward function, the agent attempts to learn the reward function value in each state and takes actions to maximize the reward. Reinforcement learning can also be realized by a direct policy search, in which the agent attempts to learn the reward functions that directly map observations to actions. Like the board game Go, radiotherapy treatment planning consists of sequential actions and long-term consequences. However, unlike board game applications in which problems are deterministic, fully observable, single objective with easy reward definition, radiotherapy treatment planning process is (semi-) stochastic, partially observable, and multiobjective with a challenging definition of reward. These characteristics have to be acknowledged for using reinforcement learning in ATP. For example, an ATP agent can allow only 1 or 2 actions for reinforcement learning (such as DVH constraint weight adjustment or auxiliary planning structure generation via Boolean operations), and the reward has to be defined in a simple way with a numerical scale (eg, target coverage percentage). In summary, to simulate the human reasoning process, reinforcement learning has to be implemented from a simple problem on a small scale before its extension to the full ATP workflow.
Another possible approach of implementing decision-making process in ATP is using generative adversarial networks (GANs), a class of algorithms that generate representative samples from a set of training data by implementing 2 competing networks in a zero-sum task.76 These 2 competing networks, the generator and the discriminator, are trained simultaneously: While the generator is trained in generating samples, the discriminator is trained to assess whether the samples are “good.” The GANs have been investigated in natural language processing and computer vision. Recently, GANs have been utilized in medical image segmentation and disease diagnosis.77,78 The GANs have also been reported for dose distribution for radiotherapy.70 To simulate decision-making for treatment planning, GANs can be used in the model-based reinforcement learning to learn about the environment so that the reinforcement learning agent can take advantage of the previously learned environment (model) instead of simply relying on interaction with the environment (trial-and-error experience). The 2 trained networks must have a competitive relationship in GANs; such a relationship can be simulated by a plan producer that utilizes the predicted plan distribution and a dose distribution predictor that requires produced treatment plans for its training.
Challenges of AI in ATP
Complexity in Treatment Planning: What Should AI Learn?
Like a self-driving vehicle, the treatment planning process can involve a large number of consequential actions. However, unlike those in a self-driving vehicle or a board game, actions in treatment planning may not have direct consequences (ie, any operation during the plan generation has to be evaluated many steps later after the final dose calculation). Thus, simulation of ATP workflow is more computationally expensive compared to board game applications.
To reduce the simulation cost, the complexity of the decision-making logic has to be settled on a manageable level. Fortunately, researchers can reduce such complexity by enforcing basic rules during ATP. The current common practice of manual treatment planning follows rules from different aspects, including machine hardware limitation (eg, forbidden zones for noncoplanar beam angle selection due to collision), radiological-based clinical preferences (eg, prioritized duodenum sparing in liver/pancreas SBRT), and institutional practice guidelines (eg, a beam setup template for standardized bilateral neck IMRT). Integration of these rules by fixing involved variables or enforcing simple “if-else” logic can reduce the ATP workflow complexity. In addition, observations following physical science rules (eg, photon dose requires build-up region with possible coverage reduction of a target near the skin surface) can be integrated into an ATP workflow to further reduce the learning complexity. As a result, building a reasonable ATP workflow for AI training requires a team effort, including radiation physicists, radiation oncologists, radiation dosimetrists, radiation therapists, and other personnel involved in radiotherapy plan generation and verification.
Working With Limited Size Data Sets
Recent progress in deep learning algorithms for imaging applications has been propelled by large-scale data sets. Compared to natural image data sets, medical image data sets have smaller sample sizes for a number of reasons: smaller sample space, patient recruitment, data acquisition variation, lack of infrastructure, and labor-intensive image processing by human experts.4,79 This limitation is compounded for ATP applications, due to the need for specific types of radiotherapy plans, a significant constraint, given the continued advancement and often short histories of modern treatment planning approaches.
So far, most reported ATP studies have included 100 or fewer patients, which is usually acknowledged as a small size data set in the discussion of these studies. Furthermore, the useful data size may get even smaller if confounding variables such as human variations have to be reduced. Relevant studies have reported that dose prediction accuracy could be improved when cases from 2 different radiation oncologists were trained separately.61 In addition, the limited size of data sets is exacerbated by the need for separated training and test data. Ideally, each data set should be separated into 3 subsets: training data, validation data, and test data. Training and validation data are used to train the model, whereas the validation data are used to tune the model during training. Model training can be carried out in a cross-validated fashion. The test data should be used to test the model performance after model validation; these data should be segregated from the model training. When using the limited size data for ATP, however, the test data set is sometimes not used and the validation results are reported as the study end point. However, it is important to note that for increasingly complex AI methods such as those discussed in section 3, a sufficiently large, well-curated, and controlled database and a rigorous model training and testing process following the 3-part data splitting described earlier are especially critical. Without them, overfitting, a modeling error where the model performance is overestimated, tends to happen when an overly complex model is developed based on a limited data set. Using data sets of practically limited small sizes for dose prediction in knowledge-based treatment planning, the more complex algorithms were indeed shown to perform inferiorly to the simpler approaches, likely due to overfitting.80
This overfitting limits model generalization when dealing with new data. One potential approach to counteract size limitations is transfer learning, which generates a model by using a small size data set to tune a model which is trained by a larger data set from another domain.81 A commonly used data set for pertaining of deep learning models for medical image studies (particularly CNN) is ImageNet, which is composed of natural scene images.81,82 Data augmentation is another approach for dealing with limited data. In general, data augmentation increases the usable number of data by adding altered versions of the original data.83 A simple example is to add affine image transformations (translations, rotations, and scaling) to the original image sets during the data training for autosegmentation. A third approach is to incorporate high-level handcrafted features in model learning, which should be treated as representative statistics from small data sets based on previous endeavors. Additional operations can be added into the network topology to avoid overfitting.84 However, this approach requires good knowledge of AI algorithms at a low hierarchy level, which can be challenging for those without a comprehensive computer science background. For ongoing deep learning–based ATP research, it will be interesting to see how the above methods can be adapted and new methods developed to overcome the critical data size limitation.
Toward Clinical Application: Regulation and Collaboration
Although the future of AI in ATP remains in motion, radiotherapy treatment planning will likely not become “driver-less” in the next coming years. The AI-based ATPs must be validated before being introduced into routine clinical use.85 Quality and efficiency improvements should be the focus when evaluating an AI-based ATP method at the current stage. Humans should remain as the center of the treatment planning process in overseeing the clinical treatment planning workflow with ultimate responsibility for plan safety and quality.
Successful validation of AI-based ATP methods requires a large patient cohort size. In addition, for each patient, multiple data types, including multimodality simulation images, treatment plan, radiogenomic tests, and clinical data, may be necessary for methodology development and validation and generalizability. Such an endeavor may require new paradigms of data regulation and supervision in addition to the standard institutional review board functions. Multicenter collaborations could augment patient cohort size for methodology development and validation. However, retrospective data with significant variations among different institutions’ practice may become a problem when investigating AI-based ATP, particularly in the simulation of reasoning logics. Prospective multi-institution studies with detailed guidelines of treatment plan generation are fundamental for future studies. Over the past decade, similar efforts on conforming structure naming to the nomenclature standardization by professional society committees, vendors, and the clinical community have made great strides that substantially facilitated radiotherapy big data research. Therefore, larger numbers of plans from recent clinical trials with rigorous planning guidelines and from the subsequent protocol adoption are expected to yield better quality data for AI-based ATP research. Meanwhile, clinical utilization of the current ATP tools may also help generate plans of more consistent quality among planners and institutions, which could in turn facilitate future AI-based treatment planning research. Finally, each step in the treatment planning workflow requires great engineering efforts to ensure accuracy and safety. For proof-of-concept studies, open software packages can be adopted when demonstrating the feasibility of new AI applications in ATP.72,86 However, when considering clinical validation, a more robust platform based on the vendor-specific automation function should be used. This may require a new model of academic/industrial cooperation to deal with potential issues in data security and intellectual proprietary conflicts and to ensure the active quality assurance that is critical during the clinical deployment of the new tool.
Conclusion
In this work, the current ATP solutions have been reviewed based on their technical characteristics and clinic workflow impacts. In reported clinical investigations, the discussed solutions have demonstrated the improved planning efficiency and plan quality consistency. Artificial intelligence in ATP is an emerging field and is rapidly developing. Recent research works of AI in ATP, particularly deep learning–based investigations, have been summarized. In addition, future research directions regarding AI in ATP have been proposed. Finally, challenges of AI research in ATP and practical issues of potential preclinical and clinical investigations have been discussed. We believe that AI technologies would eventually change the paradigm of radiotherapy treatment planning practice. While embracing the promising future, current researchers should be aware of the limitations of current practice and possible research opportunities of AI to meet health-care needs in the next or 2 decades.
Abbreviations
- AI
artificial intelligence
- ARIR
automated rule implementation and reasoning
- ATP
automatic treatment planning
- CNN
convolutional neural network
- DoseNet
dose prediction neural network
- DVH
dose–volume histogram
- DTH
distance-to-target histogram
- GANs
generative adversarial networks
- iCycle
IMRT cycle
- IMRT
intensity-modulated radiotherapy
- KBP
knowledge-based planning
- MCO
multicriteria optimization
- OAR
organ at risk
- PCA
principal component analysis
- PTV
planning target volume
- SBRT
stereotactic body radiation therapy
- SVR
support vector regression
- TPS
treatment planning system
- VMAT
volumetric modulated arc therapy
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Chunhao Wang  https://orcid.org/0000-0002-6945-7119
https://orcid.org/0000-0002-6945-7119
Julian C. Hong  https://orcid.org/0000-0001-5172-6889
https://orcid.org/0000-0001-5172-6889
Dandan Zheng  https://orcid.org/0000-0003-2259-1633
https://orcid.org/0000-0003-2259-1633
References
- 1. Russell SJ, Norvig P. Artificial Intelligence: A Modern Approach. Upper Saddle River, New Jersey: Pearson Education Limited; 2016. [Google Scholar]
- 2. Poole DL, Mackworth AK, Goebel R. Computational Intelligence: A Logical Approach. New York, NY: Oxford University Press New York; 1998. [Google Scholar]
- 3. Feng M, Valdes G, Dixit N, Solberg TD. Machine learning in radiation oncology: opportunities, requirements, and needs. Front Oncol. 2018;8:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sahiner B, Pezeshk A, Hadjiiski LM, et al. Deep learning in medical imaging and radiation therapy. Med Phys. 2019;46(1):e1–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Foy JJ, Marsh R, Ten Haken RK, et al. An analysis of knowledge-based planning for stereotactic body radiation therapy of the spine. Pract Radiat Oncol. 2017;7(5):e355–e60. [DOI] [PubMed] [Google Scholar]
- 6. Hansen CR, Bertelsen A, Hazell I, et al. Automatic treatment planning improves the clinical quality of head and neck cancer treatment plans. Clin Translat Radiat Oncol. 2016;1:2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang S, Zheng D, Zhang C, et al. Automatic planning on hippocampal avoidance whole-brain radiotherapy. Med Dosim. 2017;42(1):63–68. [DOI] [PubMed] [Google Scholar]
- 8. Zarepisheh M, Long T, Li N, et al. A DVH-guided IMRT optimization algorithm for automatic treatment planning and adaptive radiotherapy replanning. Med Phys. 2014;41(6):061711. [DOI] [PubMed] [Google Scholar]
- 9. Alongi F, Fiorentino A, Gregucci F, et al. First experience and clinical results using a new non-coplanar mono-isocenter technique (HyperArc™) for Linac-based VMAT radiosurgery in brain metastases. J Cancer Res Clin Oncol. 2019;145(1):193–200. [DOI] [PubMed] [Google Scholar]
- 10. Mori Y, Kaneda N, Hagiwara M, Ishiguchi T. Dosimetric study of automatic brain metastases planning in comparison with conventional multi-isocenter dynamic conformal arc therapy and gamma knife radiosurgery for multiple brain metastases. Cureus. 2016;8(11):e882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Y, Heijmen BJ, Petit SF. Prospective clinical validation of independent DVH prediction for plan QA in automatic treatment planning for prostate cancer patients. Radiother Oncol. 2017;125(3):500–506. [DOI] [PubMed] [Google Scholar]
- 12. Boylan C, Rowbottom C. A bias-free, automated planning tool for technique comparison in radiotherapy-application to nasopharyngeal carcinoma treatments. J Appl clin Med Phys. 2014;15(1):213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hussein M, South CP, Barry MA, et al. Clinical validation and benchmarking of knowledge-based IMRT and VMAT treatment planning in pelvic anatomy. Radiother Oncol. 2016;120(3):473–479. [DOI] [PubMed] [Google Scholar]
- 14. Wu H, Jiang F, Yue H, Zhang H, Wang K, Zhang Y. Applying a RapidPlan model trained on a technique and orientation to another: a feasibility and dosimetric evaluation. Radiat Oncol. 2016;11(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fogliata A, Wang PM, Belosi F, et al. Assessment of a model based optimization engine for volumetric modulated arc therapy for patients with advanced hepatocellular cancer. Radiat Oncol. 2014;9(1):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moore KL. Automated radiotherapy treatment planning. Semin Radiat Oncol. 2019;29(3):209–218. [DOI] [PubMed] [Google Scholar]
- 17. Berry SL, Ma R, Boczkowski A, Jackson A, Zhang P, Hunt M. Evaluating inter-campus plan consistency using a knowledge based planning model. Radiother Oncol. 2016;120(2):349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Speer S, Klein A, Kober L, Weiss A, Yohannes I, Bert C. Automation of radiation treatment planning. Strahlenthera Onkol. 2017;193(8):656–665. [DOI] [PubMed] [Google Scholar]
- 19. Gintz D, Latifi K, Caudell J, et al. Initial evaluation of automated treatment planning software. J Appl Clin Med Phys. 2016;17(3):331–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hussein M, Heijmen BJ, Verellen D, Nisbet A. Automation in intensity modulated radiotherapy treatment planning—a review of recent innovations. Br J Radiol. 2018;91(1092):20180270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ng S, Herman J. Stereotactic radiotherapy and particle therapy for pancreatic cancer. Cancers. 2018;10(3):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. System VM. VarianDeveloper.com, 2018.
- 23. Yan H, Yin F-F, Willett C. Evaluation of an artificial intelligence guided inverse planning system: clinical case study. Radiother Oncol. 2007;83(1):76–85. [DOI] [PubMed] [Google Scholar]
- 24. Xhaferllari I, Wong E, Bzdusek K, Lock M, Chen JZ. Automated IMRT planning with regional optimization using planning scripts. J Appl Clin Med Phys. 2013;14(1):176–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tol JP, Dahele M, Peltola J, Nord J, Slotman BJ, Verbakel WF. Automatic interactive optimization for volumetric modulated arc therapy planning. Radiat Oncol. 2015;10(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wilkens JJ, Alaly JR, Zakarian K, Thorstad WL, Deasy JO. IMRT treatment planning based on prioritizing prescription goals. Phys Med Bio. 2007;52(6):1675. [DOI] [PubMed] [Google Scholar]
- 27. Sheng Y, Li T, Yoo S, et al. Development of an ultra-fast, high-quality whole-breast radiation therapy treatment planning system. Int J Radiat Oncol Bio Phys. 2016;96(2):S228. [Google Scholar]
- 28. Kisling K, Zhang L, Simonds H, et al. Fully automatic treatment planning for external-beam radiation therapy of locally advanced cervical cancer: a tool for low-resource clinics. J Global Oncol. 2019;5:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yuan L, Ge Y, Lee WR, Yin FF, Kirkpatrick JP, Wu QJ. Quantitative analysis of the factors which affect the interpatient organ-at-risk dose sparing variation in IMRT plans. Med Phys. 2012;39(11):6868–6878. [DOI] [PubMed] [Google Scholar]
- 30. Zhu X, Ge Y, Li T, Thongphiew D, Yin FF, Wu QJ. A planning quality evaluation tool for prostate adaptive IMRT based on machine learning. Med Phys. 2011;38(2):719–726. [DOI] [PubMed] [Google Scholar]
- 31. Good D, Lo J, Lee WR, Wu QJ, Yin F-F, Das SK. A knowledge-based approach to improving and homogenizing intensity modulated radiation therapy planning quality among treatment centers: an example application to prostate cancer planning. Int J Rad Oncol Bio Phys. 2013;87(1):176–181. [DOI] [PubMed] [Google Scholar]
- 32. Yang Y, Ford EC, Wu B, et al. An overlap-volume-histogram based method for rectal dose prediction and automated treatment planning in the external beam prostate radiotherapy following hydrogel injection. Med Phy. 2013;40(1):011709. [DOI] [PubMed] [Google Scholar]
- 33. Schreibmann E, Fox T. Prior-knowledge treatment planning for volumetric arc therapy using feature-based database mining. J Appl Clin Med Phys. 2014;15(2):19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Munter JS, Sjölund J. Dose-volume histogram prediction using density estimation. Phys Med Bio. 2015;60(17):6923. [DOI] [PubMed] [Google Scholar]
- 35. Ahmed S, Nelms B, Gintz D, et al. A method for a priori estimation of best feasible DVH for organs-at-risk: validation for head and neck VMAT planning. Med Phys. 2017;44(10):5486–5497. [DOI] [PubMed] [Google Scholar]
- 36. Chau RMC, Leung SF, Kam MKM, et al. A broadly adaptive array of dose-constraint templates for planning of intensity-modulated radiation therapy for advanced T-stage nasopharyngeal carcinoma. Int J Radiat Oncol Bio Phys. 2009;74(1):21–28. [DOI] [PubMed] [Google Scholar]
- 37. Zhang J, Sheng Y, Wang C, et al. Modeling of Multiple Planning Target Volumes (PTVs) in Knowledge-Based Planning (KBP) Medical Physics. Hoboken, NJ: Wiley; 2018. [Google Scholar]
- 38. Zawadzka A, Nesteruk M, Brzozowska B, Kukołowicz PF. Method of predicting the mean lung dose based on a patient’s anatomy and dose-volume histograms. Med Dosim. 2017;42(1):57–162. [DOI] [PubMed] [Google Scholar]
- 39. Kuo L, Yorke ED, Dumane VA, et al. Geometric dose prediction model for hemithoracic intensity-modulated radiation therapy in mesothelioma patients with two intact lungs. J Appl Clin Med Phys. 2016;17(3):371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Petit SF, van Elmpt W. Accurate prediction of target dose-escalation and organ-at-risk dose levels for non-small cell lung cancer patients. Radiother Oncol. 2015;117(3):453–458. [DOI] [PubMed] [Google Scholar]
- 41. Tran A, Woods K, Nguyen D, et al. Predicting liver SBRT eligibility and plan quality for VMAT and 4π plans. Radiat Oncol. 2017;12(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sheng Y, Ge Y, Yuan L, Li T, Yin FF, Wu QJ. Outlier identification in radiation therapy knowledge-based planning: a study of pelvic cases. Med Phys. 2017;44(11):5617–5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McIntosh C, Welch M, McNiven A, Jaffray DA, Purdie TG. Fully automated treatment planning for head and neck radiotherapy using a voxel-based dose prediction and dose mimicking method. Phys Med Bio. 2017;62(15):5926. [DOI] [PubMed] [Google Scholar]
- 44. Shiraishi S, Moore KL. Knowledge-based prediction of three-dimensional dose distributions for external beam radiotherapy. Med Phys. 2016;43(1):378–387. [DOI] [PubMed] [Google Scholar]
- 45. Liu J, Wu QJ, Kirkpatrick JP, Yin FF, Yuan L, Ge Y. From active shape model to active optical flow model: a shape-based approach to predicting voxel-level dose distributions in spine SBRT. Phys Med Bio. 2015;60(5):N83. [DOI] [PubMed] [Google Scholar]
- 46. Zhang X, Li X, Quan EM, Pan X, Li Y. A methodology for automatic intensity-modulated radiation treatment planning for lung cancer. Phys Med Bio. 2011;56(13):3873. [DOI] [PubMed] [Google Scholar]
- 47. Yuan L, Wu QJ, Yin F, et al. Standardized beam bouquets for lung IMRT planning. Phys Med Bio. 2015;60(5):1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Monz M, Küfer K-H, Bortfeld TR, Thieke C. Pareto navigation—algorithmic foundation of interactive multi-criteria IMRT planning. Phys Med Bio. 2008;53(4):985. [DOI] [PubMed] [Google Scholar]
- 49. Lahanas M, Schreibmann E, Baltas D. Multiobjective inverse planning for intensity modulated radiotherapy with constraint-free gradient-based optimization algorithms. Phys Med Bio. 2003;48(17):2843. [DOI] [PubMed] [Google Scholar]
- 50. Thieke C, Küfer K-H, Monz M, et al. A new concept for interactive radiotherapy planning with multicriteria optimization: first clinical evaluation. Radiother Oncol. 2007;85(2):292–298. [DOI] [PubMed] [Google Scholar]
- 51. Craft D, Bortfeld T. How many plans are needed in an IMRT multi-objective plan database? Phys Med Bio. 2008;53(11):2785. [DOI] [PubMed] [Google Scholar]
- 52. Breedveld S, Storchi PR, Voet PW, Heijmen BJ. Icycle: Integrated, multicriterial beam angle, and profile optimization for generation of coplanar and noncoplanar IMRT plans. Med Phys. 2012;39(2):951–963. [DOI] [PubMed] [Google Scholar]
- 53. Breedveld S, Storchi PR, Keijzer M, Heemink AW, Heijmen BJ. A novel approach to multi-criteria inverse planning for IMRT. Phys Med Bio. 2007;52(20):6339. [DOI] [PubMed] [Google Scholar]
- 54. Buergy D, Sharfo AWM, Heijmen BJ, et al. Fully automated treatment planning of spinal metastases–a comparison to manual planning of volumetric modulated arc therapy for conventionally fractionated irradiation. Radiat Oncol. 2017;12(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Voet PW, Dirkx ML, Breedveld S, Al-Mamgani A, Incrocci L, Heijmen BJ. Fully automated volumetric modulated arc therapy plan generation for prostate cancer patients. Int J Radiat Oncol Bio Phys. 2014;88(5):1175–1179. [DOI] [PubMed] [Google Scholar]
- 56. Sharfo AWM, Voet PW, Breedveld S, Mens JWM, Hoogeman MS, Heijmen BJ. Comparison of VMAT and IMRT strategies for cervical cancer patients using automated planning. Radiother Oncol. 2015;114(3):395–401. [DOI] [PubMed] [Google Scholar]
- 57. Sharfo AWM, Stieler F, Kupfer O, et al. Automated VMAT planning for postoperative adjuvant treatment of advanced gastric cancer. Radiat Oncol. 2018;13(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436. [DOI] [PubMed] [Google Scholar]
- 59. Boutilier JJ, Lee T, Craig T, Sharpe MB, Chan TC. Models for predicting objective function weights in prostate cancer IMRT. Med Phys. 2015;42(4):1586–1595. [DOI] [PubMed] [Google Scholar]
- 60. Ma M, Kovalchuk N, Buyyounouski MK, Xing L, Yang Y. Dosimetric features-driven machine learning model for DVH prediction in VMAT treatment planning. Med Phys. 2019;46(2):857–867. [DOI] [PubMed] [Google Scholar]
- 61. Campbell WG, Miften M, et al. Neural network dose models for knowledge-based planning in pancreatic SBRT. Med Phys. 2017;44(12):6148–6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nguyen D, Long T, Jia X, et al. Dose prediction with U-net: a feasibility study for predicting dose distributions from contours using deep learning on prostate IMRT patients. arXiv preprint arXiv:170909233 2017.
- 63. Ronneberger O, Fischer P, Brox T. U-net: Convolutional networks for biomedical image segmentation In: Navab N, Hornegger J, Wells W, Frangi A, eds. International Conference on Medical Image Computing and Computer-Assisted Intervention. Cham, Switzerland: Springer; 2015:234–241. [Google Scholar]
- 64. Kearney V, Chan JW, Haaf S, Descovich M, Solberg TD. DoseNet: a volumetric dose prediction algorithm using 3D fully-convolutional neural networks. Phys Medi Bio. 2018;63(23):235022. [DOI] [PubMed] [Google Scholar]
- 65. He K, Zhang X, Ren S, Sun J. Deep residual learning for image recognition Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition; 2016; Las Vegas, NV. [Google Scholar]
- 66. Chen X, Men K, Li Y, Yi J, Dai J. A feasibility study on an automated method to generate patient-specific dose distributions for radiotherapy using deep learning. Med Phys. 2019;46(1):56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Huang G, Liu Z, Van Der Maaten L, Weinberger KQ. Densely connected convolutional networks Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition; 2017; Honolulu, HI. [Google Scholar]
- 68. Barragan-Montero AM, Nguyen D, Lu W, et al. Three-dimensional dose prediction for lung IMRT patients with deep neural networks: robust learning from heterogeneous beam configurations. arXiv preprint arXiv:181206934 2018. [DOI] [PubMed]
- 69. Long T, Chen M, Jiang S, Lu W. Threshold-driven optimization for reference-based auto-planning. Phys Med Bio. 2018;63(4):04NT1. [DOI] [PubMed] [Google Scholar]
- 70. Mahmood R, Babier A, McNiven A, Diamant A, Chan TC. Automated treatment planning in radiation therapy using generative adversarial networks. arXiv preprint arXiv:180706489 2018.
- 71. Fan J, Wang J, Chen Z, Hu C, Zhang Z, Hu W. Automatic treatment planning based on three-dimensional dose distribution predicted from deep learning technique. Med Phys. 2019;46(1):370–381. [DOI] [PubMed] [Google Scholar]
- 72. Wieser HP, Cisternas E, Wahl N, et al. Development of the open-source dose calculation and optimization toolkit matRad. Med Phys. 2017;44(6):2556–2568. [DOI] [PubMed] [Google Scholar]
- 73. Sharpe MB, Moore KL, Orton CG. Within the next ten years treatment planning will become fully automated without the need for human intervention. Med Phys. 2014;41(12):120601. [DOI] [PubMed] [Google Scholar]
- 74. Chen JX. The evolution of computing: AlphaGo. Comput Sci Eng. 2016;18(4):4–7. [Google Scholar]
- 75. Sutton RS, Barto AG. Reinforcement learning: An Introduction. Cambridge, MA: MIT press; 2018. [Google Scholar]
- 76. Finn C, Christiano P, Abbeel P, Levine S. A connection between generative adversarial networks, inverse reinforcement learning, and energy-based models. arXiv preprint arXiv:161103852 2016.
- 77. Kohl S, Bonekamp D, Schlemmer HP, et al. Adversarial networks for the detection of aggressive prostate cancer. arXiv preprint arXiv:170208014 2017.
- 78. Xue Y, Xu T, Zhang H, Long LR, Huang X. Segan: adversarial network with multi-scale l 1 loss for medical image segmentation. Neuroinformatics. 2018;16(3-4):383–392. [DOI] [PubMed] [Google Scholar]
- 79. Bibault JE, Giraud P, Burgun A. Big data and machine learning in radiation oncology: state of the art and future prospects. Cancer Lett. 2016;382(1):110–117. [DOI] [PubMed] [Google Scholar]
- 80. Landers A, Neph R, Scalzo F, Ruan D, Sheng K. Performance comparison of knowledge-based dose prediction techniques based on limited patient data. Technol Cancer Res Treat. 2018;17:1533033818811150 doi: 10.1177/1533033818811150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Shin H-C, Roth HR, Gao M, et al. Deep convolutional neural networks for computer-aided detection: CNN architectures, dataset characteristics and transfer learning. IEEE Transact Med Imaging. 2016;35(5):1285–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Deng J, Dong W, Socher R, Li LJ, Li K, Fei-Fei L. Imagenet: a large-scale hierarchical image database. IEEE Conference on Computer Vision and Pattern Recognition; 2009; Miami Beach, FL. [Google Scholar]
- 83. Wong SC, Gatt A, Stamatescu V, McDonnell MD. Understanding data augmentation for classification: when to warp? Paper presented at: 2016 International Conference on Digital Image Computing: Techniques and Applications (DICTA) Gold Coast, Australia: IEEE; 2016. [Google Scholar]
- 84. Srivastava N, Hinton G, Krizhevsky A, Sutskever I, Salakhutdinov R. Dropout: a simple way to prevent neural networks from overfitting. J Machine Learn Res. 2014;15(1):1929–1958. [Google Scholar]
- 85. Thompson RF, Valdes G, Fuller CD, et al. Artificial intelligence in radiation oncology imaging. Int J Radiat Oncol Bio Phys. 2018;102(4):1159–1161. [DOI] [PubMed] [Google Scholar]
- 86. Deasy JO, Blanco AI, Clark VH. CERR: a computational environment for radiotherapy research. Med Phys. 2003;30(5):979–985. [DOI] [PubMed] [Google Scholar]