Abstract
Background
The therapeutic potential of cardiac μ-opioid receptors in ischaemia-reperfusion (I/R) injury during opioid-modulating diseases, such as heart failure, is unknown. We aimed to explore the changes of cardiac μ-opioid receptor expression during heart failure, and its role in opioid-induced cardioprotection.
Methods
Rats received doxorubicin (DOX) or were subjected to coronary artery ligation to induce heart failure, or received normal saline (NS) as control. Hearts from NS or DOX rats were isolated and subjected to myocardial ischaemia and reperfusion in an in vitro perfusion system. The opioid [D-Ala,2N-MePhe,4 Gly-ol]-enkephalin (DAMGO), with a high μ-opioid receptor specificity, morphine, and remifentanil were administrated before I/R with or without opioid receptor antagonists, or an extracellular signal-regulated kinase (ERK) inhibitor.
Results
Cardiac μ-opioid receptor mRNA concentrations were 3.2 times elevated in DOX-treated rats compared with NS rats, while cardiac μ-opioid receptor protein concentrations showed 6.1- and 3.5-fold increases in DOX-treated and post-infarcted rats, respectively. DAMGO reduced I/R-caused infarct size, expressed as the ratio of area at risk, from 0.50 (0.04) to 0.25 (0.03) in failing rat hearts, but had no effect on infarct size in control hearts. DAMGO promoted phosphorylation of ERK and glycogen synthase kinase (GSK)-3β only in failing hearts. DAMGO-mediated cardioprotection was blocked by an ERK inhibitor. The μ-opioid receptor antagonist D-Pen-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP) prevented morphine- and remifentanil-induced cardioprotection and phosphorylation of ERK and GSK-3β in failing hearts. In contrast, δ- and κ-opioid receptor selective antagonists were less potent than CTOP in the failing hearts.
Conclusions
Cardiac μ-opioid receptors were substantially up-regulated during heart failure, which increased DAMGO-induced cardioprotection against I/R injury.
Keywords: extracellular signal-regulated MAP kinases, glycogen synthase kinases, heart failure, myocardial reperfusion injury, opioid receptors
Editor's key points.
-
•
Heart failure is associated with alterations in cardiac opioid expression, thereby modulating opioid-induced protection against myocardial reperfusion injury.
-
•
The role of cardiac μ-opioid receptors during myocardial ischaemic protection in heart failure has not been elucidated.
-
•
This study shows that heart failure induced by doxorubicin or coronary artery ligation increases cardiac μ-opioid receptor protein levels.
-
•
Heart failure further enhanced μ-opioid receptor-mediated cardioprotection in an in vitro ischaemia-reperfusion model, suggesting that disease conditions modulate the cardioprotective effects of opioids.
Heart failure is a leading cause of morbidity and mortality worldwide.1 Patients with heart failure during surgery face a higher mortality rate because of exaggerated ischaemia-reperfusion (I/R) injury.2 Ischaemia preconditioning (IPC) can confer profound protection of the reperfused myocardium after ischaemia.3 However, IPC-induced cardioprotection can be abolished in the presence of comorbidities such as hypertension, hyperlipidaemia, diabetes, and heart failure.4, 5, 6 IPC-induced cardioprotection can be mimicked or blocked by opioid receptor agonists or antagonists,7, 8, 9, 10 suggesting that opioid receptor signalling plays a role in myocardial injury during ischaemia.
The opioid receptor family mainly comprises the three μ-, δ-, κ-opioid receptor subtypes.11 Previous studies have shown that δ- and κ-receptors are expressed in adult myocardium across different mammalian species,12, 13 but the detection of the μ-opioid receptor in the adult heart has been very challenging because of absent or very low expression rates.14, 15, 16, 17 Several studies revealed up- or down-regulation of cardiac δ- and κ-opioid receptors in response to heart failure.18, 19, 20 This cardiac opioidergic system alteration might modulate cardiac function during heart failure, but whether or not cardiac μ-opioid receptors are altered in the progression of heart failure is still unknown.
To address this question, we here investigated various experimental approaches to detect the level of cardiac μ-opioid receptor in opioid-induced cardioprotection during chronic heart failure, and the involved downstream signal kinases of extracellular signal-regulated kinase (ERK) and glycogen synthase kinase (GSK)-3β.
Methods
This animal experimental study was designed according to the Animals in Research: Reporting In Vivo Experiments (ARRIVE) guidelines.21 All experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication Number 85–23, revised 1996) and approved by the Institutional Animal Care and Use Committee of Anhui Medical University.
Animal model
Adult male Sprague-Dawley rats received doxorubicin (DOX; LC laboratories, Woburn, MA, USA) weekly via the tail vein over a period of 8 weeks to induce chronic heart failure as described before.22 A second group of rats received an equal volume of normal saline (NS) as control. DOX-induced heart failure was confirmed by echocardiography, histological examination, and plasma brain natriuretic peptide (BNP) measurement as reported in the Supplementary material. Myocardial infarction was induced by ligation of the left anterior descending coronary artery (LAD), and post-infarction heart failure was confirmed 8 weeks after LAD ligation by echocardiography.23 The adequacy of anaesthesia was assessed and confirmed based on the loss of righting reflex and non-response to tail and hind limb toe pinch in rats before and during surgery. For postoperative analgesia, buprenorphine (0.05 mg kg−1) was administered s.c. every 12 h for the first 3 postoperative days.
Detection of cardiac μ-opioid receptors
Total RNA and proteins were extracted from ventricular myocardium of normal (NS) or failing (DOX) rats. Quantitative reverse transcription-polymerase chain reaction (RT-PCR) methods were performed to detect the mRNA concentrations of cardiac μ-opioid receptors. The specific binding activity of cardiac μ-opioid receptors was identified by radioligand binding assay. Western blot assay was applied to observe the protein concentrations of cardiac μ-opioid receptor in rats with DOX-induced or post-infarction heart failure. The co-localisation of cardiac μ-opioid receptors with α-sarcomeric actin (α-SA) or transient receptor potential vanilloid 1 (TRPV1) were examined by double immunofluorescence. Expanded methods are presented in the Supplementary material.
In vitro isolated heart experiments
All isolated rat hearts were stabilised for 15 min and subjected to 30 min ischaemia followed by 120 min reperfusion in a Langendorff apparatus. The synthetic opioid [D-Ala,2 N-MePhe,4 Gly-ol]-enkephalin (DAMGO; Cayman Chemical, Ann Arbor, Michigan, USA), morphine, or remifentanil preconditioning were administered in three cycles of 5 min infusion of 100 nmol litre−1 DAMGO, 1 μmol litre−1 morphine (Shenyang First Pharmaceutical Factory, Shenyang, China), or 0.1 μmol litre−1 remifentanil (Yichang Humanwell Pharmaceutical Co., Yichang, China) before I/R injury. Doses were based on preliminary experiments and previous studies.22, 24 Three opioid receptor antagonists, D-Pen-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP, 1 μmol litre−1), naltrindole hydrochloride (NTD, 5 μmol litre−1), and nor-binaltorphimine dihydrochloride (BNI, 5 μmol litre−1) (all Tocris Bioscience, Bristol, UK), or vehicle (distilled water or dimethylsulfoxide) were perfused 10 min before preconditioning and lasted for 45 min. The doses of these antagonists were based on preliminary experiments. In all groups, heart slices were stained by triphenyltetrazolium chloride for infarct size determination and the coronary effluent was collected to evaluate lactate dehydrogenase (LDH) activity. Tissue samples were obtained from left ventricular myocardium 10 min after reperfusion for western blot assay.
Statistical analysis
Data are presented as mean (standard error of the mean, sem) and analysed with GraphPad Prism version 5.0 for Windows (San Diego, CA, USA). Unpaired t-test was used for comparison between two groups and one-way analysis of variance (anova) followed by a Tukey's test or two-way anova followed by a Bonferroni's test was used for multiple comparisons of more than two groups. Repeated measures of anova followed by a Bonferroni's test were performed to analyse the LDH values and haemodynamics parameters at different time points. A P value <0.05 was considered statistically significant. The sample size in each group was calculated using a freely available software G*Power Version 3.1.7 (Franz Faul, Uiversität Kiel, Germany).25
Results
A total of 401 rats were used in the study. Among these, 290 rats were injected with DOX and 195 survived at the end of the 8th week, with a mortality of 33%. Overall, 97 rats were injected with NS and all survived during the experiment. A total of 14 rats were used in preparing the post-infarction heart failure model, and two of them with LAD ligation were discarded because of unscheduled death. In the in vitro isolated perfused heart experiments, three normal hearts were excluded from the study because of left ventricular developed pressure (LVDP) <9.31 kPa (one rat) and serious arrhythmia (two rats) during the equilibration period, and 18 failing hearts were excluded due to LVDP<9.31 kPa (four rats), heart rate <70 beats min−1 (eight rats) in the equilibration period, and cardiac arrest during reperfusion (five rats).24, 26
Up-regulation of cardiac μ-opioid receptors in rats with heart failure
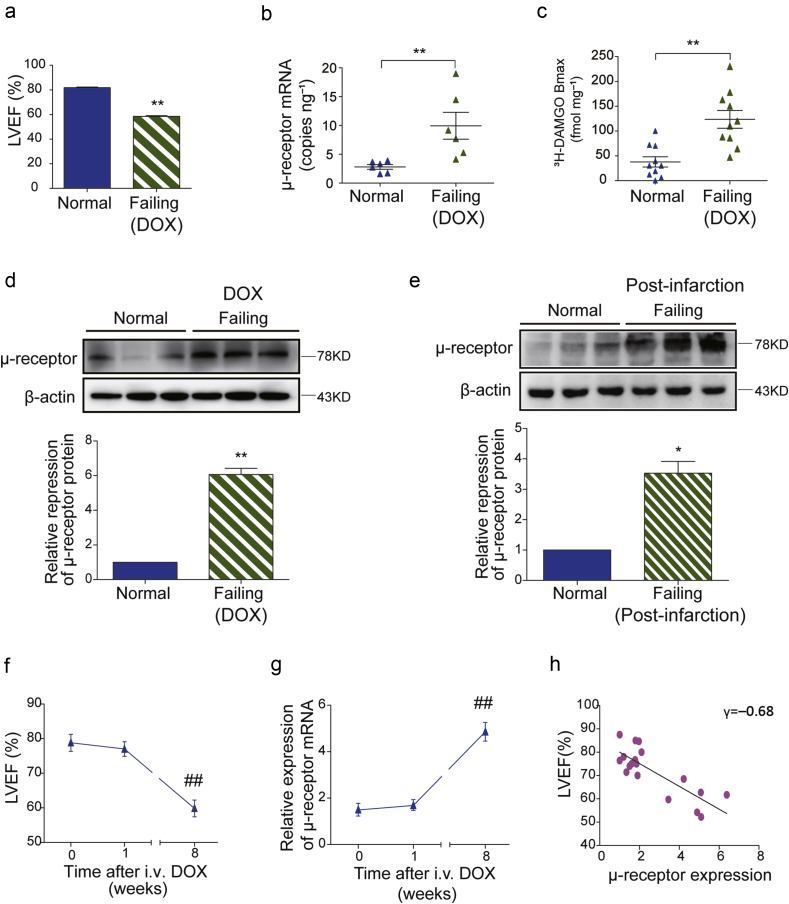
Chronic DOX treatment for 8 weeks resulted in a significant left ventricular cardiac impairment (Fig. 1a). The DOX-induced heart failure model was further confirmed by other cardiac parameters, pathological changes, and plasma BNP levels (Supplementary Fig. 1a–d). We first used absolute quantitative RT-PCR to examine the copy number of cardiac μ-opioid receptor mRNA in normal and failing hearts. This analysis was based on standard curves of μ-opioid receptor and β-actin plasmid DNA (Supplementary Fig. 2a–c).
Fig 1.
Up-regulation of cardiac μ-opioid receptors in rats with heart failure. (a) Left ventricular ejection fraction (LVEF) values of normal saline (NS, normal) or doxorubicin (DOX, failing) injected rats were measured and automatically calculated by the echocardiography system at the end of the 8th week (n=20). (b) The copy numbers of cardiac μ-opioid receptor mRNA in normal and DOX-induced failing rat heart tissues were detected by absolute quantitative RT-PCR (n=6). (c) The μ-opioid receptor specific binding capacity measured by radio-labelled specific μ-opioid receptor agonist [3H] DAMGO (n=10). (d) Representative immunoreactive bands of cardiac μ-opioid receptor protein in normal (NS) and DOX-induced failing rat heart samples. The relative expression of cardiac μ-opioid receptor protein was normalised to β-actin and the value in the normal group was assigned as 1 (n=6). (e) Representative immunoreactive bands of cardiac μ-opioid receptor protein in normal (Sham) and post-infarction failing rat heart samples. The relative expression of cardiac μ-opioid receptor protein was normalised to β-actin and the value in the normal group was assigned as 1 (n=6). *P<0.05, **P<0.01; unpaired Student's t-test. (f) LVEF was evaluated by echocardiography before (0 week), and at 1, 8 weeks after DOX injection. (g) Cardiac μ-opioid receptor mRNA concentrations were detected by relative quantitative RT-PCR at 0, 1, and 8 weeks after DOX injection. Each data point represents mean (sem) from six rats. ##P<0.01 compared with the initial data point by one-way anova followed by Tukey's test. (h) Correlational analysis of LVEF (%) and cardiac μ-opioid receptor relative expression (P<0.01 by linear regression, n=18). anova, analysis of variance; DAMGO [D-Ala,2N-MePhe,4 Gly-ol],-enkephalin; sem, standard error of the mean.
The copy number of cardiac μ-opioid receptor mRNA in the failing heart was significantly up-regulated [9.95 (2.33) copies ng−1] compared with normal hearts [2.80 (0.40) copies ng−1] (Fig. 1b), which was 3.2 times higher than that of normal hearts after normalised to reference β-actin mRNA (Supplementary Fig. 2d and e). A similar scenario was observed in experiments using relative RT-PCR in which the levels of μ-opioid receptor mRNA were substantially elevated in the failing but not normal hearts (Supplementary Fig. S2f).
We next conducted a radioactive binding assay to examine the specific binding activity of cardiac μ-opioid receptors. The maximum binding capacity (Bmax) value of μ-opioid receptor specific binding was 123 (18) fmol mg−1 protein in the failing heart membranes, higher than the value of 37 (10) fmol mg−1 protein in the normal one (Fig. 1c).
Western blot results showed that the immunoreactive signal of μ-opioid receptor protein was almost invisible in normal heart tissue, but apparent in all samples collected from DOX-induced failing rat hearts. The relative expression level of μ-opioid receptor protein in DOX failing hearts was substantially elevated by 6.1-fold from that of normal hearts (Fig. 1d). We further tested the expressional levels of cardiac μ-opioid receptor protein in rats with post-infarction heart failure. Ligation of LAD for 8 weeks induced heart failure in rats (Supplementary Fig. 3a–c). In these animals, the expression levels of cardiac μ-opioid receptor protein were increased by 3.5-fold compared with normal animals (Fig. 1e).
Correlation of cardiac μ-opioid receptors up-regulation with the development of heart failure
Next, we examined and compared the time courses of cardiac dysfunction and μ-opioid receptor up-regulation during chronic DOX treatment. While the value of left ventricular ejection fraction (LVEF) did not significantly differ from baseline levels at 1 week after DOX treatment, this value was substantially decreased at 8 weeks after DOX injection (Fig. 1f). Similarly, no significant alteration was observed in μ-opioid receptor mRNA after 1 week of DOX treatment, whereas the μ-opioid receptor mRNA concentration was markedly elevated 8 weeks after DOX treatment (Fig. 1g). There is a strong correlation between the up-regulation of cardiac μ-opioid receptor and the value of LVEF (Fig. 1h, γ=−0.68).
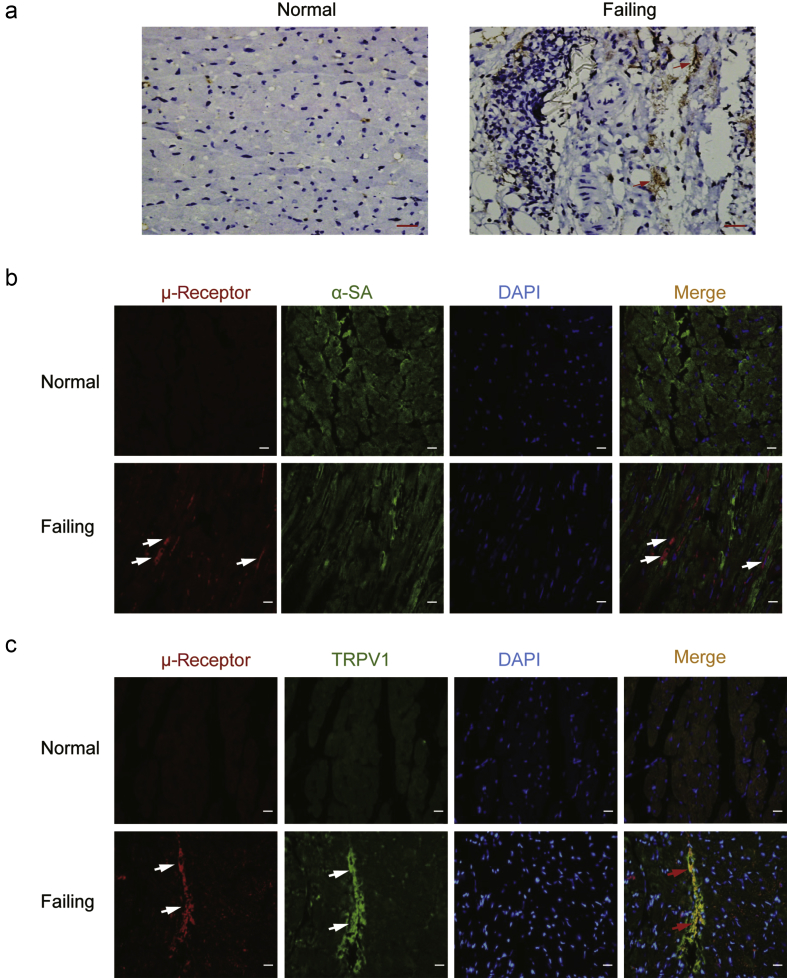
Cell-type specific localisation of cardiac μ-opioid receptors in failing rat hearts
Immunohistochemical staining showed that there was almost no detectable staining of μ-opioid receptors in the slices from normal ventricular myocardium. However, the positive expression of μ-opioid receptors (brown colour) was observed in slices prepared from DOX-induced failing hearts (Fig. 2a). We used double-immunofluorescence labelling to examine the co-localisation of the μ-opioid receptor with α-SA, a marker specific for cardiomyocyte (Fig. 2b), or TRPV1, a receptor expressed on cardiac sensory nerve terminals (Fig. 2c). Interestingly, the cardiac μ-opioid receptors (red) were not co-localised with α-SA as indicated in green. Instead, the μ-opioid receptors appeared to be predominantly co-expressed in TRPV1 positive cells (yellow).
Fig 2.
Cellular localisation of cardiac μ-opioid receptors in the ventricular myocardium. (a) The localisation of cardiac μ-opioid receptors in normal and failing rat myocardium was examined by immunohistochemistry (n=4). Red arrows indicate positive staining (brown). Bar=50 μm. Double immunofluorescence method was used to detect the co-localisation of cardiac μ-opioid receptors (red) with (b) α-sarcomeric actin (α-SA, green) or (c) transient receptor potential vanilloid type 1 (TRPV1, green) (n=4). The nuclei stained bright blue with 4′-6-diamidino-2-phenylindole (DAPI). White arrows indicate the separate positive staining and the red arrows indicates merge staining (yellow). Bar=20 μm.
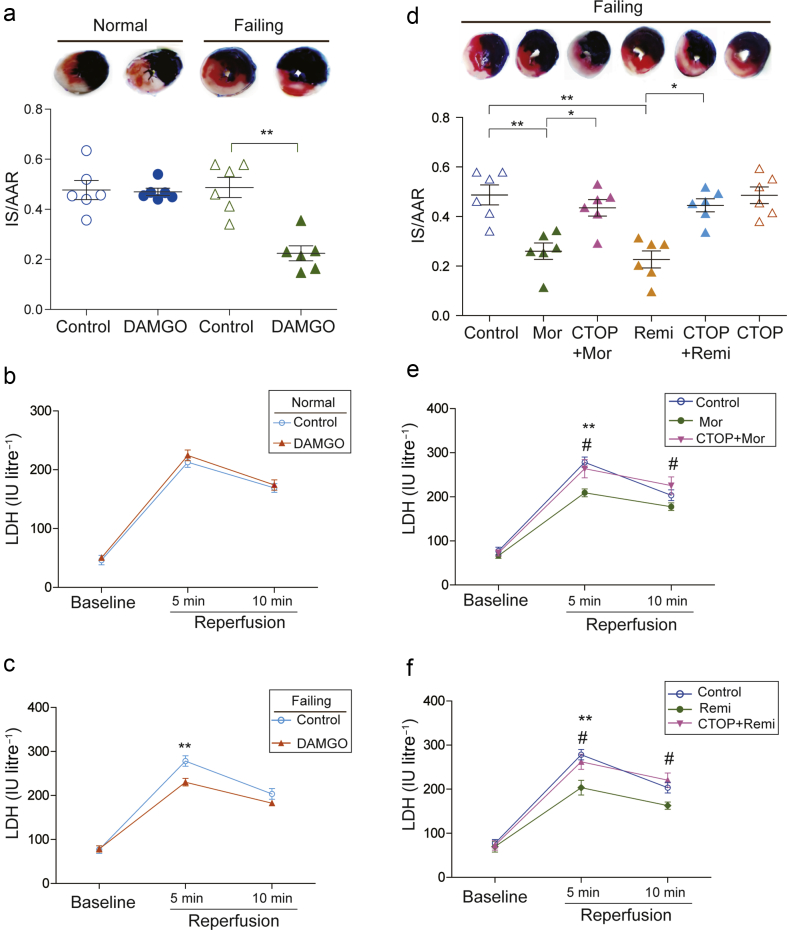
μ-Opioid receptor dependent cardioprotection in isolated failing hearts
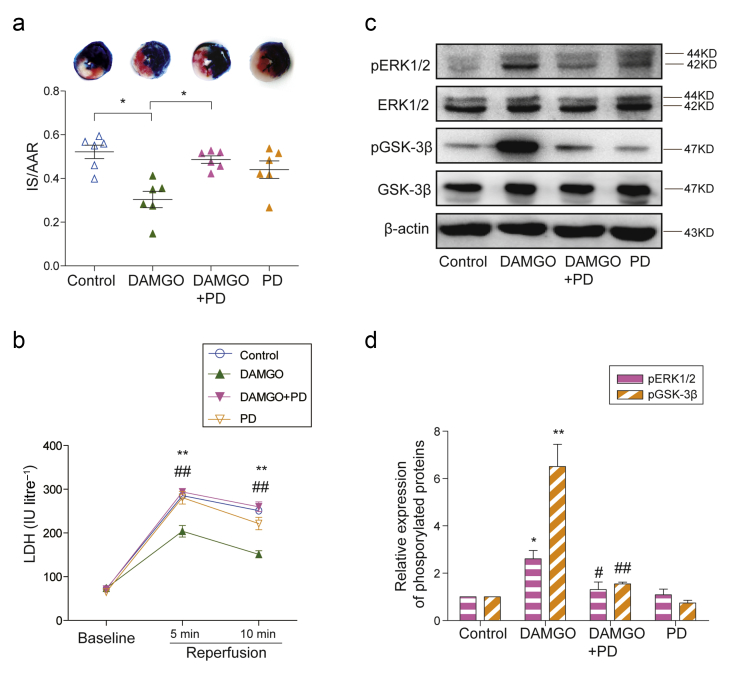
To investigate whether the activation of cardiac μ-opioid receptor could reduce myocardial I/R injury, the normal or DOX-induced failing rat hearts were pre-treated with DAMGO, the specific μ-opioid receptor agonist. DAMGO significantly reduced I/R-caused infarct size, as indicated by infarct size/area at risk (AAR), from 0.50 (0.04) to 0.25 (0.03) in failing rat hearts (Fig. 3a). DAMGO also significantly inhibited the elevated LDH activity of the coronary effluents after 5 min reperfusion (Fig. 3c). However, DAMGO did not significantly alter either the infarct size or LDH activity in the normal isolated rat hearts (Fig. 3a and b). On the contrary, pre-treatment with CTOP, a specific μ-opioid receptor antagonist, abolished morphine- and remifentanil-induced reduction of infarct size (Fig. 3d). The inhibitory effects of morphine and remifentanil on the elevated LDH activity in the isolated failing hearts were also completely prevented by CTOP (Fig. 3e and f). In all groups, the ratio of AAR to the volumes of left ventricle and right ventricle was not significantly different (Fig. 4a and b). Meanwhile, the haemodynamics parameters were not significantly altered between treated groups (Supplementary Tables S1 and S2).
Fig 3.
μ-Opioid receptor dependent cardioprotection in isolated failing hearts. (a) Representative photographs of heart sections from normal and failing hearts with or without DAMGO pre-treatment. The non-ischaemic area is stained in blue, area at risk (AAR) is in brick red, and infarct size (IS) is in white. Myocardial infarct size is expressed as a ratio of IS/AAR in normal and failing hearts (n=6, **P<0.01, two-way anova followed by Bonferroni's test). The coronary effluents were collected at baseline, 5 min, and 10 min after reperfusion from isolated (b) normal and (c) failing hearts, to measure the activity of lactate dehydrogenase (LDH). Each data point represents the average of mean (sem) from six rats. **P<0.01 compared with control group by two-way repeated anova followed by Bonferroni's test. (d) The μ-opioid receptor antagonist CTOP reversed morphine and remifentanil-reduced IS/AAR in isolated failing hearts (n=6, **P<0.01, *P<0.05, one-way anova followed by Tukey's test). The reduction of LDH activities induced by (e) morphine and (f) remifentanil were reversed by the μ-opioid receptor antagonist CTOP. Each data point represents mean (standard error of mean) from six rats. **P<0.01 compared with control group, #P<0.05 compared with morphine or remifentanil group by two-way repeated anova followed by Bonferroni's test. anova, analysis of variance; Mor, Morphine; Remi, Remifentanil; DAMGO [D-Ala,2N-MePhe,4 Gly-ol],-enkephalin; CTOP, D-Pen-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2; sem, standard error of the mean.
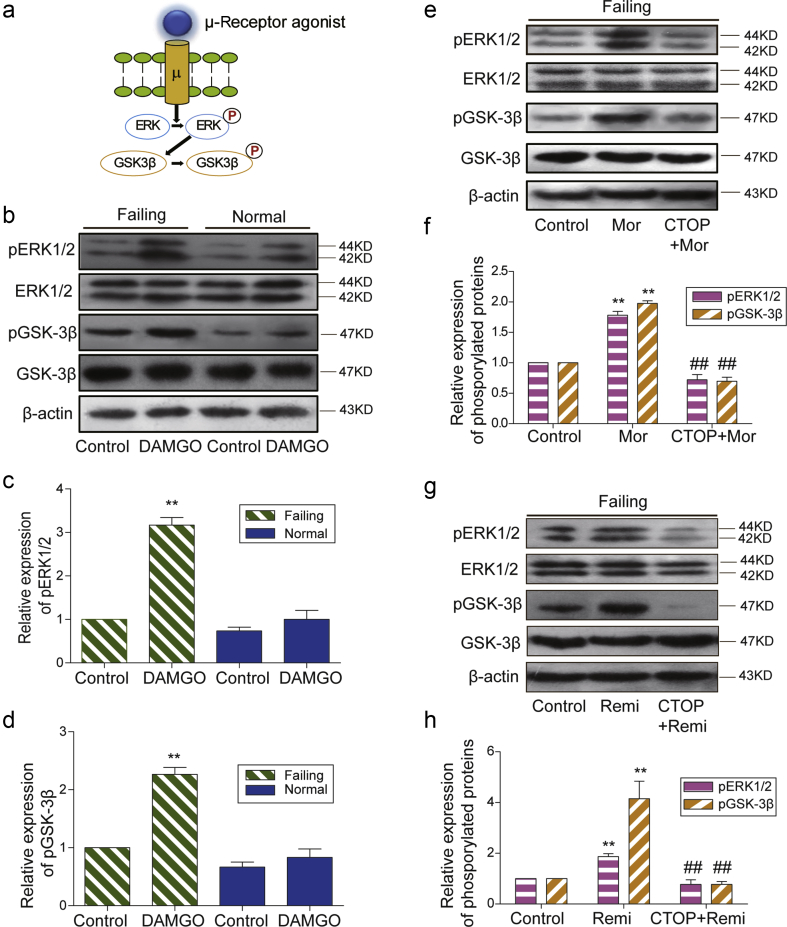
Fig 4.
μ-Opioid receptor dependent enhancement of ERK and GSK-3β phosphorylation. (a) A cartoon shows the activation of μ-opioid receptor by its agonists (DAMGO, morphine, or remifentanil) leading to phosphorylation (P) of ERK1/2 and downstream GSK-3β. (b) The μ-opioid receptor agonist DAMGO differentially induced the phosphorylation of ERK1/2 and GSK-3β in failing and normal hearts. The relative expression of (c) p-ERK1/2 or (d) p-GSK-3β was normalised to total ERK1/2 or total GSK-3β and β-actin. The value in failing control group was assigned as 1. Each bar graph represents the average of mean (sem) from four rats. **P<0.01 compared with control group by two-way anova followed by Bonferroni's test. The μ-opioid receptor antagonist CTOP inhibited (e) morphine and (g) remifentanil-induced phosphorylation of ERK1/2 and GSK-3β in failing hearts. The relative expression of (f) p-ERK1/2 or (h) p-GSK-3βwas normalised to total ERK1/2 or total GSK-3β and β-actin. The value in the control group was assigned as 1. Each bar graph represents the average of mean (sem) from four rats. **P<0.01 compared with the control group, ##P<0.01 compared with morphine or remifentanil group by one-way anova followed by Tukey's test. anova, analysis of variance; P, phosphorylation; Mor, Morphine; Remi, Remifentanil; DAMGO [D-Ala,2N-MePhe,4 Gly-ol],-enkephalin; CTOP, D-Pen-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2; ERK, extracellular signal-regulated kinase; GSK3ß, glycogen synthase kinase; sem, standard error of the mean.
μ-Opioid receptor dependent enhancement of ERK and GSK-3β phosphorylation
To explore the specific pathway downstream of cardiac μ-opioid receptor activation in isolated hearts, we first examined the effect of μ-opioid receptor agonists on the phosphorylation levels of ERK1/2 and GSK-3β (Fig. 4a). DAMGO preconditioning significantly increased the phosphorylation levels of both ERK1/2 and GSK-3β in failing hearts but not in normal hearts (Fig. 4b–d). The phosphorylation levels of ERK1/2 and GSK-3β were also enhanced by morphine and remifentanil in failing hearts, and such enhancement was completely abolished by CTOP (Fig. 4e–i). The phosphorylation levels of these kinases did not significantly differ between CTOP alone and I/R groups (Fig. 5).
Fig 5.
ERK signalling dependent mechanism of μ-opioid receptor-induced cardioprotection. (a) The ERK inhibitor PD98059 (PD) blocked the reduction of DAMGO on infarct size/area at risk (IS/AAR) in isolated failing hearts (n=6, *P<0.05, one-way anova followed by Tukey's test). (b) The coronary effluents from isolated failing hearts were collected at baseline, 5 min, and 10 min after reperfusion to measure the activity of LDH. Each data point represents the average of mean (sem) from six rats. **P<0.01 compared with control group, ## P<0.01 compared with DAMGO group by two-way repeated anova followed by Bonferroni's test. (c) The ERK inhibitor PD98059 suppressed DAMGO-induced phosphorylation levels of ERK1/2 and GSK-3β in failing hearts. (d) The relative expression of p-ERK1/2 or p-GSK-3β was normalised to total ERK1/2 or total GSK-3β and β-actin. The value in control group was assigned as 1. Each bar graph represents the average of mean (sem) from four rats. **P<0.01, *P<0.05 compared with control group, ##P<0.01, #P<0.05 compared with DAMGO group by one-way anova followed by Tukey's test. anova, analysis of variance; DAMGO [D-Ala,2N-MePhe,4 Gly-ol],-enkephalin; PD, PD98059; ERK, extracellular signal-regulated kinase; GSK3ß, glycogen synthase kinase; LDH, lactate dehydrogenase; sem, standard error of the mean.
ERK signalling dependent mechanism of μ-opioid receptor-induced cardioprotection
Next, we aimed to prove the causality between ERK1/2 phosphorylation and μ-opioid receptor-induced cardioprotection in failing rat hearts. To do this, PD98059, the specific inhibitor of ERK pathway, was added before DAMGO administration. The results showed that DAMGO-reduced infarct size was abrogated by the pre-treatment of PD98059 (Fig. 5a). Likely, DAMGO-induced decrease of LDH activities at reperfusion was restored by the addition of PD98059 (Fig. 5b). In addition, the phosphorylation levels of both ERK1/2 and GSK-3β enhanced by DAMGO were markedly suppressed by the ERK inhibitor (Fig. 5c and d). However, the ratios of AAR/left ventricle+right ventricle (Supplementary Fig. 4c) and the haemodynamics parameters (Supplementary Table S3) were not altered between treated groups.
Differential roles of cardiac opioid receptor subtypes in opioid-induced cardioprotection with and without heart failure
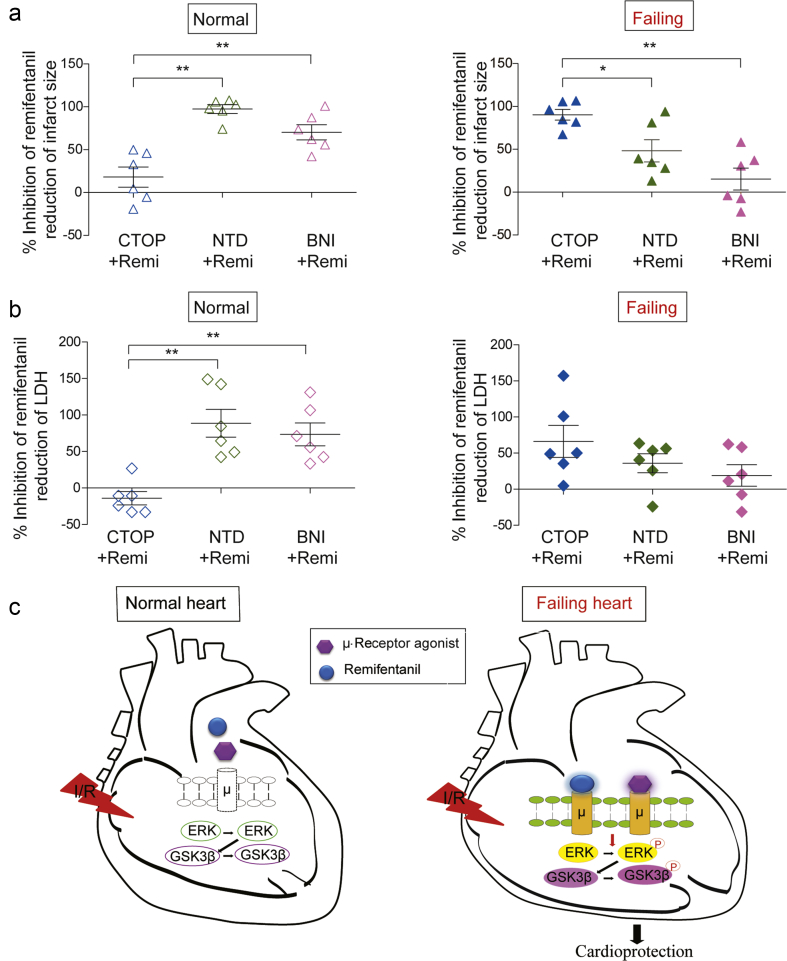
Finally, we compared in a parallel the efficacies of the antagonists selective for different opioid receptor subtypes in inhibition of remifentanil-induced cardioprotective effects in isolated normal and failing hearts. In line with previous observations,24 the antagonists selective for δ-(NTD) and κ-(BNI), but not μ-(CTOP) receptors inhibited the therapeutic effect of remifentanil on I/R injury in normal isolated hearts (left panel, Fig. 6a). Conversely, CTOP was the most potent antagonist to inhibit remifentanil-induced limitation of infarct size in failing hearts (right panel, Fig. 6a). A similar scenario was observed in remifentanil-induced reduction of elevated LDH activity at 5 min of reperfusion. While CTOP was completely ineffective in normal hearts (left panel, Fig. 6b), CTOP was more potent than NTD and BNI to inhibit remifentanil-induced reduction of LDH in failing hearts (right panel, Fig. 6b). Meanwhile, these opioid receptors alone did not affect infarct size or LDH release at reperfusion caused by I/R injury in both normal and failing hearts (Supplementary Fig. 6a and b). These observations favour a working hypothesis that cardiac μ-opioid receptors promoted by chronic heart failure can contribute to opioid-induced cardioprotection against I/R injury via an ERK/GSK-3β signalling pathway (Fig. 6c).
Fig 6.
Different inhibitory effects of opioid receptor antagonists on remifentanil-induced cardioprotection in normal and failing hearts. (a) Inhibition percentage of remifentanil reduced infarct size/area at risk by μ-opioid receptor antagonist CTOP, δ-receptor antagonist NTD or κ-receptor antagonist BNI in isolated normal (left lane) and failing (right lane) hearts (n=6). (b) Inhibition percentage of remifentanil reduced LDH activities (at 5 min of reperfusion) by μ-opioid receptor antagonist CTOP, δ-receptor antagonist NTD, or κ-receptor antagonist BNI in isolated normal (left lane) and failing (right lane) hearts (n=6). The inhibition percentage was calculated according to the formula: % Inhibitionantagonist=[1−(Valuecontrol−Valueantagonist)/(Valuecontrol−ValueRemi)] ×100%. **P<0.01, *P<0.05, one-way anova followed by Tukey's test. (c) Cartoons of a working hypothesis suggesting a dynamic regulation of cardiac μ-opioid receptor expression and the therapeutic potential of these receptors in failing hearts via an ERK/GSK-3β pathway. anova, analysis of variance; CTOP, D-Pen-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2; NTD, naltrindole hydrochloride; BNI, nor-binaltorphimine dihydrochloride; Remi, Remifentanil; ERK, extracellular signal-regulated kinase; GSK3ß, glycogen synthase kinase; I/R, ischaemia-reperfusion; LDH, lactate dehydrogenase; P, phosphorylation.
Discussion
Cardiac μ-opioid receptors have been largely ignored for decades because of a near absence of these receptors expressed in normal adult heart tissue. The data presented in this study have shown for the first time that the levels of cardiac μ-opioid receptor mRNA, protein, and ligand-binding activity were substantially elevated with the development of heart failure induced by DOX. The up-regulation of cardiac μ-opioid receptor protein was also observed in another animal model of chronic heart failure induced by infarction after ligation of coronary artery. We have provided several lines of evidence to further suggest that these cardiac μ-opioid receptors are a potential target for opioid therapeutic action in the treatment of myocardial I/R injury during DOX-induced chronic heart failure. Additionally, we have demonstrated that the cardioprotective effect mediated by cardiac μ-opioid receptors is dependent on ERK/GSK-3β signalling pathway.
DOX-induced chronic heart failure represents one popular animal model because DOX can produce dilated cardiomyopathy and heart failure in a progressive and permanent manner similar to the clinical pathological process of chronic heart failure in humans.27, 28 Moreover, there are clinical reports suggesting that DOX, when used as an anticancer agent, can increase the risk of cardiac toxicity and postoperative myocardial injury.29 In this regard, extra caution should be taken by anaesthesiologists to monitor perioperative cardiac function of the patients pre-exposure to DOX treatment.30, 31
One interesting finding from this study is the dynamic up-regulation of cardiac μ-opioid receptors during chronic heart failure induced by DOX. In line with previous studies,14, 15, 16, 17 μ-opioid receptors were nearly absent in normal heart tissues. However, the expression levels of μ-opioid receptor mRNA and protein substantially increased 8 weeks after DOX treatment. The molecular mechanism underlying up-regulation of cardiac μ-opioid receptors is unclear. It is unlikely that DOX directly increases the expression level of cardiac μ-opioid receptors because such receptor up-regulation did not occur at 1 week after DOX injection. Instead, the time course of up-regulation of μ-opioid receptors is coupled with the development of heart failure induced by DOX. This suggests that the pathophysiological process of heart failure promoted up-regulation of cardiac μ-opioid receptors. Consistent with this idea, the level of receptor up-regulation is strongly correlated with the impairment of cardiac function.
It is notable that cardiac μ-opioid receptors were co-localised with TRPV1 positive cells but not α-SA positive cardiomyocytes. TRPV1 receptors are usually found in sensory nerve endings innervating the heart to detect tissue ischemia and activate cardiac nociceptors.32 It has been proposed that the activation of peripheral opioid receptors may protect hearts through suppressing cardiac nociceptive signalling.33 A similar scenario is also observed when morphine is applied via intrathecal microinjection. Activation of central μ-opioid receptors can translate into phosphorylation of cardiomyocyte signalling through non-opioid neurotransmitters.34 Unfortunately, the exact molecular process that converts activation of μ-opioid receptors in the central and peripheral nervous system to phosphorylation of cardiomyocyte ERK/GSK3β remains elusive.
We performed myocardial I/R injury in isolated rat hearts by using the Langendorff perfusion system in order to preclude the involvement of extra-cardiac μ-opioid receptors. Preconditioning with DAMGO significantly reduced myocardial I/R injury in the failing hearts but not in normal hearts, suggesting that the μ-opioid receptors presented in failing hearts could confer efficient protective effects. More interestingly, the cardioprotective effects induced by morphine or remifentanil were markedly blocked by the antagonist specific for μ-opioid receptor but not by the antagonists specific for δ- or κ-receptor in isolated failing hearts. These results indicate that cardiac μ-opioid receptors are a primary therapeutic target for opioid-induced cardioprotection during heart failure.
The reperfusion injury salvage kinase (RISK) pathway activated by exogenous cardioprotective agonists at early reperfusion is thought to be essential for cardioprotection.35 The main components of the RISK pathway include phosphoinositide 3-kinase, protein kinase B (Akt), and ERK. Activation of these kinases is converged to the downstream GSK-3β, which suppresses the opening of mitochondrial permeability transition pore after being phosphorylated and thus inactivated.36 DAMGO, morphine, and remifentanil preconditioning were found to promote ERK and GSK-3β phosphorylation in isolated failing hearts. Moreover, the cardioprotective effects of DAMGO were abolished via inhibition of ERK and GSK-3β phosphorylation. These results strongly suggest that cardiac μ-opioid receptor-induced cardioprotection is dependent on the ERK/GSK-3β signalling pathway.
There is always a limitation from using a single animal model or single species. Previous studies have shown that the pharmacological and signalling mechanisms of opioid cardioprotection may vary between different animals.37, 38 The DOX-induced heart failure model may be relevant for cancer, but not be representative for other types of heart failure. In view of this, we tested and observed a similar up-regulation of cardiac μ-opioid receptor protein levels in rats with post-myocardial infarction heart failure. This model is thought to be more clinically relevant to the pathophysiological mechanism of human heart failure.23 This is consistent with our finding in the DOX-induced animal model and suggests that cardiac μ-opioid receptors can emerge as one primary therapeutic target during chronic heart failure induced through various pathological causes. Nevertheless, the isolated rat heart may not be representative of the in vivo situation. However, this system allows us to eliminate the influence from in vivo factors while exploring therapeutic targets within heart tissues.
In summary, we show a strong up-regulation of cardiac μ-opioid receptors with the development of chronic heart failure. These cardiac μ-opioid receptors predominantly mediate opioid-induced cardioprotection via the ERK/GSK-3β signalling pathway. This is the first evidence, to our knowledge, showing that cardiac μ-opioid receptor activation contributes to the reduction of myocardial I/R injury. A future investigation should be carried out to explore the possible therapeutic role of these cardiac μ-opioid receptors in patients with heart failure.
Authors' contributions
Conception of the project: S.F.H, Y.Z.
Design of the study: Y.Z., L.Z., S.F.H., S.Y.J.
Performance of the experiments: S.Y.J., S.F.H., W.Y., Y.L.P., J.H., S.J.Z.
Initial data collection and analysis: S.Y.J., W.Y., Y.L.P., J.H., S.J.Z.
Final data analysis and writing of the manuscript: S.Y.J., S.F.H.
Critical revision of the manuscript: L.Z., Y.Z., S.F.H., S.Y. J.
Acknowledgements
The authors thank X. Wang. (Department of Pathology, the Second Hospital of Anhui Medical University, Hefei, China), for assistance in the preparation of heart sections and histological examinations. The authors also thank S. Xu (Department of Nuclear Medicine, the Anhui Medical University, Hefei, China), for assistance in the radioactive experiments in radioligand binding assay.
Handling editor: C. Boer
Editorial decision: December 23, 2017
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.bja.2017.11.110.
Contributor Information
L. Zhang, Email: lzhang@mail.nih.gov.
Y. Zhang, Email: zhangye_hassan@sina.com.
Declaration of interest
None declared.
Funding
National Natural Science Foundation of China (81200171 to S.F.H. and 81471145 to Y.Z.) and Key Program of Natural Science Foundation of Higher Education Institutions of Anhui Province (KJ2017A172 to S.F.H.).
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Xanthakis V., Enserro D.M., Larson M.G. Prevalence, neurohormonal correlates, and prognosis of heart failure stages in the community. JACC Heart Fail. 2016;4:808–815. doi: 10.1016/j.jchf.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hausenloy D.J., Boston-Griffiths E., Yellon D.M. Cardioprotection during cardiac surgery. Cardiovasc Res. 2012;94:253–265. doi: 10.1093/cvr/cvs131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murry C.E., Jennings R.B., Reimer K.A. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 4.Hausenloy D.J., Barrabes J.A., Botker H.E. Ischaemic conditioning and targeting reperfusion injury: a 30 year voyage of discovery. Basic Res Cardiol. 2016;111:70. doi: 10.1007/s00395-016-0588-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferdinandy P., Hausenloy D.J., Heusch G., Baxter G.F., Schulz R. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev. 2014;66:1142–1174. doi: 10.1124/pr.113.008300. [DOI] [PubMed] [Google Scholar]
- 6.Andersen A., Povlsen J.A., Botker H.E., Nielsen-Kudsk J.E. Right ventricular hypertrophy and failure abolish cardioprotection by ischaemic pre-conditioning. Eur J Heart Fail. 2013;15:1208–1214. doi: 10.1093/eurjhf/hft105. [DOI] [PubMed] [Google Scholar]
- 7.Schultz J.E., Rose E., Yao Z., Gross G.J. Evidence for involvement of opioid receptors in ischemic preconditioning in rat hearts. Am J Physiol. 1995;268:H2157–H2161. doi: 10.1152/ajpheart.1995.268.5.H2157. [DOI] [PubMed] [Google Scholar]
- 8.Schultz J.J., Hsu A.K., Gross G.J. Ischemic preconditioning in the intact rat heart is mediated by delta1- but not mu- or kappa-opioid receptors. Circulation. 1998;97:1282–1289. doi: 10.1161/01.cir.97.13.1282. [DOI] [PubMed] [Google Scholar]
- 9.Schultz J.E., Hsu A.K., Gross G.J. Morphine mimics the cardioprotective effect of ischemic preconditioning via a glibenclamide-sensitive mechanism in the rat heart. Circ Res. 1996;78:1100–1104. doi: 10.1161/01.res.78.6.1100. [DOI] [PubMed] [Google Scholar]
- 10.Schultz J.J., Hsu A.K., Gross G.J. Ischemic preconditioning and morphine-induced cardioprotection involve the delta (delta)-opioid receptor in the intact rat heart. J Mol Cell Cardiol. 1997;29:2187–2195. doi: 10.1006/jmcc.1997.0454. [DOI] [PubMed] [Google Scholar]
- 11.Dhawan B.N., Cesselin F., Raghubir R. International union of pharmacology. XII. Classification of opioid receptors. Pharmacol Rev. 1996;48:567–592. [PubMed] [Google Scholar]
- 12.Ventura C., Spurgeon H., Lakatta E.G., Guarnieri C., Capogrossi M.C. Kappa and delta opioid receptor stimulation affects cardiac myocyte function and Ca2+ release from an intracellular pool in myocytes and neurons. Circ Res. 1992;70:66–81. doi: 10.1161/01.res.70.1.66. [DOI] [PubMed] [Google Scholar]
- 13.Headrick J.P., See Hoe L.E., Du Toit E.F., Peart J.N. Opioid receptors and cardioprotection - 'opioidergic conditioning' of the heart. Br J Pharmacol. 2015;172:2026–2050. doi: 10.1111/bph.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wittert G., Hope P., Pyle D. Tissue distribution of opioid receptor gene expression in the rat. Biochem Biophys Res Commun. 1996;218:877–881. doi: 10.1006/bbrc.1996.0156. [DOI] [PubMed] [Google Scholar]
- 15.Karlsson L.O., Bergh N., Li L. Dose-dependent cardioprotection of enkephalin analogue Eribis peptide 94 and cardiac expression of opioid receptors in a porcine model of ischaemia and reperfusion. Eur J Pharmacol. 2012;674:378–383. doi: 10.1016/j.ejphar.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Theisen M.M., Schlottmann S., August C. Detection and distribution of opioid peptide receptors in porcine myocardial tissue. Pharmacol Res. 2014;84:45–49. doi: 10.1016/j.phrs.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Peng J., Sarkar S., Chang S.L. Opioid receptor expression in human brain and peripheral tissues using absolute quantitative real-time RT-PCR. Drug Alcohol Depend. 2012;124:223–228. doi: 10.1016/j.drugalcdep.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Treskatsch S., Shaqura M., Dehe L. Upregulation of the kappa opioidergic system in left ventricular rat myocardium in response to volume overload: adaptive changes of the cardiac kappa opioid system in heart failure. Pharmacol Res. 2015;102:33–41. doi: 10.1016/j.phrs.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Treskatsch S., Feldheiser A., Shaqura M. Cellular localization and adaptive changes of the cardiac delta opioid receptor system in an experimental model of heart failure in rats. Heart Vessels. 2016;31:241–250. doi: 10.1007/s00380-014-0620-6. [DOI] [PubMed] [Google Scholar]
- 20.Bolte C., Newman G., Schultz Jel J. Kappa and delta opioid receptor signaling is augmented in the failing heart. J Mol Cell Cardiol. 2009;47:493–503. doi: 10.1016/j.yjmcc.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGrath J.C., Drummond G.B., McLachlan E.M., Kilkenny C., Wainwright C.L. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He S.F., Jin S.Y., Wu H. Morphine preconditioning confers cardioprotection in doxorubicin-induced failing rat hearts via ERK/GSK-3beta pathway independent of PI3K/Akt. Toxicol Appl Pharmacol. 2015;288:349–358. doi: 10.1016/j.taap.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Gomes K.M., Campos J.C., Bechara L.R. Aldehyde dehydrogenase 2 activation in heart failure restores mitochondrial function and improves ventricular function and remodelling. Cardiovasc Res. 2014;103:498–508. doi: 10.1093/cvr/cvu125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y., Irwin M.G., Wong T.M., Chen M., Cao C.M. Remifentanil preconditioning confers cardioprotection via cardiac kappa- and delta-opioid receptors. Anesthesiology. 2005;102:371–378. doi: 10.1097/00000542-200502000-00020. [DOI] [PubMed] [Google Scholar]
- 25.Faul F., Erdfelder E., Lang A.G., Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 26.Bell R.M., Mocanu M.M., Yellon D.M. Retrograde heart perfusion: the Langendorff technique of isolated heart perfusion. J Mol Cell Cardiol. 2011;50:940–950. doi: 10.1016/j.yjmcc.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 27.Houser S.R., Margulies K.B., Murphy A.M. Animal models of heart failure: a scientific statement from the American heart association. Circ Res. 2012;111:131–150. doi: 10.1161/RES.0b013e3182582523. [DOI] [PubMed] [Google Scholar]
- 28.Haidara M.A., Assiri A.S., Yassin H.Z., Ammar H.I., Obradovic M.M., Isenovic E.R. Heart failure models: traditional and novel therapy. Curr Vasc Pharmacol. 2015;13:658–669. doi: 10.2174/1570161113666150212151506. [DOI] [PubMed] [Google Scholar]
- 29.Patane S. A challenge in cardiology: the oncosurgery. Int J Cardiol. 2014;174:411–412. doi: 10.1016/j.ijcard.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 30.Ai D., Banchs J., Owusu-Agyemang P., Cata J.P. Chemotherapy-induced cardiovascular toxicity: beyond anthracyclines. Minerva Anestesiol. 2014;80:586–594. [PubMed] [Google Scholar]
- 31.Huettemann E., Junker T., Chatzinikolaou K.P. The influence of anthracycline therapy on cardiac function during anesthesia. Anesth Analg. 2004;98:941–947. doi: 10.1213/01.ANE.0000108135.52036.48. [table of contents] [DOI] [PubMed] [Google Scholar]
- 32.Pan H.L., Chen S.R. Sensing tissue ischemia: another new function for capsaicin receptors? Circulation. 2004;110:1826–1831. doi: 10.1161/01.CIR.0000142618.20278.7A. [DOI] [PubMed] [Google Scholar]
- 33.Fu L.W., Longhurst J.C. Functional role of peripheral opioid receptors in the regulation of cardiac spinal afferent nerve activity during myocardial ischemia. Am J Physiol Heart Circ Physiol. 2013;305:H76–H85. doi: 10.1152/ajpheart.00091.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong G.T., Yao L., Xia Z., Irwin M.G. Intrathecal morphine remotely preconditions the heart via a neural pathway. J Cardiovasc Pharmacol. 2012;60:172–178. doi: 10.1097/FJC.0b013e31825e2195. [DOI] [PubMed] [Google Scholar]
- 35.Kleinbongard P., Heusch G. Extracellular signalling molecules in the ischaemic/reperfused heart—druggable and translatable for cardioprotection? Br J Pharmacol. 2015;172:2010–2025. doi: 10.1111/bph.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heusch G. Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res. 2015;116:674–699. doi: 10.1161/CIRCRESAHA.116.305348. [DOI] [PubMed] [Google Scholar]
- 37.Schulz R., Gres P., Heusch G. Role of endogenous opioids in ischemic preconditioning but not in short-term hibernation in pigs. Am J Physiol Heart Circ Physiol. 2001;280:H2175–H2181. doi: 10.1152/ajpheart.2001.280.5.H2175. [DOI] [PubMed] [Google Scholar]
- 38.Skyschally A., van Caster P., Boengler K. Ischemic postconditioning in pigs: no causal role for RISK activation. Circ Res. 2009;104:15–18. doi: 10.1161/CIRCRESAHA.108.186429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.