Abstract
We aimed to develop and validate a clinical nomogram predicting bladder outlet obstruction (BOO) solely using routine clinical parameters in men with refractory nonneurogenic lower urinary tract symptoms (LUTS). A total of 750 eligible patients ≥50 years of age who had previously not responded (International Prostate Symptom Score [IPSS] improvement <4 points) to at least three different kinds of LUTS medications (including a-blocker) for the last 6 months were evaluated as subcohorts for nomogram development (n = 570) and for split-sample validation (n = 180). BOO was defined as Abrams–Griffiths number ≥40, or 20–39.9 with a slope of linear passive urethral resistance ratio >2 cmH2O ml−1 s−1. A stepwise multivariable logistic regression analysis was conducted to determine the predictors of BOO, and b-coefficients of the final model were selected to create a clinical nomogram. The final multivariable logistic regression model showed that age, IPSS, maximum urinary flow rate, postvoid residual volume, total prostate volume, and transitional zone index were significant for predicting BOO; these candidates were used to develop the final nomogram. The discrimination performance of the nomogram was 88.3% (95% CI: 82.7%–93.0%, P < 0.001), and the nomogram was reasonably well-fitted to the ideal line of the calibration plot. Independent split-sample validation revealed 80.9% (95% CI: 75.5%–84.4%, P < 0.001) accuracy. The proposed BOO nomogram based solely on routine clinical parameters was accurate and validated properly. This nomogram may be useful in determining further treatment, primarily focused on prostatic surgery for BOO, without impeding the detection of possible BOO in men with LUTS that is refractory to empirical medications.
Keywords: benign prostatic hyperplasia, bladder outlet obstruction, lower urinary tract symptoms, nomogram, urodynamics
INTRODUCTION
The estimated incidence of lower urinary tract symptoms (LUTS) includes 45.2%–72.3% of men and has been shown to increase with age.1,2 It has been suggested that symptom-oriented practices may benefit the management of LUTS,3 and it is self-evident that the clinical practices should rely on the individual diagnosis. For many reasons, prostatic surgery is sometimes necessary for men with LUTS due to benign prostatic obstruction (BPO).3 The specific diagnosis of bladder outlet obstruction (BOO) is important in real-life practice involving treatment of men with LUTS suggestive of BPO and can help guide proper treatment strategies, such as prostatic surgery for some and avoidance of unnecessary surgery for others.
Currently, urodynamic assessment with a pressure-flow study (PFS) is considered the gold standard for confirming a diagnosis of significant BOO.4 However, performing a urodynamic study requires patient cooperation for best results and is not entirely free of discomfort and invasiveness with respect to the patient; thus, it might cause several morbidities within a certain percentage of patients.5 Therefore, it may not be practical to perform a urodynamic study in all men with LUTS, especially those that are frail, elderly, or mentally impaired.
To overcome this problem, some noninvasive methods or parameters to predict the probability of BOO have been proposed in men with treatment-naïve or common LUTS;6,7,8,9,10,11 nevertheless, it has not been clarified whether these provide consistently good performance for clinical use in a large sample. In addition, the application of some methods with specific equipment is not always feasible in real-life practice.
On the other hand, most initial treatments for male LUTS/BPO are empirical medications (e.g., α-blockers, 5α-reductase inhibitors, or anticholinergics) that depend on patient symptoms and possible prostate/bladder conditions without urodynamic assessment; thus, the prediction of the presence of BOO through noninvasive methods may be more helpful for men with LUTS refractory to initial empirical medications, many of whom subsequently consider prostatic surgery. However, there have been few studies especially focused on these patients. Herein, we aimed to develop and validate a clinical nomogram to predict BOO solely using routine clinical parameters in a large cohort of men ≥50 years of age with refractory nonneurogenic LUTS.
PATIENTS AND METHODS
Study population and clinical data
A database of a consecutive series of men with LUTS, who were ≥50 years of age and who underwent a urodynamic study between May 2005 and June 2013, was created from our urodynamic database registry (SNUBH UDB); the data were prospectively collected at Seoul National University Bundang Hospital (Seongnam, Korea), and relevant clinical data were investigated using the hospital database registry. Subsequently, men were selected who had previously not responded to at least three different kinds of LUTS medications, including α-blockers, 5α-reductase inhibitor, anticholinergic, desmopressin, or cholinergic, for the prior 6 months before undergoing urodynamic testing. Nonresponse to the medication was defined as the International Prostate Symptom Score (IPSS) improvement <4 points after medication. Medications with same mechanisms of action were considered as one kind of medication, regardless of the number of medication prescribed to the patient. Men were excluded if they exhibited any of the following: anatomical deformity of the lower urinary tract, neurological disorder suggesting neurogenic bladder, diabetes mellitus with end-organ impairment, a history of pelvic surgery, pelvic radiation therapy or lower urinary tract cancers, recent hospitalization or general surgery within 1 month (possibly causing impairment of general health), urinary tract infection, interstitial cystitis, or insufficient data. The Institutional Review Board of Seoul National University Bundang Hospital approved the study protocol (B-1407/260-116), and the obtaining of informed consent was waived due to the retrospective nature of the study.
Based on the specified protocol for men with LUTS at our institution, we initially performed clinical history-taking (including the presence of acute urinary retention, AUR), physical examination, a validated Korean version of the IPSS including the quality of life question,12 3-day frequency-volume chart, prostate-specific antigen (PSA), and urine flow study (DABA, Endo tech, Seongnam, Korea) with measurement of postvoid residual (PVR; BladderScan BVI-3000, Diagnostic Ultrasound, Bothell, WA, USA) volume. The result with a higher maximum flow rate (Qmax) was selected from two sets of uroflowmetry measurements with a voided volume over 150 ml. For men ≥50 years of age, digital rectal examination (DRE) and transrectal ultrasound (TRUS) were also performed. All ultrasound measurements were performed by two experienced uroradiologists. Total prostate volume (TPV) and transitional zone volume were measured using the ellipsoid formula (π/6 × width × height × depth of prostate/transitional zone) with the use of an iU 22 or HDI 5000 ultrasound scanner (Philips Ultrasound, Bothell, WA, USA) equipped with a 9-4 MHz broadband curved array endocavitary transducer; transitional zone index (TZI) was calculated as a percentage of the ratio of transitional zone volume/TPV.
Urodynamic procedures
We perform the initial evaluation based on the probable pathophysiology inferred from patient symptoms and start to prescribe the medication according to the possible diagnosis and patient symptoms. For men with LUTS/BPO, we usually use α-blockers for 1 month to 2 months. When patient does not respond to the initial LUTS medications, we escalate doses of the same medication, change to other kind of LUTS medications, or just add another to the present medication. 5α-reductase inhibitor can be considered in symptomatic men with enlarged prostate (>30–35 ml). We perform advanced evaluations (e.g., urodynamic studies) when patients demonstrate severe LUTS in the initial evaluation, show an unsatisfactory response to several medications based on the findings of the primary evaluation, or consider prostatic surgery. In this study, we selected only men who had undergone urodynamic testing due to a previous lack of response to at least three different kinds of LUTS medications, including α-blockers, for the prior 6 months.
All urodynamic procedures, including a PFS, were performed in accordance with the guidelines of the International Continence Society13 and were supervised and interpreted by a single urologist (SJJ) with ≥10 years of relevant experience. Patients were instructed to cease medications possibly affecting micturition function at least 2 weeks prior to undergoing urodynamic testing. A multichannel urodynamic study (UD-2000, Medical Measures System, Enschede, The Netherlands) was performed; a 6-Fr double-lumen and a 9-Fr balloon catheter were utilized to measure intravesical and abdominal pressures. Intravesical pressure was measured under conditions of room-temperature saline infusion at a rate of 50 ml min−1; infusion rate was reduced to 20 ml min−1 when patients had severe storage symptoms or a lower functional capacity demonstrated in the 3-day frequency-volume chart. In a PFS, patients were instructed to void in a sitting or standing position under relaxed and comfortable circumstances. If the first voiding trial failed, several additional trials were performed to allow for the possibility that the failure occurred as a result of cortical inhibition. However, for patients who failed several voiding trials, the events were documented and excluded from the study cohort. All urodynamic procedures were conducted at least twice to acquire a reliable pressure measurement. Bladder sense and capacity, detrusor compliance and overactivity, and maximum detrusor pressure at Qmax (PdetQmax) were recorded. The degree of BOO in a PFS was evaluated using an Abrams–Griffith (AG) number, where the AG number was calculated as “PdetQmax - 2 × Qmax,” and patients with an AG number ≥40, or 20–39.9 with slope of linear passive urethral resistance ratio >2 cmH2O ml−1 s−1, were considered obstructed.14
Statistical analyses
Statistical analysis for the present study was performed through a four-step process. First, the entire cohort was randomly allocated to two subcohorts using SPSS version 19.0 (IBM Co., Armonk, NY, USA): approximately 75% of patients were allocated for developing a clinical nomogram and 25% of patients were allocated for independent split-sample validation. Clinicodemographic characteristics were compared between subcohorts using the Pearson Chi-square test and Fisher's exact test for categorical variables and Student's t-test and the Mann–Whitney U-test for continuous variables, based on normality assessment by the Kolmogorov–Smirnov normality test. Second, logistic regression analysis was conducted to determine the clinical parameters for BOO among the subcohort for the development of the nomogram. Third, β-coefficients of the final regression model were selected to create a nomogram based on the principle described by Harrell et al.15 The final model was assessed with regard to discrimination accuracy and calibration performance and was internally validated from 200 bootstrap re-samples to minimize overfit bias. Last, the proposed nomogram was independently validated by evaluating its predictive accuracy in the split-sample subcohort.
The clinical nomogram was established by the rms package in R for Windows version 2.15.0 (http://www.r-project.org/), and the discrimination accuracy of the nomogram was assessed using a concordance index. The Hosmer–Lemeshow test was used to evaluate calibration performance, in which predicted measures versus observed measures were graphically presented, which possibly facilitated further comparison of accuracy in estimation of the nomogram. Statistical analyses were two-tailed, and the level of significance was set at P < 0.05 for all tests, with the exception of multivariable logistic regression analyses of clinical parameters predicting BOO (P < 0.1). Given the diversity of previous LUTS/BPO medications and symptom durations, we set P < 0.1 as a meaningful discernment for the predictors.
RESULTS
Patient characteristics
A total of 750 men who met the inclusion criteria were enrolled for analyses; clinicodemographic characteristics of all patients are described in Table 1. Overall, mean (standard deviation) values for patient age, IPSS, Qmax, PVR volume, TPV, and TZI were 65.5 (7.5) years, 14.1 (6.9), 13.1 (5.7) ml s−1, 42.2 (73.8) ml, 36.4 (19.8) ml, and 40.2% (15.7%), respectively. Only 3.9% of patients had experienced the event of AUR. The average number of previous medications for LUTS was 3.8 during an average of 11.5 months, prior to a urodynamic test.
Table 1.
Clinicodemographics of the subcohort for developing the clinical nomogram to predict bladder outlet obstruction and of the split-sample subcohort for validation of the nomogram
| Characteristics | Total subjects | Subcohort for nomogram development | Subcohort for split-sample validation | Pa |
|---|---|---|---|---|
| Patients, n (%) | 750 (100.0) | 570 (76.0) | 180 (24.0) | |
| Age (year) | ||||
| Mean (s.d.) | 65.5 (7.5) | 65.6 (7.7) | 65.2 (6.9) | 0.956 |
| Median (range) | 66 (50–90) | 66 (50–90) | 66 (51–87) | |
| History of acute urinary retention, n (%) | 29 (3.9) | 22 (3.9) | 7 (3.9) | 0.891 |
| Number of previous LUTS medication | ||||
| Mean (s.d.) | 3.8 (0.6) | 3.8 (0.7) | 3.8 (0.5) | 0.944 |
| Median (range) | 4.0 (3.0–6.0) | 4.0 (3.0–6.0) | 4.0 (3.0–6.0) | |
| Duration of previous medication (month) | ||||
| Mean (s.d.) | 11.5 (4.2) | 11.4 (5.1) | 11.8 (3.9) | 0.796 |
| Median (range) | 11 (6–18) | 11 (6–17) | 11 (6–18) | |
| Previous LUTS medication, n (%) | ||||
| α-blocker | 750 (100.0) | 570 (100.0) | 180 (100.0) | 0.865 |
| 5α-reductase inhibitor | 541 (72.1) | 418 (73.3) | 123 (68.3) | |
| Anticholinergic | 608 (81.1) | 461 (80.9) | 147 (81.7) | |
| Desmopressin | 188 (25.1) | 142 (24.9) | 46 (25.6) | |
| Cholinergic | 178 (23.7) | 132 (23.2) | 46 (25.6) | |
| Others | 44 (5.9) | 34 (6.0) | 10 (5.6) | |
| IPSS after medication, n (%) | ||||
| 0–7 | 10 (1.3) | 7 (1.2) | 3 (1.7) | 0.902 |
| 8–19 | 507 (67.6) | 390 (68.4) | 117 (65.0) | |
| 20–35 | 233 (31.1) | 173 (30.4) | 60 (33.3) | |
| PSA (ng ml−1) | ||||
| Mean (s.d.) | 3.0 (8.5) | 3.1 (9.1) | 2.7 (8.2) | 0.806 |
| Median (range) | 1.6 (0.2–24.0) | 1.7 (0.4–24.0) | 1.6 (0.2–18.0) | |
| Qmax (ml s−1), n (%)b | ||||
| ≤5 | 50 (6.7) | 37 (6.5) | 13 (7.2) | 0.921 |
| 5.1–10.0 | 153 (20.4) | 111 (19.5) | 42 (23.3) | |
| 10.1–15.0 | 478 (63.7) | 368 (64.6) | 110 (61.1) | |
| 15.1–20.0 | 62 (8.3) | 48 (8.4) | 14 (7.8) | |
| ≥20.1 | 7 (0.9) | 6 (1.0) | 1 (0.6) | |
| PVR after medication (ml)b | ||||
| Mean (s.d.) | 42.2 (73.8) | 42.1 (77.3) | 43.0 (70.1) | 0.781 |
| Median (range) | 20 (0–400) | 20 (0–395) | 22 (0–400) | |
| TPV (ml) | ||||
| Mean (s.d.) | 36.4 (19.8) | 37.0 (20.5) | 36.1 (18.6) | 0.839 |
| Median (range) | 32.2 (9.5–100.0) | 32.8 (10.5–95.0) | 32.1 (9.5–100.0) | |
| TZI (%) | ||||
| Mean (s.d.) | 40.2 (15.7) | 40.6 (15.8) | 39.2 (15.6) | 0.897 |
| Median (range) | 37.8 (14.5–85.0) | 38.6 (15.5–82.0) | 37.1 (14.5–85.0) | |
| BOO, n (%) | 226 (30.1) | 170 (29.8) | 56 (31.1) | 0.412 |
aComparisons between the both subcohorts; bfree uroflowmetry after medication. s.d.: standard deviation; BOO: bladder outlet obstruction; LUTS: lower urinary tract symptoms; IPSS: International Prostate Symptom Score; PSA: prostate-specific antigen; Qmax: maximum flow rate; PVR: postvoid residual; TPV: total prostate volume; TZI: transitional zone index
Among all patients, 226 (30.1%) men were classified as obstructed in a PFS; as expected, Qmax, PVR volume, PSA, TPV, and TZI were significantly different between patients with and without BOO. Clinicodemographic characteristics of the 570 (76.0%) men allocated to the subcohort for nomogram development and the 180 (24.0%) men assigned to the split-sample validation are shown in Table 1; these characteristics did not differ between the subcohorts (all P > 0.05).
Logistic regression models predicting BOO
Backward stepwise multivariable logistic regression analyses in the development subcohort are demonstrated in Table 2. In the base model, all tested parameters, except for the history of AUR and PSA, were significantly correlated with the presence of BOO. The final model showed that age (P = 0.041), IPSS (P = 0.006), Qmax (P < 0.001), PVR volume (P = 0.057), TPV (P < 0.001), and TZI (P = 0.050) were significant predictors for BOO (Table 2). These predictors were incorporated to develop the final version of the clinical nomogram. The P value of the Hosmer–Lemeshow test for the final model was not statistically significant (P = 0.704), which indicated a good fit of the final model.
Table 2.
Multivariable logistic regression analyses of clinical parameters to predict bladder outlet obstruction among 590 men of the subcohort for the development of nomogram
| Clinical parameters | Base modela | Final modela | ||||
|---|---|---|---|---|---|---|
| OR | 90% CI | P | OR | 90% CI | P | |
| Age (year) | 0.961 | 0.931–0.994 | 0.050 | 0.966 | 0.933–0.999 | 0.041 |
| History of acute urinary retention | 1.229 | 0.431–3.457 | 0.703 | NI | NI | NI |
| IPSS | 0.967 | 0.943–0.990 | 0.008 | 0.964 | 0.941–0.989 | 0.006 |
| PSA (ng ml−1) | 1.029 | 0.976–1.079 | 0.297 | NI | NI | NI |
| Qmax (ml s−1) | 0.872 | 0.824–0.920 | <0.001 | 0.866 | 0.821–0.919 | <0.001 |
| PVR (ml) | 1.005 | 1.003–1.012 | 0.078 | 1.007 | 1.002–1.011 | 0.057 |
| TPV (ml) | 1.048 | 1.028–1.064 | <0.001 | 1.048 | 1.031–1.069 | <0.001 |
| TZI (%) | 1.020 | 1.005–1.041 | 0.085 | 1.022 | 1.008–1.041 | 0.050 |
aThe significance level of two-tailed P<0.1 was applied to the backward stepwise multivariable analyses. NI: not included; BOO: bladder outlet obstruction; OR: odds ratio; CI: confidence interval; IPSS: International Prostate Symptom Score; PSA: prostate-specific antigen; Qmax: maximum flow rate; PVR: postvoid residual; TPV: total prostate volume; TZI: transitional zone index
Development of the nomogram and validation
On the basis of the regression coefficients of the final model, a clinical nomogram was developed for the prediction of BOO (Figure 1). The discrimination performance of the final regression model was determined with the calculated area under the receiver-operating characteristic curve: 0.883 (95% confidence interval [CI]: 0.827–0.930, P < 0.001) for predicting BOO (Figure 2a). The bootstrap-corrected performance of the proposed nomogram was close to the ideal line of the calibration plot, with only slight deviation in the high-probability area for predicting BOO, which demonstrated reasonable calibration performance (Figure 2b). The independent split-sample (180 men) validation of the nomogram revealed 80.9% accuracy (95% CI: 75.5%–84.4%, P < 0.001; Figure 3).
Figure 1.
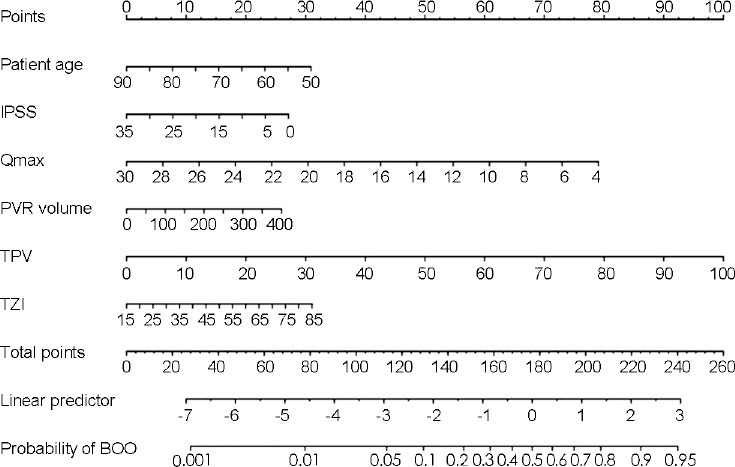
Nomogram based on the final multivariable logistic regression model to predict the probability of BOO in men ≥50 years of age with refractory LUTS. LUTS: lower urinary tract symptoms; IPSS: International Prostate Symptom Score; Qmax: maximum flow rate; PVR: postvoid residual; TPV: total prostate volume; TZI: transitional zone index; BOO: bladder outlet obstruction.
Figure 2.
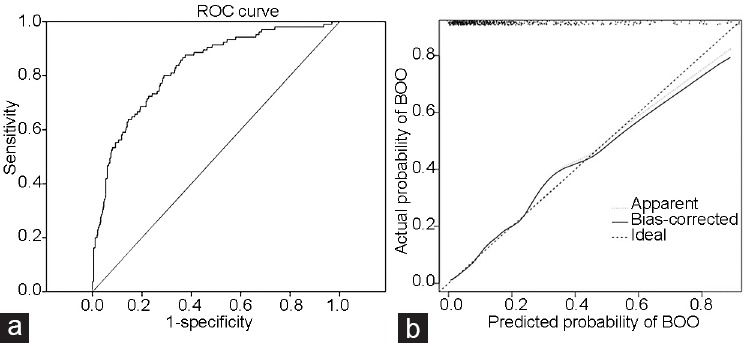
(a) ROC curve for the prediction of BOO of the proposed clinical nomogram in the development subcohort: area under the curve = 0.883 (95% CI: 0.827–0.930, P < 0.001). The curved line is the ROC curve generated from the proposed multivariable prediction model and the diagonal line is the reference line for random guessing. (b) Calibration plot of the proposed clinical nomogram: dashed line is an ideal line of the perfect nomogram and solid line is the bootstrap-corrected performance of the proposed nomogram with a scatter estimate for future accuracy. The solid line is close to the dashed line of the perfect nomogram with only slight deviation in high-probability area for predicting BOO. ROC curve: receiver-operating characteristic curve; BOO: bladder outlet obstruction; CI: confidence interval.
Figure 3.
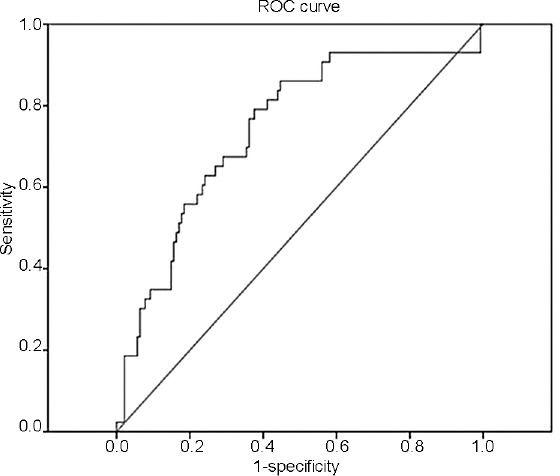
ROC curve for the prediction of BOO of the proposed clinical nomogram in the subcohort (180 men) for the independent split-sample validation: area under the curve = 0.809 (95% CI: 0.755–0.844, P < 0.001). The curved line is the ROC curve generated from the proposed multivariable prediction model and the diagonal line is the reference line for random guessing. ROC curve: receiver-operating characteristic curve; BOO: bladder outlet obstruction; CI: confidence interval.
DISCUSSION
Many researchers have investigated a noninvasive measurement to predict the probability of BOO in men with nonneurogenic LUTS. Several noninvasive methods with specific equipment or parameters have been reported as helpful in men with treatment-naïve or commonly nonneurogenic LUTS.6,7,8,9,10,11 From a clinical perspective, however, the prediction of the presence of BOO with noninvasive methods may be more helpful for men who exhibit LUTS/BPO refractory to several medications and subsequently consider prostatic surgery. In clinical practice, most initial treatments for male LUTS begin with empirical medications, such as α-blockers, 5α-reductase inhibitors, or anticholinergics, depending on patient symptoms and possible prostate/bladder conditions, without urodynamic assessment.
We aimed to develop and validate a clinical nomogram to predict BOO, using routine clinical parameters among a large number of men with refractory nonneurogenic LUTS. In general, a nomogram provides a simple graphical depiction of a statistically predictive model of a clinical outcome; it has a high accuracy rate and good discrimination properties among various prediction methods because it is built based on multivariable models with continuous parameters. Moreover, if a nomogram can be constructed solely using routine clinical parameters that are assessed in real-life practice, the nomogram would be more useful for predicting clinical events in routine practice.
Based on the guidelines from the American Urological Association and the European Association of Urology, routine clinical parameters for assessment of men with nonneurogenic LUTS are medical history, symptom score questionnaire, DRE, urinalysis, PVR volume, and uroflowmetry (mainly Qmax).3,16 Frequency-volume chart, PSA, and upper urinary tract assessment are performed if indicated. With respect to performing TRUS during the initial evaluation of LUTS, both guidelines state that TRUS is optional in selected patients (i.e., those in whom the success of certain treatments may depend on anatomical characteristics of the prostate gland)16 or should be performed if it assists in the selection of appropriate medication or surgical treatment.3 In clinical guidelines published recently in Korea, most peer reviewers agreed that TRUS had a better role than DRE for the measurement of prostate anatomy in patients with BPH, although DRE is one of the essential tests in the initial evaluation of LUTS/BPO.17 Therefore, in real-life practice, prostate imaging, such as TRUS, could be performed as an initial evaluation for the selection of the most appropriate treatment modality in most elderly men with nonneurogenic LUTS, although there remains controversy regarding this issue.
We showed that age, IPSS, Qmax, PVR volume, TPV, and TZI were significantly associated with the presence of BOO in the final multivariable regression model; these parameters were used to construct the clinical nomogram. The clinical nomogram showed good discrimination performance (88.3%) in the development cohort and 80.9% accuracy in the independent split-sample validation.
The clinical significance of Qmax and PVR volume for predicting BOO, as shown in our study, is consistent with findings from previous studies performed in men with common or treatment-naïve LUTS/BPO.10,18 However, because other conditions, such as detrusor underactivity, can also lead to lower Qmax and larger PVR volume, the presence of BOO could not be predicted properly in clinical practice with these parameters alone.4 Our final regression model also showed that TPV was a significant predictor of BOO. Similarly, previous studies reported that TPV was statistically related to the presence of BOO in large cohorts of >500 men.19,20 As a sole predictor, however, the sensitivity and specificity of this parameter have been reported as 49% and 32%;19 it was unable to reliably predict BOO.20 With respect to the ability of TZI to predict BOO shown in the present study, it has been identified as a predictor for LUTS suggestive of BPH21 and AUR22 in some previous studies, although other researchers have suggested that it was poorly correlated with BOO or clinical symptoms.23
Patient age and total IPSS score were also related to the presence of BOO in our cohort. In general, elderly men are considered to have more severe LUTS/BPH.1,2 Nevertheless, patient age alone does not reliably influence the prediction of BOO in clinical studies. Recently, De Nunzio et al.24 reported that patient age did not differ statistically with respect to the presence of BOO in men with treatment-naïve LUTS;11 moreover, it was not a predictor of BOO in another cohort. In our cohort with refractory LUTS, the mean age of men with BOO was 66.4 years, higher than that of men without BOO (mean age 65.3 years). However, the difference between groups was small (1 year) and the odds ratio for the prediction of BOO was 0.966 in the multivariable model, although it was statistically significant. A significant correlation was also demonstrated between some items of IPSS and the presence of BOO in the literature.25 However, it has also been reported that the severity of LUTS as determined by self-administered questionnaires such as IPSS was poorly correlated with the presence of BOO.26 Therefore, patient age and total IPSS score may partially guide management strategies; however, because the presence of BOO cannot be determined with respect to patient age or IPSS score alone, a nomogram constructed with other relevant parameters should be considered in clinical practice.
Although our clinical nomogram targets men with refractory LUTS, its accuracy is comparable to that of the previously published nomogram or predictive model in men with treatment-naïve or common LUTS (73.5%–83.2%).11,27 The predictive model proposed by Kim et al.27 was constructed by a combination of Qmax, PVR volume, and TPV. Notably, De Nunzio et al.11 selected only Qmax and transitional zone volume as independent statistical predictors of BOO in the development of their nomogram. Based on the final statistical model in our cohort, we added a couple of clinical parameters to the nomogram, in combination with previously proven predictors. A potential reason for the incorporation of more indicators into our nomogram may be that our cohort had failed to respond several medications for possible BOO, including α-blockers, before undergoing a urodynamic test; therefore, a considerable portion of the men might have had other (non-BOO) pathophysiologies for LUTS, such as overactive bladder, nocturnal polyuria, or detrusor underactivity. Indeed, the present cohort showed a relatively low rate of obstructed patients (30.1%), compared with the nomogram or predictive model constructed through analysis of men with treatment-naïve or common LUTS.11,27
In our cohort with a mean PSA of 3.0 ng ml−1, PSA and history of AUR did not predict the presence of BOO. In the literature, similar to our findings, PSA was not reported to be significantly associated with BOO upon multivariate analysis.18 However, among individuals with PSA between 4 ng ml−1 and 10 ng ml−1, higher PSA level was reportedly linked with increased likelihood of BOO.28 Therefore, the significance of PSA for the prediction of BOO might be further evaluated on the basis of the level of PSA. Men with previous AUR comprised a small portion (3.9%) of our cohort, and the predictive value of AUR for BOO was no longer viable in the final regression model. Indeed, AUR may be a heterogeneous symptom and its diagnosis and treatment is varied.29 Therefore, besides BOO, other pathophysiologies (e.g., detrusor underactivity) should be considered in the clinical setting when evaluating LUTS among men with previous AUR.
Thus far, several noninvasive methods for the diagnosis of BOO have been investigated in men with treatment-naïve or common LUTS, but their clinical application remains largely experimental. Oelke et al.7 reported that detrusor wall thickness was highly associated with BOO (area under the receiver-operating characteristic curve of 0.93) in 160 men with LUTS. Franco et al.8 also showed that detrusor wall thickness and intravesical prostatic protrusion could reasonably predict the presence of BOO in 100 men with LUTS. However, an increased detrusor wall thickness might be associated with other pathophysiologies, such as aging, and the measurement of intravesical prostatic protrusion is variably influenced by bladder volume and physician skill. In addition, it may be hard to conduct ultrasound while maintaining an ideal bladder volume for the evaluation of intravesical prostatic protrusion in routine practice. A recent study assessed the ability of near-infrared spectroscopy to predict BOO and demonstrated 86.2% sensitivity and 87.5% specificity in a small cohort.9 In real-life practice, however, these methods are not always readily available and their usefulness should be evaluated in a large sample.
The current study has several limitations. First, even though the clinical data were prospectively gathered and all of the urodynamic procedures were carried out with the predefined protocol based on guidelines of the International Continence Society (ICS),13 the nomogram was developed through the retrospective evaluation of collected data. Second, we selected the men who had not responded (defined as the IPSS improvement <4) previously to at least three different kinds of LUTS medications, including α-blockers, and subsequently underwent a urodynamic study. Therefore, the clinical characteristic of our cohort was different from that of patients with treatment-naïve or common LUTS. In addition, 1.3% and 9.2% of our patients showed mild symptoms and relatively good flow rate (Qmax >15.0 ml s−1), respectively, even though they were just a small portion of our cohort; this might be caused by the definition of “refractory” LUTS only based on the IPSS improvement in our study. Third, a urodynamic study is helpful to assess bladder pathophysiologies in patients with LUTS who have other confounding factors such as diabetes, neurogenic bladder, high PVR, etc., as well as BOO. However, the present nomogram has limitations in evaluating detrusor pathophysiologies other than BOO. Last, further validations with the multiracial and multicenter populations are required to generalize and utilize the proposed nomogram in the clinical setting for the patients with refractory LUTS. Moreover, more studies are needed to assess whether our nomogram would help improve the treatment algorithm for men with LUTS refractory to medication.
We desired to develop a readily accessible tool to reliably predict the possibility of BOO, without impeding the detection of possible BOO among men with refractory nonneurogenic LUTS in real-life practice. Our proposed nomogram may be useful in daily practice for the detection of BOO and provide direction regarding further management of urinary symptoms among men with LUTS refractory to empirical medications.
CONCLUSIONS
Our clinical nomogram to predict BOO on the basis of routine clinical parameters alone was accurate and validated properly in men ≥50 years of age with refractory nonneurogenic LUTS. This nomogram may be useful in determining further treatment, primarily focused on prostatic surgery for BOO, without impeding the detection of possible BOO, in populations with LUTS refractory to empirical medications.
AUTHOR CONTRIBUTIONS
YJL and SJJ carried out substantial contributions to conception of the study, data acquisition, statistical analysis, and drafting the manuscript. JKL and JJK helped gather the data and draft the manuscript. HML, JJO, SL, SWL, and JHK helped interpret the findings and revise the manuscript. SJJ supervised the process. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
We thank Mrs. Jae Kyung Oh and Mrs. Sun Mi Lee for technical assistance to this study.
REFERENCES
- 1.Coyne KS, Sexton CC, Thompson CL, Milsom I, Irwin D, et al. The prevalence of lower urinary tract symptoms (LUTS) in the USA, the UK and Sweden: results from the epidemiology of LUTS (EpiLUTS) study. BJU Int. 2009;104:352–60. doi: 10.1111/j.1464-410X.2009.08427.x. [DOI] [PubMed] [Google Scholar]
- 2.Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108:1132–8. doi: 10.1111/j.1464-410X.2010.09993.x. [DOI] [PubMed] [Google Scholar]
- 3.Gravas S, Cornu JN, Drake M, Gacci M, Gratzke C, et al. EAU guidelines on management of non-neurogenic male lower urinary tract symptoms (LUTS) [Last accessed on 2019 Jan 23]. Available from: https://uroweb.org/guideline/treatment-of-non-neurogenic-male-luts/#8 .
- 4.Abrams P, Chapple C, Khoury S, Roehrborn C, de la Rosette J. Evaluation and treatment of lower urinary tract symptoms in older men. J Urol. 2013;189:S93–101. doi: 10.1016/j.juro.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Klingler HC, Madersbacher S, Djavan B, Schatzl G, Marberger M, et al. Morbidity of the evaluation of the lower urinary tract with transurethral multichannel pressure-flow studies. J Urol. 1998;159:191–4. doi: 10.1016/s0022-5347(01)64054-0. [DOI] [PubMed] [Google Scholar]
- 6.Griffiths CJ, Harding C, Blake C, McIntosh S, Drinnan MJ, et al. A nomogram to classify men with lower urinary tract symptoms using urine flow and noninvasive measurement of bladder pressure. J Urol. 2005;174:1323–6. doi: 10.1097/01.ju.0000173637.07357.9e. [DOI] [PubMed] [Google Scholar]
- 7.Oelke M, Höfner K, Jonas U, de la Rosette JJ, Ubbink DT, et al. Diagnostic accuracy of noninvasive tests to evaluate bladder outlet obstruction in men: detrusor wall thickness, uroflowmetry, postvoid residual urine, and prostate volume. Eur Urol. 2007;52:827–34. doi: 10.1016/j.eururo.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 8.Franco G, De Nunzio C, Leonardo C, Tubaro A, Ciccariello M, et al. Ultrasound assessment of intravesical prostatic protrusion and detrusor wall thickness – new standards for noninvasive bladder outlet obstruction diagnosis? J Urol. 2010;183:2270–4. doi: 10.1016/j.juro.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Yurt M, Süer E, Gülpinar O, Telli O, Arikan N. Diagnosis of bladder outlet obstruction in men with lower urinary tract symptoms: comparison of near infrared spectroscopy algorithm and pressure flow study in a prospective study. Urology. 2012;80:182–6. doi: 10.1016/j.urology.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 10.Steele GS, Sullivan MP, Sleep DJ, Yalla SV. Combination of symptom score, flow rate and prostate volume for predicting bladder outflow obstruction in men with lower urinary tract symptoms. J Urol. 2000;164:344–8. [PubMed] [Google Scholar]
- 11.De Nunzio C, Autorino R, Bachmann A, Briganti A, Carter S, et al. The diagnosis of benign prostatic obstruction: development of a clinical nomogram. Neurourol Urodyn. 2016;35:235–40. doi: 10.1002/nau.22705. [DOI] [PubMed] [Google Scholar]
- 12.Choi HR, Chung WS, Shim BS, Kwon SW, Hong SJ, et al. Translation validity and reliability of I-PSS Korean version. Korean J Urol. 1996;37:659–65. [Google Scholar]
- 13.Schäfer W, Abrams P, Liao L, Mattiasson A, Pesce F, et al. International continence society: good urodynamic practices: uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn. 2002;21:261–74. doi: 10.1002/nau.10066. [DOI] [PubMed] [Google Scholar]
- 14.Abrams P. Bladder outlet obstruction index, bladder contractility index and bladder voiding efficiency: three simple indices to define bladder voiding function. BJU Int. 1999;84:14–5. doi: 10.1046/j.1464-410x.1999.00121.x. [DOI] [PubMed] [Google Scholar]
- 15.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 16.Abrams P, Chapple C, Khoury S, Roehrborn C, de la Rosette J. Evaluation and treatment of lower urinary tract symptoms in older men. J Urol. 2009;181:1779–87. doi: 10.1016/j.juro.2008.11.127. [DOI] [PubMed] [Google Scholar]
- 17.Yeo JK, Choi H, Bae JH, Kim JH, Yang SO, et al. Korean clinical practice guideline for benign prostatic hyperplasia. Investig Clin Urol. 2016;57:30–44. doi: 10.4111/icu.2016.57.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang MY, Ku JH, Oh SJ. Non-invasive parameters predicting bladder outlet obstruction in Korean men with lower urinary tract symptoms. J Korean Med Sci. 2010;25:272–5. doi: 10.3346/jkms.2010.25.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosier PF, de la Rosette JJ. Is there a correlation between prostate size and bladder-outlet obstruction? World J Urol. 1995;13:9–13. doi: 10.1007/BF00182658. [DOI] [PubMed] [Google Scholar]
- 20.Eckhardt MD, van Venrooij GE, Boon TA. Interactions between prostate volume, filling cystometric estimated parameters, and data from pressure-flow studies in 565 men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Neurourol Urodyn. 2001;20:579–90. doi: 10.1002/nau.1010. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan SA, Te AE, Pressler LB, Olsson CA. Transition zone index as a method of assessing benign prostatic hyperplasia: correlation with symptoms, urine flow and detrusor pressure. J Urol. 1995;154:1764–9. [PubMed] [Google Scholar]
- 22.Kurita Y, Masuda H, Terada H, Suzuki K, Fujita K. Transition zone index as a risk factor for acute urinary retention in benign prostatic hyperplasia. Urology. 1998;51:595–600. doi: 10.1016/s0090-4295(97)00685-7. [DOI] [PubMed] [Google Scholar]
- 23.Lepor H, Nieder A, Feser J, O’Connell C, Dixon C. Total prostate and transition zone volumes, and transition zone index are poorly correlated with objective measures of clinical benign prostatic hyperplasia. J Urol. 1997;158:85–8. doi: 10.1097/00005392-199707000-00023. [DOI] [PubMed] [Google Scholar]
- 24.De Nunzio C, Lombardo R, Gacci M, Milanesi M, Cancrini F, et al. The diagnosis of benign prostatic obstruction: validation of the young academic urologist clinical nomogram. Urology. 2015;86:1032–6. doi: 10.1016/j.urology.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Reynard JM, Abrams P. Bladder-outlet obstruction – assessment of symptoms. World J Urol. 1995;13:3–8. doi: 10.1007/BF00182657. [DOI] [PubMed] [Google Scholar]
- 26.Yalla SV, Sullivan MP, Lecamwasam HS, DuBeau CE, Vickers MA, et al. Correlation of American Urological Association symptom index with obstructive and nonobstructive prostatism. J Urol. 1995;153:674–9. [PubMed] [Google Scholar]
- 27.Kim M, Cheeti A, Yoo C, Choo M, Paick JS, et al. Non-invasive clinical parameters for the prediction of urodynamic bladder outlet obstruction: analysis using causal Bayesian networks. PLoS One. 2014;9:e113131. doi: 10.1371/journal.pone.0113131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laniado ME, Ockrim JL, Marronaro A, Tubaro A, Carter SS. Serum prostate-specific antigen to predict the presence of bladder outlet obstruction in men with urinary symptoms. BJU Int. 2004;94:1283–6. doi: 10.1111/j.1464-410X.2004.05158.x. [DOI] [PubMed] [Google Scholar]
- 29.Fitzpatrick JM, Kirby RS. Management of acute urinary retention. BJU Int. 2006;97:16–20. doi: 10.1111/j.1464-410X.2006.06100.x. [DOI] [PubMed] [Google Scholar]


