Abstract
Prostate cancer is the most common malignancy in the reproductive system of older males. Androgen deprivation therapy (ADT) is an important treatment for prostate cancer patients. However, almost all prostate cancer patients unavoidably progress to the castration-resistant stage after ADT treatment. Recent studies have shown that tumor-associated immune cells play major roles in the initiation, progression, and metastasis of prostate cancer. Various phenotypes of tumor-associated immune cells have tumor-promoting or antitumor functions mediated by interacting with tumor cells. Here, we review the current knowledge of tumor-associated immune cells in prostate cancer.
Keywords: immune tolerance, prostate cancer, tumor associated immune cells, tumor microenvironment
INTRODUCTION
Prostate cancer is becoming one of the most common malignancies in the male reproductive system in China. The number of estimated new cases of prostate cancer in men over 60 years of age in China was about 56 600 in 2015.1 With the improvement of living standards and diagnostic techniques, the incidence rate of prostate cancer has increased each year.1,2 Prostatectomy or radiotherapy, combined with endocrine therapy, is the first choice for the management of intermediate- and high-risk prostate cancer. Early stage prostate cancer patients receive an indolent course of treatment during the first 10–15 years, and tumor progression and aggressive metastasis may develop in the long term.3 However, for intermediate- and high-risk prostate cancer patients, most will finally develop into castration-resistant prostate cancer (CRPC) after androgen deprivation therapy (ADT) for about one and a half years. Moreover, about a third of CRPC patients will develop bone metastasis within 2 years, resulting in cancer-specific death.4,5 Therefore, it is an urgent and important issue to identify potential mechanisms for the initiation and progression of prostate cancer.
INFLAMMATION AND PROSTATE CANCER
Chronic inflammation is now considered as a major factor in the development of various malignancies.6 Accumulating studies have shown that prostatic inflammation is involved in the initiation of proliferative inflammatory atrophy (PIA), a kind of precursor lesion related to the development of prostate cancer.7,8 Bacterial infection, autoimmune responses, and other proinflammatory factors can lead to intraprostatic inflammation with consequential modification of the prostatic microenvironment. The immune cells involved in the inflammatory microenvironment can affect the initiation and progression of prostate cancer via the secretion of cytokines and growth factors. However, the roles of these inflammatory cells are still unclear because of their plasticity and phenotypes in the different stages of the tumor microenvironment.
Some studies have indicated racial differences in inflammation.9,10,11 Vidal et al.10 showed that the Asian population is more likely to experience acute prostate inflammation compared with Caucasians, which presents a higher risk of prostate cancer for Asians. The underlying mechanisms of race differences in the inflammatory response are complicated. It is generally accepted that acute prostate inflammation is considered as a protective factor, whereas chronic inflammation is significantly associated with the development of prostate cancer.
As mentioned above, inflammation is a major characteristic of human malignancies. Tumor-associated immune cells are main components in the tumor microenvironment. The infiltrating immune cells in the prostate tumor microenvironment include T regulatory cells (Tregs), tumor-associated macrophages (TAMs), neutrophils, and myeloid-derived suppressor cells (MDSCs). Many studies have shown that tumor-associated immune cells play a major role in the progression of prostate cancer.12,13 Studies concerning the development of tumor-associated immune cells have focused on drugs and the application of immune vaccines to provide a new direction for the management of prostate cancer.
TUMOR-INFILTRATING LYMPHOCYTES AND PROSTATE CANCER
Tumor-infiltrating lymphocytes (TILs) contribute to the progression of prostate cancer through a variety of mechanisms. Traditionally, effector T cells are divided into Th1 and Th2 subgroups. However, in recent decades, emerging evidence has indicated that Tregs subsets of CD4+ cells play a major role in regulating tumor progression by mediating immunosuppression.14,15
Tregs, which were first reported by Sakaguchi et al.15 as a subset of T cells with a CD4+ CD25- high phenotype, play an important role in regulating immune tolerance of the tumor microenvironment. Recent studies have found that FOXP3+ is also an important phenotype of Tregs.16,17 Zhao et al.17 found that the number of Tregs obviously increases in the bone metastatic microenvironment of prostate cancer. The infiltration of CD4+ CD25- high Tregs into bone marrow contributes to the initiation of bone metastasis in prostate cancer patients, by providing an immunosuppressive microenvironment. Akins et al.18 found that FOXP3+ Tregs in the prostate epithelium increase obviously after ADT, while cytotoxic T cells are restricted to the prostatic stroma. In addition, ADT combined with Treg clearance therapy reduced the prostate tumor burden and inhibited tumor recurrence in mice. Mo et al.19 indicated that Treg depletion enhanced the antitumor immunity of a tumor cell vaccine against prostate cancer in a subcutaneous prostate cancer mouse model. Davidsson et al.20 found that infiltration of CD4+ FOXP3+ Tregs into prostate tissue was positively correlated with Gleason scores and pathological tumor stages of prostate cancer. Therefore, Tregs may be involved in the development of prostate carcinoma from atrophic hyperplasia. Flammiger et al.21 found that an increased number of FOXP3+ cells in prostate carcinomas was obviously correlated with an elevated tumor stage and proliferation index, suggesting that the infiltration of Tregs into the tumor microenvironment contributes to the development of prostate carcinoma. Nardone et al.22 performed a retrospective study and found that the number of FOXP3+ Tregs in prostate cancer tissues was negatively correlated with overall and progression-free survival times of prostate cancer patients. Studies regarding the role of tumor-infiltrating lymphocytes in prostate cancer are summarized in Table 1.
Table 1.
Summary of studies about the role of tumor infiltrating lymphocytes in prostate cancer
| First author (year) | Study sources | Identification of TILs | Management | Outcome |
|---|---|---|---|---|
| Akins et al. 201018 | Pten knockout mice | FoxP3+ | Anti-CD25 antibody | Tregs depletion combined with in situ vaccination and ADT can reduce castration-resistant tumor burden |
| Zhao et al. 201217 | SCID mice with PC-3 intratibial injection | CD4+CD25high, CD4+Foxp3+ | Intravenously transfused with activated Tregs | Bone marrow Treg cells may facilitate cancer bone metastasis and contribute to bone deposition |
| Flammiger et al. 201321 | Patients’ samples | FoxP3+ | Immunohistochemistry analysis | Increased infiltrating of Tregs significantly involved with reduced PSA recurrence-free survival and advanced tumor stage |
| Nardone et al. 201622 | Patients’ samples | FoxP3+PD-1 | Immunohistochemistry analysis | Lower expression of PD-1/FoxP3+ correlated with prolonged PFS and OS |
| Mo et al. 201719 | Subcutaneous mice model of RM-1 prostate cancer | ICOS | Anti-ICOS antibody | ICOS blocking could deplete the infiltrated Tregs and enhance antitumor immunity of tumor cell vaccine in prostate cancer |
| Davidsson et al. 201820 | Patients’ samples | CD4+FOXP3+, CD8+FOXP3+ | Immunohistochemistry analysis | Four-fold increased risk of prostate cancer in men with epithelial CD4+ Tregs infiltration |
TILs: tumor-infiltrating lymphocytes; FOXP3: forkhead box P3; CD: cluster of differentiation; PTEN: phosphatase and tensin homolog; ICOS: inducible T cell costimulatory; SCID: severe combined immunodeficiency; PC-3: prostate cancer cell-3; ADT: androgen depletion therapy; Tregs: regulatory T cells; PSA: prostate-specific androgen; PFS: progression-free survival; OS: overall survival; PD-1: programmed cell death protein-1
Thus far, the phenotypes of Tregs that infiltrate into the prostate tumor microenvironment and their mechanisms involved in the regulation of prostate tumor initiation and in the development are still unclear.23 It is generally believed that Tregs mainly exist in secondary lymphoid organs and can be recruited into the tumor microenvironment via the induction of tumor-associated chemokines. In addition, Tregs mediate immunosuppressive effects by interacting with tumor cells and secreting related factors. For example, Tregs inhibit the function of antigen-presenting cells by interacting with CD80/CD86 on antigen-presenting cells via their surface cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) receptor, which inhibits the anticancer functions of cytotoxic and effector T cells. Moreover, Tregs directly inhibit the function of effector T cells by secreting granulase B perforating protein.23,24 Currently, Treg-related immune checkpoint inhibitors are being developed or are in clinical trials for anticancer therapy. For example, CTLA-4 is commonly expressed by Tregs, and anti-CTLA-4 monoclonal antibody drugs such as ipilimumab in combination with ADT, sipuleucel-T, or programmed cell death protein 1 (PD-1) inhibitors are being evaluated in clinical trials for prostate cancer patients.25,26
TUMOR-ASSOCIATED MACROPHAGES AND PROSTATE CANCER
Tumor-associated macrophages (TAMs) are macrophages that infiltrate into tumor tissues. Traditionally, macrophages were only considered to play an anticancer role in the tumor microenvironment. However, many recent studies have shown that macrophages in the tumor microenvironment can also be educated by tumor cells or hijacked by dead cells to exert a tumor-promoting effect.27,28 Some studies have reported that TAMs can be divided into classical activation (M1 type) macrophages and alternative activation (M2 type) macrophages, according to their different activation pathways.29,30 It is generally believed that M1-type macrophages promote inflammation, whereas M2-type macrophages promote tissue repair by inducing angiogenesis and synthesis of matrix proteins.27 It is widely accepted that there are many more M2-type macrophages than M1-type macrophages in the tumor microenvironment. Emerging evidence has indicated that increased infiltration of macrophages into the tumor microenvironment predicts a worse prognosis of breast, prostate, colorectal, and ovarian cancers.30,31,32
In recent years, the role of TAMs in the initiation, development, and metastasis of prostate cancer has become a research hotspot. Lanciotti et al.33 found that high-density TAMs in prostate cancer tissue predict poor biochemical recurrence in prostate cancer patients after radical prostatectomy, and M2-type macrophages are associated with extrafascial invasion of prostate cancer. Distant metastasis is a common and complicated issue in advanced prostate cancer patients. Some studies have reported that the total number of TAMs, especially the number of infiltrated M2-type macrophages, increases in metastatic prostate cancer, suggesting that TAM infiltration is a risk factor for distant metastasis of prostate cancer.34,35 Neuroendocrine differentiation (NED) of prostate cancer cells in the prostate tumor microenvironment is a major feature of castration-resistant prostate cancer. NED is usually positively associated with invasive prostate cancer and negatively associated with clinical outcomes of prostate cancer.35,36 Lee et al.37 found that neuroendocrine cells in human prostate cancer tissues are usually associated with BMP-6 protein expression and TAM infiltration. In addition, the removal of IL-6 or macrophages in mouse models inhibited BMP-6-induced NED. Wang et al.36 found that targeted blockade of interleukin-6 receptor (IL-6R) and high mobility protein-1 (HMGB-1) blocked the positive feedback pathway between TAMs and NED in prostate cancer, which resulted in improvement of the enzalutamide therapeutic effect in prostate cancer patients. Therefore, macrophage infiltration into the prostate tumor environment, especially M2-type TAMs, promotes the development and metastasis of prostate cancer and participates in the regulation of NED and ADT resistance in prostate cancer.
The underlying mechanisms of TAMs in the regulation of tumor initiation and progression of prostate cancer are complex. It is generally believed that TAMs influence the proliferation and migration of cancer cells via direct interactions with tumor cells or indirectly by secretion of cytokines, providing structural space, and participating in every stage of tumor progression. Chen et al.38 found that the secretion of nephroblastoma overexpressed (NOV/CCN3) from prostate cancer cells induces TAMs to express CD204, and then activates the NF-kB signaling pathway, mediates the secretion of VEGF by M2 type TAMs, and promotes tumor angiogenesis. In addition, Soki et al.39 showed that TAMs promote tumor progression via interactions with apoptotic cells through the MFG-E8 pathway in the microenvironment of prostate cancer with bone metastasis. Further exploration of the potential mechanisms and immune checkpoints of TAMs in the prostate tumor microenvironment may provide new concepts for the treatment of prostate cancer.
TUMOR-ASSOCIATED NEUTROPHILS AND PROSTATE CANCER
Tumor-associated neutrophils (TANs) specifically refer to neutrophils that infiltrate into the tumor microenvironment. The roles of TANs in various tumors are still controversial.40 Fridlender et al.41 found that TANs function like a “double-edged sword,” which can acquire a tumor-promoting phenotype via induction by TGF-β. However, after blockage of TGF-β, TANs exert an antitumor effect, suggesting that TANs can be classified into N1 and N2 phenotypes similar to TAMs. Hanahan and Weinberg.42 found that TANs promote tumor-related inflammation, thereby contributing to tumor cell proliferation, invasion, and tumor angiogenesis. Casbon et al.43 showed that TANs mediate immunosuppression by inhibiting the tumor-killing function of cytotoxic T cells. However, some studies have reported that elevated TAN infiltration is positively correlated with the prognosis of tumors. For example, Governa et al.44 found that TANs enhance the reactivity of CD8+ T cells and activate T cell receptors, contributing to the death of tumor cells and prolonging the survival time of colorectal cancer patients. Hence, the role of TAN infiltration into the tumor microenvironment is complicated and undefined.
The mechanisms involved in the role TANs in tumors still need to be elucidated. TANs promote the development of cancer via TAN-induced immunosuppression as well as changes in the tumor microenvironment caused by TAN infiltration. Furthermore, TANs function as tumor killers through the promotion of T cell-mediated tumor clearance or by secreting cytotoxic factors. The double-edged sword effects of TANs may be involved in the high heterogeneity or plasticity of neutrophils themselves. Moreover, the specific microenvironment in different tumor stages contributes to the multiple roles of TANs.30,45
TUMOR-ASSOCIATED MYELOID-DERIVED SUPPRESSOR CELLS AND PROSTATE CANCER
Myeloid-derived suppressor cells (MDSCs) are immature myeloid cells including immature granulocytes, macrophages, and dendritic cells. In humans, the phenotypes of MDSCs are mainly CD13, CD33, and CD34. Tumor-associated MDSCs are group of MDSCs in the tumor microenvironment. Recent studies have shown that MDSCs in the tumor microenvironment promote the proliferation and metastasis of tumor cells by mediating immunosuppressive effects.46,47 There is also clinical evidence that treatment with MDSCs prolongs the survival time of cancer patients. Moreover, MDSC-targeted therapy combined with other immunotherapeutic drugs are under investigation.48,49
Some studies have reported the role of MDSCs in the initiation and development of prostate cancer. Jachetti et al.50 found that multinuclear MDSCs induced immunosuppression and promoted the development of prostate cancer via interactions with mast cells through the CD40-CD40L pathway, and elevated expression of mast cell- and MDSC-related genes was obviously associated with poor prognoses of patients with prostate cancer. Chi et al.51 found that increased MDSCs in the circulating blood of prostate cancer patients were obviously associated with a poor prognosis. In recent years, preclinical trials and related clinical trials have been performed to evaluate the therapeutic efficacy of MDSC inhibitors in prostate cancer patients. Yin et al.52 indicated that phosphatidylserine-targeted therapy inhibits the differentiation and growth of MDSCs, promotes the transformation of TAMs into the M1 type, and then inhibits the growth of prostate cancer cells. The phosphatidylserine-targeted drug bavituximab is being evaluated in clinical trials. In addition, some other MDSC-targeted drugs are being evaluated in clinical trials for prostate cancer, such as verteporfin and axitinib.13,53
The mechanisms underlying the roles of MDSCs are still unclear. Ostrand-Rosenberg et al.53 reported that MDSCs in the tumor microenvironment upregulate the expression of IL-10, downregulate the expression of IL-6, IL-12, and MHC II in macrophages, and induce the differentiation of M2 type macrophages, which promotes the proliferation and metastasis of malignancies. Moreover, MDSCs prohibit the antitumor function of mature dendritic cells via impairment of their antigen presentation and migration, which facilitates the development of immune evasion and promotes the proliferation of tumor cells. The infiltration of tumor-associated MDSCs and their protumor roles were found to be associated with the PI3K/PTEN/AKT pathway in mouse models of prostate cancer.13 However, whether MDSCs are involved with PTEN loss and PI3K activation in prostate cancer patients remains to be elucidated.
SUMMARY
Immune cells in the prostate tumor microenvironment play a role as a double-edged sword. At various disease stages, tumor-associated immune cells with specific phenotypes may mediate the immune evasion or tolerance of prostate tumor cells, by direct interactions with tumor cells or indirectly by secreting cytokines to promote the initiation and progression of prostate cancer. We have summarized the relationships between tumor-associated immune cells and prostate cancer cells in Figure 1. The development of immune vaccines and immune checkpoint inhibitors may provide a new direction for the treatment of prostate cancer, especially metastatic castration-resistant prostate cancer.
Figure 1.
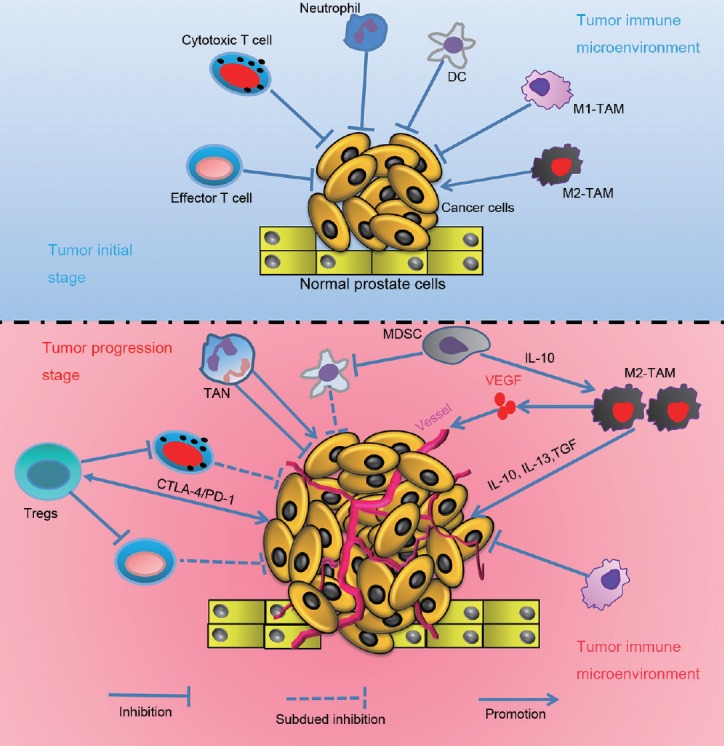
Graphical model for the role of tumor associated immune cells in the initiation and progression of prostate cancer.
AUTHOR CONTRIBUTION
SQW and HS performed literature searching and data collection. SQW and HS prepared the manuscript. XKZ and YHW participated in the design of the study and helped revise the manuscript. YHW conceived of this review and participated in its design and coordination and helped draft the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
This work was supported by the grant from the Technological Innovation Guidance Plan (No. 2017sk50123) of Hunan Province.
REFERENCES
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Zhang S, Zeng H, Xia C, et al. Cancer statistics and mortality in China, 2013. Cancer Lett. 2017;401:63–71. doi: 10.1016/j.canlet.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 3.Johansson JE, Andrén O, Andersson SO, Dickman PW, Holmberg L, et al. Natural history of early, localized prostate cancer. JAMA. 2004;291:2713–9. doi: 10.1001/jama.291.22.2713. [DOI] [PubMed] [Google Scholar]
- 4.Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: Treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630–42. doi: 10.1016/j.eururo.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Smith MR, Kabbinavar F, Saad F, Hussain A, Gittelman MC, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23:2918–25. doi: 10.1200/JCO.2005.01.529. [DOI] [PubMed] [Google Scholar]
- 6.Wang D, DuBois RN. Immunosuppression associated with chronic inflammation in the tumor microenvironment. Carcinogenesis. 2015;36:1085–93. doi: 10.1093/carcin/bgv123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gandaglia G, Zaffuto E, Fossati N, Cucchiara V, Mirone V, et al. The role of prostatic inflammation in the development and progression of benign and malignant diseases. Curr Opin Urol. 2017;27:99–106. doi: 10.1097/MOU.0000000000000369. [DOI] [PubMed] [Google Scholar]
- 8.Puhr M, De Marzo A, Isaacs W, Lucia MS, Sfanos K. Inflammation, microbiota, and prostate cancer. Eur Urol Focus. 2016;2:374–82. doi: 10.1016/j.euf.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson JF, Patel PN, Shah RY, Mulvey CK, Gadi R, et al. Race and gender variation in response to evoked inflammation. J Transl Med. 2013;11:63. doi: 10.1186/1479-5876-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vidal AC, Chen Z, Howard LE, Moreira DM, Castro-Santamaria R, et al. Racial differences in prostate inflammation: results from the REDUCE study. Oncotarget. 2016;8:71393–9. doi: 10.18632/oncotarget.10690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rybicki BA, Kryvenko ON, Wang Y, Jankowski M, Trudeau S, et al. Racial differences in the relationship between clinical prostatitis, presence of inflammation in benign prostate and subsequent risk of prostate cancer. Prostate Cancer Prostatic Dis. 2016;19:145–50. doi: 10.1038/pcan.2015.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Si TG, Wang JP, Guo Z. Analysis of circulating regulatory T cells (CD4+ CD25+ CD127-) after cryosurgery in prostate cancer. Asian J Androl. 2013;15:461–5. doi: 10.1038/aja.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez-Bujanda Z, Drake CG. Myeloid-derived cells in prostate cancer progression: phenotype and prospective therapies. J Leukoc Biol. 2017;102:393–406. doi: 10.1189/jlb.5VMR1116-491RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knochelmann HM, Dwyer CJ, Bailey SR, Amaya SM, Elston DM, et al. When worlds collide: Th17 and Treg cells in cancer and autoimmunity Naïve. Cell Mol Immunol. 2018;15:458–69. doi: 10.1038/s41423-018-0004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 16.Copland A, Bending D. Foxp3 molecular dynamics in treg in juvenile idiopathic arthritis. Front Immunol. 2018;9:2273. doi: 10.3389/fimmu.2018.02273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao E, Wang L, Dai J, Kryczek I, Wei S, et al. Regulatory T cells in the bone marrow microenvironment in patients with prostate cancer. Oncoimmunology. 2012;1:152–61. doi: 10.4161/onci.1.2.18480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akins EJ, Moore ML, Tang S, Willingham MC, Tooze JA, et al. In situ vaccination combined with androgen ablation and regulatory T-cell depletion reduces castration-resistant tumor burden in prostate-specific pten knockout mice. Cancer Res. 2010;70:3473–82. doi: 10.1158/0008-5472.CAN-09-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mo L, Chen Q, Zhang X, Shi X, Wei L, et al. Depletion of regulatory T cells by anti-ICOS antibody enhances anti-tumor immunity of tumor cell vaccine in prostate cancer. Vaccine. 2017;35:5932–8. doi: 10.1016/j.vaccine.2017.08.093. [DOI] [PubMed] [Google Scholar]
- 20.Davidsson S, Andren O, Ohlson AL, Carlsson J, Andersson SO, et al. FOXP3+ regulatory T cells in normal prostate tissue, postatrophic hyperplasia, prostatic intraepithelial neoplasia, and tumor histological lesions in men with and without prostate cancer. Prostate. 2018;78:40–7. doi: 10.1002/pros.23442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flammiger A, Weisbach L, Huland H, Tennstedt P, Simon R, et al. High tissue density of FOXP3+ T cells is associated with clinical outcome in prostate cancer. Eur J Cancer. 2013;49:1273–9. doi: 10.1016/j.ejca.2012.11.035. [DOI] [PubMed] [Google Scholar]
- 22.Nardone V, Botta C, Caraglia M, Martino EC, Ambrosio MR, et al. Tumor infiltrating T lymphocytes expressing FoxP3, CCR7 or PD-1 predict the outcome of prostate cancer patients subjected to salvage radiotherapy after biochemical relapse. Cancer Biol Ther. 2016;17:1213–20. doi: 10.1080/15384047.2016.1235666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohr A, Malhotra R, Mayer G, Gorochov G, Miyara M. Human FOXP3+ T regulatory cell heterogeneity. Clin Transl Immunol. 2018;7:e1005. doi: 10.1002/cti2.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeuchi Y, Nishikawa H. Roles of regulatory T cells in cancer immunity. Int Immunol. 2016;28:401–9. doi: 10.1093/intimm/dxw025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh BH, Gulley JL. Immunotherapy and therapeutic vaccines in prostate cancer: an update on current strategies and clinical implications. Asian J Androl. 2014;16:364–71. doi: 10.4103/1008-682X.122585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Redman JM, Gulley JL, Madan RA. Combining immunotherapies for the treatment of prostate cancer. Urol Oncol Semin Orig Investig. 2017;35:694–700. doi: 10.1016/j.urolonc.2017.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeya M, Komohara Y. Role of tumor-associated macrophages in human malignancies: friend or foe? Pathol Int. 2016;66:491–505. doi: 10.1111/pin.12440. [DOI] [PubMed] [Google Scholar]
- 28.Weigert A, Mora J, Sekar D, Syed S, Brüne B. Killing is not enough: how apoptosis hijacks tumor-associated macrophages to promote cancer progression. Adv Exp Med Biol. 2016;930:205–39. doi: 10.1007/978-3-319-39406-0_9. [DOI] [PubMed] [Google Scholar]
- 29.Rhee I. Diverse macrophages polarization in tumor microenvironment. Arch Pharm Res. 2016;39:1–9. doi: 10.1007/s12272-016-0820-y. [DOI] [PubMed] [Google Scholar]
- 30.Goswami KK, Ghosh T, Ghosh S, Sarkar M, Bose A, et al. Tumor promoting role of anti-tumor macrophages in tumor microenvironment. Cell Immunol. 2017;316:1–10. doi: 10.1016/j.cellimm.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Xiong Y, Wang K, Zhou H, Peng L, You W, et al. Profiles of immune infiltration in colorectal cancer and their clinical significant: a gene expression-based study. Cancer Med. 2018;7:4496–508. doi: 10.1002/cam4.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;8:254–65. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 33.Lanciotti M, Masieri L, Raspollini MR, Minervini A, Mari A, et al. The role of M1 and M2 macrophages in prostate cancer in relation to extracapsular tumor extension and biochemical recurrence after radical prostatectomy. Biomed Res Int. 2014;2014:486798. doi: 10.1155/2014/486798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu W, Qian Y, Yu F, Liu W, Wu Y, et al. Alternatively activated macrophages are associated with metastasis and poor prognosis in prostate adenocarcinoma. Oncol Lett. 2015;10:1390–6. doi: 10.3892/ol.2015.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sagnak L, Topaloglu H, Ozok U, Ersoy H. Prognostic significance of neuroendocrine differentiation in prostate adenocarcinoma. Clin Genitourin Cancer. 2011;9:73–80. doi: 10.1016/j.clgc.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Wang C, Peng G, Huang H, Liu F, Kong DP, et al. Blocking the feedback loop between neuroendocrine differentiation and macrophages improves the therapeutic effects of enzalutamide (MDV3100) on prostate cancer. Clin Cancer Res. 2018;24:708–23. doi: 10.1158/1078-0432.CCR-17-2446. [DOI] [PubMed] [Google Scholar]
- 37.Lee GT, Kwon SJ, Lee JH, Jeon SS, Jang KT, et al. Macrophages induce neuroendocrine differentiation of prostate cancer cells via BMP6-IL6 loop. Prostate. 2011;71:1525–37. doi: 10.1002/pros.21369. [DOI] [PubMed] [Google Scholar]
- 38.Chen PC, Cheng HC, Wang J, Wang SW, Tai HC, et al. Prostate cancer-derived CCN3 induces M2 macrophage infiltration and contributes to angiogenesis in prostate cancer microenvironment. Oncotarget. 2014;5:1595–608. doi: 10.18632/oncotarget.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soki FN, Cho SW, Kim YW, Jones JD, Park SI, et al. Bone marrow macrophages support prostate cancer growth in bone. Oncotarget. 2015;6:35782–96. doi: 10.18632/oncotarget.6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rakic A, Beaudry P, Mahoney DJ. The complex interplay between neutrophils and cancer. Cell Tissue Res. 2018;371:517–29. doi: 10.1007/s00441-017-2777-7. [DOI] [PubMed] [Google Scholar]
- 41.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–94. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 43.Casbon AJ, Reynaud D, Park C, Khuc E, Gan DD, et al. Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc Natl Acad Sci. 2015;112:E566–75. doi: 10.1073/pnas.1424927112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Governa V, Trella E, Mele V, Tornillo L, Amicarella F, et al. The interplay between neutrophils and CD8+ T cells improves survival in human colorectal cancer. Clin Cancer Res. 2017;23:3847–58. doi: 10.1158/1078-0432.CCR-16-2047. [DOI] [PubMed] [Google Scholar]
- 45.Galdiero MR, Bonavita E, Barajon I, Garlanda C, Mantovani A, et al. Tumor associated macrophages and neutrophils in cancer. Immunobiology. 2013;218:1402–10. doi: 10.1016/j.imbio.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 46.Groth C, Hu X, Weber R, Fleming V, Altevogt P, et al. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br J Cancer. 2019;120:16–25. doi: 10.1038/s41416-018-0333-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Umansky V, Blattner C, Fleming V, Hu X, Gebhardt C, et al. Myeloid-derived suppressor cells and tumor escape from immune surveillance. Semin Immunopathol. 2017;39:295–305. doi: 10.1007/s00281-016-0597-6. [DOI] [PubMed] [Google Scholar]
- 48.Tobin RP, Davis D, Jordan KR, McCarter MD. The clinical evidence for targeting human myeloid-derived suppressor cells in cancer patients. J Leukoc Biol. 2017;102:381–91. doi: 10.1189/jlb.5VMR1016-449R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Draghiciu O, Lubbers J, Nijman HW, Daemen T. Myeloid derived suppressor cells – an overview of combat strategies to increase immunotherapy efficacy. Oncoimmunology. 2015;4:e954829. doi: 10.4161/21624011.2014.954829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jachetti E, Cancila V, Rigoni A, Bongiovanni L, Cappetti B, et al. Cross-talk between myeloid-derived suppressor cells and mast cells mediates tumor-specific immunosuppression in prostate cancer. Cancer Immunol Res. 2018;6:552–65. doi: 10.1158/2326-6066.CIR-17-0385. [DOI] [PubMed] [Google Scholar]
- 51.Chi N, Tan Z, Ma K, Bao L, Yun Z. Increased circulating myeloid-derived suppressor cells correlate with cancer stages, interleukin-8 and -6 in prostate cancer. Int J Clin Exp Med. 2014;7:3181–92. [PMC free article] [PubMed] [Google Scholar]
- 52.Yin Y, Huang X, Lynn KD, Thorpe PE. Phosphatidylserine-targeting antibody induces M1 macrophage polarization and promotes myeloid-derived suppressor cell differentiation. Cancer Immunol Res. 2013;1:256–68. doi: 10.1158/2326-6066.CIR-13-0073. [DOI] [PubMed] [Google Scholar]
- 53.Ostrand-Rosenberg S, Sinha P, Beury DW, Clements VK. Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhance tumor-induced immune suppression. Semin Cancer Biol. 2012;22:275–81. doi: 10.1016/j.semcancer.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


