Abstract
Cryptorchidism is one of the most frequent causes of nonobstructive azoospermia (NOA) in adulthood. Although it is well known that spermatogenesis is more impaired in bilateral than in unilateral cryptorchidism, previous studies have only described small cohorts or inhomogeneous population. Consequently, we analyzed a cohort of 225 men with only a history of cryptorchidism as sole etiopathogenetic factor for NOA, and compared testicular sperm extraction (TESE) outcomes between men with bilateral versus unilateral cryptorchidism. Our results show no difference in follicle-stimulating hormone (FSH) levels and testicular volumes between men with a history of bilateral cryptorchidism compared to unilateral cryptorchidism (median: 21.3 IU l−1 vs 19.3 IU l−1, P = 0.306; and 7.2 ml vs 7.9 ml, P = 0.543, respectively). In addition, sperm retrieval rates were similar (66.2% vs 60.0%, P = 0.353). Using multivariate analysis, we have found that only a low inhibin B level (above the assay's detection limit) was positively associated with successful sperm retrieval (P < 0.05). Regarding intracytoplasmic sperm injection outcomes, we found that cumulative pregnancy rate and live birth rate per cycle were not statistically different between the two groups (17.4% vs 27.8%, P = 0.070; and 16.1% vs 26.4%, P = 0.067, respectively). Unexpectedly, there was no significant difference in hormonal profiles (FSH, luteinizing hormone [LH], testosterone, and inhibin B levels) and TESE outcomes between unilateral versus bilateral cryptorchidism. This suggests that a history of unilateral cryptorchidism could reflect a bilateral testicular impairment. Interestingly, inhibin B level might be a predictor of successful TESE.
Keywords: cryptorchidism, intracytoplasmic sperm injection, nonobstructive azoospermia, testicular sperm extraction
INTRODUCTION
Cryptorchidism is a very common genital disorder affecting 1%–4% of male children and representing one of the most frequent causes of nonobstructive azoospermia (NOA) in the adulthood.1 Cryptorchidism is the consequence of abnormal testicular migration, and the testis can be found anywhere along its normal migration path.2 This implies that cryptorchidism differs from ectopy, retractile testis, and anorchia. A history of cryptorchidism is reported in 20% of azoospermic or infertile men.3,4,5 In two-thirds of cases, cryptorchidism is unilateral.2 Yet, it has been suggested that the reproductive function could depend on the uni/bilaterality of cryptorchidism.6,7,8 Indeed, bilateral cryptorchidism might be more pejorative and associated with a higher risk of infertility, resulting in an increase in time to conception.9,10 In formerly cryptorchid patients, infertility is suspected in 54% of ex-bilateral cryptorchid and 9% of ex-unilateral cryptorchid men.7 In addition, spermatogenesis was reported to be more affected in bilateral cryptorchidism with up to 80% of oligozoospermia versus 50% in case of unilateral cryptorchidism.11,12 Moreover, rates of azoospermia are higher in patients who were treated for bilateral cryptorchidism in comparison with unilateral cryptorchidism.10,13,14,15
To the best of our knowledge, only few retrospective studies reported sperm retrieval rates (SRR) following testicular sperm extraction (TESE) and intracytoplasmic sperm injection (ICSI) outcomes in patients with a history of cryptorchidism.8,16,17,18,19,20 These studies focused on relatively small number of subjects, and in several cases, patients had other potential causes of azoospermia than cryptorchidism. Then, beyond these reports, no larger series have evaluated the impact of history of unilateral versus bilateral cryptorchidism on SRR.
The purpose of this study was to compare the TESE-ICSI outcomes of a large consecutive cohort of NOA patients between unilateral and bilateral cryptorchidism.
PATIENTS AND METHODS
Study population
This is a retrospective study. Data were collected from all azoospermic men (n = 1274) who referred to the Department of Andrology of Lille University Hospital (Lille, France) and who underwent a TESE procedure between 1995 and 2014. This study was conducted with the approval of the Local Ethics Committee of Lille University Hospital, and informed consent was provided by every patient. Semen analysis was performed using the World Health Organization guidelines. Azoospermia was defined by the complete absence of spermatozoa after centrifugation of total ejaculate in at least two semen analyses.21,22 Before surgical sperm extraction, each patient had a complete andrological evaluation to determine the etiology of azoospermia. A thorough medical history was recorded for every patient, as previously described.23 Only patients with a history of true cryptorchidism were included, with the exclusion of anorchia, ectopy, and retractile testis. For patients with a history of orchidopexy, the clinician examined the scar to attest that the localization was compatible with a true cryptorchidism and not an ectopic testis. When orchidopexy had occurred at young age, medical records were studied. They all had a clinical workup evaluation, including physical examination, assessment of seminal markers (including measurements of seminal plasma levels of fructose, acid phosphatase, citric acid, and α-glucosidase), ultrasound of the testes (mean testicular volume) and urogenital tract, and endocrine profile (measurements of serum follicle-stimulating hormone [FSH], inhibin B, and total testosterone). Genetic evaluations including karyotype and Y chromosome microdeletions (since 2002) were performed.24,25 NOA was defined as azoospermia without any sign of obstruction and with a FSH level >10 IU l−1 and/or mean testicular volume <16 ml.25,26,27,28 We identified 314 azoospermic men with a history of cryptorchidism. Two hundred and sixty-nine of those patients had a NOA profile. Among these 269 men, 20 had a possible additional etiology of azoospermia and were therefore excluded: 5 had a history of testicular tumor, 1 had a history of melanoma treated by chemotherapy, 1 had a history of Hodgkin lymphoma, 7 presented cytogenetic abnormalities, 4 had a Y chromosome microdeletion, and 2 had a genetic mutation. Finally, we identified 249 men with NOA, which could only be explained by a history of cryptorchidism. Among them, 225 had benefited from an orchidopexy procedure and were finally included and analyzed.
Hormone assays
Serum FSH levels were measured with a chemiluminescence assay on a multiparameter analyzer (Architect, Abbott Laboratories, Rungis, France). The assay's limit of detection was 0.05 mIU ml−1. Since 2002, serum inhibin B assay was determined using the Inhibin B Gen II ELISA kit (Beckman Coulter, Villepinte, France). The assay's limit of detection was 15 pg ml−1 (between 2002 and 2009) and 10 pg ml−1 since 2010. Below this threshold, inhibin B was defined as undetectable. Total testosterone was measured by radioimmunoassay using the Coat-A-Count kit (Siemens Diagnostics Inc., Los Angeles, CA, USA). The assay's limit of quantification was 0.14 nmol l−1 (0.04 ng ml−1).
Testicular sperm extraction
Testicular sperm retrieval was performed under general anesthesia, prior to oocyte retrieval. A centimeter scrotal incision was performed to open the skin, tunica vaginalis, and albuginea, and a small fragment of the testicular pulp was removed with scissors (one site per testis). The same procedure was performed on the contralateral testis in 147 (65.3%) of the 225 cases. Each biopsy was weighed and the testicular tissue was minced in culture medium, in order to dilacerate the seminiferous tubules. The resulting suspension was calibrated in multiple droplets under oil and incubated at 37°C in 5% CO2. After 20 min of incubation, an aliquot of each sample was examined under an inverted microscope with Hoffman optics (Leica DM IRB, Leica Microsystems, Wetzlar, Germany) at total ×400 magnification (combination of ×40 objective lens and ×10 ocular magnification). When spermatozoa were found, their number and vitality were rated. TESE was considered as successful if at least one spermatozoon was observed in the biopsy. From our experience in freezing-thawing procedure of testicular sperm, the suspension was frozen if a least >1 motile or immotile but alive sperm (using a hypo-osmotic swelling test) was detected in 10 μl. The suspension cell was then loaded into straws and frozen for further use in ICSI cycles.
During the surgery, a small testicular sample was placed into Bouin's solution (MM France, Brignais, France) for histological evaluation. Based on the most favorable histological pattern, a histological classification was done: Sertoli cell-only syndrome (SCOS) (i.e., the absence of germ cells within the seminiferous tubules), maturation arrest (i.e., an absence of the later stages of spermatogenesis due to an arrest at a particular stage), severe hypospermatogenesis (i.e., all stages of germ cells are present but in a reduced number, when <6 mature spermatids/seminiferous tubule sections were found), and normal or subnormal spermatogenesis (subnormal spermatogenesis refers to a slightly impaired spermatogenesis with a complete spermatogenesis observed in all the tubules but with a slight reduction of late spermatid number in some tubules, i.e., when 6–16 mature spermatids/seminiferous tubule sections were observed).
Ovarian stimulation
Controlled ovarian stimulation was performed with gonadotropin-releasing hormone agonist or antagonist protocols, in combination with recombinant FSH or human menopausal gonadotropin. Oocyte–cumulus complexes were retrieved 36 h after the administration of human chorionic gonadotropin (hCG; choriogonadotropin-alfa, Ovitrelle®, Merck-Serono, France) by vaginal ultrasound-guided needle aspiration.
ICSI cycles
Frozen-thawed sperm straws were used for all ICSI cycles. The most morphologically normal motile spermatozoon was selected and then injected into a metaphase II oocyte. Fertilization was assessed 16–18 h after injection (by the observation of two distinct pronuclei). Embryo quality was evaluated on day 2 or 3 according to their morphological appearance (i.e., the blastomere number, the cell symmetry, and the fragmentation). “Top-quality embryo” was defined by the presence of 4 blastomeres on day 2 or 8 blastomeres on day 3, symmetry of cleavage, and less than 10% of fragmentation.29 Embryo transfer was performed two or three days after oocyte retrieval. Supernumerary embryos of sufficient quality were frozen for further transfer. All patients received luteal phase support by vaginal micronized progesterone (Utrogestan®, Besins Healthcare, Drogenbos, Belgium) 200 mg three times a day started the evening of the oocyte retrieval.
Pregnancy follow-up
Pregnancy was diagnosed by elevated serum hCG levels 2 weeks after the embryo transfer. Clinical pregnancy was defined on ultrasound by the presence of an embryo with a cardiac activity, around 5 weeks after the embryo transfer. Implantation rate was defined by the number of gestational sacs divided by the number of embryos transferred. Live birth was defined by the delivery of a living infant beyond 20 weeks of gestation.
Outcomes measures
The primary outcome was the testicular SRR. Secondary outcomes included hormonal profiles, cumulative clinical pregnancy rate (CPR), live birth rate (LBR), and predictive factors of successful TESE. Cumulative CPR and LBR were calculated using the sum of the clinical pregnancies and live births, respectively, considering all fresh and frozen transfers from the same oocyte retrieval.
Statistical analyses
Data are presented as count (percentage) for categorical variables and median with the 5th and 95th percentiles for quantitative variables. Normality of distribution was checked graphically and using the Shapiro–Wilk test.
The search for factors associated with sperm extraction during surgical procedure was performed in bivariate analyses using Chi-square test or Fisher's exact test for categorical variables and Mann–Whitney U test for quantitative variables. Factors with values of P < 0.20 in bivariate analyses were entered into backward stepwise logistic regression analysis, with removal criteria of P > 0.05. To avoid case deletion (36.4%) in multivariate analysis due to missing data on candidate predictors (maximum rate of missing data per candidate predictors of 17.8%), missing data on candidate predictors were imputed by multiple imputations using regression-switching approach (chained equations with n = 10 obtained; m: the number of imputations). Imputation procedure was performed under the missing at random assumption using all baseline factors and positive sperm extraction with a predictive mean matching method for continuous variables and logistic regression model (binary, ordinal, or multinomial) for categorical variables. Estimates obtained in different imputed datasets were combined using the Rubin's rules.
All statistical tests were two sided, and P < 0.05 was considered statistically significant. Data were analyzed using SAS software package, release 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Andrological phenotype
The flowchart of the study population is shown in Figure 1. Andrological profiles are summarized in Table 1. Among the 225 men, 145 (64.4%) had a history of bilateral cryptorchidism and 80 (35.6%) had a history of unilateral cryptorchidism.
Figure 1.
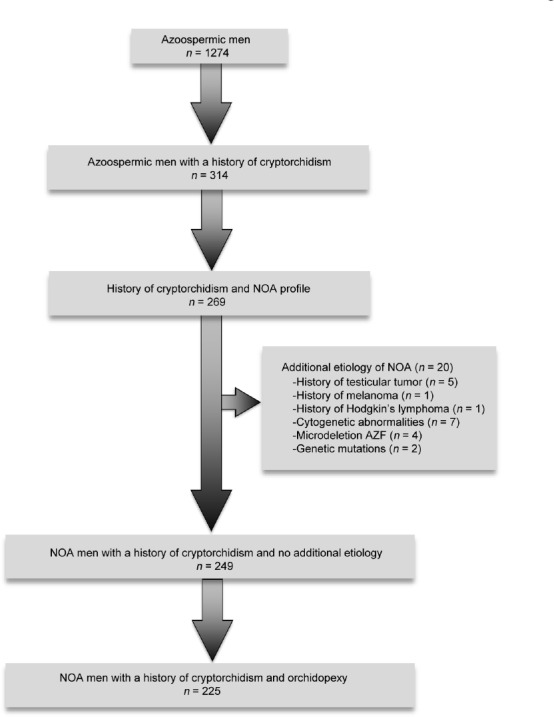
A flow chart of the studied population. NOA: nonobstructive azoospermia; AZF: azoospermia factor.
Table 1.
Phenotypic characteristics and testicular sperm extraction results
| Phenotypic characteristics | History of bilateral cryptorchidism (n=145) | History of unilateral cryptorchidism (n=80) | P |
|---|---|---|---|
| Age (year), median (5th–95th percentiles) | 31 (25–42) | 32 (24–43) | 0.287 |
| Age at orchidopexya (year), median (5th–95th percentiles) | 9 (4–23) | 10 (3–21) | 0.486 |
| Smokingb, n (%) | 51 (35.4) | 34 (42.5) | 0.295 |
| FSH levels (IU l−1), median (5th–95th percentiles) | 21.3 (6.1–53.9) | 19.3 (6.1–49.1) | 0.306 |
| Inhibin B levels (pg ml−1)c, median (5th–95th percentiles) | 36 (14–120) | 37 (13–169) | 0.155 |
| Undetectable inhibin B leveld, n (%) | 43 (37.4) | 33 (47.1) | 0.191 |
| Testosterone levels (ng ml−1), median (5th–95th percentiles) | 4.04 (2.03–6.99) | 3.83 (2.19–6.69) | 0.367 |
| Mean testicular volume (ml)e, median (5th–95th percentiles) | 7.2 (2.8–14.4) | 7.9 (3.6–16.5) | 0.543 |
| SCOS, n (%) | 72 (49.7) | 34 (42.5) | 0.303 |
| Hypospermatogenesis, n (%) | 69 (47.6) | 39 (48.8) | 0.867 |
| Maturation arrest, n (%) | 4 (2.8) | 7 (8.8) | 0.057 |
| Positive sperm retrieval, n (%) | 96 (66.2) | 48 (60.0) | 0.353 |
Chi-square test or Fisher’s exact test was performed for categorical variables and Mann–Whitney U test for quantitative variables. a33 missing values; b1 missing value; c116 missing values; d40 missing values; e9 missing values. SCOS: Sertoli cell-only syndrome
There was no significant difference between the two groups regarding median age of men at TESE (31 years vs 32 years, P = 0.287), median age at orchidopexy (9 years vs 10 years, P = 0.486), median testicular volumes (7.2 ml vs 7.9 ml, P = 0.543), and median FSH levels (21.3 IU l−1 vs 19.3 IU l−1, P = 0.306). In the bilateral cryptorchid group, spermatozoa were successfully retrieved on TESE in 66.2% (96/145) of the cases versus 60.0% (48/80) in the unilateral group (P = 0.353). Concerning the histopathological patterns, there was no normal or subnormal spermatogenesis in any group. SCOS was the most frequent diagnosis in case of bilateral cryptorchidism (49.7%) whereas hypospermatogenesis was mostly found in unilateral cryptorchidism (48.8%). However, the distribution between the groups was not significantly different (49.7% vs 42.5% for SCOS, P = 0.303; and 47.6% vs 48.8% for hypospermatogenesis, P = 0.867, respectively).
Characteristics of ICSI cycles and reproductive outcomes
There was no significant difference between the two groups regarding fertilization rates (46.8% vs 46.5%, P = 0.932, Table 2). On average and in both groups, freezing of supernumerary embryos was performed in 23.0% and 25.0% of the cases (P = 0.737). Implantation rate was significantly higher in the unilateral group (25.5% vs 11.9%, P < 0.001). Cumulative CPR and LBR per cycle were not significantly different between the two groups (27.8% vs 17.4%, P = 0.070 and 26.4% vs 16.1%, P = 0.067, respectively).
Table 2.
Intracytoplasmic sperm injection procedure results
| ICSI procedure | History of bilateral cryptorchidism | History of unilateral cryptorchidism | P |
|---|---|---|---|
| Cycles (n) | 161 | 72 | |
| Demographics, median (5th–95th percentiles) | |||
| Age of female partner (year)a | 30 (24–37) | 31 (23–38) | 0.072 |
| BMI of female partner (kg m−2) | 24.0 (18.6–33.2) | 21.0 (18.0–34.7) | 0.246 |
| AMH level of female partner (pmol l−1)b | 25.0 (4.8–57.1) | 28.7 (3.8–79.4) | 0.849 |
| Embryologic characteristics | |||
| Number of COC | 10.0 (2.0–19.0) | 11.5 (2.1–21.0) | 0.132 |
| Number of metaphase 2 oocytes, median (5th–95th percentiles) | 7 (0–14) | 7 (2–14) | 0.334 |
| Fertilization ratec (%) | 46.8 | 46.5 | 0.932 |
| Number of day 2 or 3 embryos per cycle, median (5th–95th percentiles) | 2 (0–8) | 3 (0–8) | 0.384 |
| Top quality embryos (%) | 28.7 | 25 | 0.591 |
| Number of embryos per transferc, median (5th–95th percentiles) | 2 (1–3) | 2 (1–3) | 0.172 |
| Cycles with embryo freezing (n) | 23.0 | 25.0 | 0.737 |
| Reproductive outcome | |||
| Implantation rate (%) | 11.9 | 25.5 | <0.001 |
| Cumulative CPR per cyclec, n (%) | 28 (17.4) | 20 (27.8) | 0.070 |
| Cumulative LBR per cyclec, n (%) | 26 (16.1) | 19 (26.4) | 0.067 |
Chi-square test was performed for categorical variables and Mann–Whitney U test for quantitative variables. aAge recorded on the day of the oocyte retrieval of the first ICSI cycle; bdata for AMH were available from 2010; cfresh and frozen cycles. ICSI: intracytoplasmic sperm injection; COC: cumulus oocyte complex; CPR: clinical pregnancy rate; LBR: live birth rate; AMH: anti-Müllerian hormone; BMI: body mass index
Predictors of successful sperm retrieval
On the overall population, 144 (64.0%) had a successful TESE. Patients with a history of cryptorchidism were divided into two groups according to the sperm retrieval outcome (Table 3). On univariate analysis, no difference was found in age at TESE (median: 31 years vs 32 years, P = 0.628), smoking status (39.6% vs 35.0%, P = 0.498), or testosterone levels (median: 3.96 ng ml−1 vs 3.90 ng ml−1, P = 0.873) in men with positive TESE vs negative TESE. We did not find any difference in the frequency of bilateral cryptorchidism (66.7% vs 60.5%, P = 0.353). We noted that the orchidopexy was earlier in patients with a positive TESE (P = 0.036). In addition, we found that men with successful sperm retrieval exhibited lower FSH levels and a higher testicular volume. Regarding inhibin B levels, no difference was found in patients with positive vs negative TESE (median: 40 pg ml−1 vs 36 pg ml−1, P = 0.328). However, patients with an inhibin B level below the assay's detection limit (i.e., undetectable inhibin B level) exhibited a significantly lower SRR (52.6% vs 70.6%, P = 0.012). In addition, an undetectable inhibin B level was associated with SCOS in almost two-thirds of cases (48/76). Using multivariate analysis, we have found that only an inhibin B level above the assay's detection limit was positively associated with successful sperm retrieval (P = 0.022).
Table 3.
Successful versus failed testicular sperm extraction in 225 men with history of cryptorchidism
| Phenotypic characteristics | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Positive sperm retrieval (n=144) | Negative sperm retrieval (n=81) | P | Odds ratio (95% CI) | P | |
| Age at TESE (year), median (5th–95th centiles) | 31 (25–42) | 32 (26–42) | 0.628 | ||
| Age at orchidopexya, median (5th–95th centiles) | 9 (4–17) | 10 (5–25) | 0.036 | ||
| Smokingb, n/total number (%) | 57/144 (39.6) | 28/80 (35.0) | 0.498 | ||
| Bilateral (reference) versus unilateral, n/total number (%) | 96/144 (66.7) | 49/81 (60.5) | 0.353 | ||
| FSH levels (IU l−1), median (5th–95th centiles) | 19.2 (5.2–49.9) | 23.6 (11.5–56.1) | 0.007 | ||
| Inhibin B levels (pg ml−1)c, median (5th–95th centiles) | 40 (14–167) | 36 (12–117) | 0.328 | ||
| Undetectable inhibin B leveld, n/total number (%) | 40/117 (34.2) | 36/68 (52.9) | 0.012 | 0.502 (0.279–0.905) | 0.022 |
| Testosterone levels (ng ml−1), median (5th–95th centiles) | 3.96 (2.16–6.86) | 3.90 (1.86–6.99) | 0.873 | ||
| Mean testicular volume (ml)e, median (5th–95th centiles) | 8.0 (4.1–16.0) | 7.0 (2.2–13.4) | 0.014 | ||
| SCOS | 40/144 (27.8) | 66/81 (81.5) | <0.0001 | ||
| Hypospermatogenesis, n/total number (%) | 100/144 (69.4) | 8/81 (9.9) | <0.0001 | ||
| Maturation arrest, n/total number (%) | 4/144 (2.8) | 7/81 (8.6) | 0.050 | ||
a33 missing values; b1 missing value; c116 missing values; d40 missing values; e9 missing values. SCOS: Sertoli cell-only syndrome; CI: confidence interval; TESE: testicular sperm extraction; FSH: follicle-stimulating hormone
DISCUSSION
The present study summarized our 20 years’ experience of TESE-ICSI in NOA men with a history of cryptorchidism. Our results showed similar TESE outcomes in men with a history of unilateral cryptorchidism compared to bilateral cryptorchidism, suggesting that a history of cryptorchidism could reflect a bilateral testicular impairment.
We found comparable SRR, between 52% and 66%, to those described in previous published series.17,19,20 However, two studies exhibited higher SRR,8,16 but major dissimilarities within their population could explain this observation. In these studies, some patients with coexistent pathologies, possibly responsible for spermatogenesis impairment, were included. Furthermore, in some cases, a diagnostic biopsy was performed before the TESE, which induced selection bias.
As regards ICSI outcomes, we surprisingly found a higher implantation rate in patients with a history of unilateral versus bilateral cryptorchidism. This result could suggest that bilateral cryptorchidism may be responsible for a greater alteration of the quality of the sperm, which could be the consequence of the impairment of germ cell maturation.
Furthermore, it is difficult to certify that the ovarian reserve of the partners was comparable between the two groups. The evaluation of the ovarian reserve has changed over such a long period of time. Serum AMH assay is only available since 2010, and the ultrasound equipment has drastically evolved, giving different cutoffs for antral follicular count. Thereby, the difference observed in implantation rate could also be explained by the absence of age-matched control group. Thus, it would be important in a future prospective study to control for this issue.
The main strength of our study is the studied population, because it is the largest consecutive published series of men with NOA and history of cryptorchidism. The consequence of our strict inclusion criteria (exclusion of all patients with a potential other cause of spermatogenesis impairment) was that neither normal nor subnormal spermatogenesis was observed in the groups, confirming that all obstructive azoospermic men were excluded. Indeed, it is well known that cryptorchidism is associated with a higher risk of epididymis abnormalities.30 Thus, azoospermia in patients with a history of cryptorchidism should not automatically be considered as NOA: coexistence of spermatogenesis alteration and congenital seminal duct anomaly (the latter responsible for azoospermia) is frequent and could represent up to 60% of the cryptorchid azoospermic men.8 This could explain the significant prevalence of histological diagnosis compatible with obstructive azoospermia in the literature (from 13% to 40%) and therefore better testicular sperm retrieval.8,20 SRR in obstructive profile associated with cryptorchidism is higher than 95%.18 The histological patterns observed (and more precisely the absence of normal or subnormal spermatogenesis) in the present study confirm that our clinicobiological definition of NOA (i.e., FSH >10 IU l−1 and/or mean testicular volume <16 ml) could replace the histological, postoperative definition.25
The most frequent histological diagnosis in men with bilateral cryptorchidism was SCOS, in agreement with previous studies.17,20,31 In addition, when TESE was successful, the main phenotype observed was hypospermatogenesis. This result is consistent with a previous study showing that, in ex-cryptorchid men with azoospermia, the histological pattern was inhomogeneous with foci of sperm production where SCOS was frequent.32 Regarding the two groups, we did not show any statistical difference in the distribution of the histological patterns, suggesting that cryptorchidism could reflect a primary testicular dysfunction, where both testes are affected even in cases of unilateral cryptorchidism.30,31
In addition, the majority of our cohort exhibited a history of bilateral cryptorchidism (i.e., bilateral, 64.4% and unilateral, 35.6%). This is consistent with the scarce previous literature reports16,17,19 and also with epidemiologic data.33 Indeed, a history of bilateral cryptorchidism is associated with a higher risk of azoospermia than in unilateral cases. Nevertheless, we did not find any statistical difference in inhibin B and FSH levels between patients with bilateral cryptorchidism and those with unilateral cryptorchidism, in contradiction with previous studies.10,34 Our results are in good agreement with other previous reports, showing alteration of spermatogenesis also in the normal contralateral testis in case of unilateral cryptorchidism.30,35 Scrotal testes have fewer spermatogonia than normal and both testes are affected in unilateral undescended testis.36 Some authors have also postulated that infertility in cryptorchidism is the consequence of a defective transformation of germ cell into dark spermatogonia during the mini-puberty.37 This is consistent with another study which reported a progressive loss of germ and Leydig cells in persistent undescended testes.38 The present study had several limitations. We performed a single-site TESE which may not be representative of the histology of the entire testis. Nevertheless, a single-site TESE is one of the two well-described methods of performing a conventional TESE.39 We chose this technique, despite some authors suggested that multiple-site TESE was superior to one-site single TESE, because in our experience, overall SRR in NOA population was equivalent with single-site TESE.40,41,42 In addition, single-site TESE is less invasive and can prevent testicular and vascular injuries.39 Finally, a recent study showed that in patients with previous unsuccessful TESE, neither micro-TESE nor contralateral multiple TESE improved sperm retrieval as compared to conventional single biopsy TESE.43 In this retrospective study, 28 men had normal FSH levels (<10 ml) with a mean testicular volume below 16 ml which is a sign of testicular hypotrophy. We acknowledge that testicular volume is a less valuable variable for the diagnosis of NOA than FSH, but it is also well known that a proportion of NOA men could have normal FSH values.44 This fact was confirmed in a recent study which showed that 14% of NOA men exhibited normal FSH values despite abnormal spermatogenesis.45 The patient's age at orchidopexy was very variable and could have impacted overall outcome. However, age at orchidopexy was similar between the two groups and by consequence this factor did not influence our results. Regarding TESE outcomes, age at orchidopexy was demonstrated to be an independent predictive factor of sperm retrieval in cryptorchid patients.16 In the present study, despite the orchidopexy was earlier in men with a positive TESE on univariate analysis, age at orchidopexy was not found to be a predictive factor of successful TESE on multivariate analysis. This is in accordance with a previous report.19 This could be explained by the fact the orchidopexy had been performed at a late age in our cohort (indeed none of the patients in this study had had orchiopexy earlier than a year before), which is not what is usually done in present day in clinical practice. If the patients in the present study had had orchiopexy earlier, it may improve the sperm retrieval rate. Finally, we demonstrated here for the first time in a large cohort that a history of unilateral versus bilateral cryptorchidism was not predictive of successful sperm retrieval. Moreover, we demonstrated here the value of inhibin B measurement in this context. Indeed, it has been demonstrated that serum inhibin B was a marker of seminiferous tubule function.46,47,48 However, there is still a debate as to whether serum inhibin B is predictive for TESE outcomes in NOA.42,49,50 Our study shows that inhibin B measurement may be useful as a predictive factor of success of TESE.
CONCLUSIONS
Our study showed that the unilaterality versus bilaterality of cryptorchidism did not influence the SRR, confirming that a history of unilateral cryptorchidism reflects a bilateral testicular impairment. In addition, inhibin B level might be used as a predicator of success of sperm retrieval before the TESE in NOA men with unilateral and bilateral cryptorchidism.
AUTHOR CONTRIBUTIONS
ALB contributed to study design, execution, analysis and interpretation of data, manuscript drafting, and critical discussion. A Dauvergne contributed to acquisition and analysis of data. NR performed statistical analysis. A Dumont and VM contributed to manuscript drafting and critical discussion. JMR contributed to inclusion of patients and performed TESE. FB contributed to study design, analysis and interpretation of data, manuscript drafting, and critical discussion. GR contributed to study design, execution, acquisition, analysis and interpretation of data, manuscript drafting, and critical discussion. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
We gratefully acknowledge the support and assistance provided by all the staff of Lille University Hospital's in vitro fertilization unit.
REFERENCES
- 1.Barthold JS, González R. The epidemiology of congenital cryptorchidism, testicular ascent and orchiopexy. J Urol. 2003;170:2396–401. doi: 10.1097/01.ju.0000095793.04232.d8. [DOI] [PubMed] [Google Scholar]
- 2.Averous M, Lopez C. Cryptorchidism: the point of view of a pediatric urologist. Gynecol Obstet Fertil. 2004;32:813–7. doi: 10.1016/j.gyobfe.2004.07.012. [Article in French] [DOI] [PubMed] [Google Scholar]
- 3.Hadziselimovic F. Opinion: comment on evaluation and treatment of cryptorchidism: AUA/AAP and Nordic consensus guidelines. Urol Int. 2016;96:249–54. doi: 10.1159/000443741. [DOI] [PubMed] [Google Scholar]
- 4.Olesen IA, Andersson AM, Aksglaede L, Skakkebaek NE, Rajpert-de Meyts E, et al. Clinical, genetic, biochemical, and testicular biopsy findings among 1,213 men evaluated for infertility. Fertil Steril. 2017;107:74–82.e7. doi: 10.1016/j.fertnstert.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Fedder J, Cruger D, Oestergaard B, Petersen GB. Etiology of azoospermia in 100 consecutive nonvasectomized men. Fertil Steril. 2004;82:1463–5. doi: 10.1016/j.fertnstert.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 6.Gracia J, Sánchez Zalabardo J, Sánchez García J, García C, Ferrández A. Clinical, physical, sperm and hormonal data in 251 adults operated on for cryptorchidism in childhood. BJU Int. 2000;85:1100–3. doi: 10.1046/j.1464-410x.2000.00662.x. [DOI] [PubMed] [Google Scholar]
- 7.Cortes D, Thorup J, Lindenberg S, Visfeldt J. Infertility despite surgery for cryptorchidism in childhood can be classified by patients with normal or elevated follicle-stimulating hormone and identified at orchidopexy. BJU Int. 2003;91:670–4. doi: 10.1046/j.1464-410x.2003.04177.x. [DOI] [PubMed] [Google Scholar]
- 8.Negri L, Albani E, DiRocco M, Morreale G, Novara P, et al. Testicular sperm extraction in azoospermic men submitted to bilateral orchidopexy. Hum Reprod. 2003;18:2534–9. doi: 10.1093/humrep/deg497. [DOI] [PubMed] [Google Scholar]
- 9.Coughlin MT, O’Leary LA, Songer NJ, Bellinger MF, LaPorte RE, et al. Time to conception after orchidopexy: evidence for subfertility? Fertil Steril. 1997;67:742–6. doi: 10.1016/s0015-0282(97)81376-3. [DOI] [PubMed] [Google Scholar]
- 10.Lee PA, Coughlin MT. Fertility after bilateral cryptorchidism. Evaluation by paternity, hormone, and semen data. Horm Res. 2001;55:28–32. doi: 10.1159/000049960. [DOI] [PubMed] [Google Scholar]
- 11.Caroppo E, Niederberger C, Elhanbly S, Schoor R, Ross L, et al. Effect of cryptorchidism and retractile testes on male factor infertility: a multicenter, retrospective, chart review. Fertil Steril. 2005;83:1581–4. doi: 10.1016/j.fertnstert.2005.01.088. [DOI] [PubMed] [Google Scholar]
- 12.Foresta C, Zuccarello D, Garolla A, Ferlin A. Role of hormones, genes, and environment in human cryptorchidism. Endocr Rev. 2008;29:560–80. doi: 10.1210/er.2007-0042. [DOI] [PubMed] [Google Scholar]
- 13.Chilvers C, Dudley NE, Gough MH, Jackson MB, Pike MC. Undescended testis: the effect of treatment on subsequent risk of subfertility and malignancy. J Pediatr Surg. 1986;21:691–6. doi: 10.1016/s0022-3468(86)80389-x. [DOI] [PubMed] [Google Scholar]
- 14.Chung E, Brock GB. Cryptorchidism and its impact on male fertility: a state of art review of current literature. Can Urol Assoc J. 2011;5:210–4. doi: 10.5489/cuaj.10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohayem J, Luberto A, Nieschlag E, Zitzmann M, Kliesch S. Delayed treatment of undescended testes may promote hypogonadism and infertility. Endocrine. 2017;55:914–24. doi: 10.1007/s12020-016-1178-0. [DOI] [PubMed] [Google Scholar]
- 16.Raman JD, Schlegel PN. Testicular sperm extraction with intracytoplasmic sperm injection is successful for the treatment of nonobstructive azoospermia associated with cryptorchidism. J Urol. 2003;170:1287–90. doi: 10.1097/01.ju.0000080707.75753.ec. [DOI] [PubMed] [Google Scholar]
- 17.Vernaeve V, Krikilion A, Verheyen G, Van Steirteghem A, Devroey P, et al. Outcome of testicular sperm recovery and ICSI in patients with non-obstructive azoospermia with a history of orchidopexy. Hum Reprod. 2004;19:2307–12. doi: 10.1093/humrep/deh394. [DOI] [PubMed] [Google Scholar]
- 18.Marcelli F, Robin G, Lefebvre-Khalil V, Marchetti C, Lemaitre L, et al. Results of surgical testicular sperm extractions (TESE) in a population of azoospermic patients with a history of cryptorchidism based on a 10-year experience of 142 patients. Prog Urol J. 2008;18:657–62. doi: 10.1016/j.purol.2008.04.022. Article in French. [DOI] [PubMed] [Google Scholar]
- 19.Wiser A, Raviv G, Weissenberg R, Elizur SE, Levron J, et al. Does age at orchidopexy impact on the results of testicular sperm extraction? Reprod Biomed Online. 2009;19:778–83. doi: 10.1016/j.rbmo.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 20.Haimov-Kochman R, Prus D, Farchat M, Bdolah Y, Hurwitz A. Reproductive outcome of men with azoospermia due to cryptorchidism using assisted techniques. Int J Androl. 2010;33:e139–43. doi: 10.1111/j.1365-2605.2009.00977.x. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 4th ed. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 22.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Cambridge: Cambridge University Press; 2010. [Google Scholar]
- 23.Plouvier P, Barbotin AL, Boitrelle F, Dewailly D, Mitchell V, et al. Extreme spermatogenesis failure: andrological phenotype and intracytoplasmic sperm injection outcomes. Andrology. 2017;5:219–25. doi: 10.1111/andr.12323. [DOI] [PubMed] [Google Scholar]
- 24.Huyghe E, Izard V, Rigot JM, Pariente JL, Tostain J Les membres du Comité d’andrologie de l’association française d’urologie (CCAFU) [Optimal evaluation of the infertile male.2007 French urological association guidelines] Prog En Urol. 2008;18:95–101. doi: 10.1016/j.purol.2007.12.002. Article in French. [DOI] [PubMed] [Google Scholar]
- 25.Robin G, Boitrelle F, Leroy X, Peers MC, Marcelli F, et al. Assessment of azoospermia and histological evaluation of spermatogenesis. Ann Pathol. 2010;30:182–95. doi: 10.1016/j.annpat.2010.03.015. [Article in French] [DOI] [PubMed] [Google Scholar]
- 26.Taskinen S, Taavitsainen M, Wikström S. Measurement of testicular volume: comparison of 3 different methods. J Urol. 1996;155:930–3. [PubMed] [Google Scholar]
- 27.Schiff JD, Li PS, Goldstein M. Correlation of ultrasonographic and orchidometer measurements of testis volume in adults. BJU Int. 2004;93:1015–7. doi: 10.1111/j.1464-410X.2003.04772.x. [DOI] [PubMed] [Google Scholar]
- 28.Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril. 2015;103:e18–25. doi: 10.1016/j.fertnstert.2014.12.103. [DOI] [PubMed] [Google Scholar]
- 29.Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270–83. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 30.Cortes D. Cryptorchidism–aspects of pathogenesis, histology and treatment. Scand J Urol Nephrol Suppl. 1998;196:1–54. [PubMed] [Google Scholar]
- 31.Cortes D, Thorup JM, Beck BL. Quantitative histology of germ cells in the undescended testes of human fetuses, neonates and infants. J Urol. 1995;154:1188–92. [PubMed] [Google Scholar]
- 32.Fedder J. History of cryptorchidism and ejaculate volume as simple predictors for the presence of testicular sperm. Syst Biol Reprod Med. 2011;57:154–61. doi: 10.3109/19396368.2010.550796. [DOI] [PubMed] [Google Scholar]
- 33.Toppari J, Kaleva M. Maldescendus testis. Horm Res. 1999;51:261–9. doi: 10.1159/000023412. [DOI] [PubMed] [Google Scholar]
- 34.Trsinar B, Muravec UR. Fertility potential after unilateral and bilateral orchidopexy for cryptorchidism. World J Urol. 2009;27:513–9. doi: 10.1007/s00345-009-0406-0. [DOI] [PubMed] [Google Scholar]
- 35.Zivkovic D, Bica DG, Hadziselimovic F. Effects of hormonal treatment on the contralateral descended testis in unilateral cryptorchidism. J Pediatr Urol. 2006;2:468–72. doi: 10.1016/j.jpurol.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Verkauskas G, Malcius D, Dasevicius D, Hadziselimovic F. Histopathology of unilateral cryptorchidism. Pediatr Dev Pathol. 2018 doi: 10.1177/1093526618789300. Doi: 10.1177/1093526618789300. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37.Hadziselimovic F, Herzog B. The importance of both an early orchidopexy and germ cell maturation for fertility. Lancet. 2001;358:1156–7. doi: 10.1016/S0140-6736(01)06274-2. [DOI] [PubMed] [Google Scholar]
- 38.Tasian GE, Hittelman AB, Kim GE, DiSandro MJ, Baskin LS. Age at orchiopexy and testis palpability predict germ and Leydig cell loss: clinical predictors of adverse histological features of cryptorchidism. J Urol. 2009;182:704–9. doi: 10.1016/j.juro.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 39.Janosek-Albright KJ, Schlegel PN, Dabaja AA. Testis sperm extraction. Asian J Urol. 2015;2:79–84. doi: 10.1016/j.ajur.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amer M, Haggar SE, Moustafa T, Abd El-Naser T, Zohdy W. Testicular sperm extraction: impact of testicular histology on outcome, number of biopsies to be performed and optimal time for repetition. Hum Reprod. 1999;14:3030–4. doi: 10.1093/humrep/14.12.3030. [DOI] [PubMed] [Google Scholar]
- 41.Ramasamy R, Yagan N, Schlegel PN. Structural and functional changes to the testis after conventional versus microdissection testicular sperm extraction. Urology. 2005;65:1190–4. doi: 10.1016/j.urology.2004.12.059. [DOI] [PubMed] [Google Scholar]
- 42.Tsujimura A, Matsumiya K, Miyagawa Y, Tohda A, Miura H, et al. Conventional multiple or microdissection testicular sperm extraction: a comparative study. Hum Reprod. 2002;17:2924–9. doi: 10.1093/humrep/17.11.2924. [DOI] [PubMed] [Google Scholar]
- 43.Franco G, Scarselli F, Casciani V, De Nunzio C, Dente D, et al. A novel stepwise micro-TESE approach in non-obstructive azoospermia. BMC Urol. 2016;16:20. doi: 10.1186/s12894-016-0138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society for Male Reproduction and Urology. Evaluation of the azoospermic male: a committee opinion. Fertil Steril. 2018;109:777–82. doi: 10.1016/j.fertnstert.2018.01.043. [DOI] [PubMed] [Google Scholar]
- 45.Amer MK, Ahmed AR, Abdel Hamid AA, GamalEl Din SF. Can spermatozoa be retrieved in non-obstructive azoospermic patients with high FSH level.: a retrospective cohort study? Andrologia. 2018;11:e13176. doi: 10.1111/and.13176. [DOI] [PubMed] [Google Scholar]
- 46.Pierik FH, Vreeburg JT, Stijnen T, De Jong FH, Weber RF. Serum inhibin B as a marker of spermatogenesis. J Clin Endocrinol Metab. 1998;83:3110–4. doi: 10.1210/jcem.83.9.5121. [DOI] [PubMed] [Google Scholar]
- 47.von Eckardstein S, Simoni M, Bergmann M, Weinbauer GF, Gassner P, et al. Serum inhibin B in combination with serum follicle-stimulating hormone (FSH) is a more sensitive marker than serum FSH alone for impaired spermatogenesis in men, but cannot predict the presence of sperm in testicular tissue samples. J Clin Endocrinol Metab. 1999;84:2496–501. doi: 10.1210/jcem.84.7.5855. [DOI] [PubMed] [Google Scholar]
- 48.Ballescá JL, Balasch J, Calafell JM, Alvarez R, Fábregues F, et al. Serum inhibin B determination is predictive of successful testicular sperm extraction in men with non-obstructive azoospermia. Hum Reprod. 2000;15:1734–8. doi: 10.1093/humrep/15.8.1734. [DOI] [PubMed] [Google Scholar]
- 49.Toulis KA, Iliadou PK, Venetis CA, Tsametis C, Tarlatzis BC, et al. Inhibin B and anti-Müllerian hormone as markers of persistent spermatogenesis in men with non-obstructive azoospermia: a meta-analysis of diagnostic accuracy studies. Hum Reprod. 2010;16:713–24. doi: 10.1093/humupd/dmq024. [DOI] [PubMed] [Google Scholar]
- 50.Mitchell V, Robin G, Boitrelle F, Massart P, Marchetti C, et al. Correlation between testicular sperm extraction outcomes and clinical, endocrine and testicular histology parameters in 120 azoospermic men with normal serum FSH levels. Int J Androl. 2011;34:299–305. doi: 10.1111/j.1365-2605.2010.01094.x. [DOI] [PubMed] [Google Scholar]


