Abstract
Vacuum erection device (VED), used to treat radical prostatectomy (RP)-associated erectile dysfunction, has attracted considerable attention. However, the optimal negative pressure remains to be determined. This investigation explored the optimal pressure for VED therapy in penile rehabilitation. Thirty-six 9-week-old male rats were randomly divided into six groups: control groups (sham group, bilateral cavernous nerve crush [BCNC] group) and VED therapy groups (−200 mmHg group, −300 mmHg group, −400 mmHg group, −500 mmHg group). BCNC group and VED therapy groups underwent BCNC surgery. Intracavernosal pressure (ICP)/mean arterial pressure (MAP) ratio was calculated to assess erectile function. Masson's trichrome (MT) staining, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, immunohistochemistry, and real-time polymerase chain reaction (RT-PCR) were performed to explore cellular and molecular changes of the penis. Compared to the BCNC group, ICP/MAP ratios in all VED treatment groups were improved significantly (all P < 0.05), but there were no statistically significant differences among VED therapy groups. With increased pressure, complications gradually emerged and increased in frequency. Expression of molecular indicators, such as endothelial nitric oxide synthase (eNOS) and alpha-smooth muscle actin (α-SMA), increased after VED therapy, and hypoxia-inducible factor 1α (HIF-1α) and transforming growth factor beta (TGF-β) decreased. In addition, VED therapy improved the outcomes of MT and TUNEL assay. This investigation demonstrated a pressure of −200 mmHg in a rat model is optimal for VED therapy for penile rehabilitation after RP. No further benefits were observed with increased pressure, despite an increase in complications.
Keywords: erectile dysfunction, negative pressure, penile rehabilitation, vacuum erection devices
INTRODUCTION
Prostate cancer (PCa) is the most commonly diagnosed solid cancer in men and the third leading cause of cancer-related death in developed countries.1 Although the incidence of PCa is high, likely due in part to the improvement of detection and therapeutic modalities, the 5-year survival rates have improved significantly and are close to 100% in localized PCa.2,3 Despite these improvements, several maladies associated with radical prostatectomy (RP) have also emerged, which severely impact the quality of life of these patients and may include urinary incontinence and erectile dysfunction (ED).4,5,6 With the promotion of nerve-sparing techniques, urologists see the clinical importance of recovering erectile function (EF) of patients with PCa after RP. However, several investigations have revealed that ED after RP is a complicated pathological process involving a variety of underlying mechanisms, which include neuropraxia, ischemic and hypoxic nerve insults, fibrotic remodeling, and apoptosis of the cavernous smooth muscle.7,8,9
Penile rehabilitation, which provides significant improvement in EF after RP, has attracted considerable attention and is defined as the use of medicine or device to stimulate recovery of EF after RP.10 Currently, the primary approaches of penile rehabilitation include oral phosphodiesterase type-5 inhibitors, vacuum erection device (VED), intracorporeal injection of vasoactive agents or intra-urethral suppository therapy, and a combinational approach of these therapies.11,12 VED works through negative pressure to distend the corporal sinusoids, thereby increasing blood inflow to the penis. Multiple studies have confirmed its value in preserving the penile size and incomplete facilitation of EF.6,13,14,15 Moreover, regarding the benefits from the rat model, bilateral cavernous nerve crush (BCNC) simulating ED after RP in humans, and our previous patent, the rat-specific VED, our team have verified the effects of VED therapy in antihypoxic, antiapoptotic, and antifibrotic mechanism.16,17 Soon after, Lin et al.18 also showed improvement of intracavernosal blood oxygen saturation (SO2) using VED in rats after BCNC.
Although the positive effects of VED are well documented, a uniform therapeutic regimen remains to be determined. Most of the existing approaches are based on experience, so there is a lack of evidence-based guidance for the use of VED in penile rehabilitation after RP. Current suggestions for devices used to improve EF after RP are negative pressure settings that range from −150 mmHg to −200 mmHg. Nevertheless, the impact of other negative pressure values to treat ED remains unclear. Moreover, in consideration of the widespread use of VED, it is possible that patients are surrounded by unsafe and unqualified vacuum devices that may pose danger to the user. Therefore, it is crucial to understand the influences of these different negative pressures.
Based on BCNC rat models imitating ED after RP in humans, the purpose of this investigation was to elucidate the effects of different negative pressures on the penis, which would provide scientific evidence for the clinical application of VED therapy on penile rehabilitation.
MATERIALS AND METHODS
Animals
Thirty-six 9-week-old male Sprague-Dawley rats (Dashuo Biological Technology Company, Chengdu, China) were randomly divided into six groups: sham group; BCNC group; −200 mmHg VED therapy group; −300 mmHg VED therapy group; −400 mmHg VED therapy group; and −500 mmHg VED therapy group. To mimic ED after RP, the BCNC rat model was utilized as reported previously.19 The VED (Chengdu Xinweicheng Technology Co., Ltd., Chengdu, China) was specially modified for rats, which accurately simulated the pressure created by VEDs used in humans.17 The therapy, which was started on the 7th day after BCNC surgery, was performed 5 days a week (Monday through Friday) and consisted of a 5-min session twice daily with a 2-min interval for 4 weeks.16 During this time, complications and body weight were recorded. All the animals were raised under strict guidelines, and all experimental procedures were approved by the Animal Ethics Committee of West China Hospital, Sichuan University, Chengdu, China (No. 2016057A).
Erectile functional analysis
With a washout period of 1 week after VED therapy, intracavernosal pressure (ICP) was measured in all groups to evaluate the EF in response to cavernous nerve stimulation (CNS) as described previously.20,21,22 After the induction of isoflurane anesthesia (placing rats in a chamber with 5% isoflurane for 3 min), the anesthesia was maintained at 1.0%–1.5% isoflurane through face mask. Then, the functional biological experiment system (BL-420F, Chengdu TME Technology Co. Ltd., Chengdu, China) was used to record ICP and mean arterial pressure (MAP). One side of the carotid artery was exposed and cannulated with a 24G-type detaining venipuncture with 250 IU ml−1 heparinized saline, and then, the catheter was connected to a pressure transducer to measure the MAP changes. The penis was denuded of skin; then, a 26G needle that was connected to a BL-420F system for recording of the pressure changes was inserted into one side of the penile crus. A midline abdominal incision was made to expose the bladder and prostate, and the cavernous nerve was carefully exposed and isolated. A bipolar platinum electrode (Chengdu TME Technology Co., Ltd.) attached to the electrical stimulator BL-420F was placed around the nerve for electrical stimulation. The corresponding electrical stimulation parameters used in our study for all rats were 5.0 V, 20.0 Hz, pulse width of 5.0 ms, and duration of 50 s. The ICP data were normalized with MAP values and presented as the ratio of maximal ICP to MAP, which was finally calculated and analyzed. After these assessments, all rats were euthanized, and the penises were harvested for histopathology and molecular experiments.
Histopathology
The mid-portion of each penis was fixed in a 10% formalin/phosphate-buffered solution, dehydrated in alcohol, and then cleared and embedded using paraffin. The specimens were sectioned at the 4–6-μm thickness and stained with Masson's trichrome (MT). MT staining procedures were performed according to manufacturer's instructions (Baso diagnostics Inc., Zhuhai, China), so as Sirius red stains. The mid-penile shafts were also collected for immunohistochemistry (IHC). Endothelial nitric oxide synthase (eNOS), alpha-smooth muscle actin (α-SMA), hypoxia-inducible factor 1α (HIF-1α), and transforming growth factor beta (TGF-β) were stained for IHC analysis. The primary antibodies were rabbit anti-eNOS (ab76198, 1:1000; Abcam, Cambridge, MA, USA), anti-α-SMA (ab32595, 1:100; Abcam), anti-HIF-1α (ab2185, 1:100; Abcam), and anti-TGF-β (ab50038, 1:100; Abcam). IHC staining was performed using SPlink Detection Kits (K166616F, Biotin-Streptavidin HRP Detection System; ZSbio, Beijing, China). The remaining part of the penis was immediately collected and stored at −80°C and used for a real-time polymerase chain reaction (RT-PCR) analysis. The quantitative RT-PCR analysis was conducted to detect the aforementioned four molecular indicators, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control to normalize the quantitative RT-PCR readout. Furthermore, a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay (Promega Corporation, Madison, WI, USA) was also performed following the manufacturer's instructions to assess apoptosis.
Statistical analyses
GraphPad Prism version 6.00 software (GraphPad, La Jolla, CA, USA) was used to analyze image data. All measurements are expressed as mean ± standard error of the mean, except for the safety assessment, in which the occurrences of the complications were intuitively recorded as the number of corresponding cases in a table. All data used to assess the effectiveness of VED therapy were analyzed with Student's t- test using SPSS 13.0 software (SPSS, Chicago, IL, USA). Value of P < 0.05 was considered statistically significant.
RESULTS
Effectiveness assessments
EF was assessed via tracing of the ICP and corresponding MAP to show the effectiveness of VED therapies. Our outcomes showed no difference in basal variables (ICP and MAP) before CNS assessment (Supplementary Figure 1a (125KB, tif) and 1b (125KB, tif) ), and an increase in erectile response to CNS in all groups. The BCNC group showed an impaired EF, in which the ratio of maximal ICP/MAP declined markedly when compared to that in the sham group (Figure 1). The other groups with different negative pressure treatments showed no statistically significant difference when compared to the sham group (all P > 0.05), which indicated significantly improved erectile response compared to the BCNC group (all P < 0.05) (Figure 1). Moreover, comparisons among different VED therapy groups were also explored and showed that the ICP/MAP ratio in the −200 mmHg treatment group was significantly higher than those in other VED treatment groups (−200 mmHg vs −300 mmHg, P < 0.05; vs −400 mmHg, P < 0.05; vs −500 mmHg, P < 0.05). There were no statistically significant differences observed among the remaining groups (−300 mmHg, −400 mmHg, and −500 mmHg, P > 0.05).
Figure 1.
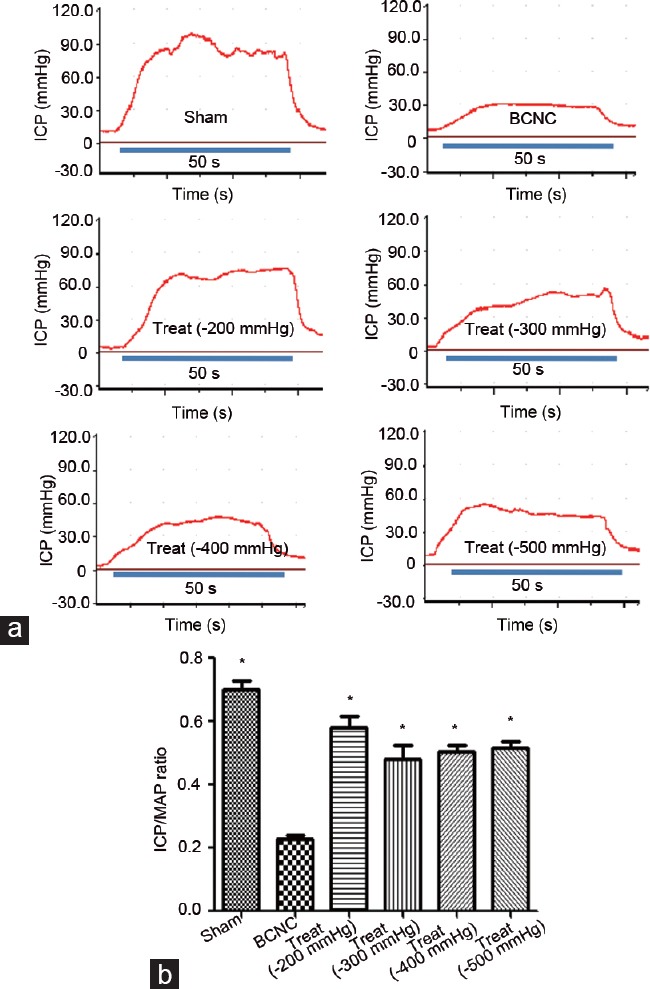
Erectile function assessment by ICP tracing under CNS. (a) Representative ICP traces for sham and BCNC, −200 mmHg, −300 mmHg, −400 mmHg, and −500 mmHg VED therapy; (b) significant differences were found in the ratios of maximum ICP/MAP between the sham and BCNC groups, as those between all VED treatment groups and the BCNC group (Student's t-test was used for each comparison, *P < 0.05, n = 6 per group; the CNS parameters for all rats were 5.0 V, 20.0 Hz pulse, width of 5.0 ms, and duration of 50 s). Among different VED therapy groups, there are no obvious differences in ICP/MAP ratios. ICP: intracavernosal pressure; CNS: cavernous nerve stimulation; VED: vacuum erection device; BCNC: bilateral cavernous nerve crush; MAP: mean arterial pressure.
Safety assessments
After a period of treatment, no significant difference in weight was observed among all groups except for the −500 mmHg VED treatment group. The weights of this group of rats were significantly lower than those in other groups. Complications of VED therapies were obtained as indicators for evaluating the safety of use and included preputial edema, ecchymoses or petechiae, bleeding, and avulsion. These data are presented in Table 1. Compared to the sham group, all VED therapy groups showed varying degrees of injuries, and the higher the negative pressure used, the greater the damage. However, in contrast, injuries induced by VED therapy with a lower negative pressure (such as −200 mmHg and −300 mmHg) recovered quickly.
Table 1.
The complications in vacuum erection device treatments
| VED treatment groups | Complications in VED treatment | |||
|---|---|---|---|---|
| Preputial edema | Ecchymoses/petechiae | Bleeding | Avulsion | |
| −200 mmHg group (n=6) | 4 | 6 | 1 | 0 |
| −300 mmHg group (n=6) | 6 | 6 | 1 | 0 |
| −400 mmHg group (n=6) | 6 | 6 | 4 | 3 |
| −500 mmHg group (n=6) | 6 | 6 | 6 | 6 |
VED: vacuum erection device
Structure and molecular assessments
MT staining was used to illustrate the change in smooth muscle/collagen ratio in each group. Our outcomes showed that the ratio in the BCNC group was the lowest in all groups, and obvious significant differences were found between this group and the remaining groups (Figure 2). Conversely, these ratios were improved in all therapeutic groups when compared to that in the BCNC group (all P < 0.05), whereas the −200 mmHg VED therapy group showed a trend superior to other VED therapy groups except for the −400 mmHg VED therapy group. The outcomes of the Sirius red staining, which was conducted to illustrate the improvement of imbalance of collagen synthesis in our study, showed that the ratios of collagen I/III improved in all VED therapy groups when compared with the BCNC group, and −200 mmHg and −300 mmHg VED therapy groups seemed to have more obvious improvements (Figure 3).
Figure 2.
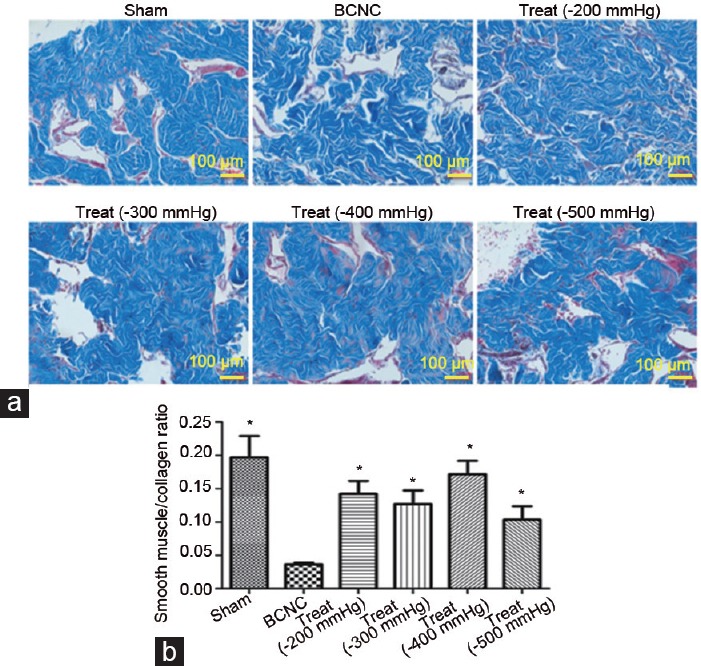
The improvements in penile smooth muscle/collagen ratios after VED therapy with different negative pressures. The data are shown according to the Masson's trichrome staining. (a) All VED therapy groups have dramatically improved penile smooth muscle/collagen ratio. (b) Significant outcomes were found in all VED therapy groups compared to BCNC group, although no obvious differences were found among different VED therapy groups (Student's t-test was used for each comparison, *P < 0.05, n = 6 per group; scale bars = 100 μm). VED: vacuum erection device; BCNC: bilateral cavernous nerve crush.
Figure 3.
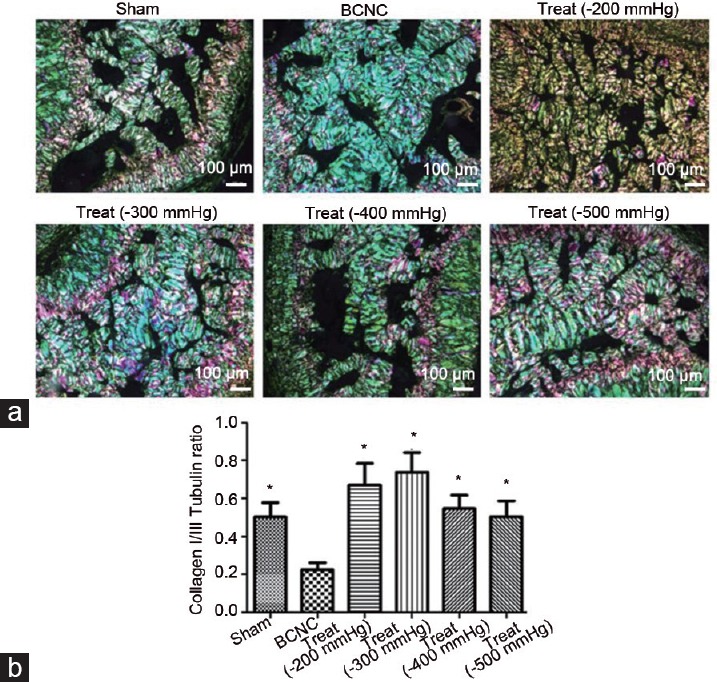
The improvements in imbalance of collagen synthesis with VED therapy under different negative pressures. (a) Sirius red staining was conducted to show the change in the ratios of collagen I/III. (b) Significant outcomes were found in all VED therapy groups compared to the BCNC group, although no obvious differences were found among different VED therapy groups (Student's t-test was used for each comparison, *P < 0.05, n = 6 per group; scale bars = 100 μm). VED: vacuum erection device; BCNC: bilateral cavernous nerve crush.
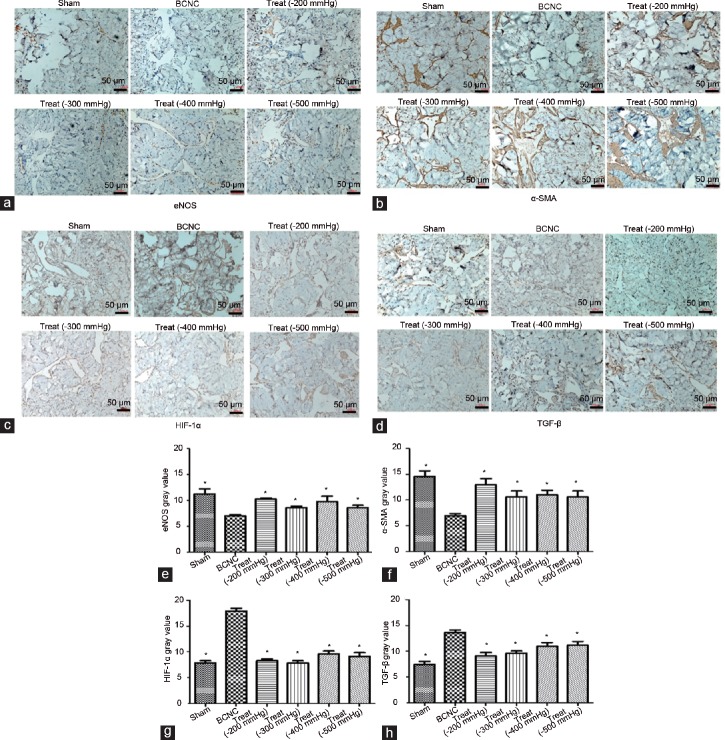
Concerning the apoptosis analysis, TUNEL assays were applied, of which the outcomes displayed an increase in apoptosis of cells in the BCNC group compared to that in the sham group. Following VED therapy, these conditions had been improved (Figure 4), in which the apoptosis had a decreasing trend in the BCNC group with VED. Moreover, expressions of multi-molecular markers, including eNOS, α-SMA, HIF-1α, and TGF-β, were also explored by IHC staining (Figure 5) and further verified by quantitative PCR analysis (Supplementary Figure 2 (207.3KB, tif) ). Markers of vascular function such as eNOS and α-SMA were reduced, whereas indicators such as HIF-1α and TGF-β were elevated in the BCNC group compared to those in the sham group (Figure 5). After the VED therapies were performed in the BCNC models, all these markers gained more or less improvements compared to those in the BCNC group without VED. In particular, although several assessments showed a slight different trend, the −200 mmHg VED therapy group still showed a greater tendency to improve than other VED therapy groups in a comprehensive consideration. However, TGF-β expression in the high-negative-pressure treatment groups (>200 mmHg) displayed an upward trend.
Figure 4.
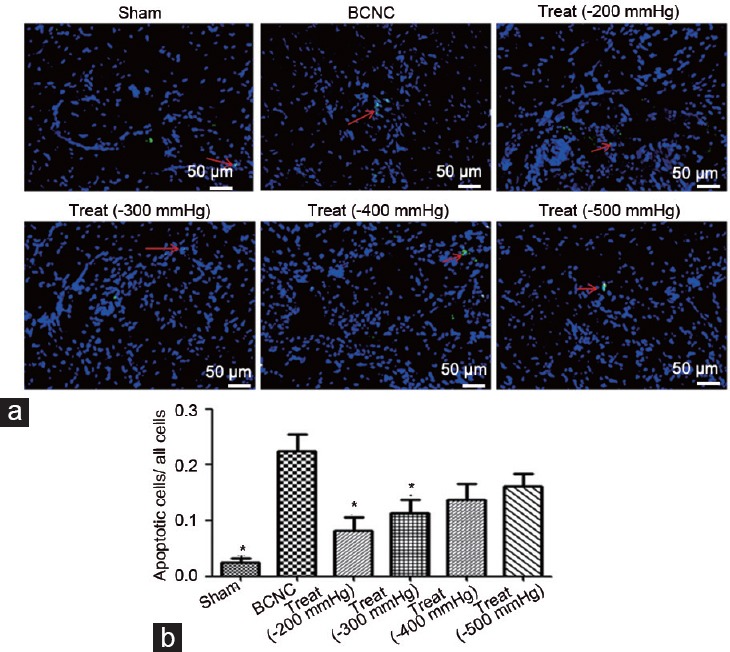
The improvements in apoptosis with VED therapy under different negative pressures. (a) The TUNEL assay was used to show these changes. (b) All VED therapy groups have significantly decreased apoptosis compared to the BCNC group; the red arrow indicated the expression of apoptosis (Student's t-test was used for each comparison, *P < 0.05, n = 6 per group; scale bars = 50 μm). VED: vacuum erection device; BCNC: bilateral cavernous nerve crush; TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling.
Figure 5.
The improvements in the expressions of eNOS, α-SMA, HIF-1α and TGF-β in the penis with VED therapy under different negative pressures. Immunohistochemistry was used to show the expression changes in these molecular indicators. The data showed that the expression of molecular indicators, such as (a) eNOS and (b) α-SMA, increased after VED therapy, and (c) HIF-1α and (d) TGF-β decreased (magnification, ×20). No obvious difference was found among different VED therapy groups in most molecular indicators except for TGF-β; the expression of TGF-β in high negative pressure treatment groups (>200 mmHg) displayed an upward tendency, and (e) eNOS, (f) α-SMA, (g) HIF1-α, and (h) TGF-β in turn (Student's t-test was used for each comparison, *P < 0.05, n = 6 per group; scale bars = 50 μm). eNOS: endothelial nitric oxide synthase; α-SMA: alpha smooth muscle action; HIF-1α: hypoxia-inducible factor 1α; TGF-β: transforming growth factor beta; VED: vacuum erection device; BCNC: bilateral cavernous nerve crush.
DISCUSSION
ED, a common complication associated with RP, severely affects the quality of life of patients with PCa. VED therapy, based on its reported ability to improve EF,23 has attracted considerable attention and is now the second leading treatment modality in penile rehabilitation.24,25 However, because of a general lack of mechanistic insight on the effects of VED and the unified guidance for its application in penile rehabilitation, its clinical utility has remained limited. Therefore, our study aimed to quantify the use of VED and was conducted to show the effects of different negative pressures on the penis with a detailed exploration of mechanisms associated with a function in the BCNC rat model.
Indeed, there are many anatomical and histological differences between the human and rat penises, and owing to the limitations of clinical research, the exploration of the corresponding mechanism of VED in penile rehabilitation after RP is inevitably hindered. Therefore, our laboratory has previously established a rat-specific VED,17 which accurately simulated the pressure created by VEDs used in humans. In addition, we had also suggested the possible mechanism of VED therapy in penile rehabilitation in the following research based on this device. The rat VED protocol employed herein fully simulates the clinical human VED regimen in penile rehabilitation. As indirect evidence for clinical uses, results from this investigation suggest that different effects of negative pressure exist, and if an excessive pressure should be used, the penile tissue may be harmed at the deeper level. Of course, we need to point out that there may be some differences between animals and humans, and further clinical research is needed to confirm our conclusion.
As the outcomes shown in the present investigation, the ICP/MAP ratios were effectively elevated in BCNC with VED therapy groups, which presented an improvement in EF. This outcome is consistent with results reported previously16 and further supports results obtained from clinical trials.13,26,27 In addition to the ICP/MAP ratios, the exploration of molecular indicators related to penile rehabilitation was also executed to verify this change. Our previous study indicated that VED facilitated penile rehabilitation based on antihypoxic, antiapoptotic, and antifibrotic mechanisms,16 which were also confirmed herein. The levels of indicators, such as eNOS and α-SMA, in all VED therapy groups, exhibited increasing trends when compared with those in the BCNC group. Moreover, HIF-1α and TGF-β, contributors to the formation of apoptosis and fibrosis, were attenuated in VED therapy groups. Furthermore, Lin et al.18 also suggested similar outcomes, in which VED therapy can effectively preserve penile size in rats with BCNC injury relating to antihypoxia by increasing cavernous blood SO2. The signal pathway associated with the molecular indicator HIF-1α is the key to antihypoxic mechanism. Although this point has been verified in our outcomes and that in Lin et al.'s study, antihypoxia is an extremely complicated process, and more detailed information on this signal pathway is needed in further studies. In addition, the effectiveness of VED therapy in improving diseases related to penile fibrosis, such as Peyronie's disease, is also worthy of our attention, which has been related to antiapoptosis, antifibrosis, and smooth muscle preservation.28 Among these mechanisms, change in TGF-β expression is important in penile fibrosis, which could bind serine and threonine kinase receptors activating Smad transcription factors, and the latter is essential in the pathogenesis of fibrotic disorder. Similar antifibrotic effects have also been mentioned in Tsambarli's study.29 These studies are basically consistent with our research. Subject to the different focuses in these studies and ours, our study focuses on the choice of negative pressure, and these signal pathways related to TGF-β and Smad transcription factors have not been explored in depth in our research, showing us a good idea to explore the detailed mechanism of VED therapy.
In this regard, our study focused on exploring the effects and influences of different negative pressure values from VED on the promotion of penile rehabilitation. Due to the lack of clinical guidance for VED use, selection of negative pressure values in VED therapy varies widely. Considering that vasodilatation occurs during exposure to −150 mmHg below atmospheric pressure,30 a −200 mmHg value was chosen as the initial treatment pressure value, and other three treatment groups were set in parallel (e.g., −300 mmHg, −400 mmHg, and −500 mmHg). In addition, for this reason, our previous outcomes suggested that −100 mmHg had no practical significance in restoring EF (Supplementary Figure 3 (55.1KB, tif) ) and thus was dismissed in our study. This pressure array covered nearly all of the standard ranges of VED used in clinical treatment (including the hazardously high value of −500 mmHg) and was aimed at illuminating the influence of different negative pressure values on EF. In this regard, these results also suggest that harmful effects of excessive negative pressure exist. Specifically, results from this investigation indicate that, with the increase in pressure values, all VED therapy groups exhibited varying degrees of improvements of EF but increased incidence of complications at high pressures. In particular, for the −500 mmHg pressure value, foreskin avulsion occurred in almost every rat. If these subjects were humans, the harm of such high pressures would likely destroy the confidence and health of patients. Owing to the inconsistent quality and improper use of VEDs, this situation has occasionally occurred in humans. Moreover, the results of this investigation suggest that the increased pressure values did not provide a more significant improvement in the structure or function of the penis. Although no significant differences in the expressions of molecular indicators related to penile rehabilitation mechanisms were found among VED therapy groups, the weakening effects gradually emerged with the elevated pressure. This was particularly evident for TGF-β, an indicator related to fibrosis, which impairs EF, as its expression in the high-negative-pressure groups (−400 and −500 mmHg) increased considerably. This occurrence may be a result of long-term inflammation,31 which stems from the damage inflicted by the excessive negative pressure. Therefore, regardless of the cause of excessive negative pressure, the damage inflicted far outweighs any potential benefit. In consideration of the effectiveness and safe use, −200 mmHg is the optimal negative pressure in VED therapy, even under special circumstances. A pressure of −300 mmHg is also worth consideration.
There are some limitations to the present investigation. Specifically, although numerous preclinical and clinical trials have supported our understanding of the positive effects of VED in preserving penile size,19,32,33 whether excessive negative pressure provides similar benefits or even worse drawbacks remains unclear and should be further confirmed in the future studies. In addition, although our study indicates that the effects of VED therapy are related to antihypoxic, antiapoptotic, and antifibrotic mechanisms, the deep related molecular signaling pathways are still unclear, and further studies are needed. Moreover, the influences of excessive negative pressure on the cavernous blood SO2– an indicator further illuminating the detailed mechanism related to penile rehabilitation with the VED – and penile vein occlusion function should also be explored in subsequent studies.
CONCLUSION
The present study shows that a pressure of −200 mmHg seems to be the optimal choice in VED therapy for penile rehabilitation based on the rat model. No additional benefits that could help preserve the EF or improve the penile tissue structure after RP can be obtained from excessive negative pressure, except for increased detrimental side effects. In addition, based on the advantages of VED therapy, multiple therapies combined with VED have emerged and achieved more remarkable successes, and the corresponding complex mechanisms are also worth our attention.
AUTHOR CONTRIBUTIONS
XLY, YY, FDF, and CJW carried out the experiments. XLY contributed to the statistical analysis, interpretation of data, and the manuscript preparation. FQ participated in the article screening, experiments design and critical revision of the manuscript. JHY conceived of this study, supervised the experiments and the manuscript drafting. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
(a) ICP and (b) MAP before CNS assessments. No significant difference was found between these groups. (*P < 0.05 versus BCNC group, the number of animals in every group: n = 6, a Student's t-test was used for each comparison). ICP: Intracavernous pressure; MAP: mean arterial pressure; CNS: cavernous nerve stimulation; BCNC: bilateral cavernous nerve crush.
The improvements in the expressions of eNOS, α-SMA, HIF-1α and TGF-β in the penis with VED therapy under different negative pressures. The data were shown based on quantitative PCR. The expression of gene such as eNOS (a) and α-SMA (b) significantly increased after VED therapy, and HIF-1α (c) and TGF-β (d) decreased (*P < 0.05 versus the BCNC group; the number of animals in every group, n = 6; Student's t-test was used for each comparison). eNOS: endothelial nitric oxide synthase; α-SMA: alpha-smooth muscle action; HIF-1α: hypoxia-inducible factor 1α; TGF-β: transforming growth factor beta; VED: vacuum erection device; BCNC: bilateral cavernous nerve crush PCR: polymerase chain reaction.
Early preliminary Erectile function assessment by ICP tracing under CNS. the ratios of maximum ICP/MAP, significant differences were found between sham group and BCNC group, except for −100 mmHg, so as between all VED treatment groups and BCNC group (*P < 0.05 versus BCNC group, the number of animals in every group: n = 4, a Student's t-test was used for each comparion. The CNS parameters for all rats were 5.0 V, 20.0 Hz pulse width of 5.0 ms and duration of 50 s.). ICP: intracavernosal pressure; CNS: cavernous nerve stimulation; VED: vacuum erection device; BCNC: bilateral cavernous nerve crush; MAP: mean arterial pressure.
ACKNOWLEDGMENTS
This work was supported by the Natural Science Foundation of China (No. 81270691 and No. 81671453), and the Sichuan Science and Technology Support Program (No. 2018TJPT0018).
Supplementary Information in linked to the online version of the paper at Asian Journal of Andrology website.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Bechis SK, Carroll PR, Cooperberg MR. Impact of age at diagnosis on prostate cancer treatment and survival. J Clin Oncol. 2011;29:235–41. doi: 10.1200/JCO.2010.30.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welch HG, Albertsen PC. Prostate cancer diagnosis and treatment after the introduction of prostate-specific antigen screening: 1986-2005. J Natl Cancer Inst. 2009;101:1325–9. doi: 10.1093/jnci/djp278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Litwin MS, Hays RD, Fink A, Ganz PA, Leake B, et al. Quality-of-life outcomes in men treated for localized prostate cancer. JAMA. 1995;273:129–35. doi: 10.1001/jama.273.2.129. [DOI] [PubMed] [Google Scholar]
- 5.Baker H, Wellman S, Lavender V. Functional quality-of-life outcomes reported by men treated for localized prostate cancer: a systematic literature review. Oncol Nurs Forum. 2016;43:199–218. doi: 10.1188/16.ONF.199-218. [DOI] [PubMed] [Google Scholar]
- 6.Pahlajani G, Raina R, Jones S, Ali M, Zippe C. Vacuum erection devices revisited: its emerging role in the treatment of erectile dysfunction and early penile rehabilitation following prostate cancer therapy. J Sex Med. 2012;9:1182–9. doi: 10.1111/j.1743-6109.2010.01881.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang R. Penile rehabilitation after radical prostatectomy: where do we stand and where are we going? J Sex Med. 2007;4:1085–97. doi: 10.1111/j.1743-6109.2007.00482.x. [DOI] [PubMed] [Google Scholar]
- 8.McCullough AR. Rehabilitation of erectile function following radical prostatectomy. Asian J Androl. 2008;10:61–74. doi: 10.1111/j.1745-7262.2008.00366.x. [DOI] [PubMed] [Google Scholar]
- 9.Clavell-Hernandez J, Wang R. The controversy surrounding penile rehabilitation after radical prostatectomy. Transl Androl Urol. 2017;6:2–11. doi: 10.21037/tau.2016.08.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mulhall JP. Penile rehabilitation following radical prostatectomy. Curr Opin Urol. 2008;18:613–20. doi: 10.1097/MOU.0b013e3283136462. [DOI] [PubMed] [Google Scholar]
- 11.Sivarajan G, Prabhu V, Taksler GB, Laze J, Lepor H. Ten-year outcomes of sexual function after radical prostatectomy: results of a prospective longitudinal study. Eur Urol. 2014;65:58–65. doi: 10.1016/j.eururo.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 12.Gandaglia G, Suardi N, Cucchiara V, Bianchi M, Shariat SF, et al. Penile rehabilitation after radical prostatectomy: does it work? Transl Androl Urol. 2015;4:110–23. doi: 10.3978/j.issn.2223-4683.2015.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raina R, Agarwal A, Ausmundson S, Lakin M, Nandipati KC, et al. Early use of vacuum constriction device following radical prostatectomy facilitates early sexual activity and potentially earlier return of erectile function. Int J Impot Res. 2006;18:77–81. doi: 10.1038/sj.ijir.3901380. [DOI] [PubMed] [Google Scholar]
- 14.Brison D, Seftel A, Sadeghi-Nejad H. The resurgence of the vacuum erection device (VED) for treatment of erectile dysfunction. J Sex Med. 2013;10:1124–35. doi: 10.1111/jsm.12046. [DOI] [PubMed] [Google Scholar]
- 15.Hoyland K, Vasdev N, Adshead J. The use of vacuum erection devices in erectile dysfunction after radical prostatectomy. Rev Urol. 2013;15:67–71. [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan J, Lin H, Li P, Zhang R, Luo A, et al. Molecular mechanisms of vacuum therapy in penile rehabilitation: a novel animal study. Eur Urol. 2010;58:773–80. doi: 10.1016/j.eururo.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Yuan J, Westney OL, Wang R. Design and application of a new rat-specific vacuum erectile device for penile rehabilitation research. J Sex Med. 2009;6:3247–53. doi: 10.1111/j.1743-6109.2009.01500.x. [DOI] [PubMed] [Google Scholar]
- 18.Lin HC, Yang WL, Zhang JL, Dai YT, Wang R. Penile rehabilitation with a vacuum erectile device in an animal model is related to an antihypoxic mechanism: blood gas evidence. Asian J Androl. 2013;15:387–90. doi: 10.1038/aja.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mullerad M, Donohue JF, Li PS, Scardino PT, Mulhall JP. Functional sequelae of cavernous nerve injury in the rat: is there model dependency. J Sex Med. 2006;3:77–83. doi: 10.1111/j.1743-6109.2005.00158.x. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Wu C, Fu F, You X, Gao L, et al. Isoflurane inhalation anesthesia should be a new requirement in intracavernosal pressure detection-the gold standard of erectile function assessment. Sci Rep. 2017;7:14949. doi: 10.1038/s41598-017-15020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulhall JP, Muller A, Donohue JF, Mullerad M, Kobylarz K, et al. The functional and structural consequences of cavernous nerve injury are ameliorated by sildenafil citrate. J Sex Med. 2008;5:1126–36. doi: 10.1111/j.1743-6109.2008.00794.x. [DOI] [PubMed] [Google Scholar]
- 22.Ning C, Wen J, Zhang Y, Dai Y, Wang W, et al. Excess adenosine A2B receptor signaling contributes to priapism through HIF-1α mediated reduction of PDE5 gene expression. FASEB J. 2014;28:2725–35. doi: 10.1096/fj.13-247833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engel JD. Effect on sexual function of a vacuum erection device post-prostatectomy. Can J Urol. 2011;18:5721–5. [PubMed] [Google Scholar]
- 24.Wang R. Vacuum erectile device for rehabilitation after radical prostatectomy. J Sex Med. 2017;14:184–6. doi: 10.1016/j.jsxm.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Tal R, Teloken P, Mulhall JP. Erectile function rehabilitation after radical prostatectomy: practice patterns among AUA members. J Sex Med. 2011;8:2370–6. doi: 10.1111/j.1743-6109.2011.02355.x. [DOI] [PubMed] [Google Scholar]
- 26.Sun L, Peng FL, Yu ZL, Liu CL, Chen J. Combined sildenafil with vacuum erection device therapy in the management of diabetic men with erectile dysfunction after failure of first-line sildenafil monotherapy. Int J Urol. 2014;21:1263–7. doi: 10.1111/iju.12564. [DOI] [PubMed] [Google Scholar]
- 27.Qi T, Ye L, Wang B, Zhang B, Chen J. Comparison of the effects of extracorporeal shock wave therapy and a vacuum erectile device on penile erectile dysfunction: a randomized clinical trial. Medicine. 2017;96:e8414. doi: 10.1097/MD.0000000000008414. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Lin H, Liu C, Wang R. Effect of penile traction and vacuum erectile device for Peyronie's disease in an animal model. J Sex Med. 2017;14:1270–6. doi: 10.1016/j.jsxm.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Tsambarlis PN, Chaus F, Levine LA. Successful placement of penile prostheses in men with severe corporal fibrosis following vacuum therapy protocol. J Sex Med. 2017;14:44–6. doi: 10.1016/j.jsxm.2016.11.304. [DOI] [PubMed] [Google Scholar]
- 30.Greenfield AD, Patterson GC. Reactions of the blood vessels of the human forearm to increases in transmural pressure. J Physiol. 1954;125:508–24. doi: 10.1113/jphysiol.1954.sp005177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–92. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 32.Kohler TS, Pedro R, Hendlin K, Utz W, Ugarte R, et al. A pilot study on the early use of the vacuum erection device after radical retropubic prostatectomy. BJU Int. 2007;100:858–62. doi: 10.1111/j.1464-410X.2007.07161.x. [DOI] [PubMed] [Google Scholar]
- 33.Trost LW, Munarriz R, Wang R, Morey A, Levine L. External mechanical devices and vascular surgery for erectile dysfunction. J Sex Med. 2016;13:1579–617. doi: 10.1016/j.jsxm.2016.09.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) ICP and (b) MAP before CNS assessments. No significant difference was found between these groups. (*P < 0.05 versus BCNC group, the number of animals in every group: n = 6, a Student's t-test was used for each comparison). ICP: Intracavernous pressure; MAP: mean arterial pressure; CNS: cavernous nerve stimulation; BCNC: bilateral cavernous nerve crush.
The improvements in the expressions of eNOS, α-SMA, HIF-1α and TGF-β in the penis with VED therapy under different negative pressures. The data were shown based on quantitative PCR. The expression of gene such as eNOS (a) and α-SMA (b) significantly increased after VED therapy, and HIF-1α (c) and TGF-β (d) decreased (*P < 0.05 versus the BCNC group; the number of animals in every group, n = 6; Student's t-test was used for each comparison). eNOS: endothelial nitric oxide synthase; α-SMA: alpha-smooth muscle action; HIF-1α: hypoxia-inducible factor 1α; TGF-β: transforming growth factor beta; VED: vacuum erection device; BCNC: bilateral cavernous nerve crush PCR: polymerase chain reaction.
Early preliminary Erectile function assessment by ICP tracing under CNS. the ratios of maximum ICP/MAP, significant differences were found between sham group and BCNC group, except for −100 mmHg, so as between all VED treatment groups and BCNC group (*P < 0.05 versus BCNC group, the number of animals in every group: n = 4, a Student's t-test was used for each comparion. The CNS parameters for all rats were 5.0 V, 20.0 Hz pulse width of 5.0 ms and duration of 50 s.). ICP: intracavernosal pressure; CNS: cavernous nerve stimulation; VED: vacuum erection device; BCNC: bilateral cavernous nerve crush; MAP: mean arterial pressure.