Abstract
Objective:
To assess the impact of MR spectroscopy (MRS) on the detection of malignancy in ovarian masses.
Methods:
This prospective work included 230 females that had 245 adnexal/ovarian masses. Tumours were spotted by preliminary pelvic ultrasound. Masses assessed by MRI, multi- or single-voxel spectroscopy. Patients’ spectra were assessed for peaks of lactate (Lac, 1.31 ppm), lipid (Lip, 1.33 ppm), N-acetyl aspartate (2.0 ppm), acetone (A, 2.05 ppm), choline (Cho, 3.23 ppm) and creatinine (Cr, 3.4 ppm) and the mean values of the (Cho/Cr) ratios were performed by a semi-quantitative approach. The operative pathology served as the standard of reference.
Results:
Cho peak twofold higher than the average noise level was detected in 72% of the malignant and only 5.4% of the benign masses with an accuracy of 83%. Adding lactate to the choline enhanced the accuracy to 93%. The mean Cho/Cr ratios of the malignant ovarian masses (2.8) were significantly higher than that of the benign ones (1.2) . We used a receiver operating characteristic curve to determine the best cut-off value (1.7) for the mean Cho/Cr ratio to discriminate malignancy with sensitivity: 81.2%, specificity: 93.3 %, positive-predictive value: 92.9 %, negative-predictive value: 82.4% and accuracy: 87.1%.
Conclusion:
The simultaneous presence of choline and lactate peaks in MRS examination of the ovarian masses minimizes the overlap between benign and malignant categories. N-acetyl aspartate and acetone are the metabolites for diagnosing complex cystic masses as benign teratoma, endomterioma and tubo- ovarian abscess.
Advances in knowledge:
MRS is a non-contrast based and fast MR sequence that gives an idea about tissue components could be used to improve the sensitivity and the accuracy of detecting malignancy in ovarian masses.
introduction
Discrimination of the nature of the pelvic/ovarian masses is more important than just identification. It is very crucial to exclude malignancy before deciding the surgical option especially if the patient is young1 Moreover, adnexal masses present numerous pathologic types and complicated features that sometimes elicit considerable debates and overlap between the benign and malignant tumours.2 Presence of a reliable method that can be used to differentiate benign from malignant adnexal/ovarian mass may reduce the number of unnecessary laparotomies or extensive surgeries done for benign diseases.3 Imaging modalities have an important role in the choice of the management, since it provides items of differentiation of the ovarian masses which vary between benign, borderline or invasive malignant.1–4 Despite the popularity and widespread acceptance of pelvic ultrasound as an initial method of examination of the ovaries, yet sometimes ultrasound is inefficient enough to distinguish the origin, relations and internal components of the pelvic masses.5 For many years, the evaluation of malignancy in an ovarian mass was performed by CT as it could provide a speedy whole body scans with minimal imaging artefacts.6 However, CT had a limited role in the characterization of the ovarian masses and exposes the patients to radiation.4 Also, there are considerable overlaps on the conventional MRI between benign and malignant adnexal tumours because of the numerous pathologic types and, consequently, the complicated morphologic features.2 MR spectroscopy (MRS) is a non-invasive modality that is free of ionizing radiation, that gives satisfactory information about the anatomy and at the same time provides quantification of the metabolic features of a cancer.7 There is paucity in the scientific research work that dealt with the use of MRS in the clinical practice and even the available studies showed limited variables and outcomes. The purpose of this work was to assess the diagnostic performance of MRS in the screening of malignancy in the ovarian masses. We discussed the significance of the different metabolites of the MR spectrum in evaluation of the ovarian masses. We estimated the credibility of the MRS to detect unobvious malignancy in benign looking masses and confirm/exclude malignancy in suspicious or malignant looking ovarian masses as featured on other imaging modalities.
patients and methods
This study is a prospective analysis that was approved by the Ethics Committee of the Radiology and Obstetrics and Gynaecology department and was approved by the international review board that is related to Cairo University. Included patients gave informed consent and were referred from the gynaecology Department to the Radiology Department in the period from March 2015 to September 2017 to confirm or exclude malignancy in the detected ovarian masses.
Patients
The study included 230 patients with 245 adnexal/ovarian masses that were detected and considered according to a preliminary pelvic ultrasound examination. The complaints of the included patients varied between lower abdominal pain, heaviness feeling in pelvis, pelvi-abdominal swelling, weight loss, unexplained lower back pain, vaginal bleeding, menstrual disorders and/or urinary symptoms.
Inclusion criteria
Adnexal/ ovarian masses with the following features: (a) Purely cystic (>4 cm) or solid masses.
(b) Complex cystic masses as endomterioma and teratoma for characterization and to detect underlying occult solid component. (c) Complex solid masses whether with small or large solid components to discriminate between borderline masses of low potential malignancy and invasive malignancy.
Exclusion criteria
Small (<4 cm) simple cystic ovarian masses (functional cysts).
Adnexal masses pathologically proven not to be ovarian in origin (e.g. subserous fibroid,… etc.)
Contraindications to MR: cardiac pacemaker, cochlear implants or other metallic prosthesis that affect the examination.
All of the included masses were surgically removed: (i) ovarian cystectomy in 68 cases, (ii) oophorectomy in 24 cases, (iii) salpingo-oophorectomy in 37 cases, and (iv) bilateral salpingo-oophorectomy with hysterectomy, omentectomy and peritoneal sampling in 95 cases.
Methods
The used MR equipment was a 1.5 T superconductive magnet device (Achieva, Philips Medical System, Best, Netherlands, Release 2.6, and Level 3), and cases were examined in the supine position with a multichannel phased-array coil (16 channels).
Scanning was done from the level of the kidneys till the level of the vulva.
Conventional pre- and post contrast MRI (Table 1)
Table 1. .
Parameters of pelvic conventional MRI
| Parameters | T 1W SE | T 2W turbo SE | T 1W with fat suppression | Dynamic post-contrast T 1W with fat suppression |
| Imaging plane | Axial | Axial and sagittal | Axial | Axial |
| TE ms | 6.5–17 | 77–103 | 6.5–17 | 9 |
| TR ms | 450–650 | 3200–4500 | 576–810 | 2.8 |
| STHK/ gap | 5/1.5 mm | 1.5/zero gap | ||
| Acquisition time in min | 2.5 | 3–3.5 | 3–3.5 | 5 |
| Matrix | 256 × 256 | |||
| FOV in cm | 32–42 | 37–40 | ||
FLASH, fast low angle shot; FOV, field of view; SE, spin echo; STHK, slice thickness; TE, echo time; TR, repetition time.
Axial T 1 weighted turbo spin echo images without and with fat saturation
T 2 weighted series: axial, coronal and sagittal turbo spin echo images.
Dynamic post contrast T 1 fat sat THRIVE (T 1 High-Resolution Isotropic Volume Examination) series: images were acquired in seven acquisitions of one pre- and six post-contrast with 40 s time interval after the intravenous injection of paramagnetic Gd-DPTA contrast media using a power injector at flow velocity of 2 ml/s and dose of 0.1 mMol/kgBW. The total duration of the contrast-based sequence was 280 s (4.5 min)
Proton MRI spectroscopy
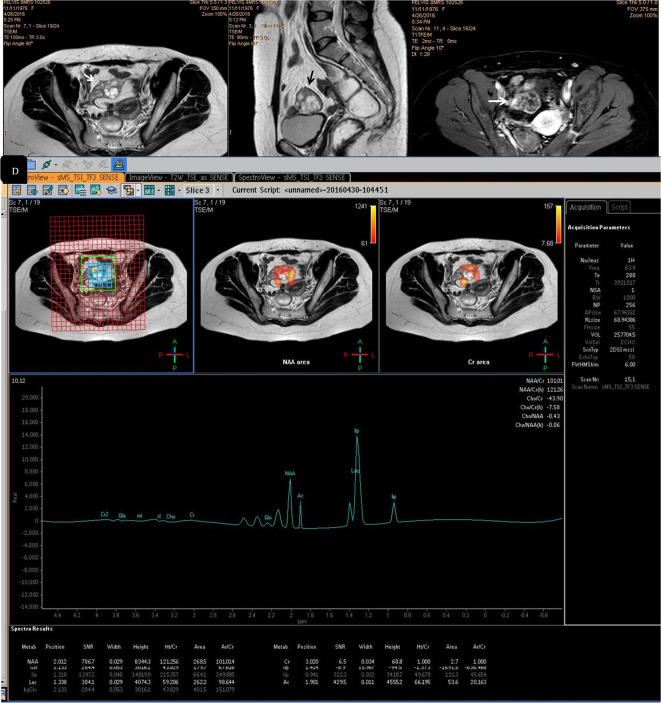
Since our main concern was the detection of malignancy and not characterization, in large cystic masses (>4 cm) with small and localized solid component; we used the single voxel spectroscopy (SVS) examination after identification of the masses on the T 1- and T 2 weighted images, where the volume of interest (VOI) was centred on the solid portion. In case of complex ovarian (cystic and multiple implanted solid components or masses with numerous papillary vegetations) and purely solid masses, we used a multivoxel spectroscopy (MVS). The localization technique was the point resolved spectroscopy (PRESS) and 192 signal averages for both types of spectral imaging (the SVS and the MVS).
*In SVS:
We used long echo time (TE) of 120 ms, repetition time (TR) of 15000 ms, flip angle 90˚, shim: iterative VOI, water suppression excitation—window (H2) 80 and second pulse angle 300.
Fat suppression was performed with inversion delay 165 ms, signal-tonoise ratio (SNR) 256, samples 512 and spectral BW (H2) 1000. Chemical shift in the anteroposterior direction was anterior [AP (A)], in the left right direction was left [LR (L)], and in the front hind direction was front [FH (F)] and the duration of scanning was 4.3 min
*In MVS:
A three-dimensional PRESS (TR = 2000 ms/TE = 135 ms) was performed. Flip angle was 90˚ and shim was PB-auto [the manufacturer’s automated shimming procedure (3D shim)]. The scanning time was longer than that of the SVS and lasted for 6.5 min The section for applying the VOI was selected based on the T 2 weighted images prior the administration of the contrast.
Image Interpretation and analysis
Interpretation and quantitative analysis were performed by two qualified consultants of radiology/readers (SM and MG of 20 and 15 years’ experience in pelvic MRI, respectively). Authors did their initial evaluation without knowledge of the tumour pathology, the tumour markers, or the diagnosis of the ultrasound examination.
*Conventional MRI findings
We assessed the MRI criteria of: (i) size of the mass, (ii) tissue content, (iii) thickness of the wall, septa and papillary vegetations in complex masses, (iv) absence/presence of necrosis in purely solid masses and (v) signal intensities on T 1- (fat and non-fat suppressed) and T 2-weighted sequences.
Following DeSouza et al, complex ovarian masses that are multilocular and present significant solid components are usually either borderline or invasive malignant tumours.8 On the other side, Sohaib et al9 and Imoka10 stated that masses with predominantly or uniformly low signal intensity on T 2 weighted images are likely benign.
*Contrast-enhanced MRI
We used dynamic post contrast MR sequence [3D T 1 high-resolution isotropic volumetric examination (THRIVE) of eight acquisitions, one before and seven after power injection of gadolinium] for the assessment of the included masses.
Based on Mansour et al;11 the parameters of evaluation were: maximum relative enhancement percentage (MRE%), the time peak of contrast uptake (T max) and the slope enhancement ratio (SER) curves. Malignant masses were those presented with MRE % of 120% or more, early T max (<120 s) and early wash out curve pattern. The plotted SER curves especially “early washout” pattern are the best parameter to predict the proper diagnosis of ovarian masses.11
*Proton MR spectroscopy
Application of the VOI and post-processing of the MRS images were also performed by two radiologists/readers who are experts in MRI of the pelvis (PN of 10 years and SM of 20 years experience). VOI was placed at the maximum diameter of the examined mass to avoid contamination signals from peripheral tissues. We performed spectral reconstruction by the aid of software at the Advantage Windows workstation of the MR device. In the included tumours, we assessed the signals of lactate (Lac, 1.31 ppm), lipid (Lip, 1.33 ppm), N acetyl aspartate (NAA, 2.0 ppm), acetone (A, 2.05 ppm), choline (Cho, 3.23 ppm) and creatinine (Cr, 3.4 ppm). Patient spectra were interpreted and qualitative assessment was performed by estimating the detected peaks. Qualitative assessment and classification of the detected metabolite peaks area was performed in our study following the technique described by Wakefield et al and Belkić and Belkić ,6,7 where we classified the peaks areas—by visual estimation—into three classes, in comparison with the noise level: that is, twofold higher than the average noise level (++), higher than the average noise level but lower than the twofold higher noise level (+), and the same as the average noise level (-). Increased choline (i.e. choline peak two fold higher than the average noise level) reflects increased membrane synthesis and/or increased number of cells (12 and 13), and so malignancy was suggested concentrations of choline and creatinine were automatically calculated on the MR machine to approach the (Cho/Cr) ntegral ratio
Statistical analysis
Surgical pathological findings served as the reference standard for assessment of the included ovarian tumours. Data were coded and entered using the statistical package SPSS (Statistical Package for the Social Sciences) v. 23. Data were summarized using mean, standard deviation, median, minimum and maximum in quantitative data and using frequency (count) and relative frequency (percentage) for categorical data.
Validity of both conventional and functional MRI (H¹ MRS) for diagnosis of ovarian lesions was tested by sensitivity, specificity, positive-predictive value (PPV), negative-predictive value (NPV) and diagnostic accuracy. We used a receiver operating characteristic (ROC) curve to determine the best cut-off value for the Cho/Cr integral ratio in differentiating benign from malignant ovarian tumours. ROC curve was constructed with area under curve analysis.
results
In the current work, we initially evaluated 245 adnexal/ovarian masses, however at the time of data analysis 19 masses have not fulfilled the inclusion criteria: large functional cysts (n = 7) and pedunclated subserous uterine fibroids (n = 11). Thus, the current work included 224 ovarian masses; 110 masses (49.1%) proved to be benign, 19 masses (8.5%) were borderline malignancy and 95 masses (42.4%) were invasive malignant Table 2.
Table 2. .
Histopathological results of the 224 ovarian masses in the study
| Count | % | |||
| Benign, n = 110 | Hemorrhagic corpus lutiem cyst | 7 | 6.40% | |
| Tubo-ovarian abscess | 18 | 16.40% | ||
| Endometriotic cyst | 15 | 13.60% | ||
| Serous cystadenoma | 24 | 21.80% | ||
| Mucinous cystadenoma | 10 | 9% | ||
| Fibroma | 6 | 5.40% | ||
| Fibrothecoma | 18 | 16.40% | ||
| Mature cystic teratoma | 12 | 11% | ||
| Malignant, n = 114 | Invasive, n = 95 | Serous cystadenocarcinoma | 29 | 30.50% |
| Mucinous cystadenocarcinoma | 29 | 30.50% | ||
| Clear cell adenocarcinoma | 7 | 7.40% | ||
| Granulosa cell tumor | 10 | 10.50% | ||
| Undifferentiated carcinoma | 5 | 5.30% | ||
| Immature teratoma | 7 | 7.40% | ||
| Dysgerminoma | 8 | 8.40% | ||
| Borderline, n = 19 | Serous cystic tumour | 9 | 47.40% | |
| Mucinous cystic tumour | 9 | 47.40% | ||
| Cystadenofibroma | 1 | 5.20% | ||
The most common benign pathology was serous cystadenoma (21.8%, n = 24/110), while the most common ovarian malignancy was the serous and the mucinous cystadenocarcinoma (30.5% each, n = 29/95).
*Pre- and postcontrast MR imaging
The morphologic MRI characteristics of each ovarian lesion were separately recorded.
Complex masses with cystic and solid components whether in the form of thick septae, papillary projections or bulky solid sheets were the most common feature (n = 72/114, 63.1%) of the malignant (borderline and invasive, n = 114) masses With respect to the signal intensity, the benign tumours showed frequently low/intermediate signal on T 1WI and high signal on T 2WI (50%, n = 55/110). On the other side, many of the malignant tumours showed low/intermediate signal on T 1WI and mixed signal on T 2WI (56.1%, n = 64/114). The number of the true positive cases was 93, the true negative was 73, the false-positive was 37, and the false-negative was 21. Conventional MRI had shown sensitivity: 81.5%, specificity: 66.3%, positive-predictive value: 71.5%, negative-predictive value: 77.6% and accuracy: 74.1% in the differentiation of benign from malignant ovarian neoplasm. When we included the dynamic post-contrast series in the evaluation of the ovarian masses; the number of the true positive became 104 masses and true negative became 90 masses. So, in distinguishing the malignant from the benign ovarian tumours, DCE showed a sensitivity of 91.2%, specificity of 81.8%, positive-predictive value of 83.8%, negativepredictive value of 90% and accuracy of 86.6%.
MRS spectrum was interpreted in correlation with the conventional MR images
High choline (Cho) peak (i.e. twofold higher than the average noise level) was detected in 82/114 of malignant masses (72%): mucinous cystadenocarcinoma (n = 18/29, 62%), papillary serous cystadenocarcinoma (n = 17/29, 58.6%), granulosa cell tumour (n = 10/10, 100%), dysgerminoma (n = 8/8, 100%), clear cell carcinoma (n = 7/7, 100%), immature teratoma (n = 7/7, 100%), undifferentiated carcinoma (n = 5/5, 100%), borderline mucinous cystic tumour (n = 5/9, 55.5%), borderline serous cystic tumour (n = 4/9, 44.4%) and borderline cystadenofibroma (n = 1/1, 100%) On the other side, only six benign masses (6/110, 5.4%) showed high Cho peak, those were fibroma (n = 2/6, 33.3 %) and fibro-thecoma (n = 4/18, 22.2 %). Presence of high Cho peak elicited a sensitivity of 72%, specificity of 94.5%, positive-predictive value of 93.2%, and negative-predictive value of 76.4% and accuracy of 83% in detecting malignancy in the included ovarian masses. 53 invasive malignant masses (55.8%) showed high creatinine (Cr) peak. Low creatinine signal was noted in all of the benign (n = 110) and the borderline malignant (n = 19) tumours. The mean Cho/Cr ratio of the malignant ovarian masses was 2.80 and it was significantly higher than that of the benign ovarian masses which was 1.2 Table 3. Regarding the discrimination of malignant from benign ovarian masses, the ROC curve analysis of Cho/Cr ratio yielded an AUC of 0.919, a threshold of 1.7 and statistical indices of sensitivity: 81.5%, specificity: 93.6%, positive-predictive value: 93%, negative-predictive value: 83%, and accuracy: 87.5% Table 4. High lactate peak (twofold higher than the average noise level) was detected in 78/114 of malignant masses (68.4%): mucinous cystadenocarcinoma (n = 22/29, 75.8%), serous cystadenocarcinoma (n = 21/29, 72.4%) , dysgerminoma (n = 8/8, 100%), granulosa cell tumour (n = 7/10, 70%), clear cell carcinoma (n = 7/7, 100%), borderline mucinous cystic tumour (n = 5/9, 55.5%), undifferentiated carcinoma (n = 3/5, 60%), borderline serous cystic tumour (n = 4/9, 44.4%) and borderline cystadenofibroma (n = 1/1, 100%). Benign masses (73/110, 66.3%) also showed high lactate peak: tubo-ovarian abscesses (n = 18/18, 100%), endomterioma (n = 15/15, 100%), fibrothecoma (n = 15/18, 83.3%), serous cystadenoma (n = 12/24, 50%) haemorrhagic cysts (n = 7/7, 100%), and mature cystic teratoma (n = 6/12, 50%) High lactate peak showed sensitivity of 68.4%, specificity of 33.6%, positive-predictive value of 51.6%, negative-predictive value of 50.7% and accuracy of 51.3% in differentiating benign from malignant ovarian masses. We combined the analysis of the choline and the lactate peaks and considered that the presence of these peaks was an indicator that the ovarian tumour was malignant in nature.
Table 3. .
Mean values of the Cho/Cr values in 224 ovarian masses
| Mean choline/creatine | |||
| Mean | |||
| Benign | Haemorrhagic corpus lutiem cyst | 1.10 | |
| Tubo-ovarian abscess | 1.30 | ||
| Endometriotic cyst | 1.20 | ||
| Serous cystadenoma | 0.75 | ||
| Mucinous cystadenoma | 0.75 | ||
| Fibroma | 3.80 | ||
| Fibro-thecoma | 1.07 | ||
| Mature cystic teratoma | 1.00 | ||
| Malignant | Invasive | Serous cystadenocarcinoma | 3.27 |
| Mucinous cystadenocarcinoma | 3.20 | ||
| Clear cell adenocarcinoma | 2.90 | ||
| Granulosa cell tumour | 2.20 | ||
| Undifferentiated carcinoma | 2.95 | ||
| Immature teratoma | 1.90 | ||
| Dysgerminoma | 4.50 | ||
| Borderline | Serous cystic tumour | 1.30 | |
| Mucinous cystic tumour | 2.30 | ||
| Cystadenofibroma | 1.17 | ||
Table 4. .
The ROC curve analysis of the “choline-to-creatine ratio” in detecting malignancy in ovarian tumours
| Area under curve | p value | 95% confidence interval | Cut-off value | Sensitivity | Specificity | PPV | NPV | Accuracy | |
| Lower bound | Upper bound | ||||||||
| 0.919 | <0.001 | 0.802 | 1.035 | 1.7 | 81.5% | 93.6% | 93% | 83% | 87.5% |
NPV, negative predictive value; PPV, positive predictive value.
Eventually, the simultaneous presence of the choline and the lactate peaks showed sensitivity of 93.8%, specificity of 90%, positive-predictive value of 92.3%, negativepredictive value of 85% and accuracy of 93%. High lipid peak was detected in 86/114 of the malignant masses (75.4%): mucinous cystadenocarcinoma (n = 20/29, 70%), serous cystadenocarcinoma (n = 19/29, 65.5%), granulosa cell tumour (n = 10/10, 100%), dysgerminoma (n = 8/8, 100%), immature teratoma (7/7, 100%), undifferentiated carcinoma (n = 3/5, 60%), borderline mucinous cystic tumour (n = 9/9, 100%), borderline serous cystic tumour (n = 9/9, 100%) and borderline cystadenofibroma (n = 1/1, 100%).
In benign masses, lipid peak was detected in 68/110 (61.8%): tubo-ovarian abscesses (n = 18/18, 100%), fibrothecoma (n = 15/18, 83.3%), serous cystadenoma (n = 12/24, 50%), endomterioma (n = 10/15, 66.7%), haemorrhagic cysts (n = 7/7, 100%), and mature cystic teratoma (n = 6/12, 50%). The peak of the NAA metabolite was noted in 50/114 (43.8%) of the malignant masses: serous cystadenocarcinoma (n = 19/29, 65.5%), mucinous cystadenocarcinoma (n = 18/29, 62%), dysgerminoma (n = 7/8, 87.5%), immature teratoma (6/7, 85.7%). NAA peak was detected in 33/110 (30%) of the benign ovarian masses: serous cystadenoma (n = 10/24, 41.6 %), mucinous cystadenoma (n = 10/10, 100%), fibroma (n = 4/6, 66.7%), mature cystic teratoma (n = 9/12, 75%).
High acetone peak was detected in 59 ovarian masses included in the study (n = 59/224, 26.3%). These masses compromised 12/114 (10.5%) malignant masses: serous cystadenocarcinoma (n = 6/29, 20.7%), immature teratoma (n = 6/7, 85.7%) and 47/110 (42.7%) benign ovarian masses: haemorrhagic cyst, (n = 5/7, 71.4%), tubo-ovarian abscess (n = 18/18, 100%),, endomterioma (n = 15/15, 100%), and mature cystic teratoma (n = 9/12, 75%). The statistical indices for the combined analysis of the traditional (pre- and post-contrast) MRI and the proton MRS in screening malignancy in ovarian masses yielded a sensitivity 97.4%, specificity 93.3%, positive-predictive value 90.2%, negativepredictive value 90%, accuracy 91.7% and positive likelihood ratio of 1.0553
In Table (5), we summed up the statistical indices of the studied spectral metabolites and the combined evaluation of the traditional MRI and the MRS. Also, we calculated the likelihood ratio per metabolite.
Table 5. .
The statistical indices of the estimated spectral metabolites in detecting malignancy in the included ovarian masses
| Metabolites | Sensitivity | Specificity | Accuracy | PPV | NPV | LHR +ve | LHR -ve |
| Choline | 72 | 94.5 | 83 | 93.2 | 76.4 | 0.7701 | 0.7513 |
| Choline/creatine (1.7) | 81.5 | 93.6 | 87.5 | 93 | 83 | 0.8801 | 0.86 |
| Lactate | 68.4 | 33.6 | 51.3 | 51.6 | 50.7 | 2.0982 | 2.006 |
| Choline + lactate | 93.8 | 90 | 93 | 92.3 | 85 | 1.0539 | 1.0311 |
| Lipid | 74.4 | 62 | 68.7 | 67.2 | 70.8 | 1.2197 | 1.1839 |
| N-acetyl aspartate | 44 | 30 | 37 | 39.4 | 34 | 1.5172 | 1.4333 |
| Acetone | 10.5 | 42.7 | 26.3 | 16 | 31.5 | 0.2518 | 0.2225 |
| Conventional MRI + MRS (All metabolites) | 97.4 | 93.3 | 91.7 | 90.2 | 90 | 1.0553 | 1.0332 |
LHR, likelihood ratio; MRS, MR spectroscopy; NPV, negative predictive value; PPV, positive predictive value.
Numbers are in percentage.
Discussion
In this study, we assessed MRS as a non-contrast based sequence that give functional data and tried to find out if it has a role in scanning the features which could be suggestive of malignancy in ovarian masses. At first, we evaluated 224 ovarian masses regarding their morphology and internal components at the pre-contrast MR sequences. Then, we performed estimation with regard the functional behaviour of the included tumours at the dynamic post-contrast and MRS sequences. The common morphologic pattern in the studied malignant masses was the complex solid (63.1%). Purely cystic pattern were not detected in any of the malignant category.
Dynamic post-contrast MRI and proton MRS are known as functional MRI.6 In our work, the use of the dynamic post-contrast series enhanced the diagnostic performance of the conventional MRI and had shown higher statistical indices: specificity of 66.3% vs 81.8%, positive-predictive value of 71.5% vs 83.8%, and accuracy of 74.1% vs 86.6%. Thomassin-Naggara et al,12 retrospectively studied complex adnexal masses in 87 females who underwent DCE MRI before surgery. They supported the addition of such sequence in the routine examination as according to them it corrected the diagnosis in 19–24% of the cases.
Also, Mansour et al11 found that dynamic contrast-enhanced MRI could be a specific sequence to differentiate ovarian masses especially those with indeterminate morphology.
In vivo MR spectroscopy
Patients' spectra were interpreted qualitatively by inspection of the choline-containing compounds, creatinine, lactate, lipid, NAA and acetone peaks and quantitatively by application of the Cho/Cr integral ratio. To save time and effort, we used a SVS in case of large cystic masses (>4 cm) with small and localized solid components. It is not definite for malignant ovarian masses to show a high choline peak, i.e. the choline metabolite is not significant in the entire solid component of these masses; and at the same time, it is difficult to sample the solid component of the complex ovarian masses that present with a large surface area of papillary vegetations or discrete solid components. That is why we had used a multivoxel spectroscopy to assess the purely solid and also for the complex solid masses (Figures 1–4). Iorio et al,13 examined the individual metabolites that comprises the total choline resonance and found a significant increase in phosphocholine and total choline levels in the cancer compared to the normal ovarian cells.
Figure 1. .
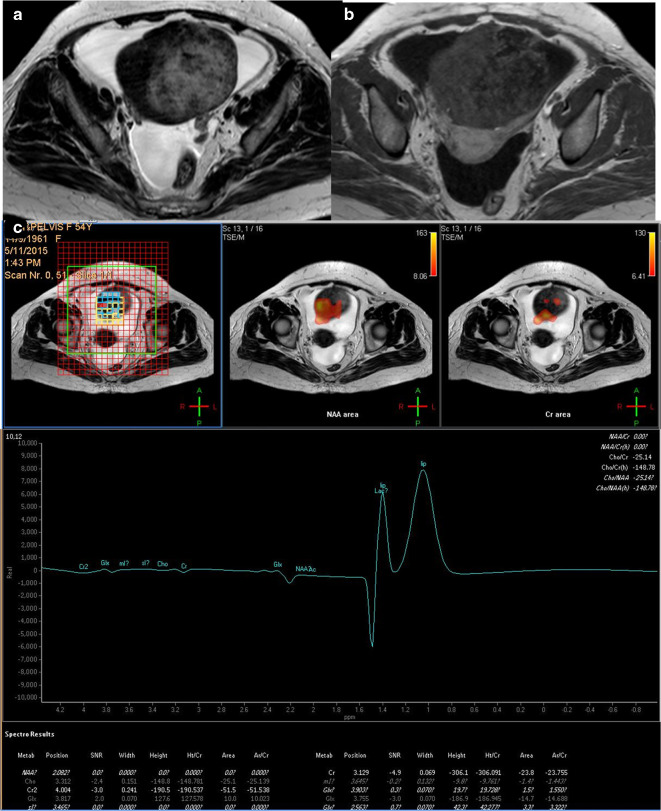
A 54 -years-old female patient with right ovarian fibrothecoma. (a) Axial T 2 weighted image showing large adnexal/ovarian purely solid mass of heterogeneous texture. (b) Axial post-contrast injection image that showed heterogeneous contrast uptake with areas devoid of contrast suggesting tissue necrosis. (c) Multivoxel MRS showing absence of choline signal and a small sharp lipid/lactate peak. The case was a true negative. MRS, MR spectroscopy; NAA, N-acetyl aspartate.
Figure 2. .
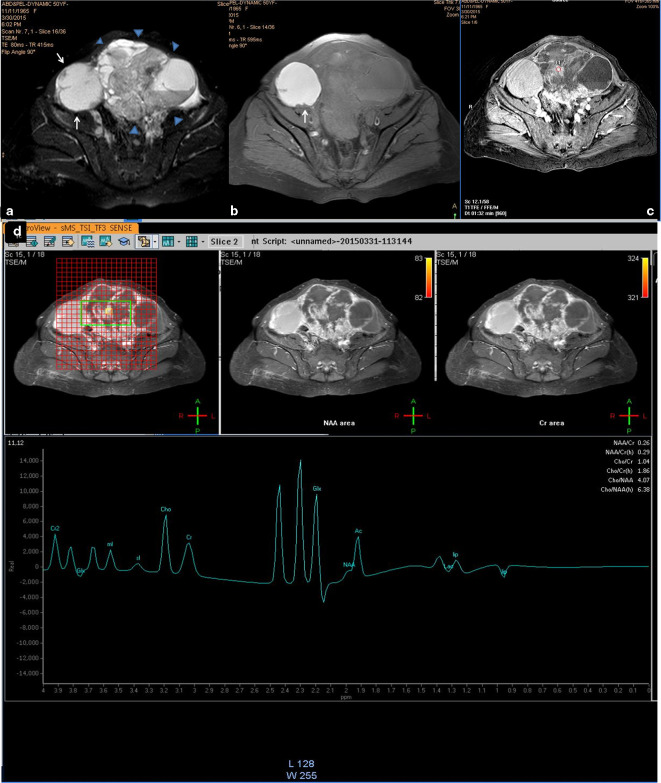
A 50-years-old female patient with bilateral ovarian clear cell adenoacarcinoma. (a, b) Axial T 2- and T 1 weighted fat suppressed images that showed bilateral complex masses adnexal/ovarian of mixed intensity; the right-sided mass is the smaller (white arrows) is mainly cystic with few mural-based nodules and the one on the left side displayed thick sepatations and papillary vegetations and wide solid sheets (arrow heads). Both cysts showed complicated content which is most likely haemorrhagic as it showed intense bright signal on the T 1-weighted image. (c) Axial contrast-enhanced image (dynamic series at 1:32 min. post-contrast injection) that showed contrast uptake of the solid component (circle). (d) Multivoxel spectrum with localization on the solid component showed sharp choline signal that present metabolic activity. Note, the multicomponents of the mass presented a complex spectral pattern in the region from 0.5 to 2.5 ppm. The right-sided ovarian mass was false negative and the left-sided one was true positive. MRS,MR spectroscopy; NAA, N-acetyl aspartate
Figure 3. .
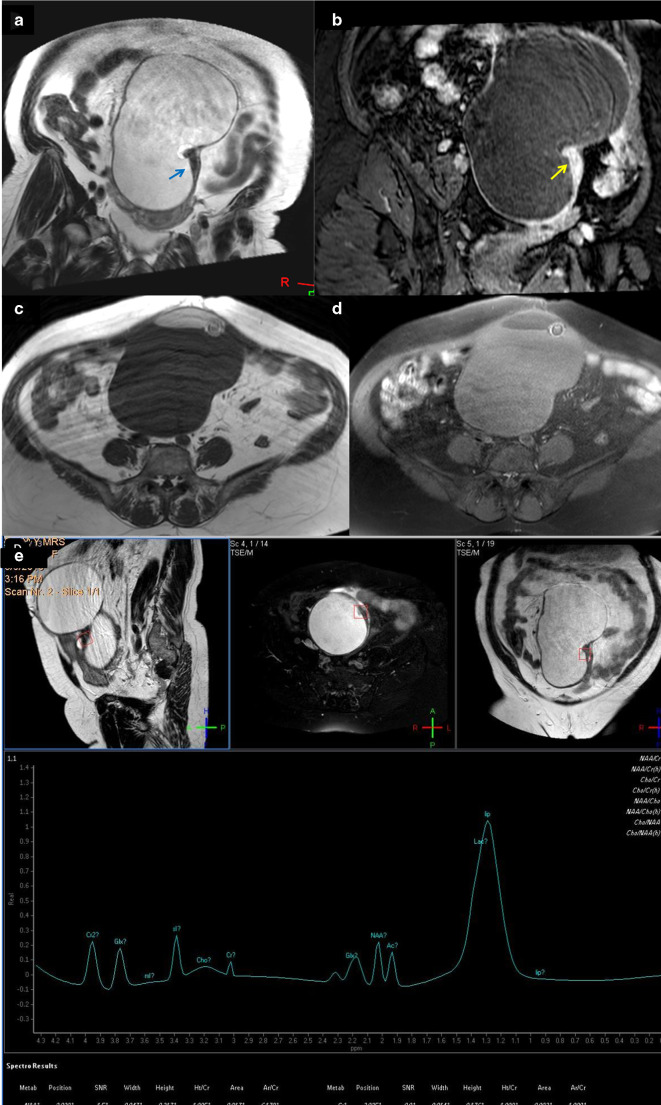
A 62-years-old female patient with immature teratoma with on top squamous cell carcinoma. (a) Coronal T 2-weighted image showing large predominantly cystic complex ovarian mass that showed localized small surface area solid sheet (arrow). (b) Contrast-enhanced coronal image that showed intense contrast uptake of the solid component (arrow). (c, d) Axial T 1- and T 1 fat suppression showed suppression of the bright T 1 signals that suggest fatty elements. (e) Single-voxel MRS, the voxel of interest focused on the solid component and there was large sharp lactate peak, N-acetyl aspartate and acetone signal and muffled choline peak (0.17). N-acetyl aspartate is attributed to the neural elements within the teratoma, the acetone presented reaction due to adherence of the different integrants within the mass. The choline peak was not significant, so it was a false negative, yet when we combined the lactate peak with the choline, malignancy was suggested and the case was considered as true positive. MRS, MR spectroscopy; SNR, signal-to-noise ratio.
Figure 4. .
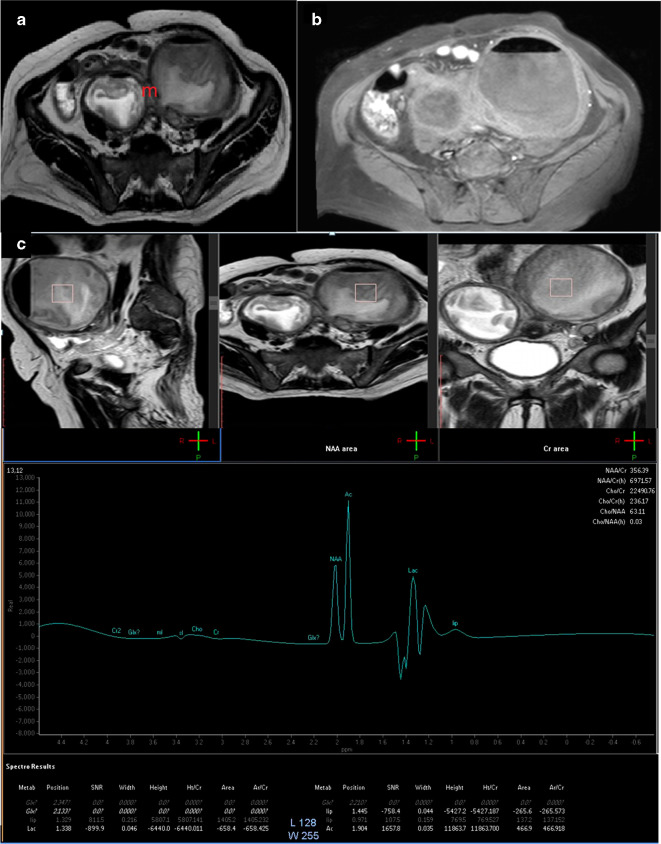
A 30-years-old female patient with bilateral tubo-ovarian abscess. (a) Axial T 2-weighted image showing bilateral cystic ovarian masses (m) that showed thickened walls, the larger on the left side, showed intracytsic indistinct solid sheets. (b) Axial post-contrast injection image showed contrast uptake of the thickened walls yet the intracystic solid component showed no uptake. (c) Single-voxel MRS, showed absence of choline peak. Lactate peak was detected. The presence of N-acetyl aspartate/acetone signal suggests tissue reaction due to turbid/complicated cystic component. The case was true negative. MRS, MR spectroscopy; SNR, signal-to-noise ratio.
In our study, a Cho peak was found in 82 of the malignant ovarian masses (72%) (Figures 2 and 3), while it was found in only six benign masses (5.4%). We found that the presence of Cho peak can predict malignancy in the scanned ovarian masses with a very high specificity and positivepredictive value of 94.4 and 92.3% respectively.
Esseridou et al14 included 23 ovarian masses, in which identification of a choline peak demonstrated 89% sensitivity and 84% specificity regarding the differentiation of the malignant from the benign ovarian pathology. Okada et al,15 measured metabolites in large tumours of the female pelvis (6 malignant and 17 benign). They found the signal of choline-containing compounds only in the solid tumours. In our study, when we relied only on the visual assessment of the choline peak, we experienced a low sensitivity (75.7%) and negative-predictive value (76.4%). This may be was attributed to the inclusion of large sector of complex masses with large cystic components as in mucinous and serous cystadenocarcinoma which may have caused noise of the spectrum. Because the spectral resolution of in vivo 1H-MRS is lower than that of in vitro 1H-MRS; studies use the ratio of peak integral to describe, analyze in a quantitative manner, and compare metabolic changes among different tumours.2 So, in our assessment we calculated the concentrations of choline and creatinine and included the integral ratios (Cho/Cr) in our assessment. We found that Cho/Cr integral ratio of greater than 1.7 was an indicator that a tumour was malignant in nature and presented a positive likelihood ratio of 0.88. Our results were in agreement with Li et al,16 who found that a threshold of 2 in the Cho/Cr integral ratio could accurately discriminate between benign and malignant adnexal tumours.
Ma et al2; examined 69 solid adnexal masses. They found that the Cho/Cr integral ratio was 5.13 in benign tumours vs 8.90 in malignant ones. The ratios previously mentioned were higher than those presented in our study; probably because they had studied double the number of our cohort and included only the solid tumours.
Stanwell et al,17 and Forstner et al18 examined ovarian masses by MRS using a 3 Tesla magnet. They stated that a Cho/Cr integral ratio of greater than three indicates that a tumour was malignant in nature, whereas a Cho/Cr integral ratio less than 1.5 implied that a tumour was benign in nature. High lactate peak presented in 78 true positive (Figures 2 and 3) and 37 true negative masses (Figures 1, 4 and 5). The resultant statistical indices were low if compared to other metabolites: sensitivity 68.4%, specificity 33.6%, positive-predictive value 51.6%, negativepredictive value 50.7% and accuracy 51.3%.
Figure 5. .
A 40-years-old female patient with right cystadenofibroma accidently discovered during the performance of MR defecagraphy functional examination. (A, B) Axial and sagittal T 2-weighted images showing right ovarian multilocular cystic mass with a small solid component (arrow). (C) Axial contrast-enhanced subtraction image (dynamic series at 1:28 min. post contrast-injection) where an enhancing nodule was detected (arrow). D: Multivoxel MRS, showed different metabolites within the mass that may suggest the diagnosis; (i) N-acetyl aspartate/acetone signal (cystic mass with turbid content), (ii) sharp lactate/lipid peak (suggest fibrosis), (iii) absence of choline signal (no metabolic activity). The case was true negative. MRS, MR spectroscopy; SNR, signal-to-noise ratio.
Our study was in agreement with the study done by Ma et al.2 Also, Kang et al,19 Hascalik et al20 and Okada et al15 had found lactate signal not only in all the malignant tumours but also in some of the benign tumours. However, they observed that lactate signals of the malignant tumours tended to form showed higher peaks than those of the benign. Detection of lactate peak should not be considered in case of pelvic abscesses.19,20 We went with that suggestion as we detected high lactate signal in all of the included pelvic abscesses (100%) (Figure 5).
We tried to study the statistical significance of simultaneous presence of choline and lactate peaks in differentiating benign from malignant ovarian masses. Interestingly, we found that there was an increase in the accuracy from 85.3% with choline alone and 50% with lactate alone, to 93% if both peaks were considered. From the above, we may suggest that lactate is not a specific metabolite of malignancy when considered alone, it requires support with the choline metabolite to enhance the accurate diagnosis of ovarian cancer (Figures 1, 3 and 5). Abramov et al21 stated that intracellular lactate levels and total choline compounds have been shown to be most prominent in the moderately to poorly differentiated histological types of ovarian neoplasm. Lipid may be observed in various malignant tumours. Cho et al,22 showed an intense lipid peak in malignant ovarian tumours and benign teratoma, whereas benign epithelial ovarian tumours showed no detectable lipid peak. In the present study, all cases of teratoma and tubo-ovarian abscess showed lipid peak. Also, 15 out of 18 masses of fibrothecoma showed sharp lipid peak. Ma et al,2 had detected a lipid peak in 11 of 12 the comas or fibrothecomas, 2 of 3 fibromas, and 6 of 11 leiomyomas vs 30 of 42 malignant tumours. NAA metabolite is detected in the spectroscopy analysis of mature cystic teratoma, denoting the presence of neural element (ectodermal tissue). It is found also in both solid and cystic parts of the mucinous cystadenoma, cyst fluid from serous cystadenoma and simple follicular cysts although devoid of neural tissue.19,20,23,24 Our results agreed with this concept, where NAA peak was detected in 83 masses: 68 of them had large cystic components and the remaining 15 were teratomas.
Takeuchi et al25 stated that the peak of NAA had 89% sensitivity and 86% specificity in diagnosis of mucinous components, and when seen together with choline, they raised the suggestion of ovarian metastases (i.e. Krukenberg tumour).
Low concentration metabolites such as acetone and acetoacetate and others can be difficult to detect, especially when they are overshadowed by the neighbouring strong metabolites (such as glutamate, glutamine and γ-aminobutyrate).26 Acetone metabolite was barely pointed to in the literature and its mention was always regarding the MRS of the brain, not the pelvis. This rare recognition may be because its detection requires high sensitivity and wide spectral window, and a high magnetic field. In our work, acetone metabolite was detected mainly in the benign masses (n = 47/110). It was noted in only 10.5% of the malignant ones (n = 12/114). All of these masses showed a common texture of complex cystic component (Figures 3–5). Acetone metabolite was noted in 100% of the tubo-ovarian abscess (n = 18/18) and the endomteriomas (n = 15/15). There are no specific early symptoms in case of the ovarian cancer and that is why most of the cases show first presentation of an advanced disease.
Upon scanning, it is very important to discriminate the low potential (borderline) malignant from the invasive malignant ovarian tumours. Stage I ovarian cancer should be precisely staged and then treated by aggressive surgery (total abdominal hysterectomy, bilateral salpingo-oophorectomy, omentectomy and lymphadenecomy). On the other side, borderline tumours do not show an infiltrative behaviour, it display a better prognosis and can be treated in a conservative way (surgical removal of the mass while leaving as much normal ovarian tissue as possible). The use of the integral Cho/Cr ratio of more than 1.7 on imaging with MRS helped us to differentiate between the benign and the malignant categories, and also discriminate between borderline (correct diagnosis in 79%, n = 15/19) and invasive malignant (correct diagnosis in 82.1%, n = 78/95) tumours.
A recent study27 which analyzed 82 ovarian tumours yet focused only on the epithelial ones; had found that the “in vivo 1H-MRS” showed a higher Cho peak for the invasive malignant category. They suggested an optimal cut-off value of 6.2 for the Cho/Cr ratio with a specificity of 100%, which is a rather a high value compared to our study but this could be explained by the fact that their cohort included only malignant tumours (36 were borderline and 46 were invasive). They concluded that NAA/Cho ratio was a reliable biomarker for differentiating borderline from malignant epithelial ovarian tumours; however, they had no statistics for the lipid and lactate peaks due to insufficient data.
We found also that the addition of the MRS to the traditional MR examination increased the overall diagnostic accuracy of the MR modality in the characterization of the ovarian neoplasm and screening of malignancy (91.7 instead of 74.1). Also, MRS was comparable to the minimally invasive contrast enhanced sequence (i.e. DCE-MRI) in the exclusion of malignancy, both presented near negative-predicative value (90 and 88.9% respectively) and took an equal time of examination, and so there is no negative impact on the clinical workflow. Moreover, MRS had the privilege over the postcontrast MR sequence in the detection of ovarian malignancy in being less expensive and a non-invasive modality which is even suitable for the pregnant cases and those with renal failure
Conclusion
Proton MRS provides good biochemical information and is a credible imaging method for the screening and diagnosis of malignancy in the different ovarian masses. The simultaneous presence of both choline and lactate peaks minimizes the overlap between benign and malignant categories with solid components. In the evaluation of complex cystic masses as benign teratoma, endomterioma and tubo- ovarian complex; NAA and acetone were the utmost specific metabolites.
Contributor Information
Sahar Mahmoud Mansour, Email: sahar_mnsr@yahoo.com.
Mohammed Mohammed Mohammed Gomma, Email: dr.s.mansr@gmail.com.
Peter Nashaat Shafik, Email: pnashat583@gmail.com.
REFERENCES
- 1. Dwivedi AND, Jain S, Shukla RC, Jain M, Srivastava A, Verma A, et al. . Mri is a state of art imaging modality in characterization of indeterminate adnexal masses. J Biomed Sci Eng 2013; 06: 309–13. doi: 10.4236/jbise.2013.63A039 [DOI] [Google Scholar]
- 2. FH M, Qiang JW, Cai SQ, et al. . Mr spectroscopy for differentiating benign from malignant solid adnexal tumors. American Journal of Roentgenology 2015; 204: 724–30. [DOI] [PubMed] [Google Scholar]
- 3. Dogheim OY, Abdel Hamid AE-DM, Barakat MS, Eid M, El-Sayed SM, et al. . Role of novel magnetic resonance imaging sequences in characterization of ovarian masses. The Egyptian Journal of Radiology and Nuclear Medicine 2014; 45: 237–51. doi: 10.1016/j.ejrnm.2013.11.008 [DOI] [Google Scholar]
- 4. Wasnik AP, et al. Multimodality imaging of ovarian cystic lesions: review with an imaging based algorithmic approach. World J Radiol 2013; 5: 113–25. doi: 10.4329/wjr.v5.i3.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anwar S, Rehan B, Hameed G. Mri for the diagnosis of ultrasonographically indeterminate pelvic masses. JPMA 2014; 64: 171–3. [PubMed] [Google Scholar]
- 6. Wakefield JC, Downey K, Kyriazi S, deSouza NM, et al. . New Mr techniques in gynecologic cancer. American Journal of Roentgenology 2013; 200: 249–60. doi: 10.2214/AJR.12.8932 [DOI] [PubMed] [Google Scholar]
- 7. Belkić D, Belkić K. In vivo magnetic resonance spectroscopy for ovarian cancer diagnostics: quantification by the fast Padé transform. J Math Chem 2017; 55: 349–405. doi: 10.1007/s10910-016-0694-8 [DOI] [Google Scholar]
- 8. deSouza NM, O'Neill R, McIndoe GA, Dina R, Soutter WP. Borderline tumors of the ovary: CT and MRI features and tumor markers in differentiation from stage I disease. American Journal of Roentgenology 2005; 184: 999–1003. doi: 10.2214/ajr.184.3.01840999 [DOI] [PubMed] [Google Scholar]
- 9. Sohaib SAA, Sahdev A, Trappen PV, Jacobs IJ, Reznek RH. Characterization of adnexal mass lesions on MR imaging. American Journal of Roentgenology 2003; 180: 1297–304. doi: 10.2214/ajr.180.5.1801297 [DOI] [PubMed] [Google Scholar]
- 10. Imaoka I, Wada A, Kaji Y, Hayashi T, Hayashi M, Matsuo M, et al. . Developing an Mr imaging strategy for diagnosis of ovarian masses. RadioGraphics 2006; 26: 1431–48. doi: 10.1148/rg.265045206 [DOI] [PubMed] [Google Scholar]
- 11. Mansour SM, Saraya S, El-faissal Y. Semi-Quantitative contrast-enhanced Mr analysis of indeterminate ovarian tumours: when to say malignancy? Br J Radiol 2015; 88: 20150099. doi: 10.1259/bjr.20150099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thomassin-Naggara I, Toussaint I, Perrot N, Rouzier R, Cuenod CA, Bazot M, et al. . Characterization of complex adnexal masses: value of adding perfusion- and diffusion-weighted MR imaging to conventional MR imaging. Radiology 2011; 258: 793–803. doi: 10.1148/radiol.10100751 [DOI] [PubMed] [Google Scholar]
- 13. Iorio E, Mezzanzanica D, Alberti P, Spadaro F, Ramoni C, D'Ascenzo S, et al. . Alterations of choline phospholipid metabolism in ovarian tumor progression. Cancer Res 2005; 65: 9369–76. doi: 10.1158/0008-5472.CAN-05-1146 [DOI] [PubMed] [Google Scholar]
- 14. Esseridou A, Di Leo G, Sconfienza LM, Caldiera V, Raspagliesi F, Grijuela B, et al. . In vivo detection of choline in ovarian tumors using 3D magnetic resonance spectroscopy. Invest Radiol 2011; 46: 377–82. doi: 10.1097/RLI.0b013e31821690ef [DOI] [PubMed] [Google Scholar]
- 15. Okada T, Masafumi H, Kenji M, et al. . Evaluation of female intra-pelvic tumors by clinical proton MR spectroscopy. J MRI, special Issue: Women's MR 2001; 13: 912–7. [DOI] [PubMed] [Google Scholar]
- 16. WH L, Chu CT, Zhang P.et al. . MRI and MRS analysis of ovarian endometrioid carcinomas. 27 China: The British Institute of Radiology.; 2008. 470–2. [Google Scholar]
- 17. Stanwell P, Russell P, Carter J, Pather S, Heintze S, Mountford C, et al. . Evaluation of ovarian tumors by proton magnetic resonance spectroscopy at three Tesla. Invest Radiol 2008; 43: 745–51. doi: 10.1097/RLI.0b013e31817e9104 [DOI] [PubMed] [Google Scholar]
- 18. Forstner R, Meissnitzer MW, Schlattau A, Spencer JA, Matthias W, et al. . Mri in ovarian cancer. Imaging Med 2012; 4: 59–75. doi: 10.2217/iim.11.69 [DOI] [Google Scholar]
- 19. Kang YH, Kim MY, Kim KT, Kim YJ, Suh CH, Kim JM, et al. . H 1 Magnetic Resonance Spectroscopy of Cystic Ovarian Lesions. Journal of the Korean Society of Magnetic Resonance in Medicine 2013; 17: 326–33. doi: 10.13104/jksmrm.2013.17.4.326 [DOI] [Google Scholar]
- 20. Hascalik S, Celik O, Sarac K, Meydanli MM, Alkan A, Mizrak B, et al. . Metabolic changes in pelvic lesions: findings at proton MR spectroscopic imaging. Gynecol Obstet Invest 2005; 60: 121–7. doi: 10.1159/000086003 [DOI] [PubMed] [Google Scholar]
- 21. Abramov Y, Carmi S, Anteby SO, Ringel I. Ex vivo1H and 31P magnetic resonance spectroscopy as a means for tumor characterization in ovarian cancer patients. Oncol Rep 2013; 29: 321–8. doi: 10.3892/or.2012.2071 [DOI] [PubMed] [Google Scholar]
- 22. Cho SW, Cho SG, Lee JH, Kim H-J, Lim MK, Kim JH, et al. . In-Vivo proton magnetic resonance spectroscopy in adnexal lesions. Korean J Radiol 2002; 3: 105–12. doi: 10.3348/kjr.2002.3.2.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. El sorogy L, El gaber NA, Omran E, Elshamy M, Youssef H, et al. . Role of diffusion MRI and proton magnetic resonance spectroscopy in characterization of ovarian neoplasms. The Egyptian Journal of Radiology and Nuclear Medicine 2012; 43: 99–106. doi: 10.1016/j.ejrnm.2011.12.008 [DOI] [Google Scholar]
- 24. Boss EA, Moolenaar SH, Massuger LFAG, Boonstra H, Engelke UFH, de Jong JGN, et al. . High-Resolution proton nuclear magnetic resonance spectroscopy of ovarian cyst fluid. NMR Biomed 2000; 13: 297–305. doi: [DOI] [PubMed] [Google Scholar]
- 25. Takeuchi M, Matsuzaki K, Harada M, et al. . Preliminary observations and clinical value of N-acetyl resonances in ovarian tumours using in-vivo proton MR spectroscopy at 3T. Eur Radiol 2011; 21: 2640–6. doi: 10.1007/s00330-011-2215-2 [DOI] [PubMed] [Google Scholar]
- 26. Wang DJ, Zimmerman RA. 3T MRS observations of acetone and beta hydroxybutyrate in an epileptic patient under ketogenic diet. Proc. Intl. Soc. Mag. Reson. Med 2006; 14: 3146. [Google Scholar]
- 27. FH M, YA L, Liu J, et al. . Role of proton MR spectroscopy in the differentiation of borderline from malignant epithelial ovarian tumors: a preliminary study. J. Magn. Reson. Imaging 2019; 49: 1684–93. [DOI] [PubMed] [Google Scholar]