Abstract
Resternotomy (RS) is a common occurrence in cardiac surgical practice. It is associated with an increased risk of injury to old conduits, cardiac structures, catastrophic hemorrhage and subsequent high morbidity and mortality rate in the operating room or during the recovery period. To mitigate this risk, we evaluated the role of multidetector CT (MDCT) in planning repeat cardiac surgery.
We evaluated sternal compartment abnormalities, sternal/ascending aorta distance, pre-reoperative assessment of the aorta (wall, diameters, lumen, valve), sternal/right ventricle distance, diaphragm insertion, pericardium and cardiac chambers, sternal/innominate vein distance, connection of the grafts to the predicted median sternotomy cut, graft patency and anatomic course, possible aortic cannulation and cross-clamping sites and additional non-cardiovascular significant findings.
Based on the MDCT findings, surgeons employed tailored operative strategies, including no-touch technique, clamping strategy and cardiopulmonary bypass (CPB) via peripheral cannulation assisted resternotomy. Our experience suggests that MDCT provides information which contributes to the safety of re-operative heart surgery reducing operative mortality and adverse outcomes. The radiologist must be aware of potential surgical options, including in the report any findings relevant to possible resternotomy complications.
Introduction
Despite significant interventional advances, the number of patients undergoing resternotomy (RS, repeat sternotomy performed at least 1 month following the prior sternotomy and requiring a saw to reopen the full length of the sternum) for re-operation of congenital and acquired cardiovascular disease continues to rise.1 According to data from the Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database (Chicago, IL, USA), re-operations account for approximately 8.6–10.3% of cardiac surgeries performed annually.2,3 While the initial sternotomy is not typically associated with risks, the successive formation of adhesions securing cardiac structures to mediastinal structures, including the sternum, increases the potential for injury during sternal re-entry. and dissection.
Development of multidetector CT (MDCT) has significantly enhanced the relevance of cardiovascular imaging.4–12 The capabilities of this modality have made it a recommended tool in the pre-operative assessment of the cardiac surgery patient.
The American College of Cardiology has stressed that the use of Cardiac CT is appropriate (use score 7) prior to a repeat sternotomy in re-operative cardiac surgery6 as well as the Working Group of the Cardiac Radiology Section of the Italian Society of Medical Radiology (Class I).7
In this article, we summarize our experience with MDCT in the preoperative evaluation of the re-operative cardiac surgery patient and at the same time we resume literature data about the subject in question. Aims of this paper are to highlight the findings that the radiologist should know, to stress the relevance of a multidisciplinary approach in order to plan a patient-tailored operative strategy and to demonstrate the effectiveness of this integrated approach in terms of surgical outcomes.
MDCT protocol of study and technical notes
Our experience refers to 274 consecutive adult patients, 175 males and 99 females, who underwent resternotomies at Azienda Ospedali dei Colli, Monaldi Hospital, a tertiary care cardiovascular Hospital, between 2009 and 2016, examined with MDCT. Among these, 36 (13%) MDCT examinations were performed in emergency cases for acute chest pain, sepsis or decrease in haemoglobin. The median age was 67.7 years (range 60.1–78 years). 225 patients underwent second, 37 third and 12 their fourth RS; 5 patients (1.8%) had prior mediastinal radiotherapy. Patients with renal failure underwent MDCT scan without contrast and haemodynamically unstable patients did not undergo a CT scan before surgery. Cardiac conditions requiring re-operation included coronary artery bypass, mitral/aortic valve surgery, ascending aorta surgery or combined procedures and adult re-operations in congenital heart diseases. Images were obtained with 64-MDCT (LightSpeed VCT 64-slice GE, GE Medical System, Milwaukee, WI, USA). The CT protocol consisted of 165 unenhanced chest CT examinations at specific request of the cardiac surgeons and 99 triphasic CT chest angiography scans; in these patients multiphasic CT scans (unenhanced, arterial and delayed contrast-enhanced phases) were considered necessary to facilitate the recognition of vascular structures and for the correct characterization of any actively bleeding vascular lesions.5 No oral contrast agent was administered.
Where feasible (in 35 selected cases) valve plane, aortic root, native coronary arteries, bypass graft and proximal ascending aorta contrast-enhanced acquisition was obtained with retrospective ECG-gating and radiation reduction technique (high pitch 100 kV imaging and electrocardiographic dose modulation).
Non-ionic contrast material (total volume of 60–100 ml of low osmolar CM) was injected at a rate of 3–6 mL/sec through a 18–20 gauge cannula in the right arm, where possible, with a dual barrel power injector (Stellant SCT 211, Medrad, Indianola, IA, USA) by adopting bolus trigger technique with a single ROI positioned in the ascending aorta or in the aortic arch; contrast infusion was routinely followed by a saline flush (20–40 ml).
Curved planar reconstructions (CPR), shaded surface display, maximum intensity projection (MIP), two-dimensional (2D) oblique and three-dimensional (3D) volumetric rendering (VR) images were routinely created. 2D reconstructions were performed by MIP or CPR. Thin-slab MIP images allowed simultaneous assessment of extended lengths of each vessel.
The study protocol was approved by the local ethical committee which waived the need for consent.
MDCT findings prior to RS (pre-RS descriptors)
Surgical risk factors linked to sternal site complications are numerous and include the following: body surface area (BSA), previous sternotomy and the number of previous operations, complexity of the surgery, type of bone saw used, type of sternal closure, blood transfusions and early re-exploration to control haemorrhage.
Chest radiography is the first-line imaging examination performed to evaluate patients prior to median resternotomy; both frontal posteroanterior and lateral views of the chest are obtained. The morphology of the thorax (as assessed by latero-lateral chest roentgenogram) and the type of valvular pathology (i.e. namely severe tricuspid lesion and aortic stenosis even without aortic aneurysm) also have a considerable impact in determining RS risk.8 However, a chest radiograph and selective angiography cannot provide the surgeon with comprehensive information on the proximity of the aorta, right ventricle, coronary arteries and grafts to the sternum, nor the extent of calcified atherosclerosis within the aorta.
Targets of MDCT imaging
1) Sternal and peristernal abnormalities (to achieve safe sternal re-entry)
Sternal compartments are classified as presternal, sternal and retrosternal.10 Soft tissues in the presternal compartment include the skin, subcutaneous tissues and muscles. In the sternal compartment, CT allows differentiation of the sternal cortex from the medulla,10,11 can depict among other variants normal spiculations and pits and allows normal variants to be differentiated from pathologic abnormalities (Figure 1).
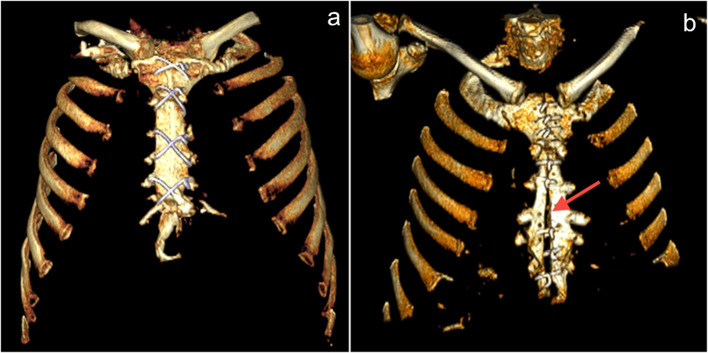
Figure 1a–b.
Normal sternotomy and midsternal lucent stripe. (a) VR reconstruction shows normal median sternotomy; (b) VR reconstruction shows midsternal lucent stripe >3 mm (arrow) 12 months after undergoing sternotomy. This finding may be indicative of dehiscence only if the stripe is thicker than 3 mm. VR, volumetric rendering.
Retrosternal adhesions were assumed to be present when the low-attenuation fat between the sternum and the sternocostal hearth surface was absent or replaced by scar tissue.11 The clinical grading of the scar tissue adhesions to the anterior chest wall was classified as follows: 0 = no adhesions; 1 = mild adhesions (“peak sign”), with only a slight scar tissue line between the sternum and the retrosternal structures, triangular in shape with apex anterior; 2 = severe adhesions (“flat sign”) retrosternal structures flattened over the sternum (Figure 2).11 Other noticeable MDCT changes at the site of adhesion between the sternum and the retrosternal structures were represented by nodular increased density of retrosternal fat, obliteration of the pericardial fat, cardiac contour change with or without nodular epicardial fat accumulation, diffuse pericardial thickening and pericardial calcification. Since the postsurgical cardiovascular structures and grafts are surrounded with connective tissue that cause their adhesion to the sternum, when the distance between the cardiovascular structure to the sternum was less than 1 cm, we defined this as adherence/contiguity. Risk of damage was determined as a distance less than or equal to 5 mm and critical contiguity or adhesion if it was less 3 mm.4–6 Consideration should be given to the fact that adhesions become less inflammatory with reduced vascularity over time; so it would be easier to re-operate after a longer period. Re-operation between 3 weeks and 6 months from the last operation is the most difficult. Previous radiotherapy alters the healing process, slowing the maturation of adhesions such that they often remain fleshy even after several years, making identification of the dissection plane and reoperations difficult in this setting.
Figure 2a–b.
Retrosternal adhesions findings. Axial MIP reconstruction (a) shows an irregularly triangular shape adhesion with anterior large apex between the sternum and the retrosternal structures (“peak sign”); MDCT axial scan (b) demonstrates severe adhesions by retrosternal structures flattened over the sternum ("flat sign“). MDCT, multidetector CT; MIP, maximum intensity projection.
2) Sternal/ascending aorta distance (S/AA)
By means of chest MDCT a mean distance of 1.7 ± 0.7 cm was found between the posterior sternal surface and the closest segment of the anterior ascending aorta;
A 1 cm cut off (distances greater than 1 cm between aorta and sternum) is regarded to be the minumim allowed for reopening;
adherence/proximity if S/AA distance <1 cm;
risk of damage was present if S/AA distance was ≤5 mm;
critical contiguity in case of distance <3 mm;
Of note, scrupulous attention should be paid if the distance is smaller than 1 cm but a layer of soft tissue can be identified throughout. In some underlying congenital disorders the aorta passes anteriorly, such as double outlet right ventricle (DORV), transposition of the great arteries (TGA), or Tetralogy of Fallot (ToF).5,13
3)Sternal/right ventricle distance (S/RV)
The most common cardiac structure that can be penetrated during resternotomy is represented by right ventricle (RV) 10-12,14.
A 1 cm cut-off (distances of more than 1 cm between right ventricle and sternum) is regarded to be uncritical for reopening;
adherence/proximity S/RV distance <1 cm;
risk of damage in case of S/RV distance ≤5 mm;
critical contiguity if S/RV distance <3 mm.
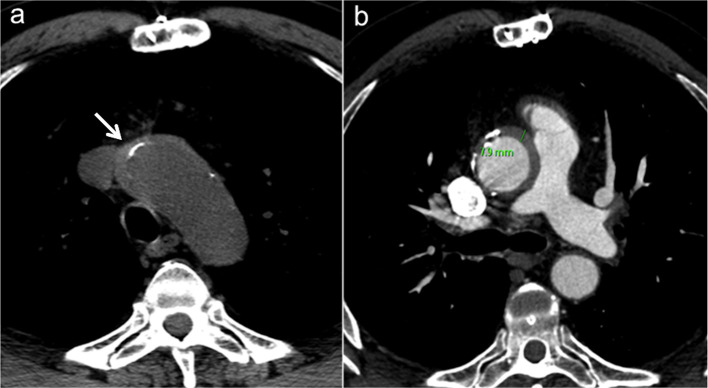
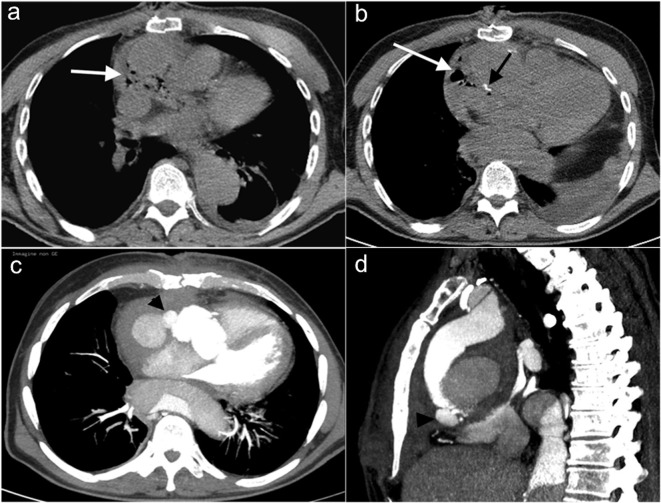
4) Innominate vein/sternum distance and systemic venous abnormalities, including persistent left superior vena cava, brachiocephalic vein stenosis andsuperior vena cava stenosis (Figure 3a-c).
Figure 3a–c.
Relations between sternum and vascular/cardiac structures. (a) Axial MIP reconstruction shows S/RV critical contiguity (<3 mm). (b) Sagittal MIP reconstruction shows risk of AA damage (S/AA ≤5 mm) (arrow). (c) Axial MIP reconstruction shows sternum/ left innominate vein critical contiguity (<3 mm). AA, ascending aorta; MIP, maximum intensity projection; RV, right ventricle; S, sternal.
5) Re-operative assessment of the ascending aorta, periaortic tissue andaortic branches
Aortic wall:
Localized calcification or sclerosis predominantly extending into anterior segments of the ascending aorta, distribution of calcified and non-calcified plaques must be reported as this may significantly complicate vessel cannulation and/or clamping, increasing the risk of stroke and distal embolism as well as the potential for dissection.
Some authors have highlighted the significant correlation between the atherosclerosis of the ascending aorta and aortic arch and adverse cardiovascular events during heart operations.5,14–16
In patients affected by aortic atherosclerosis, the risk of cerebral events was 8.7 vs 1.8% in the absence of aortic disease.15 When a significant ascending aorta atherosclerosis is not present, direct aortic cannulation can be safely performed. In patients affected by a significant ascending aortic atherosclerotic disease (Figure 4), axillary cannulation with antegrade cerebral perfusion (ACP) tecnique may be a surgical option.12
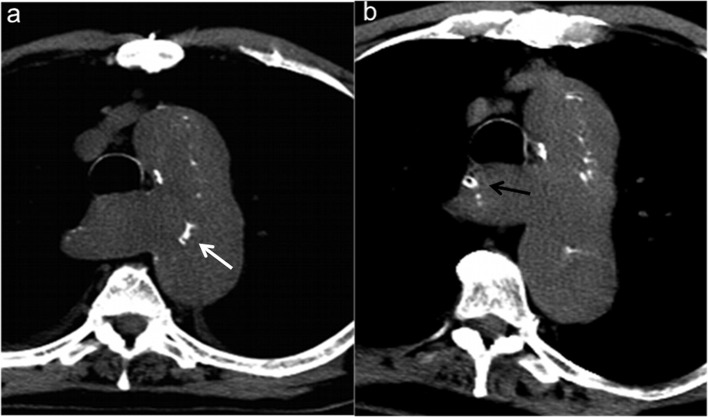
Figure 4a–b.
Diffuse atherosclerotic disease of the aorta. (a) Coronal MPR reconstruction and (b) oblique sagittal MIP reconstruction show areas of ascending aortic heavily calcifications (arrow) as at high risk for embolization with surgical clamping and manipulation. MIP, maximum intensity projection; MPR, Multiplanar reformation.
In this context, pre-operative CT imaging has been reported to lower the rates of perioperative stroke in patients undergoing cardiac re-operations by optimizing cannulation and aortic clamping strategies.17–19 It is important to identify and report potential displacement of calcifications, suggestive of intramural hematoma or displacement extending to the lusory artery in dissection (Figure 5).
Figure 5a–b.
Pre-operative assessment in a 58-year-old male with unstable angina and a history of two prior coronary artery bypass graftings. (a) Unenhanced and (b) enhanced axial MDCT images show a high density aortic wall suggestive for acute intramural hematoma (IMH) associated to displaced calcifications (white arrow). MDCT, multidetector CT.
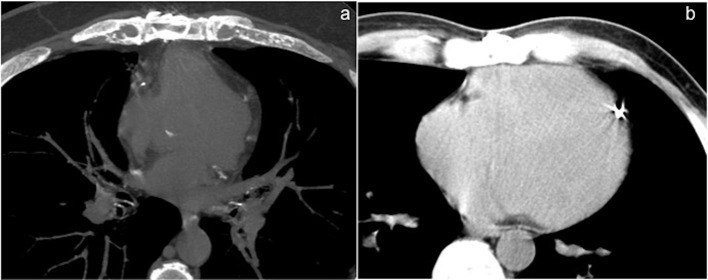
Maximal aortic diameter (AOmax)
It is important to evaluate aortic diameter in the presence of an aortic aneurysm or pseudoaneurysm. It is possible to calculate the distance between the sternum and the vertebra (D) to measure the occupying rate of ascending aorta or pulmonary artery (PA) in the mediastinal space (occupying rate = AOmax/D or PA/D).10 We measure these values in the MDCT slices where a large vessel is directly adherent to the sternum. An enlarged/pathologic aorta may displace an attached stent venous graft towards the sternum.
Aortic lumen:
Irregularities of the aortic lumen, such as an acute or chronic dissection flap or an irregular thrombus associated with ulceration, must be promptly reported14 (Figures 6–8).
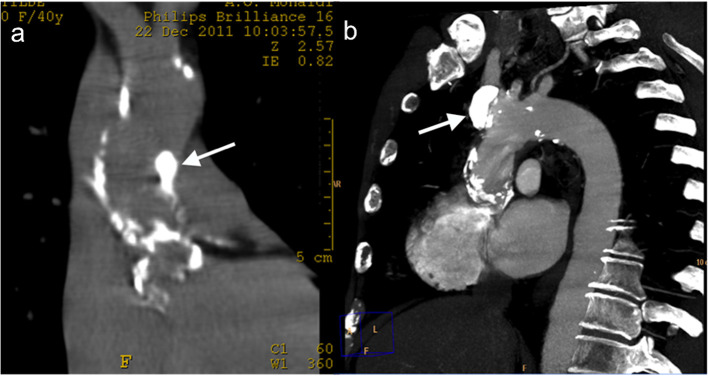
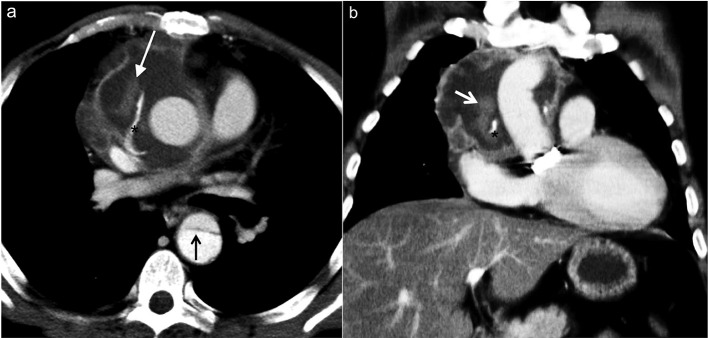
Figure 6a–b.
A 71-year-old male who presented with acute chest pain 4 months after undergoing composite graft repair for ascending aortic aneurysm. Unenhanced axial MDCT images (a, b) show displacement of calcifications (white arrow) extending to a dissected lusory artery (black arrow). MDCT, multidetector CT.
Figure 7a–b.
A 56-year-old Marfan patient contrast-enhanced MDCT after Bentall procedure for A type aortic dissection. Axial (a) an coronal (b) MIP reconstructions show hyperdense dislodged surgical material (asterisk) and a contrast medium leakage (white arrow) surrounded by a organized serosanguineous collection; note dissection flap in descending aorta (black arrow). MDCT, multidetector CT; MIP, maximum intensity projection.
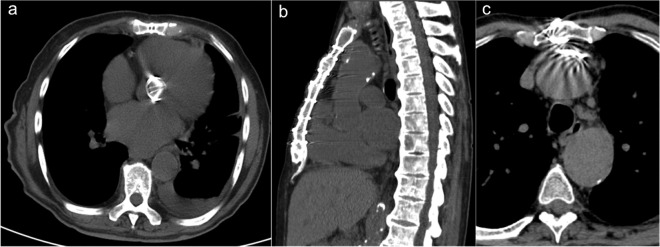
Figure 8a–b.
Assessment of a 53-year-old male with a history of CABG and subsequent development of a dissecting aneurysm of the ascending aorta who presented to the emergency department with chest pain. Axial (a) and sagittal (b) MIP reconstructions show a Stanford type A chronic slow-growing dissecting aneurysm with aortic valve insufficiency and high mediastinal occupying rate. Note the flat and fixed appearance of the fibrotic immobile and thickened flap (black arrow) characteristic of a chronic dissection and the site of the venous graft in the ascending aorta (white arrow). The patient underwent to resternotomy for button Bentall procedure. CABG, coronary artery bypass grafting; MIP, maximumintensity projection.
Aortic valve
When it is not possible to intervene with transaortic valve implantation procedure, pre-re-operative MDCT is used for assessment of aortic valve disease needing prosthetic replacement. In addition to the usual considerations of retrosternal anatomy it is useful to describe the extent and localization of valvular and perivalvular calcifications, to assess valve annulus size, sinuses and sinotubular junction, describe the morphology of the valve (bicuspid vs tricuspid), identify vegetations and highlight the relationship of coronary ostia within sinuses.18
Periaortic tissue:
Small to moderate amounts of low-attenuation perigraft material can be frequently observed during the first months following aortic graft replacement. This material probably represents a combination of seroma, liquefying chronic hematoma, fibrosis and granulation tissue. These collections do not enhance with contrast administration and tend to resolve slowly over many months or years, although some may persist.20 Chronic perigraft collections can serve as sites of secondary infection, particularly in patients with bacteraemia.
MDCT findings of an infected collection include contrast enhancement, pockets of air, increasing size of the collection, and fistulous connections to adjacent structures or extension into other compartments. Secondary findings also may include the adjacent fat and soft tissues stranding (Figures 7 and 9).
Figure 9a–d.
Infection and graft anastomotic dehiscence in a 70-year-old male who had undergone replacement of the ascending aorta with biological valve for acute type A aortic dissection 7 months earlier. A 2 months follow-up unenhanced axial CT scan examination was performed in another institution to evaluate for the aetiology of night sweats, mild chills, and fever. (a, b) Unenhanced MDCT axial scans show abnormal low-attenuation perigraft material and multiple gas bubbles (white arrows) near the graft anastomosis (black arrow); these findings suggesting purulent fluid due to graft infection were misinterpreted as a normal postoperative pattern. After other 2 months this patient came to our observation for fever and worsening dyspnoea and was performed an emergent MDCT. (c) Axial and (d) sagittal coronal MIP reconstructions show active contrast material extravasation (black arrowhead) arising from proximal graft anastomotic dehiscence. MDCT, multidetector CT; MIP, maximum intensity projection.
Perigraft air locules can persist for up to 6 weeks without reported complications, but new, persistent (>6 weeks), or increasing volume of air near the graft is suspicious for development of a fistula with an adjacent bronchus or oesophagus or an infection with a gas-producing organism.
Aortic branches:
It is advisable to comment on plaques and stenosis in the great arteries and assess the femoral and right axillary arteries as they can be used as an alternative route for CBP cannulation. Morphology of the subclavian artery particularly the left is important as it supplies the left internal mammary artery (LIMA) that will be grafted to left anterior descending (LAD).10–14
6) Assessment of RV position in relation to the xiphoid process (in cases of planned LIMA to LAD bypass).
It is important to know that in cases where at least half of the RV is located inferior to the xiphoid process, the left ventricle will be proportionally more inferior than usual; this information may help to decide the route of surgical entry (sternotomy vs thoracotomy and conventional thoracotomy at fourth intercostal space vs thoracotomy at fifth or sixth).
7) Identification of graft location and patency as well as inherent sternal distance can be assessed by use of contrast medium bolus injection. MDCT enables spatial localization of patent grafts and their closeness to the sternum, which was proved to be accurate and reliable at surgery. Although revascularization with the LIMA graft has significantly improved the patient care, almost all patients underwent LIMA grafting are “graft dependent” and LIMA injury can be devastating in redo surgery. The importance of LIMA graft preservation has been the subject of an extensive literature on protection of LIMA grafts during cardiac reoperations. In the large context of patients undergoing reoperative cardiac surgery, injury to the LIMA graft occurred in 5.3% of patients, with a peri-operative infarction rate of 50%.20–22 The authors used chest radiography and cardiac catheterization to localize LIMA grafts that were close to or adherent to the sternum. Unfortunately, these imaging technologies provide insufficient information for avoiding injury to these important grafts upon sternal re-entry. Knowledge of the exact distance between the sternotomy midline and the adherence point of the patent graft enabled safe separation of the conduit from the sternum, allowing control during cross-clamping of the aorta and positioning of the sternal retractor.
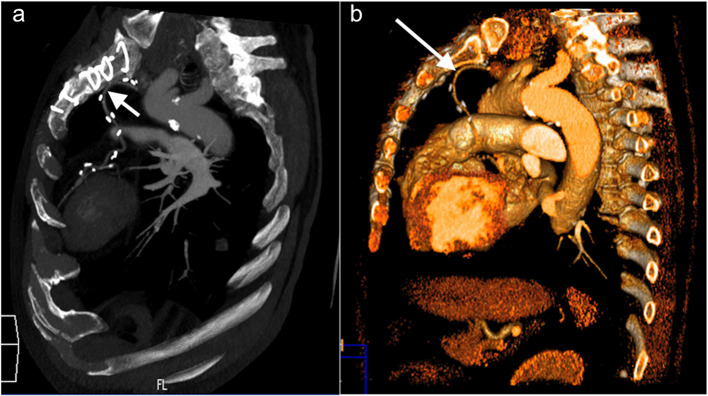
The increase of the long-term survival of patients revascularized with the use of left IMA has resulted in an improved surgical use of the right IMA.5 An in situ right-IMA must necessarily cross the median line in its path to reach the branches of the left coronary system (consisting of the anterior descending artery or the marginal branches of the circumflex artery). This course of right-IMA through the median line increases the risk of injury to this conduct during the resternotomy (Figure 10).
Figure 10a–b.
Pre-operative assessment of a 64-year-old male who had undergone a previous CABG, before repeat CABG and AVR. (a) Sagittal oblique MIP reconstructions and (b) 3D VR sagittal reconstruction shows that the left ITA is adherent to the sternum at the midline behind the manubrium, adjacent to the highest sternal suture (arrow); further down it continues with a torturous way toward its anastomosis with the LAD artery. ITA, internal thoracic artery; CABG, coronary artery bypassgrafting; LAD, left anterior descending;MIP, maximum intensity projection.
CT imaging also demonstrates the crucial relationships between saphenous venous grafts (SVG) and the midline structures. SVGs can be damaged during median sternotomy if they are located anteriorly to the right ventricle during the first surgery, as well as in patients with a dilated ascending aorta, or dilatation of the old site of proximal SVG anastomosis, without to forget a right ventricle enlarged. The manipulation of a diseased graft can also cause myocardial damage secondary to a sequence of emboli in the distal circulation. For the reasons described, a precise preoperative localization of the damaged venous grafts can help prevent a fearful damage of the myocardium.21–23
Compound 3D volume-rendered images, representing rotating anatomic structures with computer software can better delineate relationships between the sternum, ribs, and bypass grafts to help minimize the risk of injury to the graft vessel during surgical re-entry and to obtain satisfactory myocardial preservation.24,25
8) Diaphragm insertion
The diaphragm may have a high insertion at the xiphoid process level, therefore during full length median sternotomy the surgeon will enter the abdominal cavity of this normal variant 18–20 (Figure 11).
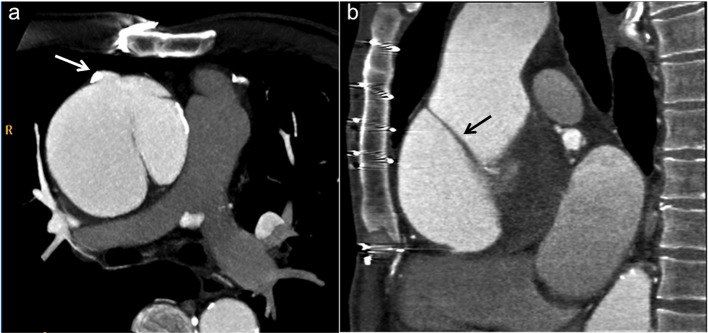
Figure 11.
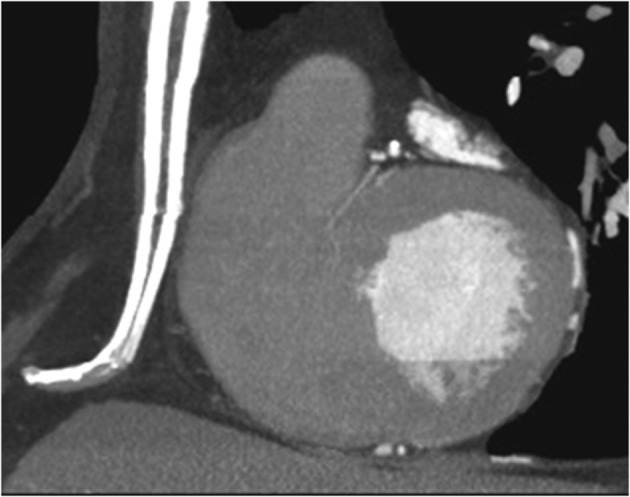
MIP sagittal reconstruction shows that, due to high insertion of the diaphragm, the sternotomy should spare the distal 2–3 cm of the sternum. MIP, maximum intensity projection.
9) Pericardium
The pericardium is visualized in 95 to –98% by CT and is best seen along the anterior aspect of the ventricular surface of the heart, where epicardial and pericardial fat is most abundant. Complete closure of the native pericardium after cardiac surgery would allow a plane of cleavage between the heart and the sternum, avoiding injuries to the heart and large vessels. Unfortunately, the primary closure is often not practicable, therefore different materials have been suggested to be used as substitutes of the pericardium to significantly limit the formation of adhesions.
Findings that should be reported are visualisation of a closed pericardium, adherence/proximity (<1 cm) of adjacent pericardium to the chest wall and pericardial thickening. Pericardial calcification usually occurs on its visceral layer and is predominantly over the less pulsatile right atrium and RV borders, atrioventricular grooves and pulmonary trunk. The left atrium has a small bare area where the pulmonary veins enter, therefore calcification of pericardium does not occur at this site. Pericardial calcification is also unusual at the cardiac apex due to vigorous contraction of the left ventricle. Approximately, 50% of patients with constrictive pericarditis demonstrate segments of pericardial calcification. All cardiac surgery requires pericardiotomy; inflammation or infection of the pericardium will cause adhesions and will pose a challenge to the procedure. Pericardial calcification, thickening more than physiological pericardial effusion, pericardial adhesion to the right ventricle in redo cases and signs of constrictive pericarditis (often well detected by echocardiography) should be reported.11–23
10) Prior pleural inflammation
Signs of prior pleural inflammation, especially near the LIMA must be highlighted because in order to harvest the LIMA the surgeon often has to enter the pleural space (passing through the parietal layer for better mobility).
It is also essential to report on important findings which may not be directly related to re-sternotomy. Among them we include: reflux of contrast into hepatic veins consistent with constrictive physiology; intracardiac thrombus which requires management prior to surgery; non-cardiovascular findings such as emphysema and fibrosis that increase operative risk, pneumonia, any focus of infection with increased risk of mediastinitis, lung cancer, retrosternal thyroid and mediastinal lymphadenopathy.14–20
Our experience, surgical strategies and role of MDCT imaging
In our population of 274 patients, MDCT findings related to the potential risks of RS were reported in 123 cases (45%). One or more high-risk CT findings were observed in 84 patients (30.6 %).
All images were commented on with the attending surgeon and in atypical cases, also discussed with the anaesthesiologist, in order to evaluate the operative risk to plan a tailored surgical strategy.
Table 1 summarises the main MDCT findings in our study.
Table 1. .
Association between high-risk findings on cardiothoracic MDCT report and preventive surgical strategies (total number of patients undergoing RS = 274)
| Preventive tailored surgical strategy (patients number and percentages) |
Present (n = 123) |
Patients (n = 84) |
| Nonmidline approach 25 (9.1 %) | 67 | 42 (15.3%) |
| Peripheral CPB 13 (4.8%) | 33 | 23 (8.4%) |
| Alternative cannulation and clamping sites 6 (2.2%) | 23 | 19 (6.9%) |
| Deep hypothermic circulatory arrest 7 (2.5%) | ||
| Cancellation of surgery 6 (2.2%) | ||
| All preventive strategies combined 57 (20.8%) | 84 (30.6%) |
MDCT, multidetector CT; RS, resternotomy.
Table 2 summarises the ‘philosophical mainstems’ of a tailored operative approach.
Table 2. .
MDCT main findings prior RS (number of times indicated in radiological reports) in 274 consecutive patients who underwent resternotomy
| Risk of damage | Critical contiguity | |
| Sternal re-entry findings | ||
| Sternal/ascending aorta distance | 16 | 9 |
| Sternal/right ventricle distance | 23 | 15 |
| Innominate vein/sternum distance | 16 | 10 |
| Patent graft | 14 | 8 |
| RV position in relation to the xiphoid | 3 | |
| High diaphragm insertion | 5 | |
| Large aortic aneurysm/pseudoaneurysm | 9 | |
| Infection and graft anastomotic dehiscence | 5 | |
| Constrictive pericarditis | 6 | |
| Cannulation site findings | ||
| Sclerotic wall | 12 | |
| Aorta/PA occupying rate too high | 14 | |
| Total c ardiovascular MDCT findings | 123 | |
| Non- c ardiovascular f indings | 21 |
CPB, cardiopulmonary;MDCT, multidetector CT; PA,pulmonary artery;RS, resternotomy; RV, right ventricle.
The adoption of preventive surgical strategies was used in 57 patients (20,8%). Six patients (2.2%) were excluded from the surgical approach because of prohibitive surgical risk and/or relevant comorbidities. In 42 (15.3%) patients MDCT found critical contiguity between the sternum and the vascular structures and in 25cases the operational strategy was changed.
No major bleeding occurred in the series and planned arterial cannulation was performed in all cases. In three cases, an antegrade dissection developed as a result of cannulation: in one case because of a technical error and in two due to underestimation of target peripheral vessel disease. In six cases, offpump myocardial revascularization was accomplished given the favourable coronary angiography pattern and the ease of surgical exposure. Planned aortic clamping site was feasible in all cases except six, in which an unplanned deep hypothermic circulatory arrest approach was required due to distal extension of aortic pathology. During surgery, all the other MDCT findings were confirmed. Our overall injury rate was low (1.3%) and rate of permanent neurologic deficit fell short of 1%. Operative mortality rate was under 2%. As already mentioned, this historical period is characterized by a progressive increase in the number of patients who need to undergo re-sternotomy for both congenital and acquired diseases.
There are different techniques that the surgeon can use according to the different cases. Conventional (“on pump”) technique, used for all types of cardiovascular surgery, includes median sternotomy, cardiopulmonary bypass and cardioplegia, allowing full access to the heart, combined surgery (bypass ± ascending aorta ± valves) and a blood free operating field. However, a large incision is required with an increased risk of complications and subsequent large post-operative scar. CPB needs at least a 4–5 cm length of ascending aorta free of calcification at the anterior and right lateral walls to allows placement of two cannulae, clamping device, cardioplegia line and 2–3 grafts for coronary bypass. The cross-clamping area must be free of calcification circumferentially and when the aorta is too calcified, CPB is performed at right axillary or common femoral vessels.26
Offpump coronary artery bypass technique is used only for CABG, involving a smaller median sternotomy, with no cardiopulmonary bypass or cardioplegia. It allows full access to the heart, avoids complications from CPB, enables surgery in those patients not able to tolerate CPB, as well as providing a faster recovery and shorter hospital stay. However, it is a more challenging technique, requiring greater displacement of the beating heart to allow full access via a smaller incision than is needed for on pump technique, but larger than that used in minimally invasive direct coronary artery bypass (MIDCAB) surgery.
Minimally invasive direct coronary artery bypass, used for LIMA to LAD bypass only, involves a left thoracotomy incision (12 cm) at the fourth intercostal space (more rarely fifth or sixth intercostal space if the heart is more caudal) to isolate the LIMA and allow access to LAD at the anterior surface of the heart for a single bypass procedure, without sternotomy, cardiopulmonary bypass or cardioplegia. It allows surgery in patients not tolerating CBP a much faster recovery and reduced hospital stay, but it is a more challenging technique due to restricted views and surgical mobility.
Early studies in RS have documented a 1–8% incidence of catastrophic haemorrhage, defined as exsanguination resulting in haemodynamic deterioration requiring emergency intervention, such as blood transfusion or rapid institution of CPB. These result in fatalities in over one-third of reported cases and are attributed to major injuries or minor lacerations to cardiac structures, grafts, and conduits.27–31 The most common structures reported to be injured during re-entry by frequency are right ventricle, venous graft, aorta, internal mammary graft and innominate vein.32
To support and improve the surgical procedure, the radiologist requires complex pre-operative evaluations, such as the analysis of the thoracic aorta, possible coronary grafts and anatomical relationships with normal cardiac structures. Also thanks to these elaborations the surgical results have improved, particularly in high-volume centers.13,26,33–37
It is common knowledge that incomplete pre-operative imaging and suboptimal utilization of alternative surgical techniques will limit preventative strategy in redo cardiac surgery. Compensatory rescue measures are not always successful; despite careful surgical planning, high mortality risk during RS correlates with increasing sternotomy number, previous radiotherapy, previous stroke and severe renal insufficiency.20 Adverse events lead to poor patient outcome and higher cost.26,30
The preventative strategies generally employed by the cardiac surgeon are different and applicable to individual cases: (1) technologies to improve visualization during sternal dissection, (2) specialized instrumentation or techniques to optimize sternal re-entry or dissection, (3) measures to prevent or avoid adhesions, 4) customized extracorporeal circulation methods.2,3,27,28,37
Three main aspects of the operative plans may be modified according to MDCT findings: route of surgical entry, cannulation site, initiation of peripheral CPB before incision and myocardial preservation technique. In our experience, multidisciplinary discussion on MDCT findings largely influenced the surgical indication and strategy. Extensive pre-operative knowledge of surgical anatomy optimized the adoption of preventative measures as seen in Table 1.
Although comparison with a pre MDCT historical series was not feasible, it is self evident from the operative case mix (Table 2) and inherent outcomes that this approach effectively impacts patient prognosis.38–41
In addition to encouraging data regarding the use of cine magnetic resonance, it would be interesting to read about other experiences regarding the use of four-dimensional CT in the assessment of patients with post-cardiac surgery retrosternal adhesions.40
Widespread adoption of innovative minimally invasive (MI) manoeuvres and instrumentation, such as the robotic-enhanced endoscopic system may further improve outcomes.2,34–37
Conclusion
Repeat median sternotomy remains a major challenge to cardiac surgeons. MDCT technique is useful in defining an optimal surgical strategy in patients who need to undergo re-operative cardiac surgery. Pre-operative imaging of coronary grafts and the other important mediastinal structures reduces the morbidity of the re-operation through change of surgical approach and has a significant impact on patient care. When radiologists use 3D techniques in planning, what lies beneath the sternum should never be a mystery. In order to configure an accurate pre-operative plan for RS, radiologist should produce a comprehensive ad exhaustive radiological report. Moreover, during the pre-surgical phase, a joint analysis of the images between the referring radiologist and the surgical team would be highly desirable in order to resolve doubts and at the same time grant any further specialist requests that may be useful or even fundamental to the cardiac. This multi disciplinary approach results in a noticeable decrease in operating risk and significant improvement of patient outcome.
Contributor Information
Tullio Valente, Email: tullio.valente@gmail.com.
Giorgio Bocchini, Email: giorgio.bocchini@gmail.com.
Giovanni Rossi, Email: giovanni.rossi@gmail.com.
Giacomo Sica, Email: sicagf@libero.it.
Hannah Davison, Email: Hannah.davison@chsft.nhs.uk.
Mariano Scaglione, Email: scaglionefun@gmail.com.
REFERENCES
- 1. Morales DLS, Zafar F, Arrington KA, Gonzalez SM, McKenzie ED, Heinle JS, et al. Repeat sternotomy in congenital heart surgery: no longer a risk factor. Ann Thorac Surg 2008; 86: 897–902. doi: 10.1016/j.athoracsur.2008.04.044 [DOI] [PubMed] [Google Scholar]
- 2. Morales D, Williams E, John R. Is resternotomy in cardiac surgery still a problem?☆. Interactive CardioVascular and Thoracic Surgery 2010; 11: 277–86. doi: 10.1510/icvts.2009.232090 [DOI] [PubMed] [Google Scholar]
- 3. American Heart Association Heart disease and stroke statistics 2009 update. A report from the American Heart Association statistics committee and stroke statistics Committee. Circulation 2009; 119: e21–181. [DOI] [PubMed] [Google Scholar]
- 4. Cremer J, Teebken OE, Simon A, Hutzelmann A, Heller M, Haverich A, et al. Thoracic computed tomography prior to redo coronary surgery. Eur J Cardiothorac Surg 1998; 13: 650–4. doi: 10.1016/S1010-7940(98)00087-6 [DOI] [PubMed] [Google Scholar]
- 5. Gilkeson RC, Markowitz AH, Ciancibello L. Multisection CT evaluation of the reoperative cardiac surgery patient. RadioGraphics 2003; 23(suppl_1): S3–S17. doi: 10.1148/rg.23si035505 [DOI] [PubMed] [Google Scholar]
- 6. Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O'Gara P, JMcB H, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. Journal of the American College of Cardiology 2010; 56: 1864–94. doi: 10.1016/j.jacc.2010.07.005 [DOI] [PubMed] [Google Scholar]
- 7. di Cesare E, Carbone I, Carriero A, Centonze M, De Cobelli F, De Rosa R, et al. Clinical indications for cardiac computed tomography. from the Working Group of the cardiac radiology section of the Italian Society of medical radiology (SIRM. Radiol med 2012; 117: 901–38. doi: 10.1007/s11547-012-0814-x [DOI] [PubMed] [Google Scholar]
- 8. Luciani N, Nasso G, Piscitelli M, Possati G, Anselmi A, et al. Computed tomography scan in Redo valvular surgery. The Annals of Thoracic Surgery 2005; 80: 2422–3. doi: 10.1016/j.athoracsur.2004.11.007 [DOI] [PubMed] [Google Scholar]
- 9. AE L, Fishman EK. Evaluations of complications after sternotomy using single- and multidetector CT with three-dimensional volume rendering. AJR 2003; 181: 1065–70. [DOI] [PubMed] [Google Scholar]
- 10. Restrepo CS, Martinez S, Lemos DF, Washington L, McAdams HP, Vargas D, et al. Imaging appearances of the sternum and sternoclavicular joints. RadioGraphics 2009; 29: 839–59. doi: 10.1148/rg.293055136 [DOI] [PubMed] [Google Scholar]
- 11. Lahtinen J, Satta J, Lähde S, Suramo I, Nissinen J, Pokela R, et al. Computed tomographic evaluation of retrosternal adhesions after pericardial substitution. The Annals of Thoracic Surgery 1998; 66: 1264–8. doi: 10.1016/S0003-4975(98)00588-8 [DOI] [PubMed] [Google Scholar]
- 12. Aviram G, Mohr R, Sharony R, et al. Open heart reoperations after coronary artery bypass grafting: the role of preoperative imaging with multidetector computed tomography. Israel Med Assoc J 2009; 11: 465–9. [PubMed] [Google Scholar]
- 13. Sabik JF, Blackstone EH, Houghtaling PL, Walts PA, Lytle BW, et al. Is reoperation still a risk factor in coronary artery bypass surgery? The Annals of Thoracic Surgery 2005; 80: 1719–27. doi: 10.1016/j.athoracsur.2005.04.033 [DOI] [PubMed] [Google Scholar]
- 14. Mizuno T, Toyama M, Tabuchi N, Kuriu K, Ozaki S, Kawase I, et al. Thickened intima of the aortic arch is a risk factor for stroke with coronary artery bypass grafting. The Annals of Thoracic Surgery 2000; 70: 1565–70. doi: 10.1016/S0003-4975(00)01925-1 [DOI] [PubMed] [Google Scholar]
- 15. Shann KG, Likosky DS, Murkin JM, Baker RA, Baribeau YR, DeFoe GR, et al. An evidence-based review of the practice of cardiopulmonary bypass in adults: a focus on neurologic injury, glycemic control, hemodilution, and the inflammatory response. The Journal of Thoracic and Cardiovascular Surgery 2006; 132: 283–90. doi: 10.1016/j.jtcvs.2006.03.027 [DOI] [PubMed] [Google Scholar]
- 16. van der Linden J, Hadjinikolaou L, Bergman P, Lindblom D, et al. Postoperative stroke in cardiac surgery is related to the location and extent of atherosclerotic disease in the ascending aorta. Journal of the American College of Cardiology 2001; 38: 131–5. doi: 10.1016/S0735-1097(01)01328-6 [DOI] [PubMed] [Google Scholar]
- 17. LaPar DJ, Ailawadi G, Irvine JN, Lau CL, Kron IL, Kern JA, et al. Preoperative computed tomography is associated with lower risk of perioperative stroke in reoperative cardiac surgery. Interactive CardioVascular and Thoracic Surgery 2011; 12: 919–23. doi: 10.1510/icvts.2010.265165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abu Saleh WK, Goswami R, Chinnadurai P, et al. Direct aortic access transcatheter aortic valve replacement: three-dimensional computed tomography planning and real-time fluoroscopic image guidance. J Heart Valve Dis 2015; 4: 420–5. [PubMed] [Google Scholar]
- 19. Yamauchi T, Miyamoto Y, Ichikawa H, Takano H, Sawa Y, et al. Large vessel — sternum adhesion after cardiac surgery; a risk-factor analysis. Surg Today 2006; 36: 596–601. doi: 10.1007/s00595-006-3187-8 [DOI] [PubMed] [Google Scholar]
- 20. Sundaram B, Quint LE, Patel HJ, Deeb GM, et al. CT findings following thoracic aortic surgery. RadioGraphics 2007; 27: 1583–94. doi: 10.1148/rg.276075004 [DOI] [PubMed] [Google Scholar]
- 21. Gillinov AM, Casselman FP, Lytle BW, Blackstone EH, Parsons EM, Loop FD, et al. Injury to a patent left internal thoracic artery graft at coronary reoperation. The Annals of Thoracic Surgery 1999; 67: 382–6. doi: 10.1016/S0003-4975(99)00009-0 [DOI] [PubMed] [Google Scholar]
- 22. Follis FM, Pett SB, Miller KB, Wong RS, Temes RT, Wernly JA, et al. Catastrophic hemorrhage on sternal reentry: still a dreaded complication? The Annals of Thoracic Surgery 1999; 68: 2215–9. doi: 10.1016/S0003-4975(99)01173-X [DOI] [PubMed] [Google Scholar]
- 23. Khan NU, Yonan N. Does preoperative computed tomography reduce the risks associated with re-do cardiac surgery? Interactive CardioVascular and Thoracic Surgery 2009; 9: 119–23. doi: 10.1510/icvts.2008.189506 [DOI] [PubMed] [Google Scholar]
- 24. Gasparovic H, Rybicki FJ, Millstine J, Unic D, Byrne JG, Yucel K, et al. Three dimensional computed tomographic imaging in planning the surgical approach for redo cardiac surgery after coronary revascularization. European Journal of Cardio-Thoracic Surgery 2005; 28: 244–9. doi: 10.1016/j.ejcts.2005.03.024 [DOI] [PubMed] [Google Scholar]
- 25. Park K-H, Lee HY, Lim C, Chung ES, Sung S-W, Choi SI, et al. Clinical impact of computerised tomographic angiography performed for preoperative evaluation before coronary artery bypass grafting. European Journal of Cardio-Thoracic Surgery 2010; 37: 1346–52. doi: 10.1016/j.ejcts.2009.12.040 [DOI] [PubMed] [Google Scholar]
- 26. Folliguet T, Vanhuyse F, Constantino X, REALLI M, LABORDE F, et al. Mitral valve repair robotic versus sternotomy☆. European Journal of Cardio-Thoracic Surgery 2006; 29: 362–6. doi: 10.1016/j.ejcts.2005.12.004 [DOI] [PubMed] [Google Scholar]
- 27. Elahi M, Dhannapuneni R, Firmin R, Hickey M, et al. Direct complications of repeat median sternotomy in adults. Asian Cardiovasc Thorac Ann 2005; 13: 135–8. doi: 10.1177/021849230501300208 [DOI] [PubMed] [Google Scholar]
- 28. Park CB, Suri RM, Burkhart HM, Greason KL, Dearani JA, Schaff HV, et al. Identifying patients at particular risk of injury during repeat sternotomy: analysis of 2555 cardiac reoperations. The Journal of Thoracic and Cardiovascular Surgery 2010; 140: 1028–35. doi: 10.1016/j.jtcvs.2010.07.086 [DOI] [PubMed] [Google Scholar]
- 29. Dobell ARC, Jain AK. Catastrophic hemorrhage during Redo sternotomy. The Annals of Thoracic Surgery 1984; 37: 273–8. doi: 10.1016/S0003-4975(10)60728-X [DOI] [PubMed] [Google Scholar]
- 30. Roselli EE, Pettersson GB, Blackstone EH, Brizzio ME, Houghtaling PL, Hauck R, et al. Adverse events during reoperative cardiac surgery: frequency, characterization, and rescue. The Journal of Thoracic and Cardiovascular Surgery 2008; 135: 316–23. doi: 10.1016/j.jtcvs.2007.08.060 [DOI] [PubMed] [Google Scholar]
- 31. Gillinov AM, Casselman FP, Lytle BW, Blackstone EH, Parsons EM, Loop FD, et al. Injury to a patent left internal thoracic artery graft at coronary reoperation. The Annals of Thoracic Surgery 1999; 67: 382–6. doi: 10.1016/S0003-4975(99)00009-0 [DOI] [PubMed] [Google Scholar]
- 32. Ghanta RK, Kaneko T, Gammie JS, Sheng S, Aranki SF, et al. Evolving trends of reoperative coronary artery bypass grafting: An analysis of the Society of Thoracic Surgeons Adult Cardiac Surgery Database. The Journal of Thoracic and Cardiovascular Surgery 2013; 145: 364–72. doi: 10.1016/j.jtcvs.2012.10.051 [DOI] [PubMed] [Google Scholar]
- 33. Launcelott S, Ouzounian M, Buth KJ, Légaré J-F, et al. Predicting in-hospital mortality after Redo cardiac operations: development of a preoperative Scorecard. The Annals of Thoracic Surgery 2012; 94: 778–84. doi: 10.1016/j.athoracsur.2012.04.062 [DOI] [PubMed] [Google Scholar]
- 34. Modi P, Rodriguez E, Chitwood WR. Robot-assisted cardiac surgery. Interactive CardioVascular and Thoracic Surgery 2009; 9: 500–5. doi: 10.1510/icvts.2009.203182 [DOI] [PubMed] [Google Scholar]
- 35. Morishita K, Kawaharada N, Fukada J, Yamada A, Masaru T, Kuwaki K, et al. Three or more median sternotomies for patients with valve disease: role of computed tomography. The Annals of Thoracic Surgery 2003; 75: 1476–80. doi: 10.1016/S0003-4975(02)04821-X [DOI] [PubMed] [Google Scholar]
- 36. Roselli EE, Surgery RC. Challenges and outcomes. Tex Heart Inst J 2011; 38: 669–71. [PMC free article] [PubMed] [Google Scholar]
- 37. Holst KA, Dearani JA, Burkhart HM, Connolly HM, Warnes CA, Li Z, et al. Risk factors and early outcomes of multiple reoperations in adults with congenital heart disease. The Annals of Thoracic Surgery 2011; 92: 122–30. doi: 10.1016/j.athoracsur.2011.03.102 [DOI] [PubMed] [Google Scholar]
- 38. Blackstone EH. Thinking beyond the risk factors☆. European Journal of Cardio-Thoracic Surgery 2006; 29: 645–52. doi: 10.1016/j.ejcts.2006.02.008 [DOI] [PubMed] [Google Scholar]
- 39. Imran Hamid U, Digney R, Soo L, Leung S, Graham ANJ, et al. Incidence and outcome of re-entry injury in redo cardiac surgery: benefits of preoperative planning. Eur J Cardiothorac Surg 2015; 47: 819–23. doi: 10.1093/ejcts/ezu261 [DOI] [PubMed] [Google Scholar]
- 40. Yoshioka I, Saiki Y, Ichinose A, Takase K, Takahashi S, Ohashi T, et al. Tagged cine magnetic resonance imaging with a finite element model can predict the severity of retrosternal adhesions prior to redo cardiac surgery. The Journal of Thoracic and Cardiovascular Surgery 2009; 137: 957–62. doi: 10.1016/j.jtcvs.2008.10.036 [DOI] [PubMed] [Google Scholar]
- 41. Kirmani BH, Brazier A, Sriskandarajah S, Azzam R, Keenan DJ, et al. A meta-analysis of computerized tomography scan for reducing complications following repeat sternotomy for cardiac surgery. Interact CardioVasc Thorac Surg 2016; 22: 472–9. doi: 10.1093/icvts/ivv367 [DOI] [PubMed] [Google Scholar]