Abstract
The number of people living with dementia is increasing, but as yet there remains no cure or disease-modifying treatment. This review aims to help readers understand the role of 18F-FDG PET/CT imaging in the investigation of cognitive impairment and how the advent of amyloid PET/CT imaging may hold the key to radically changing management of the most common form of dementia - Alzheimer’s disease.
The indications for 18F-FDG PET/CT and amyloid PET/CT imaging in cognitive impairment are outlined. Additionally, the mechanisms of action, technique, patient preparation and acquisition parameters for both are detailed. We conclude by providing a framework for interpreting 18F-FDG PET/CT and amyloid PET/CT imaging in the more common conditions that lead to cognitive impairment conditions with tips on avoiding pitfalls in interpretation.
Introduction
Dementia is a broad term and includes a variety of symptoms, the common thread being progressive cognitive decline. A number of pathologies cause dementia with Alzheimer’s disease (AD) being by far the most common subtype. One of the most frightening notions about AD is there is currently no cure and drugs to manage the disease have yet to make a real impact in terms of halting or even reversing the disease process. The number of people living with dementia is increasing. It costs the NHS £4.3 billion/year1 and its diagnosis has a life-altering impact on families and sufferers. Given its physiological, psychological and financial significance, we believe that a firm diagnosis of the underlying disease process is essential. This, however, can take years to be reached, if at all. In the United Kingdom (UK), it takes almost 3 years from when the carer first notices symptoms to the patient receiving a diagnosis of AD.2 Up to 40% of people not diagnosed with AD have evidence of it upon autopsy.3 The ability to provide the surest diagnosis antemortem is therefore crucial to aid management in terms of medications, advice, prognosis and implications for other family members.
The pathophysiology of AD is complex, but two key features include the accumulation of extracellular amyloid β (Aß) plaques and intracellular neurofibrillary tau protein tangles. These processes pre-date the onset of clinical symptoms; cognitive decline can be gradual taking years or even decades to occur and clinical presentation can be atypical, especially in younger individuals.4 Patients with early-onset disease and/or atypical/mild features are particularly vulnerable to misdiagnosis and there can be clinical overlap with other disease processes.
It can be difficult to make a firm diagnosis of AD as it requires accurate clinical assessment and evidence of pathology.3,5 Within the literature, current diagnostic criteria show a specificity of just over 80% with sensitivity closer to 60% when used by expert clinicians.3 Biomarkers have paved the way by providing surrogate information on the in vivo state of the tissue thereby allowing more confident diagnosis.6,7 Imaging is a major part of this new paradigm and advanced techniques enable cellular-level abnormalities to be visualised in the clinical setting.
Why use pet ct
Dementia is a broad term and in this broad sense, clinically not difficult to diagnose. The complexity arises when trying to determine the underlying aetiology. Knowing what pathology we are trying to manage allows for the best treatment choices to be made. Furthermore, knowing the specific subtype allows for exclusion of other factors such as medications, work environment, mood and anxiety - all of which may be modifiable.
Functional imaging, for example positron emission tomography combined with low-dose CT (PET/CT), can help to achieve an accurate diagnosis much earlier in the clinical course of the disease compared to conventional structural sequences, as functional abnormalities precede the structural changes (Figure 1). It is thought that beta-amyloid irregularities underpinning the pathology can precede clinical symptomatology by up to 20 years.8 Imaging could also help to avoid more invasive investigations such as lumbar puncture for CSF analysis when trying to confirm a diagnosis of AD. This would be of benefit as an LP is invasive and is associated with a number of side-effects including headache and pain as well as bleeding and infection risks. By being more conclusive at an earlier stage, PET imaging could lead to a reduction in the number of additional and serial investigations such as MR and/or CT imaging of the head or neuropsychological testing, the knock-on effect being fewer clinic appointments. Repeated assessment and investigation come with a time and cost premium, so savings could be made by reducing these investigations and therefore shortening the patient pathway from presentation to diagnosis.
Figure 1.
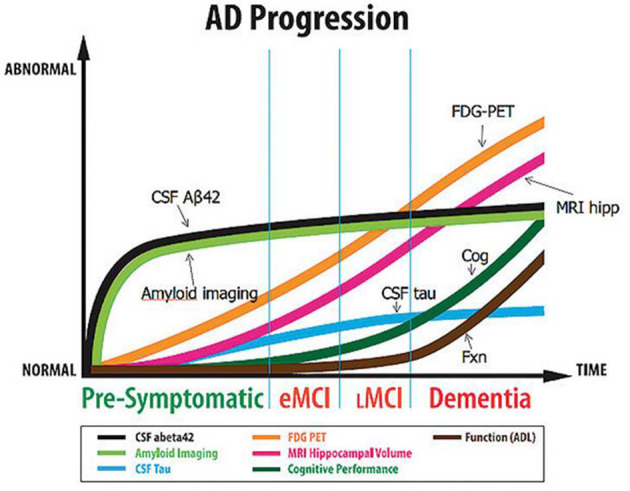
Cerebral amyloid accumulation occurs several years before functional (18F-FDG PET) and structural changes, and may precede symptoms. National Institute of Ageing. Adapted from reference.7
Critically, from a social care perspective, a definitive early diagnosis may help to activate care packages and enable patients to plan for the future. Furthermore, the need to find better treatments and potentially a cure can be aided by PET/CT scanning as it can enable patients with a more accurate diagnosis to be enrolled onto research trials. There are a multitude of Phase I, II and III trials investigating various therapies, but an increasingly common theme is the need for biomarkers to identify patients, quantitatively assess drug safety and efficacy parameters, and provide a repeatable framework for outcomes.6 It is still early days for these treatments, but it is reasonable to imagine that agents are likely to need proof of cerebral amyloid burden before commencement of therapy. It cannot be underestimated how challenging developing new and effective drugs is. A major factor in the timescale for development is the stage at which patients are recruited and the number of recruits. Enrolling patients in the pre-clinical or early stages of a disease process is difficult and typically takes longer than those with mild-moderate disease states.6
When to use 18F-FDG-PET/CT
Knowing when and how to image in dementia allows for maximum benefit. To assist with this, the American College of Radiology and the American Society for Neuroradiology published guidelines in 2016 to help clinicians decide which patients would benefit from functional imaging. In the same year, evidence-based guidelines were published in the UK by the Royal College of Radiologists in conjunction with a number of other specialties as well as the Administration of Radioactive Substance Advisory Committee (ARSAC). It regarded the indications for 18F-FDG-PET/CT for oncological and non-oncological applications with the evaluation of dementia and subtypes in selected patients being included.
18F-FDG PET/CT imaging is a reflection of glucose metabolism within a tissue with decreased activity in regions of neuronal dysfunction and degeneration; it acts as a marker for neuronal loss. At our institution, our dementia specialists find it helpful when looking for an underlying neurodegenerative brain disease in terms of neuronal loss when the MRI head appears normal. The indications for 18F-FDG PET/CT in cognitive decline and dementia would include 1) assessment of progressive dementia; distinguishing between AD and other neurodegenerative conditions by identifying underlying characteristic regional patterns of cerebral hypometabolism, 2) assessment of neurodegeneration in subjects with mild cognitive impairment (MCI) before the onset of clinically diagnosed dementia.5 It is limited by not being disease specific and it cannot reliably differentiate between underlying pathologies on an individual basis in the context of atypical appearances. Furthermore, interrogation of the structural imaging is therefore important as areas of damage from previous insults such as an infarct will also be hypometabolic (Figure 2).
Figure 2.
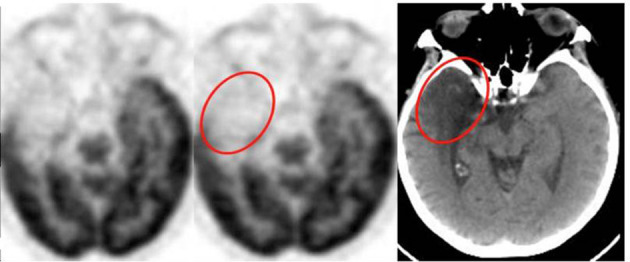
Apparent area of hypometabolism on 18F-FDG PET involving the right temporal lobe that in fact corresponds to an established infarct on the low dose non-enhanced CT component.
Why and when to use amyloid pet ct
Amyloid PET/CT imaging involves tracers that selectively bind to cerebral Aß plaques. A set of specific appropriate use criteria have been developed by the Amyloid Imaging Taskforce who were convened by the Society of Nuclear Medicine and Molecular Imaging and the Alzheimer's Association.9 It is currently most likely to be helpful when 1) the patient has objectively confirmed cognitive impairment (CI) 2) the cause of CI remains uncertain after comprehensive evaluation by a dementia expert 3) the differential diagnosis includes AD dementia and 4) the knowledge of the presence or absence of Aẞ pathology is expected to increase diagnostic certainty or alter patient management.4 Table 1 summarises appropriate and inappropriate indications:
Table 1.
Amyloid PET/CT – appropriate and inappropriate indications
| APPROPRIATE | INAPPROPRIATE |
|
|
|
|
|
|
|
|
|
|
|
|
|
Imaging algorithm
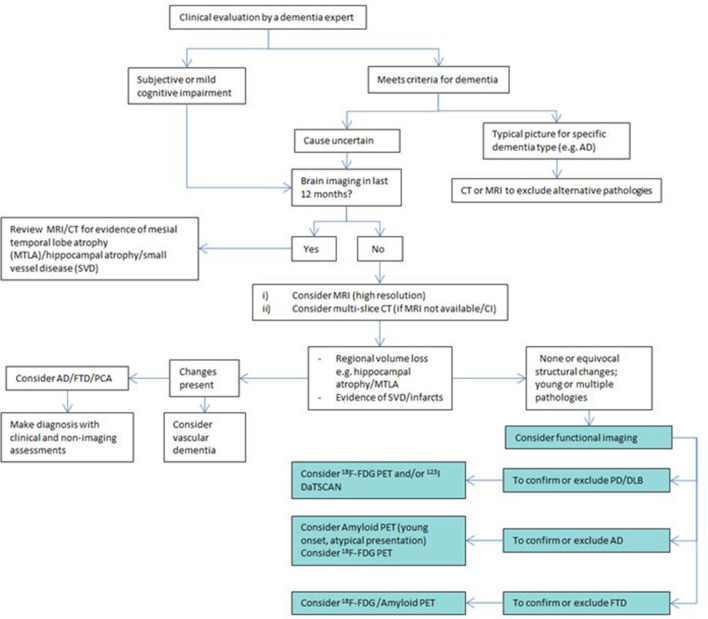
AD is a complex disorder to diagnose. Once initial clinical evaluation has been undertaken by a dementia expert, deciding what type of brain imaging to perform, if any, can be challenging. An algorithm to assess the next imaging steps has been proposed that incorporates amyloid and 18F-FDG PET/CT (Figure 3).
Figure 3.
Proposed imaging algorithm to determine whether PET/CT is useful and which subtype should be used. (AD — Alzheimer's disease, FTD — frontotemporal dementia, PCA —posterior cortical atrophy)
It is worthwhile making mention of the IDEAS study: Imaging Dementia – Evidence for Amyloid Scanning. This prospective, open-label, longitudinal cohort study aims to assess the impact of amyloid PET on short-term patient management as well as look at the impact amyloid imaging has on hospital and emergency room visits in the United States. The previously mentioned appropriate use criteria were the basis for inclusion. Preliminary results were presented at the 11th Clinical Trials on Alzheimer’s Disease conference (CTAD) held in Barcelona in 2018. Although formal results are yet to be published, Rabinovici’s group summarised that amyloid scans had their biggest impact excluding a diagnosis of AD. Treatment plans were altered and additional testing or imaging numbers fell by half as a result.
Patient preparation and acquisition
Once the appropriate imaging technique has been selected, correct preparation and image acquisition is important for accurate results (Table 2).
Table 2.
Mechanism of action, patient preparation and image acquisition.10,11
| 18F-FDG |
Amyloid Tracers
(18F-florbetapir, flutemetamol, florbetaben) |
|
| Mechanism | Glucose analogue. Undergoes neuronal trapping enabling imaging and measurement of cerebral glucose metabolic rate10 | Binds selectively to β-amyloid neuritic plaques. Biomarker of β-amyloid aggregate deposition11 |
| Patient Preparation: |
|
|
|
Activity (mBq/mCi):
Effective Dose (mSv): |
250/7 5 |
185–370/5–10 5.8–7 |
| Uptake Time (mins): | 30 | 30–90 |
| Acquisition Time (mins): | 10 | 10–20 |
Image interpretation
ALZHEIMER’S DISEASE
18F-FDG PET/CT
18F-FDG PET/CT is a reflection of cerebral glucose metabolism, which is typically symmetrical in the brain. It is important to be aware of factors that can affect this physiology; metabolism is known to change with a patient’s age,12 gender, hand dominance, diet, environmental factors, mood, medication and medical history.10
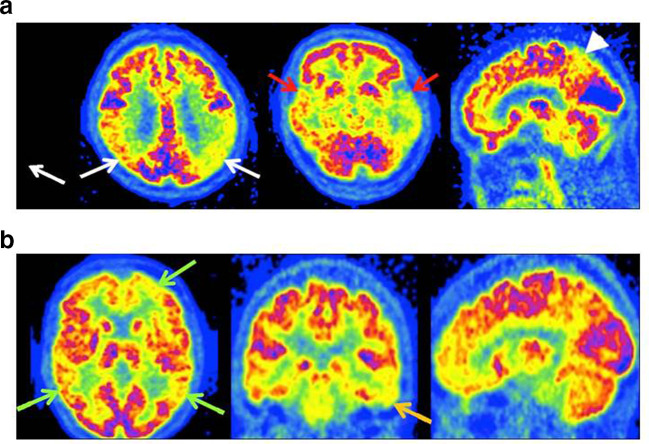
The typical spatial pattern of hypometabolism in AD involves the posterior cingulate gyrus, limbic system, temporal lobes, precuneus and parietal cortex12,13 (Figure 4A). The typical pattern of hypometabolism is bilateral and symmetrical, but can less commonly be asymmetrical. In advanced cases, the prefrontal cortices can be involved, but the anterior cingulate gyrus, precentral gyrus and occipital cortex are typically always spared.12,13 Hypometabolism can have a more atypical pattern (Figure 4B) and in these patients, amyloid PET/CT may be a useful further examination.
Figure 4.
(A) AD - typical appearances of 18F-FDG PET with severe hypometabolism of the parietal (white arrows) and temporal lobes (red arrows) - in this case left >right. There is also hypometabolism in the precuneus and posterior cingulate gyrus (arrowhead). (B) AD - atypical appearances of 18F-FDG PET with small regions of hypometabolism in the frontal and parietal lobes bilaterally (green arrows), more marked on the left, and inferior left temporal lobe (orange arrow). The appearances are not definitive for AD on 18F-FDG PET, although the subsequent amyloid PET was positive.
Amyloid pet/CT
A ligand developed by a team based at the University of Pittsburgh, and subsequently called Pittsburgh compound-B (PiB), showed on imaging the increased deposition of Aβ plaques in human trials in the context of AD. It is bound to carbon-11 (11C) and is extensively used in research, but with regard to clinical applications it has a short half-life, so analogous compounds were developed to overcome this hurdle. Currently, there are three fluorinated amyloid PET tracers in clinical and research use worldwide - 18F-florbetapir, 18F-florbetaben and 18F-flutemetamol. Interpretation of all amyloid PET scans whichever tracer is used is binary i.e. it is either positive or negative. A positive result indicates moderate or high levels of cerebral amyloid plaque deposition, which is considered to support a clinical diagnosis of AD. A negative scan indicates absent or sparse levels of cerebral amyloid plaque deposition, which would not commonly support a clinical diagnosis of AD (Figure 5A and B).
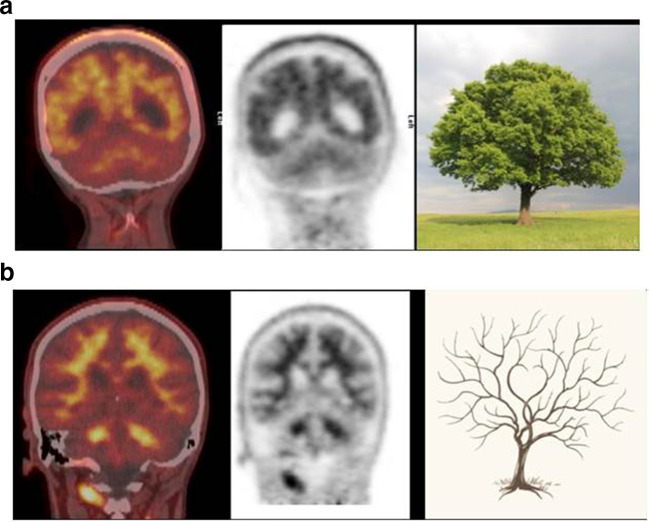
Figure 5.
(A) Typical features of a positive amyloid PET scan - “tree-in-bloom” sign with loss of cerebral grey:white matter differentiation (GWMD) indicating a positive scan.14 (B) “Branching Tree” sign with good GWMD similar to cerebellum indicating a negative amyloid PET scan.14
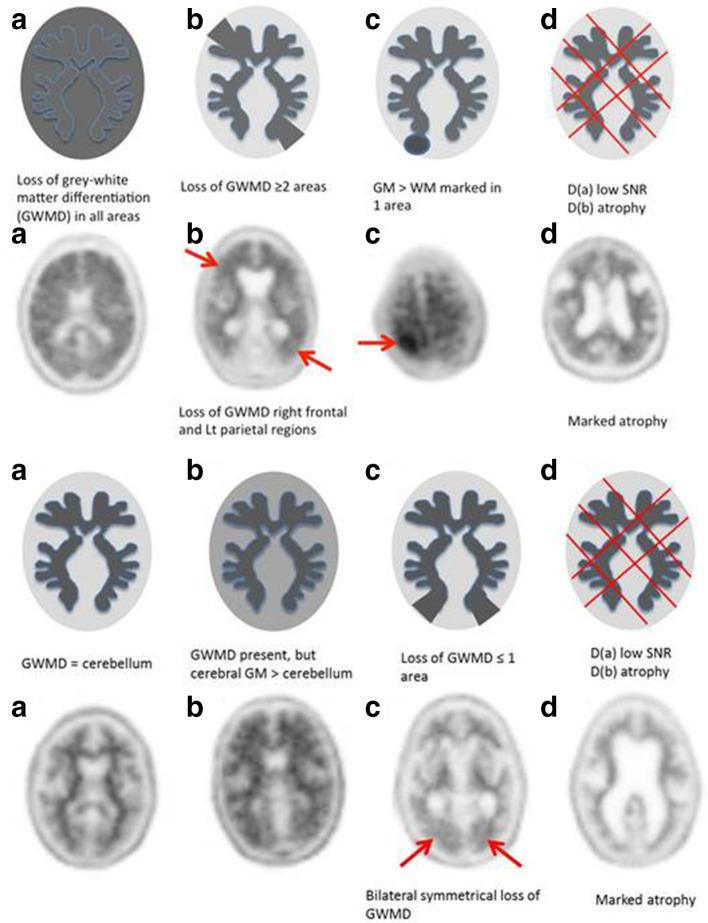
At our institution, we found differing characteristics in both the positive and negative 18F-florbetapir PET/CT scans (Figure 6A and B). According to the published 18F-florbetapir PET scans (Amyvid) read criteria14 negative scans have more activity in white matter than in grey matter, creating clear grey-white contrast. Positive scans will have either have:
Figure 6.
Positive amyloid PET imaging- type A & non-type A (B-D).14 (B) Negative amyloid PET imaging: type A and non-type A (B-D).14
Two or more brain areas (each larger than a single cortical gyrus) in which there is reduced or absent grey-white contrast; or
One or more areas in which grey matter activity is intense and clearly exceeds activity in adjacent white matter.
We further classified our first cohort of 100 18F-florbetapir studies into type A (common or typical appearance) or non-type A (atypical or less common appearance) scans according to the imaging characteristics (Figure 6A). In our experience, the majority of scans are type A (75%) and this correlates with high inter observer agreement (κ 0.75 all scan types versus a κ 0.81 for type A scans alone) and a high confidence of read. There is lower confidence of read when reporting non-type A scans.15 We concluded that the reading radiologist or physician should be aware of these subtypes, and take even more care when analysing non-type A scans to improve reader accuracy.
Amyloid pet/CT PITFALLS
Partial volume artefacts – this is commonly encountered at: inferior frontal & superior parietal lobes on axial images, extreme frontal and occipital lobes on coronal images.
Motion artefact – this can be multidirectional and often occurs early on in the scan. If head motion is present and consistent this may lead to blurring. This decreases grey-white matter differentiation and can result in a false positive read. With no motion correction the data becomes noisy. Most movements tend to be small and subtle. This means they can be imperceptible to the PET/CT technician. At our institution, to overcome these issues we have demonstrated that at least 10 min of data is necessary (for 18F-Florbetapir PET) for adequate image quality and confident reporting. List mode (LM) acquisitions in excess of 10 min are necessary to achieve a period of 10 min with reduced motion. All scans are now acquired as 20 min LM scans and first analysed for motion prior to the final reconstruction.16
Extra cerebral activity: it is important to be aware of the sites of extra cerebral activity on amyloid PET to avoid misinterpretation. Examples of sites of extra cerebral activity include parotid glands & pinna, tongue base, cervical spine, petrous portion of the temporal bone, and clivus.
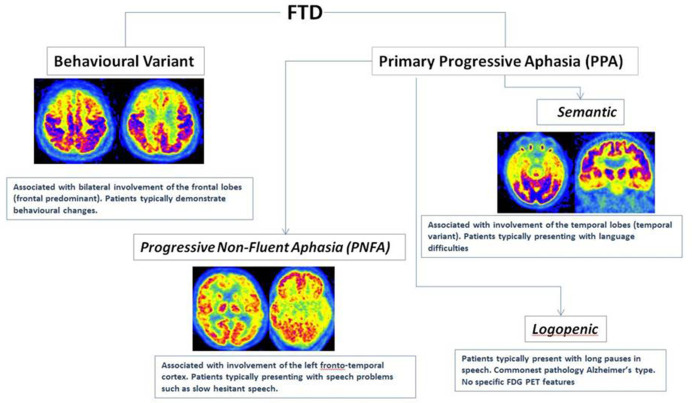
Frontotemporal dementia
Frontotemporal dementia (FTD) is common in younger (<65 years) patients when compared to age of onset in Alzheimer’s disease. It is caused by a number of pathological processes but can be further subcategorised based on the clinical features at presentation:
Behavioural variant
-
Language variant - primary progressive aphasia:
Progressive non-fluent aphasia (agrammatic variant)
Semantic dementia
As the name suggests, FTD characteristically involves changes in the frontal and temporal lobes, but the subtypes show further variation in their patterns of hypometabolism (Figure 7) particularly at the earlier stages. The frontal (behavioural variant) tends to spare the temporal lobes whilst the language variants, particularly semantic dementia, have a stronger temporal predilection.12,13,17 The progressive non-fluent aphasia subtype usually shows asymmetrical (left dominant) anterior temporal hypometabolism, but as the disease progresses changes can be seen in the frontoparietal cortex.12,13 18F-FDG PET in patients with the agrammatic variant affects the middle and inferior frontal lobes as well as the precentral gyrus.17 Amyloid PET/CT is typically negative in FTD although it should be noted that logopenic progressive aphasia (LPA) variant of primary progressive aphasia is associated with AD pathology.
Figure 7.
Different subtypes of FTD and their characteristic patterns of hypometabolism on FDG PET.
Dementia with lewy bodies
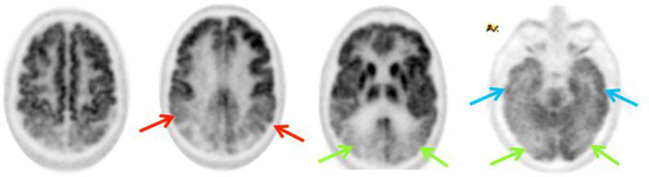
After AD, dementia with Lewy bodies (DLB) is the most common form of dementia in older people.13 Typical clinical features include visual hallucinations, fluctuating cognition, falls and Parkinsonian-like symptoms. The hypometabolism pattern on 18F-FDG PET/CT can look similar to AD with involvement of the parietal, posterior temporal region and posterior cingulate gyrus, but a distinguishing characteristic is occipital involvement (Figure 8) - occipital hypometabolism is supportive of a diagnosis of DLB in the appropriate clinical setting. It is useful to be aware of the utility of 123I-ioflupane single photon emission CT (SPECT) in dopamine transporter (DAT) scans in differentiating DLB from AD. Reduced uptake is demonstrated in the anterior striatum in DLB when compared to AD on DAT scans due to abnormalities in the neuronal pathways (Figure 9).
Figure 8.
Bilateral parietal (red arrows) and posterior temporal (blue arrows) hypometabolism in 18F-FDG PET may be seen, but in addition there is involvement of the occipital lobes (green arrows) which are typically spared in AD. Involvement of the occipital lobes either with or without metabolic changes elsewhere is supportive of a diagnosis of DLB.
Figure 9. .
MRI (top row): No focal/generalised volume loss. Hippocampal volumes within normal limits (red arrows). 18F-florbetapir (Amyvid™) PET/CT (bottom row): Global loss of grey-white differentiation within the temporal, frontal, parietal (blue arrows), and occipital lobes (green arrows) with ‘tree-in-bloom’ appearance on coronal PET. Appearances are in keeping with a positive scan (type A), supporting a clinical diagnosis of AD.
Vascular dementia
It has been suggested that vascular dementia (VD) is the second most common cause of dementia overall, after AD. If the underlying pathophysiology is sequential stroke, the clinical presentation is step-wise with decline characterised by acute episodes with periods of stability in-between. More generalised small vessel disease can have a smoother onset. 18F-FDG PET appearances tend to be territorial reflecting the arterial pattern of supply that has been affected; resultant gliosis causes well-defined hypometabolism of these regions.13,17 In addition, hypometabolism can be seen in the deep grey structures.12 A well-described phenomenon is that of crossed cerebellar diaschisis where you see damage and thus hypometabolism in the contralateral cerebellar hemisphere distant to the supratentorial region that has an infarct.13 It is secondary to degeneration through the connecting white matter tracts.
Amyloid PET is typically negative in vascular dementia unless there is co-existent AD causing a mixed dementia. A further exception to this is amyloid angiopathy–related disease, which will be discussed later.17
Corticobasal degeneration
Corticobasal degeneration (CBD) is the most common cause of corticobasal syndrome, an uncommon, progressive neurodegenerative disorder that typically presents with cognitive decline associated with apraxia, asymmetrical movement abnormality, myoclonus and “alien limb phenomenon”.13,18 On 18F-FDG PET imaging, hypometabolism involving the superior frontoparietal regions and basal ganglia contralateral to the symptomatic side-is seen. Hypermetabolism in the ipsilateral basal ganglia and cortex can be seen.18 Amyloid imaging is not well described in CBD. It should be noted that AD can rarely present as a corticobasal syndrome.
Posterior Cortical Atrophy
Posterior cortical atrophy (PCA) is characterised by progressive and severe decline in visual processing skills. The underlying pathology is normally characteristic of AD, but there is evidence to suggest a wider spectrum of disease processes can cause it including Lewy-body dementia, corticobasal degeneration and prion-based diseases.19 Parenchymal changes are typically seen in the parieto-occipital cortices.13,20 As might be expected, the pattern of 18F-FDG PET hypometabolism follows the same areas that are atrophic although interestingly, hypometabolism is also seen in the frontal eye fields bilaterally.20 Amyloid PET is positive. Although there are reports of increased deposition in the parieto-occipital regions, some studies have shown more generalised uptake and the parieto-occipital pattern seen more consistently with 18F-FDG PET is less apparent on amyloid imaging.19,20
Cerebral Amyloid Angiopathy
Cerebral amyloid angiopathy (CAA) is a vasculopathy characterised by beta-amyloid deposition within the walls of cortical, subcortical and leptomeningeal vessels. In 1995, the Boston Criteria was proposed to provide a consistent structure to diagnosis and these were modified in 2010 to incorporate the following21 : 1) definite CAA - pathological diagnosis following post-mortem histology, 2) probable CAA with pathology - (surgical specimen, not post-mortem) and clinical features, 3) probable CAA - appropriate clinical and imaging findings 4) possible CAA - appropriate clinical history but imaging findings are less conclusive. Amyloid imaging is of benefit in cases where the diagnosis is possible/uncertain and a more “conclusive” probable diagnosis is sought. A meta-analysis by Charidimou et al and Baron et al showed that a negative study essentially excludes CAA as a diagnosis.21,22 If the study is positive, further work-up is needed to differentiate it from AD.21,22 Interrogation of the distribution of uptake alone may not be enough. A meta-analysis recently published by Baron et al22 showed that the proposed concept of occipital to global uptake in CAA patients helping to differentiate from AD is not necessarily definitive. This provides a stimulus for further study and raises interesting questions around amyloid-associated neurodegenerative disorders.
Case studies
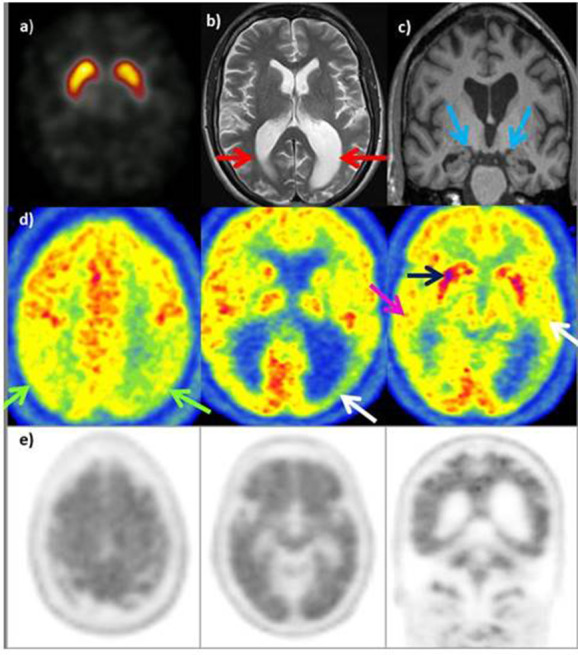
(1) History (Figure 9): A 40 year old woman. Father diagnosed with AD starting in early 30s.Scores 62/100 on Addenbrooke’s Cognitive Examination, but also struggled at school. ? AD
Tip: Amyloid PET is indicated in aiding the diagnosis of early onset progressive dementia (usually defined as ≤ 65years) with normal cross-sectional work-up.
(2) History (Figure 10): A 65 year old man presenting with widespread cognitive and language difficulties, apraxia and possible extrapyrimadal symptoms. Symptoms not classical for AD. ? DLB ? Corticobasal degeneration (CBD)
Figure 10.
123I DaT scan (image A): Normal distribution of tracer inbasal ganglia bilaterally. MRI (images B & C): Prominent lateral ventricles (red arrows) with bilateral & symmetrical low volume hippocampal formations (blue arrows). No regional volume or lobar atrophy. 18F-FDG PET (row D): Extensive hypometabolism within both parietal lobes (green arrows), left>right, extending into left occipital & posterior temporal lobes (white arrows) and right posterior temporal lobe (purple arrow). Normal metabolism in the basal ganglia (black arrows), thalamus and cingulate gyrus – these changes are not typical for CBD. Amyloid PET (row E): Complete loss of grey-white differentiation with a characteristic ‘tree-in-bloom’ appearance on coronal PET images in keeping with a positive scan (type A) supporting a clinical diagnosis of AD.
Tips:
- Amyloid PET/CT can help in the diagnosis of AD when there is an atypical clinical presentation.
- Amyloid PET/CT can help troubleshoot when cause of CI remains uncertain on conventional work-up.
(3) History (Figure 11): A 73 year old woman with a 4 year history of difficulty in dressing and colour vision. Likely posterior onset cognitive dysfunction - AD variant?
Figure 11. .
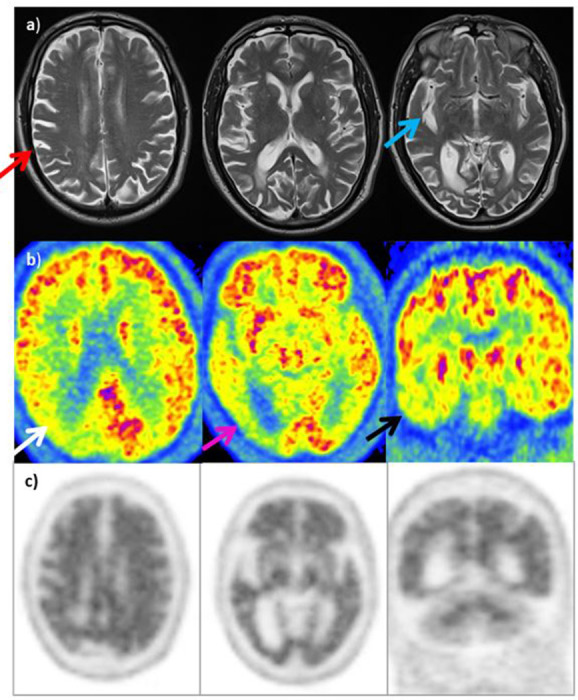
MRI (row A): Asymmetric atrophy (right>left) involving right parietal lobe (red arrow) with prominence of right sylvian fissure (blue arrow). Degree of asymmetry atypical for posterior cortical atrophy, but possibly compatible with CBD. 18F-FDG PET (row B): Marked hypometabolism in the right parietal lobe (white arrow), notably posteriorly and in the right occipital lobe (purple arrow). In addition, decreased activity in temporal lobes bilaterally, right>left (black arrow) as well as less marked reduction in activity in the left posterior parietal lobe and occipital lobe. Distribution of hypometabolism suggestive of PCA. Amyloid PET (row C): Loss of grey-white differentitation throughout entire cerebrum in keeping with a positive scan (type A) supporting a clinical diagnosis of AD-type pathology rather than CBD.
Tip: Amyloid PET/CT can help troubleshoot when cause of CI remains uncertain on conventional work-up.
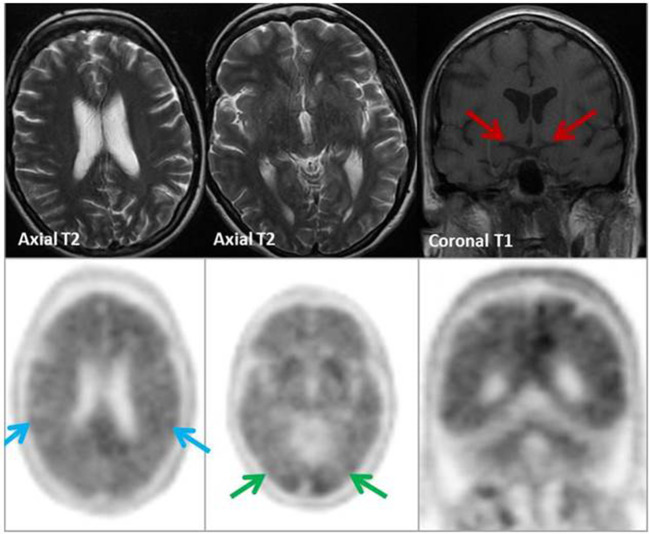
(4) History (Figure 12): A 56 year old woman with progressive CI and episodes of Parkinsonism. Partner reports occasional visual hallucinations, although subjective ? AD ? DLB
Figure 12. .
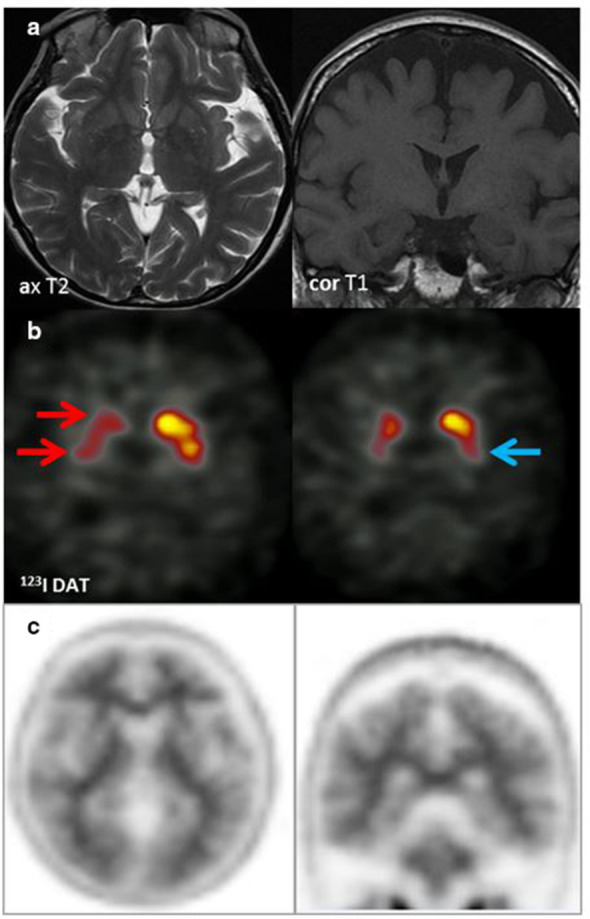
MRI (row A): No focal or regional atrophy; hippocampi within normal limits. 123I DaT scan (row B): Severely reduced uptake right caudate and putamen (red arrows) & moderately reduced uptake in the left putamen (blue arrow). Appearances suggestive of DLB. Amyloid PET (row C): Tracer uptake within the cerebral matter similar to that of cerebellar grey matter. Well-defined grey-white matter interface with good definition of the anteroposterior white matter tracts. Characteristic ‘branching-tree’ appearance on coronal PET images, which indicates normal grey-white differentiation in both the cerebrum and cerebellum in keeping with a negative scan indicating absence or sparse levels of amyloid plaque deposition. The functional imaging appearances supported a clinical diagnosis of DLB rather than AD.
Tip: Amyloid PET can help in the diagnosis of AD when there is an aetiologically mixed presentation.
Alternative approaches
It is beyond the scope of this paper to discuss imaging models outside the title. However, two techniques that may be of interest are PET/MR and Tau PET. PET/MR combines the structural detail of MRI with the physiological information from PET. Abnormal hyperphosphorylation and aggregation of tau proteins are implicated in the pathophysiology of AD. New PET tracers are being developed that can detect this intracellular abnormality, although they are not yet in routine clinical use. We would encourage the reader to look at references 23–26 for further reading on these topics.
Summary
PET/CT imaging with 18F-FDG and amyloid tracers has a vital role in the assessment of patients with cognitive impairment and dementia. Those with atypical & mixed presentations could benefit even further as the outcome of the scan has the potential to change management. The selection of optimal functional PET imaging is best achieved in the dementia multidisciplinary team (MDT)/board setting. 18F-FDG PET/CT can be used to subtype dementia/neurodegenerative syndromes, but findings can be atypical and non-specific.
Amyloid plaque accumulation usually occurs several years before structural and metabolic (18F-FDG) changes, and even before symptoms of cognitive impairment. Amyloid PET/CT is a binary read – either positive (indicating moderate to high levels of amyloid plaque) or NEGATIVE (none or sparse levels). The absence of amyloid plaque is not compatible with a clinical diagnosis of AD, and an alternative diagnosis should be sought. Evidence of the presence of amyloid plaque supports a diagnosis of AD. With future therapies being researched, conclusive evidence of this may be required prior to commencing amyloid-targeted drugs.
Footnotes
Acknowledgments: Dr P Malhotra is supported by the NIHR Imperial Biomedical Research Centre. The authors would like to acknowledge Dr Sairah Khan as joint first author for all her hard work contributing to the paper.
Contributor Information
Maureen Dumba, Email: m.dumba@nhs.net.
Sairah Khan, Email: sairah.khan@nhs.net.
Neva Patel, Email: neva.patel1@nhs.net.
Laura Perry, Email: laura.perry2@nhs.net.
Paresh Malhotra, Email: paresh.malhotra1@nhs.net.
Richard Perry, Email: richard.perry3@nhs.net.
Kuldip Nijran, Email: kuldip.nijran@nhs.net.
Tara Barwick, Email: tara.barwick@nhs.net.
Kathryn Wallitt, Email: kathryn.wallitt@nhs.net.
Zarni Win, Email: zarni.win@nhs.net.
REFERENCES
- 1. Alzheimer’s Society Dementia UK UPDATE 2014; 2014Available from. [Google Scholar]
- 2. Bond J, Stave C, Sganga A, Vincenzino O, O'connell B, Stanley RL. Inequalities in dementia care across Europe: key findings of the facing dementia survey. Int J Clin Pract 2005; 59(Suppl. 12): 8–14. doi: 10.1111/j.1368-504X.2005.00480.x [DOI] [PubMed] [Google Scholar]
- 3. Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on aging Alzheimer disease centers, 2005-2010. J Neuropathol Exp Neurol 2012; 71: 266–73. doi: 10.1097/NEN.0b013e31824b211b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cummings J. Alzheimer's Disease diagnostic criteria: practical applications. Alzheimers Res Ther 2012; 4: 35. doi: 10.1186/alzrt138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. . The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011; 7: 263–9. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cummings J, Lee G, Mortsdorf T, Ritter A, Zhong K. Alzheimer's disease drug development pipeline: 2017. Alzheimers Dement 2017; 3: 367–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. . Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 2013; 12: 207–16. doi: 10.1016/S1474-4422(12)70291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frey KA, Lodge MA, Meltzer CC, Peller PJ, Wong TZ, Hess CP, et al. . ACR-ASNR practice parameter for brain PET/CT imaging dementia. Clin Nucl Med 2016; 41: 118–25. doi: 10.1097/RLU.0000000000001037 [DOI] [PubMed] [Google Scholar]
- 9. Minoshima S, Drzezga AE, Barthel H, Bohnen N, Djekidel M, Lewis DH, et al. . SNMMI procedure Standard/EANM practice guideline for amyloid PET imaging of the brain 1.0. J Nucl Med 2016; 57: 1316–22. doi: 10.2967/jnumed.116.174615 [DOI] [PubMed] [Google Scholar]
- 10. Silverman DHS, Alavi A. PET imaging in the assessment of normal and impaired cognitive function. Radiol Clin North Am 2005; 43: 67–77 x.. doi: 10.1016/j.rcl.2004.09.012 [DOI] [PubMed] [Google Scholar]
- 11. Choi SR, Schneider JA, Bennett DA, Beach TG, Bedell BJ, Zehntner SP, et al. . Correlation of amyloid PET ligand florbetapir F 18 binding with Aβ aggregation and neuritic plaque deposition in postmortem brain tissue. Alzheimer Dis Assoc Disord 2012; 26: 8–16. doi: 10.1097/WAD.0b013e31821300bc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shivamurthy VKN, Tahari AK, Marcus C, Subramaniam RM. Brain FDG PET and the diagnosis of dementia. AJR Am J Roentgenol 2015; 204: W76–W85. doi: 10.2214/AJR.13.12363 [DOI] [PubMed] [Google Scholar]
- 13. Brown RKJ, Bohnen NI, Wong KK, Minoshima S, Frey KA. Brain PET in suspected dementia: patterns of altered FDG metabolism. Radiographics 2014; 34: 684–701. doi: 10.1148/rg.343135065 [DOI] [PubMed] [Google Scholar]
- 14. [Available from.. Available from: https://www.ema.europa.eu/documents/product-information/amyvid-epar-product-information_en.pdf.
- 15. Khan SR PN, Wallitt KL, Soneji ND, Fakhry-Darian D, Carswell CJ, Malhotra PA, et al. . First experience of 100 clinical F-18 florbetapir (Amyvid) PET/CT scans in the investigation of cognitive impairment: imaging characteristics, inter-observer agreement, confidence of read and clinical outcomes. Eur J Nucl Med Mol Imaging 2016; 43: S624. [Google Scholar]
- 16. Patel N FDD, Nijran K, Khan S, Win Z. Assessment and optimisation of Hermes amyloid brass as a quantitative diagnostic tool in reporting 18F-florbetapir (Amyvid) investigations. J Nucl Med 2018; 59: 1.29217737 [Google Scholar]
- 17. Martin-Macintosh EL, Broski SM, Johnson GB, Hunt CH, Cullen EL, Peller PJ. Multimodality imaging of neurodegenerative processes: Part 1, the basics and common dementias. AJR Am J Roentgenol 2016; 207: 871–82. doi: 10.2214/AJR.14.12842 [DOI] [PubMed] [Google Scholar]
- 18. Martin-Macintosh EL, Broski SM, Johnson GB, Hunt CH, Cullen EL, Peller PJ. Multimodality imaging of neurodegenerative processes: Part 2, atypical dementias. AJR Am J Roentgenol 2016; 207: 883–95. doi: 10.2214/AJR.14.12910 [DOI] [PubMed] [Google Scholar]
- 19. Singh TD, Josephs KA, Machulda MM, Drubach DA, Apostolova LG, Lowe VJ, et al. . Clinical, FDG and amyloid PET imaging in posterior cortical atrophy. J Neurol 2015; 262: 1483–92. doi: 10.1007/s00415-015-7732-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Crutch SJ, Lehmann M, Schott JM, Rabinovici GD, Rossor MN, Fox NC. Posterior cortical atrophy. Lancet Neurol 2012; 11: 170–8. doi: 10.1016/S1474-4422(11)70289-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Farid K, Charidimou A, Baron J-C. Amyloid positron emission tomography in sporadic cerebral amyloid angiopathy: a systematic critical update. Neuroimage Clin 2017; 15: 247–63. doi: 10.1016/j.nicl.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Charidimou A, Farid K, Tsai H-H, Tsai L-K, Yen R-F, Baron J-C. Amyloid-PET burden and regional distribution in cerebral amyloid angiopathy: a systematic review and meta-analysis of biomarker performance. J Neurol Neurosurg Psychiatry 2018; 89: 410–7. doi: 10.1136/jnnp-2017-316851 [DOI] [PubMed] [Google Scholar]
- 23. Zhang XY, Yang ZL, Lu GM, Yang GF, Zhang LJ. PET/MR imaging: new frontier in Alzheimer's disease and other dementias. Front. Mol. Neurosci. 2017; 10: 343. doi: 10.3389/fnmol.2017.00343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Broski SM, Goenka AH, Kemp BJ, Johnson GB. Clinical PET/MRI: 2018 update. American Journal of Roentgenology 2018; 211: 295–313. doi: 10.2214/AJR.18.20001 [DOI] [PubMed] [Google Scholar]
- 25. Okamura N, Harada R, Ishiki A, Kikuchi A, Nakamura T, Kudo Y. The development and validation of tau PET tracers: current status and future directions. Clin Transl Imaging 2018; 6: 305–16. doi: 10.1007/s40336-018-0290-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saint-Aubert L, Lemoine L, Chiotis K, Leuzy A, Rodriguez-Vieitez E, Nordberg A. Tau PET imaging: present and future directions. Mol Neurodegeneration 2017; 12: 19. doi: 10.1186/s13024-017-0162-3 [DOI] [PMC free article] [PubMed] [Google Scholar]