Abstract
Tumor metastasis and anticancer drug resistance are the major causes of mortality in patients with colorectal cancer (CRC). Due to the limitations of conventional biomarkers, it is urgent to identify novel and valid biomarkers to predict the progression and prognosis of CRC. Reverse transcription-quantitative polymerase chain reaction and western blotting were used to detect MAGT1 expression in CRC clinical samples or cell lines. Bioinformatics analysis was used to investigate the association between MAGT1 alteration and clinicopathological features of patients with CRC. The present study revealed that the transcription levels of magnesium transporter 1 (MAGT1) were significantly increased in CRC tissues compared with matched adjacent normal tissues. Overexpression of MAGT1 was associated with advanced tumor stage, N and M classification. In addition, for patients who underwent chemotherapy, patients in the MAGT1-low expression group exhibited a longer overall survival (OS) time than patients in the high-expression group. Patients with CRC treated with chemotherapy had a longer OS time than those treated without chemotherapy in the MAGT1-low expression group but not in the MAGT1-high expression group. Furthermore, MAGT1 was a valid but not an independent prognostic factor for CRC. Therefore, the present study highlighted that MAGT1 may serve as a valid biomarker for predicting the development, progression and poor prognosis of CRC.
Keywords: CRC, MAGT1, metastasis, prognosis, anticancer drug resistance
Introduction
Colorectal cancer (CRC) is one of the most common types of cancer worldwide, and accounted for >1 million new cases in 2014 (1). However, the mechanisms underlying the development and progression of CRC remain unclear as CRC results from co-occurrence and interaction of multiple risk factors in the majority of cases (2). In addition, previous studies reported that CRC is the fourth most common cause of cancer-associated mortality worldwide, although the mortality rate of patients with CRC has progressively declined in the last decades (1,3). Tumor metastasis and anticancer drug resistance are the major causes of the poor prognosis of CRC, which mainly result from the dysregulation of cancer-associated genes (4,5). Overall, the identification of CRC-associated genes may provide novel therapeutic targets and biomarkers for predicting the development, progression and prognosis of CRC.
In our previous study, 13 potential genes associated with the metastasis of CRC were identified via microarray screening (6). However, the effects of eight of these genes, including magnesium transporter 1 (MAGT1), on the development and progression of CRC remain unknown. MAGT1 protein is a critical regulator of the intracellular free Mg2+ levels, which serves an important role in temporally coordinating natural killer (NK) and CD8+ T cell activation (7). Notably, previous studies reported aberrant expression of MAGT1, which is associated with therapeutic effect and prognosis of cancer (8–10). However, the underlying mechanism of action of MAGT1 remains elusive.
The present study aimed to investigate the association between MAGT1 and the progression of CRC by detecting the expression levels of MAGT1 in clinical CRC samples and Gene Expression Omnibus (GEO) datasets. The results suggested that MAGT1 may be a novel predictive biomarker and feasible therapeutic target for CRC.
Materials and methods
Cell culture
CRC cell lines HT-15, HT-8, HCT116, LS174T, CACO2, SW480, SW620, LOVO and the normal epithelial cell line FHC were obtained from American Type Culture Collection. All cells were maintained as previously described (6), authenticated by short tandem repeat profiling prior to receipt and were propagated for <6 months following resuscitation. The cells were grown in RPMI-1640 medium (Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.), streptomycin (100 µg/ml; Sigma-Aldrich; Merck KGaA) and penicillin (100 U/ml; Sigma-Aldrich; Merck KGaA), and placed at 37°C in a humidified incubator containing 5% CO2.
Clinical population and public dataset analysis
Clinical data were obtained for 51 patients (18 women and 33 men, aged between 50 and 60 years) who were diagnosed with primary CRC between January 2015 and December 2017 at the Department of Pathology of Zhuajiang Hospital of Southern Medical University in Guangdong, China. The 51 pairs of CRC tissues with matched normal mucosa (isolated 10 cm from the edge of the tumor), which were obtained following surgical resection, were diagnosed by the Department of Pathology of Zhuajiang Hospital, using the Tumor-Node-Metastasis (TNM) pathological staging system (11). The analysis was restricted to population-based cases, not selected on the basis of family history. The present study was approved by the Ethics Committees of Southern Medical University, and all aspects of the present study complied with the criteria of the Declaration of Helsinki (12). MAGT1 expression profiling studies in CRC samples including relevant clinical information were identified by searching in GEO datasets GSE39852 (n=585) (13) and GSE87211 (n=363) (14).
RNA isolation and reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from tissues using TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.). To quantify the transcription of MAGT1, total RNA was subjected to polyadenylation and RT using a ThermoScript RT-PCR system (85°C for 15 min followed by 37°C for 5 sec) (Invitrogen; Thermo Fisher Scientific, Inc.). qPCR analysis was carried out using a SYBR Green PCR master mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) on an ABI 7500HT system (Thermo Fisher Scientific, Inc.). The thermocycling conditions were as follows: Stage1, 95°C for 30 sec; stage2, 95°C for 5 sec and 60°C for 34 sec for 40 cycles; and stage 3, 95°C for 15 sec, 60°C for 1 min and 95°C for 15 sec. GAPDH was used as an endogenous control. All samples were normalized to internal controls, and fold changes were calculated by relative quantification (2−ΔΔCq) (15). qPCR for target genes was performed as previously described (16). The primers used are shown in Table SI.
Western blot analysis
Cells were washed twice with cold PBS and lysed in RIPA buffer containing protease inhibitors (Sigma-Aldrich; Merck KGaA). Protein quantification was performed with bicinchoninic acid assay (Sigma-Aldrich; Merck KGaA). Proteins (40 µg) were separated on 10% SDS-PAGE and transferred onto polyvinylidene fluoride membranes (EMD Millipore). Membranes were blocked with 5% skimmed milk dissolved in TBS, incubated with primary antibodies at 4°C overnight and with horseradish peroxidase-conjugated secondary antibodies at 37°C for 1 h. The rabbit primary antibody against MAGT1 (cat. no. ab90478; 1:1,000) and the mouse primary antibody against GAPDH (cat. no. ab8245; 1:1,000) were from Abcam. The secondary antibodies goat anti-mouse (cat. no. ab205719; 1:10,000) and goat anti-rabbit (cat. no. ab6721; 1:10,000) were from Sigma-Aldrich Merck KGaA. Bands were detected using enhanced chemiluminescence substrate (EMD Millipore).
Statistical analysis
Data were analyzed using SPSS version 19.0 software (SPSS, Inc.). Unpaired Student's t-test and paired t-test were carried out to evaluate statistical differences between groups. Pearson's χ2 test, Kaplan-Meier survival analysis, log-rank test and Cox regression analysis were performed using SPSS software. All statistical tests were two-sided. Data are presented as the means ± standard deviation. Each experiment was repeated three times. P<0.05 was considered to indicate a statistically significant difference.
Results
MAGT1 is upregulated in human CRC tissues and cell lines
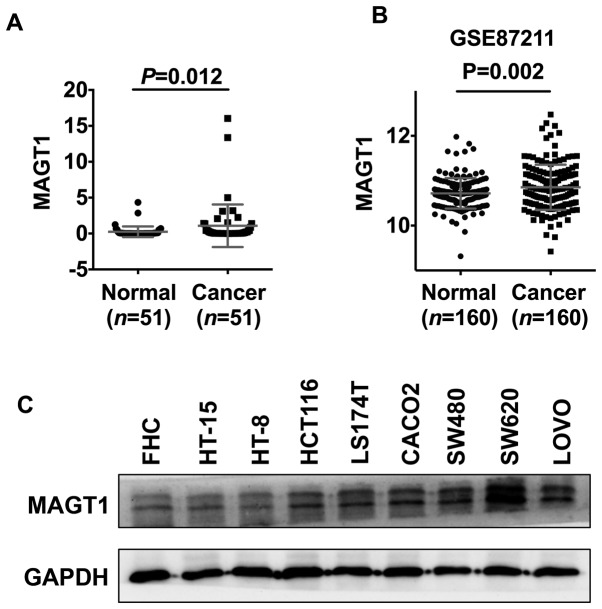
To investigate the role of MAGT1 in the development of CRC, the expression level of MAGT1 in 51 primary tumor and matched adjacent normal tissues was investigated using qPCR (Table SII). The present study revealed that the expression levels of MAGT1 were significantly upregulated in primary CRC tissues (Fig. 1A). Similar results were found in samples from GSE87211 dataset (Fig. 1B). Additionally, increased protein level of MAGT1 was observed in six out of eight CRC cell lines, including HCT116, LS174T, CACO2, SW480, SW620 and LOVO, compared with FHC cell line (Fig. 1C).
Figure 1.
MAGT1 is significantly upregulated in human CRC. (A) Reverse transcription-quantitative PCR assay for MAGT1 expression in CRC and matched normal tissues. Data were normalized to GAPDH and expressed as the mean ± STANDARD DEVIATION. (B) Gene Expression Omnibus GSE87211 dataset. (C) Western blot analysis of MAGT1 protein expression in normal epithelial cell line FHC and CRC cell lines. CRC, colorectal cancer; MAGT1, magnesium transporter 1.
MAGT1 is associated with the clinicopathological features of CRC
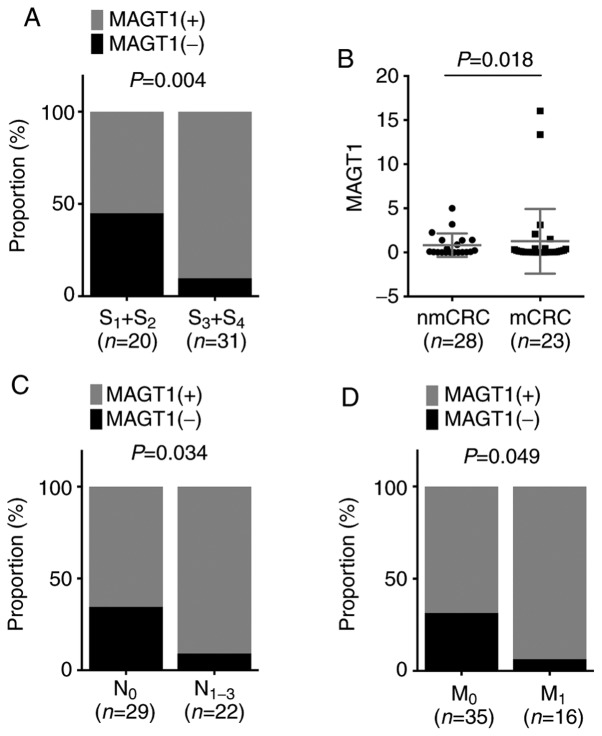
To further explore the roles of MAGT1 in CRC, the association between the overexpression of MAGT1 and clinicopathological features in 51 patients with CRC was analyzed. The present study revealed that more tumors with advanced stages were identified in the MAGT1-high expression group compared with in the low expression group (Fig. 2A). In addition, the expression levels of MAGT1 in metastatic CRC tissues were identified to be higher than those in non-metastatic CRC tissues (Fig. 2B). The MAGT1-high expression group exhibited a slightly higher proportion of patients with CRC with either lymphatic or distal metastasis compared with the low expression group (Fig. 2C and D). To further confirm the results of the present study, the GEO GSE39582 dataset was adopted to analyze the expression of MAGT1 in 566 patients with CRC. These patients with CRC were divided into MAGT1-high expression (n=283) and MAGT1-low (n=283) expression groups according to the median value of MAGT1. The present study demonstrated that there were more patients with CRC with advanced tumor stages, lymphatic and distal metastasis in the MAGT1-high expression group compared with the low expression group (Table I). In addition, the results of the present study indicated that upregulation of MAGT1 was associated with higher incidence of CRC in distal locations (Table I). These findings suggested a positive association between MAGT1 overexpression and the progression of CRC.
Figure 2.
MAGT1 expression is associated with clinicopathological features in patients with CRC. (A) Pearson's χ2 test for the association between MAGT1 expression and tumor stage. (B) Reverse transcription-quantitative PCR assay for MAGT1 expression in mCRC and nmCRC tissues. (C) Pearson's χ2 test for the association between MAGT1 expression and N classification. (D) Pearson's χ2 test for the association between MAGT1 expression and M classification. CRC, colorectal cancer; MAGT1, magnesium transporter 1; mCRC, metastatic CRC; nmCRC, non-metastatic CRC; S, tumor stage; N, node; M, metastasis.
Table I.
Association of patient characteristics and MAGT1 expression in 566 colorectal cancer samples from the GSE39582 dataset.
| MAGT1 expression | ||||
|---|---|---|---|---|
| Characteristics | Total, n | Low, n (%) | High, n (%) | P-valuea |
| Sex | 0.128 | |||
| Female | 256 | 137 (53.5) | 119 (46.5) | |
| Male | 310 | 146 (47.1) | 164 (52.9) | |
| Age at diagnosis, years | 0.332 | |||
| <50 | 76 | 34 (44.7) | 42 (55.3) | |
| ≥50 | 489 | 248 (50.7) | 241 (49.3) | |
| Tumor location | 0.025a | |||
| Proximal | 224 | 125 (55.8) | 99 (44.2) | |
| Distal | 342 | 158 (46.2) | 184 (53.8) | |
| T classification | 0.648 | |||
| Tis+T0+T1+T2 | 60 | 32 (53.3) | 28 (46.7) | |
| T3+T4 | 486 | 244 (50.2) | 242 (49.8) | |
| N classification | 0.033a | |||
| N0 | 302 | 165 (54.6) | 137 (45.4) | |
| N1+N2+N3 | 238 | 108 (45.4) | 130 (54.6) | |
| M classification | 0.001a | |||
| M0 | 482 | 255 (52.9) | 227 (47.1) | |
| M1 | 61 | 18 (29.5) | 43 (70.5) | |
| Stage | 0.015a | |||
| Stage1+Stage2 | 301 | 165 (54.8) | 136 (45.2) | |
| Stage3+Stage4 | 265 | 118 (44.5) | 147 (55.5) | |
P-value was calculated using Pearson's χ2 test of independence between covariates and MAGT11 expression. Due to incomplete data, one patient was excluded from the association between age and MAGT1 expression, 20 were excluded from the tumor classification analysis, 26 were excluded from the node classification analysis and 23 from the distal metastasis analysis.
P<0.05. MAGT1, magnesium transporter 1; T, tumor; N, node; M, metastasis.
Upregulation of MAGT1 is associated with the prognosis of patients with CRC treated with chemotherapy
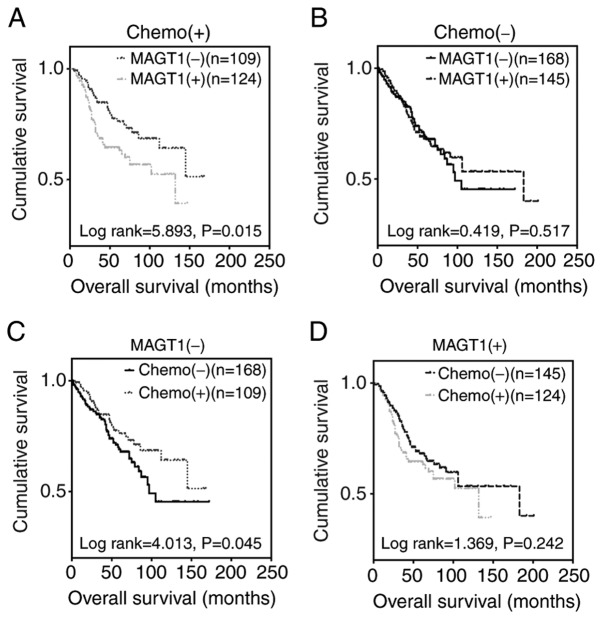
Since chemotherapy data from 19 healthy controls and 16 patients with CRC, and survival data from 4 patients with CRC were missing in the GSE39582 dataset (n=585), Kaplan-Meier and Cox regression analyses were applied to 546 patients with CRC to further investigate the predictive role of MAGT1 in the prognosis of CRC. Notably, for patients with postoperative chemotherapy, the MAGT1-low expression group had a longer overall survival (OS) time than the high expression group (Fig. 3A). However, no statistically significant difference in OS was identified between the high and low expression groups in patients who did not undergo chemotherapy (Fig. 3B). Therefore, it was hypothesized that MAGT1 may be associated with anticancer drug resistance in patients with CRC. As expected, patients treated with chemotherapy had a better prognosis only in the MAGT1-low expression group (Fig. 3C and D), which supported the aforementioned hypothesis. In addition, the 5-year overall survival rates in the MAGT1-low expression group were 38.01% (without chemotherapy) and 53.21% (with chemotherapy), while in the MAGT1-high expression group survival rates were 43.45% (without chemotherapy) and 36.29% (with chemotherapy). Therefore, univariate and multivariate Cox regression analyses were performed only for patients with CRC treated with chemotherapy. Univariate Cox regression analysis demonstrated that MAGT1, tumor stage, and T and M classification were indicators of poor prognosis, while no statistically significant difference was identified for age, sex, N classification and tumor location (Table II). However, multivariate Cox regression analysis revealed that only T and M classification, but not MAGT1, were independent factors for poor prognosis (Table III). Multivariate analysis was used to analyze the effect of independent factors on patients' survival, whereas tumor stage was determined by TNM classification. Stage was therefore excluded while applying multivariate Cox regression analysis. Collectively, MAGT1 was a valid but not an independent prognostic factor for CRC.
Figure 3.
MAGT1 expression is associated with the prognosis of patients with CRC with chemotherapy. (A) Kaplan-Meier survival analysis for MAGT1 expression and overall survival of patients with CRC. (B) Kaplan-Meier survival analysis for MAGT1 expression and overall survival of patients with CRC. (C) Kaplan-Meier survival analysis for chemotherapy and overall survival of patients with CRC and low expression of MAGT1. (D) Kaplan-Meier survival analysis for chemotherapy and overall survival of patients with CRC and high expression of MAGT1. Chemo, chemotherapy; CRC, colorectal cancer; MAGT1, magnesium transporter 1.
Table II.
Univariate Cox regression analysis of factors associated with overall survival in patients with colorectal cancer.
| 95% CI for Exp(B) | ||||
|---|---|---|---|---|
| Characteristic | Exp(B) | Lower | Upper | P-value |
| Age | 1.009 | 0.990 | 1.020 | 0.353 |
| Sex | 1.223 | 0.769 | 1.943 | 0.395 |
| T classification | 2.743 | 1.735 | 4.338 | <0.001 |
| N classification | 1.191 | 0.891 | 1.592 | 0.238 |
| M classification | 8.584 | 4.803 | 15.343 | <0.001 |
| Location | 0.767 | 0.477 | 1.233 | 0.273 |
| Stage | 2.924 | 1.846 | 4.630 | <0.001 |
| MAGT1 | 1.768 | 1.107 | 2.822 | 0.017 |
EXP(B), exponent of B; MAGT1, magnesium transporter 1; T, tumor; N, node; M, metastasis.
Table III.
Multivariate Cox regression analysis of factors associated with overall survival in patients with colorectal cancer.
| 95% CI for Exp(B) | ||||
|---|---|---|---|---|
| Factor | Exp(B) | Lower | Upper | P-value |
| T classification | 1.788 | 1.103 | 2.900 | 0.018 |
| M classification | 5.946 | 3.183 | 11.111 | <0.001 |
| MAGT1 | 1.409 | 0.849 | 2.339 | 0.185 |
EXP(B), exponent of B; MAGT1, magnesium transporter 1; T, tumor; M, metastasis.
Discussion
A previous study revealed that CRC is the third most common type of cancer (17), and the fourth most common cause of cancer-associated mortality worldwide (1,3). Furthermore, a recent study reported that the 5-year survival rate of patients with primary CRC has increased to 90%; however the 5-year survival rate of patients with advanced CRC is only 13% (4). Since tumor metastasis and drug resistance are the major reasons for the poor prognosis of patients with CRC (18,19), it is crucial to identify novel factors and mechanisms contributing to the aforementioned processes. In addition, investigation of valid biomarkers for predicting the development, progression and prognosis of CRC is also necessary.
MAGT1 is a well-known chromosome X-linked gene that encodes a highly selective Mg2+ transporter (20). An immunologic study demonstrated that mutations in MAGT1 lead to T-cell deficiency by disturbing the homeostasis of intracellular free Mg2+, which is an important second messenger among multiple cellular activities (7,21,22). However, the pathophysiological significance of MAGT1 remains elusive. The present study demonstrated that MAGT1 was upregulated in CRC tissues compared with adjacent normal tissues, indicating a positive association between the upregulation of MAGT1 and incidence of CRC. In addition, patients with CRC with high expression levels of MAGT1 had advanced tumor stage, and were more likely to exhibit lymphatic or distal metastasis. Similar results have previously been reported in hepatocellular carcinoma (8). Additionally, a previous study in breast cancer revealed that decreased MAGT1 expression serves an important role in reducing the viability of cancer cells (9). Collectively, MAGT1 may be a valid biomarker for predicting the development and metastasis of CRC. Further investigations are required to clarify the exact effects of MAGT1 on the biological activities of CRC cells.
Notably, a growing body of epidemiological studies determined that high magnesium intake is associated with a lower incidence of CRC (23–25), which seems to contradict the aforementioned association between MAGT1 and CRC. However, due to the crucial role of MAGT1 in the regulation of NK and CD8+ T-cells by mediating transient Mg2+ influx (7,21), this discrepancy may be explained by the high magnesium intake that may reduce the incidence of CRC by activating cytotoxic T-cells. In addition, the concentration of intracellular Mg2+ in epithelial and CRC cells following high magnesium intake is unknown. Therefore, the role of MAGT1-mediated alteration of Mg2+ in regulating the development and progression of CRC remains unclear.
To further investigate the association between MAGT1 and CRC, Kaplan-Meier survival and Cox regression analyses were performed in the present study. Notably, the Kaplan-Meier survival analysis revealed that MAGT1 expression was negatively associated with OS time only in patients with CRC treated with chemotherapy. Therefore, MAGT1 may be involved in regulating the mechanisms underlying anticancer drug resistance in CRC. Furthermore, univariate and multivariate Cox regression analyses performed in patients with CRC treated with chemotherapy suggested that MAGT1 was a valid but not an independent prognostic factor for CRC. Notably, patients with CRC with postoperative chemotherapy had an improved OS only in the MAGT1-low expression group. This finding suggested that MAGT1 could be a valid biomarker for predicting the chemotherapeutic efficacy in CRC.
In conclusion, the present study identified an association between CRC development and progression and MAGT1 expression level. Upregulation of MAGT1 may be associated with tumor metastasis and anticancer drug resistance, and MAGT1 could be a valid biomarker for predicting the development, progression and prognosis of CRC.
Supplementary Material
Acknowledgements
Not applicable.
Glossary
Abbreviations
- CRC
colorectal cancer
- GEO
Gene Expression Omnibus
- MAGT1
magnesium transporter 1
- OS
overall survival
Funding
This study was supported by the National Natural Science Foundation of China (grant no. 81600444).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
KZ and QY performed the experiments. LX and ZQ performed the statistical analysis. YH, YL and LT assisted in tissue sample collection. LT performed data analysis and interpretation. CC designed the study and prepared the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Southern Medical University and all aspects of the study complied with the Declaration of Helsinki.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 2.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–1502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 3.Welch HG, Robertson DJ. Colorectal cancer on the decline-why screening can't explain it all. N Engl J Med. 2016;374:1605–1607. doi: 10.1056/NEJMp1600448. [DOI] [PubMed] [Google Scholar]
- 4.Phipps AI, Robinson JR, Campbell PT, Win AK, Figueiredo JC, Lindor NM, Newcomb PA. Prediagnostic alcohol consumption and colorectal cancer survival: The colon cancer family registry. Cancer. 2017;123:1035–1043. doi: 10.1002/cncr.30446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Cancer Research Fund International (WCRF), corp-author Continuous Update Project. http://www.wcrf.org/int/cancer-facts-figures/link-between-lifestyle-cancer-risk/alcohol-cancer. [Dec 18;2017 ];Alcohol and Cancer.
- 6.Zheng K, Zhou X, Yu J, Li Q, Wang H, Li M, Shao Z, Zhang F, Luo Y, Shen Z, et al. Epigenetic silencing of miR-490-3p promotes development of an aggressive colorectal cancer phenotype through activation of the Wnt/β-catenin signaling pathway. Cancer Lett. 2016;376:178–187. doi: 10.1016/j.canlet.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Chaigne-Delalande B, Li FY, O'Connor GM, Lukacs MJ, Jiang P, Zheng L, Shatzer A, Biancalana M, Pittaluga S, Matthews HF, et al. Mg2+ regulates cytotoxic functions of NK and CD8 T cells in chronic EBV infection through NKG2D. Science. 2013;341:186–191. doi: 10.1126/science.1240094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molee P, Adisakwattana P, Reamtong O, Petmitr S, Sricharunrat T, Suwandittakul N, Chaisri U. Up-regulation of AKAP13 and MAGT1 on cytoplasmic membrane in progressive hepatocellular carcinoma: A novel target for prognosis. Int J Clin Exp Pathol. 2015;8:9796–9811. [PMC free article] [PubMed] [Google Scholar]
- 9.Uddin MB, Balaravi Pillai B, Tha KK, Ashaie M, Karim ME, Chowdhury EH. Carbonate apatite nanoparticles-facilitated intracellular delivery of siRNA(s) targeting calcium ion channels efficiently kills breast cancer cells. Toxics. 2018;6:E34. doi: 10.3390/toxics6030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willis S, Villalobos VM, Gevaert O, Abramovitz M, Williams C, Sikic BI, Leyland-Jones B. Single gene prognostic biomarkers in ovarian cancer: A meta-analysis. PLoS One. 2016;11:e0149183. doi: 10.1371/journal.pone.0149183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rice TW, Gress DM, Patil DT, Hofstetter WL, Kelsen DP, Blackstone EH. Cancer of the esophagus and esophagogastric junction-major changes in the American joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:304–317. doi: 10.3322/caac.21399. [DOI] [PubMed] [Google Scholar]
- 12.Issue Information-Declaration of Helsinki. J Bone Mineral Res. 2017;32:BMi–BMii. doi: 10.1002/jbmr.2665. [DOI] [PubMed] [Google Scholar]
- 13.Marisa L, de Reyniès A, Duval A, Selves J, Gaub MP, Vescovo L, Etienne-Grimaldi MC, Schiappa R, Guenot D, Ayadi M, et al. Gene expression classification of colon cancer into molecular subtypes: Characterization, validation, and prognostic value. PLoS Med. 2013;10:e1001453. doi: 10.1371/journal.pmed.1001453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu Y, Gaedcke J, Emons G, Beissbarth T, Grade M, Jo P, Yeager M, Chanock SJ, Wolff H, Camps J, et al. Colorectal cancer susceptibility loci as predictive markers of rectal cancer prognosis after surgery. Genes Chromosomes Cancer. 2018;57:140–149. doi: 10.1002/gcc.22512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, An H, Wang B, Liao Q, Li W, Jin X, Cui S, Zhang Y, Ding Y, Zhao L. miR-133a represses tumour growth and metastasis in colorectal cancer by targeting LIM and SH3 protein 1 and inhibiting the MAPK pathway. Eur J Cancer. 2013;49:3924–3935. doi: 10.1016/j.ejca.2013.07.149. [DOI] [PubMed] [Google Scholar]
- 17.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 18.Zarour LR, Anand S, Billingsley KG, Bisson WH, Cercek A, Clarke MF, Coussens LM, Gast CE, Geltzeiler CB, Hansen L, et al. Colorectal cancer liver metastasis: Evolving paradigms and future directions. Cell Mol Gastroenterol Hepatol. 2017;3:163–173. doi: 10.1016/j.jcmgh.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 20.de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: Implications for health and disease. Physiol Rev. 2015;95:1–46. doi: 10.1152/physrev.00012.2014. [DOI] [PubMed] [Google Scholar]
- 21.Li FY, Chaigne-Delalande B, Kanellopoulou C, Davis JC, Matthews HF, Douek DC, Cohen JI, Uzel G, Su HC, Lenardo MJ. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature. 2011;475:471–476. doi: 10.1038/nature10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takaya J, Higashino H, Kobayashi Y. Can magnesium act as a second messenger? Current data on translocation induced by various biologically active substances. Magnes Res. 2000;13:139–146. [PubMed] [Google Scholar]
- 23.Ko HJ, Youn CH, Kim HM, Cho YJ, Lee GH, Lee WK. Dietary magnesium intake and risk of cancer: A meta-analysis of epidemiologic studies. Nutr Cancer. 2014;66:915–923. doi: 10.1080/01635581.2014.922203. [DOI] [PubMed] [Google Scholar]
- 24.Gorczyca AM, He K, Xun P, Margolis KL, Wallace JP, Lane D, Thomson C, Ho GY, Shikany JM, Luo J. Association between magnesium intake and risk of colorectal cancer among postmenopausal women. Cancer Causes Control. 2015;26:1761–1769. doi: 10.1007/s10552-015-0669-2. [DOI] [PubMed] [Google Scholar]
- 25.Meng Y, Sun J, Yu J, Wang C, Su J. Dietary intakes of calcium, iron, magnesium, and potassium elements and the risk of colorectal cancer: A meta-analysis. Biol Trace Elem Res. 2019;189:325–335. doi: 10.1007/s12011-018-1474-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.