Abstract
Previous studies have demonstrated a significant difference in clinical characteristics between patients with non-small cell lung cancer (NSCLC) harboring exon 19 deletion (19-del) and an exon point mutation (21-L858R) in EGFR. The present retrospective study aimed to investigate the differential prognosis in patients with NSCLC harboring exon 19-del and 21-L858R mutations. The clinical and follow-up data of 137 patients treated at the Zhongnan Hospital of Wuhan University (Wuhan, Hubei, China) between August 2012 and August 2016, who were diagnosed with stage IIIB-IV NSCLC harboring either exon 19-del or 21-L858R mutations, were analyzed. The patients were divided into the first-line tyrosine kinase inhibitor (TKI), first-line chemotherapy and second-line TKI treatment groups. The median progression-free survival (PFS) time of patients harboring the exon 19-del mutation was significantly improved compared with that in patients harboring the 21-L858R mutation (11.3 vs. 8.8 months, respectively; P=0.017) following first-line TKI treatments. However, no significant difference in the median PFS time was observed between the exon 19-del and 21-L858R groups following the first-line chemotherapy or second-line TKI treatment. In patients with the exon 19-del, first-line TKI treatment achieved an increased objective response rate (ORR; 51.9 vs. 18.5%; P=0.004) and disease control rate (96.2 vs. 77.8%; P=0.030), and a longer PFS time (11.3 vs. 8.0 months; P=0.034) compared with that in the patients following first-line chemotherapy. First- and second-line TKI treatment achieved a similar PFS time (11.3 vs. 11.0 months, respectively; P=0.140). However, in patients with the 21-L858R mutation, the first-line TKI therapy and first-line chemotherapy groups exhibited a similar PFS time (8.8 vs. 3.5 months, respectively; P=0.063), while the second-line TKI treatment group exhibited a significantly longer PFS time compared with the first-line TKI treatment group (13.6 vs. 8.8 months, respectively; P=0.030). There was a differential sensitivity to treatment between patients harboring the exon 19-del and 21-L858R mutations. Therefore, chemotherapy may increase the sensitivity to TKIs in patients harboring the 21-L858R mutation.
Keywords: non-small cell lung cancer, tyrosine kinase inhibitors, exon 19 deletion, 21-L858R mutation, progression-free survival
Introduction
Lung cancer is the most common cause of cancer-related mortality worldwide (1), with increasing incidence and mortality rates. Non-small cell lung cancer (NSCLC) accounts for ~80% of newly diagnosed lung cancer cases annually, and the majority are diagnosed at an advanced stage (2). Chemotherapy has been recommended as the first-line treatment in patients with advanced NSCLC for the last 10 years. However, due to the toxicity and side effects, the therapeutic effect is limited and the clinical outcomes are poor, with a median overall survival (OS) time of only 8–10 months and a 5-year survival rate of only ~15% (3). Recently, treatment for patients with advanced or metastatic NSCLC has been modified. Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) have revealed significant efficacy in NSCLC patients with EGFR mutations (4,5), are associated with fewer side effects and have improved quality of life, particularly in patients harboring the exon 19 deletion (19-del) or exon 21 point mutation (21-L858R).
EGFR is a member of the ErbB receptor TK family and serves a key role in the development and progression of NSCLC (6). Overexpression of EGFR may lead to cell proliferation, promote angiogenesis, tumor invasion and metastasis, and inhibit cell apoptosis, serving an important role in the evolution of malignant tumors (7–9). Numerous studies have confirmed that EGFRs are uniquely expressed in tumor tissues, particularly NSCLC (10,11). Exon 19-del and 21-L858R mutations are common, accounting for 85% of all EGFR mutations in NSCLC (12–14). With the extensive use of TKIs in patients with NSCLC harboring EGFR mutations, accumulating evidence has demonstrated that exon 19-del and 21-L858R mutations are associated with distinguishing clinical characteristics (15). The aim of the present study was to further investigate whether these two mutations result in different prognoses in patients with NSCLC.
Materials and methods
Patient characteristics
The clinical and follow-up data of 137 patients treated at the Zhongnan Hospital of Wuhan University (Wuhan, Hubei, China) between August 2012 and August 2016, who were diagnosed with stage IIIB-IV NSCLC and harboring either the exon 19-del or 21-L858R mutations, were collected. Patient sex, age, smoking status, primary site, disease stage, type of EGFR-TKI administered (icotinib, gefitinib or erlotinib) and treatment protocol (first-line TKIs, first-line chemotherapy or second-line TKIs) were recorded during a retrospective chart review.
The inclusion criteria for the present study were as follows: i) Patients with histologically or cytologically confirmed stage IIIB-IV NSCLC according to the 7th American Joint Committee on Cancer (AJCC) staging manual (16); ii) patients harboring either the exon 19-del or 21-L858R mutation which was detected with PCR (17); iii) no serious cardiovascular disease or other diseases precluding patients from receiving chemotherapy or EGFR-TKI therapy; iv) presence of at least 1 measurable lesion assessable by computed tomography (CT) or magnetic resonance imaging (MRI); and v) Karnofsky performance status score >70 and a life expectancy ≥3 months.
The exclusion criteria were as follows: i) Patients with small cell or mixed small cell histology; ii) unknown EGFR mutation type; iii) absence of measurable lesions according to the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 (18); and iv) life expectancy <3 months.
Treatment and patient follow-up
The 137 patients were divided into first-line TKI treatment, first-line chemotherapy and second-line TKI treatment groups. A total of 89 patients were treated with first-line TKIs. First-line chemotherapy was administered to 48 patients, among who 27 received TKIs as second-line treatment following disease progression.
The oral TKIs gefitinib (250 mg/day), erlotinib (150 mg/day) or icotinib (375 mg/day) were administered as the first-line or second-line treatment for the collected patients with NSCLC until disease progression or development of intolerable adverse effects, such as severe rash, diarrhea, liver and kidney toxicity. The chemotherapy consisted of a platinum-based combination regimen, including pemetrexed (500 mg/m2; day 1) plus cisplatin or nedaplatin (75 mg/m2; day 1), gemcitabine (1,000 mg/m2; days 1 and 8) plus cisplatin or nedaplatin (75 mg/m2; day 1), docetaxel (75 mg/m2; day 1) plus cisplatin or nedaplatin (75 mg/m2; day 1), and taxol (175 mg/m2; day 1) plus cisplatin or nedaplatin (75 mg/m2; day 1), once every 21 days. A dose reduction to 80% was allowed in case of treatment-associated grade 3 or selected lengthy grade 2 toxicities.
Disease evaluation was initiated 4 weeks post-treatment and was performed every 8 weeks thereafter until disease progression or the start of new anticancer therapies. Evaluation tools included chest CT scans, brain MRI with and without contrast, abdominal CT scans, bone emission CT scans and positron emission tomography-CT scans if necessary.
Statistical analysis
Progression-free survival (PFS) was defined as the time from the first administration of first-line EGFR-TKIs, first-line chemotherapy or second-line EGFR-TKI treatment to the confirmation of disease progression or mortality from any cause. The tumor response to EGFR-TKI treatment or chemotherapy was assessed according to RECIST v1.1. Objective response rate (ORR) was defined as the sum of patients with a complete response or partial response divided by the total number of treated patients with measurable disease. Disease control rate (DCR) was defined as the number of patients with a complete response, partial response or stable disease divided by the total number of patients treated. The last follow-up date was August 31, 2017. The baseline characteristics of patients were compared between the EGFR exon 19-del and 21-L858R genotype groups using Pearson's χ2 test or Fisher's exact tests (when there were <5 expected counts in the contingency table) for categorical variables. Kaplan-Meier analysis was applied to evaluate PFS. Univariate and multivariate Cox proportional hazards analyses were conducted to identify factors associated with increased risk of disease progression. All statistical analyses were performed using SPSS (v17.0; SPSS, Inc., Chicago, IL, USA). Tests were 2-sided and P<0.05 was considered to indicate a statistically significant difference.
Results
Baseline patient characteristics
Table I presents the baseline characteristics of the involved patients. The median age was 58 years (range, 32–93 years) and there were 69 men and 68 women, of whom 39 were smokers and 98 were non-smokers. There were 71 patients with lesions in the left lung and 66 in the right lung. According to the 7th edition of the AJCC staging manual (16), there were 2 cases with stage IIIB and 135 cases with stage IV cancer. A total of 79 cases with exon 19-del mutation and 58 cases with 21-L858R mutation were identified.
Table I.
Patient characteristics.
| Characteristics | n | % |
|---|---|---|
| Age, years | ||
| ≤60 | 82 | 59.9 |
| >60 | 55 | 40.1 |
| Sex | ||
| Male | 69 | 50.4 |
| Female | 68 | 49.6 |
| Stage | ||
| IIIB | 2 | 1.5 |
| IV | 135 | 98.5 |
| Smoking | ||
| Ever | 39 | 28.5 |
| Never | 98 | 71.5 |
| Primary site | ||
| Left side | 71 | 51.8 |
| Right side | 66 | 48.2 |
| EGFR | ||
| 19-Del | 79 | 57.7 |
| 21-L858R | 58 | 42.3 |
EGFR, epidermal growth factor receptor.
The characteristics of patients harboring either the exon 19-del or 21-L858R mutations treated with different treatment protocols are presented in Table II. The percentages of patients harboring exon 19-del and 21-L858R mutations were 58.4% (52/89) and 41.6% (37/89) in the first-line EGFR-TKI treatment group, 56.3% (27/48) and 43.8% (21/48) in the first-line chemotherapy group, and 48.1% (13/27) and 51.9% (14/27) in the second-line EGFR-TKI treatment group, respectively. The patients with either the exon 19-del or 21-L858R mutations were proportionate in terms of sex, age, smoking status, primary tumor sites and stage. In the first-line and second-line EGFR-TKI treatment groups, patients with exon 19-del and 21-L858R mutations received similar types of EGFR-TKI treatment. The cycles of chemotherapy were comparable between patients with exon 19-del and 21-L858R mutations in the first-line chemotherapy group.
Table II.
Characteristics of the patients with either the exon 19-del or 21-L858R mutation receiving different treatments.
| First-line EGFR-TKIs | First-line chemotherapy | Second-line EGFR-TKIs | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Total, n (%) | 19-del, n (%) | 21-L858R, n (%) | P-value | Total, (%) | 19-del, n (%) | 21-L858R, n (%) | P-value | Total, n (%) | 19-del, n (%) | 21-L858R, n (%) | P-value |
| All patients | 89 (100.0) | 52 (58.4) | 37 (41.6) | 48 (100.0) | 27 (56.3) | 21 (43.7) | 27 (100.0) | 13 (48.1) | 14 (51.9) | |||
| Age, years | >0.999 | |||||||||||
| ≤60 | 51 (57.3) | 34 (65.4) | 17 (45.9) | 0.068 | 31 (64.6) | 18 (66.7) | 13 (61.9) | 0.732 | 18 (66.7) | 9 (69.2) | 9 (64.3) | |
| >60 | 38 (42.7) | 18 (34.6) | 20 (54.1) | 17 (35.4) | 9 (33.3) | 8 (38.1) | 9 (33.3) | 4 (30.8) | 5 (35.7) | |||
| Sex | >0.999 | |||||||||||
| Male | 41 (46.1) | 25 (48.1) | 16 (43.2) | 0.652 | 28 (58.3) | 18 (66.7) | 10 (47.6) | 0.184 | 15 (55.6) | 7 (53.8) | 8 (57.1) | |
| Female | 48 (53.9) | 27 (51.9) | 21 (56.8) | 20 (41.7) | 9 (33.3) | 11 (52.4) | 12 (44.4) | 6 (46.2) | 6 (42.9) | |||
| Stage | ||||||||||||
| IIIB | 1 (1.1) | 0 (0.0) | 1 (2.7) | 1 (2.1) | 1 (3.7) | 0 (0.0) | 1 (3.7) | 1 (7.7) | 0 (0.0) | |||
| IV | 88 (98.9) | 52 (100.0) | 36 (97.3) | 47 (97.9) | 26 (96.3) | 21 (100.0) | 26 (96.3) | 12 (92.3) | 14 (100.0) | |||
| Smoking | 0.695 | |||||||||||
| Ever | 22 (24.7) | 13 (25.0) | 9 (24.3) | 0.942 | 17 (35.4) | 10 (37.0) | 7 (33.3) | 0.790 | 10 (37.0) | 4 (30.8) | 6 (42.9) | |
| Never | 67 (75.3) | 39 (75.0) | 28 (75.7) | 31 (64.6) | 17 (63.0) | 14 (66.7) | 17 (63.0) | 9 (69.2) | 8 (57.1) | |||
| Primary site | >0.999 | |||||||||||
| Left lung | 45 (50.6) | 27 (51.9) | 18 (48.6) | 0.761 | 26 (54.2) | 15 (55.6) | 11 (52.4) | 0.827 | 15 (55.6) | 7 (53.8) | 8 (57.1) | |
| Right lung | 44 (49.4) | 25 (48.1) | 19 (51.4) | 22 (45.8) | 12 (44.4) | 10 (47.6) | 12 (44.4) | 6 (46.2) | 6 (42.9) | |||
| Drug categories | 0.660 | |||||||||||
| Gefitinib | 58 (65.2) | 34 (65.4) | 24 (64.9) | 0.815 | 16 (59.3) | 8 (61.5) | 8 (57.1) | |||||
| Icotinib | 17 (19.1) | 9 (17.3) | 8 (21.6) | 6 (22.2) | 2 (15.4) | 4 (28.6) | ||||||
| Erlotinib | 14 (15.7) | 9 (17.3) | 5 (13.5) | 5 (18.5) | 3 (23.1) | 2 (14.3) | ||||||
| Cycles of chemotherapy | 0.999 | |||||||||||
| ≤4 | 28 (58.3) | 15 (55.6) | 13 (61.9) | 0.658 | 16 (59.3) | 8 (61.5) | 8 (57.1) | |||||
| >4 | 20 (41.7) | 12 (44.4) | 8 (38.1) | 11 (40.7) | 5 (38.5) | 6 (42.9) | ||||||
EGFR, epidermal growth factor receptor; TKIs, tyrosine kinase inhibitors.
ORR and DCR
The objective response of patients harboring either the exon 19-del or 21-L858R mutations to the first-line EGFR-TKI, first-line chemotherapy and second-line EGFR-TKI treatments is presented in Table III. The ORR and DCR for patients with the exon 19-del and 21-L858R mutations were 51.9 vs. 27.0% (P=0.019) and 96.2 vs. 83.8% (P=0.102) following first-line EGFR-TKI treatment, 18.5 vs. 4.8% (P=0.322) and 77.8 vs. 52.4% (P=0.064) following first-line chemotherapy, and 15.4 vs. 42.9% (P=0.209) and 76.9 vs. 92.9% (P=0.326) following second-line EGFR-TKI treatment, respectively.
Table III.
Disease response to the different treatments in patients with either the exon 19-del or the 21-L858R mutation type.
| First-line EGFR-TKIs | First-line chemotherapy | Second-line EGFR-TKIs | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Response | 19-del | 21-L858R | P-value | 19-del | 21-L858R | P-value | 19-del | 21-L858R | P-value |
| D, n | 2 | 6 | 6 | 10 | 3 | 1 | |||
| SD, n | 23 | 21 | 16 | 10 | 8 | 7 | |||
| PR, n | 25 | 10 | 5 | 1 | 2 | 6 | |||
| CR, n | 2 | 0 | 0 | 0 | 0 | 0 | |||
| ORR, n (%) | 27 (51.9) | 10 (27.0) | 0.019 | 5 (18.5) | 1 (4.8) | 0.322 | 2 (15.4) | 6 (42.9) | 0.209 |
| DCR, n (%) | 50 (96.2) | 31 (83.8) | 0.102 | 21 (77.8) | 11 (52.4) | 0.064 | 10 (76.9) | 13 (92.9) | 0.326 |
PD, progressive disease; SD, stable disease; PR, partial response; CR, complete response; ORR, overall response rate; DCR, disease control rate; EGFR, epidermal growth factor receptor; TKIs, tyrosine kinase inhibitors.
Regardless of the mutation type, the differences between ORR and DCR in each treatment method were compared in the present study. It was revealed that the ORR and DCR were 41.6% (37/89) and 91.0% (81/89) following first-line EGFR-TKI treatment, 12.5% (6/48) and 66.7% (32/48) following first-line chemotherapy, and 29.6% (8/27) and 85.2% (23/27) following second-line TKI treatment, respectively. Compared with first-line chemotherapy treatment, first-line EGFR-TKI treatment resulted in an increased ORR (41.6 vs. 12.5%; P<0.05) and DCR (91.0 vs. 66.7%; P<0.05). Specifically, in patients with the exon 19-del mutation, first-line TKI treatment resulted in an increased ORR (51.9 vs. 18.5%; P=0.004) and DCR (96.2 vs. 77.8%; P=0.03) compared with first-line chemotherapy. Similarly, in patients with the 21-L858R mutation, first-line TKI treatment also resulted in an increased ORR (27.0 vs. 4.8%; P=0.044) and DCR (83.8 vs. 52.4%; P=0.01) compared with first-line chemotherapy.
There was a similar ORR (41.6 vs. 29.6%; P>0.05) and DCR (91.0 vs. 85.2%; P>0.05) between the first-line and second-line TKI treatment groups. According to the EGFR mutation status, in patients with the exon 19-del mutation, first-line TKI treatment resulted in an increased ORR (51.9 vs. 15.4%; P=0.018) and a similar DCR (96.2 vs. 76.9%; P=0.081) compared with second-line TKI treatment. However, in patients with the 21-L858R mutation, the first-line and second-line TKI treatment resulted in a similar ORR (27 vs. 42.9%; P=0.322) and DCR (83.8 vs. 92.9%; P=0.657).
PFS time
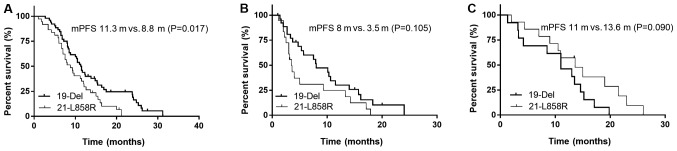
The median PFS time for patients with the exon 19-del and 21-L858R mutations was 11.3 vs. 8.8 months (P=0.017) following first-line EGFR-TKIs, 8.0 vs. 3.5 months (P=0.105) following first-line chemotherapy and 11.0 vs. 13.6 months (P=0.090) following second-line EGFR-TKIs, respectively (Fig. 1).
Figure 1.
mPFS of patients with the exon 19-del or 21-L858R mutation administered with different treatment types. (A) The mPFS times for patients harboring the 19-del and 21-L858R mutation were 11.3 and 8.8 months (P=0.017), respectively, following use of first-line TKIs. (B) The mPFS times for patients harboring the 19-del and 21-L858R mutation were 8.0 and 3.5 months (P=0.105), respectively, following first-line chemotherapy. (C) The mPFS times for patients harboring the 19-del and 21-L858R mutation were 11.0 and 13.6 months (P=0.090), respectively, following use of second-line TKIs. mPFS, median progression-free survival; TKIs, tyrosine kinase inhibitors.
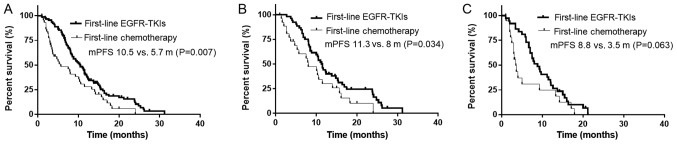
The median PFS time of the enrolled patients with NSCLC was 10.5 months following first-line TKI treatment, 5.7 months following first-line chemotherapy and 13.0 months following second-line TKI treatment. The median PFS time of patients treated with first-line TKIs was significantly improved compared with that of patients treated with first-line chemotherapy (10.5 vs. 5.7 months; P=0.007; Fig. 2A). Specifically, in patients harboring the exon 19-del, first-line TKI treatment led to the prolongation of the median PFS time (11.3 vs. 8.0 months; P=0.034) compared with that of first-line chemotherapy (Fig. 2B). However, in patients harboring the 21-L858R mutation, there was no significant difference in the median PFS time (8.8 vs. 3.5 months; P=0.063) between the exon 19-del and 21-L858R treatment groups (Fig. 2C).
Figure 2.
mPFS of patients treated with first-line TKIs versus first-line chemotherapy, and first-line versus second-line TKIs. (A) The mPFS times of patients treated with first-line TKIs and chemotherapy were 10.5 and 5.7 months (P=0.007), respectively. (B) In patients with the 19-del mutation, the mPFS times of patients treated with first-line TKIs and chemotherapy were 11.3 and 8.0 months (P=0.034), respectively. (C) In patients with the 21-L858R mutation, the PFS times of patients treated with first-line TKIs and chemotherapy were 8.8 and 3.5 months (P=0.063), respectively. mPFS, median progression-free survival; TKIs, tyrosine kinase inhibitors.
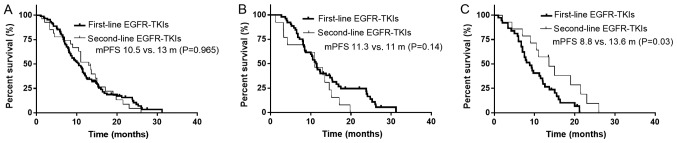
There was no significant difference in the median PFS time between patients treated with first- and second-line TKIs (13.0 vs. 10.5 months, respectively; P=0.965; Fig. 3A). In patients with the exon 19-del mutation, first- and second-line TKI treatment also led to a similar median PFS time (11.3 vs. 11.0 months; P=0.140; Fig. 3B). However, in patients with the 21-L858R mutation, second-line TKI treatment resulted in a longer median PFS time compared with that in patients with the first-line TKI treatment (8.8 vs. 13.6 months, respectively; P=0.030; Fig. 3C).
Figure 3.
mPFS of patients treated with first-line and second-line TKIs. (A) The mPFS times of patients treated with first and second-line TKIs were 10.5 and 13.0 months (P=0.965), respectively. (B) In patients with the 19-del mutation, the mPFS times of patients treated with first- and second-line TKIs were 11.3 and 11.0 months (P=0.140), respectively. (C) In patients with the 21-L858R mutation, the mPFS times of those treated with first- and second-line TKIs were 8.8 and 13.6 months (P=0.03), respectively. mPFS, median progression-free survival; TKIs, tyrosine kinase inhibitors.
Univariate and multivariate analyses
The results of the univariate and multivariate analyses of PFS time for patients with NSCLC treated with first-line EGFR-TKIs, first-line chemotherapy and second-line EGFR-TKIs indicated that the type of EGFR mutation was an independent predictor of PFS time for patients with NSCLC treated with first-line TKIs [hazard ratio (HR), 2.071; 95% confidence interval (CI), 1.120–3.480; P=0.006; Table IV]. First-line chemotherapy of >4 cycles was also associated with a longer PFS time (HR, 0.444; 95% CI, 0.214–0.921; P=0.029).
Table IV.
Univariate and multivariate analysis on PFS for NSCLC patients with either the exon 19-del or 21-L858R mutation type.
| First-line EGFR-TKIs | First-line chemotherapy | Second-line EGFR-TKIs | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | |||||||
| Characteristics | n | P-value | HR (95% CI) | P-value | n | P-value | HR (95% CI) | P-value | n | P-value | HR (95% CI) | P-value |
| EGFR | ||||||||||||
| 19-Del | 52 | 0.018 | 2.071 (1.120–3.480) | 0.006 | 27 | 0.105 | 2.086 (0.988–4.402) | 0.054 | 13 | 0.090 | 0.766 (0.275–2.587) | 0.843 |
| 21-L858R | 37 | 21 | 14 | |||||||||
| Age, years | ||||||||||||
| ≤60 | 41 | 0.629 | 0.684 (0.418–1.119) | 0.131 | 31 | 0.688 | 0.751 (0.378–1.491) | 0.423 | 18 | 0.288 | 0.777 (0.195–3.090) | 0.720 |
| >60 | 48 | 17 | 9 | |||||||||
| Sex | ||||||||||||
| Male | 41 | 0.421 | 0.891 (0.525–1.511) | 0.668 | 28 | 0.350 | 1.145 (0.48–2.732) | 0.760 | 15 | 0.786 | 1.191 (0.243–5.830) | 0.830 |
| Female | 48 | 20 | 12 | |||||||||
| Smoking | ||||||||||||
| Ever | 22 | 0.646 | 1.141 (0.604–2.155) | 0.685 | 17 | 0.715 | 0.938 (0.394–2.234) | 0.886 | 10 | 0.891 | 1.057 (0.195–5.735) | 0.948 |
| Never | 67 | 31 | 17 | |||||||||
| Primary site | ||||||||||||
| Left lung | 45 | 0.138 | 0.721 (0.452–1.151) | 0.170 | 26 | 0.804 | 1.143 0.577–2.266 | 0.701 | 15 | 0.105 | 1.28 0.334–4.893 | 0.720 |
| Right lung | 44 | 22 | 12 | |||||||||
| Drug categories | ||||||||||||
| Gefitinib | 58 | 0.517 | 0.990 (0.720–1.361) | 0.951 | 16 | 0.261 | 0.789 (0.381–1.672) | 0.550 | ||||
| Icotinib | 17 | 6 | ||||||||||
| Erlotinib | 14 | 5 | ||||||||||
| Cycles of chemotherapy | ||||||||||||
| ≤4 | 28 | 0.070 | 0.444 (0.214–0.921) | 0.029 | 16 | 0.707 | 0.905 (0.246–3.329) | 0.881 | ||||
| >4 | 20 | 11 | ||||||||||
PFS, progression-free survival; NSCLC, non-small cell lung cancer; CI, confidence interval; HR, hazard ratio; EGFR, epidermal growth factor receptor; TKIs, tyrosine kinase inhibitors.
Discussion
Lung cancer is one of the leading causes of mortality worldwide (19). Due to their tumor-targeting properties and significant therapeutic efficacy, EGFR-TKIs have been a focus of investigation and their application has lead to major advances in the treatment of NSCLC, particularly for patients with either the EGFR exon 19 del or 21-L858R mutations (10,20).
Several studies indicated that treatment with EGFR-TKIs resulted in significant improvements in PFS time, quality of life and tolerance to treatment (6,21–24). The LUX-Lung6 study (25) demonstrated that treatment with EGFR-TKIs led to a significantly longer PFS time (11.0 vs. 5.6 months; P<0.001) and increased ORR (66.9 vs. 23%; P<0.001) and DCR (92.6 vs. 76.2%; P<0.001) compared with those obtained using standard chemotherapy. As in the aforementioned studies, the present results suggested that the PFS time (10.5 vs. 5.7 months; P=0.007), ORR (41.6 vs. 12.5%; P<0.05) and DCR (91.0 vs. 66.7%; P<0.05) of patients with NSCLC harboring EGFR mutations treated with first-line TKIs were improved compared with those in patients treated with chemotherapy.
Wu et al (26) analyzed the data of 152 patients with advanced NSCLC harboring EGFR mutations in Taiwan, among whom 91 were treated with first-line gefitinib and 61 were treated with second-line gefitinib. Similar PFS and OS times were observed between the first-line and second-line gefitinib treatment groups. In addition, a Spanish Lung Cancer Group trial (27) demonstrated no significant differences in PFS and OS times between first- and second-line erlotinib treatments. Similarly, in the present study, the PFS time, ORR and DCR of patients harboring the exon 19-del and 21-L858R mutations were 10.5 vs. 13.0 months, 41.6 vs. 29.6% and 91.0 vs. 85.2% (P>0.05), respectively.
Several previous studies indicated that patients with the EGFR exon 19-del and 21-L858R mutations exhibited distinguishing clinical characteristics and different prognoses. A subgroup analysis of the IPASS (21) study indicated that, among patients with the EGFR exon 19-del, the ORR in the gefitinib group increased significantly compared with that in the chemotherapy group (84.8 vs. 43.2%). However, among patients with the 21-L858R mutation, there was no significant difference between the exon 19-del and 21-L858R mutation groups. Other studies also reported that the exon 19-del mutation was associated with an improved prognosis compared with the 21-L858R mutation in patients with stage IIIB-IV NSCLC (28,29). When treated with first-line TKIs, patients with the exon 19-del mutation exhibited an increased ORR (51.9 vs. 27%; P=0.019) and longer PFS time (11.3 vs. 8.8 months; P=0.017) compared with that in patients with the 21-L858R mutation. Accordingly, the ORR (73.0 vs. 50%; P=0.025) and PFS time (24.0 vs. 10 months; P=0.04) of patients with the exon 19-del mutation in the present study was also improved compared with those in patients with the 21-L858R mutation.
The mechanism underlying the different sensitivities to EGFR-TKI treatment between the exon 19-del and 21-L858R mutations remains to be elucidated. Previous studies suggested that, following treatment with gefitinib, the levels of G1 arrest increased in cells with the exon 19-del mutation compared with that in cells with the 21-L858R mutation (30). Sordella et al (31) found that the exon 19-del and 21-L858R mutations altered the EGFR autophosphorylation and downstream signaling pathways. For example, Y845 was highly phosphorylated in cells harboring the 21-L858R mutation compared with cells harboring the exon 19-del mutation, which may contribute to the differential sensitivities to EGFR-TKI treatments. An additional explanation may be that the exon 19-del and 21-L858R mutations may exhibit different intrinsic sensitivities to the EGFR-TKIs (11). Finally, the 21-L858R mutation is more likely to occur in combination with other rare mutations, such as 21-L861Q, 18-G719X, 18-G719X and 20-Ins, which affects the sensitivity to EGFR-TKI treatment (32).
Previous studies indicated that patients with the EGFR exon 19-del exhibited a prolonged PFS time following first-line TKI treatment compared with that in patients with the 21-L858R mutation (15,29). However, data on the difference in prognosis between the exon 19-del and 21-L858R mutations following second-line treatment with TKIs are limited. Wang et al (33) identified that patients harboring the exon 19-del experienced a prolonged PFS (8.1 vs. 6.8 months; P=0.002) and OS (17.6 vs. 12.5 months; P<0.01) time, and an increased ORR (81.1 vs. 55.6%; P=0.002) compared with those in patients harboring the 21-L858R mutation following second-line TKI treatment. However, the results obtained in the present study were different. There was no significant difference in PFS time, ORR or DCR between patients with the exon 19-del and 21-L858R mutations following second-line TKI treatment. The reason for these discrepancies may be associated with the fact that patients in the study by Wang et al (33) received at least 1 cycle of chemotherapy prior to treatment with TKIs, and it was not indicated whether disease regression was observed following chemotherapy. By contrast, the present study selected patients with disease progression following at least 2 cycles of chemotherapy followed by treatment with TKIs as second-line therapy. Further analysis indicated that, in patients with the exon 19-del mutation, there were no significant differences in PFS time (11.3 vs. 11.0 months) or DCR (96.2 vs. 76.9%) between the first- and second-line TKI treatment groups. However, in patients with the 21-L858R mutation, second-line TKI treatment significantly prolonged the PFS time (13.6 vs. 8.8 months; P=0.030) compared with that in patients with first-line TKI treatment.
The reasons for this significant difference in PFS following second-line TKI treatment between patients harboring the exon 19-del and 21-L858R mutations remain to be elucidated. Some investigators have suggested that chemotherapy and radiotherapy may modify the tumor microenvironment and modulate the sensitivity of the mutant cells to the same TKI treatment, thus resulting in different response rates (34). Furthermore, chemotherapy and radiotherapy may eliminate chemoradiation-sensitive cells, while cells resistant to chemoradiotherapy remain and repopulate the tumor. In addition, chemotherapy may reduce the EGFR mutation rate of patients with NSCLC (35), which may differentially affect the occurrence of the exon 19-del and 21-L858R mutations. Furthermore, compared with tumors with the 21-L858R mutation, tumors in the exon 19-del group exhibited an increased EGFR T790 mutation rate (36), which may be another reason for patients with 21-L858R mutation to be more sensitive to second-line TKI treatment compared with patients with the exon 19-del mutation. Finally, the spatial and temporal intratumor heterogeneity may also contribute to different response rates to TKI treatment (37).
In conclusion, the present study demonstrated that the PFS time of patients harboring the exon 19-del mutation was significantly improved compared with that in patients with the 21-L858R mutation following first-line TKI treatment, while there was no significant difference in PFS time between the exon 19-del and 21-L858R mutation groups following first-line chemotherapy and second-line TKI treatment. In patients with the exon 19-del mutation, first- and second-line TKI treatment resulted in a similar median PFS time. However, in patients harboring the 21-L858R mutation, second-line TKI treatment resulted in a longer median PFS time compared with first-line TKI treatment. Whether patients harboring the exon 19-del mutation should be administered first-line TKIs, whereas those with the 21-L858R mutation should receive second-line TKIs, requires confirmation by large prospective clinical trials.
Acknowledgements
The authors would like to thank Dr Qiuji Wu (Zhongnan Hospital of Wuhan University, China) for providing useful additions to the manuscript prior to its submission.
Funding
The present study was funded by National Natural Science Foundation of China (grant no. 81472799).
Availability of data and materials
The datasets used during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
WH, QW, JZ and YZ were responsible for the study design, data analysis and manuscript preparation. WH wrote the manuscript. The final manuscript draft was approved by all the authors.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Jiang H. Overview of gefitinib in non-small cell lung cancer: An Asian perspective. Jpn J Clin Oncol. 2009;39:137–150. doi: 10.1093/jjco/hyn139. [DOI] [PubMed] [Google Scholar]
- 3.NSCLC Meta-Analyses Collaborative Group Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: A systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol. 2008;26:4617–4625. doi: 10.1200/JCO.2008.17.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lara-Guerra H, Waddell TK, Salvarrey MA, Joshua AM, Chung CT, Paul N, Boerner S, Sakurada A, Ludkovski O, Ma C, et al. Phase II study of preoperative gefitinib in clinical stage I non-small-cell lung cancer. J Clin Oncol. 2009;27:6229–6236. doi: 10.1200/JCO.2009.22.3370. [DOI] [PubMed] [Google Scholar]
- 5.Roberts PJ, Stinchcombe TE, Der CJ, Socinski MA. Personalized medicine in non-small-cell lung cancer: Is KRAS a useful marker in selecting patients for epidermal growth factor receptor-targeted therapy? J Clin Oncol. 2010;28:4769–4777. doi: 10.1200/JCO.2009.27.4365. [DOI] [PubMed] [Google Scholar]
- 6.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Isobe H, Gemma A, Harada M, Yoshizawa H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 7.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, Kris MG, Varmus H. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2015;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciardicllo F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160–1174. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 9.Coorper WA, Lam DC, O'Toole SA, Minna JD. Molecular biology of lung cancer. J Thorac Dis. 2013;5(Suppl 5):S479–S490. doi: 10.3978/j.issn.2072-1439.2013.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 11.Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 12.Gazdar AF. Activating and resistance mutations of EGFR in non-small-cell lung cancer: Role in clinical response to EGFR tyrosine kinase inhibitors. Oncology. 2009;28(Suppl 1):S24–S31. doi: 10.1038/onc.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 14.Chintala L, Kurzrock R. Epidermal growth factor receptor mutation and diverse tumors: Case report and concise literature review. Mol Oncol. 2010;4:306–308. doi: 10.1016/j.molonc.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riely GJ, Pao W, Pham DK, Li AR, Rizvi N, Venkatraman ES, Zakowski MF, Kris MG, Ladanyi M, Miller VA. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:839–844. doi: 10.1158/1078-0432.CCR-05-1846. [DOI] [PubMed] [Google Scholar]
- 16.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. 7th. Chicago: Springer; 2010. AJCC cancer staging manual; pp. 129–143. [Google Scholar]
- 17.Taron M, Ichinose Y, Rosell R, Mok T, Massuti B, Zamora L, Mate JL, Manegold C, Ono M, Queralt C, et al. Activating mutations in the tyrosine kinase domain of the epidermal growth factor receptor are associated with improved survival in gefitinib-treated chemorefractory lung adenocarcinomas. Clin Cancer Res. 2005;11:5878–5885. doi: 10.1158/1078-0432.CCR-04-2618. [DOI] [PubMed] [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogearts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Sugio K, Uramoto H, Onitsuka T, Mizukami M, Ichiki Y, Sugaya M, Yasuda M, Takenoyama M, Oyama T, Hanagiri T, Yasumoto K. Prospective phase II study of gefitinib in non-small cell lung cancer with epidermal growth factor receptor gene mutations. Lung Cancer. 2009;64:314–318. doi: 10.1016/j.lungcan.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Schuler M, Yang JCH, Yamamoto N, O'Byrne K, Hirsh V, Mok T, Massey D, Zazulina V, Shahidi M, Sequist L. LUX-Lung 3: A randomized, open-label, phase III study of afatinib versus pemetrexed and cisplatin as first-line treatment for patients with advanced adenocarcinoma of the lung harboring EGFR-activating mutations (Subgroup Analysis) Lung Cancer. 2012;77(Suppl 1):S25–S26. doi: 10.1016/j.lungcan.2012.05.043. [DOI] [Google Scholar]
- 21.Mok TS, Wu Y, Thongprasert S, Yang C, Chu D, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 22.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 23.Zhou C, Wu Y, Chen G, Feng J, Liu X, Wang C, Zhang S, Wang J, Zhou S, Ren S, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 24.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 25.Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, Li W, Hou M, Shi JH, Lee KY, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213–222. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 26.Wu JY, Yu CJ, Yang CH, Wu SG, Chiu YH, Gow CH, Chang YC, Hsu YC, Wei PF, Shih JY, Yang PC. First- or second-line therapy with gefitinib produces equal survival in non-small cell lung cancer. Am J Respir Crit Care Med. 2008;178:847–853. doi: 10.1164/rccm.200803-389OC. [DOI] [PubMed] [Google Scholar]
- 27.Massuti B, Morán T, Porta R, Queralt C, Cardenal F, Mayo C, Camps C, Majem M, Tarón M, Rosell R. Multicenter prospective trial of customized erlotinib for advanced non-small cell lung cancer (NSCLC) patients (p) with epidermal growth factor receptor (EGFR) mutations: Final results of the Spanish lung cancer group (SLCG) trial. J Clin Oncol. 2009;27:8023. [Google Scholar]
- 28.Wang H, Huang J, Yu X, Han S, Yan X, Sun S, Zhu X. Different efficacy of EGFR tyrosine kinase inhibitors and prognosis in patients with subtypes of EGFR-mutated advanced non-small cell lung cancer: A meta-analysis. J Cancer Res Clin Oncol. 2014;140:1901–1909. doi: 10.1007/s00432-014-1825-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackman DM, Yeap BY, Sequist LV, Lindeman N, Holmes AJ, Joshi VA, Bell DW, Huberman MS, Halmos B, Rabin MS, et al. Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:3908–3914. doi: 10.1158/1078-0432.CCR-06-0462. [DOI] [PubMed] [Google Scholar]
- 30.Zhu JQ, Zhong WZ, Zhang GC, Li R, Zhang XC, Guo AL, Zhang YF, An SJ, Mok TS, Wu YL. Better survival with EGFR exon 19 than exon 21 mutations in gefitinib-treated non-small cell lung cancer patients is due to differential inhibition of downstream signals. Cancer Lett. 2008;265:307–317. doi: 10.1016/j.canlet.2008.02.064. [DOI] [PubMed] [Google Scholar]
- 31.Sordella R, Bell DW, Haber DA, Settleman J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science. 2004;305:1163–1167. doi: 10.1126/science.1101637. [DOI] [PubMed] [Google Scholar]
- 32.Hata A, Yoshioka H, Fujita S, Kunimasa K, Kaji R, Imai Y, Tomii K, Iwasaku M, Nishiyama A, Ishida T, Katakami N. Complex mutations in the epidermal growth factor receptor gene in non-small cell lung cancer. J Thorac Oncol. 2010;5:1524–1528. doi: 10.1097/JTO.0b013e3181e8b3c5. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Li RQ, Ai YQ, Zhang J, Zhao PZ, Li YF, He WJ, Xia YX, Li WH. Exon 19 deletion was associated with better survival outcomes in advanced lung adenocarcinoma with mutant EGFR treated with EGFR-TKIs as second-line therapy after first-line chemotherapy: A retrospective analysis of 128 patients. Clin Transl Oncol. 2015;17:727–736. doi: 10.1007/s12094-015-1300-4. [DOI] [PubMed] [Google Scholar]
- 34.Risbud MV, Fertala J, Vresilovic EJ, Albert TJ, Shapiro IM. Nucleus pulposus cells upregulate PI3K/Akt and MEK/ERK signaling pathways under hypoxic conditions and resist apoptosis induced by serum withdrawal. Spine (Phila Pa 1976) 2005;30:882–889. doi: 10.1097/01.brs.0000159096.11248.6d. [DOI] [PubMed] [Google Scholar]
- 35.Bai H, Wang Z, Wang Y, Zhuo M, Zhou Q, Duan J, Yang L, Wu M, An T, Zhao J, Wang J. Detection and clinical significance of intratumoral EGFR mutational heterogeneity in Chinese patients with advanced non-small cell lung cancer. PLoS One. 2013;8:e54170. doi: 10.1371/journal.pone.0054170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ke EE, Zhou Q, Zhang QY, Su J, Chen ZH, Zhang XC, Xu CR, Yang JJ, Tu HY, Yan HH, et al. A higher proportion of the EGFR T790M mutation may contribute to the better survival of patients with exon 19 deletions compared with those with L858R. J Thorac Oncol. 2017;12:1368–1375. doi: 10.1016/j.jtho.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 37.Tomonaga N, Nakamura Y, Yamaguchi H, Ikeda T, Mizoguchi K, Motoshima K, Doi S, Nakatomi K, Iida T, Hayashi T, et al. Analysis of intratumor heterogeneity of EGFR mutations in mixed type lung adenocarcinoma. Clin Lung Cancer. 2013;14:521–526. doi: 10.1016/j.cllc.2013.04.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the present study are available from the corresponding author upon reasonable request.