Abstract
Melanoma is the most malignant type of skin cancer and is resistant to numerous chemotherapeutic and radiotherapy-based treatment approaches due to the activation of rapid and reversible pro-survival signaling pathways. As a result, patients will often present with a poor prognosis. Therefore, novel preventive methods and treatments are urgently required for patients with melanoma. Vitamin C (also known as L-ascorbic acid) is a water-soluble vitamin that is widely used as a dietary additive and has been demonstrated to exhibit anti-cancer properties. In the present study, the effects of vitamin C in human melanoma A375 cells, and the mechanisms underlying these effects were investigated. Vitamin C potently suppressed human melanoma A375 cell proliferation by inducing apoptosis in A375 cells. Induction of apoptosis was related to caspase-9 and caspase-3 activation and the mitochondrial membrane potential of A375 cells significantly decreased in the presence of vitamin C. Furthermore, vitamin C induced apoptosis in A375 cells by activating the Bax- and Bcl-2-mediated mitochondrial pathway. These results indicate that vitamin C may be a potentially useful clinical anti-tumor drug for treating patients with melanoma.
Keywords: vitamin C, melanoma, A375 cell, apoptosis, mitochondrial pathway
Introduction
Melanoma is a common skin cancer that has a high mortality rate due to its propensity to metastasize (1). According to the Global Burden of Disease Study of melanoma in 2015, there were known 351,800 cases of melanoma worldwide in 2015 with an age-standardized rate of five cases per 100,000 individuals (1). Melanoma was also responsible for 59,782 global deaths with an age-standardized rate of one death per 100,000 individuals during the same year (1). Melanomas which have been diagnosed at an early stage can usually be removed by surgical resection with no further complications for the patient. However, melanomas frequently have a high metastatic potential, and once metastasis occurs, they become very difficult to successfully treat (2). Indications for radiotherapy and chemotherapy as main therapeutic approaches to treat metastatic melanoma are limited because melanoma is considered to be radio- and chemotherapy resistant (2). Therefore, the novel therapeutic options are required for treating patients with advanced melanoma.
Vitamin C is a water-soluble vitamin found in certain foodstuffs and serves as a dietary supplement (3). There are numerous studies demonstrating the effects of vitamin C as a preventative treatment for various diseases. McCormick (4) showed that vitamin C deficiency served an important role in carcinogenesis. Numerous studies have investigated the use of vitamin C alone as a potential treatment to remedy cancer. Yun et al (5) demonstrated that high doses of vitamin C may selectively kill colorectal cancer cells with mutations in KRAS or BRAF. Mark Levine's group at The National Cancer Institute (National Institutes of Health, Bethesda, MD, USA) demonstrated that vitamin C killed cancer cells whilst doing less damage to healthy cells (6–8). Furthermore, vitamin C has been shown to induce cell death in various types of cancer cells, including mesothelioma, pancreatic cancer, leukemia and renal cell carcinoma cells (9–11). Taken together, the findings of all these studies indicate that high vitamin C doses may prevent the growth of tumor cells.
Vitamin C is taken into cells through active transport and simple diffusion (5). Because there are two biological forms of vitamin C, the transport process is mediated by two families of transport proteins, including the solute carrier gene family 23 and the SLC2 family of glucose transporters. The solute carrier gene family 23, consisting of sodium-dependent vitamin C transporters, are responsible for transporting ascorbic acid (reduced form of vitamin C), and glucose transporters transport dehydroascorbic acid (oxidized form of vitamin C) (5,12). The conversion between the two forms of vitamin C is regulated by redox reactions (12). Dehydroascorbic acid is reduced back to vitamin C by glutathione (GSH), and the GSH becomes oxidized glutathione. Consequently, the cellular redox state is changed. Vitamin C is sensitive to changes in the redox potential in the internal cellular environment and also regulates the redox state in cancer cells (12). Chen et al (8) demonstrated that high vitamin C doses increased the levels of reactive oxygen species (ROS) in cancer cells resulting in the apoptosis of these cells. However, the molecular mechanisms through which vitamin C affects melanoma cells are not completely understood.
The present study demonstrated the anti-tumor effects of vitamin C in the human melanoma A375 cell line for the first time, to the best of our knowledge. Additionally, a possible underlying mechanism resulting in the apoptosis of melanoma cells following vitamin C treatment was investigated.
Materials and methods
Chemicals and reagents
Vitamin C was purchased from Selleck Chemicals (Houston, TX, USA). MTT, phenylmethylsulfonyl fluoride (PMSF), RIPA lysis buffer and 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl enzamidazolocarbocyanin iodide (JC-1) were obtained from Sigma-Aldrich; Merck KGaA, (Darmstadt, Germany). Fluorescein isothiocyanate (FITC) Annexin V Apoptosis Detection kit with propidium iodide (PI) was purchased from BioLegend, Inc. (San Diego, CA, USA) and bicinchoninic acid (BCA) protein assay kit was purchased from Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China). Mitochondria Isolation kit for Cultured Cells was purchased from Beyotime Institute of Biotechnology (Haimen, China). Cytochrome c (cat. no. 11940S), Bax (cat. no. 5023S), Bcl-2 (cat. no. 4223S), caspase-3 (cat. no. 14220S), caspase-9 (cat. no. 9502S) and β-actin (cat. no. 4970S) antibodies were purchased from CST Biological Reagents Co., Ltd. (Shanghai, China) and horseradish peroxidase-conjugated anti-rabbit Immunoglobulin G (cat. no. D110065) was purchased from Sangon Biotech Co., Ltd (Shanghai, China). Other common chemicals were purchased from Shanghai Titanchem Co., Ltd. (Shanghai, China).
Cell culture
The human melanoma cell line, A375, was purchased from American Type Culture Collection (Manassas, VA, USA) and cultured in Dulbecco's modified Eagle's medium (HyClone; GE Healthcare, Logan, UT, USA) supplemented with 10% heat-inactivated fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 U ml−1 penicillin and 100 mg ml−1 streptomycin (both from HyClone; GE Healthcare) and subsequently incubated in a cell culture incubator at 37°C and 5% CO2, and the media was changed every 2 days.
Cell viability assay
Cells were seeded into 96-well plates at a density of 5×103 cells/well at 37°C overnight, and treated with different concentrations (0, 0.6, 1 and 1.4 mM) of vitamin C for 24 h. After treatment with vitamin C, 0.5 mg/ml MTT solution was added to each well, and cells were further incubated for 2 h at 37°C. The culture media was removed and 100 µl DMSO was added to each well. The absorbance was measured at 490 nm using an Infinite M200 PRO microplate reader (Tecan Group, Ltd., Mannedorf, Switzerland). All values were normalized to the control treatment.
Apoptosis assay
Apoptosis was examined using a FITC Annexin V Apoptosis Detection kit with PI, followed by flow cytometry according to the manufacturer's protocol. Cells were treated with vitamin C for 24 h, then harvested by trypsinization, and suspended in binding buffer (BioLegend, Inc.) at a density of 1×106 cells/ml. The cell suspension was stained with Annexin V-FITC and PI in the dark for 10–20 min at room temperature (20–25°C and analyzed using a FACS Calibur flow cytometer (BD Biosciences, San Jose, CA, USA). Data were analyzed using Flow Jo Version 7.2 (Tree Star, Inc., Ashland, OR, USA).
Mitochondrial membrane potential analysis
The effects of vitamin C on the mitochondrial membrane potential were determined using JC-1. A375 cells were seeded into 6-well plates at a density of 1×106 cells/well. Cells were incubated for 24 h prior to treating with various concentrations (0, 0.6, 1 and 1.4 mM) of vitamin C for 6 h, after which the cells were washed three times with PBS and incubated with JC-1 (10 µg/ml) at 37°C for 30 min. The fluorescence intensity of vitamin C-treated cells was examined by flow cytometry.
Western blotting
After incubation with vitamin C for 6 h, cells were washed three times with PBS. Cells were harvested and homogenized in ice-cold RIPA lysis buffer supplemented with 1 mM PMSF for 30 min, followed by centrifugation at 12,000 × g for 30 min at 4°C. Cell lysates were collected, and protein concentrations were determined using the BCA Protein assay kit (Nanjing KeyGen Biotech Co., Ltd.). Proteins were mixed with 4X loading buffer (Sangon Biotech Co., Ltd.) and boiled for 15 min to denature the proteins. Equivalent quantities of protein were loaded on a 10% polyacrylamide gel for electrophoresis and subsequently transferred onto a 0.22 µm polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA, USA). The membranes were blocked with 5% non-fat dried milk dissolved in Tris-buffered saline containing 0.05% Tween-20 (TBST) for 1 h at room temperature. The blot was then probed with the following primary antibodies: Bax (1:1,000), Bcl-2 (1:1,000), caspase-3 (1:1,000), caspase-9 (1:1,000) and β-actin (1:1,000) antibodies at 37°C for 4 h. After washing three times with TBST, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody (1:1,000) for 2 h at room temperature, and the bound antibody was visualized using an enhanced chemiluminescence solution (Pierce; Thermo Fisher Scientific, Inc.). The densitometry analysis of the integrated density values of the bands was performed using Image J (National Institutes of Health), and the calculated values were normalized to those of β-actin.
Western blot analysis for cytochrome c
Untreated and drug-treated cells were harvested by centrifugation at 1,000 g for 10 min at 4°C. The cell pellets were washed once with ice-cold PBS and resuspended with ice-cold PBS for cell counting. Mitochondria Isolation kit for cultured cells (Beyotime Institute of Biotechnology) was used to isolate the mitochondria according to the manufacturer's protocol. The protein was extracted from mitochondria using the included mitochondria lysis buffer. Subsequently, mitochondrial lysates were harvested, and western blotting was performed as described above. The blot was probed with a cytochrome c antibody (1:800) at 37°C for 4 h. After washing three times with TBST, membranes were incubated with 1:1,000 dilution of horseradish peroxidase-conjugated secondary antibody for 2 h at room temperature. Signals were visualized and quantified as described above.
Statistical analysis
GraphPad PRISM version 4.0 (GraphPad Software, Inc., La Jolla, CA, USA) was used for all statistical analysis and graph plotting. All values are represented as the mean ± standard deviation (SD) from at least three independent experiments. The data were analyzed using one-way analysis of variance followed by Fisher's multiple comparisons test or an unpaired Student's t-test, as appropriate. P<0.05 was considered to indicate a statistically significant difference.
Results
Vitamin C inhibits proliferation of A375 cells
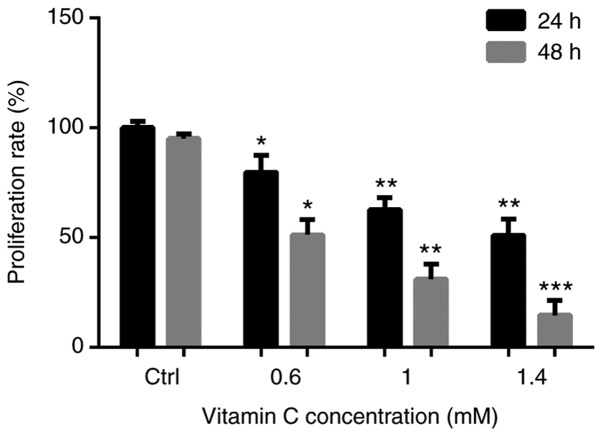
To determine the anti-proliferative activity of vitamin C on human melanoma A375 cells, cells were treated with 0, 0.6, 1 and 1.4-mM vitamin C for 24 h, and the viability of the cells was measured using an MTT assay. After treatment with vitamin C, the proliferation of A375 cells was significantly inhibited in a dose dependent manner. The proliferation rates were 79.64±7.75, 62.53±5.52 and 48.22±8.40% after treatment with 0.6, 1 and 1.4 mM of vitamin C for 24 h, respectively. Proliferation rates were 51.23±6.81, 31.02±6.79 and 14.53±6.77% after treatment with 0.6, 1 and 1.4 mM of vitamin C for 48 h, respectively (Fig. 1). As treatment with vitamin C for 48 h resulted in too many dead cells, it was decided that for all subsequent experiments, cells would be treated with vitamin C at the varying concentrations for 24 h only.
Figure 1.
Vitamin C suppresses the proliferation of the human melanoma cell line A375. Cells were incubated in the presence or absence of vitamin C (0, 0.6, 1 and 1.4 mM) for 24 and 48 h. The proliferation rate was detected by an MTT assay. Results are the representative of three independent experiments. *P<0.05, **P<0.01, ***P<0.01 vs. Ctrl treatment of the same time period. Ctrl, control.
Vitamin C induces apoptosis in A375 cells
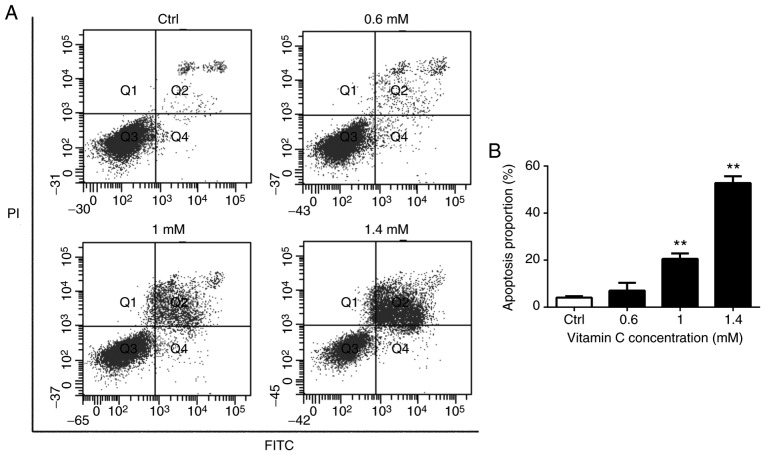
To verify the effects of vitamin C on apoptosis of A375 cells, Annexin V-FITC/PI double staining and flow cytometry was used to detect the induction of apoptosis in A375 cells treated with various concentrations of vitamin C. As presented in Fig. 2, there was a dose-dependent increase in the number of both early apoptotic cells (Q4 quadrant) and late apoptotic cells (Q2 quadrant) following treatment with vitamin C. The proportion of apoptotic cells is represented by the sum of proportion of early apoptotic cells and late apoptotic cells. Treatment with 1 and 1.4 mM vitamin C significantly increased the proportion of apoptotic cells compared with the control (P<0.01) as shown in Fig. 2B.
Figure 2.
Vitamin C induces apoptosis in human melanoma A375 cells. (A) Cells were treated with 0, 0.6, 1 and 1.4-mM vitamin C for 24 h and analyzed using Annexin V-FITC/PI by flow cytometry. (B) Histograms indicate apoptosis proportion from three separate experiments. **P<0.01 vs. Ctrl. FITC, fluorescein isothiocyanate; PI, propidium iodide; Ctrl, control.
Vitamin C decreases the mitochondrial membrane potential in A375 cells
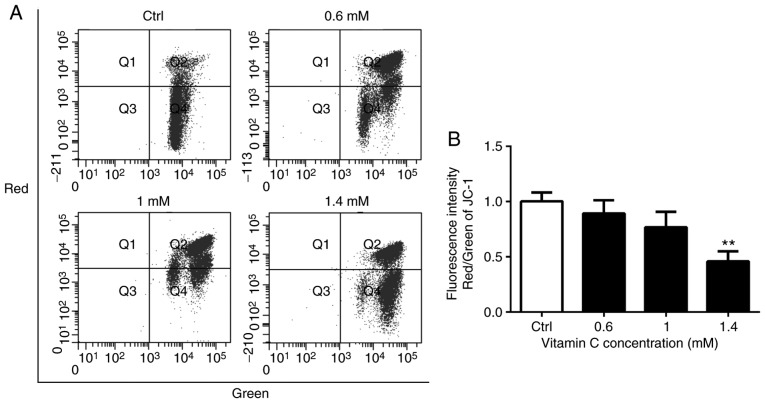
The loss of mitochondrial membrane potential is a common early event in apoptosis induced by a variety of stimuli (13,14). The integrity of the mitochondrial membrane potential was monitored during vitamin C-induced apoptosis using the mitochondrial membrane potential-sensitive dye JC-1 and flow cytometry (Fig. 3A). The vitamin C-treated cells were stained with JC-1, which accumulates in healthy mitochondria and fluoresced red, while cells with a depolarized mitochondrial membrane potential showed green fluorescence. The ratio of red to green fluorescence intensities was calculated. There was a decrease in the red/green ratio in vitamin C-treated A375 cells, indicating the loss of mitochondrial membrane potential following treatment with vitamin C (Fig. 3B). Treatment with 1.4 mM vitamin C significantly reduced the red/green ratio compared with the control (P<0.01; Fig. 3B).
Figure 3.
Vitamin C decreases mitochondrial membrane potential in A375 cells. (A) Cells were cultured and treated for 6 h in a medium containing 0, 0.6, 1 or 1.4-mM vitamin C. Cells were loaded with JC-1 and then analyzed by flow cytometry. Fluorescence intensities were measured with FACSCalibur flow cytometer. (B) Histograms indicate the ration of red to green fluorescence intensities in A375 cells after vitamin C treatment. **P<0.01 vs. Ctrl. JC-1, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl enzamidazolocarbocyanin iodide; Ctrl, control.
Effects of vitamin C on the Bax- and Bcl-2-mediated mitochondrial pathway
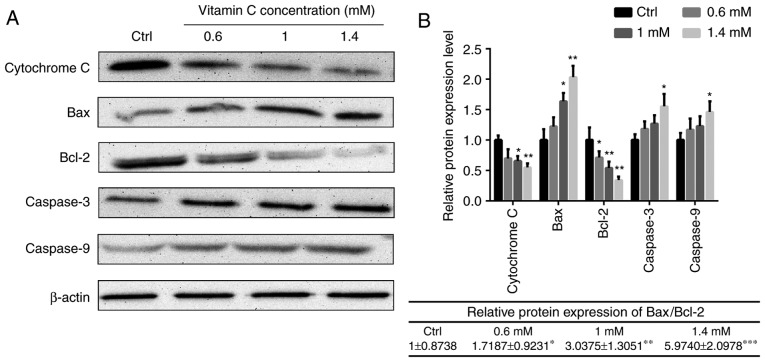
To illustrate the intracellular signaling pathway involved in the regulation of apoptosis by vitamin C, changes in the Bax- and Bcl-2-mediated mitochondrial pathway were investigated in human melanoma A375 cells. As presented in Fig. 4, the levels of cytochrome c in the mitochondrial fractions were decreased in cells treated with vitamin C compared with the control in a dose-dependent manner. The relative protein expression of Bax/Bcl-2 was 1±0.8738 in control group. The ratio was 1.7187±0.9231, 3.0375±1.3051 and 5.9740±2.0978 when treated with different concentration of vitamin C. So following vitamin C treatment, the ratio of Bax/Bcl-2 was significantly increased compared with control group in a dose-dependent manner. In addition, the protein expression levels of caspase-3 and caspase-9 were also increased after vitamin C treatment (Fig. 4).
Figure 4.
Effect of vitamin C on the expression of apoptosis-associated proteins in A375 cells. (A) Whole cell extracts and mitochondria lysis were prepared for cells treated with vitamin C for 6 h, and the expression levels of mitochondrial-cytochrome c, Bax, Bcl-2, caspase-3 and caspase-9 were determined by western blotting. (B) Relative protein expression levels of mitochondrial-cytochrome c, Bax, Bcl-2, caspase-3 and caspase-9 are shown. β-actin was used as the internal control. *P<0.05, **P<0.01 vs. Ctrl. Ctrl, control.
Discussion
Vitamin C has been reported to serve an important role in the treatment and the prevention of various types of cancer (15,16). Although there has been a considerable amount of effort devoted to discovering its mode of action, the mechanisms underlying these effects remained obscure. In the present study, an attempt at clarifying the specific mechanism of cell death induced by vitamin C was made. Vitamin C inhibited cell growth and induced cell apoptosis in the human melanoma A375 cell line, and that this occurred through a decrease in mitochondrial membrane potential and the subsequent release of cytochrome c. Furthermore, the expression of the downstream apoptotic pathway proteins caspase-9 and caspase-3 significantly increased following treatment with vitamin C. These results indicate that vitamin C induced apoptosis via the mitochondria-dependent pathway. Additionally, the expression of the apoptotic protein Bax increased, while the expression of the anti-apoptotic protein Bcl-2 decreased following treatment with vitamin C.
The mammalian Bcl-2 protein family is divided into two categories: i) Proapoptotic proteins including Bcl-2 antagonist killer 1, Bax and possibly Bcl-2-related ovarian killer; ii) anti-apoptotic proteins consisting of Bcl-2, Bcl-xL, Bclw, myeloid cell leukemia 1 and Bcl-2-related gene A1. Their functions are regulated by a third class of Bcl-2 family, the BH3-only proteins (17,18). The total intracellular ratio of Bax and Bcl-2 impacts cell fate, either apoptosis or survival (19). Bax redistributes cytochrome c to the cytosol, while Bcl-2 prevents the collapse of the mitochondrial membrane potential and prevents the release of cytochrome c (20). In the present study, vitamin C increased the ratio of Bax/Bcl-2 expression levels and this may result in an increase of cytochrome c release into the cytoplasm and subsequent activation of caspases. The activated caspases may subsequently degrade structural and nuclear proteins, resulting in the apoptosis of cells. The apoptosis effector caspase-3 and the apoptosis initiator caspase-9 were activated following treatment with vitamin C, suggesting that each of these caspases contributed to vitamin C-induced apoptosis in A375 cells.
Vitamin C is an important antioxidant and an essential nutrient (21), and the results of the present study suggest that it exhibits an anti-tumor activity through promoting apoptosis. Mata et al (22) conducted a series of non-clinical studies using different tumor cell lineages to examine the effect of different concentrations of vitamin C for the treatment and/or prevention of these types of cancer. They demonstrated that vitamin C at a concentration range of 0.1–4 mM significantly inhibited the proliferation of these cancer cell lines (22). Chen et al (6–8) additionally demonstrated that the growth and weight of ovarian, pancreatic and glioblastoma tumor xenografts in athymic nude mice decreased when intraperitoneally injected with vitamin C, and they demonstrated that mM concentrations of vitamin C killed cancer cells, but had less of an effect on normal cells (23). Clinical studies regarding the application of vitamin C have also been conducted. A previous study reported three cases of patients with cancer who received intravenous vitamin C as their primary therapy assisting additional treatments such as other minerals, botanicals and other vitamins (24). Although the histopathological examination of these cases suggested poor prognoses, patients had a longer overall survival duration following treatment compared with the control group (24). In contrast, mixed results have been reported in other studies where vitamin C was combined with other treatments. A small study consisting of 14 patients with advanced pancreatic cancer, intraperitoneal injection of vitamin C was administered alongside chemotherapy. The patients experienced very few side effects of the vitamin C treatment (25); however, other studies reported adverse side effects when vitamin C was used in combination with other drugs in patients with acute myeloid leukemia, metastatic melanoma and refractory metastatic colorectal cancer (26–28), indicating that vitamin C treatment may be associated with serious side effects and disease progression.
According to in vitro studies, vitamin C less than the concentration of 4 mM is an antioxidant that inactivates ROS. However, at higher concentrations of up to 20 mM, vitamin C acts as a pro-oxidant and generates oxidative species (22,29). Therefore, the effects of vitamin C can vary depending on its concentration. One study demonstrated that high concentrations of vitamin C induced cytotoxicity in malignant melanoma but promoted tumor growth at lower concentrations (30). In another example, at low mM concentrations, vitamin C could kill some cell lines in vitro by producing superoxide radicals and hydrogen peroxide, which are responsible for its cytotoxic activity in vivo; however, 20 mM vitamin C did not pose any risk to nonmalignant cell lines (31). Other studies have confirmed that high vitamin C doses are effective in inducing apoptosis in cancer cell in vitro as well as in vivo (32–37). However, the mechanism through which vitamin C induces cancer cell apoptosis in vitro has not been completely established. Previously, vitamin C was thought to induce apoptosis in vitro through endoplasmic reticulum stress-associated pathways and the mitochondrial pathway (38–40). In the present study, the apoptosis-inducing activity of high concentrations of vitamin C was investigated and a potential mechanism for induction of apoptosis was identified. The present study has certain limitations. The effects of lower concentrations of vitamin C on the human melanoma A375 cells were not tested. Additionally, only one human melanoma cell line was tested, and a control healthy skin cell line was not used. Therefore, further research is required to elucidate the effect of lower concentrations of vitamin C on a range of melanoma cells. The effect of vitamin C at different concentrations is being studied by our group on a range of different melanoma cell lines. Nevertheless, the present study provides an insight into the underlying mechanisms of vitamin C induced-apoptosis and provides additional evidence for the potential use of vitamin C as either a mainline or adjuvant therapy in the treatment of cancer.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- Bcl-2
BCL2 apoptosis regulator
- Bax
BCL2 associated X
- ROS
reactive oxygen species
- JC-1
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl enzamidazolocarbocyanin iodide
Funding
The present study was funded by The National Natural Science Foundation of China (grant nos. 81872162, 81602556 and 31870338), The Natural Science Foundation of Shandong Province (grant no. ZR2017JL030), Taishan Scholars Construction Engineering of Shandong Province, Yantai High-End Talent Introduction Plan ‘Double Hundred’, The Scientific Research Foundation of Binzhou Medical University (grant nos. BY2016KYQD01 and BY2018KYQD07) and The Dominant Disciplines' Talent Team Development Scheme of Higher Education of Shandong Province.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the author on reasonable request.
Authors' contributions
XYC, YC, DFL and QZ conceived and designed the study. XYC, YC, YQ and WJL performed the experiments. Statistical analysis was performed by ZHP, CJQ and XZ under the supervision of DFL and QZ. ZHP prepared the figures. All authors contributed to the analysis and interpretation of the data. XYC wrote the manuscript with contributions from all other authors. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Agarwala SS. Current systemic therapy for metastatic melanoma. Expert Rev Anticancer Ther. 2009;9:587–595. doi: 10.1586/era.09.25. [DOI] [PubMed] [Google Scholar]
- 2.Miller AJ, Mihm MC., Jr Melanoma. N Engl J Med. 2006;355:51–65. doi: 10.1056/NEJMra052166. [DOI] [PubMed] [Google Scholar]
- 3.Blake CJ. Analytical procedures for water-soluble vitamins in foods and dietary supplements: A review. Anal Bioanal Chem. 2007;389:63–76. doi: 10.1007/s00216-007-1309-9. [DOI] [PubMed] [Google Scholar]
- 4.McCormick WJ. Cancer: A collagen disease, secondary to a nutritional deficiency. Arch Pediatr. 1959;76:166–171. [PubMed] [Google Scholar]
- 5.Yun J, Mullarky E, Lu C, Bosch KN, Kavalier A, Rivera K, Roper J, Chio II, Giannopoulou EG, Rago C, et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science. 2015;350:1391–1396. doi: 10.1126/science.aaa5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Q, Espey MG, Krishna MC, Mitchell JB, Corpe CP, Buettner GR, Shacter E, Levine M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci USA. 2005;102:13604–13609. doi: 10.1073/pnas.0506390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Q, Espey MG, Sun AY, Lee JH, Krishna MC, Shacter E, Choyke PL, Pooput C, Kirk KL, Buettner GR, Levine M. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci USA. 2007;104:8749–8754. doi: 10.1073/pnas.0702854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Q, Espey MG, Sun AY, Pooput C, Kirk KL, Krishna MC, Khosh DB, Drisko J, Levine M. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci USA. 2008;105:11105–11109. doi: 10.1073/pnas.0804226105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takemura Y, Satoh M, Satoh K, Hamada H, Sekido Y, Kubota S. High dose of ascorbic acid induces cell death in mesothelioma cells. Biochem Biophys Res Commun. 2010;394:249–253. doi: 10.1016/j.bbrc.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Du J, Martin SM, Levine M, Wagner BA, Buettner GR, Wang SH, Taghiyev AF, Du C, Knudson CM, Cullen JJ. Mechanisms of ascorbate-induced cytotoxicity in pancreatic cancer. Clin Cancer Res. 2010;16:509–520. doi: 10.1158/1078-0432.CCR-09-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia L, Jia Q, Shang Y, Dong X, Li L. Vitamin C intake and risk of renal cell carcinoma: A meta-analysis. Sci Rep. 2015;5:17921. doi: 10.1038/srep17921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian W, Wang Y, Xu Y, Guo X, Wang B, Sun L, Liu L, Cui F, Zhuang Q, Bao X, et al. The hypoxia-inducible factor renders cancer cells more sensitive to vitamin C-induced toxicity. J Biol Chem. 2014;289:3339–3351. doi: 10.1074/jbc.M113.538157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burke PJ. Mitochondria, bioenergetics and apoptosis in cancer. Trends Cancer. 2017;3:857–870. doi: 10.1016/j.trecan.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 15.Lee KE, Hahm E, Bae S, Kang JS, Lee WJ. The enhanced tumor inhibitory effects of gefitinib and L-ascorbic acid combination therapy in non-small cell lung cancer cells. Oncol Lett. 2017;14:276–282. doi: 10.3892/ol.2017.6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Francesco EM, Bonuccelli G, Maggiolini M, Sotgia F, Lisanti MP. Vitamin C and Doxycycline: A synthetic lethal combination therapy targeting metabolic flexibility in cancer stem cells (CSCs) Oncotarget. 2017;8:67269–67286. doi: 10.18632/oncotarget.18428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frommel TO, Zarling EJ. Chronic inflammation and cancer: Potential role of Bcl-2 gene family members as regulators of cellular antioxidant status. Med Hypotheses. 1999;52:27–30. doi: 10.1054/mehy.1997.0621. [DOI] [PubMed] [Google Scholar]
- 18.Volkmann N, Marassi FM, Newmeyer DD, Hanein D. The rheostat in the membrane: BCL-2 family proteins and apoptosis. Cell Death Differ. 2014;21:206–215. doi: 10.1038/cdd.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raisova M, Hossini AM, Eberle J, Riebeling C, Wieder T, Sturm I, Daniel PT, Orfanos CE, Geilen CC. The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J Invest Dermatol. 2001;117:333–340. doi: 10.1046/j.0022-202x.2001.01409.x. [DOI] [PubMed] [Google Scholar]
- 20.Antonsson B, Montessuit S, Sanchez B, Martinou JC. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J Biol Chem. 2001;276:11615–11623. doi: 10.1074/jbc.M010810200. [DOI] [PubMed] [Google Scholar]
- 21.Coulter ID, Hardy ML, Morton SC, Hilton LG, Tu W, Valentine D, Shekelle PG. Antioxidants vitamin C and vitamin e for the prevention and treatment of cancer. J Gen Intern Med. 2006;21:735–744. doi: 10.1111/j.1525-1497.2006.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mata AM, Carvalho RM, Alencar MV, Cavalcante AA, Silva BB. Ascorbic acid in the prevention and treatment of cancer. Rev Assoc Med Bras (1992) 2016;62:680–686. doi: 10.1590/1806-9282.62.07.680. [DOI] [PubMed] [Google Scholar]
- 23.Frei B, Lawson S. Vitamin C and cancer revisited. Proc Natl Acad Sci USA. 2008;105:11037–11038. doi: 10.1073/pnas.0806433105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, Wesley RA, Levine M. Vitamin C pharmacokinetics: Implications for oral and intravenous use. Ann Intern Med. 2004;140:533–537. doi: 10.7326/0003-4819-140-7-200404060-00010. [DOI] [PubMed] [Google Scholar]
- 25.Monti DA, Mitchell E, Bazzan AJ, Littman S, Zabrecky G, Yeo CJ, Pillai MV, Newberg AB, Deshmukh S, Levine M. Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PLoS One. 2012;7:e29794. doi: 10.1371/journal.pone.0029794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subbarayan PR, Lima M, Ardalan B. Arsenic trioxide/ascorbic acid therapy in patients with refractory metastatic colorectal carcinoma: A clinical experience. Acta Oncol. 2007;46:557–561. doi: 10.1080/02841860601042456. [DOI] [PubMed] [Google Scholar]
- 27.Bael TE, Peterson BL, Gollob JA. Phase II trial of arsenic trioxide and ascorbic acid with temozolomide in patients with metastatic melanoma with or without central nervous system metastases. Melanoma Res. 2008;18:147–151. doi: 10.1097/CMR.0b013e3282f2a7ae. [DOI] [PubMed] [Google Scholar]
- 28.Welch JS, Klco JM, Gao F, Procknow E, Uy GL, Stockerl-Goldstein KE, Abboud CN, Westervelt P, DiPersio JF, Hassan A, et al. Combination decitabine, arsenic trioxide, and ascorbic acid for the treatment of myelodysplastic syndrome and acute myeloid leukemia: A phase I study. Am J Hematol. 2011;86:796–800. doi: 10.1002/ajh.22092. [DOI] [PubMed] [Google Scholar]
- 29.Carr A, Frei B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999;13:1007–1024. doi: 10.1096/fasebj.13.9.1007. [DOI] [PubMed] [Google Scholar]
- 30.Yang G, Yan Y, Ma Y, Yang Y. Vitamin C at high concentrations induces cytotoxicity in malignant melanoma but promotes tumor growth at low concentrations. Mol Carcinog. 2017;56:1965–1976. doi: 10.1002/mc.22654. [DOI] [PubMed] [Google Scholar]
- 31.McCarty MF, Contreras F. Increasing superoxide production and the labile iron pool in tumor cells may sensitize them to extracellular ascorbate. Front Oncol. 2014;4:249. doi: 10.3389/fonc.2014.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohwada R, Ozeki Y, Saitoh Y. High-dose ascorbic acid induces carcinostatic effects through hydrogen peroxide and superoxide anion radical generation-induced cell death and growth arrest in human tongue carcinoma cells. Free Radic Res. 2017;51:684–692. doi: 10.1080/10715762.2017.1361533. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Cruz I, Cárcamo JM, Golde DW. Caspase-8 dependent TRAIL-induced apoptosis in cancer cell lines is inhibited by vitamin C and catalase. Apoptosis. 2007;12:225–234. doi: 10.1007/s10495-006-0475-0. [DOI] [PubMed] [Google Scholar]
- 34.Hong SW, Jin DH, Hahm ES, Yim SH, Lim JS, Kim KI, Yang Y, Lee SS, Kang JS, Lee WJ, et al. Ascorbate (vitamin C) induces cell death through the apoptosis-inducing factor in human breast cancer cells. Oncol Rep. 2007;18:811–815. [PubMed] [Google Scholar]
- 35.Zou W, Yue P, Lin N, He M, Zhou Z, Lonial S, Khuri FR, Wang B, Sun SY. Vitamin C inactivates the proteasome inhibitor PS-341 in human cancer cells. Clin Cancer Res. 2006;12:273–280. doi: 10.1158/1078-0432.CCR-05-0503. [DOI] [PubMed] [Google Scholar]
- 36.Reddy VG, Khanna N, Singh N. Vitamin C augments chemotherapeutic response of cervical carcinoma HeLa cells by stabilizing P53. Biochem Biophys Res Commun. 2001;282:409–415. doi: 10.1006/bbrc.2001.4593. [DOI] [PubMed] [Google Scholar]
- 37.An SH, Kang JH, Kim DH, Lee MS. Vitamin C increases the apoptosis via up-regulation p53 during cisplatin treatment in human colon cancer cells. BMB Rep. 2011;44:211–216. doi: 10.5483/BMBRep.2011.44.3.211. [DOI] [PubMed] [Google Scholar]
- 38.Nagappan A, Park KI, Park HS, Kim JA, Hong GE, Kang SR, Lee DH, Kim EH, Lee WS, Won CK, Kim GS. Vitamin C induces apoptosis in AGS cells by down-regulation of 14-3-3σ via a mitochondrial dependent pathway. Food Chem. 2012;135:1920–1928. doi: 10.1016/j.foodchem.2012.06.050. [DOI] [PubMed] [Google Scholar]
- 39.Aumailley L, Warren A, Garand C, Dubois MJ, Paquet ER, Le Couteur DG, Marette A, Cogger VC, Lebel M. Vitamin C modulates the metabolic and cytokine profiles, alleviates hepatic endoplasmic reticulum stress, and increases the life span of Gulo-/-mice. Aging (Albany NY) 2016;8:458–483. doi: 10.18632/aging.100902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim JY, Kim D, Kim BR, Jun JS, Yeom JS, Park JS, Seo JH, Park CH, Woo HO, Youn HS, et al. Vitamin C induces apoptosis in AGS cells via production of ROS of mitochondria. Oncol Lett. 2016;12:4270–4276. doi: 10.3892/ol.2016.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the author on reasonable request.